Abstract
Background:
Pericardial fat has been associated with adverse cardiovascular outcomes through adiposity-associated inflammation and insulin resistance, which in turn are linked to cardiac dysfunction. We sought to evaluate the association between pericardial fat volume with cardiac structure and function in adults without baseline cardiovascular disease.
Methods:
We analyzed data from the Multi-Ethnic Study of Atherosclerosis (MESA). Linear regression was used to examine the association between pericardial fat volume (by cardiac CT during Exam 1; 2000–2002) with cardiac function by echocardiography, six-minute walk distance (6MWD), and symptom severity as assessed using the Kansas City Cardiomyopathy Questionnaire (KCCQ)-12 (Exam 6; 2016–2018).
Results:
Among 3,032 participants, each standard-deviation (39.3 cm3) increase in pericardial fat volume was associated with lower (worse) absolute left atrial reservoir strain (β −0.98%; 95%CI −1.29, −0.68; p<0.001), right ventricular free wall strain (β −0.75%; 95%CI −1.00, −0.51; p<0.001) and right atrial reservoir strain (β −0.59%; 95%CI −1.00, −0.19; p<0.01) after adjustment for potential confounders. Greater pericardial fat volume was associated with lower six-minute walk distances (β −5.70 m; 95%CI −10.34, −1.06; p=0.02), but not with KCCQ-12 scores or NT-proBNP after multivariable adjustment.
Conclusions:
In a population-based cohort of adults, pericardial fat volume was independently associated with subclinical atrial and right ventricular dysfunction and reduced six-minute walk distance. These distinct changes in cardiac structure and function suggests a potential mechanistic role for pericardial fat in early heart failure.
Keywords: pericardial fat, echocardiography, myocardial strain analysis, early heart failure
Graphical Abstract

Introduction:
Pericardial adipose tissue has been linked to coronary artery disease, atrial fibrillation, and abnormal cardiac structure and function[1–5]. This tissue comprises both fat superficial to the parietal pericardium (pericardial fat) and fat located between the myocardium and the visceral pericardium (epicardial fat)[6]. Epicardial fat lies in direct contact with cardiomyocytes and secretes numerous bioactive factors which have been implicated in adiposity-associated inflammation and insulin resistance[3]. This in turn contributes to myocardial fat deposition and fibrosis[5]. Epicardial adipose tissue is also metabolically active, expressing high amounts of proteins associated with lipid metabolism [3, 7].
In heart failure with preserved ejection fraction (HFpEF), pericardial adipose tissue is associated with increased cardiac filling pressures and more severe pulmonary hypertension[8]. Excessive adipose tissue around the heart has also been shown to impair ventricular filling by contributing to pericardial restraint in studies of heart failure patients[8, 9]. However, most studies of pericardial fat have included individuals with existing cardiovascular disease without using sensitive measures of cardiac dysfunction. In particular, strain imaging using speckle-tracking echocardiography is capable of detecting subclinical abnormalities in cardiac function that has yet to be applied in this context[10]. Pericardial fat is associated with an increased risk for heart failure in individuals without prior cardiac disease[11, 12], but these studies did not explore potential mechanisms. Additionally, most were performed cross-sectionally, limiting interpretations on the long-term clinical as well as cardiac structural, functional, and mechanical effects of excessive pericardial adiposity.
Therefore, we utilized data from the Multi-Ethnic Study of Atherosclerosis (MESA) to examine the association between pericardial adipose tissue volume by CT with cardiac structure and function by echocardiography. The volume of pericardial fat determined by this method has been demonstrated to approximate that of epicardial fat[2] and is independently associated with increased risk for incident heart failure[12]. We then further characterized the clinical significance of these findings by examining the association of pericardial fat with exercise capacity and heart failure symptoms. We hypothesized that increased pericardial fat volume would be independently associated with abnormal cardiac mechanics, greater symptom burden, and reduced exercise capacity.
Methods:
Study population
MESA is a multicenter prospective cohort designed to investigate the prevalence, correlates, and progression of subclinical cardiovascular disease in adults without previous clinical cardiovascular disease[13]. MESA comprises 6,814 men and women age 45–85 years old recruited from six US field sites (Baltimore, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Los Angeles County, California; New York, New York; and St. Paul, Minnesota) and followed over six Exams from 2000–2018. Standard methods were used to ascertain height, weight, body mass index (BMI), waist and hip circumference, smoking status (classified as never, former, or current), pack-years smoked, hypertension[14], diabetes[15], and metabolic syndrome[16]. Phlebotomy was performed at each Exam following a 12-hour fast. Participants provided written, informed consent and the Institutional Review Boards (IRB) at each field center approved the research. This study was determined to be exempt by the IRB at the University of Pennsylvania.
Participants were free of clinical cardiovascular disease at the time of recruitment. Standardized definitions were used to determine incident diagnoses of coronary heart disease (CHD), congestive heart failure (CHF), and atrial fibrillation (AF) (Supplement). Since right heart function may be impacted by pulmonary disease, additional covariates included spirometry and quantitated emphysema by computed tomography (CT) obtained as part of the MESA-Lung Study, which enrolled 3,965 MESA participants in 2004–2006. Spirometry testing was performed following ATS/ERS guidelines in 2004–06, 2010–12 and 2017–18 [17]. A quantitative assessment of lung density, measured as the percentage of total voxels within the lung field that fell below −950 Hounsfield units on the lung windows of cardiac CT scans was also included[18]. For those with lung measures at multiple timepoints, the reading most proximate to Exam 6 was chosen.
Exposure variable: Pericardial fat volume assessment
Pericardial fat volumes (which include both pericardial and epicardial fat) were determined using volumetric assessment of CT imaging obtained during Exam 1 (2000–2002). A subset of participants had repeated assessments at Exams 2 (2002–2004), 3 (2004–2005) and 4 (2005–2007). Image acquisition and pericardial fat volume assessment have been described in detail previously[2].
Outcome variables:
Among MESA participants, 3,032 underwent echocardiography at Exam 6 as part of the MESA-Early Heart Failure Study, an ancillary study to study the mechanisms and phenotypes of early heart failure. All study echocardiograms were performed using identical dedicated GE Vivid T8 ultrasound systems (GE Healthcare, General Electric Corp, Waukesha, WI) with 3 SC-Rs transducers (fundamental frequency 1.5–4 MHz) for M-mode, 2D, and Doppler acquisition. Participants were scanned in the left lateral decubitus position to facilitate the acquisition of clear, on-axis images as recommended by the American Society of Echocardiography (ASE).[19] A passive leg raise maneuver accompanied by 2D and Doppler imaging was also performed as part of the echo protocol. All echocardiograms were transferred from the field centers to the core laboratory in GE RAW format using a web-based PACS system (HeartIT WebPAX).
All two-dimensional, Doppler, and M-mode echocardiographic measurements were performed using GE EchoPAC software (version 201, GE Healthcare, General Electric Corp, Waukesha, WI) by two experienced research sonographers blinded to all other data. Measures of cardiac mechanics, chamber quantification and cardiac function were performed in accordance with the recommendations of the ASE[20–22]. A still image containing an overlay of the echocardiographic tracing was captured for each measurement performed and archived for review and verification by two cardiologist over-readers with expertise in echocardiography. Speckle-tracking analysis was also performed using GE EchoPAC software as described previously[23]. For ease of reporting and interpretation, all strain values were reported as absolute values (with lower absolute strain values corresponding to worse cardiac mechanics). Quality control metrics for inter- and intraobserver variability were performed on a sample of 100 studies as detailed in Supplemental Tables 1 and 2. Detailed echocardiographic methods are described in the Supplement.
Participants also completed the Kansas City Cardiomyopathy Questionnaire (KCCQ-12), a patient-reported outcome tool used to assess heart failure symptoms[24] and a subset (N=2486) underwent six-minute walk distance (6MWD) testing using a standardized protocol[25]. Log2-transformed N-terminal pro b-type natriuretic peptide (NT-proBNP) levels were obtained from fasting serum samples drawn at Exam 6 as part of the Olink Target 96 Cardiovascular III panel (Olink Proteomics, Uppsala, Sweden).
Statistical analysis
Continuous variables were expressed as means (standard deviation) for normally-distributed variables, or median with interquartile range for skewed variables. Categorical variables were expressed as frequency (percentage). To evaluate whether pericardial fat volume changed over time for participants who underwent repeated assessments of pericardial fat volume, we performed a linear mixed effects regression of pericardial fat volume over time with random slopes and intercepts.
Using linear regression, we examined the association between pericardial fat volume measured at Exam 1 (2000–2002) with cardiac function by echocardiography measured at Exam 6 (2016–2018). All models were adjusted for age, sex, race, body mass index, waist-hip ratio, smoking history (Exam 1), hypertension and atrial fibrillation status (Exam 6), FEV1/FVC and percent emphysema by CT (Exams 3–5; 2004–2011). Interactions of pericardial fat volume with baseline age, sex, and race were assessed. Multivariable models with RV parameters as dependent variables were additionally adjusted for LV ejection fraction and LV mass by echocardiography. An expanded model for pericardial fat and RV function adjusted for LV GLS, mitral E/e’ ratio, LA volume index, and LA reservoir strain. Lastly, a subset analysis was performed excluding individuals who developed AF, CHD or CHF over the follow-up period.
Linear regression was used to examine the association between Exam 1 pericardial fat volume with KCCQ-12 score, six-minute walk distance, and NT-proBNP at Exam 6 as secondary outcomes. All models were adjusted for age, sex, race, body mass index, waist-hip ratio, smoking history, FEV1/FVC, and percent emphysema. Regression analyses were performed using STATA 15.1.
Results:
Among 6,814 participants initially recruited in MESA, we included the 3,032 participants who underwent echocardiography at Exam 6 (Supplemental Table 3). At baseline, this cohort was 53% female, with mean age 57 years and 40% White, 25% Black or African American, 22% Hispanic, and 13% Asian. Compared to the excluded group, our cohort was younger, with lower baseline prevalence of hypertension, diabetes and metabolic syndrome and lower pericardial fat volume. Race, gender, BMI and waist-hip ratio were similar between the two groups. All participants were free of cardiovascular disease at Exam 1, but by Exam 6, 398 (13.1%) participants had AF, 75 (2.5%) had CHF and 198 (6.5%) had CHD.
Baseline (Exam 1) pericardial fat volume (mean 73.6 cm3, standard deviation 39.3 cm3) was positively correlated with BMI, though there was substantial variation among those with high BMI (Supplemental Figure 1). Those in the highest quartile of pericardial fat were predominantly male, older, with greater proportion of White and Hispanic participants, higher BMI and greater prevalence of baseline cardiovascular risk factors (Table 1). Among the 3032 participants, there were 6371 observations of pericardial fat volume between Exams 1–4 (average 2.1 observations per participant). Over time, mean pericardial fat volume increased by 1.19 cm3 per year (95% CI 1.03 to 1.36, p<0.001). Among those with multiple measurements of pericardial fat volume, the within-individual intraclass correlation (ICC) was high (ICC 0.95, 95% CI 0.94 to 0.95). Additional baseline participant characteristics and echocardiography measurements are summarized in Tables 1 and 2.
Table 1:
Cohort Characteristics
| Pericardial fat volume | ||||
|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | |
|
| ||||
| N | 758 | 758 | 758 | 758 |
| Pericardial fat volume, cm3 | 38.7 (31.8, 44.4) | 58.7 (54.1, 63.4) | 79.7 (73.8, 86.8) | 120.3 (105.5, 145.4) |
| Age, years | 54.0 (49.0, 61.0) | 56.5 (51.0, 64.0) | 58.0 (51.0, 66.0) | 59.0 (53.0, 66.0) |
| Female sex | 514 (67.8%) | 461 (60.8%) | 398 (52.5%) | 233 (30.7%) |
| Race/Ethnicity | ||||
| White | 296 (39.1%) | 275 (36.3%) | 288 (38.0%) | 348 (45.9%) |
| Asian | 83 (10.9%) | 129 (17.0%) | 130 (17.2%) | 65 (8.6%) |
| Black or African American | 274 (36.1%) | 215 (28.4%) | 156 (20.6%) | 116 (15.3%) |
| Hispanic | 105 (13.9%) | 139 (18.3%) | 184 (24.3%) | 229 (30.2%) |
| Body mass index, kg/m2 | 25.2 (4.3) | 27.2 (4.8) | 28.9 (5.0) | 31.6 (5.2) |
| Waist-hip ratio | 0.9 (0.1) | 0.9 (0.1) | 0.9 (0.1) | 1.0 (0.1) |
| Diabetes status | ||||
| Normal | 677 (89.3%) | 634 (83.6%) | 578 (76.3%) | 514 (67.8%) |
| Impaired fasting glucose | 43 (5.7%) | 76 (10.0%) | 104 (13.7%) | 144 (19.0%) |
| Diabetes (untreated) | 2 (0.3%) | 15 (2.0%) | 19 (2.5%) | 24 (3.2%) |
| Diabetes (treated) | 31 (4.1%) | 30 (4.0%) | 56 (7.4%) | 73 (9.6%) |
| Missing/unknown | 5 (0.7%) | 3 (0.4%) | 1 (0.1%) | 3 (0.4%) |
| Hypertension | 186 (24.5%) | 242 (31.9%) | 285 (37.6%) | 341 (45.0%) |
| Metabolic syndrome | 84 (11.1%) | 182 (24.0%) | 269 (35.5%) | 378 (49.9%) |
| FEV1, L | 2.20 (0.63) (n=740) | 2.15 (0.70) (n=739) | 2.16 (0.69) (n=729) | 2.27 (0.73) (n=726) |
| FVC, L | 3.00 (0.85) (n=740) | 2.94 (0.92) (n=738) | 2.92 (0.94) (n=729) | 3.11 (0.95) (n=726) |
| FEV1/FVC | 73.8 (8.1) (n=739) | 73.5 (8.9) (n=737) | 74.4 (8.5) (n=728) | 73.3 (8.9) (n=725) |
| Percent emphysema, % | 0.5 (0.1, 1.8) (n=758) | 0.5 (0.0, 2.0) (n=758) | 0.6 (0.1, 2.1) (n=758) | 0.6 (0.1, 2.2) (n=758) |
| Clinical events over follow-up period | ||||
| Atrial Fibrillation | 68 (9.0%) | 94 (12.4%) | 104 (13.7%) | 132 (17.4%) |
| Coronary Heart Disease | 16 (2.1%) | 23 (3.0%) | 24 (3.2%) | 37 (4.9%) |
| Congestive Heart Failure | 6 (0.8%) | 17 (2.2%) | 19 (2.5%) | 33 (4.4%) |
Table 2:
Echocardiogram Measurements
| N | 3032 |
|---|---|
|
| |
| Left ventricular parameters | |
| Global longitudinal strain, % (N=2765) | 20.0 (18.2, 21.7) |
| Ejection fraction, % (N=3032) | 63.0 (60.0, 66.0) |
| Mass index, g/m2 (N=3029) | 80.3 (68.6, 95.8) |
| Mitral E/A ratio (N=2886) | 0.9 (0.7, 1.1) |
| Average E/e’ ratio (N=2944) | 9.5 (7.8, 11.7) |
| Left atrial parameters | |
| Volume index, ml/m2 (N=2993) | 26.9 (22.4, 32.4) |
| Reservoir strain, % (N=2905) | 27.0 (23.0, 30.9) |
| Right ventricular parameters | |
| Free wall strain, % (N=2904) | 24.7 (21.4, 28.3) |
| Tricuspid annular plane systolic excursion, cm (N=3026) | 2.1 (1.9, 2.3) |
| Estimated pulmonary artery systolic pressure, mmHg (N=2382) | 32.2 (28.4, 37.0) |
| Fractional area change, % (N=3027) | 39.9 (37.2, 42.9) |
| Free wall s’ peak velocity, cm/s (N=2916) | 13.7 (11.9, 15.8) |
| Ratio of left ventricular and right ventricular end diastolic areas (N=3027) | 1.5 (1.3, 1.6) |
| Tricuspid E/A ratio (N=2848) | 1.2 (1.0, 1.4) |
| Tricuspid E/e’ ratio (N=2815) | 4.0 (3.3, 4.9) |
| Right atrial parameters | |
| End systolic area, cm2 (N=3027) | 16.3 (13.8, 19.0) |
| Reservoir strain, % (N=2940) | 32.1 (26.7, 38.0) |
For ease of reporting and interpretation, all strain values were reported as absolute values (with lower absolute strain values corresponding to worse cardiac mechanics).
In univariate analysis, higher pericardial fat volume at baseline was associated with lower (worse) absolute LV global longitudinal strain and LA reservoir strain, in addition to other indices of LV systolic and diastolic function (Table 3). The association of pericardial fat volume with left atrial (LA) reservoir strain, and greater LV mass persisted, but was attenuated by adjustment for age, sex, race, site, body mass index, waist-hip ratio, smoking status, pack-years smoked, FEV1/FVC, percent emphysema, hypertension and atrial fibrillation status (Figure 1A & Table 3). Pericardial fat volume was associated with greater mitral E/e’ ratio, lower e’ velocity, and lower LA reservoir strain following a preload challenge via passive leg-raise maneuver (Table 3).
Table 3:
Association between pericardial fat volume and left heart parameters
| Univariable β (95% CI) | Multivariable β (95% CI) | |
|---|---|---|
|
| ||
| LV parameters | ||
| Global longitudinal strain, % (N=2621) | −0.55 (−0.67, −0.42)*** | −0.10 (−0.26, 0.05) |
| Ejection fraction, % (N=2872) | −0.56 (−0.76, −0.36)*** | 0.04 (−0.22, 0.29) |
| E/A ratio (N=2736) | −0.02 (−0.03, −0.01)** | −0.01 (−0.02, 0.01) |
| E/e’ ratio (N=2790) | 0.51 (0.36, 0.65)*** | 0.16 (−0.01, 0.33) |
| Mitral e’ velocity, m/s (N=2840) | −0.23 (−0.30, −0.16)*** | −0.02 (−0.11, 0.07) |
| LV mass, grams (N=2870) | 17.63 (15.95, 19.32)*** | 3.80 (1.71, 5.89)*** |
| LA parameters | ||
| LA volume, cm3 (N=2837) | 1.17 (0.82, 1.52)*** | 1.14 (0.33, 1.95)** |
| LA reservoir strain, % (N=2755) | −1.54 (−1.77, −1.30)*** | −0.98 (−1.29, −0.68)*** |
| Preload challenge | ||
| Global longitudinal strain, % (N=2486) | −0.45 (−0.57, −0.32)*** | −0.06 (−0.23, 0.11) |
| E/A ratio (N=2727) | −0.03 (−0.04, −0.02)*** | −0.01 (−0.02, 0.01) |
| E/e’ ratio (N=2772) | 0.34 (0.22, 0.46)*** | 0.24 (0.09, 0.39)** |
| Mitral e’ velocity, m/s (N=2794) | −0.34 (−0.41, −0.26)*** | −0.20 (−0.29, −0.10)*** |
| LA reservoir strain, % (N=2697) | −1.94 (−2.24, −1.65)*** | −1.30 (−1.69, −0.91)*** |
For ease of reporting and interpretation, all strain values were reported as absolute values (with lower absolute strain values corresponding to worse cardiac mechanics). β coefficients are presented as the mean change in the outcome variable per standard-deviation increase in pericardial fat volume (1sd = 39.3 cm3). Multivariable models are adjusted age, sex, race, site, body mass index, waist-hip ratio, smoking status, pack-years smoked, FEV1/FVC, percent emphysema, hypertension and atrial fibrillation status. For preload challenge, indices were measured following a passive leg-raise maneuver
p < 0.05
p < 0.01
p < 0.001.
Figure 1:
Association between pericardial fat volume with percent absolute left atrial reservoir, with lower values indicating worse function. The model is adjusted for age, sex, race, site, body mass index, waist-hip ratio, smoking status, pack-years smoked, FEV1/FVC, percent emphysema, hypertension and atrial fibrillation status.
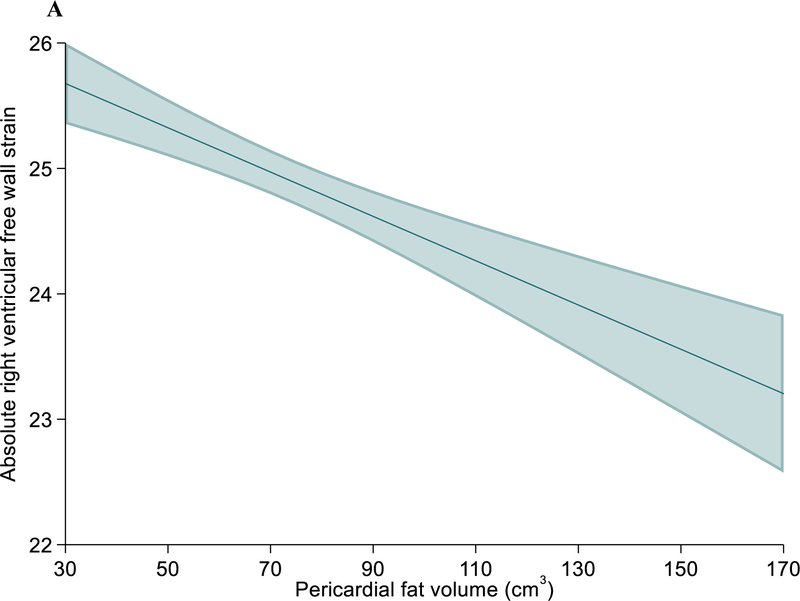
Increased pericardial fat volume was also associated with lower (worse) absolute RV free wall and right atrial (RA) reservoir strain (Table 4) in univariate analysis, which persisted, but was somewhat attenuated in multivariable models (Figure 2A & 2B). Pericardial fat volume was also associated with lower tricuspid annular plane systolic excursion (TAPSE), fractional area change (FAC) and RV s’ velocity (Table 4). These associations were largely unchanged after further adjusting for pulmonary artery systolic pressure, LV strain, E/e’ ratio, LA volume, and LA strain (Supplemental Table 4). Associations between pericardial fat volume with lower LA reservoir, RV free wall, and RA reservoir strain remained significant after excluding individuals with AF, CHD, and CHF (Supplemental Table 5). There were no significant interactions of pericardial fat volume by age, sex or race/ethnicity on any of the echo parameters tested.
Table 4:
Association between pericardial fat volume and right heart parameters
| Univariable β (95% CI) | Multivariable β (95% CI) | |
|---|---|---|
|
| ||
| RV systolic function | ||
| Free wall strain, % (N=2751) | −1.33 (−1.52, −1.14)*** | −0.69 (−0.94, −0.45)*** |
| TAPSE, cm (N=2863) | −0.03 (−0.04, −0.01)*** | −0.05 (−0.07, −0.03)*** |
| PASP, mmHg (N=2257) | 0.84 (0.51, 1.16)*** | 0.27 (−0.15, 0.69) |
| RV FAC, % (N=2864) | −0.72 (−0.88, −0.56)*** | −0.24 (−0.45, −0.03)* |
| RV s’ velocity, m/s (N=2766) | 0.01 (−0.11, 0.13) | −0.17 (−0.32, −0.02)** |
| LV:RV ratio (N=2864) | −0.03 (−0.04, −0.02)*** | −0.01 (−0.03, −0.001)* |
| RV diastolic function | ||
| Tricuspid E/A ratio (N=2701) | −0.04 (−0.05, −0.03)*** | −0.01 (−0.02, 0.01) |
| Tricuspid E/e’ ratio (N=2671) | 0.24 (0.18, 0.30)*** | 0.20 (0.12, 0.27)*** |
| RV e’ velocity, m/s (N=2751) | −0.23 (−0.36, −0.10)** | −0.21 (−0.37, −0.04)* |
| RA parameters | ||
| RA end-systolic area, cm3 (N=2864) | 1.12 (0.98, 1.27)*** | 0.38 (0.20, 0.56)*** |
| RA reservoir strain, % (N=2787) | −1.50 (−1.81, −1.20)*** | −0.59 (−1.00, −0.19)** |
For ease of reporting and interpretation, all strain values were reported as absolute values (with lower absolute strain values corresponding to worse cardiac mechanics). β coefficients are presented as the mean change in the outcome variable per standard-deviation increase in pericardial fat volume (1sd = 39.3 cm3). Multivariable models are adjusted age, sex, race, site, body mass index, waist-hip ratio, smoking status, pack-years smoked, FEV1/FVC, percent emphysema, LVEF and LV mass by echo, hypertension and atrial fibrillation status
p < 0.05
p < 0.01
p < 0.001
Figure 2:
Association between pericardial fat volume with percent absolute right ventricular free wall strain (A) and right atrial reservoir strain (B), with lower values indicating worse function. The model is adjusted for age, sex, race, site, body mass index, waist-hip ratio, smoking status, pack-years smoked, FEV1/FVC, percent emphysema, LVEF and LV mass by echo, hypertension and atrial fibrillation status.
Finally, increased pericardial fat volume was associated with lower (worse) KCCQ-12 (β per 1-SD change = −1.30, 95%CI −1.79 to −0.81, p <0.001) and higher NT-proBNP (β = 0.16, 95%CI 0.11 to 0.21, p <0.001) in univariate analysis, which were no longer significant in multivariate models adjusting for age, sex, race, site, body mass index, waist-hip ratio, smoking status, pack-years smoked, FEV1/FVC, percent emphysema, hypertension and atrial fibrillation status. NT-proBNP assay values from the Olink III assay were reported as log2-transformed, such that each standard deviation increase in pericardial fat volume was associated with a 12% increase in NT-proBNP (20.16 = 1.12). In the subset of participants who underwent walk testing, increased pericardial fat volume was associated with decreased 6MWD in both univariate (β = −19.96 m, 95%CI −23.86, −16.06, p <0.001) and multivariable-adjusted analyses (β = −5.70 m, 95%CI −10.34, −1.06, p=0.02; Figure 3).
Figure 3:
Association between pericardial fat volume with six-minute walk distance. The model is adjusted for age, sex, race, site, body mass index, waist-hip ratio, smoking status, pack-years smoked, FEV1/FVC, percent emphysema, hypertension and atrial fibrillation status.
Discussion:
In this study of a large, racially/ethnically diverse cohort of individuals, we utilized comprehensive echocardiography to identify changes in cardiac structure and function associated with excessive epicardial fat. Greater pericardial fat volume was independently associated with worse cardiac mechanics and lower exercise capacity after nearly twenty years of follow-up, which persisted after adjustment for potential confounders including overall obesity, fat distribution, lung function and other cardiovascular disease including atrial fibrillation. Pericardial fat volume remained associated with RV function after adjusting for indices of LV function, suggesting that the relationship with RV structure and function was not wholly secondary to LV morphology. While effect sizes we observed were small, we showed that increased pericardial fat was associated with sensitive and clinically-relevant indices of atrial and ventricular function which could be used to identify patients at risk for developing heart failure or to identify patients for therapies to reduce pericardial adipose tissue and associated inflammation[26].
Within MESA, pericardial fat volume is associated with increased risk of incident heart failure[12, 27]. Additionally, cross-sectional studies of pericardial fat volume with cardiac structure show an association with greater LV and RV mass [11, 28]. In our study, we found that pericardial fat volume remained associated with increased LV mass independent of BMI and fat distribution (waist-hip ratio). Additionally, pericardial fat volume remained negatively associated with LV and RV function even when restricted to individuals without CHD, CHF, or AF. In the LV, this manifested in higher E/e’ ratio and greater LA volume, suggesting higher left sided filling pressures. Associations between pericardial fat volume with worse diastolic function and LA strain were magnified following an intravascular volume challenge. This is especially compelling, as changes in LA strain in response to passive leg-raise are a distinguishing feature in HFpEF[29]. This overall pattern suggests that the effect of pericardial fat on the LV manifests primarily as diastolic dysfunction, which has been demonstrated in other studies[30, 31] and is consistent with the role of adiposity and metabolic dysfunction in HFpEF[32, 33].
In contrast, pericardial fat volume was associated with both systolic and diastolic dysfunction in the RV. In addition to standard metrics of RV function such as TAPSE and fractional area change (FAC), we also found that greater pericardial fat volume was associated with reduced RV strain. These associations also remained significant in models which further adjusted for PASP, LV strain, E/e’ ratio, LA volume, and LA strain, indicating that not all of the effect observed on the RV can be completely explained by RV loading or left-sided dysfunction. RV adaptation to disease is unique to that of the LV, owing to the differences in structure and contractile function between the two[34]. These differences may explain why we observed changes in both RV diastolic and systolic function compared to the LV. The location of epicardial fat, which primarily overlies the RV and may exert more direct paracrine effects on RV myocardium, may also help explain these findings.
Epicardial fat has been linked to atrial conduction abnormalities[35] and AF[1] by promoting cardiac remodeling and fibrosis. These same mechanisms may contribute to impaired atrial function. In our cohort, we found that pericardial fat volume also associated with decreased LA and RA strain and increased atrial volumes, which persisted even after adjusting for the presence of AF and in sensitivity analyses restricting those with cardiovascular disease, including AF. Atrial function and size may be impacted by elevated atrial pressures, which may explain some of our findings. Since atrial strain imaging is emerging as a prognostic indicator in diseases such as HFpEF[23] and pulmonary hypertension[36], and disproportionate atrial dysfunction contributes to unique pathophysiology in HFpEF[37], further studies into the effect of epicardial fat on atrial function are warranted, particularly in those populations.
Greater pericardial fat volume was also associated with a small, but significant decrease in 6MWD in both univariate and multivariable-adjusted analyses, suggesting that the observed cardiac structural and functional changes may lead to subtle changes in exercise capacity even after accounting for potential confounders such as age, body habitus, lung function, and atrial fibrillation. Pericardial fat volume was also associated with lower KCCQ-12 scores, indicating more heart failure symptoms, and higher NT-proBNP in univariate analyses, though these associations were not significant in multivariable models. The KCCQ-12 questionnaire was developed to describe and monitor symptom burden in heart failure patients and may lack the sensitivity to detect small differences in a population with mostly subclinical disease. Similarly, NT-proBNP may also not be significantly elevated in subclinical disease and might be further attenuated in patients with excess adipose tissue due to increased natriuretic peptide clearance and reduced secretion[38].
Strengths and limitations
This is the largest study of pericardial fat volume and echocardiographic indices of cardiac function to date. Our study incorporated both atrial and ventricular strain imaging, which have greater sensitivity to detect subclinical cardiac dysfunction. Additionally, the MESA cohort is ethnically diverse and is highly generalizable to the adult population in the United States. While many studies have demonstrated an association among patients with clinical cardiovascular disease, we show that this relationship is also observed in a cohort which is predominantly free of cardiovascular disease, suggesting that excessive pericardial adipose tissue is associated with a spectrum of cardiovascular dysfunction ranging from subclinical abnormalities to clinically-evident disease. The association between pericardial fat with cardiac mechanics after nearly twenty years of follow-up suggests that excess pericardial fat may be an early risk factor and a potential target for intervention.
There were several limitations to our study. Due to the lack of echocardiographic measurements for ventricular interdependence or invasive hemodynamics in our cohort, we do not know as to the role of pericardial restraint in our findings. MESA participants who died or dropped out prior to Exam 6 could not be analyzed, thus there is a potential for differential drop-out and selection bias favoring healthier participants than the general population, as evidenced by the differences in baseline characteristics between our cohort and the excluded participants. Finally, due to the time gap between exposure and outcome measures, unmeasured or residual confounding was possible. A longitudinal study incorporating multiple pericardial fat and echo measurements over time would help clarify causal relationships.
Future directions
Our study adds to the existing body of literature suggesting that excessive pericardial fat is not merely associated with cardiovascular disease risk, but contributes to the development of disease itself by highlighting the associated cardiac structural and functional changes seen on echocardiography. Pericardial fat, increased inflammation, and metabolic dysfunction play a central role in the “metabolic HFpEF phenotype”[39] and further studies are warranted to help understand the direct mechanisms by which epicardial adipose tissue impacts atrial and ventricular function and remodeling. Additionally, identifying individuals with excessive pericardial fat could further risk stratify patients and identify potential candidates for intervention. Pericardial fat volume may decrease with intensive dietary and lifestyle modification or bariatric surgery[40, 41]. Newer classes of antidiabetic therapies, such as sodium-glucose cotransporter (SGLT)-2 inhibitors and glucagon-like peptide (GLP)-1 agonists have also shown promise in decreasing pericardial fat volume[41]. Finally, among individuals in whom excessive pericardial fat volume impairs diastolic filling within the limited pericardial space, pericardiotomy has also been proposed as a therapeutic option[9].
Conclusion
In this large, diverse cohort of elderly adults free of baseline cardiovascular disease, pericardial fat volume was associated with distinct atrial and ventricular abnormalities by echocardiography after nearly twenty years of follow-up and remained independently associated with cardiac function after adjusting for overall adiposity (i.e., BMI and waist-hip ratio) and other potential confounders. These distinct changes in cardiac structure and function are accompanied by decreased 6MWD, suggesting a potential mechanistic role for pericardial fat in early heart failure.
Supplementary Material
Highlights.
Speckle tracking strain applied to 3032 elderly participants in MESA cohort
Pericardial fat volume associated with worse biatrial and right ventricular strain
Pericardial fat volume associated with lower six-minute walk distances
Pericardial fat worsens cardiac mechanics and may result in early heart failure
Acknowledgements:
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org
Funding:
This research was supported by contracts 75N92020D00001, HHSN268201500003I, N01-HC-95159, 75N92020D00005, N01-HC-95160, 75N92020D00002, N01-HC-95161, 75N92020D00003, N01-HC-95162, 75N92020D00006, N01-HC-95163, 75N92020D00004, N01-HC-95164, 75N92020D00007, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences (NCATS). Ancillary studies were funded by grants R01-HL127028, R01-HL086719, R01-HL077612, and R01-HL075476.
Individual funding:
K24-HL103844 (SK); T32-HL007891 (JM); R01 HL107577, R01 HL127028, R01 HL140731, and R01 HL149423 (SJS)
Footnotes
Disclosures: SJS has received consulting fees from Abbott, Actelion, AstraZeneca, Amgen, Axon Therapies, Bayer, Boehringer-Ingelheim, Bristol-Myers Squibb, Cardiora, CVRx, Cytokinetics, Eidos, Eisai, GSK, Ionis, Ironwood, Lilly, Merck, MyoKardia, Novartis, Novo Nordisk, Pfizer, Prothena, Sanofi, Shifamed, Tenax, and United Therapeutics. The remaining authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- [1].Al-Rawahi M, Proietti R, Thanassoulis G. Pericardial fat and atrial fibrillation: Epidemiology, mechanisms and interventions. Int J Cardiol. 2015;195:98–103. [DOI] [PubMed] [Google Scholar]
- [2].Ding J, Hsu F-C, Harris TB, Liu Y, Kritchevsky SB, Szklo M, et al. The association of pericardial fat with incident coronary heart disease: the Multi-Ethnic Study of Atherosclerosis (MESA). The American Journal of Clinical Nutrition. 2009;90:499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gaborit B, Sengenes C, Ancel P, Jacquier A, Dutour A. Role of Epicardial Adipose Tissue in Health and Disease: A Matter of Fat? Compr Physiol. 2017;7:1051–82. [DOI] [PubMed] [Google Scholar]
- [4].Iacobellis G Local and systemic effects of the multifaceted epicardial adipose tissue depot. Nature Reviews Endocrinology. 2015;11:363–71. [DOI] [PubMed] [Google Scholar]
- [5].Ng ACT, Strudwick M, Van Der Geest RJ, Ng ACC, Gillinder L, Goo SY, et al. Impact of Epicardial Adipose Tissue, Left Ventricular Myocardial Fat Content, and Interstitial Fibrosis on Myocardial Contractile Function. Circulation: Cardiovascular Imaging. 2018;11. [DOI] [PubMed] [Google Scholar]
- [6].Ong K-L, Ding J, McClelland RL, Cheung BMY, Criqui MH, Barter PJ, et al. Relationship of pericardial fat with lipoprotein distribution: The Multi-Ethnic study of atherosclerosis. Atherosclerosis. 2015;241:664–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Furuhashi M Fatty Acid-Binding Protein 4 in Cardiovascular and Metabolic Diseases. Journal of Atherosclerosis and Thrombosis. 2019;26:216–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Koepp KE, Obokata M, Reddy YNV, Olson TP, Borlaug BA. Hemodynamic and Functional Impact of Epicardial Adipose Tissue in Heart Failure With Preserved Ejection Fraction. JACC: Heart Failure. 2020;8:657–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Borlaug BA, Reddy YNV. The Role of the Pericardium in Heart Failure. JACC: Heart Failure. 2019;7:574–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Blessberger H, Binder T. NON-invasive imaging: Two dimensional speckle tracking echocardiography: basic principles. Heart. 2010;96:716–22. [DOI] [PubMed] [Google Scholar]
- [11].Shah RV, Anderson A, Ding J, Budoff M, Rider O, Petersen SE, et al. Pericardial, But Not Hepatic, Fat by CT Is Associated With CV Outcomes and Structure. JACC: Cardiovascular Imaging. 2017;10:1016–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kenchaiah S, Ding J, Carr JJ, Allison MA, Budoff MJ, Tracy RP, et al. Pericardial Fat and the Risk of Heart Failure. Journal of the American College of Cardiology. 2021;77:2638–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–81. [DOI] [PubMed] [Google Scholar]
- [14].The Sixth Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Archives of Internal Medicine. 1997;157:2413–46. [DOI] [PubMed] [Google Scholar]
- [15].Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2004;27:S5–S10. [DOI] [PubMed] [Google Scholar]
- [16].Grundy SM, Brewer HB, Cleeman JI, Smith SC, Lenfant C. Definition of Metabolic Syndrome. Circulation. 2004;109:433–8. [DOI] [PubMed] [Google Scholar]
- [17].Hankinson JL, Kawut SM, Shahar E, Smith LJ, Stukovsky KH, Barr RG. Performance of American Thoracic Society-Recommended Spirometry Reference Values in a Multiethnic Sample of Adults. Chest. 2010;137:138–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hoffman EA, Jiang R, Baumhauer H, Brooks MA, Carr JJ, Detrano R, et al. Reproducibility and Validity of Lung Density Measures from Cardiac CT Scans—The Multi-Ethnic Study of Atherosclerosis (MESA) Lung Study1. Academic Radiology. 2009;16:689–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Mitchell C, Rahko PS, Blauwet LA, Canaday B, Finstuen JA, Foster MC, et al. Guidelines for Performing a Comprehensive Transthoracic Echocardiographic Examination in Adults: Recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr. 2019;32:1–64. [DOI] [PubMed] [Google Scholar]
- [20].Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, et al. Guidelines for the Echocardiographic Assessment of the Right Heart in Adults: A Report from the American Society of Echocardiography. Journal of the American Society of Echocardiography. 2010;23:685–713. [DOI] [PubMed] [Google Scholar]
- [21].Mor-Avi V, Lang RM, Badano LP, Belohlavek M, Cardim NM, Derumeaux G, et al. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. J Am Soc Echocardiogr. 2011;24:277–313. [DOI] [PubMed] [Google Scholar]
- [22].Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Journal of the American Society of Echocardiography. 2015;28:1–39.e14. [DOI] [PubMed] [Google Scholar]
- [23].Freed BH, Daruwalla V, Cheng JY, Aguilar FG, Beussink L, Choi A, et al. Prognostic Utility and Clinical Significance of Cardiac Mechanics in Heart Failure With Preserved Ejection Fraction: Importance of Left Atrial Strain. Circ Cardiovasc Imaging. 2016;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35:1245–55. [DOI] [PubMed] [Google Scholar]
- [25].Laboratories ATSCoPSfCPF. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–7. [DOI] [PubMed] [Google Scholar]
- [26].Packer M Drugs That Ameliorate Epicardial Adipose Tissue Inflammation May Have Discordant Effects in Heart Failure With a Preserved Ejection Fraction as Compared With a Reduced Ejection Fraction. J Card Fail. 2019;25:986–1003. [DOI] [PubMed] [Google Scholar]
- [27].Kenchaiah S, Ding J, Carr JJ, Allison MA, Budoff MJ, Tracy RP, et al. Pericardial Fat and the Risk of Heart Failure. Journal of the American College of Cardiology. 2021;77:2638–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wenger DS, Kawut SM, Ding J, Bluemke DA, Hough CL, Kronmal RA, et al. Pericardial Fat and Right Ventricular Morphology: The Multi-Ethnic Study of Atherosclerosis- Right Ventricle Study (MESA-RV). 2016;11:e0157654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Obokata M, Negishi K, Kurosawa K, Arima H, Tateno R, Ui G, et al. Incremental Diagnostic Value of LA Strain With Leg Lifts in Heart Failure With Preserved Ejection Fraction. JACC: Cardiovascular Imaging. 2013;6:749–58. [DOI] [PubMed] [Google Scholar]
- [30].Cavalcante JL, Tamarappoo BK, Hachamovitch R, Kwon DH, Alraies MC, Halliburton S, et al. Association of Epicardial Fat, Hypertension, Subclinical Coronary Artery Disease, and Metabolic Syndrome With Left Ventricular Diastolic Dysfunction. The American Journal of Cardiology. 2012;110:1793–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Fontes-Carvalho R, Fontes-Oliveira M, Sampaio F, Mancio J, Bettencourt N, Teixeira M, et al. Influence of epicardial and visceral fat on left ventricular diastolic and systolic functions in patients after myocardial infarction. Am J Cardiol. 2014;114:1663–9. [DOI] [PubMed] [Google Scholar]
- [32].Shah SJ, Kitzman DW, Borlaug BA, Van Heerebeek L, Zile MR, Kass DA, et al. Phenotype-Specific Treatment of Heart Failure With Preserved Ejection Fraction. Circulation. 2016;134:73–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Paulus WJ, Dal Canto E. Distinct Myocardial Targets for Diabetes Therapy in Heart Failure With Preserved or Reduced Ejection Fraction. JACC: Heart Failure. 2018;6:1–7. [DOI] [PubMed] [Google Scholar]
- [34].Sanz J, Sánchez-Quintana D, Bossone E, Bogaard HJ, Naeije R. Anatomy, Function, and Dysfunction of the Right Ventricle. Journal of the American College of Cardiology. 2019;73:1463–82. [DOI] [PubMed] [Google Scholar]
- [35].Nalliah CJ, Bell JR, Raaijmakers AJA, Waddell HM, Wells SP, Bernasochi GB, et al. Epicardial Adipose Tissue Accumulation Confers Atrial Conduction Abnormality. Journal of the American College of Cardiology. 2020;76:1197–211. [DOI] [PubMed] [Google Scholar]
- [36].Richter MJ, Fortuni F, Wiegand MA, Dalmer A, Vanderpool R, Ghofrani HA, et al. Association of right atrial conduit phase with right ventricular lusitropic function in pulmonary hypertension. The International Journal of Cardiovascular Imaging. 2020;36:633–42. [DOI] [PubMed] [Google Scholar]
- [37].Patel RB, Lam CSP, Svedlund S, Saraste A, Hage C, Tan R-S, et al. Disproportionate left atrial myopathy in heart failure with preserved ejection fraction among participants of the PROMIS-HFpEF study. Scientific Reports. 2021;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Krauser DG, Lloyd-Jones DM, Chae CU, Cameron R, Anwaruddin S, Baggish AL, et al. Effect of body mass index on natriuretic peptide levels in patients with acute congestive heart failure: A ProBNP Investigation of Dyspnea in the Emergency Department (PRIDE) substudy. American Heart Journal. 2005;149:744–50. [DOI] [PubMed] [Google Scholar]
- [39].Salah HM, Pandey A, Soloveva A, Abdelmalek MF, Diehl AM, Moylan CA, et al. Relationship of Nonalcoholic Fatty Liver Disease and Heart Failure With Preserved Ejection Fraction. JACC: Basic to Translational Science. 2021;6:918–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Rabkin SW, Campbell H. Comparison of reducing epicardial fat by exercise, diet or bariatric surgery weight loss strategies: a systematic review and meta-analysis. Obes Rev. 2015;16:406–15. [DOI] [PubMed] [Google Scholar]
- [41].Launbo N, Zobel EH, Scholten BJ, Færch K, Jørgensen PG, Christensen RH. Targeting epicardial adipose tissue with exercise, diet, bariatric surgery or pharmaceutical interventions: A systematic review and meta-analysis. Obesity Reviews. 2021;22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.