Abstract
Objective:
Cognitive remediation approaches for early course schizophrenia are promising interventions for improving social adjustment. Premorbid sociality is a potentially important moderator of social adjustment response to cognitive remediation and may serve to personalize such interventions.
Method:
Eighty-eight early course schizophrenia outpatients with premorbid sociality scores were included in this preliminary investigation. Secondary data came from a recent 18-month multi-site confirmatory trial of Cognitive Enhancement Therapy (CET) compared to Enriched Supportive Therapy (EST). Intent-to-treat mixed effects models examined the moderating effect of premorbid sociality assessed at baseline on differential social adjustment change between CET and EST assessed at baseline, 9, and 18 months.
Results:
Premorbid sociality significantly moderated the differential effect of CET vs. EST on overall social adjustment change at 18 months, such that CET was particularly effective for patients with high premorbid sociality and EST for low premorbid sociality. This significant group x time x premorbid sociality interaction was also observed for 18-month change in interpersonal anguish, self-care, and sexual relations. Again, CET was largely favorable for higher premorbid sociality patients and EST for lower premorbid sociality for these sub-scales.
Conclusions:
The results provide initial evidence that premorbid sociality moderates differential social adjustment change during cognitive remediation in early course schizophrenia. In many, but not all cases, better social functioning prior to the development of schizophrenia was associated with a significantly better social adjustment response to CET. Data on social functioning during childhood and adolescence is possibly useful for personalizing treatment planning in the early course of schizophrenia.
Keywords: early course schizophrenia, premorbid sociality, social adjustment, Cognitive Enhancement Therapy
Schizophrenia is a complex neurodevelopmental disorder with a typical onset during late adolescence or early adulthood. Positive, negative, and cognitive symptoms are often accompanied by difficulties in functional outcomes that can lead to relapses, hospitalizations, and unsatisfactory quality of life (Eack & Newhill, 2007; Kalin et al., 2015). Cognitive remediation is one of the most promising interventions for targeting cognitive and functional recovery in the condition, and has evolved and improved over the last three decades. These interventions have shown efficacy for improving cognition and social functioning, and when applied alongside broader psychosocial treatments, the gains are reliably larger for functional recovery (McGurk et al., 2007; Revell et al., 2015; Vita et al., 2021; Wykes et al., 2011).
In order to maximize the positive outcomes and personalization of cognitive remediation, understanding the impact of heterogeneity in schizophrenia on presentation and trajectory during treatment is important. Identification of moderators in the scope of intervention research has the potential to reveal how levels of an individual’s baseline characteristics (e.g., premorbid social functioning) can influence the overall effect that treatment will have on that individual (Kraemer et al., 2002). Indeed, moderators of cognitive remediation response in schizophrenia have recently gained attention (Seccomandi et al., 2020, 2021) and are considered critical to the personalized medicine agenda outlined by the National Institute of Mental Health (Insel, 2009). In line with the advancement of cognitive remediation for schizophrenia, this is the first investigation of a potential premorbid functioning moderator during Cognitive Enhancement Therapy (CET) for early course schizophrenia. CET is a recovery-phase approach to cognitive remediation, incorporating strategy coaching and social-cognitive groups with more traditional neurocognitive computer training (Hogarty & Greenwald, 2006).
The clinical profile of individuals with schizophrenia varies widely and several factors are associated with course, symptom, and outcome, all of which could impact individual response to treatment. For example, a strong and established relationship exists in people with schizophrenia between lower social functioning during childhood and adolescence, as measured by premorbid sociality, and poorer social adjustment to the condition after onset (Addington et al., 2003; Addington & Addington, 2005; Bailer et al., 1996; Meng et al., 2006; Mueser et al., 1990). Further, previous studies have linked poor premorbid sociality to cognitive impairment, and have found these factors contribute to a lower likelihood of recovery in individuals with schizophrenia (Treen Calvo et al., 2018). Specifically, poor premorbid sociality is predictive of impairments in attention (Silverstein et al., 2002), visual learning (Levitt et al., 1996), working memory, verbal memory (Rund et al., 2004), and global neurocognition (Béchard-Evans et al., 2010; Bucci et al., 2018). Premorbid social functioning in individuals with schizophrenia has even been shown to predict global cognitive improvement after cognitive remediation (Buonocore et al., 2019). Taken together, cognitive remediation for schizophrenia seeks to improve social adjustment by way of cognitive gains, and the evidence above suggests that level of premorbid sociality may influence the degree of social adjustment response an individual has to cognitive remediation.
Given that premorbid sociality consistently predicts social adjustment and cognitive performance after onset, we sought to explore premorbid sociality as a moderator of social adjustment response to CET in patients in the early course of schizophrenia. The beneficial effects of CET on social and non-social cognition and social adjustment in the early course of schizophrenia were established previously (Eack et al., 2009). Further, both the neuro- and social-cognitive gains made during CET were identified as mechanisms of social adjustment improvement in the same early course sample (Eack et al., 2011). The current study follows up on a recently completed randomized controlled trial that sought to confirm the positive cognitive and social adjustment effects of CET in the early course of schizophrenia (Wojtalik et al., 2021). The overall effect of CET on cognitive gain was confirmed in this trial, but not the social adjustment effect, as participants in both CET and EST demonstrated improvement. Based on the evidence of premorbid sociality’s association with social adjustment after onset, such factors may explain some of the heterogeneity in social adjustment outcomes during this trial. Evidence that premorbid sociality moderates heterogeneous social adjustment response to CET will contribute to the advancement of cognitive remediation and research in personalized treatments for individuals with schizophrenia.
Methods
Participants
This research is a secondary analysis of data from a two-site (Pittsburgh, PA and Boston, MA) 18-month confirmatory randomized controlled trial of CET compared to EST for early course schizophrenia (clinicaltrials.gov identifier: NCT01561859). The parent study was reviewed and approved annually by both the Beth Israel Deaconess Medical Center and University of Pittsburgh Institutional Review Boards. Of the 102 outpatients with early course schizophrenia included in the parent trial, 88 (CET: n = 52, EST: n = 36) with premorbid sociality scores at baseline were included in the current study. Participants were randomly assigned to CET or EST by an independent database manger using a computer-generated randomization program. The parent trial allocated a higher randomization ratio to CET to facilitate the formation of social-cognitive groups, which requires 6 to 8 participations (see the description of CET below). All participants provided written informed consent prior to participation in the parent study. Eligible participants included those with a Structured Clinical Interview for the DSM-IV-TR (First et al., 2002) confirmed diagnosis of schizophrenia, schizoaffective or schizophreniform disorders, age 18-55, IQ ≥ 80, ability to read and speak fluent English, ≤ 10-year illness duration, stable positive symptoms, adherence to antipsychotic medication, and demonstration of significant social and cognitive disability. Early course schizophrenia in this study was defined as the onset of first psychotic symptoms within the past 10 years. The onset of schizophrenia typically occurs in late adolescences or early adulthood, although females tend to have a later age of onset that peaks in the early 30’s and again around the age of 45 (Li et al., 2016). There is not yet a standardized definition of early course schizophrenia, with illness durations ranging from 1 – 10 years in the early course literature (Newton et al., 2018). Wojtalik et al. (2021) provides further details regarding inclusion and exclusion criteria, study procedures, the CONSORT enrollment diagram, and the handling and limitations of attrition.
The attrition rate of the parent trial was high at 49%. The attrition rate for the current sample of 88 participants is 42%, and the rate of within group attrition did not significantly differ (p = .665) between CET (44%) and EST (39%). There were no significant baseline differences between the participants that dropped from the study (n = 37, 42%) and those who did not (n = 51, 58%) on the study measures of interest (described below): Premorbid Adjustment Scale (PAS; p = .621), Social Adjustment Scale-II (SAS-II) total score (p = .287), and the SAS-II subscales (all p’s > .084).
The baseline demographic characteristics, presented in Table 1, of the 88 participants included in the current analysis are typical of an early course schizophrenia sample. The overall sample was young in their mid-twenties, majority male, and ill for less than four years. The participants were racially diverse, with most individuals that identified as a racial minority indicating their race as African American. Almost three-quarters of the sample had some college education, although less than a third were employed at the time of enrollment. Almost half of the sample had a history of substance misuse. There were no significant differences at baseline between CET (n = 52) and EST (n = 36) on any demographic, diagnostic, or antipsychotic medication characteristics (see Table 1).
Table 1.
Baseline Demographics of Participants with Early Course Schizophrenia with Premorbid Sociality Scores
| Overall (N = 88) | CET (n = 52) | EST (n = 36) | ||
|---|---|---|---|---|
| Variable | M(SD)/N(%) | M(SD)/N(%) | M(SD)/N(%) | p c |
| Age | 24.95 (5.58) | 24.03 (4.18) | 26.28 (6.99) | .062 |
| Sex (% male) | 66 (75%) | 42 (81%) | 24 (67%) | .143 |
| Racial minority (%) | 37 (42%) | 21 (40%) | 16 (44%) | .827 |
| African American | 22 (25%) | 12 (23%) | 10 (28%) | - |
| White | 51 (58%) | 31 (60%) | 20 (55%) | - |
| Asian | 5 (6%) | 4 (7%) | 1 (3%) | - |
| Hispanic | 0 (0%) | 0 (0%) | 0 (0%) | - |
| Hawaiian/Pacific Islander | 1 (1%) | 1 (2%) | 0 (0%) | - |
| More than one race | 6 (7%) | 2 (4%) | 4 (11%) | - |
| Other | 3 (3%) | 2 (4%) | 1 (3%) | - |
| IQ | 107.91 (10.46) | 108.04 (9.63) | 107.72 (11.71) | .890 |
| Education (% some college) | 64 (73%) | 40 (77%) | 24 (67%) | .335 |
| Employment (% employed) | 26 (30%) | 13 (25%) | 13 (36%) | .343 |
| Illness Lengtha | 3.84 (2.31) | 3.86 (2.19) | 3.82 (2.51) | .940 |
| Schizophrenia Diagnosis (% with) | 70 (80%) | 43 (83%) | 27 (75%) | .427 |
| Substance Use Disorder History (% with) | 43 (49%) | 24 (46%) | 19 (53%) | .665 |
| Antipsychotic Dose (CPZ equivalent)b | 458.65 (342.93) | 466.96 (378.60) | 447.33 (292.29) | .796 |
| Antipsychotic Adherence (% adherent) | 63 (72%) | 39 (75%) | 24 (67%) | .473 |
Note. CET = Cognitive Enhancement Therapy, CPZ = Chlorpromazine, EST = Enriched Supportive Therapy
Based on a total sample of 87 with available data (CET: n = 52, EST: n = 35)
Based on a total sample of 85 with available data (CET: n = 49, EST: n = 36)
Results from independent samples t-tests or Fisher’s exact tests, two-tailed
Measures
Moderator Identification.
Moderators are critical to identify in clinical trials because they provide information about how treatment affects patients differently (Kraemer et al., 2002). Treatment moderators guide best practices to personalized medicine and are a National Institute of Mental Health priority (Insel, 2009). In this way, data about functioning during childhood and adolescence prior to the onset of schizophrenia may be informative for individualized treatment planning after illness onset. Theoretical evidence that premorbid sociality is a possible moderator of social adjustment response during cognitive remediation is supported by the long-standing associations demonstrated between premorbid sociality and social adjustment and cognitive impairment after illness onset (Addington & Addington, 2005; Bailer et al., 1996; Béchard-Evans et al., 2010; Bucci et al., 2018; Mueser et al., 1990). As such, the aim of this secondary analysis was to provide initial evidence of premorbid sociality as a moderator of the relationship between CET, compared to EST, and social adjustment response in patients with early course schizophrenia.
Premorbid Sociality (moderator).
The Premorbid Adjustment Scale (PAS) is a commonly used measure in schizophrenia research designed to capture social functioning development from childhood to adulthood prior to illness onset using a semi-structured interview format (Cannon-Spoor et al., 1982). The PAS has sound psychometric properties for assessing social functioning from childhood to adolescents prior to the onset of the schizophrenia. The PAS has been validated as a self-report measure in schizophrenia, demonstrating both predictive and concurrent validity (Brill et al., 2008), and good reliability (Krauss et al., 1998). For the current analysis, six items from the PAS assessing withdrawal (isolation), peer relationships, and interest in social activities during childhood and adolescence were selected due to their relevance to social functioning in schizophrenia and administered during social and psychiatric history interviews conducted by parent trial clinicians at baseline. The PAS items were rated on scale from 0 (i.e., “not withdrawn, active social interaction”) to 6 (i.e., “unrelated, withdrawn and isolated”). For the current analysis, a premorbid sociality total score was calculated by averaging the PAS items from the social and psychiatry history interview.
Social Adjustment (outcome).
The Social Adjustment Scale-II (SAS-II) is a structured interview-based instrument that includes 45 items assessing social adjustment to disability in the previous 2 months in the domains of social and role functioning, as well as self-care (Schooler et al., 1979). With one exception (rating 0 – 5), items are rated on a scale from 0 (i.e., “No impairment ”) to 4 (i.e., “Extreme impairment or not functioning at all”). The SAS-II has demonstrated good psychometric properties and is a field standard measure of social adjustment in schizophrenia research (Bellack et al., 1990; Glazer et al., 1980; Jaeger et al., 2003; Mueser et al., 2001). The yielded scores from the SAS-II are a mean of items and includes an overall social adjustment total score and seven subscales scores: interpersonal anguish, sexual relations, household/family relationships, social functioning, work affect, major role performance, self-care. Trained raters masked to treatment assignment completed the SAS-II with participants at baseline, 9- (mid-treatment) and 18-month (end of treatment) time points.
For the purposes of data analysis and interpretation, scores generated from both measures were reversed scored so a higher score represented better adjustment and standardized to z-scores with a mean of 0 (SD = 1). Both the SAS-II total score (α = .77) and premorbid sociality total score (α = .78) demonstrated acceptable reliability and were not correlated at baseline (r = .10, p = .348).
Treatments
All participants were on prescribed antipsychotic medication(s) approved for the treatment of schizophrenia throughout the parent trial. The same therapists provided both CET and EST to control for any potential therapist effects. See Wojtalik et al. (2021) for more details on the treatments and related procedures. CET and EST were both delivered for 18-months, with participants in both groups completing assessments at baseline, mid-treatment (9 months), and treatment completion (18 months).
Cognitive Enhancement Therapy (CET).
CET is an 18-month recovery-phase intervention that uses a comprehensive curriculum to remediate illness-related developmental impairments in social and nonsocial cognition that inhibit functional recovery (Hogarty & Greenwald, 2006). The cognitive enhancing exercises of CET are designed to be performance-based and encourage patients to engage actively in treatment. CET consists of 60 1-hour weekly neurocognitive computer-training sessions in attention, memory, and problem-solving (Ben-Yishay et al., 1987; Bracy, 1994) conducted in patient pairs that are integrated with 45 structured social-cognitive small-group sessions (i.e., 3 to 4 patient pairs). The social-cognitive groups begin after patients complete approximately 3 months of neurocognitive training sessions in attention. Subsequently, neurocognitive training and social-cognitive groups continue concurrently until the completion of treatment. The weekly social-cognitive groups are 1.5 hours in length and take advantage of experiential learning opportunities to increase social knowledge and interpersonal success. Social-cognitive group lectures and exercises (i.e., unrehearsed social exchanges, presenting homework, providing feedback) include topics such as perspective-taking, gistfulness, non-verbal communication, emotion management, and foresightfulness. Specific details on the CET protocol can be found in the manual (Hogarty & Greenwald, 2006).
Enriched Supportive Therapy (EST).
The active comparison intervention in the parent trial was EST (Wojtalik et al., 2021). EST is an 18-month intervention provided weekly on an individual basis for one hour and includes two major phases based on the basic and intermediate stages of Personal Therapy (Hogarty, 2002). The first phase focuses on psychoeducation, the relationship between stress and schizophrenia, and practices to help clients reduce or avoid stressful situations. The second phase focuses on helping clients recognize their own early cues of distress and developing personalized healthy coping techniques. The two phases are provided for roughly 9 months for a total of 18 months of treatment. Patients are able to progress through the phases at their own pace.
Data Analysis
The aim of this secondary analysis was to examine if level of premorbid sociality moderated differential effects of CET, compared to EST, on social adjustment change in the early course of schizophrenia. Intent-to-treat linear mixed-effects models were used to investigate any significant group (CET vs. EST) x time (baseline, 9, and 18 months) x baseline premorbid sociality interactions on social adjustment change. These models were executed in R using the nlme package (Pinheiro et al., 2021), adjusted for study site, included random intercept and slope parameters, and were estimated with restricted maximum likelihood (REML) (Raudenbush & Bryk, 2002). For the intent-to-treat approach, a standard expectation-maximization approach was used to estimate any missing longitudinal data (Dempster et al., 1977). After identifying any significant moderating effects of premorbid sociality on differential social adjustment change between CET and EST, predicted means within these treatment groups with premorbid sociality scores above and below 1 SD of the sample mean were created for interpretation of this three-way interaction. Hedges’ formula (Hedges, 2007) for mixed-effects models was used to calculate Cohen’s d effects sizes for the CET and EST sub-groups at the highest and lowest levels of premorbid sociality. Given the theme of this special issue to increase precision of experimental therapeutics for youth, follow-up analyses were also conducted to examine the potential moderating effects of other developmental predictors of psychopathology (i.e., IQ and age) on social adjustment response to CET vs. EST using the same mixed-effects analyses described above. To investigate the moderating effects of age, the sample was divided into two groups above and below the sample mean age of 24.95, effectively creating a group of transitional age young adults (n = 56) and a group of young-to-middle aged adults (n = 32).
Results
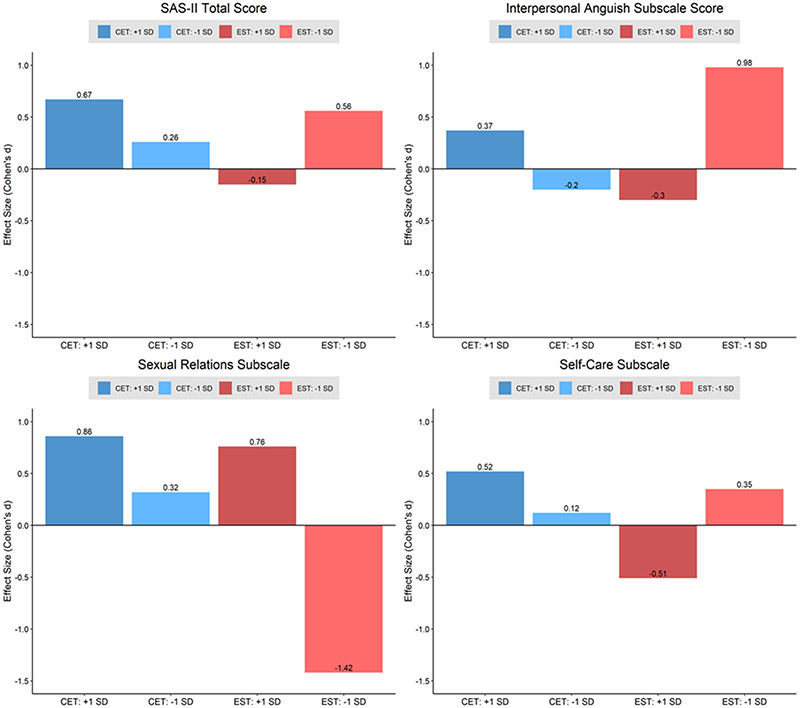
A significant group x time x premorbid sociality interaction was observed on the longitudinal trajectories of the SAS-II total score (F2,99 = 3.30, p = .041). This three-way interaction was not significant at mid-treatment (9 months), but at the completion of treatment (18 months) premorbid sociality significantly moderated differential overall social adjustment change in CET compared to EST (Table 2). Effect sizes of overall social adjustment change at 18 months among CET and EST sub-groups above and below 1 SD of the mean premorbid sociality score are presented in Figure 1. Participants with the highest premorbid sociality scores (i.e., 1 SD above the mean) benefited the most from CET, with a medium-to-large size gain in overall social adjustment at the completion of treatment (Figure 1). In contrast, EST participants with the highest premorbid sociality scores demonstrated a small reduction in overall social adjustment. EST appeared to be beneficial for participants with lower premorbid sociality scores (i.e., 1 SD below the mean), with these participants demonstrating a medium-sized improvement in overall social adjustment at 18 months (Figure 1).
Table 2.
Moderating Effects of Premorbid Sociality on Social Adjustment Change during CET vs. EST in Early Course Schizophrenia
| Moderator Effect: Group x Time x Premorbid Sociality scorea |
B | SE | df | t | p |
|---|---|---|---|---|---|
| SAS-II total score | |||||
| Month 9 | .07 | .20 | 99 | .36 | .722 |
| Month 18 | .55 | .23 | 99 | 2.39 | .019 |
| Interpersonal Anguish subscale | |||||
| Month 9 | .13 | .23 | 99 | .57 | .570 |
| Month 18 | .92 | .27 | 99 | 3.42 | .001 |
| Sexual Relations subscaleb | |||||
| Month 9 | −.56 | .29 | 91 | −1.92 | .058 |
| Month 18 | −.76 | .35 | 91 | −2.18 | .032 |
| Household/Family Relationships subscalec | |||||
| Month 9 | −.27 | .23 | 62 | −1.17 | .248 |
| Month 18 | .40 | .26 | 62 | 1.51 | .137 |
| Social Functioning subscale | |||||
| Month 9 | −.11 | .24 | 99 | −.46 | .648 |
| Month 18 | −.01 | .26 | 99 | −.04 | .969 |
| Work Affect subscaled | |||||
| Month 9 | −.35 | .43 | 34 | −.81 | .421 |
| Month 18 | .28 | .55 | 34 | .52 | .608 |
| Major Role Performance subscale | |||||
| Month 9 | .33 | .25 | 99 | 1.33 | .188 |
| Month 18 | .16 | .27 | 99 | .58 | .562 |
| Self-Care subscale | |||||
| Month 9 | .57 | .29 | 99 | 1.93 | .056 |
| Month 18 | .64 | .31 | 99 | 2.06 | .043 |
Note. SAS-II = Social Adjustment Scale, version 2 (Schooler et al., 1979), SE = standard error
Group = CET vs. EST, Time = Baseline, 9 month, and 18 month time points, Premorbid Sociality = baseline total score from the adapted Premorbid Adjustment Scale (Cannon-Spoor et al., 1982)
Not ratable for individuals living with an intimate partner
Not ratable for individuals who live independently
Not ratable for individuals who are unemployed
Figure 1, Within Sub-Group 18-Month Treatment Effect Sizes of Social Adjustment Change During CET vs. EST at Different Levels of the Premorbid Sociality Moderator.
(Note. CET = Cognitive Enhancement Therapy, EST = Enriched Supportive Therapy, +1 SD = premorbid sociality scores 1 SD above the mean, −1 SD = premorbid sociality scores 1 SD below the mean)
With respect to the subscales of the SAS-II, premorbid sociality significantly moderated the change trajectories of interpersonal anguish in the CET group relative to EST (F2,99 = 6.58, p = .002). Similar to overall social adjustment, premorbid sociality did not moderate interpersonal anguish change at 9 months, but did significantly affect change at 18 months between the treatment groups (Table 2). As seen in Figure 1, CET participants with the highest premorbid sociality had a small-to-medium sized improvement in interpersonal aguish. Conversely, the EST group with the highest premorbid sociality had a small-to-medium decrease. The largest improvement in interpersonal anguish was in the EST participants with the lowest level of premorbid sociality, with almost 1 standard deviation improvement. While not statistically significant, premorbid sociality had a moderating effect close to the conventional threshold of significance for sexual relations (F2,91 = 2.94, p = .058) and self-care (F2,99 = 2.81, p = .065). At 9 months, the group x time x premorbid sociality interaction was also trending close to significance for sexual relations and self-care change (Table 2). At 18 months, premorbid sociality significantly moderated differential sexual relations and self-care change in the CET and EST groups (Table 2). Across the premorbid sociality sub-groups presented in Figure 1, self-care change looked similar to overall social adjustment change, where the highest premorbid sociality CET group had the largest gains with a medium effect size and the highest premorbid sociality EST group had a medium decrease. The lowest premorbid sociality EST group had a small-to-medium improvement in self-care. Regarding sexual relations, participants in both CET and EST with the highest premorbid sociality had medium-to-large improvements, with CET having a slightly more favorable effect on sexual relations (Figure 1). EST was not beneficial for participants with the lowest premorbid sociality, with a large reduction in sexual relations observed in this group. For household/family relationships, although the trajectories significantly varied across the premorbid sociality moderator (F2,62 = 3.55, p = .035), there were no significant interactions at 9 or 18 months (Table 2). Premorbid sociality did not moderate differential treatment trajectories (all F’s < .88, all p’s > .418) or changes at 9 and 18 months for the social functioning, work affect, or major role performance subscales (Table 2).
For the additional follow-up moderator analyses, IQ was not a significant moderator of overall social adjustment change in CET compared to EST at neither 9 (p = .970) nor 18 months (p = .771). Non-significant moderator effects of IQ were also observed for the SAS-II subscales at both time points (all p’s > .303). This group x time x moderator interaction was also not significant for age at 9 (all p’s > .061) and 18 months (all p’s > .124), such that transitional age and older young adults did not significantly differ in their social adjustment responses (i.e., SAS-II total and subscale scores) in either the CET or EST groups.
Discussion
Poor social adjustment to schizophrenia is a common feature of the condition and present in young individuals in the early phase (Addington et al., 2003; Addington & Addington, 2005; Lenior et al., 2001). Few treatments are available that target social functioning in this population. Cognitive remediation has an established evidence-base for schizophrenia and is one of the most promising approaches for facilitating both cognitive and functional recovery (McGurk et al., 2007; Revell et al., 2015; Vita et al., 2021; Wykes et al., 2011). Premorbid and post-illness onset social functioning in schizophrenia are highly correlated (Bailer et al., 1996; Bucci et al., 2018). It is possible that level of premorbid sociality is a moderator of individual differences in social adjustment change during cognitive remediation. Such information is critical for knowing how to best adapt and personalize cognitive remediation, particularly CET, for individuals with early course schizophrenia.
The current secondary analysis examined the impact of premorbid sociality on longitudinal social adjustment change during two psychosocial interventions, CET compared to EST, in the early course of schizophrenia. The results provide initial evidence that premorbid sociality moderates differential effects of CET and EST on overall social adjustment. While not all the sub-scales of social adjustment were moderated by premorbid sociality, differential change in interpersonal anguish, self-care, and sexual relations were. The within CET and EST sub-group social adjustment effect sizes at the completion of treatment largely indicated that CET may be more beneficial for patients with higher premorbid sociality. In contrast, EST had favorable effects on social adjustment for patients with poorer premorbid sociality. This is the first premorbid moderator study of differential CET and EST effects, and to our knowledge, the first to consider premorbid sociality as a moderator of cognitive remediation outcome in schizophrenia.
The observed moderating effect of premorbid sociality on differential social adjustment outcome aligns with the neurodevelopmental theory of social-cognitive development in schizophrenia driving CET (Hogarty & Flesher, 1999a). According to this theory, neurocognitive impairments and social isolation associated with the condition significantly limit opportunities for the development of adult social-cognitive abilities, such as perspective taking, social context appraisal, foresight, cognitive flexibility, and gist abstraction (Hogarty & Flesher, 1999a). The social-cognitive abilities of adults with schizophrenia are characterized as developmentally adolescent, concrete and effortful, causing patients to miss the larger social meaning (or the gist) of social interactions. The social-cognitive group exercises of CET are designed to be active and application-based, such as in engaging in unrehearsed social exchanges, to facilitate the development of adult social-cognitive milestones (Hogarty & Flesher, 1999b). It may be that patients with higher premorbid sociality have some adult social-cognitive prerequisites that are capitalized on during the CET social cognitive group exercises. Further, in the theoretical context of CET, the interpersonal anguish, sexual relations, and self-care subscales of the SAS-II include many items that may reflect the development of such adult social-cognitive abilities listed above. Items on these subscales assess interest, distress, sensitivity, guilt, and self-appraisal in interpersonal relationships, as well as personal appearance. The remaining subscales that were not moderated by premorbid sociality included many items that may not require the use of effective adult social cognition, such as items assessing economic adequacy (i.e., major role performance subscale).
The observation that CET, in general, was more favorable for social adjustment improvement among patients with the highest premorbid sociality may also be explained by a greater cognitive reserve. A few recent studies have suggested that level of premorbid sociality is an indicator of cognitive reserve in schizophrenia (Amoretti et al., 2016; Buonocore et al., 2018; Cuesta et al., 2015; Eack et al., 2009; Herrero et al., 2020), such that people with greater cognitive reserve functioned better in their social environments prior to the onset of schizophrenia. Indeed, cortical and cognitive reserve are correlated (Pettigrew et al., 2017; Sumowski et al., 2013) and previous research has demonstrated that individuals with early course schizophrenia with greater cortical reserve have an accelerated response to CET (Keshavan et al., 2011).
Cognitive reserve may also explain the largely positive effect of EST on social adjustment among the participants with the lowest levels of premorbid sociality. Previous trials of CET compared to EST for schizophrenia (Eack et al., 2009; Hogarty et al., 2004), including the confirmatory parent trial (Wojtalik et al., 2021), demonstrate that EST participants have meaningful gains in social adjustment. EST focuses on the development of basic coping and interpersonal abilities to help patients recognize cues of distress in social contexts. As described above, CET emphases the development of more advanced, adult interpersonal effectiveness through exercises that encourage patients to apply adult-aged social-cognitive skills (i.e., perspective-taking, understanding gists) to complex social situations. Participants with low premorbid functioning, and possibly a low cognitive reserve, may find such CET exercises complex. The foundational exercises of EST are possibly a better starting point for patients that have lower premorbid social functioning. Overall, these results suggest that data on social functioning during childhood and adolescence may be useful for personalizing psychosocial treatment decisions with patients in the early course of schizophrenia with social functioning goals.
Aligned with the experimental therapeutics theme of this special issue, premorbid sociality is identified as a potential moderator of diverse trajectories of social adjustment change during cognitive remediation or supportive therapy in early course schizophrenia. Although these findings are preliminary, this research guides future investigations that have the primary aim of validating the moderating effect of premorbid sociality on social adjustment response to cognitive remediation in the early course of the condition. Future research is also needed to understand the impact of the relationship between cognitive reserve and premorbid sociality on social adjustment outcome in clinical trials of cognitive remediation for schizophrenia. Further, these findings precipitate continued moderation research of premorbid sociality on other outcomes during cognitive remediation for schizophrenia, such as social and nonsocial cognition and symptoms.
Lastly, the results of this study have implications for the use of cognitive remediation for youth at clinically high risk for developing psychosis. The provision of cognitive remediation in this population aims to either prevent transition to psychosis or reduce degree of disability after onset (Glenthøj et al., 2017). The Cognition for Learning and for Understanding Everyday Social Situations (CLUES) intervention is the modified 6-month version of CET for youth at risk of developing schizophrenia (Friedman-Yakoobian et al., 2019). A promising social functioning effect (d = .72), with continued improvement at 3-month follow-up (d = 1.04), was recently observed in a randomized feasibility trial of CLUES relative to an enriched version of Acceptance and Commitment Therapy (Friedman-Yakoobian et al., 2020). Such a finding, in combination with the observed moderating effect of premorbid sociality on social adjustment improvement during CET, informs future investigations of potential moderators of response to CLUES. Assessment of social functioning at baseline in youth at high-risk for psychosis may inform the optimization and personalization of CLUES and is an important future research direction.
Several limitations of this research require consideration. First, by nature of this analysis using secondary data and a modified version of the PAS, the analyses were exploratory and the findings preliminary. The PAS is also a self-report measure that was completed with a trained clinician at intake. This self-report nature may have introduced recall bias. Although participants needed to be clinically stable for study inclusion, condition-related impairments in memory may have inhibited their ability to accurately recall their level of social functioning during childhood and adolescence. Continued investigation with more objective, validated premorbid social functioning measures is needed to confirm the moderator effect of premorbid sociality on social adjustment change during cognitive remediation. Because this study was a secondary analysis and limited to available data in the parent trial, our analyses were only able to focus on one premorbid moderator. Other premorbid variables (Carter et al., 2002), such as genetic risk, school performance, personality, and rearing environment, are also important to consider for future research. Next, attrition was high and increased estimation of missing longitudinal data in the intent-to-treat analyses. Despite that intent-to-treat mixed effects analyses are powerful statistical models, increased estimation has the potential to elevate bias. For example, in the current subsample of 88, participants retained in the trial were significantly older (p = .004), had a higher IQ (p = .018), and were ill for a longer duration (p = .014), as well as on a higher dose of antipsychotic medication (p = .059), compared to those who were lost to attrition. These findings were similarly observed and discussed in more detail in the parent study (Wojtalik et al., 2021). While these demographic differences in study completers and non-completers indicate an important future research direction to further examine predictors of attrition in early course schizophrenia, they may also explain improvements in social functioning over the course of the trial. As such, correlations between age, IQ, illness length, antipsychotic dose, and social adjustment change scores were examined, with no significant relationships observed (all p’s > .109). Finally, considering the neurodevelopment trajectory of schizophrenia, the total score derived from the modified version of the PAS averaged premorbid sociality from childhood and adolescence. It may be the case that social functioning at distinct developmental stages has different moderating effects on outcome during cognitive remediation for early course schizophrenia. It will be important to understand how social functioning during childhood and adolescence may moderate response to cognitive remediation differently among individuals with early course schizophrenia.
In conclusion, the findings provide initial evidence that premorbid sociality may be a clinically relevant moderator of social adjustment change during cognitive remediation in early course schizophrenia. In general, meaningful social adjustment improvement was observed among participants with high premorbid sociality in CET and low premorbid sociality in EST. These findings guide future research directions to confirm premorbid sociality as a moderator of differential social adjustment outcome during cognitive remediation. Further, additional research is needed to determine the clinical utility of utilizing premorbid sociality data to inform personalized treatment for early course schizophrenia, as well as youth at clinically high-risk for psychosis.
Acknowledgements
This research was supported by NIMH grant R01 MH092440 (MSK and SME). The authors have no competing interests to report.
References
- Addington J, & Addington D (2005). Patterns of premorbid functioning in first episode psychosis: relationship to 2-year outcome. Acta Psychiatrica Scandinavica, 112(1), 40–46. 10.1111/j.1600-0447.2005.00511.x [DOI] [PubMed] [Google Scholar]
- Addington J, van Mastrigt S, & Addington D (2003). Patterns of premorbid functioning in first-episode psychosis: initial presentation. Schizophrenia Research, 62(1), 23–30. 10.1016/S0920-9964(02)00408-5 [DOI] [PubMed] [Google Scholar]
- Amoretti S, Bernardo M, Bonnin CM, Bioque M, Cabrera B, Mezquida G, Solé B, Vieta E, & Torrent C (2016). The impact of cognitive reserve in the outcome of first-episode psychoses: 2-year follow-up study. European Neuropsychopharmacology, 26(10), 1638–1648. 10.1016/j.euroneuro.2016.07.003 [DOI] [PubMed] [Google Scholar]
- Bailer J, Bräuer W, & Rey E-R (1996). Premorbid adjustment as predictor of outcome in schizophrenia: Results of a prospective study. Acta Psychiatrica Scandinavica, 93(5), 368–377. 10.1111/j.1600-0447.1996.tb10662.x [DOI] [PubMed] [Google Scholar]
- Béchard-Evans L, Iyer S, Lepage M, Joober R, & Malla A (2010). Investigating cognitive deficits and symptomatology across pre-morbid adjustment patterns in first-episode psychosis. Psychological Medicine, 40(5), 749–759. 10.1017/S0033291709991097 [DOI] [PubMed] [Google Scholar]
- Bellack AS, Morrison RL, Mueser KT, Wade JH, & Sayers SL (1990). Role play for assessing the social competence of psychiatric patients. Psychological Assessment: A Journal of Consulting and Clinical Psychology, 2(3), 248–255. 10.1037/1040-3590.2.3.248 [DOI] [Google Scholar]
- Ben-Yishay Y, Piasetsky EB, & Rattok J (1987). A systematic method for ameliorating disorders in basic attention. In Neuropsychological Rehabilitation. (pp. 165–181). Guilford Press. [Google Scholar]
- Bracy OL (1994). PSSCogRehab. Psychological Software Services Inc. [Google Scholar]
- Brill N, Reichenberg A, Weiser M, & Rabinowitz J (2008). Validity of the Premorbid Adjustment Scale. Schizophrenia Bulletin, 34(5), 981–983. 10.1093/schbul/sbm128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci P, Galderisi S, Mucci A, Rossi A, Rocca P, Bertolino A, Aguglia E, Amore M, Andriola I, Bellomo A, Biondi M, Cuomo A, dell’Osso L, Favaro A, Gambi F, Giordano GM, Girardi P, Marchesi C, Monteleone P, … Psychoses, I. N. for R. on. (2018). Premorbid academic and social functioning in patients with schizophrenia and its associations with negative symptoms and cognition. Acta Psychiatrica Scandinavica, 138(3), 253–266. 10.1111/acps.12938 [DOI] [PubMed] [Google Scholar]
- Buonocore M, Bechi M, Uberti P, Spangaro M, Cocchi F, Guglielmino C, Bianchi L, Mastromatteo AR, Bosia M, & Cavallaro R (2018). Cognitive reserve profiles in chronic schizophrenia: Effects on theory of mind performance and improvement after training. Journal of the International Neuropsychological Society, 24(6), 563–571. https://doi.org/DOI: 10.1017/S1355617718000012 [DOI] [PubMed] [Google Scholar]
- Buonocore M, Bosinelli F, Bechi M, Spangaro M, Piantanida M, Cocchi F, Bianchi L, Guglielmino C, Mastromatteo AR, Cavallaro R, & Bosia M (2019). The role of premorbid adjustment in schizophrenia: Focus on cognitive remediation outcome. Neuropsychological Rehabilitation, 29(10), 1611–1624. 10.1080/09602011.2018.1433048 [DOI] [PubMed] [Google Scholar]
- Cannon-Spoor HE, Potkin SG, & Wyatt RJ (1982). Measurement of Premorbid Adjustment in Chronic Schizophrenia. Schizophrenia Bulletin, 8(3), 470–484. 10.1093/schbul/8.3.470 [DOI] [PubMed] [Google Scholar]
- Carter JW, Schulsinger F, Parnas J, Cannon T, & Mednick SA (2002). A multivariate prediction model of schizophrenia. Schizophrenia Bulletin, 28(4), 649–682. https://academic.oup.com/schizophreniabulletin/article/28/4/649/1852822 [DOI] [PubMed] [Google Scholar]
- Cuesta MJ, Sánchez-Torres AM, Cabrera B, Bioque M, Merchán-Naranjo J, Corripio I, González-Pinto A, Lobo A, Bombín I, de la Serna E, Sanjuan J, Parellada M, Saiz-Ruiz J, & Bernardo M (2015). Premorbid adjustment and clinical correlates of cognitive impairment in first-episode psychosis. The PEPsCog Study. Schizophrenia Research, 164(1), 65–73. 10.1016/j.schres.2015.02.022 [DOI] [PubMed] [Google Scholar]
- Dempster AP, Laird NM, & Rubin DB (1977). Maximum likelihood from incomplete data via the EM algorithm. Journal of the Royal Statistical Society: Series B (Methodological), 39(1), 1–22. 10.1111/j.2517-6161.1977.tb01600.x [DOI] [Google Scholar]
- Eack SM, Greenwald DP, Hogarty SS, Cooley SJ, DiBarry AL, Montrose DM, & Keshavan MS (2009). Cognitive Enhancement Therapy for early-course schizophrenia: Effects of a two-year randomized controlled trial. Psychiatric Services, 60(11), 1468–1476. 10.1176/ps.2009.60.11.1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eack SM, & Newhill CE (2007). Psychiatric symptoms and quality of life in schizophrenia: A meta-analysis. Schizophrenia Bulletin, 33(5), 1225–1237. 10.1093/schbul/sbl071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eack SM, Pogue-Geile MF, Greenwald DP, Hogarty SS, & Keshavan MS (2011). Mechanisms of functional improvement in a 2-year trial of Cognitive Enhancement Therapy for early schizophrenia. Psychological Medicine, 41(6), 1253–1261. 10.1017/S0033291710001765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, & Williams JBW (2002). Structured Clinical Interview For DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. Biometrics Research, New York State Psychiatric Institute. [Google Scholar]
- Friedman-Yakoobian MS, Parrish EM, Eack SM, & Keshavan MS (2020). Neurocognitive and social cognitive training for youth at clinical high risk (CHR) for psychosis: A randomized controlled feasibility trial. Schizophrenia Research. 10.1016/j.schres.2020.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman-Yakoobian MS, Parrish EM, Thomas A, Lesser R, Gnong-Granato A, Eack S, & Keshavan MS (2019). An integrated neurocognitive and social-cognitive treatment for youth at clinical high risk for psychosis: Cognition for Learning and for Understanding Everyday Social Situations (CLUES). Schizophrenia Research, 208, 55–59. 10.1016/j.schres.2019.01.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazer WM, Aaronson HS, Prusoff BA, & Williams DH (1980). Assessment of social adjustment in chronic ambulatory schizophrenics. In Journal of Nervous and Mental Disease (Vol. 168, Issue: 8, pp. 493–497). Lippincott Williams & Wilkins. 10.1097/00005053-198008000-00008 [DOI] [PubMed] [Google Scholar]
- Glenthøj LB, Hjorthøj C, Kristensen TD, Davidson CA, & Nordentoft M (2017). The effect of cognitive remediation in individuals at ultra-high risk for psychosis: a systematic review. Npj Schizophrenia, 3(1), 20. 10.1038/s41537-017-0021-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges LV (2007). Effect Sizes in Cluster-Randomized Designs. Journal of Educational and Behavioral Statistics, 32(4), 341–370. 10.3102/1076998606298043 [DOI] [Google Scholar]
- Herrero P, Contador I, Stern Y, Fernández-Calvo B, Sánchez A, & Ramos F (2020). Influence of cognitive reserve in schizophrenia: A systematic review. Neuroscience & Biobehavioral Reviews, 108, 149–159. 10.1016/j.neubiorev.2019.10.019 [DOI] [PubMed] [Google Scholar]
- Hogarty GE (2002). Personal Therapy for Schizophrenia and Related Disorders: A Guide to Individualized Treatment. Guilford Press. [Google Scholar]
- Hogarty GE, & Flesher S (1999a). Developmental Theory for a Cognitive Enhancement Therapy of Schizophrenia. Schizophrenia Bulletin, 25(4), 677–692. 10.1093/oxfordjournals.schbul.a033410 [DOI] [PubMed] [Google Scholar]
- Hogarty GE, & Flesher S (1999b). Practice Principles of Cognitive Enhancement Therapy for Schizophrenia. Schizophrenia Bulletin, 25(4), 693–708. 10.1093/oxfordjournals.schbul.a033411 [DOI] [PubMed] [Google Scholar]
- Hogarty GE, Flesher S, Ulrich R, Carter M, Greenwald D, Pogue-Geile M, Keshavan M, Cooley S, DiBarry AL, Garrett A, Parepally H, & Zoretich R (2004). Cognitive Enhancement Therapy for schizophrenia: Effects of a 2-year randomized trial on cognition and behavior. Archives of General Psychiatry, 61(9), 866–876. 10.1001/archpsyc.61.9.866 [DOI] [PubMed] [Google Scholar]
- Hogarty GE, & Greenwald DP (2006). Cognitive Enhancement Therapy: The Training Manual. University of Pittsburgh Medical Center. http://www.cognitiveenhancementtherapy.com [Google Scholar]
- Insel TR (2009). Translating scientific opportunity into public health impact: A strategic plan for research on mental illness. Archives of General Psychiatry, 66(2), 128–133. 10.1001/archgenpsychiatry.2008.540 [DOI] [PubMed] [Google Scholar]
- Jaeger J, Berns SM, & Czobor P (2003). The Multidimensional Scale of Independent Functioning: A new instrument for measuring functional disability in psychiatric populations. Schizophrenia Bulletin, 29(1), 153–167. 10.1093/oxfordjournals.schbul.a006987 [DOI] [PubMed] [Google Scholar]
- Kalin M, Kaplan S, Gould F, Pinkham AE, Penn DL, & Harvey PD (2015). Social cognition, social competence, negative symptoms and social outcomes: Inter-relationships in people with schizophrenia. Journal of Psychiatric Research, 68, 254–260. 10.1016/j.jpsychires.2015.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer HC, Wilson GT, Fairburn CG, & Agras WS (2002). Mediators and moderators of treatment effects in randomized clinical trials. Archives of General Psychiatry, 59(10), 877–883. 10.1001/archpsyc.59.10.877 [DOI] [PubMed] [Google Scholar]
- Krauss H, Marwinski K, Held T, Rietschel M, & Freyberger HJ (1998). Reliability and validity of the Premorbid Adjustment Scale (PAS) in a German sample of schizophrenic and schizoaffective patients. European Archives of Psychiatry and Clinical Neuroscience, 248(6), 277–281. 10.1007/s004060050050 [DOI] [PubMed] [Google Scholar]
- Lenior ME, Dingemans PMAJ, Linszen DH, De Haan L, & Schene AH (2001). Social Functioning and the Course of Early-Onset Schizophrenia: Five-year follow-up of a psychosocial intervention. British Journal of Psychiatry, 179(1), 53–58. https://doi.org/DOI: 10.1192/bjp.179.1.53 [DOI] [PubMed] [Google Scholar]
- Levitt JJ, O’Donnell BF, McCarley RW, Nestor PG, & Shenton ME (1996). Correlations of premorbid adjustment in schizophrenia with auditory event-related potential and neuropsychological abnormalities. In The American Journal of Psychiatry (Vol. 153, Issue: 10, pp. 1347–1349). American Psychiatric Assn. 10.1176/ajp.153.10.1347 [DOI] [PubMed] [Google Scholar]
- Li R, Ma X, Wang G, Yang J, & Wang C (2016). Why sex differences in schizophrenia? Journal of Translational Neuroscience, 1(1), 37–42. 10.3868/j.issn.2096-0689.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGurk SR, Twamley EW, Sitzer DI, McHugo GJ, & Mueser KT (2007). A meta-analysis of cognitive remediation in schizophrenia. American Journal of Psychiatry, 164(12), 1791–1802. 10.1176/appi.ajp.2007.07060906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng H, Schimmelmann BG, Mohler B, Lambert M, Branik E, Koch E, Karle M, Strauss M, Preuss U, Amsler F, Riedesser P, Resch F, & Bürgin D (2006). Pretreatment social functioning predicts 1-year outcome in early onset psychosis. Acta Psychiatrica Scandinavica, 114(4), 249–256. 10.1111/j.1600-0447.2006.00773.x [DOI] [PubMed] [Google Scholar]
- Mueser KT, Bellack AS, Morrison RL, & Wixted JT (1990). Social competence in schizophrenia: Premorbid adjustment, social skill, and domains of functioning. Journal of Psychiatric Research, 24(1), 51–63. 10.1016/0022-3956(90)90024-K [DOI] [PubMed] [Google Scholar]
- Mueser KT, Salyers MP, & Mueser PR (2001). A prospective analysis of work in schizophrenia. Schizophrenia Bulletin, 27(2), 281–296. 10.1093/oxfordjournals.schbul.a006874 [DOI] [PubMed] [Google Scholar]
- Newton R, Rouleau A, Nylander A-G, Loze J-Y, Resemann HK, Steeves S, & Crespo-Facorro B (2018). Diverse definitions of the early course of schizophrenia—a targeted literature review. Npj Schizophrenia, 4(1), 21. 10.1038/s41537-018-0063-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettigrew C, Soldan A, Zhu Y, Wang M-C, Brown T, Miller M, & Albert M (2017). Cognitive reserve and cortical thickness in preclinical Alzheimer’s disease. Brain Imaging and Behavior, 11(2), 357–367. 10.1007/s11682-016-9581-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D, & R Core Team. (2021). nlme: Linear and Nonlinear Mixed Effects Models (R package version 3.1-152). https://cran.r-project.org/package=nlme [Google Scholar]
- Raudenbush DSW, & Bryk DAS (2002). Hierarchical Linear Models: Applications and Data Analysis Methods. Sage. [Google Scholar]
- Revell ER, Neill JC, Harte M, Khan Z, & Drake RJ (2015). A systematic review and meta-analysis of cognitive remediation in early schizophrenia. Schizophrenia Research, 168(1), 213–222. 10.1016/j.schres.2015.08.017 [DOI] [PubMed] [Google Scholar]
- Rund BR, Melle I, Friis S, Larsen TK, Midbøe LJ, Opjordsmoen S, Simonsen E, Vaglum P, & McGlashan T (2004). Neurocognitive dysfunction in first-episode psychosis: Correlates with symptoms, premorbid adjustment, and duration of untreated psychosis. American Journal of Psychiatry, 161(3), 466–472. 10.1176/appi.ajp.161.3.466 [DOI] [PubMed] [Google Scholar]
- Schooler N, Weissman M, & Hogarty GE (1979). Social Adjustment Scale for Schizophrenics. In Hargreaves W, Attkisson C, & Sorenson J (Eds.), Resource Material for Community Mental Health Program Evaluators. DHHS pub no (ADM) 79328. National Institute of Mental Health. [Google Scholar]
- Seccomandi B, Agbedjro D, Bell M, Keefe RSE, Keshavan M, Galderisi S, Fiszdon J, Mucci A, Cavallaro R, Ojeda N, Peña J, Müller D, Roder V, Wykes T, & Cella M (2021). Exploring the role of age as a moderator of cognitive remediation for people with schizophrenia. Schizophrenia Research, 228, 29–35. 10.1016/j.schres.2020.11.060 [DOI] [PubMed] [Google Scholar]
- Seccomandi B, Tsapekos D, Newbery K, Wykes T, & Cella M (2020). A systematic review of moderators of cognitive remediation response for people with schizophrenia. In Schizophrenia Research: Cognition (Vol. 19, p. 100160). Elsevier Inc. 10.1016/j.scog.2019.100160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein ML, Mavrolefteros G, & Close D (2002). Premorbid adjustment and neuropsychological performance in schizophrenia. Schizophrenia Bulletin, 28(1), 157–165. 10.1093/oxfordjournals.schbul.a006918 [DOI] [PubMed] [Google Scholar]
- Sumowski JF, Rocca MA, Leavitt VM, Riccitelli G, Comi G, DeLuca J, & Filippi M (2013). Brain reserve and cognitive reserve in multiple sclerosis. Neurology, 80(24), 2186 LP – 2193. 10.1212/WNL.0b013e318296e98b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treen Calvo D, Giménez-Donoso S, Setién-Suero E, Toll Privat A, Crespo-Facorro B, & Ayesa Arriola R (2018). Targeting recovery in first episode psychosis: The importance of neurocognition and premorbid adjustment in a 3-year longitudinal study. Schizophrenia Research, 195, 320–326. 10.1016/j.schres.2017.08.032 [DOI] [PubMed] [Google Scholar]
- Vita A, Barlati S, Ceraso A, Nibbio G, Ariu C, Deste G, & Wykes T (2021). Effectiveness, Core Elements, and Moderators of Response of Cognitive Remediation for Schizophrenia: A Systematic Review and Meta-analysis of Randomized Clinical Trials. JAMA Psychiatry. 10.1001/jamapsychiatry.2021.0620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtalik JA, Mesholam-Gately RI, Hogarty SS, Greenwald DP, Litschge MY, Sandoval LR, Shashidhar G, Guimond S, Keshavan MS, & Eack SM (2021). Confirmatory Efficacy of Cognitive Enhancement Therapy for Early Schizophrenia: Results from a Multi-Site Randomized Trial. Psychiatric Services. 10.1176/appi.ps.202000552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wykes T, Huddy V, Cellard C, McGurk SR, & Czobor P (2011). A meta-analysis of cognitive remediation for schizophrenia: Methodology and effect sizes. American Journal of Psychiatry, 168(5), 472–485. 10.1176/appi.ajp.2010.10060855 [DOI] [PubMed] [Google Scholar]