Abstract
The Organ Procurement and Transplant Network (OPTN) implemented a new heart allocation policy on October 18, 2018. Published estimates of lower post-transplant survival under the new policy in cohorts with limited follow-up may be biased by informative censoring. Using the Scientific Registry of Transplant Recipients, we used the Kaplan-Meier method to estimate 1-year post-transplant survival for pre-policy (November 1, 2016, to October 31, 2017) and post-policy cohorts (November 1, 2018, to October 31, 2019) with follow-up through March 2, 2021. We adjusted for changes in recipient population over time with a multivariable Cox proportional hazards model. To demonstrate the effect of inadequate follow-up on post-policy survival estimates, we repeated the analysis but only included follow-up through October 31, 2019. Transplant programs transplanted 2594 patients in the pre-policy cohort and 2761 patients in the post-policy cohort. With follow-up through March 2, 2021, unadjusted 1-year post-transplant survival was 90.6% (89.5%–91.8%) in the pre-policy cohort and 90.8% (89.7%–91.9%) in the post-policy cohort (adjusted HR = 0.93 [0.77–1.12]). Ignoring follow-up after October 31, 2019, the post-policy estimate was biased downward (1-year: 82.2%). When estimated with adequate follow-up, 1-year post-transplant survival under the new heart allocation policy was not significantly different.
1. Introduction
The Organ Procurement and Transplantation Network (OPTN) implemented a new donor heart allocation policy on October 18, 2018. Studies evaluating the impact of this new policy on post-transplant survival contain discrepant findings.1 Five reports found decreased post-transplant survival under the new policy,2,3,4,5,6 and two reports found no difference in post-transplant survival.7,8 Notably, the studies with lower estimates of post-transplant survival in the post-policy era have significantly fewer follow-up observations of post-policy recipients compared with the studies finding unchanged survival.
One proposed explanation for the conflicting results is informative censoring bias.9 A fundamental assumption of the Kaplan-Meier survival estimator is that censoring is statistically independent of survival time.10 If censored patients have longer survival times than non-censored patients, the Kaplan-Meier estimator can be biased downward. Transplant programs are required to report recipient deaths faster than routine follow-up appointments for healthy recipients.11 If a study’s data is heavily censored, this differential data submission requirement based on recipient survival status could lead to a lower Kaplan-Meier estimate than the true population survival rate. Studies that reported lower estimates of post-transplant survival in the post-policy era2,3,4,5,6 have significantly more censoring in their post-policy cohorts than the studies finding unchanged survival.7,8
This study uses more complete recipient follow-up data to evaluate the hypothesis that informative censoring biased the estimates of lower post-transplant survival in the new heart allocation system.
2. Methods
2.1. Data source and study population
This study used data from the Scientific Registry of Transplant Recipients (SRTR). The SRTR data system includes data on all donors, waitlisted candidates, and transplant recipients in the US, submitted by the members of the Organ Procurement and Transplantation Network (OPTN). The Health Resources and Services Administration (HRSA), U.S. Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors. This study was approved by the University of Chicago Medical Center institutional review board. We identified adult (aged 18 or older at the time of listing), heart-only transplant recipients who underwent transplantation between November 1, 2015, and October 31, 2019. Recipients’ date of listing for transplant was not an exclusion criterion, so the post-policy cohort includes recipients who were listed before implementation of the new policy.
2.2. Primary survival analysis
For the primary analysis, we selected recipients in two seasonally matched one-year cohorts, those transplanted from November 1, 2016, to October 31, 2017 (pre-policy) and November 1, 2018, to October 31, 2019 (post-policy). Cohorts were seasonally matched to control for known seasonal trends in deceased donor heart donation.12,13 We selected a pre-policy cohort that ended one year before policy implementation to avoid contamination from any anticipatory practice changes in the final year of the pre-policy era.14 We estimated survival for each cohort in the first year post-transplant using the Kaplan-Meier method with data through March 2, 2021. Survival data for recipients in both cohorts were administratively censored at 1 year after transplant to prevent bias from differential length of follow-up between cohorts.
To replicate previous results which were potentially biased downward by informative censoring, we repeated the primary analysis while ignoring follow-up observations that occurred after October 31, 2019. To determine the amount of follow-up required for an unbiased estimate of one-year survival, we repeated this process of truncating follow-up for each day from November 1, 2019, to March 2, 2021.
To control for changes in recipient demographics over time, we estimated the effect of the policy using a multivariable Cox proportional hazards regression controlling for the components of the Index for Mortality Prediction After Cardiac Transplantation (IMPACT) score using the entire study data range.15 Because treatment practices have changed since the new policy was implemented,16,17 we also estimated post-transplant survival before and after policy implementation by treatment support at the time of transplantation. For these subgroup analyses, high-dose inotrope support was defined by OPTN policies as “multiple inotropes or a single high-dose inotrope and has hemodynamic monitoring” (e.g., dobutamine at greater than or equal to 7.5 mcg/kg/min).11 Low-dose inotrope support is inotropic support without continuous hemodynamic monitoring. See Supporting Information for required minimum doses for each drug and category. Additionally, the pre-policy cohort was expanded to include recipients transplanted between November 1, 2015, and October 17, 2018, because of low treatment utilizations in the pre-policy era.
2.3. Sensitivity analyses and statistical analysis
We performed sensitivity analyses with different seasonally matched pre-policy cohorts to ensure our results were robust to the chosen year. These cohorts spanned from November 1, 2015, to October 31, 2016; November 1, 2017, to October 17, 2018; and November 1, 2015, to October 17, 2018. Categorical variables were compared using the chi-square test. Continuous variables were compared using the Wilcoxon rank sum test. All analyses were performed using R version 4.0.4 and RStudio (RStudio Team, 2021. RStudio: Integrated Development for R. RStudio, PBC, Boston, MA). See the Supplemental Material for access to all analysis code. All statistical tests were two-sided, and p < 0.05 was considered significant.
3. Results
3.1. Recipient characteristics
Of the 10466 heart transplant recipients in the full study period, there were 2594 in the pre-policy cohort and 2761 in the post-policy cohort (Table 1). The post-policy cohort was more likely to be treated in the ICU (28.6% pre-policy vs. 51.9% post-policy, P < 0.001) with mechanical ventilation (0.9% pre-policy vs. 2.6% post-policy, P < 0.001), extracorporeal membrane oxygenation (1.0% pre-policy vs. 5.5% post-policy, P < 0.001), and intra-aortic balloon pumps (8.3% pre-policy vs. 28.4% post-policy, P < 0.001). Recipients transplanted while supported with only low-dose inotropes (10.6% pre-policy vs. 4.3% post-policy) or high-dose inotropes (16.0% pre-policy vs. 6.2% post-policy) decreased under the new allocation policy. Bridging with left ventricular assist devices (49.2% pre-policy vs. 32.3% post-policy, P < 0.001), and median wait-list time (112 days [IQR: 30–324] pre-policy vs. 39 days [10–195] post-policy, P < 0.001) decreased after policy implementation.
Table 1.
Recipient characteristics at the time of transplant before and after implementation of the new heart allocation policy
| Pre-policy (n = 2594) | Post-policy (n = 2761) | P value | |
|---|---|---|---|
| Male | 1909 (73.6) | 1978 (71.6) | 0.116 |
| Age | 57 (46–63) | 56 (45–63) | 0.128 |
| BMI | 27.5 (24–31.5) | 27.5 (23.9–31.4) | 0.448 |
| Race/Ethnicity | |||
| White | 1661 (64) | 1780 (64.5) | 0.782 |
| Black | 595 (22.9) | 605 (21.9) | |
| Hispanic | 223 (8.6) | 245 (8.9) | |
| Asian | 90 (3.5) | 108 (3.9) | |
| Other | 25 (1) | 23 (0.8) | |
| Recipient history | |||
| Diabetes | 732 (28.2) | 745 (27) | 0.327 |
| Malignancy | 254 (9.8) | 247 (8.9) | 0.31 |
| Cerebrovascular disease | 161 (6.2) | 187 (6.8) | 0.435 |
| Heart failure etiology | |||
| Nonischemic dilated cardiomyopathy | 1489 (57.4) | 1543 (55.9) | <0.001 |
| Ischemic cardiomyopathy | 690 (26.6) | 707 (25.6) | |
| Congenital heart disease | 59 (2.3) | 104 (3.8) | |
| Restrictive cardiomyopathy | 79 (3) | 123 (4.5) | |
| Valvular heart disease | 31 (1.2) | 21 (0.8) | |
| Hypertrophic cardiomyopathy | 69 (2.7) | 93 (3.4) | |
| Failure of primary transplant | 50 (1.9) | 73 (2.6) | |
| Other etiology | 127 (4.9) | 97 (3.5) | |
| Total bilirubin (mg/dL) | 0.7 (0.4–1) | 0.7 (0.5–1.1) | 0.082 |
| Serum creatinine (mg/dL) | 1.16 (0.95–1.42) | 1.13 (0.9–1.4) | 0.016 |
| Pretransplant hospitalization status | |||
| In ICU | 743 (28.6) | 1434 (51.9) | <0.001 |
| Hospitalized, not in ICU | 381 (14.7) | 397 (14.4) | |
| Not hospitalized | 1470 (56.7) | 930 (33.7) | |
| Blood type | |||
| A | 1051 (40.5) | 1121 (40.6) | 0.68 |
| B | 370 (14.3) | 422 (15.3) | |
| AB | 135 (5.2) | 143 (5.2) | |
| O | 1037 (40) | 1068 (38.7) | |
| Pretransplant medical therapy | |||
| IV antibiotics in 2 weeks before transplant | 227 (8.8) | 288 (10.4) | 0.042 |
| High dose IV inotropes | 414 (16) | 170 (6.2) | <0.001 |
| Low dose IV inotropes | 274 (10.6) | 119 (4.3) | <0.001 |
| Mechanical ventilation | 23 (0.9) | 72 (2.6) | <0.001 |
| IABP | 216 (8.3) | 783 (28.4) | <0.001 |
| ECMO | 25 (1) | 152 (5.5) | <0.001 |
| Durable LVAD | 1276 (49.2) | 891 (32.3) | <0.001 |
| Other MCS | 71 (2.7) | 178 (6.4) | <0.001 |
| No MCS | 1022 (39.4) | 854 (30.9) | <0.001 |
| Days on wait list | 112 (30–324) | 39 (10–195) | <0.001 |
| Wait-list status at transplant | |||
| Old Status 1A | 1706 (65.8) | – | – |
| Old Status 1B | 825 (31.8) | – | |
| Old Status 2 | 63 (2.4) | – | |
| New Status 1 | – | 244 (8.8) | |
| New Status 2 | – | 1251 (45.3) | |
| New Status 3 | – | 636 (23) | |
| New Status 4 | – | 506 (18.3) | |
| New Status 6 | – | 122 (4.4) | |
Values are n (%) or median (IQR)
BMI = body mass index; ICU = intensive care unit; IV = intravenous; IABP = intra-aortic balloon pump; ECMO = extracorporeal membrane oxygenation; LVAD = left ventricular assist device; MCS = mechanical circulatory support
3.2. Post-transplant survival with complete follow-up
With follow-up through March 2, 2021, estimated 1-year post-transplant survival was not significantly different before (90.6%, 95% CI: 89.5%–91.8%) and after (90.8%, 95% CI: 89.7%–91.9%) policy implementation (log-rank P = 0.8) (Figure 1). In multivariable Cox proportional hazards regression controlling for IMPACT score risk factors, receiving a transplant after policy implementation was not associated with difference in survival (hazard ratio 0.93; 95% CI: 0.77 – 1.12, y; P = 0.45) (Table S1).
Figure 1: Survival of heart transplant recipients before and after implementation of the new heart allocation policy.
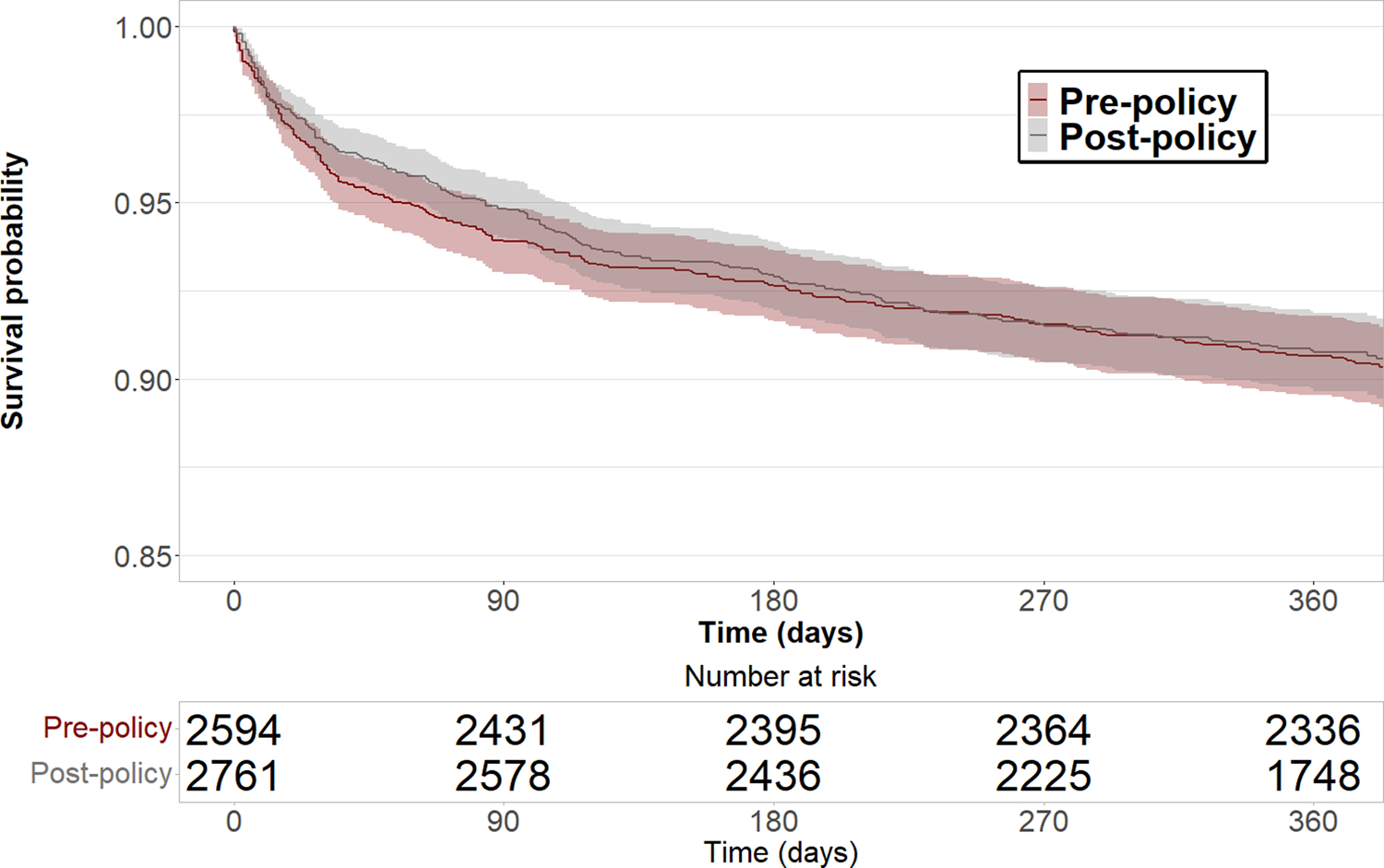
1-year post-transplant survival was not significantly different since implementation of the new heart allocation policy. Shaded regions indicate 95% confidence intervals.
3.3. Post-transplant survival estimates with limited follow-up
When ignoring observations after October 31, 2019, the median time at risk in the post-policy cohort decreased from 366 days (IQR: 335–396) to 154 days (IQR: 71–182). Repeating survival analysis on the same cohorts with truncated follow-up resulted in lower 1-year post-transplant survival in the post-policy cohort (90.6% [95% CI: 89.5%–91.8%] pre-policy vs. 82.2% [95% CI: 74.9%–90.2%] post-policy) (Figure 2). In contrast, the hazard ratio of transplant after policy implementation from an unadjusted Cox proportional hazards model was not significantly increased with incomplete follow-up (Figure S1). Cox hazard ratios are listed with follow-up truncated at November 1, 2019 (unadjusted HR = 1.04 [0.85–1.28]), May 1, 2020 (unadjusted HR = 1.04 [0.87–1.25]), November 1, 2020 (unadjusted HR = 0.99 [0.83–1.18]), and March 2, 2021 (unadjusted HR = 0.98 [0.82–1.17]).
Figure 2: Survival of heart transplant recipients before and after implementation of the new heart allocation policy with increasing follow-up.
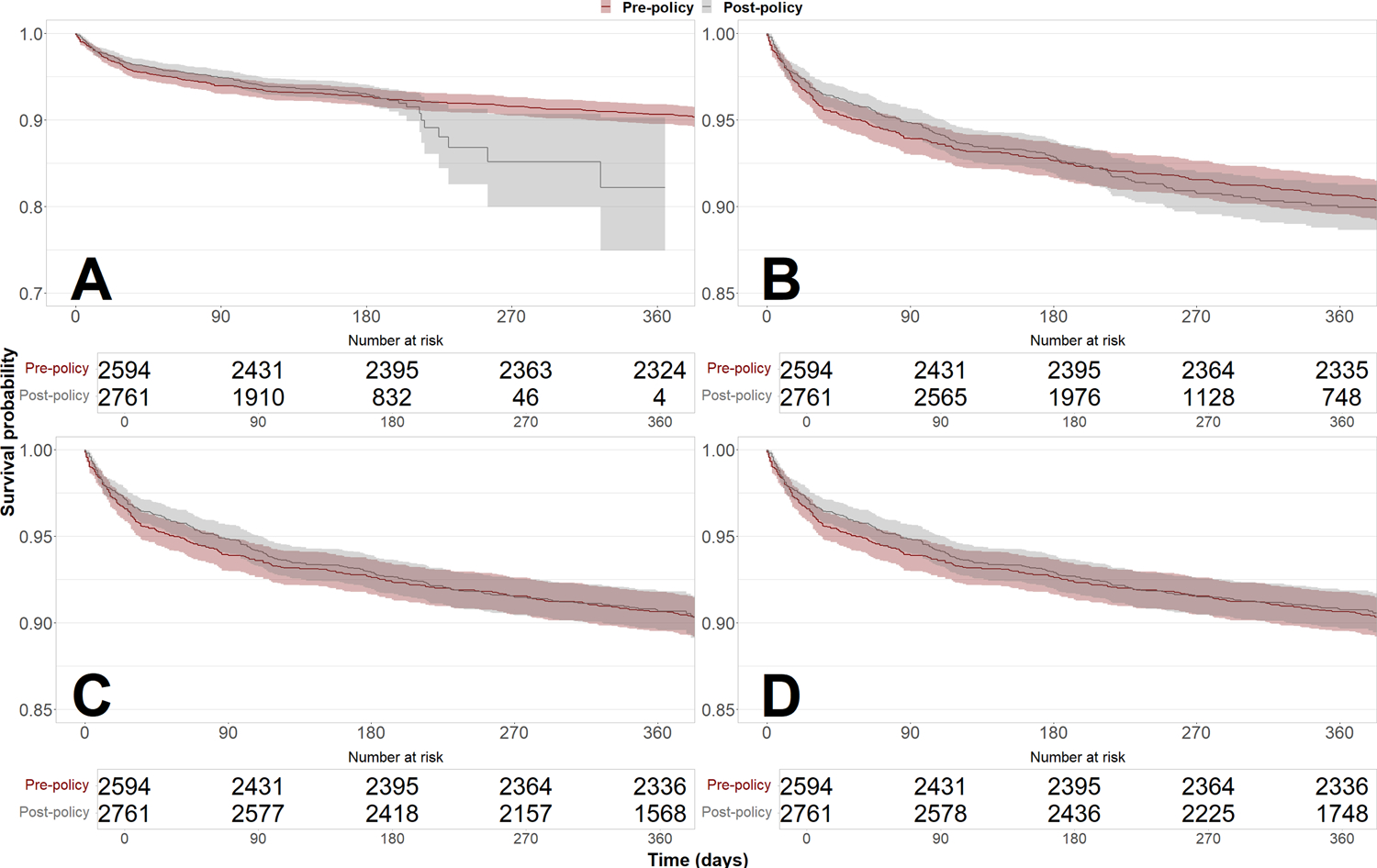
Estimates of 1-year post-transplant survival were biased downward by informative censoring with artificially truncated follow-up. However, the hazard ratio of transplant after policy implementation from an unadjusted Cox proportional hazards model was not significantly increased with truncated follow-up. Follow-up was truncated at November 1, 2019 (Panel A, log-rank P = 0.7, unadjusted HR = 1.04 [0.85–1.28]), May 1, 2020 (Panel B, log-rank P = 0.6, unadjusted HR = 1.04 [0.87–1.25]), and November 1, 2020 (Panel C, log-rank P = 0.9, unadjusted HR = 0.99 [0.83–1.18]). Panel D shows survival curves with full follow-up through March 2, 2021 (log-rank P = 0.8, unadjusted HR = 0.98 [0.82–1.17]).
3.4. Post-transplant survival by treatment
Post-transplant survival increased after policy implementation for recipients bridged with ECMO (1-year: 69.3% [59.6%–80.6%] pre-policy vs. 87.2% [81.8%–93.0%] post-policy, log-rank P < 0.001) and mechanical ventilation (68.2% [57.8%–80.4%] pre-policy vs. 82.9% [74.5%–92.2%] post-policy, log-rank P = 0.03) but was not significantly different for patients treated with IABP (log-rank P = 0.6) and durable LVAD (log-rank P = 0.3) (Figure 3). Post-transplant survival estimates were also not significantly different for patients treated with high- and low-dose inotropes, other mechanical circulatory support (MCS), and no MCS (Figure S2).
Figure 3. Survival of heart transplant recipients before and after implementation of the new heart allocation policy by treatment type.
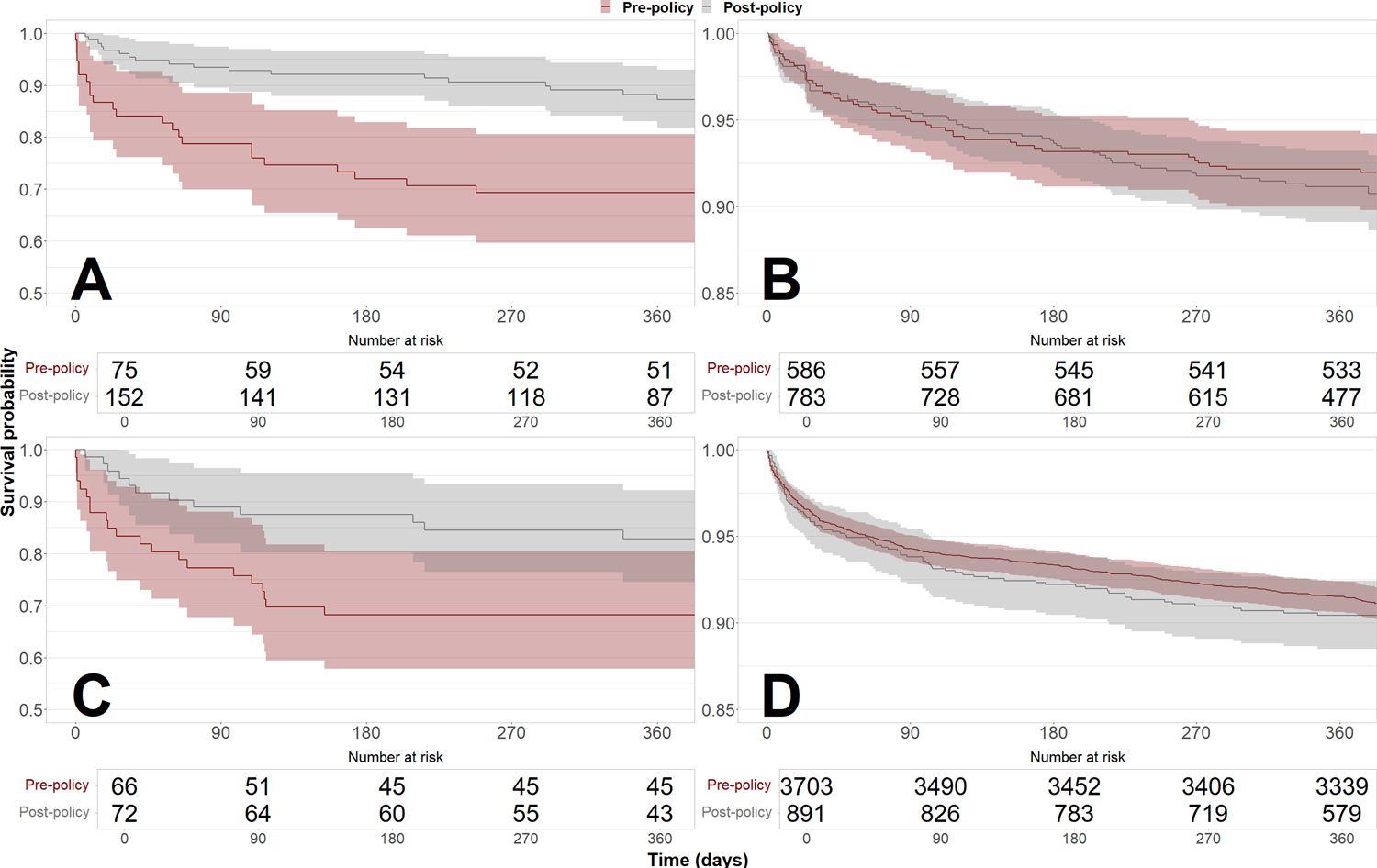
Recipients who were treated with ECMO before transplant (Panel A) experienced significantly increased 1-year survival in the post-policy cohort (69.3% [59.6%–80.6%] pre-policy vs. 87.2% [81.8%–93.0%] post-policy, log-rank P < 0.001). Recipients who were treated with IABP before transplant (Panel B) showed no significant difference in 1-year survival (92.1% [90.0%–94.3%] pre-policy vs. 91.1% [89.1%–93.2%] post-policy, log-rank P = 0.6). Recipients who were treated with mechanical ventilation before transplant (Panel C) experienced significantly increased 1-year survival in the post-policy cohort (68.2% [57.8%–80.4%] pre-policy vs. 82.9% [74.5%–92.2%] post-policy, log-rank P = 0.03). Recipients who were treated with durable LVAD before transplant (Panel D) showed no significant difference in 1-year survival (91.5% [90.6%–92.4%] pre-policy vs. 90.4% [88.5%–92.4%] post-policy, log-rank P = 0.3).
3.5. Sensitivity analyses
The estimated 1-year post-transplant survival was not significantly different for recipients transplanted November 1, 2015, to October 31, 2016 (91.8%, 95% CI: 90.7%–92.8%); November 1, 2017, to October 17, 2018 (91.8%, 95% CI: 90.8%–92.9%); and the entire pre-policy period November 1, 2015, to October 17, 2018 (91.4%, 95% CI: 90.8%–92.0%) (Tables S2 and S3).
4. Discussion
In this registry cohort study of 10,466 heart transplant recipients with median follow-up over one year, one-year post-transplant survival was not significantly different after implementation of the new heart allocation policy. Estimating post-policy recipient survival with limited follow-up biased the Kaplan-Meier estimate downwards. With adequate follow-up, one-year post-transplant survival increased for patients treated with ECMO and mechanical ventilation, and was unchanged for other treatment types.
Our findings confirm that previous reports of decreased post-transplant survival using limited follow-up were biased by informative censoring. The Kaplan-Meier survival estimator assumes that censoring is statistically independent of survival time,10 but different data submission requirements for recipient follow-up and recipient deaths can bias survival estimates when recipient follow-up is extremely limited. Transplant hospitals are required to notify the OPTN within 14 days of a recipient’s death or graft failure; in contrast, programs have until 30 days after the six-month and one-year anniversaries of a recipient’s transplant date to report survival.11 This systematic difference in post-transplant data submission leads to a downward bias on survival without adequate follow-up. These data submission requirements apply to all organ allocation systems governed by OPTN policies, so these results are potentially relevant for all evaluations of U.S. organ allocation policy changes. Our findings suggest that informative censoring bias should be considered in any cohort study with a similar design that analyzes national transplant registry data from the United States.
Differences in study design explain why some studies have found unchanged recipient survival while others have reported decreased survival after policy implementation. Studies that found decreased post-transplant survival post-policy defined the end of the post-policy cohort near or at the end of available follow-up data, creating heavy censoring. For example, the Cogswell et al. estimate of 90-day survival had follow-up beyond 50 days in less than a quarter (125 out of 539) of their post-policy cohort,3 and the Kilic et al. estimate of 1-year survival only had follow-up beyond 6 months in less than half (976 out of 2455) of the post-policy cohort.2
In contrast, Goff et al. and Hanff et al. designed their post-policy cohort end date to allow sufficient time for follow-up data to accumulate for post-policy recipients and found no significant difference in Kaplan-Meier estimated post-transplant survival.7,8 For example, Hanff et al. had follow-up beyond 100 days for 90 percent (355 out of 398) of post-policy recipients and found no significant difference in Kaplan-Meier estimated survival at 180 days. Our study provided 16 months between the end of the post-policy cohort and the last available follow-up, with follow-up through six months for nearly 90 percent (2436 out of 2761) of the post-policy cohort.
Our results show how just a few death events that occur in the context of heavy censoring can create large changes in the Kaplan-Meier survival curve estimate of a new policy change. However, when combined with administrative censoring at one year post-transplantation, we found that the hazard ratio estimate of the policy effect was not significantly different. This result can be explained by the relatively small contribution of these few events to the Cox proportional hazard model likelihood function. Our results suggest that a Cox proportional hazards model run on data administratively censored by calendar date may better evaluate early impacts of new allocation policy on post-transplant survival than Kaplan-Meier generated point estimates of specific survival times.
Previous reports proposed that observed decreases in post-transplant survival in the post-policy era were due to higher transplantation rates among high-acuity candidates.18,19 However, with adequate follow-up, we found that post-transplant survival has increased for recipients on ECMO and mechanical ventilation. More transplantation of urgent candidates with preserved post-transplant survival suggests a higher survival benefit of transplant under the new policy.20
4.1. Limitations
Though our study used more complete follow-up data than previous studies, there may have been residual informative censoring in the post-policy cohort. Even with 16 months of follow-up data after the last transplant in the post-policy cohort, 36.7% of post-policy recipients were censored prior to 1 year compared to 0.7% of recipients in the pre-policy cohort. Post-policy survival estimates may be even higher when more one-year follow-up appointments for post-policy recipients enter the SRTR dataset.
5. Conclusion
With adequate follow-up, 1-year post-transplant survival is not significantly different under the new heart allocation policy. Informative censoring can bias attempts to estimate policy effects on post-transplant survival.
Supplementary Material
Acknowledgements
We acknowledge Kevin Chung, BA; Stratton Tolmie, BA; and Sharon Zeng, BA, for assistance with study design and data analysis.
The data reported here have been supplied by the Hennepin Healthcare Research Institute (HHRI) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government.
This study was supported by career development award K08 HL150291 from the National Heart, Lung, and Blood Institute (awarded to Dr Parker) and funding from the University of Chicago Pritzker School of Medicine (awarded to Mr Lazenby).
Abbreviations:
- ECMO
extracorporeal membrane oxygenation
- IABP
intra-aortic balloon pump
- IMPACT
Index for Mortality Prediction After Cardiac Transplantation
- LVAD
left ventricular assist device
- MCS
mechanical circulatory support
- OPTN
Organ Procurement and Transplant Network
- SRTR
Scientific Registry of Transplant Recipients
- UNOS
United Network for Organ Sharing
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Supporting Information
Additional supporting information may be found online in the Supporting Information section at the end of the article.
Data Availability Statement
The data that support the findings of this study are available from the Scientific Registry of Transplant Recipients (SRTR). Restrictions apply to the availability of these data, which were used under license for this study.
References
- 1.Varshney AS, Hirji SA, Givertz MM. Outcomes in the 2018 UNOS donor heart allocation system: A perspective on disparate analyses. J Hear Lung Transplant. 2020;39(11):1191–1194. doi: 10.1016/j.healun.2020.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kilic A, Mathier MA, Hickey GW, et al. Evolving Trends in Adult Heart Transplant with the 2018 Heart Allocation Policy Change. JAMA Cardiol. 2020. doi: 10.1001/jamacardio.2020.4909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cogswell R, John R, Estep JD, et al. An early investigation of outcomes with the new 2018 donor heart allocation system in the United States. J Hear Lung Transplant. 2020;39(1):1–4. doi: 10.1016/j.healun.2019.11.002 [DOI] [PubMed] [Google Scholar]
- 4.Kilic A, Hickey G, Mathier MA, et al. Outcomes of the First 1300 Adult Heart Transplants in the United States after the Allocation Policy Change. Circulation. 2020;141(20):1662–1664. doi: 10.1161/CIRCULATIONAHA.119.045354 [DOI] [PubMed] [Google Scholar]
- 5.Jawitz OK, Fudim M, Raman V, et al. Reassessing Recipient Mortality Under the New Heart Allocation System: An Updated UNOS Registry Analysis. JACC Hear Fail. 2020;8(7):548–556. doi: 10.1016/j.jchf.2020.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trivedi JR, Slaughter MS. “Unintended” Consequences of Changes in Heart Transplant Allocation Policy: Impact on Practice Patterns. ASAIO Journal. 2020;66(2):125–127. doi: 10.1097/MAT.0000000000001128 [DOI] [PubMed] [Google Scholar]
- 7.Goff RR, Uccellini K, Lindblad K, et al. A change of heart: Preliminary results of the US 2018 adult heart allocation revision. Am J Transplant. 2020;20(10):2781–2790. doi: 10.1111/ajt.16010 [DOI] [PubMed] [Google Scholar]
- 8.Hanff TC, Harhay MO, Kimmel SE, Birati EY, Acker MA. Update to an early investigation of outcomes with the new 2018 donor heart allocation system in the United States. J Hear Lung Transplant. 2020;39(7):725–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parker WF, Churpek MM, Anderson AS. Is it too early to investigate survival outcomes of the new US heart allocation system? J Hear Lung Transplant. 2020;39(7):726. doi: 10.1016/j.healun.2020.01.1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campigotto F, Weller E. Impact of Informative Censoring on the Kaplan-Meier Estimate of Progression-Free Survival in Phase II Clinical Trials. JCO. 2014;32(27):3068–3074. doi: 10.1200/JCO.2014.55.6340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Organ Procurement and Transplant Network. OPTN Policies. Accessed August 5, 2021. https://optn.transplant.hrsa.gov/media/1200/optn_policies.pdf
- 12.Grodin JL, Ayers CR, Thibodeau JT, et al. Variation of heart transplant rates in the United States during holidays. Clin Transplant. 2014;28(8):877–882. doi: 10.1111/ctr.12396 [DOI] [PubMed] [Google Scholar]
- 13.Kamalia MA, Ramamurthi A, Rein L, Mohammed A, Joyce DL. Detection of Seasonal Trends in National Donor Heart Availability Using the UNOS Dataset. Journal of Cardiac Failure. 2019;25(8, Supplement):S174. doi: 10.1016/j.cardfail.2019.07.495 [DOI] [Google Scholar]
- 14.Parker WF, Garrity ER, Fedson S, Churpek MM. Potential impact of a shock requirement on adult heart allocation. The Journal of Heart and Lung Transplantation. 2017;36(9):1013–1016. doi: 10.1016/j.healun.2017.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kilic A, Allen JG, Weiss ES. Validation of the United States–derived Index for Mortality Prediction After Cardiac Transplantation (IMPACT) using international registry data. J Hear Lung Transplant. 2013;32(5):492–498. doi: 10.1016/j.healun.2013.02.001 [DOI] [PubMed] [Google Scholar]
- 16.Parker WF, Chung K, Anderson AS, Siegler M, Huang ES, Churpek MM. Practice Changes at U.S. Transplant Centers After the New Adult Heart Allocation Policy. Journal of the American College of Cardiology. 2020;75(23):2906–2916. doi: 10.1016/j.jacc.2020.01.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ran G, Chung K, Anderson AS, et al. Between-center variation in high-priority listing status under the new heart allocation policy. Am J Transplant. doi: 10.1111/ajt.16614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yancy CW, Fonarow GC. United Network for Organ Sharing 2018 Heart Transplant Reallocation Policy: Aiming for Evidence-Based Policy. JAMA Cardiol. 2021;6(2):168–168. doi: 10.1001/jamacardio.2020.5232 [DOI] [PubMed] [Google Scholar]
- 19.Ventura HO, Eisen H. When good intentions turn bad: A need for course correction. J Hear Lung Transplant. 2020;39(1):5–6. doi: 10.1016/j.healun.2019.12.001 [DOI] [PubMed] [Google Scholar]
- 20.Parker WF, Anderson AS, Gibbons RD, et al. Association of Transplant Center With Survival Benefit Among Adults Undergoing Heart Transplant in the United States. JAMA. 2019;322(18):1789–1798. doi: 10.1001/jama.2019.15686 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the Scientific Registry of Transplant Recipients (SRTR). Restrictions apply to the availability of these data, which were used under license for this study.


