Abstract
BAX and BAK are generally considered as fully interchangeable for mitochondrial permeabilization and consequent apoptotic cell death. Garcia-Saez and colleagues have recently documented striking kinetic differences that influence BAX and BAK oligomerization at the mitochondrial surface. These data have important implications for inflammatory responses driven by mitochondrial DNA.
Subject terms: Cell biology, Immune cell death, Inflammation
BCL2 associated X, apoptosis regulator (BAX) and BCL2 antagonist/killer 1 (BAK1, best known as BAK) are key mediators of intrinsic apoptosis, a regulated cell death (RCD) pathway that is initiated by perturbations of intracellular homeostasis, involves widespread and irreversible mitochondrial outer membrane permeabilization (MOMP), and is precipitated by cysteine proteases of the caspase family [1]. Specifically, BAX and BAK initiate MOMP by forming hetero- and homo-oligomeric pores in the outer mitochondrial membrane (OMM), in a process that is inhibited by anti-apoptotic proteins such as BCL2 apoptosis regulator (BCL2) and promoted by so-called BH3-only proteins, such as BH3 interacting domain death agonist (BID) [1]. Ultimately, MOMP results in the release of mitochondrial content into the cytosol, including not only direct and indirect caspase activators such as cytochrome c, somatic (CYCS) and diablo IAP-binding mitochondrial protein (DIABLO, best known as SMAC), respectively [1], but also molecules that may initiate inflammatory reactions, such as mitochondrial DNA (mtDNA) [2].
An abundant literature pioneered by the late Stanley Korsmeyer indicates that BAX and BAK are interchangeable for apoptotic MOMP and hence that (at least some degree of) protection against apoptotic stimuli only emerges in experimental systems lacking both proteins [3]. Recent findings from Garcia-Saez and collaborators demonstrate that in the absence of their partner in the apoptotic crime, BAX and BAK form homo-oligomers into the OMM of different size and according to strikingly distinct kinetics [4]. In physiological settings, this translates into an interplay whereby the relative availability of BAX and BAK has a major impact on the kinetic of pore formation at the OMM, ultimately influencing the release of mitochondrial content (especially mtDNA) into the cytosol [4].
Garcia-Saez and colleagues harnessed a panel of human and mouse cell lines lacking BAX, BAK or both, along with genetic strategies for the re-expression of fluorescently tagged BAX, BAK and/or SMAC and super-resolution microscopy, to investigate in detail the kinetic and structural organization of apoptotic pores elicited by canonical pro-apoptotic stimuli such as staurosporine and ABT-737. In the absence of BAX, apoptosis induction resulted in the rapid assembly of distinct BAK supramolecular architectures (namely, lines, arcs, rings, dots and aggregates) at the OMM, correlating with SMAC release into the cytosol. Similar results were obtained with supported lipid bilayers, which also revealed the existence of BAK pores displaying higher homogeneity and smaller size as compared to BAX pores achieved by the same approach in a previous work from the same team [5]. Direct comparison of BAX vs BAK oligomerization in cellula based on photon-counting confocal microscopy in combination with ratiometric analysis revealed that the latter proceeds rapidly but stops to reach a plateau of around 200 molecules shortly (10 min) after MOMP induction, while the former proceeds slowly (200 molecules were assembled in approx. 30 min after MOMP induction) but appear to steadily continue towards higher-order oligomers over time. Importantly, the possibility that such differences may reflect the differential subcellular localization of BAX (cytosolic) and BAK (mitochondrial) at baseline was discarded by using a BAX mutant that constitutively localizes to the OMM [4].
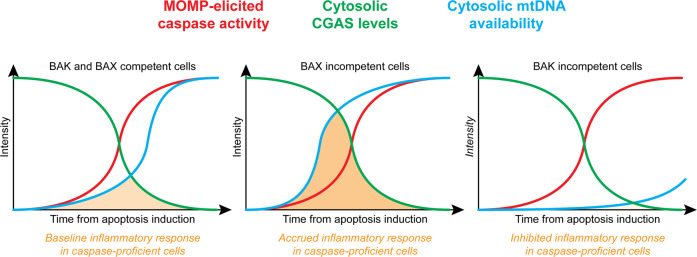
In cells co-expressing BAX and BAK, apoptotic pores formed according to a kinetic displaying intermediate features as compared to BAX-deficient and BAK-deficient settings. This suggests that BAX and BAK influence each other as they mediate MOMP, presumably as a consequence of the hetero-oligomeric nature of apoptotic pores formed in physiological conditions, and hence that the relative expression levels of BAX and BAK may influence the speed at which mitochondrial content is released during MOMP. To directly assess this hypothesis, Garcia-Saez and colleagues quantified cytosolic mtDNA in wild-type, BAX−/−, and BAK1−/− human osteosarcoma U2OS cells responding to an apoptotic stimulus, revealing that cells expressing BAK only (BAX−/−) released mtDNA much more rapidly than cells expressing BAX only (BAK1−/−), with little alterations in the kinetics of SMAC release [4].
Besides adding to our understanding of the molecular mechanisms whereby BAX and BAK regulate MOMP, these findings are particularly relevant as they suggest an unsuspected role for BAX as an inhibitor of rapid mtDNA release by BAK in cells undergoing MOMP. Cytosolic mtDNA is a potent activator of cyclic GMP-AMP synthase (CGAS), resulting in the stimulator of interferon response cGAMP interactor 1 (STING1)-dependent initiation of multiple transcriptional programs that culminate with pro-inflammatory cytokine secretion [6]. However, robust apoptotic caspase activation downstream of MOMP annihilates (rather than promotes) such inflammatory responses by a variety of mechanisms, including CGAS cleavage [7, 8].
This safeguard mechanism presumably reflects the key role of apoptotic cell death in (post-)embryonic development and the maintenance of adult tissue homeostasis [9], two settings in which uncontrolled inflammatory responses may have highly detrimental effects. However, the same pathway can be also harnessed by cancer cells undergoing apoptosis in response to therapy as a strategy to avoid immune recognition and the consequent activation of an immune response potentially targeting treatment-resistant cells [10]. Since the absence of BAX does not compromise (or even alter the kinetic of) apoptotic MOMP [3, 4], the data from Garcia-Saez and colleagues raise the intriguing possibility that inhibiting BAX in cancer cells may accelerate mtDNA release driven by apoptosis-inducing chemotherapeutics, perhaps providing CGAS with an operational window for STING1 activation and consequent secretion of inflammatory cytokines before being degraded by apoptotic caspases (Fig. 1). While apparently counterintuitive, this possibility is in line with a growing literature suggesting that multiple proteins originally discovered as cell death regulators, including caspases, may be dispensable for RCD to occur, but instead control RCD kinetic and immunological manifestations, at least in mammals [9]. Whether BAX is also one of these proteins remains to be experimentally elucidated.
Fig. 1. BAX and BAK modulation as a strategy to fine tune inflammatory responses driven by mitochondrial DNA.
In wild-type cells expressing BAX and BAK that undergo apoptosis, mitochondrial DNA (mtDNA) is released into the cytosol as a consequence of mitochondrial outer mitochondrial permeabilization (MOMP) with a kinetic that depends on relative BAX and BAK levels. This results in poor cyclic GMP-AMP synthase (CGAS) activation as it is generally preceded by active CGAS degradation by caspases. At least theoretically, the pharmacological or genetic inhibition of BAX or BAK may alter the kinetic of mtDNA release, but not that of MOMP-driven caspase activation, hence extending or restricting, respectively, the temporal window during which mtDNA co-exist with CGAS in the cytosol to drive inflammatory reactions.
From an opposite standpoint, dysregulated cytosolic mtDNA accumulation is involved in autoinflammatory conditions such as systemic lupus erythematosus (SLE) [11]. While this process has been largely ascribed to voltage-dependent anion channels (VDACs), it is tempting to speculate that BAK (rather than BAX) inhibitors may be beneficial in this setting. Indeed, VDACs interact with (and are regulated by) various Bcl-2 family members including BAX and BAK, implying that BAK inhibition may retard mtDNA release to ultimately allow for robust CGAS degradation by caspases prior to significant activation of STING1 (Fig. 1). Neither the involvement of BAX and BAK on VDAC-dependent mtDNA release, nor the impact of VDACs on the dynamic of BAX and BAK oligomerization has been investigated yet.
Despite these and other unresolved issues, the findings from Garcia-Saez and colleagues open the intriguing possibility that selectively targeting BAX or BAK may be harnessed as a strategy to fine tune inflammatory responses driven by MOMP (Fig. 1). Experimental validation of this possibility is urgently awaited.
Author contributions
TY and LG conceived the article, wrote the manuscript and prepared display items. LG addressed editorial requests.
Funding
The LG lab is supported by a Breakthrough Level 2 grant from the US DoD BRCP (#BC180476P1), by the 2019 Laura Ziskin Prize in Translational Research (#ZP-6177, PI: Formenti) from the Stand Up to Cancer (SU2C), by a Mantle Cell Lymphoma Research Initiative (MCL-RI, PI: Chen-Kiang) grant from the Leukemia and Lymphoma Society (LLS), by a startup grant from the Dept. of Radiation Oncology at Weill Cornell Medicine (New York, US), by a Rapid Response Grant from the Functional Genomics Initiative (New York, US), by industrial collaborations with Lytix Biopharma (Oslo, Norway) and Phosplatin (New York, US), and by donations from Phosplatin (New York, US), the Luke Heller TECPR2 Foundation (Boston, US), Sotio a.s. (Prague, Czech Republic), Onxeo (Paris, France), Ricerchiamo (Brescia, Italy), and Noxopharm (Chatswood, Australia).
Competing interests
LG has been holding research contracts with Lytix Biopharma and Phosplatin, and has received consulting/advisory honoraria from Boehringer Ingelheim, AstraZeneca, OmniSEQ, Onxeo, The Longevity Labs, Inzen, Phosplatin, and the Luke Heller TECPR2 Foundation. All other authors have no conflicts to declare.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Takahiro Yamazaki, Lorenzo Galluzzi.
References
- 1.Singh R, Letai A, Sarosiek K. Regulation of apoptosis in health and disease: the balancing act of BCL-2 family proteins. Nat Rev Mol Cell Biol. 2019;20:175–93. doi: 10.1038/s41580-018-0089-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McArthur K, Whitehead LW, Heddleston JM, Li L, Padman BS, Oorschot V, et al. BAK/BAX macropores facilitate mitochondrial herniation and mtDNA efflux during apoptosis. Science. 2018;359:eaao6047. doi: 10.1126/science.aao6047. [DOI] [PubMed] [Google Scholar]
- 3.Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–30. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cosentino K, Hertlein V, Jenner A, Dellmann T, Gojkovic M, Pena-Blanco A, et al. The interplay between BAX and BAK tunes apoptotic pore growth to control mitochondrial-DNA-mediated inflammation. Mol Cell. 2022;82:933–49. doi: 10.1016/j.molcel.2022.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salvador-Gallego R, Mund M, Cosentino K, Schneider J, Unsay J, Schraermeyer U, et al. Bax assembly into rings and arcs in apoptotic mitochondria is linked to membrane pores. EMBO J. 2016;35:389–401. doi: 10.15252/embj.201593384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamazaki T, Kirchmair A, Sato A, Buque A, Rybstein M, Petroni G, et al. Mitochondrial DNA drives abscopal responses to radiation that are inhibited by autophagy. Nat Immunol. 2020;21:1160–71. doi: 10.1038/s41590-020-0751-0. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez-Ruiz ME, Buque A, Hensler M, Chen J, Bloy N, Petroni G, et al. Apoptotic caspases inhibit abscopal responses to radiation and identify a new prognostic biomarker for breast cancer patients. Oncoimmunology. 2019;8:e1655964. doi: 10.1080/2162402X.2019.1655964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ning X, Wang Y, Jing M, Sha M, Lv M, Gao P, et al. Apoptotic Caspases Suppress Type I Interferon Production via the Cleavage of cGAS, MAVS, and IRF3. Mol Cell. 2019;74:19–31 e17. doi: 10.1016/j.molcel.2019.02.013. [DOI] [PubMed] [Google Scholar]
- 9.Galluzzi L, Lopez-Soto A, Kumar S, Kroemer G. Caspases Connect Cell-Death Signaling to Organismal Homeostasis. Immunity. 2016;44:221–31. doi: 10.1016/j.immuni.2016.01.020. [DOI] [PubMed] [Google Scholar]
- 10.Kroemer G, Galassi C, Zitvogel L, Galluzzi L Immunogenic cell stress and death. Nat Immunol. 2022. Online ahead of print. [DOI] [PubMed]
- 11.Kim J, Gupta R, Blanco LP, Yang S, Shteinfer-Kuzmine A, Wang K, et al. VDAC oligomers form mitochondrial pores to release mtDNA fragments and promote lupus-like disease. Science. 2019;366:1531–6. doi: 10.1126/science.aav4011. [DOI] [PMC free article] [PubMed] [Google Scholar]