Abstract
In the inner ear, the auditory and vestibular systems detect and translate sensory information regarding sound and balance. The sensory cells that transform mechanical input into an electrical signal in these systems are called hair cells. A specialized organelle on the apical surface of the hair cells called the hair bundle detects the mechanical signals. Displacement of the hair bundle causes mechanotransduction channels to open. The morphology and organization of the hair bundle, as well as the properties and characteristics of the mechanotransduction process, differ between the different hair cell types in the auditory and vestibular systems. These differences likely contribute to maximizing the transduction of specific signals in each system. This review will discuss the molecules essential for mechanotransduction and the properties of the mechanotransduction process, focusing our attention on recent data and differences between the auditory and vestibular systems.
Keywords: auditory, vestibular, hair cell, stereocilia, hair bundle, mechanotransduction, MET, MT, adaptation, frequency selectivity, cochlea, otolith
Introduction
The auditory and vestibular systems detect and process sensory information regarding sound and balance. Hair cells are the sensors of these stimuli. On the apical surface of hair cells protrudes a hair bundle composed of specialized actin-filled microvilli, termed stereocilia. Stereocilia are unique from microvilli in that they are thicker and organized in rows of ascending height (Figure 1A). The sensory inputs are mechanical in nature, but the brain processes information electrically. The hair bundle is responsible for converting the mechanical inputs into an electrical signal through the process termed mechanotransduction (also referred to as mechano-electric transduction, MT, or MET). Defects in the hair cell mechanotransduction process cause deafness and balance disorders (Petit and Richardson, 2009), demonstrating the critical role of mechanotransduction. In fact, many mouse models of deafness relating to mechanotransduction dysfunction are initially identified through an easily assessed behavioral vestibular phenotype of circling and head bobbing.
Figure 1.
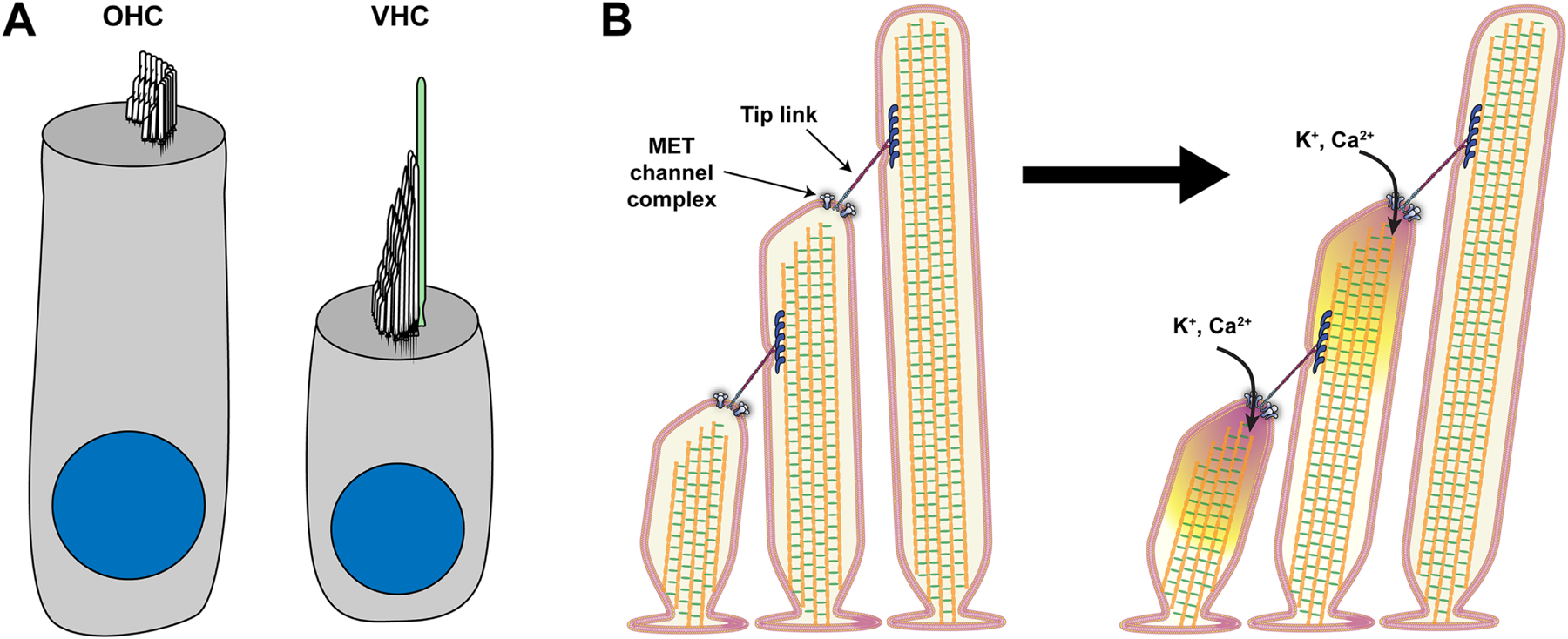
(A) Depiction of an auditory outer hair cell (OHC) and a vestibular hair cell (VHC). The actin-filled stereocilia hair bundles protrude from the hair cell’s apical surface. Vestibular hair cells also possess a kinocilium (green), a tubulin-based cilium, whereas, in auditory hair cells, the kinocilium disappears during development. (B) Taking a slice through the stereocilia rows depicts the mechanotransduction process. Tension within the tip link upon stereocilia deflection causes the opening of MET channels and the influx of potassium and calcium ions into Stereocilia.
Hair cell mechanotransduction is extremely sensitive. At the threshold of hearing, the auditory system is able to detect sound vibrations as small as the diameter of an atom (Hudspeth, 1997). This sensitivity relies on hair cells detecting and amplifying sound vibrations through the cochlear amplification process, which is a mechanical amplification in the cochlea (Hudspeth, 2008; Peng and Ricci, 2011). The hair bundle has a very intricate structure to transmit deflection of the hair bundle into a force that is detected by the mechanotransduction complex. At the core of this complex is the mechanotransduction channel itself, a mechanosensitive ion channel, which opens and closes in response to hair bundle deflection (Figure 1B). The channel complex modulates the current response depending on the stimulus size with larger current responses for larger hair bundle deflections. Adjacent rows of stereocilia are connected with extracellular proteins that form the tip links (Figure 1B). When stereocilia are deflected towards the tallest stereocilia, tension increases in tip links and is transmitted to the mechanotransduction channel complex to gate the channel. In vivo, the ions that flow through the mechanotransduction channel are K+ and Ca2+ due to the special composition of the fluid surrounding the hair bundle called endolympth (Figure 1B) (Bosher and Warren, 1971, 1978). This process is mostly studied using electrophysiology, where the hair cell is voltage-clamped, and mechanical stimuli are delivered to the hair bundle to elicit the mechanotransduction current. Studies of the mechanotransduction process have identified several properties, including activation, adaptation, and mechanics. These properties exist in both auditory and vestibular hair cells, but they differ quantitatively. The differences likely contribute to maximizing the transduction of system-specific signals.
Although there have been considerable developments in understanding hair bundle development and stereocilia structure (Pacentine et al., 2020; Richardson and Petit, 2019; Tadenev et al., 2019; Tarchini and Lu, 2019), these topics lay outside the scope of this review. In this review, we will discuss the proteins associated with the mechanotransduction channel complex and the properties of the mechanotransduction process, including the multiple timescales of adaptation. In addition, we will make an effort to highlight the differences observed between auditory and vestibular hair cells.
The molecular composition of the mechanotransduction complex
The vertebrate inner ear comprises the auditory and vestibular systems. In both systems, there are specialized hair cells able to transduce appropriate stimuli into a receptor current. Even though the complete molecular composition and structure of the mechanotransduction complex are still unknown, there has been notable progress in discovering and understanding the essential proteins for mechanotransduction in the last decade. In this section, we introduce the proteins essential for the mechanotransduction process and the differences between the auditory and vestibular systems (Table 1).
Table 1.
Summary of auditory and vestibular phenotypes of various mouse models and whether the expression is documented in the sensory hair cells.
CDH23 and PCDH15
Tip links were first discovered by electron microscopy in the mid- 1980s and hypothesized to be important for mechanotransduction channel gating (Pickles et al., 1984). Tip links rely on calcium ions to stabilize their structure and are severed by exposure to calcium chelators, causing a loss of mechanotransduction current (Assad et al., 1991; Indzhykulian et al., 2013). The tip links connect stereocilia in adjacent rows and are obliquely inclined and oriented along the hair bundle’s axis of mechanosensitivity (Figure 1B) (Pickles et al., 1984; Shotwell et al., 1981). High-resolution electron micrographs suggest that the tip link is composed of a helical dimer (Kachar et al., 2000). Cadherin 23 (CDH23) and protocadherin 15 (PCDH15) are the two proposed components of the tip links where a dimer of PCDH15 at the lower end of the tip link is suggested to interact with a dimer of CDH23 at the upper end of the tip link (Ahmed et al., 2006; Goodyear and Richardson, 2003; Kazmierczak et al., 2007; Siemens et al., 2004; Sollner et al., 2004). CDH23 and PCDH15 interact via their amino termini, and Ca2+ stabilizes this binding in a handshake-like interaction (Sotomayor et al., 2012). CDH23 and PCDH15 are important for cochlear and vestibular function; mutations in genes encoding CDH23 and PCDH15 cause Usher syndrome 1D and 1F, respectively, which is associated with hearing, vestibular, and visual dysfunction (Alagramam et al., 2001; Bolz et al., 2001; Bork et al., 2001; Di Palma et al., 2001). PCDH15 has three isoforms expressed in hair bundles (PCDH15-CD1, PCDH15-CD2, PCDH15-CD3) that differ in the C-terminal cytoplasmic domains (Ahmed et al., 2006). Mice lacking PCDH15-CD1 or PCDH15-CD3 have no auditory defects or circling behavior, whereas mice lacking PCDH15-CD2 are profoundly deaf but have normal vestibular function (Webb et al., 2011). In mice lacking PCDH15-CD2, tip links are present, MET can be recorded in immature hair cells, but hair bundles are abnormally polarized (Webb et al., 2011). During the molecular maturation process of the auditory MET machinery, there is a switch from a developmental form in which the PCDH15 isoforms are functionally redundant to a fully mature form in which PCDH15-CD2 is critical (Pepermans et al., 2014; Webb et al., 2011).
TMC1 and TMC2
The identity of the mechanotransduction channel remained elusive for many years. After first being proposed as a channel candidate, the role of transmembrane channel-like protein (TMC) in mechanotransduction continued to be debated for many years. TMC1 is associated with dominant (Beethoven – Bth mice) and recessive (Deafness – dn mice) forms of deafness (Kurima et al., 2002; Vreugde et al., 2002). Early studies in TMC1dn and TMC1Bth mice suggested a role of TMC1 in functional maturation of the hair cell, but not for mechanotransduction since mechanotransduction currents were normal at early postnatal ages (P8) (Marcotti et al., 2006). Later work showed that this was due to a switch in expression from TMC2 to TMC1 around P3 in the cochlea, and TMC2 could at least partially compensate for TMC1 deficiency (Kawashima et al., 2011). On the other hand, mice with a deletion in TMC2 are phenotypically normal (Kawashima et al., 2011). TMC1 is expressed in the auditory and vestibular hair cells, but mutations in TMC1 do not affect vestibular function (de Heer et al., 2011; Kurima et al., 2002), likely due to the fact that TMC2 expression is transient in the cochlea but persists in vestibular hair cells (Kawashima et al., 2011). TMC1 and TMC2 are both localized near the stereocilia tips of shorter-row stereocilia (Kawashima et al., 2011; Kurima et al., 2015), where transduction channels are localized (Beurg et al., 2009).
The key experiments that propelled TMCs as channel candidates were done with mutant mice lacking both TMC1 and TMC2 (Kawashima et al., 2011; Pan et al., 2013). Use of single and double knockout animals demonstrated that TMC1 and TMC2 can at least partially compensate for each other and are necessary for hair cell mechanotransduction (Kawashima et al., 2011). TMC1 point mutations resulted in altered channel properties, indicating that TMC1 was closely associated with the mechanotransduction channel, if not the mechanotransduction channel itself (Beurg et al., 2019; Beurg et al., 2015a; Beurg et al., 2021b; Corns et al., 2016; Pan et al., 2018; Pan et al., 2013).
In 2013, a second mechanosensitive current was discovered in TMC1 and TMC2 double knockout animals and after severing tip links with calcium-chelator exposure in wild-type hair cells (Kim et al., 2013; Kim and Fettiplace, 2013). This led to the possibility that TMC1 and TMC2 were not pore-forming components but rather required for the natural stimulation modality. This second mechanosensitive current was localized near the base of the hair bundle and not towards the tips of the hair bundle, and it was elicited by suction of the hair bundle regardless of direction (Beurg et al., 2016). In 2017, it was reported that this current was abolished in PIEZO2 knockout animals, whereas normal hair cell mechanotransduction was intact, indicating that the suction induced current originated from a separate channel in addition to the normal mechanotransduction channels (Wu et al., 2017). This helped to reinforce that TMC proteins were a component of the mechanotransduction channel.
Recent ideas likened TMC1 to the calcium-activated chloride channel (CaCC) TMEM16A (Hartzell et al., 2005) and facilitated a homology model of the TMC1 structure (Ballesteros et al., 2018; Pan et al., 2018). Experiments with modified amino acids to allow covalent attachment of bulky compounds showed reduced channel conductance, suggesting these amino acids are in the pore conducting pathway (Pan et al., 2018). In addition to its function in mechanotransduction, TMC1 is also suggested to have an additional role as a leak channel to modulate hair cell electrical properties (Liu et al., 2019). A further confirmation that TMCs are the pore-forming subunits of the mechanotransduction channel came from a demonstration, in a reduced system with purified proteins in liposomes, that green sea turtle TMC1 and budgerigar TMC2 are mechanically sensitive ion channels (Jia et al., 2020). To date, others have not been able to express TMC1/2 in any expression systems to demonstrate mechanosensitivity, and there are no reports of mammalian TMC1/2 in expression systems demonstrating ion channel activity. Thus, the current data indicate that TMC1 and TMC2 are pore-forming components of the mechanotransduction channel, but further work is still warranted to study the structure and mechanism of mechanosensitivity (for a more extensive review, see (Zheng and Holt, 2021)).
TMIE
In the last two decades, transmembrane inner ear (TMIE) protein has received interest in the hearing field because of its connection with hereditary deafness in humans. In particular, mutations in the TMIE gene are associated with DFNB6 characterized by a severe- to- profound non-syndromic sensorineural hearing loss. Initially, there were two mouse lines with a spontaneous deletion used as a model for the human DFNB6: spinner (sr) and circling (cir) mice. Sr and cir mice manifest with deafness, head tossing, circling, and hyperactivity (Cho et al., 2006; Deol and Robins, 1962). The TMIE gene is the only common deleted gene in the genomic deletions of sr and cir mice, suggesting TMIE is necessary for proper function in both auditory and vestibular systems. TMIE is expressed in inner hair cells (IHCs) and outer hair cells (OHCs) near the tips of the shorter rows of stereocilia (Zhao et al., 2014). Evidence showed that TMIE directly binds to the PCDH15- CD2 and can indirectly attach to LHFPL5 through PCDH15- CD1 and PCDH15- CD3 (Zhao et al., 2014). Studies in zebrafish and mice have shown that TMIE is critical for TMC1/2 localization and mechanotransduction function (Cunningham et al., 2020; Pacentine and Nicolson, 2019). Interestingly, recent studies in cochlear hair cells have shown that TMIE is an essential subunit of the mechanotransduction complex and that TMC1/2 cannot form a channel complex without TMIE (Cunningham et al., 2020). Although much work has been done regarding cochlear hair cells, very little work has approached the mammalian vestibular hair cell besides showing expression in the cells (Zhao et al., 2014).
LHFPL5/TMHS
Lipoma HMGIC fusion partner-like 5 protein (LHFPL5), also known as tetraspan membrane protein of hair cell stereocilia (TMHS), is a protein with four predicted transmembrane domains, and mutations in LHFPL5 cause deafness in humans and mice (Kalay et al., 2006; Longo-Guess et al., 2005; Shabbir et al., 2006). Mutations in LHFPL5 cause reduced macroscopic current, reduced single-channel conductance, slowed channel activation, and impaired fast adaptation (Beurg et al., 2015b; Xiong et al., 2012). LHFPL5 can bind PCDH15 and TMIE in vitro (Xiong et al., 2012; Zhao et al., 2014), and it can physically interact with and stabilize TMC1 in both heterologous expression systems and the soma and hair bundle of hair cells (Yu et al., 2020). Based on these functional and expression experiments, LHFPL5 is hypothesized to stabilize the expression of TMC1 (Beurg et al., 2015b; Yu et al., 2020). LHFPL5 mutant mice exhibit circling and head tossing, and LHFPL5 is expressed in vestibular hair cells and localized strongly to the stereocilia (Gyorgy et al., 2017; Longo-Guess et al., 2005; Longo-Guess et al., 2007; Xiong et al., 2012). Studies in zebrafish have also shown auditory and vestibular functional deficits when LHFPL5 is disrupted (Erickson et al., 2019).
CIB2 and CIB3
Ca2+- and integrin-binding (CIB) proteins are putative auxiliary mechanotransduction channel subunits. CIB2 mutations are associated with non-syndromic deafness and Usher Syndrome 1J (Patel et al., 2015; Riazuddin et al., 2012; Seco et al., 2016), although the association with Usher Syndrome 1J is questioned by another study (Booth et al., 2018). Cochlear hair cells predominantly express CIB2, whereas both CIB2 and CIB3 are expressed in vestibular hair cells (Giese et al., 2017; Liang et al., 2021). CIB2 mouse mutants mirror the non-syndromic deafness and present with hearing loss without vestibular deficits (Giese et al., 2017; Liang et al., 2021; Michel et al., 2017). The lack of a vestibular deficit is likely due to CIB3 compensating for the loss of CIB2 in vestibular hair cells (Giese et al., 2017; Liang et al., 2021). In cochlear hair cells, CIB2 and CIB3 are functionally interchangeable, and they regulate both the assembly and function of the mechanotransduction channel complex (Liang et al., 2021). Also, the expression pattern of CIB2 fits with a role in mechanotransduction, where CIB2 is localized along the stereocilia length with an accumulation toward the tip of the shorter stereocilia in IHCs, OHCs, and vestibular hair cells (Giese et al., 2017; Michel et al., 2017; Riazuddin et al., 2012).
TOMT
Transmembrane O-methyltransferase (TOMT/LRTOMT) is responsible for non-syndromic deafness DFNB63. TOMT and the TMCs interact within the secretory pathway to traffic TMC proteins to the hair bundle (Erickson et al., 2017). TOMT is critical for assembling the mechanotransduction machinery of hair cells because it is required for TMC1/2 localization in the stereocilia, but TOMT itself is not localized to stereocilia (Cunningham et al., 2017; Erickson et al., 2017). No studies have tested the importance of TOMT in the mammalian vestibular hair cells; however, a vestibular phenotype is present in zebrafish due to a lack of mechanotransduction (Erickson et al., 2017).
LOXHD1
LOXHD1 is associated with hearing loss DFNB77, and as part of a mutagenesis screen, LOXHD1 was identified as causing hearing loss in the samba mouse line (Grillet et al., 2009). LOXHD1 is localized along the plasma membrane of stereocilia in hair cells. Lack of LOXHD does not affect the development of the hair bundle but causes degeneration of hair cells with age (Grillet et al., 2009). LOXHD1 is important for mechanotransduction, but its precise role is unclear (Trouillet et al., 2021). At P11, inner hair cells have severely diminished mechanotransduction current amplitudes without showing a morphological hair bundle defect or reduction in tip-link number. Vestibular hair cells express reduced amounts of LOXHD1, but vestibular function is not affected in samba mice (Grillet et al., 2009).
Different hair cells, different hair bundle morphology
Hair cells differ in the cochlear and vestibular system (Figure 1A), but they all detect mechanical motions through the hair bundle. The two systems process different types of information, likely leading to the different hair cell types. A major property that can differentiate hair cell types is the hair bundle morphology (Figure 1A). In this section, we discuss the mode of stimulation in different sensory organs, the types of hair cells present in the inner ear, the morphological hair bundle differences, and how the morphology can affect the detection and processing of stimuli.
The cochlea is a spiral-shaped organ that detects high frequencies at the base and low frequencies at the apex. In the cochlea, there are two types of hair cells: inner hair cells (IHCs) and outer hair cells (OHCs), organized as one row of IHCs and three rows of OHCs that sit atop a basilar membrane running along the entire spiral shape. A gelatinous tectorial membrane lays atop the hair cells and is coupled to the OHC stereocilia, so the motion of the basilar membrane in relation to the tectorial membrane causes shearing of the OHC hair bundle and activation of the mechanotransduction channels to drive the cochlear amplifier (Peng and Ricci, 2011). IHCs are considered to play a purely sensory role, encoding and transmitting information via the cochlear nerve fibers to auditory nuclei in the brainstem. IHC stereocilia are generally thought to be free-standing (i.e., not attached to the tectorial membrane) and thought to be deflected by the sound-induced local motion of the endolymphatic fluid (Guinan, 2012; Hoshino, 1976, 1977; Kimura, 1966; Lenoir et al., 1987; Lim, 1972; Von Békésy, 1960). However, there has long been evidence of an attachment of the IHC stereocilia to the tectorial membrane in the base of the cochlea (Hoshino, 1976; Lenoir et al., 1987; Lim, 1986), and more recently, filamentous structures have been postulated to connect IHC stereocilia to the tectorial membrane in the apex of the cochlea (Hakizimana and Fridberger, 2021). These findings raise doubts about the stimulation mode of IHC stereocilia in vivo. The tectorial membrane is also hypothesized to act as a calcium source near stereocilia, where it can regulate calcium levels surrounding the stereocilia and affect the hair bundles’ mechanical and mechanotransduction properties (Hakizimana and Fridberger, 2021; Strimbu et al., 2019). Stereocilia attachments to structures like the tectorial membrane affect the loading and coordination of stereocilia, which determine how stereocilia move when stimulated.
The peripheral vestibular system consists of three semicircular canals that detect angular acceleration and two otolithic organs, the utricle and saccule, that detect linear acceleration. At the end of each semicircular canal, we find the ampulla containing a cluster of hair cells with stereocilia that project up into a gelatinous cupula. Rotation of the head induces inertial pressure by the fluid within the canal against the gelatinous cupula, which in turn causes displacement of the hair bundle (Goldberg, 2012). The utricle detects linear motion primarily in the horizontal plane, while the saccule detects motion primarily in the vertical plane. Atop the hair bundles of otolithic organs lies the otolithic membrane, a gelatinous material with overlying calcium carbonate crystals called otoconia. Thus, hair bundles are displaced from the inertia of the otoconia, and in this way, the hair cells can sense postural changes of head position using inertia and gravity (Goldberg, 2012). Some of the hair bundles in the utricle are tall enough to be embedded within the otolithic membrane, but other hair bundles are too short (Lim, 1976). These shorter hair bundles may be connected to the otolithic membrane via their kinocilium, which could provide a different stimulus type from embedded hair bundles; however, shorter hair bundles may also be stimulated by a meshwork that exists below the otolithic membrane or by fluid motion itself (Lim, 1984; Takumida et al., 1992). Although we have good hypotheses surrounding how hair bundles are stimulated, some uncertainties still exist which can affect the type of stimuli that various hair bundles receive.
In all five organs of the vestibular system, there are two types of hair cells: type I and II. Type II hair bundles are generally shorter and smaller (Li et al., 2008). Bundle heights affect the hair cell’s operating range and sensitivity Longer stereocilia have a greater operating range since larger displacements of the hair bundle tip can result in the same angular stimulation; therefore, a larger range of displacements can be encoded. The number of stereocilia in a hair bundle will dictate the amplitude of the mechanotransduction current since more stereocilia will generally mean a greater number of channels are available to be activated. Longer stereocilia will also have a lower hair bundle stiffness (if force is applied to the stereocilia tip). Therefore, the structural properties of the hair bundle have functional implications on stimulus encoding in the hair cell.
Cochlear hair bundles differ from vestibular bundles in a few ways. IHC and OHC bundles are organized in three rows of stereocilia in an open “V” or “W” shape, while vestibular hair bundles have more than 3 rows of stereocilia and are more cohesive (Figure 1A) (Peng et al., 2011). Vestibular hair bundles are taller than cochlear hair bundles and are specialized to detect lower frequencies. During development, the kinocilium is lost in the cochlear hair bundle, but it persists in vestibular hair bundles. The kinocilium is important during development for cell polarity; it is resorbed in mouse cochlear hair cells around ten days after birth (Kikuchi and Hilding, 1965; Kimura, 1966), however, it re-forms after mechanical injury (Sobkowicz et al., 1995). The kinocilium remains in vestibular hair bundles after development, where it could play a role during stimulus detection (e.g., being embedded in the otolithic membrane). The structural differences between cochlear and vestibular hair bundles likely reflect the differences in the type of stimuli they are tuned towards.
Mechanotransduction process: properties and kinetics
Frequency selectivity
Tonotopy in the cochlea refers to the organization of detecting decreasing frequencies from base to apex of the cochlea. The OHCs are the cochlear amplifiers, as they selectively amplify specific frequencies along the cochlear length; thus, different OHCs have become specialized to detect different frequencies. Multiple properties likely contribute to the hair cells’ ability to detect a specific frequency (Figure 2). The structure of the cochlea and the mechanical properties of the basilar membrane on which the hair cells sit have a large contribution to the hair cells’ frequency selectivity and the characteristic traveling wave of the cochlea (Altoe and Shera, 2020; Olson et al., 2012). In addition to the mechanical properties of the basilar membrane and shape of the cochlea, the morphology and the mechanical properties of the hair bundle, as well as the intrinsic properties of the mechanotransduction process, vary tonotopically. These variations can contribute to the hair cells’ ability to maximize the signal of specific frequencies.
Figure 2.
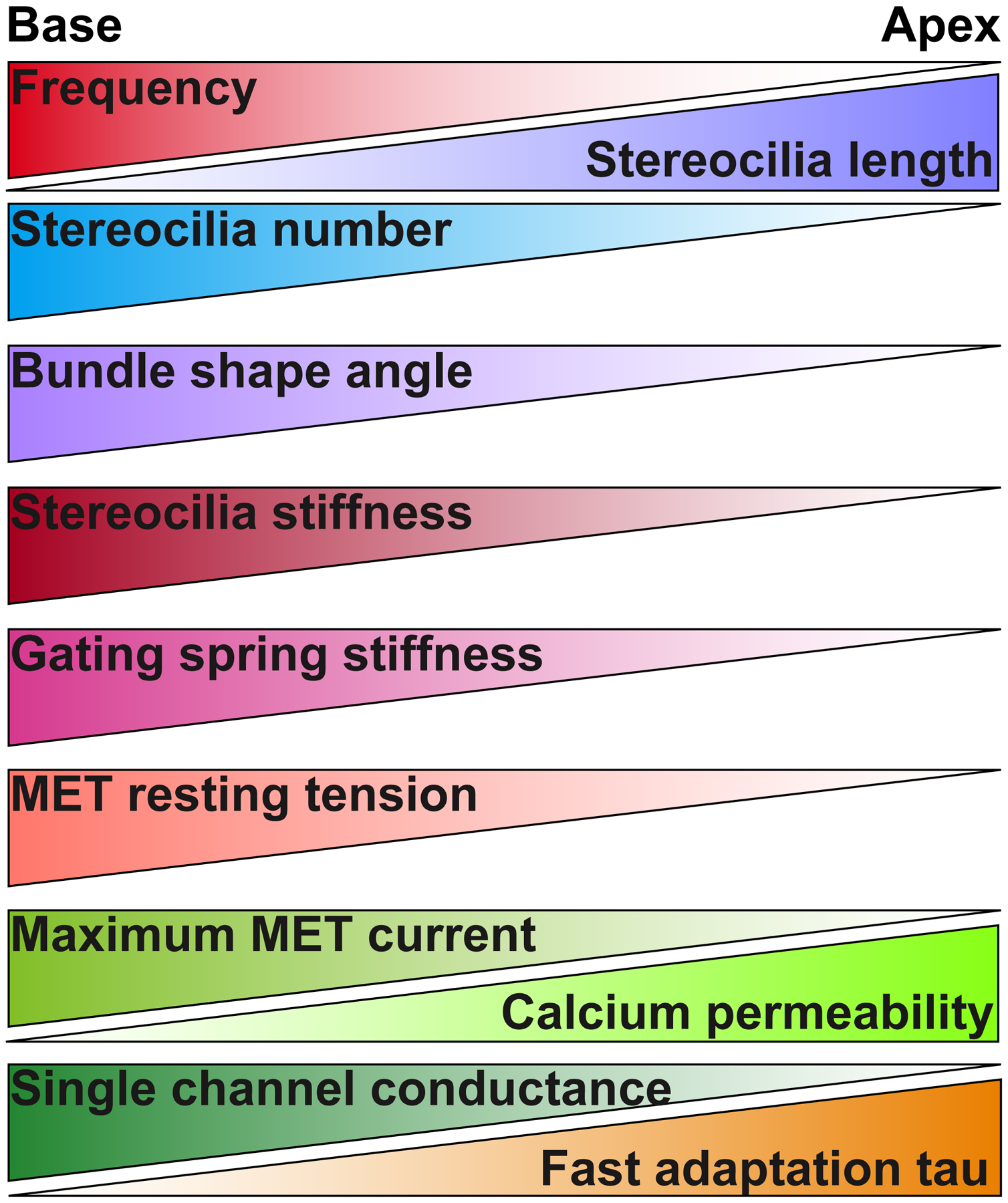
Hair bundle and mechanotransduction properties that vary tonotopically in OHCs. Stereocilia length (Garfinkle and Saunders, 1983; Kaltenbach et al., 1994). Stereocilia number (Garfinkle and Saunders, 1983; Lim, 1986). Bundle shape angle (Lim, 1986). Stereocilia stiffness (Tobin et al., 2019). Gating spring stiffness (Tobin et al., 2019). MET resting tension (Tobin et al., 2019). Maximum MET current (Kim and Fettiplace, 2013). Single channel conductance (Beurg et al., 2018; Beurg et al., 2006). Calcium permeability (Kim and Fettiplace, 2013). Fast adaptation tau (Ricci et al., 2005; Waguespack et al., 2007).
Hair bundle morphology varies along the length of the cochlea. Cochlear hair cells in various mammals can encode a frequency range from tens of Hz to over 100 kHz (Peng and Ricci, 2011). Differences in hair bundle morphology affect the mechanical properties of the hair bundle and likely help to enhance the cell’s frequency selectivity (Figure 2). Stereocilia length changes along the cochlea, decreasing from apex to base in both IHCs and OHCs (Garfinkle and Saunders, 1983; Kaltenbach et al., 1994). Longer stereocilia would exhibit less stiffness, therefore, would be sensitive to lower frequencies. Quantitative analysis of stereocilia number per hair cell shows a decrease toward the apex (Garfinkle and Saunders, 1983; Lim, 1986). Lower stereocilia numbers can also decrease the hair bundle stiffness, with fewer stereocilia contributing to the overall stiffness, although attachment to the tectorial membrane and hair bundle cohesiveness will affect this. Mammalian hair bundles have a characteristic “V” or “W” formation. Basal hair cells have a wider shape angle, while apical hair cells have a narrower shape (Lim, 1986). Some hypothesize that the characteristic shape may reduce the fluid dynamic resistance of fluid movement between the reticular lamina (i.e., the surface of the hair cell epithelium) and the tectorial membrane (Ciganovic et al., 2017; Pau and Pau, 2006), however, the reasoning for the tonotopic variations has not been explored. All these properties affect the mechanical properties of the hair bundle, which would change the type of signals maximally detected by the hair bundle.
The differences in bundle morphology discussed thus far influence the mechanical proprieties of the stereocilia; however, mechanical properties are also imparted by micro-scale structures of the mechanotransduction apparatus. The gating spring is a mechanical construct connected to mechanotransduction channels and gates the channel (i.e., opens and closes the channel) (Corey and Hudspeth, 1983b; Howard and Hudspeth, 1987). When tip links are broken, the gating spring is lost, therefore, the tip link was originally thought to be the gating spring itself (Assad et al., 1991; Gillespie and Corey, 1997). Later work suggested that the tip link itself may be too rigid based on its structure (Kachar et al., 2000; Sotomayor et al., 2012). More recently, the idea that the tip link could be the gating spring has resurfaced based on mechanical models and single-molecule force experiments demonstrating tip-link elasticity (Araya-Secchi et al., 2016; Bartsch et al., 2019; Choudhary et al., 2020; Dionne et al., 2018; Mulhall et al., 2021). However, any component connected to the tip link to convey the force from shearing/bending of the stereocilia to the mechanotransduction channels can contribute to the gating spring, including any connections to the stereocilia core or any interaction partners of tip-link components. The tip link acts as a link in the chain to transmit force to the channel, such that if a middle link in the chain is broken (e.g., the bond between Pcdh15 and Cdh23), then the whole chain no longer transmits force; therefore, any component of the chain could be contributing to the gating spring stiffness, and the total stiffness of the gating spring will be dominated by the most compliant link in the chain. The gating spring is also under resting tension, which is demonstrated by the movement of the hair bundle upon tip-link breakage (Assad et al., 1991; Tobin et al., 2019). This resting tension is critical for maintaining the sensitivity of the mechanotransduction channel and is hypothesized to be generated by myosin VIIa (Li et al., 2020). The resting tension and the gating spring stiffness are also parameters that vary tonotopically (Figure 2) (Tobin et al., 2019). It is unclear whether the gating spring itself acts as a non-linear spring where increased tension will change its stiffness. Definitively identifying the molecular components of the gating spring will allow a better understanding of the mechanotransduction process.
In addition to the mechanical properties of the hair bundle, there are intrinsic properties of the mechanotransduction process that contribute to the hair cell response. The maximum mechanotransduction current helps to determine how much the hair cell depolarizes to a given stimulus. The size of depolarization is dictated by the number of channels present in the cell, the conductance of each individual channel (i.e., the amount of current that passes through a single ion channel), and the input resistance of the cell (i.e., how leaky the membrane of the cell is since lower input resistance will require more current to depolarize the cell). The number of stereocilia and tip links in a hair bundle will determine the number of channels. It is generally thought that two channels exist per tip link based on the MET current amplitude, tip link number, and measured single-channel conductance (Beurg et al., 2006; Beurg et al., 2009). For OHCs, but not IHCs, the maximum current is greater in the base than the apex (Figure 2) (Kim and Fettiplace, 2013). This is partly contributed by the greater number of stereocilia in basal OHCs as compared to apical OHCs (Lim, 1986), partly by the gradient in single-channel conductance (Beurg et al., 2018; Beurg et al., 2006), and partly by differences in calcium permeability (Kim and Fettiplace, 2013).
There are two predominant methods used to measure single-channel conductance in hair cells. The first uses calcium chelators (e.g., EGTA or BAPTA) to break tip links such that there is only one remaining tip link in the hair bundle (Ricci et al., 2003). With this method, measured single-channel conductance is greater in basal OHCs than in apical OHCs (Beurg et al., 2018; Beurg et al., 2006). There has been an increased interest in synchronous gating of channels, potentially mediated by the lipid bilayer, which could lead to a larger apparent single-channel conductance by having more channels per tip link in the base of the cochlea that are activated synchronously (Beurg et al., 2018; Gianoli et al., 2017). While this idea is attractive, definitive proof is still lacking. The second method for determining single-channel conductance uses noise or fluctuation analysis of the macroscopic mechanotransduction current (Holton and Hudspeth, 1986). However, recent data have shown that this method, when applied to mammalian cochlear hair cells, can create artifacts and underestimate the conductance value due to the fast activation speed of the mechanotransduction channel, raising the question as to whether values determined by this method are accurate (Beurg et al., 2021a).
When analyzing the maximum mechanotransduction current and the single-channel conductance of the cell, the calcium permeability of the channel needs to be considered. In some instances, the single-channel conductance values vary across different studies (Beurg et al., 2018; Beurg et al., 2006; Beurg et al., 2015a; Pan et al., 2018; Pan et al., 2013). Calcium permeability is important to consider because calcium ions act as permeable blockers to the mechanotransduction channel (Peng et al., 2016; Ricci and Fettiplace, 1998). We state it as “calcium” permeability since this is generally what is referred to in the field, but it is important to note that other divalents also act as permeable blockers (Crawford et al., 1991; Hacohen et al., 1989; Peng et al., 2016), where magnesium is the most common other divalent ion used in experiments. Therefore, the concentration of divalent ions used in the extracellular solution in various experiments needs to be accounted for when making comparisons. As the calcium permeability of the channel changes with different TMC mutations, isoforms, and tonotopic position (Beurg et al., 2019; Beurg et al., 2018; Beurg et al., 2015a; Kim and Fettiplace, 2013; Pan et al., 2013), it could change the divalent permeable block of the channel, making it more difficult to compare single-channel conductance measurements. The best way to disentangle single-channel conductance and calcium permeability may be to record single-channel conductances in low calcium, zero magnesium extracellular solution (at least 150 μM Ca2+, since at this concentration, all of the calcium permeable block of the channel was released (Peng et al., 2016), however, this value may change based on tonotopic position and channel modifications) and measure the calcium permeability ratios as currently performed.
Vestibular epithelia are divided into striola/central and extrastriola/peripheral zones. The orientation of hair bundles varies in the utricle, making the organ sensitive to various directions. Mammalian utricular hair cells primarily detect nonperiodic signals and have a low-frequency capability, detecting a frequency range of 0 (DC, direct current) to 2 Hz (Goldberg et al., 1990). Nerve fibers that innervate hair cells in the striola are more often irregular firing afferents and transmit more phasic signals (Eatock, 2018; Eatock and Songer, 2011). Nerve fibers that innervate hair cells in the extrastriola are generally regular firing afferents and transmit tonic signals. A general organizing principle of the vestibular epithelia like the tonotopy observed in the cochlea is lacking, but recent work suggests that tuning for fast and slow responses and developmental sequence may be an organizing principle of the larval zebrafish utricle (Liu et al., 2021). Little data exist on the quantification of mammalian vestibular hair bundle ultrastructure (Bagger-Sjoback and Takumida, 1988; Lapeyre et al., 1992; Lim, 1976). One study in guinea pigs found that vestibular hair bundles have 70–100 stereocilia (Bagger-Sjoback and Takumida, 1988), similar to the number of stereocilia found in cochlear hair cells (Lim, 1986). Single-channel conductance measurements in vestibular hair cells indicate similar conductances to auditory hair cells when using similar calcium concentrations, but maximum mechanotransduction currents are less than half the amplitude in vestibular hair cells as compared to auditory hair cells (Figure 3A and C) (Beurg et al., 2006; Caprara et al., 2020; Michalski et al., 2009; Pan et al., 2013). These discrepancies warrant further investigations. Additionally, whether mechanotransduction properties differ in different regions of the utricle has not been thoroughly explored. One study of mechanotransduction properties in striolar and extrastriolar hair cells found no significant difference, but this study also had not accounted for the later modified definitions of striola and extrastriola in the mouse (Eatock and Songer, 2011; Vollrath and Eatock, 2003). More studies of vestibular ultrastructure and mechanotransduction are clearly needed.
Figure 3.
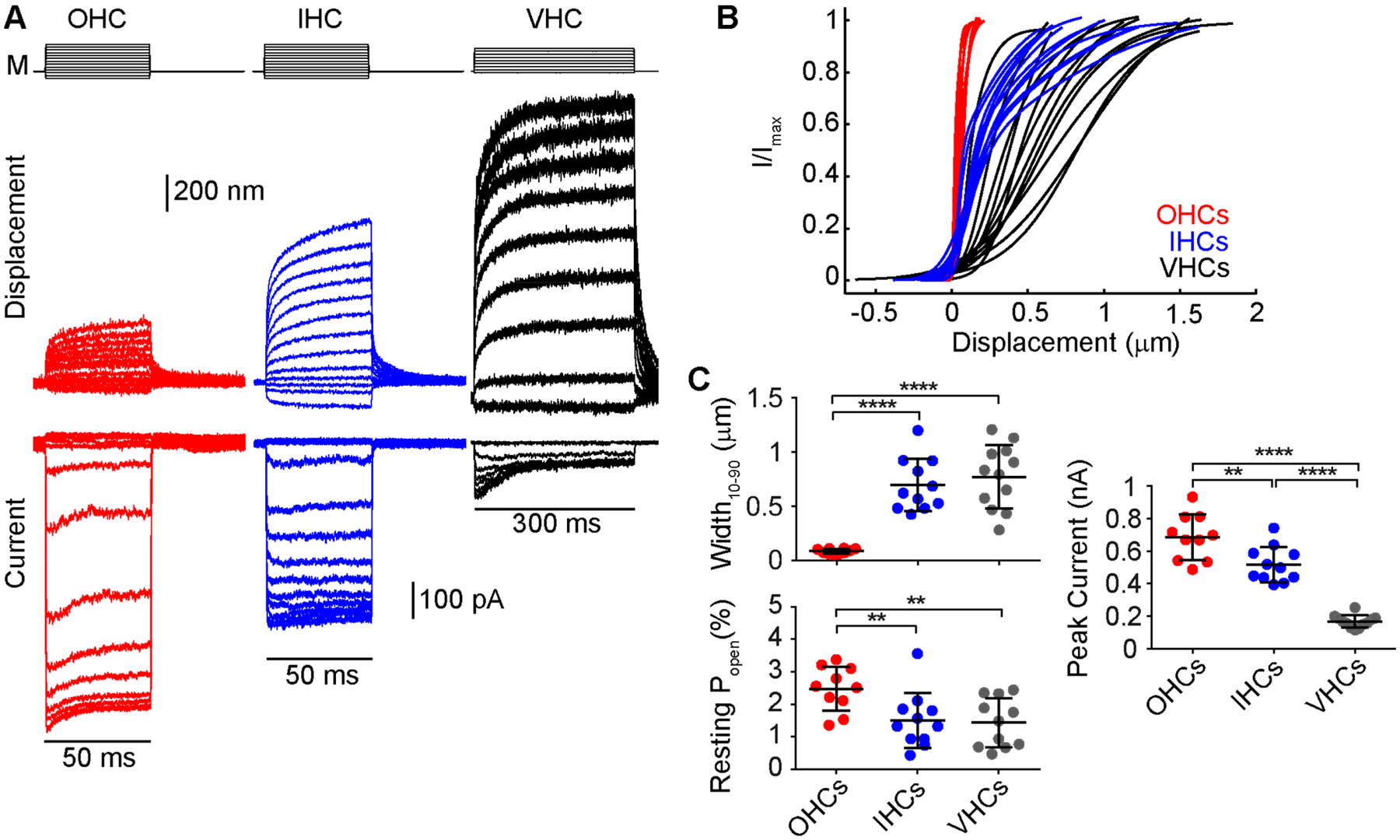
Activation curves in IHCs and vestibular hair cells are significantly wider than those of OHCs. (A) Example of a family of curves (bottom) in mouse cochlear hair cells (OHC-red and IHC-blue) and a mouse utricular type II hair cell (black) taken using a fluid-jet stimulus (M) and extracting the displacement of the hair bundle (middle) using high-speed imaging to generate the activation curves (Caprara et al., 2020). (B) Activation curves for multiple different cells in the same recording configuration across different cell types. Cells were recorded using an intracellular solution containing 0.1 mM BAPTA. n = 10 OHCs, P7-P9; n = 11 IHCs, P7-P8; n = 12 vestibular type II hair cells, P8-P11. (C) Summary plots of the width, resting open probability and maximum mechanotransduction current amplitude indicate a significantly lower peak current, wider activation curve, and lower resting probability in IHCs and vestibular hair cells. **** p < 0.0001 ** p < 0.01 Student’s t-test.
Activation curve and Operating Range
The activation curve of the mechanotransduction channel describes the sensitivity of the channel to displacements of the hair bundle. The curve takes on a sigmoidal shape and is often fit by a double Boltzmann equation (Figure 3B), which represents a two closed and one open state channel model (Corey and Hudspeth, 1983b). The operating range describes the displacement amplitudes that result in a measurable change in the mechanotransduction current, and the size of the range can be quantified as the width of the activation curve. The wider the activation curve, the less sensitive because the slope of the curve is shallower, but the channel can encode a greater range of stimuli. On the other hand, a narrower activation curve is more sensitive but encodes a smaller range of stimuli. IHCs and OHCs have different activation curve properties. IHCs have wider activation curves than OHCs (Figure 3B) (Peng et al., 2013). The resting open probability of the mechanotransduction channel quantifies the fraction of channels in the open state when no stimulus is presented to the hair bundle. The resting open probability, often expressed as a percentage, helps to describe the set point of the channel and indicates the cell’s position along the activation curve when no stimulation is present. In vivo, the resting open probability of IHCs is also lower than OHCs. OHCs have about 50% resting open probability (Beurg et al., 2010; Corns et al., 2014; Johnson et al., 2011; Peng et al., 2013), such that they are at the steepest part of the activation curve, which makes them most sensitive to small movements of the hair bundle. In contrast, IHCs have an estimated 20% resting open probability in vivo (Corns et al., 2014; Johnson et al., 2011). These differences in the activation curve may have arisen from the different functions that IHCs (detectors) and OHCs (amplifiers) have in the cochlea.
Vestibular hair cells also appear to be specialized for detecting a wide range of stimuli. The activation curve of vestibular hair cells is wider than the activation curve in OHCs, and the resting open probability is more comparable to IHCs than OHCs when recorded with similar conditions (Figure 3B) (Caprara et al., 2020; Peng et al., 2013). The sensitivity of the vestibular hair cell is also lower than OHCs. It was proposed that the activation curve was similar between OHCs and vestibular hair cells when accounting for the angular stimulation of the hair bundle (Geleoc et al., 1997), however, that is not our experience and may be a result of the previous report using neonatal hair cells where mechanotransduction properties have not fully matured (Lelli et al., 2009; Waguespack et al., 2007). We find that, on average, mouse vestibular type II cells have an activation curve nine times wider than mouse OHCs (Figure 3C, VHC: 0.77 ± 0.29 μm, OHC: 0.083 ± 0.023 μm). When compared to IHCs, the width of the activation curve is similar quantitatively, but qualitatively, the shapes of the curves are different, with IHCs having a steep initial rise, followed by a shallow roll-off (Figure 3B). The vestibular hair bundle is taller than the cochlear hair bundle, which can account for some of the difference, but not all of it. When comparing similarly processed tissue to account for artifacts of dehydration (Miller et al., 2021), mouse vestibular type II hair bundles are on the order of 6–7 μm tall, and cochlear OHC bundles are about 3 μm tall (Carlton et al., 2021; Li et al., 2008). Taller hair bundles will exhibit greater motion for the same angular movement. However, with the nine times difference in width, the vestibular hair bundles would need to be 27 μm tall to account for the sensitivity difference. Another way to measure the effect of bundle height differences is to estimate the stretch of tip links using the geometrical gain, estimated as 0.047 for vestibular hair cells and 0.12 for OHCs (Holt et al., 1997; Tobin et al., 2019). The 2–3 fold difference in geometrical gain also cannot fully account for the nine times difference in the width of the activation curve. Therefore, the mechanotransduction machinery of vestibular hair bundles must be inherently less sensitive than that of OHC hair bundles, and the mechanistic molecular differences for this are yet to be discovered.
Activation
The activation kinetics of the mammalian cochlear hair cell mechanotransduction channel is faster than most ion channels and has not been directly measured to date. The fast speed of channel activation is used to argue for a direct tethering of the tip link to the mechanotransduction channel, however, little to no data currently exist to substantiate this hypothesis (Effertz et al., 2015; Peng et al., 2011). Recently, more attention has been paid to the lipid interactions of the mechanotransduction channel, raising the possibility that channel activation may occur through the lipid bilayer like many other mechanosensors (Beurg et al., 2018; Cunningham et al., 2020; Effertz et al., 2017; Gianoli et al., 2017; Jia et al., 2020; Peng et al., 2016; Zheng and Holt, 2021). The time constant of OHC mechanotransduction activation has also been estimated recently to be as fast as a 4 μs and 10 μs time constant for basal and apical hair cells at room temperature, respectively (Beurg et al., 2021a). This would yield 10–90 rise times (i.e., time for the response to go from 10% to 90% of the maximum value) of 8.8 μs and 22 μs. With rise times this fast, direct measurements of the activation kinetics will be difficult with even the best traditional stimulation probes that have rise times of 11 μs (Peng et al., 2013). Ideally, probes would be more than two times faster than the response rise time. The use of nanomechanical force probes that have rise times of 5 μs would provide sufficient speed of stimulation for many regions of the cochlea (Doll et al., 2012), however, patch-clamp recordings are limited by clamp speed which is also required to be more than two times faster than the activation kinetics. Typically hair cells have a cell capacitance of 5–10 pF, which would require that compensated series resistance be less than 0.5 MΩ to be at least two times faster than the channel’s activation time constant Additionally when using in vitro stimulation techniques, the stimulus probe often only contacts a subset of stereocilia (Nam et al., 2015), which causes uneven stimulation of stereocilia and can result in desynchronizing channel activation across the hair bundle and slow the measured activation kinetics. Fluid jet stimuli also can cause uneven stimulation of hair bundles, requiring other stimulation methods to be developed (Peng et al., 2021). Some recent studies have developed other stimulations techniques, but more data is required to fully understand their limits (Abeytunge et al., 2021; Azimzadeh et al., 2018). These technical hurdles limit the ability to measure mechanotransduction channel activation time constants in mammalian OHCs directly.
The activation kinetics of mammalian vestibular hair cells is likely slower than cochlear hair cells since the detected frequency range is much lower. Activation kinetics of 380–750 μs have been measured in the turtle auditory hair cells (Ricci et al., 2005), and we would expect vestibular hair cells to have activation kinetics of this order or slower, which are well within the stimulation and clamp speed limitations. Vestibular hair cells are also not limited by uneven stimulation issues since the hair bundle structure is more cohesive (Figure 1A). These measurements are currently theoretically attainable with some effort.
Adaptation
Hair cells are able to adapt during sustained stimuli to shift the range of their sensitivity (Corey and Hudspeth, 1983a; Crawford et al., 1989; Eatock et al., 1987). This adaptation allows cells to maintain a high sensitivity (i.e., narrow width of the activation curve) while also encoding a larger range of stimuli effectively increasing the cell’s dynamic range Experimentally, adaptation is observed in two ways: (1) a decline in the mechanotransduction current in response to a sustained hair bundle displacement, and (2) a parallel shift in the set point of the activation curve without a change in activation curve width. In hair cells of lower vertebrates such as the frog and turtle, at least two adaptation processes have been described that differ in their kinetics (i.e., the rate that they occur), termed fast and slow adaptation with time constants of several milliseconds and tens of milliseconds, respectively (Vollrath and Eatock, 2003; Wu et al., 1999). Tonotopic variations of fast adaptation have been described, where fast adaptation is faster in the base than the apex of the cochlea (Figure 2) (Ricci et al., 2005; Waguespack et al., 2007). The gradient in adaptation time constants is hypothesized to contribute to the mechanical filtering properties of the mechanotransduction process (Ricci et al., 2005). Previously, both fast and slow adaptation processes were thought to require calcium to enter the cell to mediate the adaptation process (Assad and Corey, 1992; Crawford et al., 1991; Eatock et al., 1987; Kennedy et al., 2003; Ricci and Fettiplace, 1997; Ricci et al., 1998). For fast adaptation, it was thought that calcium bound to the channel itself, or a location very close to the channel due to the faster kinetics (Choe et al., 1998; Crawford et al., 1989; Howard and Hudspeth, 1988; Stauffer et al., 2005). For slow adaptation, it was discovered that this mechanism required myosin motor activity, and it was hypothesized that calcium bound to a myosin motor, proposed to be myosin Ic, to cause the myosin motors to slip down the side of stereocilia to release tension in the tip link; this hypothesized mechanism is often referred to as the motor model of adaptation (Assad and Corey, 1992; Holt et al., 2002; Howard and Hudspeth, 1987; Walker and Hudspeth, 1996; Yamoah and Gillespie, 1996). The motor requirement of slow adaptation led it to be sometimes referred to as slow motor adaptation. Contrary to these proposed mechanisms, fast adaptation in cochlear hair cells has been more recently shown not to require calcium entry, and this idea fits with observations that calcium permeability and adaptation rates can change in TMC variants in a manner opposite to that which would support fast adaptation relying on calcium (Caprara et al., 2019; Goldring et al., 2019; Peng et al., 2013). In cochlear hair cells, slow adaptation was confirmed to require calcium entry and myosin motor function. However, the proposed mechanism of slipping down the sides of stereocilia to release tension has been challenged as well as the specific requirement of myosin Ic function, where another myosin motor may compensate (Caprara et al., 2020). Fewer studies have been performed in mammalian vestibular hair cells on slow adaptation, but what we do know is that some mechanistic properties are the same, including the calcium reliance and lack of support for the motor model of adaptation, but others differ, where myosin Ic function is required for slow adaptation in vestibular hair cells (Caprara et al., 2020; Holt et al., 2002; Stauffer et al., 2005).
For decades, it was thought that fast and slow adaptation were the only mechanisms for regulating the set point of the mechanotransduction channel; therefore, when the set point was altered, it was assumed that adaptation was involved. However, data now indicate that set point regulation and adaptation can be decoupled, where there is an effect on either set point or adaptation but not the other (Caprara et al., 2020; Liang et al., 2021; Peng et al., 2016). These results suggest that there are other mechanisms to control the set point of the channel other than fast and slow adaptation. For example, increases in resting open probability from lowering extracellular calcium and depolarization were thought to be driven by effects of fast and slow adaptation; however, use of the tarantula toxin GsMTx4 could block the modulation without affecting the fast adaptation process, but effects on slow adaptation were not explicitly tested (Peng et al., 2016). Additionally, the rate of the resting open probability change with depolarization was on the order of 100 ms, which is slower than fast or slow adaptation, suggesting a different mechanism. Another mode of mechanotransduction channel modulation during a prolonged depolarization was found to decrease resting open probability on the order of seconds (Peng et al., 2013; Peng et al., 2016). One could argue that these are still adaptation mechanisms since it adjusts the set point, but regardless of what they are called, they are separate mechanisms from the classically thought fast and slow adaptation since they operate on timescales different from fast and slow adaptation. Other mechanisms also exist to modulate the mechanotransduction channel set point independent of adaptation like cyclic adenosine monophosphate (cAMP) (Ricci and Fettiplace, 1997). Further work is needed to elucidate these additional mechanisms.
The type of stimulation used in vitro for mechanotransduction experiments can lead to differences in the manifestation of adaptation (Caprara et al., 2019). There are two common types of stimulators: (1) stiff probes that directly couple to stereocilia and deliver step-like displacement and (2) fluid jets that deliver force stimuli by ejecting extracellular solution onto hair bundles from a pipette tip (Caprara et al., 2019; Dinklo et al., 2007; Kros et al., 1992; Peng et al., 2013; Peng and Ricci, 2016). Previous experiments showed that in rat OHCs, stiff probes with a rise time as short as 11 μs provided finer temporal resolution of the adaptation processes. Fast adaptation resulting from these fast probes during a short 5 ms duration stimulus could be fitted with two exponential decays with average time constants of about 0.2 ms and 1 ms (Peng et al., 2013). Delivering longer stimuli of 50 ms duration with the stiff probe required three time constants, with the third time constant ranging between 8 and 50 ms. We classify the two faster time constants as fast adaptation and could represent two separate fast processes, and the slowest time constant represents the slow adaptation process that requires myosin motors (i.e., slow motor adaptation).
In contrast, mechanotransduction currents elicited by a fluid jet do not show fast-current decays (with an exponential decay time constant < 5 ms) and only exhibit slow-current decays with about 20 ms time constants (Caprara et al., 2020; Caprara et al., 2019). The fluid jet stimulus uses a jet of fluid to stimulate the hair bundle, therefore delivers a force stimulus rather than a displacement stimulus delivered by the stiff probe. Imaging of the bundle motion during a step-like force stimulation using a fluid jet reveals a mechanical creep, which is a continued movement in the direction of the applied force (Caprara et al., 2019). This creep indicates an increasing effective hair bundle compliance as observed by others (Howard and Hudspeth, 1987; Kennedy et al., 2005; Kros et al., 2002; Russell et al., 1989). The creep was previously correlated with the adaptation process, therefore suggested that the myosin motors would slip down the sides of the stereocilia to release tension in tip links to mediate slow adaptation (Assad and Corey, 1992; Howard and Hudspeth, 1987). The hair bundle creep appears to mask the fast adaptation process. When the fluid-jet stimulus is shaped to deliver a step-like displacement stimulus, a fast-current decay is unmasked (Caprara et al., 2019). This result suggests that the hair bundle creep could be a manifestation of fast adaptation. The masking of fast adaptation is also advantageous when studying slow adaptation, since visualization of the slow adaptation in the current decay is much easier when fast adaptation is masked (Caprara et al., 2020; Caprara et al., 2019). Studies conducted in this way confirmed that slow adaptation requires myosin motor function, depends on calcium entry, is not correlated with the creep, and requires new models of the slow adaptation mechanism (Caprara et al., 2020).
In search of a new mechanism for slow adaptation, we have turned to the essential role of phosphatidylinositol 4,5-bisphosphate (PIP2) in slow adaptation (Hirono et al., 2004). The role of lipid constituents on ion channel properties has been increasing, and mechano-sensitive ion channels naturally interact with the lipid membrane. The most well-known signaling lipid is PIP2, and it has been shown to regulate multiple ion channels and bind to the channels themselves (Harraz et al., 2020; Hille et al., 2015; Robinson et al., 2019). PIP2 has been shown to affect mammalian hair cell mechanotransduction properties, including set point and single-channel conductance, suggesting it may also bind the mechanotransduction channel or components of the channel (Cunningham et al., 2020; Effertz et al., 2017). Studying the function of PIP2 in slow adaptation could lead to a new mechanistic understanding of the slow adaptation process.
Adaptation in mammalian vestibular hair cells has different properties as compared to cochlear hair cells. Fast adaptation in vestibular hair cells occurs with a time constant on the order of 5 ms (Songer and Eatock, 2013; Vollrath and Eatock, 2003). This time constant is more similar to the slower fast adaptation time constant observed in the cochlea and may indicate the absence of the fastest time constant on the order of 0.2 ms observed in the cochlea (Peng et al., 2013; Waguespack et al., 2007). The slow adaptation time constant observed in vestibular hair cells is on the order of 50 ms and may be comparable to the slow motor adaptation time constant in the cochlea, although, in the cochlea, it appears to be closer to about 20 ms (Caprara et al., 2020; Songer and Eatock, 2013; Stauffer and Holt, 2007; Stauffer et al., 2005; Vollrath and Eatock, 2003). Further studies in different vestibular hair cell types are required for measuring adaptation properties to help determine the types and frequency of signals that the cells are tuned towards and how vestibular hair cells process balance signals.
Concluding remarks
Although the study of hair cell mechanotransduction has occurred for many decades, some of the mechanisms still remain elusive. The discovery of the molecular components of the mechanotransduction machinery will surely fuel a better understanding of these mechanisms. Classically only two forms of adaptation were recognized, but now more mechanisms of modulating the mechanotransduction channel on different time scales are becoming apparent and require further study.
While many mechanotransduction studies have been done in cochlear hair cells, mammalian vestibular hair cells appear to have different characteristics that may be more specific to the information they encode. While it is often that findings in vestibular type hair cells are applied to mammalian cochlear hair cells, these two types of hair cells also have distinct properties and are very likely different in their implementation of specific processes on the molecular level. Studies in vestibular hair cells are an underappreciated line of research that deserves further efforts to understand how our balance system encodes signals to serve its functions.
Highlights.
Substantial progress has been made in identifying the components of the mechanotransduction machinery.
New data have revised hypotheses of how hair cell mechanotransduction mechanisms work
Differences exist between cochlear and vestibular hair cells in terms of morphology and response properties, therefore mechanistic differences may exist as well.
Acknowledgements:
Work was supported by R01 DC016868, AG073997, DC018786, DC018842, and R21 DC019701.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References:
- Abeytunge S, Gianoli F, Hudspeth AJ, Kozlov AS, 2021. Rapid mechanical stimulation of inner-ear hair cells by photonic pressure. Elife 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed ZM, Goodyear R, Riazuddin S, Lagziel A, Legan PK, Behra M, Burgess SM, Lilley KS, Wilcox ER, Griffith AJ, Frolenkov GI, Belyantseva IA, Richardson GP, Friedman TB, 2006. The tip-link antigen, a protein associated with the transduction complex of sensory hair cells, is protocadherin-15. J Neurosci 26, 7022–7034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagramam KN, Murcia CL, Kwon HY, Pawlowski KS, Wright CG, Woychik RP, 2001. The mouse Ames waltzer hearing-loss mutant is caused by mutation of Pcdh15, a novel protocadherin gene. Nat Genet 27, 99–102. [DOI] [PubMed] [Google Scholar]
- Altoe A, Shera CA, 2020. The cochlear ear horn: geometric origin of tonotopic variations in auditory signal processing. Sci Rep 10, 20528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araya-Secchi R, Neel BL, Sotomayor M, 2016. An elastic element in the protocadherin-15 tip link of the inner ear. Nat Commun 7, 13458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assad JA, Corey DP, 1992. An active motor model for adaptation by vertebrate hair cells. J Neurosci 12, 3291–3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assad JA, Shepherd GM, Corey DP, 1991. Tip-link integrity and mechanical transduction in vertebrate hair cells. Neuron 7, 985–994. [DOI] [PubMed] [Google Scholar]
- Azimzadeh JB, Fabella BA, Kastan NR, Hudspeth AJ, 2018. Thermal Excitation of the Mechanotransduction Apparatus of Hair Cells. Neuron 97, 586–595 e584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagger-Sjoback D, Takumida M, 1988. Geometrical array of the vestibular sensory hair bundle. Acta Otolaryngol 106, 393–403. [DOI] [PubMed] [Google Scholar]
- Ballesteros A, Fenollar-Ferrer C, Swartz KJ, 2018. Structural relationship between the putative hair cell mechanotransduction channel TMC1 and TMEM16 proteins. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartsch TF, Hengel FE, Oswald A, Dionne G, Chipendo IV, Mangat SS, El Shatanofy M, Shapiro L, Muller U, Hudspeth AJ, 2019. Elasticity of individual protocadherin 15 molecules implicates tip links as the gating springs for hearing. Proc Natl Acad Sci U S A 116, 11048–11056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurg M, Barlow A, Furness DN, Fettiplace R, 2019. A Tmc1 mutation reduces calcium permeability and expression of mechanoelectrical transduction channels in cochlear hair cells. Proc Natl Acad Sci U S A 116, 20743–20749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurg M, Cui R, Goldring AC, Ebrahim S, Fettiplace R, Kachar B, 2018. Variable number of TMC1-dependent mechanotransducer channels underlie tonotopic conductance gradients in the cochlea. Nat Commun 9, 2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurg M, Evans MG, Hackney CM, Fettiplace R, 2006. A large-conductance calcium-selective mechanotransducer channel in mammalian cochlear hair cells. J Neurosci 26, 10992–11000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurg M, Fettiplace R, Nam JH, Ricci AJ, 2009. Localization of inner hair cell mechanotransducer channels using high-speed calcium imaging. Nat Neurosci 12, 553–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurg M, Goldring AC, Fettiplace R, 2015a. The effects of Tmc1 Beethoven mutation on mechanotransducer channel function in cochlear hair cells. J Gen Physiol 146, 233–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurg M, Goldring AC, Ricci AJ, Fettiplace R, 2016. Development and localization of reverse-polarity mechanotransducer channels in cochlear hair cells. Proc Natl Acad Sci U S A 113, 6767–6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurg M, Nam JH, Chen Q, Fettiplace R, 2010. Calcium balance and mechanotransduction in rat cochlear hair cells. J Neurophysiol 104, 18–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurg M, Nam JH, Fettiplace R, 2021a. The speed of the hair cell mechanotransducer channel revealed by fluctuation analysis. J Gen Physiol 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurg M, Schimmenti LA, Koleilat A, Amr SS, Oza A, Barlow AJ, Ballesteros A, Fettiplace R, 2021b. New Tmc1 Deafness Mutations Impact Mechanotransduction in Auditory Hair Cells. J Neurosci 41, 4378–4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurg M, Xiong W, Zhao B, Muller U, Fettiplace R, 2015b. Subunit determination of the conductance of hair-cell mechanotransducer channels. Proc Natl Acad Sci U S A 112, 1589–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolz H, von Brederlow B, Ramirez A, Bryda EC, Kutsche K, Nothwang HG, Seeliger M, del CSCM, Vila MC, Molina OP, Gal A, Kubisch C, 2001. Mutation of CDH23, encoding a new member of the cadherin gene family, causes Usher syndrome type 1D. Nat Genet 27, 108–112. [DOI] [PubMed] [Google Scholar]
- Booth KT, Kahrizi K, Babanejad M, Daghagh H, Bademci G, Arzhangi S, Zareabdollahi D, Duman D, El-Amraoui A, Tekin M, Najmabadi H, Azaiez H, Smith RJ, 2018. Variants in CIB2 cause DFNB48 and not USH1J. Clin Genet 93, 812–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bork JM, Peters LM, Riazuddin S, Bernstein SL, Ahmed ZM, Ness SL, Polomeno R, Ramesh A, Schloss M, Srisailpathy CR, Wayne S, Bellman S, Desmukh D, Ahmed Z, Khan SN, Kaloustian VM, Li XC, Lalwani A, Riazuddin S, Bitner-Glindzicz M, Nance WE, Liu XZ, Wistow G, Smith RJ, Griffith AJ, Wilcox ER, Friedman TB, Morell RJ, 2001. Usher syndrome 1D and nonsyndromic autosomal recessive deafness DFNB12 are caused by allelic mutations of the novel cadherin-like gene CDH23. Am J Hum Genet 68, 26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosher SK, Warren RL, 1971. A study of the electrochemistry and osmotic relationships of the cochlear fluids in the neonatal rat at the time of the development of the endocochlear potential. J Physiol 212, 739–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosher SK, Warren RL, 1978. Very low calcium content of cochlear endolymph, an extracellular fluid. Nature 273, 377–378. [DOI] [PubMed] [Google Scholar]
- Caprara GA, Mecca AA, Peng AW, 2020. Decades-old model of slow adaptation in sensory hair cells is not supported in mammals. Sci Adv 6, eabb4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprara GA, Mecca AA, Wang Y, Ricci AJ, Peng AW, 2019. Hair bundle stimulation mode modifies manifestations of mechanotransduction adaptation. J Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton AJ, Halford J, Underhill A, Jeng JY, Avenarius MR, Gilbert ML, Ceriani F, Ebisine K, Brown SDM, Bowl MR, Barr-Gillespie PG, Marcotti W, 2021. Loss of Baiap2l2 destabilizes the transducing stereocilia of cochlear hair cells and leads to deafness. J Physiol 599, 1173–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho KI, Suh JG, Lee JW, Hong SH, Kang TC, Oh YS, Ryoo ZY, 2006. The circling mouse (C57BL/6J-cir) has a 40-kilobase genomic deletion that includes the transmembrane inner ear (tmie) gene. Comp Med 56, 476–481. [PubMed] [Google Scholar]
- Choe Y, Magnasco MO, Hudspeth AJ, 1998. A model for amplification of hair-bundle motion by cyclical binding of Ca2+ to mechanoelectrical-transduction channels. Proc Natl Acad Sci U S A 95, 15321–15326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary D, Narui Y, Neel BL, Wimalasena LN, Klanseck CF, De-la-Torre P, Chen C, Araya-Secchi R, Tamilselvan E, Sotomayor M, 2020. Structural determinants of protocadherin-15 mechanics and function in hearing and balance perception. Proc Natl Acad Sci U S A 117, 24837–24848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciganovic N, Wolde-Kidan A, Reichenbach T, 2017. Hair bundles of cochlear outer hair cells are shaped to minimize their fluid-dynamic resistance. Sci Rep 7, 3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey DP, Hudspeth AJ, 1983a. Analysis of the microphonic potential of the bullfrog’s sacculus. J Neurosci 3, 942–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey DP, Hudspeth AJ, 1983b. Kinetics of the receptor current in bullfrog saccular hair cells. J Neurosci 3, 962–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corns LF, Johnson SL, Kros CJ, Marcotti W, 2014. Calcium entry into stereocilia drives adaptation of the mechanoelectrical transducer current of mammalian cochlear hair cells. Proc Natl Acad Sci U S A 111, 14918–14923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corns LF, Johnson SL, Kros CJ, Marcotti W, 2016. Tmc1 Point Mutation Affects Ca2+ Sensitivity and Block by Dihydrostreptomycin of the Mechanoelectrical Transducer Current of Mouse Outer Hair Cells. J Neurosci 36, 336–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford AC, Evans MG, Fettiplace R, 1989. Activation and adaptation of transducer currents in turtle hair cells. J Physiol (Lond) 419, 405–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford AC, Evans MG, Fettiplace R, 1991. The actions of calcium on the mechano-electrical transducer current of turtle hair cells. J Physiol (Lond) 434, 369–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL, Qiu X, Wu Z, Zhao B, Peng G, Kim YH, Lauer A, Muller U, 2020. TMIE Defines Pore and Gating Properties of the Mechanotransduction Channel of Mammalian Cochlear Hair Cells. Neuron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL, Wu Z, Jafari A, Zhao B, Schrode K, Harkins-Perry S, Lauer A, Muller U, 2017. The murine catecholamine methyltransferase mTOMT is essential for mechanotransduction by cochlear hair cells. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Heer AM, Collin RW, Huygen PL, Schraders M, Oostrik J, Rouwette M, Kunst HP, Kremer H, Cremers CW, 2011. Progressive sensorineural hearing loss and normal vestibular function in a Dutch DFNB7/11 family with a novel mutation in TMC1. Audiol Neurootol 16, 93–105. [DOI] [PubMed] [Google Scholar]
- Deol MS, Robins MW, 1962. The spinner mouse. J Hered 53, 133–136. [DOI] [PubMed] [Google Scholar]
- Di Palma F, Holme RH, Bryda EC, Belyantseva IA, Pellegrino R, Kachar B, Steel KP, Noben-Trauth K, 2001. Mutations in Cdh23, encoding a new type of cadherin, cause stereocilia disorganization in waltzer, the mouse model for Usher syndrome type 1D. Nat Genet 27, 103–107. [DOI] [PubMed] [Google Scholar]
- Dinklo T, Meulenberg CJ, van Netten SM, 2007. Frequency-dependent properties of a fluid jet stimulus: calibration, modeling, and application to cochlear hair cell bundles. J Assoc Res Otolaryngol 8, 167–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionne G, Qiu X, Rapp M, Liang X, Zhao B, Peng G, Katsamba PS, Ahlsen G, Rubinstein R, Potter CS, Carragher B, Honig B, Muller U, Shapiro L, 2018. Mechanotransduction by PCDH15 Relies on a Novel cis-Dimeric Architecture. Neuron 99, 480–492 e485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll JC, Peng AW, Ricci AJ, Pruitt BL, 2012. Faster than the speed of hearing: nanomechanical force probes enable the electromechanical observation of cochlear hair cells. Nano Lett 12, 6107–6111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eatock RA, 2018. Specializations for Fast Signaling in the Amniote Vestibular Inner Ear. Integr Comp Biol 58, 341–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eatock RA, Corey DP, Hudspeth AJ, 1987. Adaptation of mechanoelectrical transduction in hair cells of the bullfrog’s sacculus. J Neurosci 7, 2821–2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eatock RA, Songer JE, 2011. Vestibular hair cells and afferents: two channels for head motion signals. Annu Rev Neurosci 34, 501–534. [DOI] [PubMed] [Google Scholar]
- Effertz T, Becker L, Peng AW, Ricci AJ, 2017. Phosphoinositol-4,5-Bisphosphate Regulates Auditory Hair-Cell Mechanotransduction-Channel Pore Properties and Fast Adaptation. J Neurosci 37, 11632–11646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effertz T, Scharr AL, Ricci AJ, 2015. The how and why of identifying the hair cell mechano-electrical transduction channel. Pflugers Arch 467, 73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson T, Morgan CP, Olt J, Hardy K, Busch-Nentwich E, Maeda R, Clemens R, Krey JF, Nechiporuk A, Barr-Gillespie PG, Marcotti W, Nicolson T, 2017. Integration of Tmc1/2 into the mechanotransduction complex in zebrafish hair cells is regulated by Transmembrane O-methyltransferase (Tomt). Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson T, Pacentine IV, Venuto A, Clemens R, Nicolson T, 2019. The lhfpl5 Ohnologs lhfpl5a and lhfpl5b Are Required for Mechanotransduction in Distinct Populations of Sensory Hair Cells in Zebrafish. Front Mol Neurosci 12, 320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkle TJ, Saunders JC, 1983. Morphology of inner hair cell stereocilia in C57BL/6J mice as studied by scanning electron microscopy. Otolaryngol Head Neck Surg 91, 421–426. [DOI] [PubMed] [Google Scholar]
- Geleoc GS, Lennan GW, Richardson GP, Kros CJ, 1997. A quantitative comparison of mechanoelectrical transduction in vestibular and auditory hair cells of neonatal mice. Proc R Soc Lond B Biol Sci 264, 611–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianoli F, Risler T, Kozlov AS, 2017. Lipid bilayer mediates ion-channel cooperativity in a model of hair-cell mechanotransduction. Proc Natl Acad Sci U S A 114, E11010–E11019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giese APJ, Tang YQ, Sinha GP, Bowl MR, Goldring AC, Parker A, Freeman MJ, Brown SDM, Riazuddin S, Fettiplace R, Schafer WR, Frolenkov GI, Ahmed ZM, 2017. CIB2 interacts with TMC1 and TMC2 and is essential for mechanotransduction in auditory hair cells. Nat Commun 8, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie PG, Corey DP, 1997. Myosin and adaptation by hair cells. Neuron 19, 955–958. [DOI] [PubMed] [Google Scholar]
- Goldberg JM, 2012. The vestibular system : a sixth sense. Oxford University Press, Oxford; New York. [Google Scholar]
- Goldberg JM, Desmadryl G, Baird RA, Fernandez C, 1990. The vestibular nerve of the chinchilla. IV. Discharge properties of utricular afferents. J Neurophysiol 63, 781–790. [DOI] [PubMed] [Google Scholar]
- Goldring AC, Beurg M, Fettiplace R, 2019. The contribution of TMC1 to adaptation of mechanoelectrical transduction channels in cochlear outer hair cells. J Physiol 597, 5949–5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodyear RJ, Richardson GP, 2003. A novel antigen sensitive to calcium chelation that is associated with the tip links and kinocilial links of sensory hair bundles. J Neurosci 23, 4878–4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillet N, Schwander M, Hildebrand MS, Sczaniecka A, Kolatkar A, Velasco J, Webster JA, Kahrizi K, Najmabadi H, Kimberling WJ, Stephan D, Bahlo M, Wiltshire T, Tarantino LM, Kuhn P, Smith RJ, Muller U, 2009. Mutations in LOXHD1, an evolutionarily conserved stereociliary protein, disrupt hair cell function in mice and cause progressive hearing loss in humans. Am J Hum Genet 85, 328–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinan JJ Jr., 2012. How are inner hair cells stimulated? Evidence for multiple mechanical drives. Hear Res 292, 35–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyorgy B, Sage C, Indzhykulian AA, Scheffer DI, Brisson AR, Tan S, Wu X, Volak A, Mu D, Tamvakologos PI, Li Y, Fitzpatrick Z, Ericsson M, Breakefield XO, Corey DP, Maguire CA, 2017. Rescue of Hearing by Gene Delivery to Inner-Ear Hair Cells Using Exosome-Associated AAV. Mol Ther 25, 379–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacohen N, Assad JA, Smith WJ, Corey DP, 1989. Regulation of tension on hair-cell transduction channels: displacement and calcium dependence. J Neurosci 9, 3988–3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakizimana P, Fridberger A, 2021. Inner hair cell stereocilia are embedded in the tectorial membrane. Nat Commun 12, 2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harraz OF, Hill-Eubanks D, Nelson MT, 2020. PIP2: A critical regulator of vascular ion channels hiding in plain sight. Proc Natl Acad Sci U S A 117, 20378–20389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzell C, Putzier I, Arreola J, 2005. Calcium-activated chloride channels. Annu Rev Physiol 67, 719–758. [DOI] [PubMed] [Google Scholar]
- Hille B, Dickson EJ, Kruse M, Vivas O, Suh BC, 2015. Phosphoinositides regulate ion channels. Biochim Biophys Acta 1851, 844–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirono M, Denis CS, Richardson GP, Gillespie PG, 2004. Hair cells require phosphatidylinositol 4,5-bisphosphate for mechanical transduction and adaptation. Neuron 44, 309–320. [DOI] [PubMed] [Google Scholar]
- Holt JR, Corey DP, Eatock RA, 1997. Mechanoelectrical transduction and adaptation in hair cells of the mouse utricle, a low-frequency vestibular organ. J Neurosci 17, 8739–8748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt JR, Gillespie SK, Provance DW, Shah K, Shokat KM, Corey DP, Mercer JA, Gillespie PG, 2002. A chemical-genetic strategy implicates myosin-1c in adaptation by hair cells. Cell 108, 371–381. [DOI] [PubMed] [Google Scholar]
- Holton T, Hudspeth AJ, 1986. The transduction channel of hair cells from the bull-frog characterized by noise analysis. J Physiol (Lond) 375, 195–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino T, 1976. Attachment of the inner sensory cell hairs to the tectorial membrane. A scanning electron microscopic study. ORL J Otorhinolaryngol Relat Spec 38, 11–18. [DOI] [PubMed] [Google Scholar]
- Hoshino T, 1977. Contact between the tectorial membrane and the cochlear sensory hairs in the human and the monkey. Arch Otorhinolaryngol 217, 53–60. [DOI] [PubMed] [Google Scholar]
- Howard J, Hudspeth AJ, 1987. Mechanical relaxation of the hair bundle mediates adaptation in mechanoelectrical transduction by the bullfrog’s saccular hair cell. Proc Natl Acad Sci U S A 84, 3064–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J, Hudspeth AJ, 1988. Compliance of the hair bundle associated with gating of mechanoelectrical transduction channels in the bullfrog’s saccular hair cell. Neuron 1, 189–199. [DOI] [PubMed] [Google Scholar]
- Hudspeth AJ, 1997. How hearing happens. Neuron 19, 947–950. [DOI] [PubMed] [Google Scholar]
- Hudspeth AJ, 2008. Making an effort to listen: mechanical amplification in the ear. Neuron 59, 530–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indzhykulian AA, Stepanyan R, Nelina A, Spinelli KJ, Ahmed ZM, Belyantseva IA, Friedman TB, Barr-Gillespie PG, Frolenkov GI, 2013. Molecular remodeling of tip links underlies mechanosensory regeneration in auditory hair cells. PLoS Biol 11, e1001583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, Zhao Y, Kusakizako T, Wang Y, Pan C, Zhang Y, Nureki O, Hattori M, Yan Z, 2020. TMC1 and TMC2 Proteins Are Pore-Forming Subunits of Mechanosensitive Ion Channels. Neuron 105, 310–321 e313. [DOI] [PubMed] [Google Scholar]
- Johnson SL, Beurg M, Marcotti W, Fettiplace R, 2011. Prestin-driven cochlear amplification is not limited by the outer hair cell membrane time constant. Neuron 70, 1143–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachar B, Parakkal M, Kurc M, Zhao Y, Gillespie PG, 2000. High-resolution structure of hair-cell tip links. Proc Natl Acad Sci U S A 97, 13336–13341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalay E, Li Y, Uzumcu A, Uyguner O, Collin RW, Caylan R, Ulubil-Emiroglu M, Kersten FF, Hafiz G, van Wijk E, Kayserili H, Rohmann E, Wagenstaller J, Hoefsloot LH, Strom TM, Nurnberg G, Baserer N, den Hollander AI, Cremers FP, Cremers CW, Becker C, Brunner HG, Nurnberg P, Karaguzel A, Basaran S, Kubisch C, Kremer H, Wollnik B, 2006. Mutations in the lipoma HMGIC fusion partner-like 5 (LHFPL5) gene cause autosomal recessive nonsyndromic hearing loss. Hum Mutat 27, 633–639. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Falzarano PR, Simpson TH, 1994. Postnatal development of the hamster cochlea. II. Growth and differentiation of stereocilia bundles. J Comp Neurol 350, 187–198. [DOI] [PubMed] [Google Scholar]
- Kawashima Y, Geleoc GS, Kurima K, Labay V, Lelli A, Asai Y, Makishima T, Wu DK, Della Santina CC, Holt JR, Griffith AJ, 2011. Mechanotransduction in mouse inner ear hair cells requires transmembrane channel-like genes. J Clin Invest 121, 4796–4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazmierczak P, Sakaguchi H, Tokita J, Wilson-Kubalek EM, Milligan RA, Muller U, Kachar B, 2007. Cadherin 23 and protocadherin 15 interact to form tip-link filaments in sensory hair cells. Nature 449, 87–91. [DOI] [PubMed] [Google Scholar]
- Kennedy HJ, Crawford AC, Fettiplace R, 2005. Force generation by mammalian hair bundles supports a role in cochlear amplification. Nature 433, 880–883. [DOI] [PubMed] [Google Scholar]
- Kennedy HJ, Evans MG, Crawford AC, Fettiplace R, 2003. Fast adaptation of mechanoelectrical transducer channels in mammalian cochlear hair cells. Nat Neurosci 6, 832–836. [DOI] [PubMed] [Google Scholar]
- Kikuchi K, Hilding D, 1965. The development of the organ of Corti in the mouse. Acta Otolaryngol 60, 207–222. [DOI] [PubMed] [Google Scholar]
- Kim KX, Beurg M, Hackney CM, Furness DN, Mahendrasingam S, Fettiplace R, 2013. The role of transmembrane channel-like proteins in the operation of hair cell mechanotransducer channels. J Gen Physiol 142, 493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KX, Fettiplace R, 2013. Developmental changes in the cochlear hair cell mechanotransducer channel and their regulation by transmembrane channel-like proteins. J Gen Physiol 141, 141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura RS, 1966. Hairs of the cochlear sensory cells and their attachment to the tectorial membrane. Acta Otolaryngol 61, 55–72. [DOI] [PubMed] [Google Scholar]
- Kros CJ, Marcotti W, van Netten SM, Self TJ, Libby RT, Brown SD, Richardson GP, Steel KP, 2002. Reduced climbing and increased slipping adaptation in cochlear hair cells of mice with Myo7a mutations. Nat Neurosci 5, 41–47. [DOI] [PubMed] [Google Scholar]
- Kros CJ, Rusch A, Richardson GP, 1992. Mechano-electrical transducer currents in hair cells of the cultured neonatal mouse cochlea. Proc R Soc Lond B Biol Sci 249, 185–193. [DOI] [PubMed] [Google Scholar]
- Kurima K, Ebrahim S, Pan B, Sedlacek M, Sengupta P, Millis BA, Cui R, Nakanishi H, Fujikawa T, Kawashima Y, Choi BY, Monahan K, Holt JR, Griffith AJ, Kachar B, 2015. TMC1 and TMC2 Localize at the Site of Mechanotransduction in Mammalian Inner Ear Hair Cell Stereocilia. Cell Rep 12, 1606–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurima K, Peters LM, Yang Y, Riazuddin S, Ahmed ZM, Naz S, Arnaud D, Drury S, Mo J, Makishima T, Ghosh M, Menon PS, Deshmukh D, Oddoux C, Ostrer H, Khan S, Riazuddin S, Deininger PL, Hampton LL, Sullivan SL, Battey JF Jr., Keats BJ, Wilcox ER, Friedman TB, Griffith AJ, 2002. Dominant and recessive deafness caused by mutations of a novel gene, TMC1, required for cochlear hair-cell function. Nat Genet 30, 277–284. [DOI] [PubMed] [Google Scholar]
- Lapeyre P, Guilhaume A, Cazals Y, 1992. Differences in hair bundles associated with type I and type II vestibular hair cells of the guinea pig saccule. Acta Otolaryngol 112, 635–642. [DOI] [PubMed] [Google Scholar]
- Lelli A, Asai Y, Forge A, Holt JR, Geleoc GS, 2009. Tonotopic gradient in the developmental acquisition of sensory transduction in outer hair cells of the mouse cochlea. J Neurophysiol 101, 2961–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenoir M, Puel JL, Pujol R, 1987. Stereocilia and tectorial membrane development in the rat cochlea. A SEM study. Anat Embryol (Berl) 175, 477–487. [DOI] [PubMed] [Google Scholar]
- Li A, Xue J, Peterson EH, 2008. Architecture of the mouse utricle: macular organization and hair bundle heights. J Neurophysiol 99, 718–733. [DOI] [PubMed] [Google Scholar]
- Li S, Mecca A, Kim J, Caprara GA, Wagner EL, Du TT, Petrov L, Xu W, Cui R, Rebustini IT, Kachar B, Peng AW, Shin JB, 2020. Myosin-VIIa is expressed in multiple isoforms and essential for tensioning the hair cell mechanotransduction complex. Nat Commun 11, 2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Qiu X, Dionne G, Cunningham CL, Pucak ML, Peng G, Kim YH, Lauer A, Shapiro L, Muller U, 2021. CIB2 and CIB3 are auxiliary subunits of the mechanotransduction channel of hair cells. Neuron 109, 2131–2149 e2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim DJ, 1972. Fine morphology of the tectorial membrane. Its relationship to the organ of Corti. Arch Otolaryngol 96, 199–215. [DOI] [PubMed] [Google Scholar]
- Lim DJ, 1976. Morphological and physiological correlates in cochlear and vestibular sensory epithelia. Scan Electron Microsc 2, 269–276. [Google Scholar]
- Lim DJ, 1984. Otoconia in health and disease. A review. Ann Otol Rhinol Laryngol Suppl 112, 17–24. [DOI] [PubMed] [Google Scholar]
- Lim DJ, 1986. Functional structure of the organ of Corti: a review. Hear Res 22, 117–146. [DOI] [PubMed] [Google Scholar]
- Liu S, Wang S, Zou L, Li J, Song C, Chen J, Hu Q, Liu L, Huang P, Xiong W, 2019. TMC1 is an essential component of a leak channel that modulates tonotopy and excitability of auditory hair cells in mice. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Hildebrand DGC, Morgan JL, Slimmon N, Bagnall MW, 2021. The organization of the gravity-sensing system in zebrafish. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo-Guess CM, Gagnon LH, Cook SA, Wu J, Zheng QY, Johnson KR, 2005. A missense mutation in the previously undescribed gene Tmhs underlies deafness in hurry-scurry (hscy) mice. Proc Natl Acad Sci U S A 102, 7894–7899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo-Guess CM, Gagnon LH, Fritzsch B, Johnson KR, 2007. Targeted knockout and lacZ reporter expression of the mouse Tmhs deafness gene and characterization of the hscy-2J mutation. Mamm Genome 18, 646–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcotti W, Erven A, Johnson SL, Steel KP, Kros CJ, 2006. Tmc1 is necessary for normal functional maturation and survival of inner and outer hair cells in the mouse cochlea. J Physiol 574, 677–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalski N, Michel V, Caberlotto E, Lefevre GM, van Aken AF, Tinevez JY, Bizard E, Houbron C, Weil D, Hardelin JP, Richardson GP, Kros CJ, Martin P, Petit C, 2009. Harmonin-b, an actin-binding scaffold protein, is involved in the adaptation of mechanoelectrical transduction by sensory hair cells. Pflugers Arch 459, 115–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel V, Booth KT, Patni P, Cortese M, Azaiez H, Bahloul A, Kahrizi K, Labbe M, Emptoz A, Lelli A, Degardin J, Dupont T, Aghaie A, Oficjalska-Pham D, Picaud S, Najmabadi H, Smith RJ, Bowl MR, Brown SD, Avan P, Petit C, El-Amraoui A, 2017. CIB2, defective in isolated deafness, is key for auditory hair cell mechanotransduction and survival. EMBO Mol Med 9, 1711–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KK, Atkinson P, Mendoza KR, D OM, Grillet N, 2021. Dimensions of a Living Cochlear Hair Bundle. Front Cell Dev Biol 9, 742529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulhall EM, Ward A, Yang D, Koussa MA, Corey DP, Wong WP, 2021. Single-molecule force spectroscopy reveals the dynamic strength of the hair-cell tip-link connection. Nat Commun 12, 849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam JH, Peng AW, Ricci AJ, 2015. Underestimated Sensitivity of Mammalian Cochlear Hair Cells Due to Splay between Stereociliary Columns. Biophys J 108, 2633–2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naz S, Giguere CM, Kohrman DC, Mitchem KL, Riazuddin S, Morell RJ, Ramesh A, Srisailpathy S, Deshmukh D, Riazuddin S, Griffith AJ, Friedman TB, Smith RJ, Wilcox ER, 2002. Mutations in a novel gene, TMIE, are associated with hearing loss linked to the DFNB6 locus. Am J Hum Genet 71, 632–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson ES, Duifhuis H, Steele CR, 2012. Von Bekesy and cochlear mechanics. Hear Res 293, 31–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacentine I, Chatterjee P, Barr-Gillespie PG, 2020. Stereocilia Rootlets: Actin-Based Structures That Are Essential for Structural Stability of the Hair Bundle. Int J Mol Sci 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacentine IV, Nicolson T, 2019. Subunits of the mechano-electrical transduction channel, Tmc1/2b, require Tmie to localize in zebrafish sensory hair cells. PLoS Genet 15, e1007635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B, Akyuz N, Liu XP, Asai Y, Nist-Lund C, Kurima K, Derfler BH, Gyorgy B, Limapichat W, Walujkar S, Wimalasena LN, Sotomayor M, Corey DP, Holt JR, 2018. TMC1 Forms the Pore of Mechanosensory Transduction Channels in Vertebrate Inner Ear Hair Cells. Neuron 99, 736–753 e736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B, Geleoc GS, Asai Y, Horwitz GC, Kurima K, Ishikawa K, Kawashima Y, Griffith AJ, Holt JR, 2013. TMC1 and TMC2 are components of the mechanotransduction channel in hair cells of the mammalian inner ear. Neuron 79, 504–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel K, Giese AP, Grossheim JM, Hegde RS, Delio M, Samanich J, Riazuddin S, Frolenkov GI, Cai J, Ahmed ZM, Morrow BE, 2015. A Novel C-Terminal CIB2 (Calcium and Integrin Binding Protein 2) Mutation Associated with Non-Syndromic Hearing Loss in a Hispanic Family. PLoS One 10, e0133082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pau HW, Pau H, 2006. Does the geometrical arrangement of the outer hair cell stereocilia perform a fluid-mechanical function? Acta Otolaryngol 126, 570–576. [DOI] [PubMed] [Google Scholar]
- Peng AW, Effertz T, Ricci AJ, 2013. Adaptation of mammalian auditory hair cell mechanotransduction is independent of calcium entry. Neuron 80, 960–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng AW, Gnanasambandam R, Sachs F, Ricci AJ, 2016. Adaptation Independent Modulation of Auditory Hair Cell Mechanotransduction Channel Open Probability Implicates a Role for the Lipid Bilayer. J Neurosci 36, 2945–2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng AW, Ricci AJ, 2011. Somatic motility and hair bundle mechanics, are both necessary for cochlear amplification? Hear Res 273, 109–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng AW, Ricci AJ, 2016. Glass Probe Stimulation of Hair Cell Stereocilia. Methods Mol Biol 1427, 487–500. [DOI] [PubMed] [Google Scholar]
- Peng AW, Salles FT, Pan B, Ricci AJ, 2011. Integrating the biophysical and molecular mechanisms of auditory hair cell mechanotransduction. Nat Commun 2, 523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng AW, Scharr AL, Caprara GA, Nettles D, Steele CR, Ricci AJ, 2021. Fluid Jet Stimulation of Auditory Hair Bundles Reveal Spatial Non-uniformities and Two Viscoelastic-Like Mechanisms. Front Cell Dev Biol 9, 725101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepermans E, Michel V, Goodyear R, Bonnet C, Abdi S, Dupont T, Gherbi S, Holder M, Makrelouf M, Hardelin JP, Marlin S, Zenati A, Richardson G, Avan P, Bahloul A, Petit C, 2014. The CD2 isoform of protocadherin-15 is an essential component of the tip-link complex in mature auditory hair cells. EMBO Mol Med 6, 984–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit C, Richardson GP, 2009. Linking genes underlying deafness to hair-bundle development and function. Nat Neurosci 12, 703–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickles JO, Comis SD, Osborne MP, 1984. Cross-links between stereocilia in the guinea pig organ of Corti, and their possible relation to sensory transduction. Hear Res 15, 103–112. [DOI] [PubMed] [Google Scholar]
- Riazuddin S, Belyantseva IA, Giese AP, Lee K, Indzhykulian AA, Nandamuri SP, Yousaf R, Sinha GP, Lee S, Terrell D, Hegde RS, Ali RA, Anwar S, Andrade-Elizondo PB, Sirmaci A, Parise LV, Basit S, Wali A, Ayub M, Ansar M, Ahmad W, Khan SN, Akram J, Tekin M, Riazuddin S, Cook T, Buschbeck EK, Frolenkov GI, Leal SM, Friedman TB, Ahmed ZM, 2012. Alterations of the CIB2 calcium- and integrin-binding protein cause Usher syndrome type 1J and nonsyndromic deafness DFNB48. Nat Genet 44, 1265–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci AJ, Crawford AC, Fettiplace R, 2003. Tonotopic variation in the conductance of the hair cell mechanotransducer channel. Neuron 40, 983–990. [DOI] [PubMed] [Google Scholar]
- Ricci AJ, Fettiplace R, 1997. The effects of calcium buffering and cyclic AMP on mechano-electrical transduction in turtle auditory hair cells. J Physiol (Lond) 501, 111–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci AJ, Fettiplace R, 1998. Calcium permeation of the turtle hair cell mechanotransducer channel and its relation to the composition of endolymph. J Physiol (Lond) 506, 159–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci AJ, Kennedy HJ, Crawford AC, Fettiplace R, 2005. The transduction channel filter in auditory hair cells. J Neurosci 25, 7831–7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci AJ, Wu YC, Fettiplace R, 1998. The endogenous calcium buffer and the time course of transducer adaptation in auditory hair cells. J Neurosci 18, 8261–8277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson GP, Petit C, 2019. Hair-Bundle Links: Genetics as the Gateway to Function. Cold Spring Harb Perspect Med 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson CV, Rohacs T, Hansen SB, 2019. Tools for Understanding Nanoscale Lipid Regulation of Ion Channels. Trends Biochem Sci 44, 795–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell IJ, Richardson GP, Kossl M, 1989. The responses of cochlear hair cells to tonic displacements of the sensory hair bundle. Hear Res 43, 55–69. [DOI] [PubMed] [Google Scholar]
- Seco CZ, Giese AP, Shafique S, Schraders M, Oonk AM, Grossheim M, Oostrik J, Strom T, Hegde R, van Wijk E, Frolenkov GI, Azam M, Yntema HG, Free RH, Riazuddin S, Verheij JB, Admiraal RJ, Qamar R, Ahmed ZM, Kremer H, 2016. Novel and recurrent CIB2 variants, associated with nonsyndromic deafness, do not affect calcium buffering and localization in hair cells. Eur J Hum Genet 24, 542–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabbir MI, Ahmed ZM, Khan SY, Riazuddin S, Waryah AM, Khan SN, Camps RD, Ghosh M, Kabra M, Belyantseva IA, Friedman TB, 2006. Mutations of human TMHS cause recessively inherited non-syndromic hearing loss. J Med Genet 43, 634–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shotwell SL, Jacobs R, Hudspeth AJ, 1981. Directional sensitivity of individual vertebrate hair cells to controlled deflection of their hair bundles. Ann N Y Acad Sci 374, 1–10. [DOI] [PubMed] [Google Scholar]
- Siemens J, Lillo C, Dumont RA, Reynolds A, Williams DS, Gillespie PG, Muller U, 2004. Cadherin 23 is a component of the tip link in hair-cell stereocilia. Nature 428, 950–955. [DOI] [PubMed] [Google Scholar]
- Sobkowicz HM, Slapnick SM, August BK, 1995. The kinocilium of auditory hair cells and evidence for its morphogenetic role during the regeneration of stereocilia and cuticular plates. J Neurocytol 24, 633–653. [DOI] [PubMed] [Google Scholar]
- Sollner C, Rauch GJ, Siemens J, Geisler R, Schuster SC, Muller U, Nicolson T, 2004. Mutations in cadherin 23 affect tip links in zebrafish sensory hair cells. Nature 428, 955–959. [DOI] [PubMed] [Google Scholar]
- Songer JE, Eatock RA, 2013. Tuning and timing in mammalian type I hair cells and calyceal synapses. J Neurosci 33, 3706–3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotomayor M, Weihofen WA, Gaudet R, Corey DP, 2012. Structure of a force-conveying cadherin bond essential for inner-ear mechanotransduction. Nature 492, 128–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer EA, Holt JR, 2007. Sensory transduction and adaptation in inner and outer hair cells of the mouse auditory system. J Neurophysiol 98, 3360–3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer EA, Scarborough JD, Hirono M, Miller ED, Shah K, Mercer JA, Holt JR, Gillespie PG, 2005. Fast adaptation in vestibular hair cells requires Myosin-1c activity. Neuron 47, 541–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strimbu CE, Prasad S, Hakizimana P, Fridberger A, 2019. Control of hearing sensitivity by tectorial membrane calcium. Proc Natl Acad Sci U S A 116, 5756–5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadenev ALD, Akturk A, Devanney N, Mathur PD, Clark AM, Yang J, Tarchini B, 2019. GPSM2-GNAI Specifies the Tallest Stereocilia and Defines Hair Bundle Row Identity. Curr Biol 29, 921–934 e924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takumida M, Harada Y, Bagger-Sjoback D, 1992. The statoconial membrane of the guinea pig utricular macula. Scanning electron microscopic investigation combined with the freeze-fracturing technique. Acta Otolaryngol 112, 643–648. [DOI] [PubMed] [Google Scholar]
- Tarchini B, Lu X, 2019. New insights into regulation and function of planar polarity in the inner ear. Neurosci Lett 709, 134373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin M, Chaiyasitdhi A, Michel V, Michalski N, Martin P, 2019. Stiffness and tension gradients of the hair cell’s tip-link complex in the mammalian cochlea. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trouillet A, Miller KK, George SS, Wang P, Ali NE, Ricci A, Grillet N, 2021. Loxhd1 Mutations Cause Mechanotransduction Defects in Cochlear Hair Cells. J Neurosci 41, 3331–3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollrath MA, Eatock RA, 2003. Time course and extent of mechanotransducer adaptation in mouse utricular hair cells: comparison with frog saccular hair cells. J Neurophysiol 90, 2676–2689. [DOI] [PubMed] [Google Scholar]
- Von Békésy G, 1960. Experiments in hearing. McGraw-Hill, New York,. [Google Scholar]
- Vreugde S, Erven A, Kros CJ, Marcotti W, Fuchs H, Kurima K, Wilcox ER, Friedman TB, Griffith AJ, Balling R, Hrabe De Angelis M, Avraham KB, Steel KP, 2002. Beethoven, a mouse model for dominant, progressive hearing loss DFNA36. Nat Genet 30, 257–258. [DOI] [PubMed] [Google Scholar]
- Waguespack J, Salles FT, Kachar B, Ricci AJ, 2007. Stepwise morphological and functional maturation of mechanotransduction in rat outer hair cells. J Neurosci 27, 13890–13902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker RG, Hudspeth AJ, 1996. Calmodulin controls adaptation of mechanoelectrical transduction by hair cells of the bullfrog’s sacculus. Proc Natl Acad Sci U S A 93, 2203–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb SW, Grillet N, Andrade LR, Xiong W, Swarthout L, Della Santina CC, Kachar B, Muller U, 2011. Regulation of PCDH15 function in mechanosensory hair cells by alternative splicing of the cytoplasmic domain. Development 138, 1607–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y-C, Ricci AJ, Fettiplace R, 1999. Two Components of Transducer Adaptation in Auditory Hair Cells. J Neurophysiol 82, 2171–2181. [DOI] [PubMed] [Google Scholar]
- Wu Z, Grillet N, Zhao B, Cunningham C, Harkins-Perry S, Coste B, Ranade S, Zebarjadi N, Beurg M, Fettiplace R, Patapoutian A, Mueller U, 2017. Mechanosensory hair cells express two molecularly distinct mechanotransduction channels. Nat Neurosci 20, 24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W, Grillet N, Elledge HM, Wagner TF, Zhao B, Johnson KR, Kazmierczak P, Muller U, 2012. TMHS Is an Integral Component of the Mechanotransduction Machinery of Cochlear Hair Cells. Cell 151, 1283–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamoah EN, Gillespie PG, 1996. Phosphate analogs block adaptation in hair cells by inhibiting adaptation-motor force production. Neuron 17, 523–533. [DOI] [PubMed] [Google Scholar]
- Yu X, Zhao Q, Li X, Chen Y, Tian Y, Liu S, Xiong W, Huang P, 2020. Deafness mutation D572N of TMC1 destabilizes TMC1 expression by disrupting LHFPL5 binding. Proc Natl Acad Sci U S A 117, 29894–29903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Wu Z, Grillet N, Yan L, Xiong W, Harkins-Perry S, Muller U, 2014. TMIE is an essential component of the mechanotransduction machinery of cochlear hair cells. Neuron 84, 954–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Holt JR, 2021. The Mechanosensory Transduction Machinery in Inner Ear Hair Cells. Annu Rev Biophys 50, 31–51. [DOI] [PMC free article] [PubMed] [Google Scholar]


