Abstract
Aminoglycosides are potent antibiotics that are commonly prescribed worldwide. Their use carries significant risks of ototoxicity by directly causing inner ear hair cell degeneration. Despite their ototoxic side effects, there are currently no approved antidotes. Here we review recent advances in our understanding of aminoglycoside ototoxicity, mechanisms of drug transport, and promising sites for intervention to prevent ototoxicity.
Introduction
Ototoxicity encompasses damage to the inner ear auditory (cochleotoxic) and/or vestibular organs (vestibulotoxic). Drug ototoxicity is defined as reversible or irreversible damage to the inner ear that results in loss of function. Although it is preferable to avoid using ototoxic drugs, their benefits often outweigh the risks. The most common ototoxic drugs include aminoglycoside (AG) antibiotics, platinum-based chemotherapeutic drugs, certain antimalarials, loop diuretics, and NSAIDs. Table 1 lists some of the common drugs from the above categories that are known to be ototoxic.
Table 1:
Common ototoxic drugs
This review focuses on the clinical importance of AGs and their side effects with a major focus on their impact in the cochlea. This review delves into existing model systems for AG research and their translational relevance, mechanisms by which the AG could enter the cochlea and the hair cells (HCs), and the potential mechanisms that could trigger HC death. Each topic is explored from the perspective of a target site for intervention. We refer the avid readers to several other recent excellent reviews on cisplatin ototoxicity (Prayuenyong et al., 2021; Steyger, 2021).
Aminoglycosides
AG-aminocyclitol antibiotics, commonly known as AGs are water-soluble, cationic molecules that exhibit broad-spectrum antibacterial properties. They are primarily used in the treatment of infections caused by both gram-positive and gram-negative bacteria, mycobacteria, and sometimes by amoeboid and protozoa (Berman and Fleckenstein, 1991). Due to their poor absorption through oral administration, they are generally administered intravenously (Edson and Terrell, 1991).
The first AG to be discovered was streptomycin (Schatz et al., 1944) followed by neomycin (Waksman and Lechevalier, 1949), which formed a base for the discovery and characterization of other similar clinically important AGs as shown in Table 2. AGs are either natural (produced by the bacteria species actinomycetes) or semisynthetic derivatives of natural AGs. For example, amikacin is a semisynthetic derivative of kanamycin B (Table 1). There are no known AGs in clinical use that are derived entirely from chemical synthesis.
Table 2:
List of aminoglycosides. Adapted and modified from (Wright et al., 1998)
| Aminoglycoside | Source | Reference |
|---|---|---|
| Streptomycin | Streptomyces griseus | (Phaniendra et al., 2015; Schatz et al., 1944) |
| Spectinomycin | Streptomyces spectabilis | (Mason et al., 1961) |
| Neomycin | Streptomyces fradiae | (Waksman and Lechevalier, 1949) |
| Kanamycin | Streptomyces kanamyceticlls | (Umezawa et al., 1957) |
| Paromomycin | Streptomyces rimasus NRRL 2234 | (TH Haskell, 1959) |
| Gentamicin | Micromonospora purpurea | (Weinstein, 1963) |
| Tobramycin | Streptomyces tenebrarius | (Higgins and Kastner, 1967) |
| Ribostamycin | Streptomyces ribosidificus | (Shomura et al., 1970) |
| Butirosin | Bacillus circulans | (Woo et al., 1971) |
| Sisomicin | Micromonospora inyoesis | (Weinstein et al., 1970) |
| Amikacin | Semisynthetic derivative of kanamycin B | (Kawaguchi et al., 1972) |
| Netilmicin | Semisynthetic derivative of sisomicin | (Kabins et al., 1976) |
| Isepamicin | Semisynthetic derivative of gentamicin B | (Nagabhushan et al., 1978) |
Types and structure of aminoglycosides
AG antibiotics can be broadly divided based on the origin as natural and semi-synthetic (Table 2). They can also be divided structurally based on their six-membered aminocyclitol rings. Based on their structure, AGs fall into two groups, those that incorporate a 2-deoxystreptamine ring and those that do not (Table 3). The first group is further subdivided into compounds that are substituted at either the 4 and 5 positions or 4 and 6 positions of the 2-deoxystreptamine ring (Table 3).
Table 3:
Types of aminoglycosides. Adapted from (Wright et al., 1998).
| 2- Deoxystreptamine aminoglycosides | Other aminoglycosides | |
|---|---|---|
| 4,5- disubstituted | 4,6- disubstituted | |
| Neomycin | Kanamycin | Hygromycin |
| Ribostamycin | Gentamicin | Streptomycin |
| Butirosin | Amikacin | Spectinomycin |
| Lividomycin | Netilmicin | Apramycin |
| Paromomycin | Tobramycin | Fortimicin |
| Isepamicin | ||
| Sisomicin | ||
| Dibekacin | ||
General use of aminoglycosides
AGs have a wide range of clinical uses. They are used to treat gram-negative and -positive bacterial infections. Streptomycin is still occasionally used in the treatment of tuberculosis despite the availability of other less ototoxic first line drug regimens (Armstrong et al., 1950; Musser, 1995). AGs block the production of HIV-1 by binding the viral regulatory protein Rev. (Wang et al., 1997; Zapp et al., 1993). AGs are frequently used in patients suffering from cystic fibrosis. AGs can effectively penetrate the sputum allowing them to effectively fight Pseudomonas aeruginosa. For example, tobramycin was found to display synergism with β-lactam antibiotics like ceftazidime (den Hollander et al., 1997). AGs are the antibiotics of choice for complicated urinary tract infections (Goodlet et al., 2018), peripartum and neonatal infections (Kankuri et al., 2003; Kent et al., 2014).
General mechanism of AG bacterial entry and action (Fig. 1)
Fig. 1. General mechanisms of aminoglycoside bacteria entry.
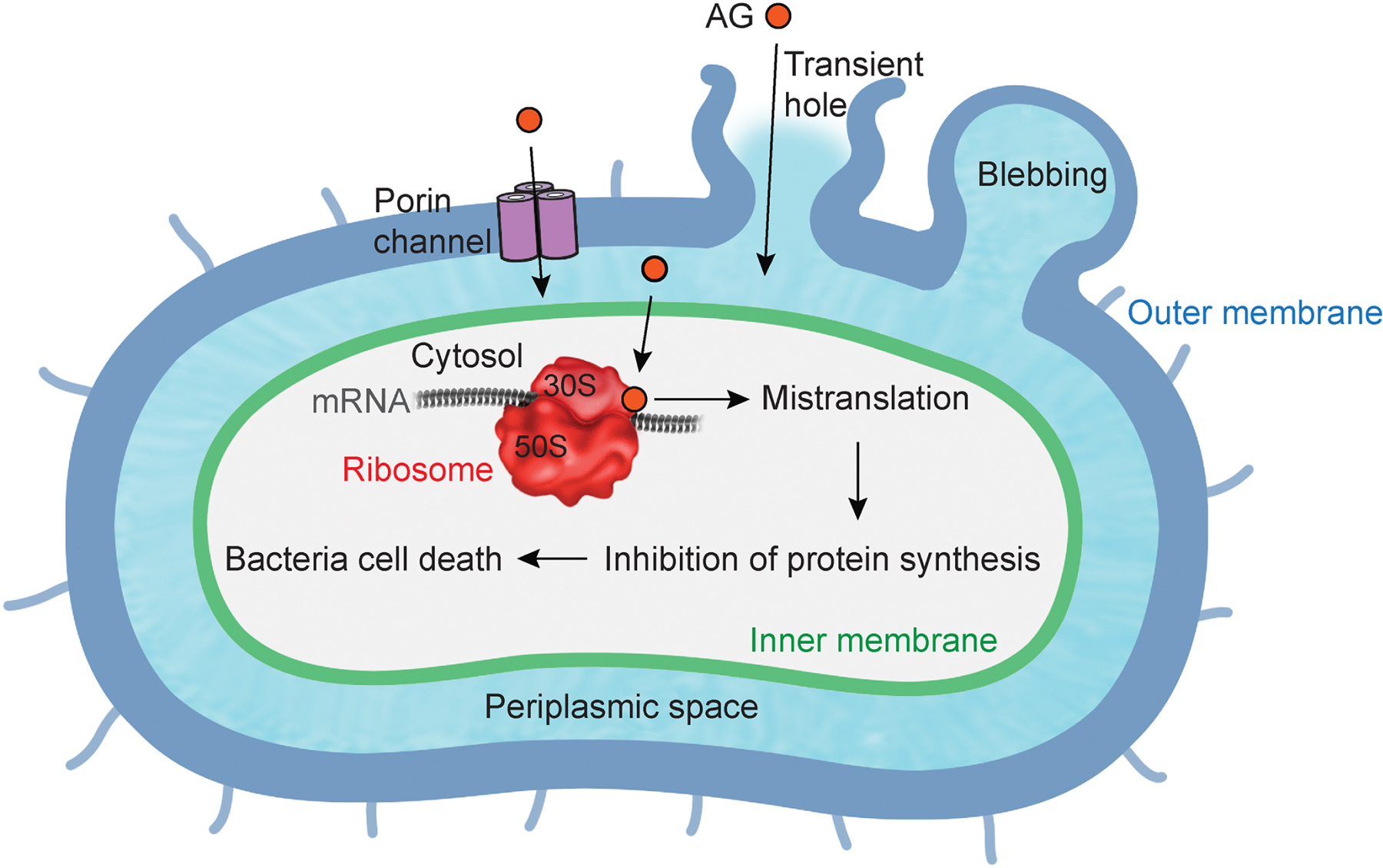
Aminoglycoside (AG) enters into a periplasmic space (light blue) through outer membrane (dark blue), and enters into cytosol through inner membrane (green). AG binding to ribosome (red) induces mistranslation, resulting in bacteria cell death.
Positively charged AG molecules promote bacterial uptake by binding to the negatively charged components of the bacterial membrane, such as the phospholipids and teichoic acids of gram-positive bacteria and the phospholipids and lipopolysaccharide of gram-negative bacteria (Davis, 1987; Hancock et al., 1991; Taber et al., 1987). Stabilized cross-links between adjacent lipid components in the outer membrane are disrupted by competitively displacing bridging divalent cations (Hancock et al., 1981; Peterson et al., 1985). The disruption induces membrane blebbing, leading to transient holes in the bacterial cell envelope (Martin and Beveridge, 1986). The holes enhance the permeability of AGs into the bacteria (Kadurugamuwa et al., 1993). Moreover, porins which are transmembrane pore-forming proteins in outer membranes, are also considered as an entry site of AGs (Nakae and Nakae, 1982). The initial entry of AGs into the periplasmic space of the bacteria occurs in a passive and energy-independent manner (EDP-I) (Bryan and Van Den Elzen, 1977). On the other hand, the ensuing steps, including entry into the cytoplasm through the inner membrane, occur in an energy-dependent manner (EDP-II) (Kislak, 1972; Martin et al., 1972; Taber et al., 1987). Various mutations in bacterial genes which are involved in energy metabolism lead to AG resistance. Once AGs access the cytoplasm, they irreversibly bind to the decoding site at the 16S rRNA in the 30S subunit of the ribosome (Garvin et al., 1974). The binding disturbs the recognition and selection of tRNA, leading to misreading during translation (Davies and Davis, 1968a). More specifically, AGs targeting translation interfere with either the assembly or the processing of the ribosome, or with the proper utilization of tRNAs and translation factors (Cabanas et al., 1978; Fredrick and Noller, 2003; Kavcic et al., 2020; Misumi et al., 1978; Wang et al., 2012). Ultimately, these perturbations either produce mistranslated proteins or inhibit protein synthesis. Furthermore, subsequent AG entry into the bacteria is facilitated by the mistranslated proteins (Davis, 1987; Davis et al., 1986; Nichols and Young, 1985). The additional uptake accelerates death of the bacteria. Interestingly, each AG has a different binding affinity for individual rRNA binding sites (Feldman et al., 2010; Fourmy et al., 1998; O’Sullivan et al., 2018; Wasserman et al., 2015; Ying et al., 2019). For antibacterial activity, AG-induced reduction in nucleotide mobility at rRNA is a more important determinant than the binding affinity (Kaul et al., 2006). These differences between multiple types of AGs are considerable for antibacterial potency and bacterial resistance.
Aminoglycoside nephro- and ototoxicity
The side effects of AGs have been established for many years (Hinshaw et al., 1947). AGs cause nephrotoxicity with repeated usage. In renal tubular cells, AGs can enter through endocytosis-dependent and independent manner (Nagai and Takano, 2014). AGs accumulate in lysosomes, leading to cell necrosis. Continued exposure can cause renal failure (Mingeot-Leclercq and Tulkens, 1999; Swan, 1997). AGs are known to enter both outer hair cells (OHCs) and inner hair cells (IHCs) with OHCs being more sensitive to AG damage (Forge and Schacht, 2000). Continued accumulation of AG antibiotics in HCs lead to their demise along the basal to apical axis (Cheng et al., 2003; Cunningham et al., 2002; Fausti et al., 1984; Forge and Schacht, 2000; Matsui et al., 2004). AG ototoxicity usually manifests itself as high frequency hearing loss consistent with an increasing apical-to-basal gradient of damage; prolonged usage extends damage to lower frequency range and apical HC loss (Zettner and Gleser, 2018).
Animal models for studying ototoxicity (Table 4)
Table 4.
Animal models for ototoxicity.
| Species | AGs (dose) | Diuretics (dose) | Route | Reference |
|---|---|---|---|---|
| C | gentamicin (10, 20, 40, 125 mg/kg; single injection) | ethacrynic acid (40 mg/kg) | i.m. | (McFadden et al., 2002) |
| M | kanamycin (1000 mg/kg; single injection) | bumetanide (50 mg/kg) | i.p. | (Taylor et al., 2008) |
| M, R | kanamycin (400–900 mg/kg; 15d, twice daily) | - | s.c. | (Wu et al., 2001) |
| C | gentamicin (125 mg/kg; single injection) | ethacrynic acid (40 mg/kg) | i.m. | (Ding et al., 2010) |
| R | kanamycin (500 mg/kg; single injection) | ethacrynic acid (75 mg/kg) | i.m. | (Liu et al., 2011) |
| GP | gentamicin (100 mg/kg; single injection) | - | i.p. | (Imamura and Adams, 2003) |
| R | amikacin (200 mg/kg; 14, 28d, once daily) | - | i.m. | (El-Anwar et al., 2018) |
| GP | gentamicin (40, 80 mg/kg; 10d, once daily) | - | s.c. | (Bezdjian et al., 2015) |
| M | kanamycin (420, 550, 600 mg/kg; 3, 5, 7, 10, 14d, once daily) | furosemide (130 mg/kg) | s.c. | (Ju et al., 2017) |
| M | kanamycin (700, 1,000 mg/kg; single injection) | furosemide (100 mg/kg) | i.p. | (Jansen et al., 2013) |
| R | amikacin (200 mg/kg; 14d, once daily) | - | i.m. | (El-Anwar et al., 2016) |
| GP | amikacin (20, 400 mg/kg; 12, 30 days, once daily) | - | i.m. | (Oliveira et al., 2004) |
| M | kanamycin (900 mg/kg; 15d, twice daily) | furosemide (50 mg/kg) | i.p. | (Hirose and Sato, 2011) |
| G | gentamicin or kanamycin (4–500 mg/kg; single injection) | bumetanide (50 mg/kg) or furosemide (100 mg/kg) | s.c. | (Abbas and Rivolta, 2015) |
| GP | kanamycin (200 mg/kg; single injection) | furosemide (100 mg/kg) | s.c. | (Havenith et al., 2013) |
| GP | amikacin (200 mg/kg; 14 days, once daily) | - | s.c. | (Dirain et al., 2018) |
| R | amikacin (600 mg/kg; 14 days, once daily) | - | i.m. | (Aksoy et al., 2014) |
| GP, R | kanamycin (400 mg/kg; single injection) | ethacrynic acid (50 mg/kg) | s.c. | (Yamasoba et al., 2003) |
| R | tobramycin (160 mg/kg; 14 days, once daily) | - | s.c. | (Asplund et al., 2009) |
| M | kanamycin (1,000 mg/kg; single injection) | furosemide (400 mg/kg) | s.c. | (Schmitz et al., 2014) |
| M | kanamycin (1,000 mg/kg; single injection) | furosemide (500 mg/kg) | s.c. | (Fernandes and Lin, 2014) |
| R | kanamycin (500 mg/kg; single injection) | furosemide (200 mg/kg) | i.p. | (Chen et al., 2020) |
| M | kanamycin (700 mg/kg; 14d, twice daily) | - | s.c | (Koo et al., 2015) |
(C, chinchilla; M, mouse; R, rat; GP, guinea pig; G, gerbil; i.m., intramuscular injection; i.p., intraperitoneal injection; s.c., subcutaneous injection)
To study mechanisms underlying AG-induced ototoxicity, various animal models have been generated with different type, dosage and application method for AGs as well as co-administration of diuretics. Animals such as mice, rats, guinea pigs, gerbils, and chinchilla were used for studying AG-induced ototoxicity. AGs are systemically delivered via intramuscular, intraperitoneal or subcutaneous injections. Most studies apply multiple lower doses of AGs on successive days rather than a single, high dose injection to induce ototoxicity. This multi-dose injection strategy can avoid animal mortality sometimes resulting from a single, high dose injection (Murillo-Cuesta et al., 2010; Taylor et al., 2008; Wu et al., 2001). Examples of animal models for the irreversible ototoxicity induced by AGs are summarized in Table 4. Doses in all of these models are typically much higher than used clinically but are needed in order to consistently generate damage.
Diuretics are generally administered simultaneously with AGs to induce robust sensory HC loss (McFadden et al., 2002). In particular, mice often need co-administration of diuretics to induce ototoxicity as they are highly resistant to AGs, presumably because of high excretion rates (Blakley et al., 2008; Murillo-Cuesta et al., 2010; Ogier et al., 2020; Taylor et al., 2008; Wu et al., 2001). Experimental studies showed the co-administration of diuretics with AGs potentiate AG-induced ototoxicity. However, how diuretics affect AG toxicity is not clear. Here are several possible mechanisms. Some studies reveal that diuretics inhibit strial enzymes Na+, K+-ATPase and adenylate cyclase (Kusakari et al., 1978; Paloheimo and Thalman, 1977). The enzyme inhibition induces swelling of the stria vascularis, leading to rapid decrease of the endocochlear potential as well as eventual loss of the cochlear microphonic, summating, and compound action potentials (Kusakari et al., 1978; Rybak et al., 1991). Due to the attenuation of the endocochlear potential, administration of diuretics induces temporary hearing loss (Ding et al., 2016). Even though swelling of the stria vascularis typically fully recovered within a day (Ding et al., 2016), simultaneous administration of diuretics and AGs can potentiate ototoxicity (Ding et al., 2010). It is possible that diuretics damage the tight cell junctions in the stria vascularis and disrupt the blood-labyrinth barrier (BLB), further increasing AG uptake into HCs (Ding et al., 2016; Liu et al., 2011). Another possible mechanism is that osmotic swelling caused by diuretic-induced inhibition of Na-K-2Cl cotransporter activates TRPV4 channel in stria vascularis (Jiang et al., 2017a). More AGs can enter the cochlea through the activated TRPV4 channels, resulting in enhanced ototoxicity. The increased endolymph AG would then promote access through MET channels (see uptake mechanisms in later sections) despite the driving force for current through the MET channel being reduced by the loss of the endocochlear potential. Another possibility is that the diuretic reduces filtration within the kidney so that AG blood levels remain elevated for longer, resulting in more time for entry into the cochlea. And finally, as diuretics can be ototoxic on their own (Table 1), it is possible that the diuretic effects are simply summing with the AGs effect to cause greater toxicity. Thus, existing models that require diuretics may be flaweds because we do not truly understand the mechanism by which the synergy is happening; however, they remain the available models of choice for small animal testing.
Mechanism of AGs transport into the cochlea (Fig. 2)
Fig. 2. Anatomy of the mammalian cochlea.
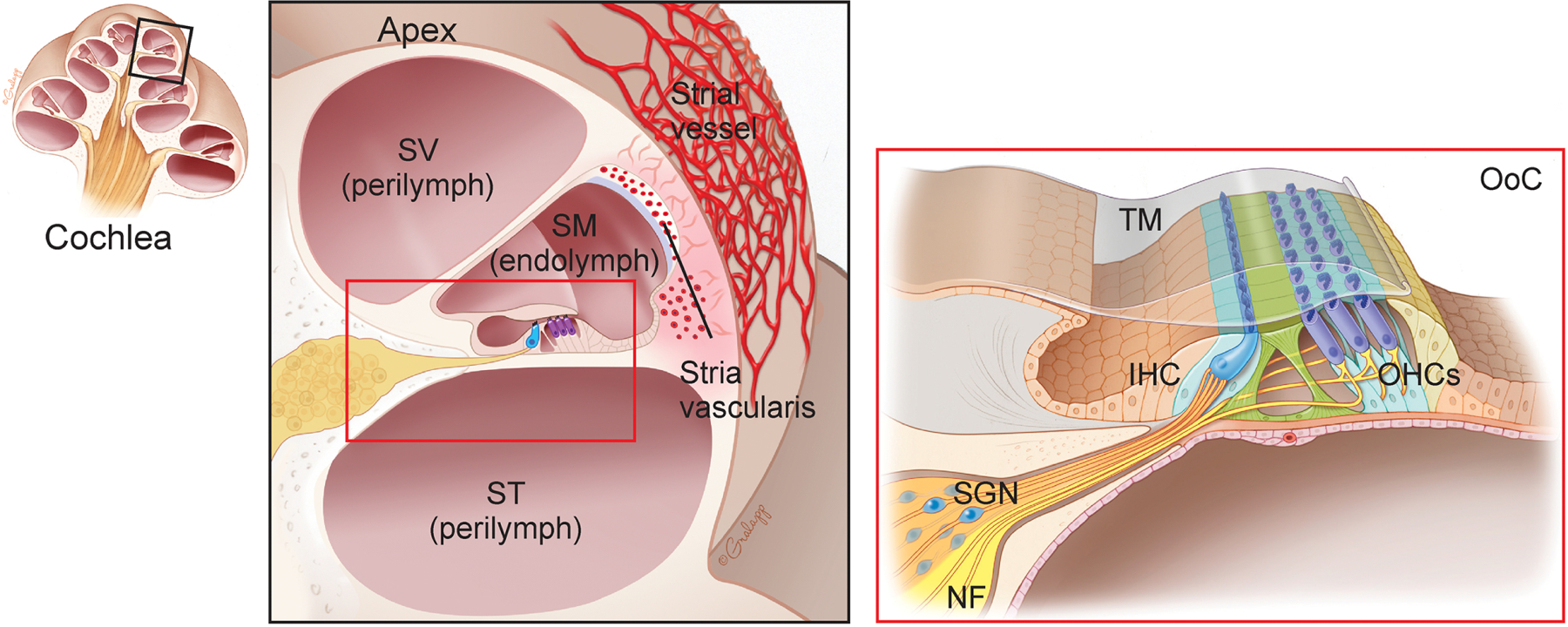
(Left) Cross section of the cochlea. (Center) apical turn of the cochlea (magnified view). Cochlea are composed of three chambers, and filled with endolymph and perilymph. Dense capillaries and transporters in the stria vascularis selectively regulate endolymph composition. SV, scala vestibuli; SM, scala media; ST, scala tympani. (Right) organ of Corti (OoC) (magnified view). OoC contains inner and outer hair cells (IHCs and OHCs) as well as supporting cells. TM, tectorial membrane; SGN, spiral ganglion neuron; NF, nerve fiber.
The cochlea has two main fluid compartments, endolymph and perilymph, which are divided into three chambers: the scala vestibuli (SV), scala media (SM), and scala tympani (ST) (Pickles, 2008). The endolymph has a unique ionic composition, high K+ and low Ca2+, while the perilymph has a similar ionic composition to extracellular fluid (Sterkers et al., 1984, 1988). The stria vascularis maintains the ionic environments which are critical for cochlear function (Wangemann, 2006). In the stria vascularis, dense capillaries and transporters selectively regulate endolymph composition (Wangemann, 2006). Furthermore, BLB, which is composed of tight junction-coupled endothelial cells, controls exchange between blood and intracochlear fluids so that entry of macromolecules into the cochlea is tightly regulated (Nyberg et al., 2019). The organ of Corti (OoC) is a specialized sensory structure that sits on the basilar membrane within the cochlea, containing IHCs and OHCs as well as at least seven types of supporting cells (Pickles, 2008). The hair bundle, comprised of actin filled stereocilia is an essential HC organelle critical for the conversion of mechanical stimulation into electrical signals (Pickles, 2008). The hair bundle sits atop the HC and faces the endolymph solution. Mechanotransducer (MET) channels at the tips of stereocilia open upon deflection of stereocilia and allow HC activation (Pickles, 2008). Furthermore, transient receptor potential (TRP) and adenosine triphosphate (ATP) receptor channels (Fettiplace, 2017; Housley et al., 1992; Housley et al., 1999) are also found on the stereocilia.
The first step in understanding mechanisms of AG-induced ototoxicity is to determine how AGs enter the cochlea and their cells. To track the AG entry route, various experimental strategies such as in vitro assays, immunolabeling and AG-conjugated to fluorescent labels have been used in neonatal or adult cochlear tissues (Alharazneh et al., 2011; Dai et al., 2006; Imamura and Adams, 2003; Kawashima et al., 2011; Marcotti et al., 2005; Wang and Steyger, 2009). Gentamicin Texas Red (GTTR) is the most often used fluorescent-conjugated AG and a powerful tool to study AG trafficking into the cochlea (Dai et al., 2006; Wang and Steyger, 2009). One caveat to GTTR is that it is a mixture of gentamicin subtypes and the labeled amine is not well targeted, so care must be taken in interpretation of results. Here, we review the findings on mechanisms of AGs entry into the cochlea and as well as their actions therein.
Mechanism and therapeutic target 1: AG access into the cochlea (Fig. 3)
Fig. 3. Proposed mechanisms of aminoglycoside entry into the endolymph.
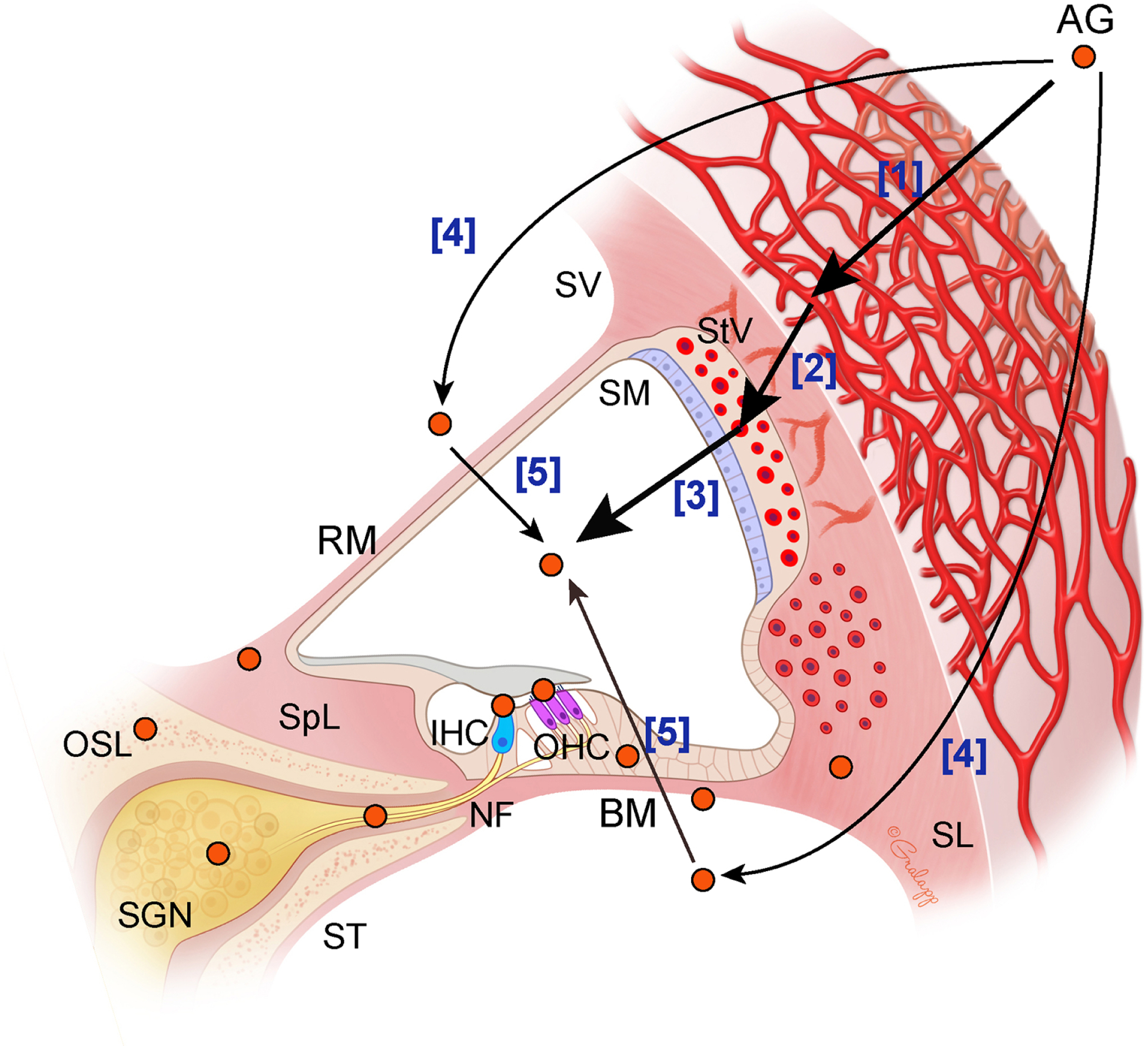
Aminoglycoside (AG) is detected in the capillaries [1] and individual cell subtypes [2] in the stria vascularis. In vivo work showed that AG enters into endolymph [3]. AG uptake into the perilymph has also been suggested [4]. Reissner’s and basilar membranes (RM and BM) are also considered as possible entry routes of AG from the perilymph to the endolymph [5]. Thick arrows indicate the AG entry routes with stronger evidences than narrow arrows. SV, scala vestibuli; SM, scala media; ST, scala tympani; StV, stria vascularis; SL, spiral ligament; SpL, spiral limbus; OSL, osseous spital lamina; SGN, spiral ganglion neuron; NF, nerve fiber.
Systemically administrated fluorescent-tagged AGs are first detected in the capillaries in stria vascularis in mice and guinea pigs (Dai et al., 2006; Imamura and Adams, 2003; Wang and Steyger, 2009) ([1] in Fig. 3). The GTTR is further detected in cell subtypes within the stria vascularis (marginal, intermediate and basal cells), suggesting drug transport across this region (Wang and Steyger, 2009) ([2] in Fig. 3). BLB is one of the initial sites of AG entry from the blood into the interstitial space in the cochlea (Dai et al., 2006; Hawkins, 1973). Multiple factors such as active and passive transports, ion channels, blood flow, inflammation, free radicals and noise exposure can influence the permeability of the BLB, possibly potentiating AG cochlear uptake (Shi, 2016). For example, many studies reveal that inflammation enhances AG uptake into the cochlea (Chai et al., 2021; Gu et al., 2021; Hirose and Li, 2019; Jiang et al., 2017b; Koo et al., 2015). In vivo work showed that AGs transport into endolymph across the strial BLB (Li and Steyger, 2011) ([3] in Fig. 3). Endolymph is considered as a predominant route for AG uptake in HCs (also see next section) compared to the uptake from the perilymph (Li and Steyger, 2011). On the other hand, one early study showed that the AG level in perilymph is higher than that in endolymph, suggesting AG uptake into the perilymph occurs (Tran Ba Huy et al., 1986) ([4] in Fig. 3). Reissner’s and basilar membranes are further considered as possible entry routes of AG from the perilymph to the endolymph (Huy et al., 1983; Tran Ba Huy et al., 1986; Tran Ba Huy et al., 1981) ([5] in Fig. 3). Furthermore, the osseous spiral lamina maybe a potential AG entry route because increasing matrix metalloproteinases can enhance the permeability to AGs between the ST and spiral ganglion neurons (Li et al., 2017). After systemic application, AGs are detected in multiple locations in the cochlea such as HCs, Deiters’ cells, interdental cells, mesenchymal cells, fibrocytes, nerve fibers, and spiral ganglion neurons (Dai et al., 2006; Imamura and Adams, 2003) (orange circles in Fig. 3). The AG uptake into multiple cochlear cells are shown based on either localization of GTTR (Dai et al., 2006; Wang and Steyger, 2009) or AG immunolabeling (Imamura and Adams, 2003). Both of these approaches relied on fixed tissues (Imamura and Adams, 2003; Wang and Steyger, 2009). Live cell imaging shows AG uptake selectively into HCs and perhaps spiral ganglia fibers (Alharazneh et al., 2011), not supporting cells. Similarly, HCs die when treated with AGs, not supporting cells. Thus, the discrepancy between fixed and live cell data needs to be reconciled in future studies. There is strong agreement that HCs uptake AGs regardless of technologies used for assessment (Dai et al., 2006; Imamura and Adams, 2003). Despite much effort, it remains unclear as to how AGs enter the endolymph from stria vascularis and target multiple cell types, with transporters, ion channels, and transcytosis serving as possible mechanisms. As described in Figure 3, the stria vascularis is considered as a first site where AGs are taken up from the bloodstream to the cochlea (Dai et al., 2006; Imamura and Adams, 2003; Wang and Steyger, 2009). BLB borders the stria vascularis and intracochlear fluid and can be a major target for blocking AG entry (Dai et al., 2006; Hawkins, 1973). As we mentioned above, the permeability of the BLB can be modulated by multiple factors such as diuretics, active and passive membrane functions, ion channels, blood flow, inflammation, free radicals and noise exposure (Chai et al., 2021; Ding et al., 2016; Hirose and Li, 2019; Koo et al., 2015; Liu et al., 2011; Shi, 2016). Numerous transporters and ion channels in the stria vascularis are potential therapeutic targets to block AG uptake (Patuzzi, 2011). Very recently, in vivo real-time observation revealed that AG trafficking into the cochlea is mediated by an endocytic receptor megalin (Kim and Ricci, 2022) which is expressed in the stria vascularis, tightly binds AGs and is required for AG transport in the kidney (Christensen and Birn, 2002; Dagil et al., 2013; Hori et al., 2017; Tauris et al., 2009). Numerous ligands, such as megalin, serve as candidate competitive inhibitors of AGs, possibly blocking AG transport into the cochlea (Christensen and Birn, 2002; Dagil et al., 2013; Hori et al., 2017; Tauris et al., 2009). Another possible target for initial AG entry route into cochlea are TRP channels such as TRPV1, TRPV4, and TRPC3, which are expressed in stria vascularis (Jiang et al., 2019; Karasawa et al., 2008; Phan et al., 2010). However, at present, the exact mechanisms governing BLB permeability are largely unknown, a knowledge gap that beckons investigation leading to discovery of novel therapeutic options for preventing AG trafficking into intracochlear spaces.
Mechanism and therapeutic target 2: AG access into cochlear hair cells (Fig. 4)
Fig. 4. Proposed mechanisms of aminoglycoside entry into hair cells.
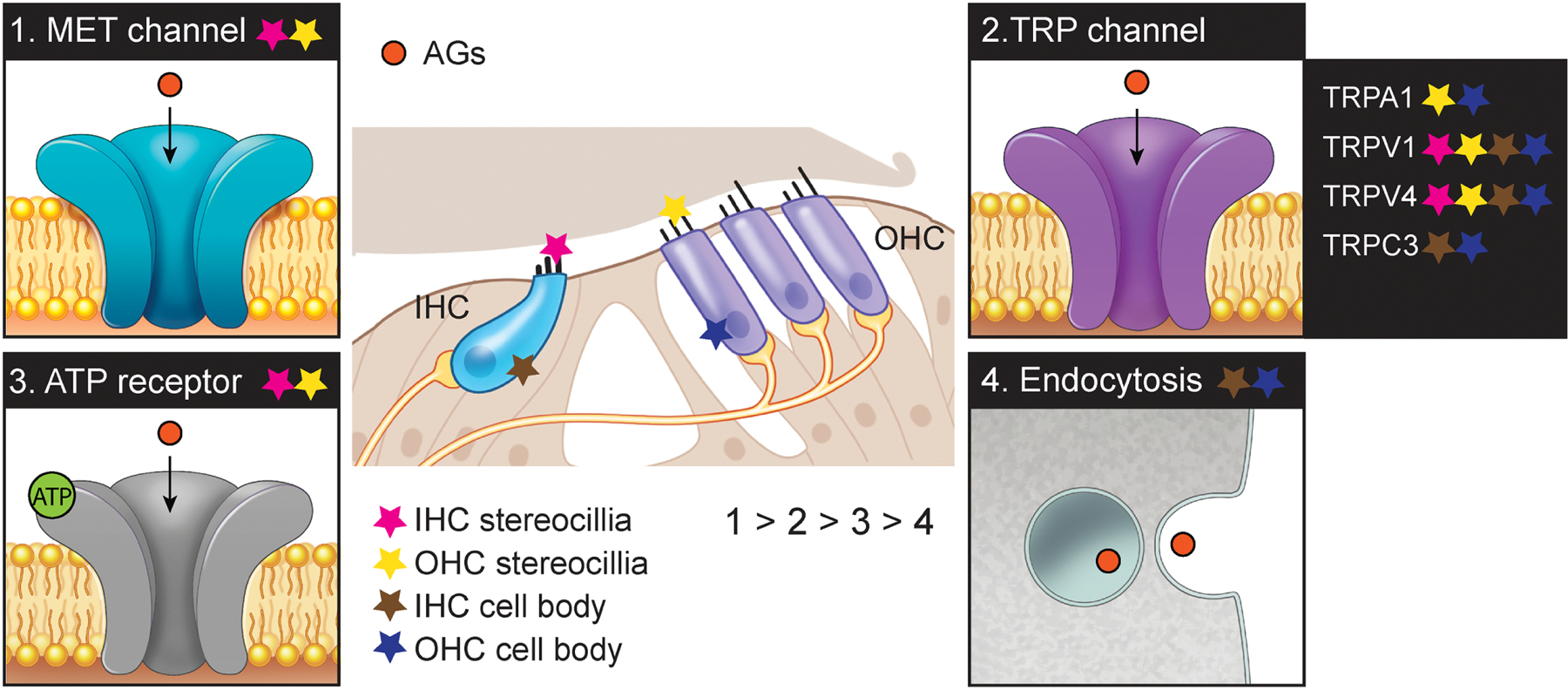
Aminoglycoside (AG) enters into hair cells (IHCs and OHCs) through mechanotransducer (MET) (1), transient receptor potential (TRP) (2), adenosine triphosphate (ATP) receptor (3) channels, and endocytosis (4). Lower number depicts stronger evidence (1 > 2 > 3 > 4). Each star indicates the location of the channels or endocytosis in hair cells (pink, IHC stereocilia; yellow, OHC stereocilia; brown, IHC cell body; blue, OHC cell body).
Once AGs enter the endolymph, OHCs and IHCs in the OoC uptake AGs (Dai et al., 2006; Imamura and Adams, 2003). Previous studies showed that not only multiple channels but also cellular process are involved in AG transport into HCs. Here, we will discuss MET, TRP, and ATP channels as well as endocytosis as entry routes of AG uptake in HCs.
The MET channel is a non-selective cation channel located at the tips of the HC stereocilia. The MET channel is unique to sensory HCs and has pore dimensions estimated to be large enough for AG permeation (Farris et al., 2004; Marcotti et al., 2005). A number of studies point to the critical role for MET channels in AG entry into HCs (Alharazneh et al., 2011; Kawashima et al., 2011; Majumder et al., 2017; Makabe et al., 2020; Marcotti et al., 2016; Marcotti et al., 2005; Ricci, 2002; Vu et al., 2013). The polycationic AGs is presumed to approach the negatively charged channel pore for HC entry. In cochlear explants, MET channel blockers such as amiloride, quinine or curare suppressed a rapid transport of AGs into HCs, leading to prevention of HC loss (Alharazneh et al., 2011; Kawashima et al., 2011; Majumder et al., 2017; Marcotti et al., 2016; Marcotti et al., 2005; Vu et al., 2013). Furthermore, high extracellular calcium levels reduce the open probability of the MET channels, which in turn reduces AGs ability to enter through the MET channels resulting in diminished ototoxicity (Coffin et al., 2009; Ou et al., 2012; Vu et al., 2013; Wang and Steyger, 2009). Reduced AG uptake and toxicity are also observed in several genetic models where MET channels are rendered non-functional such as mutants of Cadherin23, Myosin7a, and Transmembrane channel-like proteins (TMC) (Kawashima et al., 2011; Marcotti et al., 2016; Vu et al., 2013; Wang and Steyger, 2009). Taken together, MET channels are the main mediator of AG entry into the HCs. Once inside HCs, a high energy barrier in the entrance does not allow AGs to exit from the cytosol via MET channels (Farris et al., 2004; Marcotti et al., 2005; Pan et al., 2012; van Netten and Kros, 2007), thus leading to drug accumulation within the cell.
In addition to the MET channels, TRP channels may also mediate AG entry into HCs. The TRP channel is also a non-selective cation channel that is permeable to Ca2+ ions (Karasawa et al., 2008; Myrdal and Steyger, 2005; Nilius and Szallasi, 2014). The pore sizes of TRPA1 (~1.1–1.4 nm) (Karashima et al., 2010), TRPV1 (~1 nm) (Jara-Oseguera et al., 2008) and TRPC3 (~6 nm) (Mio et al., 2007) are also considered to be large enough to allow AGs entry into HCs. Several subtypes of TRP channel such as TRPA1, TRPV1, TRPV4 and TRPC3 are expressed in the cochlea (Asai et al., 2010; Cuajungco et al., 2007; Garcia-Anoveros and Duggan, 2007), and candidates for AG permeation into HCs (Karasawa et al., 2008; Myrdal and Steyger, 2005; Stepanyan et al., 2011; Zheng et al., 2003). For example, TRPA1 channels activated by lipid peroxidation products allow AG entry when the MET channel is disabled (Stepanyan et al., 2011). Noise exposure also activates TRPA1 channels, leading to an increase of AG entry into HCs (Li et al., 2015; Yamashita et al., 2004). Furthermore, TRPV1 and TRPV4 are also considered as AG-permeant channels (Karasawa et al., 2008; Myrdal and Steyger, 2005; Zheng et al., 2003). More specifically, inhibitors of TRPV1 and TRPV4 channels markedly reduce AG uptake into HCs, though these compounds also can block MET channels (Lee et al., 2013). Inflammation-induced expression of TRPV1 possibly at multiple locations lead to an overall increased in cochlear uptake of AGs (Jiang et al., 2019). The main issue with TRP channels as a primary target for AG entry is that under normal physiological conditions these channels are typically not open. However, under conditions of infections or if initial access is via MET channels and that pathology activates TRP channels, it could result in a potentiation of the toxicity response.
Some studies suggest that AGs enter HCs through ATP receptor mediated ion channels (Bongartz et al., 2010; Lin et al., 2019; Xi et al., 1993). For example, prolonged activation of ATP receptors aggravates AG-induced ototoxicity in IHCs and OHCs (Lin et al., 2019). In Xenopus laevis oocytes, AGs inhibit ATP-induced receptor currents (Bongartz et al., 2010). Moreover, AGs inhibit the ATP responses in OHCs, suggesting ATP receptors as candidate targets of AGs (Xi et al., 1993).
Endocytosis at the apical and synaptic poles of HCs is another potential route of AG entry into HCs (Hashino et al., 2000). AGs are found in membrane-bound vesicles in the HCs, and the AG-contained vesicles increase over time (Hashino et al., 2000). In the zebrafish lateral line HCs, live imaging revealed endocytic uptake of AGs (Hailey et al., 2017; Nagai and Takano, 2014). The uptake results in accumulation of AGs in lysosomes (Hailey et al., 2017; Hashino et al., 2000; Nagai and Takano, 2014). In addition to its effects on the MET channel, Myosin VIIa may be involved in endocytosis-mediated AG accumulation evidenced by its high expression at the apical end of HCs where high amounts of vesicles are found (Richardson et al., 1997). By contrast, some studies suggested that inhibition of endocytosis does not affect AG uptake into HCs (Alharazneh et al., 2011; Myrdal et al., 2005). Multiple forms of endocytosis exist and assurance of pharmacological antagonism of all pathways is difficult, possibly causing the conflicting results.
As we reviewed above, MET, TRP, ATP channels and endocytosis are proposed as the entry routes of AGs into HCs. In particular, the MET channel is a major entry route for AGs (Alharazneh et al., 2011; Kawashima et al., 2011; Majumder et al., 2017; Marcotti et al., 2016; Marcotti et al., 2005; Vu et al., 2013), as multiple types of MET channel blockers have been found to protect against AG-induced HC loss (Alharazneh et al., 2011; Kawashima et al., 2011; Marcotti et al., 2016; Marcotti et al., 2005; Vu et al., 2013). In spite of an otoprotective effect of the blocker against HC loss in vitro, some of these blockers may not be suitable for clinical use as they are rather toxic. Targeting other ion channels or endocytosis is challenging, as these pathways are neither specific nor predominant to HCs. In recent years, many analogs of MET channel blockers have been identified as promising otoprotective agents against AGs. For example, d-tubocurarine was identified as a rapid and reversible blocker of MET channel and was used to reduce AG uptake into HCs (Farris et al., 2004; Glowatzki et al., 1997; Kirkwood et al., 2017). Although the blocker induces a transient loss of hearing and remodeling of the stereocilia, those changes are reversible (Velez-Ortega et al., 2017). Berbamine is also a reversible blocker and is itself highly toxic to HCs at high concentrations; however, berbamine analogs were less toxic and maintained otoprotective effects (Hudson et al., 2020; Kirkwood et al., 2017). Another reversible MET channel blocker, phenoxybenzamine, confers significant protection to HCs, but shows ototoxicity at higher concentrations and an additive toxic effect when combined with neomycin (Majumder et al., 2017). Furthermore, large-scale screening identified several protectants such as ORC-13661 and UoS-7692, both of which reversibly block MET channels and confer otoprotection (Kenyon et al., 2021; Kenyon et al., 2017; Kitcher et al., 2019). The otoprotective effects of UoS-7692 has been verified in zebrafish in vivo and cochlear explants in vitro (Hudson et al., 2020; Kenyon et al., 2017; Kirkwood et al., 2017; Kitcher et al., 2019; Majumder et al., 2017). Furthermore, ORC-13661 has been validated to be protective in vivo in the rat cochlea. Thus, MET channel blockers are promising otoprotectants for AG-induced hearing loss in clinic. However, more studies on the potential effects of MET channel blockage on hearing function are required to determine the safety of these drugs.
Mechanism and therapeutic target 3: Intracellular mechanism (Fig. 5)
Fig. 5. Intracellular mechanism of aminoglycoside toxicity in hair cells.
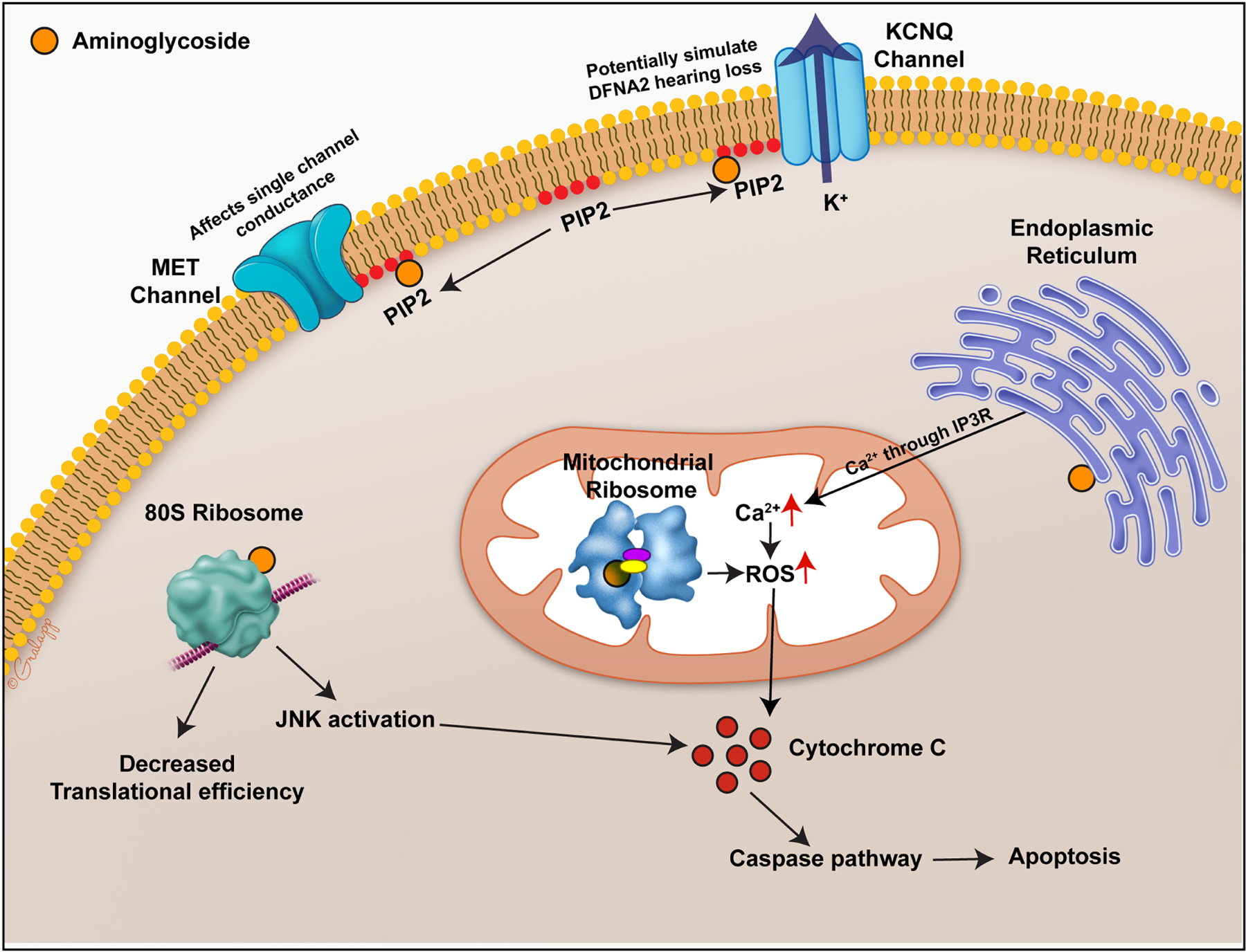
A simplified schematic of the effect of aminoglycosides (AG) in hair cells. AG interacts with organelles like mitochondria, ribosomes and endoplasmic reticulum, triggering reactive oxygen species production. Excess of reactive oxygen species within the cells can trigger hair cell death. Interaction of AGs with the lipid bilayer components like PIP2 can render the hair cells nonfunctional.
In the inner ear, after AG treatment, OHCs express more apoptotic markers than IHCs, with HCs in the basal turn showing more damage than those in the apical turn (Cheng et al., 2003; Cunningham et al., 2002; Forge and Li, 2000; Matsui et al., 2004). A large body of work has shown that AGs affect ribosomes, protein translation, ion transport, and lipids. (Forge and Schacht, 2000).
Ribotoxicity
Major clinically used antibiotics like AGs, tetracyclines, and macrolides target ribosomes thereby disrupting protein synthesis. AGs are antibiotics that specifically bind to the ribosomal RNA and cause misreading of the RNA code (Borovinskaya et al., 2007; Davies and Davis, 1968b; Feldman et al., 2010). RNA regions of the ribosomes are important for their translational activity and are conserved among organisms (Noller, 1991). Though chemically distinct, AGs bind to the conserved A-site of the 30s ribosomal subunit. This binding of AG stabilizes mRNA-tRNA binding and thereby decreases the tRNA dissociation leading to proofreading errors (Karimi and Ehrenberg, 1994). In eukaryotic cells, AG is shown to affect mitochondrial translation, which has been postulated to mediate its ototoxic side effects in humans (Böttger et al., 2001; Hobbie et al., 2008). In support of this concept, patients with mitochondrial rRNA mutations are highly sensitive to AGs. The mutations in the mitochondrial small ribosomal RNA genes A1555G and C1494T map to aminoacyl-tRNA A-site in a bacterial ribosome, resulting in inhibition of translocation of the mRNA-tRNA complex (Böttger et al., 2001; Campuzano et al., 1979; Carter et al., 2000; Davies and Davis, 1968b; Hutchin and Cortopassi, 1994; Moazed and Noller, 1987; Prezant et al., 1993; Zhao et al., 2004). This specific site is likely why AGs are more toxic to prokaryotic than eukaryotic cells (Böttger et al., 2001; Recht et al., 1999; Sander et al., 1996). A look into the molecular structure of the porcine 55S mitochondrial ribosome also shows a binding site for AGs in the A-site of the 28S subunit (Greber et al., 2015). This interaction of AGs with the mitochondrial protein synthesis may also affect the eukaryotic mitochondria and exacerbate ototoxicity (Hobbie et al., 2008) potentially through upregulation of reactive oxygen species. A recent study showed that the crystal structure of the 80S eukaryotic cytosolic ribosome in complex with multiple AG compounds (paromomycin, gentamicin, geneticin, and TC007) contains multiple AG binding sites within the large and small subunits of the ribosome; As such, AG binding affects the conformation of the pre-translational complex (Prokhorova et al., 2017). Thus, AGs can affect eukaryotic cytosolic ribosomes by multiple mechanisms and decrease their translational efficiency.
Minimizing ribotoxicity is another potential target to reduce ototoxicity. Modifying the structure of AG compounds to mitigate ribotoxicity while maintaining antibiotic effects is an efficient approach to reduce AG ototoxicity. AG antibiotics have been shown to interact with both the mitochondrial and cytosolic ribosomes in eukaryotic cells. Studies have shown that modified AGs that preferentially target cytosolic as compared to mitochondrial ribosomes displayed lower levels of ototoxicity (Kandasamy et al., 2012; Shulman et al., 2014), suggesting that the mitochondrial ribosomes may be the primary mediator of AG-induced ototoxicity. AGs with lower affinities to mitochondrial ribosomes can also potentially minimize ototoxicity (Matt et al., 2012; Perez-Fernandez et al., 2014). One study found that drug-binding of 4’−6’-acetyl and 4’-O-ether modification of 2-deoxystreptamine AG displayed less affinity to both cytosolic and mitochondrial ribosome drug binding regions and largely maintained antibacterial activity (Perez-Fernandez et al., 2014). This data suggest that exploiting the differences between the microbial and eukaryotic ribosomes is a potentially feasible approach for reducing ototoxicity. Modifications that minimize AG binding to the ribosome without compromising their antimicrobial activity should be an effective way to minimize AG-induced ototoxicity.
Free radicals
Free radicals are molecules with an unpaired electron making them unstable and highly reactive. Free radicals are normally present and participate in cell signaling and cell death pathways (Phaniendra et al., 2015). The free radical concentration in the cell is tightly regulated by an array of native antioxidants. When the antioxidant system becomes overwhelmed, free radicals can impair protein production, causing DNA and lipid damage and eventually leading to cell death. In vitro and in vivo studies have shown the production of reactive oxygen species (ROS) post AG treatment (Esterberg et al., 2016; Shulman et al., 2014). AG antibiotics have been reported to increase ROS production in avian HCs (Hirose et al., 1997) and the amount of ROS produced can be correlated with mitochondrial calcium uptake leading to HC death. A study on zebrafish lateral line HCs showed that application of the mitochondrial uniporter Ru360 reduced mitochondrial oxidation showing that mitochondrial calcium drives ROS production during AG-induced HC loss (Esterberg et al., 2016). In this study, ROS scavengers targeted the mitochondria and prevented HC death (Esterberg et al., 2016). In sum, AGs’ effects on the ribosomes and endoplasmic reticulum can lead to HC loss by ultimately increasing the ROS levels in the cell and triggering the apoptotic pathway.
Many studies have focused on minimizing the effects of ROS to mitigate ROS-induced cellular damage. Antioxidants conferred significant decrease in HC loss against AG-induced cell death. Aspirin was shown in human studies to be protective against gentamicin-induced hearing loss (Sha et al., 2006). Moreover, α-tocopherol was shown to reduce the progression of hearing loss in albino guinea pigs (Fetoni et al., 2003). In vitro screening using cell cultures showed compounds like lipoic acid, resveratrol, thiourea, deferoxamine, salicylic acid having a protective effect (Noack et al., 2017; Sha and Schacht, 2000). Corticosteroids such as dexamethasone also were shown to protect HCs by blocking nitric oxide syntheses and blocking free radicals (Himeno et al., 2002; Park et al., 2004). Hence, antioxidants have been shown to be an effective protective agent against AG ototoxicity. On the other hand, depleting glutathione, which is an intracellular free radical scavenger, did not have a significant impact on HC survival, suggesting that the involvement of free radicals in AG-ototoxicity might be more complex (Majumder et al., 2015). Scavenging ROS might possibly slow but not prevent HC death, as AGs interaction with cytosolic and mitochondrial ribosomes, lipid interactions and decreasing translational efficiency are unlikely to be protected by antioxidant treatments. The efficacy and the duration of antioxidant treatment will be determined by the rate of clearance of AGs from the HCs, as AGs have been detected in the sensory HCs 11 months post treatment (Aran et al., 1993). In summary, the applicability of and duration of treatment with antioxidants as a protectant against AGs need further investigation.
Calcium signaling
Ca2+ is a second messenger with broad physiological functions. Tight regulation of calcium is important as they have a multitude of functions from defining the open probability for the MET channels (Ricci et al., 1998) to playing a major role in exocytosis and endocytosis at IHC synapses (Beutner et al., 2001). In zebrafish, AGs first cause a loss of mitochondrial membrane potential in HCs, after which a transient increase in intracellular Ca2+ occurs prior to cell death. Chelating or caging this intracellular Ca2+ decreases the toxicity of AGs (Esterberg et al., 2013). Furthermore, there is a tight coupling of Ca2+ flow between the endoplasmic reticulum (ER) and mitochondria, with a sharp rise in Ca2+ in the ER shortly after a similar even in the mitochondria, subsequently leading to increased ROS production, intracellular damage, and cell death (Esterberg et al., 2014) (Esterberg et al., 2016).
Lipid interactions
AGs are known to interact with the plasma membrane lipids to induce membrane blebbing or phosphatidylserine externalization in the apical surface of the HCs (Goodyear et al., 2008; Richardson and Russell, 1991). AGs are also known to interact with phosphatidylinositol–4,5-bisphosphate (PIP2) and phosphatidylinositol l–3,4,5-trisphosphate (PIP3) (Gabev et al., 1989; Schacht, 1976b). PIP2 was also shown to localize in stereocilia and interact either directly or indirectly with ion channels at that location. PIP2 plays a major role in modulating MET channel (Cunningham et al., 2020; Effertz et al., 2017; Hirono et al., 2004). Free PIP2 amount in the stereocilia was shown to affect MET current adaptation and single-channel conductance (Effertz et al., 2017). AG binding to PIP2 can have an indirect effect on MET channel properties and adversely impact auditory function. PIP2 metabolism is linked to KCNQ4 potassium channel, mutations of which cause hereditary hearing loss by inducing OHC loss (Kharkovets et al., 2006; Kharkovets et al., 2000; Kubisch et al., 1999). AG binding to PIP2 may alter the KCNQ4 channels and could adversely affect the viability of the HCs by causing KCNQ4 dysfunction (Li et al., 2005; Suh et al., 2006).
Conclusions and directions for future work
AGs are potent antibiotics used for severe gram-negative bacterial infections, sepsis and tuberculosis (Schacht et al., 2012). Due to their stability, efficacy, and a low incidence of allergic reactions, AGs are among the most commonly used antibiotics despite their irreversible side effect of ototoxicity (Schacht et al., 2012; Van Boeckel et al., 2014). Understanding the mechanisms of AG uptake into the cochlea, their binding kinetics to different cellular components, and the resulting cellular response is required to identify therapeutic targets to prevent ototoxicity. Numerous efforts have revealed that AGs transport into cochlea through stria vascularis from blood vessels. Once AGs enter the endolymph, the cells in the OoC, mainly HCs, uptake AGs. MET, TRP, and ATP channels as well as endocytosis were suggested as entry routes of AG uptake in HCs. Intracellular AGs modify ribosomes, protein translation, ion transport, and lipids, leading to the cell death. We have highlighted several areas of knowledge gaps that serve as hurdles to our quest to prevent AG ototoxicity.
1) Understanding AG transport into the inner ear in human and animals. Numerous studies for elucidating the mechanisms underlying AG-induced ototoxicity were done in various animal models (e.g. mice, rats, guinea pigs, gerbils, and chinchilla). To induce AG-induced ototoxicity in animals, high and multiple doses of AGs as well as administration of diuretics are needed as most of the animals have higher resistance to AGs than human (Murillo-Cuesta et al., 2010; Taylor et al., 2008; Wu et al., 2001). The use of concurrent of diuretics and AG and high doses of AGs are both contraindicated in the clinical setting, so the clinical relevance of such paradigms needs to be considered despite their effectiveness and reproducibility as model systems. Moreover, while LPS-induced sepsis increases uptake of AGs into the inner ear, most clinical infections do not cause sepsis. How other forms of systemic infection (e.g. pneumonia or complicated urinary track infection) may affect AG uptake and ototoxicity remains elusive. Lastly, transporters responsible for AG entry from blood to the cochlea are currently unclear, and they may serve as potential targets to prevent AG uptake and ototoxicity in the long term.
2) Separation of ototoxicity and antibacterial activity. An ultimate goal of therapy is to eliminate AG-induced ototoxicity without compromising its antimicrobial effects. Understanding the structure-activity relationships of AGs can help direct our efforts in minimizing toxicity while preserving the antibacterial effects (O’Sullivan et al., 2020). Furthermore, multiple modifications of AGs have shown potential to prevent ototoxicity (Huth et al., 2015). Several validated MET channel blockers will be determined whether they are promising otoprotectants in mammalian models and in the clinic (Kenyon et al., 2021; Kitcher et al., 2019). While this co-therapy approach appears promising, it remains to be determined whether a prolonged regimen of MET channel blockers would be needed since certain AG treatment lasts weeks, and whether MET channel blockers can cause hearing loss is unclear.
3) Preventing intracellular action of AGs. Another way to decrease AG-induced ototoxicity is by targeting its intracellular action. Modifying AG structure so that it can preferentially bind to cytosolic ribosomes instead of mitochondrial ribosomes thereby delaying or preventing HC death (Kandasamy et al., 2012; Shulman et al., 2014). Designing the AGs to minimize binding to the eukaryotic ribosomes (Perez-Fernandez et al., 2014) without compromising their antimicrobial activity should be another major focus of research. Although antioxidants have been shown to prevent AG-induced HC death, its efficacy long-term and whether they interfere with antimicrobial activity remain unclear. A combinatorial approach employing modified AGs with antioxidants might prove to be a more effective approach to combat AG-induced HC loss.
In summary, the use of AGs remains vital in clinical settings worldwide. In addition to targeting individual pathways mentioned, it is conceivable to combine therapy preventing AG entry from the bloodstream in addition to blocking MET channels. It is imperative that future studies better define the mechanism of drug entry, intracellular interactions, and mechanism of HC toxicity to better identify targets in order to eliminate its side effects.
Highlights.
Aminoglycosides cause irreversible hearing loss by inducing cochlear hair cell death
Diuretics and sepsis potentiate aminoglycoside uptake into the cochlea and ototoxicity
Aminoglycoside enter hair cells via mechanotransducer channels
Blockers of mechanotransducer channels or designer aminoglycosides prevent hair cell degeneration
Acknowledgements
We thank C. Gralapp for figure illustration. This work is supported by NIDCD/NIH RO1DC014720 (A.G.C. and A.J.R.).
Abbreviation
- AG(s)
aminoglycoside(s)
- HC(s)
hair cell(s)
- OHC(s)
outer hair cell(s)
- IHC(s)
inner hair cell(s)
- OoC
organ of Corti
- SV
scala vestibuli
- SM
scala media
- ST
scala tympani
- GTTR
gentamicin texas red
- MET
mechanotransducer
- TRP
transient receptor potential
- ATP
adenosine triphosphate
- BLB
blood-labyrinth barrier
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests: The authors declare no competing interests.
References
- Abbas L, Rivolta MN, 2015. Aminoglycoside ototoxicity and hair cell ablation in the adult gerbil: A simple model to study hair cell loss and regeneration. Hear Res 325, 12–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyoshi M, Yano S, Ikeda T, 1976. Ototoxicity of spectinomycin (author’s transl). The Japanese journal of antibiotics 29, 771–782. [PubMed] [Google Scholar]
- Aksoy F, Dogan R, Ozturan O, Eren SB, Veyseller B, Pektas A, Huseyinbas O, 2014. Protective effect of trimetazidine on amikacin-induced ototoxicity in rats. Int J Pediatr Otorhinolaryngol 78, 663–669. [DOI] [PubMed] [Google Scholar]
- Alharazneh A, Luk L, Huth M, Monfared A, Steyger PS, Cheng AG, Ricci AJ, 2011. Functional hair cell mechanotransducer channels are required for aminoglycoside ototoxicity. PLoS One 6, e22347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aran J, Dulon D, Hiel H, Erre J, Aurousseau C, 1993. Ototoxicity of aminosides: recent results on uptake and clearance of gentamycin by sensory cells of the cochlea. Revue de laryngologie-otologie-rhinologie 114, 125–128. [PubMed] [Google Scholar]
- Armstrong BW, Colmore JP, Drorbaugh JE, 1950. Bed rest, collapse, and streptomycin in the treatment of pulmonary tuberculosis; clinical and roentgenologic observations; a preliminary report. Dis Chest 17, 11–32, illust. [DOI] [PubMed] [Google Scholar]
- Asai Y, Holt JR, Geleoc GS, 2010. A quantitative analysis of the spatiotemporal pattern of transient receptor potential gene expression in the developing mouse cochlea. J Assoc Res Otolaryngol 11, 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakuma S, Yoshida M, Toriya Y, Hirashima K, 1984. Experimental-Study of the Mechanism of the Decrease in Endocochlear Dc Potential after Administration of Nitrogen Mustard-N-Oxide. Acta Oto-Laryngologica 97, 273–282. [DOI] [PubMed] [Google Scholar]
- Asplund MS, Lidian A, Linder B, Takumida M, Anniko M, 2009. Protective effect of edaravone against tobramycin-induced ototoxicity. Acta Otolaryngol 129, 8–13. [DOI] [PubMed] [Google Scholar]
- Bailie GR, Neal D, 1988. Vancomycin ototoxicity and nephrotoxicity. A review. Med Toxicol Adverse Drug Exp 3, 376–386. [DOI] [PubMed] [Google Scholar]
- Berman JD, Fleckenstein L, 1991. Pharmacokinetic justification of antiprotozoal therapy. A US perspective. Clin Pharmacokinet 21, 479–493. [DOI] [PubMed] [Google Scholar]
- Beutner D, Voets T, Neher E, Moser T, 2001. Calcium dependence of exocytosis and endocytosis at the cochlear inner hair cell afferent synapse. Neuron 29, 681–690. [DOI] [PubMed] [Google Scholar]
- Bezdjian A, Mujica-Mota MA, Devic S, Daniel SJ, 2015. The effect of radiotherapy on gentamicin ototoxicity: an animal model. Otolaryngol Head Neck Surg 152, 1094–1101. [DOI] [PubMed] [Google Scholar]
- Bisht M, Bist SS, 2011. Ototoxicity: the hidden menace. Indian J Otolaryngol Head Neck Surg 63, 255–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakley BW, Hochman J, Wellman M, Gooi A, Hussain AE, 2008. Differences in Ototoxicity across Species. J Otolaryngol Head Neck Surg 37, 700–703. [PubMed] [Google Scholar]
- Bongartz EV, Rettinger J, Hausmann R, 2010. Aminoglycoside block of P2X2 receptors heterologously expressed in Xenopus laevis oocytes. Purinergic Signal 6, 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borovinskaya MA, Pai RD, Zhang W, Schuwirth BS, Holton JM, Hirokawa G, Kaji H, Kaji A, Cate JHD, 2007. Structural basis for aminoglycoside inhibition of bacterial ribosome recycling. Nature structural & molecular biology 14, 727–732. [DOI] [PubMed] [Google Scholar]
- Böttger EC, Springer B, Prammananan T, Kidan Y, Sander P, 2001. Structural basis for selectivity and toxicity of ribosomal antibiotics. EMBO reports 2, 318–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RD, Manno JE, Daigneault EA, Manno BR, 1979. Comparative acute ototoxicity of intravenous bumetanide and furosemide in the pure-bred beagle. Toxicol Appl Pharmacol 48, 157–169. [DOI] [PubMed] [Google Scholar]
- Brummett R, Fox K, Bendrick T, Himes D, 1978. Ototoxicity of tobramycin, gentamicin, amikacin and sisomicin in the guinea pig. Journal of Antimicrobial Chemotherapy 4, 73–83. [DOI] [PubMed] [Google Scholar]
- Bryan LE, Van Den Elzen HM, 1977. Effects of membrane-energy mutations and cations on streptomycin and gentamicin accumulation by bacteria: a model for entry of streptomycin and gentamicin in susceptible and resistant bacteria. Antimicrob Agents Chemother 12, 163–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabanas MJ, Vazquez D, Modolell J, 1978. Inhibition of ribosomal translocation by aminoglycoside antibiotics. Biochem Biophys Res Commun 83, 991–997. [DOI] [PubMed] [Google Scholar]
- Campuzano S, Vázquez D, Modolell J, 1979. Functional interaction of neomycin B and related antibiotics with 30S and 50S ribosomal subunits. Biochemical and biophysical research communications 87, 960–966. [DOI] [PubMed] [Google Scholar]
- Carter AP, Clemons WM, Brodersen DE, Morgan-Warren RJ, Wimberly BT, Ramakrishnan V, 2000. Functional insights from the structure of the 30S ribosomal subunit and its interactions with antibiotics. Nature 407, 340–348. [DOI] [PubMed] [Google Scholar]
- Cassidy J, Misset JL, 2002. Oxaliplatin-related side effects: characteristics and management. Semin Oncol 29, 11–20. [DOI] [PubMed] [Google Scholar]
- Cazals Y, 2000. Auditory sensori-neural alterations induced by salicylate. Prog Neurobiol 62, 583–631. [DOI] [PubMed] [Google Scholar]
- Chai Y, He W, Yang W, Hetrick AP, Gonzalez JG, Sargsyan L, Wu H, Jung TTK, Li H, 2021. Intratympanic Lipopolysaccharide Elevates Systemic Fluorescent Gentamicin Uptake in the Cochlea. Laryngoscope. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Liu Z, Yan H, Xing W, Mi W, Wang R, Li W, Chen F, Qiu J, Zha D, 2020. miR-182 prevented ototoxic deafness induced by co-administration of kanamycin and furosemide in rats. Neurosci Lett 723, 134861. [DOI] [PubMed] [Google Scholar]
- Cheng AG, Cunningham LL, Rubel EW, 2003. Hair cell death in the avian basilar papilla: characterization of the in vitro model and caspase activation. Journal of the Association for Research in Otolaryngology 4, 91–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen EI, Birn H, 2002. Megalin and cubilin: multifunctional endocytic receptors. Nat Rev Mol Cell Biol 3, 256–266. [DOI] [PubMed] [Google Scholar]
- Cianfrone G, Pentangelo D, Cianfrone F, Mazzei F, Turchetta R, Orlando M, Altissimi G, 2011. Pharmacological drugs inducing ototoxicity, vestibular symptoms and tinnitus: a reasoned and updated guide. Eur Rev Med Pharmacol Sci 15, 601–636. [PubMed] [Google Scholar]
- Coffin AB, Reinhart KE, Owens KN, Raible DW, Rubel EW, 2009. Extracellular divalent cations modulate aminoglycoside-induced hair cell death in the zebrafish lateral line. Hear Res 253, 42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuajungco MP, Grimm C, Heller S, 2007. TRP channels as candidates for hearing and balance abnormalities in vertebrates. Bba-Mol Basis Dis 1772, 1022–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings CW, 1968. Experimental observations on the ototoxicity of nitrogen mustard. Laryngoscope 78, 530–538. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Qiu X, Wu Z, Zhao B, Peng G, Kim YH, Lauer A, Muller U, 2020. TMIE Defines Pore and Gating Properties of the Mechanotransduction Channel of Mammalian Cochlear Hair Cells. Neuron 107, 126–143 e128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham LL, Cheng AG, Rubel EW, 2002. Caspase activation in hair cells of the mouse utricle exposed to neomycin. Journal of Neuroscience 22, 8532–8540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagil R, O’Shea C, Nykjaer A, Bonvin AMJJ, Kragelund BB, 2013. Gentamicin Binds to the Megalin Receptor as a Competitive Inhibitor Using the Common Ligand Binding Motif of Complement Type Repeats INSIGHT FROM THE NMR STRUCTURE OF THE 10TH COMPLEMENT TYPE REPEAT DOMAIN ALONE AND IN COMPLEX WITH GENTAMICIN. Journal of Biological Chemistry 288, 4424–4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai CF, Mangiardi D, Cotanche DA, Steyger PS, 2006. Uptake of fluorescent gentamicin by vertebrate sensory cells in vivo. Hear Res 213, 64–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal I, Edsmyr F, Stahle J, 1973. Bleomycin therapy and ototoxicity. Acta Otolaryngol 75, 323–324. [DOI] [PubMed] [Google Scholar]
- Davies J, Davis BD, 1968a. Misreading of ribonucleic acid code words induced by aminoglycoside antibiotics. The effect of drug concentration. J Biol Chem 243, 3312–3316. [PubMed] [Google Scholar]
- Davies J, Davis BD, 1968b. Misreading of ribonucleic acid code words induced by aminoglycoside antibiotics: the effect of drug concentration. Journal of Biological Chemistry 243, 3312–3316. [PubMed] [Google Scholar]
- Davis BD, 1987. Mechanism of bactericidal action of aminoglycosides. Microbiol Rev 51, 341–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis BD, Chen LL, Tai PC, 1986. Misread protein creates membrane channels: an essential step in the bactericidal action of aminoglycosides. Proc Natl Acad Sci U S A 83, 6164–6168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SN, Wu P, Camci ED, Simon JA, Rubel EW, Raible DW, 2020. Chloroquine kills hair cells in zebrafish lateral line and murine cochlear cultures: Implications for ototoxicity. Hear Res 395, 108019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Hollander JG, Horrevorts AM, van Goor ML, Verbrugh HA, Mouton JW, 1997. Synergism between tobramycin and ceftazidime against a resistant Pseudomonas aeruginosa strain, tested in an in vitro pharmacokinetic model. Antimicrob Agents Chemother 41, 95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding D, Jiang H, Salvi RJ, 2010. Mechanisms of rapid sensory hair-cell death following co-administration of gentamicin and ethacrynic acid. Hear Res 259, 16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding D, Liu H, Qi W, Jiang H, Li Y, Wu X, Sun H, Gross K, Salvi R, 2016. Ototoxic effects and mechanisms of loop diuretics. J Otol 11, 145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirain CO, Ng M, Milne-Davies B, Joseph JK, Antonelli PJ, 2018. Evaluation of Mitoquinone for Protecting Against Amikacin-Induced Ototoxicity in Guinea Pigs. Otol Neurotol 39, 111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edson RS, Terrell CL, 1991. The aminoglycosides. Mayo Clin Proc 66, 1158–1164. [DOI] [PubMed] [Google Scholar]
- Effertz T, Becker L, Peng AW, Ricci AJ, 2017. Phosphoinositol-4, 5-bisphosphate regulates auditory hair-cell mechanotransduction-channel pore properties and fast adaptation. Journal of Neuroscience 37, 11632–11646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Anwar MW, Abdelmonem S, Nada E, Galhoom D, Abdelsameea AA, 2016. Cilostazol Effect on Amikacin-Induced Ototoxicity: An Experimental Study. Audiol Neurootol 21, 250–253. [DOI] [PubMed] [Google Scholar]
- El-Anwar MW, Abdelmonem S, Nada E, Galhoom D, Abdelsameea AA, 2018. Protective effect of pentoxifylline on amikacin-induced ototoxicity. Ear Nose Throat J 97, E8–E12. [DOI] [PubMed] [Google Scholar]
- Esterberg R, Hailey DW, Coffin AB, Raible DW, Rubel EW, 2013. Disruption of intracellular calcium regulation is integral to aminoglycoside-induced hair cell death. Journal of Neuroscience 33, 7513–7525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterberg R, Hailey DW, Rubel EW, Raible DW, 2014. ER–mitochondrial calcium flow underlies vulnerability of mechanosensory hair cells to damage. Journal of Neuroscience 34, 9703–9719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterberg R, Linbo T, Pickett SB, Wu P, Ou HC, Rubel EW, Raible DW, 2016. Mitochondrial calcium uptake underlies ROS generation during aminoglycoside-induced hair cell death. The Journal of clinical investigation 126, 3556–3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farris HE, LeBlanc CL, Goswami J, Ricci AJ, 2004. Probing the pore of the auditory hair cell mechanotransducer channel in turtle. J Physiol 558, 769–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fausti SA, Schechter MA, Rappaport BZ, Frey RH, Mass RE, 1984. Early detection of cisplatin ototoxicity. Selected case reports. Cancer 53, 224–231. [DOI] [PubMed] [Google Scholar]
- Feldman MB, Terry DS, Altman RB, Blanchard SC, 2010. Aminoglycoside activity observed on single pre-translocation ribosome complexes. Nat Chem Biol 6, 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes VT, Lin VY, 2014. Development of an ototoxicity model in the adult CBA/CaJ mouse and determination of a golden window of corticosteroid intervention for otoprotection. J Otolaryngol Head Neck Surg 43, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetoni AR, Sergi B, Scarano E, Paludetti G, Ferraresi A, Troiani D, 2003. Protective effects of α-tocopherol against gentamicin-induced oto-vestibulo toxicity: an experimental study. Acta oto-laryngologica 123, 192–198. [DOI] [PubMed] [Google Scholar]
- Fettiplace R, 2017. Hair Cell Transduction, Tuning, and Synaptic Transmission in the Mammalian Cochlea. Compr Physiol 7, 1197–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forge A, Li L, 2000. Apoptotic death of hair cells in mammalian vestibular sensory epithelia. Hearing Res 139, 97–115. [DOI] [PubMed] [Google Scholar]
- Forge A, Schacht J, 2000. Aminoglycoside antibiotics. Audiology and Neurotology 5, 3–22. [DOI] [PubMed] [Google Scholar]
- Fourmy D, Recht MI, Puglisi JD, 1998. Binding of neomycin-class aminoglycoside antibiotics to the A-site of 16 S rRNA. J Mol Biol 277, 347–362. [DOI] [PubMed] [Google Scholar]
- Fredrick K, Noller HF, 2003. Catalysis of ribosomal translocation by sparsomycin. Science 300, 1159–1162. [DOI] [PubMed] [Google Scholar]
- Gabev E, Kasianowicz J, Abbott T, McLaughlin S, 1989. Binding of neomycin to phosphatidylinositol 4, 5-biphosphate (PIP2). Biochimica et Biophysica Acta (BBA)-Biomembranes 979, 105–112. [DOI] [PubMed] [Google Scholar]
- Gallagher KL, Jones JK, 1979. Furosemide-induced ototoxicity. Ann Intern Med 91, 744–745. [DOI] [PubMed] [Google Scholar]
- Garcia-Anoveros J, Duggan A, 2007. TRPA1 in Auditory and Nociceptive Organs, in: Liedtke WB, Heller S (Eds.), TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades, Boca Raton (FL). [PubMed] [Google Scholar]
- Garvin RT, Biswas DK, Gorini L, 1974. The effects of streptomycin or dihydrostreptomycin binding to 16S RNA or to 30S ribosomal subunits. Proc Natl Acad Sci U S A 71, 3814–3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glowatzki E, Ruppersberg JP, Zenner HP, Rusch A, 1997. Mechanically and ATP-induced currents of mouse outer hair cells are independent and differentially blocked by d-tubocurarine. Neuropharmacology 36, 1269–1275. [DOI] [PubMed] [Google Scholar]
- Goodlet KJ, Benhalima FZ, Nailor MD, 2018. A systematic review of single-dose aminoglycoside therapy for urinary tract infection: is it time to resurrect an old strategy? Antimicrobial agents and chemotherapy 63, e02165–02118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodyear RJ, Gale JE, Ranatunga KM, Kros CJ, Richardson GP, 2008. Aminoglycoside-induced phosphatidylserine externalization in sensory hair cells is regionally restricted, rapid, and reversible. Journal of Neuroscience 28, 9939–9952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greber BJ, Bieri P, Leibundgut M, Leitner A, Aebersold R, Boehringer D, Ban N, 2015. The complete structure of the 55S mammalian mitochondrial ribosome. Science 348, 303–308. [DOI] [PubMed] [Google Scholar]
- Greenwood GJ, 1959. Neomycin ototoxicity: Report of a case. AMA Archives of Otolaryngology 69, 390–397. [DOI] [PubMed] [Google Scholar]
- Gu R, Longenecker RJ, Homan J, Kil J, 2021. Ebselen attenuates tobramycin-induced ototoxicity in mice. J Cyst Fibros 20, 271–277. [DOI] [PubMed] [Google Scholar]
- Hailey DW, Esterberg R, Linbo TH, Rubel EW, Raible DW, 2017. Fluorescent aminoglycosides reveal intracellular trafficking routes in mechanosensory hair cells. Journal of Clinical Investigation 127, 472–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock RE, Farmer SW, Li ZS, Poole K, 1991. Interaction of aminoglycosides with the outer membranes and purified lipopolysaccharide and OmpF porin of Escherichia coli. Antimicrob Agents Chemother 35, 1309–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock RE, Raffle VJ, Nicas TI, 1981. Involvement of the outer membrane in gentamicin and streptomycin uptake and killing in Pseudomonas aeruginosa. Antimicrob Agents Chemother 19, 777–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart CW, Naunton RF, 1964. The Ototoxicity of Chloroquine Phosphate. Arch Otolaryngol 80, 407–412. [DOI] [PubMed] [Google Scholar]
- Hashino E, Shero M, Salvi RJ, 2000. Lysosomal augmentation during aminoglycoside uptake in cochlear hair cells. Brain Res 887, 90–97. [DOI] [PubMed] [Google Scholar]
- Havenith S, Klis SF, Versnel H, Grolman W, 2013. A guinea pig model of selective severe high-frequency hearing loss. Otol Neurotol 34, 1510–1518. [DOI] [PubMed] [Google Scholar]
- Hawkins E Jr., 1973. Ototoxic mechanisms. A working hypothesis. Audiology 12, 383–393. [DOI] [PubMed] [Google Scholar]
- Higgins C, Kastner R, 1967. Nebramycin, a new broad-spectrum antibiotic complex. II. Description of Streptomyces tenebrarius. Antimicrobial agents and chemotherapy 7, 324–331. [PubMed] [Google Scholar]
- Himeno C, Komeda M, Izumikawa M, Takemura K, Yagi M, Weiping Y, Doi T, Kuriyama H, Miller JM, Yamashita T, 2002. Intra-cochlear administration of dexamethasone attenuates aminoglycoside ototoxicity in the guinea pig. Hearing Res 167, 61–70. [DOI] [PubMed] [Google Scholar]
- Hinshaw HC, Feldman WH, Pfuetze KH, 1947. Streptomycin in treatment of clinical tuberculosis. Miss Valley Med J 69, 160–166. [PubMed] [Google Scholar]
- Hirono M, Denis CS, Richardson GP, Gillespie PG, 2004. Hair cells require phosphatidylinositol 4, 5-bisphosphate for mechanical transduction and adaptation. Neuron 44, 309–320. [DOI] [PubMed] [Google Scholar]
- Hirose K, Hockenbery DM, Rubel EW, 1997. Reactive oxygen species in chick hair cells after gentamicin exposure in vitro. Hearing Res 104, 1–14. [DOI] [PubMed] [Google Scholar]
- Hirose K, Li SZ, 2019. The role of monocytes and macrophages in the dynamic permeability of the blood-perilymph barrier. Hear Res 374, 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose K, Sato E, 2011. Comparative analysis of combination kanamycin-furosemide versus kanamycin alone in the mouse cochlea. Hear Res 272, 108–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie SN, Akshay S, Kalapala SK, Bruell CM, Shcherbakov D, Böttger EC, 2008. Genetic analysis of interactions with eukaryotic rRNA identify the mitoribosome as target in aminoglycoside ototoxicity. Proceedings of the National Academy of Sciences 105, 20888–20893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori Y, Aoki N, Kuwahara S, Hosojima M, Kaseda R, Goto S, Iida T, De S, Kabasawa H, Kaneko R, Aoki H, Tanabe Y, Kagamu H, Narita I, Kikuchi T, Saito A, 2017. Megalin Blockade with Cilastatin Suppresses Drug-Induced Nephrotoxicity. J Am Soc Nephrol 28, 1783–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Housley GD, Greenwood D, Ashmore JF, 1992. Localization of cholinergic and purinergic receptors on outer hair cells isolated from the guinea-pig cochlea. Proc Biol Sci 249, 265–273. [DOI] [PubMed] [Google Scholar]
- Housley GD, Kanjhan R, Raybould NP, Greenwood D, Salih SG, Jarlebark L, Burton LD, Setz VC, Cannell MB, Soeller C, Christie DL, Usami S, Matsubara A, Yoshie H, Ryan AF, Thorne PR, 1999. Expression of the P2X(2) receptor subunit of the ATP-gated ion channel in the cochlea: implications for sound transduction and auditory neurotransmission. J Neurosci 19, 8377–8388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson AM, Lockard GM, Namjoshi OA, Wilson JW, Kindt KS, Blough BE, Coffin AB, 2020. Berbamine Analogs Exhibit Differential Protective Effects From Aminoglycoside-Induced Hair Cell Death. Frontiers in Cellular Neuroscience 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchin T, Cortopassi G, 1994. Proposed molecular and cellular mechanism for aminoglycoside ototoxicity. Antimicrobial agents and chemotherapy 38, 2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huth ME, Han K-H, Sotoudeh K, Hsieh Y-J, Effertz T, Vu AA, Verhoeven S, Hsieh MH, Greenhouse R, Cheng AG, 2015. Designer aminoglycosides prevent cochlear hair cell loss and hearing loss. The Journal of clinical investigation 125, 583–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huy PTB, Manuel C, Meulemans A, Sterkers O, Wassef M, Amiel C, 1983. Ethacrynic-Acid Facilitates Gentamicin Entry into Endolymph of the Rat. Hearing Res 11, 191–202. [DOI] [PubMed] [Google Scholar]
- Imamura S, Adams JC, 2003. Distribution of gentamicin in the guinea pig inner ear after local or systemic application. J Assoc Res Otolaryngol 4, 176–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen TT, Bremer HG, Topsakal V, Hendriksen FG, Klis SF, Grolman W, 2013. Deafness induction in mice. Otol Neurotol 34, 1496–1502. [DOI] [PubMed] [Google Scholar]
- Jara-Oseguera A, Llorente I, Rosenbaum T, Islas LD, 2008. Properties of the inner pore region of TRPV1 channels revealed by block with quaternary ammoniums. J Gen Physiol 132, 547–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Karasawa T, Steyger PS, 2017a. Aminoglycoside-Induced Cochleotoxicity: A Review. Front Cell Neurosci 11, 308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Li H, Johnson A, Karasawa T, Zhang Y, Meier WB, Taghizadeh F, Kachelmeier A, Steyger PS, 2019. Inflammation up-regulates cochlear expression of TRPV1 to potentiate drug-induced hearing loss. Sci Adv 5, eaaw1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang MY, Taghizadeh F, Steyger PS, 2017b. Potential Mechanisms Underlying Inflammation-Enhanced Aminoglycoside-Induced Cochleotoxicity. Frontiers in Cellular Neuroscience 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju HM, Lee SH, Choi JS, Seo YJ, 2017. A Simple Model for Inducing Optimal Increase of SDF-1 with Aminoglycoside Ototoxicity. Biomed Res Int 2017, 4630241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung TT, Rhee CK, Lee CS, Park YS, Choi DC, 1993. Ototoxicity of salicylate, nonsteroidal antiinflammatory drugs, and quinine. Otolaryngol Clin North Am 26, 791–810. [PubMed] [Google Scholar]
- Kabins S, Nathan C, Cohen S, 1976. In vitro comparison of netilmicin, a semisynthetic derivative of sisomicin, and four other aminoglycoside antibiotics. Antimicrobial agents and chemotherapy 10, 139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadurugamuwa JL, Lam JS, Beveridge TJ, 1993. Interaction of gentamicin with the A band and B band lipopolysaccharides of Pseudomonas aeruginosa and its possible lethal effect. Antimicrob Agents Chemother 37, 715–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandasamy J, Atia-Glikin D, Shulman E, Shapira K, Shavit M, Belakhov V, Baasov T, 2012. Increased selectivity toward cytoplasmic versus mitochondrial ribosome confers improved efficiency of synthetic aminoglycosides in fixing damaged genes: a strategy for treatment of genetic diseases caused by nonsense mutations. Journal of medicinal chemistry 55, 10630–10643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kankuri E, Kurki T, Carlson P, Hiilesmaa V, 2003. Incidence, treatment and outcome of peripartum sepsis. Acta obstetricia et gynecologica Scandinavica 82, 730–735. [DOI] [PubMed] [Google Scholar]
- Karasawa T, Wang Q, Fu Y, Cohen DM, Steyger PS, 2008. TRPV4 enhances the cellular uptake of aminoglycoside antibiotics. J Cell Sci 121, 2871–2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karashima Y, Prenen J, Talavera K, Janssens A, Voets T, Nilius B, 2010. Agonist-Induced Changes in Ca2+ Permeation through the Nociceptor Cation Channel TRPA1. Biophys J 98, 773–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi R, Ehrenberg M, 1994. Dissociation rate of cognate peptidyl‐tRNA from the A‐site of hyper‐accurate and error‐prone ribosomes. European journal of biochemistry 226, 355–360. [DOI] [PubMed] [Google Scholar]
- Kaul M, Barbieri CM, Pilch DS, 2006. Aminoglycoside-induced reduction in nucleotide mobility at the ribosomal RNA A-site as a potentially key determinant of antibacterial activity. J Am Chem Soc 128, 1261–1271. [DOI] [PubMed] [Google Scholar]
- Kavcic B, Tkacik G, Bollenbach T, 2020. Mechanisms of drug interactions between translation-inhibiting antibiotics. Nat Commun 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi H, Naito T, Nakagawa S, Fujisawa K. i., 1972. BB-K8, a new semisynthetic aminoglycoside antibiotic. The Journal of antibiotics 25, 695–708. [DOI] [PubMed] [Google Scholar]
- Kawashima Y, Geleoc GS, Kurima K, Labay V, Lelli A, Asai Y, Makishima T, Wu DK, Della Santina CC, Holt JR, Griffith AJ, 2011. Mechanotransduction in mouse inner ear hair cells requires transmembrane channel-like genes. J Clin Invest 121, 4796–4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent A, Turner MA, Sharland M, Heath PT, 2014. Aminoglycoside toxicity in neonates: something to worry about? Expert review of anti-infective therapy 12, 319–331. [DOI] [PubMed] [Google Scholar]
- Kenyon EJ, Kirkwood NK, Kitcher SR, Goodyear RJ, Derudas M, Cantillon DM, Baxendale S, de la Vega de Leon A, Mahieu VN, Osgood RT, Donald Wilson C, Bull JC, Waddell SJ, Whitfield TT, Ward SE, Kros CJ, Richardson GP, 2021. Identification of a novel series of hair-cell MET channel blockers that protect against aminoglycoside-induced ototoxicity. Jci Insight. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon EJ, Kirkwood NK, Kitcher SR, O’Reilly M, Derudas M, Cantillon DM, Goodyear RJ, Secker A, Baxendale S, Bull JC, Waddell SJ, Whitfield TT, Ward SE, Kros CJ, Richardson GP, 2017. Identification of ion-channel modulators that protect against aminoglycosideinduced hair cell death. Jci Insight 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AS, Sheikh Z, Khan S, Dwivedi R, Benjamin E, 2011. Viagra deafness--sensorineural hearing loss and phosphodiesterase-5 inhibitors. Laryngoscope 121, 1049–1054. [DOI] [PubMed] [Google Scholar]
- Kharkovets T, Dedek K, Maier H, Schweizer M, Khimich D, Nouvian R, Vardanyan V, Leuwer R, Moser T, Jentsch TJ, 2006. Mice with altered KCNQ4 K+ channels implicate sensory outer hair cells in human progressive deafness. The EMBO journal 25, 642–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharkovets T, Hardelin J-P, Safieddine S, Schweizer M, El-Amraoui A, Petit C, Jentsch TJ, 2000. KCNQ4, a K+ channel mutated in a form of dominant deafness, is expressed in the inner ear and the central auditory pathway. Proceedings of the National Academy of Sciences 97, 4333–4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Ricci AJ, 2022. In vivo real-time imaging reveals megalin as the aminoglycoside gentamicin transporter into cochlea whose inhibition is otoprotective. Proceedings of the National Academy of Sciences 119, e2117946119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood NK, O’Reilly M, Derudas M, Kenyon EJ, Huckvale R, van Netten SM, Ward SE, Richardson GP, Kros CJ, 2017. d-Tubocurarine and Berbamine: Alkaloids That Are Permeant Blockers of the Hair Cell’s Mechano-Electrical Transducer Channel and Protect from Aminoglycoside Toxicity. Frontiers in Cellular Neuroscience 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kislak JW, 1972. The susceptibility of Bacteroides fragilis to 24 antibiotics. J Infect Dis 125, 295–299. [DOI] [PubMed] [Google Scholar]
- Kitcher SR, Kirkwood NK, Camci ED, Wu P, Gibson RM, Redila VA, Simon JA, Rubel EW, Raible DW, Richardson GP, Kros CJ, 2019. ORC-13661 protects sensory hair cells from aminoglycoside and cisplatin ototoxicity. Jci Insight 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinke R, Mertens M, 1988. Quantitative assessment of torasemide ototoxicity. Arzneimittelforschung 38, 153–155. [PubMed] [Google Scholar]
- Koo JW, Quintanilla-Dieck L, Jiang M, Liu J, Urdang ZD, Allensworth JJ, Cross CP, Li H, Steyger PS, 2015. Endotoxemia-mediated inflammation potentiates aminoglycoside-induced ototoxicity. Sci Transl Med 7, 298ra118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubisch C, Schroeder BC, Friedrich T, Lütjohann B, El-Amraoui A, Marlin S, Petit C, Jentsch TJ, 1999. KCNQ4, a novel potassium channel expressed in sensory outer hair cells, is mutated in dominant deafness. Cell 96, 437–446. [DOI] [PubMed] [Google Scholar]
- Kusakari J, Kambayashi J, Ise I, Kawamoto K, 1978. Reduction of the endocochlear potential by the new “loop” diuretic, bumetanide. Acta Otolaryngol 86, 336–341. [DOI] [PubMed] [Google Scholar]
- Langer T, am Zehnhoff-Dinnesen A, Radtke S, Meitert J, Zolk O, 2013. Understanding platinum-induced ototoxicity. Trends Pharmacol Sci 34, 458–469. [DOI] [PubMed] [Google Scholar]
- Lee JH, Park C, Kim SJ, Kim HJ, Oh GS, Shen AH, So HS, Park R, 2013. Different uptake of gentamicin through TRPV1 and TRPV4 channels determines cochlear hair cell vulnerability. Exp Mol Med 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Sun J, Zhao L, Guo W, Sun W, Yang S, 2017. Aminoglycoside Increases Permeability of Osseous Spiral Laminae of Cochlea by Interrupting MMP-2 and MMP-9 Balance. Neurotox Res 31, 348–357. [DOI] [PubMed] [Google Scholar]
- Li H, Steyger PS, 2011. Systemic aminoglycosides are trafficked via endolymph into cochlear hair cells. Sci Rep 1, 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HZ, Kachelmeier A, Fumess DN, Steyger PS, 2015. Local mechanisms for loud sound-enhanced aminoglycoside entry into outer hair cells. Frontiers in Cellular Neuroscience 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Gamper N, Hilgemann DW, Shapiro MS, 2005. Regulation of Kv7 (KCNQ) K+ channel open probability by phosphatidylinositol 4, 5-bisphosphate. Journal of Neuroscience 25, 9825–9835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SCY, Thorne PR, Housley GD, Vlajkovic SM, 2019. Purinergic Signaling and Aminoglycoside Ototoxicity: The Opposing Roles of P1 (Adenosine) and P2 (ATP) Receptors on Cochlear Hair Cell Survival. Front Cell Neurosci 13, 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Ding DL, Jiang HY, Wu XW, Salvi R, Sun H, 2011. Ototoxic destruction by co-administration of kanamycin and ethacrynic acid in rats. J Zhejiang Univ Sci B 12, 853–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox PT, Saunders J, Chandrasekhar SS, 2009. Sudden hearing loss from PDE-5 inhibitors: A possible cellular stress etiology. Laryngoscope 119, 1586–1589. [DOI] [PubMed] [Google Scholar]
- Majumder P, Duchen MR, Gale JE, 2015. Cellular glutathione content in the organ of Corti and its role during ototoxicity. Frontiers in cellular neuroscience 9, 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder P, Moore PA, Richardson GP, Gale JE, 2017. Protecting Mammalian Hair Cells from Aminoglycoside-Toxicity: Assessing Phenoxybenzamine’s Potential. Frontiers in Cellular Neuroscience 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makabe A, Kawashima Y, Sakamaki Y, Maruyama A, Fujikawa T, Ito T, Kurima K, Griffith AJ, Tsutsumi T, 2020. Systemic Fluorescent Gentamicin Enters Neonatal Mouse Hair Cells Predominantly Through Sensory Mechanoelectrical Transduction Channels. Jaro-J Assoc Res Oto 21, 137–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcotti W, Corns LF, Goodyear RJ, Rzadzinska AK, Avraham KB, Steel KP, Richardson GP, Kros CJ, 2016. The acquisition of mechano-electrical transducer current adaptation in auditory hair cells requires myosin VI. J Physiol 594, 3667–3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcotti W, van Netten SM, Kros CJ, 2005. The aminoglycoside antibiotic dihydrostreptomycin rapidly enters mouse outer hair cells through the mechano-electrical transducer channels. J Physiol 567, 505–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin NL, Beveridge TJ, 1986. Gentamicin interaction with Pseudomonas aeruginosa cell envelope. Antimicrob Agents Chemother 29, 1079–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin WJ, Gardner M, Washington JA 2nd, 1972. In vitro antimicrobial susceptibility of anaerobic bacteria isolated from clinical specimens. Antimicrob Agents Chemother 1, 148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason D, Dietz A, Smith R, 1961. Actino-spectacin, a New Antibiotic. I. Discovery and Biological Properties. Antibiotics & Chemotherapy 11, 118–122. [PubMed] [Google Scholar]
- Matsui JI, Gale JE, Warchol ME, 2004. Critical signaling events during the aminoglycoside‐induced death of sensory hair cells in vitro. Journal of neurobiology 61, 250–266. [DOI] [PubMed] [Google Scholar]
- Matt T, Ng CL, Lang K, Sha S-H, Akbergenov R, Shcherbakov D, Meyer M, Duscha S, Xie J, Dubbaka SR, 2012. Dissociation of antibacterial activity and aminoglycoside ototoxicity in the 4-monosubstituted 2-deoxystreptamine apramycin. Proceedings of the National Academy of Sciences 109, 10984–10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden SL, Ding D, Jiang H, Woo JM, Salvi RJ, 2002. Chinchilla models of selective cochlear hair cell loss. Hear Res 174, 230–238. [DOI] [PubMed] [Google Scholar]
- McGwin G, 2010. Phosphodiesterase Type 5 Inhibitor Use and Hearing Impairment. Archives of Otolaryngology-Head & Neck Surgery 136, 488–492. [DOI] [PubMed] [Google Scholar]
- Mingeot-Leclercq MP, Tulkens PM, 1999. Aminoglycosides: nephrotoxicity. Antimicrob Agents Chemother 43, 1003–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mio K, Ogura T, Kiyonaka S, Hiroaki Y, Tanimura Y, Fujiyoshi Y, Mori Y, Sato C, 2007. The TRPC3 channel has a large internal chamber surrounded by signal sensing antennas. Journal of Molecular Biology 367, 373–383. [DOI] [PubMed] [Google Scholar]
- Misumi M, Nishimura T, Komai T, Tanaka N, 1978. Interaction of kanamycin and related antibiotics with the large subunit of ribosomes and the inhibition of translocation. Biochem Biophys Res Commun 84, 358–365. [DOI] [PubMed] [Google Scholar]
- Moazed D, Noller HF, 1987. Interaction of antibiotics with functional sites in 16S ribosomal RNA. Nature 327, 389–394. [DOI] [PubMed] [Google Scholar]
- Mukherjee B, Shivakumar T, 2007. A case of sensorineural deafness following ingestion of sildenafil. J Laryngol Otol 121, 395–397. [DOI] [PubMed] [Google Scholar]
- Murillo-Cuesta S, Contreras J, Cediel R, Varela-Nieto I, 2010. Comparison of different aminoglycoside antibiotic treatments to refine ototoxicity studies in adult mice. Lab Anim 44, 124–131. [DOI] [PubMed] [Google Scholar]
- Musser JM, 1995. Antimicrobial agent resistance in mycobacteria: molecular genetic insights. Clin Microbiol Rev 8, 496–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrdal SE, Johnson KC, Steyger PS, 2005. Cytoplasmic and intra-nuclear binding of gentamicin does not require endocytosis. Hear Res 204, 156–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrdal SE, Steyger PS, 2005. TRPV1 regulators mediate gentamicin penetration of cultured kidney cells. Hear Res 204, 170–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagabhushan T, Cooper A, Tsai H, Daniels P, Miller G, 1978. The syntheses and biological properties of 1-N-(S-4-amino-2-hydroxybutyryl)-gentamicin B and 1-N-(S-3-amino-2-hydroxypropionyl)-gentamicin B. The Journal of antibiotics 31, 681–687. [DOI] [PubMed] [Google Scholar]
- Nagai J, Takano M, 2014. Entry of aminoglycosides into renal tubular epithelial cells via endocytosis-dependent and endocytosis-independent pathways. Biochem Pharmacol 90, 331–337. [DOI] [PubMed] [Google Scholar]
- Nakae R, Nakae T, 1982. Diffusion of Aminoglycoside Antibiotics across the Outer-Membrane of Escherichia-Coli. Antimicrobial Agents and Chemotherapy 22, 554–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols WW, Young SN, 1985. Respiration-dependent uptake of dihydrostreptomycin by Escherichia coli. Its irreversible nature and lack of evidence for a uniport process. Biochem J 228, 505–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Szallasi A, 2014. Transient Receptor Potential Channels as Drug Targets: From the Science of Basic Research to the Art of Medicine. Pharmacol Rev 66, 676–814. [DOI] [PubMed] [Google Scholar]
- Noack V, Pak K, Jalota R, Kurabi A, Ryan AF, 2017. An antioxidant screen identifies candidates for protection of cochlear hair cells from gentamicin toxicity. Frontiers in cellular neuroscience 11, 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noller HF, 1991. Ribosomal RNA and translation. Annual review of biochemistry 60, 191–227. [DOI] [PubMed] [Google Scholar]
- Nyberg S, Abbott NJ, Shi X, Steyger PS, Dabdoub A, 2019. Delivery of therapeutics to the inner ear: The challenge of the blood-labyrinth barrier. Sci Transl Med 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan ME, Poitevin F, Sierra RG, Gati C, Dao EH, Rao Y, Aksit F, Ciftci H, Corsepius N, Greenhouse R, Hayes B, Hunter MS, Liang ML, McGurk A, Mbgam P, Obrinsky T, Pardo-Avila F, Seaberg MH, Cheng AG, Ricci AJ, DeMirci H, 2018. Aminoglycoside ribosome interactions reveal novel conformational states at ambient temperature. Nucleic Acids Res 46, 9793–9804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan ME, Song Y, Greenhouse R, Lin R, Perez A, Atkinson PJ, MacDonald JP, Siddiqui Z, Lagasca D, Comstock K, Huth ME, Cheng AG, Ricci AJ, 2020. Dissociating antibacterial from ototoxic effects of gentamicin C-subtypes. Proc Natl Acad Sci U S A 117, 32423–32432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogier JM, Lockhart PJ, Burt RA, 2020. Intravenously delivered aminoglycoside antibiotics, tobramycin and amikacin, are not ototoxic in mice. Hear Res 386, 107870. [DOI] [PubMed] [Google Scholar]
- Okuyucu S, Guven OE, Akoglu E, Ucar E, Dagli S, 2009. Effect of phosphodiesterase-5 inhibitor on hearing. J Laryngol Otol 123, 718–722. [DOI] [PubMed] [Google Scholar]
- Oliveira JA, Canedo DM, Rossato M, Andrade MH, 2004. Self-protection against aminoglycoside ototoxicity in guinea pigs. Otolaryngol Head Neck Surg 131, 271–279. [DOI] [PubMed] [Google Scholar]
- Ou HC, Keating S, Wu P, Simon JA, Raible DW, Rubel EW, 2012. Quinoline ring derivatives protect against aminoglycoside-induced hair cell death in the zebrafish lateral line. J Assoc Res Otolaryngol 13, 759–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paloheimo S, Thalman R, 1977. Influence of “loop” diuretics upon Na+K+-ATPase and adenylate cyclase of the stria vascularis. Arch Otorhinolaryngol 217, 347–359. [DOI] [PubMed] [Google Scholar]
- Pan B, Waguespack J, Schnee ME, LeBlanc C, Ricci AJ, 2012. Permeation properties of the hair cell mechanotransducer channel provide insight into its molecular structure. J Neurophysiol 107, 2408–2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SK, Choi D, Russell P, John EO, Jung TT, 2004. Protective effect of corticosteroid against the cytotoxicity of aminoglycoside otic drops on isolated cochlear outer hair cells. The Laryngoscope 114, 768–771. [DOI] [PubMed] [Google Scholar]
- Patuzzi R, 2011. Ion flow in stria vascularis and the production and regulation of cochlear endolymph and the endolymphatic potential. Hear Res 277, 4–19. [DOI] [PubMed] [Google Scholar]
- Perez-Fernandez D, Shcherbakov D, Matt T, Leong NC, Kudyba I, Duscha S, Boukari H, Patak R, Dubbaka SR, Lang K, 2014. 4’-O-substitutions determine selectivity of aminoglycoside antibiotics. Nat Commun 5, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson AA, Hancock RE, McGroarty EJ, 1985. Binding of polycationic antibiotics and polyamines to lipopolysaccharides of Pseudomonas aeruginosa. J Bacteriol 164, 1256–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan PAB, Tadros SF, Kim Y, Birnbaumer L, Housley GD, 2010. Developmental regulation of TRPC3 ion channel expression in the mouse cochlea. Histochem Cell Biol 133, 437–448. [DOI] [PubMed] [Google Scholar]
- Phaniendra A, Jestadi DB, Periyasamy L, 2015. Free radicals: properties, sources, targets, and their implication in various diseases. Indian journal of clinical biochemistry 30, 11–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickles JO, 2008. An introduction to the physiology of hearing. Emerald Group Publishing Limited. [Google Scholar]
- Prayuenyong P, Baguley DM, Kros CJ, Steyger PS, 2021. Preferential cochleotoxicity of cisplatin. Frontiers in Neuroscience, 935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prezant TR, Agapian JV, Bohlman MC, Bu X, Öztas S, Qiu W-Q, Arnos KS, Cortopassi GA, Jaber L, Rotter JI, 1993. Mitochondrial ribosomal RNA mutation associated with both antibiotic–induced and non–syndromic deafness. Nature genetics 4, 289–294. [DOI] [PubMed] [Google Scholar]
- Prokhorova I, Altman RB, Djumagulov M, Shrestha JP, Urzhumtsev A, Ferguson A, Chang C-WT, Yusupov M, Blanchard SC, Yusupova G, 2017. Aminoglycoside interactions and impacts on the eukaryotic ribosome. Proceedings of the National Academy of Sciences 114, E10899–E10908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recht MI, Douthwaite S, Puglisi JD, 1999. Basis for prokaryotic specificity of action of aminoglycoside antibiotics. The EMBO journal 18, 3133–3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci A, 2002. Differences in mechano-transducer channel kinetics underlie tonotopic distribution of fast adaptation in auditory hair cells. J Neurophysiol 87, 1738–1748. [DOI] [PubMed] [Google Scholar]
- Ricci A, Wu Y, Fettiplace R, 1998. The endogenous calcium buffer and the time course of transducer adaptation in auditory hair cells. Journal of Neuroscience 18, 8261–8277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson GP, Forge A, Kros CJ, Fleming J, Brown SDM, Steel KP, 1997. Myosin VIIA is required for aminoglycoside accumulation in cochlear hair cells. Journal of Neuroscience 17, 9506–9519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson GP, Russell IJ, 1991. Cochlear cultures as a model system for studying aminoglycoside induced ototoxicity. Hearing Res 53, 293–311. [DOI] [PubMed] [Google Scholar]
- Rybak LP, Whitworth C, Scott V, 1991. Comparative acute ototoxicity of loop diuretic compounds. Eur Arch Otorhinolaryngol 248, 353–357. [DOI] [PubMed] [Google Scholar]
- Sander P, Prammananan T, Böttger EC, 1996. Introducing mutations into a chromosomal rRNA gene using a genetically modified eubacterial host with a single rRNA operon. Molecular microbiology 22, 841–848. [DOI] [PubMed] [Google Scholar]
- Sataloff J, Wagner S, Menduke H, 1964. Kanamycin ototoxicity in healthy men. Archives of Otolaryngology 80, 413–417. [DOI] [PubMed] [Google Scholar]
- Schacht J, 1976a. Biochemistry of neomycin ototoxicity. The Journal of the Acoustical Society of America 59, 940–944. [DOI] [PubMed] [Google Scholar]
- Schacht J, 1976b. Inhibition by neomycin of polyphosphoinositide turnover in subcellular fractions of guinea‐pig cerebral cortex in vitro. Journal of neurochemistry 27, 1119–1124. [DOI] [PubMed] [Google Scholar]
- Schacht J, Talaska AE, Rybak LP, 2012. Cisplatin and aminoglycoside antibiotics: hearing loss and its prevention. Anat Rec (Hoboken) 295, 1837–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz A, Bugle E, Waksman SA, 1944. Streptomycin, a Substance Exhibiting Antibiotic Activity Against Gram-Positive and Gram-Negative Bacteria.*. Proceedings of the Society for Experimental Biology and Medicine 55, 66–69. [Google Scholar]
- Schmitz HM, Johnson SB, Santi PA, 2014. Kanamycin-furosemide ototoxicity in the mouse cochlea: a 3-dimensional analysis. Otolaryngol Head Neck Surg 150, 666–672. [DOI] [PubMed] [Google Scholar]
- Sha S-H, Qiu J-H, Schacht J, 2006. Aspirin to prevent gentamicin-induced hearing loss. New England Journal of Medicine 354, 1856–1857. [DOI] [PubMed] [Google Scholar]
- Sha S-H, Schacht J, 2000. Antioxidants attenuate gentamicin-induced free radical formation in vitro and ototoxicity in vivo: D-methionine is a potential protectant. Hearing Res 142, 34–40. [DOI] [PubMed] [Google Scholar]
- Shi X, 2016. Pathophysiology of the cochlear intrastrial fluid-blood barrier (review). Hear Res 338, 52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shomura T, EZAKI N, TSURUOKA T, NIWA T, AKITA E, NIIDA T, 1970. Studies on antibiotic SF-733, a new antibiotic. I Taxonomy, isolation and characterization. The Journal of antibiotics 23, 155–161. [DOI] [PubMed] [Google Scholar]
- Shulman E, Belakhov V, Wei G, Kendall A, Meyron-Holtz EG, Ben-Shachar D, Schacht J, Baasov T, 2014. Designer aminoglycosides that selectively inhibit cytoplasmic rather than mitochondrial ribosomes show decreased ototoxicity: a strategy for the treatment of genetic diseases. Journal of Biological Chemistry 289, 2318–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanyan RS, Indzhykulian AA, Velez-Ortega AC, Boger ET, Steyger PS, Friedman TB, Frolenkov GI, 2011. TRPA1-mediated accumulation of aminoglycosides in mouse cochlear outer hair cells. J Assoc Res Otolaryngol 12, 729–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterkers O, Ferrary E, Amiel C, 1984. Intercompartmental and Intracompartmental Osmotic Gradients within the Rat Cochlea. Am J Physiol 247, F602–F606. [DOI] [PubMed] [Google Scholar]
- Sterkers O, Ferrary E, Amiel C, 1988. Production of Inner-Ear Fluids. Physiol Rev 68, 1083–1128. [DOI] [PubMed] [Google Scholar]
- Steyger PS, 2021. Mechanisms of aminoglycoside-and cisplatin-induced ototoxicity. American Journal of Audiology, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh B-C, Inoue T, Meyer T, Hille B, 2006. Rapid chemically induced changes of PtdIns (4, 5) P2 gate KCNQ ion channels. Science 314, 1454–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan SK, 1997. Aminoglycoside nephrotoxicity. Semin Nephrol 17, 27–33. [PubMed] [Google Scholar]
- Taber HW, Mueller JP, Miller PF, Arrow AS, 1987. Bacterial uptake of aminoglycoside antibiotics. Microbiol Rev 51, 439–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tange RA, Dreschler WA, Claessen FA, Perenboom RM, 1997. Ototoxic reactions of quinine in healthy persons and patients with Plasmodium falciparum infection. Auris Nasus Larynx 24, 131–136. [DOI] [PubMed] [Google Scholar]
- Tauris J, Christensen EI, Nykjaer A, Jacobsen C, Petersen CM, Ovesen T, 2009. Cubilin and megalin co-localize in the neonatal inner ear. Audiol Neurootol 14, 267–278. [DOI] [PubMed] [Google Scholar]
- Taylor RR, Nevill G, Forge A, 2008. Rapid hair cell loss: a mouse model for cochlear lesions. J Assoc Res Otolaryngol 9, 44–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TH Haskell JF, Bartz QR, 1959. PAROMOMYCIN. I. PAROMAMINE, A GLYCOSIDE OF d-GLUCOSAMINE1. Journal of the American Chemical Society 81. [Google Scholar]
- Tran Ba Huy P, Bernard P, Schacht J, 1986. Kinetics of gentamicin uptake and release in the rat. Comparison of inner ear tissues and fluids with other organs. J Clin Invest 77, 1492–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran Ba Huy P, Manuel C, Meulemans A, Sterkers O, Amiel C, 1981. Pharmacokinetics of gentamicin in perilymph and endolymph of the rat as determined by radioimmunoassay. J Infect Dis 143, 476–486. [DOI] [PubMed] [Google Scholar]
- Umezawa H, Ueda M, Maeda K, Yagishita K, Kondō S, Okami Y, Utahara R, Ōsato Y, Nitta K, Takeuchi T, 1957. Production and isolation of a new antibiotic, kanamycin. The Journal of Antibiotics, Series A 10, 181–188. [PubMed] [Google Scholar]
- Van Boeckel TP, Gandra S, Ashok A, Caudron Q, Grenfell BT, Levin SA, Laxminarayan R, 2014. Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet Infect Dis 14, 742–750. [DOI] [PubMed] [Google Scholar]
- van Netten SM, Kros CJ, 2007. Insights into the pore of the hair cell transducer channel from experiments with permeant blockers. Curr Top Membr 59, 375–398. [DOI] [PubMed] [Google Scholar]
- Velez-Ortega AC, Freeman MJ, Indzhykulian AA, Grossheim JM, Frolenkov GI, 2017. Mechanotransduction current is essential for stability of the transducing stereocilia in mammalian audiotry hair cells. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu AA, Nadaraja GS, Huth ME, Luk L, Kim J, Chai R, Ricci AJ, Cheng AG, 2013. Integrity and regeneration of mechanotransduction machinery regulate aminoglycoside entry and sensory cell death. PLoS One 8, e54794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake M, Takeno S, Ibrahim D, Harrison R, 1994. Selective inner hair cell ototoxicity induced by carboplatin. Laryngoscope 104, 488–493. [DOI] [PubMed] [Google Scholar]
- Waksman SA, Lechevalier HA, 1949. Neomycin, a new antibiotic active against streptomycin-resistant bacteria, including tuberculosis organisms. Science 109, 305–307. [DOI] [PubMed] [Google Scholar]
- Wang L, Pulk A, Wasserman MR, Feldman MB, Altman RB, Cate JH, Blanchard SC, 2012. Allosteric control of the ribosome by small-molecule antibiotics. Nat Struct Mol Biol 19, 957–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Steyger PS, 2009. Trafficking of systemic fluorescent gentamicin into the cochlea and hair cells. J Assoc Res Otolaryngol 10, 205–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Hamasaki K, Rando RR, 1997. Specificity of aminoglycoside binding to RNA constructs derived from the 16S rRNA decoding region and the HIV-RRE activator region. Biochemistry 36, 768–779. [DOI] [PubMed] [Google Scholar]
- Wangemann P, 2006. Supporting sensory transduction: cochlear fluid homeostasis and the endocochlear potential. J Physiol 576, 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman MR, Pulk A, Zhou Z, Altman RB, Zinder JC, Green KD, Garneau-Tsodikova S, Cate JHD, Blanchard SC, 2015. Chemically related 4,5-linked aminoglycoside antibiotics drive subunit rotation in opposite directions. Nat Commun 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein M, 1963. Gentamicin, a new antibiotic complex from Micromonospora. J. Med. Chem 122, 463–464. [DOI] [PubMed] [Google Scholar]
- Weinstein MJ, Marquez JA, Testa RT, Wagman GH, Oden EM, Waitz JA, 1970. Antibiotic 6640, a new micromonospora-producbd aminoglycoside antibiotic. The Journal of antibiotics 23, 551–554. [DOI] [PubMed] [Google Scholar]
- Woo PW, Dion HW, Bartz QR, 1971. Butirosins A and B, aminoglycoside antibiotics. I. Structural units. Tetrahedron Letters 12, 2617–2620. [Google Scholar]
- Wright GD, Berghuis AM, Mobashery S, 1998. Aminoglycoside antibiotics. Resolving the antibiotic paradox, 27–69. [PubMed] [Google Scholar]
- Wu WJ, Sha SH, McLaren JD, Kawamoto K, Raphael Y, Schacht J, 2001. Aminoglycoside ototoxicity in adult CBA, C57BL and BALB mice and the Sprague-Dawley rat. Hear Res 158, 165–178. [DOI] [PubMed] [Google Scholar]
- Xi L, Hume RI, Nuttall AL, 1993. Voltage-Dependent Block by Neomycin of the Atp-Induced Whole-Cell Current of Guinea-Pig Outer Hair-Cells. Journal of Neurophysiology 70, 1593–1605. [DOI] [PubMed] [Google Scholar]
- Yamashita D, Jiang HY, Schacht J, Miller JM, 2004. Delayed production of free radicals following noise exposure. Brain Res 1019, 201–209. [DOI] [PubMed] [Google Scholar]
- Yamasoba T, Kondo K, Miyajima C, Suzuki M, 2003. Changes in cell proliferation in rat and guinea pig cochlea after aminoglycoside-induced damage. Neurosci Lett 347, 171–174. [DOI] [PubMed] [Google Scholar]
- Ying LQ, Zhu HK, Shoji S, Fredrick K, 2019. Roles of specific aminoglycoside-ribosome interactions in the inhibition of translation. Rna 25, 247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapp ML, Stern S, Green MR, 1993. Small molecules that selectively block RNA binding of HIV-1 Rev protein inhibit Rev function and viral production. Cell 74, 969–978. [DOI] [PubMed] [Google Scholar]
- Zettner EM, Gleser MA, 2018. Progressive Hearing Loss among Patients with Cystic Fibrosis and Parenteral Aminoglycoside Treatment. Otolaryngol Head Neck Surg 159, 887–894. [DOI] [PubMed] [Google Scholar]
- Zhao H, Li R, Wang Q, Yan Q, Deng J-H, Han D, Bai Y, Young W-Y, Guan M-X, 2004. Maternally inherited aminoglycoside-induced and nonsyndromic deafness is associated with the novel C1494T mutation in the mitochondrial 12S rRNA gene in a large Chinese family. The American Journal of Human Genetics 74, 139–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng JF, Dai CF, Steyger PS, Kim Y, Vass Z, Ren TY, Nuttall AL, 2003. Vanilloid receptors in hearing: Altered cochlear sensitivity by vanilloids and expression of TRPV1 in the organ of Corti. Journal of Neurophysiology 90, 444–455. [DOI] [PMC free article] [PubMed] [Google Scholar]


