Abstract
Limited case series describe conflicting results regarding the safety of checkpoint inhibitors (CPI) prior to liver transplantation (LT). We reviewed single-center data on all consecutive patients who underwent LT for hepatocellular carcinoma treated with CPI between January 1, 2018, and January 30, 2021. Time from CPI to LT, immunosuppression, biopsy-proven acute cellular rejection (BPACR), graft loss and death were evaluated. Five patients with a mean age 65 (range 61–71) years underwent LT after CPI with nivolumab. Time from last CPI to LT ranged from 0.3 to 11 months. Two patients with <3 months from the last dose of CPI to LT developed BPACR and severe hepatic necrosis, one of whom required retransplantation with recurrent BPACR but without recurrent graft loss over 38 months of follow up. None of the patients who underwent LT >3 months from the last dose of CPI had BPACR. In conclusion, pretransplant use of CPIs, particularly within 90 days of LT, was associated with BPACR and immune-mediated hepatic necrosis. Future multicenter studies should consider a sufficient interval from the last dose of CPI to LT to mitigate the risk for adverse immune-mediated outcomes and graft loss.
Keywords: cancer/malignancy/neoplasia, cancer/malignancy/neoplasia: adjuvant therapy, clinical decision-making, clinical research/practice, complication: medical/metabolic, liver allograft function/dysfunction, liver transplantation/hepatology
1 |. INTRODUCTION
Immune checkpoint inhibitors (CPIs) are monoclonal antibodies that exert antitumor activity by blocking checkpoint proteins, which down-regulate immunity against tumor cells. Recently, the combination of CPI targeting programmed cell death ligand 1 (PD-L1) and vascular endothelial growth factor blockade have been approved as first-line therapy for unresectable hepatocellular carcinoma (HCC).1 Concurrently, liver transplantation (LT) has demonstrated excellent long-term outcomes in large and multifocal intrahepatic HCC beyond Milan Criteria.2 Despite the expanded use of CPIs and LT for advanced HCC, limited data are available regarding the risk of LT in patients who received CPI therapy. We hypothesized that time from CPI therapy to transplant would be associated with posttransplant rejection and critically reviewed our single-center case series to evaluate the association between CPI timing, transplantation, immunosuppression, and posttransplant outcomes.
2 |. CASE SERIES
We performed an IRB approved retrospective review of a series of all patients that received LT following treatment with CPI therapy for HCC between January 1, 2018, and January 30, 2021. All patients received nivolumab 240 mg every 2 weeks twice then 480 mg every 4 weeks administered by a referring physician. All transplants were performed with caval sparing technique and received standard maintenance immunosuppression of steroid taper with an initial dose of 1000 mg of solumedrol administered during LT, tacrolimus, and mycophenolate mofetil (1000 mg twice daily).
2.1 |. Patient A
A 60-year-old female with hepatitis C virus (HCV) cirrhosis complicated by HCC. She received a total of 18 months of nivolumab with a final dose 5 weeks prior to liver transplant. An ultrasound on post-operative day (POD) 1 demonstrated patent vasculature with normal waveforms. The immediate postoperative course was uneventful, and she was discharged on POD 4. On POD 10, the patient was readmitted for fever and elevated transaminases (Figure 1). On POD 12, treatment with methylprednisolone 1000 mg was initiated. Transaminases began to decrease on POD 14 but subsequently increased on POD 16. A liver biopsy on POD 14 revealed acute cellular rejection with sub-massive hepatic necrosis involving 60% of the core with a rejection activity index (RAI) of 4/9. The lymphocytic infiltrate was primarily CD3 positive lymphocytes with minimal CD20 positive B cells. PD-L1 immunostaining was positive only in rare inflammatory cells (<1%), and PD-1 demonstrated intermediate staining (<5% of inflammatory cells). On POD 18, a check for donor-specific antibodies (DSA) revealed antibodies against multiple human leukocyte antigen class II antigens (DR8, DR52, DQ4, and DQ7). Antibodies against DR8 and DQ7 demonstrated significant prozone inhibition in neat serum with high levels of antibodies detectable at 1:10 serum dilution and mean fluorescence intensity over 8000. Treatment was escalated with 1 mg/kg rabbit anti-thymocyte globulin (rATG); however, her transaminases increased to the 4000s with international normalized ratio >3 and bilirubin >7. Considering the positive DSA, the patient was also started on therapeutic plasma exchange (TPE) on POD 19 and she received intravenous immunoglobulin (IVIG) followed by methylprednisolone and rATG. Following TPE ×2, IVIG ×2, and rATG ×3, DSA antibodies to DR8 and DQ7 persisted at high levels. Given lack of improvement and low likelihood of graft recovery, she was re-listed for liver transplant with a biologic Model for End-Stage Liver Disease-Sodium >40.
FIGURE 1.
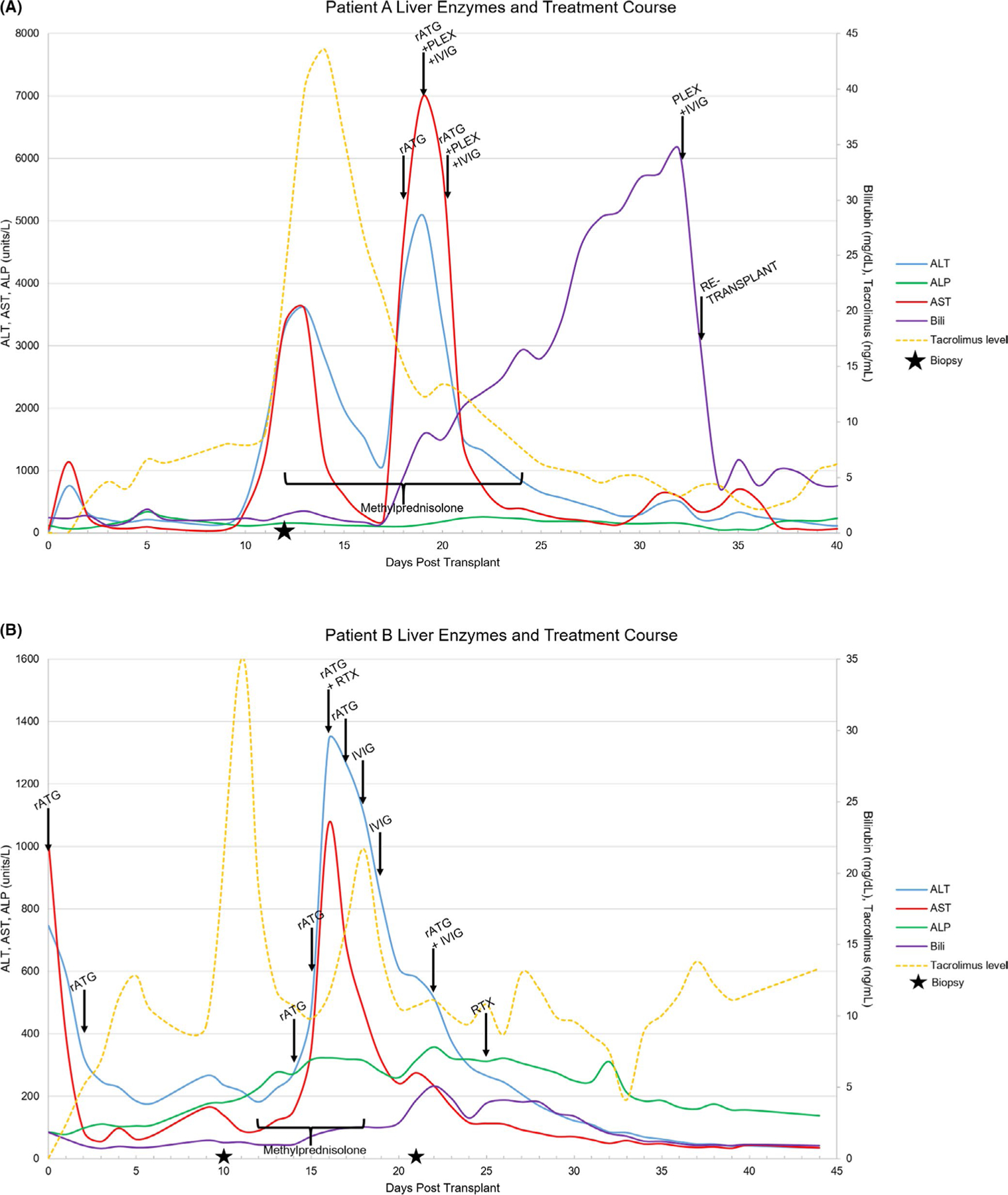
Liver function tests, tacrolimus levels, time from transplant, and timing of interventions during treatment for patients receiving checkpoint inhibitor <3 months prior to OLT (A: Patient A; B: Patient B). ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase
She underwent re-transplant from a deceased donor on POD 34. She was treated with IVIG following TPE one day prior to retransplant and she received rATG (1 mg/kg) intraoperatively. Explant of the previous allograft revealed massive hepatic necrosis with hemorrhage and organizing portal vein thrombi. DSA on POD 1 after re-transplantation was negative. On POD 9, a liver biopsy was performed for acute elevation in alkaline phosphatase. DSA remained negative. The liver biopsy demonstrated acute cellular rejection (RAI 5/9) and hepatocyte necrosis. She was successfully treated with 1000 mg methylprednisolone for 3 days with taper. She was discharged on POD 33 and recovered fully. At 38 months posttransplant, she continues to do well.
2.2 |. Patient B
This was a 65-year-old male with metabolic syndrome and HCV cirrhosis complicated by HCC. He received 8 months of nivolumab therapy with a final dose 10 days prior to liver transplant. The patient received induction with rATG 1.5 mg/kg on POD0 and POD2 for a total of 3 mg/kg rATG in addition to routine maintenance immunosuppression. Liver tests had an unremarkable decrease posttransplant and an ultrasound POD0 demonstrated normal vascular waveforms. The patient was discharged home on POD6. On routine protocol lab checks, liver tests were notably increased. An ultrasound was, again, unremarkable with normal vascular waveforms. A steroid pulse was started, and liver biopsy revealed moderate rejection expanding all of the triads (RAI 5/9). Liver tests continued to worsen and following 3 days of steroids, rATG was started POD14 (1-1.5 mg/kg/day) for a total of 4 days and total rATG dose of 5 mg/kg. Liver tests continued to rise, and aspartate aminotransferase (AST)/ alanine aminotransferase (ALT) peaked on POD16 (1341 units/L and 1074 units/L, respectively). rituximab 375 mg/m2 was administered POD16, given the experience with patient A despite the absence of measured DSAs, and IVIG 1 g/kg was given POD 18 and POD19. ALT and AST began to decline steadily after the peak on POD16. A repeat dose of rATG 1 mg/kg and IVIG 1 g/kg were given at that time. A second biopsy was obtained and showed centrizonal hepatocellular necrosis and central vein fibrinoid necrosis with rare B cells and T cells in portal tracts all corresponding with CPI injury. A second dose of rituximab 375 mg/m2 was given on POD25. The patient’s total immunosuppressive exposure from time of transplant was approximately 9 mg/kg of rATG, 3 g/kg IVIG, and rituximab 375 mg/m2 for two doses in addition to maintenance immunosuppression and steroid pulse/taper. On POD 26 liver tests began to decline and completely normalized by 50 days posttransplant and have remained normal throughout follow up. DSA were collected routinely within the first 3 months posttransplant and were negative at all time points.
All three patients who received the last dose of CPI more than 3 months prior to transplant had excellent graft function with no episodes of biopsy-proven acute cellular rejection (BPACR) (Table 1). They received 8, 12, and 12 months of nivolumab with last doses 3, 4 and 11 months prior to transplant. There was one death in the cohort from sudden cardiac death outside the hospital with a functioning graft. The other two patients are alive with functioning grafts. Follow-up time for this group ranges from 2–16 months posttransplant. There have been no episodes of rejection and no HCC recurrences. Patient D died suddenly at home, 2 days after his last follow-up visit, on POD 61 with a functioning graft and normal liver tests from a cardiac arrest.
TABLE 1.
Patient characteristics, immunosuppression, and posttransplant outcomes
| A | B | C | D | E | |
|---|---|---|---|---|---|
|
| |||||
| Age at transplant | 61 | 65 | 71 | 65 | 68 |
| Tumor stage at diagnosis | T2 | T2 | T2 | T3 | T3 |
| Gender | Female | Male | Male | Female | Male |
| Etiology | HCV, genotype la, 406 000 lU/ml prior to transplantation | HCV with SVR | HBV, undetectable viral load | HCV with SVR | HCV with SVR |
| Duration of nivolumab | 18 months | 8 months | 8 months | 12 months | 12 months |
| Time from last CPI to transplant | 5 weeks | 10 days | 3 months | 4 months | 6 months |
| Time to rising AST/ALT | 10 days | 12 days | n/a | n/a | n/a |
| Induction IS | None | rATG | rATG | rATG | None |
| Peri-transplant PRBC transfusion | 1 unit | 0 units | 2 units | 0 units | 0 units |
| Explant | |||||
| Number of viable tumors % viability | 1 viable, well differentiated tumor 0% necrosis, no microvascular invasion | 3 tumors 100% necrosis, no microvascular invasion | 1 tumor 100% necrosis, no microvascular invasion | 1 tumor 100% necrosis, no microvascular invasion | 1 tumor 100% necrosis, no microvascular invasion |
| Microvascular invasion | |||||
| Tumor differentiation | 2 tumors 100% necrosis | ||||
| Time to first biopsy | 12 days | 14 days | n/a | n/a | 7 days |
| First biopsy result | ACR (4/9) with 60% necrosis | ACR (5/9) | n/a | n/a | Cholestasis, no ACR |
| Treatment | rATG, steroid pulse, plasmapheresis, IVIG | rATG, steroid pulse, rituximab, IVIG | n/a | n/a | None |
| Subsequent pathology | Massive hepatic necrosis | 20% hepatic necrosis | n/a | n/a | Cholestasis, no ACR |
| Graft outcome | Graft failure with re-transplant | Salvaged | Stable graft function | Stable graft function | Stable graft function |
| Patient outcome | Alive | Alive | Alive | Alive | Deceased |
| Follow-up time | 38 months | 13 months | 16 months | 11 months | 2 months |
Abbreviations: ACR, acute cellular rejection; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CPI, checkpoint inhibitor; HBV, hepatitis B virus; HCV, hepatitis C virus; IS, immunosuppression; IVIG, intravenous immunoglobulin; PRBC, packed red blood cells; rATG, rabbit antithymocyte globulin; SVR, sustained virologie response.
3 |. DISCUSSION
In this study, we describe five patients who underwent LT for HCC with prior exposure to CPI and found that patients with <3 months between last CPI dose and transplant developed severe posttransplant complications including hepatic necrosis and graft loss. Importantly, in three patients with three or more months from the last dose of CPI to LT the post-operative course was benign. Overall, this suggests that the time from the last CPI dose to LT may be a key factor in the intersection between CPI use and LT.
The use of CPIs for the treatment of malignancies in solid organ transplant recipients is associated with a high rate of allograft rejection, however, should be balanced with a potentially significant oncologic response.3 Early experience with pretransplant CPI treatment has revealed a risk of graft loss and death when transplant is preceded by CPI therapy. Nordness describe acute hepatic necrosis within the first week posttransplant resulting in graft loss and death on POD 10.4 Tabrizian suggests that their favorable outcomes may be due to a wash-out effect from peri-operative blood loss.4 Our cohort did have a low transfusion requirement (Table 1); however, the difference between our experience and that described by Nordness compared to others reporting no increase in rejection or graft injury is difficult to reconcile and illuminates the need for additional, multicenter studies.4,5
In the two patients that developed CPI associated liver injury, early graft function was excellent and initial hospital stay unremarkable. Liver injury was detectable on POD 10–12 with elevated transaminases. The reason for the delay in onset of liver injury is unclear and perhaps due to initial steroid treatment or the addition of thymoglobulin induction in patient B. Unique to this series is the success of treatment in the setting of severe immune-mediated hepatic necrosis. Patient A lost the first allograft but underwent a successful re-transplant following aggressive treatment aimed at blunting subsequent immune-mediated injury, yet patient A still developed early BPACR following the second transplant. Patient A developed extremely high levels of DSA which persisted despite plasmapheresis and rituximab treatment. The mechanism of this is uncertain though PD-1 is also expressed on B cells, and inhibition with CPI may result in increased antibody production.6 This effect of PD-1 blockade together with the potential for T cell–mediated activation of the humoral response may lead to antibody-mediated rejection in solid organ transplant recipients exposed to CPIs, as well. Immunohistochemical staining did not reveal a uniform pattern though PD-1 was positive in the lymphocytes and PDL-1 in the surviving endothelial cells. Following our experience with this transplant we began screening all our CPI patients for DSAs prior to transplant and in the early post-operative period, however, we have not encountered DSAs in any additional patients.
Following our experience with patient A, we began to include induction with thymoglobulin as part of IS regimen in patients treated with CPIs. The utility of this remains unclear. We utilized this process for patient B and yet severe immune-mediated hepatic necrosis developed. That said, we were able to salvage the allograft in this patient which we were unable to do in patient A. This strategy warrants further study, particularly in patients with <3 months interval from the final CPI dose. In those patients with an interval between final CPI dose and transplant of >3 months, we did not observe acute cellular rejection and no incidence of hepatic necrosis.
This study is limited by the small number of patients treated with CPIs prior to transplant and duration of posttransplant follow up, however, it is the second largest series in patients with CPI exposure prior to LT and the first to demonstrate a trend in the timing of CPI use and posttransplant outcomes. We also applied varying approaches to immunosuppression across the group. While there are limitations, this series demonstrates the possibilities of rescue therapy and the success of re-transplantation after graft loss. We believe this series also affirms the dangers associated with a short interval between CPI treatment and transplant and also suggests that transplant can be done safely if at least 3 months have elapsed since final CPI treatment. These cases led us to alter our protocol to include a 3-month waiting period prior to transplant, screening for DSAs and thymoglobulin induction. All patients received nivolumab, and other CPIs may have different effects on posttransplant outcomes. However, nivolumab and atezolizumab, which is now approved as first-line therapy for unresectable HCC with bevacizumab, have similar half-lives. More work is needed; a multicenter study is critical with a database to evaluate pretransplant CPI use, its impact on waitlist dropout, optimal immunosuppression and posttransplant outcomes.
Abbreviations:
- ALP
alkaline phosphatase
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- CPI
checkpoint inhibitor
- CTLA-4
cytotoxic T-lymphocyte antigen 4
- DSA
donor-specific antibody
- ERCP
endoscopic retrograde cholangiopancreatography
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- IVIG
intravenous immunoglobulin
- MFI
mean fluorescence intensity
- PD-L1
programmed cell death ligand 1
- POD
post-operative day
- RAI
rejection activity index
- rATG
rabbit antithymocyte globulin
- TPE
therapeutic plasma exchange
- VEGF
vascular endothelial growth factor
Footnotes
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1.Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. [DOI] [PubMed] [Google Scholar]
- 2.Yao FY, Mehta N, Flemming J, et al. Downstaging of hepatocellu lar cancer before liver transplant: long-term outcome compared to tumors within Milan criteria. Hepatology. 2015;61(6): 1968–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.d’Izarny-Gargas T, Durrbach A, Zaidan M. Efficacy and tolerance of immune checkpoint inhibitors in transplant patients with cancer: a systematic review. Am J Transplant. 2020;20(9):2457–2465. [DOI] [PubMed] [Google Scholar]
- 4.Nordness MF, Hamel S, Godfrey CM, et al. Fatal hepatic necrosis after nivolumab as a bridge to liver transplant for HCC: are checkpoint inhibitors safe for the pretransplant patient? Am J Transplant. 2020;20(3):879–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tabrizian P, Florman SS, Schwartz ME. PD-1 inhibitor as bridge therapy to liver transplantation? Am J Transplant. 2021;21(5):1979–1980. [DOI] [PubMed] [Google Scholar]
- 6.Dong Y, Sun Q, Zhang X. PD-1 and its ligands are important immune checkpoints in cancer. Oncotarget. 2017;8(2):2171–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


