Abstract
In the coming decades, maintaining a steady food supply for the increasing world population will require high-yielding crop plants which can be productive under increasingly variable conditions. Maintaining high yields will require the successful and uniform establishment of plants in the field under altered environmental conditions. Seed vigor, a complex agronomic trait that includes seed longevity, germination speed, seedling growth, and early stress tolerance, determines the duration and success of this establishment period. Elevated temperature during early seed development can decrease seed size, number, and fertility, delay germination and reduce seed vigor in crops such as cereals, legumes, and vegetable crops. Heat stress in mature seeds can reduce seed vigor in crops such as lettuce, oat, and chickpea. Warming trends and increasing temperature variability can increase seed dormancy and reduce germination rates, especially in crops that require lower temperatures for germination and seedling establishment. To improve seed germination speed and success, much research has focused on selecting quality seeds for replanting, priming seeds before sowing, and breeding varieties with improved seed performance. Recent strides in understanding the genetic basis of variation in seed vigor have used genomics and transcriptomics to identify candidate genes for improving germination, and several studies have explored the potential impact of climate change on the percentage and timing of germination. In this review, we discuss these recent advances in the genetic underpinnings of seed performance as well as how climate change is expected to affect vigor in current varieties of staple, vegetable, and other crops.
Subject terms: Plant breeding, Plant physiology
Introduction
Increases in temperature and carbon dioxide and changes in precipitation over the 21st century pose a threat to agricultural productivity in the coming decades (Batley and Edwards 2016; Vogel et al. 2019; Wing et al. 2021). Climate change will negatively affect global food supplies, so research on improving agricultural output under deteriorating climate conditions is necessary to ensure global food security. Despite traditional breeding efforts, certain stages of the crop life cycle remain particularly sensitive to climate factors, including flowering, pollination, seed development, germination, and seedling growth. Due to reduced sequencing and genotyping costs, genomics and advanced phenotyping are transforming breeding strategies and the development of new cultivars of resilient crops (Edwards and Batley 2010; Voss-Fels and Snowdon 2016). For the majority of crops, seeds are the delivery system for transferring advanced genetics into the production field. In particular, rapid and synchronous seed germination and seedling growth are particularly important to agricultural output because they are essential for the establishment of seedlings in the field. Here, we discuss how climate change is predicted to affect seed germination and vigor and how recent advances in understanding these processes can be applied to enable high agricultural productivity under these changing conditions. In this review, we cover recent advances in identifying mechanisms that would be amenable to use for selection for improved seed vigor.
Seed vigor is a complex trait that encompasses aging tolerance, seed dormancy, viability, rapid germination, and seedling establishment, especially in suboptimal conditions. As seeds age, they progressively lose their vigor and become increasingly sensitive to stress upon germination, which occurs between imbibition and the emergence of the radicle from the seed (Bewley et al. 2013). Though in many seeds time is required to attain competency for germination in a dry after-ripening process (Fig. 1), seeds experiencing prolonged aging eventually lose the capacity to germinate (i.e., seed viability) entirely. Mechanistically, seed aging results from damage to cellular processes (Zinsmeister et al. 2020). It largely depends upon environmental storage conditions, seed genetics, and the maternal environment (Bewley et al. 2013). Although aging reduces viability, seeds are generally more resistant to environmental stressors than seedlings. To minimize both aging and the exposure of seedlings to environmental stressors, seeds must regulate germination carefully. Seed dormancy is responsible for defining the environmental conditions in which a seed can germinate (Finch-Savage and Leubner-Metzger 2006). Depth of seed dormancy determines the timing of germination, and seeds cannot germinate until dormancy is alleviated (Fig. 1). Seed viability is the percentage of seeds producing normal seedlings in an ideal growing environment, assuming removal of dormancy before testing (Basra 1995). Viability provides a user-friendly guide to growers looking to assess seed lot quality. Environmental stressors at any time from seed development to seedling establishment can reduce stand density, increase the variation in time to harvest, and reduce harvestable crop yield. Seed vigor, therefore, encompasses all of these components in the context of environment and seed genetics to assess the performance of a seed lot.
Fig. 1. Schematic illustration of the interaction of genetic, hormonal, and environmental signals for regulation of seed dormancy and germination.

During seed development, genotype and environment influence the biosynthesis of abscisic acid (ABA) in the seed, inducing differing depths of primary dormancy. Dormancy is alleviated in these seeds with light, temperature, after-ripening, or chilling. Different genotypes or environmental conditions during seed filling can cause less ABA accumulation in the seed, leading to non-dormant seeds and removing the need for breaking the primary dormancy. Non-dormant seeds in conditions meeting their water and temperature requirements for germination then shift their gibberellin (GA) to ABA ratio higher, promoting germination. Seeds in which primary dormancy has been removed or non-dormant seeds under temperature extremes, anoxia, light conditions, or aging stress can experience relative dormancy, and with extended time in these conditions, can induce secondary dormancy. Secondary dormancy and relative dormancy can be alleviated with time under proper light, temperature, after-ripening, and/or chilling conditions.
Dormancy and germination
Dormancy is an adaptive trait in wild plants for avoiding germination under conditions that would not be conducive to the survival of the seedlings (Klupczyńska and Pawłowski 2021). Dormancy can be primary, in which seeds at maturity require specific conditions, such as moist chilling or dry after-ripening, before they become capable of germinating, or secondary, in which non-dormant seeds in unfavorable environmental conditions revert to a state of dormancy (Fig. 1). Primary dormancy can be imposed through physical features (e.g. water-resistant seed coats in many pulses, which prevent hydration of zygotic tissues) (Bolingue et al. 2010), and physiologically, through hormones (Bewley et al. 2013). In crops, residual primary dormancy at sowing time can reduce germination percentages and rates (inverse of times to germination), which in turn affects stand uniformity. In bulk-harvested crops, stand uniformity is essential, because plants with delayed development may not produce a harvestable product. Therefore, domestication has selected against crop seed dormancy in many cases, favoring faster germination and an increased range of threshold temperatures and water potentials of crop species (Durr et al. 2015). Breeding programs have benefitted from investigations into the genetic networks underlying dormancy, especially when considering dormancy induced by temperature extremes; however, complete removal of dormancy is detrimental to agriculture, since this can result in pre-harvest sprouting. Pre-harvest sprouting occurs when untimely rain before harvest causes seeds to germinate before being shed from the mother plant and is due to insufficient dormancy. Pre-harvest sprouting drastically reduces seed quality and longevity (Gualano et al. 2014; Soltani et al. 2021), where longevity is defined as seed viability after dry storage (Bewley et al. 2013). Pre-harvest sprouting and its underlying genetics and regulation have been reviewed elsewhere (Rodríguez et al. 2015; Vetch et al. 2019).
The process of germination involves bidirectional interactions between the embryo and the endosperm, with the endosperm acting as an environmental sensor that regulates the growth of the embryo and the embryo controlling the degradation of the endosperm (Yan et al. 2014). Additionally, multiple interacting physical and hormonal factors control germination (Chahtane et al. 2017). Regulation of xyloglucan biosynthesis in the embryo and endosperm plays a key role in endosperm weakening, allowing germination to occur (Nonogaki 2019). Additionally, the cutin coat that lies between the endosperm and the testa negatively influences germination, while the sheath between the endosperm and the embryo facilitates germination (Nonogaki 2019). Since the embryo, endosperm, and maternal tissues have different genetic compositions that contribute to both the physical and genetic basis of dormancy, it is important to consider tissue-level differences when searching for genes associated with germination and dormancy; however, most quantitative trait locus (QTL) mapping studies ignore these tissue-level distinctions. Despite this shortcoming, work to isolate the impact of each of these tissues on dormancy has been conducted in rice (Gu et al. 2015). The researchers were able to isolate three seed dormancy loci, each one unique to either embryo, endosperm, or maternal tissues. The QTL associated with endosperm-imposed dormancy had an additive effect on germination and contained OsGA20ox2, a gibberellin synthesis gene whose expression in the endosperm is believed to control primary dormancy through a gibberellin-regulated mechanism associated with the timing of dehydration (Supplementary Table S1; Ye et al. 2015).
Hormonal control of dormancy and germination
The transition from dormancy to competency for germination is dictated by the balance between abscisic acid (ABA) and gibberellin (GA) levels in the seed, with a lower ABA/GA ratio required for alleviating dormancy and permitting germination (Fig. 1). These hormones are mutually antagonistic, with each downregulating the other’s metabolism (Fig. 2; Bewley et al. 2013). While the mechanism controlling the balance between ABA and GA and the role of this balance in seed germination remains to be fully elucidated, recent work has suggested the presence of an ABA repressor complex (Nonogaki and Zhang 2020). Such a complex could initiate germination through coordinated suppression of ABA signaling. Weighted gene correlation network analysis has shown that the genetic regulation of the ABA/GA ratio contains numerous genes involved in seed vigor that are expressed in the endosperm of tomato seeds (Bizouerne et al. 2021).
Fig. 2. Schematic illustration of hormonal regulation of seed dormancy and germination, crosstalk between hormonal signals and other regulators, and effects of climate change on these processes.
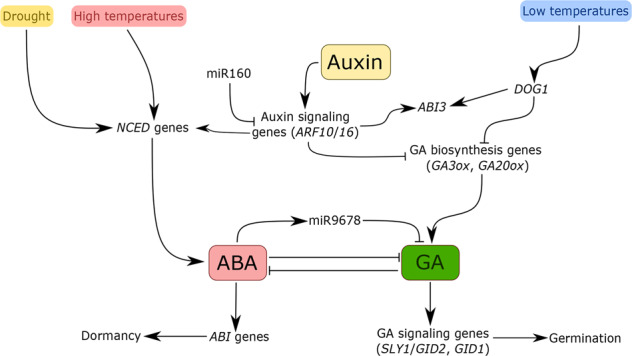
Abscisic acid (ABA) maintains seed dormancy through a gene expression network of ABSCISIC ACID INSENSITIVE (ABI) transcription factors. Gibberellins (GA) promote seed germination through GA signaling pathway genes like SLY1 and GID1. Rice GID2 is orthologous to Arabidopsis SLY1. Drought stress and high-temperature conditions can induce ABA biosynthesis, leading to elevated dormancy. Low temperatures promote ABI3 expression and downregulate GA biosynthesis through DOG1. Auxin signaling, which is under miR160 regulation through ARFs, leads to the biosynthesis of ABA and the promotion of ABA signaling genes. MiRNAs such as miR9678 in wheat can play roles in maintaining hormonal balance. DOG1 DELAY OF GERMINATION 1, NCED 9-CIS-EPOXYCAROTENOID DIOXYGENASE, ARF AUXIN RESPONSE FACTOR, SLY1 SLEEPY1, GID1 GIBBERELLIN INSENSITIVE DWARF1, GA-ox GA OXIDASE.
Response to the seed production environment involves numerous overlapping networks of genes in both maternal and zygotic tissues (Penfield and MacGregor 2017). Environmental factors associated with climate change affect the network of genes and hormones controlling germination in grains such as rice (Liu et al. 2019; Suriyasak et al. 2020) and wheat (Izydorczyk et al. 2018) and vegetables such as lettuce (Huo et al. 2013) and tomato (Geshnizjani et al. 2018). Temperature is a major environmental factor that affects the degree of dormancy, with even 1 °C difference in sensitive ranges capable of determining dormancy (Springthorpe and Penfield 2015). Dormancy is commonly greater in seeds that develop at temperature extremes (Penfield and MacGregor 2017; Toh et al. 2008). In rice, researchers have shown that high temperatures and drought stress cause ABA accumulation through the induced expression of 9-CIS-EPOXYCAROTENOID DIOXYGENASEs (NCEDs) (Liu et al. 2019; Suriyasak et al. 2020), which mediate seed dormancy, plant growth, abiotic stress tolerance, and leaf senescence (Huang et al. 2018). In many plants and tissues, ABA biosynthesis is rate-limited by NCED enzymes (Nambara and Marion-Poll 2005). As such, this class of genes presents numerous candidates for manipulating temperature-induced dormancy to maximize agricultural yield in a changing climate. In Arabidopsis, NCED genes and their functions are well-characterized. Five NCED genes (AtNCED2/3/5/6/9) are likely involved in ABA biosynthesis (Lefebvre et al. 2006). AtNCED6 is expressed specifically in the endosperm (Lefebvre et al. 2006), and its induction during seed development is sufficient for increasing dormancy (Martínez-Andújar et al. 2011). AtNCED3 and AtNCED5 are drought-inducible ABA biosynthesis genes important for regulating ABA levels during water stress (Tan et al. 2003; Ruggiero et al. 2004; Frey et al. 2012). AtNCED5, AtNCED6, and AtNCED9 synthesize ABA in the embryo and endosperm during the onset of primary dormancy (Lefebvre et al. 2006; Seo et al. 2006; Martínez-Andújar et al. 2011; Frey et al. 2012). ABA produced in the endosperm can be transported into the embryo: export of ABA from the endosperm is controlled by ATP-BINDING CASSETTE TRANSPORTERS, SUBFAMILY G (ABCG) genes AtABCG25 and AtABCG31, and import into the embryo is controlled by AtABCG30 and AtABCG40 (Kang et al. 2015). AtNCED2, AtNCED5, and AtNCED9 are induced by high temperature in imbibed seeds, and mutating them leads to a loss of thermoinhibition (Seo et al. 2006; Toh et al. 2008).
Because each NCED gene has an overlapping yet distinct function, manipulating these genes and their promoters may permit fine-tuning seed dormancy to specific field conditions (Frey et al. 2012). However, specific NCEDs are not highly conserved between crops. As such, it is difficult to identify which NCEDs to target for alleviating crop dormancy and improving germination without first characterizing these genes. One tool that has proven useful for this task is QTL mapping. For example, using QTL mapping, researchers have investigated the NCED gene family as potential targets for mitigating heat-induced dormancy in lettuce, a species highly susceptible to thermoinhibition (Huo and Bradford 2015). By using a recombinant inbred line (RIL) population from a thermotolerant lettuce cultivar and a heat-susceptible cultivar, the researchers found a QTL in which they identified LsNCED4 (Argyris et al. 2011). Gain or loss of function of LsNCED4 can mediate seed thermoinhibition (Huo et al. 2013; Bertier et al. 2018).
In addition to ABA synthesis genes, ABA response genes play key roles in seed dormancy. ABSCISIC ACID-INSENSITIVE (ABI) genes are primarily responsible for carrying the ABA signal through to the dormancy phenotype (Fig. 2), and abi mutant plants can have a significantly reduced response to ABA treatment (Söderman et al. 2000). ABI3, ABI4, and ABI5 are key ABA-related transcription factors promoting seed dormancy, and expression of ABI3, ABI4, and ABI5 is higher in dormant seeds than in non-dormant seeds (Shu et al. 2013; Huang et al. 2017; Skubacz and Daszkowska‐Golec 2017). A recent study in tomato showed that ABI4 could be associated with the acquisition of seed vigor in the embryo (Bizouerne et al. 2021). ABI5, a transcription factor shown to enhance dormancy in Arabidopsis (Wu et al. 2015), is important in downregulating PHOSPHATE1, a gene involved in reducing sensitivity to ABA (Huang et al. 2017).
While ABA and GA are the primary regulators of seed dormancy and germination, other hormones can impact the expression of key genes in a complex regulatory network. For example, exogenous auxin application in soybean represses germination by increasing the ABA/GA ratio (Fig. 2; Shuai et al. 2017). Furthermore, overexpressing the auxin signaling down-regulator microRNA 160 (miR160) or mutating auxin receptors or auxin biosynthesis genes releases seed dormancy (Liu et al. 2013). This dormancy release occurs through stimulation of ABI3 expression, and AUXIN RESPONSE FACTOR 10 and 16, which are auxin signaling genes regulated by miR160, are required for the expression of ABI3 in Arabidopsis (Fig. 2; Liu et al. 2013). ABA/GA signaling can also be affected by microRNAs. MiR9678, a miRNA specifically expressed in scutellum tissue of developing seeds, can affect seed germination: overexpression of miR9678 in wheat leads to delayed germination by reducing GA level, and miR9678 silencing improves germination (Fig. 2; Guo et al. 2018).
DELAY OF GERMINATION 1 (DOG1) is a key seed-specific regulator for the ABA and GA cross-talk that represses GA biosynthesis (Bentsink et al. 2006; Née et al. 2017) and determines the initial depth of seed dormancy (Footitt et al. 2020). DOG1 is responsive to temperature, allowing it to interface environmental signals with the ABA/GA network (Fig. 2). It is upregulated in response to cold (Kendall et al. 2011) and therefore may impact the germination of seeds planted in springtime. DOG1 also acts as a link between ABA and GA for crosstalk between ABI5 and ABI3 (Dekkers et al. 2016) and GA biosynthesis genes (Graeber et al. 2014), likely mediating the balance between these two hormones to allow a coordinated hormonal shift during dormancy alleviation and germination (Fig. 2). DOG1 also influences the levels of miR156 and miR172 that regulate the plant life cycle progression, coordinating seed dormancy and flowering phenotypes with environmental factors (Huo et al. 2016). The researchers showed that overexpression of miR172 promotes early flowering and reduces seed dormancy in Arabidopsis and that this effect requires functional DOG1. Because DOG1 determines the initial depth of seed dormancy, allelic variants can provide species growing across multiple climates the capacity to adapt dormancy and germination timing to local conditions (Kerdaffrec et al. 2016; Martínez-Berdeja et al. 2020). Orthologs of DOG1 are common to many plant species. Researchers have investigated DOG1-Like (DOG1L) genes in cereals and identified 5 DOG1L groups, four of which are functionally orthologous to AtDOG1 (Ashikawa et al. 2013). The numerous DOG1L orthologs common in many species may provide a library of possible genes for manipulating seed dormancy through transgenic approaches. This tactic has been employed in wheat using Triticeae DOG1L genes (Ashikawa et al. 2014).
Seed vigor
Defining seed vigor
While seed viability tests are user-friendly and helpful for growers looking for a rapid assessment of seed lot quality, viability is a poor predictor of a seed lot’s performance in field conditions. This is because, in an ideal growing environment, seeds of the same cultivar from different sources or production environments may have similarly high germination percentages and therefore high levels of seedling establishment; however, when placed in stressful environments like the variable conditions common in the field, these same seeds can have drastically different capacities for establishing into healthy seedlings. This capacity to germinate and emerge quickly, particularly under stressful conditions, has been termed “seed vigor”. Seed vigor is a complex agronomic trait that includes the performance in the field as well as seed storage history. The International Seed Testing Association (ISTA) has defined seed vigor as the sum of those properties that determine the activity and performance of seed lots of acceptable germination in a wide range of environments (Seed Vigour Testing 2021). This definition does not interpret seed vigor to be a single quantifiable property, but rather a trait that includes the seed’s ability to still germinate after storage and under adverse conditions, in addition to the seedlings’ ability to grow normally and uniformly. Therefore, seed vigor describes the performance potential of viable seeds in an agricultural context. Because seed vigor is based upon complex interactions between genes and the environment, it can be modified by numerous environmental factors (e.g., soil water availability, temperature, developmental stage at harvest, and harvest and storage conditions (Figs. 3 and 4; Sun et al. 2007). Some environmental stressors are common to agricultural seedbeds: for example, after sowing, soil conditions tend to deteriorate with time as moisture recedes and soil strength increases. Seeds that are genetically optimized for these common field environment conditions, and have been handled and stored properly, have higher vigor. High vigor seeds, especially in small-seeded crops, implement a stress avoidance strategy in deteriorating soil conditions through rapid seedling establishment while soil conditions are adequate. The success of this approach depends on fast germination upon sowing, rapid root growth to reach receding moisture, and upward shoot growth through the soil to reach the surface (Finch-Savage et al. 2010).
Fig. 3. Schematic diagram showing various factors associated with climate change and their effect on seed vigor.

Drought stress or heat stress during grain filling is expected to reduce seed vigor. Drought and heat stress during seed storage, seed germination, and early seedling growth will also lead to further deterioration of seed vigor. Additionally, flooding during germination can reduce seed vigor.
Fig. 4. Effects of climate change on seed vigor and possible approaches to amend it for ensuring vigorous crops.
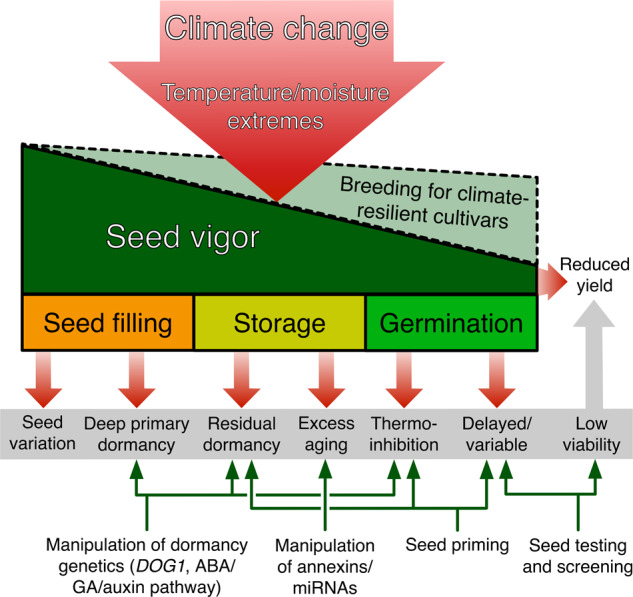
Stressors associated with climate change such as temperature and moisture extremes reduce seed vigor. If these stressors are present during seed filling, they can lead to seed variation and deep primary dormancy. During storage, they can cause residual dormancy and excess aging and during germination, they lead to thermoinhibition and delayed/variable germination. Each of these effects can reduce the viability and performance of a seed lot, reducing yield. Breeding for climate-resilient cultivars is likely to improve seed vigor in the face of climate change stressors. Manipulating genetic factors associated with dormancy provides an avenue for breeding climate change-resilient crops and may be used to directly alleviate deep primary dormancy, residual dormancy, and thermoinhibition. Manipulation of genes associated with seed longevity such as certain annexins and miRNAs can reduce excess aging. Seed priming can help remove residual dormancy and thermoinhibition and can improve the speed and uniformity of seedling establishment. Improved seed testing will better inform farmers, allowing them to more accurately predict the field performance of a seed lot and plan sowing density accordingly.
Measuring seed vigor
Historically, seed vigor has been difficult to quantify. While no single test has been developed to measure all aspects of seed vigor, and few tests apply to all crops, the performance potential of seeds can be determined in several manners. One common approach for assessing seed vigor is to first subject seeds to rapid aging under controlled conditions by elevating the temperature and seed moisture content. Differences in vigor between seed lots are enhanced under these conditions and can be detected through a standard germination test (Finch-Savage and Bassel 2016). The difference in seed germination over accelerated aging (AA) timepoints can be used to fit a survival curve and calculate a viability constant useful for assessing seed lot aging dynamics (Ellis and Roberts 1980). This approach is useful in small-seeded crops such as lettuce (Kraak and Vos 1987). Additionally, because AA reduces seed vigor, studies have used AA to manipulate the vigor of a seed lot (Baek et al. 2018). Marcos-Filho (2015) describes this and other seed vigor tests in detail. However, results at elevated moisture contents can differ from those at lower moisture contents (Schwember and Bradford 2010), and for prediction of seed storage life, moisture contents lower than those in equilibrium with 60% relative humidity (RH) are recommended (Walters et al. 2020). At these moisture contents, elevated temperatures (up to 60 °C) can be used to speed aging. Accelerated aging at higher RH, such as the test at 100% RH and (41–45 °C) approved by ISTA for soybean (TeKrony 2005), can rapidly reduce seed vigor and viability, but the mechanism of aging is distinct from that occurring during seed storage conditions. Recently, advancement for assessing viability and vigor using computerized image analysis has proven efficient in numerous crops including soybean, (Lee et al. 2017), carrot (De Marchi, Cicero (2017)), maize (Castan et al. 2018), onion (Gonçalves et al. 2017) and wheat (Fan et al. 2020). Additionally, Brassica oleracea is a species susceptible to “blindness,” which causes failure at the growing seedling apex early in seedling development, and researchers are developing tools for predicting susceptibility to this vigor-reducing condition by combining multispectral imaging, chlorophyll fluorescence, and oxygen consumption on a seed-by-seed basis (Bello and Bradford 2021). The development of rapid screening and sorting of high vigor seeds may provide a tool for ensuring that only the highest quality seeds are used for sowing (Nehoshtan et al. 2021; Seed-X 2021).
The seed germination trait most closely associated with vigor is the speed of germination (Wheeler and Ellis 1992; Bradford et al. 1993) and has long been recognized as a key trait for seed vigor testing (McDonald 1975). Vigorous seeds have high germination rates (GR), or the inverse of the time to radicle emergence of a specific percentage (GRg or 1/tg), and the values of GRg decline as seeds age and vigor decreases. Recently, GR has informed genetic analyses of seed vigor in small, direct-seeded crops such as Brassica oleracea (Bettey et al. 2000) and rice (Guo et al. 2019; Yang et al. 2021), as well as large-seeded crops such as field pea (Lamichaney, Parihar, et al. 2021). Unfortunately, this measure of seed vigor is underutilized as it requires repeated observations of germination percentages at frequent intervals, which is too labor-intensive for routine seed testing. Early counts of normal seedlings are included in ISTA rules and represent at least one point during the germination time course indicating the speed of germination (Matthews et al. 2011; Ilbi et al. 2020). Seedling growth tests are also often used as indicators of seed vigor. However, seedling growth rates after radicle emergence are often independent of germination timing (Tarquis and Bradford 1992), so differences in seedling size at a specific time after imbibition are primarily due to the differences among seeds in their times to germination, as seedlings from earlier germinating seeds have longer times to grow until the end of the test (Matthews and Khajeh-Hosseini 2007). It has also been proposed that the incidence of abnormal seedlings can be deduced from analysis of germination time courses (Bradford et al. 1993), as the lag period before germination commences indicates the extent of repair processes due to damage sustained during aging (Matthews and Khajeh-Hosseini 2007). Thus, assessing germination rates would be a single vigor index that could largely replace other labor-intensive and more subjective vigor assays. Automated imaging methods enabling repeated observations (Matthews and Powell 2011; Colmer et al. 2020) or measurements correlated with radicle emergence such as single-seed respiration rates (Bello and Bradford 2016) will likely enable wider use of seed germination time courses and of GR as a broad purpose seed vigor index soon.
Seed dormancy and vigor overlap
Seed dormancy functions to ensure germination occurs only during the right season that will allow the successful seedling establishment and thereby survival of the species (Chahtane et al. 2017). It is induced in the freshly produced seeds during the maturation process on the mother plant (Finch-Savage and Leubner-Metzger 2006). Dormancy can be released by a period of dry storage or after-ripening, most likely by the accumulation and action of reactive oxygen species which can degrade germination-inhibiting molecules like lipids, mRNAs, and proteins (El-Maarouf-Bouteau et al. 2013). Most orthodox seeds possess remarkable desiccation tolerance and can retain viability for considerable periods, even for centuries in some instances (Walters et al. 2010; Rajjou et al. 2012). Though after-ripening may be required to break primary dormancy and allow germination, prolonged aging also leads to seed deterioration and loss of viability due to oxidative damage (Schwember and Bradford 2011; Groot et al. 2012; Morscher et al. 2015). Prolonged aging can lead to delay and failure of germination, and seeds also become more sensitive to stresses during germination, resulting in poor seedling development and establishment (Roberts and Ellis 1989; De Vitis et al. 2020). Oxidation during storage plays a major role in increasing the rate of aging as seeds stored in anoxia retain viability longer (Schwember and Bradford 2011; Groot et al. 2015). Aging-driven seed longevity is also affected by the temperature and moisture content of the seeds (Roberts and Ellis 1989; De Vitis et al. 2020). Seed vigor measurements include testing for traits such as seed longevity, germination capacity, and early stress tolerance. These parameters indicate the ability of seeds to germinate and result in seedlings that can give rise to a healthy and vigorous plant. Thus, it is pertinent to stress the fact that seed dormancy and vigor represent aspects of a physiological continuum that begins on the mother plant during seed maturation, reaches peak vigor after the loss of primary dormancy, and ends eventually with the loss of seed viability.
Environmental factors affecting seed vigor
The environment directly affects seed vigor throughout seed development, storage, and pre-seedling emergence (Fig. 3). For example, high-temperature stress during seed filling reduces the germination and vigor of soybean (Egli et al. 2005) and field pea (Lamichaney, Parihar, et al. 2021). Temperature stress during seed filling can reduce seed vigor of hybrid rice, possibly by affecting starch accumulation and structure (Wang et al. 2020). High-temperature and humidity stress during soybean seed development reduce seed vigor by negatively impacting key signaling pathways (e.g., ABA-mediated, MAPK, G protein-mediated, calcium-mediated, and phosphatidylinositol), metabolic pathways, plant physiology, and biochemistry, and high seed vigor can be maintained under high-temperature and humidity conditions in part by enhancing protein synthesis and nutrient storage in the cotyledons (Wei et al. 2020). Heat stress also impacts seeds in storage (Fig. 3). For example, oat seeds stored at 50 °C versus 35 °C for less than 2 days had significantly decreased seed vigor, and subsequent proteomic analysis showed that heat-responsive protein species and mitochondrial respiration were sensitive to heat stress (Chen et al. 2016).
Like high temperatures, drought stress during seed development can severely diminish seed vigor (Fig. 3). Drought stress during seed filling reduces seed vigor in soybean (Dornbos et al. 1989; Samarah et al. 2009; Wijewardana et al. 2019) and barley (Samarah and Alqudah 2011). Crop seeds are also particularly susceptible to drought and salinity stress during germination, including maize (Khodarahmpour 2011; Liu et al. 2015), sunflower (Kaya et al. 2006) tomato (Ishola Esan et al. 2018), and cucumber (Bakhshandeh et al. 2021).
In many crops, seed vigor also declines rapidly in excess moisture. Because many crops have been bred for rapid imbibition upon sowing to hasten germination, they are particularly susceptible to flood damage, which limits the availability of oxygen (Fig. 3). This is a major issue, especially in pulses (Soltani et al. 2017), which are often grown as rainy season crops.
While the effects of other abiotic factors associated with climate change have been explored, there is relatively little published research on the effect of elevated CO2 levels on seed vigor. CO2 concentration does not significantly affect vigor during seed storage, since seeds are often stored in a CO2-rich environment for pest control, and this environment does not reduce seed quality (Shekar et al. 2018). The effects of elevated CO2 during seed filling are variable between species (Lamichaney and Maity 2021). One study showed reduced seed vigor at elevated CO2 concentrations (>610 ppm) in rice (Lamichaney et al. 2019). However, the susceptibility of seed vigor to elevated CO2 appears dependent on the species. For example, in a chickpea study, elevated CO2 concentrations (566–630 ppm) did not affect seed vigor (Lamichaney, Tewari, et al. 2021). Additional work is necessary to determine whether elevated CO2 levels could impact seed vigor more prominently in combination with other factors.
Seed vigor in the context of climate change
Traditionally, germination percentage has been used to determine the quality of a seed supply, and farmers select the time to sow their crops to maximize the growing season and the potential yield of their crop using this percentage to gauge the sowing density. However, because seeds must often be sown at suboptimal times for crop establishment, this germination percentage is not indicative of the actual seedling emergence under adverse conditions and can lead farmers to underestimate their seed requirements, leading to a loss in overall yield. Climate change is likely to worsen the conditions into which seeds must be sown in the field as farmers face temporal constraints for maximizing the growing season (Fig. 4). Therefore, seed vigor is becoming an increasingly essential agronomic trait in maintaining high seedling emergence. Additionally, seed germination under stressful conditions reduces the uniformity of establishment time within a seed lot, and this variation in time to establishment often leads to variation in the maturity of plants within a crop stand. Because stand uniformity and achieving expected plant populations per unit area are essential in obtaining a high yield in bulk-harvested crops, low vigor seeds can cause significant losses in overall harvestable yield. Climate change will likely increase the variability in seed establishment time by reducing vigor, leading to a lack of uniformity of the crop stand and losses in yield (Fig. 4). Reduction in seed vigor has been shown to detrimentally impact the uniformity, growth, development, and yield of crops such as soybean (Ebone et al. 2020). As a result, robust seeds with improved vigor are necessary for improving stand uniformity in the stressful environmental conditions encountered in the field, and improved seed vigor will therefore help to increase overall harvestable agricultural product. Successful stand establishment is also important for forming a crop canopy, which reduces weed growth and therefore herbicide usage. As such, seed vigor plays an important role in the yield and profitability of the crop and can help reduce grower costs.
Climate change is expected to have a more pronounced effect during the early stages of plant development—germination and seedling establishment—than in the adult stages (Lloret et al. 2004; Fernández-Pascual et al. 2015). Germination of imbibed seeds exposed to high temperatures is either inhibited (thermoinhibition) or prevented (thermodormancy). Thermoinhibited seeds can germinate immediately under favorable temperatures; however, thermodormancy induced by prolonged exposure to higher temperatures may not allow germination even when the temperatures are lowered. This phenomenon has been reported in several crop species like tomato (Geshnizjani et al. 2018), lettuce (Huo et al. 2013), barley (Leymarie et al. 2009), and several forest species including Pinus (Guo et al. 2020). With rising global temperatures and increasingly variable precipitation, more crops are expected to become susceptible to either thermoinhibition or thermodormancy during germination. Thus, research into understanding the genetic aspects of seed germination and vigor and characterizing the genes that can alleviate the effects of elevated temperatures and precipitation variability is critical for crop establishment and food security.
Current approaches to improve seed vigor
Seed priming/treating
One strategy that has proven effective in speeding germination is seed priming, which is a seed prehydration and drying treatment applied before sowing (Halmer 2004). Seed priming is now a widespread commercial practice to increase seed performance, primarily by reducing the time to germination and improving uniformity. Priming can also allow seeds to be sown earlier to maximize the growing season: seed priming has been reported to improve cold tolerance in maize primed with salicylic acid (Farooq et al. 2008) or chitosan (Guan et al. 2009), capsicum primed with thiourea, hydrogen peroxide (Yadav et al. 2011), and tobacco primed with putrescine (Xu et al. 2011). Seed priming has also proven useful in large-seeded crops that require time to imbibe before germination, especially in pulses (Arif et al. 2008). While seed priming can improve seedling establishment under stressful conditions, it can make seeds more susceptible to deterioration in storage, depending upon the storage conditions (Hill et al. 2007). Priming cannot compensate for poor seed vigor due to the genetic background of plants, and it should be used in coordination with seeds bred for high vigor to increase stand establishment under stressful conditions or uniformity under optimal conditions (e.g., for transplants).
Genetic dissection of seed vigor traits using linkage and association mapping
Genetic background is an essential determining factor in seed performance (Saux et al. 2020). However, because of its complexity, many aspects of seed vigor have remained elusive targets for those seeking to improve it through traditional breeding methods. Advances in genomics have sprung open the door to the improvement of seed vigor through QTL pyramiding and marker-assisted breeding.
In rice, researchers have identified and fine-mapped QTLs associated with seed vigor, providing tightly linked DNA markers for breeding (Supplementary Table S1). RILs have proven useful for identifying QTLs for seed vigor based on seed maturity stage at harvest (Liu et al. 2014; Lai et al. 2016), for seed longevity (Raquid et al. 2021), and early seedling vigor (Zhang et al. 2017). In coordination with genome-wide association studies (GWAS), RILs have helped identify specific candidate seed vigor genes (Guo et al. 2019). Differential gene expression analysis paired with QTL mapping provides an additional avenue for identifying candidate vigor genes (Yang et al. 2021). Recently, QTLs associated with vigor are also being identified in wild rice varieties and provide additional options for backcrossing seed vigor QTLs into domesticated cultivars for high yielding, high vigor rice (Jin et al. 2018).
Although most QTL studies for seed vigor have been conducted in rice, numerous studies have explored seed vigor in other cereals (Supplementary Table S1). In wheat, QTL mapping and candidate gene analysis have provided a basis for functional identification of related candidate genes for seed vigor (Shi et al. 2020). In maize, RILs have been used to identify seed vigor QTLs associated with seed maturity stage at harvest (Jing-bao et al. 2011), seed longevity (Han et al. 2018), tolerance of stressful germination conditions (Han et al. 2014), and after artificial aging (Wu et al. 2020). In barley and oat, QTLs have been paired with GWAS to identify candidate vigor genes (Thabet et al. 2018; Huang et al. 2020).
Relatively few QTL studies assessing seed vigor have been conducted outside cereal crops. However, some work has identified QTLs associated with seedling vigor in tomato (Supplementary Table S1; Khan et al. 2012) and Brassica rapa (Supplementary Table S1; Basnet et al. 2015). QTLs associated with low-temperature stress in rapeseed have also been identified (Luo et al. 2021). In common bean, a QTL analysis identified a locus associated with reduced physical seed dormancy in domesticated plants (Supplementary Table S1; Soltani et al. 2021). Numerous QTLs for longevity in soybean (Dargahi et al. 2014; Zhang et al. 2019), lettuce (Schwember and Bradford 2010), and rapeseed (Wang et al. 2018) provide a basis for breeding improved seed storage life of these crops.
Genetic engineering and gene editing
Now that significant progress has been made in the mapping of QTLs associated with seed vigor and the identification of the candidate genes involved, this data has begun to inform strategies for crop improvement. Outside of targeting seed dormancy, genome editing is an uncommon approach to improving the complex trait of seed vigor in part due to a large number of small effect loci. However, advances in understanding the genetic basis of seed vigor have proven effective at both exploring the function of specific candidate genes and for improving seed vigor. For example, among the proteins identified in a proteomic study of soybean was annexin, GmANN (Wei et al. 2020). Annexins are proteins important in plant stress responses, but they have not been found previously in developing seeds. The researchers transferred the GmANN gene to Arabidopsis, and the transgenic line had greater resistance and higher seed vitality under high-temperature and humidity stress compared to wild-type seeds (Wei et al. 2019). MiRNAs have also been targeted for improving seed vigor. MiR168 and miR164 are both important plant regulatory miRNAs, and overexpression of miR168a combined with silencing of miR164c results in higher seed germination percentages in AA seeds in rice (Zhou et al. 2020), demonstrating that the genetic manipulation of miRNAs may improve seed longevity. Additional work to improve seed vigor through genetic modification has been reviewed (Wu et al. 2017).
Conclusion
While maintaining food security in the coming decades poses a challenge due to increased demand and climate change, advancement in genetics and genomics is opening the door for improving seed vigor and other key agricultural traits involved in seed quality and marketable yield (Fig. 4). Research into the genetic underpinnings of seed dormancy and vigor will continue to identify key candidate genes associated with seed development, longevity, and field germination speed, and subsequent studies targeting these genes for manipulation will help uncover their role in improving seed vigor as well as their value for improving agricultural productivity.
Supplementary information
Acknowledgements
Research in our laboratory is supported by the Western Regional Seed Physiology Research Group (WRSPRG), the Shurl and Kay Curci Foundation (SKCF) Faculty Scholars Program award from Innovative Genomics Institute, Berkeley, and a generous start-up grant from the Department of Plant Sciences, UC Davis. We apologize to our colleagues and other researchers whose work we could not include in this review article due to space limitations.
Author contributions
IK conceptualized the review article. RCR, KJB, and IK reviewed the literature, RCR drafted the initial manuscript. All the authors contributed to writing and revising the manuscript and approved the final version of the review article.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41437-022-00497-2.
References
- Argyris J, Truco MJ, Ochoa O, McHale L, Dahal P, van Deynze A, et al. A gene encoding an abscisic acid biosynthetic enzyme (LsNCED4) collocates with the high temperature germination locus Htg6.1 in lettuce (Lactuca sp.) Theor Appl Genet. 2011;122:95–108. doi: 10.1007/s00122-010-1425-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arif M, Jan MT, Marwat KB, Khan MA. Seed priming improves emergence and yield of soybean. Pak J Bot. 2008;40:1169–1177. [Google Scholar]
- Ashikawa I, Abe F, Nakamura S. DOG1-like genes in cereals: Investigation of their function by means of ectopic expression in Arabidopsis. Plant Sci. 2013;208:1–9. doi: 10.1016/j.plantsci.2013.03.011. [DOI] [PubMed] [Google Scholar]
- Ashikawa I, Mori M, Nakamura S, Abe F. A transgenic approach to controlling wheat seed dormancy level by using Triticeae DOG1-like genes. Transgenic Res. 2014;23:621–629. doi: 10.1007/s11248-014-9800-5. [DOI] [PubMed] [Google Scholar]
- Baek JS, Cho EE, Lee DB, Chung NJ. Evaluation of seed vigor tests for predicting seedling establishment at low temperature in rice (Oryza sativa L.) J Crop Sci Biotechnol. 2018;21:155–163. doi: 10.1007/s12892-018-0079-0. [DOI] [Google Scholar]
- Bakhshandeh E, Abdellaoui R, Boughalleb F. Modeling the effects of salt stress and temperature on seed germination of cucumber using halothermal time concept. Theor Exp. Plant Physiol. 2021;33:79–93. [Google Scholar]
- Basnet RK, Duwal A, Tiwari DN, Xiao D, Monakhos S, Bucher J, et al. Quantitative trait locus analysis of seed germination and seedling vigor in Brassica rapa reveals QTL hotspots and epistatic interactions. Front Plant Sci. 2015;6:1032. doi: 10.3389/fpls.2015.01032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basra AS (1995) Seed Quality: Basic Mechanisms, 1st edn. (AS Basra, Ed.). CRC Press, New York
- Batley J, Edwards D. The application of genomics and bioinformatics to accelerate crop improvement in a changing climate. Curr Opin Plant Biol. 2016;30:78–81. doi: 10.1016/j.pbi.2016.02.002. [DOI] [PubMed] [Google Scholar]
- Bello P, Bradford KJ. Single-seed oxygen consumption measurements and population-based threshold models link respiration and germination rates under diverse conditions. Seed Sci Res. 2016;26:199–221. doi: 10.1017/S0960258516000179. [DOI] [Google Scholar]
- Bello P, Bradford KJ. Relationships of Brassica seed physical characteristics with germination performance and plant blindness. Agric. 2021;11:1–21. [Google Scholar]
- Bentsink L, Jowett J, Hanhart CJ, Koornneef M. Cloning of DOG1, a quantitative trait locus controlling seed dormancy in Arabidopsis. Proc Natl Acad Sci USA. 2006;103:17042–17047. doi: 10.1073/pnas.0607877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertier LD, Ron M, Huo H, Bradford KJ, Britt AB, Michelmore RW. High-resolution analysis of the efficiency, heritability, and editing outcomes of CRISPR/Cas9-induced modifications of NCED4 in lettuce (Lactuca sativa) G3: Genes|Genomes|Genet. 2018;8:1513–1521. doi: 10.1534/g3.117.300396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettey M, Finch-Savage WE, King GJ, Lynn JR. Quantitative genetic analysis of seed vigour and pre-emergence seedling growth traits in Brassica oleracea. N. Phytol. 2000;148:277–286. doi: 10.1046/j.1469-8137.2000.00760.x. [DOI] [Google Scholar]
- Bewley JD, Bradford KJ, Hilhorst HWM, Nonogaki H (2013) Seeds: Physiology of Development, Germination and Dormancy, 3rd edition. Springer, New York
- Bizouerne E, Buitink J, Vu BL, Vu JL, Esteban E, Pasha A, et al. Gene co-expression analysis of tomato seed maturation reveals tissue-specific regulatory networks and hubs associated with the acquisition of desiccation tolerance and seed vigour. BMC Plant Biol. 2021;21:1–23. doi: 10.1186/s12870-021-02889-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolingue W, Ly VuB, Leprince O, Buitink J. Characterization of dormancy behaviour in seeds of the model legume Medicago truncatula. Seed Sci Res. 2010;20:97–107. doi: 10.1017/S0960258510000061. [DOI] [Google Scholar]
- Bradford KJ, Tarquis AM, Durán JM. A population-based threshold model describing the relationship between germination rates and seed deterioration. J Exp Bot. 1993;44:1225–1234. doi: 10.1093/jxb/44.7.1225. [DOI] [Google Scholar]
- Castan DOC, Gomes-Junior FG, Marcos-Filho J. Vigor-S, a new system for evaluating the physiological potential of maize seeds. Sci Agric. 2018;75:167–172. doi: 10.1590/1678-992x-2016-0401. [DOI] [Google Scholar]
- Chahtane H, Kim W, Lopez-Molina L. Primary seed dormancy: a temporally multilayered riddle waiting to be unlocked. J Exp Bot. 2017;68:857–869. doi: 10.1093/jxb/erw377. [DOI] [PubMed] [Google Scholar]
- Chen L, Chen Q, Kong L, Xia F, Yan H, Zhu Y, et al. Proteomic and physiological analysis of the response of oat (Avena sativa) seeds to heat stress under different moisture conditions. Front Plant Sci. 2016;7:896. doi: 10.3389/fpls.2016.00896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmer J, O’Neill CM, Wells R, Bostrom A, Reynolds D, Websdale D, et al. SeedGerm: a cost-effective phenotyping platform for automated seed imaging and machine-learning based phenotypic analysis of crop seed germination. N. Phytol. 2020;228:778–793. doi: 10.1111/nph.16736. [DOI] [PubMed] [Google Scholar]
- Dargahi H, Tanya P, Srinives P. Mapping of the genomic regions controlling seed storability in soybean (Glycine max L.) J Genet. 2014;93:365–370. doi: 10.1007/s12041-014-0381-0. [DOI] [PubMed] [Google Scholar]
- Dekkers BJW, He H, Hanson J, Willems LAJ, Jamar DCL, Cueff G, et al. The Arabidopsis DELAY OF GERMINATION 1 gene affects ABSCISIC ACID INSENSITIVE 5 (ABI5) expression and genetically interacts with ABI3 during Arabidopsis seed development. Plant J. 2016;85:451–465. doi: 10.1111/tpj.13118. [DOI] [PubMed] [Google Scholar]
- De Marchi JL, Cicero SM. Use of the software Seed Vigor Imaging System (SVIS®) for assessing vigor of carrot seeds. Sci Agric. 2017;74:469–473. doi: 10.1590/1678-992x-2016-0220. [DOI] [Google Scholar]
- De Vitis M, Hay FR, Dickie JB, Trivedi C, Choi J, Fiegener R. Seed storage: maintaining seed viability and vigor for restoration use. Restor Ecol. 2020;28:S249–S255. doi: 10.1111/rec.13174. [DOI] [Google Scholar]
- Dornbos DL, Mullen RE, Shibles RE. Drought stress effects during seed fill on soybean seed germination and vigor. Crop Sci. 1989;29:476–480. doi: 10.2135/cropsci1989.0011183X002900020047x. [DOI] [Google Scholar]
- Dürr C, Dickie JB, Yang XY, Pritchard HW. Ranges of critical temperature and water potential values for the germination of species worldwide: contribution to a seed trait database. Agric Meteorol. 2015;200:222–232. doi: 10.1016/j.agrformet.2014.09.024. [DOI] [Google Scholar]
- Ebone LA, Caverzan A, Tagliari A, Chiomento JLT, Silveira DC, Chavarria G. Soybean seed vigor: Uniformity and growth as key factors to improve yield. Agronomy. 2020;10:545. doi: 10.3390/agronomy10040545. [DOI] [Google Scholar]
- Edwards D, Batley J. Plant genome sequencing: Applications for crop improvement. Plant Biotechnol J. 2010;8:2–9. doi: 10.1111/j.1467-7652.2009.00459.x. [DOI] [PubMed] [Google Scholar]
- Egli DB, TeKrony DM, Heitholt JJ, Rupe J. Air temperature during seed filling and soybean seed germination and vigor. Crop Sci. 2005;45:1329–1335. doi: 10.2135/cropsci2004.0029. [DOI] [Google Scholar]
- El-Maarouf-Bouteau H, Meimoun P, Job C, Job D, Bailly C. Role of protein and mRNA oxidation in seed dormancy and germination. Front. Plant Sci. 2013;4:77. doi: 10.3389/fpls.2013.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis RH, Roberts EH. Improved equations for the prediction of seed longevity. Ann Bot. 1980;45:13–30. doi: 10.1093/oxfordjournals.aob.a085797. [DOI] [Google Scholar]
- Fan Y, Ma S, Wu T. Individual wheat kernels vigor assessment based on NIR spectroscopy coupled with machine learning methodologies. Infrared Phys Technol. 2020;105:103213. doi: 10.1016/j.infrared.2020.103213. [DOI] [Google Scholar]
- Farooq M, Aziz T, Basra SMA, Cheema MA, Rehman H. Chilling tolerance in hybrid maize induced by seed priming with salicylic acid. J Agron Crop Sci. 2008;194:161–168. doi: 10.1111/j.1439-037X.2008.00300.x. [DOI] [Google Scholar]
- Fernández-Pascual E, Seal CE, Pritchard HW. Simulating the germination response to diurnally alternating temperatures under climate change scenarios: Comparative studies on Carex diandra seeds. Ann Bot. 2015;115:201–209. doi: 10.1093/aob/mcu234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch-Savage WE, Bassel GW. Seed vigour and crop establishment: extending performance beyond adaptation. J Exp Bot. 2016;67:567–591. doi: 10.1093/jxb/erv490. [DOI] [PubMed] [Google Scholar]
- Finch-Savage WE, Clay HA, Lynn JR, Morris K. Towards a genetic understanding of seed vigour in small-seeded crops using natural variation in Brassica oleracea. Plant Sci. 2010;179:582–589. doi: 10.1016/j.plantsci.2010.06.005. [DOI] [Google Scholar]
- Finch-Savage WE, Leubner-Metzger G. Seed dormancy and the control of germination. N. Phytol. 2006;171:501–523. doi: 10.1111/j.1469-8137.2006.01787.x. [DOI] [PubMed] [Google Scholar]
- Footitt S, Walley PG, Lynn JR, Hambidge AJ, Penfield S, Finch-Savage WE. Trait analysis reveals DOG1 determines initial depth of seed dormancy, but not changes during dormancy cycling that result in seedling emergence timing. N. Phytol. 2020;225:2035–2047. doi: 10.1111/nph.16081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey A, Effroy D, Lefebvre V, Seo M, Perreau F, Berger A, et al. Epoxycarotenoid cleavage by NCED5 fine-tunes ABA accumulation and affects seed dormancy and drought tolerance with other NCED family members. Plant J. 2012;70:501–512. doi: 10.1111/j.1365-313X.2011.04887.x. [DOI] [PubMed] [Google Scholar]
- Geshnizjani N, Ghaderi-Far F, Willems LAJ, Hilhorst HWM, Ligterink W. Characterization of and genetic variation for tomato seed thermo-inhibition and thermo-dormancy. BMC Plant Biol. 2018;18:1–12. doi: 10.1186/s12870-018-1455-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves NR, Cicero SM, Abud HF. Seedling image analysis and traditional tests to evaluate onion seed vigor. J Seed Sci. 2017;39:216–223. doi: 10.1590/2317-1545v39n3160444. [DOI] [Google Scholar]
- Graeber K, Linkies A, Steinbrecher T, Mummenhoff K, Tarkowská D, Turečková V, et al. DELAY OF GERMINATION 1 mediates a conserved coat-dormancy mechanism for the temperature- and gibberellin-dependent control of seed germination. Proc Natl Acad Sci USA. 2014;111:E3571–E3580. doi: 10.1073/pnas.1403851111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groot SPC, De Groot L, Kodde J, Van Treuren R. Prolonging the longevity of ex situ conserved seeds by storage under anoxia. Plant Genet Resour Characterisation Util. 2015;13:18–26. doi: 10.1017/S1479262114000586. [DOI] [Google Scholar]
- Groot SPC, Surki AA, De Vos RCH, Kodde J. Seed storage at elevated partial pressure of oxygen, a fast method for analysing seed ageing under dry conditions. Ann Bot. 2012;110:1149–1159. doi: 10.1093/aob/mcs198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu XY, Zhang J, Ye H, Zhang L, Feng J. Genotyping of endosperms to determine seed dormancy genes regulating germination through embryonic, endospermic, or maternal tissues in rice. G3 Genes, Genomes, Genet. 2015;5:183–193. doi: 10.1534/g3.114.015362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gualano NA, Del Fueyo PA, Benech-Arnold RL. Potential longevity (Ki) of malting barley (Hordeum vulgare L.) grain lots relates to their degree of pre-germination assessed through different industrial quality parameters. J Cereal Sci. 2014;60:222–228. doi: 10.1016/j.jcs.2014.03.003. [DOI] [Google Scholar]
- Guan YJ, Hu J, Wang XJ, Shao CX. Seed priming with chitosan improves maize germination and seedling growth in relation to physiological changes under low temperature stress. J Zhejiang Univ Sci B. 2009;10:427–433. doi: 10.1631/jzus.B0820373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo G, Liu X, Sun F, Cao J, Huo N, Wuda B, et al. Wheat miR9678 affects seed germination by generating phased siRNAs and modulating abscisic acid/gibberellin signaling. Plant Cell. 2018;30:796–814. doi: 10.1105/tpc.17.00842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C, Shen Y, Shi F. Effect of temperature, light, and storage time on the seed germination of Pinus bungeana Zucc. ex Endl.: The role of seed-covering layers and abscisic acid changes. Forests. 2020;11:300. doi: 10.3390/f11030300. [DOI] [Google Scholar]
- Guo T, Yang J, Li D, Sun K, Luo L, Xiao W, et al. Integrating GWAS, QTL, mapping and RNA-seq to identify candidate genes for seed vigor in rice (Oryza sativa L.) Mol Breed. 2019;39:87. doi: 10.1007/s11032-019-0993-4. [DOI] [Google Scholar]
- Halmer P (2004) Methods to improve seed performance in the field. In: Benech-Arnold RL, Sánchez RA (eds.) Handbook of Seed Physiology, Food Products Press: New York, pp 125–166
- Han Z, Bin W, Zhang J, Guo S, Zhang H, Xu L, et al. Mapping of QTLs associated with seed vigor to artificial aging using two RIL populations in maize (Zea mays L.) Agric Sci. 2018;09:397–415. [Google Scholar]
- Han Z, Ku L, Zhang Z, Zhang J, Guo SL, Liu H, et al. QTLs for seed vigor-related traits identified in maize seeds germinated under artificial aging conditions. PLoS One. 2014;9:e92535. doi: 10.1371/journal.pone.0092535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill HJ, Cunningham JD, Bradford KJ, Taylor AG. Primed lettuce seeds exhibit increased sensitivity to moisture content during controlled deterioration. HortScience. 2007;42:1436–1439. doi: 10.21273/HORTSCI.42.6.1436. [DOI] [Google Scholar]
- Huang Y, Guo Y, Liu Y, Zhang F, Wang Z, Wang H, et al. 9-cis-EPOXYCAROTENOID DIOXYGENASE 3 regulates plant growth and enhances multi-abiotic stress tolerance in rice. Front Plant Sci. 2018;9:162. doi: 10.3389/fpls.2018.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CT, Klos KE, Huang YF. Genome-wide association study reveals the genetic architecture of seed vigor in oats. G3 Genes, Genomes, Genet. 2020;10:4489–4503. doi: 10.1534/g3.120.401602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Sun MM, Ye Q, Wu XQ, Wu WH, Chen YF. Abscisic acid modulates seed germination via ABA INSENSITIVE5-mediated PHOSPHATE1. Plant Physiol. 2017;175:1661–1668. doi: 10.1104/pp.17.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo H, Bradford KJ (2015) Molecular and hormonal regulation of thermoinhibition of seed germination. In: JV Anderson, ed, Advances in Plant Dormancy, Springer, NY, pp 3–33
- Huo H, Dahal P, Kunusoth K, Mccallum CM, Bradford KJ. Expression of 9-cis-EPOXYCAROTENOID DIOXYGENASE 4 is essential for thermoinhibition of lettuce seed germination but not for seed development or stress tolerance. Plant Cell. 2013;25:884–900. doi: 10.1105/tpc.112.108902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo H, Wei S, Bradford KJ. DELAY of GERMINATION1 (DOG1) regulates both seed dormancy and flowering time through microRNA pathways. Proc Natl Acad Sci USA. 2016;113:E2199–E2206. doi: 10.1073/pnas.1600558113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilbi H, Powell AA, Alan O. Single radicle emergence count for predicting vigour of marigold (Tagetes spp.) seed lots. Seed Sci Technol. 2020;48:381–389. doi: 10.15258/sst.2020.48.3.06. [DOI] [Google Scholar]
- Ishola Esan V, Ayanniyin Ayanbamiji T, Omoyemi Adeyemo J, Oluwafemi S. Effect of drought on seed germination and early seedling of tomato genotypes using polyethylene glycol 6000. Int J Sci. 2018;4:36–43. [Google Scholar]
- Izydorczyk C, Nguyen TN, Jo SH, Son SH, Tuan PA, Ayele BT. Spatiotemporal modulation of abscisic acid and gibberellin metabolism and signalling mediates the effects of suboptimal and supraoptimal temperatures on seed germination in wheat (Triticum aestivum L.) Plant Cell Environ. 2018;41:1022–1037. doi: 10.1111/pce.12949. [DOI] [PubMed] [Google Scholar]
- Jin J, Long W, Wang L, Liu X, Pan G, Xiang W, et al. QTL mapping of seed vigor of backcross inbred lines derived from Oryza longistaminata under artificial aging. Front Plant Sci. 2018;871:1–7. doi: 10.3389/fpls.2018.01909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing-bao L, Zhi-yuan F, Hui-ling X, Yan-min H, Zong-hua L, Liu-jing D, et al. Identification of QTLs for maize seed vigor at three stages of seed maturity using a RIL population. Euphytica. 2011;178:127–135. doi: 10.1007/s10681-010-0282-0. [DOI] [Google Scholar]
- Kang J, Yim S, Choi H, Kim A, Lee KP, Lopez-Molina L, et al. Abscisic acid transporters cooperate to control seed germination. Nat Commun. 2015;6:1–10. doi: 10.1038/ncomms9113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya MD, Okçu G, Atak M, Çikili Y, Kolsarici Ö. Seed treatments to overcome salt and drought stress during germination in sunflower (Helianthus annuus L.) Eur J Agron. 2006;24:291–295. doi: 10.1016/j.eja.2005.08.001. [DOI] [Google Scholar]
- Kendall SL, Hellwege A, Marriot P, Whalley C, Graham IA, Penfield S. Induction of dormancy in Arabidopsis summer annuals requires parallel regulation of DOG1 and hormone metabolism by low temperature and CBF transcription factors. Plant Cell. 2011;23:2568–2580. doi: 10.1105/tpc.111.087643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerdaffrec E, Filiault DL, Korte A, Sasaki E, Nizhynska V, Seren Ü et al. (2016) Multiple alleles at a single locus control seed dormancy in Swedish Arabidopsis. eLife 5:e22502 [DOI] [PMC free article] [PubMed]
- Khan N, Kazmi RH, Willems LAJ, van Heusden AW, Ligterink W, Hilhorst HWM. Exploring the natural variation for seedling traits and their link with seed dimensions in tomato. PLoS One. 2012;7:43991. doi: 10.1371/journal.pone.0043991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodarahmpour Z. Effect of drought stress induced by polyethylene glycol (PEG) on germination indices in corn (Zea mays L.) hybrids. African. J Biotechnol. 2011;10:18222–18227. [Google Scholar]
- Klupczyńska EA, Pawłowski TA. Regulation of seed dormancy and germination mechanisms in a changing environment. Int J Mol Sci. 2021;22:1–18. doi: 10.3390/ijms22031357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraak HL, Vos J. Seed viability constants for lettuce. Ann Bot. 1987;59:343–349. doi: 10.1093/oxfordjournals.aob.a087323. [DOI] [Google Scholar]
- Lai Y, Cheng J, He Y, Yang B, Wang Z, Zhang H. Identification of QTLs with additive, epistatic, and QTL × seed maturity interaction effects for seed vigor in rice. Plant Mol Biol Rep. 2016;34:160–171. doi: 10.1007/s11105-015-0913-7. [DOI] [Google Scholar]
- Lamichaney A, Kumar Swain D, Biswal P, Kumar V, Pratap Singh N, Krishna, Hazra K. Elevated atmospheric carbon dioxide affects seed vigour of rice (Oryza sativa L.) Environ Exp Bot. 2019;157:171–176. doi: 10.1016/j.envexpbot.2018.10.011. [DOI] [Google Scholar]
- Lamichaney A, Maity A. Implications of rising atmospheric carbon dioxide concentration on seed quality. Int J Biometeorol. 2021;65:805–812. doi: 10.1007/s00484-020-02073-x. [DOI] [PubMed] [Google Scholar]
- Lamichaney A, Parihar AK, Hazra KK, Dixit GP, Katiyar PK, Singh D, et al. Untangling the influence of heat stress on crop phenology, seed set, seed weight, and germination in field pea (Pisum sativum L.) Front Plant Sci. 2021;12:437. doi: 10.3389/fpls.2021.635868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamichaney A, Tewari K, Basu PS, Katiyar PK, Singh NP. Effect of elevated carbon dioxide on plant growth, physiology, yield and seed quality of chickpea (Cicer arietinum L.) in Indo-Gangetic plains. Physiol Mol Biol Plants. 2021;27:251–263. doi: 10.1007/s12298-021-00928-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Huy TQ, Park E, Bae H-J, Baek I, Kim MS, et al. Machine vision technique for rapid measurement of soybean seed vigor. J Biosyst Eng. 2017;42:227–233. doi: 10.5307/JBE.2017.42.1.012. [DOI] [Google Scholar]
- Lefebvre V, North H, Frey A, Sotta B, Seo M, Okamoto M, et al. Functional analysis of Arabidopsis NCED6 and NCED9 genes indicates that ABA synthesized in the endosperm is involved in the induction of seed dormancy. Plant J. 2006;45:309–319. doi: 10.1111/j.1365-313X.2005.02622.x. [DOI] [PubMed] [Google Scholar]
- Leymarie J, Benech-Arnold RL, Farrant JM, Corbineau F. Thermodormancy and ABA metabolism in barley grains. Plant Signal Behav. 2009;4:205–207. doi: 10.4161/psb.4.3.7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Lai Y, Cheng J, Wang L, Du W, Wang Z, et al. Dynamic quantitative trait locus analysis of seed vigor at three maturity stages in rice. PLoS One. 2014;9:e115732. doi: 10.1371/journal.pone.0115732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Hasanuzzaman M, Wen H, Zhang J, Peng T, Sun H, et al. High temperature and drought stress cause abscisic acid and reactive oxygen species accumulation and suppress seed germination growth in rice. Protoplasma. 2019;256:1217–1227. doi: 10.1007/s00709-019-01354-6. [DOI] [PubMed] [Google Scholar]
- Liu M, Li M, Liu K, Sui N. Effects of drought stress on seed germination and seedling growth of different maize varieties. J Agric Sci. 2015;7:231–240. [Google Scholar]
- Liu X, Zhang H, Zhao Y, Feng Z, Li Q, Yang HQ, et al. Auxin controls seed dormancy through stimulation of abscisic acid signaling by inducing ARF-mediated ABI3 activation in Arabidopsis. Proc Natl Acad Sci USA. 2013;110:15485–15490. doi: 10.1073/pnas.1304651110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloret F, Penuelas J, Estiarte M. Experimental evidence of reduced diversity of seedlings due to climate modification in a Mediterranean-type community. Glob Chang Biol. 2004;10:248–258. doi: 10.1111/j.1365-2486.2004.00725.x. [DOI] [Google Scholar]
- Luo T, Zhang Y, Zhang C, Nelson MN, Yuan J, Guo L et al. (2021) Genome-wide association mapping unravels the genetic control of seed vigor under low-temperature conditions in rapeseed (Brassica napus L.). Plants 10:1–20 [DOI] [PMC free article] [PubMed]
- Marcos-Filho J. Seed vigor testing: An overview of the past, present and future perspective. Sci Agric. 2015;72:363–374. doi: 10.1590/0103-9016-2015-0007. [DOI] [Google Scholar]
- Martínez-Andújar C, Ordiz MI, Huang Z, Nonogaki M, Beachy RN, Nonogaki H (2011) Induction of 9-cis-epoxycarotenoid dioxygenase in Arabidopsis thaliana seeds enhances seed dormancy. Proc Natl Acad Sci USA 108:17225–17229 [DOI] [PMC free article] [PubMed]
- Martínez-Berdeja A, Stitzer MC, Taylor MA, Okada M, Ezcurra E, Runcie DE, et al. Functional variants of DOG1 control seed chilling responses and variation in seasonal life-history strategies in Arabidopsis thaliana. Proc Natl Acad Sci USA. 2020;117:2526–2534. doi: 10.1073/pnas.1912451117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews S, Khajeh-Hosseini M. Length of the lag period of germination and metabolic repair explain vigour differences in seed lots of maize (Zea mays) Seed Sci Technol. 2007;35:200–212. doi: 10.15258/sst.2007.35.1.18. [DOI] [Google Scholar]
- Matthews S, Powell A. Towards automated single counts of radicle emergence to predict seed and seedling vigour. Seed Test Int. 2011;142:44–48. [Google Scholar]
- Matthews S, Wagner M-H, Ratzeuboeck A, Khajeh-Hasseiins M, El-Khadem R, Yakhlifi M, et al. Early counts of radicle emergence during germination as a reproducible germination test for maize. Seed Test Int. 2011;141:39–45. [Google Scholar]
- McDonald MB. A review and evaluation of seed vigor tests. Proc Assoc Seed Anal. 1975;65:109–139. [Google Scholar]
- Morscher F, Kranner I, Arc E, Bailly C, Roach T. Glutathione redox state, tocochromanols, fatty acids, antioxidant enzymes and protein carbonylation in sunflower seed embryos associated with after-ripening and ageing. Ann Bot. 2015;116:669–678. doi: 10.1093/aob/mcv108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambara E, Marion-Poll A. Abscisic acid biosynthesis and catabolism. Annu Rev Plant Biol. 2005;56:165–185. doi: 10.1146/annurev.arplant.56.032604.144046. [DOI] [PubMed] [Google Scholar]
- Née G, Kramer K, Nakabayashi K, Yuan B, Xiang Y, Miatton E, et al. DELAY of GERMINATION1 requires PP2C phosphatases of the ABA signalling pathway to control seed dormancy. Nat Commun. 2017;8:1–9. doi: 10.1038/s41467-017-00113-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehoshtan Y, Carmon E, Yaniv O, Ayal S, Rotem O. Robust seed germination prediction using deep learning and RGB image data. Sci Rep. 2021;11:22030. doi: 10.1038/s41598-021-01712-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonogaki H. Seed germination and dormancy: The classic story, new puzzles, and evolution. J Integr Plant Biol. 2019;61:541–563. doi: 10.1111/jipb.12762. [DOI] [PubMed] [Google Scholar]
- Nonogaki H, Zhang S. A repressor complex silencing ABA signaling in seeds? J Exp Bot. 2020;71:2847–2853. doi: 10.1093/jxb/eraa062. [DOI] [PubMed] [Google Scholar]
- Penfield S, MacGregor DR. Effects of environmental variation during seed production on seed dormancy and germination. J Exp Bot. 2017;68:819–825. doi: 10.1093/jxb/erx021. [DOI] [PubMed] [Google Scholar]
- Rajjou L, Duval M, Gallardo K, Catusse J, Bally J, Job C, et al. Seed germination and vigor. Annu Rev Plant Biol. 2012;63:507–533. doi: 10.1146/annurev-arplant-042811-105550. [DOI] [PubMed] [Google Scholar]
- Raquid R, Kohli A, Reinke R, Dionisio-Sese M, Kwak J, Chebotarov D, et al. Genetic factors enhancing seed longevity in tropical japonica rice. Curr Plant Biol. 2021;26:100196. doi: 10.1016/j.cpb.2021.100196. [DOI] [Google Scholar]
- Roberts EH, Ellis RH. Water and seed survival. Ann Bot. 1989;63:39–52. doi: 10.1093/oxfordjournals.aob.a087727. [DOI] [Google Scholar]
- Rodríguez MV, Barrero JM, Corbineau F, Gubler F, Benech-Arnold RL. Dormancy in cereals (not too much, not so little): About the mechanisms behind this trait. Seed Sci Res. 2015;25:99–119. doi: 10.1017/S0960258515000021. [DOI] [Google Scholar]
- Ruggiero B, Koiwa H, Manabe Y, Quist TM, Inan G, Saccardo F, et al. Uncoupling the effects of abscisic acid on plant growth and water relations. Analysis of sto1/nced3, an abscisic acid-deficient but salt stress-tolerant mutant in Arabidopsis. Plant Physiol. 2004;136:3134–3147. doi: 10.1104/pp.104.046169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samarah N, Alqudah A. Effects of late-terminal drought stress on seed germination and vigor of barley (Hordeum vulgare L.) Arch Agron Soil Sci. 2011;57:27–32. doi: 10.1080/03650340903191663. [DOI] [Google Scholar]
- Samarah NH, Mullen RE, Anderson I. Soluble sugar contents, germination, and vigor of soybean seeds in response to drought stress. J N. Seeds. 2009;10:63–73. doi: 10.1080/15228860902786525. [DOI] [Google Scholar]
- Saux M, Bleys B, André T, Bailly C, El-Maarouf-bouteau H. A correlative study of sunflower seed vigor components as related to genetic background. Plants. 2020;9:1–13. doi: 10.3390/plants9030386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwember AR, Bradford KJ. Quantitative trait loci associated with longevity of lettuce seeds under conventional and controlled deterioration storage conditions. J Exp Bot. 2010;61:4423–4436. doi: 10.1093/jxb/erq248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwember AR, Bradford KJ. Oxygen interacts with priming, moisture content and temperature to affect the longevity of lettuce and onion seeds. Seed Sci Res. 2011;21:175–185. doi: 10.1017/S0960258511000080. [DOI] [Google Scholar]
- Seed Vigour Testing (2021). In: International Rules for Seed Testing. The International Seed Testing Association (ISTA), Bassersdorf Sweden, pp i-15-20(20)
- Seed-X (2021) GeNeeTM Sorter. https://www.seed-x.com/product/sorter/
- Seo M, Hanada A, Kuwahara A, Endo A, Okamoto M, Yamauchi Y, et al. Regulation of hormone metabolism in Arabidopsis seeds: Phytochrome regulation of abscisic acid metabolism and abscisic acid regulation of gibberellin metabolism. Plant J. 2006;48:354–366. doi: 10.1111/j.1365-313X.2006.02881.x. [DOI] [PubMed] [Google Scholar]
- Shekar RV, Shanti M, Reddy Shekar CV, Anil Kumar B, Padmasri A (2018) Effect of modified atmosphere with elevated levels of CO2 on Sitophilus oryzae (L.) in stored maize. J Entomol Zool Stud 6:693–700
- Shi H, Guan W, Shi Y, Wang S, Fan H, Yang J, et al. QTL mapping and candidate gene analysis of seed vigor-related traits during artificial aging in wheat (Triticum aestivum) Sci Rep. 2020;10:1–13. doi: 10.1038/s41598-019-56847-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu K, Zhang H, Wang S, Chen M, Wu Y, Tang S, et al. ABI4 regulates primary seed dormancy by regulating the biogenesis of abscisic acid and gibberellins in Arabidopsis. PLoS Genet. 2013;9:1003577. doi: 10.1371/journal.pgen.1003577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuai H, Meng Y, Luo X, Chen F, Zhou W, Dai Y, et al. Exogenous auxin represses soybean seed germination through decreasing the gibberellin/abscisic acid (GA/ABA) ratio. Sci Rep. 2017;7:1–11. doi: 10.1038/s41598-016-0028-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skubacz A, Daszkowska‐Golec A (2017) Seed Dormancy: The Complex Process Regulated by Abscisic Acid, Gibberellins, and Other Phytohormones that Makes Seed Germination Work. In: El-Esawi MA (ed) Phytohormones: Signaling Mechanisms and Crosstalk in Plant Development and Stress Responses. London: InTechOpen, pp 77–100
- Söderman EM, Brocard IM, Lynch TJ, Finkelstein RR. Regulation and function of the arabidopsis ABA-INSENSITIVE4 gene in seed and abscisic acid response signaling networks. Plant Physiol. 2000;124:1752–1765. doi: 10.1104/pp.124.4.1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltani A, MafiMoghaddam S, Walter K, Restrepo-Montoya D, Mamidi S, Schroder S, et al. Genetic architecture of flooding tolerance in the dry bean middle-American diversity panel. Front Plant Sci. 2017;8:1183. doi: 10.3389/fpls.2017.01183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltani A, Walter KA, Wiersma AT, Santiago JP, Quiqley M, Chitwood D, et al. The genetics and physiology of seed dormancy, a crucial trait in common bean domestication. BMC Plant Biol. 2021;21:1–17. doi: 10.1186/s12870-021-02837-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springthorpe V, Penfield S (2015) Flowering time and seed dormancy control use external coincidence to generate life history strategy. eLife 4:e05557 [DOI] [PMC free article] [PubMed]
- Sun Q, Wang J, Sun B. Advances on seed vigor physiological and genetic mechanisms. Agric Sci China. 2007;6:1060–1066. doi: 10.1016/S1671-2927(07)60147-3. [DOI] [Google Scholar]
- Suriyasak C, Oyama Y, Ishida T, Mashiguchi K, Yamaguchi S, Hamaoka N, et al. Mechanism of delayed seed germination caused by high temperature during grain filling in rice (Oryza sativa L.) Sci Rep. 2020;10:1–11. doi: 10.1038/s41598-020-74281-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan BC, Joseph LM, Deng WT, Liu L, Li QB, Cline K, et al. Molecular characterization of the Arabidopsis 9-cis-epoxycarotenoid dioxygenase gene family. Plant J. 2003;35:44–56. doi: 10.1046/j.1365-313X.2003.01786.x. [DOI] [PubMed] [Google Scholar]
- Tarquis AM, Bradford KJ. Prehydration and priming treatments that advance germination also increase the rate of deterioration of lettuce seeds. J Exp Bot. 1992;43:307–317. doi: 10.1093/jxb/43.3.307. [DOI] [Google Scholar]
- TeKrony DM. Accelerated aging test: principles and procedures. Seed Technol. 2005;27:135–146. [Google Scholar]
- Thabet SG, Moursi YS, Karam MA, Graner A, Alqudah AM. Genetic basis of drought tolerance during seed germination in barley. PLoS One. 2018;13:1–21. doi: 10.1371/journal.pone.0206682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh S, Imamura A, Watanabe A, Nakabayashi K, Okamoto M, Jikumaru Y, et al. High temperature-induced abscisic acid biosynthesis and its role in the inhibition of gibberellin action in Arabidopsis seeds. Plant Physiol. 2008;146:1368–1385. doi: 10.1104/pp.107.113738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetch JM, Stougaard RN, Martin JM, Giroux MJ. Review: Revealing the genetic mechanisms of pre-harvest sprouting in hexaploid wheat (Triticum aestivum L.) Plant Sci. 2019;281:180–185. doi: 10.1016/j.plantsci.2019.01.004. [DOI] [PubMed] [Google Scholar]
- Vogel E, Donat MG, Alexander LV, Meinshausen M, Ray DK, Karoly D, et al. The effects of climate extremes on global agricultural yields. Environ Res Lett. 2019;14:054010. doi: 10.1088/1748-9326/ab154b. [DOI] [Google Scholar]
- Voss-Fels K, Snowdon RJ. Understanding and utilizing crop genome diversity via high-resolution genotyping. Plant Biotechnol J. 2016;14:1086–1094. doi: 10.1111/pbi.12456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters C, Ballesteros D, Vertucci VA. Structural mechanics of seed deterioration: standing the test of time. Plant Sci. 2010;179:565–573. doi: 10.1016/j.plantsci.2010.06.016. [DOI] [Google Scholar]
- Walters C, Fleming MB, Hill LM, Dorr EJ, Richards CM. Stress-response relationships related to ageing and death of orthodox seeds: a study comparing viability and RNA integrity in soya bean (Glycine max) cv. Williams 82. Seed Sci Res. 2020;30:161–172. doi: 10.1017/S0960258520000197. [DOI] [Google Scholar]
- Wang T, Hou L, Jian H, Di F, Li J, Liu L. Combined QTL mapping, physiological and transcriptomic analyses to identify candidate genes involved in Brassica napus seed aging. Mol Genet Genomics. 2018;293:1421–1435. doi: 10.1007/s00438-018-1468-8. [DOI] [PubMed] [Google Scholar]
- Wang X, Zheng H, Tang Q, Chen Q, Mo W. Seed filling under different temperatures improves the seed vigor of hybrid rice (Oryza sativa L.) via starch accumulation and structure. Sci Rep. 2020;10:1–9. doi: 10.1038/s41598-019-56847-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Liu X, Li L, Zhao H, Liu S, Yu X, et al. Quantitative proteomic, physiological and biochemical analysis of cotyledon, embryo, leaf and pod reveals the effects of high temperature and humidity stress on seed vigor formation in soybean. BMC Plant Biol. 2020;20:1–15. doi: 10.1186/s12870-019-2170-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Shen Y, Zhao H, Liu X, Jia Y, Yu X, et al. GmANN, a glutathione-S-transferase-interacting annexin, is involved in high temperature and humidity tolerance and seed vigor formation in transgenic Arabidopsis. Plant Cell Tissue Organ Cult. 2019;138:583–595. doi: 10.1007/s11240-019-01655-x. [DOI] [Google Scholar]
- Wheeler TR, Ellis RH. Seed quality and seedling emergence in onion (Allium cepa L.) J Hortic Sci. 1992;67:319–332. doi: 10.1080/00221589.1992.11516255. [DOI] [Google Scholar]
- Wijewardana C, Raja Reddy K, Jason Krutz L, Gao W, Bellaloui N. Drought stress has transgenerational effects on soybean seed germination and seedling vigor. PLoS One. 2019;14:1–20. doi: 10.1371/journal.pone.0214977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing IS, De Cian E, Mistry MN. Global vulnerability of crop yields to climate change. J Environ Econ Manag. 2021;109:102462. doi: 10.1016/j.jeem.2021.102462. [DOI] [Google Scholar]
- Wu X, Feng F, Zhu Y, Xie F, Yang J, Gong J, et al. (2020) Construction of high-density genetic map and identification of qtls associated with seed vigor after exposure to artificial aging conditions in sweet corn using SLAF-seq. Genes 11: 1–16 [DOI] [PMC free article] [PubMed]
- Wu X, Ning F, Hu X, Wang W. Genetic modification for improving seed vigor is transitioning from model plants to crop plants. Front Plant Sci. 2017;8:1–7. doi: 10.3389/fpls.2017.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Seng S, Sui J, Vonapartis E, Luo X, Gong B, et al. Gladiolus hybridus ABSCISIC ACID INSENSITIVE 5 (GhABI5) is an important transcription factor in ABA signaling that can enhance Gladiolus corm dormancy and Arabidopsis seed dormancy. Front Plant Sci. 2015;6:1–14. doi: 10.3389/fpls.2015.00960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Hu J, Li Y, Ma W, Zheng Y, Zhu S. Chilling tolerance in Nicotiana tabacum induced by seed priming with putrescine. Plant Growth Regul. 2011;63:279–290. doi: 10.1007/s10725-010-9528-z. [DOI] [Google Scholar]
- Yadav PV, Kumari M, Ahmed Z. Seed priming mediated germination improvement and tolerance to subsequent exposure to cold and salt stress in capsicum. Res J Seed Sci. 2011;4:125–136. doi: 10.3923/rjss.2011.125.136. [DOI] [Google Scholar]
- Yan D, Duermeyer L, Leoveanu C, Nambara E. The functions of the endosperm during seed germination. Plant Cell Physiol. 2014;55:1521–1533. doi: 10.1093/pcp/pcu089. [DOI] [PubMed] [Google Scholar]
- Yang J, Guo Z, Luo L, Gao Q, Xiao W, Wang J, et al. Identification of QTL and candidate genes involved in early seedling growth in rice via high-density genetic mapping and RNA-seq. Crop J. 2021;9:360–371. doi: 10.1016/j.cj.2020.08.010. [DOI] [Google Scholar]
- Ye H, Feng J, Zhang L, Zhang J, Mispan MS, Cao Z, et al. Map-based cloning of SEED DORMANCY1-2 identified a gibberellin synthesis gene regulating the development of endosperm-imposed dormancy in rice. Plant Physiol. 2015;169:2152–2165. doi: 10.1104/pp.15.01202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Hina A, Song S, Kong J, Bhat JA, Zhao T. Whole-genome mapping identified novel “QTL hotspots regions” for seed storability in soybean (Glycine max L.) BMC Genomics. 2019;20:1–14. doi: 10.1186/s12864-018-5379-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A, Liu C, Chen G, Hong K, Gao Y, Tian P, et al. Genetic analysis for rice seedling vigor and fine mapping of a major QTL qSSL1b for seedling shoot length. Breed Sci. 2017;67:307–315. doi: 10.1270/jsbbs.16195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Zhou S, Wang L, Wu D, Cheng H, Du X, et al. miR164c and miR168a regulate seed vigor in rice. J Integr Plant Biol. 2020;62:470–486. doi: 10.1111/jipb.12792. [DOI] [PubMed] [Google Scholar]
- Zinsmeister J, Leprince O, Buitink J. Molecular and environmental factors regulating seed longevity. Biochem J. 2020;477:305–323. doi: 10.1042/BCJ20190165. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


