Abstract
Uncontrolled bleeding is a major problem in trauma and emergency medicine. While materials for trauma applications would certainly find utility in traditional surgical settings, the unique environment of emergency medicine introduces additional design considerations, including the need for materials that are easily deployed in austere environments. Ideally, these materials would be available off the shelf, could be easily transported, and would be able to be stored at room temperature for some amount of time. Both natural and synthetic materials have been explored for the development of hemostatic materials. This review article provides an overview of classes of materials used for topical hemostats and newer developments in the area of injectable hemostats for use in emergency medicine.
Keywords: hemostasis, trauma, clotting, sealant, synthetic blood product, layer-by-layer assembly
INTRODUCTION
Uncontrolled bleeding is a major problem in trauma and emergency medicine, and exsanguination after trauma remains a leading cause of death for people under the age of 45 (1). While materials for trauma applications would certainly find utility in traditional surgical settings, the unique environment of emergency medicine introduces additional design considerations, including the need for materials that are easily deployed in austere environments. Ideally, these materials would be available off the shelf, could be easily transported, and would be able to be stored at room temperature for some amount of time. Some clinically used materials meet these requirements but have much room for improvement in terms of promoting hemostasis during hemorrhage. Following a trauma or injury, various cellular mechanisms and proteins induce the wound-healing cascade. Wound healing follows a linear process of coagulation, inflammation, re-epithelialization, angiogenesis, wound contraction, and remodeling (2).
These events of the wound healing cascade are generally organized into four phases: hemostasis, inflammation, proliferation, and remodeling. Following an injury, the body’s immediate goal is to prevent blood loss which is achieved by vasoconstriction and developing a fibrin blood clot filling the wound site. The clot is initially formed from the adherence and aggregation of platelets. The generation of fibrin is then established from the activation of prothrombin to thrombin, where thrombin cleaves fibrinogen to form fibrin. During degranulation, platelets will release chemoattractants for the recruitment of inflammatory cells (2a); this allows platelets to mediate the initiation of the inflammation phase. The hemostasis and inflammation phase occur on the time scales of seconds to hours and hours to days, respectively. During inflammation, neutrophils, monocytes, and endothelial cells adhere to the fibrin clot, again attracting more cells and releasing pro-inflammatory cytokines (2b, 2c). Macrophages are a key player in the inflammation phase; limitations to macrophage function may prolong inflammation and lead to scar tissue formation. The final two phases of the wound healing cascade involve tissue formation and full development of the wound. The proliferation phase involves fibroblasts, endothelial cells, and a large array of growth factors to synthesize a provisional matrix2. Within the remodeling phase, wound contraction begins to enable wound closure and collagen remodeling to increase the tensile strength of the wound. The proliferation phase occurs on the time scales of days to weeks following the injury, while the remodeling phase can last from weeks to months.
The coagulation cascade determines the efficacy of clotting, which is hindered during significant blood loss, preexisting pathologies, and/or drug-induced effects (3). Recent research has focused on developing hemostatic biomaterials to quickly halt severe bleeding while providing an environment to promote healing. The following sections discuss these various materials
Hemostasis: cessation of bleeding at an injury site; this process can be divided into primary (platelet plug formation) and secondary (fibrin clot formation, following the conversion of fibrinogen to fibrin) hemostasis
Coagulation: formation of a blood clot
Topical hemostats, surgical sealants, adhesives, and dressings have been developed to promote cessation of bleeding; however, many of these materials are insufficient to address internal bleeding and/or noncompressible hemorrhage. Therefore, there has been great interest in recent years in developing smart injectable hemostatic materials that target sites of internal bleeding to promote hemostasis. Both natural and synthetic materials have been explored for development of hemostatic materials. This review article provides an overview of classes of materials used for topical hemostats and newer developments in the area of injectable hemostats (Figure 1).
Figure 1.
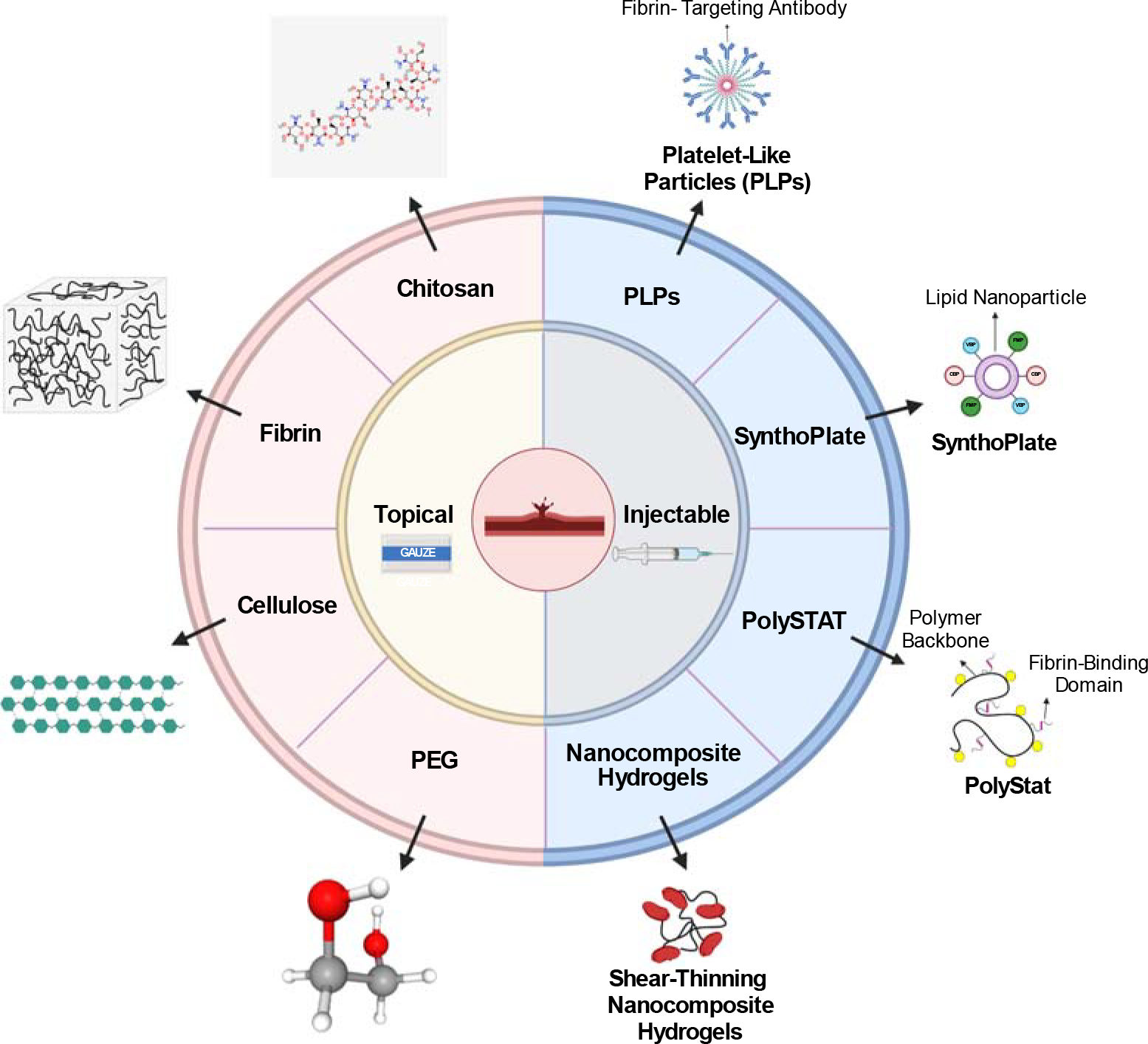
Overview of topical and injectable hemostatic materials. Topical materials include cellulose, chitosan, fibrin, and PEG products, as indicated by the red semicircle. Injectable materials include PLPs, SynthoPlate, PolySTAT, and HAPPI, as indicated by the blue semicircle. Abbreviations: HAPPI, hemostatic agent via polymer peptide infusion; PEG, polyethylene glycol; PLP, platelet-like particle. Figure adapted from image created with BioRender.com. 2D structure image of CID 71853 (chitosan): https://pubchem.ncbi.nlm.nih.gov/compound/Chitosan#section=2D-Structure. 3D structure image of CID 174 (1,2-Ethanediol): https://pubchem.ncbi.nlm.nih.gov/compound/174#section=3D-Conformer
TOPICAL HEMOSTATS
Many available topical hemostats that can be classified on the basis of their functions as mechanical barrier agents, active biological agents, flowable agents, and sealants; clinically used topical hemostats classified on the basis of these functionalities, have been recently reviewed (4). Topical hemostats are created from a wide variety of materials, from both natural and synthetic sources. Here, we review common materials utilized for topical hemostats including chitosan, cellulose, fibrin, hyaluronic acid (HA), and polyethylene glycol (PEG). Additionally, layer-by-layer (LbL) assembly–based materials, which have recently gained significant interest for hemostatic applications, are also described.
Fibrin: the activated form of fibrinogen that forms an insoluble, fibrillar clot network
Chitosan
Chitosan is a polycationic polysaccharide, derived from shrimp, crab, squid, and certain fungi (5). It is notable for its biocompatible, biodegradable, and antimicrobial properties. Previous studies proposed that the positive charge of chitosan contributes to platelet adhesion and blood coagulation (6). Currently, US Food and Drug Administration (FDA)-approved hemostatic applications of chitosan include Celox, TraumaStat, and HemCon. These products utilize the electric charge of carboxylate and amine groups (7) to promote binding with tissues and cells.
The large majority of FDA-approved chitosan-based materials are in the form of a dressing or gauze, and ongoing work in the area of chitosan materials focuses on improving these established materials and comparing their efficacy in stopping bleeding with that of standard gauze or other topical materials. As an example, Kunio et al. (8) compared standard gauze and Combat Gauze® with Celox Rapid gauze in a porcine arterial hemorrhage model. Combat Gauze is a kaolin-impregnated gauze, while Celox Rapid gauze is a chitosan granule-impregnated gauze strip. In these studies, following a 1-min hemorrhaging period, the dressing was applied with no additional compression. In the Celox Rapid gauze groups, there was a shorter dressing application but a longer initial clot formation time. However, there was significantly less posttreatment blood loss with Celox Rapid gauze in comparison with standard gauze and Combat Gauze. With this topical application, further research will address a more defined trauma model and understanding of the mechanism of a hemostatic chitosan gauze in reduced severe bleeding.
Pure chitosan has poor water solubility; therefore, it is often modified to improve solubility. An example of a modification is adding a thiol group, which has been shown to improve mucoadhesion of chitosan materials up to 140 times that of unmodified chitosan (9). An alternative modification of chitosan was explored by Huang et al. (10) to determine the efficacy of self-assembling hydrophobically modified chitosan sponges on hemostasis. Here, dodecyl groups were grafted onto the nitrogen atoms of the chitosan molecules. To evaluate the hemostatic effect in vivo, a rat femoral artery injury model was used, where hemostasis was defined as no bleeding after 10 min. When comparing unmodified and hydrophobically modified chitosan sponges, the modified chitosan increased platelet aggregation and reduced bleeding time and volume in vivo. In vitro analysis confirmed low cytotoxicity of the chitosan sponge, solidifying the modified chitosan as a biocompatible agent for hemostatic applications.
Cytotoxicity: cell death caused by a drug, material, or medical device
The applications discussed thus far employ techniques similar to those of current market applications for topical hemostatic agents. Recent work focuses on additive measures to treat severe hemorrhaging and developing chitosan films to enhance blood coagulation. Hattori et al. (11) established a photo-cross-linked chitosan hydrogel, which acts as a sealant following femoral injury. The authors found the hydrogel was completely degraded from the area of injury within 3 days following hemostasis. Unfortunately, the cross-linkable chitosan hydrogel did not prevent severe hemorrhage and demonstrated slight immunogenicity. He et al. (6) created a model using various chitosan films to investigate the relationship between the polycationic charge and blood coagulation. Previous research has shown that the procoagulant activity of chitosan is in part due to the ability to promote erythrocyte adhesion (6). When quantifying the proportion of adherent cells on films with varying degrees of protonation, there was a strong correlation between protonation degrees and the number of adherent erythrocytes. Also, fluorescence labeling of platelet-poor plasma incubated with chitosan films demonstrated an increase in fibrinogen adsorption with higher protonation degrees. Ongoing work is investigating how to balance the relationship between the positive charge of chitosan and its assistance in blood coagulation.
Immunogenicity: the ability of a drug, material, or medical device to stimulate an immune response
Fibrinogen: a protein, made by the liver and found in plasma, that is converted into fibrin by the enzymatic activity of thrombin
Other examples have shown the hemostatic ability of chitosan-loaded cyclodextrin polyester hydrogels, which are assembled with chitosan honeycomb-like monolithic mats (12). Future development within chitosan-based hemostatic materials includes developing alternatives to forming an insoluble hydrogel, avoiding formulations that inhibit the coagulation cascade, and reducing immune responses.
Cellulose
Cellulose is a polysaccharide, noted as one of the most abundant natural polymers, and is produced by plants, algae, and other organisms. Oxidized cellulose is synthesized from natural cellulose by a variety of oxidizing agents, producing an absorbable product. This product can be differentiated into oxidized regenerated cellulose (ORC) to form organized fibers and oxidized nonregenerated cellulose with unorganized fibers (13). Previous research noted that this oxidization step contributes to the biodegradability, absorbability, and antibacterial properties of cellulose as a hemostatic material. Notably, in vitro studies have demonstrated that dermal fibroblasts seeded in contact with oxidized cellulose stimulate fibroblast proliferation; adding fibroblast migration and proliferation is essential in the proliferative phase of wound healing. Current market applications include SURGICEL® (https://www.jnjmedicaldevices.com/en-US/product/surgicel-original-absorbable-hemostat), TRAUMASTEM® (https://www.cardiolinkgroup.com/en/productos/traumastem/), and RESORBA® CELL (https://www.resorba.com/region/row/product/biosurgicals/resorba-cell/). SURGICEL is an oxidized cellulose product with subproducts including a powder-based absorbable hemostat, an ORC-based material knitted into a gauze, and other hemostatic materials. Similar products are manufactured by Cardiolink Group and RESORBA Medical GmbH. Unfortunately, limitations exist with these current market cellulose-based hemostat materials. Examples of such limitations include not enhancing platelet adhesion and aggregation in the blood coagulation cascade, limited antibacterial properties, and poor biodegradability (17).
To address the immune response noted in clinical studies, Yuan et al. (17) developed a rat liver model to quantify blood loss and rate of hemostasis in vivo after treatment with oxidized bacterial nanocellulose (OBC) or a composite of OBC, collagen, and chitosan (OBC/COL/CS). The combination of OBC with chitosan-coated samples significantly reduced the proportion of bacteria (Escherichia coli, Staphylococcus aureus, and Komagataeibacter xylinus) and demonstrated an improvement of antibacterial properties. In an analysis of cytotoxicity, fibroblasts cultured on OBC/COL/CS composites displayed normal fibroblast morphology, strong adherence, and proliferation. When comparing blood loss after treatment with SURGICEL and OBC/COL/CS, the composite displayed the lowest amount of blood loss and the shortest time of hemostasis. There was no difference between the performance of SURGICEL and unmodified OBC. The composite promotes erythrocyte aggregation and clot formation due to the activity of chitosan, and collagen triggers platelet activation; therefore, the composite is a suitable hemostatic antibacterial sponge. Alternatively, Udangawa et al. (18) designed cellulose–halloysite hemostatic nanocomposite fibers (CHNFs). Halloysite is a clay mineral known to promote blood coagulation and exhibit biocompatibility. CHNFs are designed as an emergency clotting gauze, with experimental studies focusing on fluid uptake and antimicrobial activity. Using an activated partial thromboplastin time assay, CHNFs displayed faster plasma coagulation times in comparison with those of cellulose nanofibers and QuikClot®. In an analysis of the difference between halloysite and kaolin clay, both demonstrated compatibility to human fibroblast cells and no statistical difference between the number of proliferated cells. A notable limitation of this approach is a significant reduction of the procoagulant activity of CHNFs after a thorough wash with distilled water; this trend was also noted with the performance of QuikClot.
Despite the promise of current and emerging cellulose-based hemostatic materials, several key disadvantages exist, including a high cost of fabrication due to the electrospinning process, low yield, and slow production. Emerging technologies in this area should focus on addressing these limitations to improve the translation of new cellulose-based materials for use in emergency medicine situations.
Fibrin
Fibrin is the primary protein component in a blood clot and therefore represents an ideal biomaterial for hemostasis. The formation of fibrin is the last step of the coagulation cascade and occurs following the proteolytic cleavage and removal of fibrinopeptides from soluble fibrinogen via the serine protease thrombin (19). Many hemostatic applications utilize cross-linked fibrin to enhance clot stability. Clot stability and structure are largely influenced by the concentration of thrombin, fibrin(ogen), procoagulants and anticoagulants, and a variety of other blood coagulation factors; therefore, fibrin-based material properties can be modified by altering these parameters. Specifically, there is a strong dependence of thrombin concentrations on physical characteristics of fibrin fibers. At low thrombin concentrations, fibers are thinner with large networks of unbranched fibers, forming clots suspected to be more susceptible to enzymatic breakdown. Conversely, at high thrombin concentrations, dense branched fibrin fibers are formed (20).
Thrombin: a serine protease involved in the conversion of fibrinogen to fibrin
Fibrin-based hemostats are divided into liquid- and patch-based applications, with a large portion of fibrin products developed into gauze or scaffolds. Liquid-based fibrin hemostats, known as fibrin sealants or glues, include several FDA-approved materials, such as Tissel, Evicel, and Artiss, which have been reviewed elsewhere (21, 22). These liquid-based fibrin hemostats typically consist of a two-component system that uses high concentrations of fibrinogen (80–120 mg/mL) and thrombin (300–600 NIH U/mL), which are mixed immediately at the application site. These high concentrations of fibrinogen and thrombin are needed to achieve rapid hemostasis; however, they lead to very short working times and the creation of high-density fibrin networks, which can slow subsequent healing. Such liquid-based systems have additional limitations, including potential thrombosis, hypotension, high cost, and cold storage requirements (23). Current FDA-approved fibrin-based hemostatic patches include EVARREST® (https://www.jnjmedicaldevices.com/en-US/product/evarrest-fibrin-sealant-patch), a fibrin patch composed of oxidized regenerated cellulose, needle-punched Vicryl® (polyglactin 910) fibers, and embedded human fibrinogen and thrombin. Key limitations for use include the need for manual compression; the inability to control severe hemorrhaging in major arteries or veins; and the risk, though minor, of transmitting infectious diseases due to the biological components of the patch (25). Another notable fibrin-based technology is TachoSil® (https://advancedsurgery.baxter.com/tachosil), a honeycomb collagen structure coated with human coagulation factors, demonstrating strength and flexibility. Previous studies have shown that TachoSil degrades progressively in a porcine model, with products of the patch remaining after 12 months of application. Limitations similar to those mentioned for EVARREST exist for TachoSil. Therefore, there is a strong need for a fibrin-based hemostat that demonstrates similar flexibility and strength to those of current market applications but carries the ability to be applied for a variety of trauma wounds and reduces the risk of transmitting infectious diseases.
Thrombosis: formation of a clot in a blood vessel
Conventional dressings aim to reduce bleeding through manual compression, where changes of a gauze are expected after complete saturation. Removing gauze from the wound site can lead to secondary bleeding and potential exposure for infections. To address these challenges, Li et al. (27) developed a superhydrophobic (SHP) carbon nanofiber (CNF) hemostatic patch that achieves fast clotting and self-detachment from established clots, with antimicrobial properties. To analyze the hydrophobicity, a nanocomposite comprising CNFs, polytetrafluoroethylene, and polydimethylsiloxane was sprayed on a substrate surface. The generated CNF network was placed in contact with blood/platelet poor plasma, forming rapid fibrin fibers. With the sliding test, results were demonstrated as the plasma droplet moved downward, and fibrin fibers were elongated up to the fracture point. To test the abilities of the dressings to quickly promote hemostasis, blood was incubated in the presence of gauze for a fixed period and neutralized with deionized water to measure the amount of free hemoglobin. Any red blood cells not incorporated into the clot would be washed away, acting as an indirect measure of clotting ability. During a comparison of the performance of cotton with that of SHP CNF gauze, the SHP material displayed lower levels of hemoglobin and faster clotting times. Addressing the important limitation of secondary bleeding due to gauze changes in vivo, Li et al. (27) confirmed the ability of SHP CNF gauze to prevent wound tearing, whereas the control displayed large maximum peeling forces and caused secondary bleeding.
Key advantages of fibrin-based liquid and patch hemostatic formulations include the ability to modulate clot structure and stability, utility in a variety of applications, and ease of use for patch formulations. However, in clinical settings, current FDA-approved products are relatively expensive; for example, TachoSil retails at $800 per patch (28). Prime opportunities for further development exist in terms of reducing associated production costs, preventing thromboembolism from fibrin-liquid hemostats (29), balancing the relationship of the mechanical stability of fibrin patches with hemostasis assistance, and researching the relationship between fibrin structure and disease etiology (20).
Thromboembolism: obstruction of a blood vessel by a clot dislodged from another location
Etiology: the cause or origin of a disease
Hyaluronic Acid
HA is a linear polysaccharide, is a main component of extracellular matrix (ECM), and is naturally secreted by the skin and extracted from other sources (bacteria) for medical applications. Notably, HA is used to treat osteoarthritis through oral administration or injection into the synovial cavity; however, HA has also shown promise as a hemostatic material. The structural characteristics of HA are dependent on concentration; at very low concentrations, the polymer chains are mildly entangled, leading to a medium viscosity. HA solutions at high concentrations are shear dependent, whereby applied stress can alter the behavior of HA (30). HA is also involved in biological functions, such as signal transduction and gene expression; however, studies have shown that mostly low molecular weight HA is involved in promoting gene expression. A key part of wound healing is inflammation; HA participates in the increase of proinflammatory cytokines and the adhesion of lymphocytes to the endothelium (30).
To replace fibrin sealants, Luo et al. (31) developed an injectable gelatin and HA (G/HA) hydrogel, analyzing the cytocompatibility, burst strength, and hemostatic potential in a rat hemorrhaging model. Through the use of osteoblasts (3T3-E1), cells were cultured in the presence of a self-cross-linking gelatin (sc-G) hydrogel or a G/HA hydrogel. No morphological changes or adverse effects to cell viability were noted. During a comparison of the bursting strength among samples, the G/HA hydrogel displayed a strength of 13.9+/−2.4 kPa, whereas fibrin glue had the smallest strength of 2.5+/−1.6 kPa. In vivo, a 5-mm liver wound was induced, and 200 μL of the sample was applied to the bleeding site; the samples included a blank control, fibrin glue, sc-G, and G/HA (Figure 2). After 30 s, hemostasis was noted in the G/HA hydrogel and fibrin glue, indicating a similarity in hemostatic abilities.
Figure 2.
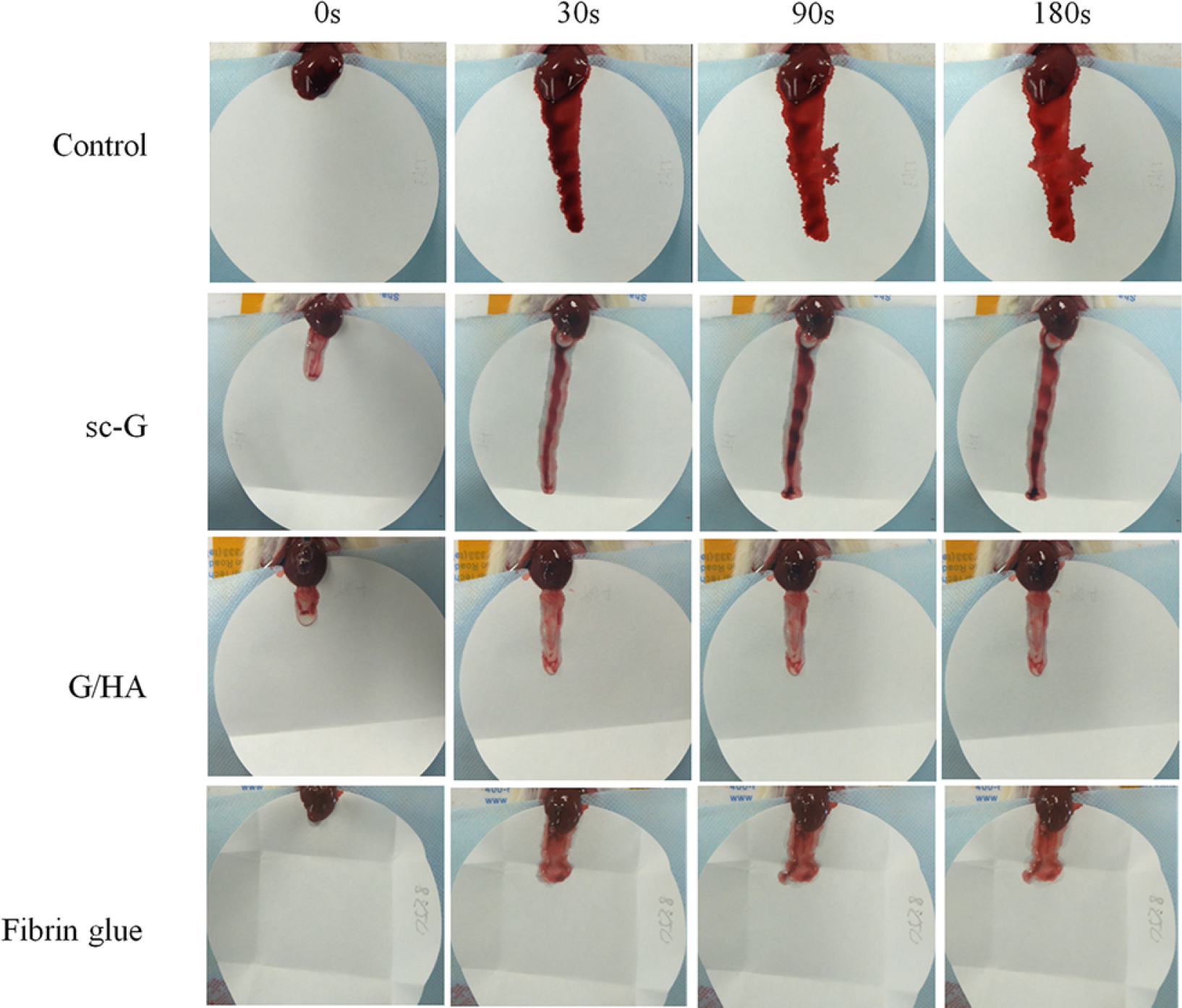
The bleeding of mouse livers with different treatments 3 min after injury. Figure adapted with permission from Reference 31. Abbreviations: G/HA, gelatin and hyaluronic acid; sc-G, self-cross-linking gelatin.
Cell viability: a quantification of viable (living) cells in a cell population
Another HA hydrogel technology was explored by Wang et al. (32). Utilizing the wet adhesion properties of scallops and the wound-healing abilities of HA, they developed a HA-catechol conjugate. The adhesive properties of scallops are visible in nature by their adhesion to marine objects (e.g., submarine substrates). These underwater adhesion properties are enhanced by scallops’ levels of dopamine. Hydrogel treatment groups were placed and cured on porcine skin, and the adhesive strength was measured using a tensile machine. At higher concentrations of H2O2 and horseradish peroxidase, the hydrogels displayed an increase in catechol oxidation and mechanical strength. In vitro, quantitative 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) analysis of rat bone marrow cells (L929) in the presence of hydrogels demonstrated good cytocompatibility. In vivo, a rat liver incision was induced, immediately after 200 μL of hydrogel (CHI-HA-R2) was applied. Filter paper was placed underneath the wound site to measure blood loss (weight) 20 min after injury. The sample with the CHI-HA-R2 hydrogel reached hemostasis within 6 s, with a significant decrease in total blood loss in comparison with that of untreated liver. To determine the wound-healing abilities of the samples, rats were treated with CHI-HA or CHI-HA-R2 and the wound closure rate was measured. Interestingly, the CHI-HA-R2 hydrogel had 97% closure within 9 days; in comparison, CHI-HA demonstrated 60% closure.
Compared with many other material-based hemostats, HA is relatively inexpensive and allows for easy preparation [as noted by Luo et al. (31) when using a dual-syringe system]. Unfortunately, limitations exist in HA hydrogel technologies using 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC)/N-hydroxysuccinimide (NHS) cross-linking, and further studies are needed to better understand if EDC/NHS cross-linking could lead to toxicity in vivo. Additionally, future research should examine any by-product degradation from HA scaffolds and performance in a variety of hemorrhaging situations.
Polyethylene Glycol
PEG is a hydrophilic polymer, utilized in medical applications as a sealant and/or adhesive. Like chitosan, PEG has key advantages that include high solubility in water (and other polar solvents), biocompatibility, and low immunogenicity. An example of a recently developed hemostatic pad for a clinical setting is the HEMOPATCH, which is composed of a PEG coating and a collagen pad to control bleeding. The active side of this hemostatic pad is covered with NHS functionalized PEG (NHS-PEG) (10). Upon contact with blood, the NHS-PEG is activated, covalently bonding to blood proteins (33). Previous studies have noted that the adherence, or activation, occurs in the presence of other fluids containing proteins (34), with a key advantage being that these fluids can consist of lymph or cerebrospinal fluid. A notable attribute involved in the increased procoagulant activity involves collagen, whereby interactions lead to thrombin generation and platelet activation.
In another example, Wei et al. (35) developed a silk fibroin (SF)-PEG sponge, with high water absorption and moisture-triggering properties. During experiments, it was noted that use of the combination SF-PEG sponge in comparison with a pure SF sponge led to an increase in gelation time and hydration. In vivo liver wound assays demonstrated a significant reduction in blood loss and hemostatic time in comparison with the results from the use of an SF and gelatin sponge. In vitro results indicated that the SF-PEG sponge induced platelet aggregation and increased the rate of platelet adhesion. These results were further confirmed by the use of an enzyme-linked immunosorbent assay to determine the conversion of fibrinogen, in the presence of stimulated platelets, to fibrin. There was no statistical difference between the fibrinogen expression of the SF-PEG sponge, the SF sponge, and adenosine diphosphate (used as a positive control). Focusing on preserving the mechanical strength of a tetrazine-armed PEG succinimidyl glutarate (Tetra-PEG-SG) hydrogel, while overcoming long degradation times, Bu et al. (36) optimized the Tetra-PEG hydrogel technology by Tetra-PEG-NH2 and tetrazine-armed PEG succinimidyl succinate (Tetra-PEG-SS). The SS and SG hydrogels are initiated by an ammonolysis reaction, via a dual-syringe mechanism. The polymerization is complete once the hydrogel ester groups interact with the amino groups of tissue proteins. Notably, the structural difference between the SS and SG hydrogels leads to a significant increase in degradation time for the Tetra-PEG-SS hydrogel. An in vivo rabbit liver injury model was used to test the hemostatic abilities of both hydrogel technologies. The SS and SG hydrogels stopped bleeding within 30 s, whereas gauze needed 5 min. Strong adherence was noted with the SG hydrogels, contributed from inflammatory responses due to long retention times. The SS hydrogels, in comparison, displayed no adverse immune response. To assess the efficacy in a severe hemorrhaging model, Bu et al. (36) utilized a 10-mm-depth porcine spleen incisional wound, measuring blood loss time. In an alternative approach, Daristotle et al. (37) developed a poly(lactic-coglycolic acid) (PLGA) and PEG polymer-silica composite, used as a one-time sealant spray deposited as a fiber using solution blow spinning. Scanning electron microscope images demonstrated a direct relationship between particle size and fiber diameter, indicating a tunable system dependent on the silica nanoparticles. In an ex vivo model, burst pressure test results indicated that larger nanoparticle polymer composites experienced a burst pressure of 171 mm Hg, in comparison with a PLGA/PEG polymer with no nanoparticles (59 mm Hg). To assess any cytotoxicity, mouse fibroblasts were cultured in the presence of sealant extracts (concentration of 50 mg of fiber/500 mL of media). After 24 h, nanoparticles of sizes 20 nm (P-20), 180 nm (P-180), and 620 nm (P-620) demonstrated only a slight difference of cell viability in comparison with PLGA or PLGA/PEG alone. To investigate the hemostatic abilities, female Yorkshire swine were induced with six wounds, where the sealant was applied immediately after injury. P-620 composites displayed a clotting time similar to control, whereas P-20 composites decreased clotting time by 25%. In comparison with PLGA/PEG, the P-620 polymer sealant used as a spray-based application remained as a sealant and achieved hemostasis after 10 min for a majority of the resection area.
Due to the hydrophilic nature of PEG, PEG-based materials are known to have good biocompatibility and protein resistance [due to the size of the polymer and chain mobility (38)], which are major advantages. However, a small proportion of individuals may develop antibodies to PEG, and recent studies have noted the existence anti-PEG antibodies in some healthy individuals who had not previously received PEGylated therapeutics (39). Preexisting anti-PEG antibodies have been linked to allergic reactions for PEGylated therapeutics that led to early termination of clinical trials for PEGylated therapeutics (40). Therefore, the potential of immune reactions needs to be carefully monitored for any PEG-based hemostatic materials.
Layer-by-Layer Assembled Films
LbL self-assembly is a powerful tool that has been utilized to fabricate hemostatic coatings. LbL assembly involves the repeated adsorption of compounds with complementary functionalities to grow a multilayered film; these films often contain polyelectrolytes as one or more of the building blocks (41–43). A spectacular breadth of applications have been explored for LbL films over the last ~30 years following the pioneering work of Decher and colleagues (44–46), ranging from antibacterial coatings (47–51) to chemotherapy (52–55) to tissue engineering (56–58). There are many advantages to this thin film fabrication approach that make LbL assembly ideal for use in hemostatic technologies. First, LbL assembly can be used to functionalize a range of existing materials at various length scales (e.g., nanoparticles, microneedles, cells, scaffolds, sponges, and gauze) while maintaining the desirable properties of the underlying substrate. Many LbL fabrication approaches have been developed over the years, from spin (59) and spray LbL (60) assembly to the more recent microfluidic LbL assembly (61); these approaches have been optimized to coat the substrates with the desired film components. Second, traditional LbL assembly is carried out under aqueous conditions, which are benign to many sensitive film components, and a range of molecules and macromolecules have been incorporated into these films. Finally, assembly conditions, including temperature, pH, and ionic strength, can be tuned to maximize loading of bioactive film components, including hemostats.
Shukla et al. (62) reported the first hemostatic LbL film in 2012. The film architecture took advantage of the hydrogen bonding capacity of tannic acid, a plant-derived polyphenol, and thrombin (clotting factor IIa) to build a hydrogen bonding–driven LbL film lacking any polymeric components. This multilayered film was applied to a commercial gelatin sponge used in bleeding control via spray LbL assembly. Using as few as 10 bilayers, (thrombin/tannic acid)10 film-coated sponges were able to arrest bleeding in less than 60 s in a porcine spleen injury model. Following this seminal work, several innovative approaches to using LbL films to promote hemostasis have been investigated. The same film components utilized by Shukla et al. were applied to a chitosan sponge substrate using the traditional dip LbL assembly approach. As expected, these films were found to have excellent dose-dependent whole blood clotting ability in vitro. Most importantly, it was demonstrated that this LbL film stabilized the thrombin protein, with 93% and 71% of thrombin activity remaining, compared with 54% and 0% for non-film-incorporated thrombin powder at 7 and 31 days, respectively (63). This preservation of bioactivity has been noted in many LbL films, including those containing other proteins [such as growth factors (64) and pharmaceutical compounds, e.g., antibiotics (65)], and appears to be a function of stabilizing interactions with complementary film components.
LbL films have also been developed free of any proteins present in the natural coagulation cascade, which is particularly useful in conditions such as coagulopathy. A recent example involved the incorporation of a hemostatic self-assembling peptide, RADARADARADARADA (RADA16-I), in spray LbL assembled films at conditions promoting peptide nanofiber assembly. Compared with direct RADA16-I coatings, these LbL films using complementary polyelectrolytes such as dextran sulfate and HA maximized loading and coverage of the peptide on the gauze and gelatin sponge substrates utilized. Upon incubation with whole blood, these films led to the formation of nanofiber clots (Figure 3), as has been observed for RADA16-I. Film release solutions were also able to form nanofiber-based clots with red blood cells, an ability that was maintained even after film storage for 5 months at an elevated temperature of 60°C, again demonstrating the stabilizing nature of LbL films. Finally, this work confirmed that gauze coated with (RADA16-I/HA)200 LbL films yielded rapid hemostasis compared with that of uncoated gauze in a porcine skin puncture injury model, with hemostasis occurring in 2 min or less after a single application of the film-coated gauze (66). Komachi et al. (67) also demonstrated another clotting factor–free approach to LbL hemostasis, using an LbL film to increase the strength of tissue adhesive nanosheets by increasing resistance to bursting during bleeding. Their unique hemostatic material was fabricated by successive spin coating of poly(L-lactic acid) (PLLA) and a mixture of sodium alginate and poly(vinyl alcohol) (PVA), followed by sodium alginate gelation using Ca2+, heat sealing, and subsequent removal of the sodium alginate and PVA from these films. The resulting structure was a stable film consisting of multiple PLLA nanosheet layers. This material demonstrated reduced tissue adhesion and hemostatic activity comparable to the commercially used TachoSil fibrin sealant in a rat liver injury model.
Figure 3.
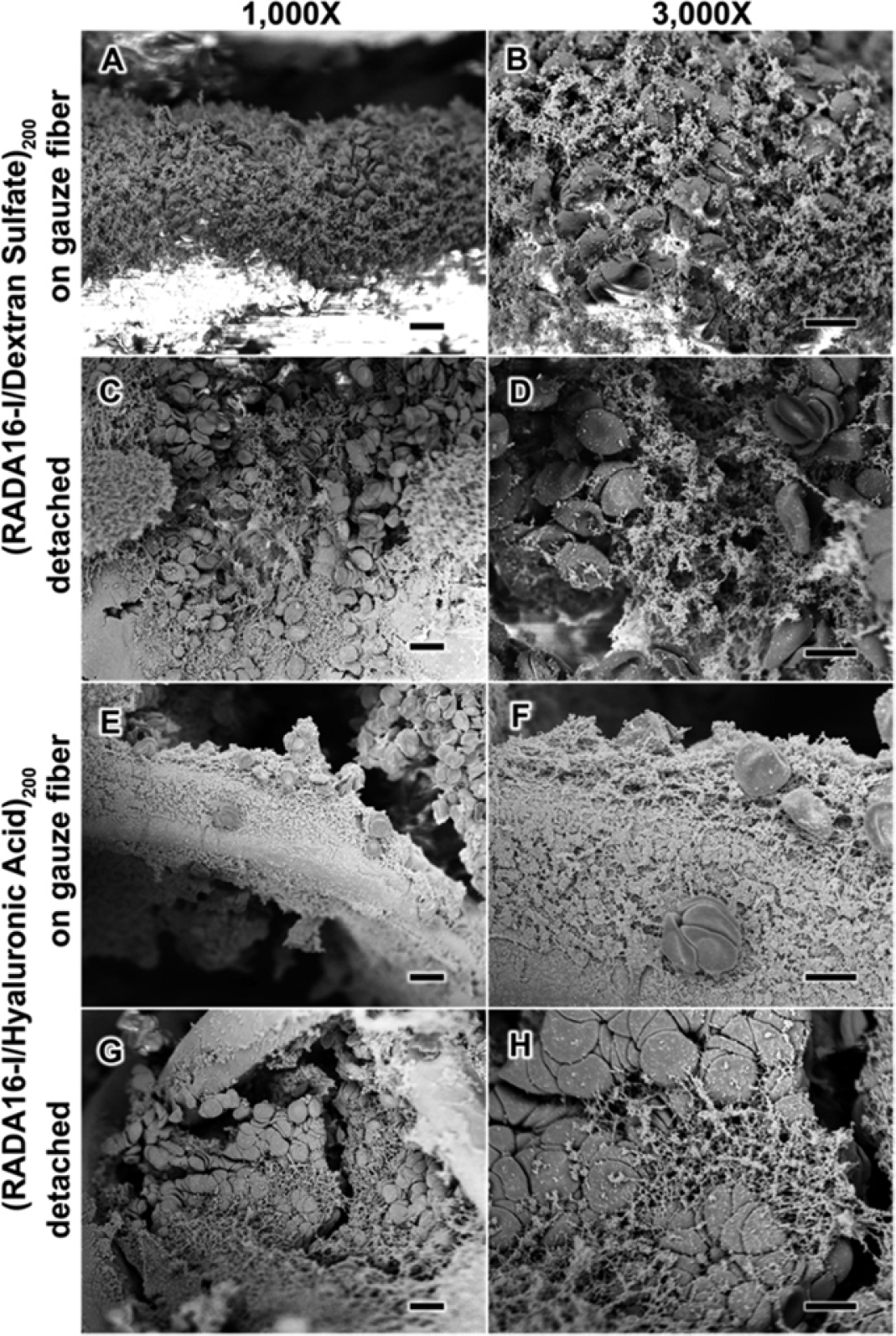
Surface characterization of anticoagulated whole blood upon contact with spray LbL assembled films. Scanning electron microscopy characterization of (RADA16-I/DS)200 films (a–d) and (RADA16/HA)200 films (e–h) in contact with anticoagulated whole blood shows that the blood components interact with the films both on the gauze fibers (a, b, e, and f) and once the films are detached from the gauze (c, d, g, and h). Figure adapted with permission from Reference 66, Copyright 2015, American Chemical Society. Abbreviations: DS, dextran sulfate; HA, hyaluronic acid; LbL, layer-by-layer; RADA16-I, RADARADARADARADA.
The versatility of LbL films has enabled researchers to explore fabrication of hemostatic coatings that can exhibit multifunctional properties. In particular, there has been a large focus on combining hemostatic and antibacterial activity, due to the common coexistence of bleeding and infection in traumatic wounds. Antibacterial properties have been imparted using several approaches, from antibiotics to natural compounds to disinfectants. In work by Hsu et al. (68), thrombin and tannic acid films were assembled via dip LbL assembly on an LbL film composed of poly-L-lysine (PLL) and an antibiotic conjugate of poly(β-L-malic acid) and vancomycin (PMLA-vanco). This (PLL/PMLA-vanco)40.5 + (thrombin/tannic acid)25 film exhibited a rapid release of thrombin over minutes, while vancomycin release persisted beyond 24 h. These composite films demonstrated excellent in vitro clotting and antibacterial efficacy and generated a robust fibrin network upon the introduction of fibrinogen, which was absent during incubation with the antibiotic film alone. In another study, tannic acid was used as the primary antibacterial component. Films were assembled by adsorption of the tannic acid on chitin nanofiber sponges, followed by a Ca2+ layer, forming an octahedral complex with the tannic acid; excessive Ca2+ at the surface enabled further adsorption of tannic acid at the next LbL adsorption step. Ca2+ (human coagulation factor IV) is known to participate in multiple processes in the coagulation cascade. Although not as potent as several common antibiotics, the (tannic acid/Ca2+) film–coated sponges exhibited dose-dependent inhibition of both gram-positive S. aureus and gram-negative E. coli. The coated sponges also exhibited excellent hemostatic behavior in a rat tail and rat liver injury model, with significant reduction in hemostasis time and blood loss compared with those of noncoated sponges. It was hypothesized that the highly porous sponge first enabled a high level of red blood cell capture, following which Ca2+ release from the LbL coating enhanced platelet activation, yielding rapid hemostasis (69).
The underlying substrate is often the primary hemostatic component, while the LbL film provides another functionality. For example, Che et al. (70) coated a hemostatic sodium alginate and gelatin composite sponge using spray LbL assembly of the disinfectant poly(hexamethylene biguanide) hydrochloride (PHMB) and HA. Here, the LbL film was only incorporated for its antibacterial properties; it was demonstrated that in the presence of bacteria that produce hyaluronidases, such as S. aureus, the LbL film caused a significant inhibition of bacterial growth. The proposed mechanism entails bacterial hyaluronidase–mediated degradation of the HA layer, thereby exposing the antibacterial agent PHMB. These LbL functionalized sponges exhibited excellent hemocompatibility and viability of murine fibroblasts as well as superior hemostatic activity in a mouse liver injury model compared with those of commercial gelatin sponges.
Along with a focus on combining hemostatic and antibacterial characteristics, the ability of LbL films to increase both wound healing and tissue repair along with hemostasis following injury is also of significant interest. Zheng et al. (71) took advantage of the attractive features of a range of a biological polymers to develop a highly biocompatible, hemostatic wound dressing based on a multilayered-film functionalized cotton gauze. The gauze was functionalized with carboxymethyl chitosan, followed by the electrostatically driven adsorption of gelatin, which is commonly used in wound-healing materials. Finally, an alginate gel was formed on top of this bilayer through the introduction of alginate and Ca2+. Use of this functionalized gauze resulted in a significant decrease in blood loss and hemostasis time after application in both mouse liver injury and tail amputation models and good wound-healing performance in a mouse full-thickness skin defect with nearly full wound closure by ~2 weeks. In another example, Liu et al. (72) developed LbL-coated microparticles for bone injury repair. They coated negatively charged porous starch microparticles with bilayers of quaternized starch and tannic acid via electrostatic interaction–driven assembly. Mouse cancellous bone defects treated with (quaternized starch/tannic acid)2. LbL film–coated microparticles had lowered intractable bleeding following injury and better bone repair than defects treated with bone wax. This treatment was also examined in a proof-of-concept tibia defect model in beagles that showed the superior bone repair induced by LbL microparticles compared with that of bone wax.
Multifunctional LbL technologies have the potential to extend far beyond the combination of hemostatic activity with antibacterial activity, wound healing, and tissue repair. In one such example, Zhang et al. (73) developed an LbL coating for the destruction of chemical warfare agents, specifically sulfur mustard, which was coated on a carboxymethylated chitosan non-woven fabric. This coated material exhibited a dose-dependent decontamination of sulfur mustard, without affecting the hemostatic abilities of the chitosan fabric. We have only just scratched the surface of possibilities for multifunctional LbL-based hemostatic technologies. Given the numerous advances that have been made in the field of LbL biomaterials, the application of these films for the treatment of injuries is expected to continue growing.
INJECTABLE MATERIALS
While numerous topical hemostats, surgical sealants, adhesives, and dressings are in use clinically, with some examples discussed above, many of these materials are not effective in treating internal bleeding and/or noncompressible bleeding. Transfusion of blood products, including whole blood or isolated blood components (platelets, fibrinogen concentrate, etc.), is typically given in these situations. However, blood products have numerous properties that limit their practicality for emergency medicine, including limited donor supply, limited shelf life, and cold storage requirements. While recent advances in blood product storage methods have improved blood product shelf life, their use in emergency medicine is still hampered due to the difficulty in transporting large volumes of blood products to austere environments. Additionally, while the risks are small, blood products still have the potential to transmit disease.
For this reason, the last decade or so has seen incredible innovation in the development of novel injectable hemostatic materials. Many of these materials engage the natural clotting cascade to promote wound-specific clot formation at the sites of injury. A range of technologies, including nanoparticle-based synthetic blood products, other nanoparticle technologies, and injectable polymers, have recently been described. This section provides an overview of recent advances in these areas (Figure 4).
Figure 4.
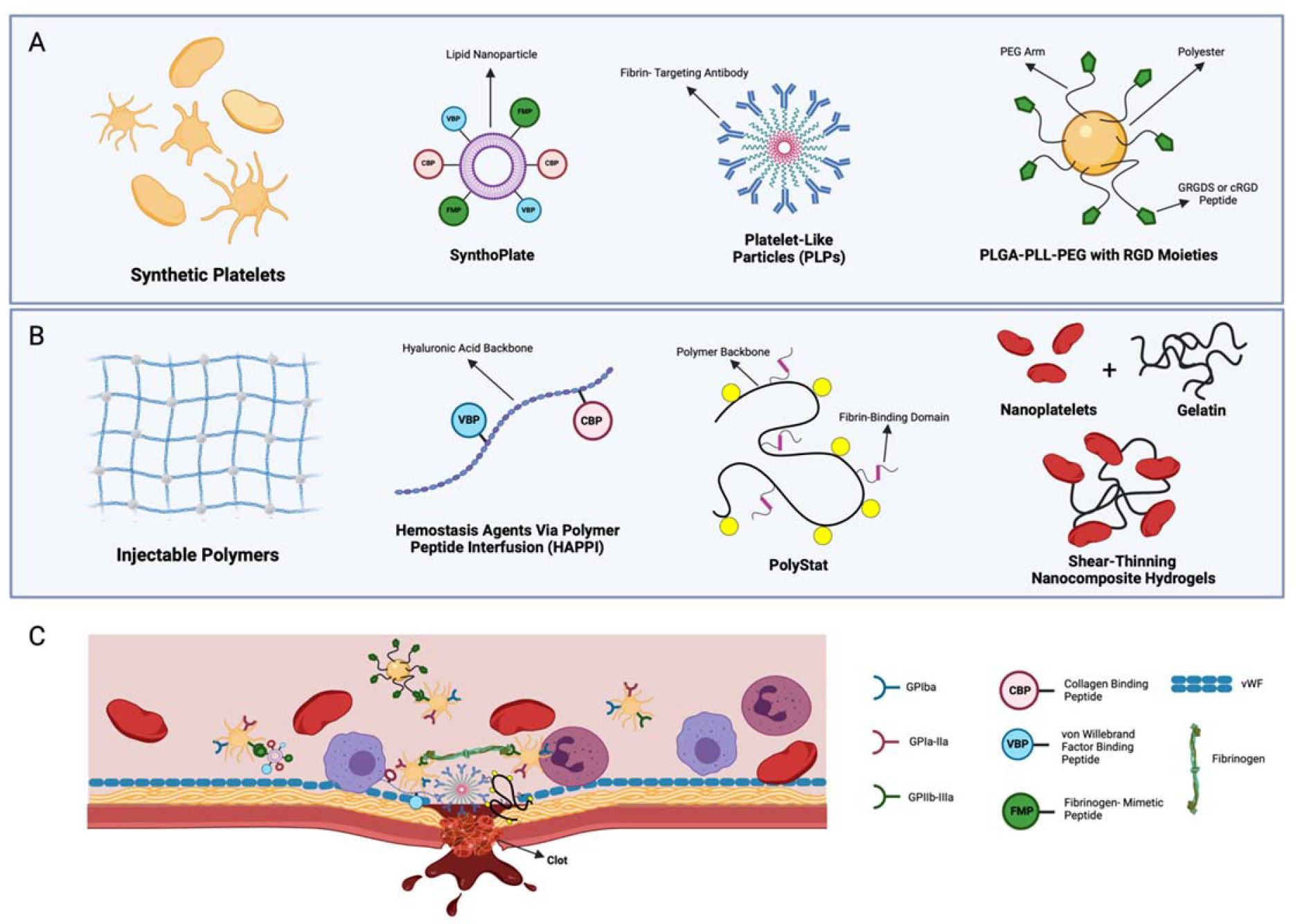
Injectable materials overview. (a) The design of applications discussed for synthetic platelets. SynthoPlate utilizes a lipid nanoparticle decorated with binding domains (VBPs, CBPs, and FMPs) to allow adherence to site of injury and enhance platelet aggregating via the binding of FMP and active GPIIb/IIIa. Similarly, hemostatic nanoparticles developed to reduce infusion reaction use a polyester degradable core, decorated with GRGDS or a cRGD peptide. PLPs are defined by a deformable pNIPAM ULC microgel coupled to a fibrin-targeting antibody. (b) In a similar manner as the technologies presented in panel a, HAPPI utilizes a hyaluronic acid backbone conjugated to VBP and CBP. PolySTAT is designed from a polymer backbone [p(HEMA-co-NHSMA)], to mimic FXIIIa-mediated fibrin stabilization, by grafting fibrin-binding peptides (74). Shear-thinning nanocomposite hydrogels are prepared from synthetic silicate nanoplatelets and type-A porcine skin gelatin. (c) The mechanism of each hemostatic agent. SynthoPlate and HAPPI utilize binding domains to attach to endothelial matrix proteins. PLPs and PolySTAT induce hemostasis by cross-linking the fibrin fibers within a clot. Notably, the low cross-linking density of ULC microgels allows for clot retraction after fibrin binding. The mechanism of shear-thinning nanocomposite hydrogels focuses on highly charged nanoparticles to induce hemostasis from concentrating clotting factors. PLGA-PLL-PEG conjugated with cRDG or GRGDS binds to receptors on activated platelets (e.g., GPIIb/IIIa. Figure adapted from images created with BioRender.com. Abbreviations: CBP, collagen-binding peptide; FMP, fibrinogen-mimetic peptide; GP, glycoprotein; HAPPI, hemostatic agent via polymer peptide infusion; PEG, polyethylene glycol; PLGA, poly(lactic-coglycolic acid); PLL, poly-L-lysine; PLP, platelet-like particle; pNIPAM, poly(N-isopropylacrylamide); ULC, ultralow cross-linked; VBP, vWF-binding peptide; vWF, von Willebrand factor.
Synthetic Platelets
During uncontrolled hemorrhaging, immediate hemostatic intervention is critical to patient survival. Current clinical strategies for halting severe bleeding include noncompressible bandages, tourniquets, transfusion of blood products, and concentrated clotting factors such as prothrombin complex concentrate and recombinant factor VIIa (NovoSeven®). Platelet transfusion is an important part of these strategies. However, shortage of platelet supply, short shelf life for donated platelets, and storage requirements demonstrate a need for platelet substitutes. To that end, a variety of platelet-mimetic particles have been developed to replicate the biochemical interactions and flexibility of native platelets. Beyond platelets, red blood cell and some white blood cell mimics have been designed and reviewed (3). Due to the major role of platelets in hemostasis, this section focuses on various strategies of synthetic nanoparticles that promote injury-specific binding, achieve hemostasis, stabilize clot networks, and facilitate clot retraction to mimic key aspects of natural platelets in hemostasis and subsequent tissue repair.
Prothrombin: a protein produced by the liver that is converted to thrombin during coagulation
To replicate the hemostatic abilities of natural platelets, many synthetic platelet designs include the use of nanoparticle platforms decorated with binding elements that interact with various stages of the clotting cascade including particles decorated with von Willebrand factor (vWF) peptides, fibrinogen-mimetic peptides (FMPs), fibrin-binding single-domain variable fragment (sdFv) antibodies, and/or collagen peptides (75). Bioconjugation allows for nanoparticles to localize at the injury site and promote hemostatic functions including platelet activation, platelet aggregation, and/or catalyzation of fibrin polymerization. Anselmo et al. (76) developed platelet-like nanoparticles (PLNs) that incorporate the discoidal shape and flexibility of native platelets to mimic targeting at vascular injury sites. When a microfluidic device was used to mimic the antigen-antibody system, under shear stress PLNs adhered to the surface as individual particles and aggregates. In vitro, PLNs decorated with vWF-binding peptides (VBPs), collagen-binding peptides (CBPs), and linear FMPs in the presence of human platelets demonstrated the ability to form a hemostatic plug. Sen Gupta and colleagues (77, 78) have described the development of a biocompatible liposomal platform decorated with VBP, CBP, and FMP SynthoPlate, which demonstrated that intravenous administration in injured pigs resulted in a reduction of blood loss rate and enhanced survival. These studies were performed within the “golden hour” of treatment, which is defined in clinical settings as 60 min after a traumatic injury (79) (Figure 5).
Figure 5:
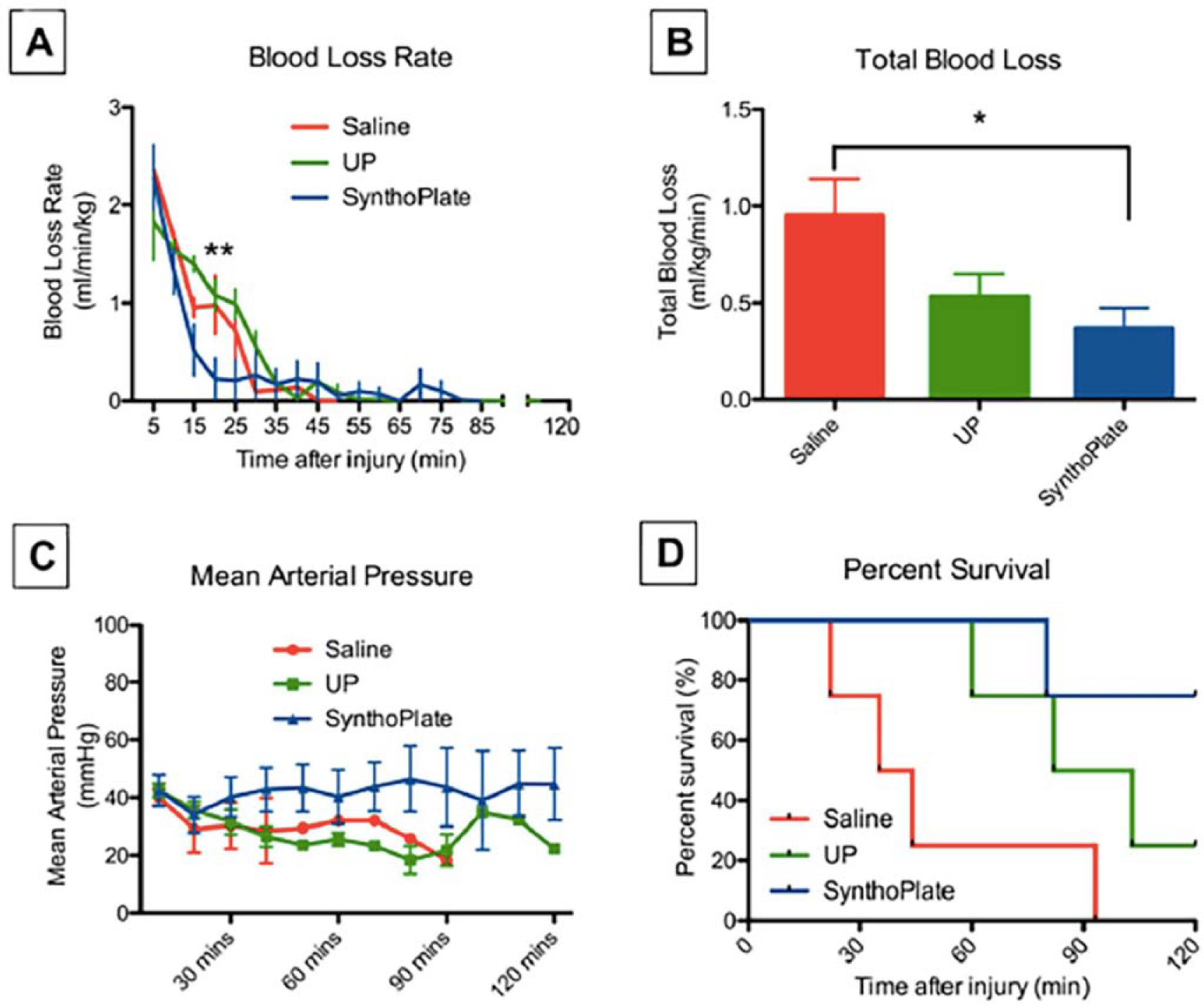
SynthoPlate particles reduce blood loss and enhance survival in a porcine model of trauma. Hemostatic efficacy analysis in injured pigs shows that (a) pigs administered SynthoPlate had a reduced blood loss rate (blue) compared with that of pigs treated with saline (red) and UP (green), especially within first 30 min of posttreatment administration, where the blood loss rate in the SynthoPlate-treated pigs was significantly lower than those treated with UP (control particles) and saline (**p < 0.01); (b) SynthoPlate administration in pigs also resulted in significantly lower total blood loss compared with that of pigs administered saline (*p < 0.05); (c) SynthoPlate administration in pigs resulted in maintenance and stabilization of a higher average mean arterial blood pressure over time (data points shown for every 10 min); and (d) SynthoPlate administration in pigs resulted in a significant enhancement of survival, with 100% surviving the first 60 min (the “golden hour”) and 75% surviving an additional 60 min (p < 0.05), compared with that of pigs given saline (25% survival by 60 min and 0% survival by ~90 min) or UP (50% survival by 90 min and 25% survival by 120 min). Figure adapted from Reference 79 (CC BY 4.0). Abbreviation: UP, unmodified particles.
von Willebrand factor (vWF): a glycoprotein that binds to FVIII, a platelet surface protein, and influences platelet adhesion to the damaged endothelium; receptors for vWF include GPIbα and GPIb-IX-V
In an alternative approach, Brown and colleagues (80–82) developed ultralow cross-linked (ULC) poly(N-isopropylacrylamide) (pNIPAM) nanoparticles coupled to a fibrin-binding sdFv antibody capable of promoting clot formation after traumatic injury in rodent models and inducing clot retraction after hemostasis, increasing clot density and stiffness The high deformability of pNIPAM nanoparticles coupled with high fibrin affinity imparted by the fibrin-binding sdFv allows for mechanical deformability in clots resembling the actin-myosin machinery of native platelets, leading to clot retraction. In vitro studies displayed a hemophilic clot model in the presence of platelet-like particles (PLPs) and showed an increase in fibroblast migration within the defect area, and in vivo experiments demonstrate that this correlates to improved wound healing when applied to topical wounds. This PLP technology has been further developed to incorporate nanosilver, to address antimicrobial activity (see the sidebar titled Sidebar 1), while retaining microgel deformability and platelet-mimetic functions (83).
SIDEBAR 1: ANTIMICROBIAL HEMOSTATIC MATERIALS
Novel research proposes hemostatic agents with antibacterial properties that demonstrate excellent biocompatibility while retaining rapid hemostasis. For example, antimicrobial versions of the PLPs discussed in the section titled Synthetic Platelets have been described. PLPs incorporated with antimicrobial metals were found to retain the original hemostatic PLP functionality with additive antimicrobial properties (83, 93). Nanosilver-containing PLPs were shown to retain microgel deformability following a composite design and significantly reduce bacterial growth in comparison with that of PLPs alone and ampicillin. Additionally, Ag-ULC microgels increased wound closure rates, an outcome that is hypothesized to be due to reduced inflammation and the promotion of fibroblast migration. In terms of topical materials, anti-infection dressings and sponges developed from HA have been described. Zhu et al. (94) displayed the hemostatic ability, wound-healing properties, and sustained release kinetics of a drug-loaded chlorhexidine diacetate, aminoethyl methacrylate HA, and methacrylated methoxy polyethylene glycol hydrogel. In another example, Che et al. (70) designed a hemostatic dressing constructed from spray LbL assembly of PHMB and HA on a sodium alginate/gelatin sponge. These novel approaches address severe hemorrhaging and provide assistance in combatting infection while achieving hemostasis.
While most work in this area has focused on the ability of synthetic platelet technologies to stop hemostasis following trauma, emerging areas of interest include the evaluation of other outcomes following trauma. For example, VandeVord and colleagues (84, 85) designed hemostatic nanoparticles loaded with dexamethasone (hDNPs) to investigate adverse effects from a body blast trauma, focusing on reducing anxiety behavior following a blast injury. It was found through a rodent behavioral test that hDNPs mitigated anxiety behavior. Previous research has demonstrated that rodents with anxiety-like behavior spend less time within the center of an open-field-testing box. After treatment with hDNPs, the track pattern for these rodents displayed higher exploration within all regions of the arena. This work highlights the potential utility of synthetic platelets in modulating outcomes beyond promoting hemostasis.
Promising developments exist in platelet substitutes that promote hemostasis, demonstrated by binding to activated platelets, increasing survival for in vivo trauma models, and inducing clot retraction for tissue repair and remodeling after cessation of bleeding. However, most synthetic platelet technologies are still in the relatively early stages of development, with many of these technologies having only been validated in small-animal models. Several technologies have progressed to larger-animal studies, with initial promising results, which are critical steps toward translation. However, critically important work by Lavik and colleagues (86) has demonstrated that certain polymeric designs of hemostatic nanoparticles have the potential to cause infusion-associated complement response in porcine models, indicating that complement responses should be carefully characterized and considered in synthetic platelet design moving forward. Additionally, future studies in this area should focus on (a) developing in vivo studies for understanding the hemostasis response in severe trauma models and (b) creating a scale-up design for synthesizing synthetic platelets. Also, to date, most in vivo studies analyzing synthetic platelet outcomes have had terminal outcomes. Future studies should focus on longer term responses following administration and potential immune response from any degradation products.
Injectable Polymers
Beyond synthetic platelets, other injectable strategies for inducing clotting and promoting hemostasis for internal injuries, including various injectable polymers and other nanoparticle systems, have been described. Requirements for such therapeutics include quick control of bleeding, solidification within the wound environment, high biocompatibility, and a simple design for clinical applications (87).
As described in previous sections, during hemostasis the proteolytic cleavage of fibrinopeptides by thrombin forms fibrin monomers. The aggregation of fibrin fibers is essential in clot formation and stability. Pun and colleagues (74) utilized the architectural structure produced by fibrin networks to develop an injectable synthetic polymer binding to fibrin monomers, termed PolySTAT, to enhance clot strength. In vitro, confocal imaging with fluorescently labeled fibrinogen confirmed the integration of PolySTAT within the fibrin fibers. During comparison of the turbidities of fibrin, PolySTAT demonstrated great turbidity, indicating changes to the fibrin nanostructure. To investigate the potential use of PolySTAT for patients with low fibrinogen levels in vitro, various concentrations of fibrin clots were formed with and without PolySTAT. For all concentrations, there was a significant increase in storage moduli, displaying the ability of PolySTAT to reverse the effects of fibrinogen depletion. In vivo, PolySTAT was intravenously administered in a femoral artery incision, and the total blood loss was measured from gauze collection. The PolySTAT treatment groups displayed a 100% survival rating (n = 3), in comparison with the PolySCRAM (nonbinding scrambled polymer), rat albumin, and human coagulation factor XIIIa groups. In another example, Mitragotri and colleagues (88) designed a hemostatic agent via polymer peptide infusion (HAPPI) by conjugating CBP and VBP to an HA backbone. This combination conjugation mediates the binding of exposed collagen, and vWF that has been immobilized on activated platelets, to the polymer peptide. Analysis of the pharmacokinetics and distribution of HAPPI demonstrated a circulation time of 1 h, with concentration in the liver and spleen after 6 h. To investigate hemostatic potential in vivo, a mouse tail vein laceration model was used to measure blood loss for HA alone, HA-VBP, HA-CBP, and HAPPI. In comparison with HA alone, there was a significant reduction in blood loss for HAPPI and HA-VBP (99% and 97%, respectively). Interestingly, using fluorescence-activated cell sorting, HAPPI was noted to selectively bind to activated platelets at a higher affinity in comparison with controls, potentially due to the interaction of the polymer peptide to GPIIb/IIIa receptors.
Lokhande et al. developed an injectable-based polymer, incorporating nanosilicates and kappa carrageenan (kCA) to form mechanically strong hydrogel networks (88a). Previous studies have demonstrated the in vivo ability of nanosilicates to improve hemostasis, but this synthetic clay limited the mechanical stiffness of the hydrogel network. At specific temperatures, kCA undergoes gelation, enhancing the mechanical stiffness of the injectable hydrogels. To investigate the physiochemical characteristic, the combination nanocomposite (kCA-nanosilicate) hydrogels were subjected to varying shear rates (0.1–100 s−1) and strain. There was a significant increase in shear-recovery ability and prepolymer viscosity when pure kCA was compared to kCA with the addition of nanosilicates. To analyze the blood-clotting ability of kCA-nanosilicate hydrogels, bovine blood and coagulation-activated blood were incubated with 1% kCA and 1% kCA–2% nanosilicate. Compared with previous literature values for clotting times of bovine blood (8 min), 1% kCA–2% nanosilicate displayed a reduction to less than 3 min. With the negative potential of kCA, this is hypothesized to influence the activation of platelets and Hageman factor (FXII). Opportunities exist for tunability of the kCA-nanosilicate hydrogels by embedding therapeutic biomolecules to aid in wound healing or assist antimicrobial performance. Utilizing the blood coagulation properties of synthetic silicates and the ECM-mimetic ability of gelatin, Khademhosseini, Olsen, and colleagues (89) investigated gelatin and silicate nanoplatelets for hemostatic potential. Whole blood was incubated with the surface of nanocomposites on a 96-well plate to investigate the in vitro response. With an increase in the concentration of nanoplatelets 9NC25, 9NC50, and 9NC75 (describing the ratio to total weight and total solid weight of nanocomposites), all treatment groups exhibited a reduction in clotting time. To investigate the ability of nanocomposites to enhance platelet aggregation, channels with nanocomposites or control (gelatin or plastic) surfaces were filled with blood. For both platelet-rich and platelet-poor plasma samples, aggregates influenced by the charged surfaces were formed around silicate structures. To investigate the hemostatic potential in vivo, a 1-cm dorsal skin incision was induced where nanocomposites were injected or QuikClot was implanted. Neither material resulted in an inflammatory response, and both materials were easily detected within the wound environment. Also, a liver bleeding model demonstrated that both samples were able to stop severe hemorrhaging within seconds. These results display the ability of gelatin silicate nanocomposites to promote hemostasis.
The approaches discussed thus far have addressed injectable materials promoting the blood coagulation cascade, but little research has focused on identifying the bleeding source. Olsen et al. (90) established therapeutically active PLGA-b-PEG nanoparticles to assist in diagnosis for severe injury models. Functionalized particles, with GRGDS moieties, displayed an increase in platelet aggregation in comparison with control (adenosine diphosphate and platelet-rich plasma) and unfunctionalized peptides. In vivo, GRGDS nanoparticles injected intravenously following a liver resection significantly increased survival rates among rodents. The addition of a tracer, hydrophobic or hydrophilic, did not interfere with survival rates. Diagnosis was successful using near-infrared dye–labeled nanoparticles via confocal microscopy and CT imaging. These studies confirm the usability of GRGDS functionalized nanoparticles as diagnostic tracers and hemostatic agents.
GRGDS: a peptide sequence involved in many cell integrin interactions; this sequence is found in the cell-binding region of fibronectin, an extracellular matrix protein involved in cell adhesion and migration
In a different approach, Kastrup and colleagues (91) established self-propelling particles that utilize propulsion forces to deliver therapeutic agents upstream against blood flow. This design consists of calcium carbonate microparticles that effervesce strongly when in contact with blood. In vivo self-propelling particles demonstrated efficiency in halting hemorrhaging, without causing thrombosis or toxicity. These particles can be loaded with thrombin and tranexamic acid. These self-propelling formulations of thrombin and tranexamic acid have shown efficacy in stopping bleeding in a surgical carotid injury sheep model (92) (Figure 6).
Figure 6.
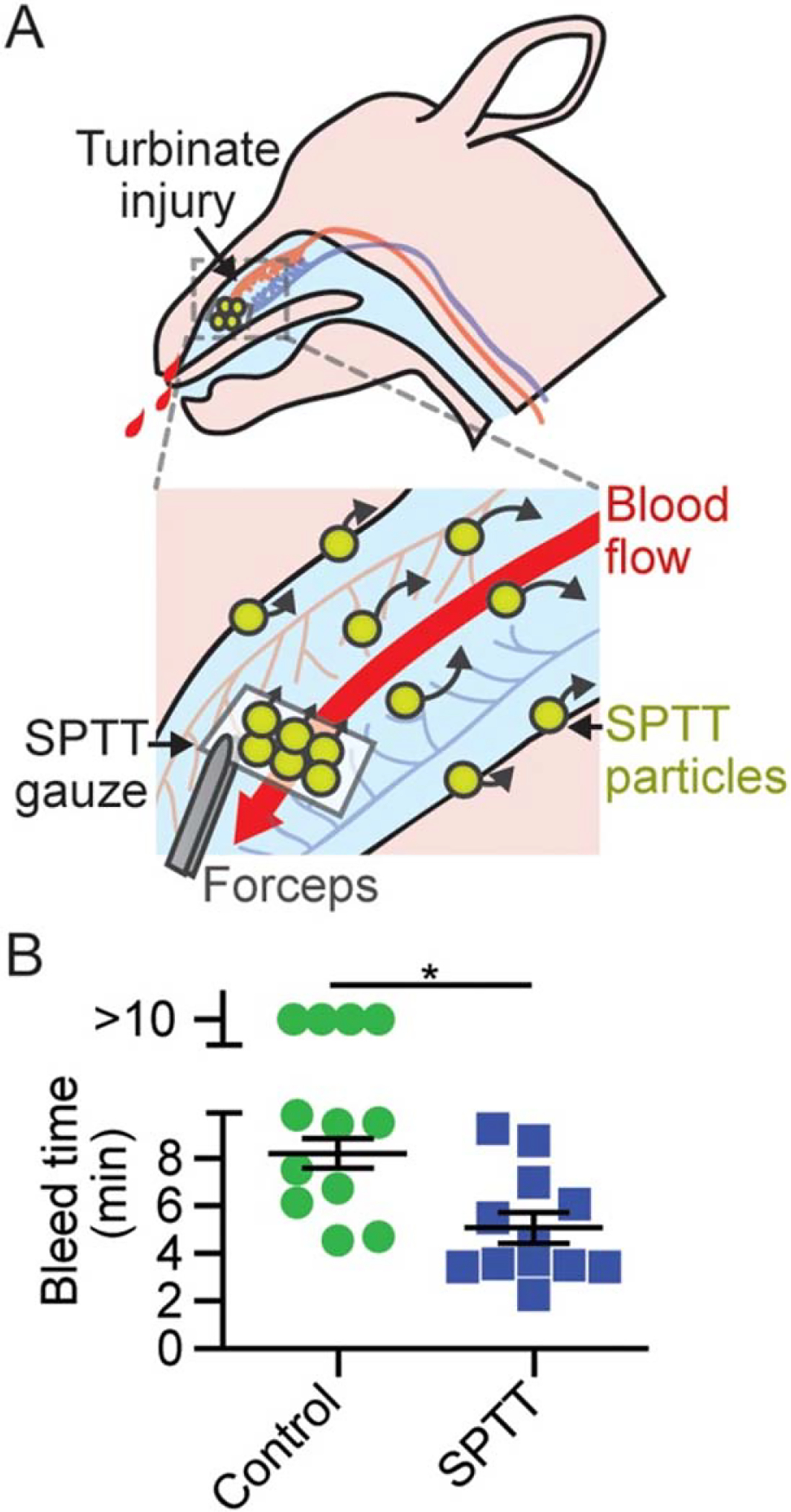
SPTT decreases bleed times following turbinate injury. (a) Paranasal application of SPTT-loaded gauze and rapid delivery of cargo via SPTT particles through blood flow. (b) Bleed times following turbinate injury and application of hemostatic agent (n = 12, *P < 0.01). Error bars represent standard error of the mean. Figure adapted with permission from Reference 92. Abbreviation: SPTT, self-propelling formulation of thrombin and tranexamic acid.
Injectable polymers and nanoparticles that interface with coagulation can be used to enhance hemostatic performance and clot formation. The technologies presented above exist as additive measures to promote coagulation and provide diagnostic techniques during internal bleeding. Future studies should focus on addressing the biodistribution of these materials over time, developing in vitro and in vivo models to understand the long-term effects, and increasing the versatility of applications [e.g., removing PolySTAT during surgical repair of injury (74)].
CONCLUSIONS AND FUTURE OUTLOOK
In this review, we discuss both topical and injectable biomaterials for promoting hemostasis after trauma. Topical materials are more developed and many clinically used materials exist. However, numerous opportunities exist for design improvement, though the specifics of these opportunities depend on the material. For example, LbL assembled coatings have been applied to many of these topical materials to improve outcomes. Despite their widespread clinical use, topical hemostatic materials are limited in their utility for treating internal bleeding, particularly for noncompressible wounds. Therefore, great innovation has been seen in recent years in the area of injectable biomaterials for treating bleeding. However, most of these technologies are still in the early stages of development, and much remains to be determined regarding the safety and efficacy of these materials in humans. Nonetheless, early studies in large-animal models are demonstrating the promise of injectable materials in stopping bleeding after trauma, and it is to be hoped that these studies will motivate clinical trials in the coming years. Overall, both topical and injectable hemostatic biomaterials have the potential to greatly improve patient outcomes and improve survival after trauma.
ACKNOWLEDGMENTS
Funding for this work was provided by R01HL146701 and the National Science Foundation DMR CAREER 1847488 (A.C.B.). Funding for this work from the Office of Naval Research N000141712120 (A.S.) is also gratefully acknowledged.
Footnotes
DISCLOSURE STATEMENT
Dr. Brown is co-founder and CEO of Selsym Biotech Inc, a start-up company focused on developing novel wound care technologies. Dr. Shukla and Ms. Simpson are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Kauvar DS, Lefering R, Wade CE. 2006. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J. Trauma Acute Care Surg. 60(6):S3–11 [DOI] [PubMed] [Google Scholar]
- 2.Dickinson LE, Gerecht S. 2016. Engineered biopolymeric scaffolds for chronic wound healing. Front. Physiol. 7:341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thiruvoth FM, Mohapatra DP, Kumar D, Chittoria SRK, Nandhagopal V. 2015. Current concepts in the physiology of adult wound healing. Plast. Aesthetic Res. 2:250–56 [Google Scholar]
- 4.Ozgok Kangal MK, Regan JP. 2022. WoundHealing. Treasure Island, FL: StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK470443/ [Google Scholar]
- 5.Wallace HA, Basehore BM, Zito PM. 2022. Wound Healing Phases. Treasure Island, FL: StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK470443/ [PubMed] [Google Scholar]
- 6.Sen Gupta A 2017. Bio-inspired Nanomedicine Strategies for Artificial Blood Components. Wiley Inter-discip. Rev. Nanomed. Nanobiotechnol. 9(6):e1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tompeck AJ, Gajdhar AUR, Dowling M, Johnson SB, Barie PS, et al. 2020. A comprehensive review of topical hemostatic agents: the good, the bad, and the novel. J. Trauma Acute Care Surg. 88(1):e1–21 [DOI] [PubMed] [Google Scholar]
- 8.Hu Z, Zhang D-Y, Lu S-T, Li P-W, Li S-D. 2018. Chitosan-based composite materials for prospective hemostatic applications. Mar. Drugs 16(8):273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He Q, Gong K, Ao Q, Ma T, Yan Y, et al. 2013. Positive charge of chitosan retards blood coagulation on chitosan films. J. Biomater. Appl. 27(8):1032–45 [DOI] [PubMed] [Google Scholar]
- 10.Nainggolan I, Nasution TI, Putri SRE, Azdena D, Balyan M, Agusnar H. 2018. Study on chitosan film properties as a green dielectric. IOP Conf. Ser. Mater. Sci. Eng. 309:012081 [Google Scholar]
- 11.Kunio NR, Riha GM, Watson KM, Differding JA, Schreiber MA, Watters JM. 2013. Chitosan based advanced hemostatic dressing is associated with decreased blood loss in a swine uncontrolled hemorrhage model. Am. J. Surg. 205(5):505–10 [DOI] [PubMed] [Google Scholar]
- 12.Wang X, Zheng C, Wu Z, Teng D, Zhang X, et al. 2009. Chitosan-NAC nanoparticles as a vehicle for nasal absorption enhancement of insulin. J. Biomed. Mater. Res. B Appl. Biomater 88B(1):150–61 [DOI] [PubMed] [Google Scholar]
- 13.Huang Y, Feng L, Zhang Y, He L, Wang C, et al. 2017. Hemostasis mechanism and applications of N-alkylated chitosan sponge. Polym. Adv. Technol. 28(9):1107–14 [Google Scholar]
- 14.Hattori H, Amano Y, Nogami Y, Takase B, Ishihara M. 2010. Hemostasis for severe hemorrhage with photocrosslinkable chitosan hydrogel and calcium alginate. Ann. Biomed. Eng. 38(12):3724–32 [DOI] [PubMed] [Google Scholar]
- 15.Leonhardt EE, Kang N, Hamad MA, Wooley KL, Elsabahy M. 2019. Absorbable hemostatic hydrogels comprising composites of sacrificial templates and honeycomb-like nanofibrous mats of chitosan. Nat. Commun. 10(1):2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhong YT, Hu HY, Min NN, Wei YF, Li XD, Li XR. 2021. Application and outlook of topical hemostatic materials: a narrative review. Ann. Transl. Med. 9(7):577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuan H, Chen L, Hong FF. 2020. A biodegradable antibacterial nanocomposite based on oxidized bacterial nanocellulose for rapid hemostasis and wound healing. ACS Appl. Mater. Interfaces 12(3):3382–92 [DOI] [PubMed] [Google Scholar]
- 18.Udangawa RN, Mikael PE, Mancinelli C, Chapman C, Willard CF, et al. 2019. Novel cellulose-halloysite hemostatic nanocomposite fibers with a dramatic reduction in human plasma coagulation time. ACS Appl. Mater. Interfaces 11(17):15447–56 [DOI] [PubMed] [Google Scholar]
- 19.Kattula S, Byrnes JR, Wolberg AS. 2017. Fibrinogen and fibrin in hemostasis and thrombosis. Arterioscler. Thromb. Vasc. Biol. 37(3):e13–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown AC, Barker TH. 2014. Fibrin-based biomaterials: modulation of macroscopic properties through rational design at the molecular level. Acta Biomater. 10(4):1502–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quinn JV. 2005. Tissue Adhesives in Clinical Medicine. Hamilton, Ontario, Canada: PMPH-USA. 2nd ed. [Google Scholar]
- 22.Chiara O, Cimbanassi S, Bellanova G, Chiarugi M, Mingoli A, et al. 2018. A systematic review on the use of topical hemostats in trauma and emergency surgery. BMC Surg. 18(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.US Food Drug Admin. 2019. EVARREST (Fibrin Sealant Patch). Regul. Doc, US Food Drug Admin., Silver Spring, MD. https://www.fda.gov/vaccines-blood-biologics/approved-blood-products/ [Google Scholar]
- 24.Li Z, Milionis A, Zheng Y, Yee M, Codispoti L, et al. 2019. Superhydrophobic hemostatic nanofiber composites for fast clotting and minimal adhesion. Nat. Commun. 10(1):5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spotnitz WD. 2014. Fibrin sealant patches: powerful and easy-to-use hemostats. Open Access Surg. 7:71–79 [Google Scholar]
- 26.Chandrashekar A, Singh G, Garry J, Sikalas N, Labropoulos N. 2018. Mechanical and biochemical role of fibrin within a venous thrombus. Eur. J. Vasc. Endovasc. Surg. 55(3):417–24 [DOI] [PubMed] [Google Scholar]
- 27.Fakhari A, Berkland C. 2013. Applications and emerging trends of hyaluronic acid in tissue engineering, as a dermal filler, and in osteoarthritis treatment. Acta Biomater. 9(7):7081–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo J-W, Liu C, Wu J-H, Zhao D-H, Lin L-X, et al. 2020. In situ forming gelatin/hyaluronic acid hy- drogel for tissue sealing and hemostasis. J. Biomed. Mater. Res. B Appl. Biomater. 108(3):790–97 [DOI] [PubMed] [Google Scholar]
- 29.Wang D, Xu P, Wang S, Li W, Liu W. 2020. Rapidly curable hyaluronic acid-catechol hydrogels inspired by scallops as tissue adhesives for hemostasis and wound healing. Eur. Polym. J. 134:109763 [Google Scholar]
- 30.Lewis KM, Kuntze CE, Gulle H. 2015. Control of bleeding in surgical procedures: critical appraisal of HEMOPATCH (Sealing Hemostat). Med. Devices (Auckl). 9:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lewis KM, Spazierer D, Slezak P, Baumgartner B, Regenbogen J, Gulle H. 2014. Swelling, sealing, and hemostatic ability of a novel biomaterial: a polyethylene glycol-coated collagen pad. J. Biomater. Appl. 29(5):780–88 [DOI] [PubMed] [Google Scholar]
- 32.Wei W, Liu J, Peng Z, Liang M, Wang Y, Wang X. 2020. Gellable silk fibroin-polyethylene sponge for hemostasis. Artif. Cells Nanomed. Biotechnol. 48(1):28–36 [DOI] [PubMed] [Google Scholar]
- 33.Bu Y, Zhang L, Sun G, Sun F, Liu J, et al. 2019. Tetra-PEG based hydrogel sealants for in vivo visceral hemostasis. Adv. Mater. 31(28):1901580. [DOI] [PubMed] [Google Scholar]
- 34.Daristotle JL, Zaki ST, Lau LW, Torres L, Zografos A, et al. 2019. Improving the adhesion, flexibility, and hemostatic efficacy of a sprayable polymer blend surgical sealant by incorporating silica particles. Acta Biomater. 90:205–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu L-C, Bauer J, Siedlecki CA. 2014. Proteins, platelets, and blood coagulation at biomaterial interfaces. Colloids Surf. B Biointerfaces 124:49–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lubich C, Allacher P, de la Rosa M, Bauer A, Prenninger T, et al. 2016. The mystery of antibodies against polyethylene glycol (PEG)—what do we know? Pharm. Res. 33(9):2239–49 [DOI] [PubMed] [Google Scholar]
- 37.Ganson NJ, Povsic TJ, Sullenger BA, Alexander JH, Zelenkofske SL, et al. 2016. Pre-existing anti- polyethylene glycol antibody linked to first-exposure allergic reactions to pegnivacogin, a PEGylated RNA aptamer. J. Allergy Clin. Immunol. 137(5):1610–13.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park S, Han U, Choi D, Hong J. 2018. Layer-by-layer assembled polymeric thin films as prospective drug delivery carriers: design and applications. Biomater. Res. 22(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alkekhia D, Hammond PT, Shukla A. 2020. Layer-by-layer biomaterials for drug delivery. Annu. Rev. Biomed. Eng. 22:1–24 [DOI] [PubMed] [Google Scholar]
- 40.Shukla A, Almeida B. 2014. Advances in cellular and tissue engineering using layer-by-layer assembly. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 6(5):411–21 [DOI] [PubMed] [Google Scholar]
- 41.Decher G, Hong JD. 1991. Buildup of ultrathin multilayer films by a self-assembly process: II. Consec- utive adsorption of anionic and cationic bipolar amphiphiles and polyelectrolytes on charged surfaces. Berichte Bunsenges. Phys. Chem 95(11):1430–34 [Google Scholar]
- 42.Decher G, Hong JD. 1991. Buildup of ultrathin multilayer films by a self-assembly process, 1: Consecutive adsorption of anionic and cationic bipolar amphiphiles on charged surfaces. Makromol. Chem. Macromol. Symp. 46(1):321–27 [Google Scholar]
- 43.Decher G 1997. Fuzzy nanoassemblies: toward layered polymeric multicomposites. Science 277(5330):1232–37 [Google Scholar]
- 44.Alkekhia D, Shukla A. 2019. Influence of poly-l-lysine molecular weight on antibacterial efficacy in poly- mer multilayer films. J. Biomed. Mater. Res. A 107(6):1324–39 [DOI] [PubMed] [Google Scholar]
- 45.Zhuk I, Jariwala F, Attygalle AB, Wu Y, Libera MR, Sukhishvili SA. 2014. Self-defensive layer-by-layer films with bacteria-triggered antibiotic release. ACS Nano 8(8):7733–45 [DOI] [PubMed] [Google Scholar]
- 46.Shukla A, Fang JC, Puranam S, Hammond PT. 2012. Release of vancomycin from multilayer coated absorbent gelatin sponges. J. Control. Release 157(1):64–71 [DOI] [PubMed] [Google Scholar]
- 47.Shukla A, Avadhany SN, Fang JC, Hammond PT. 2010. Tunable vancomycin releasing surfaces for biomedical applications. Small Weinh. Bergstr. Ger. 6(21):2392–404 [DOI] [PubMed] [Google Scholar]
- 48.Shukla A, Fuller RC, Hammond PT. 2011. Design of multi-drug release coatings targeting infection and inflammation. J. Control. Release 155(2):159–66 [DOI] [PubMed] [Google Scholar]
- 49.Choi KY, Correa S, Min J, Li J, Roy S, et al. 2019. Binary targeting of siRNA to hematologic cancer cells in vivo using layer-by-layer nanoparticles. Adv. Funct. Mater. 29(20):1900018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang S, Battigelli A, Alkekhia D, Fairman A, Antoci V, et al. 2020. Controlled delivery of a protein tyrosine phosphatase inhibitor, SHP099, using cyclodextrin-mediated host-guest interactions in poly- electrolyte multilayer films for cancer therapy. RSC Adv. 10(34):20073–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Deng ZJ, Morton SW, Ben-Akiva E, Dreaden EC, Shopsowitz KE, Hammond PT. 2013. Layer-by-layer nanoparticles for systemic codelivery of an anticancer drug and siRNA for potential triple-negative breast cancer treatment. ACS Nano 7(11):9571–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kapadia CH, Ioele SA, Day ES. 2020. Layer-by-layer assembled PLGA nanoparticles carrying miR-34a cargo inhibit the proliferation and cell cycle progression of triple-negative breast cancer cells. J. Biomed. Mater. Res. A 108(3):601–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zeng J, Matsusaki M. 2019. Layer-by-layer assembly of nanofilms to control cell functions. Polym. Chem. 10(23):2960–74 [Google Scholar]
- 54.Shah NJ, Hyder MN, Quadir MA, Courchesne N-MD, Seeherman HJ, et al. 2014. Adaptive growth factor delivery from a polyelectrolyte coating promotes synergistic bone tissue repair and reconstruction. PNAS 111(35):12847–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang S, Xing M, Li B. 2018. Biomimetic layer-by-layer self-assembly of nanofilms, nanocoatings, and 3D scaffolds for tissue engineering. Int. J. Mol. Sci. 19(6):1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kharlampieva E, Kozlovskaya V, Chan J, Ankner JF, Tsukruk VV. 2009. Spin-assisted layer-by-layer as- sembly: variation of stratification as studied with neutron reflectivity. Langmuir 25(24):14017–24 [DOI] [PubMed] [Google Scholar]
- 57.Krogman KC, Lowery JL, Zacharia NS, Rutledge GC, Hammond PT. 2009. Spraying asymmetry into functional membranes layer-by-layer. Nat. Mater. 8(6):512–18 [DOI] [PubMed] [Google Scholar]
- 58.Castleberry SA, Li W, Deng D, Mayner S, Hammond PT. 2014. Capillary flow layer-by-layer: a mi- crofluidic platform for the high-throughput assembly and screening of nanolayered film libraries. ACS Nano 8(7):6580–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shukla A, Fang JC, Puranam S, Jensen FR, Hammond PT. 2012. Hemostatic multilayer coatings. Adv. Mater. 24(4):492–96 [DOI] [PubMed] [Google Scholar]
- 60.Huang X, Jia J, Wang Z, Hu Q. 2015. A novel chitosan-based sponge coated with self-assembled thrombin/tannic acid multilayer films as a hemostatic dressing. Chin. J. Polym. Sci. 33(2):284–90 [Google Scholar]
- 61.Guillot R, Gilde F, Becquart P, Sailhan F, Lapeyrere A, et al. 2013. The stability of BMP loaded poly- electrolyte multilayer coatings on titanium. Biomaterials 34(23):5737–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shukla A, Puranam S, Hammond PT. 2012. Vancomycin storage stability in multilayer thin film coatings for on-demand care. J. Biomater. Sci. Polym. Ed. 23(15):1895–902 [DOI] [PubMed] [Google Scholar]
- 63.Hsu BB, Conway W, Tschabrunn CM, Mehta M, Perez-Cuevas MB, et al. 2015. Clotting mimicry from robust hemostatic bandages based on self-assembling peptides. ACS Nano 9(9):9394–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Komachi T, Sumiyoshi H, Inagaki Y, Takeoka S, Nagase Y, Okamura Y. 2017. Adhesive and robust mul- tilayered poly(lactic acid) nanosheets for hemostatic dressing in liver injury model. J. Biomed. Mater. Res. B Appl. Biomater. 105(7):1747–57 [DOI] [PubMed] [Google Scholar]
- 65.Hsu BB, Hagerman SR, Jamieson K, Castleberry SA, Wang W, et al. 2015. Multifunctional self-assembled films for rapid hemostat and sustained anti-infective delivery. ACS Biomater. Sci. Eng. 1(3):148–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tan L, Zhou X, Wu K, Yang D, Jiao Y, Zhou C. 2020. Tannic acid/CaII anchored on the surface of chitin nanofiber sponge by layer-by-layer deposition: integrating effective antibacterial and hemostatic performance. Int. J. Biol. Macromol. 159:304–15 [DOI] [PubMed] [Google Scholar]
- 67.Che C, Liu L, Wang X, Zhang X, Luan S, et al. 2020. Surface-adaptive and on-demand antibacterial sponge for synergistic rapid hemostasis and wound disinfection. ACS Biomater. Sci. Eng. 6(3):1776–86 [DOI] [PubMed] [Google Scholar]
- 68.Zheng W, Chen C, Zhang X, Wen X, Xiao Y, et al. 2021. Layer-by-layer coating of carboxymethyl chitosan-gelatin-alginate on cotton gauze for hemostasis and wound healing. Surf. Coat. Technol. [Google Scholar]
- 69.Liu J-Y, Hu Y, Li L, Wang C, Wang J, et al. 2020. Biomass-derived multilayer-structured microparticles for accelerated hemostasis and bone repair. Adv. Sci. 7(22):2002243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang L, Sun J, Zhou Y, Zhong Y, Ying Y, et al. 2017. Layer-by-layer assembly of Cu3(BTC)2 on chitosan non-woven fabrics: a promising haemostatic decontaminant composite material against sulfur mustard. J. Mater. Chem. B 5(30):6138–46 [DOI] [PubMed] [Google Scholar]
- 71.Chan LW-G, Wang X, Wei H, Pozzo LD, White NJ, Pun SH. 2015. A synthetic fibrin-crosslinking polymer for modulating clot properties and inducing hemostasis. Sci. Transl. Med. 7(277):277ra29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nandi S, Brown AC. 2016. Platelet-mimetic strategies for modulating the wound environment and in- flammatory responses. Exp. Biol. Med. 241(10):1138–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Anselmo AC, Modery-Pawlowski CL, Menegatti S, Kumar S, Vogus DR, et al. 2014. Platelet-like nanoparticles: mimicking shape, flexibility, and surface biology of platelets to target vascular injuries. ACS Nano 8(11):11243–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dyer MR, Hickman D, Luc N, Haldeman S, Loughran P, et al. 2018. Intravenous administration of synthetic platelets (SynthoPlate) in a mouse liver injury model of uncontrolled hemorrhage improves hemostasis. J. Trauma Acute Care Surg. 84(6):917–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Girish A, Sekhon U, Sen Gupta A. 2020. Bioinspired artificial platelets for transfusion applications in traumatic hemorrhage. Transfusion 60(2):229–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hickman DA, Pawlowski CL, Shevitz A, Luc NF, Kim A, et al. 2018. Intravenous synthetic platelet (SynthoPlate) nanoconstructs reduce bleeding and improve ‘golden hour’ survival in a porcine model of traumatic arterial hemorrhage. Sci. Rep. 8:3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nandi S, Sproul EP, Nellenbach K, Erb M, Gaffney L, et al. 2019. Platelet-like particles dynamically stiffen fibrin matrices and improve wound healing outcomes. Biomater. Sci. 7(2):669–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nandi S, Sommerville L, Nellenbach K, Mihalko E, Erb M, et al. 2020. Platelet-like particles improve fibrin network properties in a hemophilic model of provisional matrix structural defects. J. Colloid Interface Sci. 577:406–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brown AC, Stabenfeldt SE, Ahn B, Hannan RT, Dhada KS, et al. 2014. Ultrasoft microgels displaying emergent platelet-like behaviours. Nat. Mater. 13(12):1108–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chee E, Nandi S, Nellenbach K, Mihalko E, Snider DB, et al. 2020. Nanosilver composite pNIPAm mi- crogels for the development of antimicrobial platelet-like particles. J. Biomed. Mater. Res. B Appl. Biomater. 108(6):2599–609 [DOI] [PubMed] [Google Scholar]
- 81.Hubbard WB, Lashof-Sullivan MM, Lavik EB, VandeVord PJ. 2015. Steroid-loaded hemostatic nanopar- ticles combat lung injury after blast trauma. ACS Macro Lett. 4(4):387–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hubbard WB, Lashof-Sullivan M, Greenberg S, Norris C, Eck J, et al. 2018. Hemostatic nanoparticles increase survival, mitigate neuropathology and alleviate anxiety in a rodent blast trauma model. Sci. Rep. 8:10622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Onwukwe C, Maisha N, Holland M, Varley M, Groynom R, et al. 2018. Engineering intravenously ad- ministered nanoparticles to reduce infusion reaction and stop bleeding in a large animal model of trauma. Bioconjug. Chem. 29(7):2436–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Peng HT. 2020. Hemostatic agents for prehospital hemorrhage control: a narrative review. Mil. Med. Res. 7(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gao Y, Sarode A, Kokoroskos N, Ukidve A, Zhao Z, et al. 2020. A polymer-based systemic hemostatic agent. Sci. Adv. 6(31):eaba0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lokhande G, Carrow JK, Thakur T, Xavier JR, Parani M, et al. 2018. Nanoengineered injectable hydro- gels for wound healing application. Acta Biomater. 70:35–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gaharwar AK, Avery RK, Assmann A, Paul A, McKinley GH, et al. 2014. Shear-thinning nanocomposite hydrogels for the treatment of hemorrhage. ACS Nano 8(10):9833–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gkikas M, Peponis T, Mesar T, Hong C, Avery RK, et al. 2019. Systemically administered hemostatic nanoparticles for identification and treatment of internal bleeding. ACS Biomater. Sci. Eng. 5(5):2563–76 [DOI] [PubMed] [Google Scholar]
- 89.Baylis JR, Chan KYT, Kastrup CJ. 2016. Halting hemorrhage with self-propelling particles and local drug delivery. Thromb. Res. 141:S36–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Baylis JR, Finkelstein-Kulka A, Macias-Valle L, Manji J, Lee M, et al. 2017. Rapid hemostasis in a sheep model using particles that propel thrombin and tranexamic acid. Laryngoscope 127(4):787–93 [DOI] [PubMed] [Google Scholar]
- 91.Sproul EP, Nandi S, Chee E, Sivadanam S, Igo BJ, et al. 2020. Development of biomimetic antimicrobial platelet-like particles comprised of microgel nanogold composites. Regen. Eng. Transl. Med. 6:299–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhu J, Li F, Wang X, Yu J, Wu D. 2018. Hyaluronic acid and polyethylene glycol hybrid hydrogel en- capsulating nanogel with hemostasis and sustainable antibacterial property for wound healing. ACS Appl. Mater. Interfaces 10(16):13304–16 [DOI] [PubMed] [Google Scholar]


