Abstract
Background:
A clinical hallmark of aneurysmal SAH (aSAH) is headache. Little is known about post-aSAH headache factors, which may point to underlying mechanisms. In this study, we aimed to characterize the severity and trajectory of headaches in relation to clinical features of patients with aSAH.
Methods:
This is a retrospective longitudinal study of adult patients admitted to an academic tertiary care center between 2012–2019 with aSAH who could verbalize pain scores. Additional factors recorded included demographics, aneurysm characteristics, analgesia, daily morning serum sodium concentration, and occurrence of vasospasm. Group-based trajectory modeling was used to identify headache pain trajectories, and clinical factors were compared between trajectories.
Results:
Of 91 patients included in the analysis, the mean age was 57 years and 20 (22%) were male. Headache score trajectories clustered into two groups: patients with mild-moderate and moderate-severe pain. Patients in the moderate-severe pain group were younger (P<0.05), received more opioid analgesia (P<0.001), and had lower sodium concentrations (P<0.001) than patients in the mild-moderate pain group.
Conclusion:
We identified two distinct post-aSAH headache pain trajectory cohorts and an association with age, analgesia, and sodium levels was identified. Future prospective studies considering sodium homeostasis and volume status under standardized analgesic regimens are warranted.
Keywords: aneurysmal subarachnoid hemorrhage, headache, vasospasm, sodium, hyponatremia, SIADH, opioids
Introduction
One of the main clinical features after aneurysmal rupture (aneurysmal SAH, aSAH) are relentless headaches (1). Severe headaches occur in up to 90% of patients admitted to the intensive care unit (ICU) with aSAH (2) and contribute significantly to subjective health impairment (3). The etiology of post-aSAH headache is incompletely understood but likely involves chemical irritation of the meninges by subarachnoid blood, oxidative damage from blood breakdown products, elevations of intracranial pressure (ICP), inflammation, hydrocephalus, cerebral edema, vasoreactivity and impairment thereof (4–7). Headaches may be exacerbated by complications occurring in the acute phase following an aSAH such as rebleeding, seizures, and delayed cerebral ischemia (3,8). Although clinical factors associated headache such as aneurysm development and rupture have received much attention, there is limited literature regarding specific factors associated with post-aSAH headache severity and trajectory over time (8,9).
Acute headaches following aSAH typically last approximately two weeks but can persist for months or even years in select patients (2,10). Prior work has shown mixed relationships between headache and demographics such as age, race, and sex in the acute period (2). For example, some studies have shown that younger patients may have greater long-term headache burden likely due to lack of brain atrophy and increased steepness of compliance curve (10), whereas others have found no relationship between headache and age (11). However, few studies have explored clinical factors that contribute to variability in pain scores in the immediate post-aSAH period. Herein, we examine a large database of patients with aSAH to evaluate the hypothesis that there exist distinct post-aSAH headache pain trajectories that relate to specific demographic as well as clinical factors.
Materials and Methods
Study design, setting, and patient selection
In this retrospective observational study, patients who were admitted to the University of Florida (UF) Health Shands Neurointensive Care Unit (NeuroICU) between January 2012 and January 2019 with a diagnosis of aSAH were included when 1) age was ≥18 years, 2) SAH was of non-traumatic, aneurysmal etiology (repaired by clipping or coiling). Patients were excluded if they could not verbally report pain scores using the standard 11-point numeric rating scale (NRS) (range, no pain = 0 to worst pain imaginable = 10) for 4 or more days during the first 12 days of ICU admission (12). The NRS is a validated, guideline-based pain assessment tool for non-intubated patients. Ability to verbalize pain was selected to ensure response to interventions could be accurately assessed. We selected the time cutoff because headache scores were sparse after this timepoint due to institutional practice of reduced frequency of neurological checks including pain assessments to either every 2 or every 4 hours. NRS pain scores were documented by the ICU nurse every hour according to standard of care in our unit. We categorized NRS scores as mild (0–3), moderate (4–7), and severe (8–10). All NeuroICU patients received guideline-recommended post-aSAH care (8,13) including nimodipine, blood pressure control, and short-term seizure prophylaxis (levetiracetam 500–1000mg orally or intravenously twice daily for 3 days). Per institutional practice, serum sodium goals were targeted for eunatremia (i.e., 135–145 mmol/l), with concomitant euvolemia as assessed by the care team based on features of clinical exam and fluid balance status, and utilization of hyperosmolar sodium infusion, oral salt tablets and/or fludrocortisone in order to attain these goals.
The study was approved by the local Institutional Review Board (IRB201900190), including a waiver of informed consent.
Data collection and management
Data extracted from electronic medical records included demographics, NRS headache pain scores, analgesic medications and dosage administered, and occurrence of vasospasm as measured by transcranial Doppler criteria (i.e., mean flow velocity ≥120 cm/sec and/or Lindegaard ratio ≥3) (12), clinical symptoms (9), or both. The daily maximal (max) and mean headache pain scores were charted and calculated, respectively. Frequency of vasospasm for each post-aSAH day was defined as the number of patients with recorded sonographic vasospasm divided by total number of patients examined on that post-aSAH day. All opioid drugs administered (e.g., methadone, fentanyl, oxycodone, hydrocodone, hydromorphone, codeine, tramadol) were converted to the equianalgesic oral morphine equivalent (OME) dosage in milligrams (mg) using an accepted conversion scale (14). Other analgesic medications recorded included acetaminophen and butalbital-acetaminophen-caffeine tablets (butalbital 50 mg, acetaminophen 325 mg and caffeine 40 mg). Additional clinical variables collected included morning sodium levels (mmol/L), presence of radiographic hydrocephalus reported on head CT studies, presence of a ventriculostomy, whether initial aneurysm treatment involved clipping or coiling, and radiologic and symptomatic measures of aneurysm severity at presentation including Hunt and Hess grade, modified Fisher score, aneurysm size, and whether intraventricular hemorrhage occurred during admission. Subjects with ventriculostomy had ICP values charted hourly. All data were stored in the institutional REDCap™ database (15,16).
Statistical analysis
A mixed-effects model with a random effects term for the effect of each subject was used to evaluate whether headache severity depended on post-aSAH day. Headache trajectories were developed using group-based trajectory modeling via maximum likelihood estimating using 3rd degree polynomials. In an exploration step, this algorithm appeared to converge on two groups based on information criteria, hence the selection of two as the number of fixed groups. Patients were assigned to groups based on the largest probability of group assignment. Once the trajectories were determined, linear regression analysis was conducted to assess the relationship between the output trajectories and post-aSAH day.
Unpaired student t-test or Wilcoxon signed rank test was used for hypothesis generation to compare factors defined by continuous variables between the two trajectory groups for normal and non-normal data, respectively. Categorical data were compared using chi-squared tests. Normality was determined using Shapiro Wilk test.
All statistical analyses were conducted using R 3.5.2 (r-project.org) software. Graphical data are presented as the mean ± 2 standard errors (SE) unless otherwise stated. Statistical significance was determined by P<0.05.
Data availability
Anonymized aggregate data will be shared with any qualified investigator upon request.
Results
Cohort characteristics
There were 344 patients with a diagnosis of non-traumatic SAH identified within the examined timeframe. A total of 253 patients were excluded, 42 patients with non-aneurysmal SAH, 8 patients for whom relevant data could not be retrieved, and 203 patients were excluded due to inability to verbalize pain scale scores for ≥4 days. The demographics and clinical characteristics of the analyzed cohort comprising 91 patients are summarized in Table 1. The mean age was 57.0 ± 1.6 years, the proportion of male patients was 22.0% (N=20), and 70.3% (N=64) of patients self-identified racially as white. The mean NeuroICU length of stay was 15.1 ± 0.5 days, mean Fisher scale was 3 (rounded), and mean Hunt & Hess grade was 2 (rounded).
Table 1.
Demographics and Clinical factors and association with headache trajectory groups
| Total Cohort | Mild-Moderate Cohort (N=26) | Moderate-Severe Cohort (N=65) | T, X2, or Wilcoxon Test | |
|---|---|---|---|---|
| Age (years) | 57.0 ± 1.6 | 63.4 ± 3.1 | 54.4 ± 1.8 | P<0.05 |
| Sex | 71 F, 20 M | 19 F, 7 M | 52 F, 13 M | P=0.66 |
| Race | 64 W, 18 AA, 9 Other | 16 W, 6 AA, 4 Other | 48 W, 12 AA, 5 Other | P=0.58 |
| Hx of Headache | 6 Y, 82 N | 3 Y, 21 N | 3 Y, 61 N | P=0.41 |
| Hx of Pain | 10 Y, 81 N | 2 Y, 24 N | 8 Y, 57 N | P=0.79 |
| Avg Max Headache Score | 6.1 ± 0.3 | 2.5 ± 0.3 | 7.6 ± 0.2 | P<0.001 |
| Aneurysm Size | 5.3 ± 0.3 | 5.2 ± 0.4 | 5.4 ± 0.4 | P=0.91 |
| Aneurysm Shape | 79 sacc, 4 fus, 6 mix | 21 sacc, 3 fus, 2 mix | 58 sacc, 1 fus, 4 mix | P=0.15 |
| Aneurysm Location | 26 Acom, 13 Basilar, 5 ICA, 6 MCA, 23 Pcom, 18 Other | 10 Acom, 1 Basilar, 1 ICA, 1 MCA, 6 Pcom, 7 Other | 16 Acom, 12 Basilar, 4 ICA, 5 MCA, 17 Pcom, 11 Other | P=0.31 |
| Fisher | 2.8 ± 0.1 | 2.9 ± 0.1 | 2.8 ± 0.1 | P=0.92 |
| Hunt & Hess | 2.2 ± 0.1 | 2.4 ± 0.7 | 2.2 ± 0.1 | P=0.38 |
| Hydrocephalus | 66 Y, 25 N | 20 Y, 6 N | 46 Y, 19 N | P=0.74 |
| Ventriculostomy | 63 Y, 28 N | 18 Y, 8 N | 45 Y, 20 N | P=1.0 |
| Interventricular Hemorrhage | 40 Y, 51 N | 13 Y, 13 N | 27 Y, 38 N | P=0.62 |
| Aneurysm Management | 26 clip, 61 coil, 1 both | 8 clip, 17 coil, 1 both | 18 clip, 44 coil, 0 both | P=0.44 |
| Any Vasospasm | 29 Y, 62 N | 8 Y, 18 N | 21 Y, 44 N | P=1.0 |
| Percent Days Vasospasm | 6.6 ± 1.4 | 7.8 ± 3.3 | 6.1 ± 1.5 | P=1.0 |
| LOS | 15.1 ± 0.5 | 16.3 ± 1.4 | 14.8 ± 0.5 | P=0.66 |
| Avg OME (mg) | 21.7 ± 3.2 | 15.2 ± 6.1 | 24.4 ± 3.7 | P<0.01 |
| Avg Tylenol (mg) | 1072.0 ± 93.0 | 998.0 ± 156.5 | 1101.6 ± 114.6 | P=0.68 |
| Avg butalbital-acetaminophen-caffeine (tabs) | 3.7 ± 0.23 | 1.6 ± 0.3 | 4.5 ± 0.2 | P<0.001 |
| Avg Ibuprofen (mg) | 30.3 ± 17.6 | 26.9 ± 26.9 | 31.6 ± 22.3 | P=0.69 |
| Avg Celecoxib (mg) | 6.7 ± 3.1 | 0.6 ± 0.6 | 9.1 ± 4.2 | P=0.37 |
| Avg Na+ | 138.0 ± 0.3 | 139.3 ± 0.5 | 137.5 ± 0.3 | P<0.01 |
| Sodium augmentation | 38 Y, 53 N | 9 Y, 17 N | 29 Y, 36 N | P=0.52 |
| Avg Min ICP | 2.8 ± 0.4 | 3.6 ± 1.4 | 2.5 ± 0.3 | P=0.83 |
| Avg Max ICP | 13.9 ± 0.8 | 15.3 ± 1.7 | 13.4 ± 0.9 | P=0.80 |
Mean ± two standard errors are provided for continuous variables.
Hx = history, Avg = average, LOS = length of stay, OME = oral morphine equivalents, Min = minimum, Max = maximum, ICP = intracranial pressure, W = white, AA = African American, Y = yes, N = no, sacc = saccular, fus = fusiform, Acom = anterior communicating artery, ICA = internal carotid artery, MCA = middle cerebral artery, Pcom = posterior communicating artery.
Aneurysm characteristics and management
The average aneurysm size was 5.3 ± 0.3 mm, and the anterior communicating artery comprised the largest proportion of aneurysm location (N=26) (Table 1). Hydrocephalus was seen in 73% (N=66) of patients, and 69% (N=63) required ventriculostomy. Three patients had ventriculostomy placed for neurological exam decline without documented hydrocephalus. Aneurysm management included coiling in 61 patients and clipping in 26 patients; one patient underwent both clipping and coiling.
Headache scores
From ICU admission through post-aSAH day 12, the average daily max NRS headache pain score was 6.1 ± 0.3 (Figure 1a). Mixed model analyses showed that overall, headache scores slightly decreased over time (Estimate=−0.049, F=5.78, P<0.05) (Figure 1a).
Figure 1.
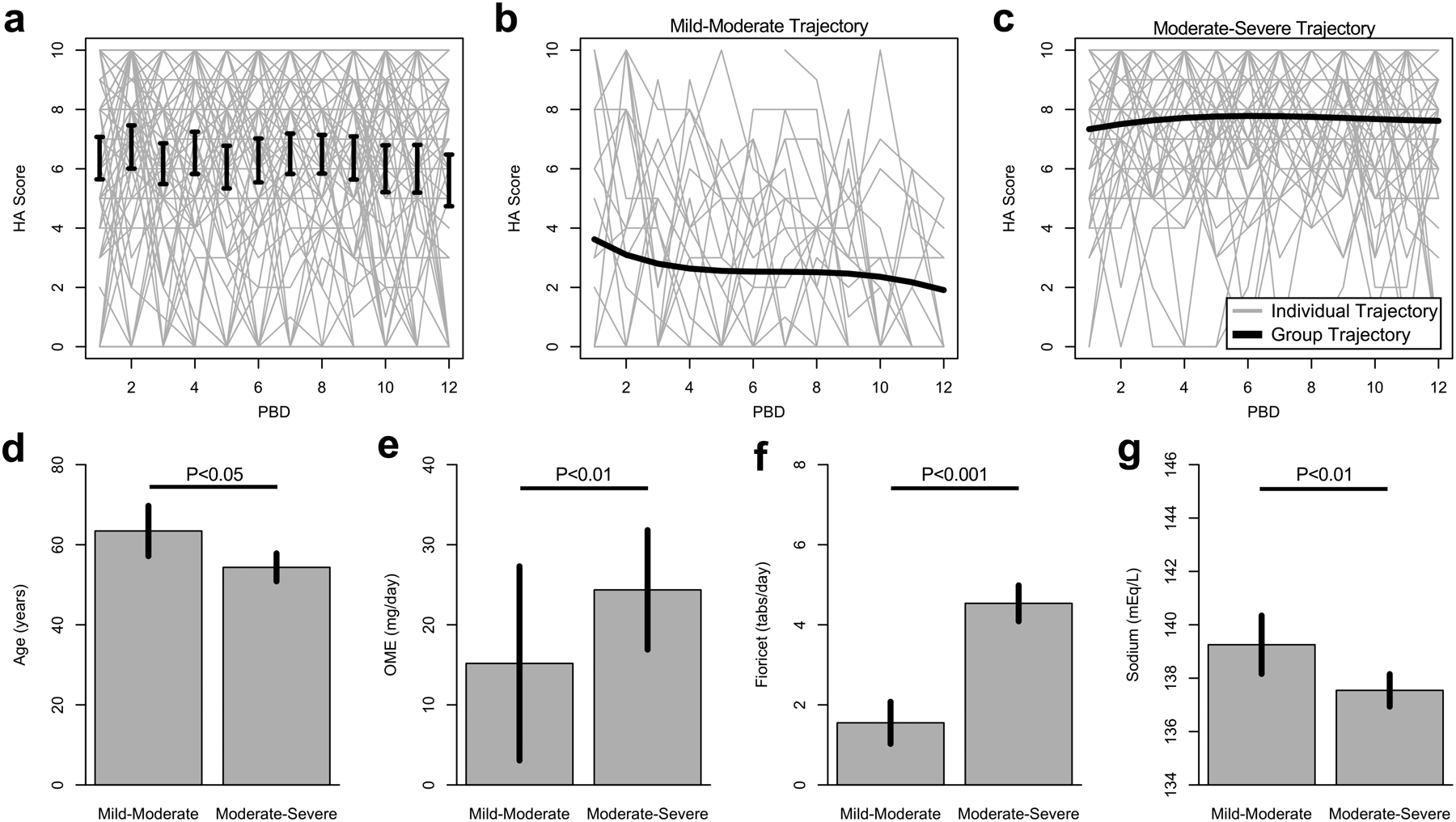
HA = headache; PBD = post bleed day; OME = oral morphine equivalents. (a) Average maximum daily headache scores across participants with plus and minus two standard errors. Light gray lines indicate the raw data. (b-c) The two trajectory groups, mild-moderate and moderate-severe, and their corresponding average headache trajectory as well as raw subject-level data. (d-g) Comparison of age, OME, fioricet tabs, and sodium in patients assigned to the mild-moderate versus the moderate-severe headache groups.
Trajectory analyses revealed two post-aSAH headache courses across patients, indicative of a mild-moderate pain group (29% of the cohort) and a moderate-severe pain group (71% of the cohort). In the first group (Figure 1b), headache score trajectory averaged 2.5 ± 0.3 and significantly decreased during the 12 days of follow-up (linear regression, Estimate=−0.11, P<0.001). In the second group (Figure 1c), headache score trajectory averaged 7.6 ± 0.2, and scores remained stable during the 12 days of follow-up (linear regression, P=0.15). Severe headache scores comprised 9.7% of all scores in the mild-moderate group, and mild headache scores comprised 5.3% of scores in the moderate-severe group. Analgesic medications administered and associated dosing information is provided in Table 1.
Relationship between headache and other clinical factors
Table 1 also provides a comparison of clinical factors between the two identified headache trajectory groups. Regarding demographics, the patients in the moderate-severe group were younger than those in the mild-moderate group (54.4 ± 1.8 vs 64.3 ± 3.1 years old, P<0.05, Figure 1d); no significant differences regarding sex (P=0.66) and race (P=0.58) were found. There were also no significant differences between the two groups in terms of history of headache or pain, aneurysm characteristics (including size, shape, location, severity, and management), presence of intraventricular hemorrhage and ventriculostomy placement, or vasospasm. Acetaminophen use was similar across groups. Opioid use was higher in the moderate-severe group with an average OME 24.4 ± 3.7 versus 15.2 ± 6.1 mg/day (P<0.01) in the mild-moderate group (Figure 1e). Similarly, in the moderate-severe group, more acetaminophen-butalbital-caffeine was used than in the mild-moderate group (4.5 ± 0.2 vs 1.6 ± 0.3 tabs, P<0.001, Figure 1f). Average morning sodium was lower in the moderate-severe group compared to the mild-moderate group (137.5 ± 0.3 vs 139.3 ± 0.5, P<0.01, Figure 1g), while sodium augmentation treatment (use of either sodium tablets, hyperosmolar sodium infusions or fludrocortisone) was similar between both groups (P=0.52). For patients with EVD, both minimum (P=0.83) and maximum (P=0.80) ICPs were similar between the two groups.
Discussion
In this retrospective study of adult patients with aSAH admitted to the neuroICU whose neurologic status allowed for verbalization of pain scores, we identified two distinct headache severity trajectories: mild-moderate and moderate-severe. Significant differences between the two groups were discovered regarding analgesia use, age, and serum sodium levels.
The identification of a relationship between lower morning serum sodium levels and increased headache severity is novel. Dysnatremia in aSAH can be multifactorial: syndrome of inappropriate anti-diuretic hormone (SIADH), steroid deficiency, cerebral salt wasting (CSW), and central diabetes insipidus are possible etiologies (17). Hyponatremia is commonly observed in the course after SAH (18). aSAH has been linked to both CSW and SIADH for anterior communicating artery aneurysms (19). Furthermore, pain itself is a potent stimulus for ADH release, and may thereby contribute to a relative hyponatremia among those with more severe headache (20), with low sodium representing a signal for poorly controlled pain. However, ADH levels may also be affected by other factors including medications (e.g., tramadol), surgery, other symptoms (e.g., nausea), and infection (20,21).
While an association of lower sodium levels with headache has so far not been described in SAH, disturbed sodium homeostasis and the role of sodium in other headache disorders have been examined with mixed results. In migraine, elevated plasma vasopressin levels have been described (22), and odds of migraine decreased in some series with increasing sodium intake (23), while reduced sodium intake has been found to be associated with reduced occurrence of headache in others (24). Etiologic factors contributing to post-aSAH headache have been largely elusive. Few studies have examined headache pain and related clinical factors, but so far have failed to identify a confirmed relationship between headache severity and the majority of demographic, aneurysmal, and other clinical factors. Sympathetic upregulation has also been postulated, but further studies for validation are needed (25).
We compared patient characteristics between the mild-moderate and moderate-severe trajectory and, like most studies, found no difference in several of the hypothesized factors including aneurysm characteristics, treatment strategy, and vasospasm. Few studies have found relationships between headache pain and variables such as vasospasm (5), history of headache (26), as well as factors that were not described here such as magnesium use (27) and anxiety (28). While some data indicate a possible relationship of clinical characteristics such as aneurysm location with sodium dyshomeostasis (19), prior data do not suggest that the clinical grade of SAH is associated with sodium dyshomeostasis (39). Given that in our multi-factor analysis, we found no significant difference between headache trajectory groups when comparing Fisher grade, Hunt Hess grade, occurrence of hydrocephalus and IVH, cumulative days with vasospasm, and utilization of sodium augmentation, it is unlikely that this difference in sodium levels is simply a surrogate for SAH severity or frequency of disturbance in sodium homeostasis, but rather indicates a potential difference in severity of disturbance in sodium homeostasis. Hence, our finding of lower sodium levels in the more severe headache group may provide a signal that could provide more insight into headache generation and trajectory after SAH. Furthermore, baseline sodium levels prior to aneurysmal rupture would aid in determining the threshold of change needed for headache correlation. Whether lower serum sodium constitutes an intervenable metric or a byproduct of headache pain or administered pain medications (i.e., opioids) remains to be determined, as does the mechanism underlying its association with headache trajectory. Of particular note is that there is a lack of data-driven guidelines for management of post-SAH headaches – this is likely also due to the incompletely understood variety of headache severity, trajectories and interindividual headache phenotypes. The only guideline mentioning post-SAH headaches suggest that pain is commonly so severe that opioids are often needed (40) for its treatment. As discussed above, the interrelation between disturbed sodium homeostasis and headache severity may also be affected by the amount of opioids administered. Future dedicated post-aSAH sodium studies are needed to investigate more closely factors such as fluid status, sodium-affecting medications, and sodium fluctuations as they relate to headache severity and outcomes. Additionally, the select group of patients with intraoperative rupture during treatment of elective aneurysms may provide important insights into understanding this mechanism.
We further found that patients in the moderate-severe group were approximately 10 years younger on average than the mild-moderate group. This finding aligns with prior work, where younger age has been associated with more severe and persistent headache after aSAH (1,10,26).
As expected, patients in the moderate-severe group required more narcotic-based analgesia (both opioids and butalbital-acetaminophen-caffeine) than patients in the mild-moderate group. This association is a likely product of headache intensity, but may also be related to a prolonged relentless pain trajectory from a medication overuse phenomenon, commonly seen with around-the-clock analgesic use notably butalbital-caffeine-acetaminophen (29). Another potential confounding variable is the association of sleep deprivation with headache scores given the requirement of frequent neurologic checks in this population. Sleep deprivation and headaches have been well reported in the literature (31,33) although given the standard of frequent neurological checks in the neurocritically ill, this would not explain a difference within the cohort of patients with SAH. Our findings are further confirmatory of numerous prior reports that documented high analgesia requirements in patients with severe headache post-aSAH (1,2,5,13,30), and further showed that despite aggressive analgesia, headache pain did not substantially improve during follow-up. Unfortunately, despite attempts to introduce agents such as gabapentin and multimodal analgesia, this finding has not changed over the past years (32), motivating the need for improved or alternative medical management of patients who have suffered aSAH (27,34). Importantly, opioid use itself can affect ADH secretion (20, 21); thus, higher opioid requirements may be an important factor, as proxy for headache severity, contributing to sodium dyshomeostasis after aSAH.
In our study, headache severity decreased in those with mild-moderate post-aSAH headache, whereas those with moderate-severe post-aSAH headache sustained elevated pain scores. Prior studies have revealed mixed longitudinal trends in post-aSAH headache, likely heavily influenced by the sample inclusion criteria. For example, we strictly examined patients who could verbalize pain scores, while others included physiologic pain scores, the type of pain score, and frequency of scoring. Our study stands out by the high frequency of hourly score documentation with verbal confirmation of scores. Prior headache characterization has depended on whether mean, median, or maximum headache score is used as an endpoint (1,5,11,27). One study showed increasing group-level median values of the maximum headache pain within the first seven post-aSAH days (5); however, individual fluctuations in headache scores were not evaluated, and the average age of the cohort was younger (average, 52 years old) than ours (average, 57 years old). Other studies demonstrated that, during the first week, pain is moderate-severe without significant change over time following hemorrhage (1,2,27); however, distinct trajectory groups were not evaluated in these studies. One recent study by Jaffa et al. used a combination of verbal pain scores whenever possible and a physiology-based assessment tool in intubated patients to identify five distinct headache trajectories; surprisingly, most patients experienced overall much less headache pain within the first two weeks post-aSAH in this cohort (11). One of the five pain trajectories did show an increase in pain from minimal to mild-moderate pain by two weeks post-aSAH, while the other trajectories showed either unchanged or decreased pain over time. Contributive to this might be the inclusion of intubated patients, who oftentimes receive analgosedation; hence, physiologic, nonverbal pain scores, may indicate residual but not absolute pain. Additionally, as applicable to most mentioned studies, which are, like our study, single center studies, there are differences in proportions of sex, racial distribution and age. These are factors that have been documented to influence not only occurrence but also perception of pain (35–37). Especially with regard to race, American Blacks and Hispanics are disproportionally more highly affected by aSAH (38), but the majority of the scarce data available on headache after aSAH, including our own, are not able to shed sufficient light on this aspect, due to the majority of subject data relating to patients of white race. This, in addition to differences in local pain regimen practices, might also explain differences in pain trajectories found between our and Jaffa et al. cohorts, where roughly half of their cohort was of racial/ethnic minority groups (11).
Limitations
The present study has several important limitations. The retrospective design carries inherent limitations, including the inability to control for unmeasurable confounders and factors which we could not retrieve such as other medications. The single-center nature of the study including the local composition of our cohort limits the generalizability of our findings, especially as our patient demographic differs from those of other parts of the country. Additionally, analgesic prescription patterns and standard of care practices for eunatremia may reflect the bias of our institution as well as management strategies for when patients become hyponatremic. Our sample size, though larger than that of many other studies, was limited in both size and included clinical grades, which may affect the trajectory outputs. It is possible that other trajectories may emerge with larger and differently composed (i.e., with regard to race, age distribution, sex) samples.
Conclusions
In conclusion, our study revealed a novel association of sodium levels with headache severity post-SAH by identification of two headache trajectories in the acute post-aSAH period. Whether this is causative or a reflection of headache severity cannot be fully elucidated with retrospective data. Future studies should further develop statistics-based characterization of headache trajectories in a prospective manner, and focus on expanding the described relationship between headache and sodium, as this may point to possible headache mechanism amenable to treatment. The importance of this novel finding should be validated across institutions with different patient demographics.
Acknowledgements and Funding
All coauthors have reviewed and approve of the content of this manuscript. The requirements for authorship have been met. This submission is not under review by any other publication. We confirm adherence to ethical guidelines with IRB approval.
RSE, ZAS contributed to conceptualization, investigation, and manuscript writing. BLW, SZ contributed to conceptualization, investigation, data curation, methodology, resources, supervision. BH, BB contributed critical revision of the manuscript. CM contributed to conceptualization, data interpretation and critical revision of the manuscript. KB contributed to conceptualization, data curation, methodology, resources, supervision, manuscript writing and review. RSE is funded by NIH F30NS111841, ZAS is funded by NIH F30AG063446, BL is funded by NIH 1R25NS108939-01, BH is funded by NIH U01 NS117450, NIH R01 NS110710, NIH R25 NS108939. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. BH reports the following COI: Proprio Vision: investor in startup company; Progressive Neuro: investor and advisor in startup company; Galaxy Therapeutics: investor and advisor in startup company. CM has received funding from Claude D. Pepper Older Americans Independence Center and American Heart Association.
Disclosure statement
No potential conflict of interest relevant to this study was reported by the author(s). BH reports the following COI: Proprio Vision: investor in startup company; Progressive Neuro: investor and advisor in startup company; Galaxy Therapeutics: investor and advisor in startup company. CM has received funding from Claude D. Pepper Older Americans Independence Center and American Heart Association. KB has received compensation for consulting for Guidepoint Global and Techspert
References
- 1.Glisic EK, Gardiner L, Josti L, Dermanelian E, Ridel S, Dziodzio J, et al. (2016): Inadequacy of headache management after subarachnoid hemorrhage. Am J Crit Care 25: 136–143. [DOI] [PubMed] [Google Scholar]
- 2.Morad AH, Tamargo RJ, Gottschalk A (2016): The Longitudinal Course of Pain and Analgesic Therapy Following Aneurysmal Subarachnoid Hemorrhage: A Cohort Study. Headache 56. 10.1111/head.12908 [DOI] [PubMed] [Google Scholar]
- 3.Gerner ST, Reichl J, Custal C, Brandner S, Eyüpoglu IY, Lücking H, et al. (2020): Long-Term Complications and Influence on Outcome in Patients Surviving Spontaneous Subarachnoid Hemorrhage. Cerebrovasc Dis 49: 307–315. [DOI] [PubMed] [Google Scholar]
- 4.Kwon OK (2019): Headache and Aneurysm. Neuroimaging Clin N Am 29: 255–260. [DOI] [PubMed] [Google Scholar]
- 5.Swope R, Glover K, Gokun Y, Fraser JF, Cook AM (2014): Evaluation of headache severity after aneurysmal subarachnoid hemorrhage. Interdiscip Neurosurg 1: 119–122. [Google Scholar]
- 6.Bederson JB, Connolly E. Sander J, Batjer HH, Dacey RG, Dion JE, Diringer MN, et al. (2009): Guidelines for the Management of Aneurysmal Subarachnoid Hemorrhage. Stroke 40: 994–1025. [DOI] [PubMed] [Google Scholar]
- 7.Baron EP (2015): Headache, cerebral aneurysms, and the use of triptans and ergot derivatives. Headache 55: 739–747. [DOI] [PubMed] [Google Scholar]
- 8.Neifert SN, Chapman EK, Martini ML, Shuman WH, Schupper AJ, Oermann EK, et al. (2021): Aneurysmal Subarachnoid Hemorrhage: the Last Decade. Translational Stroke Research, vol. 12. 10.1007/s12975-020-00867-0 [DOI] [PubMed] [Google Scholar]
- 9.Li K, Barras CD, Chandra RV., Kok HK, Maingard JT, Carter NS, et al. (2019, June 1): A Review of the Management of Cerebral Vasospasm After Aneurysmal Subarachnoid Hemorrhage. World Neurosurgery, vol. 126. World Neurosurg, pp 513–527. [DOI] [PubMed] [Google Scholar]
- 10.Huckhagel T, Klinger R, Schmidt NO, Regelsberger J, Westphal M, Czorlich P (n.d.): The burden of headache following aneurysmal subarachnoid hemorrhage: a prospective single-center cross-sectional analysis. 10.1007/s00701-020-04235-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaffa MN, Jha RM, Elmer J, Kardon A, Podell JE, Zusman BE, et al. (2021): Pain Trajectories Following Subarachnoid Hemorrhage are Associated with Continued Opioid Use at Outpatient Follow-up. Neurocrit Care 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Newell DW, Winn HR (1990): Transcranial Doppler In Cerebral Vasospasm. Neurosurg Clin 1: 319–328. [PubMed] [Google Scholar]
- 13.Connolly ES, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, et al. (2012, June): Guidelines for the management of aneurysmal subarachnoid hemorrhage: A guideline for healthcare professionals from the american heart association/american stroke association. Stroke, vol. 43. Stroke, pp 1711–1737. [DOI] [PubMed] [Google Scholar]
- 14.Chung KC, Barlev A, Braun AH, Qian Y, Zagari M (2014): Assessing analgesic use in patients with advanced cancer: Development of a new scale-the analgesic quantification algorithm. Pain Med (United States) 15: 225–232. [DOI] [PubMed] [Google Scholar]
- 15.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG (2009): Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42: 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. (2019, July 1): The REDCap consortium: Building an international community of software platform partners. Journal of Biomedical Informatics, vol. 95. J Biomed Inform. 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okazaki T, Hifumi T, Kawakita K, Shishido H, Ogawa D, Okauchi M, et al. (2017): Target Serum Sodium Levels during Intensive Care Unit Management of Aneurysmal Subarachnoid Hemorrhage. Shock 48: 558–563. [DOI] [PubMed] [Google Scholar]
- 18.Fraser JF, Stieg PE (2006): Hyponatremia in the Neurosurgical PatientEpidemiology, Pathophysiology, Diagnosis, and Management. Neurosurgery 59: 222–229. [DOI] [PubMed] [Google Scholar]
- 19.Hoffman H, Ziechmann R, Gould G, Chin LS (2018): The Impact of Aneurysm Location on Incidence and Etiology of Hyponatremia Following Subarachnoid Hemorrhage. World Neurosurg 110: e621–e626. [DOI] [PubMed] [Google Scholar]
- 20.Cuzzo B, Padala SA, Lappin SL (2020): Physiology, Vasopressin (Antidiuretic Hormone, ADH). StatPearls. Retrieved July 31, 2021, from http://europepmc.org/books/NBK526069 [Google Scholar]
- 21.Schrier RW, Goldberg JP (1980): The Physiology of Vasopressin Release and the Pathogenesis of Impaired Water Excretion in Adrenal, Thyroid, and Edematous Disorders. YALE J Biol Med 53: 525–541. [PMC free article] [PubMed] [Google Scholar]
- 22.Hampton KK, Grant PJ, Esack A, Peatfield RC (1991): Elevation of plasma vasopressin in spontaneous migraine. Cephalalgia 11: 249–250. [DOI] [PubMed] [Google Scholar]
- 23.Pogoda JM, Gross NB, Arakaki X, Fonteh AN, Cowan RP, Harrington MG (2016): Severe Headache or Migraine History Is Inversely Correlated with Dietary Sodium Intake: NHANES 1999–2004. Headache 56: 688–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen L, Zhang Z, Chen W, Whelton PK, Appel LJ (2016): Lower sodium intake and risk of headaches: Results from the trial of Nonpharmacologic Interventions in the Elderly. Am J Public Health 106: 1270–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garg R, Bar B (2017): Systemic Complications Following Aneurysmal Subarachnoid Hemorrhage. Curr Neurol Neurosci Rep 17. 10.1007/S11910-017-0716-3 [DOI] [PubMed] [Google Scholar]
- 26.Hwang G, Jeong E-A, Sohn JH, Park H, Bang JS, Jin S-C, et al. (2012): The Characteristics and Risk Factors of Headache Development after the Coil Embolization of an Unruptured Aneurysm. Am J Neuroradiol 33: 1676–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Treatment of headache in aneurysmal subarachnoid hemorrhage_ Multimodal approach | Elsevier Enhanced Reader (n.d.): Retrieved July 9, 2021, from https://reader.elsevier.com/reader/sd/pii/S2214751920304187?token=012524B8D3DE3080ACBE817A78E82CDE5D05AF7F77B0B28AD809484DDC94A1C5005511ADCC65D8FB9AC328C1123ADBB6&originRegion=us-east-1&originCreation=20210708131300
- 28.Magalhães JE, Azevedo-Filho HRC, Rocha-Filho PAS (2013): The risk of headache attributed to surgical treatment of intracranial aneurysms: A cohort study. Headache 53: 1613–1623. [DOI] [PubMed] [Google Scholar]
- 29.Tepper SJ (2012): Medication-overuse headache. Contin Lifelong Learn Neurol 18: 807–822. [DOI] [PubMed] [Google Scholar]
- 30.Dorhout Mees SM, Bertens D, Van Der Worp HB, Rinkel GJE, Van Den Bergh WM (n.d.): Magnesium and headache after aneurysmal subarachnoid haemorrhage. 10.1136/jnnp.2009.181404 [DOI] [PubMed] [Google Scholar]
- 31.Bertisch SM, Li W, Buettner C, Mostofsky E, Rueschman M, Kaplan ER, et al. (2020): Nightly sleep duration, fragmentation, and quality and daily risk of migraine. Neurology 94: E489–E496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hile GB, Cook AM (2020): Treatment of headache in aneurysmal subarachnoid hemorrhage: Multimodal approach. Interdiscip Neurosurg 22: 100857. [Google Scholar]
- 33.Stark CD, Stark RJ (2015): Sleep and chronic daily headache. Curr Pain Headache Rep 19. 10.1007/S11916-014-0468-6 [DOI] [PubMed] [Google Scholar]
- 34.Smith CR, Fox WC, Robinson CP, Garvan C, Babi MA, Pizzi MA, et al. (2021): Pterygopalatine Fossa Blockade as Novel, Narcotic-Sparing Treatment for Headache in Patients with Spontaneous Subarachnoid Hemorrhage. Neurocrit Care 35: 241–248. [DOI] [PubMed] [Google Scholar]
- 35.Morrison R, Jesdale B, Dube C, Forrester S, Nunes A, Bova C, Lapane KL (2021): Racial/Ethnic Differences in Staff-Assessed Pain Behaviors Among Newly Admitted Nursing Home Residents. J Pain Symptom Manage 61: 438–448.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lillis TA, Burns J, Aranda F, Burgess HJ, Purim-Shem-Tov YA, Bruehl S, et al. (2019): Race-related differences in acute pain complaints among inner-city women: the role of socioeconomic status. J Behav Med 2019 435 43: 791–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ng BW, Nanavaty N, Mathur VA (2019): The influence of Latinx American identity on pain perception and treatment seeking. J Pain Res 12: 3025–3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Labovitz DL, Halim AX, Brent B, Boden-Albala B, Hauser WA, Sacco RL (2006): Subarachnoid Hemorrhage Incidence among Whites, Blacks and Caribbean Hispanics: The Northern Manhattan Study. Neuroepidemiology 26: 147–150. [DOI] [PubMed] [Google Scholar]
- 39.Kieninger M, Kerscher C, Bründl E, Bele S, Proescholdt M, et al. (2021): Acute hyponatremia after aneurysmal subarachnoid hemorrhage: Frequency, treatment, and outcome. J Clin Neurosci 88:237–242. doi: 10.1016/j.jocn.2021.04.004 [DOI] [PubMed] [Google Scholar]
- 40.Steiner T, Juvela S, Unterberg A, Jung C, Forsting M, et al. (2013): European Stroke Organization guidelines for the management of intracranial aneurysms and subarachnoid haemorrhage. Cerebrovasc Dis. 35(2):93–112. doi: 10.1159/000346087 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized aggregate data will be shared with any qualified investigator upon request.


