Abstract
Objective
Prior research has shown that cancer survivors often report positive psychological changes from the experience of cancer, or posttraumatic growth (PTG). However, few studies have focused on PTG in cancer patients recovering from hematopoietic cell transplantation (HCT). The present study measured PTG at specific milestones during the year following HCT and investigated psychosocial and treatment-related factors that may hinder or facilitate PTG.
Methods
Participants (N = 430) completed assessments of PTG, social support, and coping pre-transplant. PTG was also assessed at 1, 3, 6, and 12 months post-transplant. Information about treatment regimen and post-transplant complications was abstracted from medical records. Mixed-effects linear regression models were used to evaluate the extent to which pre-transplant social support, coping approaches, treatment intensity, and post-transplant complications predicted PTG.
Results
Compared to pre-transplant, PTG scores were significantly higher at 6- and 12-months post-transplant. Greater pre-transplant social support significantly predicted greater PTG across the assessment points. Use of emotional engagement coping strategies also strongly predicted post-transplant PTG. Conversely, coping styles characterized by emotional avoidance generally were not predictive of PTG. No treatment-related factors or post-transplant complications were predictive of PTG.
Conclusions
Findings indicate that supportive social relationships and coping by engaging with difficult emotions may facilitate PTG following HCT. Moreover, these factors were more important than medical characteristics in explaining PTG. Findings may guide the development of interventions to enhance positive psychological outcomes after HCT.
Keywords: cancer, hematopoietic cell transplant, posttraumatic growth, coping, social support, oncology, hematology, psycho-oncology
Hematopoietic stem cell transplantation (HCT) is a potentially curative treatment for advanced hematologic cancers, such as leukemia, lymphoma, and multiple myeloma. Although HCT can extend survival, the intensive treatment carries a significant risk of morbidity and mortality that may impair quality of life. Patients frequently experience post-transplant complications, such as infection and graft-versus-host disease (GVHD), and remain at risk for disease relapse (1). In addition, patients experience the burdens of a long hospitalization, need for frequent follow-up care, persistent side effects, and related life disruptions (2). It is therefore not surprising that HCT patients frequently experience depressed mood, heightened anxiety, and fatigue. Indeed, most research examining the psychological sequelae of HCT has focused on measures of distress and maladjustment. There is little research on indicators of resilience or positive psychological outcomes following HCT (2).
Posttraumatic growth (PTG) describes positive psychological changes associated with challenging life circumstances and adversity (3). When a significant event challenges an individual’s core beliefs, PTG can emerge as a result of gaining new insight through navigating pain, suffering, or loss (3). Among cancer patients, PTG has been associated with improved psychological and physical outcomes, including lower levels of depression (4) and enhanced immune functioning following chemotherapy (5). These factors, in turn, are associated with higher survival rates (5). Despite the growing body of research suggesting that cancer survivors often experience PTG (6-10), less is known about cancer patients recovering from HCT. One study found that long-term HCT survivors, as compared to a reference sample, reported more psychological and interpersonal growth (11), and another found evidence of PTG at 9 years post-transplant (12). However, few HCT studies have tracked PTG pre- and post-transplant to understand the longitudinal trajectories relative to post-transplant milestones.
Tedeschi and Calhoun’s (1998) model of PTG suggests that active coping, social support, and acceptance most strongly facilitate PTG (3). PTG does not occur as a direct result of a traumatic or stressful experience, but rather develops as an individual relates to the experience over time via adaptive coping (3,10). Active efforts to overcome adversity by seeking emotional support and processing difficult emotions have been found to be key facilitators of PTG among women with breast cancer (8,14,15) and patients with other types of cancer (16,17). Social relationships and acceptance- or approach-oriented coping strategies are psychosocial factors that are most strongly and consistently associated with PTG (15,17). Individuals who have close, supportive relationships may be better able to cognitively engage with their experience in a way that facilitates PTG (11,18). Research also suggests that individuals who are more open to and accepting of difficult emotions and spend time processing emotions are more likely to experience PTG (8,14,19-21). In contrast, disengaging from or avoiding painful emotional experiences may hinder PTG (14,19-21). However, these relationships have not been well studied among HCT patients.
The nature of the adverse experience may also affect the occurrence of PTG. A more threatening experience may lead to a greater capacity for growth (22,23). Among HCT recipients, those who have a more adverse experience, for example, those with extensive disease or more toxic treatment regimens, may be more likely to experience PTG than those with limited disease or less intensive treatment. Patients who receive allogeneic transplants (i.e., stem cells from a donor) typically experience a longer, more complicated recovery and have a greater risk of treatment-related mortality compared to those who receive autologous transplants (i.e., their own stem cells; 12,23). Allogeneic transplant recipients often require longer hospitalizations and isolation precautions (12,23). Similarly, HCT recipients who receive treatment that completely ablates the bone marrow typically have more severe side effects, complications, and a longer recovery than those who receive less intensive regimens (1,22,23). Finally, individuals who have other medical comorbidities are at greater risk for a complicated recovery and poorer physical functioning after HCT (1,22,23).
The present study used a prospective, longitudinal design to investigate PTG in a sample of adults with hematologic cancers undergoing HCT pre-transplant and at 1, 3, 6, and 12 months post-transplant. The first aim was to examine the trajectory of PTG prior to HCT and at specific milestones during the first year following transplant. The second aim was to evaluate psychosocial factors that may hinder or facilitate PTG. We hypothesized that patients who perceived close, supportive social relationships and those who reported greater acceptance of and ability to process painful thoughts and emotions would experience higher PTG post-transplant, whereas patients who used avoidant coping strategies would have lower PTG post-transplant. Finally, we evaluated whether medical and treatment-related factors that confer a more adverse recovery from HCT (allogeneic transplant, ablative treatment regimen, presence of post-transplant complications, medical comorbidities) predicted PTG. We hypothesized that patients who experienced a more stressful and adverse recovery would report greater PTG.
Method
Participants
The study sample included 430 adults who underwent HCT and received treatment at the University of Wisconsin Carbone Cancer Center. Participants were part of a larger, IRB-approved study investigating quality of life following HCT (UW Health Sciences IRB #2013-1062). Prior to transplant, participants provided written informed consent and completed assessments of quality of life and psychological well-being, including measures of perceived social support and use of coping strategies. Posttraumatic growth was also assessed at pre-transplant and at 1, 3, 6, and 12 months post-transplant. Questionnaires were administered at clinic appointments or mailed if the participant did not have an appointment near the evaluation point. Follow-up phone calls were made if participants did not return the questionnaires within 2 weeks.
During the study timeframe, 871 adults underwent HCT at our center, of which 568 (65%) provided informed consent and were enrolled in the parent study. Data from participants who completed the pre-transplant assessment and at least one post-transplant assessment were included in the present analyses (N = 430). Because of mortality and other attrition, data were available for fewer patients at the 1 (86%), 3 (80%), 6 (72%), and 12 (61%) month follow-up assessments. We examined missing data patterns using independent-samples t-tests and chi-square analyses to evaluate differences on pre-transplant characteristics between individuals who completed all assessment time points and those with missing data for at least one post-transplant time point. Completers were more likely than noncompleters to report coping strategies characterized by greater self-distraction, t(1558) = 2.33, p = .020, d = 0.12, 95% CI [0.03, 0.41]; substance use, t(1553) = 2.74, p = .006, d = 0.14, 95% CI [0.05, 0.27]; and positive reframing, t(1553) = 2.42, p = .016, d = 0.13, 95% CI [0.04, 0.41]. In addition, participants with missing data were more likely to be diagnosed with chronic GVHD, X2 (1) = 49.10, p < .001. There were no other significant differences between completers and noncompleters on predictor or outcome variables, including pre-transplant PTG (all p values > .05).
Most participants had been diagnosed with leukemia (33.1%), lymphoma (25.5%), or multiple myeloma (31.4%). Patients receiving autologous (52.8%) and allogeneic (38.4%) transplants participated. A majority of the sample were non-Hispanic White (96.5%) and male (60.0%), with a mean age of 53 years (range: 19–74). A complete description of demographic and clinical characteristics of the sample is displayed in Tables 1 and 2.
Table 1.
Demographic Characteristics of the Patient Sample (N = 430)
| Characteristic | n (%) |
|---|---|
| Age (in years) | |
| 19–29 | 27 (6.3%) |
| 30–39 | 38 (8.9%) |
| 40–49 | 70 (16.3%) |
| 50–59 | 150 (34.9%) |
| 60–69 | 126 (29.4%) |
| 70–79 | 19 (4.4%) |
| Gender | |
| Men | 258 (60.0%) |
| Women | 172 (40.0%) |
| Ethnicity | |
| White (non-Hispanic) | 413 (96.5%) |
| Native American | 6 (1.4%) |
| African American/Black | 4 (0.9%) |
| Hispanic/Latino | 2 (0.5%) |
| Other | 3 (0.7%) |
| No response | 2 (0.5%) |
| Relationship status | |
| Married/Living with Partner | 354 (82.3%) |
| Single | 36 (8.4%) |
| Divorced/Separated | 33 (7.7%) |
| Widowed | 7 (1.6%) |
| Education | |
| Less than 12 years | 14 (3.3%) |
| High school | 114 (26.8%) |
| Trade school/Some college | 127 (29.8%) |
| College graduate | 101 (23.7%) |
| Post-graduate degree | 70 (16.5%) |
| No response | 4 (0.9%) |
| Employment status | |
| Work full-time | 148 (34.9%) |
| Work part-time | 34 (8.0%) |
| Student | 4 (0.9%) |
| Disabled | 121 (28.5%) |
| Homemaker | 15 (3.5%) |
| Retired | 102 (24.1%) |
| No response | 6 (1.3%) |
| Annual Household Income | |
| <$25,000 | 56 (13.0%) |
| $25,000–$55,000 | 123 (30.5%) |
| $55,000–$85,000 | 113 (28.0%) |
| >$85,000 | 112 (27.7%) |
| No response | 26 (6.0%) |
Table 2.
Clinical Characteristics of the Patient Sample (N = 430)
| Characteristic | n (%) |
|---|---|
| Diagnosis | |
| Leukemia | 140 (32.9%) |
| ALL | 27 (19.3%) |
| AML | 66 (47.1%) |
| CLL | 10 (7.1%) |
| CML | 5 (3.6%) |
| MDS | 32 (22.9%) |
| Lymphoma | 134 (31.5%) |
| Non-Hodgkin | 107 (79.9%) |
| Hodgkin | 27 (20.1%) |
| Multiple myeloma | 130 (30.5%) |
| Aplastic anemia | 5 (1.2%) |
| Other | 17 (4.0%) |
| Transplant type | |
| Autologous | 243 (57.0%) |
| Allogeneic | 183 (43.0%) |
| Conditioning regimen a | |
| Fully ablative | 123 (67.2%) |
| Reduced intensity | 60 (32.8%) |
| Number of comorbid conditions | |
| 0 | 83 (26.9%) |
| 1 | 46 (14.9%) |
| 2 | 71 (23.1%) |
| 3 | 57 (18.5%) |
| 4 | 28 (9.1%) |
| 5 | 10 (3.2%) |
| 6 | 8 (2.6%) |
| 7 | 2 (0.6%) |
| 10 or more | 3 (1.0%) |
| Chronic graft-versus-host-disease (cGVHD) a | 92 (55.8%) |
| Acute graft-versus-host disease (aGVHD) a | 92 (52.6%) |
| Infections b | 181 (48.3%) |
Note. Numbers for some clinical characteristics add up to less than 430 due to data that were unavailable in the patient record. CML = chronic myeloid leukemia; CLL = chronic lymphocytic leukemia; AML = acute myeloid leukemia; ALL = acute lymphoblastic leukemia; MDS = myelodysplastic syndrome.
Allogeneic stem cell recipients only (n = 183)
Presence of infection(s) pretransplant to 1-month posttransplant
Measures
Posttraumatic Growth.
The Posttraumatic Growth Inventory (PTGI) is a 21-item questionnaire that assesses PTG in the domains of relating to others, the capacity to find new possibilities, a sense of personal strength, spiritual change, and appreciation of life (3). Each item is rated on a 6-point Likert-type scale ranging from 0 (I did not experience this change as a result of my crisis) to 5 (I experienced this change to a very great degree as a result of my crisis), with higher scores indicating greater PTG. In this study, participants were asked to consider changes as a result of “my illness.” The PTGI has been extensively used in cancer populations (e.g., 5,6,8,12,13). The six PTGI subscales demonstrated strong reliability in our sample across the pre-transplant and all post-transplant time points (α = .95–.96).
Social Support.
Participants completed the 24-item Social Provisions Scale (SPS) prior to transplant (24), which examines the following dimensions of social support: social integration (sense of belonging to a group), guidance (opportunity to receive information or advice from others), nurturance (opportunity to receive physical or emotional assistance from others), attachment (emotional closeness from which one derives a sense of security), reliable alliance (assurance that others can be counted upon for tangible assistance), and reassurance of worth (recognition of one’s competence, skills, and value by others). Each item is rated on a 4-point Likert-type scale ranging from 1 (strongly disagree) to 4 (strongly agree), with higher scores indicating greater social support. The SPS has documented validity in measuring social support among cancer patients (25) and good internal consistency in the current sample (α = .86).
Coping.
Prior to transplant, participants completed the Brief Coping Orientations to Problems Experienced Scale (Brief COPE), which evaluates 13 different coping strategies (26). The Brief COPE assesses how frequently a participant engages in a particular strategy to deal with an illness-related symptom or concern. Using methodology used by Hoyt et al. (27) and Costanzo et al. (19), coping dimensions were categorized and evaluated as emotional engagement strategies (active coping, positive reframing, emotional processing, acceptance, and planning) and emotional avoidance strategies (distraction, denial, behavioral disengagement, and substance use). Items are rated on a 4-point scale, with higher scores signifying greater use of the corresponding coping strategy. The measure has been used with adult cancer patients (17) and had good internal consistency in the current sample (α = .86).
Treatment-Related Factors.
Treatment-related information was abstracted from patient medical records and is displayed in Table 2. The predictors of interest in this study included graft type (allogeneic vs. autologous stem cells); pre-transplant conditioning regimen (fully ablative vs. reduced intensity); occurrence of post-transplant complications, including infections occurring during the month after transplant and occurrence of acute and chronic GVHD. GVHD occurrence was evaluated only within the subset of participants who received an allogeneic HCT, as autologous HCT recipients do not experience this complication.
Statistical Methods
STATA/SE 17.0 was used to analyze the data. All statistical tests were two-sided, and results were considered significant with p values < .05. A repeated-measures analysis of variance (ANOVA) with post-hoc pairwise comparisons was used to analyze changes in PTGI from pre-transplant through 1, 3, 6, and 12 months post-transplant.
Linear mixed-effects regression models were used to determine the extent to which pre-transplant psychosocial factors (SPS, COPE scales) and treatment-related factors predicted PTG scores at 1, 3, 6, and 12 months post-transplant. Separate models were run for each predictor using STATA ‘xtmixed’ routine. Age was centered at the sample mean (Mage = 52.93, SD = 12.37), and coping and social support variables were standardized based on means and standard deviations. Time since transplant was included in models as a factor variable to account for nonlinear changes of outcome measures over time. The models analyzed main effects of each predictor, as well as interactions between the predictor and time since transplant, to evaluate whether each predictor was associated with different PTGI trajectories over time. All models covaried for participant age and sex given known relationships between these variables and PTG (21).
Due to multiple tests, we used the Benjamini and Hochberg False Discovery Rate (FDR) procedure to control for both the false discovery rate and the family-wise error rate. In brief, the FDR procedure involves ranking p values from smallest to largest and requires increasingly low p values to reject the null hypothesis as the p-value rank decreases (28).
Results
Changes in PTGI Over Time
Table 3 shows mean PTGI scores across the assessment points. PTGI scores significantly increased over time, F(4,1252) = 8.24, p < .001. Pairwise comparisons indicated that that PTGI remained stable from pre-transplant to 1 month post-transplant (p = .440) as well as between 1 and 3 months post-transplant (p = .078). PTGI increased significantly between 3 and 6 months post-transplant (p < .001; d = 0.25) and remained stable between 6 and 12 months post-transplant (p = .892). Relative to pre-transplant scores, PTGI scores were significantly higher at 6 and 12 months post-transplant (effect sizes in Table 3).
Table 3.
Changes in PTGI Scores Over Time
| Assessment point | M (SD) | p | d |
|---|---|---|---|
| Pre-transplant (n = 430) | 57.62 (23.45) | – | – |
| 1 month post-transplant (n = 370) | 56.76 (23.65) | .44 | .04 |
| 3 months post-transplant (n = 346) | 59.23 (23.13) | .31 | .07 |
| 6 months post-transplant (n = 308) | 62.66 (22.73) | < .001 | .22 |
| 12 months post-transplant (n = 261) | 61.20 (22.78) | < .001 | .15 |
Note. p values and effect sizes (d) are provided for comparisons with the pre-transplant mean.
Psychosocial Factors and PTGI
Coefficients, test statistics, and p values for models evaluating the extent to which each pre-transplant social support and coping measures predicted PTGI scores across the assessment points are provided in Table 4. Interaction terms were removed in the final model if not statistically significant. Regarding social support dimensions, social integration, guidance, and nurturance scores significantly predicted PTGI scores across assessment points, although nurturance was no longer a significant predictor after the FDR procedure was applied. In contrast, reassurance of worth, attachment, and reliable alliance did not predict PTGI. There were no significant interactions between social support measures and time in the models, indicating that social support did not predict different trajectories of PTGI scores across the time points.
Table 4.
Mixed-Effects Linear Regression Models Examining Pre-Transplant Social Support Dimensions and Coping Strategies as Predictors of PTG
| Predictor | Coefficient | z | p |
|---|---|---|---|
| Social support | |||
| Social integration | 2.38 | 2.38 | .017* |
| Guidance | 3.11 | 3.12 | .002* |
| Nurturance | 2.02 | 2.01 | .044 |
| Reassurance of worth | 0.67 | 0.67 | .501 |
| Attachment | 1.78 | 1.77 | .076 |
| Reliable alliance | 1.07 | 1.06 | .290 |
| Engagement coping strategies | |||
| Active coping | 4.78 | 4.59 | < .001* |
| Positive reframing | 8.50 | 8.55 | < .001* |
| Planning | 5.83 | 5.62 | < .001* |
| Acceptance | 4.73 | 4.31 | < .001* |
| Emotional Processing | 10.55 | 6.83 | < .001* |
| Avoidant coping strategies | |||
| Self-distraction | 5.49 | 5.20 | < .001* |
| Denial | 0.07 | 0.06 | .950 |
| Substance use | 0.86 | 0.78 | .436 |
| Behavioral disengagement | 0.04 | 0.04 | .968 |
p value is statistically significant after FDR procedure.
Note. PTGI scores were assessed pre-HCT and 1-, 3-, 6-, and 12-months post-HCT. All models covaried for time since transplant, age, and gender. Social support and coping strategies scores were standardized. Therefore, coefficients represent change in PTGI score for each one standard deviation change in coping strategy or social support measure.
Emotional engagement strategies—including active coping, positive reframing, acceptance, emotional processing, and planning—predicted greater PTGI scores across the assessment points. Most coping styles characterized by emotional avoidance—including behavioral disengagement, denial, and substance use—did not predict PTGI. The exception was use of self-distraction, which significantly predicted higher PTGI scores. There was a significant interaction between acceptance and time since transplant (see Figure 1). Upon probing the interaction by quartile, the findings indicated that patients in the lowest quartile of acceptance had low PTGI scores from pre- to post-transplant. The pattern of results suggested that patients with the lowest acceptance scores prior to transplant also had especially low PTGI scores at 12 months post-transplant. There were no other significant interactions between coping measures and time, suggesting a relatively stable influence of most pre-HCT coping strategies on PTGI across the assessment points.
Figure 1. Acceptance as a Moderator of Post-Transplant Changes in PTG.
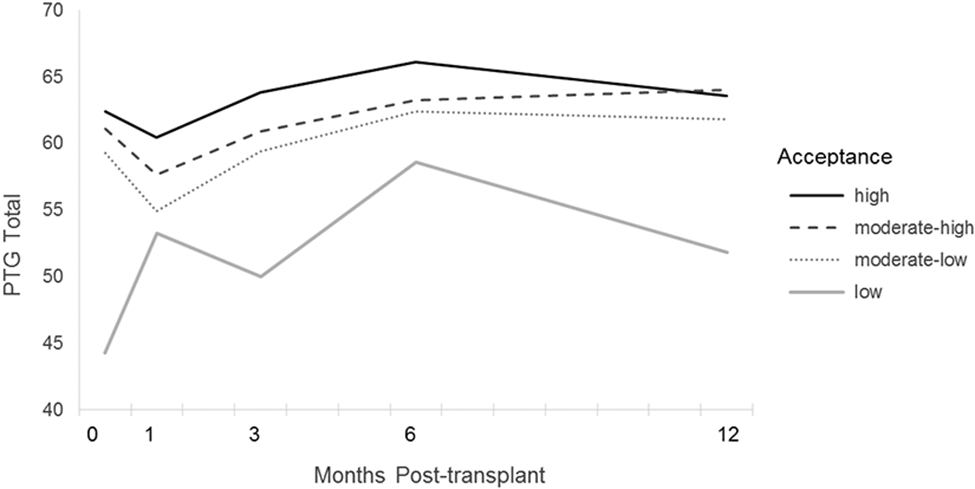
Note. Changes over time in mean PTG scores for participants in upper, upper-middle, lower-middle, and lower quartiles on acceptance. A significant interaction between acceptance and time was found, in which participants in the lowest quartile on acceptance showed markedly lower PTGI scores at the pre-transplant and 12-months post-transplant assessment points to participants reporting moderate or high acceptance.
Treatment-Related Factors and PTGI Scores
After covarying for age, sex, and time since transplant, no significant relationships were observed between treatment-related factors and PTGI scores (see Table 5). There were also no significant interactions between treatment-related factors and time since transplant in the models predicting PTGI scores.
Table 5.
Mixed-Effects Linear Regression Models Examining Treatment-Related Factors as Predictors of PTG
| Predictor | Coefficient | z | p |
|---|---|---|---|
|
Graft type: Autologous (reference) vs. allogeneic |
0.144 | 0.07 | .946 |
|
Conditioning regimen: Reduced intensity (reference) vs. ablative |
−3.656 | −1.04 | .298 |
|
Occurrence of acute GVHD: No (reference) vs. yes |
−1.810 | −0.56 | .576 |
|
Occurrence of chronic GVHD: No (reference) vs. yes |
1.882 | 0.57 | .576 |
|
Occurrence of infections: No (reference) vs. yes |
0.773 | 0.36 | .720 |
| Number of comorbid conditions | 0.280 | 0.46 | .646 |
Note. All models covaried for time since transplant, age, and gender. Coefficients represent the difference in PTGI scores between the reference and comparison group. Occurrence of acute and chronic GVHD was examined among those receiving an allogeneic transplant only.
Discussion
Although prior research has focused on the negative psychological impact of HCT, our findings suggest that individuals recovering from HCT may also experience psychological growth. With most prior work focusing on cross-sectional and/or long-term outcomes, to our knowledge, this is the first study to prospectively and longitudinally evaluate changes in PTG during post-transplant recovery. This approach enabled us not only to examine the predictive power of individual characteristics on post-transplant PTG but also to understand changes over time within participants. We found that that HCT recipients had significantly higher PTGI scores at 6 and 12 months post-transplant as compared to the pre-transplant baseline assessment. Results suggest that PTGI scores are lowest during the acute post-transplant recovery phase, when the physical and psychological burden of the hospital stay, treatment side effects, and risk for early complications are greatest. Scores indicated that, on average, patients experienced positive changes reflective of PTG between “a small degree” and “a medium degree.” PTGI scores increased by 6-months post-transplant, when most patients are returning to a more normal routine. Patients reported “a medium degree” of positive change, on average. Scores then remained stable between 6 and 12 months post-transplant, suggesting that these positive psychological effects are not short-lived, but rather, persistent. PTGI scores were with the same range as other studies of cancer patients and survivors, including patients with breast, liver, and hematologic cancers (5, 29, 30). Of note, allogeneic transplant survivors had scores that were about 10 points higher than the present sample at 9 years post-transplant (12), so growth may continue beyond the study timeframe.
The general trend of PTGI scores belies significant individual variation in both the extent of PTG and its trajectory over time. Our findings suggest that individual differences in social support and coping approach explain some of these differences. Consistent with previous findings in cancer samples (13,14), supportive social relationships appeared to facilitate HCT survivors’ ability to find meaning and grow from experiences. In particular, HCT recipients appear to benefit from experiencing a sense of belonging and being able to rely on others for advice or assistance (13,14). It may be that interactions with supportive others stimulate therapeutic cognitive processes and search for meaning (14). According to Tedeschi and Calhoun’s (2004) model, supportive others may help patients positively alter perceptions and adjust schemas to changing situations. Social relationships may provide a supportive context for exploring and accepting painful internal experiences and receiving validation. Input and advice from others may help patients find meaning in their experience. The empathetic response of others during self-disclosure may also enhance patients’ self-perceptions of strength, confidence, and efficacy (14). Interestingly, reassurance of worth, attachment, and reliable alliance did not predict PTG. It is notable that social integration, guidance, and nurturance are the subscales whose items focus on engagement with social supports, and this may explain the stronger association with PTG. The items from the reassurance of worth, attachment, and reliable alliance, on the other hand, focus on the degree to which supportive others value the patient’s skills or abilities, intimacy with supports, and reliability of supports (24).
Individual differences in coping strategies measured prior to transplant also predicted post-transplant PTG. Consistent with previous findings (14,16), coping approaches that involved active engagement with stressors (e.g., planning, active coping) and difficult internal experiences (e.g., emotional processing) appeared to facilitate PTG. In contrast, avoidance of difficult thoughts and emotions (e.g., denial, disengagement) generally did not either hinder or facilitate PTG, with the exception of distraction. These findings are consistent with some prior research evaluating PTG in cancer patients suggesting that distraction may serve as an adaptive strategy, especially early on (14, 17). Distraction may indeed be adaptive during the most challenging weeks following transplant. Later, openness and an ability to reflect on difficult thoughts and emotions may allow patients to discover meaning from a very difficult experience. Tedeschi and Calhoun (2004) theorized that active coping may facilitate PTG when an individual engages in cognitive processing of the situation to reconstruct basic assumptions and create new goals, schemas, and meanings (20,32). Effective active coping has been associated with meaning-making, in which HCT patients used positive appraisal to find meaning in their transplant experience, minimize distress associated with uncertainty, and more effectively manage physical symptoms (20,32).
Treatment regimen and development of complications were not associated with PTGI scores. The findings herein indicate that coping and social support appear to play a more salient role in predicting PTG for HCT patients than do characteristics of the stressful experience. Social support and coping styles are thought to be stable over time (33), so it may be that PTG is more strongly shaped by longstanding traits in combination with adversity. It may also be that PTG is more strongly influenced by patients’ subjective appraisals of the adversity than objective measures, which is consistent with prior research among breast cancer patients (33). In another study of HCT patients, subjective concerns about mortality were positively associated with PTG, whereas objective measures of mortality risk given by clinicians were unrelated to PTG (21). An additional possibility is that the severely physically taxing experience of HCT functions as a ceiling effect for treatment intensity in this sample; results may be different if the sample included cancer patients with greater variation in treatment intensity and impact.
Study Limitations
Limitations of the study include the ethnic, racial, and religious homogeneity of the sample (i.e., primarily Caucasian participants with protestant or Catholic religious affiliations); while consistent with the demographics of the catchment area of our cancer center, our ability to generalize the findings to other demographic groups is restricted. Chronic GVHD cases were slightly underrepresented in our sample, which may also affect generalizability. Participants who did not complete the study reported more use of distraction, positive reframing, and substance use, affecting variation on these measures; however, there were no differences in their PTGI scores. In addition, as this is an observational study, we cannot draw definite conclusions or determine mechanisms of the relations among study variables. Because social support and coping were only assessed pre-transplant, we could not evaluate them as time-varying predictors. Interventional study designs may help to tease apart the likely complex, bidirectional relationships between psychosocial factors and PTG.
Clinical Implications and Conclusions
In conclusion, results suggest that HCT patients experience small effect size increases in PTG during the first year following transplant, with the primary gains occurring during the first 6 months post-transplant and remaining stable thereafter. Supportive social relationships, including both receiving and providing support to others, as well as engagement with difficult thoughts and emotions, may facilitate PTG after transplant. Our findings indicate that potentially modifiable psychosocial factors, rather than medical characteristics of the treatment or post-transplant recovery, are most important for PTG. A modest increase in PTG was observed in the absence of any intervention, highlighting the potential for interventions that foster social support and adaptive coping to yield a larger and more clinically meaningful effect on PTG. Mindfulness-based interventions, which facilitate PTG by helping HCT recipients engage with difficult private experiences, and interventions that encourage HCT recipients to utilize their support networks may be particularly fruitful in facilitating PTG after HCT. Furthermore, given the stable influence of coping responses on PTG observed here, it may be prudent to identify coping responses early in the HCT recovery. Integration of mental health care into cancer treatment could facilitate early assessment and intervention to improve coping responses and promote engagement and increased social support among HCT patients.
Acknowledgements:
This research was supported by NIH National Cancer Institute (NCI) grants K07 CA136966 and R21 CA133343, the University of Wisconsin Carbone Cancer Center Support Grant P30 CA014520, the Clinical and Translational Science Award (CTSA) program through the NIH National Center for Advancing Translational Sciences (NCATS) grant UL1 TR002372, the Forward Lymphoma Research Foundation, and a University of Wisconsin Hilldale Award.
Footnotes
Conflict of Interest Statement: The authors have no conflicts of interest to declare.
Data Availability Statement:
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
- 1.Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006; 354: 1813–1826. doi: 10.1056/nejmra052638 [DOI] [PubMed] [Google Scholar]
- 2.Bishop MM, Wingard JR. Thriving after hematopoietic stem cell transplant: A focus on positive changes in quality of life. Expert Rev Pharmacoecon Outcomes Res. 2004; 4: 111–123. doi: 10.1586/14737167.4.1.111 [DOI] [PubMed] [Google Scholar]
- 3.Tedeschi RG, Calhoun LG. The post-traumatic growth inventory: Measuring the positive legacy of trauma. J Trauma Stress.1996; 9: 455–471. doi: 10.1007/bf02103658 [DOI] [PubMed] [Google Scholar]
- 4.Helgeson VS, Reynolds KA, Tomich PL. A meta-analytic review of benefit finding and growth. J Consult Clin Psychol. 2006; 74: 797–816. doi: 10.1037/0022-006X.74.5.797 [DOI] [PubMed] [Google Scholar]
- 5.Dunigan J, Carr T, Steel B. Posttraumatic growth, immunity and survival in patients with hepatoma. Dig Dis Sci. 2007; 52: 2452–2459. doi: 10.1007/s10620-006-9477-6 [DOI] [PubMed] [Google Scholar]
- 6.Bellizzi KM, Blank TO. Predicting posttraumatic growth in breast cancer survivors. Health Psychol. 2006; 25: 47–56. doi: 10.1002/pon.3380 [DOI] [PubMed] [Google Scholar]
- 7.Connerty TJ, Knott V. Promoting positive change in the face of adversity: Experiences of cancer and post-traumatic growth. European J Cancer Care. 2013; 22: 334–344. doi: 10.1111/ecc.12036 [DOI] [PubMed] [Google Scholar]
- 8.Danhauer SC, Case LD, Tedeschi R, et al. Predictors of posttraumatic growth in women with breast cancer. Psycho-Oncol. 2013; 22: 2672–2783. doi: 10.1002/pon.3298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pat-Horenczyk R, Perry S, Hamama-Raz Y, Ziv Y, Schramm-Yavin S, Stemmer S. Posttraumatic growth in breast cancer survivors: Constructive and illusory aspects. J Trauma Stress. 2015; 28: 214–222. doi: 10.1002/jts.22014 [DOI] [PubMed] [Google Scholar]
- 10.Stanton AL, Bower JE, Low CA. Posttraumatic growth after cancer. In: Calhoun LG, Tedeschi RG, eds. Handbook of Posttraumatic Growth: Research and Practice. New York, NY: Lawrence Erlbaum Associates; 2006: 138–175. [Google Scholar]
- 11.Andrykowski MA, Bishop MM, Hahn EA, et al. Long-term health-related quality of life, growth, and spiritual well-being after hematopoietic stem-cell transplantation. J Clin Oncol. 2005; 23: 599–608. doi: 10.1200/jco.2005.03.189 [DOI] [PubMed] [Google Scholar]
- 12.Tallman B, Shaw K, Schultz J, Altmaier E. Well-being and posttraumatic growth in unrelated donor marrow transplant survivors: A nine-year longitudinal study. Rehab Psychol. 2010; 55: 204–210. doi: 10.1037/a0019541 [DOI] [PubMed] [Google Scholar]
- 13.Nenova M, DuHamel K, Zemon V, Rini C, Redd WH. Posttraumatic growth, social support, and social constraint in hematopoietic stem cell transplant survivors. Psycho-Oncol. 2013; 22: 195–202. doi: 10.1002/pon.2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lelorain S, Tessier P, Florin A, Bonnaud-Antignac A. Posttraumatic growth in long term breast cancer survivors: Relation to coping, social support and cognitive processes. J Health Psychol. 2012; 17: 627–639. doi: 10.1177/1359105311427475 [DOI] [PubMed] [Google Scholar]
- 15.McDonough MH, Sabiston CM, Wrosch C. Predicting changes in posttraumatic growth and subjective well-being among breast cancer survivors: The role of social support and stress. Psycho-Oncol. 2014; 23: 114–120. doi: 10.1002/pon.3380 [DOI] [PubMed] [Google Scholar]
- 16.Schmidt SD, Blank TO, Bellizzi KM, Park CL. The relationship of coping strategies, social support, and attachment style with posttraumatic growth in cancer survivors. J Health Psychol. 2011; 17: 1033–1040. doi: 10.1177/1359105311429203 [DOI] [PubMed] [Google Scholar]
- 17.Scrignaro M, Barni S, Magrin ME. The combined contribution of social support and coping strategies in predicting post-traumatic growth: A longitudinal study on cancer patients. Psycho-Oncol. 2011; 20: 823–831. doi: 10.1002/pon.1782 [DOI] [PubMed] [Google Scholar]
- 18.Schroevers MJ, Helgeson VS, Sanderman R, Ranchor AV. Type of social support matters for prediction of posttraumatic growth among cancer survivors. Psycho-Oncol. 2010; 19: 46–53. doi: 10.1002/pon.1501 [DOI] [PubMed] [Google Scholar]
- 19.Costanzo ES, Lutgendorf SK, Rothrock NE, Anderson B. Coping and quality of life among women extensively treated for gynecologic cancer. Psycho-Oncol. 2006; 15: 132–142. doi: 10.1002/pon.930 [DOI] [PubMed] [Google Scholar]
- 20.Rajandram RK, Jenewein J, McGrath C, Zwahlen RA. Coping processes relevant to posttraumatic growth: An evidence-based review. Support Care Cancer. 2011; 19: 583–589. doi: 10.1007/s00520-011-1105-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Widows MR, Jacobsen PB, Booth-Jones M, Fields KK. Predictors of posttraumatic growth following bone marrow transplantation for cancer. Health Psychol. 2005; 24: 266–273. doi: 10.1037/0278-6133.24.3.266 [DOI] [PubMed] [Google Scholar]
- 22.Lechner SC, Zakowski SG, Antoni MH, Greenhawt M, Block K, Block P. Do sociodemographic and disease-related variables influence benefit-finding in cancer patients? Psycho-Oncol. 2003; 12: 491–499. doi: 10.1002/pon.671 [DOI] [PubMed] [Google Scholar]
- 23.Bush NE, Donaldson GW, Haberman MH, Dacanay R, Sullivan KM. Conditional and unconditional estimation of multidimensional quality of life after hematopoietic stem cell transplantation: A longitudinal follow-up of 415 patients. Biol Blood Marrow Transplant. 2000; 6: 576–591. doi: 10.1016/s1083-8791(00)70067-x [DOI] [PubMed] [Google Scholar]
- 24.Cutrona CE, Russell D. The provisions of social relationships and adaptation to stress. In: Jones WH, Perlman D, eds. Advances in Personal Relationships (Vol. 1). Greenwich, CT: JAI Press; 1987: 37–67. [Google Scholar]
- 25.Mallinckrodt B, Armer JM, Heppner PP. A threshold model of social support, adjustment, and distress after breast cancer treatment. J Couns Psychol. 2012; 59: 150–160. doi: 10.1037/a0026549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carver CS. You want to measure coping but your protocol’s too long: Consider the Brief COPE. Int J Behav Med. 1997; 4: 92–100. doi: 10.1207/s15327558ijbm0401_6 [DOI] [PubMed] [Google Scholar]
- 27.Hoyt MA, Marin-Chollom AM, Bower JE, Thomas KS, Irwin MR, Stanton AL. Approach and avoidance coping: Diurnal cortisol rhythm in prostate cancer survivors. Psychoneuroendocrinology. 2014; 49: 182–186. doi: 10.1016/j.psyneuen.2014.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benjamini Y, Hochberg Y. Controlling the false discovery rate - A practical and powerful approach to multiple testing. J Royal Statist Soc., Series B. 1995; 57: 289–300. doi: 10.2307/2346101. [DOI] [Google Scholar]
- 29.Danhauer SC, Russell GB, Tedeschi RG, Jesse MT, Vishnevsky T, Daley K, Carroll S, Triplett KN, Calhoun LG, Cann A, Powell BL. A longitudinal investigation of posttraumatic growth in adult patients undergoing treatment for acute leukemia. J Clin Psychol Med Settings. 2013; 20: 13–24. doi: 10.1007/s10880-012-9304-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sears SR, Stanton AL, Danoff-Burg S. Yellow brick road and the emerald city: Benefit finding, positive reappraisal coping and posttraumatic growth in women with early-stage breast cancer. H Psychol. 2003; 22: 487–497. doi: 10.1037/0278-6133.22.5.487 [DOI] [PubMed] [Google Scholar]
- 31.Adelstein KE, Anderson JG, Taylor AG. Importance of meaning-making for patients undergoing hematopoietic stem cell transplantation. Oncol Nursing Forum. 2014; 41: E172–E184. doi: 10.1188/14.onf.e172-e184 [DOI] [PubMed] [Google Scholar]
- 32.Lelorain S, Bonnaud-Antignac A, Florin A. Long term posttraumatic growth after breast cancer: Prevalence, predictors and relationships with psychological health. J Clin Psycol Med Settings. 2010; 17: 14–22. doi: 10.1007/s10880-009-9183-6 [DOI] [PubMed] [Google Scholar]
- 33.Koutrouli N, Anagnostopoulos F, Potamianos G. Posttraumatic stress disorder and posttraumatic growth in breast cancer patients: A systematic review. Women & Health. 2012; 52: 503–516. doi: 10.1080/03630242.2012.679337 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


