Abstract
Significance.
Children are being fitted at younger ages with soft contact lenses for myopia control. This three-year investigation of adverse events related to contact lens wear in 7–11 year old participants helps optometrists understand what to expect when fitting children with soft contact lenses.
Purpose.
The purpose of this paper is to report the frequency and type of ocular and non-ocular adverse events related to soft contact lens wear in children.
Methods.
Seven to 11 year old children wore soft contact lenses for three years. Adverse events were defined by a slit lamp examination finding of grade 3 or worse; parental report of a clinically meaningful change (determined by the examiner) in eyes, vision, or health; or a clinically meaningful response (determined by examiner) to a symptom checklist. Adverse events were categorized and reported by examiners and finalized by the executive committee. The presence or absence of an infiltrate and a list of diagnoses was determined at the conclusion of the study.
Results.
The 294 participants wore their contact lenses 73.0 ± 26.5 hours per week, and 220 (74.8%) encountered at least one adverse event. Of the 432 adverse events, 75.2% were ocular and 24.8% were non-ocular. Contact lens wear was probably or definitely related to 60.6% of the ocular and 2.8% of the non-ocular adverse events. None of the ocular adverse events were serious, severe, or caused permanent contact lens discontinuation. The corneal infiltrate incidence was 185 cases/10,000 patient-years of wear (95% CI: 110—294). The incidence of moderate ocular adverse events that were definitely or probably related to contact lens wear was 405 cases/10,000 patient-years of wear (95% CI: 286—557).
Conclusions.
The adverse events experienced by 7–11 year old myopic children rarely required meaningful treatment and never led to permanent discontinuation of contact lens wear or loss of best-corrected vision.
Today, children are fit with contact lenses more often and at earlier ages, due primarily to the availability of daily disposable contact lenses.1, 2 As growing evidence supports contact lens-based treatments to slow eye growth and with the first myopia control indication approved by the Food and Drug Administration in November 2019, contact lenses prescribed for myopia control in young children are likely to increase even more.3–5
Safety is one of the primary concerns of both parents and practitioners when fitting a child with soft contact lenses, although the rates of non-infectious inflammatory events has been shown to be comparable between children6 and adults.7 A recent paper combined data from retrospective chart reviews and a prospective clinical trial of children between the ages of 8 and 16 years to estimate the risk of ocular adverse events.6 A total of 2,713 patient-years of wear elicited an annualized rate of non-infectious inflammatory adverse events of 660 cases per 10,000 years of wear (95% CI: 390—1,050) and two cases of presumed or probable microbial keratitis resulted in a rate of 7.4 cases per 10,000 years of wear.6 A subset of 144 of those children who were 8 to 12 years old at baseline and were prospectively examined for 6 years exhibited a corneal infiltrative event rate of 61 cases per 10,000 patient-years of wear, with no serious adverse events reported.8
The Contact Lens Assessment in Youth (CLAY) Study Group conducted retrospective chart reviews and showed that the prevalence of corneal infiltrative events and of contact lens discontinuation due to adverse events are lower in 8–17 year old children than in 18 to 25 year old adults,9, 10 likely due to 18–25 year old adults participating in more risky behaviors related to contact lens wear than the 8 to 17 year old children.11
A two-year prospective study of 240 Chinese children between 7 and 14 years of age reported 55 adverse events, which yielded an adverse event incidence of 0.14 cases per 10,000 patient-years. However, almost one-third of the participants discontinued participation in the study, and half of the discontinuations were within the first 3 months of the study.12
A comprehensive examination of the literature evaluating soft contact lens wear in children concluded that the risk of corneal infiltrative events is similar to, if not lower than, adults.13 The paper also called for prospective studies of myopia control, particularly those with low rates of losses to follow-up, to rigorously document adverse events and to report the results. The purpose of this paper is to report the frequency and type of ocular and non-ocular adverse events related to soft contact lens wear in children.
METHODS
A total of 294 myopic children, ages 7 to 11 years were enrolled in the Bifocal Lenses In Nearsighted Kids (BLINK) Study between September 2014 and June 2016 at either the University of Houston College of Optometry or The Ohio State University College of Optometry. The BLINK Study was a double-masked, 3-year, randomized clinical trial to determine if children wearing multifocal soft contact lenses have a slower rate of myopic progression compared to those wearing single vision contact lenses. At enrollment, participants had −0.75 to −5.00 D spherical component and less than 1.00 D astigmatism in each eye, as measured by cycloplegic autorefraction, and no more than 2.00 D of anisometropia. At the time of enrollment, all participants were free of eye disease, binocular vision problems, and systemic conditions (including use of chronic medications), that could affect vision, vision development, or contact lens wear. This research was reviewed by an independent ethical review board and conforms with the principles and applicable guidelines for the protection of human subjects in biomedical research. The study design and protocols also adhered to the tenets of the Declaration of Helsinki. The child’s assent and the permission from the parent were obtained in an informed consent process, using procedures and documentation that were reviewed and approved by the Institutional Review Board. Complete entry criteria, baseline characteristics, methods, and primary study results were previously reported.14, 15 The methods relevant to the current analyses are described below. The trial was registered with ClinicalTrials.gov (NCT02255474).
During the baseline visit, eligibility was verified, a comprehensive eye examination was conducted, and all outcome variables, including cycloplegic autorefraction and axial length, were measured. Participants attended visits one week (contact lens dispense) and three weeks (contact lens check) after the baseline visit, then every six months for three years. They also attended unscheduled visits as needed. Participants were randomly assigned to wear Biofinity single vision, Biofinity Multifocal “D” with a +1.50 D add power, or Biofinity Multifocal “D” with a +2.50 D add power soft contact lenses (CooperVision; Pleasanton, CA) on a daily wear-only basis for three years. If the fit was deemed clinically poor, the participant was fitted with Proclear or Proclear Multifocal “D” contact lenses as the primary backup lens. Other contact lenses were used if deemed clinically necessary to obtain a satisfactory fit. All participants initially received Biotrue contact lens solution (Bausch + Lomb; Rochester, NY). OptiFree Puremoist or Clear Care solutions (Alcon; Ft. Worth, TX) were provided when the examiner decided it was clinically necessary. All contact lens materials were provided at no charge, and back-up glasses were provided either at discount or no charge, depending on the frame chosen by the participant.
At the baseline visit and every six months, conjunctival redness, limbal redness, corneal neovascularization, corneal staining, papillary conjunctivitis, blepharitis, meibomian gland dysfunction, and corneal infiltrates were graded using the Efron Grading Scales.16 For corneal staining, fluorescein was applied by touching a saline-moistened strip to the superior bulbar conjunctiva. Observations were made with a blue light and yellow barrier filter.
Adverse Events
An adverse event was identified in the BLINK Study by three different methods at each study visit:
The participant experienced or presented with ocular signs or symptoms worse than those encountered during routine contact lens wear (e.g., a slit lamp sign of ≥ grade 3 on the Efron Grading Scales). An unmasked examiner determined whether an Adverse Event Form was completed.
- At every visit, an unmasked examiner asked the participant’s parent or guardian, “Has your child experienced any changes in his or her eyes, vision, or health since the last visit?” After asking follow-up questions, an unmasked examiner documented an adverse event if:
- the condition was chronic, not previously documented, and required a change in medications or change in daily activities over an extended period, or
- the examiner felt the event was acute and warranted documentation, such as a broken bone or head trauma (not warranted were cold, flu, ear infection, etc.).
At every visit after the baseline visit, an unmasked examiner asked the parent, “Have you or your child noticed any of the following symptoms related to his or her eyes since the last visit?” The symptoms listed were burning or stinging, itching, dryness, poor comfort at the end of the day, excessive tearing or discharge, blurry vision, and headache. The parent or participant answered “yes” or “no” to each symptom. If the answer was “yes,” then follow-up questions were asked to determine whether the symptom warranted completion of an Adverse Event Form.
Categorization of an Adverse Event
Potential adverse events were categorized by an unmasked examiner according to the category definitions in Table 1. It should be noted that the definition of a serious adverse event differs from the International Organization for Standardization guidance for clinical investigations of contact lenses (ISO 11980), which states, “Serious adverse events are those events that result in, or have potential to cause, either permanent impairment of an ocular function or damage to an ocular structure…” Our definition, “Fatal, life threatening, permanently disabling (≥2-line loss of best-corrected visual acuity) or requires or prolongs hospitalization” could result in a difference in the number of serious adverse events based on each of the definitions.
Table 1.
Categorization of adverse events.
| Type | Ocular: Pertaining to the eye and adnexa |
| Non-ocular: All other events | |
|
| |
| Significance | Serious: Fatal, life threatening, permanently disabling (≥2 line loss of best-corrected visual acuity), or requires or prolongs hospitalization |
| Non-serious: all other events | |
|
| |
| Severity | Mild: Easily tolerated signs or symptoms that do not change activities or require prescription |
| Moderate: Leads to signs or symptoms that interfere with daily activities or are treated with prescription | |
| Severe: Incapacitating or sight-threatening and generally requires prescription | |
|
| |
| Discontinuation | No: Doctor would not have discontinued contact lens wear |
| Temporarily: Doctor would have interrupted contact lens wear | |
| Permanently: Doctor would have permanently discontinued contact lens wear | |
|
| |
| Related | Definitely: timely relationship to contact lenses and follows known pattern of response for which no alternative cause is present |
| Probably: timely relationship to contact lenses and follows known pattern of response, but alternative cause may be present | |
| Possibly: timely relationship to contact lenses and follows no known pattern of response, but no potential alternative cause | |
| Unrelated: definitely related to cause other than contact lenses | |
Final Acceptance of an Adverse Event
Final acceptance of whether each submitted event was an adverse event was decided by a majority vote of the Executive Committee (JJW, DOM, DAB, LAJ) during weekly conference calls. If deemed necessary, the Executive Committee also discussed the unmasked examiner’s categorization of each adverse event.
Diagnosis and Infiltrates
At the conclusion of the study, after reading through all the Adverse Event Forms, a list of diagnoses was established by one investigator (JJW). Two investigators (JJW and DOM) used the list to independently place a diagnosis on each adverse event. All mismatched diagnoses were discussed to reach agreement, and a few more diagnoses were added to the list to improve matched diagnoses between the two investigators. Finally, all adverse events that still did not match diagnoses from the two investigators were adjudicated by the Executive Committee. At the conclusion of the study, a new category was created to indicate whether corneal infiltrates were present based solely on the case description written by the examiner in the Adverse Event Form. Solution-induced corneal staining was not defined a priori; it was diagnosed by examiners based on clinical acumen.
Contact Lens Wearing Time
Wearing time was calculated from the parents’ report of the typical time the child inserted and removed contact lenses on weekdays and weekends, as well as the number of weekdays and number of weekend days that the child wore the contact lenses. These data were collected every six months from the six-month visit until the three-year visit. The hours per day were determined by calculating the number of hours between application and removal for weekdays and weekends. The number of hours per day was multiplied by the total number of weekdays and weekend days the parent reported that the participant typically wore his or her contact lenses. These numbers were then averaged across all the visits.
Patient-years of contact lens wear were calculated by determining the number of years from initiation of contact lens wear to the date of crossover or last examination in the study.
Statistical Analyses
All statistics were calculated using SAS 9.4 (SAS Institute Inc.; Cary, NC). Mean and standard deviations were calculated for all continuous variables, and frequencies were reported for categorical variables. The average hours of contact lens wear time per week were compared between the three treatment groups using a one-way analysis of variance (ANOVA). Observed frequencies of ocular, non-ocular, and total adverse events were compared to expected frequencies in each age and treatment group using a chi-squared test. Based on a median split (–3.75 D) of the participants’ lens power at the final study visit, the proportion of adverse events in participants whose lens power at the final visit was more myopic than the sample median was compared to the proportion of adverse events in participants whose lens power was less myopic than the median at their last visit using a chi-squared test. The Mid-P exact test was used to calculate confidence intervals for incidence rates.17
RESULTS
The average (± standard deviation) age at baseline of the 294 participants was 10.3 ± 1.2 years, 60.2% were female, 68% were white, 10% were black, 9% were Asian, and 26% indicated Hispanic ethnicity.14 After three years in the study, 91.8% of the participants were still wearing their originally assigned contact lens modality. The average contact lens wearing time was 73.0 ± 26.5 hours per week. The wearing time includes participants who switched to glasses (contact lens wearing time = 0 hours) and those wearing contact lenses other than the ones to which they were randomly assigned, but excludes one participant in the single vision group who did not attend any visits after the one-week visit (Table 2). Of the 273 participants wearing contact lenses at the three-year visit, 15 (5.5%) were wearing Proclear and two (0.7%) were wearing other materials. Four (1.5%) of the participants were wearing toric contact lenses. The mean ± standard deviation wearing time (hours per week) was 73.0 ± 27.5 for the single vision group, 72.3 ± 27.1 for the +1.50 D add group, and 73.8 ± 25.2 for the +2.50 D add group (ANOVA, P = .92).
Table 2.
The number of participants who were originally fit in each contact lens type, and what they were wearing at the end of the three-year study.
| Single Vision | +1.50 D add | +2.50 D add | |
|---|---|---|---|
| Originally fit in (n=294) | 98 | 98 | 98 |
| Withdrew (n=2) | 1 | 1 | 0 |
| Lost to follow-up (n=5) | 1 | 1 | 3 |
| Wearing spectacles (n=14) | 7 | 3 | 4 |
| Wearing other contact lens than assigned (n=3) | 0 | 0 | 3 |
| Wearing assigned contact lenses (n=270) | 89 | 93 | 88 |
Over the course of three years, 220 (74.8%) of the 294 participants experienced at least one adverse event, and 151 (51.3%) experienced at least one adverse event probably or definitely related to contact lens wear. While most of the non-ocular adverse events occurred in the third year (n = 49, 45.8%), 32 (29.9%) occurred during the first year and 26 (24.3%) occurred during the second year. On the other hand, more than half (n = 163, 50.2%) of the ocular adverse events happened during the first year, 90 (27.7%) during the second year, and 72 (22.1%) during the third year. A total of 432 adverse events were documented, including 70 participants who experienced two adverse events, 33 participants who experienced three adverse events, and 22 participants who experienced four or more adverse events. Of the 432 adverse events, 107 (24.8%) were non-ocular and 325 (75.2%) were ocular. The proportion of ocular, non-ocular, and all adverse events by category is shown in Table 3. The majority of adverse events were ocular, non-serious, mild events that did not result in discontinuation of contact lens wear.
Table 3.
The number (percent) of ocular, non-ocular, and total adverse events by category.
| Ocular n (%) |
Non-Ocular n (%) |
All n (%) |
|
|---|---|---|---|
| Total | 325 | 107 | 432 |
| Significance | |||
| Non-Serious | 325 (100) | 101 (94) | 426 (99) |
| Serious | 0 (0) | 6 (6) | 6 (1) |
|
| |||
| Severity | |||
| Mild | 240 (74) | 39 (36) | 279 (65) |
| Moderate | 85 (26) | 62 (58) | 147 (34) |
| Severe | 0 (0) | 6 (6) | 6 (1) |
|
| |||
| Discontinuation | |||
| No | 225 (69) | 105 (98) | 330 (76) |
| Interruption | 100 (31) | 2 (2) | 102 (24) |
| Permanently | 0 (0) | 0 (0) | 0 (0) |
|
| |||
| Relationship | |||
| Definitely | 158 (49) | 0 (0) | 158 (37) |
| Probably | 39 (12) | 1 (1) | 40 (9) |
| Possibly | 44 (13) | 2 (2) | 46 (11) |
| Unrelated | 56 (17) | 97 (91) | 153 (35) |
| Unknown | 28 (9) | 7 (6) | 35 (8) |
The proportion of participants in each baseline age group who experienced either an ocular or a non-ocular adverse event is presented in Figure 1. Each age group experienced a similar proportion of ocular (Chi-square, P = .20), non-ocular (Chi-square, P = .68), or all adverse events (Chi-square, P = .57). Adverse events occurred at rates similar to the proportion of children in each age group. For example, about 15% of the children were between 7 and 8 years of age at baseline and about 15% of ocular and non-ocular adverse events were experienced by this group.
Figure 1.
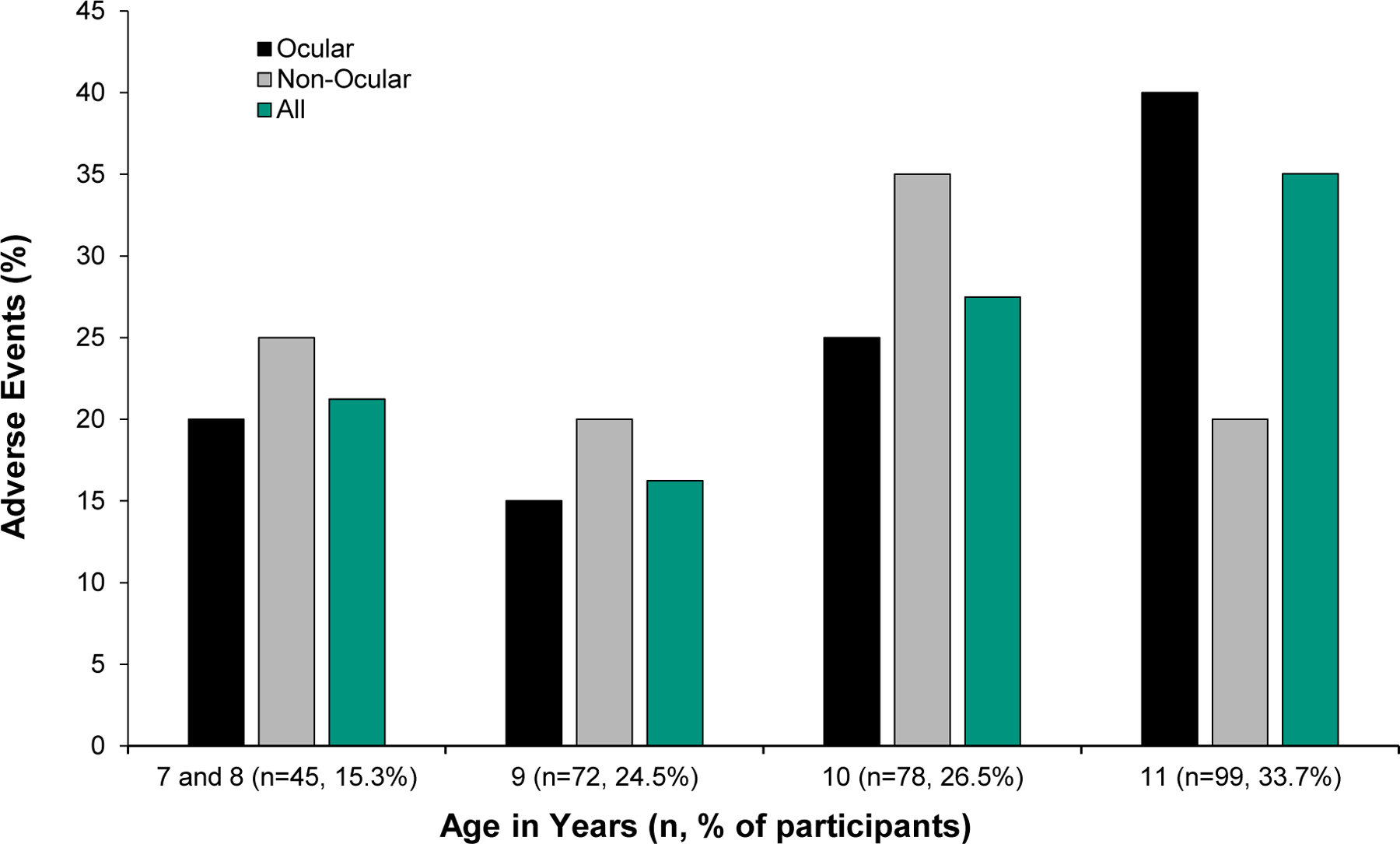
Percentage of participants by baseline age group that experienced ocular, non-ocular, and all adverse events. The percent of the total number of children in each age group is indicated in the x-axis labels. Age is not associated with adverse events, so the proportion of adverse events in each age group is similar to the proportion of participants in each age group.
Ocular Adverse Events
None of the 325 ocular adverse events were serious, severe, or caused permanent contact lens discontinuation. The relationship of the event to contact lens wear was unknown in 28 (8.6%), definite in 158 (48.6%), probable in 39 (12.0%), possible in 44 (13.6%), and unrelated in 56 (17.2%). Of the ocular adverse events, 225 (69.2%) required no contact lens discontinuation and 100 (30.8%) required interruption of contact lens wear. Nearly three-quarters (73.8%) of the adverse events were mild and 85 (26.2%) were moderate. All the cases were expected except two: one case of “letters moving” during reading and one case of unexplained vision loss that eventually resolved. Sixteen (4.9%) of the adverse events involved corneal infiltrates.
The three most common diagnoses were 91 (28.0%) cases of solution-induced corneal staining, 46 (14.2%) cases of ocular allergies, and 23 (7.1%) cases of hordeola/chalazia. See the Appendix table A1, available at [LWW insert link] for a record of all the ocular adverse events. Of the adverse events, 120 were experienced by the +2.50 D add group, 98 were experienced by the +1.50 D add group, and 107 were experienced by the single vision group (Chi-square, P = .32).
Neither the total number of ocular adverse events nor the proportion of ocular adverse events in each category differed by treatment group (Table 4).
Table 4.
Categories of ocular adverse events by treatment group.
| +2.50 Add n (%) |
+1.50 Add n (%) |
Single Vision n (%) |
Χ2 P-values |
|
|---|---|---|---|---|
| Total | 120 | 98 | 107 | .32 |
|
| ||||
| Significance | ||||
| Non-Serious | 120 (100) | 98 (100) | 107 (100) | |
| Serious | 0 | 0 | 0 | |
|
| ||||
| Severity | .19 | |||
| Mild | 85 (71) | 79 (81) | 76 (71) | |
| Moderate | 35 (29) | 19 (19) | 31 (29) | |
| Severe | 0 (0) | 0 (0) | 0 (0) | |
|
| ||||
| Discontinuation | .53 | |||
| No | 82 (68) | 72 (73) | 71 (66) | |
| Interruption | 38 (32) | 26 (27) | 36 (34) | |
| Permanently | 0 (0) | 0 (0) | 0 (0) | |
|
| ||||
| Relationship | .11 | |||
| Definitely | 52 (43) | 52 (53) | 54 (51) | |
| Probably | 18 (16) | 14 (14) | 7 (7) | |
| Possibly | 22 (18) | 11 (11) | 11 (10) | |
| Unrelated | 16 (13) | 15 (16) | 25 (23) | |
| Unknown | 12 (10) | 6 (6) | 10 (9) | |
Examination of the ocular adverse events (n = 325) indicates 240 were mild and 85 were moderate (none were severe). The most common diagnoses in the mild category were solution-induced corneal staining (n = 91), ocular allergy (n = 27), and new corneal scar (n = 14). The most common diagnoses in the moderate category were ocular allergy (n = 19), infiltrative keratitis (n = 11), blepharitis (n = 10), and giant papillary conjunctivitis (n = 10).
The median power of the right contact lens was –3.75 D at the three-year visit. Participants with a contact lens prescription at least as myopic as the median did not experience more ocular adverse events (224) than participants with contact lens prescription less myopic than the median (208 adverse events, Chi-square, P = .78).
Non-Ocular Adverse Events
Of the 107 non-ocular adverse events, six were serious: two cases were for psychiatric observation, two for injuries, one for possible meningitis, and one for fibromyalgia. Of the adverse events, 97 were unrelated to contact lens wear, two were possibly related (headache and visual symptoms with unknown cause), one was probably related (headache), and seven were unknown. Two of these non-ocular adverse events required interruption of contact lens wear: one was diagnosed with bronchitis and given steroid treatment and one was injury-related. Thirty-nine (36.5%) of the adverse events were mild, 62 (57.9%) were moderate, and 6 (5.6%) were severe. Four of the severe cases were broken bones, one was for possible meningitis, and one was for a Rathke’s cleft cyst.
Of the adverse events, 97 were unrelated to contact lens wear, two were possibly related (headache and visual symptoms with unknown cause), one was probably related (headache), and seven were unknown. Two of these non-ocular adverse events required interruption of contact lens wear: one was diagnosed with bronchitis and given steroid treatment and one was injury-related.
The most common diagnoses included 43 (40.2%) non-ocular trauma cases, 29 (27.1%) new systemic disease diagnoses, and 15 (14.0%) cases of headaches that were not vision-related, based on clinical acumen. The diagnoses are categorized by the assigned treatment group in Table 5. There was not a significant difference in the total number of adverse events based on treatment group (Chi-square, P = .25).
Table 5.
Diagnoses of non-ocular adverse events during the BLINK Study, by treatment category.
| +2.50 D add n (%) |
+1.50 D add n (%) |
Single Vision n (%) |
|
|---|---|---|---|
| Visual symptoms, no obvious cause | 1 (2.9) | 0 (0.0) | 0 (0.0) |
| Non-ocular allergy | 0 (0.0) | 1 (3.4) | 3 (7.0) |
| Non-ocular trauma | 10 (28.6) | 12 (41.4) | 21 (48.8) |
| Headaches (not vision-related) | 6 (17.1) | 3 (10.3) | 6 (14.0) |
| Concussion | 4 (11.4) | 1 (3.5) | 1 (2.3) |
| Systemic disease | 12 (34.3) | 9 (31.0) | 8 (18.6) |
| Mental health issue | 2 (5.7) | 2 (6.9) | 4 (9.3) |
| Can’t categorize | 0 (0.0) | 1 (3.5) | 0 (0.0) |
|
| |||
| Total | 35 | 29 | 43 |
Incidence Rates
The 294 participants contributed 864.9 patient-years of contact lens wear. Sixteen cases of corneal infiltrates (13 infiltrative keratitis, 1 probable microbial keratitis, 1 toxic reaction to gasoline, and 1 sterile corneal ulcer) were detected in 14 participants (two participants exhibited two distinct cases of infiltrates), so the incidence of corneal infiltrates was 185 cases / 10,000 patient-years of wear (95% CI: 110—294).
There were 35 moderate ocular adverse events that were definitely or probably related to contact lens wear. The incidence of these events was 405 cases / 10,000 patient-years of wear (95% CI: 286—557).
Microbial Keratitis
One adverse event was described as ‘probable microbial keratitis.’ The participant presented with pain, redness, and light sensitivity of the right eye that started one day prior to the examination, after wearing the contact lenses overnight. The pain began as 4/10 but progressed to 6/10 at presentation. The patient denied tearing or discharge, as well as any symptoms of the left eye. Slit lamp examination revealed pinpoint corneal staining with underlying infiltrate and stromal haze in the mid-peripheral superior cornea, mild anterior chamber reaction, diffuse bulbar injection, and mild lid edema. Following successful treatment with Moxeza, then Tobradex, then Lotemax, the participant exhibited a 0.3 mm anterior stromal scar near the superior pupil margin, with no loss of best-corrected visual acuity. The participant resumed contact lens wear.
DISCUSSION
Reporting both ocular and non-ocular adverse events aids in understanding the experience to be expected in practice when fitting 7–11-year-old children in soft contact lenses. Most children (74.8%) experienced an adverse event during the three years they were randomized to wear either single vision, +1.50 D add, or +2.50 D add daily wear soft contact lenses for myopia control. While the most common diagnoses for ocular adverse events were relatively innocuous, (solution-induced staining, ocular allergy, and hordeola/chalazion), others of greater concern (corneal infiltrates and new corneal scars) also occurred, although at a low rate. None of the 325 ocular adverse events was serious, severe, or caused permanent contact lens discontinuation. These results are in general agreement with past reports in the literature.
A three-year myopia control study using daily disposable contact lenses in 144 children between the ages of 8 and 12 years, inclusive, also reported no serious (vision-threatening and result in permanent impairment) or significant (usually are symptomatic but non–vision-threatening and result in temporary impairment) events or loss of best-corrected visual acuity. In that study, adverse events were defined as “any unfavorable and unintended sign, symptom, or disease temporally associated with the use of an investigational product, whether or not considered related to the investigational product.”18 That study reported a total of 11 adverse events that were related to contact lens wear.19 Our study reported 35 moderate ocular adverse events that were definitely or probably related to contact lens wear.15 The definitions of adverse events were similar, but not identical, between the two studies, possibly explaining the difference in number of adverse events. The MiSight study reported 4 cases of infiltrates, and our study reported 16 cases in 14 participants. The higher number of adverse events and corneal infiltrative events in our study could potentially be explained by the larger number of participants, higher rate of retention of participants over time, or replacement schedules of the contact lenses. However, there is remarkable similarity in meaningful adverse event rates related to contact lens wear between the two studies.
In the same children as reported in the three-year study of 144 children, 40 ocular adverse events were experienced in 30 participants over six years. Only one case was serious: the participant reported with uveitis related to herpes zoster. No new corneal infiltrative events were reported over the final three years of follow-up.8 The BLINK Study participants continue to be examined as part of the BLINK2 Study, so comparisons of adverse events over 6 years will also be possible in the future.
Bullimore reported a corneal infiltrative event rate of 136 cases per 10,000 years of wear (95% CI: 50—300), based upon aggregation of data from three prospective studies of children wearing soft contact lenses between the ages of 8 and 14 years.13 The six-year MiSight follow-up study found a corneal infiltrative event rate of 61 cases per 10,000 patient-years (95% CI: 24—157).8 Our study found an incidence rate of 197 cases per 10,000 patient-years of wear (95% CI: 118—308). The MiSight study may have reported lower corneal infiltrative event rates because all of the children wore daily disposable contact lenses, whereas the studies reported by Bullimore wore a mixture of daily disposable and frequent replacement,13 and the BLINK Study participants wore frequent replacement contact lenses.
This was the first longitudinal myopia control study to examine non-ocular adverse events. The largest proportion of the events were non-ocular injury, explaining approximately two-out-of-every-five cases. There was not a significant difference between the three treatment groups in terms of the number of either ocular or non-ocular adverse events that were reported.
There was no difference in the number of ocular adverse events based on age or on the amount of myopia. There was no evidence that younger children experienced more adverse events, but that may be related to the narrow age range (7–11 years) of children enrolled in the BLINK Study. As noted earlier, the CLAY Study group reported that corneal infiltrative events and contact lens discontinuation due to adverse events are actually lower in 8–17-year-old children than in 18- to 25-year-old adults.9, 10 We also found no evidence that participants with higher myopia were more likely to experience more adverse events, potentially because they are required to wear their vision correction more often. That conflicts with a previous report that showed higher myopes are more likely to experience contact lens-related complications,20 possibly because they defined higher myopia as more myopic than −5.00 D, but our definition of higher myopia was greater than −3.75 D.
Limitations
This study provides the first report of non-ocular adverse events in pediatric contact lens wearers, and a more thorough assessment of ocular adverse events, including events that didn’t did not require an intervention, than other longitudinal studies of pediatric contact lens wear. However, no study is free of limitations. Although data in this study were collected prospectively, the tabulation of cases with corneal infiltrates and categorization of diagnoses were conducted retrospectively. All free text records describing the cases were searched for ‘infiltrate,’ ‘inf,’ ‘CIE,’ ‘SEI,’ and manually examined to tabulate the number of participants who experienced infiltrates. If a different term was used or if infiltrates were not mentioned, then the cases may have been missed. Although the categorization of diagnoses occurred retrospectively, two independent diagnoses were made based on the open text box describing the adverse event, and all disagreements were adjudicated by the Executive Committee. Furthermore, all contact lens materials, solution, and eye care were provided on a regular basis and at no charge, which may have improved compliance. Related to solutions, a record of the solution used when an adverse event occurred was not made in this study, so we cannot examine whether the solution influenced adverse events, as was reported by Carnt, et al.21 Finally, this was not an FDA clinical trial, so definitions used by industry partners, such as the definition of a serious adverse event by ISO 11980, may differ from those used in this study.
CONCLUSIONS
The number of adverse events indicates that 7–11-year-old contact lens wearers should be examined on a routine basis to ensure continued good ocular health. However, the adverse events experienced rarely required medical treatment and never led to permanent discontinuation of contact lens wear or loss of best-corrected vision. Contact lenses provide a safe option for children who do not want to wear spectacles or want to try daily wear contact lenses as a method to slow the progression of myopia.
ACKNOWLEDGMENTS
NIH U10 EY023204, 023206, 023208, 023210, P30 EY007551, UL1 TR001070.
APPENDIX
Appendix table A1, available at [LWW insert link]), lists the ocular adverse events associated with three years of contact lens wear by 7–11-year-old myopic research participants. This shows that eye care practitioners should carefully evaluate entire eye of young contact lens wearers, particularly the cornea, as well ask pertinent questions about ocular comfort that may elicit adverse events.
BLINK Study Group
Executive Committee
Jeffrey J. Walline (Study Chair, The Ohio State University College of Optometry), David A. Berntsen (UH Clinic Principal Investigator, University of Houston College of Optometry), Donald O. Mutti, (OSU Clinic Principal Investigator, The Ohio State University College of Optometry), Lisa A. Jones-Jordan (Data Coordinating Center Director, The Ohio State University College of Optometry), Donald F. Everett (NEI Program Official), Jimmy Le (NEI Program Official, 2019-present)
Chair’s Center
Kimberly J. Shaw (Study Coordinator, The Ohio State University College of Optometry), Juan Huang (Investigator, The Ohio State University College of Optometry), Bradley E. Dougherty (Survey Consultant, The Ohio State University College of Optometry)
Data Coordinating Center
Loraine T. Sinnott (Biostatistician, The Ohio State University College of Optometry)
University of Houston Clinic Site (University of Houston College of Optometry)
Laura Cardenas (Clinic Coordinator), Krystal L. Schulle (Unmasked Examiner, 2014–2019), Dashaini V. Retnasothie (Unmasked Examiner, 2014–2015), Amber Gaume Giannoni (Masked Examiner), Anita Tićak (Masked Examiner), Maria K. Walker (Masked Examiner), Moriah A. Chandler (Unmasked Examiner, 2016-present), Mylan Nguyen (Data Entry, 2016–2017), Lea Hair (Data Entry, 2017-present), Augustine N. Nti (Data Entry, 2019-present)
Ohio State University Clinic Site (The Ohio State University College of Optometry)
Jill A. Myers (Clinic Coordinator), Alex D. Nixon (Unmasked Examiner, 2014–2019), Katherine M. Bickle (Unmasked Examiner), Kathleen S. Reuter (Masked Examiner, 2014–2019), Andrew D. Pucker (Masked Examiner, 2015–2016), Matthew Kowalski (Masked Examiner, 2016–2017), Ann Morrison (Masked Examiner, 2017–2019)
Data Safety and Monitoring Committee
Janet T. Holbrook (Chair, Johns Hopkins Bloomberg School of Public Health), Jane Gwiazda (Member, New England College of Optometry), Timothy B. Edrington (Member, Southern California College of Optometry at Marshall B. Ketchum University), John Mark Jackson (Member, Southern College of Optometry), Charlotte E. Joslin (Member, University of Illinois at Chicago)
Appendix Table A1.
Ocular adverse events associated with three years of contact lens wear by 7–11 year old myopic research participants.
| Part of the eye | Diagnoses | # of adverse events |
|---|---|---|
| Adnexa n = 28 8.6% |
Blepharitis | 3 |
| Hordeolum/chalazion | 23 | |
| Eyelash issue | 1 | |
| Ptosis | 1 | |
|
| ||
| Cornea n = 153 47.1% |
Solution induced corneal staining | 91 |
| Superior epithelial arcuate lesion | 5 | |
| Contact lens-associated red eye | 4 | |
| Corneal epithelial defect/erosion | 9 | |
| Corneal scar, new | 15 | |
| Infiltrative keratitis | 13 | |
| Probable microbial keratitis | 1 | |
| Sterile corneal ulcer | 2 | |
| Dry eye | 13 | |
|
| ||
| Conjunctiva n = 34 10.5% |
Conjunctivitis (any type) | 15 |
| Subconjunctival hemorrhage | 8 | |
| Giant papillary conjunctivitis | 11 | |
|
| ||
| Posterior Segment n = 13 4.0% |
Retinal issue | 10 |
| Optic nerve issue | 2 | |
| Uveitis | 1 | |
|
| ||
| Other n = 97 29.8% |
Poor contact lens fit | 2 |
| Foreign body | 3 | |
| Toxic: gas, soap, etc. in eye | 7 | |
| Solution issue | 10 | |
| Ocular allergy | 46 | |
| Ocular trauma | 5 | |
| Binocular vision/accommodation issue | 11 | |
| Color vision defect | 1 | |
| Transient visual disturbance | 2 | |
| Unspecified contact lens issue | 6 | |
| Visual symptoms, no obvious cause | 1 | |
| Headaches | 2 | |
| Can’t categorize | 1 | |
REFERENCES
- 1.Wolffsohn JS, Calossi A, Cho P, et al. Global Trends in Myopia Management Attitudes and Strategies in Clinical Practice - 2019 Update. Cont Lens Anterior Eye 2020;43:9–17. [DOI] [PubMed] [Google Scholar]
- 2.Sindt CW, Riley CM. Practitioner Attitudes on Children and Contact Lenses. Optometry 2011;82:44–5. [DOI] [PubMed] [Google Scholar]
- 3.Nichols JJ, Starcher L. Contact Lenses 2019. Contact Lens Spectrum 2020;35:18, 9, 21–5. [Google Scholar]
- 4.Eydelman M. Misight Premarket Approval Letter https://www.accessdata.fda.gov/cdrh_docs/pdf18/P180035A.pdf. Accessed: March 15, 2021.
- 5.Efron N, Morgan PB, Woods CA, et al. International Survey of Contact Lens Fitting for Myopia Control in Children. Cont Lens Anterior Eye 2020;43:4–8. [DOI] [PubMed] [Google Scholar]
- 6.Chalmers RL, McNally JJ, Chamberlain P, Keay L. Adverse Event Rates in the Retrospective Cohort Study of Safety of Paediatric Soft Contact Lens Wear: The ReCSS Study. Ophthalmic Physiol Opt 2021;41:84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stapleton F, Keay L, Edwards K, et al. The Incidence of Contact Lens-Related Microbial Keratitis in Australia. Ophthalmology 2008;115:1655–62. [DOI] [PubMed] [Google Scholar]
- 8.Woods J, Jones D, Jones L, et al. Ocular Health of Children Wearing Daily Disposable Contact Lenses over a 6-Year Period. Cont Lens Anterior Eye 2021;44:101391. [DOI] [PubMed] [Google Scholar]
- 9.Wagner H, Chalmers RL, Mitchell GL, et al. Risk Factors for Interruption to Soft Contact Lens Wear in Children and Young Adults. Optom Vis Sci 2011;88:973–80. [DOI] [PubMed] [Google Scholar]
- 10.Chalmers RL, Wagner H, Mitchell GL, et al. Age and other Risk Factors for Corneal Infiltrative and Inflammatory Events in Young Soft Contact Lens Wearers from the Contact Lens Assessment in Youth (CLAY) Study. Invest Ophthalmol Vis Sci 2011;52:6690–6. [DOI] [PubMed] [Google Scholar]
- 11.Wagner H, Richdale K, Mitchell GL, et al. Age, Behavior, Environment, and Health Factors in the Soft Contact Lens Risk Survey. Optom Vis Sci 2014;91:252–61. [DOI] [PubMed] [Google Scholar]
- 12.Sankaridurg P, Chen X, Naduvilath T, et al. Adverse Events during 2 Years of Daily Wear of Silicone Hydrogels in Children. Optom Vis Sci 2013;90:961–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bullimore MA. The Safety of Soft Contact Lenses in Children. Optom Vis Sci 2017;94:638–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walline JJ, Gaume Giannoni A, Sinnott LT, et al. A Randomized Trial of Soft Multifocal Contact Lenses for Myopia Control: Baseline Data and Methods. Optom Vis Sci 2017;94:856–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walline JJ, Walker MK, Mutti DO, et al. Effect of High Add Power, Medium Add Power, or Single-Vision Contact Lenses on Myopia Progression in Children: The BLINK Randomized Clinical Trial. JAMA 2020;324:571–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Efron N. Grading Scales for Contact Lens Complications. Ophthalmic Physiol Opt 1998;18:182–6. [DOI] [PubMed] [Google Scholar]
- 17.Biddle DA, Morris SB. Using Lancaster’s Mid-P Correction to the Fisher’s Exact Test for Adverse Impact Analyses. J Appl Psychol 2011;96:956–65. [DOI] [PubMed] [Google Scholar]
- 18.CooperVision Inc. A Multicentre Dispensing Clinical Evaluation of Misight Lenses Available at: https://clinicaltrials.gov/ProvidedDocs/08/NCT01729208/Prot_000.pdf. Accessed: April 29, 2021.
- 19.Chamberlain P, Peixoto-de-Matos SC, Logan NS, et al. A 3-Year Randomized Clinical Trial of Misight Lenses for Myopia Control. Optom Vis Sci 2019;96:556–67. [DOI] [PubMed] [Google Scholar]
- 20.Zadnik K, Mutti DO, Cutter GR, Chalmers RL. The Effect of Degree of Refractive Error on Hydrogel Contact Lens-Induced Complications and Patient Self-Management Behaviors. Optom Vis Sci 2001;78:652–6. [DOI] [PubMed] [Google Scholar]
- 21.Carnt NA, Evans VE, Naduvilath TJ, et al. Contact Lens-related Adverse Events and the Silicone Hydrogel Lenses and Daily Wear Care System Used. Arch Ophthalmol 2009;127:1616–23. [DOI] [PubMed] [Google Scholar]


