Abstract
Introduction:
Vitamin E supplementation is recommended for the treatment of non-alcoholic fatty liver disease (NAFLD) for nondiabetic patients, but its preventative effects are unclear.
Methods:
We assessed dietary Vitamin E intake with disease phenotypes and evaluated Vitamin E levels with the development of NAFLD.
Results:
Data from >210,000 participants demonstrate that increased dietary Vitamin E associates with reduced rates of several gastrointestinal diseases and reduced overall mortality. Diabetic and overweight subjects with increased Vitamin E intake have fewer NAFLD diagnoses.
Conclusion:
Our findings reveal the relevance of Vitamin E consumption for several gastrointestinal diseases and warrant further mechanistic and therapeutic investigations.
Keywords: Vitamin E, nutrition, NAFLD, gastrointestinal diseases
Graphical Abstract
Introduction
Non-alcoholic fatty liver disease (NAFLD) is characterised by lipid droplet accumulation in the hepatocytes and affects approximately 20–30% of the general population (1). NAFLD is a multisystem disease and is a risk factor for developing type 2 diabetes (T2D), cardiovascular disease, insulin resistance, and kidney disease (2). The most effective therapeutic intervention for NAFLD is lifestyle modification involving a healthy diet, weight loss, and increased physical activity (3–5). However, currently, there are no approved pharmacological agents available to treat NAFLD directly.
Growing evidence supports the potential benefits of Vitamin E on NAFLD development. Vitamin E is a lipid-soluble vitamin with antioxidant, anti-inflammatory, and anti-apoptotic properties and has shown promise in treating NAFLD and NASH (6–9). The American Association for the Study of Liver Disease (10) and the European Association for the Study of the Liver (11) guidelines recommend Vitamin E supplementation in patients with biopsy-proven NASH without diabetes. However, current evidence is lacking to support the use of Vitamin E for other NAFLD patient subgroups or as a preventative measure in NAFLD risk populations. Here, we studied the associations between dietary intake of Vitamin E and 1495 medically defined phenotypes (PheCodes) available for >210,000 UK Biobank (UKB) study participants. Participants with increased dietary Vitamin E intake were less likely to be diagnosed with digestive diseases, including NAFLD, as well as cardiovascular diseases and other digestive disorders.
Methods
For this study, we used the UKB dataset reference number 71300. Information about the UKB cohort (Suppl. Table 1), dietary intake information, and the study endpoints is described in the Supplementary Materials.
Phenome-Wide Association Study (PheWAS)
A PheWAS was performed to examine causal linkage between dietary Vitamin E intake and clinical phenotypic outcomes. Average Vitamin E intake data was extracted from participant dietary questionnaires divided by daily energy intake(Suppl. Methods) and corrected for age, sex, BMI, smoking, alcohol consumption/total daily energy intake, fat/total daily energy intake, carbohydrates/total daily energy intake, protein/total daily energy intake, and Mediterranean Diet Score (MDS, Suppl. Table 2). Participants with reported use of Vitamin E supplements were excluded from the study. Adjusted Vitamin E intake was then analyzed for phenotype associations using coding for clinical diagnoses with the ICD-10 coding systems. ICD-10 codes from electronic health records were collated throughout the study period and removed duplicates for each study subject. ICD-10 codes were converted to n=1495 associated PheCodes using the PheWAS package version 0.99.5–5 in R. The association of increased dietary intake of % Vitamin E/total energy consumption with the development of each PheCode was analysed by a continuous model.
Statistics
All continuous variables were presented as mean ± standard deviation (SD) (normal distribution). Categorical variables were displayed as relative (%) frequencies. Univariate ANOVA was used to determine overall differences between the four quartiles of Vitamin E consumption. For PheWAS analysis, odds ratios (ORs) are presented with their corresponding 95% confidence intervals (CI) given in brackets. Multivariable-adjusted Cox regression was used to estimate Hazard rations (HRs) for the associations between Vitamin E intake and mortality. All multivariable analyses were corrected for age, sex, BMI, smoking, daily energy intake, daily alcohol consumption/ energy intake, daily fat/energy intake, daily carbohydrates/energy intake, daily protein/energy intake and Mediterranean Diet Score. Differences were considered to be statistically significant if p<0.05. For PheWAS analyses, False discovery rate correction was performed to account for multiple testing for the number of the major ICD categories analyzed (p<.0.0032) (12). The data were analyzed using R version 4.0.2 (R Foundation for Statistical Computing; Vienna, Austria), SPSS Statistics version 26 (IBM; Armonk, NY, USA), and Prism version 8 (GraphPad, LaJolla, CA, USA).
Results
We investigated the associations between increased dietary Vitamin E intake and disease occurrence using the UKB dataset consisting of 210,667 individuals with valid information on nutritional intake (Suppl. Table 3). Multivariable PheWAS analysis indicated that increased Vitamin E intake was most strongly associated with decreased occurrence of chronic airway obstruction (OR= 0.80; p=4.6×10−26) followed by tobacco use disorder (OR=0.81, p=5.0×10−22) and diseases of the oesophagus (OR= 0.92, p= 1.1 ×10−19), and oesophagitis, gastroesophageal reflux disease (GERD) (OR=0.92; p=6.6×10−19) (Figure 1, Suppl. Table 4). Interestingly, “Chronic liver disease and cirrhosis” and “other non-alcoholic liver diseases” were among the digestive PheCodes with the strongest effect per SD Vitamin E increase (Suppl. Table 4). A SD (0.038%) increase in percent dietary Vitamin E per kJ energy consumed was associated with decreased occurrence of “other non-alcoholic liver diseases”, the PheCode for NAFLD (OR= 0.91; p=1.3×10−03) (Suppl. Table 4). Of the 1495 PheCodes assessed, five were significantly overrepresented per SD increase in dietary Vitamin E intake, including Malaise, Chronic Fatigue Syndrome, Schmorl’s nodes disease (spinal disc herniation), anaemia in neoplastic disease, and eating disorder (Suppl. Table 4), suggesting higher Vitamin E intake is associated with increased occurrence of fatigue symptoms (Suppl Table 4). Importantly, we found that a SD increase in percent dietary Vitamin E per kJ energy consumed reduced overall mortality by 5.3% (p<.0001) (Suppl. Table 5).
Figure 1: Multivariable PheWAS of the effect of increased Vitamin E intake on disease occurrence.
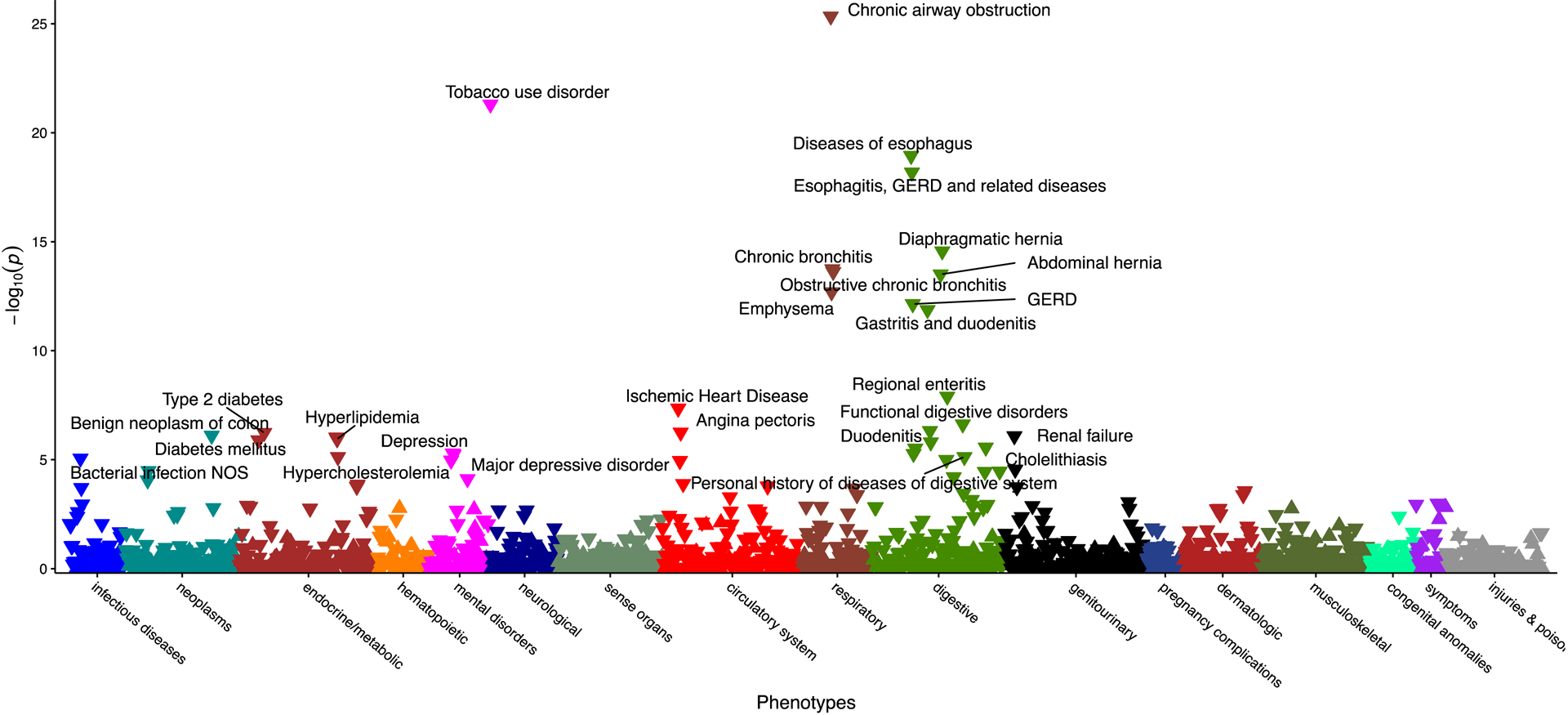
Manhattan plot of adjusted −log10 (P-values) for all PheCodes comparing their occurrence per SD increase in Vitamin E consumption/total daily energy corrected for age, sex, BMI, smoking, alcohol consumption/total daily energy, fat/total daily energy, carbohydrates/total daily energy, protein/total daily energy, and Mediterranean Diet Score (MDS). Highlighted are associations results with p-values <10−5.
Dietary Vitamin E intake and NAFLD
Because the PheWAS analysis supported a protective role of Vitamin E intake against digestive diseases, we next investigated the possible association between dietary Vitamin E and NAFLD occurrence based on ICD-10 and liver image-proven diagnoses. Each SD Vitamin E increase per kJ energy intake reduced the risk of NAFLD ICD-10 diagnosis by 12.5 % (Suppl. Table 6) and a reduced MRI diagnosis of NAFLD by 24.3%, in a random subset of participants 1.5 years after the first dietary questionnaire (Suppl. Table 7). Lastly, we studied the effect of increased Vitamin E intake on NAFLD development in at-risk subpopulations (Figure 2). The protective effect of increased Vitamin E intake on NAFLD development was more pronounced in the presence of T2D and modestly evident in overweight patients (Figure 2). Patients with T2D with higher Vitamin E intake were, on average, 20% less likely to develop ICD10-coded or imaging diagnosed NAFLD than their low Vitamin E consuming counterparts.
Figure 2: Increased Vitamin E intake protects from future ICD10 NAFLD diagnosis and risk of liver fat on imaging in patients that are at risk of metabolic outcomes.
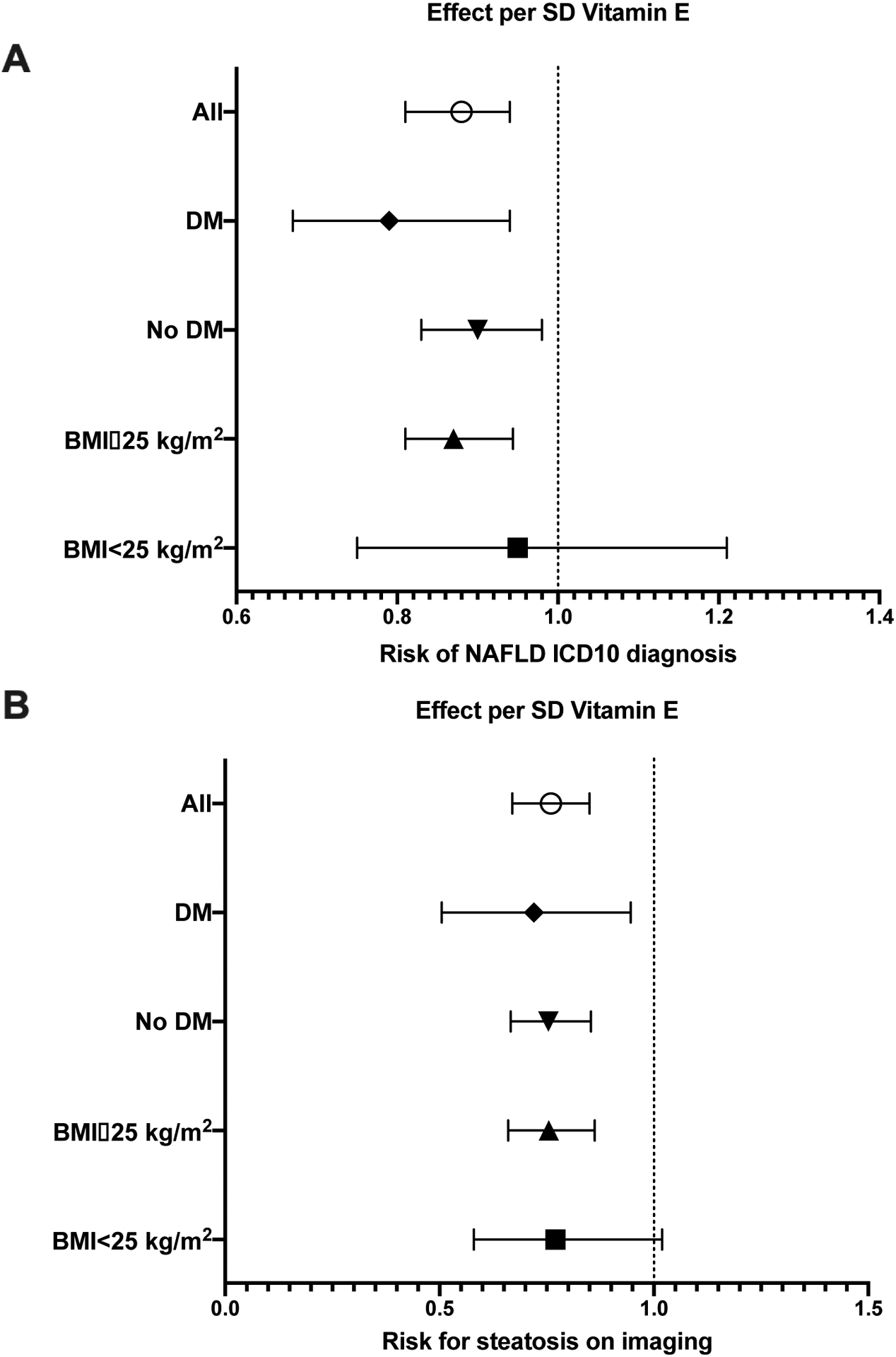
Subjects were stratified by Vitamin E intake levels and assessed for NAFLD diagnosis with either diabetes mellitus (DM) or overweight (BMI≥25kg/m2). Analyses were calculated per SD increase in Vitamin E consumption/total daily energy corrected for age, sex, BMI, smoking, alcohol consumption/total daily energy, fat/total daily energy, carbohydrates/total daily energy, protein/total daily energy, and MDS. A) Each SD increase in Vitamin E/kJ resulted in approximately 20% decrease in ICD10 NAFLD diagnosis in subjects with T2D, and approximately 13% in overweight subjects. B) Per SD Vitamin E/kJ increase, risk of steatosis on MRI decreased by 28% in subjects with T2D and approximately by 25% in overweight subjects.
Discussion
Vitamin E supplementation for NAFLD treatment has shown beneficial outcomes in nondiabetic patients but has not yet been fully evaluated for preventative effects, especially in at-risk populations (13, 14). We analysed the UKB database to delineate the relevance of dietary Vitamin E for human health and discovered >75 PheCodes that are significantly associated with increased Vitamin E intake. Among other digestive diseases, our study revealed a strong inverse association with NAFLD diagnosis. We then validated this association by assessing the effect of increased Vitamin E intake on NAFLD diagnosed by MRI. Interestingly, the protective effect of Vitamin E was most pronounced in overweight or diabetic subjects. Additionally, our analyses revealed that increased dietary Vitamin E intake is associated with reduced overall mortality. This contrasts with prior reports suggesting high Vitamin E intake, particularly from supplementation, may increase all-cause mortality (15, 16). These associations were strongest among users of high-dose supplementation (>400 IU/day), and more recent meta-analyses of these data failed to replicate these negative outcomes (17, 18). Although our analyses are only correlative, our data clearly suggest that increasing dietary Vitamin E might have a long-term beneficial effect with a reduction in overall mortality, and dietary Vitamin E intake is unlikely to reach the potentially dangerous levels associated with increased mortality. This study is limited by the sample population characteristics, potential misclassification or underdiagnosis of conditions by ICD codes, and the reliance on self-reported dietary questionnaires; however, the analyses are strengthened by the large number of study subjects and long follow-up period. Therefore, this study presents the largest analysis published to date on the effects of dietary Vitamin E on NAFLD and related cardiometabolic diseases. In conclusion, this study demonstrates that increased dietary intake of Vitamin E may be protective from developing NAFLD and other cardiometabolic diseases. Further studies are needed to identify the underlying mechanisms of Vitamin E on NAFLD pathophysiology and prevention.
Supplementary Material
Acknowledgements:
This research has been conducted using the UK Biobank Resource under Application Number 71300. C.V.S is supported by Walter-Benjamin Fellowship from German Research Foundation (SCHN-1640/1-1). K.M.S is supported by the German Research Foundation (DFG) consortium (SCHN 1626/1-1). K.T.C. is supported by NIH grants R01DK114291-01A1 and UM1DK126194-02. E.S. is supported by the AFDHAL/MCHUTCHISON LIFER Award.
Abbreviations:
- NAFLD
Non-alcoholic fatty liver disease
- NASH
Non-alcoholic steatohepatitis
- T2D
Type 2 Diabetes
- UKB
UK Biobank
- PheWAS
Phenome-Wide Association Study
- Mediterranean Diet
Footnotes
Conflicts of Interest: All authors report no conflict of interest for this study.
Competing Interests: None declared.
References
- 1.Scorletti E, Carr RM. A new perspective on NAFLD: focusing on lipid droplets. Journal of hepatology. 2021. [DOI] [PubMed] [Google Scholar]
- 2.Byrne CD, Targher G. NAFLD: a multisystem disease. Journal of hepatology. 2015;62(1 Suppl):S47–64. [DOI] [PubMed] [Google Scholar]
- 3.Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, et al. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology. 2015;149(2):367–78.e5; quiz e14–5. [DOI] [PubMed] [Google Scholar]
- 4.Eslamparast T, Tandon P, Raman M. Dietary Composition Independent of Weight Loss in the Management of Non-Alcoholic Fatty Liver Disease. Nutrients. 2017;9(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Houghton D, Thoma C, Hallsworth K, Cassidy S, Hardy T, Burt AD, et al. Exercise Reduces Liver Lipids and Visceral Adiposity in Patients With Nonalcoholic Steatohepatitis in a Randomized Controlled Trial. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2017;15(1):96–102.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. The New England journal of medicine. 2010;362(18):1675–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lavine JE, Schwimmer JB, Van Natta ML, Molleston JP, Murray KF, Rosenthal P, et al. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. Jama. 2011;305(16):1659–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dufour JF, Oneta CM, Gonvers JJ, Bihl F, Cerny A, Cereda JM, et al. Randomized placebo-controlled trial of ursodeoxycholic acid with vitamin e in nonalcoholic steatohepatitis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2006;4(12):1537–43. [DOI] [PubMed] [Google Scholar]
- 9.Bril F, Biernacki DM, Kalavalapalli S, Lomonaco R, Subbarayan SK, Lai J, et al. Role of Vitamin E for Nonalcoholic Steatohepatitis in Patients With Type 2 Diabetes: A Randomized Controlled Trial. Diabetes care. 2019;42(8):1481–8. [DOI] [PubMed] [Google Scholar]
- 10.Ando Y, Jou JH. Nonalcoholic Fatty Liver Disease and Recent Guideline Updates. Clin Liver Dis (Hoboken). 2021;17(1):23–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. Journal of hepatology. 2016;64(6):1388–402. [DOI] [PubMed] [Google Scholar]
- 12.Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res. 2001;125(1–2):279–84. [DOI] [PubMed] [Google Scholar]
- 13.Abdel-Maboud M, Menshawy A, Menshawy E, Emara A, Alshandidy M, Eid M. The efficacy of vitamin E in reducing non-alcoholic fatty liver disease: a systematic review, meta-analysis, and meta-regression. Therapeutic advances in gastroenterology. 2020;13:1756284820974917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perumpail BJ, Li AA, John N, Sallam S, Shah ND, Kwong W, et al. The Role of Vitamin E in the Treatment of NAFLD. Diseases. 2018;6(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller ER 3rd, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Annals of internal medicine. 2005;142(1):37–46. [DOI] [PubMed] [Google Scholar]
- 16.Lock M, Loblaw A. Vitamin E might increase risk of death. Can Fam Physician. 2005;51(6):829–31. [PMC free article] [PubMed] [Google Scholar]
- 17.Abner EL, Schmitt FA, Mendiondo MS, Marcum JL, Kryscio RJ. Vitamin E and all-cause mortality: a meta-analysis. Curr Aging Sci. 2011;4(2):158–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Traber MG, Head B. Vitamin E: How much is enough, too much and why! Free Radic Biol Med. 2021;177:212–25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


