Abstract
Rationale & objectives
±3,4-Methylenedioxymethamphetamine (MDMA) and psilocybin are currently moving through the US Food and Drug Administration’s phased drug development process for psychiatric treatment indications: posttraumatic stress disorder and depression, respectively. The current standard of care for these disorders involves treatment with psychiatric medications (e.g., selective serotonin reuptake inhibitors), so it will be important to understand drug-drug interactions between MDMA or psilocybin and psychiatric medications.
Methods
In accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, we queried the MEDLINE database via PubMed for publications of human studies in English spanning between the first synthesis of psilocybin (1958) and December 2020. We used 163 search terms containing 22 psychiatric medication classes, 135 specific psychiatric medications, and 6 terms describing MDMA or psilocybin.
Results
Forty publications were included in our systematic review: 26 reporting outcomes from randomized controlled studies with healthy adults, 3 epidemiologic studies, and 11 case reports. Publications of studies describe interactions between MDMA (N = 24) or psilocybin (N = 5) and medications from several psychiatric drug classes: adrenergic agents, antipsychotics, anxiolytics, mood stabilizers, NMDA antagonists, psychostimulants, and several classes of antidepressants. We focus our results on pharmacodynamic, physiological, and subjective outcomes of drug-drug interactions.
Conclusions
As MDMA and psilocybin continue to move through the FDA drug development process, this systematic review offers a compilation of existing research on psychiatric drug-drug interactions with MDMA or psilocybin.
Keywords: N-Methyl-3,4-methylenedioxyamphetamine; Psilocybin; Hallucinogens; Drug interactions; Stress disorders; Post-traumatic; Depression; Serotonin uptake inhibitors; Psychopharmacology
Introduction
Preliminary evidence for the use of ± 3,4-methylenedioxymethamphetamine (MDMA) (Jerome et al. 2020; Mitchell et al. 2021) and psilocybin (Carhart-Harris et al. 2021; Castro Santos and Gama Marques 2021; Davis et al. 2021) in the treatment of psychiatric disorders has been promising. Both drugs have been studied as augmentation to psychotherapy and are typically only administered one to three times during a treatment course (Mithoefer et al. 2016). This represents a departure from traditional pharmacological treatment in psychiatry. MDMA and psilocybin were researched as clinical interventions beginning in the 1970s and 1960s, respectively. However, the field of psychiatry has a limited understanding of their therapeutic use due to their Schedule I categorization (i.e., drugs determined to lack safety even under medical supervision, have no currently accepted medical use, and are deemed to have a high potential for abuse). Despite MDMA and psilocybin’s categorization as Schedule I substances, the US Food and Drug Administration (FDA) has more recently granted Breakthrough Therapy designation to MDMA-assisted psychotherapy (MAP) for Posttraumatic Stress Disorder (PTSD) as well as psilocybin-assisted psychotherapy (PAP) for both Major Depressive Disorder (MDD) and Treatment-Resistant Depression (TRD). Breakthrough Therapy designation is a process designed to expedite the development and review of drugs intended to treat a serious or life-threatening condition for which preliminary clinical evidence suggests substantial improvement over available options. MAP and PAP are currently being studied in Phase III and Phase II manufacturer-sponsored clinical trials, respectively. Upon successful completion of Phase III trials, a manufacturer may submit a New Drug Application asking the FDA to consider a drug for marketing approval.
MAP and PAP have shown transdiagnostic therapeutic potential beyond PTSD and depression, including as treatments for anxiety disorders (Danforth et al. 2018; Moreno et al. 2006; Vargas et al. 2020) and substance use disorders (Bogenschutz et al. 2015; Johnson et al. 2014; Sessa et al. 2021). All of the above-mentioned indications are frequently treated with psychiatric medications, such as: adrenergic agents, antipsychotics, anxiolytics, mood stabilizers, psychostimulants, and antidepressants. In fact, a combination of multiple psychiatric medications (i.e., polypharmacy) is common in the clinical management of MDD (Blier et al. 2010; Carpenter et al. 2002) and PTSD (Khachatryan et al. 2016; Krystal et al. 2011). With the number of clinical trials of MAP and PAP increasing exponentially, and potential FDA approval in the pipeline, it is important to compile what we know about the potential drug-drug interactions between psychiatric medications and MDMA or psilocybin.
MDMA
MDMA is a phenethylamine compound with psychostimulant effects. It promotes social engagement (Kirkpatrick and de Wit 2015), openness (Wagner et al. 2017), receptiveness to positive affect (Hysek et al. 2012b), heightened empathy (Hysek et al. 2014a), and increased disclosure of emotional content in dialogue (Baggott et al. 2015), leading to its characterization as an “entactogen-empathogen.” These effects distinguish MDMA from “classic psychedelics.” The active dose range of MDMA in studies is 75–225 mg administered orally (Multidisciplinary Association for Psychedelic Studies 2021). The onset of pharmacological effects usually occurs 30–60 min after ingestion, with peak effects appearing 75–120 min after ingestion and a total duration of 3–6 h (Multidisciplinary Association for Psychedelic Studies 2021). The elimination half-life is 8–9 h.
MDMA reverses the action of monoamine transporters, which include the serotonin transporter (SERT), norepinephrine transporter (NET), and dopamine transporter (DAT) (Rudnick and Wall 1992). MDMA also competes with monoamines for sites on the vesicular monoamine transporter-2 (VMAT-2) (Partilla et al. 2006), suggesting that MDMA leads to the displacement of monoamines from presynaptic vesicular stores as well. Together, transporter reversal and emptying of vesicular stores results in increased concentrations of intrasynaptic monoamines: predominantly serotonin (5-HT), to a lesser degree norepinephrine (NE), and to the least degree dopamine (DA) (Bogen et al. 2003; Oeri 2021). Additionally, MDMA displays some binding affinity as an agonist at 5-HT1A, 5-HT2A, 5-HT2B and 5-HT2C serotonin receptors; α1-, α2A- and β-adrenergic receptors; D1- and D2-dopamine receptors; M1- and M2-muscarinic receptors; H1-histamine receptor; and minimally at the human trace amine-associated receptor 1 (TAAR1) (Battaglia et al. 1988; Oeri 2021). The S( +)-MDMA enantiomer has a stronger binding affinity to radiolabeled brain serotonin and dopamine binding sites than R(−)-MDMA (Lyon et al. 1986; Shulgin 1986). Recent MAP clinical trials have used a racemic mixture of MDMA. MDMA’s downstream effects include increased secretion of arginine vasopressin (Simmler et al. 2011), adrenocorticotropic hormone (ACTH) (Grob et al. 1995), cortisol, prolactin, and oxytocin (Hysek et al. 2014a). MDMA is metabolized by cytochrome P450 enzymes (Table 1), primarily CYP2D6 followed by catechol-O-methyltransferase to its main inactive metabolite 4-hydroxy-3-methoxymethamphetamine (HMMA) (Abraham et al. 2009). MDMA also has a minor metabolism pathway via CYP3A4, CYP1A2, CYP2B6, and CYP2C19 to its only active metabolite 3,4-methylenedioxyamphetamine (MDA) (Vizeli et al. 2017). MDMA is also thought to lead to auto-inhibition of CYP2D6, resulting in nonlinear pharmacokinetics (Kolbrich et al. 2008). MDMA and its metabolites are primarily excreted as conjugates, such as sulfates and glucuronides (Vizeli et al. 2017).
Table 1.
Main enzymes involved in MDMA & psilocybin metabolism
| Metabolic enzymes | |
|---|---|
|
| |
| MDMA |
CYP2D6 (auto inhibitor) CYP3A4 CYP2B6 CYP2C19 CYP1A2 COMT |
| Psilocybin/Psilocin |
Alkaline phosphatase UGT1A10 UGT1A9 Aldehyde dehydrogenase MAO-A |
Bold = primary metabolic enzyme(s)
Common physiological effects of MDMA include increased heart rate, blood pressure, temperature, pupil size, as well as adverse acute transient physical effects such as nausea, vomiting, bruxism, muscle aches, headache, sweating, fatigue, dizziness, and dry mouth (Multidisciplinary Association for Psychedelic Studies 2021). MDMA increases plasma epinephrine and NE (Hysek and Liechti 2012; Pacifici et al. 2004). Common psychological effects of MDMA include euphoria, anxiolysis, enhanced fear-extinction learning, feelings of closeness or connectedness, an expanded emotional range (Carhart-Harris et al. 2014; Sessa 2017), as well as adverse subjective effects such as fearfulness, dysphoria, confusion, sleeplessness, and decreased appetite (Baylen and Rosenberg 2006). MDMA’s properties as an ‘empathogen-entactogen’ may have specific advantages in the treatment of PTSD including promoting increases in the personality feature of openness (Wagner et al. 2017), large magnitudes of posttraumatic growth (i.e. positive changes in self-perception, interpersonal relationships, or philosophy of life) (Gorman et al. 2020), fear extinction (Feduccia and Mithoefer 2018), widened window of tolerance (Mithoefer et al. 2011), and reopening of the critical period for social reward learning possibly mediated by oxytocin release (in mice, Nardou et al. 2019).
In recreational settings, MDMA is colloquially known as “ecstasy” or “molly.” Past year illicit use among US persons aged 12 or older was approximately 1.0% of the population between 2015 and 2019 (Center for Behavioral Health Statistics and Quality 2020). Unverified recreational ecstasy pills often consist of variable doses ranging from no detectable MDMA to 280 mg or more (Morefield et al. 2011; Wood et al. 2011). Ecstasy is frequently adulterated with other substances prior to sale (Saleemi et al. 2017; Togni et al. 2015). Additionally, ecstasy use is often intentionally co-ingested with other substances (Rigg and Sharp 2018), and involves situational risk factors such as heavy physical exertion, excessive heat, low water intake or water intoxication, and lack of screening for medical risk factors. In these settings, several types of short and longer-term adverse effects have been reported, including seizures, rhabdomyolysis, cardiovascular events, neurocognitive dysfunction, psychiatric crisis, habituation and addiction, and death (Grob and Grigsby 2021). Case reports and epidemiologic analyses of drug interactions from these recreational settings are much less reliable and present major limitations. In contrast to recreational use, clinical trials to date have involved carefully screened participants ingesting laboratory-grade MDMA at precise dosages. There has only been one reported serious adverse reaction to MDMA within a clinical trial setting: one participant experienced exacerbated ventricular extrasystoles after 125 mg of MDMA, which resulted in overnight hospital monitoring and full recovery within one day (Multidisciplinary Association for Psychedelic Studies 2021). Moderate doses utilized in MAP clinical trials, administered only two or three times during a course of treatment, have not resulted in persistently detectable deficits in neurocognitive function (Grob and Grigsby 2021).
Psilocybin
Psilocybin (4-phosphoryloxy-N,N-dimethyltryptamine) is a psychoactive tryptamine that naturally occurs in over 200 species of mushrooms, most notably from the genus Psilocybe (Nichols 2020). It is often referred to as a “classic psychedelic,” since the drug-induced alterations of perception, emotion, and cognition are primarily related to 5-HT2A receptor agonism (Johnson et al. 2019), though it also binds to other 5-HT receptor subtypes (Nichols 2020). Pooled data from 579 oral psilocybin doses administered across completed clinical trials involved a broad range of dosing for psilocybin from “very low dose” (0.014 mg/kg) to “high dose” (0.6 mg/kg), corresponding to 3.15–42 mg in a 70-kg human (Usona Institute 2021). The subjective effects of psilocybin usually begin 20–40 min after ingestion, peak after 60–90 min, and have a total duration of 6 h (Hasler et al. 2004).
Psilocybin is a prodrug, rapidly dephosphorylated upon ingestion by alkaline phosphatase or non-specific esterases, to its active metabolite, psilocin (Thomas et al. 2017). Psilocybin and psilocin lack clinically significant inhibitor activity at monoamine reuptake pumps, such as SERT or VMAT-2, thus do not result in substantially increased intrasynaptic serotonin (Rickli et al. 2016). Due to minimal intrasynaptic serotonin release, psilocybin’s mechanism of action is unlikely, even in high doses, to result in serotonin toxicity (Malcolm and Thomas 2021). Psilocin metabolism primarily occurs via UDP-glucuronyltransferase enzymes, UGT1A9 and UGT1A10 (Table 1) (Dinis-Oliveira 2017; Manevski et al. 2010). Psilocin also undergoes deamination and demethylation to form the inactive metabolite 4-hydroxy-indole-3-acetic acid (4HIAA) (Manevski et al. 2010).
Physiologically, psilocybin is known to cause mild increases in blood pressure and heart rate (Thomas et al. 2017). Common physical adverse effects include headache, nausea, dizziness, and fatigue (Usona Institute 2021). Psilocybin can elicit subjective experiences with mystical-type qualities, such as an increased sense of unity, transcendence of time and space, loss of self, and euphoria (Griffiths et al. 2006; Studerus et al. 2010). Mystical-type experiences are correlated in the laboratory with diminished activity of functionally connected brain circuits collectively termed the default mode network (DMN) as well as long-term improvements in wellbeing, psychosocial function, symptoms of clinical illness, and mindfulness-related capacities (Barrett and Griffiths 2018; Griffiths et al. 2008). Psilocybin lacks dopamine-mediated reinforcement, and there is no evidence supporting potential for habituation or addiction despite development of rapid tolerance with repeated administration (Johnson et al. 2018). Common adverse psychological effects include transient anxiety, dysphoria, impaired sleep, paranoia, grief, and preoccupation with death (Barrett et al. 2016, Usona Institute 2021).
While laboratory-made synthetic psilocybin is typically used in clinical trials, psilocybin is often ingested for personal use as “magic mushrooms.” Pooled data from the 2015–2018 National Survey on Drug Use and Health shows that approximately 9.68% of US adults have ever used psilocybin in their lifetime (Yockey and King 2021). Psilocybin is known to have a wide margin of safety, with lethal doses of psilocybin estimated to be more than 1000-fold higher than therapeutic doses (Gable 2004; Johnson et al. 2018). There have been no serious adverse events related to psilocybin in clinical trials (Thomas et al. 2017). Compared to personal use of other psychedelics, psilocybin appears to have the lowest rates of adverse events reported to poison control centers and very few reports of cardiovascular events (Borowiak et al. 1998; Leonard et al. 2018).
Drug interaction potential
Given that both MDMA and psilocybin modulate serotonin neurotransmission, there is a potential for drug-drug interactions with medications that also modulate the serotonin system, including Selective Serotonin Reuptake Inhibitors (SSRIs), Serotonin-Norepinephrine Reuptake Inhibitors (SNRIs), Tricyclic Antidepressants (TCAs), Monoamine Oxidase Inhibitors (MAOIs), mirtazapine, trazodone, lithium, buspirone, atypical antipsychotics, and others. Some of these interactions may increase the risk of toxidromes such as serotonin syndrome (Malcolm and Thomas 2021). Another pharmacodynamic interaction that may occur is the attenuation of MDMA’s subjective effects or reduced efficacy of MAP for PTSD in participants with recent exposure to antidepressant drugs that inhibit reuptake transporters (Hysek et al. 2012d; Feduccia et al. 2020). While a history of SSRI usage ever in one’s lifetime does not seem to be associated with reduced MAP efficacy (Mitchell et al. 2021), the adequate washout period between SSRI use and MAP has not yet been delineated. Due to MDMA also acting on the NE and DA neurotransmitter systems, there are potential interactions with drugs that target these neurotransmitters as well, such as SNRIs, bupropion, and psychostimulants. There may also be relevant drug-drug interactions between psychiatric medications and MDMA or psilocybin through more indirect mechanisms. For example, GABAergic agents such as benzodiazepines have been advocated as an intervention to mitigate acute psychological or physiological adverse effects related to psychedelics (Johnson et al. 2008). From a pharmacokinetic perspective, other medications may be substrates, inhibitors (e.g., CYP2D6, which could result in competitive interactions with MDMA), or inducers of drug-metabolizing enzymes. While interactions with UGTs are not as common, there are some medications that inhibit or induce UGT enzymes, which may theoretically affect the metabolism of psilocybin (English et al. 2012).
Objective of systematic review
Although reviews of drug-drug interactions (DDI) with MDMA have been published (Papaseit et al. 2020), none have been systematic, and there have been no reviews of DDIs with psilocybin. Therefore, the objective of this systematic review is to summarize all existing data related to pharmacokinetic, physiological, and subjective effects of drug-drug interactions between medications prescribed in psychiatry and MDMA or psilocybin.
Methods
This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. PROSPERO 2021 registration ID: CRD42021233519. The PICOS (Participants-Intervention/Exposure-Comparison-Outcomes-Study) framework was used to guide the methods of this review. Participants are people of any age who were exposed to, or ingested, MDMA or psilocybin in combination with a psychiatric medication. For clinical trials, comparison is made between MDMA or psilocybin in combination with a psychiatric drug versus MDMA or psilocybin alone. Outcomes include measures of pharmacokinetics (e.g., plasma drug and metabolite levels), physiology (e.g., vital signs, circulating catecholamines), and subjective outcomes (e.g., psychological effects, list of complaints). Study type was defined broadly to include meta-analyses, systematic reviews, randomized controlled trials (RCTs), retrospective studies, cross-sectional studies, case–control studies, cohort studies, and case reports.
A list of psychiatric medications (see Table 2) was compiled using the APA Textbook of Psychopharmacology, 5th Edition, Appendix for the psychiatric pharmacopoeia in the United States (Schatzberg and Nemeroff 2017). Given that clinical research investigating MDMA and psilocybin is also being conducted outside of the United States (e.g., NCT01689740, NCT04670081), we referenced Martindale: The Complete Drug Reference (Scriba 2011) and included any internationally-approved pharmacotherapy for the treatment of PTSD and MDD that we had not already included. Lastly, we referred to the Department of Veterans Affairs and Department of Defense practice guidelines for PTSD and MDD to include additional medications that are not typically categorized as psychiatric drugs, but are used in the treatment of these disorders (e.g., prazosin, liothyronine) (VA/DOD Clinical Practice Guideline for the Management of Posttraumatic Stress Disorder and Acute Stress Disorder 2017).
Table 2.
Psychiatric medication and medication class search terms
| Antidepressants | MAOI | Chlorpromazine | Psychostimulants for treatment of ADHD and narcolepsy | Naltrexone |
| Antidepressant | Monoamine oxidase inhibitor | Fluphenazine | Varenicline | |
| TCA | Haloperidol | Methadone | ||
| Tricyclic antidepressant | Isocarboxazid | Loxapine | Psychostimulant | Buprenorphine |
| Desipramine | Phenelzine | Perphenazine | Stimulant | MDD & PTSD EU/International pharmacotherapy |
| Nortriptyline | Selegiline | Pimozide | Amphetamine | |
| Protriptyline | Tranylcypromine | Thioridazine | Dextroamphetamine | |
| Amitriptyline | NMDA antagonist | Thiothixene | Armodafinil | reboxetine |
| Clomipramine | Ketamine | Trifluoperazine | Modafinil | viloxazine |
| Doxepin | Esketamine | Second generation antipsychotic | Dextroamphetamine | dibenzepin |
| Imipramine | Anxiolytics | Lisdexamfetamine | dimetacrine | |
| Trimipramine | Anxiolytics | Atypical antipsychotic | Methamphetamine | dosulepin |
| Amoxapine | Benzodiazepine | Aripiprazole | Dexmethylphenidate | lofepramine |
| Maprotiline | Alprazolam | Asenapine | Methylphenidate | melitracen |
| SSRI | Chlordiazepoxide | Brexpiprazole | Non-psychostimulants for treatment of ADHD | nitroxazepine |
| Serotonin reuptake inhibitor | Clonazepam | Cariprazine | noxiptiline | |
| Clorazepate | Clozapine | Atomoxetine | pipofezine | |
| Citalopram | Diazepam | Iloperidone | Clonidine | maprotiline |
| Escitalopram | Lorazepam | Lurasidone | Guanfacine | mianserin |
| Fluoxetine | Oxazepam | Olanzapine | Agents for cognitive disorders | setiptiline |
| Fluvoxamine | Buspirone | Paliperidone | moclobemide | |
| Paroxetine | Agents for treatment of insomnia | Invega Sustenna | Donepezil | pirlindole |
| Sertraline | Invega Trinza | Galantamine | toloxatone | |
| SNRI | Estazolam | Quetiapine | Memantine | agomelatine |
| Serotonin norepinephrine reuptake inhibitor | Flurazepam | Risperidone | Rivastigmine | tandospirone |
| Quazepam | Ziprasidone | Agents for treatment of antipsychotic-induced extrapyramidal side effects | tianeptine | |
| Desvenlafaxine | Temazepam | Mood stabilizers | Additional Adjunctive PTSD & MDD pharmacotherapy | |
| Duloxetine | Triazolam | Mood stabilizer | ||
| Levomilnacipran | Eszopiclone | Carbamazepine | ||
| Milnacipran | Ramelteon | Gabapentin | Amantadine | PTSD |
| Venlafaxine | Suvorexant | Lamotrigine | Benztropine | Prazosin |
| Norepinephrine dopamine reuptake inhibitor | Tasimelteon | Lithium | Diphenhydramine | MDD |
| Zaleplon | Oxcarbazepine | Propranolol | Liothyronine | |
| NDRI | Zolpidem | Pregabalin | Trihexyphenidyl | St. Johns Wort |
| Bupropion | Antipsychotics | Topiramate | Agents for treatment of substance use disorders | |
| Aplenzin | First generation antipsychotic | Valproate | ||
| Nefazodone | Divalproex | Acamprosate | ||
| Trazodone | Antipsychotic | Depakote | Disulfiram | |
| Vortioxetine | Typical antipsychotic | |||
| Vilazodone | ||||
| Mirtazapine |
Search strategy
We searched the MEDLINE database via PubMed for publications in English between 1958 and 2020. This date range was chosen to capture relevant scientific literature beginning with the synthesis and formal research use of psilocybin (Hofmann et al. 1958) as well as the early use of MDMA’s therapeutic properties in psychotherapy starting in the 1970’s (Passie 2018). We utilized the PubMed Advanced Search Builder to conduct a query comprising 163 terms included in the Title or Abstract. A total of 22 search terms reflecting psychopharmacology classes and 135 search terms reflecting psychiatric medications (e.g., Tricyclic antidepressant OR Desipramine OR Nortriptyline) were combined using the logical operator AND with 6 search terms aimed to capture MDMA OR psilocybin (“psilocybin” OR “mdma” OR “ecstasy” OR “psychedelic” OR “entheogen” OR “3 4 methylenedioxymethamphetamine”). See Tables 2 and 3 for the full list of search terms. A total of 21 filters were applied: case reports, classical article, clinical conference, clinical study, clinical trial, clinical trial protocol, phase 1 clinical trial, phase 2 clinical trial, phase 3 clinical trial, phase 4 clinical trial, comparative study, controlled clinical trial, historical article, meta-analysis, multicenter study, observational study, practice guideline, pragmatic clinical trial, randomized controlled trial, systematic review, humans. This resulted in a total of 455 abstracts at the time of query (December 21st, 2020).
Table 3.
Psychedelic-related search terms
| Psilocybin |
| MDMA |
| Ecstasy |
| Psychedelic |
| Entheogen |
| 3 4 methylenedioxymethamphetamine |
Study selection
Each abstract was screened for inclusion by two independent reviewers. Abstracts were excluded if they were an editorial, a duplicate, not available in the English language, or otherwise did not address the objective of this systematic review. Any discrepancies between independent reviewers were discussed among the authors of this systematic review, leading to a final decision to include or exclude a specific article based on unanimous consensus. We assessed the remaining full-text articles in detail, during which 12 additional hand-selected articles arose that had not been captured by our database query. Hand-selected articles were reviewed and met all study selection criteria.
Ultimately, 40 articles were included in our systematic review. See Fig. 1 for a PRISMA flow diagram. 26 RCTs and 3 epidemiologic studies were captured. We corresponded with any researchers who published more than one of the resulting RCT articles to seek unpublished or uncaptured data; no additional data were obtained this way. We included the 3 epidemiologic studies in this review due to their large sample sizes and implications for future hypothesis generation, despite their methodological limitations. In addition, 11 case studies were reviewed and placed in the Supplemental Materials.
Fig. 1.
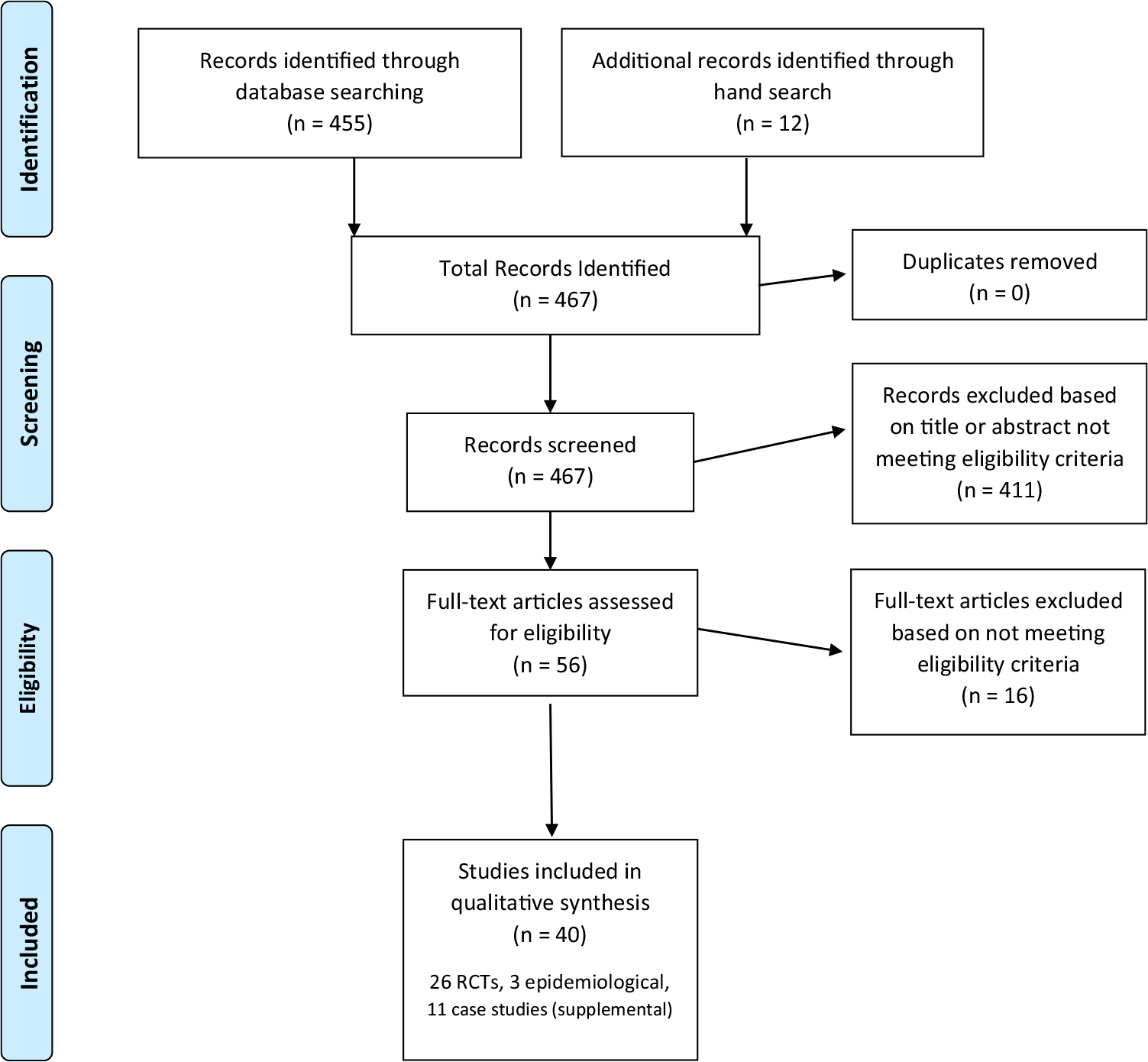
PRISMA study selection flow diagram
Percent change calculation
The following formula was utilized to standardize comparison of outcome measures (e.g., Epinephrine nmol/l) when ingestion of MDMA or psilocybin was preceded by ingestion of another drug (e.g., carvedilol) versus preceded by placebo, resulting in a percentage change (e.g., ↑204.3%) in the outcome of interest. VPBO-MDMA refers to the value of the outcome of interest for the MDMA (or psilocybin) pretreatment with placebo condition, and Vmed-MDMA refers to the value of the outcome of interest for MDMA (or psilocybin) pretreatment with the psychiatric medication.
Results
This review includes 26 publications of RCTs and 3 epidemiologic studies (1 post-marketing surveillance association study, 1 cross-sectional survey, and 1 quantitative analysis). RCTs (Table 4) include combinations of MDMA with SSRIs (Farré et al. 2007; Liechti and Vollenweider 2000a; Tancer and Johanson 2006), SNRIs (Hysek et al. 2011; Hysek et al. 2012d), bupropion (Schmid et al. 2015), methylphenidate (Hysek et al. 2014b), haloperidol (Liechti and Vollenweider 2000b), memantine (de Sousa Fernandes Perna et al. 2014), and adrenergic agents (Hasler et al. 2009; Hysek et al. 2010, 2013; Hysek et al. 2012a, b, c); and combinations of psilocybin with ergotamine or buspirone (Pokorny et al. 2016), haloperidol or risperidone (Vollenweider et al. 1998), chlorpromazine (Keeler 1967), and escitalopram (Becker et al. 2021). Our results include primary outcome publications for 17 original RCTs along with 9 additional analyses of secondary or exploratory outcome measures using the same sample from a previously-published RCT. Exploratory analyses were on drug-drug interactions with MDMA and their effects on: immune response (Bigler et al. 2015; Pacifici et al. 2004), drug metabolism (Pacifici et al. 2004; Steuer et al. 2016), plasma copeptin (Simmler et al. 2011), pupillary response (Hysek and Liechti 2012), psychological effects (Liechti 2000), and habituation of startle response (Liechti et al. 2001).
Table 4.
Summary of drug-drug interaction randomized controlled trials
| Medication Class | MDMA RCTsa | Psilocybin RCTsa |
|---|---|---|
|
| ||
| Adrenergic Agents | carvedilol | ergotamine |
| pindolol | ||
| clonidine ER | ||
| doxazosin XL | ||
| Antipsychotics | haloperidol | chlorpromazine |
| haloperidol | ||
| risperidone | ||
| Anxiolytics | buspirone | |
| MAOIs | ||
| Mood Stabilizers | ||
| NDRIs | bupropion XR | |
| NMDAR Antagonists | memantine | |
| Psychostimulants | methylphenidate | |
| SSRIs | citalopram | escitalopram |
| fluoxetine | ||
| paroxetine | ||
| SNRIs | duloxetine | |
| reboxetine | ||
MAOI monoamine oxidase inhibitor, NDRI norepinephrine-dopamine reuptake inhibitor, NMDAR N-methyl-D-aspartate receptor, RCT randomized controlled trial, SNRI serotonin-norepinephrine reuptake inhibitor, SSRI selective serotonin reuptake inhibitor
See Table 5 for randomized controlled trial citations, study designs, and sample description
Not included: MDMA/ecstasy co-ingestion from cross-sectional studies (Copeland et al. 2006; Cohen et al. 2021) or epidemiologic data from online “trip reports” (Nayak et al. 2021)
All of the RCTs enrolled only “healthy” adults, often recruited from a university campus, and had limited sample sizes (range of 8–23 participants). Several trials also investigated pharmacogenetic biomarkers, such as CYP2D6 genotype, and categorized participants as extensive, intermediate, or poor metabolizers. Common exclusion criteria across clinical trials were pregnancy or nursing, age < 18 or > 45, BMI < 18.5 or > 25 kg/m2, presence of psychiatric illness in self or first-degree relatives, and active substance use. Many studies required female participants to be in the follicular phase of their menstrual cycle (day 2–14) during drug dosing due the potential for cyclic change in subjective reactivity to amphetamines (White et al. 2002). Refer to Table 5 for additional details on study designs and study participants and to Tables 6 and 7 for pharmacokinetic, physiological, and subjective outcomes of the RCTs included in our systematic review.
Table 5.
Randomized controlled trial designs and participants
| Citation(s) | Study Design | Study Participants |
|---|---|---|
|
| ||
| MDMA | ||
| ADRENERGIC AGENTS | ||
|
Hysek et al. 2012c
* Hysek and Liechti 2012 |
DB, placebo-controlled, within-subjects, counterbalanced, crossover (> 10-day washout) 1. Carvedilol 50 mg + MDMA 125 mg 2. Carvedilol 50 mg + PBO 3. PBO + MDMA 125 mg 4. PBO + PBO Carvedilol/PBO p.o. given 1 h prior to MDMA/PBO p.o |
N = 16, healthy 8F, 8 M Mean age 24.2 ± 2.2 1 had used ecstasy previously CYP2D6 metabolism: 9 extensive, 6 intermed, 1 poor |
|
Hasler et al. 2009
* Hysek et al. 2010 |
DB, placebo-controlled, within-subjects, counterbalanced, crossover (14-day washout) 1. Pindolol 20 mg + MDMA 1.6 mg/kg 2. Pindolol 20 mg + PBO 3. PBO + MDMA 1.6 mg/kg 4. PBO + PBO Pindolol/PBO p.o. given 1 h prior to MDMA/PBO p.o |
N = 16, healthy 100% male Mean age 25 ± 4 (range 20–36) 2 had used ecstasy previously |
|
Hysek et al. 2012a
* Hysek and Liechti 2012 |
DB, placebo-controlled, within-subjects, counterbalanced, crossover (10- to 14-day washout) 1. Clonidine 0.15 mg + MDMA 125 mg 2. Clonidine 0.15 mg + PBO 3. PBO + MDMA 125 mg 4. PBO + PBO Clonidine/PBO p.o. given 1 h prior to MDMA/PBO p.o |
N = 16, healthy 8F, 8 M Mean age 25.4 ± 4.9 2 had used ecstasy previously CYP2D6 metabolism: 8 extensive, 7 intermed, 1 poor |
|
Hysek et al. 2013
* Hysek and Liechti 2012 |
DB, placebo-controlled, within-subjects, counterbalanced, crossover (≥ 7-day washout) 1. Doxazosin XL 8 mg + MDMA 125 mg 2. Doxazosin XL 8 mg + PBO 3. PBO + MDMA 125mg 4. PBO + PBO Doxazosin mesylate XL 4 mg/PBO p.o. × 1 day, then 8 mg/PBO × 2 days During testing days, Doxazosin XL/PBO given 16 h prior to MDMA/PBO p.o |
N = 16, healthy 8F, 8 M Mean age 25.8 ± 3.3 CYP2D6 metabolism: 13 extensive, 2 intermediate, and 1 poor |
| ANTIPSYCHOTICS | ||
|
Liechti and Vollenweider 2000b
* Liechti et al. 2001 |
DB, placebo-controlled, within-subjects, counterbalanced, crossover (≥ 10-day washout) 1. Haloperidol 1.4 mg + MDMA 1.5 mg/kg 2. Haloperidol 1.4 mg + PBO 3. PBO + MDMA 1.5 mg/kg 4. PBO + PBO Haloperidol/PBO i.v. given 10 min prior to MDMA (mean 100 mg) p.o |
N = 14, healthy 5F, 9 M 1 had used ecstacy previously Mean age 26 (range 21–38) Scoring > 2 standard deviations from mean normative values on the Neuroticism scale of the Freiburger Personality Inventory was exclusionary |
| NOREPINEPHRINE-DOPAMINE REUPTAKE INHIBITORS | ||
|
Schmid et al. 2015
* Steuer et al. 2016 |
DB, placebo-controlled, crossover (≥ 10-day washout) 1. Bupropion XR 300 mg + MDMA 125 mg 2. Bupropion XR 300 mg + PBO 3. PBO. + MDMA 125 mg 4. PBO + PBO Bupropion XR 150 mg/PBO p.o. daily × 3 days, then 300 mg/PBO × 4 days During testing days, Bupropion XR/PBO given 2 h prior to MDMA/PBO p.o |
N = 16, healthy 8F, 8 M, 100% white Mean age 23.3 ± 2.2 6 had used ecstacy once previously CYP2D6 metabolism: 13 extensive, 3 intermediate, and 0 poor |
| NMDA ANTAGONISTS | ||
| de Sousa Fernandes Perna et al. 2014 | DB, placebo-controlled, within-subjects, counterbalanced, crossover (≥ 7-day washout) 1. Memantine 20 mg + MDMA 75 mg 2. Memantine 20 mg + PBO 3. PBO + MDMA 75 mg 4. PBO + PBO Memantine/PBO p.o. given 2 h prior to MDMA/PBO p.o |
N = 15, healthy 4F, 11 M Mean age 22.9 ± 1.9 (range 20–28) Mean previous ecstasy use 18.8 times |
| PSYCHOSTIMULANTS | ||
|
Hysek et al. 2014b
* Bigler et al. 2015 |
DB, placebo-controlled, within-subjects, counterbalanced crossover (10-day washout) 1. Methylphenidate 60 mg + MDMA 125 mg 2. Methylphenidate 60 mg + PBO 3. PBO + MDMA 125 mg 4. PBO + PBO Immediate-release methylphenidate/PBO p.o. given 1 h prior to MDMA/PBO p.o |
N = 16, healthy 8F, 8 M Mean age 24.8 ± 2.6 6 had used ecstasy previously CYP2D6 metabolism: 12 extensive, 2 intermed, 1 poor, 1 unknown (Bigler et al. 2015: n = 12, 8F, 4 M, mean age 24.9) |
| SELECTIVE SEROTONIN REUPTAKE INHIBITORS | ||
|
Liechti and Vollenweider 2000a
* Liechti 2000 * Liechti et al. 2001 |
DB, placebo-controlled, within-subjects, counterbalanced, crossover (≥ 14-day washout) 1. Citalopram 40 mg + MDMA 1.5 mg/kg 2. Citalopram 40 mg + PBO 3. PBO + MDMA 1.5 mg/kg 4. PBO + PBO Citalopram/PBO i.v. infused over 90 min prior to MDMA/PBO (dose range 80–120 mg) p.o |
N = 16, healthy 4F, 12 M Mean age 27.4 ± 4.4 (range 21–39) 3 had used ecstasy previously Scoring > 2 standard deviations from mean normative values on the Neuroticism scale of the Freiburger Personality Inventory (Fahrenberg et al., 1984) was exclusionary |
| Tancer and Johanson 2006 | DB, placebo-controlled, within-subjects, crossover (≥ 48-h MDMA-PBO washout) 1. Fluoxetine 20 mg + MDMA 1.5 mg/kg 2. Fluoxetine 20 mg + PBO 3. PBO + MDMA 1.5 mg/kg 4. PBO + PBO Fluoxetine 20 mg/PBO p.o. daily × 5–11 days PBO pre-treatment was always tested first During testing days, Fluoxetine/PBO given 1 h prior to MDMA/PBO p.o |
N = 8, recreational MDMA users (mean 28.6 times, range 4–66) 2F, 6 M, 100% white Mean age 23.9 (range 19–33) Drug or alcohol dependence in the past year -OR- lifetime recreational use of stimulants, opiates, phencyclidine, or sedative exceeding 50 times were exclusionary |
|
Farré et al. 2007
* Pacifici et al. 2004 * Segura et al. 2005 |
DB, placebo-controlled, within-subjects, counterbalanced, crossover (15-day washout) 1. Paroxetine 20 mg + MDMA 100 mg 2. PBO + MDMA 100 mg Paroxetine 20 mg/PBO p.o. daily × 3 days During testing days, Paroxetine/PBO given 3 h prior to MDMA p.o |
N = 12, healthy 100% male Mean age 24 years (range 19–34) Previously used ecstasy ≥ 5 times 100% extensive CYP2D6 metabolizers (Segura n = 7) |
| SEROTONIN-NOREPINEPHRINE REUPTAKE INHIBITORS | ||
|
Hysek et al. 2012d
* Simmler et al. 2011 * Hysek and Liechti 2012 |
DB, placebo-controlled, within-subjects, counterbalanced, crossover (≥ 10-day washout) 1. Duloxetine 120 mg + MDMA 125 mg 2. Duloxetine 120 mg + PBO 3. PBO + MDMA 125 mg 4. PBO + PBO Duloxetine 120 mg/PBO given 16 h & 4 h prior to MDMA/PBO p.o |
N = 16, healthy 8F, 8 M Mean age 26.1 ± 6.0 Ecstasy-naive CYP2D6 metabolism: 13 extensive, 2 intermed, and 1 poor |
|
Hysek et al. 2011
* Hysek and Liechti 2012 |
DB, placebo-controlled, within-subjects, counterbalanced, crossover (10- to 14-day washout) 1. Reboxetine 8 mg + MDMA 125 mg 2. Reboxetine 8 mg + PBO 3. PBO + MDMA 125 mg 4. PBO + PBO Reboxetine 8 mg/PBO p.o. given 12 h & 1 h prior to MDMA/PBO p.o |
N = 16, healthy 8F, 8 M Mean age 25.7 ± 5.5 3 had used ecstasy previously CYP2D6 metabolism: 10 extensive, 4 intermed, 2 poor |
| PSILOCYBIN | ||
| ADRENERGIC & ANXIOLYTIC AGENTS | ||
| Pokorny et al. 2016 | DB, placebo-controlled, randomized, within-subjects, crossover (≥ 2-week washout) A) 1. Buspirone 20 mg + Psilocybin 0.17 mg/kg 2. Buspirone 20 mg + PBO 3. PBO + Psilocybin 0.17 mg/kg 4. PBO + PBO Buspirone/PBO p.o. given once 1 h prior to Psilocybin/PBO B) 1. Ergotamine 3 mg + Psilocybin 0.17 mg/kg 2. Ergotamine 3 mg + PBO 3. PBO + Psilocybin 0.17 mg/kg 4. PBO + PBO Ergotamine/PBO p.o. given 100 min prior to Psilocybin/PBO p.o |
A. Buspirone Group N = 19, healthy 9F, 10 M Mean age 24.9 ± 4.0 B. Ergotamine Group N = 17, healthy Mean age 23.8 ± 3.7 |
| ANTIPSYCHOTICS | ||
| Keeler 1967 | Placebo-controlled, crossover (2-week washout) 1. Chlorpromazine 50 mg + Psilocybin 0.2 mg/kg 2. PBO + Psilocybin 0.2 mg/kg Chlorpromazine/Placebo p.o. given once 2 h prior to Psilocybin p.o |
N = 8, healthy “free from evidences of major psychopathology, had stable academic and work histories and did not conduct themselves in ways actively contradictory to conventional mores” |
| Vollenweider et al. 1998 | Placebo-controlled, within-subjects, crossover (1-month washout) A) 1. Haloperidol 0.021 mg + Psilocybin 0.25 mg/kg 2. Haloperidol 0.021 mg + PBO 3. PBO + Psilocybin 0.25 mg/kg 4. PBO + PBO Haloperidol/PBO i.v. given 75 min prior Psilocybin/PBO p.o B) 1. Risperidone 0.5 mg + Psilocybin 0.25 mg/kg 2. Risperidone 1 mg + Psilocybin 0.25 mg/kg 3. PBO + Psilocybin 0.25 mg/kg 4. Risperidone 0.5 mg + PBO 5. Risperidone 1 mg + PBO 6. PBO + PBO Risperidone/PBO p.o. given once 90 min prior to Psilocybin/PBO p.o |
A. Haloperidol Group N = 5, healthy B. Risperidone Group N = 5, healthy 7F, 8 M University staff History of “illicit drug abuse” exclusionary |
| SELECTIVE SEROTONIN REUPTAKE INHIBITORS | ||
| Becker et al. 2021 | DB, placebo-controlled, within-subjects, counterbalanced, crossover (≥ 2-day washout) 1. Escitalopram 20 mg + Psilocybin 25 mg 2. PBO + Psilocybin 25 mg Escitalopram 10 mg/PBO p.o. daily × 7 days, followed by Escitalopram 20 mg/PBO p.o. daily × 7 days During testing days, Escitalopram/PBO given 2 h prior to Psilocybin p.o |
N = 23, healthy 11F, 12 M Mean age 34 ± 10 (range 25–55) 6 had used psilocybin-containing mushrooms previously |
Secondary or exploratory outcomes. DB double-blind, F female, h hour(s), kg kilogram, M male, mg milligram, min minute(s), PBO placebo, p.o. per os (by mouth)
Table 6.
Randomized controlled trial outcomes—MDMA
| Measures (Timing from MDMA Administration) | Outcomes | Citation |
|---|---|---|
| ADRENERGIC AGENTS | ||
| Carvedilol-MDMA vs Placebo-MDMA | Hysek et al. 2012c | |
| PHARMACOKINETIC | ||
| Drug & metabolite levels (Cmax) | MDMA ↑4.7%, MDA ↑1.6% | |
| Drug & metabolite levels (AUC0–6 h) | MDMA ↑6.4%, MDA ↑6.5% | |
| Drug & metabolite levels (tmax) | MDMA ↓3.4%, MDA ↓9.1% | |
| PHYSIOLOGICAL | ||
| Vitals (ΔEmax) | Heart rate ↓79.0%***, SBP ↓76.9%***, DBP ↓39.2%*, Temp ↓42.0%* | |
| Circulating catecholamines (ΔEmax) | Epinephrine (nmol/1) ↑204.3%***, Norepinephrine (nmol/1) ↑789.7%*** | |
| Pupil function (Emax) | Pupil size (mm) ↑0.4%, Pupil size after light (mm) ↑3.2%, Constriction amplitude (mm) ↓27.9%, Latency (seconds) ↓0.3%** | (Hysek and Leichti 2012) |
| SUBJECTIVE | ||
| Visual Analog Scale (ΔEmax) | Any drug effect ↑7.4%, Good drug effect ↑8.0%, Bad drug effect ↑86.0%, Drug liking ↑1.5%, Drug high ↑11.6%, Stimulated ↑6.7% | |
| 5-Dimensions of Altered States of Consciousness Rating Scale (ΔEmax) | Global ↑19.7%, Oceanic boundlessness ↑21.8%, Anxious ego dissolution ↓16.1%, Visionary restructuralization ↑69.7% | |
| List of Complaints (3 h & 24 h) | Acute (3 h) Global ↑17.9%, Subacute (24 h) Global ↓7.5% | |
| Pindolol-MDMA vs Placebo-MDMA | Hasler et al. 2009 | |
| PHYSIOLOGICAL | ||
| Vitals (Emax) | Heart rate ↓ ∼ 17.6%***, MAP ↓ ∼ 0.9%, Temp ↔ | (Hysek et al. 2010) |
| SUBJECTIVE | ||
| 5-Dimensions of Altered States of Consciousness Rating Scale (150 m) | Global ↓ ∼ 26.7%**, Oceanic boundlessness ↓ ∼ 31.9%**,Anxious ego dissolution ↑ ∼ 14.3%, Visionary Restructuralization ↓ ∼ 30.0% | |
| Adjective Mood Rating Scale (75 m & 110 m) | Dreaminess ↓95.4%* (75 min) & ↓97.6%* (110 min), no significant differences in any other domains | |
| State Anxiety (75 m & 110 m, 24 h) | Anxiety ↔ | |
| Cambridge Neuropsychological Test Automated Battery (120 min) | Rapid visual processing – total hits ↑4.8%, Paired Associates Learning – total trials ↔, Dimensional Set Shifting – stages completed ↑2.3% | |
| List of Complaints (75 m) | Global ↔ | (Hysek et al. 2010) |
| Clonidine-MDMA vs Placebo-MDMA | Hysek et al. 2012a | |
| PHARMACOKINETIC | ||
| Drug & metabolite levels (Cmax) | MDMA ↓2.9%, MDA ↑2.8% | |
| Drug & metabolite levels (AUC0–6) | MDMA ↑1.1%, MDA ↑4.7% | |
| Drug & metabolite levels (tmax) | MDMA ↔, MDA ↓1.9% | |
| Drug & metabolite levels (t1/2) | MDMA ↑22.1% | |
| PHYSIOLOGICAL | ||
| Vitals (Emax) | Heart rate ↓3.9%, MAP ↓27.2%, SBP ↓24.9%, DBP ↓26.4%, Temp ↔ | |
| Vitals (AUEC0–6 h) | Heart rate ↓2.6%, MAP ↓4.5%*, SBP ↓3.9%*, DBP ↓5.2%*, Temp ↑0.1% | |
| Pupil function (Emax) | Pupil size (mm) ↓2.5%, Pupil size after light (mm) ↓2.6%, Constriction amplitude (mm) ↓13.8%, Latency (seconds) ↑3.7% | (Hysek and Leichti 2012) |
| Circulating catecholamines (Emax) | Epinephrine ↓29.4%, Norepinephrine ↓109.8%** | |
| SUBJECTIVE | ||
| Visual Analog Scale (Emax) | Any drug effect ↓9.0%, Good ↓12.9%, Bad ↑18.3%, Liking ↓11.2%, Drug high ↓11.9%, Stimulated ↓8.9%, Tiredness ↓2.9%, Closeness to others ↑2.8%, Open ↓0.3% | |
| Adjective Mood Rating Scale (Emax) | Emotional excitation ↑2.2%, Well being ↑1.2%, Extroversion ↑18.2%, Dreaminess ↓24.4%, Activity ↑61.9%, Inactivation ↓10.2%, Anxiety-depression ↑162.5% | |
| State-Trait Anxiety Inventory (Emax) | State-anxiety ↑29.7% | |
| 5-Dimensions of Altered States of Consciousness Rating Scale | Oceanic boundlessness ↓ ∼9%, Anxious ego dissolution ↑ ∼ 26%, Visionary restructuralization ↔, Insightfulness ↓ ∼ 52%*, Global ↔ | |
| List of Complaints (3 h & 24 h) | Acute (3 h) Global ↑23.3%, Subacute (24 h) Global ↑85.7% | |
| Doxazosin-MDMA vs Placebo-MDMA | Hysek et al. 2013 | |
| PHARMACOKINETIC | ||
| Drug & metabolite levels (Cmax) | MDMA ↓1.6%, MDA ↓12.1%*, HMMA inc 0.6% | |
| Drug & metabolite levels (AUC0–6) | MDMA ↓3.6%, MDA ↓14.6%**, HMMA ↑ 1.8% | |
| Drug & metabolite levels (tmax) | MDMA ↑ 16.0%, MDA ↓5.3%, HMMA ↑ 10.5% | |
| PHYSIOLOGICAL | ||
| Vitals (Emax) | Heart rate ↑ 9.6%*, MAP ↓ 6.8%*, Temp ↓ 26.8% | |
| Circulating catecholamines (Emax) | Epinephrine (nmol/l) ↑28.2%, Norepinephrine (nmol/l) ↑102.8%*** | |
| Pupil function (Emax) | Pupil size (mm) ↓3.2%, Pupil size after light (mm) ↓1.0%, Constriction amplitude (mm) ↓3.7%, Latency (seconds) ↓10.5% | (Hysek and Leichti 2012) |
| SUBJECTIVE | ||
| Visual Analog Scale (ΔEmax) | Any drug effect ↓0.1%, Good drug effect ↓1.7%, Bad drug effect ↓9.5%, Drug liking ↓1.2%, Drug high ↓1.6%, Stimulated ↑9.7% | |
| Adjective Mood Rating Scale (Emax) | Emotional excitation ↓1.9%, Self-reported activation ↓38.5%, Heightened mood ↓40%, Extroversion ↓23.3%, Self-confidence ↓14.3%, Dreaminess ↓2.6% | |
| Adjective Mood Rating Scale (AUEC0–6 h) | Self-reported activation ↓84.9%, Heightened mood ↓85.7* | |
| 5-Dimensions of Altered States of Consciousness Rating Scale (Emax) | Global ↓ ∼ 15%, Oceanic boundlessness ↓ ∼ 9%, Anxious ego dissolution ↔, Visionary restructuralization ↓ ∼ 36%, Auditory Alterations ↓ ∼ 20%, Vigilance reduction ↓ ∼ 16% | |
| List of Complaints (3 h & 24 h) | Acute (3 h) Global ↑9.5%; Subacute (24 h) Global ↑1.5% | |
| ANTIPSYCHOTICS | ||
| Haloperidol-MDMA vs Placebo-MDMA | Liechti and Vollenweider 2000b | |
| PHYSIOLOGICAL | ||
| Vitals (120 m) | Heart rate ↑13.7%, SBP ↑5.0%, DBP ↑2.3%, Temp ↑1.4% | |
| Startle Response (90 m) | Mean startle magnitude: First block of startle testing ↔; Last block of startle testing ↑ ∼ 5% Mean percent prepulse inhibition of startle response ↑ ∼ 6% |
(Liechti et al 2001) |
| SUBJECTIVE | ||
| Adjective Mood Scale (EWL) (2 h) | Well-being ↓ ∼ 31%*, Efficiency-activation ↓ ∼ 25%, Inactivation ↑ ∼ 100%, Extro-/Introversion ↓ ∼ 20%, Emotional excitability ↑ ∼ 20% | |
| State-Trait Anxiety Inventory (75 & 120 m) | 75 min: ↑ ∼ 47%**, 120 min: ↑ ∼ 29% | |
| Altered State of Consciousness Scale Rating (2 h) | Oceanic boundlessness ↓ ∼ 59%*, Anxious ego-dissolution ↑ ∼ 100%, Visionary restructuralization ↔ | |
| List of Complaints (on drug, 1 day, 3 days) | Global: on drug ↑ ∼ 14%, 1 day ↓ ∼ 14%, 3 days ↓ ∼ 33% | |
| NOREPINEPHRINE-DOPAMINE REUPTAKE INHIBITORS | ||
| Bupropion-MDMA vs Placebo-MDMA | Schmid et al 2015 | |
| PHARMACOKINETIC | ||
| Drug & metabolite levels (Cmax) | MDMA ↑14.3%**, MDA ↓14.6%**, HMMA ↓75.9%***, bupropion ↑18.0%# | |
| Enantiomer Drug & metabolite levels (Cmax) | R-MDMA ↑9%*, S-MDMA ↑16%*; R-MDA ↓27%*, S-MDA ↓24%* | (Steuer et al 2016) |
| Drug & metabolite levels (AUC0–24 h) | MDMA ↑ 33.1%***, MDA ↓12.4%, HMMA ↓66.8%***, bupropion ↑27.5%## | |
| Enantiomer levels (AUC0–24 h) | R-MDMA ↑25%*, S-MDMA ↑38%*; R-MDA ↓26%*, S-MDA ↓20%* | (Steuer et al 2016) |
| PHYSIOLOGICAL | ||
| Vitals (ΔEmax) | Heart rate ↓38.7%***, SBP ↓16.3%, DBP ↓4.5%, Temp ↑4.76% | |
| Pupillometry (Emax) | Pupil size (mm) ↑0.3% | |
| Circulating Catecholamines/Hormones (Emax) | Norepinephrine (nmol/l) ↓65.6%*, Prolactin (mU/l) ↓12.0%, Cortisol (nmol/l) ↓29.3%, Oxytocin (pg/ml) ↓8.1%, Epinephrine (nmol/l) ↓20.0%, Dopamine (nmol/l) ↓25.0% | |
| SUBJECTIVE | ||
| Visual Analog Scale (ΔEmax) | Any drug effect ↑12.0%, Good drug effect ↑11.6%, Drug high ↑17.1%, Drug liking ↑5.6%, Stimulated ↑11.5% | |
| Visual Analog Scale (ΔAUEC0–8 h) | Any drug effect ↑36.7%*, Good drug effect ↑46.1%*, Drug high ↑43.5%*, Drug liking ↑41.6%*, Stimulated ↑34.0% | |
| List of Complaints (5 h & 24 h) | Acute (5 h) ↓5.0%, Subacute (24 h) ↓6.0% | |
| NMDA ANTAGONISTS | ||
| Memantine-MDMA vs Placebo-MDMA | de Sousa Fernandes Perna et al. 2014 | |
| PHARMACOKINETIC | ||
| Drug levels (1 h) | MDMA ↓1.0% | |
| SUBJECTIVE | ||
| Memory (95–165 m) | Visual Verbal Learning Task – total immediate recall ↓6.0% and delayed recall ↓0.8%, Prospective Memory Task – reaction time ↓3.5% and accuracy no-go ↑1.3%, Sternberg Memory Test – reaction time ↑0.4% and correct ↓1.4%, Abstract Visual Pattern Learning Task – reaction time ↑13.6% and correct ↓1.8% | |
| Profile of Mood States (90 m) | Arousal ↑0.7%, Elation ↓13.1%, Positive Mood ↓41.1%, Vigor ↓16.0, Anxiety ↑7.1% | |
| Visual Analog Scale (90 m and 120 m) | Subjective high ↑20.9% (90 min) and ↑70.3% (120 min) | |
| Psychomotor Performance (95–165 m) | Critical Tracking Task ↓1.4%, Divided Attention Task – tracking error ↓2.8% and reaction time ↑0.1% | |
| PSYCHOSTIMULANTS | ||
| Methylphenidate-MDMA vs Placebo-MDMA | Hysek, et al. 2014b | |
| PHARMACOKINETIC | ||
| Drug & metabolite levels (Cmax) | MDMA ↓3.5%, MDA ↓3.6%, HMMA ↓12.1%, Methylphenidate# ↑0.7% | |
| Drug & metabolite levels (AUC0–24) | MDMA ↑3.2%, MDA ↓3.4%, HMMA ↓4.2%, Methylphenidate# ↓0.1% | |
| Drug & metabolite levels (tmax) | MDMA ↑45.8%*, MDA ↑13.8%*, HMMA ↑16.7%, Methylphenidate# ↑4.3% | |
| Drug & metabolite levels (t1/2) | MDMA ↔, MDA ↑2.5%, HMMA ↑5.2%, Methylphenidate# ↔ | |
| PHYSIOLOGICAL | ||
| Vitals (Emax) | SBP ↑2.6%, DBP ↔, Heart Rate ↑15.1%***, Rate Pressure Product ↑16.8%***, Temp change ↑6.0% | |
| Pupillometry (Emax) | Pupil size (mm) ↑1.8% & after light reflex ↓2.2% | |
| Hormones (ΔEmax) | Cortisol (nmol/l) ↑26.8%, Prolactin(mU/l) ↓29.5% | |
| Circulating catecholamines; (ΔEmax) | Epinephrine (nmol/l) ↑54.6%**, Norepinephrine (nmol/l) ↓41.1%, Dopamine (nmol/l) ↓52.9% | |
| SUBJECTIVE | ||
| Visual Analog Scale (ΔEmax) | Any drug effect ↓1.4%, Drug liking ↓8.4%, Drug high ↓13.7%, Stimulated ↑2.1%, Happy ↓29.8%, Close to others ↓20.4% | |
| Adjective Mood Rating Scale (ΔEmax) | Emotional excitation ↑59.5%, Well-being ↓12.5%, Extroversion ↔, Activity ↑16.7%, Concentration ↑500.0%*, Anger ↑166.7% | |
| Addictions Research Center Inventory (ΔEmax) | Amphetamine ↑6.4%, Benzedrine ↑7.4%, Morphine-benzedrine ↓10.2%, Phenobarbital-alcohol ↑8.7%, LSD ↑15.5% | |
| Altered State of Consciousness Rating Scale | Global ↓10.0%, Oceanic boundlessness ↓22.2%, Anxious ego dissolution ↑23.3%, Visionary restructuralization ↓3.5% | |
| Facial emotion recognition task (1.5 h) | Global ↓1.8%, Neutral ↑0.9%, Happy ↓11.4%*, Sad ↑11.4%, Anger ↓5.3%, Fear ↔ | |
| List of Complaints (5 h & 24 h) | Acute (5 h) Global ↑86.5%**, Dry mouth (n = 15v13), Lack of appetite (n = 16v8), Palpitations (n = 9v4), Headache (n = 7v4), Nausea (n = 5v1); Subacute (24 h) Global ↑191.9%*** | |
| SELECTIVE SEROTONIN REUPTAKE INHIBITORS | ||
| Citalopram-MDMA vs Placebo-MDMA | Liechti and Vollenweider 2000a | |
| PHYSIOLOGICAL | ||
| Vitals (60 m) | Heart rate ↓12.3%*, SBP ↓4.2%**, DBP ↓1.2%, Temp ↓0.3% | |
| Vitals (120 m) | Heart rate ↓9.6%, SBP↓6.5%**, DBP↓5.8%*, Temp ↓0.3% | |
| Startle response (90 m) | Mean startle magnitude: First block of startle testing ↑ ∼ 3%, Last block of startle testing ↔ Mean percent prepulse inhibition of startle response ↓ ∼ 10%* |
(Liechti et al 2001) |
| SUBJECTIVE | ||
| Altered State of Consciousness Rating Scale (Emax) | Oceanic boundlessness ↓ ∼ 60%*, Anxious ego dissolution ↓ ∼ 63%*, Visionary restructuralization ↓ ∼ 70%* | (Liechti 2000) |
| Adjective Mood Rating Scale (Emax) | Efficiency-activation ↓ ∼ 52%*, Self-confidence ↓ ∼ 30%*, Heightened mood ↓ ∼ 21%, Apprehension-anxiety ↓ ∼ 25%, Depressiveness ↓ ∼ 6%, Thoughtfulness-contemplativeness ↓ ∼ 23%, Extroversion ↓ ∼ 32%**, Introversion ↓ ∼ 3%, Inactivation ↑ ∼ 20%, Dazed state ↑ ∼ 15%, Tiredness ↑ ∼ 25%, Sensitivity ↓ ∼ 5%, Aggression-anger ↑ ∼ 23%, Emotional excitability ↔ | |
| List of Complaints, acute (during session, n drug combo versus n MDMA alone) | Global ↓ ∼ 46%**, Difficulty concentrating (7v10), Impaired balance (5v8), Lack of appetite (6v8), Dizziness (4v8), Palpitations (4v7), Restlessness (5v7), Bruxism (3v7), Being cold (1v6), Thirst (3v6), Hot flashes (1v5), Paresthesias (1v5), Fatigue (0v3), Nausea (3v3), Tremor (0v3), Inner tension (8v3), Fear (0v2), Headache (3v1) | |
| List of Complaints, sub-acute (24 h; n drug combo versus n MDMA alone) | Global ↑ ∼ 14%, Headache (6v7), Fatigue (6v6), Lack of appetite (8v6), Difficulty concentrating (4v4), Brooding (4v3), Decreased libido (0v3), Bad dreams (2v3), Thirst (4v3), Forgetfulness (1v3), Inner tension (5v2), Gloomy thoughts (0v2), Insomnia (6v2), Increased need to sleep (4v2), Dizziness (4v2), Bruxism (0v2), Palpitations (3v1), Private/job related worries (1v1), Tremor (3v1), Irritability (2v1), Nausea (3v0) | |
| Fluoxetine-MDMA vs Placebo-MDMA | Tancer and Johanson 2006 | |
| PHYSIOLOGICAL | ||
| Vitals (Emax) | Heart rate ↓14.6%*, SBP ↓4.2%, DBP ↓2.1% | |
| SUBJECTIVE | ||
| Visual Analog Scale (Emax) | Drug Liking ↓31.2%*, High ↓47.6%*, Stimulated ↓40.1%*, Anxious ↓51.9%, Friendly ↓19.2%, Good Drug Effect ↓28.1%, Talkative ↓30% | |
| Addictions Research Center Inventory (Emax) | Morphine-benzedrine ↓33.7%*, Amphetamine ↓14.3%, LSD ↓9.2%, Benzedrine ↓7.9%, Pentobarbital-Chlorpromazine-Alcohol ↑53.3% | |
| Profile of Mood States (Emax) | Arousal ↓41.7%*, Elation ↓29.2%*, Positive Mood ↓33.3%*, Vigor ↓40.9%*, Anxiety ↓30% | |
| Hallucinogen Rating Scale (Emax) | Affect ↓33.3%*, Soma ↓41.7%*, Intensity ↓38.1%, Cognition ↓14.3%, Perception ↓50% | |
| Multiple-Choice Procedure (8 h) | ↓63.9% | |
| End-of-Session Liking Questionnaire (8 h) | ↓29.3% * | |
| Paroxetine-MDMA vs Placebo-MDMA | Farré et al 2007 | |
| PHARMACOKINETIC | ||
| Drug & metabolite plasma levels (Cmax) | MDMA ↑16.0%**, HMMA ↓49.3%** | |
| Drug & metabolite plasma levels (AUC0–21 h) | MDMA ↑21.6%**, HMMA ↓38.2%** | |
| Drug & metabolite plasma levels (tmax) | MDMA ↑16.7%, HMMA ↑33.3% | |
| Drug & metabolite plasma levels (t1/2) | MDMA ↓5.8%, HMMA ↑37.3%** | |
| PHYSIOLOGICAL | ||
| Vitals (Emax) | Heart rate ↓ ∼ 42%*, SBP ↓ ∼ 41%***, Temp ↓ ∼ 40%*, Pupillary diameter (mm) ↓63.1%*** | |
| Psychomotor Performance (Emax) | Simple reaction time errors ↓88.9%*, Esophoria (Maddox-wing, diopters) ↑54.9%* | |
| MDMA-induced immune response (Emax) | CD4/CD3 suppression ↓ ∼ 33%**, CD8 ↔, Natural killer cell increase ↓ ∼ 55%**, IL-2 reduction ↓67%**, IL-10 & TGFβ1 increase ↓ > 90%**, phytohemagglutinin suppression ↓3.9%**, Concanavalin A suppression ↓2.0%** | (Pacifici et al 2004) |
| Hormones (AUC0–9 h) | Cortisol stimulation ↓21.5%**, Prolactin stimulation ↓24.1%** | |
| SUBJECTIVE | ||
| Visual Analog Scale (Emax) | Stimulated ↓ ∼ 65%**, High ↓ ∼ 68%***, Good effects ↓ ∼ 62%***, Liking ↓ ∼ 62%***, Different body sensation ↓ ∼ 53%** | |
| Addictions Research Center Inventory (Emax) | Morphine-benzedrine ↓45.0%**, Amphetamine ↓30.4%**, LSD ↓21.1% | |
| Evaluation of the Subjective Effects of Substances with Abuse Potential | Pleasure and sociability ↓80.7%*, Psychosomatic anxiety ↓51.4%**, Activity and energy ↓79.5%** | |
| SEROTONIN-NOREPINEPHRINE REUPTAKE INHIBITORS | ||
| Duloxetine-MDMA vs Placebo-MDMA | Hysek et al. 2012d | |
| PHARMACOKINETIC | ||
| Drug & metabolite plasma levels (Cmax) | MDMA ↑14.6%**, MDA ↓9.2%, HMMA ↓40.5%***, duloxetine# ↑4.6% | |
| Drug & metabolite plasma levels (AUC0–6 h) | MDMA ↑16.2%**, MDA ↓10.0%, HMMA ↓40.0%***, duloxetine# ↑1.8% | |
| Drug & metabolite plasma levels (tmax) | MDMA ↑13.7%, MDA ↓4.5%, HMMA ↑2.7%, duloxetine# ↑15.8% | |
| Drug & metabolite plasma levels (t1/2) | MDMA ↓12.6%, duloxetine# ↑3.6% | |
| Ex vivo: monoamine transporter binding | NET ↓41.5%***, SERT ↓ > 94.4%***, DAT ↔ | |
| Osmolality (120 m) | Women: Copeptin (pmol/l, 60 min) ↓ ∼ 96%***, arginine vasopressin (pmol/l) ↓ ∼ 60%, Urinary osmolality (mmol/kg) ↓ ∼ 22%, Urinary sodium (mmol/l) ↓ ∼ 12%, Plasma osmolality (mmol/kg) ↓ ∼ 2%, plasma sodium (mmol/l) ↓ ∼ 1% Men: Copeptin (60 min) ↔, arginine vasopressin ↑ ∼ 18%, Urinary osmolality ↑ ∼7%, Urinary sodium ↓ ∼ 27%, Plasma osmolality ↓ ∼ 1%, plasma sodium ↓ ∼ 1% |
(Simmler et al 2011) |
| PHYSIOLOGICAL | ||
| Vitals (Emax) | Heart rate ↓57.4%***, MAP ↓60.8%***, SBP ↓63.5%***, DBP ↓58.3%***, Temp ↓27.8% | |
| Pupil function (Emax) | Pupil size (mm) ↑1.7%, Pupil size after light (mm) ↓8.1%**, Constriction amplitude (mm) ↑126.9***, Latency (seconds) ↓35.0% | (Hysek and Leichti 2012) |
| Circulating catecholamines (Emax) | Epinephrine (nmol/l) ↓48.0%, Norepinephrine (nmol/l) ↓143.2%*** | |
| SUBJECTIVE | ||
| Visual Analog Scale (Emax) | Any drug effect ↓61.7%***, Good drug effect ↓54.6%***, Drug liking ↓57.7%***, Drug high ↓67.0%***, Stimulated ↓70.8%***, Closeness to others ↓83.1%***, Talkative ↓63.0%***, Open ↓81.3%*** | |
| Adjective Mood Rating Scale (Emax) | Well-being ↓49.6%**, Emotional excitation ↓73.5%***, Extroversion ↓58.9%***, Introversion ↓35.5%, Dreaminess ↓38.4%, Activity ↑40.1% | |
| 5-Dimensions of Altered States of Consciousness Rating Scale (4 h) | Global ↓ ∼ 84%***, Oceanic Boundlessness ↓ ∼ 87%***, Anxious Ego Dissolution ↓ ∼ 71%*, Visionary Restructuralization ↓ ∼ 92%*** | |
| List of Complaints (3 h & 24 h) | Acute (3 h) Global ↓122.5%**, Sub-acute (24 h) Global ↓109.8%* | |
| Reboxetine-MDMA vs Placebo-MDMA | Hysek et al. 2011 | |
| PHARMACOKINETIC | ||
| Drug & metabolite levels (Cmax) | MDMA ↑17.8%**, MDA ↑21.1%, Reboxetine# ↑12.2%* | |
| Drug & metabolite levels (AUC0–24 h) | MDMA ↑8.1%*, MDA ↑44.1%**, Reboxetine# ↑8.3% | |
| Drug & metabolite levels (tmax) | MDMA ↑7.7%, MDA ↑48.9%, Reboxetine# ↑9.1% | |
| Drug & metabolite levels (t1/2) | MDMA ↓27.1%, MDA ↑7.5%, Reboxetine# ↓3.6% | |
| Ex vivo: monoamine transporter binding (1 h) | NET ↓67.8%***, SERT ↔, DAT ↔ | |
| PHYSIOLOGICAL | ||
| Vitals (Emax) | Heart rate ↓26.2%*, MAP ↓37.2%**, SBP ↓46.5%***, DBP ↓24.0%, Temp ↓31.2% | |
| Circulating catecholamines (1 h) | Epinephrine (nmol/l) ↓40%, Norepinephrine (nmol/1) ↓36.1%** | |
| Pupil function (Emax) | Pupil size (mm) ↑10.4%***, Pupil size after light (mm) ↑10.8%***, Constriction amplitude (mm) ↑11.7%, Latency (seconds) ↑1.0% | (Hysek and Leichti 2012) |
| SUBJECTIVE | ||
| Visual Analog Scale (Emax) | Any drug effect ↓20.7%**, Drug high ↓24.3%*, Stimulated ↓28.9%*, Closeness ↓38.6%*, Good drug effect ↓15.2%, Liking ↓10.0% | |
| Adjective Mood Rating Scale (Emax) | Activity ↑10.0%, Inactivation ↓20.6%, Extroversion 13.3%, Introversion ↑60.3%**, Well-being ↓3.9%, Emotional excitation ↓47.5%*, Anxiety-depression ↓38.7%, Dreaminess ↓20.3% | |
| State-Trait Anxiety Inventory (Emax) | State-anxiety ↓62.6% * | |
| 5-Dimensions of Altered States of Consciousness Rating Scale (4 h) | Global ↓ ∼ 33%**, Oceanic boundlessness ↓ ∼ 42%**, Anxious ego dissolution ↓ ∼ 24%, Visionary restructuralization ↓ ∼ 26%*, | |
| List of Complaints (3 h & 24 h) | Acute (3 h) Global ↓80.8%*; Lack of appetite (n = 8v12), Tremor (n = 3v9), Restlessness (n = 4v8), Dizziness (n = 4v6); Subacute (24 h) Global ↓91.8%* | |
Percent change reported for [psych med] + MDMA vs. placebo + MDMA
= [psych med] + MDMA vs [psych med] + placebo
↔ no change in response, ∼ = estimated percentage change approximated from graphical representation of outcome data
p < .05
p < .01, and
p < .001 (in bold)
AUC area under the curve, AUEC area under the effect-time curve, Cmax peak plasma concentration, DAT dopamine transporter, DBP diastolic blood pressure (mmHg), Emax peak effects, HMMA 4-hydroxy-3-methoxymethamphetamine, MAP mean arterial pressure, MDA 3,4-methylenedioxyamphetamine, MDMA 3,4-Methylenedioxymethamphetamine, NET norepinephrine transporter, SBP systolic blood pressure (mmHg), SERT sertraline transporter, Temp temperature (C°), t1/2 half-life, tmax time to maximum plasma concentration, Δ change from baseline
Table 7.
Randomized controlled trial outcomes—psilocybin
| Measures | Outcomes | Citation |
|---|---|---|
|
| ||
| (Timing from Psilocybin Administration) | ||
| ADRENERGIC AGENTS | ||
| Ergotamine-Psilocybin vs Placebo-Psilocybin | Pokorny et al. 2016 | |
| SUBJECTIVE | ||
| 5-Dimensions of Altered States of Consciousness Rating Scale (180 m) | Oceanic boundlessness ↓ ∼ 9%, Anxious ego dissolution ↓ ∼ 17%, Visionary restructuralization ↑ ∼ 8%, Auditory alterations ↔, Vigilance Reduction ↑ ∼ 27% | |
| ANTIPSYCHOTICS | ||
| Chlorpromazine-Psilocybin vs Placebo-Psilocybin | Keeler 1967 | |
| PHYSIOLOGICAL | ||
| Pupil function (2 h) | Photography measure (mm) ↓91.8%*, After-image effect measure (mm) ↓95.0%* | |
| SUBJECTIVE | ||
| Unusual visual experiences (2 h) | Major visual response: ↓52.9%*, Minor visual response: ↓35.1%* | |
| Haloperidol-Psilocybin vs Placebo-Psilocybin | Vollenweider et al 1998 | |
| SUBJECTIVE | ||
| Altered States of Consciousness Rating Scale (80 m) | Oceanic boundlessness ↓ ∼ 14%**, Dread of ego dissolution ↑ ∼ 27%*, Visionary restructuralization ↓ ∼ 9% | |
| Delayed response task (80 m) | Reaction time (ms) ↓ ∼ 2% | |
| Risperidone-Psilocybin vs Placebo-Psilocybin | Vollenweider et al 1998 | |
| SUBJECTIVE | ||
| Altered States of Consciousness Rating Scale (80 m) |
Oceanic boundlessness ↓ ∼ 23% (risperidone 0.5 mg) & ↓ ∼ 31%**
(risperidone 1 mg) Visionary restructuralization ↓ ∼ 19%* (risperidone 0.5 mg) & ↓ ∼ 27%** (risperidone 1 mg) Dread of ego dissolution ↓* (risperidone 0.5 mg) & ↓** (risperidone 1 mg) |
|
| Delayed response task (80 m) | Reaction time (ms) ↓ ∼ 15% (risperidone 0.5 mg) & ↓ ∼ 24%** (risperidone 1 mg) | |
| ANXIOLYTICS | ||
| Buspirone-Psilocybin vs Placebo-Psilocybin | Pokorny et al 2016 | |
| SUBJECTIVE | ||
| 5-Dimensions of Altered States of Consciousness Rating Scale (180 m) | Oceanic boundlessness ↓ ∼ 29%, Anxious ego dissolution ↓ ∼ 33%, Visionary restructuralization ↓ ∼ 44%***, Auditory alterations ↑20%, Vigilance Reduction ↓ ∼ 26% | |
| 5-Dimensions of Altered States of Consciousness Rating Scale; Visionary Restructuralization Item Clusters (180 m) | Elementary hallucinations ↓ ∼ 48%**, Complex hallucinations ↓ ∼ 53%**, Synaesthesia ↔, Changing meaning of percepts ↓ ∼ 41%**, Facilitated autobiographic memory recollection ↓ ∼ 50%*, Facilitated imagination ↓ ∼ 33%* | |
| SELECTIVE SEROTONIN REUPTAKE INHIBITORS | ||
| Escitalopram-Psilocybin vs Placebo-Psilocybin | Becker et al 2021 | |
| PHARMACOKINETIC | ||
| Drug level (Cmax) | Psilocin ↑1.0% | |
| PHYSIOLOGICAL | ||
| Vitals (ΔEmax) | Heart rate ↓33.3, SBP ↓43.8%***, DBP ↓23.5%*, MAP ↓37.5%**, Temp ↓11.1% | |
| QTc interval (150 m) | ↑2.3% | |
| Pupil function (Emax) | Pupil dilation (mm) ↓66.7% ** | |
| Plasma Brain-Derived Neutrophic Factor level (Cmax) | ↓6.6% | |
| Gene expression levels | SLC6A4 ↑7.0%, HTR2A ↓6.5% | |
| SUBJECTIVE | ||
| 5-Dimensions of Altered States of Consciousness Rating Scale (Emax) | Oceanic boundlessness ↔, Anxious ego dissolution ↓40%, Visionary restructuralization ↓4.7% | |
| Visual Analog Scales (ΔEmax) | Any drug effects ↓14.1%*, Good drug effects ↓3.8%, Bad drug effects ↓53.8%**, High ↓2.7%, Fear ↓50%**, Talkative ↓47.1%*, Open ↓25%*, Happy ↔, Concentration ↓20% | |
| Adjective Mood Rating Scale (ΔEmax) | Concentration ↓233.3%, Activity ↑133.3%, Extroversion ↓35.7%, Introversion ↑2.8%, General well-being ↔, Emotional Excitation ↓8.6%, Anxiety ↓80%** | |
| Mystical Effects Questionnaire | MEQ30 total score ↓7.7%, no significant differences in subscales except ineffability ↓19.3%* | |
| List of Complaints (0–7 h) | Global ↓25% * | |
Percent change reported for [psych med] + psilocybin vs. placebo + psilocybin
= [psych med] + psilocybin vs [psych med] + placebo; ↔ no change in response, ∼ = estimated percentage change approximated from graphical representation of outcome data
p < .05
p < .01, and
p < .001 (in bold)
MDMA
Adrenergic agents & MDMA
RCTs were conducted combining MDMA with four different adrenergic agents: carvedilol, pindolol, clonidine, and doxazosin. These studies were among those hand-selected for inclusion as these medications, or medications from the same class, are occasionally used in clinical psychiatric practice as augmentation treatment (e.g., α1-adrenergic receptor blockers for the treatment of PTSD) and may be relevant clinically in the management of common transient effects of MDMA such as elevated blood pressure (De Jong et al. 2010; Kinzie and Leung 1989; Scherrer et al. 2019).
Hasler et al. (2009) hypothesized that pindolol (20 mg p.o.), a mixed β-adrenergic and 5HT1A receptor antagonist, administered one hour prior to MDMA (1.6 mg/kg p.o.) would affect MDMA-induced affective, cognitive, and cardiovascular responses (Hasler et al. 2009; Hysek et al. 2010). It was found that pindolol pretreatment on average affected experience for only a few subscales of the affective measures (i.e., reduction in MDMA-induced “positive basic mood,” “mania-like experience,” and “dreaminess”) and had no significant effect on MDMA-induced impairments when performing neurocognitive tasks (Hasler et al. 2009). Pindolol pretreatment reduced MDMA-induced increases in peak heart rate, but had no effect on MDMA-induced change in mean arterial pressure, body temperature, or adverse effects (Hysek et al. 2010).
Hysek et al. (2012c) examined the combination of carvedilol (50 mg p.o.), an α1- and β-adrenoreceptor antagonist, with MDMA (125 mg p.o.) and found a large reduction in MDMA’s cardiostimulant and hyperthermic effects without significantly affecting MDMA’s subjective effects. Self-reported acute and subacute complaints from MDMA were not significantly affected by carvedilol. Despite the attenuation of MDMA’s physiological effects, there was a significant rise in circulating epinephrine and NE. Carvedilol and MDMA are both CYP2D6 substrates, while MDMA is also an autoinhibitor (O’Mathúna et al. 2008); however, MDMA and MDA plasma levels were unchanged when co-administered with carvedilol compared to MDMA alone.
Hysek et al. (2012a) hypothesized that the α2-adrenergic receptor agonist clonidine (150 μg p.o.), when administered one hour prior to MDMA (125 mg p.o.), would modulate the exocytotic release of NE and thus reduce MDMA’s effects. It was found that clonidine did indeed reduce MDMA-induced elevation in circulating NE and blood pressure, but decreased by the same magnitude as clonidine alone. Body temperature, mydriasis, self-reported mood effects and adverse effects from MDMA were not significantly affected by clonidine pretreatment (Hysek and Liechti 2012).
Hysek et al. (2013) hypothesized that pretreatment with the α1-adrenergic receptor antagonist doxazosin (8 mg p.o. ~ 16 h prior) would reduce MDMA-induced (125 mg p.o.) increases in blood pressure and positive mood. Indeed, doxazosin pretreatment, on average, reduced MDMA-induced increases in mean arterial pressure (MAP), despite enhancing circulating NE and tachycardia. Doxazosin pretreatment also attenuated MDMA-induced heightened mood ratings as predicted, and moderately attenuated increased body temperature. Self-reported adverse effects and mydriasis from MDMA were not significantly affected by doxazosin pretreatment (Hysek and Liechti 2012).
Antipsychotics & MDMA
One RCT was identified that combined MDMA with an antipsychotic (Liechti and Vollenweider 2000b). Researchers hypothesized that the D2 antagonism effects of haloperidol (1.4 mg i.v.) would attenuate some of the stimulant-like effects of MDMA (1.5 mg/kg p.o.). No changes were observed in MDMA-induced cardiovascular or thermogenic effects, nor responsiveness to startle (Liechti et al. 2001). In contrast to their hypothesis, this drug combination resulted in reduced well-being, reduced “oceanic boundlessness”, and a higher rate of state anxiety. Overall, researchers noted an alteration in the psychological profile of MDMA’s usually pleasurable state to a dysphoric one.
Bupropion & MDMA
Schmid et al. (2015) studied the combination of bupropion XR (titrated to 300 mg p.o. over 7 days) with MDMA (125 mg p.o. administered concurrently with bupropion XR 300 mg on day 7). The researchers hypothesized that DAT and NET blockade by bupropion pretreatment would attenuate the mood and cardiostimulant effects of MDMA. There was a moderate reduction in NE as well as an attenuation in heart rate elevation. No other significant cardiovascular, mydriatic, or hormonal changes occurred compared to MDMA alone. Bupropion did affect self-reported subjective complaints from MDMA, although bupropion significantly prolonged the positive mood effects of MDMA. There was an increase in MDMA levels and reduction in the primary metabolites, DHMA and HMMA, suggesting inhibition of CYP2D6 by bupropion (Steuer et al. 2016). There was also reduction in MDA levels, suggesting inhibition of CYP2B6 by bupropion (Steuer et al. 2016). MDMA increased bupropion levels when co-administered due to MDMA’s autoinhibition of CYP2D6.
Memantine & MDMA
de Sousa Fernandes Perna et al. (2014) studied pretreatment with the NMDA-antagonist memantine (20 mg p.o. 2 h prior) to determine if this would alter MDMA’s (75 mg p.o.) effects on memory and mood. The researchers hypothesized that memantine pretreatment may potentially reverse MDMA-induced memory impairment since this had been demonstrated in animal models. The study determined that memantine pretreatment had no effect on MDMA-induced acute memory impairment or effects on mood.
Psychostimulants & MDMA
Hysek et al. (2014b) conducted an RCT combining MDMA with methylphenidate. Researchers hypothesized that MDMA and methylphenidate may have pharmacodynamic interactions given that they both act on NET and DAT. MDMA and methylphenidate levels and half-lives were unaffected by co-administration; however, researchers found that methylphenidate delayed the time it took for MDMA to reach maximum concentration. The researchers remark this may be due to methylphenidate reducing the absorption of MDMA. There was an increase in circulating epinephrine, heart rate, and rate pressure product. Subjectively, methylphenidate attenuated MDMA’s effect on “happy” affect recognition and increased mental concentration. The co-administration of methylphenidate and MDMA increased acute and subacute subjective complaints. This suggested to researchers that “the combined use of methylphenidate and MDMA would not result in additional psychoactive effects compared with MDMA alone, but such a combination would enhance cardiovascular and adverse effects”.
Serotonin reuptake inhibitors & MDMA
Given that MDMA increases intrasynaptic 5HT, partially by inhibiting SERT, researchers hypothesized that the SERT-blocking effects of SSRIs carry the potential for pharmacodynamic interactions when co-administered with MDMA. In addition, several SSRIs are also substrates or inhibitors of cytochrome P450 enzymes, which may result in pharmacokinetic interactions with MDMA. Three primary RCTs, and four additional analyses of exploratory outcomes from the primary RCTs, have been published looking at the combination of MDMA with citalopram (Liechti 2000; Liechti and Vollenweider 2000a; Liechti et al. 2001) fluoxetine (Tancer and Johanson 2006) and paroxetine (Farré et al. 2007; Pacifici et al. 2004; Segura et al. 2005). All three SSRIs attenuated the subjective effects of MDMA by ~ 30– ~ 80%, while physiological effects were attenuated by ~ 6– ~ 14% (with the exception of paroxetine, which was on the order of ~ 40– ~ 60%). Both fluoxetine and paroxetine are strong inhibitors of CYP2D6, and plasma concentrations of MDMA were increased despite the effects of MDMA being attenuated. Exploratory studies demonstrated that paroxetine both blunted MDMA-induced immunosuppression, especially cytokine release (Pacifici et al. 2004), and may interact with multiple cytochrome P450 enzymes involved in MDMA metabolism (Segura et al. 2005).
Hysek et al. (2012d) studied the effect of duloxetine, an SNRI, on MDMA because of its potent inhibition of both SERT and NET, without substantial activity at DAT. Compared to MDMA alone, the addition of duloxetine reduced circulating NE and attenuated MDMA-induced increases in heart rate and blood pressure. There was a near complete elimination of MDMA’s effects on the pupillary light reflex (Hysek and Liechti 2012). Exploratory hormone measures with female participants found that MDMA-induced elevations in copeptin were reduced by duloxetine, while there was no significant change in males (Simmler et al. 2011). Subjectively, many of MDMA’s effects, including those on mood—such as well-being, extroversion, closeness, openness, as well as alterations in consciousness—were significantly reduced by duloxetine. Participants given MDMA alone had 5.56 more acute complaints than baseline, while duloxetine co-administered with MDMA resulted in having less acute and subacute complaints than their pre-drug baseline. These reductions of MDMA’s pharmacodynamic, physiological, and subjective effects occurred despite increased MDMA plasma levels due to CYP2D6 inhibition by duloxetine, suggesting that pharmacokinetics are not responsible for these attenuating effects.
Reboxetine & MDMA
Hysek et al. (2011) were interested in studying the effects of a NE specific reuptake inhibitor, reboxetine (8 mg p.o.), on MDMA (125 p.o.). Researchers hypothesized that reboxetine inhibition of NET would outcompete MDMA’s effects on NET, resulting in attenuation of MDMA’s effects. Confirming their hypothesis, they found reductions in circulating NE and attenuation of the cardiovascular stimulant effects of MDMA. Subjectively, the addition of reboxetine attenuated MDMA’s psychostimulant properties, such as emotional excitation, feeling “stimulated,” state anxiety, and blissful feelings such as closeness and boundlessness. There were no significant effects on other psychological parameters such as drug “liking” or “good drug effect.” Reboxetine reduced MDMA-induced acute and subacute complaints, such as tremor and restlessness. These attenuations in MDMA’s effects occurred despite increased plasma MDMA, likely due to reboxetine’s CYP2D6 inhibition. Ex vivo plasma examination showed that reboxetine co-administered with MDMA reduced NET binding and had no effect on SERT or DAT binding.
Psilocybin
Antipsychotics & psilocybin
RCTs were conducted combining psilocybin with three antipsychotics: chlorpromazine, haloperidol, and risperidone. An early study co-administered the typical antipsychotic chlorpromazine (50 mg p.o), which antagonizes both D2 and 5HT2A receptors, with psilocybin (0.2 mg/kg p.o.), resulting in attenuation in psilocybin-induced mydriasis and visual perceptual changes (Keeler 1967).Vollenweider et al. (1998) examined the role of the D2 antagonist haloperidol (0.021 mg/kg i.v.) and the mixed 5 HT2A/D2 antagonist risperidone (0.5 mg p.o., 1 mg p.o.) when separately combined with psilocybin (0.25 mg/kg p.o.). In contrast to risperidone, haloperidol had no effects on psilocybin-induced perceptual changes but did increase the “dread of ego dissolution” parameter of the Altered States of Consciousness (APZ-OAV) scale. Risperidone reduced all the parameters of the APZ-OAV, demonstrating attenuation in psilocybin-induced alterations in consciousness, including a reduction in dread of ego dissolution. Working memory was tested using the delayed-response task, where a brief visual stimulus is presented then withdrawn, and after a several second delay, the participant is asked to identify the location of the visual stimulus. Psilocybin alone delayed reaction time during its peak effects, though co-administration with risperidone attenuated the delay in reaction time.
Serotonin agonists & psilocybin
We found only one RCT combining psilocybin with serotonin agonists (Pokorny et al. 2016). Due to psilocybin’s binding affinity for 5-HT1A and 5-HT2A, the researchers independently examined the effects of combining psilocybin (170 μg/kg p.o.) with buspirone (20 mg p.o.) or ergotamine (3 mg p.o.), both of which have 5-HT1A and 5-HT2A affinity. It was found that buspirone reduced visionary restructuralization, a subscale of the 5-Dimensions of Altered States of Consciousness Rating Scale (5D-ASC). This suggests that buspirone attenuated psilocybin-induced visual perceptual changes. Ergotamine had no effect on psilocybin-induced subjective effects, perhaps due to different functional selectivity profiles at these receptors.
Serotonin reuptake inhibitors & psilocybin
To date, there has been only one RCT combining psilocybin with an SSRI (Becker et al. 2021). Participants were titrated to 20 mg of escitalopram over 14 days and then administered one dose of psilocybin (25 mg p.o., day 14) 2 h after final escitalopram administration. Due to prior case reports on SSRI-induced attenuation of the effects of LSD (Bonson 1996; Strassman 1992) researchers hypothesized a significant reduction in the subjective effects of psilocybin. Contrary to their hypothesis, escitalopram pretreatment did not significantly attenuate ratings of altered states of consciousness from psilocybin. Escitalopram pretreatment was associated with significant reductions in subjective ratings of bad drug effects, fear, talkativeness, openness, anxiety, ineffability, and global adverse effects. Physiologically, escitalopram pretreatment also significantly attenuated psilocybin-induced elevations in blood pressure and pupil dilation. Psilocybin alone did not alter the QTc interval and its co-administration with escitalopram had no effect on QTc. Escitalopram did not alter levels of psilocin.
Epidemiologic studies
Cohen et al. (2021) conducted a post-marketing surveillance association study that was hand-selected by the authors for inclusion in this systematic review as it was published shortly after the initial Pubmed query, met inclusion criteria, and is clinically relevant. Researchers analyzed 946 unique recreational ecstasy use reports from the FDA Adverse Event Reporting System (FAERS) database from 2000–2020. The mean age of the mostly male (70.1%) sample was 28 years. Several concomitant drug class ingestions were associated with an increased multivariate adjusted odds ratios of death: MDMA metabolites or analogs [aOR 8.71], muscle relaxants [aOR 8.17], anesthetics [aOR 7.13], amphetamines and stimulants [aOR 3.05], benzodiazepines [aOR 2.25], opioids [2.05], ethanol [2.03], and antidepressants [aOR 1.62]. Five psychiatric medications analyzed individually demonstrated increased univariate unadjusted odds ratios of death: bupropion [OR 2.82], sertraline [OR 2.36], venlafaxine [OR 1.97], citalopram [OR 1.92], and olanzapine [OR 1.86]. All 18 reported cases of metoclopramide resulted in death. Risperidone and lithium odds ratios were incalculable due to no fatal cases.
Copeland et al. (2006) conducted a cross-sectional survey study of 216 adults who co-ingested ecstasy and a pharmaceutical drug within the previous 6 months. The mean age of the participants was 26 years, mostly male (63%), with an average of 11 days of ecstasy use in the prior 6 months. 28% of the participants intentionally co-ingested a pharmaceutical drug to augment the effects of ecstasy, in particular sildenafil (77% reported use was to gain or maintain an erection) and benzodiazepines. 76% of participants who concomitantly used ecstasy and antidepressants still experienced euphoria; however, they experienced higher rates of adverse effects, including: muscle rigidity (0% vs 45%), nystagmus (0% vs 33%), dizziness (19% vs 42%), headache (21% vs 62%, and profuse sweating (28% vs 53%). Notably, antidepressants were not analyzed based on pharmacologic class and included MAOIs which may have contributed to the frequency of the aforementioned adverse effects.
Nayak et al. (2021) conducted an epidemiologic quantitative analysis, which was hand-selected by the authors as it was pre-published online shortly after the initial PubMed query and met inclusion criteria. Analysis of 96 first- or second-person accounts posted online (i.e., Erowid, the Shroomery, and Reddit) involving co-ingestion of a psychedelic and a mood stabilizer (i.e., lithium, lamotrigine, valproic acid, carbamazepine, and oxcarbazepine). The researchers looked at numerous psychedelic substances, including psilocybin, however most reports involved lysergic acid diethylamide (LSD), another “classic psychedelic.” Of the mood stabilizers, there was only data on lamotrigine and lithium being co-ingested with psilocybin. They found that 2 of the 6 reports of lithium plus psilocybin resulted in seizures. None of the 10 reports of lamotrigine plus psilocybin resulted in seizures. “Low quality evidence due to lack of standardization, selection bias, and lack of clinical verification” was acknowledged by the study authors.
Discussion
To our knowledge, this is the first systematic review of drug-drug interactions between psychiatric medications and MDMA or psilocybin. Forty publications met our inclusion criteria, including 22 RCTs of MDMA administered with and without a psychiatric medication and 4 RCTs of psilocybin administered with and without a psychiatric medication. Cumulatively, these clinical trials included over 200 participants. We also identified 3 epidemiologic studies: one large cross-sectional survey, one large post-marketing surveillance association study involving ecstasy co-ingestion with medications, and one quantitative analysis of online “trip reports.”
This review demonstrates that the existing data on drug-drug interactions between a psychiatric medication and MDMA or psilocybin predominantly pertains to MDMA. There are only 2 studies on psilocybin captured in this review that were published within the last decade, highlighting a limited body of scientific literature related to drug-drug interactions with psilocybin. It is important to note that the clinical trials in this review were primarily designed to ascertain the mechanisms of MDMA or psilocybin. Therefore, all the study participants were young healthy adults who were either administered a psychiatric medication only once or, rarely, over several days for a maximum of 14 days (Becker et al. 2021). Therefore, these studies are limited in their extrapolation to real world clinical settings where psychiatric medications are often taken daily for months or years. Other factors that limit the extrapolation of these studies to clinical settings is that some studies instituted less common routes of administration of psychiatric medications, such as intravenous haloperidol (Vollenweider et al. 1998) or intravenous citalopram (Liechti and Vollenweider 2000a). Some studies had unique exclusion criteria that may limit the generalizability of outcomes further, such as high levels of neuroticism (Liechti and Vollenweider 2000a, 2000b)—which recently has been shown to predict negative psychological responses to MDMA (Studerus et al. 2021)—or inclusion of only individuals with prior recreational ecstasy use (Farré et al. 2007; Tancer and Johanson 2006).
MDMA psychopharmacology
Pharmacokinetic interactions—MDMA
MDMA’s pharmacokinetics are complex, involving several cytochrome P450 enzymes, which makes MDMA particularly susceptible to drug-drug interactions with strong pan-CYP inhibitors like ritonavir—which has resulted in significant toxicity (Papaseit et al. 2012). Many psychiatric medications have significant inhibitory effects on CYP2D6, and it is also an enzyme that displays a wide range in metabolic capacity throughout the population (Bertilsson et al. 2002). The studies included in this review had numerous instances of CYP2D6 interactions with MDMA including paroxetine, fluoxetine, duloxetine, reboxetine, and bupropion.
Pharmacodynamic interactions—MDMA
MDMA’s effects on monoamine transporters, namely SERT and NET, are unique. MDMA acts as a SERT and NET substrate, resulting in reverse transport and exocytotic release of 5HT and NE (Hilber et al. 2005; Verrico et al. 2007). In contrast: antidepressants (i.e., SSRIs, SNRIs, NRIs, and NDRIs) capture the monoamine transporter (i.e., SERT, NET, and DAT) in an inhibitor-bound conformation, preventing substrate binding (Coleman et al. 2016; Penmatsa et al. 2013, 2015) and resulting in higher monoamine concentrations in the synaptic cleft.
Researchers primarily from the same laboratory group systematically conducted a series of clinical trials on drug-drug combinations of MDMA with various monoamine reuptake blocking agents to ascertain relative contributions of different monoamine systems to MDMA’s mechanism of action. SERT inhibitors (SSRIs), including citalopram (Liechti 2000; Liechti et al. 2001; Liechti and Vollenweider 2000a), paroxetine (Farré et al. 2007; Pacifici et al. 2004; Segura et al. 2005), and fluoxetine (Tancer and Johanson 2006), reduce MDMA’s physiological and subjective effects broadly. NET-specific inhibition by reboxetine reduced MDMA’s NE-mediated cardiostimulant properties and some psychological outcomes (Hysek et al. 2011; Hysek and Liechti 2012); while combined NET and SERT inhibition by duloxetine broadly attenuated physiological and psychological outcomes, similar to outcomes from SSRI + MDMA combinations (Hysek et al. 2012d; Hysek and Liechti 2012; Simmler et al. 2011). Overall, monoamine reuptake inhibitors resulted in attenuation of MDMA’s physiological and subjective effects, even in instances of higher MDMA plasma levels by means of CYP2D6 inhibition. This consistent outcome may suggest that, when administered together, monoamine reuptake inhibitors outcompete MDMA by binding to monoamine transporters (i.e., SERT, NET, DAT), preventing the efflux of monoamines into the synaptic cleft, thus attenuating the effects of MDMA. Exceptions to this trend include the combined NET and DAT inhibitor, bupropion, which prolonged the subjective effects and heightened the positive mood effects of MDMA. In this case, CYP2D6 and CYP2B6 pharmacokinetic interactions and bupropion’s relatively low ability to inhibit NET or DAT (Schmid et al. 2015; Steuer et al. 2016) may explain this outcome. While no serious adverse effects of these combinations were observed in clinical trial settings, there are risks to combining SSRIs or bupropion with MDMA in uncontrolled settings. For example, a recent large-scale surveillance study of data from the FDA’s Adverse Event Reporting Systems (FAERS) reviewed reports of co-ingestion of MDMA in non-clinical settings with medications, finding that several antidepressant + MDMA combinations increased mortality, with bupropion having the highest odds ratio for death of any antidepressant [OR 2.82] (Cohen et al. 2021). It is plausible that the combination of bupropion and MDMA increases the risk of seizures, stimulant toxicity, or serotonin syndrome given that both drugs are stimulants capable of lowering seizure thresholds and that they increase blood concentrations of each other when taken together.
Relevant to the co-administration of monoamine reuptake inhibitors and MDMA, there has been ongoing attention paid to the risk of serotonin syndrome with the combination of SSRIs and MDMA, theorized to be related to a rise of 5HT in the central nervous system (Copeland et al. 2006; Dobry et al. 2013). The RCTs in this review show attenuation of MDMA’s physiological effects, suggesting a reduced odds of serotonin syndrome, at least with acute SSRI dosing. This is corroborated by at least one other scientific review positing SSRIs, SNRIs, and TCAs are not likely to increase serotonin to life-threatening toxicity when combined with MDMA (Silins et al. 2007). This may be due to the fact that MDMA’s reversal of monoamine transporters is negated by the inhibition of monoamine transporters by most antidepressants; therefore, there is not an increase in intrasynaptic serotonin which is the hallmark mechanism for serotonin toxicity to occur. A notable downside of SSRI or SNRI use in conjunction with MDMA is attenuation of subjective effects and the potential for reduced MAP treatment efficacy (Hysek et al. 2012d; Feduccia et al. 2020). However, discontinuation of psychiatric medications poses a risk of antidepressant discontinuation syndromes or relapse of psychiatric symptoms that must be weighed in clinical decision-making by the prescribing provider. An important outlier when it comes to serotonergic agents and MDMA is the MAOI class. MAOIs prevent breakdown of monoamines and, when combined with MDMA, result in dangerous elevations in intrasynaptic serotonin. This has contributed to the majority of deaths cited in published case studies (see summary of case studies in Supplementary Materials).
Stimulants have been proposed to induce exocytotic release of monoamines via DAT and NET, similar to MDMA (Fleckenstein and Hanson 2003), or to only inhibit DAT and NET reuptake similar to methylphenidate (Hysek et al. 2014a, b). In fact, the combination of methylphenidate and MDMA resulted in synergistic effects: higher rates of self-reported complaints and heightened cardiostimulant effects (Hysek et al. 2014a, b). Dopamine blockade via co-administration of haloperidol did not alter MDMA’s physiological effects (Liechti and Vollenweider 2000b), which is unsurprising given MDMA’s limited impact on the dopamine system. However, it is intriguing that MDMA’s typically euphoric effects were absent, while trait anxiety and dysphoria were exaggerated, potentially related to haloperidol’s dysphoric effects.
Regarding adrenergic agents and MDMA: carvedilol (Hysek et al. 2012c) dramatically reduced MDMA-induced cardiostimulant and hyperthermic effects without influencing MDMA’s psychological effects, while clonidine and doxazosin had a minimal effect on physiology (Hysek et al. 2012a; Hysek et al. 2013). It is not fully clear if combining an adrenergic agent with MDMA reflects additive effects or a pharmacodynamic interaction. The notion of additive effects is supported by the fact that carvedilol (Hysek et al. 2012c), pindolol (Hysek et al. 2010), and clonidine (Hysek et al. 2012a) reduced adrenergic activity but did not significantly alter the subjective effects of MDMA (Hasler et al. 2009; Hysek et al. 2010, 2012a, c). However, doxazosin is an outlier as it attenuated attenuated MDMA’s mood heightening effects (Hysek et al. 2013). Doxazosin also increased MDMA-induced tachycardia which may be a result of its α1 specific blockade resulting in compensatory activation of other α- and β-adrenoreceptors as described elsewhere in the literature (Richards et al. 2017). Regardless, the interaction between adrenergic agents and MDMA would be a valuable area of additional research, as well as clinically useful information in choosing an antihypertensive or antipyretic to address MDMA-related adverse effects in the setting of MAP treatment without compromising the potentially beneficial affective effects of MDMA.
Pharmacogenetics & MDMA
It has been demonstrated that CYP2D6 poor metabolizers carrying only *3, *4, or *5 alleles administered MDMA reached an average maximum concentration (Cmax) that is 19% higher and an initial drug exposure (AUC0–6 h) that is 25% higher than extensive metabolizers carrying only *1 and *2 alleles, which corresponded to a more rapid onset of subjective effects and elevated blood pressure (Schmid et al. 2016). Recent pooled RCT data demonstrated that the physiological and psychological effects of MDMA were most strongly dependent on plasma concentration, while higher activity scores for CYP2D6 metabolizer status predicted a lower MDMA plasma concentration (Studerus et al. 2021).
Psilocybin psychopharmacology
Pharmacokinetic interactions—psilocybin
Compared to MDMA, psilocybin is less likely to contribute to pharmacokinetic drug-drug interactions given that its metabolic pathway predominantly involves UGT1A10 and UGT1A9 (Manevski et al. 2010). Diclofenac and probenecid are examples of UGT1A9/10 inhibitors that may hypothetically alter psilocybin metabolism if co-ingested; however, there is no evidence to confirm this hypothesis beyond shared metabolism. There are few pharmacologic agents that influence UGT1A9 and UGT1A10 (Liu et al. 2011). Furthermore, the pharmacokinetics of psilocin, the active metabolite of psilocybin, is linear and is minimally affected by renal clearance (Brown et al. 2017).
Pharmacodynamic interactions—psilocybin
Research into drug interactions with psilocybin is sparse yet paints an interesting picture of its mechanism. When 5 HT2A is blocked pharmacologically, such as by buspirone (Pokorny et al. 2016; Bonhaus et al. 1997), chlorpromazine (Keeler 1967), ketanserin, or risperidone (Vollenweider et al. 1998), there is a consistent pattern of attenuation of psilocybin’s subjective effects. However, 5HT2A and 5HT1A agonism, such as from ergotamine (Pokorny et al. 2016) does not seem to have an effect on psilocybin nor LSD’s subjective effects (Mattusek and Halbach 1964). D2 antagonism alone, in the case of haloperidol, does not attenuate psilocybin’s effects, and, in fact, exacerbates anxiety and produces dysphoria (Vollenweider et al. 1998). Interestingly, when blocking SERT pharmacologically with escitalopram (Becker et al. 2021), there is no attenuation of psilocybin’s psychedelic effects and in fact reduced measures of anxious distress and participant complaints. Collectively, this data points towards psilocybin’s unique mechanism of action which is contingent on direct serotonin receptor agonism that does not compete nor rely on SERT activity. We would hypothesize that other SERT blockers, such as sertraline, in combination with psilocybin would reproduce similar findings as Becker et al. (2021) while 5HT2A blockade would attenuate psilocybin’s effects, both subjectively and physiologically.
Trends in case studies
We identified 11 case study manuscripts (Akhondzadeh and Hampa 2005; Bingham 1998; Delgado et al. 2004; Kaskey 1992; Lauerma et al. 1998; McCann and Ricaurte 1993; Pilgrim et al. 2012; Ramcharan et al. 1998; Smilkstein et al. 1987; Stein and Rink 1999; Vuori et al. 2003), all of which involve the recreational ingestion of ecstasy and provide helpful insights on the implications of co-ingestion in the context of illicit use. However, these data are limited by the inability to ascertain drug levels of MDMA or other potential adulterants in most cases; therefore, case studies are described in Supplemental Materials. These case studies reveal a trend that the highest rates of morbidity occurred from the combination of MDMA with MAOIs, such as moclobemide or phenelzine. A number of these cases involved significant hyperthermia, seizure-like activity, and altered mental status, suggestive of unrecognized symptoms of serotonin syndrome, many of which resulted in fatality. In several cases, coronary artery disease was noted upon autopsy and was a suggested risk factor in the cause of death. In one notable case of a young man with bipolar disorder prescribed moclobemide and lithium who ingested ecstasy, the symptoms of serotonin syndrome were misattributed by his peers to a “psychotic episode” whereupon he was left unaccompanied for 4 h and after which he was found deceased (Pilgrim et al. 2012). The combination of SSRIs with ecstasy in case reports are consistent with lab data, suggesting attenuation of MDMA’s effects. However, the attenuated subjective effects of MDMA from SSRIs may drive individuals to take escalating drug doses to achieve effects, resulting in the elevated odds-ratio for mortality observed by Cohen et al. (2021)) in instances of concomitant MDMA use with SSRIs.
Limitations
This review focuses on MDMA and psilocybin, thus did not include literature on other psychedelic substances, such as: lysergic acid diethyladmide (LSD), ayahuasca or N, N-demethyltryptamine (DMT), mescaline, or designer drugs. Ketamine can be used in combination with psychotherapy for its psychedelic effects (Dore et al. 2019); we included ketamine in our search as an antidepressant rather than a psychedelic, but did not find any literature on interaction with MDMA or psilocybin. We also did not include the effects of repeated dosing of MDMA (Farré et al. 2004; Peiró et al. 2013; Farré et al. 2015) or psilocybin on subsequent effects. Additionally, our scope was limited to psychiatric medications rather than other medications that may have the propensity for drug-drug interactions with MDMA and psilocybin. Although this review attempts to capture all relevant clinical data to date on co-administration of a psychiatric medication with MDMA or psilocybin, the window of permissible scientific study on psychedelics has been narrow in comparison to groups who have implemented psychedelics into their cultural and spiritual practices for longer than psychiatric medications have been around (e.g., the Mazatec people of Oaxaca) or in comparison to other forms of collective knowledge (e.g., the Erowid Experience Vaults).
Considerations for future research
Modern research protocols for MAP and PAP have either excluded participants taking psychiatric medications or discontinued psychiatric medications prior to administration of MDMA or psilocybin. Tapering psychiatric medications before MAP or PAP poses unique challenges and vulnerabilities to those suffering from psychiatric illnesses by way of increased risk of illness relapse, serotonin discontinuation syndrome, and reduced treatment efficacy (Feduccia et al. 2020). It is apparent that developing optimal strategic approaches for patients who may benefit from MAP or PAP, but are currently prescribed psychiatric medications, will require additional clinical research. In particular, there is a dearth of studies on psilocybin drug-drug interactions, signaling a need for additional research on how psychiatric medications and psilocybin interact. Additionally, of the psilocybin RCTs in this review, none included measures of physiological outcomes. Additional studies of psilocybin drug-drug interactions are pending completion, including a cross-sectional online survey being conducted by the Johns Hopkins Center for Psychedelic and Consciousness Research investigating psilocybin mushrooms taken with antidepressants (https://hopkinspsychedelic.org/adsurvey).
Certain groups have been excluded from the clinical trial samples described in this systematic review, including those with particular psychiatric symptoms or medical illnesses such as uncontrolled hypertension, cardiovascular disease, and liver disease. It remains unclear how to safely administer MAP or PAP to medically ill individuals, including individuals with hepatic impairment that would affect drug metabolism. At this time, research is underway to ascertain how hepatic impairment may affect MDMA’s pharmacokinetics (NCT03606538), which is an FDA requirement for drug development and may broaden inclusion in future studies. Additionally, none of the included studies reported on the race or ethnicity of participants nor commented on the inclusion of sexual or gender minorities. This corresponds to the lack of diverse participants in MAP and PAP clinical trials (Fogg et al. 2021). Additionally, there is evidence that estradiol up-regulates expression of UGT1A9 resulting in increased glucuronidation of UGT1A9 substrates (Cho et al. 2016). This suggests that hormone replacement therapy, oral contraceptives, and cyclical hormonal changes may affect the metabolism of psilocybin, highlighting the need for more inclusive research.
There are a number of psychiatric medications commonly used in the management of acute psychological distress sometimes experienced acutely with MDMA or psilocybin administration. Benzodiazepines have been suggested by guidelines, informed by research on management of LSD-related psychological distress, to address extremely challenging psychedelic experiences that don’t respond to behavioral interventions (Johnson et al. 2008). We did not find any studies on the concomitant use of benzodiazepines with MDMA nor psilocybin. In fact, Cohen et al. (2021) found that, among several other drug class co-ingestions with recreational MDMA, benzodiazepines were associated with an increased multivariate adjusted odds ratio of death—higher than that of co-ingested opioids, ethanol, or antidepressants. Online communities have encouraged using olanzapine as a “trip terminator”; in other words, a pharmacologic technique for self-management of psychedelic crises (Valeriani et al. 2015). However, it remains unclear if atypical antipsychotics would be an appropriate approach to manage psychological distress related to MDMA or psilocybin. Recent data from Cohen et al. (2021) demonstrated that olanzapine had an increased univariate unadjusted odds ratio of death among adverse reports of MDMA co-ingestion, while there were no cases of death with risperidone co-ingestion. It is notable that the study by Cohen et al. (2021) was in regards to unregulated ecstasy use in uncontrolled non-clinical settings. The absence of clinical trials on the combination of benzodiazepines or atypical antipsychotics with MDMA, and mixed data regarding heightened risk with psilocybin, suggest that it would be valuable to investigate these drug-drug interactions in future research.
The mechanism in which MAP or PAP lead to psychiatric recovery remains unclear as is the mechanism in which psychiatric drugs affect MAP or PAP outcomes. Of the current body of literature, 5HT2A serotonin receptor modulation (Carhart-Harris and Nutt 2017), changes in resting state functional connectivity in the default mode network (Carhart-Harris et al. 2014; Müller et al. 2021; Roseman et al. 2014), and neuroplasticity (Artin et al. 2021; Harmer et al. 2017) are among some of the prevailing hypotheses. Whether or not combinations of psychiatric medications with MDMA or psilocybin would interfere with these processes specifically has not been studied. However, there are surprising results from a sub-analysis of pooled Phase 2 MAP studies demonstrating that participants who recently tapered off of prescription antidepressants (e.g. SSRIs, SNRIs, etc.) had a higher PTSD symptom burden and were more likely than the non-taper group to still meet PTSD criteria at the primary study endpoint (63.6% vs. 25.0%), despite completing the taper by an average of 25.1 days before their first MDMA administration session (Feduccia et al. 2020). This suggests that mechanisms beyond direct and immediate pharmacodynamic interactions may have an influence on how drug-drug interactions affect MAP and PAP outcomes. It would be valuable to further study how lifelong psychiatric medication adherence or enduring psychedelic use would affect MAP or PAP outcomes.
Conclusions
As MDMA and psilocybin continue to move through the FDA drug development process, this systematic review offers a compilation of existing research on psychiatric drug-drug interactions with MDMA or psilocybin. There are clear acute pharmacokinetic and pharmacodynamic interactions between many common psychiatric medications and MDMA or psilocybin which affect their physiological and subjective effects and help us better understand the mechanisms of action of these substances. However, the existing body of literature is not readily applicable to the clinical setting, prompting the need for additional research on the topic of drug-drug interactions with MDMA or psilocybin.
Supplementary Material
Acknowledgements
The authors thank Paul Bonney and Jason Jarria for assisting in abstract review. Salary support for CSS provided by the Department of Veterans Affairs, Clinical Science Research & Development, Federal Award Identification Number IK2CX001495.
Footnotes
Declarations
Conflict of interest CSS has received payment from the MAPS Public Benefit Corporation for Training clinicians in MAP. BM runs www.spiritpharmacist.com, which offers a monthly membership service for access to drug interactions guides, courses, and consultation services. AS and KT have no conflicts of interest to report.
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s00213-022-06083-y.
References
- Abraham TT, Barnes AJ, Lowe RH, Spargo EAK, Milman G, Pirnay SO, Gorelick DA, Goodwin RS, Huestis MA (2009) Urinary MDMA, MDA, HMMA, and HMA Excretion Following Controlled MDMA Administration to Humans. J Anal Toxicol 33(8):439–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhondzadeh S, Hampa AD (2005) Topiramate prevents ecstasy consumption: A case report. Fundam Clin Pharmacol 19(5):601–602. 10.1111/j.1472-8206.2005.00355.x [DOI] [PubMed] [Google Scholar]
- Artin H, Zisook S, Ramanathan D (2021) How do serotonergic psychedelics treat depression: The potential role of neuroplasticity. World J Psychiatr 11(6):201–214. 10.5498/wjp.v11.i6.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggott MJ, Kirkpatrick MG, Bedi G, de Wit H (2015) Intimate insight: MDMA changes how people talk about significant others. J Psychopharmacol 29(6):669–677. 10.1177/0269881115581962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett FS, Bradstreet MP, Leoutsakos JMS, Johnson MW, Griffiths RR (2016) The Challenging Experience Questionnaire: Characterization of challenging experiences with psilocybin mushrooms. J Psychopharmacol 30(12):1279–1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett FS, Griffiths RR (2018) Classic Hallucinogens and Mystical Experiences: Phenomenology and Neural Correlates. Curr Top Behav Neurosci 36:393–430. 10.1007/7854_2017_474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia G, Brooks BP, Kulsakdinun C, De Souza EB (1988) Pharmacologic profile of MDMA (3,4-methylenedioxymethamphetamine) at various brain recognition sites. Eur J Pharmacol 149(1–2):159–163. 10.1016/0014-2999(88)90056-8 [DOI] [PubMed] [Google Scholar]
- Baylen CA, Rosenberg H (2006) A review of the acute subjective effects of MDMA/ecstasy. Addiction 101(7):933–947. 10.1111/j.1360-0443.2006.01423.x [DOI] [PubMed] [Google Scholar]
- Becker AM, Holze F, Grandinetti T, Klaiber A, Toedtli VE, Kolaczynska KE, Duthaler U, Varghese N, Eckert A, Grünblatt E, Liechti ME (2021) Acute Effects of Psilocybin After Escitalopram or Placebo Pretreatment in a Randomized, Double-Blind, Placebo-Controlled, Crossover Study in Healthy Subjects. Clin Pharmacol Ther n/a(n/a). 10.1002/cpt.2487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertilsson L, Dahl M-L, Dalén P, Al-Shurbaji A (2002) Molecular genetics of CYP2D6: Clinical relevance with focus on psychotropic drugs: Molecular genetics of CYP2D6. Br J Clin Pharmacol 53(2):111–122. 10.1046/j.0306-5251.2001.01548.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigler MB, Egli SB, Hysek CM, Hoenger G, Schmied L, Baldin FS, Marquardsen FA, Recher M, Liechti ME, Hess C, Berger CT (2015) Stress-Induced In Vivo Recruitment of Human Cytotoxic Natural Killer Cells Favors Subsets with Distinct Receptor Profiles and Associates with Increased Epinephrine Levels. PLoS ONE 10(12):e0145635. 10.1371/journal.pone.0145635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham C (1998) Necrotizing renal vasculopathy resulting in chronic renal failure after ingestion of methamphetamine and 3,4-methylenedioxymethamphetamine (‘ecstasy’). Nephrol Dial Transplant 13(10):2654–2655. 10.1093/ndt/13.10.2654 [DOI] [PubMed] [Google Scholar]
- Blier P, Ward HE, Tremblay P, Laberge L, Hébert C, Bergeron R (2010) Combination of Antidepressant Medications From Treatment Initiation for Major Depressive Disorder: A Double-Blind Randomized Study. Am J Psychiatry 167(3):281–288. 10.1176/appi.ajp.2009.09020186 [DOI] [PubMed] [Google Scholar]
- Bogen IL, Haug KH, Myhre O, Fonnum F (2003) Short- and long-term effects of MDMA (“ecstasy”) on synaptosomal and vesicular uptake of neurotransmitters in vitro and ex vivo. Neurochem Int 43(4–5):393–400. 10.1016/S0197-0186(03)00027-5 [DOI] [PubMed] [Google Scholar]
- Bogenschutz MP, Forcehimes AA, Pommy JA, Wilcox CE, Barbosa P, Strassman RJ (2015) Psilocybin-assisted treatment for alcohol dependence: A proof-of-concept study. J Psychopharmacol 29(3):289–299. 10.1177/0269881114565144 [DOI] [PubMed] [Google Scholar]
- Bonhaus DW, Weinhardt KK, Taylor M, Desouza A, Mcneeley PM, Szczepanski K, Fontana DJ, Trinh J, Rocha CL, Dawson MW, Flippin LA, Eglen RM (1997) RS-102221: A Novel High Affinity and Selective, 5-HT 2C Receptor Antagonist. Neuropharmacology 36(4–5):621–629. 10.1016/S0028-3908(97)00049-X [DOI] [PubMed] [Google Scholar]
- Bonson K (1996) Chronic Administration of Serotonergic Antidepressants Attenuates the Subjective Effects of LSD in Humans. Neuropsychopharmacology 14(6):425–436. 10.1016/0893-133X(95)00145-4 [DOI] [PubMed] [Google Scholar]
- Borowiak KS, Ciechanowski K, Waloszczyk P (1998) Psilocybin mushroom (Psilocybe semilanceata) intoxication with myocardial infarction. J Toxicol Clin Toxicol 36(1–2):47–49. 10.3109/15563659809162584 [DOI] [PubMed] [Google Scholar]
- Brown RT, Nicholas CR, Cozzi NV, Gassman MC, Cooper KM, Muller D, Thomas CD, Hetzel SJ, Henriquez KM, Ribaudo AS, Hutson PR (2017) Pharmacokinetics of Escalating Doses of Oral Psilocybin in Healthy Adults. Clin Pharmacokinet 56(12):1543–1554. 10.1007/s40262-017-0540-6 [DOI] [PubMed] [Google Scholar]
- Carhart-Harris R, Giribaldi B, Watts R, Baker-Jones M, Murphy-Beiner A, Murphy R, Martell J, Blemings A, Erritzoe D, Nutt DJ (2021) Trial of Psilocybin versus Escitalopram for Depression. N Engl J Med 384(15):1402–1411. 10.1056/NEJMoa2032994 [DOI] [PubMed] [Google Scholar]
- Carhart-Harris R, Leech R, Hellyer PJ, Shanahan M, Feilding A, Tagliazucchi E, Chialvo DR, Nutt D (2014) The entropic brain: A theory of conscious states informed by neuroimaging research with psychedelic drugs. Front Hum Neurosci 8. 10.3389/fnhum.2014.00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris R, Nutt D (2017) Serotonin and brain function: A tale of two receptors. J Psychopharmacol 31(9):1091–1120. 10.1177/0269881117725915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter LL, Yasmin S, Price LH (2002) A double-blind, placebo-controlled study of antidepressant augementation with mirtazapine. Biol Psychiat 51(2):183–188. 10.1016/S0006-3223(01)01262-8 [DOI] [PubMed] [Google Scholar]
- Castro Santos H, Gama Marques J (2021) What is the clinical evidence on psilocybin for the treatment of psychiatric disorders? A Systematic Review Porto Biomedical Journal 6(1):e128. 10.1097/j.pbj.0000000000000128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Behavioral Health Statistics and Quality (2020) National Survey on Drug Use and Health—2019. Substance Abuse and Mental Health Services Administration. https://www.samhsa.gov/data/ [PubMed] [Google Scholar]
- Cho S, Ning M, Zhang Y, Rubin LH, Jeong H (2016) 17β-Estradiol up-regulates UDP-glucuronosyltransferase 1A9 expression via estrogen receptor α. Acta Pharmaceutica Sinica B 6(5):504–509. 10.1016/j.apsb.2016.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen IV, Makunts T, Abagyan R, Thomas K (2021) Concomitant drugs associated with increased mortality for MDMA users reported in a drug safety surveillance database. Sci Rep 11(1):5997. 10.1038/s41598-021-85389-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman JA, Green EM, Gouaux E (2016) X-ray structures and mechanism of the human serotonin transporter. Nature 532(7599):334–339. 10.1038/nature17629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland J, Dillon P, Gascoigne M (2006) Ecstasy and the concomitant use of pharmaceuticals. Addict Behav 31(2):367–370. 10.1016/j.addbeh.2005.05.025 [DOI] [PubMed] [Google Scholar]
- Danforth AL, Grob CS, Struble C, Feduccia AA, Walker N, Jerome L, Yazar-Klosinski B, Emerson A (2018) Reduction in social anxiety after MDMA-assisted psychotherapy with autistic adults: A randomized, double-blind, placebo-controlled pilot study. Psychopharmacology 235(11):3137–3148. 10.1007/s00213-018-5010-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis AK, Barrett FS, May DG, Cosimano MP, Sepeda ND, Johnson MW, Finan PH, Griffiths RR (2021) Effects of Psilocybin-Assisted Therapy on Major Depressive Disorder: A Randomized Clinical Trial. JAMA Psychiat 78(5):481–489. 10.1001/jamapsychiatry.2020.3285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jong J, Wauben P, Huijbrechts I, Oolders H, Haffmans J (2010) Doxazosin treatment for posttraumatic stress disorder. J Clin Psychopharmacol 30(1):84–85. 10.1097/JCP.0b013e3181c827ae [DOI] [PubMed] [Google Scholar]
- Perna DSF EB, Theunissen EL, Kuypers KP, Heckman P, de la Torre R, Farre M, & Ramaekers JG (2014) Memory and mood during MDMA intoxication, with and without memantine pretreatment. Neuropharmacology 87:198–205. 10.1016/j.neuropharm.2014.03.008 [DOI] [PubMed] [Google Scholar]
- Delgado JH, Caruso MJ, Waksman JC, Honigman B, Stillman D (2004) Acute, transient urinary retention from combined ecstasy and methamphetamine use. J Emerg Med 26(2):173–175. 10.1016/j.jemermed.2003.05.007 [DOI] [PubMed] [Google Scholar]
- Dinis-Oliveira RJ (2017) Metabolism of psilocybin and psilocin: Clinical and forensic toxicological relevance. Drug Metab Rev 49(1):84–91. 10.1080/03602532.2016.1278228 [DOI] [PubMed] [Google Scholar]
- Dobry Y, Rice T, Sher L (2013) Ecstasy use and serotonin syndrome: A neglected danger to adolescents and young adults prescribed selective serotonin reuptake inhibitors. Int J Adolesc Med Health 25(3):193–199. 10.1515/ijamh-2013-0052 [DOI] [PubMed] [Google Scholar]
- Dore J, Turnipseed B, Dwyer S, Turnipseed A, Andries J, Ascani G, Monnette C, Huidekoper A, Strauss N, Wolfson P (2019) Ketamine Assisted Psychotherapy (KAP): Patient Demographics, Clinical Data and Outcomes in Three Large Practices Administering Ketamine with Psychotherapy. J Psychoactive Drugs 51(2):189–198. 10.1080/02791072.2019.1587556 [DOI] [PubMed] [Google Scholar]
- English BA, Dortch M, Ereshefsky L, Jhee S (2012) Clinically Significant Psychotropic Drug-Drug Interactions in the Primary Care Setting. Curr Psychiatry Rep 14(4):376–390. 10.1007/s11920-012-0284-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farré M, de la Torre R, Mathúna BO, Roset PN, Peiró AM, Torrens M, Ortuño J, Pujadas M, Camí J (2004) Repeated doses administration of MDMA in humans: pharmacological effects and pharmacokinetics. Psychopharmacology (Berl) 173(3–4):364–75 [DOI] [PubMed] [Google Scholar]
- Farré M, Abanades S, Roset PN, Peiró AM, Torrens M, O’Mathúna B, Segura M, de la Torre R (2007) Pharmacological Interaction between 3,4-Methylenedioxymethamphetamine (Ecstasy) and Paroxetine: Pharmacological Effects and Pharmacokinetics. J Pharmacol Exp Ther 323(3):954–962. 10.1124/jpet.107.129056 [DOI] [PubMed] [Google Scholar]
- Feduccia AA, Jerome L, Mithoefer MC, Holland J (2020) Discontinuation of medications classified as reuptake inhibitors affects treatment response of MDMA-assisted psychotherapy. Psychopharmacology. 10.1007/s00213-020-05710-w [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Feduccia AA, Mithoefer MC (2018) MDMA-assisted psychotherapy for PTSD: Are memory reconsolidation and fear extinction underlying mechanisms? Prog Neuropsychopharmacol Biol Psychiatry 84:221–228. 10.1016/j.pnpbp.2018.03.003 [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Hanson GR (2003) Impact of psychostimulants on vesicular monoamine transporter function. Eur J Pharmacol 479(1):283–289. 10.1016/j.ejphar.2003.08.077 [DOI] [PubMed] [Google Scholar]
- Fogg C, Michaels TI, de la Salle S, Jahn ZW, Williams MT (2021) Ethnoracial health disparities and the ethnopsychopharmacology of psychedelic-assisted psychotherapies. Exp Clin Psychopharmacol. 10.1037/pha0000490 [DOI] [PubMed] [Google Scholar]
- Gable RS (2004) Comparison of acute lethal toxicity of commonly abused psychoactive substances. Addiction 99(6):686–696. 10.1111/j.1360-0443.2004.00744.x [DOI] [PubMed] [Google Scholar]
- Gorman I, Belser AB, Jerome L, Hennigan C, Shechet B, Hamilton S, Yazar-Klosinski B, Emerson A, Feduccia AA (2020) Post-traumatic Growth After MDMA-Assisted Psychotherapy for Posttraumatic Stress Disorder. J Trauma Stress 33(2):161–170. 10.1002/jts.22479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Richards WA, Johnson MW, McCann UD, Jesse R (2008) Mystical-type experiences occasioned by psilocybin mediate the attribution of personal meaning and spiritual significance 14 months later. Journal of Psychopharmacology (oxford, England) 22(6):621–632. 10.1177/0269881108094300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Richards WA, McCann U, & Jesse R (2006). Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual significance. Psychopharmacology, 187(3), 268–283 284–292. 10.1007/s00213-006-0457-5 [DOI] [PubMed] [Google Scholar]
- Grob CS, & Grigsby J (Eds.). (2021). Handbook of medical hallucinogens. The Guilford Press. [Google Scholar]
- Grob CS, Poland RE, Chang L, Ernst T (1995) Psychobiologic effects of 3,4-methylenedioxymethamphetamine in humans: Methodological considerations and preliminary observations. Behav Brain Res 73(1):103–107. 10.1016/0166-4328(96)00078-2 [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Duman RS, Cowen PJ (2017) How do antidepressants work? New perspectives for refining future treatment approaches. The Lancet Psychiatry 4(5):409–418. 10.1016/S2215-0366(17)30015-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler F, Grimberg U, Benz MA, Huber T, Vollenweider FX (2004) Acute psychological and physiological effects of psilocybin in healthy humans: a double-blind, placebo-controlled dose-effect study. Psychopharmacology (Berl) 172(2):145–56 [DOI] [PubMed] [Google Scholar]
- Hasler F, Studerus E, Lindner K, Ludewig S, Vollenweider F (2009) Investigation of serotonin-1A receptor function in the human psychopharmacology of MDMA. J Psychopharmacol 23(8):923–935. 10.1177/0269881108094650 [DOI] [PubMed] [Google Scholar]
- Hilber B, Scholze P, Dorostkar MM, Sandtner W, Holy M, Boehm S, Singer EA, Sitte HH (2005) Serotonin-transporter mediated efflux: A pharmacological analysis of amphetamines and non-amphetamines. Neuropharmacology 49(6):811–819. 10.1016/j.neuropharm.2005.08.008 [DOI] [PubMed] [Google Scholar]
- Hofmann A, Frey A, Ott H, Petrzilka Th, Troxler F (1958) Konstitutionsaufklärung und Synthese von Psilocybin. Experientia 14(11):397–399. 10.1007/BF02160424 [DOI] [PubMed] [Google Scholar]
- Hysek CM, Brugger R, Simmler LD, Bruggisser M, Donzelli M, Grouzmann E, Hoener MC, Liechti ME (2012a) Effects of the α 2 -Adrenergic Agonist Clonidine on the Pharmacodynamics and Pharmacokinetics of 3,4-Methylenedioxymethamphetamine in Healthy Volunteers. J Pharmacol Exp Ther 340(2):286–294. 10.1124/jpet.111.188425 [DOI] [PubMed] [Google Scholar]
- Hysek CM, Domes G, Liechti ME (2012b) MDMA enhances “mind reading” of positive emotions and impairs “mind reading” of negative emotions. Psychopharmacology 222(2):293–302. 10.1007/s00213-012-2645-9 [DOI] [PubMed] [Google Scholar]
- Hysek CM, Fink AE, Simmler LD, Donzelli M, Grouzmann E, Liechti ME (2013) Α1-Adrenergic receptors contribute to the acute effects of 3,4-methylenedioxymethamphetamine in humans. J Clin Psychopharmacol 33(5):658–666. 10.1097/JCP.0b013e3182979d32 [DOI] [PubMed] [Google Scholar]
- Hysek CM, Liechti ME (2012) Effects of MDMA alone and after pretreatment with reboxetine, duloxetine, clonidine, carvedilol, and doxazosin on pupillary light reflex. Psychopharmacology 224(3):363–376. 10.1007/s00213-012-2761-6 [DOI] [PubMed] [Google Scholar]
- Hysek CM, Schmid Y, Rickli A, Simmler L, Donzelli M, Grouzmann E, Liechti ME (2012c) Carvedilol inhibits the cardiostimulant and thermogenic effects of MDMA in humans. Br J Pharmacol 166(8):2277–2288. 10.1111/j.1476-5381.2012.01936.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hysek CM, Schmid Y, Simmler LD, Domes G, Heinrichs M, Eisenegger C, Preller KH, Quednow BB, Liechti ME (2014a) MDMA enhances emotional empathy and prosocial behavior. Social Cognitive and Affective Neuroscience 9(11):1645–1652. 10.1093/scan/nst161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hysek CM, Simmler LD, Ineichen M, Grouzmann E, Hoener MC, Brenneisen R, Huwyler J, Liechti ME (2011) The Norepinephrine Transporter Inhibitor Reboxetine Reduces Stimulant Effects of MDMA (“Ecstasy”) in Humans. Clin Pharmacol Ther 90(2):246–255. 10.1038/clpt.2011.78 [DOI] [PubMed] [Google Scholar]
- Hysek CM, Simmler LD, Nicola VG, Vischer N, Donzelli M, Krähenbühl S, Grouzmann E, Huwyler J, Hoener MC, Liechti ME (2012d) Duloxetine Inhibits Effects of MDMA (“Ecstasy”) In Vitro and in Humans in a Randomized Placebo-Controlled Laboratory Study. PLoS ONE 7(5):e36476. 10.1371/journal.pone.0036476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hysek CM, Simmler LD, Schillinger N, Meyer N, Schmid Y, Donzelli M, Grouzmann E, Liechti ME (2014b) Pharmacokinetic and pharmacodynamic effects of methylphenidate and MDMA administered alone or in combination. Int J Neuropsychopharmacol 17(03):371–381. 10.1017/S1461145713001132 [DOI] [PubMed] [Google Scholar]
- Hysek CM, Vollenweider FX, Liechti ME (2010) Effects of a beta-blocker on the cardiovascular response to MDMA (Ecstasy). Emergency Medicine Journal : EMJ 27(8):586–589. 10.1136/emj.2009.079905 [DOI] [PubMed] [Google Scholar]
- Jerome L, Feduccia AA, Wang JB, Hamilton S, Yazar-Klosinski B, Emerson A, Mithoefer MC, Doblin R (2020) Long-term follow-up outcomes of MDMA-assisted psychotherapy for treatment of PTSD: A longitudinal pooled analysis of six phase 2 trials. Psychopharmacology 237(8):2485–2497. 10.1007/s00213-020-05548-2 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Johnson MW, Garcia-Romeu A, Cosimano MP, Griffiths RR (2014) Pilot study of the 5-HT2AR agonist psilocybin in the treatment of tobacco addiction. J Psychopharmacol 28(11):983–992. 10.1177/0269881114548296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Griffiths RR, Hendricks PS, Henningfield JE (2018) The abuse potential of medical psilocybin according to the 8 factors of the Controlled Substances Act. Neuropharmacology 142:143–166. 10.1016/j.neuropharm.2018.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Hendricks PS, Barrett FS, Griffiths RR (2019) Classic psychedelics: An integrative review of epidemiology, therapeutics, mystical experience, and brain network function. Pharmacol Ther 197:83–102. 10.1016/j.pharmthera.2018.11.010 [DOI] [PubMed] [Google Scholar]
- Johnson MW, Richards WA, Griffiths RR (2008) Human Hallucinogen Research: Guidelines for Safety. Journal of Psychopharmacology (oxford, England) 22(6):603–620. 10.1177/0269881108093587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaskey G (1992) Possible interaction between an MAOI and “ecstasy.” Am J Psychiatry 149(3):411a–4412. 10.1176/ajp.149.3.411a [DOI] [PubMed] [Google Scholar]
- Keeler MH (1967). Chlorpromazine Antagonism of Psilocybin Effect. International Journal of Neuropsychiatry, 3, 66–71. https://bibliography.maps.org/bibliography/default/citation/1972 [Google Scholar]
- Khachatryan D, Groll D, Booij L, Sepehry AA, Schütz CG (2016) Prazosin for treating sleep disturbances in adults with posttraumatic stress disorder: A systematic review and meta-analysis of randomized controlled trials. Gen Hosp Psychiatry 39:46–52. 10.1016/j.genhosppsych.2015.10.007 [DOI] [PubMed] [Google Scholar]
- Kinzie JD, Leung P (1989) Clonidine in Cambodian patients with posttraumatic stress disorder. J Nerv Ment Dis 177(9):546–550. 10.1097/00005053-198909000-00005 [DOI] [PubMed] [Google Scholar]
- Kirkpatrick MG, de Wit H (2015) MDMA: A social drug in a social context. Psychopharmacology 232(6):1155–1163. 10.1007/s00213-014-3752-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolbrich EA, Goodwin RS, Gorelick DA, Hayes RJ, Stein EA, Huestis MA (2008) Plasma pharmacokinetics of 3,4-methylenedioxymethamphetamine after controlled oral administration to young adults. Ther Drug Monit 30(3):320–332. 10.1097/FTD.0b013e3181684fa0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Rosenheck RA, Cramer JA, Vessicchio JC, Jones KM, Vertrees JE, Horney RA, Huang GD, Stock C, & Veterans Affairs Cooperative Study No. 504 Group (2011) Adjunctive risperidone treatment for antidepressant-resistant symptoms of chronic military service-related PTSD: A randomized trial. JAMA 306(5):493–502. 10.1001/jama.2011.1080 [DOI] [PubMed] [Google Scholar]
- Lauerma H, Wuorela M, Halme M (1998) Interaction of serotonin reuptake inhibitor and 3,4-methylenedioxymethamphetamine? Biol Psychiat 43(12):929. [PubMed] [Google Scholar]
- Leonard JB, Anderson B, Klein-Schwartz W (2018) Does getting high hurt? Characterization of cases of LSD and psilocybin-containing mushroom exposures to national poison centers between 2000 and 2016. Journal of Psychopharmacology (oxford, England) 32(12):1286–1294. 10.1177/0269881118793086 [DOI] [PubMed] [Google Scholar]
- Liechti ME (2000) Acute Psychological Effects of 3,4-Methylenedioxymethamphetamine (MDMA, “Ecstasy”) are Attenuated by the Serotonin Uptake Inhibitor Citalopram. Neuropsychopharmacology 22(5):513–521. 10.1016/S0893-133X(99)00148-7 [DOI] [PubMed] [Google Scholar]
- Liechti ME, Geyer MA, Hell D, & Vollenweider FX (2001). Effects of MDMA (ecstasy) on prepulse inhibition and habituation of startle in humans after pretreatment with citalopram, haloperidol, or ketanserin. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 24(3), 240–252. 10.1016/S0893-133X(00)00199-8 [DOI] [PubMed] [Google Scholar]
- Liechti ME, Vollenweider FX (2000a) The serotonin uptake inhibitor citalopram reduces acute cardiovascular and vegetative effects of 3, 4-methylenedioxymethamphetamine (‘Ecstasy’) in healthy volunteers. J Psychopharmacol 14(3):269–274. 10.1177/026988110001400313 [DOI] [PubMed] [Google Scholar]
- Liechti ME, Vollenweider FX (2000b) Acute psychological and physiological effects of MDMA (“Ecstasy”) after haloperidol pretreatment in healthy humans. Eur Neuropsychopharmacol 10(4):289–295. 10.1016/S0924-977X(00)00086-9 [DOI] [PubMed] [Google Scholar]
- Liu Y, She M, Wu Z, Dai R (2011) The inhibition study of human UDP-glucuronosyltransferases with cytochrome P450 selective substrates and inhibitors. J Enzyme Inhib Med Chem 26(3):386–393. 10.3109/14756366.2010.518965 [DOI] [PubMed] [Google Scholar]
- Lyon RA, Glennon RA, Titeler M (1986) 3,4-Methylenedioxymethamphetamine (MDMA): Stereoselective interactions at brain 5-HT1 and 5-HT2 receptors. Psychopharmacology 88(4):525–526. 10.1007/BF00178519 [DOI] [PubMed] [Google Scholar]
- Malcolm B, Thomas K (2021) Serotonin toxicity of serotonergic psychedelics. Psychopharmacology. 10.1007/s00213-021-05876-x [DOI] [PubMed] [Google Scholar]
- Manevski N, Kurkela M, Höglund C, Mauriala T, Court MH, Yli-Kauhaluoma J, Finel M (2010) Glucuronidation of psilocin and 4-hydroxyindole by the human UDP-glucuronosyltransferases. Drug Metabolism and Disposition: the Biological Fate of Chemicals 38(3):386–395. 10.1124/dmd.109.031138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattusek N, & Halbach A (1964). Über den Einfluss von Ergotamin auf die LSD-”Psychose” beim Menschen. [On the Influence of Ergotamine on LSD-”Psychosis” in Man]. In Psychopharmacologia (Vol. 5, pp. 158–160). 10.1007/BF00413052 [DOI] [PubMed] [Google Scholar]
- McCann UD, Ricaurte GA (1993) Reinforcing subjective effects of (+/−) 3,4-methylenedioxymethamphetamine (“ecstasy”) may be separable from its neurotoxic actions: Clinical evidence. J Clin Psychopharmacol 13(3):214–217 [PubMed] [Google Scholar]
- Mitchell JM, Bogenschutz M, Lilienstein A, Harrison C, Kleiman S, Parker-Guilbert K, G MO, Garas W, Paleos C, Gorman I, Nicholas C, Mithoefer M, Carlin S, Poulter B, Mithoefer A, Quevedo S, Wells G, Klaire SS, van der Kolk B, … Doblin R. (2021) MDMA-assisted therapy for severe PTSD: A randomized, double-blind, placebo-controlled phase 3 study. Nat Med 27(6):1025–1033. 10.1038/s41591-021-01336-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mithoefer MC, Grob CS, Brewerton TD (2016) Novel psychopharmacological therapies for psychiatric disorders: Psilocybin and MDMA. The Lancet Psychiatry 3(5):481–488. 10.1016/S2215-0366(15)00576-3 [DOI] [PubMed] [Google Scholar]
- Mithoefer MC, Wagner MT, Mithoefer AT, Jerome L, Doblin R (2011) The safety and efficacy of ±3,4-methylenedioxymethamphetamine-assisted psychotherapy in subjects with chronic, treatment-resistant posttraumatic stress disorder: The first randomized controlled pilot study. Journal of Psychopharmacology (oxford, England) 25(4):439–452. 10.1177/0269881110378371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morefield KM, Keane M, Felgate P, White JM, Irvine RJ (2011) Pill content, dose and resulting plasma concentrations of 3,4-methylendioxymethamphetamine (MDMA) in recreational ‘ecstasy’ users. Addiction 106(7):1293–1300. 10.1111/j.1360-0443.2011.03399.x [DOI] [PubMed] [Google Scholar]
- Moreno FA, Wiegand CB, Taitano EK, Delgado PL (2006) Safety, tolerability, and efficacy of psilocybin in 9 patients with obsessive-compulsive disorder. J Clin Psychiatry 67(11):1735–1740. 10.4088/jcp.v67n1110 [DOI] [PubMed] [Google Scholar]
- Müller F, Holze F, Dolder P, Ley L, Vizeli P, Soltermann A, Liechti ME, Borgwardt S (2021) MDMA-induced changes in within-network connectivity contradict the specificity of these alterations for the effects of serotonergic hallucinogens. Neuropsychopharmacology 46(3):545–553. 10.1038/s41386-020-00906-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Multidisciplinary Association for Psychedelic Studies. (2021). MAPS MDMA Investigator’s Brochure. [Google Scholar]
- Nardou R, Lewis EM, Rothhaas R, Xu R, Yang A, Boyden E, Dölen G (2019) Oxytocin-dependent reopening of a social reward learning critical period with MDMA. Nature 569(7754):116–120. 10.1038/s41586-019-1075-9 [DOI] [PubMed] [Google Scholar]
- Nayak S, Gukasyan N, Barrett FS, Erowid E, Erowid F, & Griffiths RR (2021). Classic psychedelic coadministration with lithium, but not lamotrigine, is associated with seizures: An analysis of online psychedelic experience reports. PsyArXiv. 10.31234/osf.io/r726d [DOI] [PubMed] [Google Scholar]
- Nichols DE (2020) Psilocybin: From ancient magic to modern medicine. J Antibiot 73(10):679–686. 10.1038/s41429-020-0311-8 [DOI] [PubMed] [Google Scholar]
- Oeri HE (2021) Beyond ecstasy: Alternative entactogens to 3,4-methylenedioxymethamphetamine with potential applications in psychotherapy. J Psychopharmacol 35(5):512–536. 10.1177/0269881120920420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Mathúna B, Farré M, Rostami-Hodjegan A, Yang J, Cuyàs E, Torrens M, Pardo R, Abanades S, Maluf S, Tucker GT, Torre R (2008) The consequences of 3,4-methylenedioxymethamphetamine induced CYP2D6 inhibition in humans. J Clin Psychopharmacol 28(5):523–529. 10.1097/JCP.0b013e318184ff6e [DOI] [PubMed] [Google Scholar]
- Pacifici R, Pichini S, Zuccaro P, Farré M, Segura M, Ortuño J, Di Carlo S, Bacosi A, Roset PN, Segura J, de la Torre R (2004) Paroxetine Inhibits Acute Effects of 3,4-Methylenedioxymethamphetamine on the Immune System in Humans. J Pharmacol Exp Ther 309(1):285–292. 10.1124/jpet.103.061374 [DOI] [PubMed] [Google Scholar]
- Papaseit E, Pérez-Mañá C, Torrens M, Farré A, Poyatos L, Hladun O, Sanvisens A, Muga R, Farré M (2020) MDMA interactions with pharmaceuticals and drugs of abuse. Expert Opin Drug Metab Toxicol 16(5):357–369. 10.1080/17425255.2020.1749262 [DOI] [PubMed] [Google Scholar]
- Papaseit E, Vázquez A, Pérez-Mañá C, Pujadas M, de la Torre R, Farré M, Nolla J (2012) Surviving life-threatening MDMA (3,4-methylenedioxymethamphetamine, ecstasy) toxicity caused by ritonavir (RTV). Intensive Care Med 38(7):1239–1240. 10.1007/s00134-012-2537-9 [DOI] [PubMed] [Google Scholar]
- Partilla JS, Dempsey AG, Nagpal AS, Blough BE, Baumann MH, Rothman RB (2006) Interaction of Amphetamines and Related Compounds at the Vesicular Monoamine Transporter. J Pharmacol Exp Ther 319(1):237–246. 10.1124/jpet.106.103622 [DOI] [PubMed] [Google Scholar]
- Passie T (2018) The early use of MDMA (‘Ecstasy’) in psychotherapy (1977–1985). Drug Science, Policy and Law 4:2050324518767442. 10.1177/2050324518767442 [DOI] [Google Scholar]
- Penmatsa A, Wang KH, Gouaux E (2013) X-ray structure of dopamine transporter elucidates antidepressant mechanism. Nature 503(7474):85–90. 10.1038/nature12533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penmatsa A, Wang KH, Gouaux E (2015) X-ray structures of Drosophila dopamine transporter in complex with nisoxetine and reboxetine. Nat Struct Mol Biol 22(6):506–508. 10.1038/nsmb.3029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilgrim JL, Gerostamoulos D, Woodford N, Drummer OH (2012) Serotonin toxicity involving MDMA (ecstasy) and moclobemide. Forensic Sci Int 215(1–3):184–188. 10.1016/j.forsciint.2011.04.008 [DOI] [PubMed] [Google Scholar]
- Pokorny T, Preller KH, Kraehenmann R, Vollenweider FX (2016) Modulatory effect of the 5-HT1A agonist buspirone and the mixed non-hallucinogenic 5-HT1A/2A agonist ergotamine on psilocybin-induced psychedelic experience. Eur Neuropsychopharmacol 26(4):756–766. 10.1016/j.euroneuro.2016.01.005 [DOI] [PubMed] [Google Scholar]
- Ramcharan S, Meenhorst P, Otten J, Koks C, de Boer D, Maes R, Beijnen J (1998) Survival After Massive Ecstasy Overdose. J Toxicol Clin Toxicol 36(7):727–731. 10.3109/15563659809162623 [DOI] [PubMed] [Google Scholar]
- Richards JR, Hollander JE, Ramoska EA, Fareed FN, Sand IC, Gómez MMI, Lange RA (2017) β-Blockers, Cocaine, and the Unopposed α-Stimulation Phenomenon. J Cardiovasc Pharmacol Ther 22(3):239–249. 10.1177/1074248416681644 [DOI] [PubMed] [Google Scholar]
- Rickli A, Moning OD, Hoener MC, Liechti ME (2016) Receptor interaction profiles of novel psychoactive tryptamines compared with classic hallucinogens. Eur Neuropsychopharmacol 26(8):1327–1337. 10.1016/j.euroneuro.2016.05.001 [DOI] [PubMed] [Google Scholar]
- Rigg KK, Sharp A (2018) Deaths related to MDMA (ecstasy/molly): Prevalence, root causes, and harm reduction interventions. Journal of Substance Use 23(4):345–352. 10.1080/14659891.2018.1436607 [DOI] [Google Scholar]
- Roseman L, Leech R, Feilding A, Nutt DJ, Carhart-Harris RL (2014) The effects of psilocybin and MDMA on between-network resting state functional connectivity in healthy volunteers. Front Hum Neurosci 8. 10.3389/fnhum.2014.00204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnick G, Wall SC (1992) The molecular mechanism of “ecstasy” [3,4-methylenedioxy-methamphetamine (MDMA)]: Serotonin transporters are targets for MDMA-induced serotonin release. Proc Natl Acad Sci 89(5):1817–1821. 10.1073/pnas.89.5.1817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleemi S, Pennybaker SJ, Wooldridge M, Johnson MW (2017) Who is “Molly”? MDMA adulterants by product name and the impact of harm-reduction services at raves. Journal of Psychopharmacology (oxford, England) 31(8):1056–1060. 10.1177/0269881117715596 [DOI] [PubMed] [Google Scholar]
- Schatzberg AF, & Nemeroff CB (2017). The American Psychiatric Association Publishing Textbook Of Psychopharmacology. American Psychiatric Pub. [Google Scholar]
- Scherrer JF, Salas J, Cohen BE, Schnurr PP, Schneider FD, Chard KM, Tuerk P, Friedman MJ, Norman SB, van den Berk-Clark C, & Lustman PJ (2019). Comorbid Conditions Explain the Association Between Posttraumatic Stress Disorder and Incident Cardiovascular Disease. Journal of the American Heart Association: Cardiovascular and Cerebrovascular Disease, 8(4). 10.1161/JAHA.118.011133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid Y, Rickli A, Schaffner A, Duthaler U, Grouzmann E, Hysek CM, Liechti ME (2015) Interactions between Bupropion and 3,4-Methylenedioxymethamphetamine in Healthy Subjects. J Pharmacol Exp Ther 353(1):102–111. 10.1124/jpet.114.222356 [DOI] [PubMed] [Google Scholar]
- Schmid Y, Vizeli P, Hysek CM, Prestin K, Meyer zu Schwabedissen HE, & Liechti ME (2016) CYP2D6 function moderates the pharmacokinetics and pharmacodynamics of 3,4-methylenedioxymethamphetamine in a controlled study in healthy individuals. Pharmacogenet Genomics 26(8):397–401. 10.1097/FPC.0000000000000231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scriba GKE (2011). Sweetman Sean C.(Ed.): Martindale: The Complete Drug Reference. Chromatographia, 74(7), 647–648. 10.1007/s10337-011-2101-8 [DOI] [Google Scholar]
- Segura M, Farre M, Pichini S, Peiro AM, Roset PN, Ramirez A, Ortuno J, Pacifici R, Zuccaro P, Segura J, de la Torre R (2005) Contribution of Cytochrome P450 2D6 to 3,4-Methylenedioxymethamphetamine Disposition in Humans: Use of Paroxetine as a Metabolic Inhibitor Probe. Clin Pharmacokinet 44(6):649–660. 10.2165/00003088-200544060-00006 [DOI] [PubMed] [Google Scholar]
- Sessa B (2017) MDMA and PTSD treatment: “PTSD: From novel pathophysiology to innovative therapeutics.” Neurosci Lett 649:176–180. 10.1016/j.neulet.2016.07.004 [DOI] [PubMed] [Google Scholar]
- Sessa B, Higbed L, O’Brien S, Durant C, Sakal C, Titheradge D, Williams TM, Rose-Morris A, Brew-Girard E, Burrows S, Wiseman C, Wilson S, Rickard J, Nutt DJ (2021) First study of safety and tolerability of 3,4-methylenedioxymethamphetamine-assisted psychotherapy in patients with alcohol use disorder. J Psychopharmacol 35(4):375–383. 10.1177/0269881121991792 [DOI] [PubMed] [Google Scholar]
- Shulgin AT (1986) The background and chemistry of MDMA. J Psychoactive Drugs 18(4):291–304. 10.1080/02791072.1986.10472361 [DOI] [PubMed] [Google Scholar]
- Silins E, Copeland J, Dillon P (2007) Qualitative Review of Serotonin Syndrome, Ecstasy (MDMA) and the use of Other Serotonergic Substances: Hierarchy of Risk. Aust N Z J Psychiatry 41(8):649–655. 10.1080/00048670701449237 [DOI] [PubMed] [Google Scholar]
- Simmler LD, Hysek CM, Liechti ME (2011) Sex Differences in the Effects of MDMA (Ecstasy) on Plasma Copeptin in Healthy Subjects. J Clin Endocrinol Metab 96(9):2844–2850. 10.1210/jc.2011-1143 [DOI] [PubMed] [Google Scholar]
- Smilkstein MJ, Smolinske SC, Rumack BH (1987) A Case of Mao Inhibitor/MDMA Interaction: Agony After Ecstasy. J Toxicol Clin Toxicol 25(1–2):149–159. 10.3109/15563658708992620 [DOI] [PubMed] [Google Scholar]
- Stein DJ, & Rink J (1999). Effects of “Ecstasy” Blocked by Serotonin Reuptake Inhibitors. The Journal of Clinical Psychiatry, 60(7), 485–485. https://www.psychiatrist.com/JCP/article/Pages/effects-ecstasy-blocked-serotonin-reuptake-inhibitors.aspx [DOI] [PubMed] [Google Scholar]
- Steuer AE, Schmidhauser C, Tingelhoff EH, Schmid Y, Rickli A, Kraemer T, Liechti ME (2016) Impact of Cytochrome P450 2D6 Function on the Chiral Blood Plasma Pharmacokinetics of 3,4-Methylenedioxymethamphetamine (MDMA) and Its Phase I and II Metabolites in Humans. PLoS ONE 11(3):e0150955. 10.1371/journal.pone.0150955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassman R (1992). Human Hallucinogen Interactions with Drugs Affecting Serotonergic. Neuropsychopharmacol, 7(3). [PubMed] [Google Scholar]
- Studerus E, Gamma A, & Vollenweider FX (2010). Psychometric Evaluation of the Altered States of Consciousness Rating Scale (OAV). PLoS ONE, 5(8). 10.1371/journal.pone.0012412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studerus E, Vizeli P, Harder S, Ley L, Liechti ME (2021) Prediction of MDMA response in healthy humans: A pooled analysis of placebo-controlled studies. J Psychopharmacol 0269881121998322. 10.1177/0269881121998322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tancer M, Johanson C-E (2006) The effects of fluoxetine on the subjective and physiological effects of 3,4-methylenedioxymethamphetamine (MDMA) in humans. Psychopharmacology 189(4):565–573. 10.1007/s00213-006-0576-z [DOI] [PubMed] [Google Scholar]
- Thomas K, Malcolm B, Lastra D (2017) Psilocybin-Assisted Therapy: A Review of a Novel Treatment for Psychiatric Disorders. J Psychoactive Drugs 49(5):446–455. 10.1080/02791072.2017.1320734 [DOI] [PubMed] [Google Scholar]
- Togni LR, Lanaro R, Resende RR, Costa JL (2015) The variability of ecstasy tablets composition in Brazil. J Forensic Sci 60(1):147–151. 10.1111/1556-4029.12584 [DOI] [PubMed] [Google Scholar]
- Usona Institute. (2021). Usona Institute Psilocybin Investigator’s Brochure (Vol. 4). [Google Scholar]
- VA/DOD Clinical Practice Guideline for the Management of Posttraumatic Stress Disorder and Acute Stress Disorder. (2017) 200. [DOI] [PMC free article] [PubMed]
- Valeriani G, Corazza O, Bersani FS, Melcore C, Metastasio A, Bersani G, Schifano F (2015) Olanzapine as the ideal “trip terminator”? Analysis of online reports relating to antipsychotics’ use and misuse following occurrence of novel psychoactive substance-related psychotic symptoms. Hum Psychopharmacol 30(4):249–254. 10.1002/hup.2431 [DOI] [PubMed] [Google Scholar]
- Vargas AS, Luís Â, Barroso M, Gallardo E, Pereira L (2020) Psilocybin as a New Approach to Treat Depression and Anxiety in the Context of Life-Threatening Diseases—A Systematic Review and Meta-Analysis of Clinical Trials. Biomedicines 8(9):331. 10.3390/biomedicines8090331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrico CD, Miller GM, Madras BK (2007) MDMA (Ecstasy) and human dopamine, norepinephrine, and serotonin transporters: Implications for MDMA-induced neurotoxicity and treatment. Psychopharmacology 189(4):489–503. 10.1007/s00213-005-0174-5 [DOI] [PubMed] [Google Scholar]
- Vizeli P, Schmid Y, Prestin K, Schwabedissen MZ, H E, & Liechti ME. (2017) Pharmacogenetics of ecstasy: CYP1A2, CYP2C19, and CYP2B6 polymorphisms moderate pharmacokinetics of MDMA in healthy subjects. European Neuropsychopharmacology: the Journal of the European College of Neuropsychopharmacology 27(3):232–238. 10.1016/j.euroneuro.2017.01.008 [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Vollenweider-Scherpenhuyzen MFI, Bäbler A, Vogel H, & Hell D (1998). Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action: NeuroReport, 9(17), 3897–3902. 10.1097/00001756-199812010-00024 [DOI] [PubMed] [Google Scholar]
- Vuori E, Henry JA, Ojanperä I, Nieminen R, Savolainen T, Wahlsten P, Jäntti M (2003) Death following ingestion of MDMA (ecstasy) and moclobemide: Fatal MDMA-moclobemide interaction. Addiction 98(3):365–368. 10.1046/j.1360-0443.2003.00292.x [DOI] [PubMed] [Google Scholar]
- Wagner MT, Mithoefer MC, Mithoefer AT, MacAulay RK, Jerome L, Yazar-Klosinski B, Doblin R (2017) Therapeutic effect of increased openness: Investigating mechanism of action in MDMA-assisted psychotherapy. Journal of Psychopharmacology (oxford, England) 31(8):967–974. 10.1177/0269881117711712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TL, Justice AJH, de Wit H (2002) Differential subjective effects of D-amphetamine by gender, hormone levels and menstrual cycle phase. Pharmacol Biochem Behav 73(4):729–741. 10.1016/s0091-3057(02)00818-3 [DOI] [PubMed] [Google Scholar]
- Wood DM, Stribley V, Dargan PI, Davies S, Holt DW, Ramsey J (2011) Variability in the 3,4-methylenedioxymethamphetamine content of “ecstasy” tablets in the UK. Emergency Medicine Journal: EMJ 28(9):764–765. 10.1136/emj.2010.092270 [DOI] [PubMed] [Google Scholar]
- Yockey A, King K (2021) Use of psilocybin (“mushrooms”) among US adults: 2015–2018. Journal of Psychedelic Studies 5(1):17–21. 10.1556/2054.2020.00159 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


