Abstract
BACKGROUND:
Isocitrate dehydrogenase (IDH) mutations are present in 70% of World Health Organization grade II and III gliomas. IDH mutation induces accumulation of the oncometabolite 2-hydroxyglutarate. Therefore, therapies targeting reversal of epigenetic dysregulation in gliomas have been suggested. However, the utility of epigenetic treatments in gliomas remains unclear. Here, we present the first clinical systematic review of epigenetic therapies in treatment of IDH-mutant gliomas and highlight their safety and efficacy.
METHODS:
We conducted a systematic search of electronic databases from 2000 to January 2021 following PRISMA guidelines. Articles were screened to include clinical usage of epigenetic therapies in case reports, prospective case series, or clinical trials. Primary and secondary outcomes included safety/tolerability of epigenetic therapies and progression-free survival/overall survival, respectively.
RESULTS:
A total of 133 patients across 8 clinical studies were included in our analysis. IDH inhibitors appear to have the best safety profile, with an overall grade 3/grade 4 adverse event rate of 9%. Response rates to IDH-mutant inhibitors were highest in nonenhancing gliomas (stable disease achieved in 55% of patients). In contrast, histone deacetylase inhibitors demonstrate a lower safety profile with single-study adverse events as high as 28%.
CONCLUSION:
IDH inhibitors appear promising given their benign toxicity profile and ease of monitoring. Histone deacetylase inhibitors appear to have a narrow therapeutic index, as lower concentrations do not appear effective, while increased doses can produce severe immunosuppressive effects. Preliminary data suggest that epigenetic therapies are generally well tolerated and may control disease in certain patient groups, such as those with nonenhancing lesions.
Keywords: Epigenetics, Glioma, Glioblastoma, Histone deacetylase inhibitor, HDAC, IDH
INTRODUCTION
Despite maximal safe resection and adjuvant chemoradiation, gliomas often harbor a poor prognosis due to tumor heterogeneity, treatment resistance, and infiltrating tumor cells on top of the limitations related to the blood-brain barrier.1,2 However, recent advances in molecular phenotyping of gliomas have demonstrated that isocitrate dehydrogenase (IDH) mutation (IDHm) serves as a molecular prognosticator for certain gliomas, conferring improved outcomes compared to IDH wild-type gliomas.1,2 Two forms of IDH are considered especially relevant to glioblastoma multiforme (GBM) classification: IDH1, which is primarily found in peroxisomes or in the cytoplasm, and IDH2, which is primarily mitochondrial. Within IDH-mutated gliomas, the IDH1 R132 mutation is also a more prevalent mutation than IDH2 mutations. IDHm is considered to be an early event in glioma pathophysiology that disrupts cellular epigenetic regulation by formation of 2-hydroxyglutarate (2-HG).3 The wild-type IDH enzyme catalyzes conversion of isocitrate to α-ketoglutarate (α-KG) during oxidative phosphorylation; however, in IDHm, α-KG is converted to an oncometabolite, 2-HG, which triggers a oncogenic cascade through inhibition of α-KG—dependent enzymes.1,3 As a result, α-KG—dependent dioxygenases such as a histone/DNA demethylases and 5-methlycytosine hydroxylase do not properly function, thereby facilitating epigenetic dysregulation through histone/DNA hypermethylation.4 Thus, IDHm-mutant tumors are not synonymous with GBM but should rather be considered a unique subtype of gliomas with a distinct biochemical etiology. These characteristic epigenetic marks define IDHm gliomas and provide a potential avenue for novel treatment modalities.
Recently, several therapeutics have been developed that aim to disrupt the activity of epigenetic regulators in IDH-mutant gliomas. Currently, 5 classes of therapy have undergone or are currently undergoing clinical investigation for gliomas specifically: direct mutant IDH inhibitors, histone deacetylase inhibitors, bromodomain inhibitors, poly-ADP ribose polymerase inhibitors, and nicotinamide phosphoribosyl transferase inhibitors. However, despite these advances, there are no current systematic reviews of published clinical studies regarding the safety and efficacy of these treatment modalities for IDH-mutant gliomas. Here we present the first comprehensive systematic review of clinical studies investigating epigenetic therapies in management of IDH-mutant gliomas.
METHODS
Literature Search and Selection
A systematic literature search of the English literature from 1997 to 2020 in the PubMed and EMBASE databases was performed. The Medical Subject Headings system was queried for all articles containing the terms epigenetic therapy and glioma: (’histone deacetylase inhibitor’ OR ’bromodomain inhibitor’ OR ’DNA methyltransferase inhibitor’ OR ’IDH inhibitor’ OR ‘epigenetic therapy’) AND (’glioma’ OR ’low grade glioma’ OR ’glioblastoma’) AND ’IDH1 mutant’. The objective was to screen for articles containing series of patients with IDH-mutant gliomas who have undergone any form of epigenetic-modifying treatments.
Inclusion and Exclusion Criteria
Article search was limited to English with humans as the only study participants. All articles were specified as retrospective or prospective patient studies, clinical trials, randomized clinical trials, or post hoc analysis of clinical trials. Reviews, editorials, commentaries, and case reports were excluded. Studies evaluating chemotherapy clinical and radiological outcomes in human patients with IDH-mutant gliomas or glioblastomas were included. The exclusion criteria were as follows: 1) studies that did not involve human subjects, 2) cohorts that did not incorporate pharmacologic management, 3) studies that did not evaluate outcomes following administration of therapy, and 4) studies with data duplicated in a previous clinical study.
RESULTS
Study Selection and Characteristics
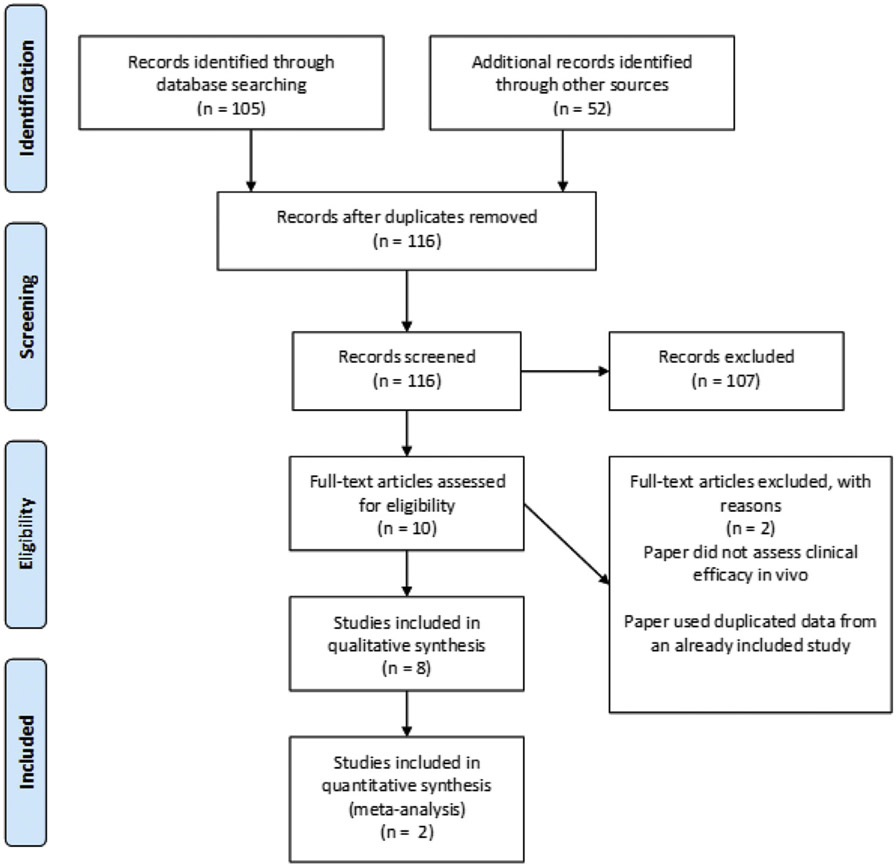
After applying the inclusion criteria, 23 studies were included for data analysis, where 2 articles were excluded because of inadequate data description, reaching the final number of 21 studies retrieved. A total of 157 published abstracts or manuscripts were identified. Through the PRISMA flow chart (Figure 1), and applying the inclusion and exclusion criteria, a total of 8 studies were included in the qualitative analysis. Selection and data extraction of the studies was performed by 3 authors (VG, AHS, and LD).
Figure 1.
PRISMA diagram summarizing systematic review process.
Eight articles met the criteria for inclusion from an initial pool of 157 articles. All articles were performed in humans and evaluated the impact of epigenetic therapies radiographically, biochemically, histologically, clinically, or a combination thereof. Given the limited data set and their heterogeneity, we did not perform any quantitative analyses with these articles. Key differences between articles are summarized in Table 1. Three studies evaluated IDH1 inhibitors, 3 studies evaluated histone deacetylase (HDAC) inhibitors, and one study investigated a bromodomain inhibitor. In our included studies, there were also several key divisions regarding tumor classification. First, several articles make distinctions between high-grade gliomas (World Health Organization [WHO] classification of grade III or IV) and low-grade gliomas (WHO classification of grade II or lower). Furthermore, several articles refer to differences in response between enhancing and nonenhancing lesions. Seven ongoing clinical trials were also included. However, these trials are not complete and have not provided any preliminary efficacy or safety data. All 7 of these trials investigate IDH1 inhibitors and are summarized in Table 2.
Table 1.
Summary of Published Clinical Studies Investigating Epigenetic Therapies
| Author | Drug | Number of Patients |
Dose | Treatment Duration | Follow-up | Primary Outcomes |
Secondary Outcomes | Adverse Events |
|---|---|---|---|---|---|---|---|---|
| Andronesi et al., 20189 | IDH305 | 8 (R132H mutation) WHO grade II (n = 2), Grade III (n = 5) Grade IV (n = 1) | 550 mg IDH305 | IDH305 twice daily for 7 days | 1 week | Decreased 2-HG levels at 1 week (P < 0.05) | Significant inverse correlation between 2-HGglutamine + glutamate ratio and cell density (P = 0.03) | |
| Mellinghoff et al., 202010 | Ivosidenib | 66 (86.4% R132H) 1p19q (33.3%) | Dose escalation: 100 mg BID; 1200 mg QD; 500 mg daily for expansion cohort. | 18.4 months (nonenhancing lesions); 1.9 months (enhancing lesions) | Efficacy assessed every 2 cycles (56 ± 3 days) | Median PFS 13.9 months (nonenhancing) and 1.4 months (enhancing lesions) | Stable disease in 85.7% of nonenhancing lesions, 45.2% in enhancing lesions. Tumor growth rate of −14% after treatment with ivosidenib | 3% grade ≥3 and treatment-related adverse effects |
| Tejera et al., 202011 | Ivosidenib | 1 (IDH1m) | 500 mg daily in continuous 28-day cycles | 4.08 years | 5.16 years | Stable disease for duration of follow-up | Improved seizure control | |
| Gurbani et al., 202020 | Belinostat | 1 IDH1 R132H MGMT-nonco-deleted | 500 mg/m2/day | Five days prior to RT followed by 2 additional 5-day cycles at 3-week intervals | 1.5 years | Reduction in tumor size and mass effect. Stable disease for 16 months | Improved neurocognition | |
| Moreno et al., 202024 | CC-90010 | 1 IDH1m MGMT+ | 30 mg/day | 4 days followed by 24 days without drug (28-day cycles) | 10 mo. | Complete response | Disappearance of enhancing and nonenhancing lesions within 6 cycles | |
| Gurbani et al., 201918 | Belinostat | 2 (IDH1m) | Control arm: Daily TMZ with RT Treatment arm: 500 or 750 mg/m2/day | 42 days of TMZ (control arm), 5 consecutive days across 3 cycles, every 3 weeks | 1 year/until progression | No significant difference in PFS (P = 0.09) | Statistically significant improvement in depression among belinostat patients (P = 0.04) | Hematological toxicity at 700 mg/m2 dose |
| Galanis et al., 201819 | Vorinostat | 3 (IDH1m) | Initial cycle of 300 or 400 mg/day followed by 400 mg/day | Initial cycle on days 1—5, followed by 12 cycles of vorinostat on days 1—7 and days 15—21 of 28-day cycle | 16 months | Median OS of 16.1 months 55.1% survival at 15 months | Statistically significant improvement in PFS among patients with vorinostat | Leukopenia, thrombocytopenia, or neutropenia seen in 9—28% of patients |
| Hanna et al., 202044 | Olaparib | 39 (several had unknown IDHm status, unknown how many) | 200 mg or dose escalation protocol: 100 mg to 150 mg | Twice daily for 7 days or 42 days per cycle for 3 56-day cycles | 6 months | Tumor penetration found in all 21 tumor core samples | 39% of patients remained progression-free at 6-month follow-up | 7 patients experienced severe anemia, lymphopenia, thrombocytopenia, or neutropenia |
RT, radiation therapy; TMZ, temozolomide; 2-HG, 2-hydroxyglutarate; PFS, progression-free survival; WHO, World Health Organization.
Table 2.
Summary of Ongoing Clinical Trials Investigating Epigenetic Therapies
| Author | Recruitment Status |
Intervention | Control | Randomized? | Estimated Enrollment |
Primary Outcomes | Secondary Outcomes |
|---|---|---|---|---|---|---|---|
| Luke et al. | Not yet recruiting | Ivosidenib | None | No | 35 participants | Dose-limiting toxicity Best overall response PFS | Adverse events OS Tumor progression |
| Servier Pharmaceuticals | Active, not recruiting | AG-120, AG-881 prior to surgery | No treatment prior to surgery | Yes | 49 participants | 2-HG concentration in surgically resected tumors | Safety, pharmacodynamics, pharmacokinetics, clinical activity |
| Active, not recruiting | AG-881 postoperatively | Placebo | Yes | 340 participants | Progression-free survival | Adverse events, clinical response, overall survival | |
| Active, not recruiting | AG-881 | None | No | 93 participants (52 with glioma) | Safety/tolerability, Maximum tolerated dose | Pharmacokinetics and pharmacodynamics | |
| Novartis Pharmaceuticals | Active, not recruiting | IDH305 | None | No | 166 patients | Dose-limiting toxicity Maximum tolerated dose | Adverse events, pharmacodynamics, pharmacokinetics, overall response rate |
| Loxo Oncology, Eli Lilly | Recruiting | LY3410738 | None | No | 180 patients | Identification of recommended phase 2 dose | Pharmacodynamics, pharmacokinetics, safety, objective response rate |
| Forma Pharmaceuticals | Active, not recruiting | FT-2102 | None | No | 200 patients | Dose confirmation | Preliminary efficacy data |
OS, overall survival; 2-HG, 2-hydroxyglutarate; PFS, progression-free survival.
Mutant IDH Inhibitors
IDH converts isocitrate to α-KG; however, mutations in the IDH have been noted in several cancers including chondrosarcoma, cholangiocarcinoma, acute myeloid leukemia (AML), and gliomas.4,5 Mutant IDH produces excess quantities of 2-HG, which inhibits several key demethylases, resulting in marked histone and DNA methylation.4 The resulting methylation pattern resembles that of progenitor cells, with inhibition of cellular differentiation and enrichment of stem cell markers.4 Furthermore, inhibition of mutant IDH can promote differentiation in tumorigenic cells, making IDHm a promising drug target.6
Currently, a variety of IDH inhibitors are under clinical investigation in gliomas. A recent study by Mellinghoff et al. using ivosidenib (an IDH1 inhibitor) demonstrated stable disease in 86% of all nonenhancing gliomas with an acceptable tolerability (3% grade 3—4 adverse event rate).7 Vorasidenib, a dual IDH1/2 inhibitor, possesses enhanced brain penetration relative to ivosidenib.7 In a phase 1 study of vorasidenib in recurrent IDH-mutant glioma, the drug was well tolerated with a median progression-free survival (PFS) of 31 months and an overall response rate of 18%, primarily in patients with nonenhancing lesions.7 Olutasidenib, a brain penetrant, IDH 1 inhibitor, was also well tolerated as a single agent in recurrent, mainly enhancing gliomas, with a disease control rate of 45% in an ongoing phase 1b/2 study.8
Overall, the median age across all studies evaluating IDH inhibitors was 43 years. The overall response rate to IDH inhibitors across all studies was 55% (primarily nonenhancing lesions), with an overall grade 3—4 adverse event rate of 9%.9,10 The mechanism of IDHm and its inhibitors is illustrated in Figure 2A. Additionally, 2-HG has been demonstrated as a specific biomarker of IDH1 inhibitor efficacy and can be utilized to monitor treatment response.5
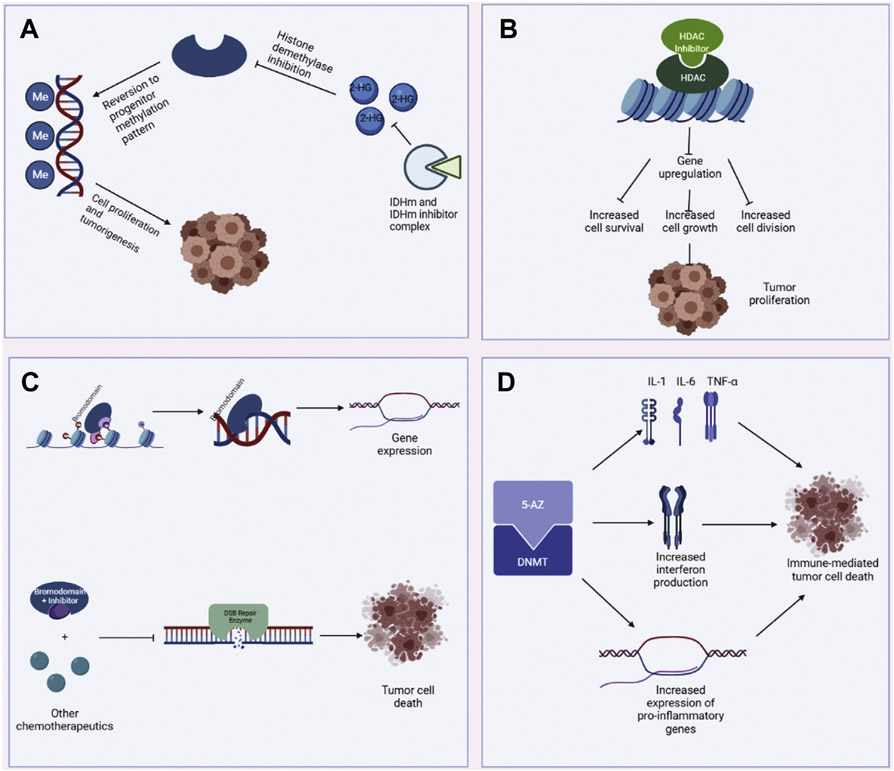
Figure 2.
(A) Mechanism of IDH1m and IDH1m inhibitors. IDH1m produces 2-hydroxyglutarate (2-HG), which in turn inhibits histone demethylases. The loss of histone demethylases reverts DNA methylation patterns to those of progenitor cells, promoting tumorigenesis. IDH1m inhibitors aim to disrupt this process by directly inhibiting IDH1m. (B) Mechanism of histone deacetylase (HDAC) and HDAC inhibitors. HDAC decondenses chromatin, thereby allowing for gene expression. Mutant HDACs are products of gain-of-function mutations that upregulate multiple genes associated with increased cell survival, growth, and division, contributing to tumor formation. (C) Mechanism of bromodomains and their inhibitors. Bromodomains bind acetylated lysine residues and subsequently act as scaffold for transcription machinery. Bromodomain inhibitors may not have direct cytotoxic effects but inhibit double-strand break (DSB) repair, predisposing tumor cells to the cytotoxic effects of other chemotherapeutics. (D) Description of 5-azacitidine (5-AZ) mechanism of action. 5-AZ inhibits DNA methyltransferase (DNMT), leading to upregulation of immunomodulatory genes, namely cytokines and interferon. These gene products may leverage the host immune response to target tumor cells. IDH, isocitrate dehydrogenase.
HDAC Inhibitors
Currently, 3 clinical studies have investigated the utility of HDAC inhibitors in the treatment of IDH-mutant brain tumors (median age: 50 years, n = 6 IDHm patients). In one study, Gurbani et al. were unable to demonstrate a difference in the PFS between patients who received the HDAC inhibitor belinostat (P = 0.09), though only 2 patients had IDHm gliomas.9 However, a similar study by Galanis et al. demonstrated a median overall survival (OS) of 16.1 months among patients with IDH1-mutant glioblastoma receiving vorinostat (n = 3).10 Furthermore, the authors demonstrated that patients with specific vorinostat sensitivity signatures (collections of putatively connected genes that mediate cellular response to chemotherapeutics) had significantly higher PFS (PFS, P < 0.0001).10 Lastly, belinostat, another HDAC inhibitor, had demonstrated some efficacy for IDH1-mutant glioblastoma with disease stabilization at the 16-month follow-up.9,11 Several adverse events have been recorded across the available studies, mostly hematologic toxicities (leukopenia, thrombocytopenia, or neutropenia).9,10 The mechanism of HDAC and its inhibitors is illustrated in Figure 2B.
Bromodomain Inhibitors
Bromodomains are highly conserved structures that are associated with histone acetylation. Primarily, bromodomains “read” and bind acetylated lysine residues on histones which then mark these regions for transcription.12 Bromodomains then form a scaffold for transcription factors and requisite transcription machinery, thus serving as a critical link between epigenetic modifications and gene expression.13,14 Only one clinical study investigating the effects of a bromodomain inhibitor has been conducted. Moreno et al. investigated the utility of the bromodomain inhibitor CC-90010 in a series of 69 patients, one of whom had an IDH1-mutant glioma.15 The patient demonstrated a complete clinical response to treatment, with loss of enhancing and nonenhancing areas of the lesion within 6 cycles of CC-90010.15 A description of bromodomains and their inhibitors can be found in Figure 2C.
5-Azacitidine
5-azacitidine (5-AZ) is a DNA methyltransferase (DNMT) inhibitor (DNMTi). Members of this chemotherapy class act by direct, irreversible inhibition of DNMT, which prevents further methylation of downstream DNA.16 One study by Federici et al. investigated the utility of 5-AZ in 12 patients with IDHm glioma. All patients received 6 cycles of 5-AZ, and at a median follow-up period of 20 months, all patients had demonstrated disease progression; however, 3 (25%) patients had prolonged disease stabilization on treatment.16 The median PFS for this cohort was 4.7 months, with a median OS of 25.2 months. Furthermore, 7 patients experienced grade 3 or grade 4 leukopenia and 9 patients experienced grade 3/4 neutropenia.16 The mechanism of 5-AZ is displayed in Figure 2D. Previously, it has been demonstrated that systemic 5-AZ can penetrate the blood-cerebrospinal fluid barrier and achieve adequate deposition in the cerebrospinal fluid16; however, systematic analyses of 5-AZ penetration into tumors have yet to be performed.
Poly-ADP Ribose Polymerase Inhibitors
Poly-ADP ribose polymerase (PARP) has been identified as a key regulator of several DNA repair processes, including both single-strand break and double-strand break (DSB) repair, homologous end-joining, and nonhomologous end-joining.17 PARP inhibitors (PARPIs) induce accumulation of DSBs, which have demonstrated cytotoxicity in the absence of complementary repair mechanisms.18 This feature of PARPIs is particularly useful in treating cancers with BRCA1/2 mutations or mutations in other homologous recombination (HR)—associated genes.18 However, PARPIs have also demonstrated efficacy in tumors with wild-type HR genes when combined with DNA-damaging agents.19 A summary of PARP and its inhibitor’s mechanisms of action is shown in Figure 3A. Currently, the PARPI olaparib is being investigated in a phase 2 study to determine its use in gliomas and other solid tumors (NCT03212274).
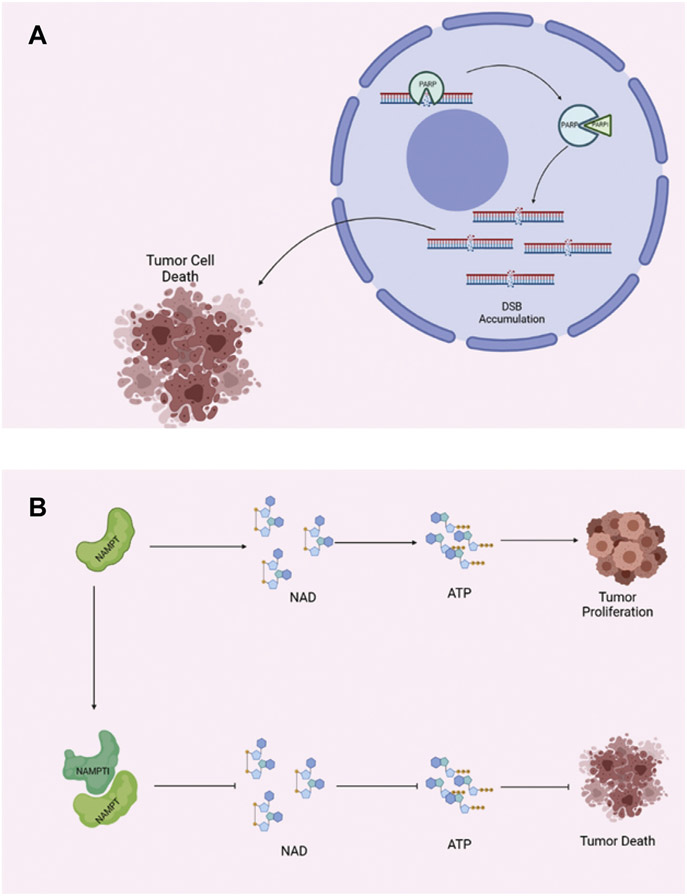
Figure 3.
(A) Mechanism of PARP and PARP inhibitors. PARP normally repairs several mechanisms of DNA damage, including double-strand breaks (DSBs). In tumor cells that lack other forms of DNA repair (nonhomologous end-joining, homologous recombination, etc.), PARP is critical for their continued survival and proliferation. PARP inhibitors (PARPIs) take advantage of this fact, leading to cytotoxic accumulation of DSBs, ultimately leading to tumor cell death. (B) Characterized by a high metabolic rate, cancer cells often rely on. PARP, poly-ADP ribose polymerase.
Nicotinamide Phosphoribosyl Transferase Inhibitors
Cancers are generally characterized by high rates of metabolic activity and therefore require large quantities of NAD to meet their metabolic demands (Figure 3B). Nicotinamide phosphoribosyl transferase (NAMPT) appears to be particularly relevant in brain tumorigenesis, as NAMPT overexpression has been previously associated with brain tumor formation and may potentially serve as a biomarker.20,21 Furthermore, it has been previously established that NAMPT prevents cell death in neural lineage cells, which are also characterized by high metabolic activity.22 NAMPT facilitates this process by augmenting NAD regeneration, thus making NAMPT a prime target for novel chemotherapeutics. NAMPT inhibitors (NAMPTIs) prevent NAD regeneration, ultimately depleting ATP and inducing cell death.20 Initially, 3 NAMPTIs underwent phase I clinical trials; however, all were discontinued due to drug toxicity concerns.20
DISCUSSION
Considerations of the 2021 WHO Guidelines
While IDH mutation status has been a longstanding means of differentiating high- and low-grade gliomas, recent changes to the WHO Classification of Tumors of the Central Nervous System have further increased the nuance of GBM classification. For example, recent scholarship has suggested that there exists a group of low-grade IDHwt gliomas that lack conventional GBM signatures and are quite similar to neoplasms commonly found in the pediatric population.23 These recent results and guideline changes may require a deeper analysis of low-grade versus high-grade gliomas, namely, in addition to IDH mutations, other significant molecular events must be analyzed, which may offer additional therapeutic targets. For example, Ahrendsen et al. recently demonstrated alterations in FGFR, BRAF, and NTRK in patients with IDHm liomas, suggesting that IDH assessment alone may be insufficient to properly characterize gliomas.13,15,24
Assessment of Existing Epigenetic Therapies
With respect to safety, across all current studies, IDH inhibitors demonstrated an overall aceptable safety profile.4,6,25 The best responders to IDH inhibitors seem to be patients with slowly growing or nonenhancing gliomas, suggesting that timing of therapy remains vital for clinical decision-making. IDH inhibitors also offer a means of noninvasively and objectively monitoring efficacy via measurement of 2-HG.26 The benign safety profile coupled with convenient monitoring may make IDH inhibitors promising candidates for combination therapy with other chemotherapeutics, especially given their ease of monitoring via serum sampling.
HDAC Inhibitors
Histone deacetylation is a key process in cellular proliferation and growth. Namely, histone deacetylation decondenses chromatin, thereby allowing for the expression of genes responsible for cellular growth and development; therefore, HDACs may act as drivers of oncogenesis.27-31 As such, HDAC inhibitors have been developed as a class of chemotherapeutics aimed at inducing cell cycle arrest and tumor cell apoptosis.32
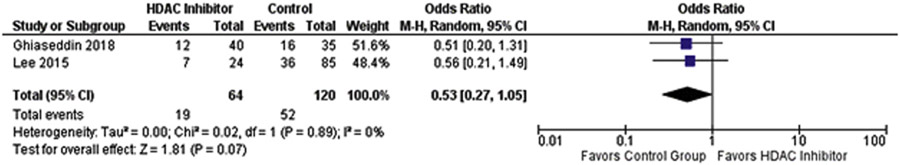
HDAC inhibitors are one of the few medications that have been investigated in both IDH wild-type and IDH-mutant glioma and glioblastoma, and its efficacy in IDH wild-type tumors may give insight into their efficacy in IDH-mutant tumors. We performed a brief meta-analysis that demonstrated that patients receiving HDAC inhibitors had lower PFS at 6 months, though this was not significantly significant (P = 0.07, OR = 0.53, 95% confidence interval [0.27—1.05]) Figure 4. Additionally, HDAC inhibitors must be titrated carefully due to their pronounced side-effect profile, with some studies quoting adverse event rates up to 28%.10,11 This potentially poor survival benefit coupled with the relatively severe side-effect profile may limit the use of these medications in the future. Furthermore, these documented toxicities may severely limit their potential use in combination chemotherapy regimens.
Figure 4.
Comparison of percent progression-free patients at 6 months (PFS6) in patients receiving HDAC inhibitors relative to historical controls. Patients receiving HDAC inhibitors were less likely to be progression-free at 6 months post treatment though this was not significant (P = 0.07, OR = 0.53, 95% CI [0.27–1.05]). CI, confidence interval; HDAC, histone deacetylase; OR, odds ratio.
Bromodomain Inhibitors
Bromodomain inhibitors are currently the least well-studied epigenetic chemotherapy in management of IDH1-mutant gliomas. There have been several preliminary studies that have investigated its use in other solid tumors, with varying results.33,34 Given their relatively new arrival to the pharmaceutical market, there is a general paucity of clinical data using bromodomain inhibitors in gliomas.35,36 Therefore, more studies are required before any judgments regarding their utility can be made.
DNMT Inhibitors
5-AZ is a DNMTi that was formerly used as a salvage therapy for myelodysplastic syndromes and AML.37 5-AZ primarily exerts its effects by upregulating several immunomodulatory genes, namely cytokines, interferon, and proinflammatory genes, and has shown efficacy in both solid tumors and AML.38 Given the relative lack of large systematic studies using 5-AZ for gliomas, its safety and effiacacy cannot be determined at this time, although there are some data to suggest that 5-AZ may improve outcomes in a subset of patients.37,39 Systemic 5-AZ must be titrated carefully due to its risk of developing severe leukopenia. Additional investigations using intracavitary or intraventricular 5-AZ are also currently underway for select CNS tumors.
Limitations of Current Therapies
Despite the development of these novel therapies, several limitations exist that may explain their relatively modest efficacy. Primarily, epigenetic therapies must be designed to efficiently cross the blood-brain barrier with minimal neurotoxicity.40 Some HDAC inhibitors may have poor BBB permeability due to hydroxyamic acid—, amino, and zinc-binding groups.40 Therefore, utilizing native BBB transport proteins may be a promising strategy to augment BBB penetration of novel HDAC inhibitors.41
Another issue surrounding IDHm tumors is that the sequence of acquired mutations directly impacts the utility of any direct epigenetic therapies. It has been previously demonstrated that IDHms are drivers of further mutation, producing DNA damage and methylation by producing 2-HG.4,5 Thus, early inhibition of IDHm is critical in preventing the acquisition of further mutations, thereby mitigating tumor invasion. Furthermore, it has been previously demonstrated that any epigenetic changes produced by IDHm are preserved and transmitted through successive divisions of tumor cell lines.42
Overall Assessment and Future Directions
We have reviewed the most common epigenetic chemotherapeutics used in the treatment of IDH-mutant glioma, as summarized in Table 3. In summary, most available data are limited to small samples, which greatly limits our ability to perceive possible benefits. Thus, there is currently little consensus regarding the true benefits of these agents. However, there are multiple ongoing multicenter clinical trials that can potentially demonstrate the true utility of several of these agents.
Table 3.
Summary of Different Mechanisms, Strengths, and Weaknesses of Current Epigenetic Therapies
| Therapeutic | Mechanism of Action | Strengths | Weaknesses |
|---|---|---|---|
| IDHm inhibitors |
|
|
|
| HDAC inhibitors |
|
|
|
| Bromodomain ininhibitors |
|
|
|
| 5-Azacitidine |
|
|
|
| PARP inhibitors |
|
|
|
| NAMPT inhibitors |
|
|
|
BBB, blood-brain barrier; TMZ, temozolomide; 2-HG, 2-hydroxyglutarate; IDHm, IDH-mutant; IDHw, IDH wild-type; NAMPT, nicotinamide phosphoribosyl transferase; PARP, poly-ADP ribose polymerase inhibitor; DSB, double-strand break.
Study Limitations
Due to the nature of the studies included in our report, this study is based on a summary of heterogeneous clinical trials and constitutes class III evidence. The trials included in this systemtatic review may be subject to selection bias and reporting bias. Additionally, the heterogenous patient cohorts in all the studies may also complicate pooled analyses and comparisons across medications. A direct comparison of HDAC inhibitors and IDH1m inhibitors among patients matched for age is necessary to resolve this potential confounder. Furthermore, most studies did not have sufficiently large cohorts of patients with IDHm tumors; thus, results of these studies may not be generalizable to this subset of patients.
Combination therapy may be an alternative to current monotherapy regimens, particularly if used early in the disease course. For example, one clinical trial investigating combination therapy in AML has demonstrated a 78% response rate among patients receiving a combination of ivosidenib and 5-AZ and a 67% response rate among patients receiving a combination of enasidenib and 5-AZ.41 Unfortunately, similar research for use in IDHm glioma and glioblastoma has largely been preclinical.43 However, our own review of the current literature suggests that many of these epigenetic agents may have narrow therapeutic indices, and thus, combination therapies must be balanced by their potential toxicities.
CONCLUSIONS
We performed the first-known systematic review investigating current epigenetic therapies in management of IDH-mutant gliomas. Among current therapies demonstrated in clinical studies, IDH inhibitors appear to be the most promising given their benign safety profile and the presence of 2-HG as a potential biomarker. In contrast, HDAC inhibitors appear to be more limited as current studies have not demonstrated a clinically significant benefit. Furthermore, HDAC inhibitors appear to have a narrow therapeutic index, given the relatively high rate of adverse events documented in previous studies. Bromodomain inhibitors may be a promising third class of chemotherapeutics; however, more research is necessary to elucidate their true utility. Future research in combination epigenetic therapies may be necessary as demonstrated in several ongoing clinical trials in patients with AML; however, this strategy must be tailored to limit dose-related toxicities of the respective drugs. This may be particularly true of the newer NAMPT and PARP inhibitors that may require combination therapy to demonstrate efficacy, while limiting toxicities. Generally speaking, epigenetic therapies may have a particular value in a subset of patients with nonenhancing gliomas, though further efficacy studies in agents aside from IDHm inhibitors will be valuable in determineing the scope of epigenetic therapy utility.
Abbreviations and Acronyms
- α-KG
α-ketoglutarate
- 2-DG
2-hydroxyglutarate
- 5-AZ
5-azacitidine
- AML
Acute myeloid leukemia
- BBB
Blood-brain barrier
- DNMT
DNA methyltransferase
- GBM
Glioblastoma multiforme
- HDAC
Histone deacetylase
- IDH
Isocitrate dehydrogenase
- IGHm
Isocitrate dehydrogenase mutant
- NAMPT
Nicotinamide phosphoribosyl transferase
- NAMPTI
Nicotinamide phosphoribosyl transferase inhibitor
- OS
Overall survival
- PARP
Poly-ADP ribose polymerase
- PARPI
Poly-ADP ribose polymerase inhibitor
- PFS
Progression-free survival
Footnotes
Conflicts of interest statement: MID is a member of the advisory board of Agios and Forma Therapeutics.
Data Availability:
Data sharing is not applicable to this article as no data sets were generated or analysed during the current study.
REFERENCES
- 1.Ohba S, Hirose YJNm-c. Biological significance of mutant isocitrate dehydrogenase 1 and 2 in gliomagenesis. Neurol Med Chir (Tokyo). 2016;56:170–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. [DOI] [PubMed] [Google Scholar]
- 3.Xu B, Ma R, Russell L, et al. An oncolytic herpesvirus expressing E-cadherin improves survival in mouse models of glioblastoma. Article. Nat Biotechnol. 2019;37:45–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Figueroa ME, Abdel-Wahab O, Lu C, et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell. 2010;18:553–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dang L, White DW, Gross S, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462:739–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rohle D, Popovici-Muller J, Palaskas N, et al. An inhibitor of mutant IDH1 delays growth and promotes differentiation of glioma cells. Science (New York, NY). 2013;340:626–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mellinghoff IK, Ellingson BM, Touat M, et al. Ivosidenib in isocitrate dehydrogenase 1—mutated advanced glioma. J Clin Oncol. 2020;38:3398–3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuente MIDL, Colman H, Rosenthal M, et al. A phase Ib/II study of olutasidenib in patients with relapsed/refractory IDH1 mutant gliomas: safety and efficacy as single agent and in combination with azacitidine. J Clin Oncol. 2020;38(suppl):2505. [Google Scholar]
- 9.Gurbani SS, Yoon Y, Weinberg BD, et al. Assessing treatment response of glioblastoma to an HDAC inhibitor using Whole-brain Spectroscopic MRI. Tomography (Ann Arbor, Mich). 2019;5:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galanis E, Anderson SK, Miller CR, et al. Phase I/II trial of vorinostat combined with temozolomide and radiation therapy for newly diagnosed glioblastoma: results of Alliance N0874/ABTC02. Neuro Oncol. 2017;20:546–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gurbani SS, Weinberg BD, Salgado E, et al. Remarkable response of a patient with secondary glioblastoma to a histone deacetylase inhibitor. Oxf Med Case Rep. 2020;2020:omaa006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kulikowski E, Rakai BD, Wong NCW. Inhibitors of bromodomain and extra-terminal proteins for treating multiple human diseases. Med Res Rev. 2021;41:223–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donati B, Lorenzini E, Ciarrocchi A. BRD4 and Cancer: going beyond transcriptional regulation. Mol Cancer. 2018;17:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferri E, Petosa C, McKenna CE. Bromodomains: Structure, function and pharmacology of inhibition. Biochem Pharmacol. 2016;106:1–18. [DOI] [PubMed] [Google Scholar]
- 15.Moreno V, Sepulveda JM, Vieito M, et al. Phase I study of CC-90010, a reversible, oral BET inhibitor in patients with advanced solid tumors and relapsed/refractory non-Hodgkin’s lymphoma. Ann Oncol. 2020;31:780–788. [DOI] [PubMed] [Google Scholar]
- 16.Federici L, Capelle L, Annereau M, et al. 5-Azacitidine in patients with IDH1/2-mutant recurrent glioma. Neuro Oncol. 2020;22:1226–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ray Chaudhuri A, Nussenzweig A. The multifaceted roles of PARP1 in DNA repair and chromatin remodelling. Nat Rev Mol Cell Biol. 2017;18:610–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lord CJ, Ashworth A. PARP inhibitors: Synthetic lethality in the clinic. Science (New York, NY). 2017;355:1152–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ning J-F, Stanciu M, Humphrey MR, et al. Myc targeted CDK18 promotes ATR and homologous recombination to mediate PARP inhibitor resistance in glioblastoma. Nat Commun. 2019;10:2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lucena-Cacace A, Otero-Albiol D, Jiménez-García MP, Peinado-Serrano J, Carnero A. NAMPT overexpression induces cancer stemness and defines a novel tumor signature for glioma prognosis. Oncotarget. 2017;8:99514–99530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vachher M, Arora K, Burman A, Kumar B. NAMPT, GRN, and SERPINE1 signature as predictor of disease progression and survival in gliomas. J Cell Biochem. 2020;121:3010–3023. [DOI] [PubMed] [Google Scholar]
- 22.Wang S, Xing Z, Vosler PS, et al. Cellular NAD replenishment confers marked neuroprotection against ischemic cell death: role of enhanced DNA repair. Stroke. 2008;39:2587–2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Komori T Grading of adult diffuse gliomas according to the 2021 WHO classification of tumors of the Central Nervous system. Lab Invest. 2022;102:126–133. [DOI] [PubMed] [Google Scholar]
- 24.Ahrendsen JT, Torre M, Meredith DM, et al. IDH-mutant gliomas with additional class-defining molecular events. Mod Pathol. 2021;34:1236–1244. [DOI] [PubMed] [Google Scholar]
- 25.Andronesi OC, Arrillaga-Romany IC, Ly KI, et al. Pharmacodynamics of mutant-IDH1 inhibitors in glioma patients probed by in vivo 3D MRS imaging of 2-hydroxyglutarate. Nat Commun. 2018;9:1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tadimety A, Closson A, Li C, Yi S, Shen T, Zhang JXJ. Advances in liquid biopsy on-chip for cancer management: Technologies, biomarkers, and clinical analysis. Crit Rev Clin Lab Sci. 2018;55:140–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi JH, Kwon HJ, Yoon BI, et al. Expression profile of histone deacetylase 1 in gastric cancer tissues. Jpn J Cancer Res. 2001;92:1300–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Halkidou K, Gaughan L, Cook S, Leung HY, Neal DE, Robson CN. Upregulation and Nuclear Recruitment of HDAC1 in Hormone refractory Prostate cancer. Prostate. 2004;59:177–189. [DOI] [PubMed] [Google Scholar]
- 29.Li G, Margueron R, Hu G, Stokes D, Wang YH, Reinberg D. Highly compacted chromatin formed in vitro reflects the dynamics of transcription activation in vivo. Mol Cel. 2010;38:41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oehme I, Deubzer HE, Wegener D, et al. Histone deacetylase 8 in neuroblastoma tumorigenesis. Clin Cancer Res. 2009;15:91–99. [DOI] [PubMed] [Google Scholar]
- 31.Richon VM, Sandhoff TW, Rifkind RA, Marks PA. Histone deacetylase inhibitor selectively induces p21WAF1 expression and gene-associated histone acetylation. Proc Natl Acad Sci USA. 2000;97:10014–10019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwon SH, Ahn SH, Kim YK, et al. Apicidin, a histone deacetylase inhibitor, induces apoptosis and Fas/Fas ligand expression in human acute promyelocytic leukemia cells. J Biol Chem. 2002;277:2073–2080. [DOI] [PubMed] [Google Scholar]
- 33.Piha-Paul SA, Sachdev JC, Barve M, et al. First-in-Human study of Mivebresib (ABBV-075), an oral Pan-inhibitor of bromodomain and extra terminal proteins, in patients with relapsed/refractory solid tumors. Clin Cancer Res. 2019;25:6309–6319. [DOI] [PubMed] [Google Scholar]
- 34.Postel-Vinay S, Herbschleb K, Massard C, et al. First-in-human phase I study of the bromodomain and extraterminal motif inhibitor BAY 1238097: emerging pharmacokinetic/pharmacodynamic relationship and early termination due to unexpected toxicity. Eur J Cancer (Oxford, Engl 1990). 2019;109:103–110. [DOI] [PubMed] [Google Scholar]
- 35.Falchook G, Rosen S, LoRusso P, et al. Development of 2 bromodomain and extraterminal inhibitors with distinct pharmacokinetic and pharmacodynamic profiles for the treatment of advanced Malignancies. Clin Cancer Res. 2020;26:1247–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lewin J, Soria JC, Stathis A, et al. Phase Ib trial with Birabresib, a small-Molecule inhibitor of bromodomain and extraterminal proteins, in patients with selected advanced solid tumors. J Clin Oncol. 2018;36:3007–3014. [DOI] [PubMed] [Google Scholar]
- 37.Castelli G, Pelosi E, Testa U. Targeting histone methyltransferase and demethylase in acute myeloid leukemia therapy. OncoTargets Ther. 2018;11:131–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li H, Chiappinelli KB, Guzzetta AA, et al. Immune regulation by low doses of the DNA methyltransferase inhibitor 5-azacitidine in common human epithelial cancers. Oncotarget. 2014;5:587–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wong KK, Lawrie CH, Green TM. Oncogenic roles and inhibitors of DNMT1, DNMT3A, and DNMT3B in acute myeloid Leukaemia. Biomarker insights. 2019;14, 1177271919846454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hiranaka S, Tega Y, Higuchi K, et al. Design, Synthesis, and blood-brain barrier transport study of Pyrilamine Derivatives as histone deacetylase inhibitors. ACS Med Chem Lett. 2018;9:884–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.DiNardo CD, Propert KJ, Loren AW, et al. Serum 2-hydroxyglutarate levels predict isocitrate dehydrogenase mutations and clinical outcome in acute myeloid leukemia. Blood. 2013;121:4917–4924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dixit D, Xie Q, Rich JN, Zhao JC. Messenger RNA methylation Regulates glioblastoma tumorigenesis. Cancer Cell. 2017;31:474–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamashita AS, da Costa Rosa M, Borodovsky A, Festuccia WT, Chan T, Riggins GJ. Demethylation and epigenetic modification with 5-azacytidine reduces IDH1 mutant glioma growth in combination with temozolomide. Neuro Oncol. 2019;21:189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hanna C, Kurian KM, Williams K, et al. Pharmacokinetics, safety, and tolerability of olaparib and temozolomide for recurrent glioblastoma: results of the phase I OPARATIC trial. Neuro Oncol. 2020;22:1840–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no data sets were generated or analysed during the current study.