Abstract
Relative abundance of fibroblast growth factor-23 (FGF23) measured by the C-terminal (cFGF23, which measures both intact FGF23 & c-terminal fragments) vs intact (iFGF23, measures only intact hormone) assays varies by kidney function in humans. Differential kidney clearance may explain this finding. We measured cFGF23 and iFGF23 in the aorta and bilateral renal veins of 162 patients with essential hypertension undergoing renal angiography. Using multivariable linear regression, we examined factors associated with aorta to renal vein reduction of FGF23 using both assays. Similar parameters and with addition of urine concentrations of cFGF23 and iFGF23 were measured in 6 Wistar rats.
Mean age was 54±12 years, 54% percent were women, and mean creatinine clearance was 72±48 ml/min/100g. The human kidney reduced the concentrations of both cFGF23 (16±12%) and iFGF23 (21±16 %) compared to the aorta, but reduction was higher for iFGF23. Greater kidney creatinine and PTH reductions were each independently associated with greater reductions of both cFGF23 and iFGF23. The greater kidney reduction of iFGF23 compared to cFGF23 appeared stable and consistent across the range of creatinine clearance evaluated. Kidney clearance was similar, and urine concentrations of both assays were low in the rat models, suggesting kidney metabolism of both cFGF23 and iFGF23.
Renal reduction of iFGF23 is higher than that of creatinine and cFGF23. Our data suggest that FGF23 is metabolized by the kidney. However, the major cell types involved in metabolization of FGF23 requires future study. Kidney clearance of FGF23 does not explain differences in C-terminal and intact moieties across the range of kidney function.
Keywords: FGF23, mineral metabolism
Introduction
Fibroblast growth factor (FGF)-23 is a 32 kilodalton (kDa) circulating peptide that is produced mainly by osteocytes.1 It plays a central role in phosphate homeostasis through its influences on the kidney to promote phosphate excretion and limit calcitriol production.2 Beyond its physiologic effects on the kidney, FGF23 may also induce pathological cardiac myocyte hypertrophy.3 The FGF23 polypeptide has a complex biology including transcription, post-translational modification and cleavage, which regulate circulating FGF23 concentrations. After transcription and translation, FGF23 can be shuttled into different post-translational modification pathways involving O-glycosylation, or phosphorylation by the extracellular serine/threonine protein kinase FAM20C.4 FGF23 that is not modified by O-glycosylation is cleaved within the osteocyte near the carboxy--terminus.5 Thus, the balance between post-translational glycosylation and phosphorylation may determine the net amount of full length FGF23 versus C-terminal FGF23 fragments that are produced and subsequently secreted into circulation. There are two main types of assays used to measure FGF23 in humans. Intact assays utilize antibodies that capture epitopes on amino- and carboxy termini o either side of the cleavage site, thus they capture only intact, biologically active FGF23.6 In contrast, the C-terminal assay recognizes two epitopes on the C-terminus aspect of the hormone, capturing both intact FGF23 and its smaller C-terminal fragments that are formed when the active hormone is cleaved.7
We and others have shown that higher FGF23 concentrations are linked with risk of cardiovascular disease and mortality in multiple large epidemiological studies, both in the general population and in CKD populations.8,9,10,11 However, strengths of associations differ dramatically by the assay used to measure FGF23. Relationships of FGF23 with clinical endpoints including heart failure and mortality are stronger when a C-terminal FGF23 assay is used compared with the intact FGF assay.12,13 We recently demonstrated that kidney function completely extinguished the relationship of intact FGF23 with mortality independent of other key effectors of FGF23 cleavage including iron status, and inflammation. The effects of adjustment for kidney function were much less when the C-terminal FGF23 assay was used.14 Thus, understanding the kidneys’ influence on the concentrations of both the intact and C-terminal FGF23 assays is critical to determine mechanisms of clearance and provide understanding to mechanisms linking FGF23 with clinical endpoints.
Relative abundance of C-terminal fragments vs. the intact FGF23 hormone varies by the level of kidney function in humans. In individuals with normal kidney function, concentrations of both C-terminal and intact FGF23 are relatively low and the resulting ratio of intact and C-terminal FGF23 is simply consistent with their baseline FGF23 transcription and cleavage. However, as kidney function declines in chronic kidney disease (CKD), there is a dramatic albeit unequal increase in both intact and C-terminal FGF23. While concentrations of both C-terminal and intact FGF23 increase with progressive CKD, their relative abundance becomes more similar as eGFR declines.15 The reasons for these changes in relative abundance in relation to eGFR is uncertain. Some have hypothesized that uremia leads to accumulation of a heretofore undiscovered inhibitor of FGF23 cleavage.16 Alternatively, we hypothesized the kidney itself may have differential clearance of the intact FGF23 hormone vs. its C-terminal fragments. These hypotheses have not previously been evaluated.
Prior studies in animals and humans demonstrate that the kidney clears intact FGF23 and may be a key organ involved in regulation of its blood levels.17,18 Yet, whether the kidney is more or less efficient in clearing C-terminal FGF23, as compared to the intact hormone, from circulation is unexplored. These differences in kidney clearance may explain different concentrations of the two FGF23 moieties in different stages of CKD, and may also provide insights into why associations of C-terminal and intact FGF23 with clinical endpoints differs dramatically when adjusted for kidney function.14 To directly investigate the effects of the kidney on renal reduction of C-terminal and intact FGF23, here, we measured both assays in 162 humans in specimens obtained from the aorta and bilateral renal veins. We investigate the renal reduction of both, and how they differ across the range of renal function. A priori, we hypothesized that kidney clearance of intact FGF23 would be less than that of C-terminal FGF23 given its larger size, and that this effect would be more pronounced at lower levels of kidney function, accounting for more similar intact to C-terminal FGF23 plasma concentrations in those with lower GFR, reported in prior studies. In addition, as our study in humans did not have available urine data, we repeated experiments to evaluate renal reduction and excretion of FGF23 in rats.
Materials & Methods
Human Studies
Study Participants & Setting
Details on this protocol have been published previously.19 Patients with suspected renal artery stenosis (RAS) were referred for angiography to Maastricht University Hospital, the Netherlands and were asked about participation in an observational research protocol. Eligibility required 1 or more of the following criteria: difficult-to-treat hypertension (blood pressure remaining above goal despite use of ≥ 3 adequately dosed antihypertensive medications) or clinical suspicion on renovascular abnormalities (for instance, the presence of an abdominal bruit, peripheral vascular disease, or a rise in serum creatinine >20% during treatment with renin angiotensin inhibitors). For this study, we included all participants who were found to have only essential hypertension at time of angiography and excluded those with RAS. All anti-hypertensive medications were halted 21 days before the investigation, thus providing reliable estimates without confounding effects of fixed renal artery lesions or medication effects. None of the participants were using vitamin D supplements, calcitriol or phosphate binders. During the angiography study to evaluate for RAS, simultaneous catheterization of the aorta and both renal veins was performed, with blood samples drawn at the same time and stored at −80 degrees Celsius until subsequent thawing for determination of intact and C-terminal FGF23 along with PTH, α klotho, calcium, and phosphorus.
Subsequently, renal blood flow was measured selectively in both kidneys by the 133Xenon washout technique as described previously.20 Mean renal blood flow was calculated from the initial slope analysis and expressed as ml/min/100 g renal tissue.
Measurements
We measured intact FGF23 in previously unthawed serum utilizing the Kainos ELISA (Kainos Laboratories, Japan) with reported inter-assay coefficients of variation (CV) of ≤4%.21 We measured C-terminal FGF23 in EDTA plasma using the Immutopics C-terminal FGF23 ELISA (San Clemente, California), with an inter-assay CV of 3.4-6.5%. Serum PTH was measured using an intact PTH immunoassay (Beckman DxI 800, Le Brea, CA), with inter-assay CV of 2.7-4.2% and an analytical measurement range of 1-3,100 pg/mL. Measurements were made at the University of Washington, Seattle, WA. Serum α Klotho was determined by immunoprecipitation-immunoblot assay described previously,22,23 with intra-assay and inter-assay CVs of 4.8% to 16.6% at the O’Brien Kidney Research Center at the University of Texas Southwestern Medical Center, Dallas, Tx.
Animal Studies
Six adult male Wistar rats weighing 200–300 gm (purchased from Envigo, Indianapolis, IN) were included in the study. The rats were housed in a temperature-controlled environment with a 12-h light/dark circle with free access to water and food, a standard diet containing 2.0 % calcium, 1.0% phosphorus, and 3.0 IU/gm vitamin D. The rats were anesthetized with Inactin (100 mg/kg) intraperitoneally. 3H-inulin was infused to measure GFR. Renal artery and vein sampling was performed by left femoral artery and left renal vein puncture 3 times separated by two 45 min urine collections (using a bladder catheter) per rat. Renal plasma flow was computed from inulin extraction by Fick equation.
EDTA plasma was used to measure both intact FGF23 and C-terminal FGF23 using rodent intact FGF23 and C-terminal enzyme-linked immunosorbent assay respectively (Quidel, San Diego, CA). Urine intact and C-terminal FGF23 concentrations were measured according to the manufacturer’s instructions using the same assays. We calculated renal reduction of intact and C-terminal FGF23 by two methods- 1) Product of renal plasma flow with renal reduction ((metabolite concentration in the artery minus its concentration in the vein)/ concentration in the artery). As femoral artery and renal vein measurements were made 3 times per rat, the clearance was calculated and averaged across these three measurements to improve precision 2) Clearance= UV/P where U is the urine concentration of the metabolite, V is the volume of urine excreted per time and P is the plasma concentration of the metabolite.
Study Approvals
The human renal angiography studies were approved by the medical ethical committee of the Maastricht University Hospital and adhered to Declaration of Helsinki principles, and all participants provided written informed consent prior to inclusion in the study. Animal studies were performed in accordance with the National Institutes for Health’s Guide for Care and Use of Laboratory Animals, and the experimental protocols had gained approval by the Veterans Affairs San Diego Health Care Systems and conducted under their guidelines.
Statistical Analyses
We calculated renal reduction (%) of intact and C-terminal FGF23 = {(Ao-RV)/Ao}*100 where Ao is the metabolite concentration in the Aorta and RV is concentration in renal vein (average of both right and left). We examined correlations between reduction of the two FGF-23 assays with one another, and with the reduction of creatinine, phosphate, calcium, α Klotho and PTH using Spearman correlation coefficients. We used multivariate linear regression to evaluate factors associated with the reduction of intact and C-terminal FGF23. Our predictors of interest included the reduction of creatinine, PTH, calcium, phosphate, and α Klotho. Missing data for calcium (n=102) and α Klotho (n=104) were imputed with multivariate imputation by chained equations which were combined using Rubin’s rules to account for variability in the imputation procedure.24,25All covariates from the fully adjusted model were used in the imputation.
All analyses were performed using R Core Team (2019) (R Foundation for Statistical Computing, Vienna, Austria) and SPSS statistical software (IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corp 2019). A two-sided p-value <0.05 was considered statistically significant for all analyses.
Results
Among the 162 study participants who were hypertensive, but found not to have RAS at the time of angiography, the mean age was 54 ±12 years, 46% were male, and the mean arterial blood pressure was 120 ± 14 mmHg. The mean creatinine clearance was 72 ± 48 ml/min/100g and 27% had CKD (eGFR < 60ml/min/1.73m2). Mean (±SD) values of serum creatinine, calcium, and phosphate in the renal artery were: 0.86 ±0.26 mg/dl, 8.8 ± 0.4 mg/dl, and 2.7±0.5 mg/dl respectively. Median [IQR] values of C-terminal FGF23, intact FGF23, and PTH in the renal artery were 82 [59,105] RU/ml, 47 [37, 65] pg/ml, and 56 [42, 76] pg/ml respectively. Finally, mean α Klotho was 15.6 ± 4.4 pM (Table 1).
Table 1:
Baseline Characteristics of Participants Who Underwent Renal Angiography
| Demographics (N=162) | |
|---|---|
| Age (years) | 54 ± 12 |
| Males | 75 (46%) |
| Cardiovascular &Kidney Risk Factors | |
| BMI (kg/m2) | 27.7 ± 4.6 |
| Mean arterial blood pressure (mmHg) | 120 ± 14 |
| Serum creatinine (mg/dl) | 0.86 ± 0.26 |
| Creatinine clearance (ml/min/100g) | 72 ± 48 |
| C-terminal FGF23 (RU/ml) | 82 [59, 105]* |
| Intact FGF23 (pg/ml) | 47 [37, 65]* |
| Calcium (g/dl)** | 8.7 ± 1.0 |
| Phosphate (mg/dl) | 2.7 ± 0.5 |
| Intact PTH (pg/ml) | 56 [42,76]* |
| α Klotho (pM)** | 15.6 ± 4.4 |
Median [IQR]
indicates missing values for variables
The renal reduction of creatinine in a single pass from aorta to renal vein was 17 ± 10 %. The renal reduction of intact FGF23 was significantly higher at 21 ± 16 % (P<0.001) compared to renal reduction of creatinine. Intact FGF23 reduction was also found to be higher than that of C-terminal FGF23 (16 ± 12%; P<0.001). PTH had the highest renal reduction of all metabolites evaluated, at 35 ± 17 % (Table 2). The mean concentration of calcium and phosphate were similar in the renal vein compared to the aorta while α Klotho concentration was 6% higher in the renal vein relative to the aorta, a finding that approached but did not reach statistical significance (p=0.06).
Table 2:
Renal Reduction (%) of Metabolites Among 162 Participants Who Underwent Renal Angiography
| Metabolite | Renal Reduction, % (SD)* |
|---|---|
| Creatinine | 17% (10%) |
| Intact FGF23 | 21% (16%) |
| C-terminal FGF23 | 16% (12%) |
| PTH | 35% (17%) |
| α Klotho | −6% (16%) |
| Calcium | 1.4% (4.1%) |
| Phosphate | −0.1% (4.6%) |
Positive% reflect a lower concentration in the renal vein compared to the renal artery, and negative % reflect a higher concentration in the renal vein compared to the renal artery
Renal Reduction of Intact and C-terminal FGF23 in Humans
We found that the % creatinine reduction correlated moderately with renal reduction of FGF23, and that this effect appeared similar using either the intact or C-terminal FGF23 assays (r=0.389 and 0.392, respectively). The percent reduction of intact and C-terminal FGF23 reduction themselves were moderately correlated with one another (r=0.45). Finally, the percent reduction of intact and C-terminal FGF23 reduction with PTH reduction were r= 0.44 and 0.45, respectively while the percent reduction of calcium and phosphate were only weakly correlated with FGF23 reduction (Table 3). These univariate relationships may have been driven by mutual effects of GFR on the parameters, so we evaluated multivariable models to identify independent predictors of FGF23 renal clearance.
Table 3:
Correlations between Renal Reduction of Creatinine and Mineral Metabolism Markers in Humans
| Creatinine | Calcium | Phosphate | intact PTH | α Klotho | Intact FGF23 | C-term. FGF23 | |
|---|---|---|---|---|---|---|---|
| Creatinine | 1.00 | −0.185 | 0.212** | 0.375** | −0.038 | 0.389** | 0.392** |
| Calcium | 1.000 | −0.614** | −0.197* | 0.104 | −0.173 | -0.230* | |
| Phosphate | 1.000 | 0.200* | 0.011 | 0.140 | 0.153 | ||
| intact PTH | 1.000 | −0.052 | 0.441** | 0.451** | |||
| α Klotho | 1.000 | −0.123 | -0.206* | ||||
| Intact FGF23 | 1.000 | 0.455** | |||||
| C-term. FGF23 | 1.000 |
Correlation is significant at the 0.01 level (2-tailed).
Correlation is significant at the 0.05 level (2-tailed).
In the univariate model, variables associated with greater intact FGF23 reduction were greater creatinine reduction and PTH reduction. Both of these variables remained statistically significantly associated with greater intact FGF23 reduction in the multivariable model, demonstrating that the relationship of PTH reduction with intact FGF23 reduction was independent of creatinine reduction and vice versa (Table 4).
Table 4:
Univariate and Multivariate Factors Associated with Renal Reduction of Intact FGF23, C-terminal FGF23, and Intact to C-terminal Ratio
| Intact FGF23 Reduction | ||||
|---|---|---|---|---|
| Variable | Univariate Analysis | Multivariate Analysis | ||
| β (95% CI) | P-value | β (95% CI) | P-value | |
| Creatinine reduction | 4.84 (2.47, 7.21) | <0.001 | 3.08 (0.93, 5.24) | 0.005 |
| Intact PTH reduction | 9.15 (7.11, 11.19) | <0.001 | 8.47 (6.43, 10.52) | <0.001 |
| Calcium reduction | −1.48 (−4.38, 1.42) | 0.313 | −0.01 (−2.99, 2.98) | 0.996 |
| Phosphate reduction | 1.77 (−0.69, 4.24) | 0.159 | 0.45 (−2.09, 2.99) | 0.729 |
| α Klotho reduction | −0.07 (−0.25, 0.10) | 0.405 | −0.54 (−3.43, 2.36) | 0.714 |
| C-terminal FGF23 Reduction | ||||
| Creatinine reduction | 4.64 (2.92, 6.36) | <0.001 | 3.14 (1.66, 4.61) | <0.001 |
| Intact PTH reduction | 7.56 (6.12, 9.00) | <0.001 | 6.89 (5.50, 8.28) | <0.001 |
| Calcium reduction | −1.75 (−4.18, 0.69) | 0.158 | −0.34 (−2.29, 1.61) | 0.735 |
| Phosphate reduction | 1.24 (−0.61, 3.08) | 0.191 | −0.16 (−1.87, 1.56) | 0.858 |
| α Klotho reduction | −2.05 (−4.45, 0.34) | 0.092 | −1.05 (−2.65, 0.55) | 0.198 |
| Intact / C-terminal FGF23 Reduction | ||||
| Creatinine reduction | 0.07 (−1.20, 1.35) | 0.912 | −0.05 (−1.42, 1.32) | 0.94 |
| Intact PTH reduction | 0.07 (−1.25, 1.35) | 0.913 | 0.04 (−1.28, 1.36) | 0.955 |
| Calcium reduction | −0.45 (−1.73, 0.84) | 0.497 | −0.59 (−2.25, 1.07) | 0.485 |
| Phosphate reduction | 0.35 (−0.93, 1.62) | 0.595 | 0.07 (−1.50, 1.63) | 0.935 |
| α Klotho reduction | 0.06 (−0.07, 0.18) | 0.369 | 0.66 (−0.67, 1.99) | 0.331 |
β* reflects % change in FGF23 clearance per 1SD higher of each variable
Variables associated with C-terminal FGF23 reduction were similar to that of intact FGF23. Again, greater creatinine reduction and PTH reduction were associated with C-terminal FGF23 reduction both in univariate and mutually adjusted models (Table 4).
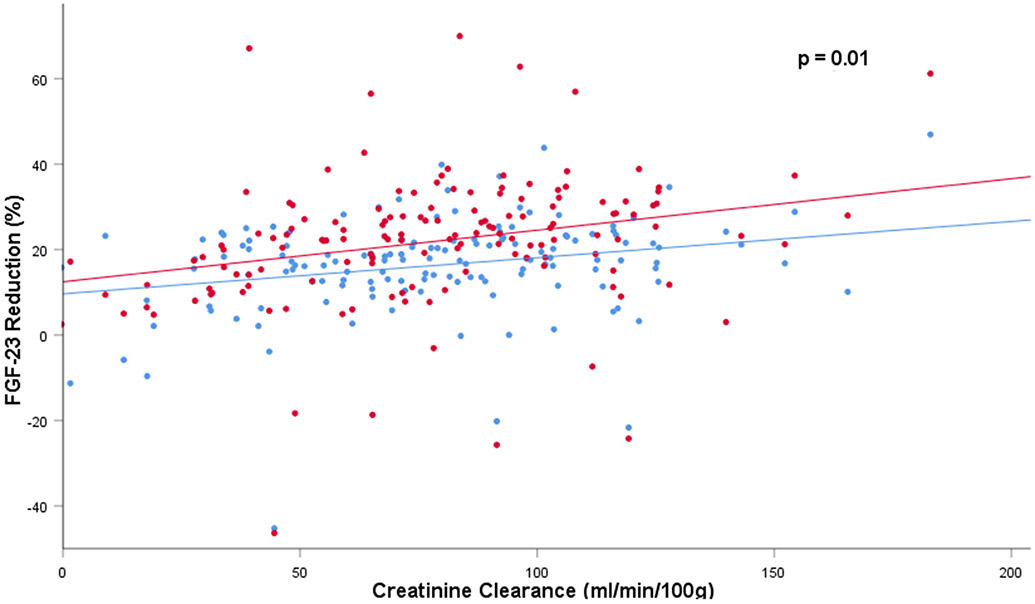
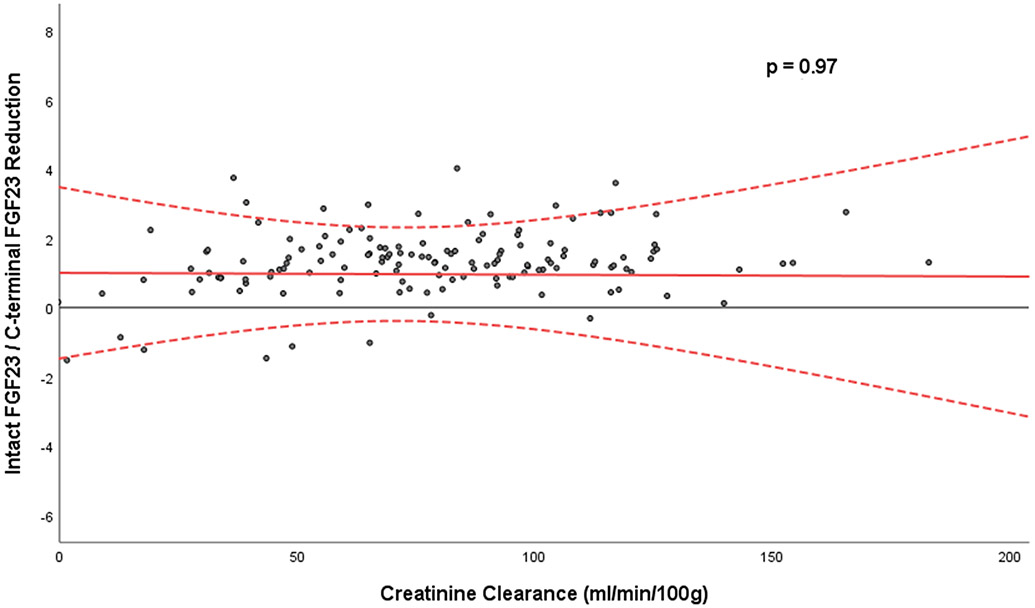
Next, given prior studies demonstrating plasma C-terminal FGF23 concentrations are higher than intact FGF23 concentrations in persons with normal kidney function, but approach similar concentrations in persons with advanced CKD, we were interested in the relative reduction of intact vs. C-terminal FGF23 in individual patients, and whether kidney function influenced it. We created an empirical ratio of the % reduction of intact to C-terminal FGF23 using conventional clinical units for each participant, and examined whether creatinine reduction or other variables was related to this ratio. We found that neither creatinine reduction, nor reduction of PTH or the other mineral biomarkers were related to the relative reduction of intact to C-terminal FGF23 (Table 4). Consistent with this, while reduction of both intact and C-terminal FGF23 were heavily influenced by creatinine reduction (Figure 1), the relative renal reduction of intact to C-terminal FGF23 appeared stable and consistent across the range of creatinine clearance that was evaluated (Figure 2).
Figure 1:
Intact and C-terminal FGF23 reduction across the range of creatinine clearance evaluated; Red dots indicate intact FGF23 reduction and blue dots indicate C-terminal FGF23 reduction
Figure 2:
Relative concentration of intact to C-terminal FGF23 across the range of creatinine clearance evaluated
Clearance of Intact and C-terminal FGF23 in Rats
The findings above suggest that the human kidney extracts both intact and C-terminal FGF23 from the circulation. We next investigated whether FGF23 was metabolized by the kidney or excreted in urine. Our study in humans provided blood in the aorta and renal veins, but urine specimens were not available. Thus, we repeated experiments in a rodent model, measuring both intact and C-terminal FGF23 in the femoral artery, renal vein, and urine, in 6 healthy rats. Mean renal plasma flow was 13.6 ± 6.9 ml/min and GFR was 2.5 ± 0.4 ml/min, for a mean filtration fraction of 0.2 ± 0.05. Mean intact FGF23 reduction was 21.5 ± 12.6 % and mean C-terminal FGF23 reduction was 32.0 ± 13.5%. Both C-terminal and intact FGF23 concentrations were very low in the urine and calculated intact and C-terminal FGF23 clearance using UV/P was at least an order of magnitude lower than what we measured using the product of renal reduction and renal plasma flow (Table 5). Thus, most of the intact and C-terminal FGF23 removed by the kidney is likely metabolized by the kidney rather than excreted unchanged in the urine in rats.
Table 5:
Intact and C-terminal FGF23 Clearance in Rats
| Variable | Mean ± SD |
|---|---|
| Intact FGF23 | |
| Renal Artery Intact FGF23 (pg/ml) | 203 ± 48 |
| Renal Vein Intact FGF23 (pg/ml) | 160 ± 44 |
| Urine Intact FGF23 (pg/ml) | 2 ± 0 |
| % Renal Reduction Intact FGF23 | 21.5% ± 12.6% |
| Renal Clearance of Intact FGF23 (Renal Artery vs. Renal Vein) uL/min | 3.0 ± 1.3 |
| Renal Clearance of Intact FGF23 (UV/P) uL/min | 0.2 ± 0.4 |
| C-terminal FGF23 | |
| Renal Artery C-terminal FGF23 (pg/ml) | 1589 (420, 4794)* |
| Renal Vein C-terminal FGF23 (pg/ml) | 901 (225, 3706)* |
| Urine C-terminal FGF23 (pg/ml) | 3.8 (2.0, 8.3)* |
| % Renal Reduction C-terminal FGF23 | 32.0% ± 13.5% |
| Renal Clearance of C-terminal FGF23 (Renal Artery vs. Renal Vein) uL/min | 4.3 ± 1.4 |
| Renal Clearance of C-terminal FGF23 (UV/P) uL/min | 0.01 ± 0.01 |
Median [IQR]
Discussion
Our aim was to assess the role of the human kidney in clearance of both intact and C-terminal FGF23, and to investigate whether differences in kidney function explain why the relative concentrations of intact and C-terminal FGF23 become more similar in persons with lower GFR. Using blood samples directly obtained from aorta and bilateral renal veins among participants with hypertension and a range of kidney function from normal to moderate CKD, we found that the human kidney extracts both C-terminal and intact FGF23 from the circulation. However, the kidney appears to be similarly efficient at removing intact and C-terminal FGF23 across the range of kidney function evaluated. Furthermore, in a rodent model, we found that the vast majority of extracted intact and C-terminal FGF23 are not found in the urine, suggesting that most of the extracted intact and C-terminal FGF23 is metabolized by the kidney. Finally, we found that, independent of creatinine clearance, the human kidney’s ability for both intact and C-terminal FGF23 reduction was correlated with how much PTH it removed. These findings give important new insights to the kidney’s role in regulating blood FGF23 concentrations.
The recognition of FGF23’s central role in regulating phosphate and vitamin D metabolism, its potential role in promoting left ventricular hypertrophy,3 and its strong associations with heart failure8,10 and mortality risk26,27 have led to considerable interest in understanding its regulation. While much is known about FGF23 production in bone, and processes that regulate its cleavage, very little is known about intact FGF23 clearance from circulation, and even less is known about the kidney’s role in clearance of C-terminal FGF23 fragments. Understanding the regulation of circulating C-terminal FGF23 and its similarities or differences from intact FGF23 is becoming increasingly important, as epidemiologic studies evaluating relationships of FGF23 with clinical endpoints provide different results when evaluating intact vs. C-terminal FGF23 assays. We recently reported that kidney function is the key factor responsible for this difference.14 C-terminal FGF23 appears much more strongly associated with clinical endpoints than intact FGF23 once kidney function is accounted for. This is surprising, as most existing literature on FGF23 suggests that intact FGF23 is the biologically active hormone. However, emerging data suggest that the C-terminal fragments may have their own biological role in regulating iron utilization,28 and possibly as a natural competitive inhibitor of full length FGF23 binding to its FGFR1-Klotho coreceptors.29
Beyond the important role that kidney function has in influencing associations of the two FGF23 measurements with clinical outcomes, other studies suggest that the kidney may have an important role in regulation of both C-terminal and intact FGF-23. Smith and colleagues studied healthy volunteers, persons with stage 3-5 CKD, and dialysis patients and found that, while both intact and C-terminal FGF23 concentrations were elevated in those with more advanced CKD, C-terminal FGF23 concentrations were much higher relative to intact FGF23 in healthy controls.15 In another study among humans with acute kidney injury, C-terminal FGF23 fragments were detected in the urine while intact FGF23 was not, consistent with potential renal catabolism. Higher urine concentrations of FGF23 fragments predicted death after acute kidney injury, suggesting that this catabolic pathway may be clinically important.30 These studies led us to explore the role of the human kidney in C-terminal FGF23 clearance, and to compare it to that of intact FGF23.
Our findings are consistent with, and expand on, prior studies conducted in experimental animals.17 By injecting recombinant FGF23 and blocking FGF23 receptors, investigators estimated that renal reduction of intact FGF23 was responsible for about 40% of its clearance. While they could not rule out the possibility of renal FGF23 excretion and do not compare renal reduction of intact vs C-terminal FGF23, they found clearance of intact FGF23 to be unrelated to calcium, phosphate, or FGF receptor signaling in their rodent acute uremia model.
To our knowledge, only one prior study has directly evaluated the role of the human kidney in clearance of intact FGF23. Van Ballegooijen et al. evaluated 17 humans undergoing angiography and demonstrated that intact FGF23 concentrations were 17% lower in the renal vein relative to the aortal,18 similar to our study. C-terminal FGF23 was not evaluated in this study. Importantly, we were able to investigate clearance of both C-terminal and intact FGF23 and found that the relative renal reduction of both were similar across a wide range of GFR evaluated. As summarized, prior studies demonstrate that both C-terminal and intact FGF23 are increased in persons with lower GFR, persons with normal renal function have higher C-terminal FGF23 relative to intact FGF23; a relationship that becomes less apparent in persons with more advanced CKD. Our findings suggest that this change in relative abundance may not be explained by differential renal clearance. Instead, it is plausible that FGF23 cleavage may be downregulated or impaired secondary to dysregulation of cleavage enzymes as renal function declines causing an increase in intact FGF23.15
Our study also provides insights to mechanism of renal reduction of FGF23. The aforementioned study by Van Ballegooijen demonstrated a 17% reduction of intact FGF23 by a single pass through the kidney. They also measured intact FGF23 in urine, finding very low concentrations, thereby suggesting that most was metabolized in the kidney.18 Based on their published data, we estimated intact FGF23 clearance using UV/P to be only approximately 0.2ml/min, much lower than anticipated by a 17% renal reduction assuming a typical renal plasma flow. We lacked urine data in our human studies, but confirm this finding for intact FGF23 using our rodent model. We expand on this finding in important ways. First, we demonstrate that C-terminal FGF23 is also removed by the human and rodent kidney, and that very little C-terminal FGF23 is found in the urine in rodents. This suggests that C-terminal FGF23 is also likely to be metabolized by the kidney. Our larger sample size provides greater precision in the estimates of FGF23 reduction. Finally, the larger sample size allowed us to explore how the relative clearance of intact vs. C-terminal FGF23 may differ across the spectrum of kidney function, providing insights to mechanisms responsible for the more similar concentrations observed in persons with lower GFR.
We found that the relative renal reduction of intact FGF23 was significantly higher than for C-terminal FGF23 in humans. As the C-terminal FGF23 assay measures both the intact hormone and cleaved C-terminal fragments, this finding suggests that the human kidney may be more effective at clearing the intact FGF23 hormone than smaller fragments, which is contrary to our a priori hypothesis. Potential mechanisms by which intact FGF23 reduction is greater than C-terminal FGF23 fragment clearance may involve differences in protein binding, or in tubular uptake mechanisms and subsequent metabolism. Intact FGF23 may be reabsorbed by cubilin mediated endocytosis in the proximal tubule as noted by Nielsen et al.31 Although FGF23 acts chiefly on the proximal tubule and reduces renal absorption of phosphate by downregulating the sodium-phosphate cotransporter Npt2a, immunofluorescent analyses following FGF23 injection in mice show robust staining for FGF23 bioactivity within the distal tubule.32 Therefore, it is also plausible that the intact hormone is reabsorbed in the distal tubules, which would also be consistent with our data wherein the relative levels of C-terminal FGF23 are higher in the renal vein compared to the intact hormone.
An unexpected, but important finding of this study is that the renal reduction of both C-terminal and intact FGF23 were related to renal reduction of PTH, and that this was independent of creatinine reduction. This finding was not a pre-specified hypothesis and requires confirmation. However, if confirmed, it may provide new insights into a complex regulatory feedback loop between FGF23 and PTH. FGF23 is known to decrease PTH gene expression and secretion.33 On the other hand, PTH increases FGF23 gene expression in bone.34 This has led to recognition of a classic negative feedback loop between the parathyroid gland and bone. Our findings suggest that the degree of renal reduction of FGF23 may be dependent upon the degree of PTH reduction or vice versa, and that this effect is independent of creatinine clearance. In a study testing the effect of PTH on renal clearance of intact FGF23 in a rodent model, investigators demonstrated that intact FGF23 clearance was similar in animals after parathyroidectomy.17 Therefore, we hypothesize that intact FGF23 may drive PTH reduction rather than the opposite. It is possible that additional factors such as 1,25-dihydroxyvitamin D may be involved in this pathway, as reported during PTH infusion in healthy human subjects.35 However, the direction of these effects remains unclear. The cross-sectional nature of our data does not allow us to determine if greater FGF23 reduction may induce greater PTH reduction, the opposite, or whether a third factor may induce greater reduction of both. Nonetheless, because both PTH and FGF23 are key regulators of phosphate homeostasis, and because this relationship was independent of creatinine clearance, this finding suggests that the feedback loops involved between FGF23 and PTH may be more complex than simply between bone and the parathyroid gland. This exciting new hypothesis requires future study.
Our findings are also consistent with prior human and animal studies demonstrating robust renal clearance of PTH. Among nine patients with hyperparathyroidism, the mean reduction of PTH was 44% in the liver, 34% by the kidney, and 16% across the lower extremity.36 Experimental dog models have been used to understand PTH reduction mechanisms. Glomerular filtration was halted by ureteral obstruction, following which mean creatinine reduction decreased from 20% to 4%, whereas PTH reduction only decreased from 22% to 15%, indicating that the kidney still appears to extract PTH from the circulation even when the GFR is acutely halted. This suggests tubular metabolism of PTH.37 We found a modestly higher concentration of α klotho in the renal vein compared to the aorta in humans. This finding is consistent with rodent data demonstrating that the kidney secretes α klotho into the circulation38,39 and studies in humans demonstrating increased klotho concentrations in the infrarenal compared to suprarenal venous system.38 Since α Klotho is passed from blood to urine, taken up and metabolized by the kidney, and synthesized by the kidney and released into the blood, the net effect on the plasma concentration from traversing the kidney is complex. Without knowledge of the renal venous plasma flow, our data do not allow definitive evaluation of the net effects of the kidney on systemic αKlotho concentrations, but our data are compatible with net addition of αKlotho into the renal vein by the kidney.
This study has several strengths. We leveraged the unique Maastricht Renal Artery Stenosis Study, which allowed us to assess renal clearance of multiple metabolites including both C-terminal and intact FGF23 by obtaining blood samples directly during renal angiography in a large number of participants. Thus, we had ample statistical power to test differences in renal reduction of total and intact FGF23 and to compare how these parameters are influenced by level of kidney function. Participants had a 3-week wash-out from anti-hypertensive medications at the time of angiography diminishing any influence of these drugs on renal hemodynamics at the time of blood collection. The concurrent measurement of PTH, α klotho, and other metabolites provided unique new discoveries which will open exciting new areas of investigation.
The study also has several important limitations. We lack data on urine concentrations of FGF23 among humans in our study. On the basis of the observational nature of our study, we cannot determine whether greater FGF23 cleavage by a renal proteolytic apparatus or differences in receptor mediated endocytosis is the key driver responsible for less renal reduction of C-terminal vs. intact FGF23 by the human kidney. Therefore, additional experiments are required to understand the exact mechanisms by which the presumed tubular metabolism of intact and C-terminal FGF23 occurs. Finally, we did not study participants with advanced CKD, all were European reflecting the predominant race of persons in the Netherlands, and all had essential hypertension. Generalizability of our findings to other populations is uncertain.
In conclusion, we directly demonstrate that the human kidney extracts both C-terminal and intact FGF23 from the circulation and the renal reduction of intact FGF23 is higher than that of creatinine. In parallel, we demonstrate that the rat kidney also extracts both intact as well as C-terminal FGF23 and very low quantities of intact FGF23 were found in the urine. Both these findings suggest that intact FGF23 is metabolized and cleared through the human kidney tubules, above and beyond the glomerular filtration. While persons with lower GFR have lower renal clearance of both C-terminal and intact FGF23, the relative clearance efficiency of C-terminal and intact FGF23 appears similar across the range of GFR we evaluated here. Thus, differential kidney clearance of FGF23 does not explain why persons with normal kidney function have higher C-terminal compared to intact FGF23, whereas those with lower renal function have more similar blood concentrations.
Acknowledgements/Support
This work was supported by National Institute of Diabetes and Digestive Kidney Diseases (NIDDK) Grants R01DK119528 and K24DK110427 (J.H.I). P30 DK079328, R01 DK091392, R01 DK092461 and R01 DK119528 (O.W.M). Veterans Health Administration IK2-CX002195 (SS).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosure Statement
Dr. Ix is principal investigator of an investigator-initiated research grant from Baxter International, serves as a data safety monitoring board member for Sanifit International, and has served on advisory boards for Alpha Young, AstraZeneca, Ardelyx Inc., and Jnana Inc. Dr. Moe has served on advisory boards and received compensations in the last 5 years for Allena, Alnylam, Ardelyx, Applied Therapeutics, Dicerna, Janssen, Genzyme-Sanofi, and Tricida.
Remaining authors have declared no conflicts of interest.
References
- 1.Martin A, David V, Quarles LD. Regulation and function of the FGF23/klotho endocrine pathways. Physiol Rev. 2012;92(1):131–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kovesdy CP, Quarles LD. FGF23 from bench to bedside. Am J Physiol Renal Physiol. 2016;310(11):F1168–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Faul C, Amaral AP, Oskouei B, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121(11):4393–4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ichikawa S, Gray AK, Padgett LR, et al. Genetic rescue of glycosylation-deficient Fgf23 in the Galnt3 knockout mouse. Endocrinology. 2014;155(10):3891–3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edmonston D, Wolf M. FGF23 at the crossroads of phosphate, iron economy and erythropoiesis. Nat Rev Nephrol. 2020;16(1):7–19. [DOI] [PubMed] [Google Scholar]
- 6.Shimada T, Urakawa I, Isakova T, et al. Circulating fibroblast growth factor 23 in patients with end-stage renal disease treated by peritoneal dialysis is intact and biologically active. J Clin Endocrinol Metab. 2010;95(2):578–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jonsson KB, Zahradnik R, Larsson T, et al. Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N Engl J Med. 2003;348(17):1656–1663. [DOI] [PubMed] [Google Scholar]
- 8.Ix JH, Katz R, Kestenbaum BR, et al. Fibroblast growth factor-23 and death, heart failure, and cardiovascular events in community-living individuals: CHS (Cardiovascular Health Study). J Am Coll Cardiol. 2012;60(3):200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kestenbaum B, Sachs MC, Hoofnagle AN, et al. Fibroblast growth factor-23 and cardiovascular disease in the general population: the Multi-Ethnic Study of Atherosclerosis. Circ Heart Fail. 2014;7(3):409–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lutsey PL, Alonso A, Selvin E, et al. Fibroblast growth factor-23 and incident coronary heart disease, heart failure, and cardiovascular mortality: the Atherosclerosis Risk in Communities study. J Am Heart Assoc. 2014;3(3):e000936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Isakova T, Xie H, Yang W, et al. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA. 2011;305(23):2432–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qin Z, Liu X, Song M, et al. Fibroblast growth factor 23 as a predictor of cardiovascular and all-cause mortality in prospective studies. Atherosclerosis. 2017;261:1–11. [DOI] [PubMed] [Google Scholar]
- 13.Xiao Y, Luo X, Huang W, Zhang J, Peng C. FGF 23 and risk of all-cause mortality and cardiovascular events: a meta-analysis of prospective cohort studies. Int J Cardiol. 2014;177(2):575–577. [DOI] [PubMed] [Google Scholar]
- 14.Sharma S, Katz R, Bullen AL, et al. Intact and C-Terminal FGF23 Assays-Do Kidney Function, Inflammation, and Low Iron Influence Relationships With Outcomes? J Clin Endocrinol Metab. 2020;105(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith ER, Cai MM, McMahon LP, Holt SG. Biological variability of plasma intact and C-terminal FGF23 measurements. J Clin Endocrinol Metab. 2012;97(9):3357–3365. [DOI] [PubMed] [Google Scholar]
- 16.Stubbs JR, He N, Idiculla A, et al. Longitudinal evaluation of FGF23 changes and mineral metabolism abnormalities in a mouse model of chronic kidney disease. J Bone Miner Res. 2012;27(1):38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mace ML, Gravesen E, Hofman-Bang J, Olgaard K, Lewin E. Key role of the kidney in the regulation of fibroblast growth factor 23. Kidney Int. 2015;88(6):1304–1313. [DOI] [PubMed] [Google Scholar]
- 18.van Ballegooijen AJ, Rhee EP, Elmariah S, de Boer IH, Kestenbaum B. Renal Clearance of Mineral Metabolism Biomarkers. J Am Soc Nephrol. 2016;27(2):392–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Onna M, Houben AJ, Kroon AA, et al. Asymmetry of renal blood flow in patients with moderate to severe hypertension. Hypertension. 2003;41(1):108–113. [DOI] [PubMed] [Google Scholar]
- 20.Wierema TK, Houben AJ, Kroon AA, et al. Nitric oxide dependence of renal blood flow in patients with renal artery stenosis. J Am Soc Nephrol. 2001;12(9):1836–1843. [DOI] [PubMed] [Google Scholar]
- 21.Burnett SM, Gunawardene SC, Bringhurst FR, Juppner H, Lee H, Finkelstein JS. Regulation of C-terminal and intact FGF-23 by dietary phosphate in men and women. J Bone Miner Res. 2006;21(8):1187–1196. [DOI] [PubMed] [Google Scholar]
- 22.Barker SL, Pastor J, Carranza D, et al. The demonstration of alphaKlotho deficiency in human chronic kidney disease with a novel synthetic antibody. Nephrol Dial Transplant. 2015;30(2):223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neyra JA, Moe OW, Pastor J, et al. Performance of soluble Klotho assays in clinical samples of kidney disease. Clin Kidney J. 2020;13(2):235–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.P R. Multiple imputation of missing values. The Stata Journal. 2004;4(3):227–241. [Google Scholar]
- 25.DB R Multiple Imputation for Nonresponse in Surveys. Wiley Classics Library 2004. [Google Scholar]
- 26.Arnlov J, Carlsson AC, Sundstrom J, et al. Higher fibroblast growth factor-23 increases the risk of all-cause and cardiovascular mortality in the community. Kidney Int. 2013;83(1):160–166. [DOI] [PubMed] [Google Scholar]
- 27.Westerberg PA, Tivesten A, Karlsson MK, et al. Fibroblast growth factor 23, mineral metabolism and mortality among elderly men (Swedish MrOs). BMC Nephrol. 2013;14:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.David V, Martin A, Isakova T, et al. Inflammation and functional iron deficiency regulate fibroblast growth factor 23 production. Kidney Int. 2016;89(1):135–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goetz R, Nakada Y, Hu MC, et al. Isolated C-terminal tail of FGF23 alleviates hypophosphatemia by inhibiting FGF23-FGFR-Klotho complex formation. Proc Natl Acad Sci U S A. 2010;107(1):407–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leaf DE, Jacob KA, Srivastava A, et al. Fibroblast Growth Factor 23 Levels Associate with AKI and Death in Critical Illness. J Am Soc Nephrol. 2017;28(6):1877–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nielsen R, Christensen EI, Birn H. Megalin and cubilin in proximal tubule protein reabsorption: from experimental models to human disease. Kidney Int. 2016;89(1):58–67. [DOI] [PubMed] [Google Scholar]
- 32.Farrow EG, Davis SI, Summers LJ, White KE. Initial FGF23-mediated signaling occurs in the distal convoluted tubule. J Am Soc Nephrol. 2009;20(5):955–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silver J, Naveh-Many T. FGF23 and the parathyroid glands. Pediatr Nephrol. 2010;25(11):2241–2245. [DOI] [PubMed] [Google Scholar]
- 34.Lavi-Moshayoff V, Wasserman G, Meir T, Silver J, Naveh-Many T. PTH increases FGF23 gene expression and mediates the high-FGF23 levels of experimental kidney failure: a bone parathyroid feedback loop. Am J Physiol Renal Physiol. 2010;299(4):F882–889. [DOI] [PubMed] [Google Scholar]
- 35.Burnett-Bowie SM, Henao MP, Dere ME, Lee H, Leder BZ. Effects of hPTH(1-34) infusion on circulating serum phosphate, 1,25-dihydroxyvitamin D, and FGF23 levels in healthy men. J Bone Miner Res. 2009;24(10):1681–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corvilain J, Manderlier T, Struyven J, et al. Metabolism of human PTH by the kidney and the liver. Horm Metab Res. 1977;9(3):239–242. [DOI] [PubMed] [Google Scholar]
- 37.Martin KJ, Hruska KA, Lewis J, Anderson C, Slatopolsky E. The renal handling of parathyroid hormone. Role of peritubular uptake and glomerular filtration. J Clin Invest. 1977;60(4):808–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu MC, Shi M, Zhang J, et al. Renal Production, Uptake, and Handling of Circulating alphaKlotho. J Am Soc Nephrol. 2016;27(1):79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lindberg K, Amin R, Moe OW, et al. The kidney is the principal organ mediating klotho effects. J Am Soc Nephrol. 2014;25(10):2169–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]