Abstract
Inhalation of ambient PM2.5, shown to be able to cross the placenta, has been linked to adverse obstetric and postnatal metabolic health outcomes. The placenta regulates fetal growth and influences postnatal development via fetal programming. Placental gene expression may be influenced by intrauterine exposures to PM2.5. Herein, we explore whether maternal PM2.5 exposure during pregnancy alters placental gene expression related to lipid and glucose metabolism in a U.S. birth cohort, the Rhode Island Child Health Study (RICHS). Average PM2.5 exposure level was estimated linking residential addresses and satellite data across the three trimesters using spatio-temporal models. Based on Gene Ontology annotations, we curated a list of 657 lipid and glucose metabolism genes. We conducted a two-staged analysis by leveraging placental RNA-Seq data from 148 subjects to identify top dysregulated metabolic genes associated with PM2.5 (Phase I) and then validated the results in placental samples from 415 participants of the cohort using RT-qPCR (Phase II). Associations between PM2.5 and placental gene expression were explored using multivariable linear regression models in the overall population and in sex-stratified analyses. The average level of PM2.5 exposure across pregnancy was 8.0μg/m3, which is below the national standard of 12μg/m3. Phase I revealed that expression levels of 32 out of the curated list of 657 genes were significantly associated with PM2.5 exposure (FDR P <0.01), 28 genes showed differential expression modified by sex of the infant. Five of these genes (ABHD3, ATP11A, CLTCL1, ST6GALNAC4 and PSCA) were validated using RT-qPCR. Associations were stronger in placentas from male births compared to females, indicating a sex-dependent effect. These genes are involved in inflammation, lipid transport, cell-cell communication or cell invasion. Our results suggest that gestational PM2.5 exposure may alter placental metabolic function. However, whether it confers long-term programming effects postnatally, especially in a sex-specific matter, warrants further studies.
Keywords: PM2.5, air pollution, placenta, pregnancy, sex differences, metabolism
1. Introduction
Exposure to fine-sized ambient particles has been identified as a leading risk factor for global disease burden (Brauer et al., 2016). Fine-sized particles with an aerodynamic diameter of less than 2.5μm (i.e., particulate matter or [PM2.5]), have been shown to penetrate into the alveolar region of the lungs via inhalation (Shimada et al., 2006; Sun et al., 2009; Franklin et al., 2013), and can translocate into the systemic circulation, leading to a myriad of diseases over time, such as cardiovascular and respiratory illnesses (Lepeule et al., 2012; Caiazzo et al., 2013).
Numerous epidemiologic and animal studies have demonstrated that chronic exposure to PM2.5 can cause systemic inflammation, and is associated with metabolic syndrome, obesity and diabetes, suggesting that inflammation is a possible underlying mechanism (Xu et al., 2017; Mao et al., 2017; Li et al., 2018, 2017; Shamy et al., 2018; Yang et al., 2018; Orioli et al., 2018). Pregnancy represents a state of increased vulnerability to diabetes and hypertension (Wilson et al., 1980; Bosio et al., 1999; Songara et al., 2014), especially in those who have pre-existing risk factors (i.e., obesity prior to pregnancy). Thus, pregnant women may be more susceptible to the effects of PM2.5 exposure and adverse health outcomes, potentially putting the conceptus at increased risk for metabolic diseases.
PM2.5 exposure during the gestational period has been linked with adverse obstetric outcomes, such as poor placental function, altered fetal development and detrimental postnatal health outcomes (Kingsley et al., 2017a, 2017b, 2016; Clemente et al., 2017; Provost et al., 2017). The placenta plays a key role in mediating the in utero environment and regulating fetal growth (Fowden et al., 2006; Sandovici et al., 2012). The placenta is a metabolically active organ with the unique ability to adapt to changes within its environment in response to maternal and/or fetal needs (Fowden et al., 2006). While placental plasticity serves to maintain fetal growth and ensure survival at birth, disruption by environmental toxicants may lead to adverse effects on fetal programming. As per ex vivo human placenta perfusion studies, the placenta is permeable to fine-sized particles up to 240nm in diameter (Wick et al., 2010), which may cause direct tissue injury and alter fetal development. Bove et al., reported similarly that black carbon particles were detected on the fetal side of the placenta, indicating penetration across the human placental barrier (2019). Gestational PM2.5 exposure has also been shown to cause placental maladaptations that increase fetal oxygen and nutrient delivery in an attempt to optimize fetal growth, which may lead to detrimental effects on fetal development and postnatal health (Veras et al., 2008; de Melo et al., 2015; Liu et al., 2016; Fajersztajn and Veras 2017; Soto et al., 2017).
Sex-specific outcomes following prenatal PM2.5 exposure have also been shown in the literature (Chiu et al., 2017; Virjens et al., 2017; Brunst et al., 2018). Positive associations were observed between PM2.5 exposure and adiponectin and leptin levels in cord blood from female infants only, and both hormones were linked with increased weight in infancy (Lavigne et al., 2016; Alderete et al., 2017). Health effects resulting from prenatal PM2.5 exposure have demonstrated that males exhibit a higher body mass index, whereas increased waist-to-hip ratios were observed in girls per μg/m3 increase in PM2.5 level (Chiu et al., 2017). While sex-specific effects of PM2.5 exposure are evident in neonates, children and adults, the genetic bases and mechanisms underlying these outcomes are not well-characterized.
From the same study population, Rhode Island Child Health Study (RICHS), we previously reported that maternal PM2.5 exposure alters placental imprinted gene expression and birthweight (Kingsley et al., 2017b, 2016; Deyssenroth et al., 2017). More recently, Deyssenroth et al., 2021 reported that pre-conception and first trimester PM2.5 exposure was a critical window associated with growth restriction. Furthermore, a transcriptome-wide analysis revealed that amino acid transport and cellular respiration pathways may underlie susceptibility to postnatal cardio-metabolic health outcomes (Deyssenroth et al., 2021). Herein, we use a pathway approach to examine the sex-specific effects of PM2.5 on the expression of a panel of metabolic genes within the placenta. We assess associations between placental gene expression and maternal PM2.5 exposure averaged across pregnancy based on the assumption that placental gene expression at the time of delivery reflects the culmination of environmental impacts throughout gestation and is a functional marker for the in utero environment.
2. Materials and Methods
2.1. Study Population
The study utilizes the resources of the RICHS, which have been described in detail previously (Paquette et al., 2014; Lesseur et al., 2014). In brief, the study population was oversampled for large for gestational age (LGA) and small for gestational age (SGA). Each LGA or SGA infant enrolled into the study was matched to an appropriate for gestational age (AGA) infant based on gestational age at birth and infant sex. The birthweight group assignments were determined by the 2013 Fenton Birthweight Chart revision, which is harmonized with the WHO growth chart and can be used for term and pre-term infants (Fenton and Kim 2013). The study collected placental samples from singleton, term (>37 weeks gestation) pregnancies, excluding any pregnancies diagnosed for congenital and/or chromosomal abnormalities or life-threatening obstetric complications. A total of 799 subjects were enrolled into the study between March 2009 and May 2013 (Kingsley et al., 2017), among which 415 provided residential information for PM2.5 assessment and were used in the present investigation.
2.2. Exposure Assessment
Participant addresses at time of delivery were geocoded using ArcMap 10.1 (ESRI; Redlands, CA) as previously described by Kingsley et al. (2017a; 2017b). Daily PM2.5 levels were estimated for each maternal residential address with a hybrid spatial-temporal model that utilizes land-use regression and satellite measurements of aerosol optical depth (AOD) as described in detail by Kloog et al. (2014). The land use regression and satellite-based AOD (1 km resolution) accurately fit a calibration regression using PM2.5 ground-level measurements for the entire pregnancy. Finer scale exposure was estimated based on the differences in AOD and PM2.5 measured at ground-level that were regressed against local features (i.e., source emission points, traffic density) at a finer resolution of 200m x 200m. Daily ground-level PM2.5 measurements were gathered from six RI Department of Environmental Management-operated stationary monitors. Overall, PM2.5 exposure concentrations were estimated from modeling and monitoring data for any participant deliveries occurring between 2009 – 2013 that were geocoded. All pregnancy-average pollutant levels across nine months were calculated for the participants during the entirety of the study period. Data on address changes during participant pregnancies were not collected for the RICHS population.
2.3. Placental collection and existing RNA-Seq data
As described previously (Kappil et al., 2015), placental biopsies from RICHS subjects were taken within two hours of delivery. Biopsies were free of maternal decidua and retrieved from four quadrants (2cm from the cord insertion site) of the fetal portion. Each biopsy was stored in RNALater (4°C) for 72 hours before snap-freezing and homogenization. Samples were stored at −80°C until RNA sequencing analysis. The placenta transcriptome was profiled by RNAseq in a subset of 200 participants from the RICHS population in a separate study (Deyssenroth et al., 2017), of which 148 participants had available PM2.5 data.
2.4. Curation of metabolic gene list
We compiled a curated list of lipid and glucose metabolic genes based on the biological process (BP) domain of the Gene Ontology (GO) annotations of human genes (Ashburner et al., 2000). We used the GO.db and BiomaRt R Bioconductor packages to compile a list of genes (n = 1,409) annotated to GO BP terms related to lipid (n = 111 GO terms) and glucose (n = 103 GO terms) metabolism (Carlson, 2009; Durinck et al., 2009) [Supplemental Table 1]. This list was further filtered to: 1) Genes expressed within the placenta, expression >2 normalized logCPM in at least 15% of the samples (30/200) from the RNA-Seq subset; 2) Genes with variances between >20% and <80% across samples; and 3) Genes in autosomes only. The final metabolic and lipid gene set used in the discovery analyses comprised 657 genes in Phase I. This process is summarized in Supplemental Figure 1.
2.5. Quantitative real-time PCR
Placental RNA were converted to cDNA (iScript cDNA Conversion Kit; Bio-Rad Laboratories, Inc., CN:1708891, Hercules, CA, USA). The reverse transcriptase reaction was run (ThermoCycler; Bio-Rad Laboratories, Inc.) with the 415 samples across five batches, in 96-well plates by the same operator (blinded to exposure status) in 1-week period. Five genes were selected (ABHD3, Hs01128760_m1; ATP11A, Hs00392589_m1; CLTCL1, Hs01047204_m1; PSCA, Hs00194665_m1; ST6GALNAC4, Hs01082656_m1) as per the selection criteria described below for qPCR analysis (TaqMan Gene Expression Assays; Thermo Fisher Scientific, Inc., Waltham, MA, USA). Samples were run in triplicate as duplex reactions on the ABI 7900HT with the Sequence Detection Systems V 2.4 software (Applied Biosystems, Foster City, CA, USA), with RPL19 (Hs02338565_gH) serving as the housekeeping gene. Changes in relative gene expression were calculated using the ΔΔCt method (Livak and Schmittgen 2001).
2.6. Statistical Analyses
A two-staged analysis plan was employed for this study. Our Phase I subset capitalized on existing data of the placental transcriptome of 148 participants. Subsequently, Phase II was carried out in the 415 participants who have both placenta collection and PM exposure information (including the 148 participants in Phase I). We used the limma R package (Ritchie et al., 2015) to identify genes with a false discovery rate (FDR) of 1% (q value= 0.1) and absolute effect size of >0.2 among the 657 curated genes.
For both Phases I and II, linear regression models were used to assess overall and sex-stratified associations between placental gene expression and maternal PM2.5 exposure averaged across pregnancy. We also performed trimester-specific sensitivity analyses for each placental gene target and PM2.5 exposure by trimester in the overall and sex-specific models. Models have all been adjusted for maternal smoking during pregnancy, PCR batch effect and infant sex was accounted for in the overall model.
A moderate beta coefficient (>∣0.2∣) and a significant p-value (<0.05) were indicative of a significant association between placental gene expression and PM2.5 exposure. If the beta coefficients between the male and female stratified analyses were observed to be in opposite directions (i.e., positive vs negative coefficients), the relationship between placental gene expression and exposure to PM2.5 was considered to be sexually-dimorphic. However, if the beta coefficients shared directionality, but demonstrated a stronger effect size and p-value in one of the infant sexes, it was considered to be a sex-dependent effect.
Sex-stratified linear regression analyses were performed for each of the gene targets selected for qPCR in Phase II. Linear regression models were adjusted for maternal smoking during pregnancy and the batch effect. All analyses were performed in R version 3.6.0 (R Core Team, 2019).
3. Results
The demographic characteristics for the Phase I subset (148 subjects), Phase II subset (415 subjects) and overall RICHS population (799 subjects) are described in Table 1. The average daily concentrations of PM2.5 across nine months of pregnancy are shown in Supplemental Figure 2. The study population was predominantly Caucasian (77%) with very few smokers (5%).
Table 1. Demographic characteristics.
Phase I (n=148), Phase II (n=415) and total study Rhode Island Child Health Study population (n=799) subsets.
| Phase I Subset (n=148) | Phase II Subset (n=415) | Total Study Population (n=799) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Covariates | SGA | AGA | LGA | SGA | AGA | LGA | SGA | AGA | LGA |
| 21 (14%) |
82 (55%) |
45 (30%) |
68 (16%) |
231 (56%) |
116 (28%) |
157 (20%) |
456 (57%) |
186 (23%) |
|
| Maternal | |||||||||
| Mean Age (years) | 32.14 | 30.98 | 31.36 | 29.5 | 29.82 | 30.56 | 28.39 | 29.83 | 30.58 |
| Ethnicity | |||||||||
| White | 13 | 67 | 67 | 40 | 180 | 96 | 89 | 110 | 33 |
| Other | 8 | 15 | 15 | 26 | 48 | 18 | 68 | 344 | 152 |
| Smoking During Pregnancy | |||||||||
| Yes | 1 | 1 | 1 | 9 | 7 | 3 | 20 | 18 | 4 |
| No | 18 | 79 | 44 | 56 | 220 | 113 | 132 | 432 | 182 |
| Infant | |||||||||
| Sex | |||||||||
| Female | 13 (62%) |
41 (50%) |
17 (38%) |
41 (60%) |
108 (47%) |
50 (43%) |
93 (59%) |
220 (48%) |
88 (47%) |
| Male | 8 (38%) | 41 (50%) |
28 (62%) |
27 (40%) |
123 (53%) |
66 (57%) |
64 (41%) |
236 (52%) |
98 (53%) |
| Mean Birthweight (g) | 2572 | 3406 | 4274 | 2591 | 3476 | 4303 | 2609 | 3440 | 4301 |
| Delivery Method | |||||||||
| Vaginal | 13 | 52 | 17 | 41 | 129 | 34 | 94 | 250 | 47 |
| C-Section | 8 | 30 | 28 | 27 | 102 | 82 | 63 | 206 | 139 |
For Phase I analyses, volcano plots were generated to show the overall association between the 657 metabolic genes and PM2.5 exposure [Figure 1] and in each sex stratum [Figures 2A and 2B]. When analyzed in a non-sex specific fashion, a total of 32 genes were significantly associated (FDR adjusted p<0.01) with maternal PM2.5 exposure. When assessing sex-specific effect, 4 genes were significantly downregulated with PM2.5 exposure in female placentas, whereas 24 genes (14 up-regulated and 10 down-regulated) were significantly associated with PM2.5 exposure in male placentas [Figures 2A and 2B, respectively]. Only lipid metabolic GO terms, rather than glucose were related to PM2.5 exposure in the overall analysis [Supplemental Table 1].
Figure 1. Placental gene expression in relation to Particulate Matter <2.5 microns (PM2.5) exposure.
Volcano plot of association PM2.5 association results for 657 placental metabolic genes (n=148). Log 2 fold changes are in the y-axis and - log10 p-values in the y-axis. Models adjusted for maternal smoking. Red and blue dots (n=32) are genes associated with PM2.5 (False Discovery Rate q<0.01).
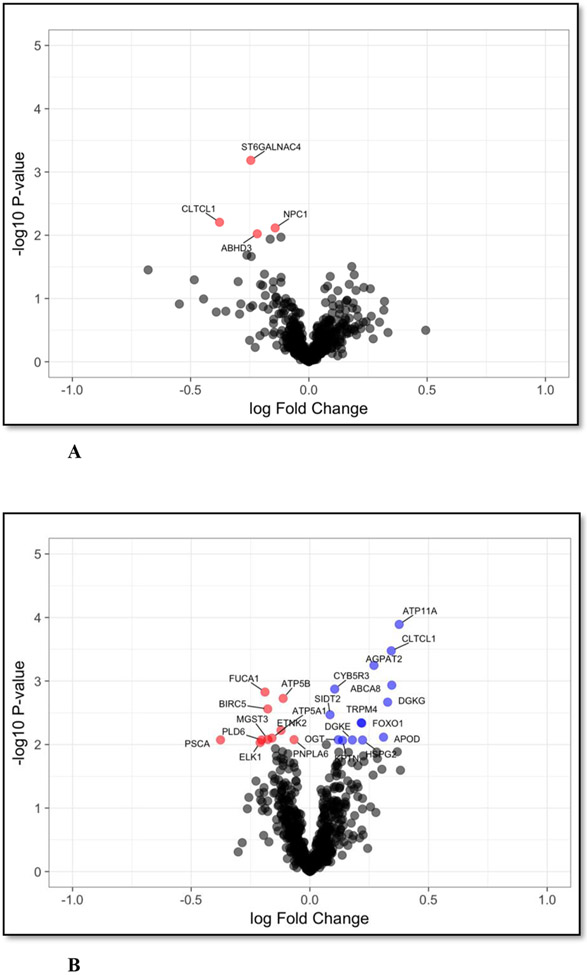
Figures 2. A and B – Sex-stratified Particulate Matter <2.5 microns (PM2.5) -associated placental metabolic genes.
Volcano plots demonstrating placental metabolic genes significantly associated with PM2.5 exposure during pregnancy, stratified by the sex of the infant. A linear regression was performed using Ribonucleic acid sequencing data and PM2.5 exposure monitoring data for each infant sex (n=148; n=71 female, n=77 male). Models were adjusted for maternal smoking. Linear regression model revealed genes significantly (False Discovery Rate adjusted P<0.01) associated with PM2.5 exposure, stratified by infant sex to assess top hit genes. A more pronounced sex-dependent effect is evident in male placental gene expression is association with PM2.5. Plot A demonstrates results from female infant placentas, n=4 genes are significantly associated with PM whereas in Plot B, male infant placentas demonstrate n=24 genes are associated significantly with PM.
Figure 2A – Particulate Matter <2.5 microns (PM2.5) -associated placental genes in female infant placentas
Figure 2B – Particulate Matter <2.5 microns (PM2.5) associated placental genes in male infant placentas
For the Phase II, placental RNA from 415 subjects, (including the previous Phase I subset of 148 subjects) were analyzed by qRT-PCR [Supplemental Figures 3A, 3B and 3C; Supplemental Table 2]. Four genes (ABHD3, ATP11A, PSCA, ST6GALNAC4) were selected for Phase II based on criteria of low p-value (FDR adjusted <0.01) and large effect size (> 0.2). An additional gene, CLTCL1 was selected because it was the only significant overlapping gene between male and female placentas. Results from multivariable-adjusted linear regression models are summarized in Table 2.
Table 2. Multivariable-adjusted linear regression models for placental genes significantly associated with pregnancy-average Particulate Matter <2.5 microns (PM2.5) exposure.
Linear regression models for the pooled subset and the sex-stratified subsets summarizing the Beta estimates, standard error and P-values for each placental gene assessed via polymerase chain reaction (PCR) with a significant association with pregnancy-average PM2.5 exposure. Models have all been adjusted for maternal smoking, the PCR batch effect and the sex of the infant.
| Beta Estimate | Standard Error | P-value | ||
|---|---|---|---|---|
| ABHD3 (n = 412) | ||||
| PM2.5 | −0.23 | 0.06 | 0.0003* | |
| Smoking | −0.03 | 0.14 | 0.85 | |
| Female (n = 199) | ||||
| PM2.5 | −0.17 | 0.06 | 0.07 | |
| Male (n = 213) | ||||
| PM2.5 | −0.27 | 0.06 | 0.0009* | |
| ATP11A (n = 413) | ||||
| PM2.5 | 0.23 | 0.1 | 0.02* | |
| Smoking | 0.25 | 0.23 | 0.27 | |
| Female (n = 199) | ||||
| PM2.5 | 0.12 | 0.14 | 0.38 | |
| Male (n = 214) | ||||
| PM2.5 | 0.31 | 0.15 | 0.04* | |
| PSCA (n = 412) | ||||
| PM2.5 | 0.31 | 0.2 | 0.045* | |
| Smoking | −0.36 | 0.3 | 0.31 | |
| Female (n = 199) | ||||
| PM2.5 | 0.1 | 0.21 | 0.65 | |
| Male (n = 213) | ||||
| PM2.5 | 0.47 | 0.2 | 0.04* | |
| ST6GALNAC4 (n = 406) | ||||
| PM2.5 | 0.30 | 0.09 | 0.0009* | |
| Smoking | −0.07 | 0.2 | 0.72 | |
| Female (n = 196) | ||||
| PM2.5 | 0.28 | 0.13 | 0.04* | |
| Male (n = 210) | ||||
| PM2.5 | 0.33 | 0.12 | 0.006* | |
| CLTCL1 (n = 398) | ||||
| PM2.5 | 0.03 | 0.1 | 0.77 | |
| Smoking | 0.27 | 0.2 | 0.23 | |
| Female (n = 193) | ||||
| PM2.5 | −0.008 | 0.1 | 0.94 | |
| Male (n = 205) | ||||
| PM2.5 | 0.1 | 0.1 | 0.48 |
p < 0.05 and Beta estimates in bold indicate a relationship of moderate correlation between PM2.5 exposure and gene expression.
Consistent with the results from Phase I, placental ABHD3 expression remained inversely associated with PM2.5 exposure (beta = −0.23, p = 0.0003). The sex-stratified regression model indicates that this association was stronger in male placentas (beta = −0.27, p = 0.0009), however this sex difference was not evident in Phase I. Placental ATP11A, PSCA and ST6GALNAC4 expression was positively associated with pregnancy-average PM2.5 exposure; stronger associations were also observed in male placentas for ATP11A and PSCA, consistent with Phase I outcomes. Inconsistent with data from Phase I, no overall nor sex-specific association between placenta CLTCL1 expression and PM2.5 was observed in Phase II analyses.
We assessed whether a specific window or trimester is more sensitive to PM2.5–related gene expression changes in the placenta [Supplemental Table 4]. The results did not reveal any significant relationships (moderate beta coefficient >∣0.2∣ and a significant p-value <0.05) between the three trimester-specific PM2.5 levels and gene expression, also when assessed for sex differences [Supplemental Table 4].
4. Discussion
Despite extensive data supporting the adverse health effects of inhaled particulate air pollution exposure and sex differences in these effects (Ebisu and Bell 2012; Yue et al., 2020), few studies have investigated whether maternal exposure to air pollution during gestation may alter placental metabolic gene expression and whether the influence differs by infant sex. Considering the growing human health and economic burden of metabolic diseases, understanding their underlying molecular mechanisms is critical to better understand how early environmental exposures influence adverse metabolic outcomes later in life, possibly through fetal programming at the feto-placental interface. This study investigates the overall and sex-specific impact of exposure to fine-sized ambient air pollution (PM2.5) during pregnancy on placental metabolic gene expression from a well-characterized US-based birth cohort.
Overall the PM2.5 in the study area of Rhode Island and southeastern Massachusetts during the study period (2009 – 2014) was below the National Ambient Air Quality Standards (NAAQS) action level of 12μg/m3 annual mean (EPA 2012). Data from the monitoring and modeling exposure assessment for this cohort that measured daily PM2.5 levels during pregnancy reveal that the average PM2.5 level was 8μg/m3, ranging from 7 – 10μg/m3 for the Phase II subset of 415 subjects analyzed in this study. The mean PM2.5 level for both the Phase I subset of 148 subjects and the total population of 799 subjects was also 8μg/m3.
Sexually dimorphic (i.e., opposing outcomes/phenotypes in males versus females) effects on health outcomes, such as metabolic diseases, are well-characterized in the literature and are especially evident following PM2.5 exposure (Xia et al., 2018; Brunst et al., 2018; Chiu et al., 2017; Virejns et al., 2017; Winckelmans et al., 2017; Lee et al., 2017, 2016; Hsu et al., 2015; Lakshmanan et al., 2015). This present study was formulated with an interest in exploring sexually dimorphic effects following PM2.5 exposure during pregnancy. While a true dimorphic effect is not evident in the data reported herein, a stronger sex-dependent effect is evident in placentas from male infants with respect to gene expression following PM2.5 exposure during pregnancy. We found that out of the five genes selected for Phase II (ABHD3, ATP11A, PSCA, ST6GALNAC4, CLTCL1), four genes (ABHD3, ATP11A, PSCA, ST6GALNAC4) were significantly associated with PM2.5 exposure across pregnancy. Furthermore, male placentas consistently demonstrated stronger associations for the aforementioned genes, compared to female placentas. Our trimester-specific sensitivity analysis did not reveal any strong associations between the five target genes and PM2.5 levels by trimester. Perhaps this is in part due to the overall low levels of PM2.5 in this area of RI, thus acute exposure within trimester windows did not confer a significant impact on placental gene expression that were evident when PM2.5 was averaged across the entire pregnancy.
As pregnancy-average PM2.5 increased, placental expression of ABHD3 was significantly decreased. ABHD3-gene regulated oxidized phosphatidylcholine enzymatic breakdown may be a method by which damaged lipids are removed from cell membranes to reduce chronic inflammatory disease states (Long et al., 2011; Thomas et al., 2014). Altered levels of circulating phospholipids have been implicated in dyslipidemia, type 2 diabetes and cardiovascular disease (Demirkan et al., 2012). Placental ATP11A expression was positively associated with PM2.5 in this study. ATP11A is a P4-ATPase, an integral membrane protein involved in attenuating inflammatory responses (Takatsu et al., 2014; van der Mark et al., 2017). ATP11A−/− mouse embryos presented with defects in placentation and vascular development (Perez-Garcia et al., 2018; Tsuchiya et al., 2018; Carvalho-Silva et al., 2019). PSCA expression and PM2.5 exposure were positively associated. This gene is a glycosylphosphatidylinositol-anchored cell surface protein that regulates cell growth through the p53 signaling pathway (Feng et al., 2008). PSCA overexpression was observed in cases of hyatidiform mole progressing into neoplasia. Another study of human placentas identified that PSCA was hypomethylated in early-onset preeclampsia compared to control (Yuen et al., 2010). ST6GALNAC4 expression was significantly increased with PM2.5 exposure during pregnancy. This gene is highly expressed in fetal tissues, indicating a possible regulatory role during rapid fetal growth (Dall’olio 1990). A genome-wide transcriptional analysis identified ST6GALNAC4 as a downregulated transcript following volatile organic compound exposure (Gostner et al., 2016).
While lifestyle factors, hormones and gene expression can all impact sex-specific outcomes of exposure, the placenta offers a unique and distinct snapshot prior to the influence of the offspring’s lifestyle factors and changing hormonal milieu. Since the placenta is a fetal tissue and develops early in pregnancy, sex chromosomes play a role in the trajectory of placental function and growth, ranging from morphology, transcription and methylation (Gonzalez et al., 2018; Gong et al., 2018). While many factors can affect child cardiometabolic outcomes induced by prenatal environmental exposures, the placenta can further influence the developing metabolism (Bellone et al., 2004). The male-fetus derived placenta is smaller in size than the female placenta and more efficient in supporting male fetal growth; yet male placentas lack reserve capacity in changing environments (Eriksson et al., 2010). Perhaps this impacts the ability of males to adapt to environmental insult from PM2.5 constituents due to the lower levels of X-linked gene O-GlcNAc transferase (OGT) (Howerton and Bale, 2014; Sandman et al., 2013). OGT is key in placental epigenetic processes, thus male placentas may have reduced histone repressive marks (H3K2me3) and increased susceptibility to environmental toxicants.
We acknowledge some limitations of our study. First, data on address changes during participant pregnancies were not collected for the RICHS population, which reduces the accuracy of PM2.5 exposure modeling. Second, the placenta is comprised of mixed cell populations that vary in prevalence as the dominant cell type present throughout pregnancy; these cells also differ in their gene expression profile and metabolic capability. Heterogeneous cell types can influence exposure-related metabolic gene expression and may confer cell-specific toxicity that cannot be reflected accurately in the present study’s sampling methodology. Third, because of limited sample size and high cost of RNAseq, a pathway-driven approach was employed to reduce multiple comparison. This may have led to bias and/or exclusion of genes that may have proven to also be significantly associated with PM exposure in downstream analyses. Lastly, PM2.5 data was bio-monitored at 200m x 200m resolution only at the residential address, which may introduce misclassification of exposure and is not as accurate as personal air monitoring. Perhaps future studies can implement personal air monitoring for more accurate exposure assessment.
5. Conclusion
Overall, our study indicates that even low levels of PM2.5 exposure, below the National Ambient Air Quality Standards (NAAQS), may impact the expression of placental genes involved in metabolism; and such effects may be in a sex-specific manner. This study adds to the weight of evidence relating the effects of air pollution on the placenta as a potential pathway of fetal programming. These changes may have relevance to understanding how the fetus can be predisposed to altered growth in utero, increased susceptibility to postnatal and possibly even later life metabolic diseases, but confirmatory studies are warranted.
Supplementary Material
Acknowledgements
The authors thank the RICHS team for collecting and processing all biospecimens, the Chen laboratory members (Drs. James Wetmur, Vasily Aushev, Yula Ma and Qian Li) for their lab support and data feedback, NYU Langone Health Departments of Environmental Medicine and Ob/Gyn professors (Drs. Frederick Naftolin, Judith Zelikoff, Alan Arslan) for their support and feedback on the project and the Mount Sinai Dean’s Core Facility (Drs. Nada Marjanovic and Venugopalan Nair) for the PCR experiments.
Funding
This study was supported by the NIEHS-NIH R24ES028507 fund for RICHS. The Mount Sinai Transdisciplinary Center on Early Environmental Exposures NIEHS P30ES023515 and P30ES109776. The NIEHS 2T32ES007324-16 funded K. Kaur. The authors retain responsibility for the content, it does not necessarily the official views of the National Institutes of Health.
Abbreviations:
- AGA
appropriate for gestational age
- AOD
aerosol optical depth
- BP
biological process
- cDNA
complementary deoxyribonucleic acid
- ΔΔCt
delta delta cycle threshold
- EPA
Environmental Protection Agency
- FDR
false discovery rate
- GO
gene ontology
- LGA
large for gestational age
- LIMMA
linear models for microarray data
- logCPM
log counts per million
- NAAQS
National Ambient Air Quality Standards
- PM2.5
particular matter <2.5 microns
- RI
Rhode Island
- RICHS
Rhode Island Child Health Study
- RNAseq
ribonucleic acid sequencing
- RT-qPCR
real time quantitative polymerase chain reaction
- SGA
small for gestational age
- WHO
World Health Organization
Footnotes
Declaration of competing interest
The authors declare that they do not have any known competing financial interests nor personal relationships that could have influenced the research reported herein.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alderete TL, Song AY, Bastain T et al. (2018). Prenatal traffic-related air pollution exposures, cord blood adipokines and infant weight. Pediatric obesity, 13, 6, 348–356. 10.1111/ijpo.12248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner M, Ball C, Blake J et al. (2000). Gene Ontology: tool for the unification of biology. Nat Genet, 25, 25–29. 10.1038/75556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellone S, Rapa A, Petri A et al. (2004). Leptin levels as function of age, gender, auxological and hormonal parameters in 202 healthy neonates at birth and during the first month of life. J Endocrinol Invest, 27, 18–23. 10.1007/BF03350905 [DOI] [PubMed] [Google Scholar]
- Bosio PM, McKenna PJ, Conroy R et al. (1999). Maternal central hemodynamics in hypertensive disorders of pregnancy. Obstet Gynecol, 94, 978–984. 10.1016/S0029-7844(99)00430-5 [DOI] [PubMed] [Google Scholar]
- Bové H, Bongaerts E, Slenders E et al. (2019). Ambient black carbon particles reach the fetal side of human placenta. Nat Commun, 10, 3866. 10.1038/s41467-019-11654-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauer M, Freedman G, Frostad J et al. (2016). Ambient Air Pollution Exposure Estimation for the Global Burden of Disease 2013. Environ Sci Technol, 50, 79–88. 10.1021/acs.est.5b03709 [DOI] [PubMed] [Google Scholar]
- Brunst KJ, Sanchez-Guerra M, Chiu YM et al. (2018). Prenatal particulate matter exposure and mitochondrial dysfunction at the maternal-fetal interface: Effect modification by maternal lifetime trauma and child sex. Environment International, 112, 49–58. 10.1016/j.envint.2017.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caiazzo F, Ashok A, Waitz IA et al. (2013). Air pollution and early deaths in the United States. Part I: Quantifying the impact of major sectors in 2005. Atmospheric Environment, 79, 198–208. 10.1016/j.atmosenv.2013.05.081 [DOI] [Google Scholar]
- Carlson M (2019). GO.db: A set of annotation maps describing the entire Gene Ontology. R package version 3.8.2. [Google Scholar]
- Carvalho-Silva D, Pierleoni A, Pignatelli M et al. (2019). Open Targets Platform: new developments and updates two years on, Nucleic Acids Research, 47, D1, 08, D1056–D1065. 10.1093/nar/gky1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu YM, Hsu HL, Wilson A et al. (2017). Prenatal particulate air pollution exposure and body composition in urban preschool children: Examining sensitive windows and sex-specific associations. Environl Res, 158, 798–805. 10.1016/j.envres.2017.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemente DBP, Casas M, Janssen BG et al. (2017). Prenatal ambient air pollution exposure, infant growth and placental mitochondrial DNA content in the INMA birth cohort. Envrion Res, 157, 96–102. 10.1016/j.envres.2017.05.018 [DOI] [PubMed] [Google Scholar]
- Dall’Olio F (1990). Sialyltransferases of developing rat brain. Glycoconjugate J, 7, 301–310. 10.1007/BF01073374 [DOI] [PubMed] [Google Scholar]
- de Melo JO, Soto SF, Katayama IA et al. (2015). Inhalation of fine particulate matter during pregnancy increased IL-4 cytokine levels in the fetal portion of the placenta. Toxicol Lett, 232, 2, 475–480. 10.1016/j.toxlet.2014.12.001 [DOI] [PubMed] [Google Scholar]
- Demirkan A, van Duijn CM, Ugocsai P, et al. (2012). Genome-wide association study identifies novel loci associated with circulating phospho- and sphingolipid concentrations. PLoS genetics, 8(2), e1002490. 10.1371/journal.pgen.1002490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deyssenroth MA, Rosa MJ, Eliot MN, Kelsey KT, Kloog I, Schwartz JD et al. (2021). Placental gene networks at the interface between maternal PM2.5 exposure early in gestation and reduced infant birthweight. Environ. Res 199: 111342. DOI: 10.1016/j.envres.2021.111342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deyssenroth MA, Peng S, Hao K, Lambertini L, Marsit CJ, Chen J (2017). Whole-transcriptome analysis delineates the human placenta gene network and its associations with fetal growth. BMC Genomics. 18(1):520. Published 2017 Jul 10. DOI: 10.1186/s12864-017-3878-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durinck S, Spellman P, Birney E and Huber W (2009). Mapping identifiers for the integration of genomic datasets with the R/Bioconductor package biomaRt. Nature Protocols, 4, pp. 1184–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebisu K, Bell ML (2012). Airborne PM2.5 chemical components and low birth weight in the northeastern and mid-Atlantic regions of the United States. Environ health perspectives. 120(12):1746–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Environmental Protection Agency (2012). Revised Air Quality Standards for Particulate Pollution and Updates to the Air Quality Index (AQI). https://www.epa.gov/sites/production/files/2016-04/documents/2012_aqi_factsheet.pdf
- Fajersztajn L, Veras MM (2017). Hypoxia: From Placental Development to Fetal Programming. Birth Defects Res. 109(17): 1377–1385. DOI: 10.1002/bdr2.1142. [DOI] [PubMed] [Google Scholar]
- Feng HC, Tsao SW, Ngan HYS, Xue WC, Kwan HS, Siu MKY, et al. , (2008). Overexpression of prostate stem cell antigen is associated with gestational trophoblastic neoplasia. Histopathology. 52: 167–174. DOI: 10.1111/j.1365-2559.2007.02925.x. [DOI] [PubMed] [Google Scholar]
- Fenton TR and Kim JH (2013). A systemic review and meta-analysis to revise the Fenton growth chart for pre-term infants. BMC Pediatrics. 13(59). DOI: 10.1186/1471-2431-13-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowden AL, Ward JW, Woods FPB, Forhead AJ, Constancia M (2006). Programming placental nutrient transport capacity. J Physiol. 572: 5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin M, Koutrakis P and Schwartz P (2013). The role of particle composition on the association between PM2.5 and mortality. Epidemiology. 19(5): 680–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GO Term Mapper - The Generic GO Term Mapper. http://go.princeton.edu/cgi-bin/GOTermMapper (Accessed: March 2020).
- Gong S, Johnson MD, Dopierala J, Gaccioli F, Sovio U, Constância M, Smith GC, Charnock-Jones DS. Genome-wide oxidative bisulfite sequencing identifies sex-specific methylation differences in the human placenta. Epigenetics. 2018;13(3):228–239. doi: 10.1080/15592294.2018.1429857. Epub 2018 Feb 21. PMID: 29376485; PMCID: PMC5989156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez TL, Sun T, Koeppel AF, Lee B, Wang ET, Farber CR, Rich SS, Sundheimer LW, Buttle RA, Chen YI, Rotter JI, Turner SD, Williams J 3rd, Goodarzi MO, Pisarska MD. Sex differences in the late first trimester human placenta transcriptome. Biol Sex Differ. 2018. Jan 15;9(1):4. doi: 10.1186/s13293-018-0165-y. PMID: 29335024; PMCID: PMC5769539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gostner JM, Zeisler J, Alam MT, Gruber P, Fuchs D, Becker K, et al. , (2016). Cellular reactions to long-term volatile organic compound (VOC) exposures. Sci Rep. 6: 37842. DOI: 10.1038/srep37842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HGNC Database, HUGO Gene Nomenclature Committee (HGNC), European Molecular Biology Laboratory, European Bioinformatics Institute (EMBL-EBI), Wellcome Genome Campus, Hinxton, Cambridge CB10 1SD, United Kingdom: www.genenames.org. (Accessed: March 2020). [Google Scholar]
- Howerton CL, Bale TL. Targeted placental deletion of OGT recapitulates the prenatal stress phenotype including hypothalamic mitochondrial dysfunction. Proc Natl Acad Sci U S A. 2014. Jul 1;111(26):9639–44. doi: 10.1073/pnas.1401203111. Epub 2014 Jun 16. PMID: 24979775; PMCID: PMC4084439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu HH, Chiu YH, Coull BA, Kloog I, Schwartz J, Lee A, Wright RO, Wright RJ (2015). Prenatal Particulate Air Pollution and Asthma Onset in Urban Children. Identifying Sensitive Windows and Sex Differences. American Journal of Respiratory and Critical Care Medicine. 192(9) DOI: 10.1164/rccm.201504-0658OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappil MA, Green BB, Armstrong DA, Sharp AJ, Lambertini L, Marsit CJ, & Chen J (2015). Placental expression profile of imprinted genes impacts birth weight. Epigenetics. 10(9), 842–849. 10.1080/15592294.2015.1073881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsley SL, Eliot MN, Glazer K, Awad YA, Schwartz JD, Savitz DA, Kelsey KT, Marsit CJ, Wellenius GA (2017a). Maternal ambient air pollution, preterm birth and markers of fetal growth in Rhode Island: results of a hospital-based linkage study. J Epidemiol Community Health. 71(12):1131–1136. DOI: 10.1136/jech-2017-208963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsley SL, Deyssenroth MA, Kelsey KT, Awad YA, Kloog I, Schwartz JD, Lambertini L, Chen J, Marsit CJ, Wellenius GA (2017b). Maternal residential air pollution and placental imprinted gene expression. Environment International. 108:204–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsley SL, Eliot MN, Whitsel EA, Huang YT, Kelsey KT, Marsit CJ, Wellenius GA (2016). Maternal residential proximity to major roadways, birth weight, and placental DNA methylation. Environment International. 92-93, 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloog I, Chudnovsky AA, Just AC, Nordio F, Koutrakis P, Coull BA, Lyapustin A, Wang Y, Schwartz J (2014). A new hybrid spatio-temporal model for estimating daily multi-year PM2.5 concentrations across northeastern USA using high resolution aerosol optical depth data. Atmos. Environ 95, 581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshmanan A, Chiu YHM, Coull BA, Just AC, Maxwell SL, Schwartz J, Gryparis A, Kloog I, Wright RJ, Wright RO (2015). Associations between prenatal traffic-related air pollution exposure and birth weight: Modification by sex and maternal pre-pregnancy body mass index. Environ Res. 137: 268–277. DOI: 10.1016/j.envres.2014.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepeule J, Laden F, Dockery D, Schwartz J (2012). Chronic exposure to fine particles and mortality: an extended follow-up of the Harvard Six Cities study from 1974 to 2009. Environ health perspectives. 120(7):965–970. DOI: 10.1289/ehp.1104660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesseur C, Armstrong DA, Paquette AG, Li Z, Padbury JF, Marsit CJ. Maternal obesity and gestational diabetes are associated with placental leptin DNA methylation. Am J Obstet Gynecol. 2014;211(6):654.e1–654.e6549. doi: 10.1016/j.ajog.2014.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wang L, Wang F, Li C (2016). Effect of Fine Particulate Matter (PM2.5) on Rat Placenta Pathology and Perinatal Outcomes. Med Sci Monit. 22: 3274–3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ and Schmittgen TD (2001). Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods. 25(4): 402–408. [DOI] [PubMed] [Google Scholar]
- Long JZ, Cisar JS, Milliken D, Niessen S, Wang C, Trauger SA, Siuzdak G, Cravatt BF (2011). Metabolomics annotates ABHD3 as a physiological regulator of medium-chain phospholipids. Nat Chem Biol. 7(11): 763–765. DOI: 10.1038/nchembio.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao G, Nachman RM, Sun Q, Zhang X, Koehler K, Chen Z, Hong X, Wang G, Caruso D, Zong G, Pearson C, Ji H, Biswal S, Zuckerman B, Wills-Karp M, Wang X (2017). Individual and Joint Effects of Early-Life Ambient Exposure and Maternal Prepregnancy Obesity on Childhood Overweight or Obesity. Environ health perspectives. 125(6): 067005. DOI: 10.1289/EHP261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsit CJ, Maccani MA, Padbury JF, Lester BM (2012). Placental 11-beta hydroxysteroid dehydrogenase methylation is associated with newborn growth and a measure of neurobehavioral outcome. PLoS One. 7, e33794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquette AG, Lester BM, Koestler DC, Lesseur C, Armstrong DA, Marsit CJ (2014). Placental FKBP5 genetic and epigenetic variation is associated with infant neurobehavioral outcomes in the RICHS cohort. PLoS One. 2014;9(8):e104913. DOI: 10.1371/journal.pone.0104913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Garcia V, Fineberg E, Wilson R, Murray A, Mazzeo C, Tudor C et al. , (2018). Placentation defects are highly prevalent in embryonic lethal mouse mutants. Nature. 555(7697): 463–468. DOI: 10.1038/nature26002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2019). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/ [Google Scholar]
- Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK (2015). “limma powers differential expression analyses for RNA-sequencing and microarray studies.” Nucleic Acids Research, 43(7), e47. DOI: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandman CA, Glynn LM, Davis EP. Is there a viability-vulnerability tradeoff? Sex differences in fetal programming. J Psychosom Res. 2013;75(4):327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandovici I, Hoelle K, Angiolini E, Constancia M (2012). Placental adaptations to the maternal-fetal environment: implications for fetal growth and developmental programming. Reprod BioMed Online. 25: 68–89. [DOI] [PubMed] [Google Scholar]
- Shamy M, Alghamdi M, Khoder MI, Mohorjy AM, Alkhatim AA, Alkhalaf AK, Brocato J, Chen LC, Thurston GD, Lim CC, Costa M (2018). Association between Exposure to Ambient Air Particulates and Metabolic Syndrome Components in a Saudi Arabian Population. Int J Environ Res Public Health. 15(1) pii:E27. DOI: 10.3390/ijerph15010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada A, Kawamura N, Okajima M, Kaewamatawong T, Inoue H, Morita T (2006). Translocation pathway of the intratracheally instilled ultrafine particles from the lung into the blood circulation in the mouse. Toxicol Pathol. 34(7):949–957. DOI: 10.1080/01926230601080502. [DOI] [PubMed] [Google Scholar]
- Sonagra AD, Biradar SM, K D, & Murthy D S J (2014). Normal pregnancy- a state of insulin resistance. Journal of clinical and diagnostic research: JCDR, 8(11), CC01–CC3. 10.7860/JCDR/2014/10068.5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto SF, Melo JO, Marchesi GD, Lopes KL, Veras MM, Oliveira IB, Souza RM, de Castro I, Furukawa LNS, Saldiva PHN, Heimann JC (2017). Exposure to fine particulate matter in the air alters placental structure and to renin-angiotensin system. PLoS One. 12(8): e0183314. DOI: 10.1371/journal.pone.0183314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Yue P, Deiuliis J, Lumeng C, Kampfrath T, Mikolaj M, Caj Y, Ostrowsky M, Parthasarathy S, Brook R, et al. , (2009). Ambient air pollution exaggerates adipose inflammation and insulin resistance in a mouse model of diet-induced obesity. Circulation. 119, 538–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatsu J, Tanaka G, Segawa K, Suzuki J, Nagata S, Nakayama K, Shin H-W (2014). Phospholipid Flippase Activites and Substrate Specificities of Human Type IV P-type ATPases Localized to the Plasma Membrane. The Journal of Biological Chemistry. 289(48): 33543033556. DOI: 10.1074/jbc.M114.593012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G, Brown AL, Brown JM (2014). In vivo metabolite profiling as a means to identify uncharacterized lipase function: recent success stories within the alpha beta hydrolase domain (ABHD) enzyme family. Biochem Biphys Acta. 1841(8): 1097–1101. DOI: 10.1016/j.bbalip.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Mark V, Ghiboub M, Marsman C, Zhao J, van Dijk R, Hiralall J, Ho-Mok K, Castricum Z, de Jonge W, Alferink R, Paulusma (2017). Phospholipid flippases attenuate LPS-induced TLR4 signaling by mediating endocytic retrieval of Toll-like receptor 4. Cell Mol. Life Sci 74: 715–730. DOI: 10.1007/s00018-016-2360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veras MM, Damaceno-Rodrigues NR, Caldini EG, Maciel Ribeiro AA, Mayhew TM, Saldiva PH, Dolhnikoff M (2008). Particulate urban air pollution affects the functional morphology of mouse placenta. Biol Reprod. 79(3): 578–548. DOI: 10.1095/biolreprod.108.069591. [DOI] [PubMed] [Google Scholar]
- Virejns K, Winckelmans E, Tsamou M, Baeyens W, De Boever P, Jennen D, de Kok TM, Den Hond E, Lefebvre W, Plusquin M, Reynders H, Schoeters G, Van Larebeke N, Vanpoucke C, Kleinjans J, Nawrot TS (2017). Sex-Specific Associations between Particulate Matter Exposure and Gene Expression in Independent Discovery and Validation Cohorts of Middle-Aged Men and Women. Environmental health perspectives, 125(4): 660–669. DOI: 10.1289/EHP370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick P, Malek A, Manser P, Meili D, Maeder-Althaus X, Diener L, Diener PA, Zisch A, Krug HF, & von Mandach U (2010). Barrier capacity of human placenta for nanosized materials. Environmental health perspectives, 118(3), 432–436. DOI: 10.1289/ehp.0901200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M, Morganti AA, Zervoudakis I et al. (1980). Blood pressure, the renin-aldosterone system and sexsteroids throughout normal pregnancy. Am J Med. 68:97–104. [DOI] [PubMed] [Google Scholar]
- Winckelmans E, Cox B, Martens E, Fierens F, Nemery B, Nawrot TS (2015). Fetal growth and maternal exposure to particulate air pollution – More marked effects at lower exposure and modification by gestational duration. Environ Res. 140: 611–618. DOI: 10.1016/j.envres.2015.05.015. [DOI] [PubMed] [Google Scholar]
- Xia B, Wang Y, Wang X, Wu J, Song Q, Sun Z, Zhang Y (2018). In utero and lactational exposure of DEHP increases the susceptibility of prostate carcinogenesis in male offspring through PSCA hypomethylation. Toxicology Letters. 292: 78–84. DOI: 10.1016/j.toxlet.2018.04.022. [DOI] [PubMed] [Google Scholar]
- Xu J, Zhang W, Lu Z, Zhang F, Ding W (2017). Airborne PM2.5-Induced Hepatic Insulin Resistance by Nrf2/JNK-Mediated Signaling Pathway. Int J Environ Res Public Health. 14(7): 787. DOI: 10.3390/ijerph14070787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang BY, Qian ZM, Li S, Fan S, Chen G, Syberg KM, Xian H, Wang SQ, Ma H, Chen DH, Yang M, Liu KK, Zeng XW, Hu LW, Guo Y, Dong GH (2018). Long-term exposure to ambient air pollution (including PM1) and metabolic syndrome: The 33 Communities Chinese Health Study (33CCHS). Environ Res. 164:204–211. DOI: 10.1016/j.envres.2018.02.029. [DOI] [PubMed] [Google Scholar]
- Yue H, Ji X, Ku T, Li G, Sang N (2020). Sex difference in bronchopulmonary dysplasia of offspring in response to maternal PM2.5 exposure. J Hazard Mater. 5; 389:122033. [DOI] [PubMed] [Google Scholar]
- Yuen RKC, Penaherrera MS, von Dadelszen P, McFadden DE, Robinson WP (2010). DNA methylation profiling of human placentas reveals promoter hypomethylation of multiple genes in early-onset preeclampsia. Eur J Hum Genet. 18(9): 1006–1012. DOI: 10.1038/ejhg.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Wang F and Hou M (2015). Expression of stem cell markers nanog and PSCA in gastric cancer and its significance. Oncology Letters. 11: 442–448. DOI: 10.3892/ol.2015.3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.