SUMMARY
Neurological disorders encompass an extremely broad range of conditions, including those that present early in development and those that progress slowly or manifest with advanced age. Although these disorders have distinct underlying etiologies, the activation of shared pathways, e.g., integrated stress response (ISR) and the development of shared phenotypes (sleep deficits) may offer clues toward understanding some of the mechanistic underpinnings of neurologic dysfunction. While it is incontrovertibly complex, the relationship between sleep and persistent stress in the brain has broad implications in understanding neurological disorders from development to degeneration. The convergent nature of the ISR could be a common thread linking genetically distinct neurological disorders through the dysregulation of a core cellular homeostasis pathway.
Keywords: Sleep, Neurodevelopmental disorders, Neurodegenerative disorders, Fragile X syndrome, Autism spectrum disorder, Alzheimer’s disease, Integrated stress response, DNA damage response
Introduction
The brain is subject to unique stresses. Post-mitotic neurons are constrained in their ability to undergo cell death and replenish their population. The central neuronal network is an extremely metabolically demanding system, requiring approximately 20% of total basal oxygen consumption in adult humans, and as much as 50% in children [1–3]. This demand is dependent on mitochondrial oxidative phosphorylation, which supplies much of the energy and maintains calcium and redox homeostasis to support key processes including neurogenesis, cytoskeleton assembly, signal transmission, and plasticity [4–9]. Thus, the brain has a highly developed mitochondrial network, which may function to support the intricate synaptic networks and signal transmission necessary to sustain brain function [10]. This high metabolic load also produces high levels of reactive oxygen species (ROS) and reactive nitrogen species (RNS) as a biproduct of ATP synthesis. While the brain produces significant levels of antioxidants, stress and genetics can perturb the balance of oxidation and reduction, which along with other susceptibility features in the brain, increases the risk of persistent oxidative damage [11]. Together, these factors contribute to a brain environment that is rife with free radicals, which can lead to the accumulation of misfolded proteins and persistent DNA damage [12–15].
In post-mitotic cells such as neurons, constant repair is required since cell replacement is not an option for maintaining cellular function in the brain. Sleep likely plays a critical role during development and aging in reducing the metabolic demand of the brain [15,16] and repair of wake-induced cellular damage [17,18]. Sleep alters the translational profile of the brain to facilitate synaptic normalization and homeostasis [19–21]. Furthermore, sleep increases the clearing of metabolites accumulated during wake including misfolded proteins and proteolytic byproducts such as amyloid beta (Aβ) [22]. Wake-mediated free radicals also induce DNA lesions, which comprise a major class of DNA damage in neurons, leading to base pair modification and double-stranded DNA breakage (DSB) [13,18]. Sleep plays a direct role in repairing this DNA damage. The repair of enriched wake DSBs and gamma-irradiation induced DSBs is delayed or inhibited by sleep deprivation, with repair resuming upon the restoration of sleep [18]. In a study of overnight on-call doctors, expression of several key DNA repair genes was decreased after acute sleep deprivation [23]. Those genes include 8-oxoguanine glycosylase (OGG1), X-ray repair cross complementing 1 (XRCC1), and excision repair cross-complementing group 1 (ERCC1) in the base excision repair (BER) pathway, the primary mechanism for repairing oxidative base pair modification in neurons [24–26]. Furthermore, the study demonstrated that DNA breaks and oxidized purines were increased, and blood plasma antioxidant capacity was reduced, reflecting the role of sleep in DNA damage and repair [23].
Sleep deficiency and persistent oxidative stress leads to the accumulation of damage to proteins and DNA, which can further induce cellular stress [12–15]. Cells respond to stress through a versatile mechanism called the integrated stress response (ISR). The ISR is a signaling network found in all eukaryotic cells and is critical for cellular adaptation and homeostasis in response to external and internal stressors. Through the ISR, cells activate response programs to alleviate stress induced by misfolded proteins, DNA damage and metabolic pressure [27–30]. This includes the preferential activation of gene networks that repair and promote cell survival in the brain [31], as neurons must favor prosurvival solutions to stress. Wake is energy intensive and stressful [14,15,32,33]. Sleep provides a respite from wake and a time to activate homeostatic and repair mechanisms [18,19,22]. In fact, brain oxidation and the accumulation of DNA damage during wake play a role in triggering the induction of sleep to promote DNA repair [34–37]. Whether the ISR is functionally involved in the restorative function of sleep remains to be fully studied, however PERK signaling, a core feature of ISR activation, promotes sleep [38]. Parp1, a key factor in the initiation of DNA repair, also promotes sleep and the repair of DNA damage by inducing repair protein activity and chromosome mobility [36].
Metabolic stress and biomolecule damage is increased under conditions of sleep fragmentation [15,17,39], and inefficient and insufficient sleep are common underlying features of many neurological disorders [40–50]. Neurological disorders are highly comorbid with sleep abnormalities, suggesting that functions at the intersection of the ISR and sleep could contribute to the synaptic and behavioral deficits observed in these disorders. Despite the widely shared dysregulation of the ISR and sleep among neurological disorders, there is still little clarity on the mechanistic relationship between cellular stress and sleep dysregulation in neurological diseases. Evidence of persistent stress and stress-related damage to biomolecules along with the manifestation of sleep phenotypes is observed in neurological conditions arising by both genetic mutation and injury to the nervous system, underscoring the central nature of this relationship (Table 1). The goal of this review is to discuss our current understanding of the ISR and sleep, focusing on three neurological diseases (Alzheimer’s disease, autism spectrum disorder, and Fragile X syndrome) and propose future avenues of research to examine how these processes interact to contribute to the progression of neurological dysfunction (Fig. 1).
Table 1.
Sleep deficits and cellular stress in neurological disorders with various etiologies.
| Onset of delay | Onset of sleep difficulties | Prevalence of sleep deficits | Sleep phenotypes | Cellular stress and damage in the brain | Cognitive and behavioral phenotypes | |
|---|---|---|---|---|---|---|
|
| ||||||
| Neurodevelopment | ||||||
| Autism spectrum disorder (ASD) | 12–18 months | 0–6 months [122] | 86% [88] | Insomnia, bedtime resistance, parasomnias, sleep disordered breathing, morning rise problems, daytime sleepiness, increased sleep latency, decreased sleep efficiency, decreased REM, increased late-stage NREM [88] | ER stress, altered expression of ER stress genes, accumulation of reactive oxygen species, decreased antioxidant capacity, lipid peroxidation, increased levels of 8-oxo-dG [72–76,80–84] | Restrictive and repetitive behaviors, avoiding physical contact, communication deficits, sometimes non-verbal, social interaction deficits [123] |
| Fragile X syndrome (FXS) | 12–16 months | ≤3 years [50] | 32% [50] | Increased sleep latency, sleep fragmentation, reduced REM duration, fewer REM bouts, disrupted NREM [50,124] | Decreased expression of DNA repair genes, elevated Aβ levels, elevated NADPH-oxidase activity, altered antioxidant activity, increased lipid and protein oxidation [93,98–101] | Cognitive impairment, hyperactivity, anxiety, social avoidance, hyperarousal to stimuli, attention deficits, increased risk of ASD [125] |
| Neurodegeneration | ||||||
| Alzheimer’s disease (AD) | ∼65 years | often precedes, risk factor (1.49-fold) [70] | 45% [126] | Insomnia, sleep fragmentation, sleep disordered breathing, disrupted circadian rhythms, excessive daytime sleepiness, reduced REM and NREM [44,70,127,128] | Cellular oxidative stress, ER stress, mitochondrial dysfunction, upregulation of BACE1, nuclear and mitochondrial DNA oxidation, reduced activity of base excision repair proteins [53–58,60,62–67] | Sundowning (agitation/confusion beginning around dusk), dementia, memory loss, impaired communication, disorientation/confusion, poor judgement, behavioral changes, difficulty swallowing, speaking, and walking [52] |
| Parkinson’s disease (PD) | ∼65–70 years | RBD onset often precedes PD (12.7 ± 7.3 years) [129] | 90% [130] | REM sleep behavior disorder (RBD), daytime sleepiness, insomnia, restless leg syndrome, decreased total sleep time, decreased sleep efficiency, decreased NREM and REM, increased wake time after sleep onset, increased REM latency, sleep apnea [130] | Oxidative stress, elevated 8-oxo-G, abasic sites, and nuclear DNA strand breaks, persistent mtDNA abasic sites, impaired mitochondrial complex I, reduced ATP synthesis, increased ROS production, increased mitochondrial mutations and defective mitochondrial repair pathways, decreased GSH levels [131–133] | Problems with movement (tremor, rigidity, bradykinesia, postural instability), dementia, depression, sensory dysfunction, cognitive changes, behavioral changes, autonomic dysfunction [134] |
| Neurological injury | ||||||
| Traumatic brain injury (TBI) | - | - | 30–70% [135] | Sleep apnea, excessive daytime sleepiness, circadian rhythm misalignment, sleep-wake disturbances, fatigue, insomnia, hypersomnia, REM behavior disorder [135–138] | Oxidative stress, ER stress, elevated reactive oxygen species, neuroinflammation, disrupted brain energy metabolism, lipid peroxidation, impaired energy homeostasis, increased Aβ, BACE1, and APP [139–141] | Centralized pain, headaches, negative mood and emotional impacts, depression, anxiety, memory impairment, increased risk of neurodegenerative disease [138,142] |
| Stroke | - | Risk factor and outcome [143] | 20–69% (insomnia) [144] | Insomnia, sleep disordered breathing, circadian rhythm dysfunctions, sleep-related movement disorders, decreased REM, prolonged REM latency, decreased NREM, reduced total sleep time, lower sleep efficiency [145–147] | Excessive ROS production, decreased ROS scavenging (decreased SODs, CATs, GPx, and glutathione), depletion of cellular energy, inflammation, DNA damage (apurinic/apyrimidinic sites, oxidative base modifications, singlestrand breaks, and double strand breaks) [148–150] | Depression, anxiety, impaired mobility, cognition and memory, and speaking, emotional difficulties, social isolation, fatigue [151] |
Abbreviations: ROS, reactive oxygen species; REM, rapid eye movement; NREM, non-REM; RBD, REM sleep behavior disorder; ER, endoplasmic reticulum; Aβ, Amyloid beta; GSH, glutathione; BACE1, Beta-secretase 1; APP, Amyloid beta precursor protein; SOD, superoxide dismutase; CAT, catalase; GPx, glutathione peroxidase.
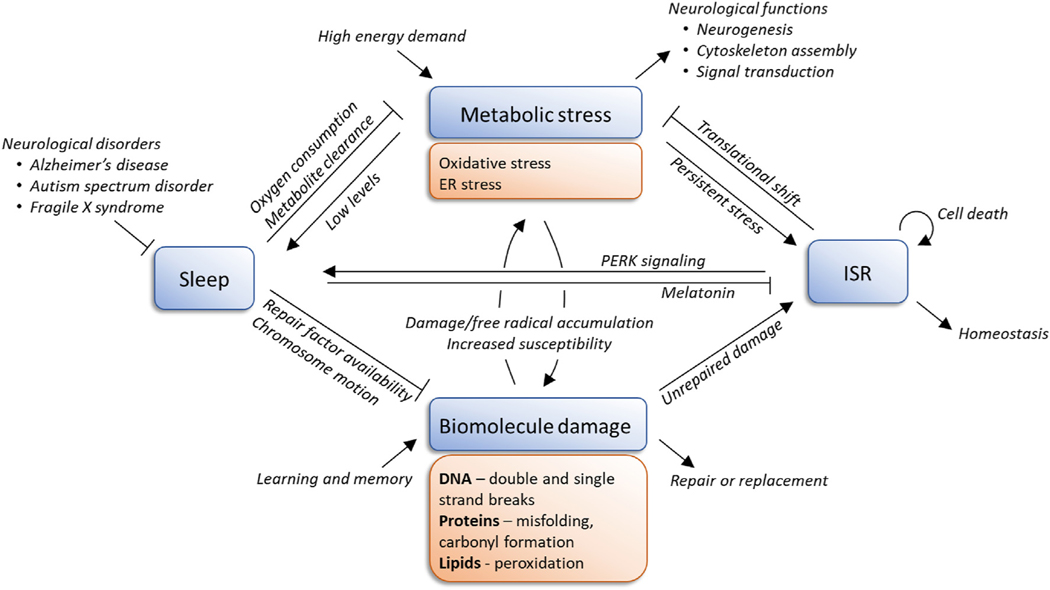
Fig. 1.
A proposed model of the relationship between sleep and the integrated stress response (ISR), drawn from observations described in the literature. Sleep deficiency, a common phenotype among neurological disorders, may lead to persistent activation of the stress response through these pathways, driving a positive feedback loop of stress and damage in the brain.
Alzheimer’s disease
Aging may be associated with a mild decline in mental acuity, however significant cognitive decline or memory loss is not a typical feature of healthy aging. Alzheimer’s disease (AD) is a progressive disorder, which often initially presents in older adults and worsens with age. AD is the most common cause of dementia, and the fifth leading cause of death in adults over 65 [51]. Great strides have been made in understanding the etiology and progression of AD, however our knowledge is still incomplete and our efforts to slow AD progression have yielded little success. The involvement of the ISR in neurodegeneration is widely supported and activation of the ISR has been described in both animal models [52–55] of AD as well as in brain tissue from individuals with AD [52,54–57]. An abundance of oxidative stress, ER stress, and mitochondrial dysfunction are well documented in AD. This cellular oxidative stress in AD likely contributes to accumulation of protein and DNA damage that feeds back into ISR activation leading to persistent ISR activation in AD. Among the genes that are translationally upregulated by the ISR is beta-secretase 1 (BACE1), which has important implications in a variety of neurological and neurodegenerative diseases [52,54]. BACE1 is involved in the initiation of the amyloidogenic pathway and the buildup of Aβ, which is relevant to the pathogenesis of AD.
The awake brain operates at an elevated baseline of oxidative stress [3,11,58]. Elevated oxidative damage is highly implicated as a major contributor to cell death and the progression of AD potentially due to the pro-oxidative effect of Aβ accumulation [59], protein misfolding [60], and/or activation of the inflammatory response [61]. Concurrent with elevated levels of oxidative stress, increased oxidative damage, including nuclear and mitochondrial DNA oxidation is observed in the brain of individuals with AD [62–64]. Reduced activity of OGG1, Uracil-DNA glycosylase (UDG), and DNA polymerase beta (POLB), key factors in the BER pathway, is observed in the brains of AD patients, leading to a BER deficiency [65,66]. Additionally, BER function is impaired in individuals with amnestic mild cognitive impairment, which represents a transitional phase between normal aging and the development of AD [65]. Both nuclear and mitochondrial DNA oxidative damage is apparent in this early stage of cognitive impairment, reflecting this BER deficiency [67,68], and suggesting that BER deficiency may be an early indicator in the development of AD. This impairment is observed in both the cerebellum and inferior parietal lobule, which correspond to the least and most highly affected regions of the brain, respectively [65], indicating that BER deficiency may be a susceptibility feature of the AD brain, rather than directly contributing to neuronal cell death, which is not observed in the cerebellum.
Even in normal aging, sleep quantity and quality progressively decline with age and the association between sleep disturbances, cognitive decline, and the risk of developing dementia is widely documented. In a meta-analysis, a random effect model predicted that individuals experiencing sleep disturbances had a 1.49-fold increased risk of developing AD [69]. Common sleep problems experienced with age include insomnia, sleep fragmentation, sleep disordered breathing, disrupted circadian rhythms, and excessive daytime sleepiness, which are exacerbated with the development and progression of AD [43,70,71]. The role of sleep in the underlying etiology of AD is not understood, however it is likely that the progressive development of sleep abnormalities contributes to, or at least aggravates the cognitive and behavioral characteristics of AD and neurodegeneration in general.
Autism spectrum disorder
Autism spectrum disorder (ASD) is a behaviorally defined group of neurodevelopmental disorders (NDD) characterized by social and cognitive deficits, and is increasingly prevalent, with an estimated one in 59 children diagnosed with ASD worldwide [72]. Because ASD is currently diagnosed based only on behavioral criteria, there is no single underlying etiology, and many genetically distinct disorders are grouped together based on shared or similar cellular dysfunctions and phenotypic presentations. Studies in children with ASD increasingly implicate oxidative stress, and its deleterious effects on brain and metabolic processes, as an important feature of ASD pathophysiology. ER stress is a major contributor to ISR activation in ASD. Several genetic models of ASD have been shown to induce ER stress [73–76], however because ASD is an extremely heterogeneous disorder, any single copy number variant or genetic mutation is found in only a small fraction of ASD cases. Using a multivariate model, a recent study showed that ASD status was able to predict mRNA levels of ER stress genes including PKR-like ER kinase (PERK), activating transcription factor 4 (ATF4), activating transcription factor 6 (ATF6), X-box binding protein 1 (XBP1), C/EBP homologous protein (CHOP), and inositol-requiring enzyme 1 (IRE1). Expression of these genes were significantly upregulated in the middle frontal gyrus in individuals with ASD. Additionally, ER stress genes were positively associated with stereotyped behavior classified by the Autism Diagnostic Interview-Revised (ADI-R) [77]. Not only does protein oxidation lead to the accumulation of damaged and unfolded proteins, but mistranslated or mutated proteins are also more susceptible to oxidation, thus eliciting a cycle of damage and stress [60].
The effects of oxidative stress are potentially more damaging during early development due to low glutathione levels and an immature antioxidant system [78–80]. Thus, children are more susceptible to damage from oxidative stress at typical levels, even before considering the elevated levels observed in children with NDD. Elevated oxidative stress has more recently become an area of interest in neurodevelopment and the development of intellectual disability disorders such as ASD. Accumulation of ROS, decreased antioxidant capacity, and damage to biomolecules have been demonstrated in the blood or postmortem brain tissue of children with ASD [81–85]. Children with ASD have an elevated concentration of 8-oxo-dG in the cerebellum and temporal cortex compared to typically developing children [81]. BTBR T+ Itpr3tf/J (BTBR) mice, which exhibit autism-like behavioral phenotypes, also exhibit elevated 8-oxo-dG levels in the cerebellum, which is inversely correlated with a 70–73% decrease in Oggl expression. Male BTBR mice also exhibit significantly more mitochondrial DNA damage [86]. It is important to note that BTBR mice differ genetically from their C57BL/6J controls, including single nucleotide polymorphisms between the strains in the coding and noncoding regions of Oggl. Genomic 8-oxo-dG enrichment is also observed in human post-mortem cerebellar samples, providing confidence in the translation of these results.
Abnormal sleep is a common feature of NDDs including ASD and related intellectual disability disorders [40,41,44–49]. In fact, sleep difficulties are included as a diagnostic criterion of many NDDs, and have been reported in 80% of children with intellectual disability in general [87] and 44–83% of children with ASD [44,88]. The types of sleep disturbances experienced by children with ASD are quite abundant and variable, potentially reflecting the underlying variability in etiology, however in one study, 86% of children were found to experience at least one sleep problem every day, with insomnia being the most commonly reported at 56% [89]. In addition to their prevalence, sleep problems in children with ASD also increase over time [46]. Children with ASD who are considered “poor sleepers” are more likely to have more affective problems and poorer social interactions than “good sleepers” or typically developing children [40]. These persistent sleep deficits and their correlation with behavior and social interactions suggest that sleep intervention has the potential to improve developmental outcomes of children with ASD.
Fragile X syndrome
Fragile X syndrome (FXS) is a neurodevelopmental disorder characterized by anxiety, social behavioral deficits, cognitive impairment, and sleep abnormalities. Individuals with FXS have an increased risk of developing attention deficit disorder (ADD) and ASD. In fact, FXS is the most common monogenic cause of inherited intellectual disability and ASD [90]. FXS is caused by the loss of fragile x mental retardation 1 (FMR1) gene expression. Its encoded protein, fragile x mental retardation protein (FMRP), has well-studied functions as a translational regulator [91], however its roles within the nucleus are much less understood. FMRP has recently been identified to have roles in gene expression and genome function, including the DNA damage response [92,93]. Individuals with FXS express important DNA repair genes at lower levels than their typically developing counterparts, including key BER factors OGGI and XRCC1 [94]. Some of these genes, and oxidative stress itself, are also implicated in trinucleotide expansion, the most common cause of FXS in humans [95–98].
The role of the ISR in the brain in FXS is an active area of investigation. Aβ levels are elevated in the brains of FXS patients and Fmr1 KO mice, which also exhibit elevated amyloid precursor protein (APP), thus BACE1 inhibitors have been proposed as a potential therapeutic strategy for FXS [99,100]. Fmr1-deficient mice exhibit elevated NADPH-oxidase activity, altered antioxidant activity, and increased oxidation of lipids and proteins. Interestingly, elevated ROS levels are developmentally dependent, detected at 4 months of age but not early (newborn and 1 mo old) or late (8 and 12 months old) in development [101,102]. FXS phenotypes and deficits at the molecular, cellular, and synaptic levels are also highly developmentally regulated [103–106]. Whether the interaction of sleep, the DNA damage response, and cellular stress plays a role in orchestrating the developmental trajectory of FXS poses an exciting question that remains to be addressed.
Sleep difficulties are a prevalent phenotype of children with FXS, and are detected very early in development, suggesting that sleep has the potential to contribute significantly to the manifestation or aggravation of other FXS phenotypes. According to a large caregiver survey, 32% of children with FXS suffer from sleep difficulties, with sleep latency and fragmented sleep being the most common difficulties [49]. Additionally, sleep problems in children with FXS were most highly reported to occur in early development, before the age of three (71% of males and 64% of females who experience sleep problems), with progressively diminishing reports of sleep problems with age (10% of males and 21% of females after the age of eleven) [49]. If sleep plays a role in the precipitation or aggravation of damage accumulation and persistent cellular stress response, these phenotypes may also exhibit a variable or developmentally dependent pattern in FXS. Consequently, this relationship presents the possibility that the alleviation of sleep deficits may have a significant impact on FXS developmental outcomes. Insufficient or poor sleep has far-reaching impacts on both physical and mental health including the development of metabolic disorders, cancer, cognition and learning deficits, and depression, which can in turn, negatively impact sleep [107–110].
Discussion
The relationship between sleep and activation of the ISR involves an interconnected web of bidirectional effects, which can escalate through feedback loops to drive neurological impairment. The ISR is an elaborate signaling network that integrates intrinsic and extrinsic stimuli to moderate the normal cellular stress of a functional organism. Thus, disruptions in a wide variety of pathways, which contribute to many different disorders, converge upon this central pathway. In this review, we have focused on ISR activation in the brain, which is particularly susceptible to oxidative stress. Persistent activation of the ISR in the brain has been demonstrated in neurodegenerative and neurodevelopmental disorders of diverse etiologies. We present sleep deficiency as another shared feature among these disorders, which can activate the ISR through the accumulation of unrepaired damage to biomolecules such as DNA and proteins.
Sleep deprivation induces ER stress through the unfolded protein response in the cortex [12,111,112]. Due to high metabolic demand during wake, extended wake likely leads to the depletion of ATP, inhibiting protein folding and leading the accumulation of misfolded proteins [12]. In addition to damage by ROS in the highly oxidative environment of the brain during sleep deprivation [23,113], the accumulation of aberrant proteins causes further protein oxidation, promoting a positive feedback loop of stress and damage, which may be exacerbated by sleep deficits [12,60]. The connection between sleep and the repair of DNA damage has only been demonstrated in recent years and there is still much that remains to be understood about how sleep promotes the maintenance of a healthy genome. Current evidence supports a role for sleep in mediating the levels and activity of key repair enzymes [18,23,36] and regulating chromosome dynamics [36,37] in the repair of DNA damage. Deficiencies in sleep-mediated repair or clearance of damaged biomolecules can potentially lead to elevated levels of cellular stress, persistently activating the ISR. While the effect of sleep deprivation on oxidative stress in the brain is not uniform [113–116], dysregulation of the ISR and the accumulation of biomolecular damage may shed light on the mechanisms underlying the development of cognitive impairment observed in many neurological disorders.
Although neurological disorders are heterogenous in genetic etiology, environmental interactions, and phenotypic presentation, sleep disruption is a pervasive feature central to disorders across the spectrum [41,69,117,118]. Sleep abnormalities were once considered a side effect rather than a central phenotype in these patients, however studies of disorders with known genetic etiologies, including FXS, have offered insight into the molecular basis of sleep physiology and homeostasis in maintaining a healthy and balanced synaptic network [118–121]. Impaired sleep manifests as a variety of deleterious stresses and dysfunction at the molecular, cellular, and synaptic levels.
Pharmacological modulation of the ISR has become an area of great interest in the treatment of a variety of neurological disorders given its central role in cellular homeostasis. Beneficial effects of both inhibitors and enhancers targeting different levels of the ISR pathway have been observed, especially in neurodegenerative disorders including AD [16]. However, unexpected and undesirable side-effects are of concern when targeting the ISR in heterogeneous cell populations. Additionally, modulation of the ISR must be carefully regulated, as cells must maintain the ability to respond efficiently to other sources of normal stress stimuli. Given the relationship between sleep deficiency and cellular stress, a combinatorial approach leveraging both pharmacological and sleep intervention therapies presents a potentially more moderate and adaptable mechanism for modulating the ISR in a wide variety of neurological disorders, while also providing the many benefits of healthy sleep. While we have focused on the brain in this review, sleep deficiency, biomolecule damage, and conditions of high cellular stress pose threats to the health of all systems in the body and gaining a deeper knowledge of these processes and their relationship will be invaluable to our understanding of human health.
Practice points
Sleep deficits are a prevalent phenotype among neurodevelopmental and neurodegenerative disorders, and in some cases sleep phenotypes are included as a diagnostic criterion.
The brain is a highly oxidative environment due to the high metabolic load, especially during waking activity.
Sleep deprivation may activate the ISR through the accumulation of biomolecular damage and prolonged cellular stress.
The expression and activity of DNA repair genes is downregulated under conditions of sleep deprivation.
Research agenda
Further elucidate the mechanisms underlying sleep-dependent regulation of biomolecule repair.
Examine how persistent activation of the integrated stress response in the brain may contribute to synaptic dysfunction in neurological disorders.
Assess combinatorial approaches leveraging pharmacological and sleep intervention as an adaptable therapeutic strategy for neurological disorders.
Acknowledgements
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development F32 HD103451, FRAXA Research Foundation, National Institute on Aging K01 AG061230, National Institute of Neurological Disorders and Stroke R01 NS104950, John Merck Fund, Brain and Behavior Research Foundation, and SUMS Seed Grant.
Abbreviations
- Aβ
Amyloid beta
- AD
Alzheimer’s disease
- ADD
Attention deficit disorder
- ADI-R
Autism Diagnostic Interview-Revised
- APP
Amyloid precursor protein
- ASD
Autism spectrum disorder
- ATF4
Activating transcription factor 4
- ATF6
Activating transcription factor 6
- BACE1
Beta-secretase 1
- BER
Base excision repair
- BTBR
BTBR T+ Itpr3tf/J
- CHOP
C/EBP homologous protein
- DSB
Double-strand break
- ERCC1
Excision repair cross-complementing group 1
- FMR1
Fragile X mental retardation 1
- FMRP
Fragile X mental retardation protein
- FXS
Fragile X syndrome
- IRE1
Inositol-requiring enzyme 1
- ISR
Integrated stress response
- NDD
Neurodevelopmental disorders
- OGG1
8-oxoguanine glycosylase
- PERK
PKR-like ER kinase
- POLB
Polymerase beta
- RNS
Reactive nitrogen species
- ROS
Reactive oxygen species
- UDG
Uracil-DNA glycosylase
- XBP1
X-box binding protein 1
- XRCC1
X-ray repair cross complementing 1
Footnotes
Conflicts of interest
The authors do not have any conflicts of interest to disclose.
References
* The most important references are denoted by an asterisk.
- [1].Kety SS, Schmidt CF. The nitrous oxide method for the quantitative determination of cerebral blood flow in man: theory, procedure and normal values. J Clin Invest 1948;27:476–83. 10.1172/JCI101994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kennedy C, Sokoloff L. An adaptation of the nitrous oxide method to the study of the cerebral circulation in children; normal values for cerebral blood flow and cerebral metabolic rate in childhood.J Clin Invest 1957;36: 1130–7. 10.1172/JCI103509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Clarke DD, Sokoloff L. Circulation and energy metabolism of the brain. Chem Fac Publ 1999;81:638–69. [Google Scholar]
- [4].Cheng A, Hou Y, Mattson MP. Mitochondria and neuroplasticity. ASN Neuro 2010;2:243–56. 10.1042/AN20100019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hall CN, Klein-Flügge MC, Howarth C, Attwell D. Oxidative phosphorylation, not glycolysis, powers presynaptic and postsynaptic mechanisms underlying brain information processing. J Neurosci 2012;32:8940–51. 10.1523/JNEUR0SCI.0026-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Seta K, Sansur M, Lajtha A. The rate of incorporation of amino acids into brain proteins during infusion in the rat. Biochim Biophys Acta 1973;294: 472–80. [DOI] [PubMed] [Google Scholar]
- [7].Dunlop DS, van Elden W, Lajtha A. A method for measuring brain protein synthesis rates in young and adult rats. J Neurochem 1975;24:337–44. 10.1111/j.1471-4159.1975.tb11885.x. [DOI] [PubMed] [Google Scholar]
- [8].Astrup J, Sørensen PM, S0rensen HR. Oxygen and glucose consumption related to Na+-K+ transport in canine brain. Stroke 1981;12:726–30. 10.1161/01.STR.12.6.726. [DOI] [PubMed] [Google Scholar]
- [9].Whittam R The dependence of the respiration of brain cortex on active cation transport. Biochem J 1962;82:205–12. 10.1042/bj0820205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Popov V, Medvedev NI, Davies HA, Stewart MG. Mitochondria form a filamentous reticular network in hippocampal dendrites but are present as discrete bodies in axons: a three-dimensional ultrastructural study. J Comp Neurol 2005;492:50–65. 10.1002/cne.20682. [DOI] [PubMed] [Google Scholar]
- [11].Halliwell B Role of free radicals in the neurodegenerative diseases: therapeutic implications for antioxidant treatment. Drugs Aging 2001;18: 685–716. 10.2165/00002512-200118090-00004. [DOI] [PubMed] [Google Scholar]
- [12].Naidoo N, Giang W, Galante RJ, Pack AI. Sleep deprivation induces the unfolded protein response in mouse cerebral cortex. J Neurochem 2005;92:1150–7. 10.1111/j.1471-4159.2004.02952.x. [DOI] [PubMed] [Google Scholar]
- [13].Suberbielle E, Sanchez PE, Kravitz AV, Wang X, Ho K, Eilertson K, et al. Physiologic brain activity causes DNA double-strand breaks in neurons, with exacerbation by amyloid-β. Nat Neurosci 2013;16:613–21. 10.1038/nn.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Nikonova EV, Naidoo N, Zhang L, Romer M, Cater JR, Scharf MT, et al. Changes in components of energy regulation in mouse cortex with increases in wakefulness. Sleep 2010;33:889–900. 10.1093/sleep/33.7.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zhang J, Zhu Y, Zhan G, Fenik P, Panossian L, Wang MM, et al. Extended wakefulness: compromised metabolics in and degeneration of locus ceruleus neurons. J Neurosci 2014;34:4418–31. 10.1523/JNEUROSCI.5025-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lee DY, Lee KS, Lee HJ, Kim DH, Noh YH, Yu K, et al. Activation of PERK signaling attenuates Aβ-mediated ER stress. PLoS One 2010;5:1–8. 10.1371/journal.pone.0010489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Andersen ML, Ribeiro DA, Bergamaschi CT, Alvarenga TA, Silva A, Zager A, et al. Distinct effects of acute and chronic sleep loss on DNA damage in rats. Prog Neuro-Psychopharmacol Biol Psychiatry 2009;33:562–7. 10.1016/j.pnpbp.2009.02.014. [DOI] [PubMed] [Google Scholar]
- [18]. Bellesi M, Bushey D, Chini M, Tononi G, Cirelli C. Contribution of sleep to the repair of neuronal DNA double-strand breaks: evidence from flies and mice. Sci Rep 2016;6:1–13. 10.1038/srep36804. *
- [19].Tononi G, Cirelli C. Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration. Neuron 2014;81:12–34. 10.1016/j.neuron.2013.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Noya SB, Colameo D, Brüning F, Spinnler A, Mircsof D, Opitz L, et al. The forebrain synaptic transcriptome is organized by clocks but its proteome is driven by sleep. Science 2019;366. 10.1126/science.aav2642. [DOI] [PubMed] [Google Scholar]
- [21].Seibt J, Dumoulin MC, Aton SJ, Coleman T, Watson A, Naidoo N, et al. Protein synthesis during sleep consolidates cortical plasticity in vivo. Curr Biol 2012;22:676–82. 10.1016/j.cub.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, et al. Sleep drives metabolite clearance from the adult brain. Sci Rep 2018;8:8868. 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. Cheung V, Yuen VM, Wong GTC, Choi SW. The effect of sleep deprivation and disruption on DNA damage and health of doctors. Anaesthesia 2019;74:434–40. 10.1111/anae.14533. *
- [24].Lindahl T Instability and decay of the primary structure of DNA. Nature 1993;362:709–15. 10.1038/362709a0. [DOI] [PubMed] [Google Scholar]
- [25].Slupphaug G, Kavli B, Krokan HE. The interacting pathways for prevention and repair of oxidative DNA damage. Mutat Res Fund Mol Mech Mutagen 2003;531:231–51. 10.1016/j.mrfmmm.2003.06.002. [DOI] [PubMed] [Google Scholar]
- [26].Barnes DE, Lindahl T. Repair and genetic consequences of endogenous DNA base damage in mammalian cells. Annu Rev Genet 2004;38:445–76. 10.1146/annurev.genet.38.072902.092448. [DOI] [PubMed] [Google Scholar]
- [27].Baker BM, Nargund AM, Sun T, Haynes CM. Protective coupling of mitochondrial function and protein synthesis via the eIF2α kinase GCN-2. PLoS Genet 2012;8. 10.1371/journal.pgen.1002760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell 2000;5:897–904. 10.1016/S1097-2765(00)80330-5. [DOI] [PubMed] [Google Scholar]
- [29].Legrand AJ, Poletto M, Pankova D, Clementi E, Moore J, Castro-Giner F, et al. Persistent DNA strand breaks induce a CAF-like phenotype in normal fibroblasts. Oncotarget 2018;9:13666–81. 10.18632/oncotarget.24446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30]. Clementi E, Inglin L, Beebe E, Gsell C, Garajova Z, Markkanen E. Persistent DNA damage triggers activation of the integrated stress response to promote cell survival under nutrient restriction. BMC Biol 2020;18: 1–15. *
- [31].Sun X, Liu J, Crary JF, Malagelada C, Sulzer D, Greene LA, et al. ATF4 protects against neuronal death in cellular Parkinson’s disease models by maintaining levels of parkin. J Neurosci 2013;33:2398–407. 10.1523/JNEUR0SCI.2292-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].De Vivo L, Nelson AB, Bellesi M, Noguti J, Tononi G, Cirelli C. Loss of sleep affects the ultrastructure of pyramidal neurons in the adolescent mouse frontal cortex. Sleep 2016;39:861–74. 10.5665/sleep.5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Attwell D, Gibb A. Neuroenergetics and the kinetic design of excitatory synapses. Nat Rev Neurosci 2005;6:841–9. 10.1038/nrn1784. [DOI] [PubMed] [Google Scholar]
- [34].Honda K, Komoda Y, Inoué S. Oxidized glutathione regulates physiological sleep in unrestrained rats. Brain Res 1994;636:253–8. 10.1016/0006-8993(94)91024-3. [DOI] [PubMed] [Google Scholar]
- [35]. Ikeda M, Ikeda-Sagara M, Okada T, Clement P, Urade Y, Nagai T, et al. Brain oxidation is an initial process in sleep induction. Neuroscience 2005;130: 1029–40. 10.1016/j.neuroscience.2004.09.05. *
- [36].Zada D, Sela Y, Matosevich N, Monsonego A, Lerer-Goldshtein T, Nir Y, et al. Parp1 promotes sleep, which enhances DNA repair in neurons. Mol Cell 2021;81:4979–4993.e7. 10.1016/j.molcel.2021.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zada D, Bronshtein I, Lerer-Goldshtein T, Garini Y, Appelbaum L. Sleep increases chromosome dynamics to enable reduction of accumulating DNA damage in single neurons. Nat Commun 2019;10. 10.1038/s41467-019-08806-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ly S, Lee DA, Strus E, Prober DA, Naidoo N. Evolutionarily conserved regulation of sleep by the protein translational regulator PERK. Curr Biol 2020;30:1639–1648.e3. 10.1016/j.cub.2020.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Everson CA, Henchen CJ, Szabo A, Hogg N. Cell injury and repair resulting from sleep loss and sleep recovery in laboratory rats. Sleep 2014;37: 1929–40. 10.5665/sleep.4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Malow BA, Marzec ML, McGrew SG, Wang L, Henderson LM, Stone WL. Characterizing sleep in children with autism spectrum disorders: a multidimensional approach. Sleep 2006;29:1563–71. [DOI] [PubMed] [Google Scholar]
- [41].Gibbs S, Wiltshire E, Elder D. Nocturnal sleep measured by actigraphy in children with Prader-Willi syndrome. J Pediatr 2013;162:765–9. 10.1016/j.jpeds.2012.09.019. [DOI] [PubMed] [Google Scholar]
- [42].Loewenstein RJ, Weingartner H, Gillin JC, Kaye W, Ebert M, Mendelson WB. Disturbances of sleep and cognitive functioning in patients with dementia. Neurobiol Aging 1982;3:371–7. 10.1016/0197-4580(82)90025-2. [DOI] [PubMed] [Google Scholar]
- [43].Vitiello MV, Prinz Pn, Williams DE, Frommlet MS, Ries RK. Sleep disturbances in patients with mild-stage Alzheimer’s disease. J Gerontol 1990;45:131–8. [DOI] [PubMed] [Google Scholar]
- [44].Patzold LM, Richdale AL, Tonge BJ. An investigation into sleep characteristics of children with autism and Asperger’s Disorder. J Paediatr Child Health 1998;34:528–33. 10.1046/j.1440-1754.1998.00291.x. [DOI] [PubMed] [Google Scholar]
- [45].Richdale AL. Sleep problems in autism: prevalence, cause, and intervention. Dev Med Child Neurol 1999;41:60–6. [DOI] [PubMed] [Google Scholar]
- [46].Sivertsen B, Posserud MB, Gillberg C, Lundervold AJ, Hysing M. Sleep problems in children with autism spectrum problems: a longitudinal population-based study. Autism 2012;16:139–50. 10.1177/1362361311404255. [DOI] [PubMed] [Google Scholar]
- [47].Limoges É, Mottron L, Bolduc C, Berthiaume C, Godbout R. Atypical sleep architecture and the autism phenotype. Brain 2005;128:1049–61. 10.1093/brain/awh425. [DOI] [PubMed] [Google Scholar]
- [48].Miano S, Bruni O, Leuzzi V, Elia M, Verrillo E, Ferri R. Sleep polygraphy in Angelman syndrome. Clin Neurophysiol 2004;115:938–45. 10.1016/j.clinph.2003.11.004. [DOI] [PubMed] [Google Scholar]
- [49]. Kronk R, Bishop EE, Raspa M, Bickel JO, Mandel DA, Bailey DB. Prevalence, nature, and correlates of sleep problems among children with fragile X syndrome based on a large scale parent survey. Sleep 2010;33:679–8. *
- [50].Galbiati A, Carli G, Hensley M, Ferini-Strambi L. REM sleep behavior disorder and Alzheimer’s disease: definitely no relationship? J Alzheim Dis 2018;63:1–11. 10.3233/JAD-171164. [DOI] [PubMed] [Google Scholar]
- [51].2021 Alzheimer’s disease facts and figures. Alzheimer’s Dementia 2021;17:327–406. 10.1002/alz.12328. [DOI] [PubMed] [Google Scholar]
- [52].O’Connor T, Sadleir KR, Maus E, Velliquette RA, Zhao J, Cole SL, et al. Phosphorylation of the translation initiation factor eIF2α increases BACE1 levels and promotes Amyloidogenesis. Neuron 2008;60:988–1009. 10.1016/j.neuron.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Page G, Rioux Bilan A, Ingrand S, Lafay-Chebassier C, Pain S, Perault Pochat MC, et al. Activated double-stranded RNA-dependent protein kinase and neuronal death in models of Alzheimer’s disease. Neuroscience 2006;139:1343–54. 10.1016/j.neuroscience.2006.01.047. [DOI] [PubMed] [Google Scholar]
- [54].Mouton-Liger F, Paquet C, Dumurgier J, Bouras C, Pradier L, Gray F, et al. Oxidative stress increases BACE1 protein levels through activation of the PKR-eIF2α pathway. Biochim Biophys Acta (BBA) - Mol Basis Dis 2012;1822:885–96. 10.1016/j.bbadis.2012.01.009. [DOI] [PubMed] [Google Scholar]
- [55].Kim H-S, Choi Y, Shin K-Y, Joo Y, Lee Y-K, Jung SY, et al. Swedish amyloid precursor protein mutation increases phosphorylation of eIF2α in vitro and in vivo. J Neurosci Res 2007;85:1528–37. 10.1002/jnr. [DOI] [PubMed] [Google Scholar]
- [56].Hoozemans JJM, Veerhuis R, Van Haastert ES, Rozemuller JM, Baas F, Eikelenboom P, et al. The unfolded protein response is activated in Alzheimer’s disease. Acta Neuropathol 2005;110:165–72. 10.1007/s00401-005-1038-0. [DOI] [PubMed] [Google Scholar]
- [57].Chang RCC, Wong AKY, Ng HK, Hugon J. Phosphorylation of eukaryotic initiation factor-2α (eIF2α) is associated with neuronal degeneration in Alzheimer’s disease. Neuroreport 2002;13:2429–32. 10.1097/00001756-200212200-00011. [DOI] [PubMed] [Google Scholar]
- [58].Cobley JN, Fiorello ML, Bailey DM. 13 reasons why the brain is susceptible to oxidative stress. Redox Biol 2018;15:490–503. 10.1016/j.redox.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Harris ME, Hensley K, Butterfield DA, Leedle RA, Carney JM. Direct evidence of oxidative injury produced by the Alzheimer’s β-Amyloid peptide (1–40) in cultured hippocampal neurons. Exp Neurol 1995;131:193–202. 10.1016/0014-4886(95)90041-1. [DOI] [PubMed] [Google Scholar]
- [60].Dukan S, Farewell A, Ballesteros M, Taddei F, Radman M, Nyström T. Protein oxidation in response to increased transcriptional or translational errors. Proc Natl Acad Sci U S A 2000;97:5746–9. 10.1073/pnas.100422497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Della Bianca V, Dusi S, Bianchini E, Dal Pra I, Rossi F. β-amyloid activates the O2/- forming NADPH oxidase in microglia, monocytes, and neutrophils. A possible inflammatory mechanism of neuronal damage in Alzheimer’s disease. J Biol Chem 1999;274:15493–9. 10.1074/jbc.274.22.15493. [DOI] [PubMed] [Google Scholar]
- [62].Gabbita SP, Lovell MA, Markesbery WR. Increased nuclear DNA oxidation in the brain in Alzheimer’s disease. J Neurochem 2002;71:2034–40. 10.1046/j.1471-4159.1998.71052034.x. [DOI] [PubMed] [Google Scholar]
- [63].Mecocci P, MacGarvey U, Beal MF. Oxidative damage to mitochondrial DNA is increased in Alzheimer’s disease. Ann Neurol 1994;36:747–51. 10.1002/ana.410360510. [DOI] [PubMed] [Google Scholar]
- [64].Lyras L, Cairns NJ, Jenner A, Jenner P, Halliwell B. An Assessment of oxidative damage to proteins, lipids, and DNA in brain from patients with Alzheimer’s disease. J Neurochem 2002;68:2061–9. 10.1046/j.1471-4159.1997.68052061.x. [DOI] [PubMed] [Google Scholar]
- [65]. Weissman L, Jo DG, Sørensen MM, de Souza-Pinto NC, Markesbery WR, Mattson MP, et al. Defective DNA base excision repair in brain from individuals with Alzheimer’s disease and amnestic mild cognitive impairment. Nucleic Acids Res 2007;35:5545–55. 10.1093/nar/gkm605. *
- [66].Lovell MA, Xie C, Markesbery WR. Decreased base excision repair and increased helicase activity in Alzheimer’s disease brain. Brain Res 2000;855:116–23. 10.1016/S0006-8993(99)02335-5. [DOI] [PubMed] [Google Scholar]
- [67].Migliore L, Fontana I, Trippi F, Colognato R, Coppedè F, Tognoni G, et al. Oxidative DNA damage in peripheral leukocytes of mild cognitive impairment and AD patients. Neurobiol Aging 2005;26:567–73. 10.1016/j.neurobiolaging.2004.07.016. [DOI] [PubMed] [Google Scholar]
- [68].Wang J, Markesbery WR, Lovell MA. Increased oxidative damage in nuclear and mitochondrial DNA in mild cognitive impairment. J Neurochem 2006;96:825–32. 10.1111/j.1471-4159.2005.03615.x. [DOI] [PubMed] [Google Scholar]
- [69]. Shi L, Chen SJ, Ma MY, Bao YP, Han Y, Wang YM, et al. Sleep disturbances increase the risk of dementia: a systematic review and meta-analysis. Sleep Med Rev 2018;40:4–16. 10.1016/j.smrv.2017.06.010. *
- [70].Moe KE, Vitiello MV, Larsen LH, Prinz PN. Sleep/wake patterns in Alzheimer’s disease: relationships with cognition and function. 1995. p. 15–20. [DOI] [PubMed]
- [71].Bliwise DL, Hughes M, McMahon PM, Kutner N. Observed sleep/wakefulness and severity of dementia in an Alzheimer’s disease special care unit. J Gerontol 1995;50A:M303–6. 10.1093/gerona/50A.6.M303. [DOI] [PubMed] [Google Scholar]
- [72].Baio J, Wiggins L, Chirstensen DL, Maenner MJ, Daniels J, Warren Z, et al. Prevalence of autism spectrum disorder among children aged 8 Years-autism and developmental disabilities monitoring network, 11 sites, United States, 2014. MMWRSurveill Summ 2014;67:1–23. 10.15585/mmwr.ss6706a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Fujita-Jimbo E, Tanabe Y, Yu Z, Kojima K, Mori M, Li H, et al. The association of GPR85 with PSD-95-neuroligin complex and autism spectrum disorder: a molecular analysis. Mol Autism 2015;6:1–10. 10.1186/s13229-015-0012-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Fujita E, Dai H, Tanabe Y, Zhiling Y, Yamagata T, Miyakawa T, et al. Autism spectrum disorder is related to endoplasmic reticulum stress induced by mutations in the synaptic cell adhesion molecule, CADM1. Cell Death Dis 2010;1:1–7. 10.1038/cddis.2010.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Falivelli G, De jaco A, Favaloro FL, Kim H, Wilson J, Dubi N, et al. Inherited genetic variants in autism-related CNTNAP2 show perturbed trafficking and ATF6 activation. Hum Mol Genet 2012;21:4761–73. 10.1093/hmg/dds320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Ulbrich L, Favaloro FL, Trobiani L, Marchetti V, Patel V, Pascucci T, et al. Autism-associated R451C mutation in neuroligin3 leads to activation of the unfolded protein response in a PC12 Tet-On inducible system. Biochem J 2016;473:423–34. 10.1042/BJ20150274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77]. Crider A, Ahmed AO, Pillai A. Altered expression of endoplasmic reticulum stress-related genes in the middle frontal cortex of subjects with autism spectrum disorder. Mol Neuropsychiatr 2017;3:85–91. 10.1159/000477212. *
- [78].Erden-nal M, Sunal E, Kanbak G. Age-related changes in the glutathione redox system. Cell Biochem Funct 2002;20:61–6. 10.1002/cbf.937. [DOI] [PubMed] [Google Scholar]
- [79].Perry SW, Norman JP, Litzburg A, Gelbard HA. Antioxidants are required during the early critical period, but not later, for neuronal survival. J Neurosci Res 2004;78:485–92. 10.1002/jnr.20272. [DOI] [PubMed] [Google Scholar]
- [80].Meagher EA, Fitzgerald GA. Indices of lipid peroxidation in vivo: strengths and limitations. Free Radic Biol Med 2000;28:1745–50. 10.1016/S0891-5849(00)00232-X. [DOI] [PubMed] [Google Scholar]
- [81]. Rose S, Melnyk S, Pavliv O, Bai S, Nick TG, Frye RE, et al. Evidence of oxidative damage and inflammation associated with low glutathione redox status in the autism brain. Transl Psychiatry 2012;2:e134–8. 10.1038/tp.2012.6. *
- [82].González-Fraguela M Oxidative stress markers in children with autism spectrum disorders. Br J Med Med Res 2013;3:307–17. 10.9734/bjmmr/2013/2335. [DOI] [Google Scholar]
- [83].Söğüt S, Zoroğlu SS, Özyurt H, Yilmaz HR, Özuğurlu F, Sivasli E, et al. Changes in nitric oxide levels and antioxidant enzyme activities may have a role in the pathophysiological mechanisms involved in autism. Clin Chim Acta 2003;331:111–7. 10.1016/S0009-8981(03)00119-0. [DOI] [PubMed] [Google Scholar]
- [84].Sajdel-Sulkowska EM, Xu M, Koibuchi N. Increase in cerebellar neurotrophin-3 and oxidative stress markers in Autism. Cerebellum 2009;8:366–72. 10.1007/s12311-009-0105-9. [DOI] [PubMed] [Google Scholar]
- [85].Chauhan A, Chauhan V, Brown WT, Cohen I. Oxidative stress in autism: increased lipid peroxidation and reduced serum levels of ceruloplasmin and transferrin - the antioxidant proteins. Life Sci 2004;75:2539–49. 10.1016/j.lfs.2004.04.038. [DOI] [PubMed] [Google Scholar]
- [86].Shpyleva S, Ivanovsky S, De Conti A, Melnyk S, Tryndyak V, Beland FA, et al. Cerebellar oxidative DNA damage and altered DNA methylation in the BTBR T+tf/J mouse model of autism and similarities with human post mortem cerebellum. PLoS One 2014;9:1–18. 10.1371/journal.pone.0113712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Bartlett LB, Rooney V, Spedding S. Nocturnal difficulties in a population of mentally handicapped children. Br J Ment Subnorm 1985;31:54–9. 10.1179/bjms.1985.009. [DOI] [Google Scholar]
- [88].Richdale AL, Prior MR. The sleep/wake rhythm in children with autism. Eur Child Adolesc Psychiatr 1995;4:175–86. 10.1007/BF01980456. [DOI] [PubMed] [Google Scholar]
- [89].Liu X, Hubbard JA, Fabes RA, Adam JB. Sleep disturbances and correlates of children with autism spectrum disorders. Child Psychiatr Hum Dev 2006;37:179–91. 10.1007/s10578-006-0028-3. [DOI] [PubMed] [Google Scholar]
- [90].Kelleher RJ, Bear MF. The Autistic neuron: troubled translation? Cell 2008;135:401–6. 10.1016/j.cell.2008.10.017. [DOI] [PubMed] [Google Scholar]
- [91].Brown V, Jin P, Ceman S, Darnell JC, O’donnell WT, Tenenbaum SA, et al. Microarray identification of FMRP-associated brain mRNAs and altered mRNA translational profiles in fragile X syndrome. Cell 2001;107: 477–8. [DOI] [PubMed] [Google Scholar]
- [92].Feng Y, Gutekunst CA, Eberhart DE, Yi H, Warren ST, Hersch SM. Fragile X mental retardation protein: nucleocytoplasmic shuttling and association with somatodendritic ribosomes. J Neurosci 1997;17:1539–47. 10.1523/jneurosci.17-05-01539.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Alpatov R, Lesch BJ, Nakamoto-Kinoshita M, Blanco A, Chen S, Stützer A, et al. A chromatin-dependent role of the fragile X mental retardation protein FMRP in the DNA damage response. Cell 2014;157:869–81. 10.1016/j.cell.2014.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94]. Xu H, Rosales-Reynoso MA, Barros-Núñez P, Peprah E. DNA repair/replication transcripts are down regulated in patients with Fragile X Syndrome. BMC Res Notes 2013;6. 10.1186/1756-0500-6-90. *
- [95].Kovtun IV, Liu Y, Bjoras M, Klungland A, Wilson SH, McMurray CT. OGG1 initiates age-dependent CAG trinucleotide expansion in somatic cells. Nature 2007;447:447–52. 10.1038/nature05778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Entezam A, Lokanga AR, Le W, Hoffman G, Usdin K. Potassium bromate, a potent DNA oxidizing agent, exacerbates germline repeat expansion in a fragile X premutation mouse model. Hum Mutat 2010;31:611–6. 10.1002/humu.21237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Entezam A, Usdin K. ATM and ATR protect the genome against two different types of tandem repeat instability in Fragile X premutation mice. Nucleic Acids Res 2009;37:6371–7. 10.1093/nar/gkp666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Entezam A, Usdin K. ATR protects the genome against CGGCCG-repeat expansion in Fragile X premutation mice. Nucleic Acids Res 2008;36: 1050–6. 10.1093/nar/gkm1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Westmark CJ, Westmark PR, O’Riordan KJ, Ray BC, Hervey CM, Salamat MS, et al. Reversal of fragile X phenotypes by manipulation of AβPP/Aβ levels in Fmr1 KO mice. PLoS One 2011;6:1–11. 10.1371/journal.pone.0026549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Westmark CJ, Berry-Kravis EM, Ikonomidou C, Yin JCP, Puglielli L. Developing BACE-1 inhibitors for FXS. Front Cell Neurosci 2013;7:1–8. 10.3389/fncel.2013.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].El Bekay R, Romero-Zerbo Y, Decara J, Sanchez-Salido L, Del Arco-Herrera I, Rodríguez-De Fonseca F, et al. Enhanced markers of oxidative stress, altered antioxidants and NADPH-oxidase activation in brains from Fragile X mental retardation 1-deficient mice, a pathological model for Fragile X syndrome. Eur J Neurosci 2007;26:3169–80. 10.1111/j.1460-9568.2007.05939.x. [DOI] [PubMed] [Google Scholar]
- [102].Davidovic L, Navratil V, Bonaccorso CM, Catania MV, Bardoni B, Dumas AE. A metabolomic and systems biology perspective on the brain of the Fragile X syndrome mouse model. Genome Res 2011;21:2190–202. 10.1101/gr.116764.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Nimchinsky EA, Oberlander AM, Svoboda K. Abnormal development of dendritic spines in FMR1 knock-out mice. J Neurosci 2001;21:5139–46. 10.1523/jneurosci.21-14-05139.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Galvez R, Greenough WT. Sequence of abnormal dendritic spine development in primary somatosensory cortex of a mouse model of the fragile X mental retardation syndrome. Am J Med Genet 2005;135 A:155–60. 10.1002/ajmg.a.30709. [DOI] [PubMed] [Google Scholar]
- [105].Suresh A, Dunaevsky A. Relationship between synaptic AMPAR and spine dynamics: impairments in the FXS mouse. Cerebr Cortex 2017;27: 4244–56. 10.1093/cercor/bhx128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Hodges JL, Yu X, Gilmore A, Bennett H, Tjia M, Perna JF, et al. Astrocytic contributions to synaptic and learning abnormalities in a mouse model of fragile X syndrome. Biol Psychiatr 2017;82:139–49. 10.1016/j.biopsych.2016.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Cespedes EM, Rifas-Shiman SL, Redline S, Gillman MW, Pena M-M, Taveras EM. Longitudinal associations of sleep curtailment with metabolic risk in mid-childhood. Obesity 2018;176:139–48. 10.1016/j.physbeh.2017.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Hjorth MF, Chaput JP, Damsgaard CT, Dalskov SM, Andersen R, Astrup A, et al. Low physical activity level and short sleep duration are associated with an increased cardio-metabolic risk profile: a longitudinal study in 811 year old Danish children. PLoS One 2014;9. 10.1371/journal.pone.010467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Lim J, Dinges DF. A meta-analysis of the impact of short-term sleep deprivation on cognitive variables. Psychol Bull 2010;136:375–89. 10.1037/a0018883.A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Lowe CJ, Safati A, Hall PA. The neurocognitive consequences of sleep restriction: a meta-analytic review. Neurosci Biobehav Rev 2017;80: 586–604. 10.1016/j.neubiorev.2017.07.010. [DOI] [PubMed] [Google Scholar]
- [111].Mackiewicz M, Shockley KR, Romer MA, Galante RJ, Zimmerman JE, Naidoo N, et al. Macromolecule biosynthesis: a key function of sleep. Physiol Genom 2007;31:441–57. 10.1152/physiolgenomics.00275.2006. [DOI] [PubMed] [Google Scholar]
- [112].Terao A, Steininger TL, Hyder K, Apte-Deshpande A, Ding J, Rishipathak D, et al. Differential increase in the expression of heat shock protein family members during sleep deprivation and during sleep. Neuroscience 2003;116:187–200. 10.1016/S0306-4522(02)00695-4. [DOI] [PubMed] [Google Scholar]
- [113].Villafuerte G, Miguel-Puga A, Murillo Rodríguez E, Machado S, Manjarrez E, Arias-Carrión O. Sleep deprivation and oxidative stress in animal models: a systematic review. Oxid Med Cell Longev 2015. 10.1155/2015/234952. [DOI] [PMC free article] [PubMed]
- [114].D’Almeida V, Hipólide DC, Azzalis LA, Lobo LL, Junqueira VBC, Tufik S. Absence of oxidative stress following paradoxical sleep deprivation in rats. Neurosci Lett 1997;235:25–8. 10.1016/S0304-3940(97)00706-4. [DOI] [PubMed] [Google Scholar]
- [115].Gopalakrishnan A, Ji LL, Cirelli C. Sleep deprivation and cellular responses to oxidative stress. Sleep 2004;27:27–35. 10.1093/sleep/27.1.27. [DOI] [PubMed] [Google Scholar]
- [116].Singh R, Kiloung J, Singh S, Sharma D. Effect of paradoxical sleep deprivation on oxidative stress parameters in brain regions of adult and old rats. Biogerontology 2008;9:153–62. 10.1007/s10522-008-9124-z. [DOI] [PubMed] [Google Scholar]
- [117].Esbensen AJ, Schwichtenberg AJ. Sleep in neurodevelopmenal disorders. Int Rev Res Dev Disabil 2016:153–91. 10.1016/bs.irrdd.2016.07.005. [DOI] [PMC free article] [PubMed]
- [118].Colas D, Wagstaff J, Fort P, Salvert D, Sarda N. Sleep disturbances in Ube3a maternal-deficient mice modeling Angelman syndrome. Neurobiol Dis 2005;20:471–8. 10.1016/j.nbd.2005.04.003. [DOI] [PubMed] [Google Scholar]
- [119].Ehlen JC, Jones KA, Pinckney L, Gray CL, Burette S, Weinberg RJ, et al. Maternal Ube3a loss disrupts sleep homeostasis but leaves circadian rhythmicity largely intact. J Neurosci 2015;35:13587–98. 10.1523/JNEUROSCI.2194-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Bushey D, Tononi G, Cirelli C. Sleep and synaptic homeostasis: structural evidence in Drosophila. Science 2011;332:1576–81. 10.1126/science.1202839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Lassi G, Priano L, Maggi S, Garcia-Garcia C, Balzani E, El-Assawy N, et al. Deletion of the snord116/SNORD116 alters sleep in mice and patients with prader-willi syndrome. Sleep 2016;39:637–44. 10.5665/sleep.5542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Richdale AL, Schreck KA. Sleep problems in autism spectrum disorders: prevalence, nature, & possible biopsychosocial aetiologies. Sleep Med Rev 2009;13:403e11. 10.1016/j.smrv.2009.02.003. [DOI] [PubMed] [Google Scholar]
- [123].Saxena A, Chahrour M. Autism spectrum disorder. Genomic Precis Med Prim Care Third Ed 2017;392:301–16. 10.1016/B978-0-12-800685-6.00016-3. [DOI] [Google Scholar]
- [124].Miano S, Bruni O, Elia M, Scifo L, Smerieri A, Trovato A, et al. Sleep phenotypes of intellectual disability: a polysomnographic evaluation in subjects with Down syndrome and Fragile-X syndrome. Clin Neurophysiol 2008;119:1242–7. 10.1016/j.clinph.2008.03.004. [DOI] [PubMed] [Google Scholar]
- [125].Lozano R, Rosero CA, Hagerman RJ. Fragile X spectrum disorders. Intractable Rare Dis Res 2014;3:134–46. 10.5582/irdr.2014.01022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Bubu OM, Brannick M, Mortimer J, Umasabor-Bubu O, Sebastiao YV, Wen Y, et al. Sleep, cognitive impairment, and Alzheimer’s disease: a systematic review and meta-analysis. Sleep 2017;40:1–18. [DOI] [PubMed] [Google Scholar]
- [127].Prinz PN, Peskind ER, Vitaiano PP, Raskind MA, Eisdorfer C, Zemcuznikov N, et al. Changes in the sleep and waking EEGs of nondemented and demented elderly subjects. J Am Geriatr Soc 1982;30: 86–92. 10.1111/j.1532-5415.1982.tb01279.x. [DOI] [PubMed] [Google Scholar]
- [128].Prinz PN, Vitaliano PP, Vitiello MV, Bokan J, Raskind M, Peskind E, et al. Sleep, EEG and mental function changes in senile dementia of the Alzheimer’s type. Neurobiol Aging 1982;3:361–70. 10.1016/0197-4580(82)90024-0. [DOI] [PubMed] [Google Scholar]
- [129].Schenck CH, Bundlie SR, Mahowald MW. Delayed emergence of a parkinsonian disorder in 38% of 29 older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder. Neurology 1996;46:388–93. [DOI] [PubMed] [Google Scholar]
- [130].Zhang Y, Ren R, Sanford LD, Yang L, Zhou J, Tan L, et al. Sleep in Parkinson’s disease: a systematic review and meta-analysis of polysomnographic findings. Sleep Med Rev 2020;51:101281. 10.1016/j.smrv.2020.101281. [DOI] [PubMed] [Google Scholar]
- [131].Smeyne M, Smeyne RJ. Glutathione metabolism and Parkinson’s disease. Free Radic Biol Med 2013;62:13–25. 10.1016/j.freeradbiomed.2013.05.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Subramaniam SR, Chesselet MF. Mitochondrial dysfunction and oxidative stress in Parkinson’s disease. Prog Neurobiol 2013;106–107:17–32. 10.1016/j.pneurobio.2013.04.004. [DOI] [PMC free article] [PubMed]
- [133].Gonzalez-Hunt CP, Sanders LH. DNA damage and repair in Parkinson’s disease: recent advances and new opportunities. J Neurosci Res 2021;99: 180–9. 10.1002/jnr.24592. [DOI] [PubMed] [Google Scholar]
- [134].Beitz JM. Parkinson’s disease: a review. Front Biosci 2014;6:65–74. [DOI] [PubMed] [Google Scholar]
- [135].Sandsmark DK, Elliott JE, Lim MM. Sleep-wake disturbances after traumatic brain injury: synthesis ofhuman and animal studies. Sleep 2017;40. 10.1093/sleep/zsx044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Miles SR, Silva MA, Lang B, Hoffman JM, Venkatesan UM, Sevigny M, et al. Sleep apnea and posttraumatic stress after traumatic brain injury (TBI): a veterans affairs TBI model systems study. Rehabil Psychol 2021;66: 450–60. 10.1037/rep0000389. [DOI] [PubMed] [Google Scholar]
- [137].Hull B, Karabon P, Alpiner N. Insufficient sleep following pediatric mild traumatic brain injury correlates with neurocognitive dysfunction. Neurology 2022;98. 10.1212/wnl.0000000000013046.S1.S1-S1. [DOI] [Google Scholar]
- [138].Lavigne G, Khoury S, Chauny JM, Desautels A. Pain and sleep in postconcussion/mild traumatic brain injury. Pain 2015;156:S75–85. 10.1097/j.pain.0000000000000111. [DOI] [PubMed] [Google Scholar]
- [139].Rehman SU, Ikram M, Ullah N, Alam SI, Park HY, Badshah H, et al. Neurological enhancement effects of melatonin against brain injury-induced oxidative stress, neuroinflammation, and neurodegeneration via AMPK/CREB signaling. Cells 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Yin Y, Sun G, Li E, Kiselyov K, Sun D. ER stress and impaired autophagy flux in neuronal degeneration and brain injury. Ageing Res Rev 2017;34:3–14. 10.1016/j.arr.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Wang HC, Lin YJ, Shih FY, Chang HW, Su YJ, Cheng BC, et al. The role of serial oxidative stress levels in acute traumatic brain injury and as predictors of outcome. World Neurosurg 2016;87:463–70. 10.1016/j.wneu.2015.10.010. [DOI] [PubMed] [Google Scholar]
- [142].Mortimer JA, French LR, Hutton JT, Schuman LM. Head injury as a risk factor for Alzheimer’s disease. Neurology 1985;35. [DOI] [PubMed] [Google Scholar]
- [143].Wang X, Ji X. Interactions between remote ischemic conditioning and post-stroke sleep regulation. Front Med 2021;15:867–76. 10.1007/s11684-021-0887-9. [DOI] [PubMed] [Google Scholar]
- [144].Baylan S, Griffiths S, Grant N, Broomfield NM, Evans JJ, Gardani M. Incidence and prevalence of post-stroke insomnia: a systematic review and meta-analysis. Sleep Med Rev 2020;49. 10.1016/j.smrv.2019.101222. [DOI] [PubMed] [Google Scholar]
- [145].Terzoudi A, Vorvolakos T, Heliopoulos I, Livaditis M, Vadikolias K, Piperidou H. Sleep architecture in stroke and relation to outcome. Eur Neurol 2008;61:16–22. 10.1159/000165344. [DOI] [PubMed] [Google Scholar]
- [146].Pace M, Camilo MR, Seiler A, Duss SB, Mathis J, Manconi M, et al. Rapid eye movements sleep as a predictor of functional outcome after stroke: a translational study. Sleep 2018;41:1–11. 10.1093/sleep/zsy138. [DOI] [PubMed] [Google Scholar]
- [147].Bassetti CL, Aldrich MS. Sleep electroencephalogram changes in acute hemispheric stroke. Sleep Med 2001;2:185–94. 10.1016/S1389-9457(00)00071-X. [DOI] [PubMed] [Google Scholar]
- [148].Dumitrescu L, Popescu-olaru I, Cozma L, Tulb D, Hinescu ME, Ceafalan LC, et al. Review article oxidative stress and the microbiota-gut-brain axis. 2018. [DOI] [PMC free article] [PubMed]
- [149].Zhang R, Xu M, Wang Y, Xie F, Zhang G, Qin X. Nrf2–a promising therapeutic target for defensing against oxidative stress in stroke. Mol Neurobiol 2017;54:6006–17. 10.1007/s12035-016-0111-0. [DOI] [PubMed] [Google Scholar]
- [150].Li P, Stetler RA, Leak RK, Shi Y, Li Y, Yu W, et al. Oxidative stress and DNA damage after cerebral ischemia: potential therapeutic targets to repair the genome and improve stroke recovery. Neuropharmacology 2018;134: 208–17. 10.1016/j.neuropharm.2017.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].McKevitt C, Fudge N, Redfern J, Sheldenkar A, Crichton S, Rudd AR, et al. Self-reported long-term needs after stroke. Stroke 2011;42:1398–403. 10.1161/STROKEAHA.110.598839. [DOI] [PubMed] [Google Scholar]