Abstract
Introduction. COVID-19 is an infection caused by severe acute respiratory syndrome corona virus-2 (SARS-CoV-2) which may be associated with a wide range of bacterial and fungal co-infections. Mucormycosis is an opportunistic fungal infection occurring in post COVID-19 patients. Objectives. To study the role of histopathology in mucormycosis and the predisposing factors associated in development of mucormycosis in post COVID-19 patients. Materials and methods. A prospective observational study was conducted in our hospital in the pathology department over a period of 3 months on 200 patients with mucormycosis who were infected with SARS-CoV-2 virus. Results. Out of the 200 patients with mucormycosis studied in post COVID-19 patients, age ranged from 21–80 years, of which 132 were men and 68 were women. Sites involved by mucormycosis were sinuses, orbit, cranium, and cutaneous. Ethmoid sinus was most involved, followed by maxillary sinus. Diabetes was present in 162 patients and hypertension in 92 patients. On histopathological examination, fungal load was severe in 49 patients, angioinvasion was present in 48 patients, perineural invasion was present in 32 patients, and necrosis was present in 121 patients. The number of patients discharged after surgery was 169, whereas 31 died. Conclusion. Histopathological features of mucormycosis like angioinvasion, perineural invasion, severe fungal load, and large areas of necrosis were directly proportional to the mortality rate. Thus, histopathologists can help in assessing prognosis at the time of tissue diagnosis, so that clinicians can optimize treatment accordingly. Diabetes and history of corticosteroid intake for treatment of COVID-19 were the two commonest predisposing factors for development of mucormycosis.
Keywords: COVID-19, mucormycosis, sinuses, diabetes, corticosteroids
Introduction
Corona virus disease 2019 (COVID-19) is caused by SARS-CoV-2 (Severe acute respiratory syndrome corona virus 2). It was first identified in December 2019 in Wuhan, China.1 The disease quickly spread to other parts of the world yielding a global pandemic. Now there is an increasing number of cases of mucormycosis, which was not reported in the beginning of the COVID-19 pandemic. Mucormycosis is an acute invasive and life-threatening fungal infection that affects immunocompromised patients with diabetes mellitus, systemic corticosteroid use, haematological malignancy, neutropenia and organ transplantation.2
Mucormycosis is characterized by direct tissue invasion and necrosis followed by rapid progression, angioinvasion from nasal and sinus mucosa into the orbit and brain. The infection spreads rapidly from sinuses to orbit, cavernous sinus, and cranium, and if not diagnosed early, results in death.3 Clinically, rhinocerebral mucormycosis can present with nasal block, crusting, facial pain, edema, proptosis, ophthalmoplegia, headache, fever, and neurological signs if intracranial extension is present.4,5
Histological features of mucormycosis include infiltration of the fungus into mucosa, sub mucosa, blood vessels, thrombosis, tissue infarction, and haemorrhage.6
Vascular invasion and perineural invasion are the common pathological features of invasive mucormycosis.7 Histopathological examination plays a critical role in establishing diagnosis and evidence of tissue invasion by mucor. Rhino-orbito-cerebral mucormycosis causes high mortality and morbidity due to the angioinvasive properties of the fungus, causing vascular occlusion and consequently resulting in extensive tissue necrosis, which is a hallmark of mucormycosis.8
Histopathological features can predict the patients progress by assessing prognosis and guide the clinician to optimize treatment accordingly. Without early diagnosis and treatment, rapid progression of the disease occurs leading to mortality due to intra-orbital and intracranial complications.9
Materials and Methods
A prospective observational study was conducted at our hospital over a period of 3 months from May to July 2021.
Two hundred post COVID-19 patients were included in the study, whose tissue biopsy samples included functional endoscopic sinus surgery, maxillectomy, orbital exenteration, intracranial excision of space occupying lesion, and cutaneous excision of lesion, were submitted to the histopathology department. Post COVID patients were those who developed symptoms of mucormycosis between the second to fourth week after testing positive for COVID.
Demographic data including age and sex of the patient, presenting complaints, comorbidities, imaging findings, history of corticosteroid treatment for COVID-19 were obtained. Sites of involvement by mucor and the type of surgery performed, were collected and analyzed.
Tissue samples sent to histopathology were examined grossly, processed as routine paraffin sections and were stained with Haematoxylin & Eosin (H&E), Periodic Acid Schiff (PAS), Gomori Methanamine Silver (GMS) when necessary and studied.
Tissue samples received were studied for the following microscopic details.
Fungal morphology
Fungal load, graded as mild, moderate and severe
Presence and absence of tissue necrosis
Angioinvasion and perineural invasion
Presence/absence of granuloma
Invasive fungal sinusitis was diagnosed when hyphal forms were present in sinus mucosa or submucosa, blood vessel or bone. Fungal load was graded on the basis of number of microscopic fields showing fungal hyphae in 400x objective. It is graded as mild when hyphae were noted in <4 microscopic fields, moderate when hyphae were noted in 5–7 microscopic fields, and severe when hyphae noted in >8–10 microscopic fields. The grading was done by examining the area with maximum load.
Samples were sent to microbiology department for culture. Growth of mucormycosis was seen in 172 patients (86%) and in the remaining 28 patients (14%), no growth was identified. The probable factors could be low fungal load, lack of representative tissue.
A short follow up of patients was done to know whether the patient was discharged or succumbed to death. Patients with recurrence were not included in the study.
Results
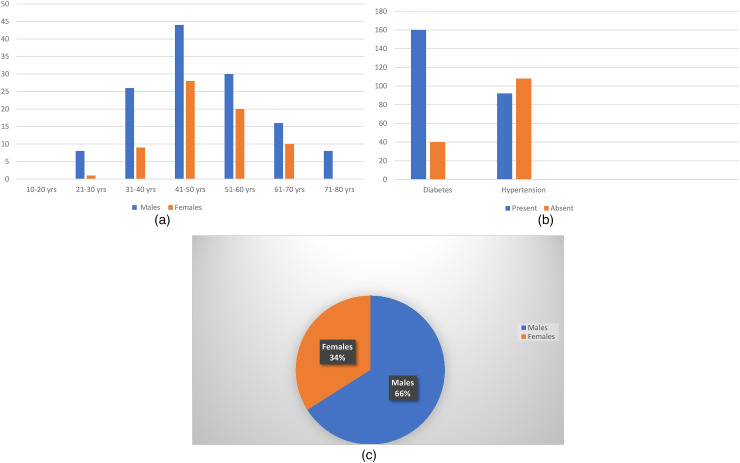
A total of 200 post COVID patients who were clinically diagnosed as mucormycosis were confirmed with histopathology. Out of these 132 (66%) patients were men and 68 (34%) patients were women. Male to female ratio (Figure 1c) was 1.9:1. Age (Figure 1a) of the patients ranged from 21 to 80 years, with higher incidence in 41–50 years of age in both sexes. Youngest age was 28 years in men and 30 years in women. Oldest age was 75 years in men and 70 years in women. Diabetes and hypertension (Figure 1b) were seen in 162 and 70 patients respectively.
Figure 1.
(a) Age and sex distribution; (b) diabetes and hypertension; (c) sex distribution.
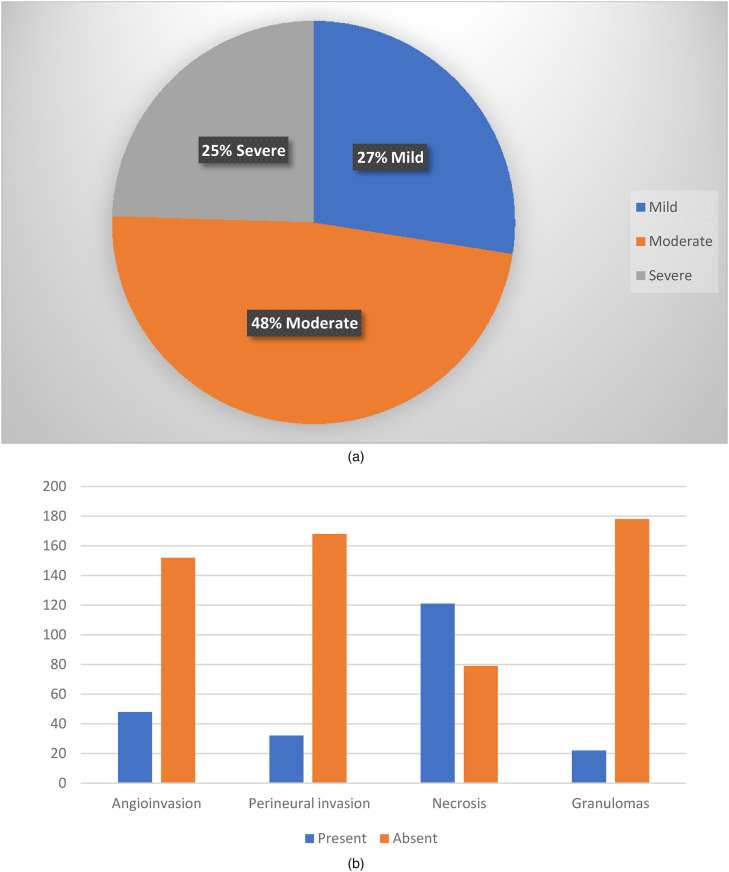
Facial pain, swelling and nasal discharge were the commonest complaints. Mucormycosis affected sinuses and orbit in 197 patients, cranium in 2, and skin in 1 (Figure 5c). Tissue samples were stained with H&E and special stains like Periodic Acid Schiff (PAS) and Gomori Methanamine Silver (GMS) stains when necessary to demonstrate fungal hyphae. Out of the 200 patients studied, the characteristic fungal hyphae were broad, aseptate, and ribbon like, with wide angled branching. Fungal load (Figure 2a) was mild in 55 (27%), moderate in 96 (48%) and severe in 49 (25%) patients. Necrosis (Figure 2b) was present in 121 (61%). Granulomas were present in 22 (11%). Angio invasion was present in 48 (24%) patients. Perineural invasion was noted in 32 (16%) patients. Fungal culture yielded growth of Mucor in 172 patients (86%).
Figure 2.
(a) Fungal load; (b) presence and absence of angioinvasion, perineural invasion, necrosis, granulomas.
Surgeries performed (Table 1) were functional endoscopic sinus surgery in 145 (73%) patients, functional endoscopic sinus surgery and orbital exenteration in 9 (5%), functional endoscopic sinus surgery and maxillectomy in 17 (9%), functional endoscopic sinus surgery + orbital exenteration + maxillectomy in 1 (0.5%) patient, orbital exenteration and maxillectomy in 16 (8%) patients, maxillectomy in 6 (3%) patients, orbital exenteration in 3 (1.5%) patients, craniotomy and excision of lesion in 2 (1%) patients, and cutaneous excision of lesion in 1 (0.5%) patient.
Table 1.
Types of Surgeries Performed.
| Sl No | Types of surgeries performed | No. of patients (%) |
|---|---|---|
| 1. | Functional endoscopic sinus surgery | 145 (73%) |
| 2. | Functional endoscopic sinus surgery and orbital exenteration | 9 (5%) |
| 3. | Functional endoscopic sinus surgery and maxillectomy | 17 (9%) |
| 4. | Functional endoscopic sinus surgery + orbital exenteration + maxillectomy | 1 (<1%) |
| 5. | Orbital exenteration and maxillectomy | 16 (8%) |
| 6. | Maxillectomy | 6 (3%) |
| 7. | Orbital exenteration | 3 (2%) |
| 8. | Craniotomy and excision of lesion | 2 (1%) |
| 9. | Cutaneous excision of lesion | 1 (<1%) |
CT findings showed involvement of the following sinuses (Table 2) pan sinus (frontal + ethmoid + maxillary + sphenoid) in 58 (29%) patients, maxillary + ethmoid + sphenoid in 35 (18%) patients, frontal + ethmoid + maxillary in 18 (9%) patients, ethmoid + maxillary in 47 (24%) patients, ethmoid + sphenoid in 2 (1%) patients, ethmoid in 8 (4%) patients, maxillary in 29 (15%) patients. Ethmoid sinus was the commonest sinus involved, followed by maxillary.
Table 2.
Types of Sinuses Involved.
| Sl. No | Types of Sinuses | No. of patients |
|---|---|---|
| 1. | Pan sinus (frontal + ethmoid + maxillary + sphenoid) | 58 (29%) |
| 2. | Maxillary + ethmoid + sphenoid | 35 (18%) |
| 3. | Frontal + ethmoid + maxillary | 18 (9%) |
| 4. | Ethmoid + maxillary | 47 (24%) |
| 5. | Ethmoid + sphenoid | 2 (1%) |
| 6. | Ethmoid | 8 (4%) |
| 7. | Maxillary | 29 (15%) |
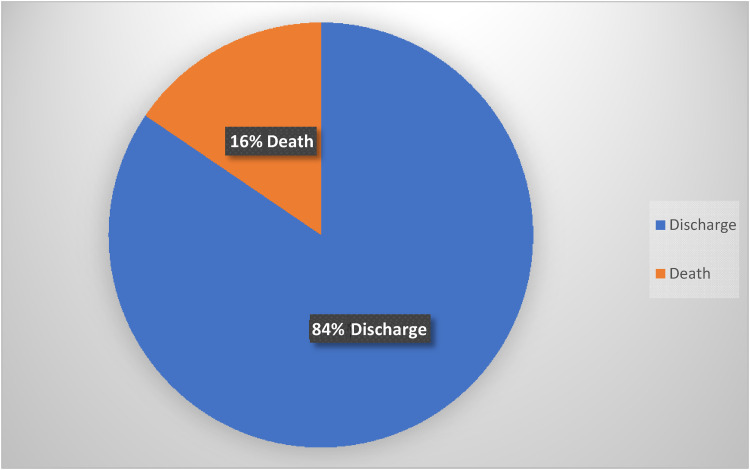
Corticosteroids were used for treatment of COVID (Table 3) in 172 patients (86%). Number of patients discharged after surgery were 169 (85%), and 31(16%) died (Figure 3).
Figure 3.
Discharge vs death.
Table 3.
Corticosteroid Intake for Treatment of COVID-19.
| No. of patients taken | No. of patients not taken |
|---|---|
| 172 | 28 |
Discussion
Mucormycosis is an opportunistic fulminant fungal infection occurring in post COVID-19 patients. The conditions that predispose to it are uncontrolled diabetes mellitus with or without diabetic ketoacidosis, hematological and other malignancies, organ transplantation, neutropenia, immunosuppressive and corticosterioid therapy, and acquired immunodeficiency syndrome (AIDS).10
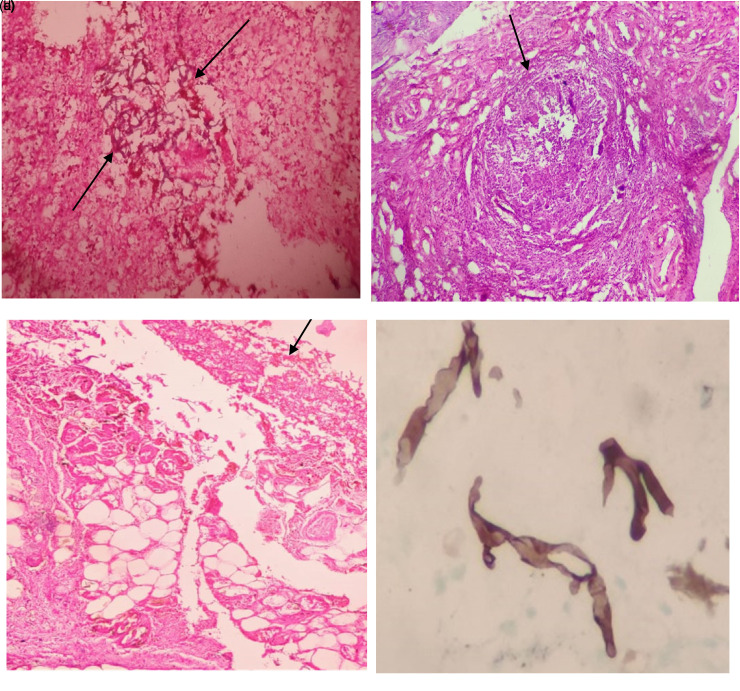
Figure 4.
(a) Numerous broad aseptate fungal hyphae (H&E 40x); (b) Fungal hyphae invading blood vessel (H&E 40x); (c) Perineural invasion by fungal hyphae (H&E 100x); (d) Microabscess in glial tissue with fungal hyphae (H&E 40x).
Figure 5.
(a) Glial tissue invaded by numerous fungal hyphae (H&E 40x); (b) Granuloma with giant cells (H&E 10x); (c) Fungal hyphae in subcutaneous tissue in cutaneous mucormycosis (H&E 40x); (d) Gomori methenamine silver stain showing fungal hyphae (H&E 40x).
Based on its anatomic location, mucormycosis is classified into isolated nasal, rhino-orbital or rhino-orbital cerebral, pulmonary, cutaneous, disseminated, gastrointestinal mucormycosis, and miscellaneous.11 The fungus gains entry into host through nose and sinuses directly or through vascular occlusion.12 From the paranasal sinuses enters the orbit via ethmoid and maxillary sinuses or via the nasolacrimal duct.13 Intracranial involvement occurs by invasion through superior orbital fissure ophthalmic vessels, cribriform plate, carotid artery or possible via perineural route.12,14
Mucormycosis gains entry through the respiratory tract and exhibits a remarkable affinity for arteries. Fungus invades arteries and forms thrombi within the blood vessels that reduce blood supply and cause necrosis of hard and soft tissue.15 Thus, angioinvasion (Figure 4b) with vessel thrombosis and tissue necrosis is the pathological hallmark of mucormycosis.
Histopathological evaluation of mucormycosis is the main stay of diagnosis and shows non-septate, broad, ribbon like fungal hyphae (Figure 4a) (10–20 micron). In our study patients with severe fungal load showed poorer prognosis based on survival rate as compared to mild and moderate fungal load. So, as the fungal load increases the survival rate decreases. The patients who have high fungal load have a state that favors fungal growth and are relatively more immunocompromised. Ashina Goel et al showed similar association in non COVID patients in their study on role of histopathology as an aid to prognosis in rhino-orbito-cerebral zygomycosis.16
Mucor patients showing large areas of necrosis were associated with high density of fungal organisms thus increasing the fungal load in necrotic areas. Hence necrotic areas must be sampled well. In our study fungal hyphae invading blood vessel (Figure 4b) (angioinvasion) was seen in 48 patients (24%) and these patients succumbed to death, indicating a poorer prognosis. Thus angioinvasion was directly proportional to mortality rate, a similar finding noted in study by Ashina Goel et al16
Perineural invasion (Figure 4c) was seen in 36 (16%) patients which were mostly found in orbital exenteration specimens. Histopathology showing combined angioinvasion and perineural invasion by fungal hyphae showed high mortality rate. In a study by Sravani et al in non-covid patients showed that perineural invasion was associated with advanced extent of invasion by mucor.17
Two patients showed intracranial involvement in the form of brain abscesses (Figure 4d) involving frontal and temporal lobe. Glial tissue showed invasion by numerous fungal hyphae (Figure 5a) along with angioinvasion and these cases showed increased mortality. Ethmoid sinus was the commonest sinus involved followed by maxillary sinus in our study. S Sharma et al in their study showed similar findings.18 Granulomatous response (Figure 5b) was present in 22 patients and they showed a good prognosis.
Fungal hyphae were seen in subcutaneous tissue in cutaneous mucormycosis (Figure 5c). Special stains like Gomori methanamine silver stain demonstrated fungal hyphae (Figure 5d) when the load was less. In our study, post COVID mucormycosis patients had a strong association with diabetes mellitus. Most of the patients received corticosteroid therapy for treatment for COVID-19 and the combination of corticosteroid therapy and diabetes mellitus increases the risk of mucormycosis mediated hyperglycemia induced immunosuppression.19 John et al20, Kumar et al21 and Sharma et al18 concluded that there is a strong association between diabetes mellitus and use of corticosteroids in the treatment of COVID-19 and development of mucormycosis. The possible mechanisms for increased growth of mucor would be uncontrolled hyperglycemia and precipitation of diabetic keto acidosis due to corticosteroid intake, free available iron, high glucose, low pH which decrease the phagocytic activity of white blood cells.22 Thus, efforts should be made to maintain optimal glucose levels and use of corticosteroids should be done cautiously.
Conclusion
Histopathological features of mucormycosis showing severe fungal load, large areas of necrosis, presence of angioinvasion and perineural invasion were associated with poorer prognosis and decreased survival rate. Thus, histopathologists can help in assessing prognosis at the time of tissue diagnosis so that clinicians could optimize treatment accordingly.
The association between diabetes mellitus and use of corticosteroids for treatment of COVID-19 are also the two main factors which aggravate the illness and should be properly checked. Hence it is essential to maintain optimal glucose levels and guarded use of corticosteroids to reduce the burden of mucormycosis in post covid patients. Early diagnosis and treatment can reduce progression of disease and decrease mortality rate.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical Approval: Not applicable, because this article does not contain any studies with human or animal subjects.
Informed Consent: Not applicable, because this article does not contain any studies with human or animal subjects.
Trial Registration: Not applicable, because this article does not contain any clinical trials.
ORCID iD: B. Swapna Kumari https://orcid.org/0000-0002-8597-1185
References
- 1.Park M, Cook AR, Lim JT, Sun Y, Dickens BL. A systematic review of COVID-19 epidemiology based on current evidence. J Clin Med. 2020;9(4):967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirabayashi KE, Idowu OO, Kalin-Hajdu E, et al. Invasive fungal sinusitis: risk factors for visual acuity outcomes and mortality. Ophthalmic PlastReconstrSurg. 2019;35(6):535-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walsh TJ, Gamaletsou MN, McGinnis MR, Hayden RT, Kontoyiannis DP. Early clinical and laboratory diagnosis of invasive pulmonary, extrapulmonary and disseminated mucormycosis (zygomycosis). Clin Infect Dis 2012;54Suppl (1):S55-S60. [DOI] [PubMed] [Google Scholar]
- 4.Scheckenbach K, Cornely O, Hoffmann TK, et al. Emerging therapeutic options in fulminant invasive rhinocerebralmucormycosis. Auris Nasus Larynx. 2010;37(3):322-328. [DOI] [PubMed] [Google Scholar]
- 5.Vairaktaris E, Moschos MM, Vassiliou S, et al. Orbital cellulitis, orbital subperiosteal and intraorbital abscess. Report of three cases and review of the literature. J Craniomaxillofac Surg. 2009;37(3):132-136. [DOI] [PubMed] [Google Scholar]
- 6.DeShazo RD, Chapin K, Swain RE. Fungal sinusitis. N Engl J Med. 1997;337(4):254-259. [DOI] [PubMed] [Google Scholar]
- 7.Morace G, Borghi E. Invasive mold infections: virulence and pathogenesis of mucorales. Int J Microbiol. 2012;2012:349278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parfrey NA. Improved diagnosis and prognosis of mucormycosis: a clinicopathologic study of 33 cases. Medicine (Baltimore). 1986;65(2):113-123. [DOI] [PubMed] [Google Scholar]
- 9.Gillespie MB, O’Malley BW. An algorithmic approach to the diagnosis and management of invasive fungal rhinosinusitis in the immunocompromised patient. Otolaryngol Clin North Am. 2000;33(2):323-334. [DOI] [PubMed] [Google Scholar]
- 10.AM. Sugar mucormycosis. Clin Infect Dis. 1992;14:S126-S(1)29. [DOI] [PubMed] [Google Scholar]
- 11.Safi M, Ang MJ, Patel P, Silkiss RZ. Rhino-orbital-cerebral mucormycosis (ROCM) and associated cerebritis treated with adjuvant retrobulbar amphotericin B. Am J Ophthalmol Case Rep. 2020;19:100771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bawankar P, Lahane S, Pathak P, Gonde P, Singh A. Central retinal artery occlusion as the presenting manifestation of invasive rhino-orbital-cerebral mucormycosis. Taiwan J Ophthalmol. 2020;10(1):62-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abramson E, Wilson D, Arky RA. Rhinocerebralphycomycosis in association with diabetic ketoacidosis. Ann Intern Med. 1967;66(4):735-742. [DOI] [PubMed] [Google Scholar]
- 14.Parsi K, Itgampalli RK, Vittal R, Kumar A. Perineural spread of rhino-orbitocerebral mucormycosis caused by Apophysomyces elegans. Ann Indian Acad Neurol. 2013;16(3):414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta S, Goyal R, Kaore NM. Rhino-Orbital-Cerebral mucormycosis: battle with the deadly enemy. Indian J Otolaryngol Head Neck Surg. 2020;72(1):104-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ashina G, Usha K, Subhas Chandra S. Role of histopathology as an aid to prognosis in rhino-orbito-cerebral zygomycosis. Indian J Pathol Microbiol. 2010;53(2):253-257. [DOI] [PubMed] [Google Scholar]
- 17.Sravani T, Shantveer G, Megha U, Sundaram C. Rhinocerebralmucormyosis: pathology revisited with emphasis on perineural spread. Neurol India. 2014;62(4):383-386. [DOI] [PubMed] [Google Scholar]
- 18.Sharma S, Grover M, Bhargava S, Samdani S, Kataria T. Post coronavirus disease mucormycosis: a deadly addition to the pandemic spectrum. J Laryngol Otol. 2021;135(5):442-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmadikia K, Hashemi SJ, Khodavaisy S, et al. The double-edged sword of systemic corticosteroid therapy in viral pneumonia: a case report and comparative review of influenza-associated mucormycosis versus COVID-19 associated mucormycosis. Mycoses. 2021;64(8):798-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.John TM, Jacob CN, KontoyiannisDP. When uncontrolled diabetes mellitus and severe COVID-19 converge: the perfect storm for mucormycosis J Fimgo (Basel) 2021 Apr 15;7:(4): 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Awadesh Kumar S, Ritu S, Shashank R, Anoop M. Mucormycosis in COVID-19: a systematic review of cases reported worldwide in India. Diabetes Metab Syndr: Clin Res Rev. 2021;15(4):102146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baldin C, Ibrahim AS. Molecular mechanisms of muormycos-the bitter and the sweet. PLoSPathog. 2017;13(8):e1006408. [DOI] [PMC free article] [PubMed] [Google Scholar]