Abstract
Introduction
The role of artificial intelligence (AI) is currently increasing in terms of diagnosing diseases and planning treatment in endodontics. However, findings from individual research studies are not systematically reviewed and compiled together. Hence, this study aimed to systematically review, appraise, and evaluate neural AI algorithms employed and their comparative efficacy to conventional methods in endodontic diagnosis and treatment planning.
Methods
The present research question focused on the literature search about different AI algorithms and models of AI assisted endodontic diagnosis and treatment planning. The search engine included databases such as Google Scholar, PubMed, and Science Direct with search criteria of primary research paper, published in English, and analyzed data on AI and its role in the field of endodontics.
Results
The initial search resulted in 785 articles, exclusion based on abstract relevance, animal studies, grey literature and letter to editors narrowed down the scope of selected articles to 11 accepted for review. The review data supported the findings that AI can play a crucial role in the area of endodontics, such as identification of apical lesions, classifying and numbering teeth, detecting dental caries, periodontitis and periapical disease, diagnosing different dental problems, helping dentists make referrals, and also helping them make plans for treatment of dental disorders in a timely and effective manner with greater accuracy.
Conclusion
AI with different models or frameworks and algorithms can help dentists to diagnose and manage endodontic problems with greater accuracy. However, endodontic fraternity needs to provide more emphasis on the utilization of AI, provision of evidence based guidelines and implementation of the AI models.
Keywords: Artificial intelligence, Neural artificial intelligence, Artificial neural networks, Treatment planning, Endodontics
1. Introduction
The complexity of the neural networks in a functional human brain have always intrigued physicians, technologists, and scientists (Richardson, 2017). With evolution of time, multiple advanced technologies have emerged in several fields of science which have mimicked the functions of the human brain (Lebedev and Nicolelis, 2017). However, it is still not feasible for scientists to simulate the human brain in a wholesome manner (Khanna and Dhaimade, 2017). Despite this challenge, in recent times “artificial intelligence” (AI) has gained immeasurable importance in all walks of life. (Stuart and Peter, 2016, Ishwarya et al., 2017). “Artificial intelligence (AI) is the ability of a digital computer or computer-controlled robot to perform tasks commonly associated with intelligent beings.”(Alexander and John, 2018, Yadav and Sehrawat, 2018). Thus, AI can be considered as a broader field that is concerned with the electronic models that can think and behave or undertake jobs and assigned activities (Deshmukh, 2018, Park and Park, 2018). Moreover, AI structures can help researchers, scientists and health care providers to obtain required knowledge with higher and desirable accuracy and results (Akerkar, 2019, Shankar et al., 2019).
In addition, artificial neural networks (ANN) are greatly inspired by the human brain. It consists of machine learning algorithms called neurons (nodes) that focus on recognizing underlying patterns or connections from a dataset and imitate brain’s data processing function. (Da Silva et al., 2017). Neural signal transmission is stimulated by these ANNs and the human brain functions as a crucial part of AI (Kalappanavar et al., 2018, Yaji, 2019). Applied work in this field of ANN shows that neural networks being a computational cognitive model can perform complex tasks at human or even super-human performance level. These deep neural networks explain a significant amount of variance across sensory cortex and approximation with the compositional structure of physical processes. Evidence based literature reveals that recurrent ANN’s can also explain decision-related processes (Ojha et al., 2017). One of the imperative benefits of ANNs is that this structure resolves problems that are too multifarious and intricate to orthodox methods (Klyuchko, 2017, Miller and Brown, 2018). Furthermore, these ANNs are utilized in innumerable specialties of medicine ranging from diagnosis of a disease, development of medicines to the analysis of an image or scan (Talari et al., 2019).
The last decade has witnessed a noticeable progression in the use of AI in the field of dentistry (Chen et al., 2020). Thus, AI has its effective implications in the field of dentistry, facilitating precise diagnosis in oral medicine and radiology (Hung et al., 2020). AI-enabled shared data storage uses modern analytics for evaluation of genetic information and AI workloads enabling accurate diagnosis and treatment planning. Despite its widespread applications, AI is yet to influence the standard protocols adopted in the field of dentistry (Singh et al., 1956). AI and ANNs will not only aid in accurate diagnosis of Dental anomalies and conditions but also improve work efficiency and final outcome of the planned treatment (Naik and de at, 2016).
Presently, AI is being popularly used to plan treatment in the area of endodontics (Orhan et al., 2020). Although there is a dearth of literature regarding the role of neural AI for treatment planning in the field of dentistry, there are some studies that provide substantial evidence on the role of AI and ANN mainly in the field of endodontics. However, findings from these individual research studies are not systematically reviewed and reported. Thus, till date, no systematic reviews have aggregated and synthesized the findings about neural AI aided treatment planning in endodontics. Hence, the purpose of this study is to systematically review, appraise, and evaluate the neural AI algorithms employed in endodontic diagnosis and treatment planning. Moreover, the present review was conducted on the premise that comprehensive and systematic knowledge regarding influence of AI in Endodontic treatment planning ensures enhanced and precise patient care.
2. Material and methods
This systematic review aimed to appraise and evaluate the available literature on the neural AI aided treatment planning in endodontics. The review focused to extract and analyze data on endodontic diagnostic application of AI, the algorithms and models employed (CNN [convolutional neural network], DCNN [deep convolutional neural network] and ANN), datasets utilized and comparative efficacy reported. This review was conducted according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines for reporting the systematic review (Moher et al., 2010).
2.1. Inclusion and exclusion criteria
An electronic search was carried out on the chosen topic of neural AI aided treatment planning in endodontics. A study article was considered eligible if it was a primary research paper, published in English, and had provided information on neural AI assisted treatment planning in the field of endodontics. Articles were excluded if they were in vitro studies; animal studies; reports from ‘grey literature’ (conference abstracts, unpublished studies); letters to the editors; and reviews and studies for which full-texts were not available or accessible.
2.2. Information sources and search strategy
A systematic search of published articles on the chosen topic was conducted in 2021 with inclusion criteria of articles published between January 2010 and December 2020. The comprehensive search was conducted using databases such as PubMed, Google Scholar, and Science Direct. The titles of the articles were first screened, followed by abstract screening, and full-text article assessment. Finally, studies that did not meet the inclusion criteria were excluded.
An amalgamation of Medical Subject Headings (MeSH) keywords along with text words were used and assembled as search terms. These search terms were distributed into four major groups, namely, population, intervention, comparison, and outcome using PICO framework (Schardt et al., 2007). Most common search terms included “Artificial intelligence AND endodontics”, “neural artificial intelligence AND endodontics”, “endodontics AND Artificial intelligence”, “endodontics AND neural artificial intelligence”, “neural artificial intelligence assisted treatment AND endodontics” “Artificial intelligence assisted treatment AND endodontics” and “neural artificial intelligence assisted treatment planning AND endodontics OR artificial intelligence assisted treatment planning AND endodontics”, which appeared in abstracts, keywords and titles.
Further, diverse wordings of main concepts such as Artificial Intelligence vs neural artificial intelligence assisted treatment planning and endodontics, etc. were also used to obtain pertinent research papers. These major concepts were then combined (using Boolean operators AND, OR) relevant to the research question. Moreover, to detect more research articles, truncation (*) with the same root word was utilized. The search strategy based on the four categories of the PICO framework is given in Table 1.
Table 1.
Search strategy for the potential studies according to PICO criteria.
| Population | ‘adults*’ [Mesh] OR *human beings*’ OR * women* OR Men* OR ‘Women 18 years old*’ OR ‘ Men 18 years old’ OR Men, OR women, OR adults, OR adults, OR Men, OR overweight Women ‘Men AND women 18 years and older’[Mesh]) AND |
| Intervention/Exposure (Artificial Network) |
“Artificial intelligence” OR “neural artificial intelligence OR Artificial intelligence”, OR neural artificial intelligence” OR “neural artificial intelligence assisted treatment OR “Artificial intelligence assisted treatment and neural artificial intelligence assisted treatment planning OR “artificial intelligence assisted treatment planning” |
| Comparison group | Not Applicable |
| Outcome | “dental caries* OR dental decay OR teeth classification, OR “segmentation” OR “ location of apical foramen”, OR dentistry, OR dental problems OR “endodontics” |
2.3. Data abstraction
After importing all the appropriate research studies into a reference manager software (EndnoteTM), titles were screened for duplicates and the same were eliminated. The abstracts which did not explicitly measure the study objective were not considered for further review. Finally the full-text articles were examined followed by abstracting and summarizing the articles that met the eligibility criteria using a standardized pro forma. Besides, the bibliography of remaining studies were also checked and scrutinized to avoid missing any useful studies.
The data extraction tables were compared to ensure inclusion of the imperative findings of the eligible studies. The abstracted data table comprised comprehensive information including author name(s), publication year, country of origin, sample size and characteristics, type of artificial network used, study objectives, main metrics used in the study, main findings, strengths, limitations, and future directions.
3. Results
3.1. Findings of the search strategy
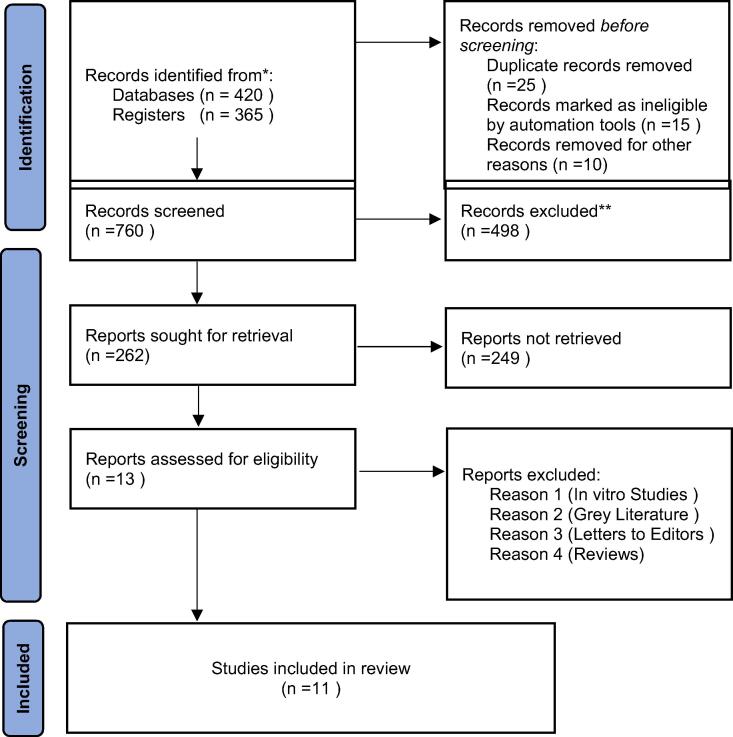
After the initial search 785 citations were identified relevant to the review title, however 20 articles were removed due to duplication. The abstract relevance narrowed down the article number to 262. Further scrutinizing the articles based on the inclusion and exclusion criteria, resulted in 11 studies for systematic reviewing of the implications of AI in endodontic diagnosis and treatment planning. The full texts retrieved for the 11 research articles that were assessed and included in the quantitative synthesis are shown in Fig. 1.
Fig. 1.
Screening, identification, and selection of research articles for systematic review.
3.2. Characteristics of the eligible studies
The tabulated review data (Table 2) shows the features of the finally eligible and relevant studies. Of the 11 studies, 9 studies were conducted between 2017 and 2020, and only two studies were undertaken in 2012. Most of these studies had been conducted in developed countries such as the USA, Japan, and Turkey. This was in contrast to the very few studies conducted in developing countries like Iran and India. While the sample sizes of the studies were predominantly based on the number of teeth, in a few studies it was also based on the number of patients enrolled in the study (Saghiri et al., 2012a, Saghiri et al., 2012b, Miki et al., 2017a). However, there was a wide variability in the sample size based on the number of teeth, ranging from 21 to 3000 teeth, and mainly examined through radiographs of the patients being evaluated in the study (Table 2).
Table 2.
Characteristics of studies included in the systematic review.
| Authors (Year) | Country | Sample size and characteristics | Outcome evaluated |
Study objective | Methodology | Type of AI Network | Study parameter(s) | Main Findings | Study limitation | Future directions |
|---|---|---|---|---|---|---|---|---|---|---|
| Saghiri et al., 2012a | Iran | 50 single rooted extracted teeth (mandibular incisor and 2nd premolar) placed within extraction sockets of dried skull. | Locating anatomic position of minor apical foramen. | “To develop a new approach for locating the minor apical foramen using feature-extracting procedures from radiographs and then processing data using ANN as a decision making system.” | Following access cavity preparation, a file was placed and radiograph was taken to evaluate the location of the file in relation to the minor apical foramen and further checked after retrieving the tooth from the alveolar socket. This was evaluated by two endodontists, who assessed the position of the files on the radiographs and then visualized the root apices under a stereomicroscope, considered as the gold standard. |
ANN – Multilayer perceptron model | Accuracy of identifying the minor apical foramen. | Analysis of the images from radiographs by ANN showed that in 93% of the samples, the location of the foramen had been determined correctly by false rejection and acceptation error methods.Significant differences were observed in data obtained from endodontists and ANN (p < 0.01). Comparing with stereomicroscope examination for determining anatomic position of minor apical foramen, ANN had greater accuracy (96%) than endodontists (76%). |
Small sample size | Larger studies with more sample size. |
| Saghiri et al., 2012b | Iran | “To evaluate the accuracy of the ANN to simulate the clinical situation of working length determination.” | ||||||||
| Miki et al., 2017a | Japan | 52 CBCT volumes divided into training (n = 42) and test (n = 10) cases. | Identification and classification of teeth from CBCT for forensic applications. | “To investigate the application of a DCNN for classifying tooth types on dental CBCT images and to automate the dental filing process using dental x-ray images.” | 52 CBCT datasets with a field of view ranging from 51 to 200 mm and voxel resolution in the range of 0.1–0.39 mm, were used in a DCNN for identifying and classifying 7 types of teeth (central incisors, lateral incisors, canines, first premolars, second premolars, first molars and second molars).The 52 volumes were randomly divided into training (n = 42) and test (n = 10) datasets and were evaluated for possible application in automated filing of post-mortem dental charts. |
DCNN - As a component of automated dental charting | Teeth classification accuracy | The average classification accuracy using the augmented training data by CBCT image rotation and intensity transformation was 88.8%. | Limited number of CBCT datasets. | Classification accuracy can be improved by combining the results for a tooth, or by applying 3D convolution. |
| Lee et al., 2018a | South Korea | 3000 dental periapical radiographs including maxillary premolars (n = 778) and molar (n = 769), and mandibular premolars (n = 722) and molars (n = 731) | Detection of dental caries from periapical radiographic images. | “To evaluate the efficacy of DCNN algorithms for detection and diagnosis of dental caries on periapical radiographs” | 3000 periapical radiographic images were divided into training and validationdataset (n = 2400) and test dataset (n = 600). |
DCNN | Diagnostic accuracy, sensitivity, specificity, positive/negative predictive values, ROC curve, and AUC for detection of dental caries by DCNN. |
DCNN based premolar model provided the best diagnostic accuracy (89%) and AUC (0.917), which was significantly greater (p < 0.001) molar model (accuracy – 88%, AUC – 0.890) and combined molar premolar model (accuracy – 82%, AUC – 0.845). | Only permanent teeth were included. Number of periapical radiographs with/without dental caries were too small to perform optimal DCNN learning. |
Enhance diagnostic accuracy through inclusion of history, clinical examination, percussion and tactile evaluation in DCNN algorithm. |
| Bouchahma et al., 2019 | Tunisia | 200 dental periapical radiographs. | Predicting treatment options for dental decay. | “To propose an automatic method using DCNN to detect the decay from dental X-Ray images and to predict the needed treatment.” | DCNN model was designed based on dental periapical radiographs obtained from patients. The model was evaluated in a subset of 200 radiographs to identify dental decay and predict treatment either as fluoride application, root canal treatment or simple dental restoration. |
DCNN | Treatment prediction accuracy | The DCNN based dental decay treatment prediction model had an overall accuracy of 87% (fluoride application – 98%, root canal treatment – 88%, simple dental restoration – 77%). | Small sample size | Further studies with larger sample size. |
| Ekert et al., 2019b | Germany | Dental panoramic radiographs from 85 patients (median age – 51 years) including 2001 teeth. | Detection of apical Lesions | “To apply DCNN to detect apical lesions on panoramic dental radiographs.” | DCNN model was tested against an ordinal reference scale inferred through majority values assigned by 6 independent examiners who assessed the panoramic dental radiographs.Reference scale values were, no apical lesion (0), widened PDL space or uncertain apical lesion (1), and certain apical lesion (2) . |
DCNN | Sensitivity, specificity, positive and negative predictive values, and AUC for ROC. | The DCNN had an overall AUC of 0.85 ± 0.04 for detection of apical lesions. The model showed greater specificity (0.87 ± 0.04) than sensitivity (0.65 ± 0.12). This correlated with a greater negative predictive value (0.93 ± 0.03) than positive predictive value (0.49 ± 0.10). The sensitivity was significantly higher for molars than for other tooth types. |
Smaller training datasets |
Further studies to consider the impact of factors such as image Projection and contrast quality on the discrimination ability of DCNN. |
| Hu et al., 2019 | USA | 21 patients (mean age – 27.6 ± 3.5 years) | AI based real time pain detection and localization using neuro-imaging. | “To test the feasibility of a mobile neuroimaging-based clinical augmented reality and AI framework for objective pain detection and also localization direct from the patient's brain in real time.” | Cortical brain activity during acute pain (cold stimulation of hypersensitive teeth) was recorded in real time using a portable optical neuroimaging technology (functional near-infrared spectroscopy). ANN and DCNN based AI algorithm was used to classify the hemodynamic data into pain and non-pain brain states. |
ANN and DCNN based AI algorithm | Classification accuracy of pain and non-pain states. | For pain/non-pain discrimination, the AI algorithm achieved 80.37% classification accuracy and a positive likelihood ratio of 2.35. Using the same algorithm for a left/right localization task 74.23% accuracy and a positive likelihood ratio of 2.02 were observed. | Small sample size | Extensive validation is still required for clinical translation. |
| Tuzoff et al., 2019 | Russia | 1574 dental panoramic radiographs. | Teeth detection and numbering. | “To propose and evaluate a novel solution based on CNN for automatically performing the tasks of teeth detection and numbering from dental panoramic radiographs.” | 1574 randomly selected dental panoramic radiographs were divided into training (n = 1352) and testing (n = 22) subsets. Comparison of sensitivity, specificity and precision of tooth detection and numbering by the CNN based algorithm was done with information provided by five experts in oral radiology. |
CNN | Sensitivity, specificity and precision of tooth detection and numbering. | CNN based algorithm achieved a sensitivity of 0.9941 and precision of 0.9945 for teeth detection, and sensitivity of 0.9800 and specificity of 0.9994 for teeth numbering. These results were comparable to that of the experts (teeth detection – sensitivity of 0.9980/precision of 0.9998; teeth numbering – sensitivity of 0.9893/specificity of 0.9997). | Majority of the misclassifications were observed among teeth neighboring edentulous spaces. | Further scope for improvement of in terms of advanced augmentation techniques, extended datasets and use of more recent CNN architectures. |
| Fukuda et al., 2020 | Japan | 300 dental panoramic radiographs, including 330 teeth with VRF. | Detecting vertical root fracture in teeth. | “To evaluate the use of a CNN system for detecting VRF on panoramic radiography.” | 300 dental panoramic radiographs were randomly divided into training (n = 240) and testing (n = 60) images. For comparison, the presence of VRF line was confirmed by 3 experts (2 radiologists and 1 endodontist). | CNN | Recall, precision, and F measure for diagnostic performance. | 267 teeth with VRF (80.9%) were accurately detected by the CNN algorithm. In addition, 20 teeth without VRF were falsely diagnosed. Values for recall, precision and F measure were 0.75, 0.93, and 0.83, respectively. | Inadequate training items, inclusion of only radiographs with clear VRF lines and single center study. | Further large scale studies to address limitations. |
| Mallishery et al., 2020 | India | 500 root canal treatment cases. | Difficulty level and decision for referral in root canal treatment cases. | “To generate a machine learning algorithm which can help predict the difficulty level of the root canal treatment case and decide about a referral, with the help of the standard AAE endodontic case difficulty assessment form.” | 500 root canal treatment cases recorded using AAE endodontic case difficulty assessment form, were assessed by 2 pre-calibrated endodontists (3rd endodontist was consulted for conflicting opinions). This was compared with the algorithm generated through ANN. | ANN | Sensitivity of the ANN algorithm for identifying difficulty level and deciding referral. | ANN algorithm achieved a sensitivity of 94.96%. | No analysis of alternative sources of data. | Further ANN algorithms should include alternative sources of data such as radiographs and clinical findings in conjunction with the AAE case difficulty assessment form. |
| Orhan et al., 2020 | Turkey | 153 CBCT images of periapical lesions acquired from 109 patients. | Detection, localization and volume determination of periapical Lesions. |
“To verify the diagnostic performance of an artificial intelligence system based on the DCNN method to detect periapical pathosis CBCT images.” | 153 CBCT images showing periapical lesions were evaluated by an expert human observer using manual segmentation in a medical imaging software. DCNN was trained and evaluated for detecting, localizing and determining volume of the periapical lesions from CBCT images. | DCNN | Reliability of DCNN for detection, localization and volume determination of periapical Lesions. |
The DCNN detected and localized teeth with a reliability of 92.8%. 142 of 153 periapical lesions were correctly detected and only tooth was wrongly localized. Comparing volume determination of the lesions by DCNN and manual segmentation, there was no significant difference (p > 0.05). | DCNN based measurements may have been altered by the presence of perio-endo lesions, PDL tissue loss and alveolar bone defects. | Further studies to address algorithms which include variation of normal dentoalveolar anatomy. |
ANN – Artificial neural network, DCNN – Depp convolutional neural network, CBCT – Cone beam computed tomography, ROC - Receiver operating characteristics, AUC – Area under curve, PDL – Periodontal ligament, AI – Artificial intelligence, CNN – Convolutional neural network, VRF – Vertical root fracture, AAE – American association of endodontists.
Research methodologies of the reviewed studies used various AI models and methods with specified architectures or frameworks. Although the most common AI network architecture used in the studies were based on ANNs, DCNN based AI architecture were also reported (Saghiri et al., 2012c, Saghiri et al., 2012a, Miki et al., 2017a). The main purposes of these networks or frameworks, as reported in the studies, were either to classify the teeth or detect the anatomic position of the teeth or find the location of the apical lesion or diagnose the dental problems such as dental caries, dental decay, or hypersensitive teeth (Lee et al., 2018a, Bouchahma et al., 2019). Furthermore, these networks were also used by endodontists to make decisions for timely referrals or management plans for the patients (Mallishery et al., 2020). A wide range of statistical parameters were utilized as markers of validity, reliability, and accuracy to compare the results obtained by these AI methods (Bouchahma et al., 2019, Mallishery et al., 2020), as opposed to those achieved by experts in the field of endodontics as shown in Table 2.
3.3. Findings reported in different studies regarding the validity of artificial intelligence methods in the field of endodontics
The review elicited scarcity of researches on assessing whether AI or ANN can help to diagnose or treat dental problems and assist dentists in the field of endodontics. However, the findings synthesized and appraised in this systematic review demonstrates that there is a vital role played by these artificial neural networks to assist the treatment and diagnoses of dental issues.
3.3.1. Evaluating studies utilizing ANN
The study conducted by Hu et al. (2019) tested the practical application of a mobile neuroimaging-based clinical amplified reality and AI structure, to detect the pain and also locate it from the patient’s brain in real-time (Hu et al., 2019). Impressively, ANN associated analysis performed on split data history segments demonstrated appreciable accuracy (Hu et al., 2019). More specifically, the authors found that the prediction accuracy of ANN was 80.37%, with a positive likelihood ratio of 2.35 having a sensitivity of 32.6%, and a specificity of 86.1%. Similarly, ANN, which was performed on split data history blocks with reweighted loss function obtained the sensitivity of 40.9% and specificity of 80.1% and a positive likelihood of 2.06. Likewise, another study conducted by Saghiri et al., 2012a, Saghiri et al., 2012b, Saghiri et al., 2012c found that the ANN framework was able to locate the apical foramen in >90% of the samples. (Saghiri et al., 2012b). Hence the authors recommended that ANN frameworks can help to improve the accuracy of dental diagnosis in the future and thus can lead to better treatment outcomes in endodontics (Saghiri et al., 2012c) (Table 2).
3.3.2. Studies explaining the implications of CNN
Interestingly, the previously mentioned study conducted by Hu, Nascimento et al., in addition to using an ANN architecture also utilized CNN and showed a specificity of 89.6%, but with a positive likelihood of 1.39 and a sensitivity of 14.4% (Hu et al., 2019). Another study conducted by Ekert et al. (2019a) applied CNN on panoramic radiographs to identify the apical lesions to prove the hypothesis that usage of CNN may increase discriminatory ability and reliability (Ekert et al., 2019b). The research data revealed that a moderately CNN trained on a very restricted image data demonstrated sufficient discriminatory ability to identify apical lesions on panoramic radiographs (Ekert et al., 2019b). Hence, these CNN can improve the reliability and assist dentists to obtain comparative results as that of expert dentists (Ekert et al., 2019b). (Table 2).
3.3.3. Studies emphasizing the use ANN, CNN and DCNN
The study conducted in South Korea by Lee, Kim, Jeong, & Choi, found that deep algorithm developed based on DCNN had high potential to correctly identify and diagnose dental caries (Lee et al., 2018a). Overall, DCNN algorithm delivered significant output in identifying dental caries on periapical radiographs (Lee et al., 2018b). Likewise, Tuzoff et al. (2019) conducted a study in Russia and found that DCNN are 99.4% sensitive and elaborated that these findings are comparable to the validity and reliability results of the expert dentists in numbering and detecting teeth on panoramic radiographs with analogous sensitivity and specificity. On the other hand, similar study conducted in 2012 in Iran found significant differences between data acquired from endodontists and ANN by stereomicroscope after extraction. More specifically, endodontists obtained correct and accurate measurements in 76% of the cases, while ANN were able to locate the correct position of teeth in 96% of the cases. (Saghiri et al., 2012). Similarly, another study conducted in Turkey found higher reliability (92.8%) of the DCNN system to detect a periapical lesion, and the same framework also identified teeth and numbered them correctly except missing one tooth (Orhan et al., 2020). The AI based system identified 142 out of 153 periapical lesions. The authors also found a significant and positive correlation between the measurements made by the radiologists and by a DCNN system. However, measurements made by DCNN and manual segmentation were not significantly different (P > 0.05) (Orhan et al., 2020).
These findings are consistent with studies conducted by Fukuda et al. (2019) where authors developed an AI framework for identifying vertical tooth fracture on panoramic radiography and authors found that the CNN model was able to detect vertical tooth fracture on panoramic images (Fukuda et al., 2019). Moreover, a study conducted by Miki et al. (2017b) investigated the utility of the DCNN to classify teeth, and the authors found an improved classification performance with high accuracy of 91.0% (Miki et al., 2017b). Authors also alluded that one of the benefits of this DCNN is that it does not require the precise tooth segmentation as opposed to conventional feature-based classification. Authors also illustrated that ANN can successfully predict for dental decay with an accuracy of 72% to 98%. These findings are also supported by a similar research report conducted in 2019 where authors found a sensitivity of 94.96% by using a machine learning algorithm for making decisions and making referrals (Mallishery et al., 2019).
4. Discussion
The current systematic review about the role of neural AI in treatment planning and diagnosis in the field of endodontics appraised and synthesized findings on the pre-defined objective. In General, the review results reveal that most of the available literature was from developed countries with very few studies from the developing countries. Overall, there is a dearth of the research on assessing whether AI or associated ANN, CNN or DCNN can help to diagnose or treat dental problems and assist dentists in the field of endodontics. Almost all of the studies in this review supported the findings that artificial intelligence can play a crucial role in diagnosing various dental issues and even can classify the number of teeth and anatomical location of the same with the highest accuracy. Hence the AI with different models or frameworks and algorithms can help dentists to diagnose and manage the dental problems with greater accuracy.
These findings are analogous to the existing literature where AI has been used to assist the health expert to a larger extent. The ANN is primarily a mixture of soft and hardware imitation of a human brain, which learns to recognize patterns from the input of the data followed by making extrapolations by identifying comparable arrangements in future data (Prieto et al., 2016). For instance, the literature highlights that virtual dental assistants developed using the machine algorithm of AI can undertake a plethora of activities with substantial accuracy in the dental clinics or office with the lower workforce when compared to human beings (Zhuet al., 2018). More specifically, these AI-based frameworks and algorithms can help to manage insurance claims, arrange appointments and help health experts in the diagnosis and management of patients in diverse dental fields such as oral radiology, oral pathology, and oral medicine (Drozdzal et al., 2018). AI-based networks are also capable of assisting dentists to complete history and examination of the patients as well as other history related to diet, alcoholism, and smoking (Drozdzal et al., 2018). Interestingly, AI-based networks are also successfully used in emergency Tele-assistance, when the doctor is not physically available to attend the patient (Drozdzal et al., 2018).
Similar to the results in the present review, findings from the existing literature also reveal that results regarding accuracy are more significant for AI-based networks when compared to conventional methods (Drozdzal et al., 2018). These networks are also used successfully in the field of orthodontics where patients are diagnosed and managed by using radiographs and photographs by scanners in the oral cavity, which works on the codes of AI. Nevertheless, the movement of teeth and final treatment outcomes can also be anticipated with the help of these AI algorithms (Drozdzal et al., 2018). For effective and accurate clinical practice, a precise diagnosis is considered as a robust basis. Competently skilled neural networks can be a valuable asset for dentists, chiefly in the management of illnesses and disorders with numerous causes or factors (Jha and Topol, 2016). In this regard, AI can be considered as one of the best measures in diagnosing and treatment planning or treating dental problems. Even nominal to minuscule variations at the level of single-pixel which might go unnoticeable by the human eye can be distinguished using AI based networks (Topol, 2019).
4.1. Strengths and limitations of the study
Despite the fact that this is the first systematic review on the role of neural AI in treatment planning and diagnosis in the field of endodontics from this geographic region, the present review has some potential limitations. Firstly, this review did not include the grey literature and there is a possibility that unpublished studies on the same topic might have different results. Secondly, most of the reviewed studies were from developed countries with very few studies conducted in the developing countries, thereby making the results difficult to be generalized. Thirdly, this review included papers only in the English language and might have overlooked the important literature published in local languages. Lastly, the heterogeneity in the sample size of the studies included in the systematic review with a fewer number of cases included in the majority of the studies.
5. Conclusion and future research
To summarize, AI models described in the research articles of this systematic review demonstrated numerous promising uses in the field of endodontics, such as identification of apical lesions, classifying and numbering the teeth, detecting dental caries, periodontitis/periapical disease, diagnosing different dental problems, helping dentists to make referrals, and also helping them to make plans for the treatment of dental disorders in a timely and effective way. However, given the limitations of the studies, future studies should be conducted with larger sample sizes to assess the validity and specificity of AI in daily dental practice, especially from a perspective of making short term and long term plans for patients with dental disorders. It is also important to consider that all the algorithms used on the principles of AI may not be appropriate for clinical application, and further studies are recommended to evaluate the analytic programs for different scenarios. Besides, the diagnostic performance of the AI networks is not similar across all algorithms used, therefore, it is crucial to validate the reliability and external validity of these networks by using representative and larger samples from several settings before extrapolating and applying these frameworks and algorithms, in their entirety, into the field of endodontics.
Ethical Statement
Not applicable.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors would like to thank the Deanship of Scientific Research at Majmaah University for all the support.
Footnotes
Peer review under responsibility of King Saud University.
References
- Akerkar R. Artificial Intelligence for Business. Springer; 2019. Introduction to artificial intelligence; pp. 1–18. [Google Scholar]
- Alexander B., John S. Artificial intelligence in dentistry: Current concepts and a peep into the future. Int. J. Adv. Res. 2018;6(12):1105–1108. [Google Scholar]
- Bouchahma M., et al. 2019 IEEE/ACS 16th International Conference on Computer Systems and Applications (AICCSA) IEEE; 2019. An Automatic Dental Decay Treatment Prediction using a Deep Convolutional Neural Network on X-Ray Images. [Google Scholar]
- Chen Y.-W., et al. Artificial intelligence in dentistry: Current applications and future perspectives. Quintessence Int. 2020;51:248–257. doi: 10.3290/j.qi.a43952. [DOI] [PubMed] [Google Scholar]
- Da Silva I.N., et al. Springer International Publishing; Cham: 2017. Artificial Neural Networks; p. 39. [Google Scholar]
- Deshmukh S.V. Artificial intelligence in dentistry. J. Int. Clin. Dental Res. Organ. 2018;10(2):47. [Google Scholar]
- Drozdzal M., et al. Learning normalized inputs for iterative estimation in medical image segmentation. Medical Image Analysis. 2018;44:1–13. doi: 10.1016/j.media.2017.11.005. [DOI] [PubMed] [Google Scholar]
- Ekert T., et al. Deep learning for the radiographic detection of apical lesions. J. Endodont. 2019;45(7) doi: 10.1016/j.joen.2019.03.016. 917-922. e915. [DOI] [PubMed] [Google Scholar]
- Ekert T., et al. Deep Learning for the Radiographic Detection of Apical Lesions. Jo. Endodontics. 2019;45(7) doi: 10.1016/j.joen.2019.03.016. 917-922 e915. [DOI] [PubMed] [Google Scholar]
- Fukuda M., et al. Evaluation of an artificial intelligence system for detecting vertical root fracture on panoramic radiography. Oral Radiol. 2019:1–7. doi: 10.1007/s11282-019-00409-x. [DOI] [PubMed] [Google Scholar]
- Fukuda M., et al. Evaluation of an artificial intelligence system for detecting vertical root fracture on panoramic radiography. Oral. Radiol. 2020;36(4):337–343. doi: 10.1007/s11282-019-00409-x. [DOI] [PubMed] [Google Scholar]
- Hu X.-S., et al. Feasibility of a Real-Time Clinical Augmented Reality and Artificial Intelligence Framework for Pain Detection and Localization From the Brain. J. Medical Internet Res. 2019;21(6):e13594. doi: 10.2196/13594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung K., et al. The use and performance of artificial intelligence applications in dental and maxillofacial radiology: A systematic review. Dentomaxillofacial Radiol. 2020;49(1):20190107. doi: 10.1259/dmfr.20190107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishwarya T.A., et al. A modern approach to design and integrate conceptual methods in video games with artificial intelligence. Mater. Today: Proc. 2017;4(8):9100–9106. [Google Scholar]
- Jha S., Topol E.J. Adapting to artificial intelligence: radiologists and pathologists as information specialists. JAMA. 2016;316(22):2353–2354. doi: 10.1001/jama.2016.17438. [DOI] [PubMed] [Google Scholar]
- Kalappanavar A., et al. Artificial intelligence: A dentist's perspective. J. Med., Radiol., Pathol. Surgery. 2018;5(2):2–4. [Google Scholar]
- Khanna S.S., Dhaimade P.A. Artificial intelligence: Transforming dentistry today. Indian J. Basic Appl. Med. Res. 2017;6(4):161–167. [Google Scholar]
- Klyuchko O. Application of artificial neural networks method in biotechnology. Biotechnol. Acta. 2017;10:4. [Google Scholar]
- Lebedev M.A., Nicolelis M.A. Brain-machine interfaces: From basic science to neuroprostheses and neurorehabilitation. Physiol. Rev. 2017;97(2):767–837. doi: 10.1152/physrev.00027.2016. [DOI] [PubMed] [Google Scholar]
- Lee J.-H., et al. Detection and diagnosis of dental caries using a deep learning-based convolutional neural network algorithm. J. Dentistry. 2018;77:106–111. doi: 10.1016/j.jdent.2018.07.015. [DOI] [PubMed] [Google Scholar]
- Lee J.H., et al. Detection and diagnosis of dental caries using a deep learning-based convolutional neural network algorithm. J. Dent. 2018;77:106–111. doi: 10.1016/j.jdent.2018.07.015. [DOI] [PubMed] [Google Scholar]
- Mallishery S., et al. The precision of case difficulty and referral decisions: an innovative automated approach. Clin. Oral Investigat. 2019:1–7. doi: 10.1007/s00784-019-03050-4. [DOI] [PubMed] [Google Scholar]
- Mallishery S., et al. The precision of case difficulty and referral decisions: an innovative automated approach. Clin. Oral Investigat. 2020;24(6):1909–1915. doi: 10.1007/s00784-019-03050-4. [DOI] [PubMed] [Google Scholar]
- Miki Y., et al. Classification of teeth in cone-beam CT using deep convolutional neural network. Comput Biol Med. 2017;80:24–29. doi: 10.1016/j.compbiomed.2016.11.003. [DOI] [PubMed] [Google Scholar]
- Miki Y., et al. Classification of teeth in cone-beam CT using deep convolutional neural network. Comput. Biol. Med. 2017;80:24–29. doi: 10.1016/j.compbiomed.2016.11.003. [DOI] [PubMed] [Google Scholar]
- Miller D.D., Brown E.W. Artificial Intelligence in Medical Practice: The Question to the Answer? Am. J. Med. 2018;131(2):129–133. doi: 10.1016/j.amjmed.2017.10.035. [DOI] [PubMed] [Google Scholar]
- Moher D., et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int. J. Surg. 2010;8(5):336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Naik, M., I. d. N. de At, 2016. Future of Endodontics. Int. J. Curr. Res. 8, 25610–25616.
- Ojha V.K., et al. Metaheuristic design of feedforward neural networks: A review of two decades of research. Eng. Appl. Artif. Intell. 2017;60:97–116. [Google Scholar]
- Orhan K., et al. Evaluation of artificial intelligence for detecting periapical pathosis on cone-beam computed tomography scans. Int. Endod. J. 2020 doi: 10.1111/iej.13265. [DOI] [PubMed] [Google Scholar]
- Park W.J., Park J.-B. History and application of artificial neural networks in dentistry. Eur. J. Dent. 2018;12(04):594–601. doi: 10.4103/ejd.ejd_325_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto A., et al. Neural networks: An overview of early research, current frameworks and new challenges. Neurocomputing. 2016;214:242–268. [Google Scholar]
- Richardson K. Columbia University Press; 2017. Genes, brains, and human potential: the science and ideology of intelligence. [Google Scholar]
- Saghiri M., et al. A new approach for locating the minor apical foramen using an artificial neural network. Int. Endodontic J. 2012;45(3):257–265. doi: 10.1111/j.1365-2591.2011.01970.x. [DOI] [PubMed] [Google Scholar]
- Saghiri M.A., et al. A new approach for locating the minor apical foramen using an artificial neural network. Int. Endod. J. 2012;45(3):257–265. doi: 10.1111/j.1365-2591.2011.01970.x. [DOI] [PubMed] [Google Scholar]
- Saghiri M.A., et al. The reliability of artificial neural network in locating minor apical foramen: a cadaver study. J. Endodont. 2012;38(8):1130–1134. doi: 10.1016/j.joen.2012.05.004. [DOI] [PubMed] [Google Scholar]
- Schardt C., et al. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med. Informat. Decis. Making. 2007;7(1):16. doi: 10.1186/1472-6947-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar R.S., et al. 2019 IEEE International Conference on System, Computation, Automation and Networking (ICSCAN) IEEE; 2019. The Source of Growing Knowledge by Cognitive Artificial Intelligence. [Google Scholar]
- Singh, S., et al., 1956. Artificial Intelligence in Dentistry: The Way Forward.
- Stuart, R., Peter, N., 2016. Artificial intelligence-a modern approach, 3rd ed., Berkeley.
- Talari A.C., et al. Advancing cancer diagnostics with artificial intelligence and spectroscopy: identifying chemical changes associated with breast cancer. Expert Rev. Mol. Diagnost. 2019;19(10):929–940. doi: 10.1080/14737159.2019.1659727. [DOI] [PubMed] [Google Scholar]
- Topol E.J. High-performance medicine: the convergence of human and artificial intelligence. Nature Med. 2019;25(1):44–56. doi: 10.1038/s41591-018-0300-7. [DOI] [PubMed] [Google Scholar]
- Tuzoff D.V., et al. Tooth detection and numbering in panoramic radiographs using convolutional neural networks. Dento Maxillo Facial Radiol. 2019;48(4):20180051. doi: 10.1259/dmfr.20180051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav D., Sehrawat R. Artificial intelligence integration in healthcare and Medicine. Artif. Intell. 2018;7:4. [Google Scholar]
- Yaji A. Artificial Intelligence in Dento-Maxillofacial Radiology. Acta Scientific Dental Sci. 2019;3:116–121. [Google Scholar]
- Zhu J., et al. Bayesian inference of phylogenetic networks from bi-allelic genetic markers. PLoS Comput. Biol. 2018;14(1):e1005932. doi: 10.1371/journal.pcbi.1005932. [DOI] [PMC free article] [PubMed] [Google Scholar]