Abstract
Malaria transmission-blocking vaccines induce antibodies that target Plasmodium in the mosquito vector. We recently reported that Pfs230 vaccine achieves superior activity to Pfs25 in humans. Here, we describe clonal expansion in the variable region of immunoglobulin heavy chains (VH) of antigen-specific single B cells collected from humans immunized with Pfs230D1-EPA or Pfs25-EPA conjugate vaccines formulated in Alhydrogel®. Based on studies of CD27+ memory B cells following Pfs230 vaccination, clonal expansion and somatic hypermutation was seen in four of five subjects. Pfs25 did not induce sufficient CD27+ cells for sorting; based instead on CD19+ Pfs25-reactive B cells, clonal expansion was only seen in two of five subjects. Clonal expansions and mutations in Pfs230-specific single B cells combined with the enhanced activity of Pfs230 antibodies by complement, might justify the outstanding activity of Pfs230D1 as a TBV candidate.
Keywords: Pfs230, Pfs25, Malaria transmission-blocking vaccines, B cell sequencing, Clonal expansion, VH mutation
Graphical Abstract
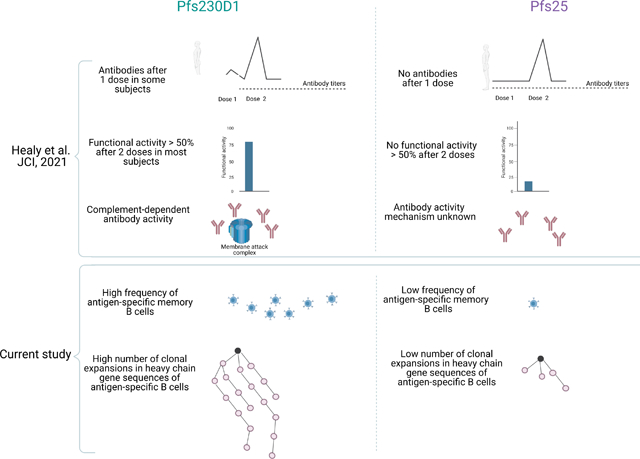
Recent advances in malaria vaccine development have expanded the array of Plasmodium falciparum antigens in clinical trials (Duffy and Patrick Gorres, 2020), such as sexual-stage proteins expressed by the parasite in the mosquito vector. By targeting sexual-stage proteins, vaccines seek to reduce the rate of parasite transmission in the community. Malaria transmission-blocking vaccines (TBVs) act primarily by eliciting antibodies that neutralize sexual stage parasites (Sagara et al., 2018; Coelho et al., 2021) but mechanisms that promote antibody diversification during TBV immunization are not yet clear. Among the leading TBV candidates, P. falciparum surface 230 kD protein Pfs230 and Pfs25 have shown promising activity in preclinical and clinical studies (Goodman et al., 2011; Talaat et al., 2016; Sagara et al., 2018; Singh et al., 2019; Tachibana et al., 2019). Our group previously demonstrated that when Pfs25 or the first domain of Pfs230 (Pfs230D1) are conjugated to the Exoprotein A (EPA) carrier and formulated with the Th2 adjuvant Alhydrogel®, both vaccine candidates induce functional antibodies in murine and non-human primate models (Healy et al., 2021). However, Pfs25-EPA has shown modest activity in humans (Talaat et al., 2016; Sagara et al., 2018), while Pfs230 induces superior activity in healthy volunteers (Healy et al., 2021). Furthermore, antibody responses to Pfs230 vaccine occur earlier than for Pfs25 in humans: Pfs25 antibody arises after the second dose, while Pfs230D1 antibody does so after the first dose in some recipients (Healy et al., 2021). The functionality of both vaccines is strongly dependent on antibody activity (Sagara et al., 2018; Healy et al., 2021) and complement enhances the activity of Pfs230 antibodies (Coelho et al., 2021), but a full understanding of the superior functional immunogenicity of Pfs230 over Pfs25 remains to be elucidated.
The success of a malaria vaccine that is mediated by antibody is determined by the capacity of immunoglobulins secreted by B cells to recognize and bind to an antigen, and then to neutralize the parasite. A diverse B cell receptor (BCR) repertoire is generated by the variable, diversity and joining regions (VDJ) recombination process that occurs in early stages of B cell maturation, and by somatic hypermutation and selection of B cells with higher affinity for a given antigen during immunization or infection. Thus, deep sequencing of BCRs can be used as a tool to measure changes in the BCR repertoire following immunization, and to determine how these changes are related to functional activity (Galson et al., 2015, 2016; Truck et al., 2015). Although antibodies are encoded by sequences of light and heavy chains, analyses of the heavy chain are considered sufficient to determine the signature in response to vaccination (Xu and Davis, 2000; Zhou and Kleinstein, 2019), since most of the sequence variation and antigen binding is mediated by the heavy chain.
Here, we sought to analyze heavy chain variable (VH) regions of Pfs230- or Pfs25-specific single B cells (henceforth called Pfs25 or Pfs230 B cells) from 10 Malian adults receiving either Pfs25-EPA/Alhydrogel® or Pfs230D1-EPA/Alhydrogel® vaccine (ClinicalTrials.gov NCT02334462). Subjects received four 40 μg doses of Pfs230D1 or four 47 μg doses of Pfs25 at days 0, 28, 168, 530 of the study. Peripheral blood mononuclear cells (PBMCs) were collected 14 days after the last dose, at day 554. The clinical study was approved by the ethics review boards from the Faculté de Médecine, de Pharmacie et d’OdontoStomatologie (FMPOS) Bamako, Mali and the US National Institute of Allergy and Infectious Diseases (NIH, Bethesda, Maryland, USA). The safety and immunogenicity results of the vaccinations in US malaria-naïve subjects are reported elsewhere (Healy et al., 2021). B cell staining, gating strategy and flow cytometry sorting were performed as previously described (Coelho et al., 2021).
The less potent immunogenicity of Pfs25-EPA/Alhydrogel® compared with Pfs230-EPA/Alhydrogel® was reflected by the low proportion of Pfs25 B cells among CD27+ memory B cells, resulting in an insufficient number for flow cytometry sorting. Instead, we sorted Pfs25 B cells from the CD19+CD20+ total B cell population, while we sorted the more abundant Pfs230 B cells from the CD19+CD20+CD27+ population. The average percentage of Pfs230 B cells was 1.34% (min= 0.03%; max= 2.70%) among CD27+ memory B cells, while the average percentage of Pfs25 B cells was 2.18% among the CD19+CD20+ total B cell population (min= 0.70%; max= 3.90%) (Supplementary Fig. S1). Nested PCR targeting the VDJ region and sequencing of single B cells were performed by iRepertoire as previously described (Coelho et al., 2021). We obtained 105 VH sequences from Pfs230 B cells and 101 VH sequences from Pfs25 B cells from 10 subjects in total (five subjects in each of the two vaccine groups) (Supplementary Table S1).
B cells that acquire mutations during germinal center reactions can improve antibody-antigen binding and are selected for further expansion, leading to affinity maturation of the antigen-specific B cell population (Kepler and Perelson, 1993). Thus, analyses of somatic hypermutation can reveal the expansion of clones that are related to higher antibody affinity. We performed analyses to evaluate clonal expansion in those BCR sequences using the R package Alakazam (Nouri and Kleinstein, 2020). Sequences were defined as being part of the same clonal expansion if they shared the same V and J genes, and were >90% similar in their CDR3 sequence. Data were submitted to Mendeley Data (doi: 10.17632/4xhwkd3jfx.1),
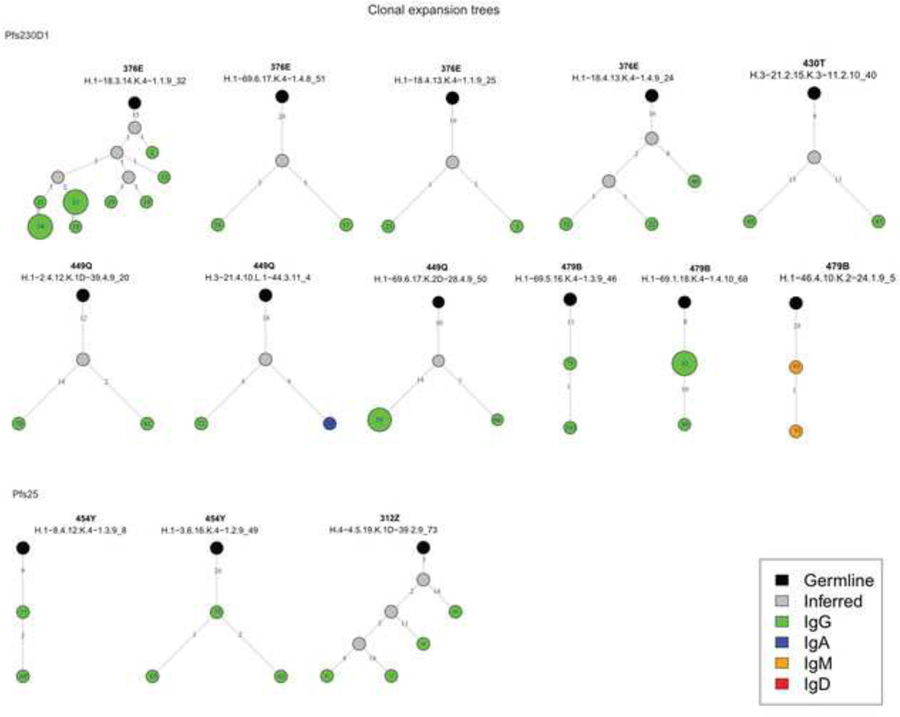
As expected by the sorting of CD27+ cells for Pfs230 and CD19+CD20+ for Pfs25, our results revealed that clonal expansions occurred with more frequency among Pfs230 B cells than for Pfs25 B cells, evidenced by a higher number of clusters (Fig. 1) and larger sizes of clusters that diverged from germline sequences (Fig. 2A). In addition, the number of mutations in the VH region was higher in Pfs230 B cells than Pfs25 B cells (Fig. 2B).
Fig. 1.
Lineages of Plasmodium falciparum surface 230 kD protein Pfs230 or Pfs25-specific clonal expansions. Sequences were defined as being part of the same clonal expansion if they shared the same V and J genes, and were >90% similar in their CDR3 sequence. Bold numbers represent subject ID and are shown above the cluster name. Black circles represent germline sequences, gray circles inferred sequences, and green circles the ID number of the heavy chain variable domain (VH) sequences identified. Numbers in the lines represent number of somatic hypermutations compared with the ancestor. Maximum parsimony tree was drawn using the R package alakazam.
Fig. 2.
Clonal expansion analyses of antigen-specific single B cells from subjects immunized with Plasmodium falciparum surface 230 kD protein Pfs230D1-ExoProtein A (EPA)/Alhydrogel Pfs25-EPA/Alhydrogel. (A) Cluster sizes and isotypes of the sequences. (B) Numbers of mutations in the heavy chain variable domain (VH) region. nt, nucleotide.
Pfs230 vaccine yielded a higher number of mutations in the IgM repertoire of CD19+CD20+CD27+ Pfs230 B cells compared with CD19+CD20+ Pfs25 B cells (Fig. 2, although this could be explained by the sorting of different populations. Three sequences from Pfs230 B cells were determined to be IgA.
Age groups differ in their IgM and IgA repertoire responses to pneumococcal vaccination (Wu et al., 2012), but here we used samples from subjects within a similar age range (Pfs230 = 25–40 years, average= 34; Pfs25= 25–51 years, average= 42). Thus, we believe the differing mutation profiles within antibody isotypes are the result of antigen-specific stimulation effects. IgA responses can be elicited in response to malaria vaccines, but as we determined here, they usually comprise a small proportion of the antibody repertoire (Biswas et al., 2014; Feng et al., 2018).
Three subjects from the Pfs25 group and one from the Pfs230 group showed no evidence of clonal expansion, although the only Pfs230 subject without evidence of clonal expansion had just two sequences analyzed (Supplementary Table S1). Previous data showed that immunization with infectious P. falciparum sporozoites under cover of chloroquine prophylaxis promoted strong B cell clonal expansion in five out of eight subjects (Murugan et al., 2018). All clusters determined in the Pfs230 B cells from subject 1 were shared with other subjects, totaling four convergent clusters (Supplementary Table S2), versus three convergent clusters for Pfs25 (Supplementary Table S3). We did not find a direct correlation between antibody titers or functional activity and clonal expansion, although subject 1 developed the highest number of clusters (Fig. 1) and had the highest antibody titers among Pfs230 vaccinees (Supplementary Table S4).
A caveat for interpretation of our study involves the comparison between Pfs25 B cells from the CD19+CD20+ population with Pfs230 B cells from the CD19+CD20+CD27+ population. We would expect higher mutation rates in CD27+ cells compared with CD19+20+ cells. However, this reflected the dearth of CD27+ cells induced in response to Pfs25. Further, we used antigen tetramers to sort antigen-specific B cells and confirmed the specificity of the probe for both antigens (Coelho et al., 2020, 2021).
In conclusion, Pfs230D1 induces a greater number of CD27+ cells than Pfs25, and a clonal expansion of antigen-specific B cells in four out of five subjects. This, allied to the enhanced activity of Pfs230 antibodies by complement, might contribute to the excellent activity of Pfs230D1 as a TBV candidate (Healy et al., 2021).
Supplementary Material
Supplementary Fig. S1. Percentage of Plasmoidum falciparum surface 230 kD protein Pfs230-specific B cells among memory B cell population (CD19+CD20+CD27+) and of Pfs25-specific B cells among total B cells (CD19+CD20+). Tetramer staining and sorting were performed as previously described (Coelho et al., 2020).
Highlights.
Previously, we showed that immunogenicity and activity of Pfs230D1 vaccine is superior to Pfs25 in humans.
Clonal expansion in IG heavy chains (VH) of Pfs230 B cells occurs with high frequency in response to Pfs230D1 vaccine.
A stronger B cell response might contribute to superior Pfs230D1 activity.
Acknowledgements
CHC and PED are supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health, USA. JDG is an employee of Alchemab Therapeutics Limited.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Biswas S, Choudhary P, Elias SC, Miura K, Milne KH, de Cassan SC, Collins KA, Halstead FD, Bliss CM, Ewer KJ, Osier FH, Hodgson SH, Duncan CJ, O’Hara GA, Long CA, Hill AV, Draper SJ, 2014. Assessment of humoral immune responses to blood-stage malaria antigens following ChAd63-MVA immunization, controlled human malaria infection and natural exposure. PLoS One 9, e107903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho CH, Nadakal ST, Gonzales Hurtado PA, Morrison R, Galson JD, Neal J, Wu Y, King CR, Price V, Miura K, Wong-Madden S, Dortichamou JY, Narum DL, MacDonald NJ, Snow-Smith M, Vignali M, Taylor JJ, Lefranc MP, Truck J, Long CA, Sagara I, Fried M, Duffy PE, 2020. Antimalarial antibody repertoire defined by plasma IG proteomics and single B cell IG sequencing. JCI Insight 5(22):e143471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho CH, Tang WK, Burkhardt M, Galson JD, Muratova O, Salinas ND, Alves ESTL, Reiter K, MacDonald NJ, Nguyen V, Herrera R, Shimp R, Narum DL, Byrne-Steele M, Pan W, Hou X, Brown B, Eisenhower M, Han J, Jenkins BJ, Doritchamou JYA, Smelkinson MG, Vega-Rodriguez J, Truck J, Taylor JJ, Sagara I, Renn JP, Tolia NH, Duffy PE, 2021. A human monoclonal antibody blocks malaria transmission and defines a highly conserved neutralizing epitope on gametes. Nat Commun 12, 1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy PE, Patrick Gorres J, 2020. Malaria vaccines since 2000: progress, priorities, products. NPJ Vaccines 5, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng G, Boyle MJ, Cross N, Chan JA, Reiling L, Osier F, Stanisic DI, Mueller I, Anders RF, McCarthy JS, Richards JS, Beeson JG, 2018. Human Immunization With a Polymorphic Malaria Vaccine Candidate Induced Antibodies to Conserved Epitopes That Promote Functional Antibodies to Multiple Parasite Strains. J Infect Dis 218, 35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galson JD, Truck J, Clutterbuck EA, Fowler A, Cerundolo V, Pollard AJ, Lunter G, Kelly DF, 2016. B-cell repertoire dynamics after sequential hepatitis B vaccination and evidence for cross-reactive B-cell activation. Genome Med 8, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galson JD, Truck J, Fowler A, Clutterbuck EA, Munz M, Cerundolo V, Reinhard C, van der Most R, Pollard AJ, Lunter G, Kelly DF, 2015. Analysis of B Cell Repertoire Dynamics Following Hepatitis B Vaccination in Humans, and Enrichment of Vaccine-specific Antibody Sequences. EBioMedicine 2, 2070–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman AL, Blagborough AM, Biswas S, Wu Y, Hill AV, Sinden RE, Draper SJ, 2011. A viral vectored prime-boost immunization regime targeting the malaria Pfs25 antigen induces transmission-blocking activity. PLoS One 6, e29428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy SA, Anderson CF, Swihart BJ, Mwakingwe-Omari A, Gabriel EE, Decederfelt H, Hobbs CV, Rausch KM, Zhu D, Muratova O, Herrera R, Scaria PV, MacDonald NJ, Lambert LE, Zaidi I, Coelho CH, Renn JP, Wu Y, Narum DL, Duffy PE, 2021. Pfs230 yields higher malaria transmission-blocking vaccine activity than Pfs25 in humans but not mice. J Clin Invest 131(7), e146221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepler TB, Perelson AS, 1993. Somatic hypermutation in B cells: an optimal control treatment. J Theor Biol 164, 37–64. [DOI] [PubMed] [Google Scholar]
- Murugan R, Buchauer L, Triller G, Kreschel C, Costa G, Pidelaserra Marti G, Imkeller K, Busse CE, Chakravarty S, Sim BKL, Hoffman SL, Levashina EA, Kremsner PG, Mordmuller B, Hofer T, Wardemann H, 2018. Clonal selection drives protective memory B cell responses in controlled human malaria infection. Sci Immunol 3, eaap8029. [DOI] [PubMed] [Google Scholar]
- Nouri N, Kleinstein SH, 2020. Somatic hypermutation analysis for improved identification of B cell clonal families from next-generation sequencing data. PLoS Comput Biol 16, e1007977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagara I, Healy SA, Assadou MH, Gabriel EE, Kone M, Sissoko K, Tembine I, Guindo MA, Doucoure M, Niare K, Dolo A, Rausch KM, Narum DL, Jones DL, MacDonald NJ, Zhu D, Mohan R, Muratova O, Baber I, Coulibaly MB, Fay MP, Anderson C, Wu Y, Traore SF, Doumbo OK, Duffy PE, 2018. Safety and immunogenicity of Pfs25H-EPA/Alhydrogel, a transmission-blocking vaccine against Plasmodium falciparum: a randomised, double-blind, comparator-controlled, dose-escalation study in healthy Malian adults. Lancet Infect Dis 18, 969–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SK, Thrane S, Chourasia BK, Teelen K, Graumans W, Stoter R, van Gemert GJ, van de Vegte-Bolmer MG, Nielsen MA, Salanti A, Sander AF, Sauerwein RW, Jore MM, Theisen M, 2019. Pfs230 and Pfs48/45 Fusion Proteins Elicit Strong Transmission-Blocking Antibody Responses Against Plasmodium falciparum. Front Immunol 10, 1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana M, Miura K, Takashima E, Morita M, Nagaoka H, Zhou L, Long CA, Richter King C, Torii M, Tsuboi T, Ishino T, 2019. Identification of domains within Pfs230 that elicit transmission blocking antibody responses. Vaccine 37, 1799–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talaat KR, Ellis RD, Hurd J, Hentrich A, Gabriel E, Hynes NA, Rausch KM, Zhu D, Muratova O, Herrera R, Anderson C, Jones D, Aebig J, Brockley S, MacDonald NJ, Wang X, Fay MP, Healy SA, Durbin AP, Narum DL, Wu Y, Duffy PE, 2016. Safety and Immunogenicity of Pfs25-EPA/Alhydrogel(R), a Transmission Blocking Vaccine against Plasmodium falciparum: An Open Label Study in Malaria Naive Adults. PLoS One 11, e0163144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truck J, Ramasamy MN, Galson JD, Rance R, Parkhill J, Lunter G, Pollard AJ, Kelly DF, 2015. Identification of antigen-specific B cell receptor sequences using public repertoire analysis. J Immunol 194, 252–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YC, Kipling D, Dunn-Walters DK, 2012. Age-Related Changes in Human Peripheral Blood IGH Repertoire Following Vaccination. Front Immunol 3, 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu JL, Davis MM, 2000. Diversity in the CDR3 region of V(H) is sufficient for most antibody specificities. Immunity 13, 37–45. [DOI] [PubMed] [Google Scholar]
- Zhou JQ, Kleinstein SH, 2019. Cutting Edge: Ig H Chains Are Sufficient to Determine Most B Cell Clonal Relationships. J Immunol 203, 1687–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. S1. Percentage of Plasmoidum falciparum surface 230 kD protein Pfs230-specific B cells among memory B cell population (CD19+CD20+CD27+) and of Pfs25-specific B cells among total B cells (CD19+CD20+). Tetramer staining and sorting were performed as previously described (Coelho et al., 2020).