Abstract
Rhizobium tropici forms nitrogen-fixing nodules on the roots of the common bean (Phaseolus vulgaris). Like other legume-Rhizobium symbioses, the bean-R. tropici association is sensitive to the availability of phosphate (Pi). To better understand phosphorus movement between the bacteroid and the host plant, Pi transport was characterized in R. tropici. We observed two Pi transport systems, a high-affinity system and a low-affinity system. To facilitate the study of these transport systems, a Tn5B22 transposon mutant lacking expression of the high-affinity transport system was isolated and used to characterize the low-affinity transport system in the absence of the high-affinity system. The Km and Vmax values for the low-affinity system were estimated to be 34 ± 3 μM Pi and 118 ± 8 nmol of Pi · min−1 · mg (dry weight) of cells−1, respectively, and the Km and Vmax values for the high-affinity system were 0.45 ± 0.01 μM Pi and 86 ± 5 nmol of Pi · min−1 · mg (dry weight) of cells−1, respectively. Both systems were inducible by Pi starvation and were also shock sensitive, which indicated that there was a periplasmic binding-protein component. Neither transport system appeared to be sensitive to the proton motive force dissipator carbonyl cyanide m-chlorophenylhydrazone, but Pi transport through both systems was eliminated by the ATPase inhibitor N,N′-dicyclohexylcarbodiimide; the Pi transport rate was correlated with the intracellular ATP concentration. Also, Pi movement through both systems appeared to be unidirectional, as no efflux or exchange was observed with either the wild-type strain or the mutant. These properties suggest that both Pi transport systems are ABC type systems. Analysis of the transposon insertion site revealed that the interrupted gene exhibited a high level of homology with kdpE, which in several bacteria encodes a cytoplasmic response regulator that governs responses to low potassium contents and/or changes in medium osmolarity.
Nitrogen fixation in legume nodules involves a complex exchange of nutrients between the plant and bacteroids. This exchange involves transport across the bacteroid membrane and the plant-derived envelope surrounding the bacteroid, the peribacteroid membrane. In its simplest terms, this symbiosis is often viewed as an exchange of reduced carbon for reduced nitrogen. However, it is clear that optimum nodule function also involves a balanced flow of other nutrients (33). One nutrient that has been shown to be important for this symbiosis is phosphorus. Low phosphorus availability in soils is common and limits legume production worldwide; however, phosphorus metabolism in this plant-microbe interaction has not been well characterized. Given the significant metabolic activity of bacteroids, the phosphorus supply may be critical for optimum symbiotic functioning of bacteroids, and understanding the mechanisms by which bacteroids acquire phosphorus should provide useful information concerning phosphorus exchange between the symbionts and phosphorus flow in the symbiosis.
Phosphate (Pi) uptake has been investigated in various bacteria. In some microorganisms only a single transport system has been found. This is the case for Micrococcus lysodeikticus (19) and for several Rhizobium species (41). In other bacteria, two Pi transport systems have been found. Examples of such bacteria include Escherichia coli (34, 53), Acinetobacter johnsonii (48), and Pseudomonas aeruginosa (26). In each of the latter bacteria, a constitutively expressed low-affinity transport system and a Pi-repressible high-affinity permease have been identified. In E. coli, the low-affinity Pi transport system (LATS) is energized by the proton motive force (Δp) and consists of a single membrane component (17). In contrast, the high-affinity Pi transport system (HATS) is a multicomponent system consisting of proteins associated with the cytoplasmic membrane, an ATP-binding protein, and a periplasmic solute-binding protein (reviewed in reference 51).
Recently, Sinorhizobium meliloti has been reported to have at least two Pi transport systems, consistent with the high-affinity–low-affinity model described above (49). The high-affinity system is encoded by the phoCDET operon, and the low-affinity system is encoded by pit (in the orfA-pit operon) (6). Previously published evidence strongly suggests that expression of the genes coding for both Pi transport systems in S. meliloti is controlled by PhoB (6). PhoB (presumably phosphorylated PhoB) positively regulates the phoCDET operon but negatively controls orfA-pit. Under nonlimiting Pi conditions, the low-affinity Pit permease is expressed and is primarily responsible for Pi uptake. When S. meliloti is grown under Pi-limiting conditions, the Pit system is repressed, while the high-affinity PhoCDET system is induced and becomes the primary mechanism of Pi transport.
Some of our efforts to characterize and understand phosphorus metabolism and exchange in the Rhizobium-legume association have focused on the Rhizobium tropici-bean symbiosis (1), with initial work aimed at characterizing Pi assimilation and regulation in the microbial partner. As observed with other gram-negative bacteria (51), R. tropici induces alkaline phosphatase, and its Pi transport rate increases significantly in response to Pi limit-limiting conditions (1). The induction occurs when the medium Pi concentration is approximately 1 μM (1). R. tropici bacteroids isolated from nodules of bean plants grown in the presence of nonlimiting phosphorus concentrations contain extremely high levels of alkaline phosphatase, as well as a Pi stress-inducible acid phosphatase (1). This implies that under normal growth conditions a bean plant provides very low levels of Pi to the bacteroids in its nodules. In order to determine the importance of Pi supply for the bean-bacteroid symbiotic system, we are now assessing R. tropici Pi transport systems and estimating their kinetic properties. In this report, the Pi transport systems of R. tropici are described. Like S. meliloti (49), this bacterium has two distinct functional Pi transport systems. However, R. tropici appears to differ from S. meliloti and all other bacteria investigated previously since both Pi transport systems are inducible by Pi stress, are shock sensitive, and are energized by phosphate bond energy. In addition, in this paper we also describe a mutant that lacks high-affinity Pi transport activity.
MATERIALS AND METHODS
Strains and culture conditions.
Strains CIAT899 and CAP45 were used in all experiments. CIAT899 is the type strain of R. tropici type IIB (29), and CAP45 is a Pi transport mutant derived from CIAT899 (see below). CIAT899 was maintained on the minimal mannitol-ammonium agar (MMNH4) (pH 7.2) described previously (42, 43). CAP45 was maintained on the same medium, except that β-glycerolphosphate (βGP) replaced mannitol as the sole carbon source and gentamicin was included at a final concentration of 25 mg · liter−1. The other antibiotics used in the experiments were ampicillin (100 mg · liter−1) and tetracycline (25 mg · liter−1). In experiments in which Pi-starved cells (−Pi cells) were used, the cells were incubated in MMNH4 which lacked added phosphorus but was buffered to pH 7.2 with 5 mM MES (morpholineethanesulfonic acid) and 10 mM MOPS (morpholinepropanesulfonic acid) (MMNH4-OP) (43).
Mutant isolation.
Pho regulatory mutants that are constitutive for the Pi-repressible alkaline phosphatase often also do not express a high-affinity Pi transporter (5; reviewed in reference 51). We used the strategy and methods of Torriani and Rothman (44) to isolate R. tropici mutants that expressed alkaline phosphatase constitutively and then screened these mutants for a Pi transport phenotype. Briefly, R. tropici CIAT899 was mutated with transposon Tn5B22 (40) as previously described (2, 15). E. coli S17-1 (39) was used to mobilize the transposon into CIAT899 in mating mixtures, which were plated onto βGP-gentamicin agar. In order to be used as a carbon source, βGP must first be dephosphorylated at rates sufficient to supply glycerol for growth. One candidate phosphatase in CIAT899 is alkaline phosphatase (1). However, because of the high concentration of Pi in the medium, growth would require phoA expression under conditions where this gene is normally repressed. Transconjugants were isolated from the βGP-gentamicin agar plates by streaking twice to obtain pure cultures, and then constitutive expression of alkaline phosphatase activity was measured by comparing alkaline phosphatase activities in cells grown under high-Pi conditions (MMNH4 broth) and in cells after incubation under zero-Pi conditions (MMNH4-OP broth). Periplasm proteins were extracted and alkaline phosphatase was assayed by using previously described methods (1). Subsamples of the mutants were then screened for Pi uptake to identify mutants that were defective in Pi transport.
Transport assays.
Early-stationary-phase MMNH4 cultures were washed twice in MMNH4-OP and resuspended in MMNH4-OP to an optical density of 0.60 at an absorbance of 595 nm. To obtain −Pi cells, washed cells were incubated in MMNH4-OP at 30°C for 7 h in order to allow for maximum induction of Pi transport (1). Chloramphenicol (50 mg · liter−1) was then added to stop further protein synthesis. Cells not starved for Pi (+Pi cells) were prepared in the same way except that chloramphenicol was added immediately after washing and the cells were used within 1 h. In preliminary experiments, we found that chloramphenicol did not interfere with Pi transport (results not shown) but did inhibit the synthesis of alkaline phosphatase for at least 5 h. Thus, we concluded that de novo protein synthesis in +Pi cells did not occur during the experiments performed with +Pi cells.
The standard transport assay was conducted in an orbital shaker water bath at 30°C. Washed cells were diluted with MMNH4-OP to a concentration of 0.025 mg (dry weight) of cells · ml−1 for −Pi cells. Because the Pi transport rates were much lower in +Pi cells, the cell concentration used in +Pi cell assays was 0.125 mg (dry weight) of cells · ml−1 to ensure that sensitive and accurate uptake measurements were obtained. After 5 min of preincubation in MMNH4-OP, the transport assay was initiated by adding Pi (at concentrations specified below) as [32P]KH2PO4 (specific activity, 22.5 μCi · μmol−1). The [32P]KH2PO4-containing solution was filtered prior to use in order to remove any extraneous particles that had adsorbed label. Cell samples (0.5 ml) were withdrawn at 20-s intervals (unless otherwise specified); each sample was collected on a 0.3-μm-pore-size glass fiber filter (Gelman Sciences, Ann Arbor, Mich.) and washed with 20 ml of transport rinse buffer, which contained 20 mM MES and 5 mM KH2PO4 (pH 6.5). The filters were placed in counting vials, 20 ml of H2O was added to each vial, and the radioactivity retained on the filters was measured as Cerenkov radiation (21). All counts were corrected for background values and were standardized by using similarly prepared spiked standard samples.
Phosphate exchange and efflux.
The methods of Medveczky and Rosenberg (30) were modified slightly for use with R. tropici. Briefly, −Pi cells were loaded for 4 min with [32P]KH2PO4 (either 5 or 400 μM; specific activity, 22.5 μCi · μmol−1) at 30°C and then diluted 100-fold with MMNH4-OP without unlabeled potassium phosphate (efflux experiments) or with either 25 μM or 2 mM unlabeled potassium phosphate (exchange experiments). At time intervals, 0.5-ml samples were filtered and washed with transport rinse buffer as described above for the transport experiments.
Osmotic shock treatment.
An osmotic shock procedure similar to that described by Neu and Heppel (31) was used. Cells were washed twice with 30 mM Tris (pH 8.0) and resuspended to a density of 5 mg (dry weight) of cells · ml−1 in 30 mM Tris (pH 8.0) containing 1 M sucrose and 10 mM EDTA. Following 15 min of incubation at room temperature, cells were collected by centrifugation for 4 min at 14,000 × g, and then periplasmic proteins were released by resuspending the pellet in 0.1 mM MgSO4 at room temperature. The shock-treated cells were collected by centrifugation, gently resuspended in MMNH4-OP, and then used for Pi transport assays.
To verify that periplasmic enzymes were released, the protein concentrations and levels of activity of the periplasm marker enzyme alkaline phosphatase in the supernatant of the pelleted shock-treated cells were determined. In addition, the cytoplasm marker enzyme malate dehydrogenase was assayed to determine if cell lysis had occurred. We also determined the alkaline phosphatase and protein levels in supernatants of pelleted control cells and in cleared extracts of sonicated samples that contained equivalent amounts of shocked cells. Alkaline phosphatase activity was measured as described above, and malate dehydrogenase activity was assayed at 340 nm by determining the rate of NADH oxidation (1). Each 350-μl reaction mixture contained 1.5 mM oxalacetic acid, 0.25 mM NADH, 10 mM K2HPO4 (pH 7.5), and 50 μl of shock fluid or cell extract (1). Both assays were conducted with a Bio-Rad model 3550-UV microplate reader. Protein concentrations were determined by using a Bio-Rad protein assay kit.
EDTA treatment of cells.
Like previous investigators (23, 25, 48), we found that it was necessary to use a mild EDTA treatment to permeabilize the outer membrane in order to use the ATPase inhibitor N,N′-dicyclohexylcarbodiimide (DCCD), the protonophore carbonyl cyanide m-chlorophenylhydrazone (CCCP), and the Δp probe tetraphenylphosphonium bromide (TPP+). −Pi cells were washed twice with 30 mM Tris (pH 8.0) and resuspended to a density of 5 mg (dry weight) of cells · ml−1 in 30 mM Tris, and then 1 mM EDTA (pH 7.0) was added. After 5 min of incubation at room temperature, the cells were centrifuged for 4 min at 14,000 × g, washed twice with 30 mM Tris, and resuspended in MMNH4-OP to a density of 0.5 mg (dry weight) of cells · ml−1. Alkaline phosphatase was not released by this procedure (data not shown), which indicated that the EDTA treatment did not result in release of periplasmic proteins.
Energy coupling. (i) Qualitative determination of membrane potential.
CCCP was used to dissipate all components of the Δp (23, 24). Δp probes, such as TPP+, are passively distributed between the cell and the medium depending on the membrane potential (25) and can be used to assess the effect of CCCP on Δp (23, 25, 35). TPP+ uptake was measured with and without CCCP by using the medium and conditions described above for Pi uptake, except that choline chloride was added to a final concentration of 50 mM. Choline chloride reduces binding of TPP+ to anionic groups at the cell surface (28) but does not interfere with Pi uptake (data not shown).
For the TPP+ uptake assays we used MMNH4-OP with or without CCCP (final concentration, 1 μmol of CCCP per 0.025 mg [dry weight] of EDTA-treated −Pi cells per ml). Two types of CCCP addition experiments were performed. In the first type, CCCP was added to a cell suspension 5 min before [3H]TPP+ (final concentration, 18 μM; specific activity, 27.5 μCi · μmol−1) was added. After [3H]TPP+ was added, 0.5-ml cell samples were removed at specific times and then collected and washed with 0.3-μm-pore-size glass fiber filters as described above for the Pi transport experiments. In the second type of experiment, [3H]TPP+ was added to initiate the transport assay, the cells were allowed to accumulate [3H]TPP+ for 4 min, and then CCCP was added after 4.5 min; this was followed by cell sampling. For both types of experiments, the [3H]TPP+ content of the cells was measured by placing the filters in counting vials, adding 20 ml of scintillation cocktail (Scintisafe Plus 50%; Fisher Chemical) to each vial, and measuring the radioactivity with a Tri-Carb liquid scintillation analyzer (model 4430; Packard Instrument Co.). All counts were corrected for background values and were standardized by using similarly prepared spiked standard samples.
In experiments performed to determine the effect of CCCP on Pi transport, Pi transport assays were performed as described above for the routine assays, except that the cells were incubated in the presence of CCCP (1 μmol of CCCP per 0.025 mg [dry weight] of EDTA-treated −Pi cells per ml) for 5 min at 30°C with constant shaking before [32P]KH2PO4 (specific activity, 22.5 μCi · μmol−1; same concentration as described above) was added. At specific times, cell samples were removed and filtered, and radioactivity was quantified as described above. [3H]TPP+ was not included in the assay mixtures.
To separately manipulate ATP pools and Δp for Pi transport assays, mixtures containing CCCP were preincubated for 5 min, which resulted in dissipation of the membrane potential without appreciable reductions in the ATP pool size (i.e., we avoided reductions in the ATP pool size via loss of H+-ATPase function). To facilitate exhaustion of intracellular ATP pools, cells were preincubated in the presence of DCCD for 45 min (see below). Short incubations (5 min) in transport suspension media that contained ethanol (final concentration, 0.8% [vol/vol]; ethanol was required to solubilize CCCP and DCCD) were found to have negative effects on the rates of uptake by both the HATS and LATS in R. tropici (compare the rates in Table 1 to the estimated Vmax values in Table 2). Prolonged incubation (45 min) further reduced the rates of uptake by the HATS but appeared to have no additional effect on the LATS (Table 1).
TABLE 1.
Effect of Δp dissipation and ATP depletion on Pi transport in R. tropici CIAT899 and CAP45a
| Treatment | Strain | Pi uptake (nmol of Pi/min/mg [dry wt] of cells)
|
ATP concn (nmol of ATP/mg [dry wt] of cells) | |
|---|---|---|---|---|
| 5 μM Pi | 400 μM Pi | |||
| Ethanol control | CIAT899 | 26.7 ± 3.2 | 41.8 ± 3.1 | 2.20 ± 0.20 |
| CAP45 | 2.4 ± 0.3 | 8.8 ± 0.1 | 1.41 ± 0.31 | |
| CCCP (1 μM) | CIAT899 | 23.8 ± 1.4 (11)b | 44.9 ± 2.1 (0) | 1.84 ± 0.08 (18) |
| CAP45 | 2.1 ± 0.1 (12) | 12.2 ± 0.1 (0) | 1.35 ± 0.26 (4) | |
| Ethanol control | CIAT899 | 10.2 ± 2.2 | 11.76 ± 1.97 | 0.305 ± 0.005 |
| CAP45 | 2.2 ± 0.1 | 7.06 ± 0.37 | 1.03 ± 0.16 | |
| DCCD (100 μM) | CIAT899 | 0 ± 0 (100) | 0.43 ± 0.08 (96) | 0.02 ± 0.01 (93) |
| CAP45 | 0.034 ± 0.001 (98) | 0.29 ± 0.02 (96) | 0.09 ± 0.04 (91) | |
−Pi cells were treated with EDTA as described in the text, preincubated with an inhibitor and then assayed to determine Pi uptake and the intracellular ATP concentration. Values are means ± standard errors based on values from two separate experiments. Cells were preincubated for 5 min in the presence of the Δp dissipator CCCP or for 45 min in the presence of the ATPase inhibitor DCCD. Using ethanol to solubilize CCCP and DCCD did have a negative effect on the overall transport rates, and therefore an ethanol control was included in all experiments.
The values in parentheses are percentages of reduction in Pi transport or ATP concentration compared to the controls.
TABLE 2.
Kinetic parameters of Pi uptake in R. tropici CIAT899 and CAP45a
| Strain | Growth conditions | High-affinity uptake
|
Low-affinity uptake
|
||
|---|---|---|---|---|---|
| Km (μM Pi) | Vmax (nmol of Pi/min/mg [dry wt] of cells) | Km (μM Pi) | Vmax (nmol of Pi/min/mg [dry wt] of cells) | ||
| CIAT899 | −Pi | 0.45 ± 0.01 | 86.2 ± 4.9 | 9.6 ± 1.0 | 153.8 ± 13.6 |
| +Pi | 0.34 ± 0.02 | 0.22 ± 0.01 | 35.7 ± 1.0 | 1.33 ± 0.01 | |
| CAP45 | −Pi | NDb | ND | 34.3 ± 2.7 | 118.0 ± 7.5 |
| +Pi | ND | ND | 33.1 ± 1.3 | 0.41 ± 0.02 | |
The kinetics of Pi uptake were analyzed by using Eadie-Hofstee plots. The initial velocities in +Pi and −Pi cells were determined for the first 20 and 10 s, respectively. The values are means ± standard errors based on values from three separate experiments; each Pi concentration was replicated three times in each experiment.
ND, not detected.
(ii) Determination of ATP concentrations.
Intracellular ATP concentrations were determined in experiments in which the effects of CCCP and DCCD were examined. To determine ATP concentrations, the reaction mixtures used were identical to the Pi transport assay reaction mixtures, except that no radioisotope was added. After incubation (see below), cellular ATP was extracted as described by Joshi et al. (23). ATP concentrations were determined by using the luciferase assay, measuring light emission with a Turner model TD-20e luminometer, and employing the internal standard technique (47). Each ATP assay mixture contained 0.05 ml of perchloric acid-treated supernatant, 0.1 ml of 10 mM Tris buffer (pH 8.0), and 0.1 ml of luciferase-luciferin (Promega). After luciferase was injected into the sample and light was measured, an ATP standard was added to the same cuvette and the light was measured again. The amount of ATP in the sample was calculated by using the following equation: ATP concentration = [(RU − RB)/(RIS − RU)] × ATP concentration in the standard, where RU is the luminescence value for the sample, RB is the luminescence value for the blank, and RIS is the luminescence value after addition of the internal standard.
Nucleic acid manipulations.
The protocols of Sambrook et al. (36) were used for all routine manipulations of plasmid and chromosomal DNA. The Tn5B22 insertion site in the mutant was characterized by selectively subcloning the transposase portion of Tn5B22 (40) containing the gentamicin resistance gene along with the balance of the transposon and flanking chromosomal DNA. The transposon-chromosome junction was then sequenced, and the resulting nucleotide sequence data were used to conduct a BLASTX search to identify a possible match (4, 16). Briefly, total chromosomal DNA was harvested from the mutant, digested with XmaI, and then ligated into pBluescript KS(+) (Stratagene). The ligation mixture was transformed into E. coli DH5α (36), and plasmids from transformants that were resistant to ampicillin and gentamicin were analyzed by restriction analysis to verify that each contained a single cloned fragment. Southern blotting was then used to verify that the cloned fragment was identical to the fragment in the genome of mutant CAP45. The flanking DNA was sequenced by using an ABI Prism BigDye kit (PE Applied Biosystems, Foster City, Calif.) and an ABI model 310 genetic analyzer (PE Applied Biosystems). The primer 5′-CCATGTTAGGAGGTCACATGGAAGT-CAG-3′ was used to initiate sequencing from the transposase terminal (40).
RESULTS
Isolation of phosphate transport mutant CAP45.
Tn5B22 mutagenesis of R. tropici CIAT899 and selection on minimal βGP-gentamicin agar resulted in several gentamicin-resistant mutants that were found to be constitutive for expression of alkaline phosphatase. Enzyme assays of periplasmic extracts obtained from these mutants revealed that they had alkaline phosphatase specific activities of about 800 U (1 U = 1 nmol of p-nitrophenylphosphate hydrolyzed · min−1 · mg of protein−1) when +Pi cells were used. Typically, the alkaline phosphatase activity of CIAT899 +Pi cells is approximately 30 U, and the alkaline phosphatase activity of CIAT899 −Pi cells is approximately 1,500 U (1). Presumably, constitutive expression of alkaline phosphatase in the mutants was due to a lack of normal repressive regulatory mechanisms. By screening a subset of the mutants for a Pi transport phenotype we identified isolates that had reduced Pi transport rates. Southern blot analysis of chromosomal DNA prepared from the Pi transport mutants verified that Tn5B22 was present, and all of the blot patterns appeared to be identical, suggesting that the insertion sites were very similar or that the mutants were siblings (results not shown). One representative isolate of these transport mutants was selected for further study; this isolate was designated CAP45.
Kinetic parameters of phosphate uptake.
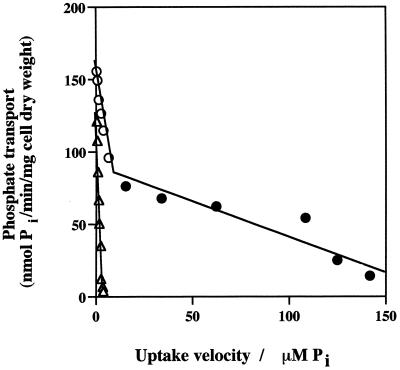
Kinetic plots of Pi transport in both +Pi cells and −Pi cells of CIAT899 revealed that two separate transport systems were present. Eadie-Hofstee plots of Pi transport in −Pi cells of CIAT899 and CAP45 are shown in Fig. 1. As measured at Pi concentrations of 0.1 to 500 μM and calculated from a linear regression analysis, the estimated Km values for two transport systems differed by approximately 2 orders of magnitude. In addition to being expressed under high-Pi growth conditions, both systems were induced in response to Pi deprivation, as shown by the increases in the Vmax values of −Pi cells (Table 2).
FIG. 1.
Eadie-Hofstee plots of initial Pi uptake velocities in R. tropici CIAT899 and CAP45. Symbols: ●, CIAT899 HATS; ○, CIAT899 LATS; ▵, CAP45 LATS. Transport rates were determined with −Pi cells as described in the text at Pi concentrations between 0.1 and 500 μM. Each point is the mean of values from three independent experiments; for each experiment three replicate values were obtained at each Pi concentration. The standard error for each data point did not exceed 10% of the mean, and the standard errors are not shown to simplify presentation.
Only a single transport system was evident in CAP45 (Fig. 1 and Table 2), providing an opportunity to study it in the absence of the other system that would otherwise influence overall Pi transport behavior. The Km for this Pi permease was found to be 34 μM, which suggested that the system was the low-affinity system present in CIAT899. The Vmax for the low-affinity system present in CAP45 was similar to the Vmax obtained for CIAT899 under both +Pi and −Pi growth conditions (Fig. 1 and Table 2). However, the proportional increase in the estimated Vmax for CAP45 −Pi cells suggested that the increase in Pi transport by the low-affinity system in response to Pi stress was more substantial than the increase observed in wild-type strain CIAT899. Based on the estimated Km values, Pi concentrations of 5 and 400 μM were used in subsequent experiments to evaluate Pi uptake by the HATS and the LATS, respectively, when the effects of various treatments or inhibitors were determined.
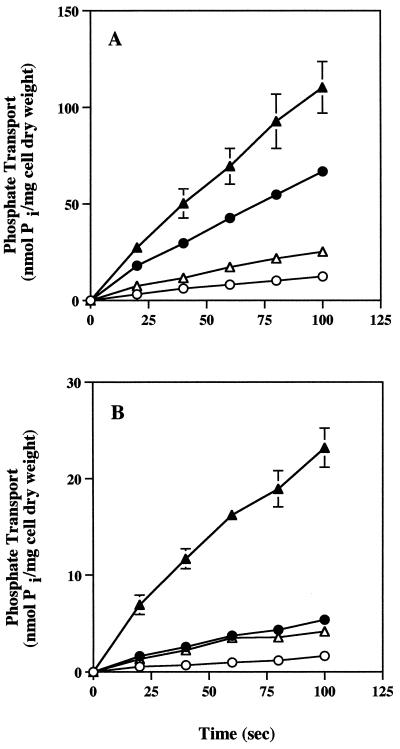
Effect of osmotic shock on phosphate uptake.
Osmotic shock release of periplasmic proteins was used to determine if either Pi transport system required a periplasmic solute-binding protein to exhibit the maximal transport rate. Pi uptake was dramatically reduced in osmotic shock-treated cells (Fig. 2). As determined with high and low Pi levels that were saturating for either Pi transport system, osmotic shock reduced the Pi transport rates by approximately 80%. This was the case for both strains and suggested that both the HATS and the LATS depend on a Pi-binding protein for maximal Pi translocating activity.
FIG. 2.
Effect of osmotic shock on the uptake of Pi in −Pi cells of R. tropici CIAT899 and CAP45. Pi uptake was determined for both control cells and shocked cells at Pi concentrations of 5 μM and 400 μM. (A) Pi uptake in wild-type strain CIAT899. (B) Pi uptake in mutant CAP45. Symbols: ○, 5 μM Pi with shocked cells; ●, 5 μM Pi with control cells; ▵, 400 μM Pi with shocked cells; ▴, 400 μM Pi with control cells. The value for each time point is the mean of values from three independent experiments for CIAT899 or two independent experiments for CAP45. The error bars indicate the standard errors of the means.
Periplasmic protein release and the structural integrity of osmotically shocked cells were verified by assaying for the marker enzymes alkaline phosphatase and malate dehydrogenase, respectively. The supernatant of pelleted shocked −Pi cells contained 19% of the total cellular protein and 16% of the total alkaline phosphatase activity (Table 3). The protein concentration and alkaline phosphatase activity in the supernatant obtained from the same quantity of pelleted nonshocked control cells were less than 1% of the values in the supernatant of pelleted shocked cells. The combination of relatively high levels of alkaline phosphatase and the presence of proteins in the supernatant of the shock-treated cells was taken as evidence that the cells lost significant amounts of periplasmic proteins during the osmotic shock treatment. The complete lack of detectable malate dehydrogenase activity in the shock fluids also demonstrated that the shock treatment did not lyse the cells (Table 3).
TABLE 3.
Release of periplasmic proteins from CIAT899 by osmotic shock treatmenta
| Treatment | Protein concn (μg of protein/ ml of extract) | Alkaline phosphatase activity (nmol/min/ml of extract)b | Malate dehydrogenase activity (nmol/min/ml of extract)c |
|---|---|---|---|
| Control cells | 0.5 (0.1)d | 15 (0.8) | 0 (0) |
| Osmotically shocked cells | 54.5 (19.3) | 182 (15.6) | 0 (0) |
| Sonicated cells (control) | 354.3 (99.9) | 1,880 (99.2) | 321 (100) |
| Sonicated cells (shocked) | 228.6 (80.7) | 983 (84.4) | 284 (100) |
−Pi cells were shocked as described in the text. Control cells were treated like the shocked cells were treated, except that neither EDTA nor sucrose was used. The data are data from a single representative experiment.
Nanomoles of p-nitrophenyl phosphate hydrolyzed per minute per milliliter of extract.
Nanomoles of NADH oxidized per minute per milliliter of extract.
The values in parentheses are percentages of the total cellular protein or enzyme activity.
Energy coupling to phosphate transport.
To assess the roles of Δp and ATP in energizing Pi transport, CIAT899 and CAP45 were treated with CCCP and DCCD. The protonophore CCCP dissipates the energized membrane and inhibits processes that use the Δp directly as a source of energy (i.e., secondary transport systems). However, reactions driven directly by phosphate bond energy should be relatively resistant to the action of this compound. Conversely, the ATPase inhibitor DCCD should significantly reduce ATP levels, and thus ATP-dependent transport activity should also be significantly reduced when DCCD is added. On the basis of these criteria, we examined energy coupling to Pi transport in both CIAT899 and CAP45. Under the conditions used in the assays (pH 7.2), neutrophilic bacteria, such as rhizobia, do not generate a significant chemical potential (ΔpH), and therefore the Δp consists primarily of the electrical membrane component (ΔΨ) (25), which in cowpea rhizobia has been shown to be unaffected by changes in pH (20).
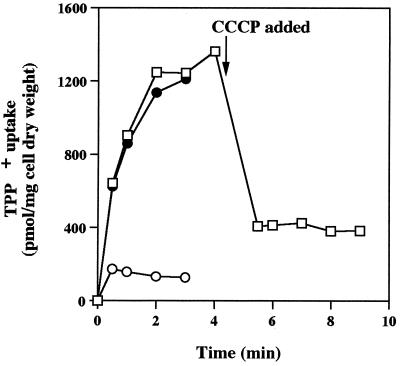
The effects of CCCP on Δp, as measured by uptake and accumulation of the Δp probe [3H]TPP+, are shown in Fig. 3. In one set of experiments, CCCP was included in each cell suspension before [3H]TPP+ was added during the uptake assay (Fig. 3A). These experiments showed that CCCP dissipated Δp, which resulted in significantly reduced [3H]TPP+ uptake and accumulation. In other experiments, CCCP was added to cells that were in the process of accumulating [3H]TPP+. This addition resulted in the immediate release of [3H]TPP+; again, the data showed that CCCP treatment dissipated a significant portion of the Δp (interior negative) but also demonstrated that [3H]TPP+ did not simply bind to cell components, as it was readily released when the Δp was dissipated. On the basis of several such experiments in which CCCP treatment consistently either resulted in the release of [3H]TPP+ or inhibited [3H]TPP+ uptake and accumulation by 50 to 90% compared to control cells, we concluded that CCCP largely eliminated the Δp and could be used to assess the importance of the Δp as the driving force for Pi transport in R. tropici.
FIG. 3.
Effect of CCCP on TPP+ uptake and accumulation in R. tropici CIAT899. CCCP (5 μmol · 0.125 mg [dry weight]−1 · ml−1) was (○) or was not (●) added before [3H]TPP+ (18 μM; specific activity, 27.5 μCi · μmol−1) was added. In other experiments (□), CIAT899 was allowed to take up [3H]TPP+ for 4 min, CCCP was added after 4.5 min, and then sampling commenced at 5 min. Cell samples were taken at the times shown and as described in Materials and Methods. The data show the typical effect of CCCP on [3H]TPP+ uptake and accumulation and are from one of the three independent assays performed.
As shown in Table 1, under Pi transport assay conditions identical to the conditions used in the experiments whose results are shown in Fig. 3A (which verified that CCCP significantly reduced the Δp), CCCP treatment of cells had no effect on Pi transport with either transport system compared to cells not treated with CCCP. As expected, the ATP levels in cells treated with CCCP under these conditions were also not affected. In contrast to CCCP treatment, DCCD treatment reduced the ATP levels to near zero and eliminated Pi transport in both CIAT899 (which contains both transport systems) and CAP45 (which contains only the LATS) (Table 1). Pi transport rates were highly positively correlated with ATP levels in the cell (r2 = 0.95 for the HATS in CIAT899; r2 = 0.89 for the LATS in CAP45). These results indicate that the Δp per se is not involved in energizing Pi transport by either system. Rather, the correlation between ATP levels and Pi transport suggests that ATP is involved in energizing both Pi transport systems.
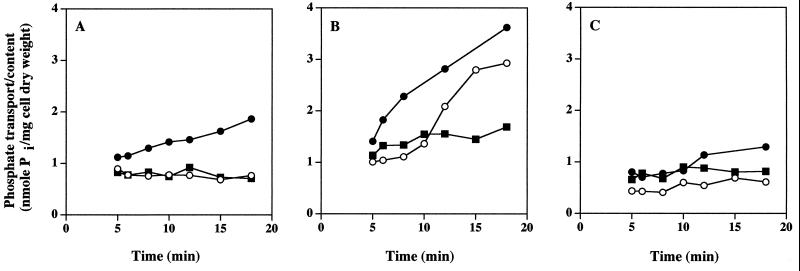
Exchange and efflux of phosphate.
After dilution of preloaded cells with media containing no Pi or with media containing excess unlabeled Pi, the level of radioactivity in CIAT899 remained constant, implying that neither Pi transport system mediated Pi efflux or exchange of internal Pi with external Pi (Fig. 4A and B). Mutant strain CAP45 behaved similarly (Fig. 4C). CIAT899 cells preloaded with 400 μM [32P]KH2PO4 (to evaluate both transport systems) and diluted 100-fold with medium containing no Pi exhibited high levels of phosphate uptake (Fig. 4B). We assume that this resulted from diluted [32P]KH2PO4 in the medium that was still saturating the HATS and theoretically half-saturating the LATS. In contrast, after cells preloaded in the presence of 5 μM [32P]KH2PO4 were diluted 100-fold with medium containing no Pi, neither the HATS in CIAT899 (Fig. 4A) nor the LATS in CAP45 (Fig. 4C) was saturated with respect to the solute substrate, and therefore the cells exhibited very reduced or no uptake activity.
FIG. 4.
Absence of Pi exchange and efflux in R. tropici CIAT899 and CAP45. (A) −Pi cells of CIAT899 were loaded with 5 μM [32P]KH2PO4 (specific activity, 22.5 μCi · μmol−1) for 4 min and then diluted 100-fold with a medium containing 5 μM [32P]KH2PO4. Symbols: ●, uptake control; ○, no Pi (efflux); ■, 25 μM unlabeled Pi (exchange). (B and C) −Pi cells of CIAT899 (B) and CAP45 (C) were loaded with 400 μM [32P]KH2PO4 (specific activity, 22.5 μCi · μmol−1) for 4 min and then diluted 100-fold with a medium containing 400 μM [32P]KH2PO4. Symbols: ●, uptake control; ○, no Pi (efflux); ■, 2 mM unlabeled Pi (exchange). The results are typical of the results of two experiments in which this response was documented.
Characterization of the transposon insertion site.
A sequence analysis of the chromosomal DNA adjacent to the transposase end of Tn5B22 revealed a 151-bp segment immediately adjacent to Tn5B22 that exhibited 48 to 52% identity and 74 to 78% similarity to KdpE of E. coli (50), Clostridium acetobutylicum (45, 46), and Mycobacterium tuberculosis (11). KdpE is the cytoplasmic response regulator that is paired with the sensor kinase KdpD, and together these proteins govern expression of the high-affinity potassium transport system in response to changes in medium osmolarity or to potassium-limiting conditions.
DISCUSSION
Kinetic analysis showed that R. tropici CIAT899 has two Pi transport systems whose kinetic properties differ significantly. In contrast, CAP45 had a single Pi transport system that exhibited low affinity for Pi. At the solute substrate concentrations used in our assays, the apparent lack of transport activity via a HATS in this mutant allowed us to characterize the LATS. The kinetic properties of the R. tropici HATS suggest that it is not atypical. Its apparent Km (0.45 μM) is very similar to the apparent Km values reported for the HATS of E. coli (30), P. aeruginosa (26), and A. johnsonii (48). While it exhibited a Vmax that is appreciably higher than the Vmax values measured for the HATS of E. coli (30) and P. aeruginosa (26), it is very similar to the Vmax observed for the HATS of A. johnsonii (48). The Km of the LATS is much higher and indeed is more consistent with the range of values reported for secondary Pi transport systems in these bacteria (26, 48, 53).
Both Pi transport systems have characteristics that are consistent with ABC-type transporters (8). Both are shock sensitive, losing roughly 80% of their transport activity when periplasmic proteins are lost (Fig. 2 and Table 3). In addition, the ATPase inhibitor DCCD (Table 1) eliminated the transport activities of both systems. In contrast, the Δp dissipator CCCP, which has been shown to strongly inhibit secondary transport systems (7, 14), affected neither system (Table 1). Under the assay conditions used in routine Pi transport experiments, CCCP either significantly reduced TPP+ uptake or caused the release of TPP+ that had accumulated in response to an intact membrane potential (Fig. 3). Finally, the unidirectional uptake activity, as shown by the lack of apparent efflux and exchange activity observed with both systems (Fig. 4), also indicates that both the HATS and the LATS belong to the traffic ATPase class of solute transport systems. To summarize, the data obtained in this study suggest that R. tropici CIAT899 has two Pi transport systems. These transport systems differ in their affinities for Pi (Table 2) but are otherwise similar. Both are inducible by Pi limitation (Table 2), are shock sensitive (Fig. 2 and Tables 3), and utilize ATP to engage Pi transport (Table 1). The presence of two Pi transport systems in R. tropici is in contrast to the single Pi transport system reported for some rhizobia (41), and the presence of two functional traffic ATPase primary Pi transporters has not been reported for any of the other bacteria studied thus far (26, 34, 48, 54), including S. meliloti (49).
Additional, but indirect, evidence suggesting that at least the HATS of R. tropici is a multicomponent ABC type of solute transport system comes from the complex Pho phenotype of CAP45. In addition to the absence of a HATS, CAP45 also expresses alkaline phosphatase constitutively. These two traits also occur together in E. coli (12, 13, 52) and S. meliloti (5) mutants whose multicomponent HATS are affected. In both of the latter species, an operon arrangement is involved, and the operon typically includes genes coding for a periplasmic solute-binding protein, two integral membrane proteins, and an ATP-binding protein. Mutations in these operons result in a loss of the high affinity Pi transport function and also result in a loss of normal Pho regulation (i.e., constitutive expression of alkaline phosphatase, the marker enzyme for the Pi stress response). Analysis of the transposon insertion site in CAP45 revealed that the interrupted gene is kdpE. In both E. coli and C. acetobutylicum (46, 50), KdpE has been shown to be the cytoplasmic response regulator of a two-component regulatory pair which includes the sensor KdpD. Also in both of these bacteria, genes coding for KdpDE are arranged in an operon and are located immediately adjacent to the kdp operon, which codes for an ABC-type high-affinity K+ transport system that is upregulated in response to low potassium concentrations in the medium or to low osmotic conditions (for reviews see references 3 and 38). In order to assess the effect (if any) of the affected region of the chromosome on the Pho and Pi transport phenotypes of CAP45, efforts to clone and fully characterize this region are currently under way and will be the subject of a subsequent report.
Functional duplication has been found previously in R. tropici (22, 32), and indeed reiteration is not uncommon in members of the Rhizobiaceae (18, 37). Therefore, the presence of two functional Pi stress-inducible Pi transport systems in CIAT899 is not without precedent. Two separate ABC type Pi transport operons that exhibit homology to the E. coli pst operon have been identified in the unrelated organism M. tuberculosis (10, 11, 27), and this finding implies that perhaps there are at least two traffic ATPase Pi transporters in mycobacteria (27). To our knowledge, these systems have not been characterized at the physiological level, and therefore it is not known if they are functional or to what extent they differ in their kinetic properties.
As discussed above, in broth culture R. tropici does not express alkaline phosphatase until the medium Pi concentration decreases to approximately 1 μM (1). The high levels of alkaline phosphatase in R. tropici bacteroids (1) suggest that under normal growth conditions the host plant perhaps distributes small amounts of Pi to the bacteroids and that the Pi concentration in the peribacteroid space may be quite low. Under such conditions, the HATS may be important to Pi acquisition by R. tropici bacteroids. Initial studies on the symbiotic properties of the mutant isolated in this study have shown that in situ Pi acquisition by CAP45 bacteroids is reduced during symbiosis and that the symbiotic competence of this mutant is also reduced (9).
ACKNOWLEDGMENTS
This work was supported by National Science Foundation grant IBN-9420798.
We thank Mark Burr and Dan Hassett for carefully reading the manuscript and for making critical comments.
REFERENCES
- 1.Al-Niemi T S, Kahn M L, McDermott T R. P metabolism in the bean-Rhizobium tropici symbiosis. Plant Physiol. 1997;113:1233–1242. doi: 10.1104/pp.113.4.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Niemi T S, Summers M L, Elkins J G, Kahn M L, McDermott T R. Regulation of the phosphate stress response in Rhizobium meliloti by PhoB. Appl Environ Microbiol. 1997;63:4978–4981. doi: 10.1128/aem.63.12.4978-4981.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altendorf K, Epstein W. Kdp-ATPase of Escherichia coli. Cell Physiol Biochem. 1993;4:160–168. [Google Scholar]
- 4.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D L. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bardin S D, Finan T M. Regulation of phosphate assimilation in Rhizobium (Sinorhizobium) meliloti. Genetics. 1998;148:1689–1700. doi: 10.1093/genetics/148.4.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bardin S D, Voegele R T, Finan T M. Phosphate assimilation in Rhizobium (Sinorhizobium) meliloti: identification of a pit-like gene. J Bacteriol. 1998;180:4219–4226. doi: 10.1128/jb.180.16.4219-4226.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berger E A, Heppel L A. Different mechanisms of energy coupling for the shock-sensitive and shock-resistant amino acid permeases of Escherichia coli. J Biol Chem. 1974;249:7747–7755. [PubMed] [Google Scholar]
- 8.Boos W, Lucht J M. Periplasmic binding protein-dependent ABC transporters. In: Neidhardt F C, Curtiss R, Ingraham J L, Lin E C C, Brooks Low K, Magasanik B, Resnikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Vol. 1. Washington, D.C.: American Society for Microbiology; 1996. pp. 1175–1209. [Google Scholar]
- 9.Botero, L. M., and T. R. McDermott. Unpublished data.
- 10.Braibant M, Lefevre P, de Wit L, Ooms J, Peirs P, Huygen K, Wattiez R, Content J. Identification of a second Mycobacterium tuberculosis gene cluster encoding proteins of an ABC phosphate transporter. FEBS Lett. 1996;394:206–212. doi: 10.1016/0014-5793(96)00953-2. [DOI] [PubMed] [Google Scholar]
- 11.Cole S T, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon S V, Eiglmeier K, Gas S, Barry III C E, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Krogh A, McLean J, Moule S, Murphy L, Oliver K, Osborne J, Quail M A, Rajandream M-A, Rogers J, Rutter S, Seeger K, Skelton J, Squares R, Sulston J E, Taylor K, Whitehead S, Barrell B G. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 12.Cox G B, Web D, Godovac-Zimmermann J, Rosenberg H. Arg-220 of the PstA protein is required for phosphate transport through the phosphate-specific transport system in Escherichia coli but not for alkaline phosphatase repression. J Bacteriol. 1988;170:2283–2286. doi: 10.1128/jb.170.5.2283-2286.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cox G B, Web D, Rosenberg H. Specific amino acid residues in both the PstB and PstC proteins are required for phosphate transport by the Escherichia coli Pst system. J Bacteriol. 1989;171:1532–1534. doi: 10.1128/jb.171.3.1531-1534.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daruwalla K R, Paxton A T, Henderson P J F. Energization of the transport systems for arabinose and comparison with galactose transport in Escherichia coli. Biochem J. 1981;200:611–627. doi: 10.1042/bj2000611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Bruijn F J, Lupski J R. The use of transposon Tn5 mutagenesis in the rapid generation of correlated physical and genetic map segments cloned into multicopy plasmids—a review. Gene. 1984;27:131–149. doi: 10.1016/0378-1119(84)90135-5. [DOI] [PubMed] [Google Scholar]
- 16.Devereux J, Haeberli V, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elvin C M, Hardy C M, Rosenberg H. Molecular studies on the phosphate inorganic transport system of Escherichia coli. In: Torriani-Gorini A, Rothman F G, Silver S, Wright A, Yagil E, editors. Phosphate metabolism and cellular regulation in microorganisms. Washington, D.C.: American Society for Microbiology; 1987. pp. 156–158. [Google Scholar]
- 18.Espin G, Moreno S, Guzman J. Molecular genetics of the glutamine synthetases in Rhizobium species. Crit Rev Microbiol. 1994;20:117–123. doi: 10.3109/10408419409113551. [DOI] [PubMed] [Google Scholar]
- 19.Friedberg I. Phosphate transport in Micrococcus lysodeikticus. Biochim Biophys Acta. 1977;466:451–460. doi: 10.1016/0005-2736(77)90338-8. [DOI] [PubMed] [Google Scholar]
- 20.Gober J W, Kashket E R. H+/ATP stoichiometry of cowpea Rhizobium sp. strain 32H1 cells grown under nitrogen-fixing and nitrogen-nonfixing conditions. J Bacteriol. 1984;160:216–221. doi: 10.1128/jb.160.1.216-221.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haviland R T, Bieber L L. Scintillation counting of 32P without added scintillator in aqueous solutions and organic solvents and on dry chromatographic media. Anal Biochem. 1970;33:323–334. doi: 10.1016/0003-2697(70)90303-9. [DOI] [PubMed] [Google Scholar]
- 22.Hernandez-Lucas I, Pardo M A, Segovia L, Miranda J, Martinez-Romero E. Rhizobium tropici chromosomal citrate synthase gene. Appl Environ Microbiol. 1995;61:3992–3997. doi: 10.1128/aem.61.11.3992-3997.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joshi A K, Ahmed S, Ames G F-L. Energy coupling in bacterial periplasmic transport systems: studies in intact Escherichia coli cells. J Biol Chem. 1989;264:2126–2133. [PubMed] [Google Scholar]
- 24.Kadner R J. Cytoplasmic membrane. In: Neidhardt F C, Curtiss R, Ingraham J L, Lin E C C, Brooks Low K, Magasanik B, Resnikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: American Society for Microbiology; 1996. pp. 58–87. [Google Scholar]
- 25.Kashket E R. The proton motive force in bacteria: a critical assessment of methods. Annu Rev Microbiol. 1985;39:219–242. doi: 10.1146/annurev.mi.39.100185.001251. [DOI] [PubMed] [Google Scholar]
- 26.Lacoste A-M, Cassaigne A, Neuzil E. Transport of inorganic phosphate in Pseudomonas aeruginosa. Curr Microbiol. 1981;6:115–120. [Google Scholar]
- 27.Lefevre P, Braibant M, de Wit L, Kalay M, Roeper D, Grotzinger J, Delville J-P, Peirs P, Ooms J, Huygen K, Content J. Three different putative phosphate transport receptors are encoded by the Mycobacterium tuberculosis genome and are present at the surface of Mycobacterium bovis BCG. J Bacteriol. 1997;179:2900–2906. doi: 10.1128/jb.179.9.2900-2906.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maloney P C. Relationship between phosphorylation potential and electrochemical H+ gradient during glycolysis in Streptococcus lactis. J Bacteriol. 1983;153:1461–1470. doi: 10.1128/jb.153.3.1461-1470.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinez-Romero E, Segovia L, Mercante F M, Franco A A, Graham P, Pardo M A. Rhizobium tropici, a novel species nodulating Phaseolus vulgaris L. beans and Leucaena sp. trees. Int J Syst Bacteriol. 1991;41:417–426. doi: 10.1099/00207713-41-3-417. [DOI] [PubMed] [Google Scholar]
- 30.Medveczky N, Rosenberg H. Phosphate transport in Escherichia coli. Biochim Biophys Acta. 1971;241:494–506. doi: 10.1016/0005-2736(71)90048-4. [DOI] [PubMed] [Google Scholar]
- 31.Neu H C, Heppel L A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965;240:3685–3692. [PubMed] [Google Scholar]
- 32.Pardo M A, Lagunez J, Miranda J, Martinez E. Nodulating ability of Rhizobium tropici is conditioned by a plasmid-encoded citrate synthase. Mol Microbiol. 1994;11:315–321. doi: 10.1111/j.1365-2958.1994.tb00311.x. [DOI] [PubMed] [Google Scholar]
- 33.Robson A D. Mineral nutrition. In: Broughton W J, editor. Nitrogen fixation. 3. Legumes. Oxford, Great Britain: Oxford University Press; 1983. pp. 36–55. [Google Scholar]
- 34.Rosenberg H, Gerdes R G, Chegwidden K. Two systems for the uptake of phosphate in Escherichia coli. J Bacteriol. 1977;131:505–511. doi: 10.1128/jb.131.2.505-511.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rottenberg H. The measurement of membrane potential and ΔpH in cells, organelles, and vesicles. Methods Enzymol. 1979;55:547–569. doi: 10.1016/0076-6879(79)55066-6. [DOI] [PubMed] [Google Scholar]
- 36.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 37.Shatters R G, Liu Y, Kahn M L. Isolation and characterization of a novel glutamine synthetase from Rhizobium meliloti. J Biol Chem. 1993;268:1–7. [PubMed] [Google Scholar]
- 38.Siebers A, Altendorf K. K+-translocating Kdp-ATPases and other bacterial P-type ATPases. In: Bakker E P, editor. Alkali cation transport systems in prokaryotes. Boca Raton, Fla: CRC Press; 1992. pp. 205–224. [Google Scholar]
- 39.Simon R, Priefer U, Puhler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Bio/Technology. 1983;1:784–791. [Google Scholar]
- 40.Simon R, Quandt J, Klipp W. New derivatives of transposon Tn5 suitable for mobilization of replicons, generation of operon fusions, and induction of genes in Gram-negative bacteria. Gene. 1989;80:161–169. doi: 10.1016/0378-1119(89)90262-x. [DOI] [PubMed] [Google Scholar]
- 41.Smart J B, Dilworth M J, Robson A D. Effect of phosphorus supply on phosphate uptake and alkaline phosphatase activity in rhizobia. Arch Microbiol. 1984;140:281–286. [Google Scholar]
- 42.Somerville J E, Kahn M L. Cloning of the glutamine synthetase I gene from Rhizobium meliloti. J Bacteriol. 1983;156:168–176. doi: 10.1128/jb.156.1.168-176.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Summers M L, Elkins J G, Elliot B A, McDermott T R. Expression and regulation of phosphate stress inducible genes in Sinorhizobium meliloti. Mol Plant-Microbe Interact. 1998;11:1094–1101. doi: 10.1094/MPMI.1998.11.11.1094. [DOI] [PubMed] [Google Scholar]
- 44.Torriani A, Rothman F. Mutants of Escherichia coli constitutive for alkaline phosphatase. J Bacteriol. 1961;81:835–836. doi: 10.1128/jb.81.5.835-836.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Treuner-Lange A, Durre P. Molecular biological analysis of kdpD/E, a sensor histidine kinase/response regulator system in Clostridium acetobutylicum. Anaerobe. 1996;2:351–363. [Google Scholar]
- 46.Treuner-Lange A, Kuhn A, Durre P. The kdp system of Clostridium acetobutylicum: cloning, sequencing, and transcriptional regulation in response to potassium concentration. J Bacteriol. 1997;179:4501–4512. doi: 10.1128/jb.179.14.4501-4512.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Turner Designs. Turner luminescence review. Bulletin no. 204. Sunnyvale, Calif: Turner Designs; 1983. [Google Scholar]
- 48.Van Veen H W, Abee T, Kortstee G J J, Koninigs W N, Zehnder A J B. Characterization of two phosphate transport systems in Acinetobacter johnsonii 210A. J Bacteriol. 1993;175:200–206. doi: 10.1128/jb.175.1.200-206.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Voegele R T, Bardin S, Finan T M. Characterization of the Rhizobium (Sinorhizobium) meliloti high- and low-affinity phosphate uptake systems. J Bacteriol. 1997;179:7226–7232. doi: 10.1128/jb.179.23.7226-7232.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walderhaug M O, Polarek J W, Voelkner P, Daniel J M, Hesse J E, Altendorf K, Epstein W. KdpD and KdpE, proteins that control expression of the kdpABC operon, are members of the two-component sensor-effector class of regulators. J Bacteriol. 1992;174:2152–2159. doi: 10.1128/jb.174.7.2152-2159.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wanner B L. Phosphorus assimilation and control of the phosphate regulon. In: Neidhardt F C, Curtiss R, Ingraham J L, Lin E C C, Brooks Low K, Magasanik B, Resnikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: American Society for Microbiology; 1996. pp. 1357–1381. [Google Scholar]
- 52.Web D C, Rosenberg H, Cox G B. Mutational analysis of the Escherichia coli phosphate-specific transport system, a member of the traffic ATPase (or ABC) family of membrane transporters. A role for proline residues in transmembrane helices. J Biol Chem. 1992;267:24661–24668. [PubMed] [Google Scholar]
- 53.Willsky G R, Malamy M H. Characterization of two genetically separable inorganic phosphate transport systems in Escherichia coli. J Bacteriol. 1980;144:356–365. doi: 10.1128/jb.144.1.356-365.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yashphe J, Chikarmane H, Iranzo M, Halvorson H O. Inorganic phosphate transport in Acinetobacter lwoffi. Curr Microbiol. 1992;24:275–280. [Google Scholar]