Abstract
Toxoplasmosis is a globally parasitic zoonotic disease transmitted by Toxoplasma gondii protozoa. This infection in its chronic form can cause a change in its host's specific behavior and is also associated with developing neuropsychological symptoms in humans. Changes in neurotransmitters' levels, especially dopamine, have been identified as a behavior change factor in the infected host. This study aimed to evaluate serum dopamine levels in acute murine toxoplasmosis. In this study, 50 mice infected with Toxoplasma were studied in 5 separate groups, and ten healthy mice were considered as negative control. For five consecutive days after parasite injection, blood sampling and serum isolation were performed daily from one of the groups. Serum dopamine levels were measured by HPLC method. Statistical studies showed that serum dopamine on the first to the fourth day after parasite inoculation was the same as the negative control, but the fifth day began to increase. The present study results indicate that dopamine production in mice infected with Toxoplasma gondii increases from day five after infection. This result suggests that in acute toxoplasmosis, dopamine production is low, and the trend of chronic disease increases dopamine production.
Keywords: Toxoplasma gondii, Dopamine, HPLC, BALB/c mice
Introduction
Toxoplasmosis is a common parasitic disease among humans and animals. About one-third of the world's population is chronically infected with this protozoal infection (Robert-Gangneux and Dardé 2012; Saadatnia and Golkar 2012). Felidae are the final host, containing sexual parasite stages and produces infective step (Oocyst) in their small intestine enterocytes(Innes 2010). Birds and a wide variety of mammals, including humans, are intermediate hosts and carriers of parasitic tissue cysts in their brains and muscles (Dubey 2020). Humans become infected by eating contaminated meat with tissue cysts, water, or vegetables contaminated with the Oocyst and congenitally (Asgari et al. 2011; Dubey 2020; Innes 2010). Infection is often asymptomatic but is especially important in immunosuppressed individuals and congenital forms (Omidian et al. 2020; Robert-Gangneux and Dardé 2012; Shiadeh et al. 2020). Chronic Toxoplasmosis was initially thought to have no clinical significance, but in recent decades the potential role of Toxoplasma brain cysts in causing mental disorders in humans has become controversial (Flegr 2013a).
Since the 1950s, researchers have drawn attention to the relationship between Toxoplasma and mental disorders (Torrey and Yolken 2003). The high prevalence of Toxoplasmosis, the high affinity of Toxoplasma parasites to the brain in the chronic phase, and the significant concurrency between anti-Toxoplasma antibodies and mental disorders have raised researcher's doubts about the causal relationship between this parasites with mental and mood disorders (Del Grande et al. 2017; Fekadu et al. 2010; Pearce et al. 2012; Xiao et al. 2018). Toxoplasma can manipulate the host brain cells and consequently behavioral changes in their host (Boillat et al. 2020). It seems that the parasite's ability to induce behavioral changes in the intermediate host leads to its predation by the final and other intermediate host and facilitates parasite transmission (Boillat et al. 2020; Hammoudi and Soldati-Favre 2017).
The main suspect for behavioral change in latent Toxoplasmosis in intermediate hosts is dopamine (Flegr 2013a; b). Dopamine is made in mammals by the adrenal glands as well as dopaminergic cells in the brain. This catecholamine is made by removing a carboxyl group from its precursor levodopa (L-dopa). Also, L-dopa is synthesized from L-Tyrosine, by the tyrosine hydroxylase enzyme (Berke 2018; Iversen and Iversen 2007). It's reported that Toxoplasma parasites can express two aromatic amino acid hydroxylases (AAH1 and AAH2) enzymes. These enzymes are responsible for catalyzing phenylalanine conversion to tyrosine and tyrosine to L-dopa (Gaskell et al. 2009). In addition, the parasite synthesizes L-dopa to build its Oocyst wall (Wang et al. 2017).
Several studies have been performed on the Toxoplasma cyst stage's role in altering neurotransmitters' levels, especially dopamine, subsequently induce behavioral changes (Johnson and Johnson 2020). Toxoplasma also has been shown capable of changing testosterone levels in addition to neurotransmitters (Kaňková et al. 2011, Lim et al. 2013, Bahreini et al. 2020). Various roles have been proposed for dopamine, outside the central nervous system, including local paracrine messenger, vasodilator function (in average concentrations), help to excretion of sodium and urine, reduces insulin production, reduces gastrointestinal motility, reduces the activity of lymphocytes (Carey 2001, Eisenhofer et al. 2004, Sarkar et al. 2010, R Buttarelli et al. 2011, Bucolo et al. 2019).
Studies have shown that the chronic stage of the Toxoplasma parasite can stimulate dopaminergic cells and induce dopamine production in the host. In addition, there is some evidence that this parasite in the acute phase can stimulate host cells to produce more dopamine. (Gaskell et al. Showed that encoding genes) AAH1 and AAH2enzymes are activated in the chronic phase when tachyzoites converted to bradyzoite form (Gaskell et al. 2009). On the other hand, Carruthers et al. demonstrated that the Toxoplasma parasite could effectively develop schizophrenia, even in the acute phase (Carruthers and Suzuki 2007). Increasing in Toxoplasma tachyzoites proliferation was observed in human fibroblast cell culture media after adding dopamine volumes (Strobl et al. 2012), which indicates the crucial role of this catecholamine in parasite physiology.
Numerous studies have focused on the role of toxoplasmosis in dopamine level changes in the chronic stage of the disease. However, the parasite's role in changing dopamine levels during the acute phase and its possible effects remains unclear. In this regard, the current study aimed to investigate the acute stage effect of the Toxoplasma parasite on changing blood dopamine levels in mice model.
Materials and methods
Ethics approval
This work was aimed to evaluate the serum dopamine levels in acute murine Toxoplasmosis in 2018 in Shiraz, Iran. The present study is based on guidelines for the care and use of laboratory animals (Council 2011). The Ethics Committee of Animal Experiments of the Shiraz University of Medical Sciences approved this research project (permit number IR.SUMS.REC.1398.023).
Parasite's preparation
Toxoplasma Parasites were prepared as previously described (Asgari et al. 2013). In brief T. gondii parasite RH strain was injected intraperitoneally into BALB/c mice. After 72 h, the mice were euthanized according to ethical standards. The peritoneal area was flushed with physiological saline via a 5 cc syringe, and the parasites were collected from the peritoneal site and then washed with PBS. The parasites were mechanically isolated from the host cells bypassing the aspirated fluid through high gauge needles. The solution was then centrifuged at 200 g for 10 min to remove cell debris. The supernatant was separated and centrifuged at 800 g for 10 min. The sediment was washed three times with Phosphate-buffered saline (PBS) at a pH of 7.2 and prepared as a pure tachyzoite.
Animals
Sixty female BALB/c mice aged 6 weeks and weighing 30–35 g were obtained from the Comparative Medical Institute of Shiraz University of Medical Sciences. All animals were kept in standard conditions: temperature of 22 ± 2 °C, the humidity of 60–40%, dark–light cycles of 12 h, proper ventilation, and access to adequate water and food. Sixty mice were divided into 6 groups of 10. Each group was kept in separate cages (Council 2011). The parasites were subcutaneously injected into groups 1 to 5 (105 tachyzoites per mice). The sixth group was considered as the negative control (only PBS was injected). After 24 h from parasite injection, the sampling began so that a group of 10 mice was sampled daily after anesthesia for five consecutive days. Moreover, uninfected mice were excluded from the study if they were Toxoplama negative by touch smear test or died before blood sampling.
The sera samples were taken to the Medical School of the Shiraz University of Medical Science. The serum was isolated and kept at 70 °C until the test.
HPLC
Chromatography conditions
HPLC was used to determine serum tyrosine levels (Waters, USA). Chromatographed Samples on an inverted phase column (Spherisorb C1; Waters) with a C18 column in isocratic mode. A 5% water-soluble acetonitrile at a 1 ml/min flow rate was used as the mobile phase. The absorption of diluted serum and control samples was reading at 225 nm in a UV detector (LC 95; Perkin-Elmer, U ‘Berlin, Germany).
Standard curve preparation
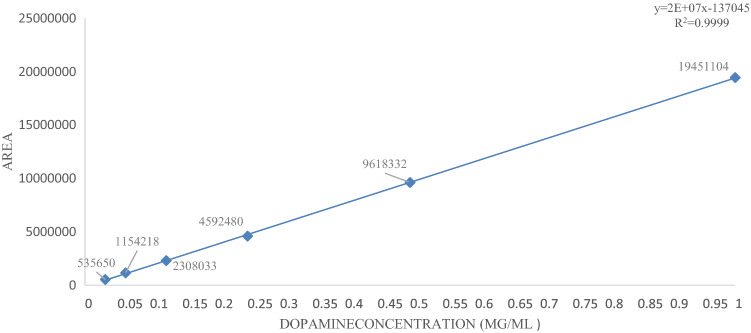
The HPLC apparatus was set up to draw a Standard curve by 1, 0.5, 0.25, 0.125, 0.0625, and 0.03125 μg/ml concentration of standard dopamine solution. An amount of 0.01 mg of dopamine was dissolved in 1 cc of 5% perchloric acid to obtain a Homogeneous solution, increased the volume solution to 10 cc, and passing through the syringe filter. Then by Serial dilution was performed using 5% perchloric acid to obtain the desired concentrations. Finally, Determined concentrations of dopamine solutions were made by absolute ethanol solvent. Each density was run three times in the HPLC device, and the standard curve was drawn using software (SQS 98; Perkin-Elmer) by different concentrations (Fig. 1).
Fig. 1.
drawn curve by HPLC corresponding to different standard concentrations of dopamine
Sample examination
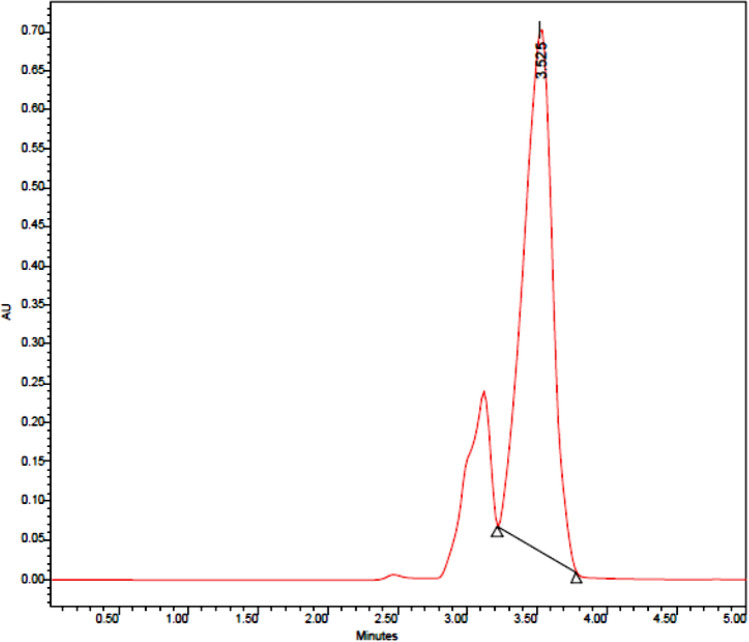
The sera were taken out of the freezer, and after thawing at room temperature, 50 μl of each serum was mixed with an equal amount of 5% (v/v) perchloric acid solution. This step was performed for all 60 samples, injected 50 μl of each sera sample into the device by an HPLC needle. Finally, by applying a retention time of 3.5 min, each sample's curve was drawn at a wavelength of 236 nm. Dopamine peaks of sera samples were determined by comparing the retention times of standard dopamine. (Fig. 2).
Fig. 2.
Drawn Standard dopamine curve by HPLC. (concentration: 0.5 μg/ml), (Retention time: 3.45), (Flow: 1 ml/minute), (Absorbance = 236 A°)
Statistical analysis
ANOVA and Post Hoc test evaluated statistical data in software SPSS version 22 (Chicago, IL, USA). P value ≤ 0.05 was considered as statistical differences.
Results
Dopamine concentration in serum samples was calculated based on the standard curve. After preparing standard dopamine solutions in different concentrations and injecting them into the device, the relevant curves were plotted. The standard equation was obtained based on the area below the curve. Then, based on the area below the calculated curve for each unknown sample and the equation's slope, the samples' dopamine concentration was calculated. The mean serum dopamine level on the first, second, third, and fourth days after injection of the parasite was similar and unmeasurable with its level in the negative control. The mean serum dopamine level in the fifth group was raised, and significant difference from that in the negative control (P = 0.042). (Fig. 3).
Fig. 3.
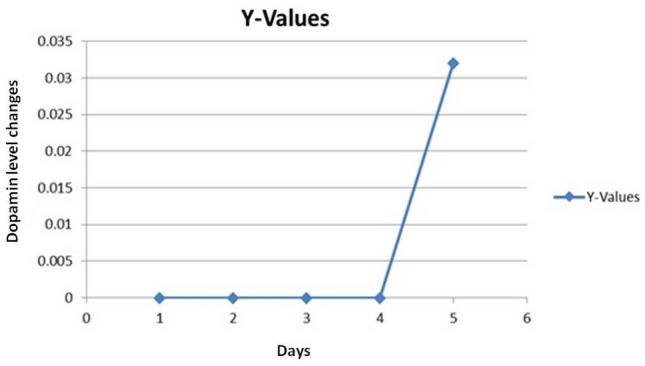
Mean serum dopamine concentrations on different days after parasite inoculation (mg/ml)
Discussion
The present study evaluated the Toxoplasma parasite's effect on changes in blood dopamine levels in acute murine toxoplasmosis. For this purpose, serum dopamine levels of 50 mice as a positive control and 10 mice as a negative control were measured on 5 consecutive days post-infection. Dopamine levels in the first to fourth days were not different in the positive and negative control groups, but on the Late acute phase (fifth day of infection), dopamine levels increased. Our previous study, which focused on tyrosine production (one of the dopamine precursors) in acute murine toxoplasmosis, showed the highest and lowest tyrosine levels in the second and fifth-day post-infection, respectively (Asgari et al. 2020). While in the current study, dopamine production began on 5th day after infection. It considered that, decreaseing the tyrosine levels and increasing the dopamine may be correlated to acute toxoplasmosis. It has been shown that the Toxoplasma parasite has a tyrosine hydroxylase enzyme that catalyzes L-dopa from L-tyrosine (Gaskell et al. 2009).
Mirzaeipour (2020) have examined tyrosine and dopamine levels in chronic toxoplasmosis (days 40, 50, 60, 70, and 80 post-infection) in mice models; They showed the highest levels of tyrosine on The 40th day. Also, an increasing trend in dopamine levels was observed from day 40 to 70 (Mirzaeipour et al. 2020). The mentioned study results showed a reducing and increasing trend in tyrosine and dopamine levels, respectively, in the late acute phase of murine toxoplasmosis (5th day). The present study results are in line with Mirzaeipour (2020), and suggest the possible role of the Toxoplasma parasite in converting tyrosine to dopamine in toxoplasmosis via the tyrosine hydroxylase enzyme.
Stibbs (1985) showed a similar dopamine production pattern in the negative control and positive control in acute murine toxoplasmosis. But dopamine production in chronic Toxoplasmosis was 14% higher in the case group than in the negative control (Mirzaeipour et al. 2020).
In the present study, we used the parasite's RH strain (lethal strain) to observe dopamine changes only in the acute phase. This strain is killing mice due to its high pathogenicity (Asgari et al. 2013).
Several studies have been performed to understand the potential of Toxoplasma's induction of behavioral disorders in the intermediate host (Fekadu et al. 2010). Many studies agree on the parasite's ability in its chronic form to alter the levels of neurotransmitters (Flegr 2013a, b). It has been concluded that the parasite in the chronic stage causes mood and physiological changes by manipulating the nervous system functions. For example, mice with Toxoplasmosis do not respond to cat urine and its presence; consequently, they are easier to hunt (Berdoy et al. 2000). According to the manipulation hypothesis, Toxoplasma parasite has been evolved to alter the intermediate host's nervous function system. In doing so, the parasite increases its chances of transmission to the next host and its survival (Flegr 2013b). Unlike the acute phase, many studies have been conducted to highlight the relation between chronic toxoplasmosis and dopamine brain levels in animal models and humans because of dopamine's importance in behavioral changes (Babaie et al. 2017; Berenreiterová et al. 2011; Parlog et al. 2015; Xiao et al. 2014, 2018). So, the present study was conducted in BALB/c mice to survey the dopamine level in acute toxoplasmosis. Our result suggests that blood dopamine level is negligible in the acute phase of infection and raise in part as we approach this phase’s end.
Conclusion
Measurement of dopamine levels in the serum of Toxoplasma mice infected in five consecutive days after parasite inoculation showed that dopamine production did not change on days one to four compared to the negative control group. Only on day 5 in some mice dopamine production increased. This result indicates that dopamine production is deficient in Toxoplasmosis's acute period and that dopamine production increases chronically over time.
Acknowledgements
The authors would like to thank the Office of Vice-Chancellor for Research of Shiraz University of Medical Sciences, Shiraz, Iran, to support this project. This article was extracted from an MD thesis by Shokoufeh Moshgi.
Declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Asgari Q, Mehrabani D, Motazedian M, Kalantari M, Nouroozi J, Adnani Sadati S. The viability and infectivity of Toxoplasma gondii tachyzoites in dairy products undergoing food processing. Asian J Anim Sci. 2011;5(2):1–6. [Google Scholar]
- Asgari Q, Keshavarz H, Shojaee S, Motazedian MH, Mohebali M, Miri R, Mehrabani D, Rezaeian M. In vitro and in vivo potential of RH strain of Toxoplasma gondii (Type I) in tissue cyst forming. Iran J Parasitol. 2013;8(3):367–375. [PMC free article] [PubMed] [Google Scholar]
- Asgari Q, Sisakht MM, Shahabadi SN, Karami F, Omidian M. Serum tyrosine level in acute murine toxoplasmosis. Iran J Parasitol. 2020;15(4):568–575. doi: 10.18502/ijpa.v15i4.4866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babaie J, Sayyah M, Fard-Esfahani P, Golkar M, Gharagozli K. Contribution of dopamine neurotransmission in proconvulsant effect of Toxoplasma gondii infection in male mice. J Neurosci Res. 2017;95(10):1894–1905. doi: 10.1002/jnr.24036. [DOI] [PubMed] [Google Scholar]
- Bahreini MS, Zarei F, Dastan N, Sami Jahromi S, Pourzargham P, Asgari Q. The relationship between Toxoplasma gondii infection in mothers and neonate’s gender. J Matern Fetal Neonatal Med. 2020 doi: 10.1080/14767058.2020.1849103. [DOI] [PubMed] [Google Scholar]
- Berdoy M, Webster JP, Macdonald DW. Fatal attraction in rats infected with Toxoplasma gondii. Proc Soc Exp Biol Med. 2000;267(1452):1591–1594. doi: 10.1098/rspb.2000.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenreiterová M, Flegr J, Kuběna AA, Němec P. The distribution of Toxoplasma gondii cysts in the brain of a mouse with latent toxoplasmosis: implications for the behavioral manipulation hypothesis. PloS one. 2011;6(12):e28925. doi: 10.1371/journal.pone.0028925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berke JD. What does dopamine mean? Nat Neurosci. 2018;21(6):787–793. doi: 10.1038/s41593-018-0152-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boillat M, Hammoudi PM, Dogga SK, Pagès S, Goubran M, Rodriguez I, Soldati-Favre D. Neuroinflammation-associated aspecific manipulation of mouse predator fear by Toxoplasma gondii. Cell reports. 2020;30(2):320–334. doi: 10.1016/j.celrep.2019.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucolo C, Leggio GM, Drago F, Salomone S. Dopamine outside the brain: The eye, cardiovascular system and endocrine pancreas. Pharmacol Ther. 2019;203:107392. doi: 10.1016/j.pharmthera.2019.07.003. [DOI] [PubMed] [Google Scholar]
- Buttarelli RF, Fanciulli A, Pellicano C, Pontieri E, F, The dopaminergic system in peripheral blood lymphocytes: from physiology to pharmacology and potential applications to neuropsychiatric disorders. Curr Neuropharmacol. 2011;9(2):278–288. doi: 10.2174/157015911795596612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey RM. Renal dopamine system: paracrine regulator of sodium homeostasis and blood pressure. Hypertension. 2001;38(3):297–302. doi: 10.1161/hy0901.096422. [DOI] [PubMed] [Google Scholar]
- Carruthers VB, Suzuki Y. Effects of Toxoplasma gondii infection on the brain. Schizophr Bull. 2007;33(3):745–751. doi: 10.1093/schbul/sbm008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Council NR (2011) Guide for the care and use of laboratory animals, National Academies Press, Washington [PubMed]
- Del Grande C, Galli L, Schiavi E, Dell’osso L, Bruschi F, Is Toxoplasma gondii a trigger of bipolar disorder? Pathogens. 2017;6(1):3. doi: 10.3390/pathogens6010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey JP. The history and life cycle of Toxoplasma gondii. USA: Elsevier; 2020. [Google Scholar]
- Eisenhofer G, Kopin IJ, Goldstein DS. Catecholamine metabolism: a contemporary view with implications for physiology and medicine. Pharmacol Rev. 2004;56(3):331–349. doi: 10.1124/pr.56.3.1. [DOI] [PubMed] [Google Scholar]
- Fekadu A, Shibre T, Cleare AJ. Toxoplasmosis as a cause for behaviour disorders-overview of evidence and mechanisms. Folia Parasitol. 2010;57(2):105. doi: 10.14411/fp.2010.013. [DOI] [PubMed] [Google Scholar]
- Flegr J. How and why Toxoplasma makes us crazy. Trends Parasitol. 2013;29(4):156–163. doi: 10.1016/j.pt.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Flegr J. Influence of latent Toxoplasma infection on human personality, physiology and morphology: pros and cons of the Toxoplasma–human model in studying the manipulation hypothesis. J Exp Biol. 2013;216(1):127–133. doi: 10.1242/jeb.073635. [DOI] [PubMed] [Google Scholar]
- Gaskell EA, Smith JE, Pinney JW, Westhead DR, Mcconkey GA. A unique dual activity amino acid hydroxylase in Toxoplasma gondii. PloS one. 2009;4(3):e4801. doi: 10.1371/journal.pone.0004801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammoudi P-M, Soldati-Favre D. Insights into the molecular basis of host behaviour manipulation by Toxoplasma gondii infection. Emerging Top Life Sci. 2017;1(6):563–572. doi: 10.1042/ETLS20170108. [DOI] [PubMed] [Google Scholar]
- Innes E. A brief history and overview of Toxoplasma gondii. Zoonoses Public Health. 2010;57(1):1–7. doi: 10.1111/j.1863-2378.2009.01276.x. [DOI] [PubMed] [Google Scholar]
- Iversen SD, Iversen LL. Dopamine: 50 years in perspective. Trends Neurosci. 2007;30(5):188–193. doi: 10.1016/j.tins.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Johnson SK, Johnson PTJ. Toxoplasmosis: recent advances in understanding the link between infection and host behavior. Annu Rev Anim Biosci. 2020;9(1):249–264. doi: 10.1146/annurev-animal-081720-111125. [DOI] [PubMed] [Google Scholar]
- Kaňková Š, Kodym P, Flegr J. Direct evidence of Toxoplasma-induced changes in serum testosterone in mice. Exp Parasitol. 2011;128(3):181–183. doi: 10.1016/j.exppara.2011.03.014. [DOI] [PubMed] [Google Scholar]
- Lim A, Kumar V, Hari Dass SA, Vyas A. Toxoplasma gondii infection enhances testicular steroidogenesis in rats. Mol Ecol. 2013;22(1):102–110. doi: 10.1111/mec.12042. [DOI] [PubMed] [Google Scholar]
- Mirzaeipour M, Mikaeili F, Asgari Q, Nohtani M, Rashidi S, Bahreini MS (2020) Evaluation of the tyrosine and dopamine serum level in experimental infected BALB/c mice with chronic toxoplasmosis. bioRxiv. [DOI] [PMC free article] [PubMed]
- Omidian M, Ganjkarimi AH, Asgari Q, Hatam G. Molecular and serological study on congenital toxoplasmosis in newborn of Shiraz, Southern Iran. E Environ Sci Pollut Res Int. 2020;28(3):16122–16128. doi: 10.1007/s11356-020-11707-x. [DOI] [PubMed] [Google Scholar]
- Parlog A, Schlüter D, Dunay IR. Toxoplasma gondii-induced neuronal alterations. Parasite Immunol. 2015;37(3):159–170. doi: 10.1111/pim.12157. [DOI] [PubMed] [Google Scholar]
- Pearce BD, Kruszon-Moran D, Jones JL. The relationship between Toxoplasma gondii infection and mood disorders in the third national health and nutrition survey. Biol Psychiatry. 2012;72(4):290–295. doi: 10.1016/j.biopsych.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert-Gangneux F, Dardé M-L. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin Microbiol Rev. 2012;25(2):264–296. doi: 10.1128/CMR.05013-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadatnia G, Golkar M. A review on human toxoplasmosis. Scand J Infect Dis. 2012;44(11):805–814. doi: 10.3109/00365548.2012.693197. [DOI] [PubMed] [Google Scholar]
- Sarkar C, Basu B, Chakroborty D, Dasgupta PS, Basu S. The immunoregulatory role of dopamine: an update. Brain Behav Immun. 2010;24(4):525–528. doi: 10.1016/j.bbi.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiadeh MN, Esfandyari S, Ashrafmansouri M, Mirzapour A, Taghipour A, Spotin A, Arefkhah N, Gamble R, Safa A, Rostami A. The prevalence of latent and acute toxoplasmosis in HIV-infected pregnant women: a systematic review and meta-analysis. Microb Pathog. 2020 doi: 10.1016/j.micpath.2020.104549. [DOI] [PubMed] [Google Scholar]
- Stibbs H. Changes in brain concentrations of catecholamines and indoleamines in Toxoplasma gondii infected mice. Ann Trop Med Parasitol. 1985;79(2):153–157. doi: 10.1080/00034983.1985.11811902. [DOI] [PubMed] [Google Scholar]
- Strobl JS, Goodwin DG, Rzigalinski BA, Lindsay DS. Dopamine stimulates propagation of Toxoplasma gondii tachyzoites in human fibroblast and primary neonatal rat astrocyte cell cultures. J Parasitol. 2012;98(6):1296–1299. doi: 10.1645/GE-2760.1. [DOI] [PubMed] [Google Scholar]
- Torrey EF, Yolken RH. Toxoplasma gondii and schizophrenia. Emerg Infect Dis. 2003;9(11):1375. doi: 10.3201/eid0911.030143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZT, Verma SK, Dubey JP, Sibley LD. The aromatic amino acid hydroxylase genes AAH1 and AAH2 in Toxoplasma gondii contribute to transmission in the cat. PLoS pathogens. 2017;13(3):e1006272. doi: 10.1371/journal.ppat.1006272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J, Li Y, Prandovszky E, Karuppagounder SS, Talbot CC, Jr, Dawson VL, Dawson TM, Yolken RH. MicroRNA-132 dysregulation in Toxoplasma gondii infection has implications for dopamine signaling pathway. J Neurosci. 2014;268:128–138. doi: 10.1016/j.neuroscience.2014.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J, Prandovszky E, Kannan G, Pletnikov MV, Dickerson F, Severance EG, Yolken RH. Toxoplasma gondii: biological parameters of the connection to schizophrenia. Schizophr Bull. 2018;44(5):983–992. doi: 10.1093/schbul/sby082. [DOI] [PMC free article] [PubMed] [Google Scholar]