Abstract
Use of anabolic androgenic steroids (AAS) is associated with adverse health effects. The factors that predispose to AAS use among athletes are poorly understood, but attention deficit/hyperactivity disorder (ADHD), which is known to occur among athletes more often than in the general population, is associated with risk behaviors, including substance abuse. We aimed to see if AAS use in male weightlifters was associated with ADHD symptoms, and test the link between ADHD symptoms and cognitive performance. Hundred and forty male weightlifters, 72 AAS users and 68 weightlifting controls (WLC), completed the Achenbach system of empirically based assessment (ASEBA) for ADHD symptoms and underwent cognitive examination. Self-reported ADHD symptom scores were significantly higher among AAS users compared to WLC, and scores in the range indicating clinically important ADHD was significantly more common in the AAS-using group. Age of onset of AAS use correlated inversely with ADHD scale score (r = − 0.35; p = 0.003). ADHD score correlated inversely with cognitive scores for working memory (r = − 0.25, p < 0.001), processing speed (r = − 0.24, p < 0.001), verbal learning and memory (r = − 0.19, p = 0.03), and problem solving (r = − 0.20, p = 0.02). AAS use among weightlifters is associated with ADHD symptoms and corresponding lower cognitive performance. Recognising a relationship between ADHD symptoms and AAS use may guide drug prevention strategies in sports.
Subject terms: Neuroscience, Psychology, Endocrinology, Neurology, Risk factors
Introduction
Use of anabolic androgenic steroids (AAS) is a serious abuse problem among professional and recreational athletes1–4. AAS have anabolic properties, stimulating muscle growth5, and androgenic properties inducing masculine secondary sexual characterisics, and augments cognitive features like alertness6–9. However, AAS use may have serious psychological and physiological consequences, such as major mood syndromes and cardiovascular disease10,11. The main activity of AAS in the brain occurs via activation of widely distributed cytoplasmic androgen receptors, as has been shown in animal studies12–15. This may explain the various effects that AAS have on cognition and mental state10,11,16,17. Long term AAS use is associated with both structural brain abnormalities18–21 and cognitive and behavioral abnormalities20,22,23. Several studies suggest an association between AAS use and aggressiveness, hostility, mood swings, and violent crime3,18,24–31. Still, its massive impact on muscle growth has made AAS popular among athletes worldwide32–34.
The impact of AAS doses may be difficult to determine for several reasons. More than 100 different AAS compounds have been synthesised, with three major classes that differ in molecular structure and metabolic half-lives, and hence physiologic effects. AAS include testosterone and its various synthetic derivatives with the three most common forms being (1) 19-nortestosterone derivatives (nandrolone phenylpropriate, nandrolone decanote, methenolone enanthate), (2) C-17 β-ester derivatives (testosterone propionate, cypionate, enanthate, or undecanoate), and (3) 17 α-alkyl derivatives (stanozolol, oxymetholone, norethandrolone, danazol). Weightlifters commonly coadminister various AAS and administer drugs in cycles of use and nonuse lasting from weeks to months1,22,35–37.
The factors that predispose to AAS use are poorly understood. However, attention deficit/hyperactivity disorder (ADHD) occurs among athletes at different levels, from any organized sport to the elite, with a prevalence between 7 and 11%, higher than in the general population38–40. Moreover, in a longitudinal study of 100 AAS users, 17% reported a history of psychiatric illness at inclusion, where ADHD was the most common diagnosis reported by 7%30. Persons with ADHD have increased risk of substance use41–46, which, theoretically, could include AAS use. ADHD implies inattention, and/or impulsivity and hyperactivity at a disabling level. Symptoms at levels that do not meet the diagnostic criteria for ADHD may still affect a person’s cognition and behavior. The severity and number of ADHD symptoms are associated with the degree of psychiatric comorbidity and disability, including cognitive abnormalities47. Cognitive domains commonly affected in ADHD include attention, working memory and problem solving48–50. As mental health issues may be neglected among athletes51, adverse symptoms, like neurocognitive deficits, may be present despite the lack of diagnosis and treatment. While the sporting context might serve as an outlet for certain symptoms, these athletes may suffer significantly in other contexts like in social relationships or working life. As ADHD is a risk factor for overall drug use41–46, and ADHD symptoms are common yet often undetected among athletes38–40, it is possible that ADHD symptoms could predispose to AAS use.
The aim of our study was to see whether use of AAS among male weightlifters is associated with symptoms of ADHD. To identify ADHD symptoms participants were asked to complete the Achenbach System of Empirically Based Assessment (ASEBA) questionnaire. We further examined how self-reported ADHD symptoms were associated with cognitive performance as evaluated by cognitive examination.
Methods
Study population
The study was conducted at the Department of Physical Medicine and Rehabilitation, Oslo University Hospital, Oslo. Two groups of weightlifters over 18 years of age were recruited to the study: those with (1) current or previous use of AAS, with at least 1 year of cumulative AAS exposure (n = 89), or (2) no previous or current exposure to AAS or other muscle- and performance-enhancing drugs (n = 72). Cognitive data was obtained from 159 participants, including 89 current or previous AAS users, and 70 weightlifting controls (WLC). Of those, 24 were not included in the current study due to missing data on the ASEBA questionnaire, and one was excluded due to a neuroradiological finding and one due to epilepsy. The present study comprises 134 male weightlifters; thereof 72 AAS users and 62 WLC with complete datasets including cognitive tests and the ASEBA questionnaire for ADHD symptoms. The sample is partly overlapping with the one described in our previous work19,22.
The recruitment was done through websites or online forums associated with heavy resistance training or AAS use, as well as through posters and flyers distributed in selected gyms in Oslo. Every participants received a written description of the study prior to participation and written formal consent was obtained from all participants. They were compensated with 1000 NOK (approx. 125 USD) for their participation. The study was approved by the Regional Committees for Medical and Health Research Ethics South East of Norway (approval # 2013/601), and all research was carried out in accordance with the Declaration of Helsinki.
Cognitive assessment
Participants underwent eight neuropsyhological tests covering a broad range of cognitive domains, including the Wechsler Abbreviated Scale of Intelligence52, the California Verbal Learning Test53, Letter Memory Task54,55, the Delis-Kaplan Executive Functioning’s Color-Word Interference Test (CWIT) and Trail Making Test (TMT) from the Delis-Kaplan Executive Functioning (D-KEFS) test battery56, and Corsi Block Test from the PEBL (Psychology Experiment Building Language) Version 0.13 test battery57,58. The twenty-five subtests were divided into six cognitive domains with acceptable reliability as described by Bjørnebekk et al.22. Of those, five were considered relevant for the current study; (1) Speed, (2) Working memory, (3) Learning and memory, (4) Problem Solving, and (5) Executive functioning. Overview of the cognitive tests administered, and the cognitive domains are shown in Table 1.
Table 1.
Tests used for cognitive assessment and what functions they assess.
| Test name | Abbreviation | Measures included | Function measured |
|---|---|---|---|
| California learning test-second edition | CVLT-II | Total learning, delayed recall 30 min, and false positives | Learning and memory |
| Wechsler abbreviated scale of intelligence | WASI | Matrix reasoning | Problem solving |
| Stroop color-word interference test—Delis Kaplan | CWIT—D-KEFS | CWIT1-4 (color naming speed, word reading speed, inhibiton, switching), contrast and flexibility | Executive function, Processing speed |
| Trail making test—Delis Kaplan executive function | TMT—D-KEFS | TMT1-4 (visual scanning, number sequencing, letter sequencing, number-letter switching) |
Executive function, Processing speed |
| Letter memory task | Letter memory | Letter memory | Working memory |
| Corsi block—tapping task—psychology experiment building | Corsi, PEBL | Block span and memory span | Working memory |
| Attention network task | ANT | Response time | Processing speed |
Assessment of ADHD symptoms
The Adult Self Report (ASR) ASEBA is a revision of the Young Adult Self-Report protocol for adults aged 18–59 originally derived from the widely used Child Behavior Checklist59. The ASEBA assesses emotional and behavioral problems in a standardised format and has performed well in validation studies (sensitivity = 68.7% and specificity = 99.5%) with high concordance with clinician diagnosis42,59–63. The ASR ASEBA contains 126 items to assess behavior that have occurred over the past 6 months with the total score for each scale being the sum of the scores for scale items. On all scales a T score > 65 is clinicaly concerning, while a T score > 70 is indicative of diagnosis59.
Data presentation and statistics
Data are given as mean and standard deviation (SD) values or as number of participants and percentage, as appropriate. Statistical analyses were performed using SPSS version 2564 and violin plot using R ggplot265 Group differences in demographic data and ADHD scores were evaluated with two-tailed independent sample t tests ot assuming equal variance and Fisher’s exact tests for categorical data. Differences in ADHD scores were evaluated with Wilcoxon–Mann–Whitney tests, to account for the non-normal distributions. To explore whether ADHD symptoms seems to be influenced by current use of AAS, a similar analysis within the AAS-group was conducted comparing current and past AAS users (defined as more than 1 year since past AAS use). Lastly, Spearman’s rank-order correlation (rs) was used to investigate relationships between ADHD symptoms, cognitive performance, and parameters of AAS use. For cognitive performance z-transformed residuals were used, removing variability associated with age and education.
Results
The group of AAS users (n = 72) and WLC (n = 62) weightlifters did not differ significantly with respect to age or height (Table 2). The two groups did however differ on weight, time spent excercising, and bench press record where AAS users were significantly heavier, and had higher bench press records compared to the WLC even though they spent less time per week on strength training. They also differed on average IQ score and years of education: WLC had significantly longer education and higher IQ scores.
Table 2.
Demographic and clinical data.
| Sample characteristics | AAS (n = 72) | WLC (n = 62) | t | p | ||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Age, years | 33.2 | 8.2 | 31.0 | 9.3 | − 1.34 | 0.091 |
| Education, years | 14.3 | 2.5 | 15.8 | 2.7 | 3.26 | < 0.001 |
| IQ | 105.6 | 11.8 | 113.3 | 9.5 | 4.15 | < 0.001 |
| Alcohol, units/week | 1.6 | 3.2 | 3.4 | 5.0 | 2.34 | 0.020 |
| Height, cm | 180.9 | 6.6 | 181.0 | 6.9 | 0.09 | 0.842 |
| Weight, kg | 97.6 | 13.7 | 90.9 | 14.6 | − 2.72 | 0.007 |
| BMI | 29.7 | 4.1 | 27.7 | 4.1 | − 2.8 | 0.6 |
| Cigarettes per day | 1.5 | 1.5 | 0.3 | 2.6 | − 1.95 | 0.051 |
| Strength training, min/week | 346.1 | 184.7 | 479.7 | 246.8 | 3.55 | 0.037 |
| Bench press record, kg | 169 | 31 | 135.4 | 33 | − 5.88 | < 0.001 |
Male weightlifters who used AAS (n = 72) were compared to a group of weightlifters who had not used AAS (n = 62). Data are number of participants and mean values (SD) for all variables.
AAS: anabolic androgenic steroid, WLC: weightlifting control subjects, IQ: intelligence quotient, BMI: body mass index.
The two groups differed on substance use other than AAS. Alcohol consumption was lower among AAS users, while tobacco use was more frequent among AAS users (Table 2). Use of anxiolytics and antidepressants were more common among AAS users, as 32% (n = 23) reported to have used these medications compared to only 3% (n = 2) of WLC (p < 0.001).
Participants’ AAS use typically started in their early twenties (mean age 21.5 years, SD = 5.3, range 12–39) and on average AAS had been used for 9.5 years (SD = 5.6, range = 1.5–30).
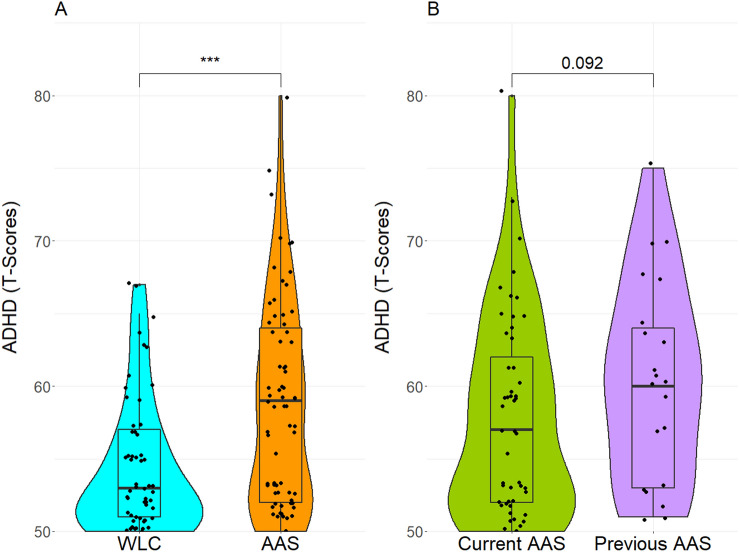
ADHD symptom scores were higher among AAS users (Mdn = 59.0) than among WLC (Mdn = 53.0). This difference was statistically significant, U (NAAS = 72, NWLC = 61,) = 1360.00, z = − 3.79, p < 0.001 (Fig. 1A). Also, there was a significant group difference in the frequency of clinically concerning ADHD symptoms (t-score > 65), where twelve (16.7%) AAS-users had scores within the borderline or clinical range, compared to two (3.3%) of the WLC (p = 0.02). A negative correlation was found between age of onset of AAS use and self-reported ADHD symptoms (rs = − 0.35, p = 0.003), whereas years of AAS use were not related to ADHD scores (rs = 0.08, p = 0.50). Furthermore, analyses within the AAS sample, showed that ADHD scores of current users (Mdn = 57.0) were lower than scores of previous users (Mdn = 60.0), however not significantly, U (NAAS CURRENT = 51, NAAS PAST = 21,) = 399.50, z = − 1.69, p = 0.09 (Fig. 1B).
Figure 1.
ADHD symptoms among AAS users and WLC. AAS-using male weightlifters (n = 72) and WLC (n = 62) complete the ASEBA questionnaire on ADHD symptoms. (A) Shows ADHD symtoms presented as T scores in AAS users and controls. (B) Shows ADHD symptoms presented as T scores in current and previous users of AAS. ADHD: attention deficit/hyperactivity disorder, WLC: weightlifting controls, AAS: anabolic androgenic steroids.
Self-reported ADHD symptoms correlated inversely with cognitive scores on working memory, processing speed, verbal learning and memory, and problem solving (Table 3). In contrast, no correlation was found between self-reported ADHD symptoms and executive function.
Table 3.
Spearman’s correlation between self-reported ADHD symptoms and neuropsychological test scores.
| ADHD | Working memory | Speed | Verbal learning and memory | Problem solving | Executive function |
|---|---|---|---|---|---|
| Rho | − 0.25 | − 0.24 | − 0.19 | − 0.20 | 0.11 |
| P | 0.00 | 0.00 | 0.03 | 0.02 | 0.21 |
The participants neuropsychological test scores within five cognitive domains were correlated to their ADHD T scores. Correlation is corrected for age and education using standardised residuals. The data are Spearman’s correlation coefficients (Rho) and their corresponding p values.
Discussion
The main finding of the present study was the higher occurence of ADHD symptoms among AAS-using male weightlifters compared to WLC. This prevalence is likely an underestimation (due to ASEBA’s low sensitivity (68.7%) for ADHD symptoms66. Other studies have found that ADHD entails a risk for substance abuse46,67–70. Our finding suggests that this risk also includes use of AAS. As the psychological and physiological effects of AAS use include adverse effects like major mood syndromes, hostility, structural and functional brain abnormalities19,21,22, violent crime, and cardiovascular diseases10,11, prevention programs are needed. Treatment and medication for ADHD have been shown to prevent substance abuse44,71,72. In-person brief motivational interventions, programs with discussion of sports nutrition, exercise alternatives to AAS, drug refusal role-playing, and the creation of health promotion messages have been shown effective in drug prevention among athletes73,74. Recognising the relationship between ADHD symptoms and AAS use can inform such prevention programs in sports medicine.
We found that ADHD symptoms correlated inversely with age of onset of AAS use. This cross-sectional study is not able to determine whether the ADHD symptoms were the cause or the consequence of AAS use, or whether AAS use caused ADHD symptoms. On the one hand, ADHD is present from childhood75, whereas AAS-exposure occurs later in life, an observation suggesting that ADHD may be the primary factor for AAS use. On the other hand, AAS at high doses are known to cause impulsivity and aggressiveness9, two symptoms that are common in ADHD76. In the present study, three observations suggested a primary role for ADHD as predisposing to AAS use. First, the degree of self-reported ADHD symptoms did not increase with the number of years of AAS use, suggesting that greater length of AAS use does not increase ADHD symptom score. Second, the age of onset of AAS use was inversely correlated with ADHD symptom level, suggesting that the more severe the ADHD symptomatology, the greater the likelihood of early AAS onset. Third, previous users of AAS scored equally high as current users, suggesting that current AAS use does not increase the severity of ADHD-like symptoms. This conclusion fits the notion that ADHD predisposes to substance abuse in general41–46. However, prospective studies are needed to determine to what degree ADHD predisposes to AAS use and whether AAS use may cause the appearance of ADHD symptoms.
We found a higher use of antidepressants and anxiolytics among AAS-using male weightlifters than among WLC with respect to. This finding is in accordance with previous studies reporting high rates of psychiatric comorbidity in ADHD77–83. It should be noted, however, that the majority of AAS users did not use prescription drugs, whether for physical og psychological conditions. Thus, the use of psychotropic medication among the AAS users was not a major confounder in our study.
ADHD scores correlated inversely with scores on several tests of cognitive domains related to ADHD. This finding indicates that the self-reported symptoms of ADHD were reliable and that the ADHD symptoms had implications for the participants’ cognitive function. Specifically, we found that self-reported ADHD symptoms correlated inversely with cognitive scores on working memory, processing speed, verbal learning and memory, and problem solving. These findings are consistent with previous findings on cognitive deficits among persons with ADHD41,84–88. Executive functioning was the only cognitive domain measured that did not correlate with ADHD symptoms. Deficits in executive function is considered a central underlying mechanism of ADHD89,90. However, our findings are in accordance with studies of other patients and samples of AAS users, in which performance-based executive functions and self-reported measures of executive functions in everyday life are unrelated23,91,92.
Some limitations should be noted. First, the cross-sectional study design implies that we cannot draw definite conclusions about whether ADHD causes AAS use or vice versa. Because we limited ourselves to the study of male weight lifters, our findings are not generalizable to female AAS users. Further, as we have recruited participants from online forums, social media and gyms, targeting heavy resistance training and AAS use, we risk having a skewed selection of AAS users. Therefore, we cannot generalize from our study to subpopulations such as prisoners93, substance use patients94, and sexual minority males95, among whom AAS use also occurs. It is also possible that our offering financial compensation for participation could introduce recruitment bias. However, the modest sum of money that participants received was intended to compensate for their use of time and their travel expenses when going to the hospital. Finally, whereas we did ask about the use of medications, we did not ask about ADHD medication specifically. Therefore, we do not know to what degree use of ADHD medication influenced our results.
Conclusion
Our findings suggest that ADHD symptoms are more common among weightlifters who use AAS. Correspondence between ADHD symptoms and cognitive test performance substantiated this finding. Recognising a relationship between ADHD symptoms and AAS use may guide prevention strategies against AAS use in sports.
Abbreviations
- AAS
Anabolic androgenic steroids
- WLC
Weightlifting controls
- ADHD
Attention deficit/hyperactivity disorder
- ASEBA
Achenbach system of empirically based assessment
Author contributions
E.K. conceived the hypothesis. E.K. and A.B. developed the study and performed the computations. B.H. supervised the findings. E.K. wrote the manuscript with support from B.H. and A.B. All authors discussed the results and contributed to the final manuscript.
Funding
Funding was provided by Helse Sør-Øst RHF (grant Nos 2013087, 2016049, 2017025, and 2018075).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kanayama G, Brower KJ, Wood RI, Hudson JI, Pope HG., Jr Anabolic-androgenic steroid dependence: An emerging disorder. Addiction. 2009 doi: 10.1111/j.1360-0443.2009.02734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mullen C, Whalley BJ, Schifano F, Baker JS. Anabolic androgenic steroid abuse in the United Kingdom: An update. Br. J. Pharmacol. 2020 doi: 10.1111/bph.14995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pope HG, Jr, Kouri EM, Hudson JI. Effects of supraphysiologic doses of testosterone on mood and aggression in normal men: A randomized controlled trial. Arch. Gen. Psychiatry. 2000;57:133–140. doi: 10.1001/archpsyc.57.2.133. [DOI] [PubMed] [Google Scholar]
- 4.de Ronde W, Smit DL. Anabolic androgenic steroid abuse in young males. Endocr. Connect. 2020 doi: 10.1530/EC-19-0557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herbst KL, Bhasin S. Testosterone action on skeletal muscle. Curr. Opin. Clin. Nutr. Metab. Care. 2004 doi: 10.1097/00075197-200405000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Fang H, Li X, Wu Y, Peng W. Single dose testosterone administration modulates the temporal dynamics of distractor processing. Psychoneuroendocrinology. 2020 doi: 10.1016/j.psyneuen.2020.104838. [DOI] [PubMed] [Google Scholar]
- 7.Frye CA, Seliga AM. Testosterone increases analgesia, anxiolysis, and cognitive performance of male rats. Cogn. Affect. Behav. Neurosci. 2001 doi: 10.3758/cabn.1.4.371. [DOI] [PubMed] [Google Scholar]
- 8.Frye CA, Edinger K, Sumida K. Androgen administration to aged male mice increases anti-anxiety behavior and enhances cognitive performance. Neuropsychopharmacology. 2008 doi: 10.1038/sj.npp.1301498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hildebrandt T, Langenbucher JW, Flores A, Harty S, Berlin HA. The influence of age of onset and acute anabolic steroid exposure on cognitive performance, impulsivity, and aggression in men. Psychol. Addict. Behav. 2014 doi: 10.1037/a0036482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pope HG, Katz DL. Psychiatric and medical effects of anabolic-androgenic steroid use. A controlled study of 160 athletes. Arch. Gen. Psychiatry. 1994 doi: 10.1001/archpsyc.1994.03950050035004. [DOI] [PubMed] [Google Scholar]
- 11.Baggish AL, et al. Cardiovascular toxicity of illicit anabolic-androgenic steroid use. Circulation. 2017 doi: 10.1161/CIRCULATIONAHA.116.026945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clark AS, Henderson LP. Behavioral and physiological responses to anabolic-androgenic steroids. Neurosci. Biobehav. Rev. 2003 doi: 10.1016/s0149-7634(03)00064-2. [DOI] [PubMed] [Google Scholar]
- 13.Pomerantz SM, Fox TO, Sholl SA, Vito CC, Goy RW. Androgen and estrogen receptors in fetal rhesus monkey brain and anterior pituitary. Endocrinology. 1985 doi: 10.1210/endo-116-1-83. [DOI] [PubMed] [Google Scholar]
- 14.Roselli CE. The effect of anabolic-androgenic steroids on aromatase activity and androgen receptor binding in the rat preoptic area. Brain Res. 1998 doi: 10.1016/s0006-8993(98)00148-6. [DOI] [PubMed] [Google Scholar]
- 15.Simerly RB, Swanson LW, Chang C, Muramatsu M. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: An in situ hybridization study. J. Compar. Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- 16.Kanayama G, Kean J, Hudson JI, Pope HG., Jr Cognitive deficits in long-term anabolic-androgenic steroid users. Drug Alcohol Depend. 2013 doi: 10.1016/j.drugalcdep.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanayama G, Kaufman MJ, Pope HG., Jr Public health impact of androgens. Curr. Opin. Endocrinol. Diabetes Obes. 2018 doi: 10.1097/MED.0000000000000404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hauger LE, Havnes IA, Jørstad ML, Bjørnebekk A. Anabolic androgenic steroids, antisocial personality traits, aggression and violence. Drug Alcohol Depend. 2021 doi: 10.1016/j.drugalcdep.2021.108604. [DOI] [PubMed] [Google Scholar]
- 19.Bjørnebekk A, et al. Structural brain imaging of long-term anabolic-androgenic steroid users and nonusing weightlifters. Biol. Psychiatry. 2017 doi: 10.1016/j.biopsych.2016.06.017. [DOI] [PubMed] [Google Scholar]
- 20.Kaufman MJ, Janes AC, Hudson JI, Brennan BP, Kanayama G, Kerrigan AR, Jensen JE, Pope HG., Jr Brain and cognition abnormalities in long-term anabolic-androgenic steroid users. Drug Alcohol Depend. 2015 doi: 10.1016/j.drugalcdep.2015.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hauger LE, Westlye LT, Fjell AM, Walhovd KB, Bjørnebekk A. Structural brain characteristics of anabolic-androgenic steroid dependence in men. Addiction. 2019 doi: 10.1111/add.14629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bjørnebekk A, et al. Cognitive performance and structural brain correlates in long-term anabolic-androgenic steroid exposed and nonexposed weightlifters. Neuropsychology. 2019 doi: 10.1037/neu0000537. [DOI] [PubMed] [Google Scholar]
- 23.Hauger LE, Westlye LT, Bjørnebekk A. Anabolic androgenic steroid dependence is associated with executive dysfunction. Drug Alcohol Depend. 2020 doi: 10.1016/j.drugalcdep.2020.107874. [DOI] [PubMed] [Google Scholar]
- 24.Lundholm L, Frisell T, Lichtenstein P, Långström N. Anabolic androgenic steroids and violent offending: Confounding by polysubstance abuse among 10,365 general population men. Addiction. 2015 doi: 10.1111/add.12715. [DOI] [PubMed] [Google Scholar]
- 25.Beaver KM, Vaughn MG, Delisi M, Wright JP. Anabolic-androgenic steroid use and involvement in violent behavior in a nationally representative sample of young adult males in the United States. Am. J. Public Health. 2008 doi: 10.2105/AJPH.2008.137018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hall RC, Chapman MJ. Psychiatric complications of anabolic steroid abuse. Psychosomatics. 2005 doi: 10.1176/appi.psy.46.4.285. [DOI] [PubMed] [Google Scholar]
- 27.Horwitz H, Andersen JT, Dalhoff KP. Health consequences of androgenic anabolic steroid use. J. Intern. Med. 2019 doi: 10.1111/joim.12850. [DOI] [PubMed] [Google Scholar]
- 28.Klötz F, Petersson A, Hoffman O, Thiblin I. The significance of anabolic androgenic steroids in a Swedish prison population. Compr. Psychiatry. 2010 doi: 10.1016/j.comppsych.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 29.Christoffersen T, Andersen JT, Dalhoff KP, Horwitz H. Anabolic-androgenic steroids and the risk of imprisonment. Drug Alcohol Depend. 2019 doi: 10.1016/j.drugalcdep.2019.04.041. [DOI] [PubMed] [Google Scholar]
- 30.Smit DL, de Hon O, Venhuis BJ, den Heijer M, de Ronde W. Baseline characteristics of the HAARLEM study: 100 male amateur athletes using anabolic androgenic steroids. Scand. J. Med. Sci. Sports. 2020 doi: 10.1111/sms.13592. [DOI] [PubMed] [Google Scholar]
- 31.Smit D, de Ronde W. Outpatient clinic for users of anabolic androgenic steroids: An overview. Neth. J. Med. 2018;76:167. [PubMed] [Google Scholar]
- 32.Kokkevi A, Fotiou A, Chileva A, Nociar A, Miller P. Daily exercise and anabolic steroids use in adolescents: A cross-national European study. Subst. Use Misuse. 2008 doi: 10.1080/10826080802279342. [DOI] [PubMed] [Google Scholar]
- 33.Pallesen S, Jøsendal O, Johnsen BH, Larsen S, Molde H. Anabolic steroid use in high school students. Subst. Use Misuse. 2006 doi: 10.1080/10826080601006367. [DOI] [PubMed] [Google Scholar]
- 34.Pope HG, Jr, Kanayama G, Athey A, Ryan E, Hudson JI, Baggish A. The lifetime prevalence of anabolic-androgenic steroid use and dependence in Americans: Current best estimates. Am. J. Addict. 2014 doi: 10.1111/j.1521-0391.2013.12118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brower KJ. Anabolic steroid abuse and dependence. Curr. Psychiatry Rep. 2002 doi: 10.1007/s11920-002-0086-6. [DOI] [PubMed] [Google Scholar]
- 36.Barnett MJ, Tenerowicz MJ, Perry PJ. The Anabolic 500 survey: Characteristics of male users versus nonusers of anabolic-androgenic steroids for strength training. Pharmacotherapy. 2011 doi: 10.1592/phco.31.8.757. [DOI] [PubMed] [Google Scholar]
- 37.Chandler, M. & Mcveigh, J. (2014).
- 38.Han DH, et al. Attention-deficit/hyperactivity disorder in elite athletes: A narrative review. Br. J. Sports Med. 2019 doi: 10.1136/bjsports-2019-100713. [DOI] [PubMed] [Google Scholar]
- 39.Reardon CL, Factor RM. Considerations in the use of stimulants in sport. Sports Med. 2016 doi: 10.1007/s40279-015-0456-y. [DOI] [PubMed] [Google Scholar]
- 40.Poysophon P, Rao AL. Neurocognitive deficits associated with ADHD in athletes: A systematic review. Sports health. 2018 doi: 10.1177/1941738117751387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holst Y, Thorell LB. Neuropsychological functioning in adults with ADHD and adults with other psychiatric disorders. J. Atten. Disord. 2017 doi: 10.1177/1087054713506264. [DOI] [PubMed] [Google Scholar]
- 42.Kessler RC, et al. The prevalence and correlates of adult ADHD in the United States: Results from the National Comorbidity Survey Replication. Am. J. Psychiatry. 2006 doi: 10.1176/ajp.2006.163.4.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Howard AL, et al. Early substance use in the pathway from childhood attention-deficit/hyperactivity disorder (ADHD) to young adult substance use: Evidence of statistical mediation and substance specificity. Psychol. Addict. Behav. 2020 doi: 10.1037/adb0000542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Quinn PD, et al. ADHD medication and substance-related problems. Am. J. Psychiatry. 2017 doi: 10.1176/appi.ajp.2017.16060686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dirks H, Scherbaum N, Kis B, Mette C. ADHD in adults and comorbid substance use disorder: Prevalence, clinical diagnostics and integrated therapy. Fortschr. Neurol. Psychiatr. 2017 doi: 10.1055/s-0043-100763. [DOI] [PubMed] [Google Scholar]
- 46.Wilens T, et al. Presenting ADHD symptoms, subtypes, and comorbid disorders in clinically referred adults with ADHD. J. Clin. Psychiatry. 2009 doi: 10.4088/JCP.08m04785pur. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vogel SWN, et al. Distribution of ADHD symptoms, and associated comorbidity, exposure to risk factors and disability: Results from a general population study. Psychiatry Res. 2018 doi: 10.1016/j.psychres.2018.06.017. [DOI] [PubMed] [Google Scholar]
- 48.Lange KW, et al. Utility of cognitive neuropsychological assessment in attention-deficit/hyperactivity disorder. Atten. Deficit Hyperact. Disord. 2014 doi: 10.1007/s12402-014-0132-3. [DOI] [PubMed] [Google Scholar]
- 49.Marshall P, Hoelzle J, Nikolas M. Diagnosing Attention-Deficit/Hyperactivity Disorder (ADHD) in young adults: A qualitative review of the utility of assessment measures and recommendations for improving the diagnostic process. Clin. Neuropsychol. 2019 doi: 10.1080/13854046.2019.1696409. [DOI] [PubMed] [Google Scholar]
- 50.Wang LJ, et al. Validity of visual and auditory attention tests for detecting ADHD. J. Atten. Disord. 2019 doi: 10.1177/1087054719887433. [DOI] [PubMed] [Google Scholar]
- 51.Xanthopoulos MS, Benton T, Lewis J, Case JA, Master CL. Mental health in the young athlete. Curr. Psychiatry Rep. 2020 doi: 10.1007/s11920-020-01185-w. [DOI] [PubMed] [Google Scholar]
- 52.Weschler, D. (The Psychological Corporation, 1999).
- 53.Woods SP, Delis DC, Scott JC, Kramer JH, Holdnack JA. The California Verbal Learning Test–second edition: Test-retest reliability, practice effects, and reliable change indices for the standard and alternate forms. Arch. Clin. Neuropsychol. 2006 doi: 10.1016/j.acn.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 54.Miyake A, et al. The unity and diversity of executive functions and their contributions to complex "Frontal Lobe" tasks: A latent variable analysis. Cogn. Psychol. 2000 doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- 55.Miyake A, Friedman NP. The nature and organization of individual differences in executive functions: Four general conclusions. Curr. Dir. Psychol. Sci. 2012 doi: 10.1177/0963721411429458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Delis, D. C., Kaplan, E. & Kramer, J. H. (The Psychological Corporation, 2001).
- 57.Pebl-Personality, Emotion, and Behaviour Lab, https://www2.psych.ubc.ca/~klonsky/home.html.
- 58.Mueller ST, Piper BJ. The psychology experiment building language (PEBL) and PEBL test battery. J. Neurosci. Methods. 2014 doi: 10.1016/j.jneumeth.2013.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Achenbach, T. (University of Vermont, Department of Psychiatry, 1990).
- 60.Achenbach TM, Ivanova MY, Rescorla LA. Empirically based assessment and taxonomy of psychopathology for ages 1½-90+ years: Developmental, multi-informant, and multicultural findings. Compr. Psychiatry. 2017 doi: 10.1016/j.comppsych.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 61.Rescorla LA. Assessment of young children using the Achenbach System of Empirically Based Assessment (ASEBA) Ment. Retard. Dev. Disabil. Res. Rev. 2005 doi: 10.1002/mrdd.20071. [DOI] [PubMed] [Google Scholar]
- 62.Rescorla L, et al. Behavioral and emotional problems reported by parents of children ages 6 to 16 in 31 societies. J. Emot. Behav. Disord. 2007;15:130–142. doi: 10.1177/10634266070150030101. [DOI] [Google Scholar]
- 63.Mcconaughy, S. H. (2001).
- 64.IBM SPSS Statistics v. 25.
- 65.R: a language and environment for statistical computing (R Foundation for Statistical Computing, 2014).
- 66.Achenbach T. Adult Self Report. University of Vermont, Department of Psychiatry; 1990. [Google Scholar]
- 67.Turner C, McClure R, Pirozzo S. Injury and risk-taking behavior—A systematic review. Accid. Anal. Prev. 2004 doi: 10.1016/s0001-4575(02)00131-8. [DOI] [PubMed] [Google Scholar]
- 68.Chang Z, Lichtenstein P, Larsson H. The effects of childhood ADHD symptoms on early-onset substance use: A Swedish twin study. J. Abnorm. Child Psychol. 2012 doi: 10.1007/s10802-011-9575-6. [DOI] [PubMed] [Google Scholar]
- 69.Elkins IJ, McGue M, Iacono WG. Prospective effects of attention-deficit/hyperactivity disorder, conduct disorder, and sex on adolescent substance use and abuse. Arch. Gen. Psychiatry. 2007 doi: 10.1001/archpsyc.64.10.1145. [DOI] [PubMed] [Google Scholar]
- 70.Upadhyaya HP. Substance use disorders in children and adolescents with attention-deficit/hyperactivity disorder: Implications for treatment and the role of the primary care physician. Primary Care Companion J. Clin. Psychiatry. 2008 doi: 10.4088/pcc.v10n0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chamakalayil S, et al. Methylphenidate for attention-deficit and hyperactivity disorder in adult patients with substance use disorders: Good clinical practice. Front. Psychiatry. 2021 doi: 10.3389/fpsyt.2020.540837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Biederman J, Wilens T, Mick E, Spencer T, Faraone S. Pharmacotherapy of attention-deficit/hyperactivity disorder reduces risk for substance use disorder. Pediatrics. 1999 doi: 10.1542/peds.104.2.e20. [DOI] [PubMed] [Google Scholar]
- 73.Dennhardt AA, Murphy JG. Prevention and treatment of college student drug use: A review of the literature. Addict. Behav. 2013;38:2607–2618. doi: 10.1016/j.addbeh.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 74.Goldberg L, et al. The adolescents training and learning to avoid steroids program: Preventing drug use and promoting health behaviors. Arch. Pediatr. Adolesc. Med. 2000;154:332–338. doi: 10.1001/archpedi.154.4.332. [DOI] [PubMed] [Google Scholar]
- 75.(World Health Organization (WHO), 1993).
- 76.Connor DF, Chartier KG, Preen EC, Kaplan RF. Impulsive aggression in attention-deficit/hyperactivity disorder: Symptom severity, co-morbidity, and attention-deficit/hyperactivity disorder subtype. J. Child Adolesc. Psychopharmacol. 2010 doi: 10.1089/cap.2009.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bozkurt M, Evren C, Umut G, Evren B. Relationship of attention-deficit/hyperactivity disorder symptom severity with severity of alcohol-related problems in a sample of inpatients with alcohol use disorder. Neuropsychiatr. Dis. Treat. 2016 doi: 10.2147/NDT.S105190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Evren B, Evren C, Dalbudak E, Topcu M, Kutlu N. Relationship of internet addiction severity with probable ADHD and difficulties in emotion regulation among young adults. Psychiatry Res. 2018 doi: 10.1016/j.psychres.2018.08.112. [DOI] [PubMed] [Google Scholar]
- 79.Van Ameringen M, Mancini C, Simpson W, Patterson B. Adult attention deficit hyperactivity disorder in an anxiety disorders population. CNS Neurosci. Ther. 2011 doi: 10.1111/j.1755-5949.2010.00148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Piñeiro-Dieguez B, Balanzá-Martínez V, García-García P, Soler-López B. Psychiatric comorbidity at the time of diagnosis in adults with ADHD: The CAT study. J. Atten. Disord. 2016 doi: 10.1177/1087054713518240. [DOI] [PubMed] [Google Scholar]
- 81.Nelson JM, Liebel SW. Anxiety and depression among college students with attention-deficit/hyperactivity disorder (ADHD): Cross-informant, sex, and subtype differences. J. Am. Coll. Health. 2018 doi: 10.1080/07448481.2017.1382499. [DOI] [PubMed] [Google Scholar]
- 82.Gnanavel S, Sharma P, Kaushal P, Hussain S. Attention deficit hyperactivity disorder and comorbidity: A review of literature. World J. Clin. Cases. 2019 doi: 10.12998/wjcc.v7.i17.2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Reale L, et al. Comorbidity prevalence and treatment outcome in children and adolescents with ADHD. Eur. Child Adolesc. Psychiatry. 2017 doi: 10.1007/s00787-017-1005-z. [DOI] [PubMed] [Google Scholar]
- 84.Gao Y, Li H, Luo Y. An empirical study of wearable technology acceptance in healthcare. Ind. Manag. Data Syst. 2015;115:1704–1723. doi: 10.1108/IMDS-03-2015-0087. [DOI] [Google Scholar]
- 85.Johnson KA, et al. Impaired conflict resolution and alerting in children with ADHD: Evidence from the Attention Network Task (ANT) J. Child Psychol. Psychiatry Allied Discipl. 2008 doi: 10.1111/j.1469-7610.2008.01936.x. [DOI] [PubMed] [Google Scholar]
- 86.Mogg K, et al. Attention network functioning in children with anxiety disorders, attention-deficit/hyperactivity disorder and non-clinical anxiety. Psychol. Med. 2015 doi: 10.1017/S0033291715000586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vakil E, Mass M, Schiff R. Eye movement performance on the stroop test in adults with ADHD. J. Atten. Disord. 2019 doi: 10.1177/1087054716642904. [DOI] [PubMed] [Google Scholar]
- 88.Wodka EL, et al. Prediction of ADHD in boys and girls using the D-KEFS. Arch. Clin. Neuropsychol. 2008 doi: 10.1016/j.acn.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Barkley A. Differential diagnosis of adults with ADHD: The role of executive function and self-regulation. J. Clin. Psychiatry. 2010 doi: 10.4088/JCP.9066tx1c. [DOI] [PubMed] [Google Scholar]
- 90.Silverstein MJ, et al. The relationship between executive function deficits and DSM-5-defined ADHD symptoms. J. Atten. Disord. 2020 doi: 10.1177/1087054718804347. [DOI] [PubMed] [Google Scholar]
- 91.Hauger LE, et al. Anabolic androgenic steroid dependence is associated with impaired emotion recognition. Psychopharmacology. 2019 doi: 10.1007/s00213-019-05239-7. [DOI] [PubMed] [Google Scholar]
- 92.Lovstad M, et al. Behavior rating inventory of executive function adult version in patients with neurological and neuropsychiatric conditions: Symptom levels and relationship to emotional distress. J. Int. Neuropsychol. Soc. 2016;22:682–694. doi: 10.1017/s135561771600031x. [DOI] [PubMed] [Google Scholar]
- 93.Havnes IA, Bukten A, Rognli EB, Muller AE. Use of anabolic-androgenic steroids and other substances prior to and during imprisonment—Results from the Norwegian Offender Mental Health and Addiction (NorMA) study. Drug Alcohol Depend. 2020;217:108255. doi: 10.1016/j.drugalcdep.2020.108255. [DOI] [PubMed] [Google Scholar]
- 94.Havnes IA, Jørstad ML, McVeigh J, Van Hout MC, Bjørnebekk A. The anabolic androgenic steroid treatment gap: A national study of substance use disorder treatment. Subst. Abuse. 2020;14:1178221820904150. doi: 10.1177/1178221820904150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Griffiths S, Murray SB, Dunn M, Blashill AJ. Anabolic steroid use among gay and bisexual men living in Australia and New Zealand: Associations with demographics, body dissatisfaction, eating disorder psychopathology, and quality of life. Drug Alcohol Depend. 2017;181:170–176. doi: 10.1016/j.drugalcdep.2017.10.003. [DOI] [PubMed] [Google Scholar]