Abstract
Membranous nephropathy (MN) is the most common cause of nephrotic syndrome among adults, which is the leading glomerular disease that recurs after kidney transplantation. Treatment for MN remained controversial and challenging, partly owing to absence of sensitive and specific biomarkers and effective therapy for prediction and diagnosis of disease activity. MN starts with the formation and deposition of circulating immune complexes on the outer area in the glomerular basement membrane, leading to complement activation. The identification of autoantibodies against the phospholipase A2 receptor (PLA2R) and thrombospondin type-1 domain-containing protein 7A (THSD7A) antigens illuminated a distinct pathophysiological rationale for MN treatments. Nowadays, detection of serum anti-PLA2R antibodies and deposited glomerular PLA2R antigen can be routinely applied to MN. Anti-PLA2R antibodies exhibited much high specificity and sensitivity. Measurement of PLA2R in immune complex deposition allows for the diagnosis of PLA2R-associated MN in patients with renal biopsies. In the review, we critically summarized newer diagnosis biomarkers including PLA2R and THSD7A tests and novel promising therapies by using traditional Chinese medicines such as Astragalus membranaceus, Tripterygium wilfordii, and Astragaloside IV for the treatment of MN patients. We also described unresolved questions and future challenges to reveal the diagnosis and treatments of MN. These unprecedented breakthroughs were quickly translated to clinical diagnosis and management. Considerable advances of detection methods played a critical role in diagnosis and monitoring of treatment.
Keywords: chronic kidney disease, idiopathic membranous nephropathy, membranous nephropathy, traditional Chinese medicine, Astragalus membranaceus, Tripterygium wilfordii, Astragaloside IV, Shenqi particle
1 Introduction
Membranous nephropathy (MN) is one of the most common causes of formation of nephrotic syndrome in adults, accounting for 30% incidence of patients (1.7/100000/year), with a 67% male preponderance and a high incidence in humans aged 30–50 years (Bally et al., 2016). It is unwonted in children (Liu et al., 2020a; Tamura, 2021). MN mainly affects renal glomerulus, particularly podocytes in glomerulus, indicating that podocytes play a critical role in regulating renal permeability to various molecules including proteins (Ronco and Debiec, 2020). In healthy individuals, albumin and macromolecule proteins are not filtered, while in a milieu called nephrotic syndrome, many proteins are leaked and excreted through urine, leading to a reduction in serum albumin and generalized edema development (Ronco and Debiec, 2020; Medina Rangel et al., 2021).
This condition can be “primary” or “idiopathic” for patients that did not present disease association (70–80% of patients), or for patients that present disease association, such as infections, lupus erythematosus, malignancy, or drug toxicity (Moroni and Ponticelli, 2020; Cravedi et al., 2019). Exogenous antigens may pass via the glomerular basement membrane (GBM), become planted under the podocyte surface layer, and following the combination with circulating antibodies (Cravedi et al., 2019) (Figure 1). Circulating immune complexes separate and reform in the subepithelial space (Moroni and Ponticelli, 2020). Idiopathic membranous nephropathy (IMN) is a kidney-specific non-inflammatory autoimmune disease, and circulating autoantibodies bind to autoantigens on the podocyte surface layer (Moszczuk et al., 2021). About 40% of IMN patients could suffer spontaneous remission. However, the rest of 30% showed a poor outcome to immunosuppressive treatment and finally reached end-stage renal disease treated by dialysis and transplantation (Tesar and Hruskova, 2021; Passerini et al., 2019; Xipell et al., 2018; Molina Andújar et al., 2022; da Silva et al., 2018), which were two leading therapies for patients with end-stage renal disease (Zhang et al., 2020a; Sawhney and Gill, 2020; Wu et al., 2021a; Bacharaki et al., 2021; Chang et al., 2021; Chuengsaman et al., 2021; Gambino et al., 2021). Approximately, 40% patients accept kidney grafts that lead to recurrence, and about 45% patients lose the graft (Passerini et al., 2019; Robson and Kitching, 2021; Uffing et al., 2021). Treatment with costly drugs and potential adverse effects of drugs remain challenging (Gauckler et al., 2021). The most important points of precision therapy are the discovery of the accurate etiology and pathogenesis in IMN.
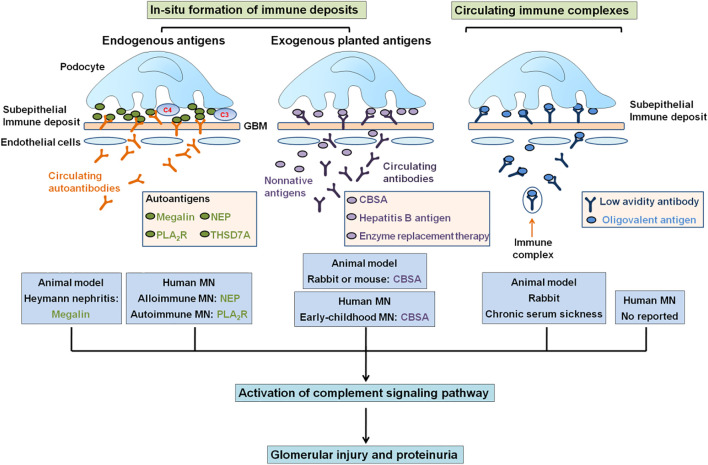
FIGURE 1.
Mechanisms of formation of subepithelial immune complex deposits. The depositions of subepithelial immune complexes might form either in situ immune complexes or circulating immune complexes that contained circulating antibodies binding to endogenous antigens (megalin, NEP, PLA2R, and THSD7A) in podocytes or to exogenous antigens (CBSA, hepatitis B antigen, and enzyme replacement therapy) planted in GBM. In total, four endogenous antigenic targets have been identified, including megalin in Heymann nephritis, NEP in alloimmune neonatal MN, and PLA2R and THSD7A in IMN. In total, four circulating exogenous antibodies can target surface-exposed intrinsic podocyte antigens to cause capping and shedding of in situ antigen–antibody complex into underlying GBM. CBSA, enzyme from enzyme replacement therapy, and hepatitis B antigen may traverse GBM and bind under podocyte as planted antigens and also as target for circulating antibodies. In addition, circulating immune complexes deposited in the subepithelial region.
The feature of IMN is immune complex deposition along the subepithelial region of GBM, which leads to a membrane-like thickening of GBM (Liu et al., 2020b; Gu et al., 2021) (Figure 1). The immune complexes are composed of several components, such as IgG4, antigens that are eluded, and membrane attack complex, which is formed by complement components to produce C5b–9 (Gu et al., 2021; Hu et al., 2021) (Figure 2). IgG4 is the most main IgG subclass deposited in IMN, although altered IgG1 is also involved in the deposited immune complexes, IgG1-3 exceeds IgG4 deposition in secondary MN patients (Lönnbro-Widgren et al., 2015; Xu et al., 2020; Ronco et al., 2021). Subepithelial immune complex formation deposits and activation of complement are directly associated with functional lesion of the glomerular capillary wall, which leads to urine protein in absence of inflammatory cells (Xu et al., 2020; Gu et al., 2021) (Figure 2).
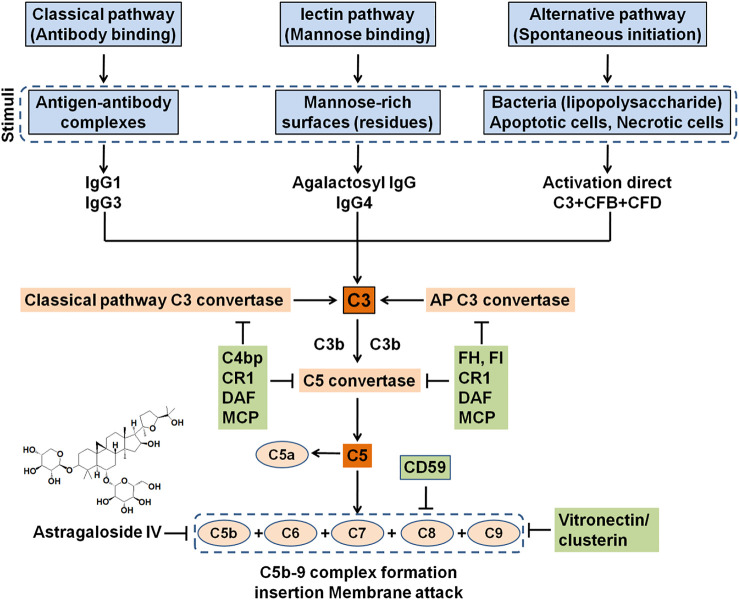
FIGURE 2.
Activation of complement system-induced formation of MAC (C5b-9). Complement could be activated by three pathways including classical, lectin, and alternative signaling pathways, which came together toward production of C5 convertase and formation of C5b that bound to C6, C7, C8, and multiple C9 to generate the MAC. This process could be mostly inhibited by circulating inhibitors, podocyte-positive regulatory proteins, and natural products such as Astragaloside IV. However, this inhibitory effect might be devastated at high levels of complement. MAC could insert into the podocyte membrane, even in sublytic quantity, evoked cell injury under a lack of inflammation condition.
Based on the natural IMN history, IMN has a classic rule of thirds including one-third patients with spontaneous remission, one-third patients with sustaining proteinuria, and one-third patients with progression of kidney failure (McQuarrie et al., 2012). Although spontaneous remission is a common feature of IMN, IMN causes end-stage renal disease in nearly 40% of patients after 10 years (Ronco et al., 2021). Interventions remain controversial and challenging owing to adverse effect using immunosuppressive treatment, and, apart from proteinuria, few sensitive and reliable biomarkers are used for the prediction of disease activity and outcome because of the fact that antigens targeted by antibodies remain enigmatic (Moszczuk et al., 2021; Tesar and Hruskova, 2021). Taken together, an essential improvement of IMN diagnosis, monitoring, treatment, and prognosis is the elucidation of pathogenic mechanisms.
In the past several decades, important advances have been achieved in the illumination of the mechanisms of molecular pathogenesis of human MN (Gu et al., 2021; Xu et al., 2020). In addition, several latest publications have shown that MN was associated with the dysbiosis of gut microbiota and dysregulation of long non-coding RNAs (Zhang et al., 2020b; Dong et al., 2020; Luan et al., 2022; Jin et al., 2019). These advances were inspired by using animal models of IMN, including Heymann nephritis and cationic bovine serum albumin (CBSA)–induced MN (Jiang et al., 2020). The model of Heymann nephritis put forward the concept that a podocyte antigen, namely, megalin, was a target of antibody-forming in situ immune complexes, whereas CBSA-induced MN first reflected the case of planted antigen (Figure 1). Jones (1957) first reported MN as a specific disease entity. Subsequently, the recognition that autoimmune response to antigen-induced MN was first reported in an animal model in 1959. The rats were injected by using extracts from proximal tubular cells that mediated immune complex deposition on the subepithelial capillary wall region in the glomerular that were similar to those found in MN patients, strongly indicated the possibility of an immune-induced pathogenesis mechanism (Heymann et al., 1959). These immunocomplexes included IgG antibody–targeting megalin, which was expressed on both rat podocytes and tubuli (Kerjaschki and Farquhar, 1982), but not on podocytes in human. In humans, progress in MN started in 2002 with the discovery of target antigen, namely, neutral endopeptidase (NEP, also known as neprilysin) as a targeting antigen in baby of a woman with NEP deficiency (Debiec et al., 2002). Anti-NEP alloantibodies was generated by the mother-passed placenta and bound to NEP expressed in fetal podocytes (Debiec et al., 2002), which showed the role of autoantibody in human MN pathogenesis. This result showed the proof of concept that podocyte antigens were associated with human MN, as is the paradigm for megalin in rats, and laid the foundation for the identification of a novel causal antigen M-type phospholipase A2 receptor (PLA2R), which is the first podocyte autoantigen identified in human IMN in 2009 (Beck et al., 2009). In 2014, this was followed by the discovery of a second antigen, namely, thrombospondin type-1 domain-containing 7A (THSD7A) in human IMN (Tomas et al., 2014). PLA2R-related and THSD7A-related MN accounted for about 70% and 1–5% of IMN patients, respectively (Zhang et al., 2021a). A genome-wide association study indicated that single nucleotide polymorphisms in the PLA 2 R gene were closely related to IMN, which again revealed the involvement of this antigen by using an untargeted genetic approach (Xu et al., 2020; Yoshikawa and Asaba, 2020). Other antigens including aldose reductase, superoxide dismutase-2, and α-enolase were also found in human, although their function remains to be established because they were not detected on normal podocyte surfaces (Prunotto et al., 2010). In addition, endogenous podocyte antigens and exogenous antigens including CBSA were also involved in patients with early-childhood MN (Ayalon and Beck, 2015; Jiang et al., 2020). Taken together, these studies exhibited a new era for the diagnosis, monitoring, and prognosis of MN from early infancy to adulthood. In this article, we summarize the traditional diagnostic method for MN and review recent improvements in diagnostics and the treatment of MN.
2 Diagnosis of Membranous Nephropathy
2.1 Traditional Diagnosis Approach
On the patients with nephrotic syndrome, after diagnosis for secondary causes, a renal biopsy was subsequently performed, which was a gold standard for MN diagnosis. The membranous summarized microscopic characteristic of capillary wall thickening in glomeruli, which led to subepithelial IgG accumulation. Immune complex depositions produced a spiked appearance and formed granular lines. Electron microscopy analysis further confirmed electron-dense subepithelial immune complexes, which were often accompanied by foot process effacement in podocyte. If the diagnosis was established, Kidney Disease: Improving Global Outcomes advised a 6-month observation, as there are many patients of spontaneous remission of MN. However, it takes more than a year to reach spontaneous remission.
2.2 Newer Diagnosis Approach
Renal biopsy is a gold standard in the analysis and detection of the pattern of MN damage. However, standard light and electron microscopic results could not reflect nature of MN (Xie et al., 2020). The distinction between IMN and secondary MN is the main challenge in the diagnosis of MN, in particular malignancy-related MN in patients. It is a common practice to exclude secondary causes of the lesion of MN on the basis of physical examination, pathological analysis, and laboratory examination. An assessment of the immunopathologic features from the biopsy specimen may get discriminative information (Ronco and Debiec, 2015). A sole subepithelial location of the immune complex deposits is typical characteristics of IMN. The deposition of C1q was hardly observed in IMN but could be found in other secondary causes, especially in systemic lupus erythematosus. IgG subclass staining could help to classify MN. The deposits of IgG1, IgG2, and IgG3 usually distribute in secondary MN, while many amounts of IgG4 are characteristic for IMN, indicating the fact that PLA2R antibody and THSD7A antibody are mostly of the IgG4 subclass.
2.2.1 Phospholipase A2 Receptor Test for Diagnosis and Monitoring
Although the underlying mechanism of PLA2R in the pathogenesis of IMN is still unknown, the presence of anti-PLA2R antibodies is highly specific for IMN (Logt et al., 2021; Nieto-Gañán et al., 2021). A low occurrence of PLA2R antibodies was found in secondary MN (Porcelli et al., 2021), but in these patients, coincidental occurrence of IMN with related disease might be included. The level of PLA2R antibodies was detected in patients with other cause-induced nephrotic syndrome or healthy controls (Tomas et al., 2021). Several findings suggest that anti-PLA2R antibodies were associated with disease activity (Jurubită et al., 2021; Logt et al., 2021).
The commercial immunofluorescent test (Euroimmun, Lübeck, Germany) was applied to diagnosis. Anti-PLA2R antibodies occurred in serum of 52–86% of MN patients (Hofstra and Wetzels, 2012). Detections by using either Western blot or immunofluorescent approaches, and immunofluorescent tests have a lower sensitivity than the Western blot technique (Hoxha et al., 2017). The studies included patients with different ethnicity, long-standing disease, and treated in remission. It was also reported that initially antibodies against PLA2R lacked in serum of patients with PLA2R-related MN. An earlier study showed 10 patients with PLA2R antigen staining positive in immune complexes in the renal biopsy specimen from a cohort of 42 patients, with no measurable serum antibodies (Debiec and Ronco, 2011). Subsequent results showed that antibodies against PLA2R indeed were absent at disease the onset and could be detected during follow-up analysis (Ramachandran et al., 2015; van de Logt et al., 2015). We hypothesized that antibodies combined with antigen of podocytes with high affinity and only be detected when binding sites in renal tissues are saturated (van de Logt et al., 2015). Of note, the same research group also reported three patients with a high circulating concentration of antibodies against PLA2R who did not have detectable PLA2R in the glomeruli (Debiec and Ronco, 2011). This phenomenon attracts little attention. The immunofluorescent analysis only allowed semi-quantitative evaluation of antibodies against PLA2R by dilution steps. The enzyme-linked immunosorbent assay (ELISA) provided a precise quantitation for antibodies against PLA2R. A commercially available ELISA (Euroimmun) was introduced to clinical practice and widespread application (Dähnrich et al., 2013; Zhu et al., 2022). This ELISA determined total antibodies against PLA2R IgG. However, PLA2R antigen was observed in immune deposits in some patients, which suggested rapid clearance of antibodies and deposition in glomeruli. Conversely, determination of circulating antibodies was not always related to the presence of the antigen in the immune complex deposits, which indicated that not all antibodies to PLA2R are pathogenic. Evaluation of both circulating anti-PLA2R antibodies and PLA2R in kidney biopsy could better select patients for accurate therapy. In addition, anti-PLA2R antibodies showed a high diagnostic ability on IMN for the population with diabetic kidney disease (Wang et al., 2020a).
2.2.2 Thrombospondin Type-1 Domain-Containing Protein 7A Test for Diagnosis and Monitoring
So far, THSD7A antibodies were not detected in healthy individuals or patients with other renal and systemic diseases (Tomas et al., 2014), presenting a 100% specificity for the damage of MN. However, another study has demonstrated that circulating autoantibodies against human podocyte antigen THSD7A was found in 5–10% of MN patients who did not present circulating anti-PLA2R autoantibodies (Tomas et al., 2014). The percentages of THSD7A-related IMN range from 3% to 9% in Europe and the United States. Notably, in large-scale THSD7A-associated MN patients, a tumor was found from diagnosis of MN (Hoxha et al., 2016). Of note, chemotherapy initiation caused a decreasing THSD7A antibody followed by a reduction of proteinuria (Hoxha et al., 2016). These findings showed that the immune system discerned cancer THSD7A as a foreign antigen mediating THSD7A antibody production, the latter binding to THSD7A on the surface of podocyte in situ.
2.2.3 Combined Phospholipase A2 Receptor and Thrombospondin Type-1 Domain-Containing Protein 7A Test for Diagnosis and Monitoring
It is presently uncertain whether there are patients with dual PLA2R and THSD7A antibody positivity (Zhang et al., 2021a). When PLA2R antibodies were detected in serum and there is no evidence for secondary MN, one may consider that patients do not undergo a kidney biopsy sample, provided that patients presented normal or only mild renal function decline. In patients with kidney function injury, a biopsy could help us to exclude a crescentic form of MN or concurrence of other diseases and evaluate the chronic damage degree.
3 Treatment of Membranous Nephropathy
Currently, IMN patients are mainly treated with either calcineurin inhibitors, alkylating cytotoxic agents, B-cell depleting monoclonal antibody, or rituximab (Wang et al., 2018a; Hamilton et al., 2018; Yu et al., 2018). The use of calcineurin inhibitors led to a high relapse rate on their withdrawal. Alkylating cytotoxic agents were effective, but their use led to severely adverse effects. Rituximab-treated MN patients showed a decrease in anti-PLA2R antibody levels in the follow-up period (Ramachandran et al., 2018). However, it is worth noting that some natural products exhibit an excellent efficacy for MN treatment (Feng et al., 2020; Lang et al., 2020; Lu et al., 2021).
3.1 Immunosuppressive Treatment
Kidney Disease: Improving Global Outcomes guidelines recommend that corticosteroids and alkylating agents including cyclophosphamide or chlorambucil are prescribed for 6 months for MN patients with nephrotic syndrome after 6–12 months as a conservative treatment or with decreased baseline renal function (Stevens and Levin, 2013). However, these therapies showed some severe adverse effects. The severity of side effects had important drawbacks in this therapy. Therefore, the classical combined therapy should be optimized (Chen et al., 2020a). The latest results indicated that remission at first 3 months were higher in the steroid–tacrolimus group than in the steroid–cyclophosphamide group over an 18-month period (Zou et al., 2020). Although the incidence of adverse effects was not different between two groups, the incidence after first 3 months was decreased in the steroid–tacrolimus group. Levels of 24-h urinary protein and serum albumin improved in the steroid–tacrolimus group more than those in the steroid–cyclophosphamide group (Zou et al., 2020).
Hoxha et al. (2014)reported that antibody levels were significantly reduced after 3 months of immunosuppressive treatment by 81% and proteinuria by 39% in 133 MN patients with positive anti-PLA2R antibody. Patients with remission after 12 months presented decreased levels of anti-PLA2R antibodies at baseline compared to patients with no remission. Moreover, patients with high anti-PLA2R antibody concentrations achieved remission later than patients with low concentrations. The antibody concentrations remained increased in patients who did not achieve proteinuria remission.
Alternatively, decreasing proteinuria concentrations without an immunological response could not be interpreted as real remission. There is an increasing risk of relapse when treatment by frequently using cyclosporine is withdrawn. Persistence of the high anti-PLA2R antibody titer is a sign of ongoing immunologic activity that was associated with ongoing podocyte injury despite decreased proteinuria concentrations. Therefore, the efficacy of any immunosuppressive treatment need to be assessed based on immunologic remission induction (Bomback and Fervenza, 2018). Anti-PLA2R positivity reappearance and/or an elevation in the previously lower titer of anti-PLA2R antibody was a clear indicator of the impending relapse of MN, sometimes preceding the rise of proteinuria concentrations. Serial detection methods of anti-PLA2R antibodies during immunosuppressive treatment could improve personalized treatment of MN.
3.2 Rituximab
After nearly 40 years of empirical treatment, the identification of newer anti-PLA2R and anti-THSD7A autoantibodies was an unprecedented breakthrough for the understanding of underlying pathophysiological mechanisms of MN and its interventions specifically aimed at preventing antibody production (Figure 3). Rituximab was one of the first-line drugs for the treatment of moderate and high-risk IMN. The latest study demonstrated that 91 patients treated by rituximab achieved anti-PLA2R antibody depletion at 6 months; 58.2% of patients showed clinical remission at 12 months (Gao et al., 2021). Further analysis indicated that high proteinuria levels and persistent positive anti-PLA2R antibodies could be independent risk factors for no remission. The remission rate treated using rituximab as an initial regimen was increased compared to rituximab as an alternative regimen. In addition, 45 adverse events occurred in 37 patients. Rituximab effect was determined for the patients with PLA2R-associated MN and stage four or five chronic kidney disease (CKD) (Hanset et al., 2020). In total, 10 treatment courses caused an increase in an estimated glomerular filtration rate and remission of nephrotic syndrome. In contrast, four patients treated by rituximab were unsuccessful and required chronic hemodialysis within 1 year. Immunological remission was observed after 11 treatments and was related to response. However, three patients showed severe adverse events (Hanset et al., 2020). These findings suggest that rituximab showed effective and reasonably safe in PLA2R-related MN with stage four or five CKD. Immunological remission is related to a beneficial clinical outcome.
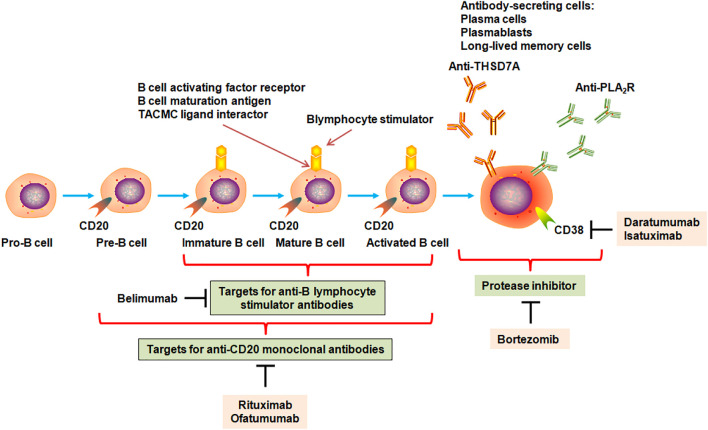
FIGURE 3.
Targets for monoclonal antibodies in B cells for the MN therapy strategy. B cells originated from bone marrow stem cells as pro-B cells and mature to pre-B cells, immature B cells, mature B cells, and activated B cells, which could form plasma cells, plasmablasts, and long-lived memory plasma cells that could secrete antibodies such as immunoglobulin A, immunoglobulin E, immunoglobulin G, and immunoglobulin M. Autoreactive B-cell clones could generate anti-PLA2R and anti-THSD7A antibodies. When B cells became mature, they generated many biomarkers that were considered targets for monoclonal antibodies. Monoclonal antibodies rituximab and ofatumumab could bind and inhibit a number of CD20-positive B cells but not mature plasma cells, plasmablasts, and memory plasma cells that could not produce CD20 antigen. Plasma cells produced CD38 and could be a target for anti-CD38 antibodies by daratumumab and isatuximab. Immature, mature, and activated B cells produced receptors for B-lymphocyte stimulator including B-cell activating factor receptor, B-cell maturation antigen, transmembrane activator, and calcium-modulating cyclophilin (TACMC) ligand interactor. Belimumab could inhibit B-cell differentiation into plasma cells by suppressing interaction between B-lymphocyte stimulator and its receptors. Bortezomib, a proteasome inhibitor, could inhibit antibody production by mediating plasma cell apoptosis, while anti-CD38 antibodies daratumumab and isatuximab could directly mediate plasma cell cytolysis.
Prior to the finding that anti-PLA2R autoantibodies affected MN pathogenesis, in vivo animal experiments had consistently demonstrated that antibodies generated by autoreactive B-cell clones triggered events that led to glomerular barrier damage and proteinuria development (Brglez et al., 2020). Consistent with these findings, cyclophosphamide showed an inhibitory effect on production of B-cell antibody in MN, adding to the non-specific antimitotic and immunosuppressive nature that induced some side effects. A monoclonal antibody against B-cell surface antigen CD20 has performed whether targeted B-cell depletion with inhibition of autoantibody production ameliorated MN patients while reduced side effects of immunosuppressants and steroids. Therefore, rituximab, as an anti-CD20 monoclonal antibody (Figure 3), was used in MN patients. MN patients treated by rituximab showed reduced levels of circulating anti-PLA2R antibody and proteinuria within several months (Gao et al., 2021; Gauckler et al., 2021). Ruggenenti et al. (2015) demonstrated that rituximab-treated patients with IMN showed a partial or complete remission, whereas anti-PLA2R antibody-positive patients showed a remission demonstrated by a decrease in antibody titer before treatment. These patients presented complete antibody depletion 6 months after the beginning of rituximab regimen. The finding from all patients showed that depletion of anti-PLA2R antibodies preceded complete remission. Early decreasing of anti-PLA2R antibody titer by 50% was in line with a reduction in proteinuria levels by 50% by 10 months, whereas rituximab was not associated with polymorphisms of PLA2R1.
Recently, it was reported that early response to either cyclosporine or rituximab was complete, and partial remission was observed in 130 patients treated by cyclosporine or rituximab, which showed similar results. However, partial or complete remission was faster in rituximab-treated patients than cyclosporine-treated patients at 24 months Fervenza et al. (2019). However, cyclosporine was withdrawn at 12 months, which reflected the risk of early relapse after cyclosporine withdrawal. Of note, the reduction of anti-PLA2R antibodies was faster in rituximab-treated patients than the cyclosporine-treated patients (Fervenza et al., 2019). These findings illuminated that immunological remission by depleting anti-PLA2R antibodies was promising for clinical remission.
Recently, a randomized and open-label controlled experiment was performed on 86 patients with IMN and nephrotic syndrome and assigned 43 each to receive 6-month cyclical intervention with the corticosteroid–cyclophosphamide group or sequential intervention with the tacrolimus–rituximab group (Fernández-Juárez et al., 2021). The results showed 83.7% of patients treated by the corticosteroid–cyclophosphamide group and 51.8% of patients treated by the tacrolimus–rituximab group exhibited complete or partial remission at 24 months. Complete remission occurred in 60% of patients treated by the corticosteroid–cyclophosphamide group and 26% of patients treated by the tacrolimus–rituximab group (Fernández-Juárez et al., 2021). Anti-PLA2R titers were significantly decreased in both groups, but the proportion of anti-PLA2R-positive patients who showed anti-PLA2R antibody depletion was higher at 3 and 6 months in the corticosteroid–cyclophosphamide group than the tacrolimus–rituximab group (Fernández-Juárez et al., 2021). Severe adverse effects were similar in both groups. Therefore, the corticosteroid–cyclophosphamide treated remission in many patients with IMN compared to tacrolimus–rituximab. In addition, Tian et al. (2022)assessed the efficacy and safety of tacrolimus combined with corticosteroids in patients with IMN and reported that 75 patients with renal biopsy MN and nephrotic syndrome were treated by rituximab or non-immunosuppressant. The depletion of anti-PLA2R was demonstrated at 6 months in 50% of patients intervened by rituximab and only in 12% of patients intervened by non-immunosuppressant Dahan et al. (2017).
3.3 Pharmacological Effects of Natural Products on Membranous Nephropathy
Natural products or traditional Chinese medicines have been long used in patients and considered an alternative therapeutic strategy for prevention and treatment of glomerular-associated diseases including MN (Feng et al., 2020), glomerulonephritis (Gianassi et al., 2019; Wang et al., 2021), diabetes (Chen et al., 2020b; Wang et al., 2020b; Fang et al., 2021; Su et al., 2021; He et al., 2022), and diabetic nephropathy (Li et al., 2020a; Yang et al., 2020; Wang, 2021; Xuan et al., 2021; Yang and Wu, 2021; Zhou et al., 2021; Liu et al., 2022). Earlier finding have demonstrated that a 77-year-old woman with IMN was treated by Astragalus membranaceus and achieved clinical remission without using immunosuppressive therapy (Ahmed et al., 2007). A multicenter randomized controlled clinical study assessed efficacy and safety of Shenqi particle for patients with IMN. Shenqi particle showed a beneficial effect on patients with IMN and nephrotic syndrome (Chen et al., 2013). In addition, Jian Pi Qu Shi formula treatment showed improvement in 15 patients, who failed to respond immunosuppressive therapy, and showed that 80% of the patients achieved clinical remission, whereas no obvious adverse effects were observed after 1-year follow-up (Shi et al., 2018). Similarly, Shulifenxiao formula as a clinical cocktail therapy also showed a beneficial intervention effect on steroid and immunosuppressant-resistant refractory IMN patients (Cui et al., 2021). Therefore, these formulas might be an alternative therapy for steroid and general immunosuppressant-resistant IMN patients. The combination of Tripterygium wilfordii multi-glycosides and prednisone is considered an effective therapy for IMN. The remission probability was similar for both Tripterygium wilfordii multi-glycosides and tacrolimus (Jin et al., 2020). Traditional Chinese medicines also could improve immunosuppressant efficacy. Wuzhi capsule could increase blood FK506 concentration in patients with IMN (Zhang et al., 2019). These studies have indicated that traditional Chinese medicines can effectively improve IMN and reduce proteinuria, but the underlying mechanism is still elusive.
Mechanistically, Wu et al. recently reported that 24-h urine protein level was significantly decreased, and kidney histological injury was restored in the CBSA-induced rats treated by Wenyang Lishui decoction. Similarly, an in vitro experiment showed that the apoptosis rate was increased in CBSA-induced mouse podocytes, while it was decreased when treated by Wenyang Lishui decoction, which was associated with downregulation of p53 mRNA and protein expression and upregulation of Bcl-2 mRNA and protein expression (Lu et al., 2020). An earlier study has revealed that Astragaloside IV might ameliorate complement attack complex-mediated podocyte lesion via inhibiting extracellular-regulated protein kinase expression (Zheng et al., 2012) (Figure 2). Recently, Tian et al. (2019)revealed that administration of Sanqi oral solution mitigated MN by lowering proteinuria, increasing serum albumin, and retarding renal damages in the experimental rat model of MN induced by CBSA. Sanqi oral solution also inhibited depositions of C3 and IgG and restored the protein expressions of podocin and synaptopodin, which were associated with the nuclear factor-κB (NF-κB) signaling pathway (Tian et al., 2019). The NF-κB signaling pathway plays an important role in immune modulation. Recent studies revealed that the NF-κB pathway was involved in the pathogenesis of MN (Sutariya et al., 2017). Liu et al. (2019)demonstrated that Zhenwu decoction reduced urine protein levels and alleviated kidney damage in the rat model of MN; furthermore, treatment with Zhenwu decoction could downregulate the protein expressions of IgG, C3, and desmin as well as upregulate podocin expression in glomerulus. The same research group demonstrated that Zhenwu decoction inhibited the advanced glycation end by suppressing the expression of receptor for advanced glycation end products in podocyte, which reduced oxidative stress in podocyte (Wu et al., 2016). These findings revealed that natural products ameliorate MN through targeting inflammation. However, MN is a non-inflammatory autoimmune disease of the kidney glomerulus. Taken together, the underlying mechanism should be investigated in the future.
In addition, Yu et al. (2020) reported that Chinese herbal injections were demonstrated to be superior to treatment of chemical drugs alone in the treatment of primary nephrotic syndrome and might be beneficial for patients with primary nephrotic syndrome. The combination of chemical drugs and Yinxingdamo injection and chemical drugs and Danhong injection had the potential to be the best Chinese herbal injections relative to total clinical effectiveness, serum albumin, and 24-h urinary protein excretion. Moreover, Li et al. (2020b) and Li et al. (2020c) demonstrated that Zhen-Wu-Tang could ameliorate immunoglobulin A nephropathy in rats.
3.4 Future Therapy and Directions
The integrated assessment of the levels of autoantibody and albumin in serum and proteinuria in patients could provide MN diagnosis and individually tailored treatment protocols. Traditional, toxic, and non-specific immunosuppressant will be replaced by safe and disease-specific agents, such as B-cell-targeting anti-CD20 antibodies (Figure 3), providing a new treatment paradigm based on the principles of precision medicine and personalized therapy. Although great advances were achieved in MN pathogenesis, a number of critical issues remain unsolved. For example, how the immune response is triggered and spreads remains unknown; the conditions that resulted in the appearance of PLA2R epitope of podocytes and the events that mediated immunization are elusive; the aim of antigen-driven therapy has still not been fulfilled. Although anti-PLA2R and anti-THSD7A antibodies as the leading diagnosis approaches were extensively applied to MN patients, some questions including autoimmune response, antigenic epitopes, and podocyte injury-associated with signaling pathways remain unresolved. In addition, when the patients have renal biopsy of MN, the clinicians need to answer two key issues. If patients are PLA2R-positive, then what are the risk factors of progression to renal failure? If patients are PLA2R-negative, then the causes of disease development and progression are primary or secondary?
Owing to the adverse effects of currently available immunosuppressive agents, a better understanding of MN pathomechanisms will provide more specific concept-directed therapy strategies. Based on the critical role of IgG antibodies in MN, targeting B lymphocytes by anti-CD20 antibody might be a specific and effective therapy to ameliorate MN by blocking antibody formation (Figure 3). However, responses are wide, and further research is needed to identify those patients who are benefitted by rituximab treatment and those who are non-effective. A clinical study showed that adrenocorticotropic hormone exerted its role through activated melanocortin receptors (MC1R–MC5R) that existed in the whole body were effective therapy for IMN patients who failed immunosuppressive treatment (Markell et al., 2019; Wu et al., 2021b). Melanocortin one receptor occurred in B cells, T cells, podocytes, and antigen-presenting cells. Melanocortin one receptor agonists could lower proteinuria, improve glomerular morphology, and retard oxidative stress in passive Heymann nephritis rats, which were associated with decrease in production of nephritogenic antibodies, direct targeting effect on podocytes, and stabilizing glomerular architecture. Further experiment should be performed to adrenocorticotropic hormone effect. The pathophysiological mechanisms have an important effect on patient care, including kidney biopsy, diagnosis, monitoring, and therapy.
Intriguingly, natural products are one of the most promising therapies for a broad spectrum of refractory diseases including coronavirus disease 2019 and its complications (Chen et al., 2018a; Chen et al., 2018b; Izzo et al., 2020; Yang et al., 2021a; Yang et al., 2021b; Zhang et al., 2021b; Singla et al., 2021; Zuo et al., 2021). Mounting natural products have been demonstrated to exhibit excellent efficacy for CKD treatment (Wang et al., 2018b; Zhang et al., 2020c; Geng et al., 2020; Meng et al., 2020; Miao et al., 2020; Lan, 2021; Li et al., 2021; Luo et al., 2021; Miao et al., 2022; Yu et al., 2022). Although a number of natural products could mitigate MN, the natural products in MN application are still in its infancy compared with CKD. Therefore, whether natural products can abolish MN, for which the study should be carried out on the animal models and patients with MN in the future.
Data Availability Statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Author Contributions
Y-YZ designed and wrote the review. Y-NW, H-YF, XN, Y-MZ, LZ, XL, and X-YY revised the manuscript. All authors accepted the final version of the manuscript.
Funding
This study was supported by the National Key Research and Development Project (No. 2019YFC1709405) and National Natural Science Foundation of China (Nos. 82074002 and 81872985).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviation
CBSA, cationic bovine serum albumin; CKD, chronic kidney disease; ELISA, enzyme-linked immunosorbent assay; GBM, glomerular basement membrane; IMN, idiopathic membranous nephropathy; MN, membranous nephropathy; NEP, neutral endopeptidase; NF-κB, nuclear factor-κB; PLA2R, M-type phospholipase A2 receptor; THSD7A, thrombospondin type-1 domain-containing protein 7A.
References
- Ahmed M. S., Hou S. H., Battaglia M. C., Picken M. M., Leehey D. J. (2007). Treatment of Idiopathic Membranous Nephropathy with the Herb Astragalus Membranaceus. Am. J. Kidney Dis. 50 (6), 1028–1032. 10.1053/j.ajkd.2007.07.032 [DOI] [PubMed] [Google Scholar]
- Ayalon R., Beck L. H., Jr. (2015). Membranous Nephropathy: Not Just a Disease for Adults. Pediatr. Nephrol. 30 (1), 31–39. 10.1007/s00467-013-2717-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacharaki D., Chrysanthopoulou E., Grigoropoulou S., Giannakopoulos P., Simitsis P., Frantzeskaki F., et al. (2021). Siblings with Coronavirus Disease 2019 Infection and Opposite Outcome-The Hemodialysis's Better Outcome Paradox: Two Case Reports. World J. Nephrol. 10 (2), 21–28. 10.5527/wjn.v10.i2.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bally S., Debiec H., Ponard D., Dijoud F., Rendu J., Fauré J., et al. (2016). Phospholipase A2 Receptor-Related Membranous Nephropathy and Mannan-Binding Lectin Deficiency. J. Am. Soc. Nephrol. 27 (12), 3539–3544. 10.1681/ASN.2015101155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck L. H., Jr., Bonegio R. G., Lambeau G., Beck D. M., Powell D. W., Cummins T. D., et al. (2009). M-type Phospholipase A2 Receptor as Target Antigen in Idiopathic Membranous Nephropathy. N. Engl. J. Med. 361 (1), 11–21. 10.1056/NEJMoa0810457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomback A. S., Fervenza F. C. (2018). Membranous Nephropathy: Approaches to Treatment. Am. J. Nephrol. 47 (Suppl. 1), 30–42. 10.1159/000481635 [DOI] [PubMed] [Google Scholar]
- Brglez V., Boyer-Suavet S., Zorzi K., Fernandez C., Fontas E., Esnault V., et al. (2020). Personalized Medicine for PLA2R1-Related Membranous Nephropathy: A Multicenter Randomized Control Trial. Front. Med. (Lausanne) 7, 412. 10.3389/fmed.2020.00412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang T.-Y., Wu H.-H., Li Y.-J., Liu H.-L., Yeh C.-H., Jian H.-S., et al. (2021). Changes of Brain Functional Connectivity in End-Stage Renal Disease Patients Receiving Peritoneal Dialysis without Cognitive Decline. Front. Med. 8, 734410. 10.3389/fmed.2021.734410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D. Q., Feng Y. L., Cao G., Zhao Y. Y. (2018). Natural Products as a Source for Antifibrosis Therapy. Trends Pharmacol. Sci. 39 (11), 937–952. 10.1016/j.tips.2018.09.002 [DOI] [PubMed] [Google Scholar]
- Chen D. Q., Hu H. H., Wang Y. N., Feng Y. L., Cao G., Zhao Y. Y. (2018). Natural Products for the Prevention and Treatment of Kidney Disease. Phytomedicine 50, 50–60. 10.1016/j.phymed.2018.09.182 [DOI] [PubMed] [Google Scholar]
- Chen S., Ren S., Wang A. Y., Tran H., Li Z., Cheng X., et al. (2020). Comparison of the Efficacy and Safety of Tacrolimus Monotherapy and Cyclophosphamide Combined with Glucocorticoid in the Treatment of Adult Primary Membranous Nephropathy: Protocol of a Multicenter, Randomized, Controlled, Open Study. Trials 21 (1), 219. 10.1186/s13063-020-4144-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Deng Y., Ni Z., Chen N., Chen X., Shi W., et al. (2013). Efficacy and Safety of Traditional Chinese Medicine (Shenqi Particle) for Patients with Idiopathic Membranous Nephropathy: a Multicenter Randomized Controlled Clinical Trial. Am. J. Kidney Dis. 62 (6), 1068–1076. 10.1053/j.ajkd.2013.05.005 [DOI] [PubMed] [Google Scholar]
- Chen Z. Q., Sun X. H., Li X. J., Xu Z. C., Yang Y., Lin Z. Y., et al. (2020). Polydatin Attenuates Renal Fibrosis in Diabetic Mice through Regulating the Cx32-Nox4 Signaling Pathway. Acta Pharmacol. Sin. 41 (12), 1587–1596. 10.1038/s41401-020-0475-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuengsaman P., Narenpitak S., Sritippayawan S. (2021). Efficacy and Safety of Recombinant Human Erythropoietin (Hema-Plus®) for Management of Anemia in Thai Patients on Peritoneal Dialysis. World J. Nephrol. 10 (6), 109–121. 10.5527/wjn.v10.i6.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravedi P., Jarque M., Angeletti A., Favà À., Cantarelli C., Bestard O. (2019). Immune-monitoring Disease Activity in Primary Membranous Nephropathy. Front. Med. 6, 241. 10.3389/fmed.2019.00241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui H., Fu F. Q., Liu B., Liu W. J., Liu Y. N. (2021). Herbal Medicine "Shulifenxiao" Formula for Nephrotic Syndrome of Refractory Idiopathic Membranous Nephropathy. Front. Pharmacol. 12, 675406. 10.3389/fphar.2021.675406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva A. Q. B., de Sandes-Freitas T. V., Mansur J. B., Medicina-Pestana J. O., Mastroianni-Kirsztajn G. (2018). Clinical Presentation, Outcomes, and Treatment of Membranous Nephropathy after Transplantation. Int. J. Nephrol. 2018, 3720591. 10.1155/2018/3720591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahan K., Debiec H., Plaisier E., Cachanado M., Rousseau A., Wakselman L., et al. (2017). Rituximab for Severe Membranous Nephropathy: a 6-month Trial with Extended Follow-Up. J. Am. Soc. Nephrol. 28 (1), 348–358. 10.1681/ASN.2016040449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dähnrich C., Komorowski L., Probst C., Seitz-Polski B., Esnault V., Wetzels J. F., et al. (2013). Development of a Standardized ELISA for the Determination of Autoantibodies against Human M-type Phospholipase A2 Receptor in Primary Membranous Nephropathy. Clin. Chim. Acta 421, 213–218. 10.1016/j.cca.2013.03.015 [DOI] [PubMed] [Google Scholar]
- Debiec H., Guigonis V., Mougenot B., Decobert F., Haymann J. P., Bensman A., et al. (2002). Antenatal Membranous Glomerulonephritis Due to Anti-neutral Endopeptidase Antibodies. N. Engl. J. Med. 346 (26), 2053–2060. 10.1056/NEJMoa012895 [DOI] [PubMed] [Google Scholar]
- Debiec H., Ronco P. (2011). PLA2R Autoantibodies and PLA2R Glomerular Deposits in Membranous Nephropathy. N. Engl. J. Med. 364 (7), 689–690. 10.1056/NEJMc1011678 [DOI] [PubMed] [Google Scholar]
- Dong R., Bai M., Zhao J., Wang D., Ning X., Sun S. (2020). A Comparative Study of the Gut Microbiota Associated with Immunoglobulin a Nephropathy and Membranous Nephropathy. Front. Cell Infect. Microbiol. 10, 557368. 10.3389/fcimb.2020.557368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang C. Y., Lou D. Y., Zhou L. Q., Wang J. C., Yang B., He Q. J., et al. (2021). Natural Products: Potential Treatments for Cisplatin-Induced Nephrotoxicity. Acta Pharmacol. Sin. 42 (12), 1951–1969. 10.1038/s41401-021-00620-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z., Liu W., Jiang H. X., Dai H., Gao C., Dong Z., et al. (2020). How Does Herbal Medicine Treat Idiopathic Membranous Nephropathy? Front. Pharmacol. 11, 994. 10.3389/fphar.2020.00994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Juárez G., Rojas-Rivera J., Logt A. V., Justino J., Sevillano A., Caravaca-Fontán F., et al. (2021). The STARMEN Trial Indicates that Alternating Treatment with Corticosteroids and Cyclophosphamide Is Superior to Sequential Treatment with Tacrolimus and Rituximab in Primary Membranous Nephropathy. Kidney Int. 99 (4), 986–998. 10.1016/j.kint.2020.10.014 [DOI] [PubMed] [Google Scholar]
- Fervenza F. C., Appel G. B., Barbour S. J., Rovin B. H., Lafayette R. A., Aslam N., et al. (2019). Rituximab or Cyclosporine in the Treatment of Membranous Nephropathy. N. Engl. J. Med. 381 (1), 36–46. 10.1056/NEJMoa1814427 [DOI] [PubMed] [Google Scholar]
- Gambino G., Catalano C., Marangoni M., Geers C., Moine A. L., Boon N., et al. (2021). Case Report: Homozygous Pathogenic Variant P209L in the TTC21B Gene: a Rare Cause of End Stage Renal Disease and Biliary Cirrhosis Requiring Combined Liver-Kidney Transplantation. A Case Report and Literature Review. Front. Med. 8, 795216. 10.3389/fmed.2021.795216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S., Cui Z., Wang X., Zhang Y. M., Wang F., Cheng X. Y., et al. (2021). Rituximab Therapy for Primary Membranous Nephropathy in a Chinese Cohort. Front. Med. (Lausanne) 8, 663680. 10.3389/fmed.2021.663680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauckler P., Shin J. I., Alberici F., Audard V., Bruchfeld A., Busch M., et al. (2021). Rituximab in Membranous Nephropathy. Kidney Int. Rep. 6 (4), 881–893. 10.1016/j.ekir.2020.12.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng X. Q., Ma A., He J. Z., Wang L., Jia Y. L., Shao G. Y., et al. (2020). Ganoderic Acid Hinders Renal Fibrosis via Suppressing the TGF-β/Smad and MAPK Signaling Pathways. Acta Pharmacol. Sin. 41 (5), 670–677. 10.1038/s41401-019-0324-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianassi I., Allinovi M., Caroti L., Cirami L. C. (2019). Broad Spectrum of Interferon-Related Nephropathies-Glomerulonephritis, Systemic Lupus Erythematosus-like Syndrome and Thrombotic Microangiopathy: A Case Report and Review of Literature. World J. Nephrol. 8 (7), 109–117. 10.5527/wjn.v8.i7.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y., Xu H., Tang D. (2021). Mechanisms of Primary Membranous Nephropathy. Biomolecules 11 (4), 513. 10.3390/biom11040513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton P., Kanigicherla D., Venning M., Brenchley P., Meads D. (2018). Rituximab versus the Modified Ponticelli Regimen in the Treatment of Primary Membranous Nephropathy: a Health Economic Model. Nephrol. Dial. Transpl. 33 (12), 2145–2155. 10.1093/ndt/gfy049 [DOI] [PubMed] [Google Scholar]
- Hanset N., Esteve E., Plaisier E., Johanet C., Michel P. A., Boffa J. J., et al. (2020). Rituximab in Patients with Phospholipase A2 Receptor-Associated Membranous Nephropathy and Severe CKD. Kidney Int. Rep. 5 (3), 331–338. 10.1016/j.ekir.2019.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J. Y., Hong Q., Chen B. X., Cui S. Y., Liu R., Cai G. Y., et al. (2022). Ginsenoside Rb1 Alleviates Diabetic Kidney Podocyte Injury by Inhibiting Aldose Reductase Activity. Acta Pharmacol. Sin. 43 (2), 342–353. 10.1038/s41401-021-00788-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymann W., Hackel D. B., Harwood S., Wilson S. G., Hunter J. L. (1959). Production of Nephrotic Syndrome in Rats by Freund's Adjuvants and Rat Kidney Suspensions. Proc. Soc. Exp. Biol. Med. 100 (4), 660–664. 10.3181/00379727-100-24736 [DOI] [PubMed] [Google Scholar]
- Hofstra J. M., Wetzels J. F. (2012). Anti-PLA₂R Antibodies in Membranous Nephropathy: Ready for Routine Clinical Practice? Neth J. Med. 70 (3), 109–113. [PubMed] [Google Scholar]
- Hoxha E., Beck L. H., Wiech T., Tomas N. M., Probst C., Mindorf S., et al. (2017). An Indirect Immunofluorescence Method Facilitates Detection of Thrombospondin Type 1 Domain-Containing 7A-specific Antibodies in Membranous Nephropathy. J. Am. Soc. Nephrol. 28 (2), 520–531. 10.1681/ASN.2016010050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoxha E., Thiele I., Zahner G., Panzer U., Harendza S., Stahl R. A. (2014). Phospholipase A2 Receptor Autoantibodies and Clinical Outcome in Patients with Primary Membranous Nephropathy. J. Am. Soc. Nephrol. 25 (6), 1357–1366. 10.1681/ASN.2013040430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoxha E., Wiech T., Stahl P. R., Zahner G., Tomas N. M., Meyer-Schwesinger C., et al. (2016). A Mechanism for Cancer-Associated Membranous Nephropathy. N. Engl. J. Med. 374 (20), 1995–1996. 10.1056/NEJMc1511702 [DOI] [PubMed] [Google Scholar]
- Hu W., Li G., Lin J., Dong W., Yu F., Liu W., et al. (2021). M2 Macrophage Subpopulations in Glomeruli Are Associated with the Deposition of IgG Subclasses and Complements in Primary Membranous Nephropathy. Front. Med. (Lausanne) 8, 657232. 10.3389/fmed.2021.657232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzo A. A., Teixeira M., Alexander S. P. H., Cirino G., Docherty J. R., George C. H., et al. (2020). A Practical Guide for Transparent Reporting of Research on Natural Products in the British Journal of Pharmacology: Reproducibility of Natural Product Research. Br. J. Pharmacol. 177 (10), 2169–2178. 10.1111/bph.15054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H. X., Feng Z., Zhu Z. B., Xia C. H., Zhang W., Guo J., et al. (2020). Advances of the Experimental Models of Idiopathic Membranous Nephropathy (Review). Mol. Med. Rep. 21 (5), 1993–2005. 10.3892/mmr.2020.11014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L. W., Pan M., Ye H. Y., Zheng Y., Chen Y., Huang W. W., et al. (2019). Down-regulation of the Long Non-coding RNA XIST Ameliorates Podocyte Apoptosis in Membranous Nephropathy via the miR-217-TLR4 Pathway. Exp. Physiol. 104 (2), 220–230. 10.1113/EP087190 [DOI] [PubMed] [Google Scholar]
- Jin Y., Zhang J., Wang Y., Xiao X., Zhang Q. (2020). Tripterygium Wilfordii Multiglycosides Combined with Prednisone in the Treatment of Idiopathic Membranous Nephropathy: A Protocol for a Systematic Review and Meta-Analysis. Med. Baltim. 99 (5), e18970. 10.1097/MD.0000000000018970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. B. (1957). Nephrotic Glomerulonephritis. Am. J. Pathol. 33 (2), 313–329. [PMC free article] [PubMed] [Google Scholar]
- Jurubită R., Obrișcă B., Sorohan B., Achim C., Micu G. E., Mircescu G., et al. (2021). Clinical Phenotypes and Predictors of Remission in Primary Membranous Nephropathy. J. Clin. Med. 10 (12), 2624. 10.3390/jcm10122624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerjaschki D., Farquhar M. G. (1982). The Pathogenic Antigen of Heymann Nephritis Is a Membrane Glycoprotein of the Renal Proximal Tubule Brush Border. Proc. Natl. Acad. Sci. U. S. A. 79 (18), 5557–5561. 10.1073/pnas.79.18.5557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan H.-y. (2021). The Yin and Yang Role of Transforming Growth Factor-β in Kidney Disease. Integr. Med. Nephrol. Androl. 8 (1), 1. 10.4103/imna.imna_17_21 [DOI] [Google Scholar]
- Lang R., Wang X., Liang Y., Yan L., Shi B., Yu R. (2020). Research Progress in the Treatment of Idiopathic Membranous Nephropathy Using Traditional Chinese Medicine. J. Transl. Int. Med. 8 (1), 3–8. 10.2478/jtim-2020-0002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Lu R., Pang Y., Li J., Cao Y., Fu H., et al. (2020). Zhen-Wu-Tang Protects IgA Nephropathy in Rats by Regulating Exosomes to Inhibit NF-Κb/nlrp3 Pathway. Front. Pharmacol. 11, 1080. 10.3389/fphar.2020.01080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Cao Y., Lu R., Li H., Pang Y., Fu H., et al. (2020). Integrated Fecal Microbiome and Serum Metabolomics Analysis Reveals Abnormal Changes in Rats with Immunoglobulin A Nephropathy and the Intervention Effect of Zhen Wu Tang. Front. Pharmacol. 11, 606689. 10.3389/fphar.2020.606689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N., Zhao T., Cao Y., Zhang H., Peng L., Wang Y., et al. (2020). Tangshen Formula Attenuates Diabetic Kidney Injury by Imparting Anti-pyroptotic Effects via the TXNIP-NLRP3-GSDMD axis. Front. Pharmacol. 11, 623489. 10.3389/fphar.2020.623489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S. S., Sun Q., Hua M. R., Suo P., Chen J. R., Yu X. Y., et al. (2021). Targeting the Wnt/β-Catenin Signaling Pathway as a Potential Therapeutic Strategy in Renal Tubulointerstitial Fibrosis. Front. Pharmacol. 12, 719880. 10.3389/fphar.2021.719880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B., Lu R., Li H., Zhou Y., Zhang P., Bai L., et al. (2019). Zhen-Wu-tang Ameliorates Membranous Nephropathy Rats through Inhibiting NF-Κb Pathway and NLRP3 Inflammasome. Phytomedicine 59, 152913. 10.1016/j.phymed.2019.152913 [DOI] [PubMed] [Google Scholar]
- Liu W., Gao C., Liu Z., Dai H., Feng Z., Dong Z., et al. (2020). Idiopathic Membranous Nephropathy: Glomerular Pathological Pattern Caused by Extrarenal Immunity Activity. Front. Immunol. 11, 1846. 10.3389/fimmu.2020.01846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X. Q., Jiang L., Li Y. Y., Huang Y. B., Hu X. R., Zhu W., et al. (2022). Wogonin Protects Glomerular Podocytes by Targeting Bcl-2-Mediated Autophagy and Apoptosis in Diabetic Kidney Disease. Acta Pharmacol. Sin. 43 (1), 96–110. 10.1038/s41401-021-00721-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z. N., Cui Z., He Y. D., Zhang Y. M., Wang F., Wang X., et al. (2020). Membranous Nephropathy in Pregnancy. Am. J. Nephrol. 51 (4), 304–317. 10.1159/000505175 [DOI] [PubMed] [Google Scholar]
- Logt A. V., Justino J., Vink C. H., van den Brand J., Debiec H., Lambeau G., et al. (2021). Anti-PLA2R1 Antibodies as Prognostic Biomarker in Membranous Nephropathy. Kidney Int. Rep. 6 (6), 1677–1686. 10.1016/j.ekir.2021.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lönnbro-Widgren J., Ebefors K., Mölne J., Nyström J., Haraldsson B. (2015). Glomerular IgG Subclasses in Idiopathic and Malignancy-Associated Membranous Nephropathy. Clin. Kidney J. 8 (4), 433–439. 10.1093/ckj/sfv049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H., Luo Y., Su B., Tang S., Chen G., Zhang L., et al. (2020). Wenyang Lishui Decoction Ameliorates Podocyte Injury in Membranous Nephropathy Rat and Cell Models by Regulating P53 and Bcl-2. Evid. Based Complement. Altern. Med. 2020, 6813760. 10.1155/2020/6813760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z., Liu W., Gao H., Chen W., Ge W., Li F., et al. (2021). Traditional Chinese Medicine as an Adjunct Therapy in the Treatment of Idiopathic Membranous Nephropathy: A Systematic Review and Meta-Analysis. PLoS One 16 (5), e0251131. 10.1371/journal.pone.0251131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan S., Zhang S., Pan L., Hu W., Cui H., Wei X., et al. (2022). Salivary Microbiota Analysis of Patients with Membranous Nephropathy. Mol. Med. Rep. 25 (5), 190. 10.3892/mmr.2022.12706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L. P., Suo P., Ren L. L., Liu H. J., Zhang Y., Zhao Y. Y. (2021). Shenkang Injection and its Three Anthraquinones Ameliorates Renal Fibrosis by Simultaneous Targeting IƙB/NF-Ƙb and Keap1/Nrf2 Signaling Pathways. Front. Pharmacol. 12, 800522. 10.3389/fphar.2021.800522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markell M., Brar A., Bhela S., Patel A., Salifu M. (2019). Use of Repository Corticotropin Gel (Acthar) in Progressive Nephrotic Syndrome Secondary to Transplant Glomerulopathy: a Report of Three Cases. Kidney Med. 1 (1), 31–35. 10.1016/j.xkme.2018.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuarrie E. P., Stirling C. M., Geddes C. C. (2012). Idiopathic Membranous Nephropathy and Nephrotic Syndrome: Outcome in the Era of Evidence-Based Therapy. Nephrol. Dial. Transpl. 27 (1), 235–242. 10.1093/ndt/gfr220 [DOI] [PubMed] [Google Scholar]
- Medina Rangel P. X., Priyadarshini A., Tian X. (2021). New Insights into the Immunity and Podocyte in Glomerular Health and Disease: From Pathogenesis to Therapy in Proteinuric Kidney Disease. Integr. Med. Nephrol. Androl. 28, 1707. 10.1681/ASN.2017010027 [DOI] [Google Scholar]
- Meng J., Sai-Zhen W., He J. Z., Zhu S., Huang B. Y., Wang S. Y., et al. (2020). Ganoderic Acid A Is the Effective Ingredient of Ganoderma Triterpenes in Retarding Renal Cyst Development in Polycystic Kidney Disease. Acta Pharmacol. Sin. 41 (6), 782–790. 10.1038/s41401-019-0329-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao H., Cao G., Wu X. Q., Chen Y. Y., Chen D. Q., Chen L., et al. (2020). Identification of Endogenous 1-aminopyrene as a Novel Mediator of Progressive Chronic Kidney Disease via Aryl Hydrocarbon Receptor Activation. Br. J. Pharmacol. 177 (15), 3415–3435. 10.1111/bph.15062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao H., Wu X. Q., Wang Y. N., Chen D. Q., Chen L., Vaziri N. D., et al. (2022). 1-Hydroxypyrene Mediates Renal Fibrosis through Aryl Hydrocarbon Receptor Signalling Pathway. Br. J. Pharmacol. 179 (1), 103–124. 10.1111/bph.15705 [DOI] [PubMed] [Google Scholar]
- Molina Andújar A., Lucas A., Escudero V. J., Rovira I., Matute P., Ibañez C., et al. (2022). Antiphospholipase A2 Receptor Antibody-Positive Membranous Nephropathy in the Kidney Donor: Lessons from a Serendipitous Transplantation. Am. J. Transpl. 22 (1), 299–303. 10.1111/ajt.16813 [DOI] [PubMed] [Google Scholar]
- Moroni G., Ponticelli C. (2020). Secondary Membranous Nephropathy. A Narrative Review. Front. Med. 7, 611317. 10.3389/fmed.2020.611317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moszczuk B., Kiryluk K., Pączek L., Mucha K. (2021). Membranous Nephropathy: from Research Bench to Personalized Care. J. Clin. Med. 10 (6), 1205. 10.3390/jcm10061205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto-Gañán I., Iturrieta-Zuazo I., Rita C., Carrasco-Sayalero Á. (2021). Comparison of 3 Anti-pla2r Inmmunoassaysfor the Diagnosis of Idiopathic Membranous Nephropathy in an European Population. A Pilot Study. Clin. Immunol. 227, 108729. 10.1016/j.clim.2021.108729 [DOI] [PubMed] [Google Scholar]
- Passerini P., Malvica S., Tripodi F., Cerutti R., Messa P. (2019). Membranous Nephropathy (MN) Recurrence after Renal Transplantation. Front. Immunol. 10, 1326. 10.3389/fimmu.2019.01326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcelli B., Guarnieri A., Ferretti F., Garosi G., Terzuoli L., Cinci F., et al. (2021). Diagnostic Accuracy of Anti-phospholipase A2 Receptor (PLA2R) Antibodies in Idiopathic Membranous Nephropathy: an Italian Experience. J. Nephrol. 34 (2), 573–579. 10.1007/s40620-020-00888-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prunotto M., Carnevali M. L., Candiano G., Murtas C., Bruschi M., Corradini E., et al. (2010). Autoimmunity in Membranous Nephropathy Targets Aldose Reductase and SOD2. J. Am. Soc. Nephrol. 21 (3), 507–519. 10.1681/ASN.2008121259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran R., Kumar V., Nada R., Jha V. (2015). Serial Monitoring of Anti-pla2r in Initial PLA2R-Negative Patients with Primary Membranous Nephropathy. Kidney Int. 88 (5), 1198–1199. 10.1038/ki.2015.310 [DOI] [PubMed] [Google Scholar]
- Ramachandran R., Yadav A. K., Kumar V., Inamdar N., Nada R., Gupta K. L., et al. (2018). Temporal Association between PLA2R Antibodies and Clinical Outcomes in Primary Membranous Nephropathy. Kidney Int. Rep. 3 (1), 142–147. 10.1016/j.ekir.2017.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson K. J., Kitching A. R. (2021). Recurrent Membranous Nephropathy after Transplantation: Donor Antigen and HLA Converge in Defining Risk. Kidney Int. 99 (3), 545–548. 10.1016/j.kint.2020.10.044 [DOI] [PubMed] [Google Scholar]
- Ronco P., Debiec H. (2020). Molecular Pathogenesis of Membranous Nephropathy. Annu. Rev. Pathol. 15, 287–313. 10.1146/annurev-pathol-020117-043811 [DOI] [PubMed] [Google Scholar]
- Ronco P., Debiec H. (2015). Pathophysiological Advances in Membranous Nephropathy: Time for a Shift in Patient's Care. Lancet 385 (9981), 1983–1992. 10.1016/S0140-6736(15)60731-0 [DOI] [PubMed] [Google Scholar]
- Ronco P., Plaisier E., Debiec H. (2021). Advances in Membranous Nephropathy. J. Clin. Med. 10 (4), 607. 10.3390/jcm10040607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggenenti P., Debiec H., Ruggiero B., Chianca A., Pellé T., Gaspari F., et al. (2015). Anti-Phospholipase A2 Receptor Antibody Titer Predicts Post-Rituximab Outcome of Membranous Nephropathy. J. Am. Soc. Nephrol. 26 (10), 2545–2558. 10.1681/ASN.2014070640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawhney H., Gill S. S. (2020). Renal Transplant Recipient Seizure Practical Management. World J. Nephrol. 9 (1), 1–8. 10.5527/wjn.v9.i1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi B., Zhang R. R., Liang Y., Wang X. H., Lang R., Yu R. H. (2018). Efficacy of Traditional Chinese Medicine Regimen Jian Pi Qu Shi Formula for Refractory Patients with Idiopathic Membranous Nephropathy: a Retrospective Case-Series Study. Evid. Based Complement. Altern. Med. 2018, 5854710. 10.1155/2018/5854710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singla R. K., He X., Chopra H., Tsagkaris C., Shen L., Kamal M. A., et al. (2021). Natural Products for the Prevention and Control of the COVID-19 Pandemic: Sustainable Bioresources. Front. Pharmacol. 12, 758159. 10.3389/fphar.2021.758159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens P. E., Levin A. (2013). Evaluation and Management of Chronic Kidney Disease: Synopsis of the Kidney Disease: Improving Global Outcomes 2012 Clinical Practice Guideline. Ann. Intern Med. 158 (11), 825–830. 10.7326/0003-4819-158-11-201306040-00007 [DOI] [PubMed] [Google Scholar]
- Su J., Gao C., Xie L., Fan Y., Shen Y., Huang Q., et al. (2021). Astragaloside II Ameliorated Podocyte Injury and Mitochondrial Dysfunction in Streptozotocin-Induced Diabetic Rats. Front. Pharmacol. 12, 638422. 10.3389/fphar.2021.638422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutariya B., Taneja N., Saraf M. (2017). Betulinic Acid, Isolated from the Leaves of Syzygium Cumini (L.) Skeels, Ameliorates the Proteinuria in Experimental Membranous Nephropathy through Regulating Nrf2/NF-Κb Pathways. Chem. Biol. Interact. 274, 124–137. 10.1016/j.cbi.2017.07.011 [DOI] [PubMed] [Google Scholar]
- Tamura H. (2021). Trends in Pediatric Nephrotic Syndrome. World J. Nephrol. 10 (5), 88–100. 10.5527/wjn.v10.i5.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesar V., Hruskova Z. (2021). Autoantibodies in the Diagnosis, Monitoring, and Treatment of Membranous Nephropathy. Front. Immunol. 12, 593288. 10.3389/fimmu.2021.593288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian R., Wang L., Chen A., Huang L., Liang X., Wang R., et al. (2019). Sanqi Oral Solution Ameliorates Renal Damage and Restores Podocyte Injury in Experimental Membranous Nephropathy via Suppression of NFκB. Biomed. Pharmacother. 115, 108904. 10.1016/j.biopha.2019.108904 [DOI] [PubMed] [Google Scholar]
- Tian Z., Wang M., Huang C., Gao W., Zhu Y., Zhang F., et al. (2022). Efficacy and Safety of Tacrolimus Combined with Corticosteroids in Patients with Idiopathic Membranous Nephropathy: a Systematic Review and Meta-Analysis of Randomized Controlled Trials. Int. Urol. Nephrol. 1, 1. 10.1007/s11255-022-03169-6 [DOI] [PubMed] [Google Scholar]
- Tomas N. M., Beck L. H., Meyer-Schwesinger C., Seitz-Polski B., Ma H., Zahner G., et al. (2014). Thrombospondin Type-1 Domain-Containing 7A in Idiopathic Membranous Nephropathy. N. Engl. J. Med. 371 (24), 2277–2287. 10.1056/NEJMoa1409354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomas N. M., Huber T. B., Hoxha E. (2021). Perspectives in Membranous Nephropathy. Cell Tissue Res. 385 (2), 405–422. 10.1007/s00441-021-03429-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uffing A., Hullekes F., Riella L. V., Hogan J. J. (2021). Recurrent Glomerular Disease after Kidney Transplantation: Diagnostic and Management Dilemmas. Clin. J. Am. Soc. Nephrol. 16 (11), 1730–1742. 10.2215/CJN.00280121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Logt A. E., Hofstra J. M., Wetzels J. F. (2015). Serum Anti-pla2r Antibodies Can Be Initially Absent in Idiopathic Membranous Nephropathy: Seroconversion after Prolonged Follow-Up. Kidney Int. 87 (6), 1263–1264. 10.1038/ki.2015.34 [DOI] [PubMed] [Google Scholar]
- Wang M., Chen D. Q., Chen L., Cao G., Zhao H., Liu D., et al. (2018). Novel Inhibitors of the Cellular Renin-Angiotensin System Components, Poricoic Acids, Target Smad3 Phosphorylation and Wnt/β-Catenin Pathway against Renal Fibrosis. Br. J. Pharmacol. 175 (13), 2689–2708. 10.1111/bph.14333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Dong Z. Y., Zhang W. G., Liu X. M., Qu Y. L., Duan S. W., et al. (2020). Diagnostic Efficacy of Serum Anti-phospholipase A2 Receptor Antibodies for Idiopathic Membranous Nephropathy in Patients with Diabetic Kidney Disease. Clin. Chim. Acta 502, 222–226. 10.1016/j.cca.2019.11.004 [DOI] [PubMed] [Google Scholar]
- Wang W., Long H., Huang W., Zhang T., Xie L., Chen C., et al. (2020). Bu-Shen-Huo-Xue Decoction Ameliorates Diabetic Nephropathy by Inhibiting Rac1/PAK1/p38MAPK Signaling Pathway in High-Fat Diet/Streptozotocin-Induced Diabetic Mice. Front. Pharmacol. 11, 587663. 10.3389/fphar.2020.587663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Cui Z., Zhang Y. M., Qu Z., Wang F., Meng L. Q., et al. (2018). Rituximab for Non-responsive Idiopathic Membranous Nephropathy in a Chinese Cohort. Nephrol. Dial. Transpl. 33 (9), 1558–1563. 10.1093/ndt/gfx295 [DOI] [PubMed] [Google Scholar]
- Wang Y. W., Zeng Q., Yu R. H. (2021). Treatment of Membranoproliferative Glomerulonephritis with Traditional Chinese Medicine and Rituximab: A Case Report. Integr. Med. Nephrol. Androl. 8, 3. [Google Scholar]
- Wang Y. (2021). Xiaochaihu Decoction in Diabetic Kidney Disease: A Study Based on Network Pharmacology and Molecular Docking Technology. Integr. Med. Nephrol. Androl. 8, 13. [Google Scholar]
- Wu J., Liu B., Liang C., Ouyang H., Lin J., Zhong Y., et al. (2016). Zhen-Wu-tang Attenuates Cationic Bovine Serum Albumin-Induced Inflammatory Response in Membranous Glomerulonephritis Rat through Inhibiting AGEs/RAGE/NF-κB Pathway Activation. Int. Immunopharmacol. 33, 33–41. 10.1016/j.intimp.2016.01.008 [DOI] [PubMed] [Google Scholar]
- Wu L., Lai J., Ling Y., Weng Y., Zhou S., Wu S., et al. (2021). A Review of the Current Practice of Diagnosis and Treatment of Idiopathic Membranous Nephropathy in China. Med. Sci. Monit. 27, e930097. 10.12659/MSM.930097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Zhong Y., Meng T., Ooi J. D., Eggenhuizen P. J., Tang R., et al. (2021). Patient Survival between Hemodialysis and Peritoneal Dialysis Among End-Stage Renal Disease Patients Secondary to Myeloperoxidase-ANCA-Associated Vasculitis. Front. Med. (Lausanne) 8, 775586. 10.3389/fmed.2021.775586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J., Liu L., Mladkova N., Li Y., Ren H., Wang W., et al. (2020). The Genetic Architecture of Membranous Nephropathy and its Potential to Improve Non-invasive Diagnosis. Nat. Commun. 11 (1), 1600. 10.1038/s41467-020-15383-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xipell M., Rodas L. M., Villarreal J., Molina A., Reinoso-Moreno J., Blasco M., et al. (2018). The Utility of Phospholipase A2 Receptor Autoantibody in Membranous Nephropathy after Kidney Transplantation. Clin. Kidney J. 11 (3), 422–428. 10.1093/ckj/sfx128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Chen L., Xiang H., Zhang C., Xiong J. (2020). Advances in Pathogenesis of Idiopathic Membranous Nephropathy. Kidney Dis. (Basel) 6 (5), 330–345. 10.1159/000507704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan C., Xi Y. M., Zhang Y. D., Tao C. H., Zhang L. Y., Cao W. F. (2021). Yiqi Jiedu Huayu Decoction Alleviates Renal Injury in Rats with Diabetic Nephropathy by Promoting Autophagy. Front. Pharmacol. 12, 624404. 10.3389/fphar.2021.624404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F., Zhang Q., Yuan Z., Teng S., Cui L., Xue F., et al. (2021). Signaling Potential Therapeutic Herbal Medicine Prescription for Treating COVID-19 by Collaborative Filtering. Front. Pharmacol. 12, 759479. 10.3389/fphar.2021.759479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K., Bai Y., Yu N., Lu B., Han G., Yin C., et al. (2020). Huidouba Improved Podocyte Injury by Down-Regulating NOX4 Expression in Rats with Diabetic Nephropathy. Front. Pharmacol. 11, 587995. 10.3389/fphar.2020.587995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Wu C. (2021). Traditional Chinese Medicine in Ameliorating Diabetic Kidney Disease via Modulating Gut Microbiota. Integr. Med. Nephrol. Androl. 8, 8. 10.4103/imna.imna_28_21 [DOI] [Google Scholar]
- Yang Z.-H., Wang B., Ma Q., Wang L., Lin Y.-X., Yan H.-F., et al. (2021). Potential Mechanisms of Action of Chinese Patent Medicines for COVID-19: a Review. Front. Pharmacol. 12, 668407. 10.3389/fphar.2021.668407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa M., Asaba K. (2020). Single-nucleotide Polymorphism Rs4664308 in PLA2R1 Gene Is Associated with the Risk of Idiopathic Membranous Nephropathy: a Meta-Analysis. Sci. Rep. 10 (1), 13119. 10.1038/s41598-020-70009-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Han M., Lin W., Wang L., Liu P., Yang K., et al. (2020). Efficacy of Chinese Herbal Injections for the Treatment of Primary Nephrotic Syndrome: a Bayesian Network Meta-Analysis of Randomized Controlled Trials. Front. Pharmacol. 11, 579241. 10.3389/fphar.2020.579241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Cai J., Jiao X., Zhang S., Liu H., Ding X. (2018). Response Predictors to Calcineurin Inhibitors in Patients with Primary Membranous Nephropathy. Am. J. Nephrol. 47 (4), 266–274. 10.1159/000488728 [DOI] [PubMed] [Google Scholar]
- Yu X. Y., Sun Q., Zhang Y. M., Zou L., Zhao Y. Y. (2022). TGF-β/Smad Signaling Pathway in Tubulointerstitial Fibrosis. Front. Pharmacol. 13, 860588. 10.3389/fphar.2022.860588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Luo D., Lin Z., Zhou W., Rao J., Li Y., et al. (2020). Dysbiosis of Gut Microbiota in Adult Idiopathic Membranous Nephropathy with Nephrotic Syndrome. Microb. Pathog. 147, 104359. 10.1016/j.micpath.2020.104359 [DOI] [PubMed] [Google Scholar]
- Zhang P., Huang W., Zheng Q., Tang J., Dong Z., Jiang Y., et al. (2021). A Novel Insight into the Role of PLA2R and THSD7A in Membranous Nephropathy. J. Immunol. Res. 2021, 8163298. 10.1155/2021/8163298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q. H., Huang H. Z., Qiu M., Wu Z. F., Xin Z. C., Cai X. F., et al. (2021). Traditional Uses, Pharmacological Effects, and Molecular Mechanisms of Licorice in Potential Therapy of COVID-19. Front. Pharmacol. 12, 719758. 10.3389/fphar.2021.719758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Bian S. Z., Yang K., Wang Y., Tang S., Wang W., et al. (2020). Baseline Soluble Anti-erythropoietin Antibody Level Is an Independent Associated Factor for Follow-Up Erythropoietin Demand in Maintenance Dialysis Patients with End-Stage Renal Disease: a Prospective Cohort Study. Front. Med. (Lausanne) 7, 109. 10.3389/fmed.2020.00109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Lu X., Dong L., Ma J., Fan X. (2019). Clinical Observation on the Effect of Wuzhi Soft Capsule on FK506 Concentration in Membranous Nephropathy Patients. Med. Baltim. 98 (48), e18150. 10.1097/MD.0000000000018150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z. H., He J. Q., Zhao Y. Y., Chen H. C., Tan N. H. (2020). Asiatic Acid Prevents Renal Fibrosis in UUO Rats via Promoting the Production of 15d-PGJ2, an Endogenous Ligand of PPAR-γ. Acta Pharmacol. Sin. 41 (3), 373–382. 10.1038/s41401-019-0319-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng R., Deng Y., Chen Y., Fan J., Zhang M., Zhong Y., et al. (2012). Astragaloside IV Attenuates Complement Membranous Attack Complex Induced Podocyte Injury through the MAPK Pathway. Phytother. Res. 26 (6), 892–898. 10.1002/ptr.3656 [DOI] [PubMed] [Google Scholar]
- Zhou X. F., Wang Y., Luo M. J., Zhao T. T. (2021). Tangshen Formula Attenuates Renal Fibrosis by Downregulating Transforming Growth Factor β1/Smad3 and LncRNA-MEG3 in Rats with Diabetic Kidney Disease. Integr. Med. Nephrol. Androl. 8, 1. 10.4103/imna.imna_22_21 [DOI] [Google Scholar]
- Zhu H., Xu L., Liu X., Liu B., Zhai C., Wang R., et al. (2022). Anti-PLA2R Antibody Measured by ELISA Predicts the Risk of Vein Thrombosis in Patients with Primary Membranous Nephropathy. Ren. Fail 44 (1), 594–600. 10.1080/0886022X.2022.2057861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou H., Jiang F., Xu G. (2020). Effectiveness and Safety of Cyclophosphamide or Tacrolimus Therapy for Idiopathic Membranous Nephropathy. Intern Med. J. 50 (5), 612–619. 10.1111/imj.14446 [DOI] [PubMed] [Google Scholar]
- Zuo H. L., Lin Y. C., Huang H. Y., Wang X., Tang Y., Hu Y. J., et al. (2021). The Challenges and Opportunities of Traditional Chinese Medicines against COVID-19: a Way Out from a Network Perspective. Acta Pharmacol. Sin. 42 (6), 845–847. 10.1038/s41401-021-00645-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.