Abstract
Background
Sarcopenia is an age‐related chronic condition that can lead to mobility disabilities. This study aimed to evaluate the risk factors for incident sarcopenia in older Korean adults.
Methods
The Korean Frailty and Aging Cohort Study (KFACS) is a multicentre prospective study with a baseline examination in 2016–2017. A prospective follow‐up study was conducted in 2018–2019. Changes in muscle‐related variables were evaluated for subjects aged 70–84 years lacking sarcopenia at baseline. Sarcopenia was diagnosed according to the 2019 updated Asian Working Group for Sarcopenia consensus.
Results
Among the 1636 participants (54.4% women, age 75.9 ± 3.7) who did not have sarcopenia at baseline, 101 men (13.5%) and 104 women (11.7%) developed sarcopenia by the follow‐up. Those who developed sarcopenia were older (men, 77.9 ± 3.9 vs. 75.7 ± 3.5, P < 0.001; women, 77.5 ± 4.0 vs. 75.5 ± 3.6, P < 0.001) with a lower body mass index at baseline (men, 23.9 ± 2.4 vs. 24.5 ± 2.9 kg/m2, P = 0.025; women, 23.7 ± 2.8 vs. 25.2 ± 2.9 kg/m2, P < 0.001) compared with older adults who remained nonsarcopenic; levels of glycated haemoglobin (men, 6.2 ± 1.0% vs. 5.9 ± 0.8%, P = 0.029) and the homeostasis model assessment of insulin resistance (men, 2.0 ± 1.3 vs. 1.7 ± 1.2, P = 0.022) were higher in men who progressed to sarcopenia but not in women. Development of sarcopenia was associated with older age and the frequency of resistance training (≥2 per week) after adjusting for potential risk factors in men [age, odds ratio (OR) 1.17, 95% confidence interval (CI) 1.10–1.25; frequent resistance training, OR 0.50, 95% CI 0.30–0.82]. In women, advanced age, poor nutritional status, and physical inactivity contributed to the development of sarcopenia (age, OR 1.14, 95% CI 1.08–1.21; mini nutritional assessment short form, OR 0.79, 95% CI 0.70–0.90; moderate to high physical activity, OR 0.57, 95% CI 0.34–0.95).
Conclusions
In this 2 year KFACS follow‐up, modifiable risk factors for incident sarcopenia differed between genders. Resistance training (≥2 per week) helped to prevent sarcopenia in these community‐dwelling older men. In older women, adequate nutritional support and being physically active might play a role in preventing progression to sarcopenia.
Keywords: Sarcopenia, Resistance training, Insulin resistance, Malnutrition, Exercise
Introduction
Over the past several decades, there has been a major demographic shift to older population distributions. The percentage of elderly persons of 65 years or older in Korea has more than doubled since 2000 and exceeded 16% in 2020. 1 Sarcopenia is defined as age‐related progressive reduction in skeletal muscle mass and strength, leading to poor musculoskeletal health outcomes. 2 It interferes with successful aging and impairs the quality of life, in addition to generating a profound health‐related effect on aging communities. 3 To date, there are no specific drugs approved for the treatment of sarcopenia. Once it develops, it becomes extremely challenging to counteract the loss of muscle mass and progressive functional decline.
Therefore, it is of utmost importance to recognize persons who are vulnerable to developing sarcopenia and to assess the factors contributing to its development. The incidence of sarcopenia increases with aging, and pathologic conditions closely linked to cachexia are regarded as an epiphenomenon of sarcopenia. 4 Other frequent underlying causes of sarcopenia include nutritional factors, circumstances associated with inactivity, and iatrogenic situations such as drug‐related problems or hospital admission. Because sarcopenia is multifactorial in aetiology, equally sarcopenic individuals could be characterized by markedly different risk factor profiles depending on gender and ethnicity. It is unarguable that primary interventions to prevent the progression to sarcopenia should focus on amendable components regarding these factors.
It is generally acknowledged that men lose skeletal muscle mass and strength twice as rapidly as women during the aging process, 5 , 6 , 7 , 8 but to date, there are few longitudinal studies that explored the remediable risk factors for sarcopenia by gender. In this study, we have assessed the 2 year follow‐up data of the Korean Frailty and Aging Cohort Study (KFACS) to evaluate the preventable measures in progression to new onset sarcopenia according to gender in older Korean men and women.
Methods
Study population
The KFACS is a multicentre, longitudinal study, with the baseline survey and examination conducted in 2016–2017 and the first follow‐up examination in 2018–2019. The KFACS cohort consisted of community‐dwelling older adults aged 70–84 years stratified by age and gender, recruited from 10 urban, suburban, and rural regions nationwide. Details on enrolment into the KFACS have been described. 9 , 10
Of the 3014 enrolled participants, 2403 whose appendicular skeletal muscle mass (ASM) was measured by dual‐energy X‐ray absorptiometry (DXA) were included initially in the cross‐sectional analysis, after excluding 611 participants from 2 centres that measured ASM using bioelectrical impedance analysis, not DXA. 10 Those exclusions were made considering the systematic bias in appendicular lean mass measurements between the two methods. Of these, 1983 participants were followed up after excluding 409 participants who were lost to follow‐up, or who did not have the follow‐up DXA or hand grip strength (HGS) measurement data (Figure 1). This study was conducted in accordance with the ethical standards of the Declaration of Helsinki. The study protocol was approved by the Institutional Review Board of Seoul National University Bundang Hospital (B‐1607/354‐402), and informed consent was obtained from all participants included in the study.
Figure 1.
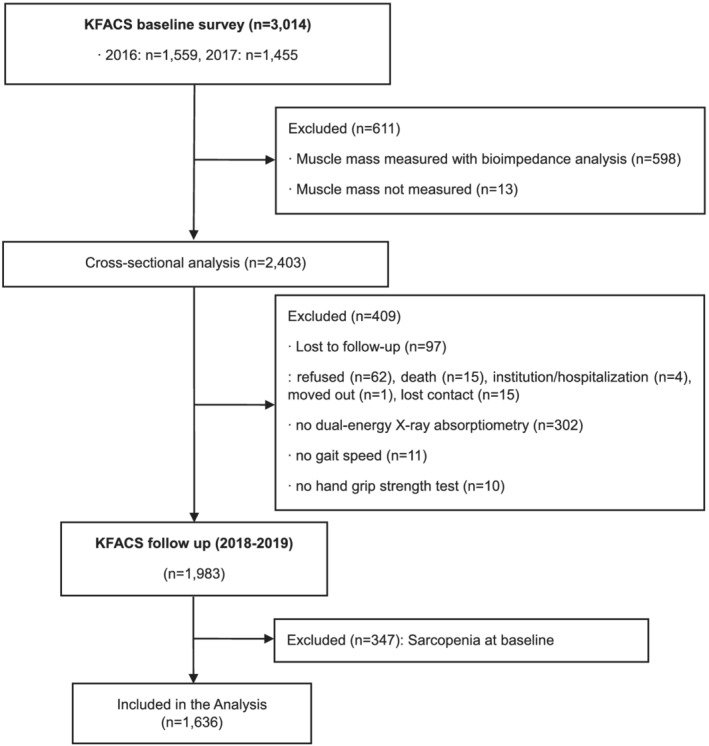
Consort diagram of participants enrolled in the Korean Frailty and Aging Cohort Study (KFACS).
Definition of disease status
Sarcopenia
Each subject's ASM was assessed by DXA. Muscle strength was evaluated using HGS measured on a digital grip strength dynamometer (TTK‐5401, Takei Ltd, Tokyo, Japan) as described. 10 The short physical performance battery (SPPB) comprised the sum of scorings obtained from three tests: standing‐balance measures, chair‐stand time measures, and gait speed. 11 For the chair‐stand time test, participants were instructed to fully sit on the chair between each stand five times without using their arms. A timed up and go (TUG) test was also performed to assess physical performance.
Diagnosis of sarcopenia was made based on the updated definition of the Asian Working Group for Sarcopenia (AWGS) 2019. 12 Sarcopenia is defined as a combination of low ASM index and low muscle strength or physical performance. Specifically, a low ASM index was defined as ASM/height2 < 7.0 kg/m2 for men and <5.4 kg/m2 for women. HGS values of <28 kg for men and <18 kg for women were used as cut‐offs for defining low muscle strength. Low physical performance was defined as a usual gait speed of <1.0 m/s, five‐times sit‐to‐stand test of ≥12 s, or an SPPB score of ≤9 for both genders.
Comorbidities
To assess for comorbidity, the Charlson comorbidity index (CCI), a well‐validated prognostic indicator for 1 year mortality, was applied. 13 The CCI comprised 17 comorbid conditions, of which the individual comorbid diseases were divided into 10 classes: myocardial, vascular, pulmonary, neurologic, endocrine, renal, liver, gastrointestinal, cancer/immune, and miscellaneous, including rheumatologic and diseases involving coagulopathy. Additional weights in the CCI were assigned according to disease severity.
Measurements
Physical activity
The extent of physical activity was assessed based on the scoring of the short‐form International Physical Activity Questionnaire (IPAQ). 14 The IPAQ comprises four sets of questions about the duration (in minutes) and frequency (times per week) of physical activities. Total physical activity level was determined by multiplying the duration (in minutes) and frequency of physical activities with the metabolic equivalent of task (MET), expressed as MET‐min/week. Participants were classified into three levels of physical activities: low (<600 MET‐min/week), moderate (600–2999 MET‐min/week), and high activity (≥3000 MET‐min/week). Moderate to high levels of physical activity on the IPAQ score were considered to reflect subjects who were physically active.
Resistance training
The level of resistance training (RT) was determined by self‐reported questionnaires. Those participants who engaged in at least two weekly sessions of exercising with weight machines (barbells, dumbbells, and/or elastic bands) or with free weights (push‐ups, sit‐ups, and/or squats) were defined to be active in RT. 15
Nutritional assessment
The short‐form version of the mini nutritional assessment (MNA‐SF) screening score was used to assess nutritional status. 16 , 17 The six items in this well‐validated MNA are used conventionally as a short screening tool in classifying geriatric patients at risk of malnutrition and malnutrition.
Laboratory measurements
All blood samples were obtained at around 8 AM after at least 8 h of overnight fasting and were analysed accordingly as described. 10 , 18 All blood tests were carried out in a core laboratory (Seegene Co., Seoul, Korea). The homeostasis model assessment of insulin resistance (HOMA‐IR) was used to estimate insulin resistance. HOMA‐IR was calculated as glucose (mg/dL) × insulin (μU/mL)/405.
Statistical analysis
Data are described as the mean ± standard deviation for continuous variables, and numbers and percentages for categorical variables. Differences in means and proportions by sarcopenia status were tested using Student's t‐tests or analysis of variance for continuous variables and χ 2 test for categorical variables. In comparing three consecutive subgroups, P for trend was calculated using polynomial regression for continuous variables and the Cochran–Armitage test for categorical variables. For post hoc analysis, Tukey's honest significant difference method or Holm's adjustment was applied for continuous and categorical variables, respectively. For continuous variables that did not follow normal distribution, either logarithmic transformation (Ln) was applied to achieve normality or the Mann–Whitney non‐parametric U‐test was applied for comparison. To analyse longitudinal data by time and the status of sarcopenia, or by time and gender, generalized linear models were applied using generalized estimating equations.
Factors attributed to the development of sarcopenia in participants who did not have sarcopenia at baseline were assessed by binary logistic regression analysis. Univariable logistic regression analysis was performed to identify the potential risk factors for the onset of sarcopenia. Multivariable logistic regression analysis was followed to determine the independent association between the risk factors and incident sarcopenia. All following analyses were stratified by gender. All analyses were performed using IBM SPSS Statistics Version 27.0 (IBM Corp., Armonk, NY, USA) and R programming Version 4.1.2 (The R Foundation for Statistical Computing, Vienna, Austria, http://www.R‐project.org). A two‐sided P value of ≤0.05 was considered statistically significant.
Results
Baseline characteristics of participants who developed sarcopenia at the 2 year follow‐up
Among the 1983 older adults with a complete 2 year follow‐up, 194 men (20.4%) and 153 women (14.6%) had sarcopenia at baseline (Figure 1). Participants excluded for such underlying sarcopenia or follow‐up loss were generally older, with lower body mass index (BMI) and muscle mass, and inferior muscle function (Supporting Information, Table S1).
For all participants with follow‐up examination, biennial changes of muscle mass and function are described in Table S2. Of the 746 men and 890 women who did not have sarcopenia at baseline, 101 men (13.5%) and 104 women (11.7%) developed sarcopenia during the 2 years of follow‐up (P = 0.292, men vs. women for incident sarcopenia; Table 1). Both genders who developed sarcopenia were older with a lower BMI at baseline. The men who developed sarcopenia had more comorbidities including hypertension, diabetes mellitus, and atherosclerotic cardiovascular disease compared with those who did not. Of note, none of the participants had disabled conditions leading to hemiplegia or paraplegia during the follow‐up.
Table 1.
Baseline characteristics of subjects according to incident sarcopenia
| Men (N = 746) | Women (N = 890) | |||||
|---|---|---|---|---|---|---|
| Sarcopenia (+) | Sarcopenia (−) | P | Sarcopenia (+) | Sarcopenia (−) | P | |
| N = 101 | N = 645 | N = 104 | N = 786 | |||
| Age | 77.9 ± 3.9 | 75.7 ± 3.5 | <0.001 | 77.5 ± 4.0 | 75.5 ± 3.6 | <0.001 |
| Height (cm) | 163.9 ± 4.8 | 165.4 ± 5.4 | 0.009 | 150.5 ± 5.0 | 152.5 ± 5.2 | <0.001 |
| Weight (kg) | 64.2 ± 7.0 | 67.0 ± 8.6 | <0.001 | 53.7 ± 7.2 | 58.7 ± 8.0 | <0.001 |
| BMI (kg/m2) | 23.9 ± 2.4 | 24.5 ± 2.9 | 0.025 | 23.7 ± 2.8 | 25.2 ± 2.9 | <0.001 |
| Waist circumference (cm) | 88.0 ± 7.8 | 89.3 ± 8.2 | 0.139 | 84.1 ± 7.9 | 87.6 ± 8.3 | <0.001 |
| Hypertension (%) | 64 (63.4) | 326 (50.6) | 0.017 | 65 (62.5) | 460 (58.5) | 0.439 |
| SBP (mmHg) | 129.7 ± 14.0 | 131.4 ± 15.0 | 0.287 | 130.5 ± 16.4 | 131.6 ± 15.3 | 0.512 |
| DBP (mmHg) | 76.4 ± 9.5 | 78.6 ± 8.8 | 0.019 | 75.5 ± 9.2 | 77.4 ± 9.0 | 0.044 |
| Diabetes mellitus (%) | 40 (40.0) | 177 (27.5) | 0.010 | 31 (29.8) | 189 (24.1) | 0.206 |
| Dyslipidaemia (%) | 24 (24.0) | 158 (24.7) | 0.882 | 37 (35.9) | 322 (42.8) | 0.182 |
| ASCVD (%) | 26 (25.7) | 92 (14.3) | 0.003 | 12 (11.5) | 79 (10.1) | 0.644 |
| Charlson comorbidity index | 0.94 ± 1.16 | 0.69 ± 0.99 | 0.024 | 0.93 ± 0.98 | 0.81 ± 0.94 | 0.206 |
| MNA‐SF | 13.16 ± 1.05 | 13.07 ± 1.39 | 0.540 | 12.36 ± 1.91 | 13.02 ± 1.34 | 0.013 |
| Alcohol (%) | 60 (68.9) | 459 (76.5) | 0.163 | 10 (0.2) | 155 (3.5) | 0.072 |
| Current smoker (%) | 12 (17.2) | 61 (12.2) | 0.330 | 2 (0.0) | 4 (0.0) | 0.148 |
| Moderate to high physical activity (%) | 85 (85.0) | 573 (90.2) | 0.112 | 74 (72.5) | 652 (83.5) | 0.007 |
| Resistance training (≥2 per week) | 26 (25.7) | 272 (42.2) | 0.002 | 17 (16.3) | 186 (23.7) | 0.093 |
| Biochemical variables | ||||||
| Fasting glucose (mg/dL) | 110 ± 28 | 104 ± 21 | 0.032 | 102 ± 20 | 102 ± 21 | 0.895 |
| HbA1c (%) | 6.15 ± 0.97 | 5.93 ± 0.77 | 0.029 | 6.07 ± 0.81 | 6.04 ± 0.79 | 0.698 |
| HOMA‐IR | 1.97 ± 1.29 | 1.72 ± 1.24 | 0.022 a | 2.01 ± 1.34 | 2.15 ± 1.42 | 0.347 a |
| Total cholesterol (mg/dL) | 163 ± 33 | 168 ± 35 | 0.168 | 179 ± 38 | 181 ± 35 | 0.544 |
| Triglyceride (mg/dL) | 121 ± 66 | 114 ± 64 | 0.295 | 124 ± 57 | 124 ± 58 | 0.896 |
| HDL‐C (mg/dL) | 47 ± 13 | 51 ± 14 | 0.004 | 55 ± 15 | 54 ± 13 | 0.352 |
| LDL‐C (mg/dL) | 102 ± 30 | 104 ± 32 | 0.453 | 109 ± 35 | 113 ± 33 | 0.236 |
| BUN (mg/dL) | 17.7 ± 5.1 | 16.8 ± 4.9 | 0.060 | 15.5 ± 4.4 | 15.9 ± 4.4 | 0.465 |
| Creatinine (mg/dL) | 1.00 ± 0.23 | 0.96 ± 0.35 | 0.292 | 0.74 ± 0.17 | 0.71 ± 0.16 | 0.207 |
| AST (IU/L) | 22.9 ± 6.9 | 23.8 ± 11.3 | 0.475 | 22.5 ± 10.7 | 22.0 ± 6.8 | 0.640 |
| ALT (IU/L) | 20.2 ± 12.0 | 20.6 ± 12.3 | 0.795 | 17.4 ± 10.8 | 18.6 ± 9.3 | 0.218 |
| CRP (mg/L) | 1.51 ± 2.38 | 1.52 ± 2.73 | 0.975 | 1.17 ± 1.57 | 1.33 ± 2.30 | 0.471 |
ALT, alanine aminotransferase; ASCVD, atherosclerotic cardiovascular disease, defined as myocardial infarction, angina pectoris, peripheral artery disease, or cerebrovascular disease; AST, aspartate aminotransferase; BMI, body mass index; BUN, blood urea nitrogen; CRP, C‐reactive protein; DBP, diastolic blood pressure; HbA1c, glycated haemoglobin; HDL‐C, high‐density lipoprotein‐cholesterol; HOMA‐IR, homeostasis model assessment of insulin resistance; LDL‐C, low‐density lipoprotein‐cholesterol; MNA‐SF, mini nutritional assessment‐short form; SBP, systolic blood pressure.
Data are shown as mean ± standard deviation, interquartile ranges, or number (percentage). Alcohol (%), number (%) of men who drink alcohol one or more times a month; moderate to high physical activity was assessed using the International Physical Activity Questionnaire short form.
P value is for the T‐test of the log transformed value.
Whereas the total physical activity levels were higher in men than women regardless of progression to sarcopenia, the proportion of participants in the moderate to high physical activity group was significantly lower only in women out of those who developed sarcopenia (Tables 1 and S3). The proportion of participants who reported to be engaged in RT twice or more per week were lower only in men out of those who developed sarcopenia. The baseline MNA‐SF was lower only in women who developed sarcopenia compared with those who remained nonsarcopenic.
Notably, the men who developed sarcopenia at follow‐up had higher fasting glucose, glycated haemoglobin (HbA1c), and HOMA‐IR values at baseline. In contrast, these variables were similar in women regardless of any development of sarcopenia at follow‐up (Table 1).
Comparison of the changes in muscle mass and function according to incident sarcopenia
In conformity with the definition of sarcopenia, participants who developed sarcopenia at the 2 year follow‐up had lower muscle mass and strength at baseline (Table 2). In men, those who developed sarcopenia at follow‐up had had worse scores on all physical performances including gait speed, SPPB, TUG test, and five‐times sit‐to‐stand test at baseline. In women, gait speed, TUG test, and five‐times sit‐to‐stand test had been significantly inferior at baseline in those who developed sarcopenia at the follow‐up.
Table 2.
Biennial changes in muscle mass, strength, and physical performance
| Incident sarcopenia (+) | Incident sarcopenia (−) | |||||||
|---|---|---|---|---|---|---|---|---|
| 2016–2017 | 2018–2019 | P a | 2016–2017 | 2018–2019 | P a | P b | P c | |
| Men | ||||||||
| ASM/height2 (kg/m2) | 7.04 ± 0.75 | 6.31 ± 0.55 | <0.001 | 7.31 ± 0.80 | 7.03 ± 0.75 | <0.001 | <0.001 | 0.111 |
| Hand grip strength (kg) | 30.4 ± 4.2 | 28.2 ± 5.2 | <0.001 | 34.4 ± 5.1 | 34.4 ± 5.2 | 0.635 | <0.001 | 0.013 |
| Gait speed (m/s) | 1.07 ± 0.21 | 0.99 ± 0.21 | <0.001 | 1.25 ± 0.25 | 1.23 ± 0.20 | 0.058 | 0.006 | 0.785 |
| SPPB (score) | 11.0 ± 1.2 | 10.7 ± 1.4 | 0.015 | 11.4 ± 0.9 | 11.5 ± 0.9 | 0.229 | 0.007 | 0.536 |
| Timed up and go test (s) | 10.5 ± 2.3 | 12.5 ± 2.9 | 0.015 | 9.6 ± 2.1 | 10.0 ± 2.3 | <0.001 | <0.001 | 0.782 |
| Five‐times sit‐to‐stand test (s) | 10.8 ± 2.8 | 11.5 ± 3.3 | 0.034 | 10.0 ± 2.9 | 9.5 ± 2.7 | <0.001 | 0.004 | 0.852 |
| Women | ||||||||
| ASM/height2 (kg/m2) | 5.58 ± 0.55 | 4.99 ± 0.33 | <0.001 | 6.02 ± 0.68 | 5.83 ± 0.63 | <0.001 | <0.001 | — |
| Hand grip strength (kg) | 19.2 ± 3.7 | 18.2 ± 3.3 | 0.002 | 22.0 ± 3.7 | 21.9 ± 3.9 | 0.305 | 0.008 | — |
| Gait speed (m/s) | 1.03 ± 0.20 | 0.94 ± 0.19 | <0.001 | 1.13 ± 0.23 | 1.11 ± 0.21 | 0.003 | 0.003 | — |
| SPPB (score) | 10.8 ± 1.5 | 10.4 ± 1.6 | 0.031 | 10.9 ± 1.4 | 10.9 ± 1.5 | 0.614 | 0.135 | — |
| Timed up and go test (s) | 11.2 ± 3.5 | 13.0 ± 4.6 | <0.001 | 10.1 ± 2.3 | 10.8 ± 2.8 | <0.001 | <0.001 | — |
| Five‐times sit‐to‐stand test (s) | 12.4 ± 4.7 | 13.1 ± 5.8 | 0.127 | 11.4 ± 3.5 | 11.1 ± 3.7 | 0.040 | 0.073 | — |
ASM, appendicular skeletal muscle mass; SPPB, short physical performance battery.
Data are shown as mean ± standard deviation.
P statistical significance between the baseline value (2016–2017) and the follow‐up value (2018–2019).
P for interaction of time and sarcopenic status.
P for interaction of time and sex in participants with incident sarcopenia.
The extent of muscle mass decline was considerably higher in participants who developed sarcopenia compared with those who did not (Figure 2 and Table 2). All muscle function parameters deteriorated more expeditiously in men with incident sarcopenia compared with men without; HGS, gait speed, and TUG test retrogressed more significantly in women who developed sarcopenia compared with women who did not develop sarcopenia, but the SPPB and five‐times sit‐to‐stand test did not. Intriguingly, the rate of muscle mass decline over the 2 years of follow‐up was similar between genders in participants with incident sarcopenia. Similar findings were observed for physical performances except HGS.
Figure 2.
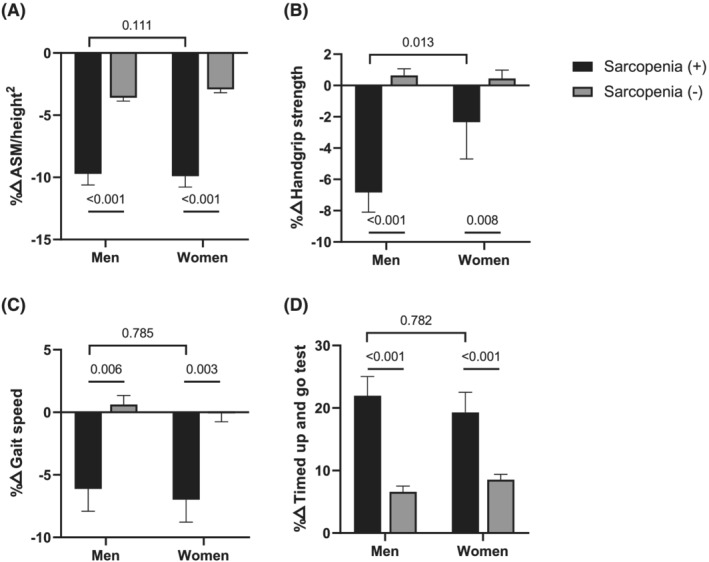
Changes in muscle mass and function according to the development of incident sarcopenia by gender. Black rectangles and grey rectangles indicate incident sarcopenia and no sarcopenia, respectively. (A) Relative percentage change of appendicular skeletal muscle mass (ASM) divided by height2. (B) Relative percentage change of hand grip strength. (C) Relative percentage change of gait speed. (D) Relative percentage change in the timed up and go test.
The only parameter that differed in the magnitude of reduction between men and women was HGS; it was greater in men than women with incident sarcopenia (P = 0.013; Table 2).
Risk factors for the development of sarcopenia
To investigate the potential determinants of sarcopenia, logistic regression was performed with the variables presumed to contribute to the progression to sarcopenia along with the established risk factors. 2 The following variables were selected for analysis: age, CCI, MNA‐SF, moderate to high physical activity, active RT, Ln(HOMA‐IR), and HbA1c. In the univariable analysis, age, CCI, active RT, Ln(HOMA‐IR), and HbA1c contributed to the development of sarcopenia in men (Table 3). In women, age, MNA‐SF, and moderate to high physical activity were associated with incident sarcopenia, but not CCI, active RT, or Ln(HOMA‐IR).
Table 3.
Logistic regression of variables associated with incident sarcopenia
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Men | ||||
| Age | 1.18 (1.11–1.25) | <0.001 | 1.17 (1.10–1.25) | <0.001 |
| Charlson comorbidity index | 1.23 (1.02–1.48) | 0.026 | 1.15 (0.94–1.42) | 0.172 |
| MNA‐SF | 1.05 (0.89–1.24) | 0.539 | 1.04 (0.87–1.25) | 0.678 |
| Moderate to high physical activity | 0.61 (0.33–1.13) | 0.115 | 0.84 (0.43–1.63) | 0.604 |
| Resistance training (≥2 per week) | 0.48 (0.30–0.76) | 0.002 | 0.50 (0.30–0.82) | 0.006 |
| Ln(HOMA‐IR) | 1.45 (1.05–1.99) | 0.022 | 1.26 (0.87–1.82) | 0.222 |
| HbA1c | 1.35 (1.07–1.70) | 0.010 | 1.15 (0.86–1.54) | 0.335 |
| Women | ||||
| Age | 1.15 (1.09–1.21) | <0.001 | 1.14 (1.08–1.21) | <0.001 |
| Charlson comorbidity index | 1.14 (0.93–1.40) | 0.207 | 1.08 (0.86–1.34) | 0.507 |
| MNA‐SF | 0.77 (0.68–0.87) | <0.001 | 0.79 (0.70–0.90) | <0.001 |
| Moderate to high physical activity | 0.52 (0.33–0.84) | 0.007 | 0.57 (0.34–0.95) | 0.030 |
| Resistance training (≥2 per week) | 0.63 (0.37–1.09) | 0.096 | 0.72 (0.41–1.26) | 0.249 |
| Ln(HOMA‐IR) | 0.85 (0.61–1.19) | 0.346 | 0.79 (0.54–1.16) | 0.231 |
| HbA1c | 1.05 (0.82–1.35) | 0.698 | 1.06 (0.80–1.40) | 0.701 |
CI, confidence interval; HbA1c, glycated haemoglobin; HOMA‐IR, homeostasis model assessment of insulin resistance; MNA‐SF, mini nutritional assessment‐short form; Moderate to high physical activity, 600 MET‐min/week or higher score in the International Physical Activity Questionnaire short form; OR, odds ratio.
When concurrently adjusted for multiple variables, the significance for CCI, Ln(HOMA‐IR), and HbA1c were attenuated, and only age and active RT were significant in men (Table 3). In women, age, MNA‐SF, and moderate to high physical activity all remained as independent risk factors for progression to sarcopenia in the fully adjusted model.
Next, we classified the men without sarcopenia at baseline based on the frequency of RT: individuals who performed one or less sessions of RT per week as the ‘Infrequent RT’ group (n = 453, 60%) and those who engaged in two or more sessions per week as the ‘Frequent RT’ group (n = 302, 40%) (Figure 3 and Table S4). Men in the Infrequent RT group had lower HGS, SPPB scores, and functional mobility measured by the five‐times sit‐to‐stand test whereas their muscle masses were similar at baseline, and they developed sarcopenia at a significantly higher rate compared with men in the Frequent RT group (16.7% vs. 8.7%; P = 0.002).
Figure 3.
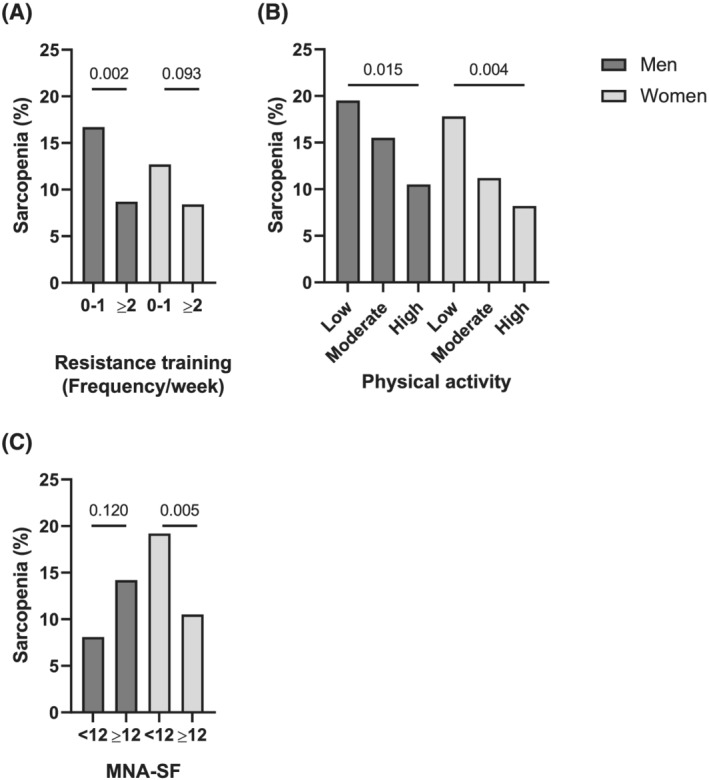
Incidence of sarcopenia according to risk factors by gender. Dark grey rectangles and light grey rectangles indicate men and women who developed incident sarcopenia, respectively. (A) Men who are engaged in resistance training for 0–1 times per week vs. ≥2 times per week. (B) Men and women grouped according to low/moderate/high levels of physical activity. (C) Women with mini nutritional assessment‐short form (MNA‐SF) of <12 vs. ≥12.
The decreasing trend in the prevalence of incident sarcopenia was observed for higher physical activity levels in both men and women, with statistical significance retained for women in the subgroup analysis (men, P for trend = 0.015; women, P for trend = 0.004; Table S5). Despite the decreasing trend for BMI and waist circumference with higher physical activity levels, baseline muscle mass indices as well as MNA‐SF screening scores were similar between the groups. Baseline muscle strength and physical performances were better preserved in men and women with higher physical activity levels. When women were categorized according to the MNA‐SF screening score of 12 or higher (Well‐nourished) and <12 (Malnutrition at risk), the latter indubitably demonstrated lower BMI values; however, similar baseline muscle mass indices were observed between the two groups (Table S6). In line with other indices, baseline muscle strength and physical performance were inferior for women in the Malnutrition at risk group and were antecedents of incident sarcopenia.
Discussion
Sarcopenia poses a serious threat to independence and quality of life in elderly populations, which could lead to major healthcare burdens. In this longitudinal cohort study at 2 year follow‐up, we found 13.5% and 11.7% of incident sarcopenia in men and women. Amendable determinants differed for the development of new sarcopenia in older Korean persons by gender: thus, an RT of <2 times per week was a risk factor for the development of sarcopenia in men; in women, malnutrition and physical inactivity played a role in the progression to sarcopenia.
Gender differences in the prevalence of sarcopenia using various definitions were reported recently using cross‐sectional data of the KFACS cohort. 10 , 19 , 20 Accordingly, all analyses were stratified by gender in this study. However, studies on the prevalence of new onset sarcopenia are scarce, and to the best of our knowledge, none have investigated gender‐based differences in incident sarcopenia. The cumulative incidence of sarcopenia was 13.7% during the 12 year follow‐up of the English Longitudinal Study of Ageing (ELSA) with participants with a mean age of 69.1 ± 6.7 years, 21 13.7% during the 4 year follow‐up of the sarcopenia and physical impairment with advancing age (SarcoPhAge) cohort with participants with a mean age of 72.5 ± 5.8 years, 22 and 3.6% during the 6.7 year follow‐up of Taiwanese participants with a mean age of 60.5 years. 23 The prevalence of incident sarcopenia varied considerably by age, ethnicity, comorbidities, and the follow‐up duration. Of note, the threshold for diagnosing sarcopenia were heterogeneous: the sum of only the low HGS and low skeletal muscle mass was applied for the ELSA and the SarcoPhAge study. In the Taiwanese study, the diagnosis of sarcopenia was made based on an insurance claim database, which might explain the particularly low incidence rates of new onset sarcopenia, as the validity of the diagnosis codes for sarcopenia is unknown. The rate of 2 year incident sarcopenia using the AWGS 2019 criteria was 13.5% and 11.7% for men and women in this KFACS cohort, respectively, but this incidence did not differ between men and women during the 2 years of follow‐up.
Here, the rate of decline in muscle mass did not differ between men and women who developed incident sarcopenia. This finding was contrary to those of previous longitudinal studies that demonstrated that age‐related decline in height‐adjusted muscle mass is less pronounced in women than in men. 24 , 25 Decreases in physical performances were also similar between men and women over the 2 years although a longer follow‐up is warranted. Of note, the biennial decline in HGS was higher in men than in women. More expeditious hand grip weakness in men in comparison with women is in concordance with a 22 year follow‐up study that evaluated the accelerated decline in HGS in men 26 and a 4 year prospective study from China that revealed a particularly faster decline in HGS compared with other measures for sarcopenia. 27
While the effectiveness of RT to combat muscle wasting and weakness is legitimately underscored by a series of landmark randomized clinical studies, 28 , 29 , 30 few epidemiological studies conducted in the field of geriatric medicine have revealed RT as a major modifiable risk factor for preventing sarcopenia. A plausible explanation is that information regarding RT might not have been explored extensively through cohort studies. Here, we demonstrated that the most important modifiable factor in the development of sarcopenia in elderly men was engaging in RT at least two times per week. Importance of RT is recently being revitalized, as it has been proposed that while aerobic exercise may attenuate the loss of skeletal muscle during energy restriction in obese adults, a single mode of aerobic exercise alone does not promote muscle hypertrophy to the same extent as RT. 31 , 32 , 33 , 34 , 35 Along the same line, while there was an increasing trend in the onset of sarcopenia according to a decrease in the physical activity level, this association was modest and did not independently contribute to the development of sarcopenia in men.
In the current study, men with or without the development of sarcopenia were relatively insulin sensitive and nonobese at baseline. That this cohort mainly consisted of metabolically healthy phenotypes partly explains why higher fasting glucose, HbA1c, and HOMA‐IR values only demonstrated meagre roles in the progression to sarcopenia. Furthermore, we adopted ASM/height2 as a muscle mass index in this study, as recommended by the contemporary AWGS guidelines, but it has been implied that muscle mass index adjusted by body weight is more closely associated with insulin resistance when compared with ASM/height2. 36 The effects of hyperglycaemia and insulin resistance were completely abolished after adjusting for potential risk factors that could negatively influence muscle mass and function, belying the apparent association shown with sarcopenia development in the crude analysis.
In women, insulin resistance, comorbidities, and engagement in RT had negligible effects on sarcopenia development. On the contrary, malnutrition conferred an appreciable risk, consistent with a previous cross‐sectional study. 37 This is in line with a 9 week study that provided either an adequate‐protein or a low‐protein diet to groups of healthy elderly women. 38 Despite the small sample size, women fed with the low‐protein diet had negative nitrogen balance and this resulted in worse functional capacity, while those in the adequate‐protein diet group were able to maintain both a lean body mass and muscle function. In addition, accumulating evidence suggests that immobility induces anabolic resistance and that increasing physical activity might enhance the muscle protein anabolic response upon protein intake by improving blood flow and nutrient delivery to muscle. 39 In this cohort, self‐reported physical activity levels were considerably lower in women than men. Whether there is a threshold level of physical inactivity that could impair functional capacity in older adults should be elucidated; however, there is a possibility that sedentary behaviour in older Korean women might have been closely linked to the deteriorations in muscle indices. Collectively, avoiding malnutrition and being physically active could be fundamental modifiable factors to preserve muscle mass and function in Korean older women.
It should be noted that in women, the rate of decline in SPPB and five‐times sit‐to‐stand test did not differ regarding incident sarcopenia status. Musculoskeletal complaints linked to less competent use of knee extensor muscles may have overtaken the sole impact of sarcopenia on scoring of the SPPB and five‐times sit‐to‐stand test. Indeed, the prevalence of osteoarthritis was higher in women compared with men in this study. 40
This study had some inherent limitations. First, our analysis was conducted after the first biennial visit of this cohort, which made the duration of follow‐up 2 years. A longer follow‐up might distinguish the currently subtle gender‐based differences in the annual deterioration rate of muscle mass and function, as well as detecting other potential factors that may contribute to the development of sarcopenia. Second, as most participants in this cohort had relatively low HOMA‐IR values, the impact of insulin resistance might have been underestimated. Third, the frequency of RT and physical activity levels were based on a self‐reported questionnaire, and individual intensities and durations of exercise may have been diverse. While admitting that the optimal intensity of RT is yet to be determined, RT, especially if performed by physically frail elderly persons who have been habitually sedentary, should be individualized periodically.
With these caveats in mind, we surmise that understanding gender‐based differences of potential modifiable factors in progression to sarcopenia is imperative. An adequate amount of RT may be a safeguard to elderly men in retaining muscle mass and function. Performing moderate to high physical activity and improving nutritional status could be effective strategies for preventing incident sarcopenia in women.
Funding
This study was supported by a grant from the Korea Health Technology R&D Project through the Korean Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant numbers: HI15C3153 and HI15C3207), and the Research Program funded by the National Institute of Health, Korea Disease Control and Prevention Agency (2021‐ER0605‐00).
Conflict of interest
None declared.
Supporting information
Table S1. Characteristics of participants who were not included in the current analysis
Table S2. Biennial changes of muscle mass and function in participants with follow‐up examination
Table S3. Comparison of physical activity levels according to incident sarcopenia
Table S4. Clinical characteristics and muscle indices at baseline according to the frequency of resistance training
Table S5. Clinical characteristics and muscle indices at baseline according to physical activity levels
Table S6. Clinical characteristics and muscle indices at baseline according to nutritional status
Acknowledgements
We would like to thank all the participants of this study and the staff of the KFACS field centres who helped with the recruitment of participants. The authors acknowledge Division of Statistics in Medical Research Collaborating Center at Seoul National University Bundang Hospital for assistance in statistical analysis.
Choe H. J., Cho B. L., Park Y. S., Roh E., Kim H. J., Lee S.‐G., Kim B. J., Kim M., Won C. W., Park K. S., and Jang H. C. (2022) Gender differences in risk factors for the 2 year development of sarcopenia in community‐dwelling older adults, Journal of Cachexia, Sarcopenia and Muscle, 13, 1908–1918, 10.1002/jcsm.12993
Contributor Information
Chang Won Won, Email: chunwon@khmc.or.kr.
Hak Chul Jang, Email: janghak@snu.ac.kr.
References
- 1. KOSTAT . Explore Korea through Statistics 2020. https://kostat.go.kr/portal/korea/kor_nw/1/1/index.board?bmode=read&aSeq=403253. Accessed 09 Jan 2022.
- 2. Cruz‐Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Batsis JA, Villareal DT. Sarcopenic obesity in older adults: aetiology, epidemiology and treatment strategies. Nat Rev Endocrinol 2018;14:513–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cruz‐Jentoft AJ, Sayer AA. Sarcopenia. Lancet 2019;393:2636–2646. [DOI] [PubMed] [Google Scholar]
- 5. Gallagher D, Visser M, De Meersman RE, Sepulveda D, Baumgartner RN, Pierson RN, et al. Appendicular skeletal muscle mass: effects of age, gender, and ethnicity. J Appl Physiol (1985) 1997;83:229–239. [DOI] [PubMed] [Google Scholar]
- 6. Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci 2006;61:1059–1064. [DOI] [PubMed] [Google Scholar]
- 7. Visser M, Pahor M, Tylavsky F, Kritchevsky SB, Cauley JA, Newman AB, et al. One‐ and two‐year change in body composition as measured by DXA in a population‐based cohort of older men and women. J Appl Physiol (1985) 2003;94:2368–2374. [DOI] [PubMed] [Google Scholar]
- 8. Hughes VA, Frontera WR, Roubenoff R, Evans WJ, Singh MA. Longitudinal changes in body composition in older men and women: role of body weight change and physical activity. Am J Clin Nutr 2002;76:473–481. [DOI] [PubMed] [Google Scholar]
- 9. Won CW, Lee Y, Choi J, Kim KW, Park Y, Park H, et al. Starting construction of frailty cohort for elderly and intervention study. Ann Geriatr Med Res 2016;20:114–117. [Google Scholar]
- 10. Kang S, Oh TJ, Cho BL, Park YS, Roh E, Kim HJ, et al. Sex differences in sarcopenia and frailty among community‐dwelling Korean older adults with diabetes: the Korean Frailty and Aging Cohort Study. J Diabetes Investig 2021;12:155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self‐reported disability and prediction of mortality and nursing home admission. J Gerontol 1994;49:M85–M94. [DOI] [PubMed] [Google Scholar]
- 12. Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc 2020;21:300–307, e2. [DOI] [PubMed] [Google Scholar]
- 13. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 14. Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, et al. International Physical Activity Questionnaire: 12‐country reliability and validity. Med Sci Sports Exerc 2003;35:1381–1395. [DOI] [PubMed] [Google Scholar]
- 15. Fragala MS, Cadore EL, Dorgo S, Izquierdo M, Kraemer WJ, Peterson MD, et al. Resistance training for older adults: position statement from the National Strength and Conditioning Association. J Strength Cond Res 2019;33:2019–2052. [DOI] [PubMed] [Google Scholar]
- 16. Vellas B, Guigoz Y, Garry PJ, Nourhashemi F, Bennahum D, Lauque S, et al. The Mini Nutritional Assessment (MNA) and its use in grading the nutritional state of elderly patients. Nutrition 1999;15:116–122. [DOI] [PubMed] [Google Scholar]
- 17. Rubenstein LZ, Harker JO, Salva A, Guigoz Y, Vellas B. Screening for undernutrition in geriatric practice: developing the short‐form mini‐nutritional assessment (MNA‐SF). J Gerontol A Biol Sci Med Sci 2001;56:M366–M372. [DOI] [PubMed] [Google Scholar]
- 18. Won CW, Lee S, Kim J, Chon D, Kim S, Kim CO, et al. Korean Frailty and Aging Cohort Study (KFACS): cohort profile. BMJ Open 2020;10:e035573. 10.1136/bmjopen-2019-035573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim S, Kim M, Won CW. Validation of the Korean version of the SARC‐F questionnaire to assess sarcopenia: Korean Frailty and Aging Cohort Study. J Am Med Dir Assoc 2018;19:40–45, e1. [DOI] [PubMed] [Google Scholar]
- 20. Kim M, Won CW. Sarcopenia in Korean community‐dwelling adults aged 70 years and older: application of screening and diagnostic tools from the Asian Working Group for Sarcopenia 2019 update. J Am Med Dir Assoc 2020;21:752–758. [DOI] [PubMed] [Google Scholar]
- 21. Veronese N, Smith L, Cereda E, Maggi S, Barbagallo M, Dominguez LJ, et al. Multimorbidity increases the risk for sarcopenia onset: longitudinal analyses from the English Longitudinal Study of Ageing. Exp Gerontol 2021;156:111624. 10.1016/j.exger.2021.111624 [DOI] [PubMed] [Google Scholar]
- 22. Beaudart C, Sanchez‐Rodriguez D, Locquet M, Reginster JY, Lengele L, Bruyere O. Malnutrition as a strong predictor of the onset of sarcopenia. Nutrients 2019;11 (12):2883. 10.3390/nu11122883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lin MH, Chiu SY, Chang PH, Lai YL, Chen PC, Ho WC. Hyperlipidemia and statins use for the risk of new diagnosed sarcopenia in patients with chronic kidney: a population‐based study. Int J Environ Res Public Health 2020;17:1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu LK, Lee WJ, Liu CL, Chen LY, Lin MH, Peng LN, et al. Age‐related skeletal muscle mass loss and physical performance in Taiwan: implications to diagnostic strategy of sarcopenia in Asia. Geriatr Gerontol Int 2013;13:964–971. [DOI] [PubMed] [Google Scholar]
- 25. Shimokata H, Ando F, Yuki A, Otsuka R. Age‐related changes in skeletal muscle mass among community‐dwelling Japanese: a 12‐year longitudinal study. Geriatr Gerontol Int 2014;14:85–92. [DOI] [PubMed] [Google Scholar]
- 26. Stenholm S, Harkanen T, Sainio P, Heliovaara M, Koskinen S. Long‐term changes in handgrip strength in men and women—accounting the effect of right censoring due to death. J Gerontol A Biol Sci Med Sci 2012;67:1068–1074. [DOI] [PubMed] [Google Scholar]
- 27. Auyeung TW, Lee SW, Leung J, Kwok T, Woo J. Age‐associated decline of muscle mass, grip strength and gait speed: a 4‐year longitudinal study of 3018 community‐dwelling older Chinese. Geriatr Gerontol Int 2014;14:76–84. [DOI] [PubMed] [Google Scholar]
- 28. Fiatarone MA, Marks EC, Ryan ND, Meredith CN, Lipsitz LA, Evans WJ. High‐intensity strength training in nonagenarians. Effects on skeletal muscle. JAMA 1990;263:3029–3034. [PubMed] [Google Scholar]
- 29. Fiatarone MA, O'Neill EF, Ryan ND, Clements KM, Solares GR, Nelson ME, et al. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med 1994;330:1769–1775. [DOI] [PubMed] [Google Scholar]
- 30. Singh NA, Quine S, Clemson LM, Williams EJ, Williamson DA, Stavrinos TM, et al. Effects of high‐intensity progressive resistance training and targeted multidisciplinary treatment of frailty on mortality and nursing home admissions after hip fracture: a randomized controlled trial. J Am Med Dir Assoc 2012;13:24–30. [DOI] [PubMed] [Google Scholar]
- 31. Yoshimura E, Kumahara H, Tobina T, Matsuda T, Watabe K, Matono S, et al. Aerobic exercise attenuates the loss of skeletal muscle during energy restriction in adults with visceral adiposity. Obes Facts 2014;7:26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Grgic J, McLlvenna LC, Fyfe JJ, Sabol F, Bishop DJ, Schoenfeld BJ, et al. Does aerobic training promote the same skeletal muscle hypertrophy as resistance training? A systematic review and meta‐analysis. Sports Med 2019;49:233–254. [DOI] [PubMed] [Google Scholar]
- 33. Villareal DT, Aguirre L, Gurney AB, Waters DL, Sinacore DR, Colombo E, et al. Aerobic or resistance exercise, or both, in dieting obese older adults. N Engl J Med 2017;376:1943–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Colleluori G, Aguirre L, Phadnis U, Fowler K, Armamento‐Villareal R, Sun Z, et al. Aerobic plus resistance exercise in obese older adults improves muscle protein synthesis and preserves myocellular quality despite weight loss. Cell Metab 2019;30:261–273, e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Waters DL, Aguirre L, Gurney AB, Sinacore DR, Fowler K, Gregori G, et al. Effect of aerobic or resistance exercise, or both, on intermuscular and visceral fat and physical and metabolic function in older adults with obesity while dieting. J Gerontol A Biol Sci Med Sci 2022;77:131–139. 10.1093/gerona/glab111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lim S, Kim JH, Yoon JW, Kang SM, Choi SH, Park YJ, et al. Sarcopenic obesity: prevalence and association with metabolic syndrome in the Korean Longitudinal Study on Health and Aging (KLoSHA). Diabetes Care 2010;33:1652–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tay L, Ding YY, Leung BP, Ismail NH, Yeo A, Yew S, et al. Sex‐specific differences in risk factors for sarcopenia amongst community‐dwelling older adults. Age (Dordr) 2015;37:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Castaneda C, Charnley JM, Evans WJ, Crim MC. Elderly women accommodate to a low‐protein diet with losses of body cell mass, muscle function, and immune response. Am J Clin Nutr 1995;62:30–39. [DOI] [PubMed] [Google Scholar]
- 39. Burd NA, Gorissen SH, van Loon LJ. Anabolic resistance of muscle protein synthesis with aging. Exerc Sport Sci Rev 2013;41:169–173. [DOI] [PubMed] [Google Scholar]
- 40. Kim M, Won CW. Prevalence of sarcopenia in community‐dwelling older adults using the definition of the European Working Group on Sarcopenia in Older People 2: findings from the Korean Frailty and Aging Cohort Study. Age Ageing 2019;48:910–916. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Characteristics of participants who were not included in the current analysis
Table S2. Biennial changes of muscle mass and function in participants with follow‐up examination
Table S3. Comparison of physical activity levels according to incident sarcopenia
Table S4. Clinical characteristics and muscle indices at baseline according to the frequency of resistance training
Table S5. Clinical characteristics and muscle indices at baseline according to physical activity levels
Table S6. Clinical characteristics and muscle indices at baseline according to nutritional status


