Abstract
Background
Efforts to enhance diagnostic measures for sarcopenia have led to an increased focus on the screening utility of blood‐based biomarkers. In this regard, circulating neurofilament light chain (NfL) levels are a potent indicator of axonal damage and have been linked with several neurological disorders. However, despite the strong neurogenic contribution to skeletal muscle health, no studies have explored the relevance of NfL concentrations to sarcopenia. With that in mind, this study aimed to examine the association between plasma NfL concentration and sarcopenic domains.
Methods
Three hundred adults aged between 50 and 83 years participated to this study (male participants, n = 150; mean age: 64.2 ± 8.7 years and female participants, n = 150; mean age: 63.9 ± 8.3 years). Body composition was assessed using dual‐energy X‐ray absorptiometry, and a skeletal muscle index (SMI) was calculated. Muscle strength was assessed with hand dynamometry. Sarcopenia was classified using the European Working Group on Sarcopenia in Older People criteria. Plasma NfL concentration was determined using a highly sensitive, enzyme‐linked immunosorbent assay.
Results
Neurofilament light chain levels were associated with grip strength and SMI (P = 0.005 and P = 0.045, respectively) and were significantly elevated in sarcopenic individuals, compared with non‐sarcopenic participants (P < 0.001). Individuals with pre‐sarcopenia (either low grip strength or low SMI) had significantly higher NfL levels, compared with healthy controls (P = 0.001 and P = 0.006, respectively). Male participants with either low grip strength or low SMI had significantly raised NfL levels (P = 0.006 and P = 0.002, respectively), while in female participants, NfL concentrations were significantly elevated only in those with low grip strength (P = 0.049). NfL concentration displayed acceptable diagnostic accuracy for sarcopenia (area under the curve = 0.726, P < 0.001).
Conclusions
Our study clearly demonstrates the indicative pertinence of circulating NfL levels to sarcopenic domains, supporting its potential use as a biomarker of sarcopenia. More studies are needed, however, to further illuminate the diagnostic value of circulating NfL. Future research should explore whether NfL levels are more powerfully linked with muscle strength than mass and whether sex mediates the relevance of NfL concentrations to sarcopenic phenotypes.
Keywords: Muscle wasting, Muscle strength, Sarcopenia, Screening, Diagnosis, Biomarker
Introduction
‘Sarcopenia’, a geriatric syndrome diagnosed according to the presence of low muscle mass and strength, 1 , 2 is a major public health concern due to its high prevalence and adverse health, and economic consequences. 3 In particular, sarcopenia is a principal contributor to the development of cognitive and mobility impairments, 4 , 5 which ultimately lead to a loss of independence and institutionalization among older populations. 6 Despite advances in knowledge in recent years, the prevalence of sarcopenia remains high, affecting over 40% of those aged ≥80 years. 3 This prevalence, coupled with the array of unfavourable correlates, demonstrates the urgent need to establish proficient screening and therapeutic strategies. Currently, the incorporation of diagnostic and treatment measures into standard medical practice is primarily impeded by two critical factors.
The first, and perhaps most important factor, is the absence of a universally accepted operational definition for sarcopenia. The current ambiguity among definitions is driven by efforts to enhance the ability of the sarcopenic phenotype in identifying individuals with an increased risk of clinical outcomes such as falls, functional disability, and hospitalization. In that regard, although low muscle mass has been a primary component of early definitions, 1 more recently, muscle strength has gained credence as a cardinal criterion. 7 This change in focus is supported by recent findings demonstrating that muscle strength has a strong diagnostic and prognostic value for multiple clinical outcomes, while the relevance of muscle mass to such outcomes has been questioned. 7 , 8 Nonetheless, despite such recent advances in knowledge, there remains uncertainty surrounding an ‘optimal’ definition, with the need to establish a consensus definition a high priority.
The second limiting factor relates to difficulties in ascertaining precise muscle mass and strength data, the two most frequently studied phenotypes of sarcopenia. Despite ongoing debates surrounding a ‘gold standard’ imaging technique, methods such as dual‐energy X‐ray absorptiometry (DXA), magnetic resonance imaging, and computed tomography are frequently used to assess lean mass. 9 While these techniques are suitably reliable, intermodal disparities, relating to the underpinning algorithms by which lean mass is estimated, 9 impede the potential for data comparison. Additionally, these techniques are costly, and not readily available in community settings, further limiting their practicality in routine clinical assessments. Similarly, although hand dynamometry is a well‐established proxy for muscle strength, the precision of measurement can be strongly affected by comorbidities and joint disorders, 10 which are prevalent in sarcopenic populations. In light of this, the efficacy of such methods for muscle mass and strength quantification is limited. However, blood‐based biomarkers may provide an alternative and readily accessible screening method for sarcopenia, 11 potentially facilitating a more consistent implementation of preservative measures targeting skeletal muscle.
Although the aetiological mechanisms driving sarcopenia are considered to be multifactorial, 12 , 13 there is increasing evidence to suggest skeletal muscle health is powerfully mediated by neurological processes, 14 , 15 particularly in older adulthood. Similarly, although sarcopenia assessment is often a multifaceted process involving several skeletal muscle properties, decreased muscle strength remains a fundamental component. Interestingly, while myofibre atrophy was originally considered a primary contributor towards age‐related declines in muscle strength, 4 mounting evidence indicates this loss to be more potently modulated by neurodegenerative changes. 14 , 15 , 16 Hence, the regulatory pertinence of neurological processes for skeletal muscle integrity appears to be of central importance.
Although early studies reported a significant loss of cortical neurons (up to 50%) during ageing, 17 later evidence suggested that no more than 10% of total cortical neurons are likely to be lost. 18 Instead, increasing evidence of axonal degeneration and a progressive regression of the dendritic tree of cortical and lower motor neurons has emerged. 18 , 19 Hence, exploring the relevance of biomarkers of neuronal health for sarcopenia and sarcopenic phenotypes may be particularly beneficial.
In this regard, neurofilaments (NFs) have emerged as biomarkers of neurodegeneration, whereby increased circulating concentrations are indicative of neuronal damage and/or death. 20 NFs are structural proteins of the neuronal cytoskeleton that serve an indispensable role in mediating axonal integrity and nerve conduction velocity. 21 In adults, NFs consist of four main subunits, heavy‐chain (~200 kDa), medium‐chain (~150 kDa), light‐chain [neurofilament light chain (NfL)] (~70 kDa), and either a‐internexin (~66 kDa) in the central nervous system or peripherin (~57 kDa) in the peripheral nervous system (Figure 1). 21 Damage to, or degeneration of, axons causes a release of NFs into cerebrospinal fluid and blood, where they can be easily measured. 20 In particular, the pertinence of NfL as a blood‐based biomarker has been suggested, given its low molecular weight and corresponding enhanced diffusive capacity. 22
Figure 1.
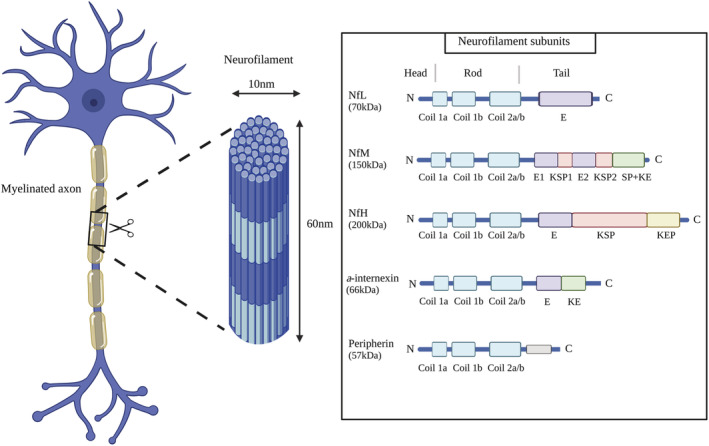
Neurofilament structure and subunits. E, glutamic acid‐rich segments; KE, lysine‐glutamic acid; KEP, lysine‐glutamic acid‐proline; KSP, lysine‐serine‐proline; NfH, neurofilament heavy chain; NfL, neurofilament light chain; NfM, neurofilament medium chain; SP, serine‐proline; created with BioRender.com.
Indeed, recent studies have demonstrated serum, or plasma NfL concentrations to be significantly elevated in patients with several neurological disorders, such as Alzheimer's disease, 23 Parkinson's disease, 24 multiple sclerosis, 25 and amyotrophic lateral sclerosis. 26 However, despite growing evidence of a strong neurological contribution to ageing muscle phenotypes, no studies have explored the potential relevance of plasma NfL concentration to sarcopenia. With that in mind, examining the relationship between NfL and sarcopenic phenotypes may help illuminate the importance of neural health in preserving skeletal muscle health, and ultimately, support the development of targeted screening and therapeutic protocols. Accordingly, this study aimed to assess the pertinence of plasma NfL concentration as a biomarker of muscle mass and strength in a large sample of healthy middle‐aged and older adults.
Methods
Participant characteristics
Participants were recruited as part of the GenoFit study, a large, dual‐site cross‐sectional study that took place in Ireland between September 2017 and October 2020 that aimed to examine the effect of genetics, fitness and lifestyle, on health. Three hundred participants aged between 50 and 83 years were randomly chosen from a pool of 1728 individuals aged ≥50 years (male participants, n = 150; mean age: 64.2 ± 8.7 years and female participants, n = 150; mean age: 63.9 ± 8.3 years). To be eligible, participants had to be aged ≥50 years, be free from musculoskeletal injuries and/or neurological pathologies and be able to provide informed consent. The study protocol was approved by University College Dublin's Human Research Ethics Committee, and written informed consent was obtained from all participants upon enrolment.
Anthropometry
Height and body mass were determined using a SECA (SECA, Hamburg, Germany) stadiometer and weighing scales, respectively. Participants were dressed lightly and without shoes. Body mass index (BMI) was defined as body mass divided by height squared (kg/m2).
Body composition analysis
Body composition was assessed using DXA (Lunar Prodigy, GE Healthcare Technologies, USA). Appendicular lean mass was calculated as the sum of lean mass of the arms and legs. Skeletal muscle index (SMI) was defined as appendicular lean mass divided by height squared (kg/m2). All DXA scans were referred by a registered physician.
Grip strength testing
Grip strength was assessed using a digital Jamar hand‐held dynamometer (JLW Instruments, Chicago, IL, USA) according to a previously described protocol. 8 Briefly, while standing, participants performed two maximal attempts with each hand with their arm positioned straight by their side. The dynamometer was adjusted for each participant so that a 90° angle was formed between the handle and middle phalanx. The mean of the greatest score from each hand was incorporated in the analysis.
Sarcopenia classification
Sarcopenia was classified according to the European Working Group on Sarcopenia in Older Persons (EWGSOP2) recommendations, while modified cut‐off points were established to ensure relevance to the study population. 2 Correspondingly, the presence of both low muscle strength and low SMI indicated sarcopenia, while the presence of either low muscle strength or low SMI indicated pre‐sarcopenia. Diagnostic thresholds were calculated using the sex‐specific lowest quintile of grip strength and SMI measurements from the study sample. With that in mind, low grip strength was defined as <32.95 kg and <20.40 kg, for male and female participants, respectively, and low SMI was defined as <7.86 kg/m2 and <5.91 kg/m2, for male and female participants, respectively.
Blood sampling and plasma neurofilament light chain determination
Trained phlebotomists collected blood samples by venepuncture of the median cubital vein and vacutainers containing the anticoagulant, ethylenediaminetetraacetic acid (BD Vacutainer®). Samples were processed according to a previously described protocol. 11 Briefly, samples were left to rest for 30 min, then centrifuged at 4000g for 10 min at 4°C. Plasma was then pipetted into aliquots and immediately stored at −80°C until analysis. Plasma NfL concentrations were determined using a commercially available, high sensitivity enzyme‐linked immunosorbent assay (ELISA) kit (#OKCD01380, Aviva Systems Biology, San Diego, CA, USA) according to the manufacturer's instructions. Briefly, 100 μL of standards and diluted sample (10‐fold dilution) were added to the pre‐coated microplate and incubated at 37°C for 60 min. Next, 100 μL of NfL detector antibody was added to each well and incubated at 37°C for a further 60 min. Then, after the microplate was washed three times, 100 μL of Avidin‐HRP Conjugate was added into each well and incubated at 37°C for 30 min. The microplate was then washed five times. Next, 90 μL of TMB substrate was added into each well and incubated at 37°C for 15 min in the dark. Finally, 50 μL of stop solution was added to each well, and the measurements were taken at 450 nm (CLARIOStar BMG Labtech Microplate reader).
Statistical analysis
Data are presented as mean ± standard deviation, unless stated otherwise. Plasma NfL was the only non‐normally distributed variable and was therefore, log transformed to achieve normality. Participant characteristics were compared according to sarcopenia status using a chi‐square test for categorical variables and analysis of variance with Tukey HSD post hoc analysis for continuous variables. Independent sample Student's t‐test was performed to assess differences in NfL concentrations between sexes. Pearson's correlation coefficient was used to examine the association between NfL levels and age. Multiple linear regression models were used to investigate the relationship between NfL concentrations, grip strength, and SMI, while adjusting for potential confounders, sex, age, and BMI. Analysis of covariance was performed to compare mean plasma NfL concentrations between those with sarcopenia or pre‐sarcopenia and healthy controls, with age and BMI as covariates. Finally, receiver operating characteristic (ROC) analyses were conducted to assess the clinical value of plasma NfL levels for diagnosing sarcopenia, low grip strength, and low SMI, with optimal cut‐off points determined by the greatest Youden's index. All statistical analyses were performed using the spss software (Version 26, IBM SPSS Inc., Chicago, IL, USA) with the exception of ROC analyses for which MedCalc was used (Version 20.011, MedCalc Software, Ostend, Belgium). The significance level was set at P < 0.05 for all tests.
Results
Study sample
Participant characteristics are presented in Table 1. Three hundred individuals aged between 50 and 83 years participated in this study (male participants, n = 150 and female participants, n = 150). Sarcopenia was identified in 22 participants (7.3% prevalence; male participants, n = 10 and female participants n = 12) while pre‐sarcopenia was recorded in 76 individuals (25.3% prevalence; male participants, n = 40 and female participants, n = 36; low SMI, n = 38 and low grip strength, n = 38). The remaining 202 participants were non‐sarcopenic. When compared with non‐sarcopenic subjects, those with sarcopenia displayed significantly lower body mass, BMI, SMI, and grip strength (all P < 0.001) and significantly higher concentrations of plasma NfL (P < 0.001). Those with pre‐sarcopenia had significantly lower body mass (P = 0.044), SMI and grip strength (both P < 0.001), and significantly higher plasma NfL levels (P = 0.006), compared with non‐sarcopenic subjects. Compared with pre‐sarcopenic individuals, those with sarcopenia displayed significantly lower body mass (P = 0.013), BMI (P = 0.001), SMI (P = 0.006) and grip strength (P = 0.038), and significantly higher plasma NfL concentrations (P = 0.035).
Table 1.
Participant characteristics according to sarcopenia status
| Total (n = 300) | Sarcopenic (n = 22) | Pre‐sarcopenic (n = 76) | Healthy (n = 202) | P value a | |
|---|---|---|---|---|---|
| Sociodemographic characteristics | |||||
| Age (years) | 64.0 ± 8.5 | 65.3 ± 10.8 | 67.3 ± 8.0 | 62.7 ± 8.1 | <0.001 |
| Sex | |||||
| Male, n (%) | 150 (50) | 10 (6.6) | 40 (26.7) | 100 (66.7) | 0.814 |
| Female, n (%) | 150 (50) | 12 (8.0) | 36 (24.0) | 102 (68.0) | |
| Anthropometric characteristics | |||||
| Height (cm) | 168.5 ± 9.7 | 166.7 ± 9.3 | 167.6 ± 9.8 | 169.1 ± 9.6 | 0.353 |
| Body mass (kg) | 74.6 ± 13.8 | 63.1 ± 9.8 | 72.3 ± 14.0 | 76.7 ± 13.4 | <0.001 |
| BMI (kg/m2) | 26.1 ± 3.6 | 22.6 ± 2.4 | 25.7 ± 4.2 | 26.7 ± 3.2 | <0.001 |
| Plasma biomarkers | |||||
| NfL (pg/mL) b | 24.5 [18.3–33.9] | 33.6 [28.6–42.1] | 25.3 [19.8–37.9] | 23.3 [17.3–31.9] | <0.001 |
| logNfL (pg/mL) | 1.40 ± 0.21 | 1.54 ± 0.15 | 1.45 ± 0.23 | 1.37 ± 0.20 | <0.001 |
| Sarcopenia phenotypes | |||||
| SMI (kg/m2) | 7.5 ± 1.3 | 6.2 ± 1.0 | 7.1 ± 1.2 | 7.8 ± 1.2 | <0.001 |
| Grip strength (kg) | 33.7 ± 11.6 | 22.4 ± 6.8 | 28.7 ± 10.0 | 36.7 ± 11.2 | <0.001 |
BMI, body mass index; NfL, neurofilament light chain; SMI, skeletal muscle index.
Between group differences assessed using chi‐squared test for categorical variables and analysis of variance for continuous variables.
Data presented as median and interquartile range.
Plasma neurofilament light chain levels, age, and sex
The association between plasma NfL concentrations, age, and sex is displayed in Figure 2. Significant positive correlations were observed between plasma NfL levels and age for both sexes, although a stronger correlation was noted for female participants (r = 0.355, P < 0.001 vs. r = 0.280, P = 0.001). Overall, male participants had significantly higher levels of plasma NfL compared with female participants (1.43 log pg/mL vs. 1.38 log pg/mL, P = 0.039).
Figure 2.
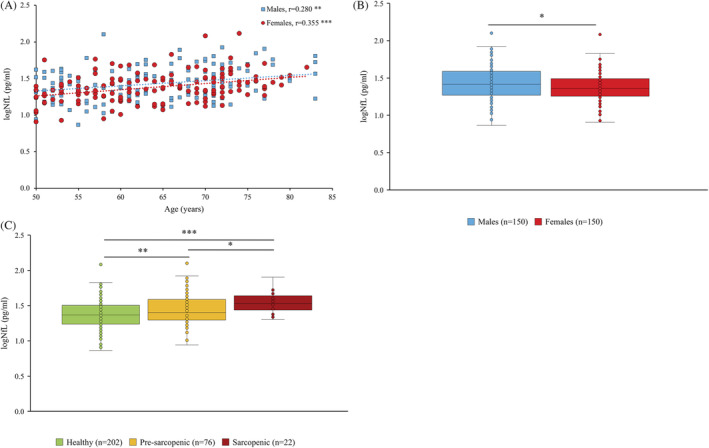
Association between plasma neurofilament light chain (NfL) levels and age (A), NfL levels and sex (B) and NfL levels and sarcopenia status (C) (*P < 0.05, **P < 0.01, ***P < 0.001).
Plasma neurofilament light chain levels, muscle mass, and strength
Multiple regression revealed significant negative associations between NfL levels and SMI (P = 0.045) and NfL levels and grip strength (P = 0.005), while controlling for potential confounders including sex, age, and BMI (Table 2). Interestingly, plasma NfL levels were also associated with dichotomisations of the individual sarcopenic phenotypes. Overall, those with either low grip strength, or low SMI displayed significantly raised NfL concentrations, compared with healthy controls (1.49 log pg/mL vs. 1.39 log pg/mL, P = 0.001 and 1.48 log pg/mL vs. 1.39 log pg/mL, P = 0.006, respectively; Tables 3 and 4). Male participants with low grip strength or low SMI also displayed significantly elevated NfL levels compared to healthy subjects (1.53 log pg/mL vs 1.41 log pg/mL, P = 0.006 and 1.52 log pg/mL vs. 1.41 log pg/mL, P = 0.002, respectively), after adjusting for age and BMI (Tables 3 and 4). In the female population, after adjusting for the same covariates, those with low grip strength had significantly higher NfL levels compared with healthy controls (1.44 log pg/mL vs. 1.36 log pg/mL, P = 0.049), while higher, although non‐significant NfL levels were reported in those with low SMI (1.43 log pg/mL vs. 1.37 log pg/mL, P = 0.179; Tables 3 and 4).
Table 2.
Multiple regression examining the association between (1) skeletal muscle index (SMI) and neurofilament light chain (NfL) and (2) grip strength and NfL
| Variables | Unstandardized coefficients | 95%CI | P value | R 2 |
|---|---|---|---|---|
| SMI a | ||||
| logNfL | −0.325 | −0.644–0.007 | 0.045 | 0.816 |
| Grip strength a | ||||
| logNfL | −6.331 | −10.723–1.938 | 0.005 | 0.575 |
N = 300; dependent variables: Model 1 = SMI, Model 2 = grip strength; adjusted R 2: Model 1 = 0.814, Model 2 = 0.569.
Adjusted for sex, age, and body mass index.
Table 3.
Unadjusted and adjusted association between plasma neurofilament light chain (NfL) and pre‐sarcopenia (low grip strength)
| Low grip (n = 38) | Normal grip (n = 240) | P value | Low grip (n = 38) | Normal grip (n = 240) | P value | |
|---|---|---|---|---|---|---|
| Unadjusted means (SEM) | Adjusted means (SEM) a | |||||
| logNfL (pg/mL) | ||||||
| All subjects | 1.51 (0.03) | 1.38 (0.01) | <0.001 | 1.49 (0.03) | 1.39 (0.01) | 0.001 |
| Men | 1.56 (0.04) | 1.40 (0.02) | <0.001 | 1.53 (0.04) | 1.41 (0.02) | 0.006 |
| Women | 1.47 (0.04) | 1.36 (0.02) | 0.007 | 1.44 (0.04) | 1.36 (0.02) | 0.049 |
SEM, standard error of mean.
Adjusted for age and body mass index.
Table 4.
Unadjusted and adjusted association between plasma neurofilament light chain (NfL) and pre‐sarcopenia (low skeletal muscle index; SMI)
| Low SMI (n = 38) | Normal SMI (n = 240) | P value | Low SMI (n = 38) | Normal SMI (n = 240) | P value | |
|---|---|---|---|---|---|---|
| Unadjusted means (SEM) | Adjusted means (SEM) a | |||||
| logNfL (pg/mL) | ||||||
| All subjects | 1.46 (0.03) | 1.39 (0.01) | 0.034 | 1.48 (0.03) | 1.39 (0.01) | 0.006 |
| Men | 1.48 (0.04) | 1.42 (0.02) | 0.143 | 1.52 (0.04) | 1.41 (0.02) | 0.002 |
| Women | 1.43 (0.04) | 1.37 (0.02) | 0.126 | 1.43 (0.04) | 1.37 (0.02) | 0.179 |
SEM, standard error of mean.
Adjusted for age and body mass index.
Plasma neurofilament light chain levels and sarcopenia
Interestingly, analysis of covariance indicated those with sarcopenia to have significantly elevated NfL concentrations, compared with healthy controls (1.56 log pg/mL vs. 1.39 log pg/mL, P < 0.001; Table 5). Importantly, this association remained significant for male and female participants, after adjustment for age and BMI (1.60 log pg/mL vs. 1.42 log pg/mL, P = 0.009 and 1.51 log pg/mL vs. 1.37 log pg/mL, P = 0.012, respectively; Table 5). It is worth noting, however, that despite being significantly different, the increase in NfL concentration between sarcopenic and non‐sarcopenic subjects is mild.
Table 5.
Unadjusted and adjusted association between plasma neurofilament light chain (NfL) and sarcopenia
| Sarcopenia (n = 22) | No sarcopenia (n = 202) | P value | Sarcopenia (n = 22) | No sarcopenia (n = 202) | P value | |
|---|---|---|---|---|---|---|
| Unadjusted means (SEM) | Adjusted means (SEM) a | |||||
| logNfL (pg/mL) | ||||||
| All subjects | 1.54 (0.04) | 1.39 (0.01) | 0.001 | 1.56 (0.04) | 1.39 (0.01) | <0.001 |
| Men | 1.57 (0.07) | 1.42 (0.02) | 0.035 | 1.60 (0.07) | 1.42 (0.02) | 0.009 |
| Women | 1.52 (0.06) | 1.37 (0.02) | 0.013 | 1.51 (0.06) | 1.37 (0.02) | 0.012 |
SEM, standard error of mean.
Adjusted for age and body mass index.
Receiver operating characteristic analysis
Figure 3 presents the ROC curves for sarcopenia, low grip strength, and low SMI. Plasma NfL concentrations displayed acceptable diagnostic accuracy for sarcopenia with an area under the curve (AUC) of 0.726 (95%CI = 0.643–0.810, P < 0.001) and optimal cut‐point of 1.45 log pg/mL (77.3% sensitivity and 64.4% specificity). The AUCs for low grip strength and low SMI were 0.677 (95%CI = 0.602–0.753, P < 0.001) and 0.588 (95%CI = 0.511–0.666, P = 0.025), respectively. For low grip strength assessment, the optimal NfL threshold was 1.33 log pg/mL (83.3% sensitivity and 42.5% specificity), while for low SMI diagnosis, the optimal cut‐point was 1.36 log pg/mL (66.7% sensitivity and 47.1% specificity).
Figure 3.
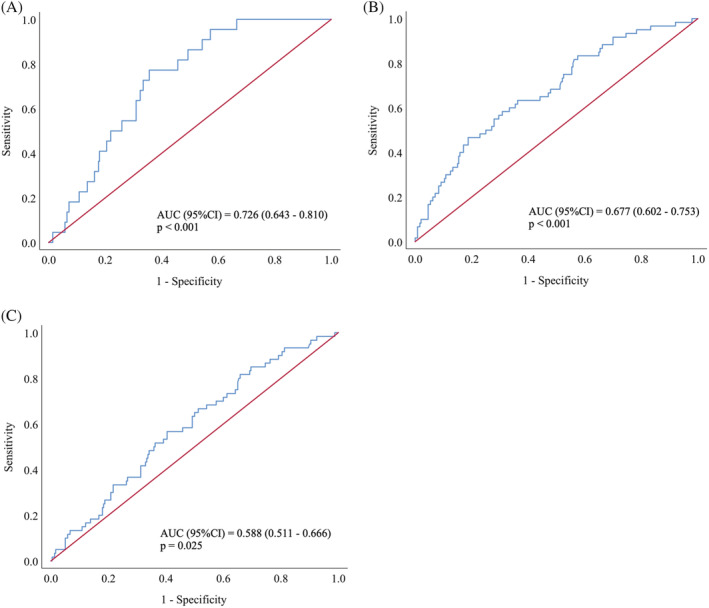
Receiver operating characteristic analysis of neurofilament light chain for: (A) sarcopenia, (B) low grip strength, and (C) low skeletal muscle index. AUC, area under the curve.
Discussion
Despite advances in knowledge and technology in recent decades, sarcopenia remains a highly prevalent disease that imposes profound health and financial consequences globally. Efforts to enhance diagnostic measures have stimulated interest in the potential screening utility of blood‐based biomarkers. Considering the increasing support for a strong neurological contribution to skeletal muscle regulation in older adulthood, 14 , 15 examining markers of neuronal health may be of particular benefit. In that regard, NFs have recently gained credence as a strong candidate biomarker of several neurodegenerative disorders. 23 , 24 , 25 , 26 However, no studies to date have assessed its relevance to sarcopenia. Accordingly, our study aimed, for the first time, to explore the relationship between plasma NfL levels and sarcopenia domains in a large sample of healthy middle‐aged and older adults.
The principle findings of our study are as follows: (1) plasma NfL concentrations are significantly higher in those with sarcopenia and pre‐sarcopenia (low SMI or low grip strength), when compared with healthy controls; (2) plasma NfL is a stronger indicator of muscle strength than muscle mass; (3) sex alters the association between NfL concentrations and sarcopenic domains, such that the indicative relevance of circulating NfL to skeletal muscle health is greater in male participants, than in female participants; and (4) plasma NfL levels demonstrate acceptable diagnostic accuracy for sarcopenia.
Our study indicates positive associations between plasma NfL concentrations and age for both sexes, with a slightly stronger association being observed in the female population (r = 0.355, P < 0.001 vs. r = 0.280, P = 0.001; Figure 2). Such findings are in accordance with previous reports demonstrating positive associations between NfL and age, both in heathy control groups, 27 , 28 , 29 and in diseased groups. 24 , 25 , 28 Presumably, the positive associations consistently observed between circulating NfL concentrations and age reflect the increasing levels of axonal degeneration that occur during normal ageing or disease progression. 19 While the impact of age on NfL levels is consistently reported, research pertaining to the effect of sex on NfL concentrations is conflicting. Interestingly, we found that overall, male participants had significantly higher levels of plasma NfL compared with female participants (1.43 log pg/mL vs. 1.38 log pg/mL, P = 0.039; Figure 2). While this finding is in agreement with some reports, 30 , 31 others have observed female participants to have higher concentrations than male participants, 24 , 26 and still others have found there to be no significant difference between sexes. 27 , 29 It is important to note however, that heterogeneity in relation to participant health status impedes the potential for direct comparison between studies and is a likely contributor to such inconsistent findings. It is clear, therefore, that additional research is needed to further elucidate the potential mediating effect of sex on circulating NfL concentrations.
Importantly, our study provides novel evidence that plasma NfL concentrations are significantly elevated not only in sarcopenic individuals but also in those with pre‐sarcopenia, when compared with healthy controls. Intriguingly, circulating NfL levels appear to be progressively associated with sarcopenia status, such that healthy individuals have the lowest quantities, followed by pre‐sarcopenic subjects, followed by sarcopenic subjects (Figure 2). This graded association is particularly noteworthy as it is a strong biological indicator of a causal relationship. Moreover, ROC analysis revealed plasma NfL levels to have acceptable diagnostic accuracy for sarcopenia (AUC = 0.726, P < 0.001; Figure 3), supporting its potential screening utility. It should be noted, however, that despite being significant, the increase in NfL concentration is mild between sarcopenia status. Such finding reinforces the need for future studies to use more sensitive analytical methods to confirm these data. While multiple studies have demonstrated associations between serum or plasma NfL concentration and severe neurodegenerative disorders, 23 , 24 , 25 , 26 no research has examined the pertinence of circulating NfL levels to sarcopenia. Interestingly, sarcopenia is often prevalent among patients with neurodegenerative disorders such as Alzheimer's disease and Parkinson's disease and has been shown to increase the risk of disease progression. 32 , 33 While the casual mechanisms underpinning this relationship remain unknown, it is reasonable to postulate that sarcopenia and other neurodegenerative disorders share common biological pathways. In that regard, there is a clear rationale underpinning the elevated levels of NfL observed in sarcopenic subjects in the present study. Indeed, considering the regulatory pertinence of neuronal integrity towards skeletal muscle health, coupled with the well‐established evidence of NfL concentration as a marker of axonal damage, there is underlying reasoning to support the potential of NfL as a biomarker for sarcopenia.
Furthermore, it is worth mentioning that multiple studies have demonstrated significant associations between other markers of neural or neuromuscular health and sarcopenia. For example, over the last decade, multiple studies have shown that circulating levels of C‐terminal agrin fragment, a marker of neuromuscular junction integrity, are significantly elevated among sarcopenic and pre‐sarcopenic individuals, when compared with healthy controls. 11 Furthermore, plasma levels of brain derived neurotrophic factor, a potent mediator of neuronal formation and synaptic plasticity, 34 were recently shown to be significantly associated with sarcopenia and physical function in older adults. 35 Thus, circulating indicators of neural health appear to be particularly promising candidates for sarcopenic biomarkers. Indeed, our findings support the potency of the neurogenic contribution to sarcopenia and provide encouraging evidence of the potential relevance of plasma NfL levels.
Although our study demonstrates promising associations between plasma NfL levels, sarcopenia, and each pre‐sarcopenic domain, it should be noted that circulating NfL appears to be a particularly potent indicator of muscle degradation in male participants. Indeed, while significantly elevated NfL concentrations were observed in male participants with either low grip strength or low SMI, in female participants, NfL levels were only significantly raised in those with low grip strength. Currently, the mechanisms underpinning these differences between sexes remain unknown. Besides the complete paucity of research relating to NfL levels and sarcopenia, studies that have assessed the relationship between blood‐based NfL concentrations and other neurodegenerative disorders have not examined the effect of sex on diagnostic proficiency. Nevertheless, evidence for a mediating effect of sex on cerebrospinal fluid NfL concentrations has been observed. For example, a recent meta‐analysis found male participants had higher cerebrospinal fluid NfL levels than female participants in 33.3% of diagnoses, which included multiple sclerosis, Alzheimer's disease, and Parkinson's disease. 36 Notwithstanding such findings, more studies are needed to elucidate whether sex alters the indicative relevance of circulating NfL to sarcopenia and to illuminate the causative pathways driving this effect.
In addition to sex‐specific differences, NfL concentrations appear to be more robustly linked with muscle strength than muscle mass. Indeed, in the present study, despite significant associations between circulating NfL and each pre‐sarcopenic domain, ROC analysis revealed stronger associations between NfL and grip strength, compared with SMI (AUC = 0.677 vs. AUC = 0.588; Figure 3). This is a particularly interesting finding for two principle reasons. Firstly, mounting evidence indicates that age‐related changes in muscle strength are largely independent of myofibre atrophy, but rather a consequence of an impaired neural activation of skeletal muscle, 14 , 15 , 16 which may help explain the stronger associations between plasma NfL and grip strength, compared with SMI, observed in our study. Secondly, as mentioned, recent efforts to enhance the prognostic efficiency of the sarcopenic phenotype in identifying those at risk of clinically relevant outcomes, such as falls, fractures, and hospitalization, have led to muscle strength becoming a cardinal criterion, while the relevance of muscle mass has been scrutinized. With this in mind, research seeking to establish biomarkers of sarcopenia may particularly benefit by focusing on markers of neural health.
There are several limitations to this study that should be acknowledged. Firstly, while ELISAs have been used in previous studies to determine blood NfL levels, 28 , 37 using a more sensitive analytical method such as single molecule array or electrochemiluminescence 38 may have been useful. Nonetheless, the ELISA used in the present study was highly sensitive (<0.61 pg/mL), enhancing the accuracy of results. Going forward, however, future studies seeking to determine blood NfL concentrations should utilize single molecule array or electrochemiluminescence where possible. Secondly, incorporating a measure of gait speed may have provided more insight into the relevance of circulating NfL levels to muscle function. Thirdly, despite the large sample size incorporated in the present study, replication using an additional dataset may have further strengthened our findings. Also, using a more accurate method of lean mass determination such as creatine dilution or magnetic resonance imaging 39 may have further enhanced the quality of findings in the present study. It is also worth mentioning that the cross‐sectional design of this study and subsequent lack of longitudinal data impedes the potential to infer causal relationships. Finally, while the purpose of this study was to explore the relationship between NfL levels and sarcopenia, we recognize the potential benefits of using a combination of biomarkers in screening for sarcopenia. Future research, therefore, may benefit from incorporating a measure of NfL concentrations into combination models.
In conclusion, our study provides novel evidence that plasma NfL concentrations are significantly associated with muscle mass and strength in middle‐aged and older adults. Moreover, plasma NfL levels achieved an encouraging level of accuracy in discriminating between sarcopenic individuals and healthy controls. Nevertheless, while our study provides promising early findings, more research is needed to further elucidate the diagnostic potential of plasma NfL levels for sarcopenia assessment. In particular, future studies should explore whether NfL levels are more potently linked with muscle strength than mass and whether NfL levels are more indicative of muscle health in male participants than female participants. Additionally, research should examine how axonal integrity, as inferred from NfL concentration, is affected by lifestyle.
Conflicts of interests
All authors declare that they have no conflict of interest.
Acknowledgements
This work was supported by a scholarship awarded by the Irish Research Council (EBPPG/2019/9) to JP. The authors would like to thank Judith Conroy for her feedback on the manuscript. The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. 40
Pratt J., De Vito G., Segurado R., Pessanha L., Dolan J., Narici M., and Boreham C. (2022) Plasma neurofilament light levels associate with muscle mass and strength in middle‐aged and older adults: findings from GenoFit, Journal of Cachexia, Sarcopenia and Muscle, 13, 1811–1820, 10.1002/jcsm.12979
Marco Narici and Colin Boreham contributed equally to this work and should be considered joint final authors.
References
- 1. Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr 1997;127:990S–991S. [DOI] [PubMed] [Google Scholar]
- 2. Cruz‐Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shafiee G, Keshtkar A, Soltani A, Ahadi Z, Larijani B, Heshmat R. Prevalence of sarcopenia in the world: a systematic review and meta‐analysis of general population studies. J Diabetes Metab Disord 2017;16:21, 10.1186/s40200-017-0302-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc 2002;50:889–896. [DOI] [PubMed] [Google Scholar]
- 5. Chang KV, Hsu TH, Wu WT, Huang KC, Han DS. Association between sarcopenia and cognitive impairment: a systematic review and meta‐analysis. J Am Med Dir Assoc 2016;17:1164.e7‐.e15.121164.e15, 10.1016/j.jamda.2016.09.013 [DOI] [PubMed] [Google Scholar]
- 6. Hirani V, Blyth F, Naganathan V, Le Couteur DG, Seibel MJ, Waite LM, et al. Sarcopenia is associated with incident disability, institutionalization, and mortality in community‐dwelling older men: the Concord Health and Ageing in Men project. J Am Med Dir Assoc 2015;16:607–613. [DOI] [PubMed] [Google Scholar]
- 7. Bhasin S, Travison TG, Manini TM, Patel S, Pencina KM, Fielding RA, et al. Sarcopenia definition: the position statements of the Sarcopenia Definition and Outcomes Consortium. J Am Geriatr Soc 2020;68:1410–1418. [DOI] [PubMed] [Google Scholar]
- 8. Pratt J, De Vito G, Narici M, Segurado R, Dolan J, Conroy J, et al. Grip strength performance from 9431 participants of the GenoFit study: normative data and associated factors. Geroscience 2021;43:2533–2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Buckinx F, Landi F, Cesari M, Fielding RA, Visser M, Engelke K, et al. Pitfalls in the measurement of muscle mass: a need for a reference standard. J Cachexia Sarcopenia Muscle 2018;9:269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cheung CL, Nguyen US, Au E, Tan KC, Kung AW. Association of handgrip strength with chronic diseases and multimorbidity: a cross‐sectional study. Age (Dordr) 2013;35:929–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pratt J, De Vito G, Narici M, Segurado R, Pessanha L, Dolan J, et al. Plasma C‐terminal agrin fragment as an early biomarker for sarcopenia: results from the GenoFit study. J Gerontol A Biol Sci Med Sci 2021;76:2090–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pratt J, Boreham C, Ennis S, Ryan AW, De Vito G. Genetic associations with aging muscle: a systematic review. Cell 2019;9:12, 10.3390/cells9010012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pratt J, De Vito G, Narici M, Boreham C. Neuromuscular junction aging: a role for biomarkers and exercise. J Gerontol A Biol Sci Med Sci 2020;76:576–585. [DOI] [PubMed] [Google Scholar]
- 14. Clark BC. Neuromuscular changes with aging and sarcopenia. J Frailty Aging 2019;8:7–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Carson RG. Get a grip: individual variations in grip strength are a marker of brain health. Neurobiol Aging 2018;71:189–222. [DOI] [PubMed] [Google Scholar]
- 16. Clark BC, Taylor JL, Hong SL, Law TD, Russ DW. Weaker seniors exhibit motor cortex hypoexcitability and impairments in voluntary activation. J Gerontol A Biol Sci Med Sci 2015;70:1112–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brody H. Organization of the cerebral cortex. III. A study of aging in the human cerebral cortex. J Comp Neurol 1955;102:511–516. [DOI] [PubMed] [Google Scholar]
- 18. Pannese E. Morphological changes in nerve cells during normal aging. Brain Struct Funct 2011;216:85–89. [DOI] [PubMed] [Google Scholar]
- 19. Salvadores N, Sanhueza M, Manque P, Court FA. Axonal degeneration during aging and its functional role in neurodegenerative disorders. Front Neurosci 2017;11:451, 10.3389/fnins.2017.00451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Khalil M, Teunissen CE, Otto M, Piehl F, Sormani MP, Gattringer T, et al. Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol 2018;14:577–589. [DOI] [PubMed] [Google Scholar]
- 21. Yuan A, Rao MV, Veeranna N, RA. Neurofilaments and neurofilament proteins in health and disease. Cold Spring Harb Perspect Biol 2017;9:a018309, 10.1101/cshperspect.a018309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kušnierová P, Zeman D, Hradílek P, Čábal M, Zapletalová O. Neurofilament levels in patients with neurological diseases: a comparison of neurofilament light and heavy chain levels. J Clin Lab Anal 2019;33:e22948, 10.1002/jcla.22948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gaiottino J, Norgren N, Dobson R, Topping J, Nissim A, Malaspina A, et al. Increased neurofilament light chain blood levels in neurodegenerative neurological diseases. PLoS ONE 2013;8:e75091, 10.1371/journal.pone.0075091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mollenhauer B, Dakna M, Kruse N, Galasko D, Foroud T, Zetterberg H, et al. Validation of serum neurofilament light chain as a biomarker of Parkinson's disease progression. Mov Disord 2020;35:1999–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cantó E, Barro C, Zhao C, Caillier SJ, Michalak Z, Bove R, et al. Association between serum neurofilament light chain levels and long‐term disease course among patients with multiple sclerosis followed up for 12 years. JAMA Neurol 2019;76:1359–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lu CH, Macdonald‐Wallis C, Gray E, Pearce N, Petzold A, Norgren N, et al. Neurofilament light chain: a prognostic biomarker in amyotrophic lateral sclerosis. Neurology 2015;84:2247–2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rübsamen N, Maceski A, Leppert D, Benkert P, Kuhle J, Wiendl H, et al. Serum neurofilament light and tau as prognostic markers for all‐cause mortality in the elderly general population‐an analysis from the MEMO study. BMC Med 2021;19:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Donath H, Woelke S, Schubert R, Kieslich M, Theis M, Auburger G, et al. Neurofilament light chain is a biomarker of neurodegeneration in ataxia telangiectasia. Cerebellum 2021;1–9, 10.1007/s12311-021-01257-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Khalil M, Pirpamer L, Hofer E, Voortman MM, Barro C, Leppert D, et al. Serum neurofilament light levels in normal aging and their association with morphologic brain changes. Nat Commun 2020;11:812, 10.1038/s41467-020-14612-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lin YS, Lee WJ, Wang SJ, Fuh JL. Levels of plasma neurofilament light chain and cognitive function in patients with Alzheimer or Parkinson disease. Sci Rep 2018;8:17368, 10.1038/s41598-018-35766-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Skillbäck T, Blennow K, Zetterberg H, Shams S, Machado A, Pereira J, et al. Sex differences in CSF biomarkers for neurodegeneration and blood‐brain barrier integrity. Alzheimers Dement (Amst) 2021;13:e12141, 10.1002/dad2.12141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ogawa Y, Kaneko Y, Sato T, Shimizu S, Kanetaka H, Hanyu H. Sarcopenia and muscle functions at various stages of Alzheimer disease. Front Neurol 2018;9:710, 10.3389/fneur.2018.00710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vetrano DL, Pisciotta MS, Laudisio A, Lo Monaco MR, Onder G, Brandi V, et al. Sarcopenia in Parkinson disease: comparison of different criteria and association with disease severity. J Am Med Dir Assoc 2018;19:523–527. [DOI] [PubMed] [Google Scholar]
- 34. Bathina S, Das UN. Brain‐derived neurotrophic factor and its clinical implications. Arch Med Sci 2015;11:1164–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Miyazaki S, Iino N, Koda R, Narita I, Kaneko Y. Brain‐derived neurotrophic factor is associated with sarcopenia and frailty in Japanese hemodialysis patients. Geriatr Gerontol Int 2021;21:27–33. [DOI] [PubMed] [Google Scholar]
- 36. Bridel C, van Wieringen WN, Zetterberg H, Tijms BM, Teunissen CE, Alvarez‐Cermeño JC, et al. Diagnostic value of cerebrospinal fluid neurofilament light protein in neurology: a systematic review and meta‐analysis. JAMA Neurol 2019;76:1035–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gong ZY, Lv GP, Gao LN, Lu Y, Guo J, Zang DW. Neurofilament subunit L levels in the cerebrospinal fluid and serum of patients with amyotrophic lateral sclerosis. Neurodegener Dis 2018;18:165–172. [DOI] [PubMed] [Google Scholar]
- 38. Kuhle J, Barro C, Andreasson U, Derfuss T, Lindberg R, Sandelius Å, et al. Comparison of three analytical platforms for quantification of the neurofilament light chain in blood samples: ELISA, electrochemiluminescence immunoassay and Simoa. Clin Chem Lab Med 2016;54:1655–1661. [DOI] [PubMed] [Google Scholar]
- 39. Clark RV, Walker AC, Miller RR, O'Connor‐Semmes RL, Ravussin E, Cefalu WT. Creatine (methyl‐d(3)) dilution in urine for estimation of total body skeletal muscle mass: accuracy and variability vs. MRI and DXA. J Appl Physiol (1985) 2018;124:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2021. J Cachexia Sarcopenia Muscle 2021;12:2259–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]


