Abstract
Background
Tiotropium via the HandiHaler device is an established long-acting, anticholinergic bronchodilator that prevents exacerbations and improves lung function in patients with chronic obstructive pulmonary disease. We hypothesised that tiotropium would reduce pulmonary exacerbations and improve lung function in patients with stable bronchiectasis and airflow limitation, and assessed the effect of tiotropium on these outcomes.
Methods
In a randomised, double-blind, two-period crossover trial, we recruited adult patients from three hospitals in New Zealand. Patients were excluded if they had a smoking history of >20 pack-years. Patients were assigned to either the tiotropium–placebo or placebo–tiotropium sequence in a 1:1 ratio, using randomly permuted blocks stratified by centre. Participants and investigators were masked to treatment allocation. Eligible patients received tiotropium 18 μg via HandiHaler daily for 6 months followed by 6 months of placebo, or vice versa, with a washout period of 4 weeks. The primary end-point was rate of event-based exacerbations during the 6-month period. Primary analyses were carried out in an intention-to-treat set.
Results
90 patients were randomly assigned and 85 completed both treatment cycles. The rate of exacerbations was 2.17 per year under the tiotropium treatment and 2.27 per year under placebo (rate ratio 0.96, 95% CI 0.72–1.27; p=0.77). Tiotropium, compared with placebo, improved forced expiratory volume in 1 s by 58 mL (95% CI 23–92 mL; p=0.002). Adverse events were similar under both treatments.
Conclusions
Tiotropium via HandiHaler over 6 months significantly improved lung function but not frequency of exacerbations. Further research is required to understand the clinical context and significance of these findings.
Short abstract
This trial assessed tiotropium in adults with bronchiectasis. Daily tiotropium via HandiHaler over 6 months did not reduce exacerbations but improved lung function. More studies are required to identify those patients who will benefit from tiotropium. https://bit.ly/30oTuvz
Introduction
Despite significant advances in the diagnosis of bronchiectasis with the advent of high-resolution computed tomography (HRCT) chest scans, treatment options are limited and predominantly involve daily sputum clearance techniques and antibiotic therapy (including prolonged macrolide therapy) to both treat and prevent exacerbations [1, 2].
In recent years there has been increasing research into alternative therapies to improve both patient-related outcomes, including exacerbations, and to prevent deterioration in lung function. Trialled therapies include mucolytics, inhaled antibiotics, and combined inhaled corticosteroid and bronchodilators [3]. Unlike smoking-related chronic obstructive pulmonary disease (COPD), a disorder defined by respiratory symptoms and irreversible airflow limitation on spirometry where the role of inhaled long-acting bronchodilator therapy is clearly proven, the clinical usefulness of this treatment in patients with bronchiectasis is anecdotal, limited to small studies and not supported by current treatment guidelines [4, 5].
There is, however, biological plausibility for the role of inhaler therapy, especially anticholinergic inhaler therapy such as tiotropium, in bronchiectasis. Drug inhibition of the parasympathetic nervous system has been shown to result in bronchodilatation via muscarinic receptors within the airway muscle and to reduce the submucosal gland production of sputum [6]. Recent in vitro studies have elegantly demonstrated that anticholinergic therapy increases the clearance of mucin bundles and the trapped bacteria within the trachea [7], and attenuates induced airway inflammation in both human epithelial cell lines and in animal models [8].
In clinical practice, tiotropium bromide via the HandiHaler device has been prescribed for >15 years for patients with COPD, for whom it is has been shown to be highly effective in improving symptoms, exacerbations, quality of life, lung function and possibly survival through airway bronchodilatation and reduction in lung hyperinflation [9, 10]. While >40% of patients with bronchiectasis have documented airflow obstruction [11, 12], trials examining the usefulness of tiotropium in bronchiectasis are lacking. Open-label studies of tiotropium in patients with bronchiectasis have been of short duration (≤3 months), small sample size (≤22 patients), and included patients with and without airflow limitation. These studies have shown improvement in symptoms accompanied by a variable increase in lung function [13–15].
The aim of this larger study was to evaluate whether inhaled tiotropium via HandiHaler, similar to COPD, reduced exacerbations and improved lung function in adult patients with bronchiectasis and airflow limitation.
Methods
Study design
This randomised, double-blind, placebo-controlled, two-period crossover trial (comprised of 6 months for each period) was undertaken at three centres (Middlemore Hospital, Auckland; Auckland Hospital, Auckland; and Waikato Hospital, Hamilton) in New Zealand. A washout interval of 4 weeks between the two periods was considered sufficient to exclude a treatment carryover effect and to ensure a return to baseline clinical stability.
An independent data and safety monitoring committee of the Health Research Council of New Zealand oversaw the study. The study was approved by our Regional Ethics Committee (NTY/11/10/104). The study was conducted according to the principles of the World Medical Association Declaration of Helsinki. The study is registered with the Australian New Zealand Clinical Trials Registry with identifier number ACTRN12612000206820.
Participants and data collection
Eligible participants were aged ≥18 years. All had had at least one pulmonary exacerbation requiring antibiotic treatment in the past year and had a diagnosis of bronchiectasis defined by HRCT chest scan. All HRCT scans were reviewed centrally by one respiratory radiologist (D.M.) to verify the diagnosis of bronchiectasis. All participants had evidence of airflow limitation (FEV1/forced vital capacity (FVC) ratio <70%) on screening spirometry and were clinically stable at entry into the study. Key exclusion criteria were: a history of cystic fibrosis, smoking history >20 pack-years, a primary diagnosis of asthma, unstable or life-threatening cardiac arrhythmia, narrow-angle glaucoma and symptomatic prostatic hyperplasia, short- and long-acting anticholinergics, and antibiotics (including macrolides) within 6 weeks prior to randomisation. Long-acting β-agonist (LABA) and LABA/inhaled corticosteroid (ICS) combinations were permitted for the study duration. All participants provided written informed consent.
Randomisation and masking
Eligible patients were randomly assigned to either placebo–tiotropium (sequence A) or tiotropium–placebo (sequence B) in a 1:1 ratio, using randomly permuted blocks with a random block size of either four or six, stratified by centre. Participants, research assistants and investigators were masked to treatment allocation, with the exception of the trial statistician, who generated the randomisation schedule but did not take part in day-to-day operations. Both tiotropium and placebo HandiHalers were identically packaged. Furthermore, tiotropium capsules with logos were re-encapsulated to prevent identification and were identical to placebo capsules.
Procedures
Patients were given either 18 μg tiotropium or matching placebo capsules delivered by a HandiHaler dry powder inhalation device (Boehringer Ingelheim, Ingelheim am Rhein, Germany) daily for 6 months. A first clinic visit occurred 2–4 weeks before randomisation to assess eligibility. Clinic visits then occurred at weeks 0, 4, 13 and 26 (period 1) followed by a washout period of 4 weeks to ensure clinical stability. Participants then crossed over to the alternate treatment for a further 6 months with clinic visits at weeks 30, 34, 43 and 56 (period 2). Patients completed a daily symptom diary card. At each visit the following were undertaken: review of the daily symptom diary card, and spirometry pre- and post-salbutamol (MicroLab; Micro Direct, Lewiston, ME, USA) performed to American Thoracic Society/European Respiratory Society standards with predicted values from NHANES III [16]. The same spirometer was used for the same subject during the entire study period. Compliance was monitored at each visit and measured by keeping a record of the number of capsules issued and returned. Health-related quality of life and symptom questionnaires were administered at screening and at weeks 26, 30 and 56. They consisted of the St George's Respiratory Questionnaire (SGRQ), a questionnaire measuring the impact of disease on quality of life (scored from 0 to 100, where 0 indicates no health impairment and 100 reflects significant health impairment [17]), the COPD Assessment Test (CAT), a concise questionnaire consisting of eight questions assessing and monitoring the impact of symptoms in COPD, and the Leicester Cough Questionnaire (LCQ), validated for use in bronchiectasis to assess the impact of cough severity, a major symptom of bronchiectasis [18, 19]. The 6-min walk test (6MWT) was also performed according to American Thoracic Society guidelines at the same visits [20]. Full blood count, C-reactive protein and erythrocyte sedimentation rate measurements were undertaken at weeks 0, 26, 30 and 56. Spontaneously expectorated sputum samples were collected at weeks 0, 26, 30 and 56, and assessed for total and differential white cell count and fluid-phase markers using standardised methods [21]. Adverse events were ascertained and recorded at each visit.
Outcomes
The primary end-point was rate of event-based exacerbations during each 6-month period. An event-based exacerbation was based on the EMBRACE study [11] and was defined as a sustained worsening of the patient's respiratory condition from a stable state with the presence of any one of increased sputum volume, increased sputum purulence or increased dyspnoea, necessitating treatment with oral or intravenous antibiotics. This definition preceded the consensus document on exacerbations in bronchiectasis developed in 2017 [22]. All exacerbations were reviewed and adjudicated by two blinded investigators at the central site (Middlemore Hospital), who confirmed that the exacerbations met the study definition and were independent of any previous events.
Secondary outcomes included FEV1, time to first exacerbation, health-related quality of life (as measured by SGRQ, LCQ and CAT), FVC, exercise capacity (6MWT), frequency of adverse events and assessment of inflammatory markers.
Statistical analysis
Sample size and power computations were based on the EMBRACE trial examining the efficacy of oral azithromycin on exacerbations [11]. Extensive simulations indicated that a sample size of 45 participants per arm with target follow-up of 6 months per period yielded an estimated minimal power (across different attrition scenarios) of 81% to detect a relative rate reduction of exacerbations of 0.33 between treatment and placebo. This sample size, accounting for 10% loss to follow-up, and based on the pooled standard deviation of FEV1 among treatment and control group participants in the EMBRACE trial, was also sufficiently powered, at a minimum of 80% power, to detect a change of 269 mL in FEV1.
Primary analyses were carried out in an intention-to-treat set, consisting of all randomised participants. All analyses except that of time to first exacerbation were carried out using mixed models accounting for centre and participants as random effects. Measurements from all visits were included as dependent variables in the models, with the first visit within a period assigned a neutral treatment identifier. All models were adjusted for period and treatment allocation was fitted in interaction with visit number within the period, treated as a factor. Possible carryover effects were tested by including an interaction between period and treatment in all cases. Adherence was calculated as the total number of nonmissed doses over the total number of days of follow-up.
Statistical analyses for secondary outcome measures are detailed in the supplementary material.
Statistical analyses were carried out using SAS/STAT version 9.4 of the SAS System for Windows (SAS Institute, Cary, NC, USA), as well as R version 3.4.0 and above (www.r-project.org).
Results
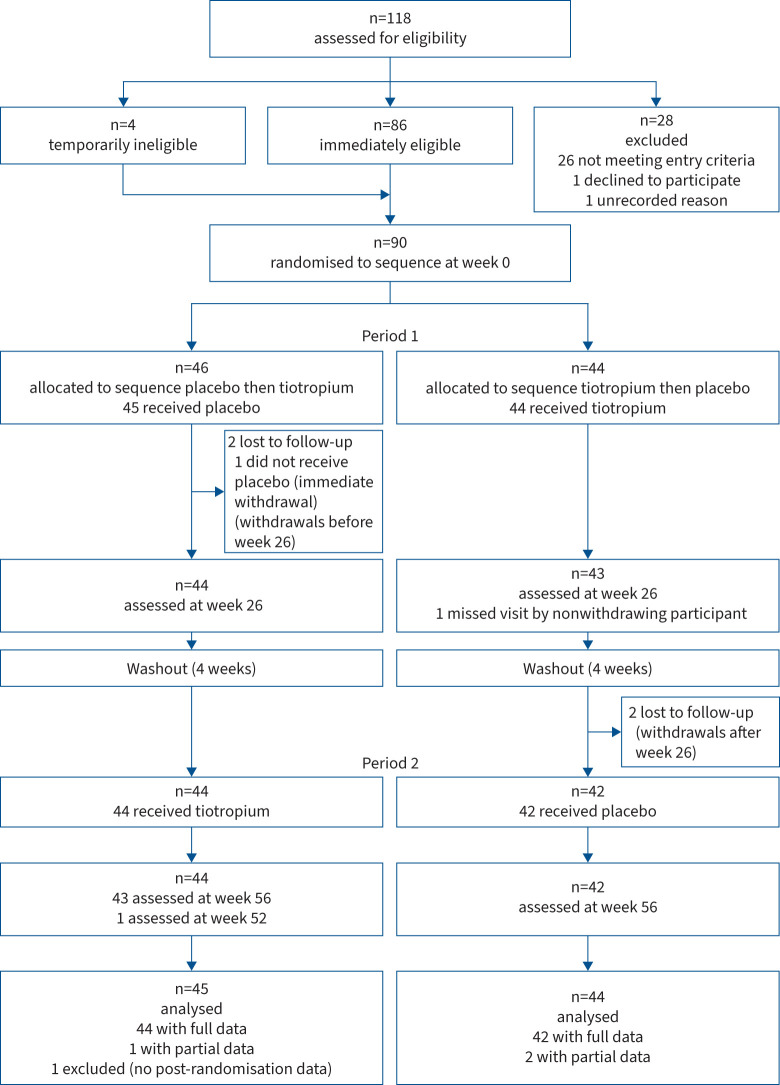
Between March 2012 and May 2015, 118 potential participants were screened and 90 were randomised in this two-period crossover study (figure 1). The trial was completed by 85 (94%) participants, accounting for a total follow-up time of 86.2 person-years. Table 1 shows baseline characteristics and concomitant use of respiratory medications. A count of the tiotropium capsules at each visit demonstrated excellent adherence recorded at >95.6% for the study duration. Participants were predominantly female with a mean age of 60 years. Most were never-smokers (58.9%) or ex-smokers (38.9%) and the mean±sd smoking history of current/ex-smokers was light at 6.0±5.4 pack-years. Participants had mild symptoms based on the SGRQ and an exercise capacity near or within the normal range based on the 6MWT. Average pre-bronchodilator FEV1 was moderately reduced at 61% predicted. Sputum cell counts were within the normal range. Most had had two to three exacerbations in the preceding year. Nearly 48% (43 out of 90) were on both LABA/ICS therapy at the time of study enrolment and continued with this during the study.
FIGURE 1.
Trial profile.
TABLE 1.
Baseline characteristics
| Sequence A: placebo–tiotropium (n=46) | Sequence B: tiotropium–placebo (n=44) | |
| Male | 22 (48) | 12 (27) |
| Age (years) | 59.3±13.0 | 62.0±11.3 |
| Smoking status | ||
| Current/ex-smoker | 21 (46) | 16 (37) |
| Smoking history (pack-years)# | 6.0±5.7 | 6.0±5.3 |
| Asthma | 11 (24) | 11 (25) |
| Medical conditions (n ) | 3.8±2.4 | 4.0±2.1 |
| Body mass index (kg·m−2) | 28.3±7.7 | 28.8±9.8 |
| Ethnic origin | ||
| European | 29 (63) | 25 (57) |
| Pasifika | 6 (13) | 7 (16) |
| Māori | 8 (17) | 10 (23) |
| Other | 3 (7) | 2 (5) |
| Exacerbations in past year (n) | 2.4±1.4 | 3.2±1.6 |
| Spirometry | ||
| Pre-bronchodilator | ||
| FEV1 (L) | 1.78±0.53 | 1.67±0.45 |
| FEV1 (% pred) | 59.4±14.2 | 64.2±17.2 |
| FVC (L) | 3.02±0.83 | 2.82±0.67 |
| FVC (% pred) | 75.3±15.1 | 81.6±16.9 |
| Post-bronchodilator | ||
| FEV1 (L) | 1.88±0.55 | 1.76±0.50 |
| FEV1 (% pred) | 63.1±14.4 | 68.3±18.6 |
| FVC (L) | 3.06±0.83 | 2.87±0.72 |
| FVC (% pred) | 77.5±14.9 | 84.2±16.5 |
| SGRQ domain scores | ||
| Symptoms | 52.8±20.9 | 45.4±24.6 |
| Activity | 40.3±24.7 | 37.6±22.0 |
| Impacts | 28.3±15.6 | 24.3±16.3 |
| Total | 35.9±16.9 | 31.7±17.5 |
| 6MWT (m) | 536.0±69.8 | 500.6±99.2 |
| Peripheral blood cells | ||
| White blood cells (×109 mL−1) | 7.7±2.2 | 8.2±2.4 |
| Neutrophils (×109 mL−1) | 4.8±2.1 | 5.1±2.1 |
| Eosinophils (×109 mL−1) | 0.22±0.15 | 0.28±0.23 |
| Sputum cells | ||
| Total cells (×109 mL−1) | 15.2±23.9 | 21.1±34.5 |
| Neutrophils (×109 mL−1) | 14.2±23.2 | 20.2±34.4 |
| Eosinophils (×109 mL−1) | 0.04±0.18 | 0.18±0.48 |
| Bronchial epithelial cells (×109 mL−1) | 0.12±0.24 | 0.08±0.16 |
| Respiratory drugs | ||
| Any | 33 (72) | 29 (66) |
| Inhaled anticholinergic | ||
| Short- or long-acting | 0 (0) | 0 (0) |
| Inhaled β2-agonists | ||
| Short-acting, alone | 9 (17) | 4 (7) |
| Long-acting, alone | 0 (0) | 0 (0) |
| ICS | ||
| Alone | 2 (4) | 5 (11) |
| Mucolytic agent | 5 (11) | 1 (2) |
| Leukotriene receptor antagonist | 0 (0) | 0 (0) |
| Combination inhalers | ||
| LABA/ICS | 5 (11) | 1 (2) |
| LABA combined with ICS in two separate inhalers | 20 (43) | 17 (39) |
| Aetiology | ||
| Idiopathic | 36 (78) | 31 (70) |
| Inflammatory bowel disease | 3 (7) | 2 (5) |
| Pink disease (infantile mercury exposure) | 0 (0) | 1 (2) |
| Post-infective | 5 (11) | 6 (14) |
| Post-tuberculous | 1 (2) | 4 (9) |
| Primary ciliary dyskinesia | 1 (2) | 0 (0) |
Data are presented as mean±sd or n (%), unless otherwise stated. FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; SGRQ: St George's Respiratory Questionnaire; 6MWT: 6-min walk test distance; ICS: inhaled corticosteroids; LABA: long-acting β-agonist. #: among current and ex-smokers only.
Primary outcome
The annual rate of exacerbations was 2.17 per patient under tiotropium treatment and 2.27 per patient under placebo (rate ratio 0.96, 95% CI 0.72–1.27; p=0.77).
Secondary outcomes
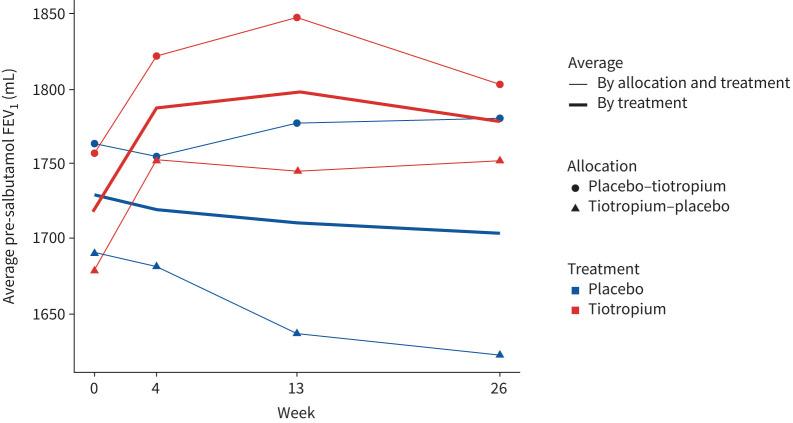
Lung function improved significantly from baseline to 6 months between treatment arms with a 58 mL (95% CI 23–92 mL; p=0.002) difference in pre-bronchodilator FEV1 and a 56 mL (95% CI 17–92 mL; p=0.005) difference in post-bronchodilator FEV1 favouring tiotropium (table 2 and figure 2). The greatest improvement in mean FEV1 of up to 83 mL (95% CI 42–124 mL) between treatment arms was noted at 13 weeks of therapy (figure 2) with an overall improvement of 3%. Pre-bronchodilator FVC also improved similarly and significantly to 6 months under tiotropium treatment compared with placebo (table 2).
TABLE 2.
Lung function at 26 weeks
| Placebo# | Tiotropium# | Mean difference (95% CI )¶ | p-value | |
| Absolute (mL) | ||||
| Pre-BD FEV1 | 1704±528 | 1778±526 | 58 (23–92) | 0.002 |
| Post-BD FEV1 | 1798±555 | 1871±574 | 56 (17–92) | 0.005 |
| Pre-BD FVC | 2880±805 | 2956±767 | 78 (25–131) | 0.004 |
| Post-BD FVC | 2974±827 | 3011±785 | 34 (−22–90) | 0.24 |
| Percentage predicted (%) | ||||
| Pre-BD FEV1 | 61.5 | 64.4 | 2.67 (1.36–3.98) | 0.00006 |
| Post-BD FEV1 | 65.0 | 67.7 | 2.69 (1.36–4.03) | 0.00006 |
| Pre-BD FVC | 79.2 | 81.2 | 2.09 (0.55–3.64) | 0.008 |
| Post-BD FVC | 81.3 | 82.5 | 1.38 (−0.26–3.01) | 0.10 |
BD: bronchodilator; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity. #: absolute values presented as mean±sd; ¶: adjusted for baseline value at start of period.
FIGURE 2.
Pre-bronchodilator (salbutamol) forced expiratory volume in 1 s (FEV1) by treatment and allocation. For graphing purposes, weeks 30, 34, 43 and 56 are represented by weeks 0, 4, 13 and 26, respectively
Other secondary outcomes did not improve significantly with tiotropium treatment compared with placebo (table 3). The mean change in SGRQ total and component scores at 6 months did not differ significantly No significant treatment effects on the LCQ score, CAT score or 6MWT distance were found between the treatment modalities. Blood and sputum inflammatory outcomes were similar between treatments and did not alter significantly from baseline values. Tiotropium was well tolerated with an adverse event profile similar to placebo (table 4).
TABLE 3.
Secondary outcome measures at 26 weeks
| Placebo | Tiotropium | Difference in change adjusted for period (95% CI ) | p-value | |
| Exacerbation duration (days) | 19.6±14.6# | 21.7±16.9# | 2.4 (−1.5–6.2) | 0.49 |
| Time to first exacerbation (days) | 104 (80–136)¶ | 74 (50–156)¶ | 1.00 (0.68–1.46)+ | 0.98 |
| 6MWT (m) | 522±91 | 526±79 | −0.3 (−8.0–7.3) | 0.93 |
| SGRQ domain score | ||||
| Symptoms | 45.5±232.3 | 46.0±23.1 | 0.7 (−3.5–4.8) | 0.31 |
| Activity | 34.7±22.8 | 33.5±20.9 | −0.9 (−3.7–2.0) | 0.54 |
| Impacts | 23.1±16.5 | 24.0±16.3 | 0.5 (−2.1–3.0) | 0.72 |
| Total | 30.0±17.2 | 30.5±16.4 | 0.3 (−2.0–2.6) | 0.81 |
| LCQ domain score | ||||
| Physical | 5.21±1.08 | 5.08±1.19 | −0.12 (−0.34–0.10) | 0.28 |
| Psychological | 5.34±1.47 | 5.29±1.50 | −0.09 (−0.37–0.18) | 0.50 |
| Social | 5.41±1.36 | 5.33±1.41 | −0.12 (−0.37–0.13) | 0.35 |
| Total | 16.0±3.8 | 15.7±3.9 | −0.33 (−1.01,0.35) | 0.34 |
| CAT score | 14.7±6.9 | 14.6±7.6 | −0.17 (−1.47–1.14) | 0.80 |
| Peripheral blood cells | ||||
| White blood cells (×109 mL−1) | 8.04±2.53 | 7.65±1.99 | 0.97§ (0.91–1.03) | 0.27 |
| Neutrophils (×109 mL−1) | 5.03±2.26 | 4.73±1.72 | 0.97§ (0.89–1.05) | 0.47 |
| Eosinophils (×109 mL−1) | 0.247±0.184 | 0.311±0.601 | 1.08§ (0.94–1.24) | 0.30 |
| Sputum cells | ||||
| Total cells (×109 mL−1) | 21.3±28.8 | 22.9±38.3 | 0.87§ (0.78–0.97) | 0.016 |
| Neutrophils (×109 mL−1) | 21.0±28.7 | 22.2±37.6 | 0.90§ (0.62–1.30) | 0.45 |
| Eosinophils (×109 mL−1) | 0.15±0.44 | 0.33±1.38 | 0.84ƒ (0.32–2.21) | 0.76¶¶ |
| 0.83## (0.35–1.95) | ||||
| Bronchial epithelial cells (×109 mL−1) | 0.13±0.28 | 0.16±0.27 | 0.92ƒ (0.40–2.16) | 0.87¶¶ |
| 1.25## (0.74–2.10) |
Data are presented as mean±sd, unless otherwise stated. 6MWT: 6-min walk test; SGRQ: St George's Respiratory Questionnaire; LCQ: Leicester Cough Questionnaire; CAT: COPD Assessment Test. #: sd indicative only, as not all observations independent; ¶: median (95% CI); +: hazard ratio; §: ratio of means; ƒ: odds ratio of value being 0; ##: ratio of means when value >0; ¶¶: overall p-value.
TABLE 4.
Adverse events# over 26 weeks
| Placebo (n=213) | Tiotropium (n=205) | |
| Respiratory¶ | 116 (49) | 113 (47) |
| Asthma | 3 | 3 |
| Lower respiratory tract infection | 102 | 93 |
| Upper respiratory tract infection | 3 | 5 |
| Influenza | 3 | 4 |
| Nontuberculous mycobacteria | 1 | 0 |
| Other | 4 | 8 |
| Ear, nose and throat | 21 (9.0) | 25 (10.5) |
| Rhinosinusitis | 8 | 12 |
| Laryngitis/pharyngitis | 11 | 7 |
| Other | 2 | 6 |
| Gastrointestinal | 15 (6.4) | 7 (2.9) |
| Dermatology | 14 (6.0) | 6 (2.5) |
| Genitourinary | 13 (5.6) | 6 (2.5) |
| Musculoskeletal | 9 (3.9) | 12 (5.0) |
| Dental | 6 (2.6) | 6 (2.5) |
| Injury | 6 (2.6) | 8 (3.3) |
| Neurological | 4 (1.7) | 8 (3.4) |
| Other | 9 (3.8) | 14 (5.9) |
Data are presented as n (%) or n. #: any adverse event in >2% of participants; ¶: includes one instance of haemoptysis under each of placebo and tiotropium.
Treatment carryover effects were not demonstrated (p=0.35 for exacerbation rate and p=0.22 for FEV1).
Discussion
This randomised, double-blind, placebo-controlled study examined the effect of tiotropium in bronchiectasis and airflow limitation. It demonstrated that tiotropium did not significantly reduce exacerbations but did improve lung function (FEV1 and FVC).
Exacerbations did not decline with tiotropium compared with placebo despite the improvement in lung function. Tiotropium primarily targets airway smooth muscle and bronchoconstriction but the magnitude of the effect in bronchiectasis does not appear to be sufficient to prevent exacerbations, which are primarily driven by infection and airway inflammation. The absence of an anti-inflammatory effect is supported by the lack of response in blood and sputum inflammatory markers with tiotropium.
These findings support previous nonrandomised studies assessing the efficacy of long-acting bronchodilators on lung function in patients with chronic bronchitis and mucus hypersecretion. In a small, open-label, Japanese study in 22 ex-smokers with chronic bronchitis (including two with bronchiectasis), spirometry-based evidence of airflow limitation and mucus hypersecretion, daily tiotropium via HandiHaler over 8 weeks significantly increased FEV1 by a clinically meaningful 150 mL, and improved symptoms of cough, sputum and dyspnoea [13]. In a second open-label study involving 13 participants with chronic mucus hypersecretion (including five with bronchiectasis) that had not resolved with macrolide therapy, daily tiotropium via HandiHaler over 3 months significantly improved symptoms, and FEV1 absolute and FEV1 % pred by 100 mL and 6%, respectively [14].
Other studies, unlike ours, have demonstrated improvement in symptoms but not in lung function. A nonrandomised trial involving 22 patients with bronchiectasis and a mean FEV of 1.63 L demonstrated that while daily tiotropium over a 28-day period significantly improved clinical symptoms of cough and breathlessness, 6MWT, and BODE index (body mass index, airflow obstruction, dyspnoea, exercise capacity; a validated score reflecting morbidity and mortality in COPD but not bronchiectasis), it did not improve lung function [15]. Similarly, another single-blind randomised trial involving 40 patients with bronchiectasis, a combination LABA/ICS inhaler (formoterol/budesonide) given for 3 months compared with ICS alone showed clinically and statistically significant benefits in symptoms and quality of life but not in lung function [23].
While lung function improvement reached statistical significance in our study, with post-bronchodilator differences in FEV1 of 58 mL (3%) and FVC of 78 mL over the 6-month period reflecting that achieved in the landmark UPLIFT trial [24] which established the benefits of tiotropium in COPD, the clinical relevance of these results is unclear, given the lack of improvement in other patient-related outcome measures. The minimally clinically important difference (MCID) in FEV1 in bronchiectasis is yet to be determined. The traditionally used MCID extrapolated from patients with COPD [25], defined as a 5–10% or 100 mL improvement in FEV1 from baseline, was not achieved at 26 weeks (6 months). Improvements of up to 83 mL were, however, noted between tiotropium and placebo at 13 weeks (3 months). Additionally, nearly half of our patients (47%) received combined LABA alone or in combination with ICS therapy (LABA/ICS) (table 1) for the duration of the study. This too may have contributed to the smaller than anticipated increase in FEV1. Owing to the small numbers it was not possible to further delineate the effects of tiotropium alone on lung function compared with either ICS or LABA, or both. Similarly, we were not able to delineate the effect of tiotropium on mild, moderate and severe airway limitation.
Time to first exacerbation, symptoms, quality of life, exercise capacity (measured by the 6MWT) were also similar between treatment arms. Our participants had mild symptoms based on the SGRQ and had minimal exercise limitation when compared with grouped normative data for healthy subjects (mean (range) 659 (484–820) m) [26], and this may have contributed to the aforementioned findings. Further studies to evaluate the clinical benefits in patients with more severe symptoms, greater functional limitation and more severe disease are warranted, as it may be these patients that benefit most from tiotropium.
Adverse events were comparable in both treatment arms (table 4). No cardiovascular events were noted with tiotropium, given previous concerns with this medication [27]. Patients were rigorously screened and those with unstable or life-threatening cardiac arrhythmia were excluded from the study as in previous large randomised controlled trials involving tiotropium. The relative risk of all adverse effects (whether related to the study medication or not) for tiotropium compared with placebo was minimal and comparable between treatment groups at 1.02 (95% CI 0.68–1.51; p=0.87).
Together, these findings indicate that daily tiotropium via HandiHaler taken over 6 months does not reduce exacerbations, or improve symptoms, quality of life or exercise capacity, compared with placebo. Tiotropium does improve lung function significantly compared with placebo. The clinical relevance of this result, however, is unclear in light of the other study findings and the lack of a specific MCID for lung function variables in bronchiectasis. Our study included patients who were taking ICS and concerns have been raised that inhaled steroids may increase the risk of infection. In those who do not have coexisting eosinophilic airways disease, tiotropium could have a role as single-inhaler therapy or in combination with a LABA.
The heterogeneity of our population may be perceived as a limitation of our study. By not phenotyping participants [28] we may have missed an opportunity to assess who may most benefit from tiotropium and this should be examined in future studies. Inclusion of current smokers may have raised the possibility of concurrent emphysema confounding the results. This was mitigated by strict inclusion criteria, which capped the smoking history and included a respiratory radiologist diagnosis of bronchiectasis as the primary disease. A strength was the crossover design which defused the possibility of chance confounding, as each participant served as their own control. The placebo-controlled rather than open-label design in assessing the efficacy of bronchodilator therapy in bronchiectasis, a first as far as we are aware, added to the strength of this study [5]. Similarly, the concomitant use of dual bronchodilators and ICS in a large proportion of our patients, and the paucity of patients with more symptomatic and severe bronchiectasis, may have underestimated the beneficial effects of tiotropium. This needs to be addressed in future studies.
Conclusions
In patients with bronchiectasis and coexisting airflow limitation, tiotropium does not reduce exacerbations or improve symptoms, but improves lung function significantly by an average of nearly 60 mL or 3% over a 6-month period. The clinical relevance of this degree of improvement needs to be explored. Further studies phenotyping responders to tiotropium and exploring the relationship between physiological benefits and targeted clinical and inflammatory measures are required. Based on these findings, and until there is further evidence, an empirical trial of tiotropium may be an option to consider in those with fixed airflow limitation and respiratory symptoms. Using tiotropium in the real world may provide additional information regarding its role in bronchiectasis, similar to that noted with COPD and severe asthma [29]. While we do not recommend the routine use of tiotropium in bronchiectasis, patients could be given a trial of treatment if they have persistent symptoms such as breathlessness, cough and daily sputum production, since treatment options remain limited for this condition and the risk of adverse effects is low.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material: statistical analyses for secondary outcome measures ERJ-02184-2021.Supplement (195.2KB, pdf)
Shareable PDF
Acknowledgements
We thank the Data Monitoring Committee of the Health Research Council of New Zealand and Middlemore Clinical Trials for their assistance and support. We would like to acknowledge Christin Coomarasamy (Middlemore Hospital, Auckland, New Zealand) for data entry, data monitoring and statistical support.
Footnotes
This article has an editorial commentary: https://doi.org/10.1183/13993003.03127-2021
This study is registered with the Australian New Zealand Clinical Trials Registry with identifier number ACTRN12612000206820. The data used in this study are stored on a secure drive at Counties Manukau District Health Board facilities. All requests for data sharing will be considered on an individual basis and data will be made available to facilitate replication of the results.
Conflict of interest: All authors have no conflicts of interest to declare.
Support statement: Funding was provided by the Health Research Council of New Zealand (grant 11-694). The sponsor had no role in study design, data collection, data analyses, or data interpretation. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.McShane PJ, Naureckas ET, Tino G, et al. . Non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med 2013; 188: 647–656. doi: 10.1164/rccm.201303-0411CI [DOI] [PubMed] [Google Scholar]
- 2.Khoo JK, Venning V, Wong C, et al. . Bronchiectasis in the last five years: new developments. J Clin Med 2016; 5: 115. doi: 10.3390/jcm5120115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chalmers JD, Chortimall SH. Bronchiectasis: new therapies and new perspectives. Lancet Respir Med 2018; 6: 715–726. doi: 10.1016/S2213-2600(18)30053-5 [DOI] [PubMed] [Google Scholar]
- 4.Goyal V, Chang AB. Combination inhaled corticosteroids and long-acting beta2-agonists for children and adults with bronchiectasis. Cochrane Database Syst Rev 2014; 6: CD010327. doi: 10.1002/14651858.CD010327.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lasserson T, Holt K, Evans D, et al. . Anticholinergic therapy for bronchiectasis. Cochrane Database Syst Rev 2001; 4: CD002163. doi: 10.1002/14651858.CD002163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bateman ED, Rennard S, Barnes PJ, et al. . Alternative mechanisms for tiotropium. Pulm Pharmacol Ther 2009; 22: 533–542. doi: 10.1016/j.pupt.2009.06.002 [DOI] [PubMed] [Google Scholar]
- 7.Ermund A, Meiss LN, Dolan B, et al. . The mucus bundles responsible for airway cleaning are retained in cystic fibrosis and by cholinergic stimulation. Eur Respir J 2018; 52: 1800457. doi: 10.1183/13993003.00457-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toumpanakis D, Loverdos K, Tzouda V, et al. . Tiotropium bromide exerts anti-inflammatory effects during resistive breathing, an experimental model of severe airway obstruction. Int J Chron Obstruct Pulmon Dis 2017; 12: 2207–2220. doi: 10.2147/COPD.S137587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karner C, Chong J, Poole P. Tiotropium versus placebo for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2014; 7: CD009285. doi: 10.1002/14651858.CD009285.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mathioudakis AG, Kanavidis P, Chatzimavridou-Grigoriadou V, et al. . Tiotropium HandiHaler improves the survival of patients with COPD: a systematic review and meta-analysis. J Aerosol Med Pulm Drug Deliv 2014; 27: 43–50. doi: 10.1089/jamp.2012.1012 [DOI] [PubMed] [Google Scholar]
- 11.Wong C, Jayaram L, Karalus N, et al. . Azithromycin for prevention of exacerbations in non-cystic fibrosis bronchiectasis (EMBRACE): a randomised, double-blind, placebo-controlled trial. Lancet 2012; 380: 660–667. doi: 10.1016/S0140-6736(12)60953-2 [DOI] [PubMed] [Google Scholar]
- 12.Chalmers JD, Boersma W, Lonergan M, et al. . Long-term macrolide antibiotics for the treatment of bronchiectasis in adults: an individual participant data meta-analysis. Lancet Respir Med 2019; 7: 845–854. doi: 10.1016/S2213-2600(19)30191-2 [DOI] [PubMed] [Google Scholar]
- 13.Tagaya E, Yagi O, Sato A, et al. . Effect of tiotropium on mucus hypersecretion and airway clearance in patients with COPD. Pulm Pharmacol Ther 2016; 39: 81–84. doi: 10.1016/j.pupt.2016.06.008 [DOI] [PubMed] [Google Scholar]
- 14.Saito Y, Azuma A, Morimoto T, et al. . Tiotropium ameliorates symptoms in patients with chronic airway mucus hypersecretion which is resistant to macrolide therapy. Intern Med 2008; 47: 585–591. doi: 10.2169/internalmedicine.47.0568 [DOI] [PubMed] [Google Scholar]
- 15.Li XL, Cai S, Zhao H, et al. . [Therapeutic effect of tiotropium bromide powder inhalation in patients with stable bronchiectasis.] Nan Fang Yi Ke Da Xue Bao 2010; 30: 1072–1074. [PubMed] [Google Scholar]
- 16.Miller MR, Hankinson J, Brusasco V, et al. . Standardisation of spirometry. Eur Respir J 2005; 26: 319–338. doi: 10.1183/09031936.05.00034805 [DOI] [PubMed] [Google Scholar]
- 17.Jones PW. St. George's Respiratory Questionnaire: MCID. COPD 2005; 2: 75–79. doi: 10.1081/COPD-200050513 [DOI] [PubMed] [Google Scholar]
- 18.Murray MP, Turnbull K, MacQuarrie S, et al. . Validation of the Leicester Cough Questionnaire in non-cystic fibrosis bronchiectasis. Eur Respir J 2009; 34: 125–131. doi: 10.1183/09031936.00160508 [DOI] [PubMed] [Google Scholar]
- 19.Spinou A, Fragkos KC, Lee KK, et al. . The validity of health-related quality of life questionnaires in bronchiectasis: a systematic review and meta-analysis. Thorax 2016; 71: 683–694. doi: 10.1136/thoraxjnl-2015-207315 [DOI] [PubMed] [Google Scholar]
- 20.American Thoracic Society . ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002; 166: 111–117. doi: 10.1164/ajrccm.166.1.at1102 [DOI] [PubMed] [Google Scholar]
- 21.Djukanovic R, Sterk PJ, Fahy JV, et al. . Standardised methodology of sputum induction and processing. Eur Respir J Suppl 2002; 37: 1s–2s. doi: 10.1183/09031936.02.00000102 [DOI] [PubMed] [Google Scholar]
- 22.Hill AT, Haworth CS, Aliberti S, et al. . Pulmonary exacerbation in adults with bronchiectasis: a consensus definition for clinical research. Eur Respir J 2017; 49: 1700051. doi: 10.1183/13993003.00051-2017 [DOI] [PubMed] [Google Scholar]
- 23.Martinez-Garcia MA, Soler-Cataluña JJ, Catalán-Serra P, et al. . Clinical efficacy and safety of budesonide-formoterol in non-cystic fibrosis bronchiectasis. Chest 2012; 141: 461–468. doi: 10.1378/chest.11-0180 [DOI] [PubMed] [Google Scholar]
- 24.Tashkin DP, Celli B, Senn S, et al. . A 4 year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med 2008; 359: 1543–1554. doi: 10.1056/NEJMoa0805800 [DOI] [PubMed] [Google Scholar]
- 25.Jones PW. Estimation and application of the minimum clinically important difference in COPD. Lancet Respir Med 2014; 2: 167–169. doi: 10.1016/S2213-2600(14)70038-4 [DOI] [PubMed] [Google Scholar]
- 26.Camarri B, Eastwood PR, Cecins NM, et al. . Six minute walk distance in healthy subjects aged 55–75 years. Respir Med 2006; 100: 658–665. doi: 10.1016/j.rmed.2005.08.003 [DOI] [PubMed] [Google Scholar]
- 27.Celli B, Decramer M, Leimer I, et al. . Cardiovascular safety of tiotropium in patients with COPD. Chest 2010; 137: 20–30. doi: 10.1378/chest.09-0011 [DOI] [PubMed] [Google Scholar]
- 28.Aliberti S, Lonni S, Dore S, et al. . Clinical phenotypes in adult patients with bronchiectasis. Eur Respir J 2016; 47: 1113–1122. doi: 10.1183/13993003.01899-2015 [DOI] [PubMed] [Google Scholar]
- 29.Price D, Kaplan A, Jones R, et al. . Long-acting muscarinic antagonist use in adults with asthma: real-life prescribing and outcomes of add-on therapy with tiotropium bromide. J Asthma Allergy 2015; 8: 1–13. doi: 10.2147/JAA.S76639 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material: statistical analyses for secondary outcome measures ERJ-02184-2021.Supplement (195.2KB, pdf)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-02184-2021.Shareable (336.3KB, pdf)