Abstract
Background
Elbow supracondylar fractures are common, with treatment decisions based on fracture displacement. However, there remains controversy regarding the best treatments for this injury.
Objectives
To assess the effects (benefits and harms) of interventions for treating supracondylar elbow fractures in children.
Search methods
We searched CENTRAL, MEDLINE, and Embase in March 2021. We also searched trial registers and reference lists. We applied no language or publication restrictions.
Selection criteria
We included randomised and quasi‐randomised controlled trials comparing different interventions for the treatment of supracondylar elbow fractures in children. We included studies investigating surgical interventions (different fixation techniques and different reduction techniques), surgical versus non‐surgical treatment, traction types, methods of non‐surgical intervention, and timing and location of treatment.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. We collected data and conducted GRADE assessment for five critical outcomes: functional outcomes, treatment failure (requiring re‐intervention), nerve injury, major complications (pin site infection in most studies), and cosmetic deformity (cubitus varus).
Main results
We included 52 trials with 3594 children who had supracondylar elbow fractures; most were Gartland 2 and 3 fractures. The mean ages of children ranged from 4.9 to 8.4 years and the majority of participants were boys. Most studies (33) were conducted in countries in South‐East Asia.
We identified 12 different comparisons of interventions: retrograde lateral wires versus retrograde crossed wires; lateral crossed (Dorgan) wires versus retrograde crossed wires; retrograde lateral wires versus lateral crossed (Dorgan) wires; retrograde crossed wires versus posterior intrafocal wires; retrograde lateral wires in a parallel versus divergent configuration; retrograde crossed wires using a mini‐open technique or inserted percutaneously; buried versus non‐buried wires; external versus internal fixation; open versus closed reduction; surgical fixation versus non‐surgical immobilisation; skeletal versus skin traction; and collar and cuff versus backslab.
We report here the findings of four comparisons that represent the most substantial body of evidence for the most clinically relevant comparisons.
All studies in these four comparisons had unclear risks of bias in at least one domain. We downgraded the certainty of all outcomes for serious risks of bias, for imprecision when evidence was derived from a small sample size or had a wide confidence interval (CI) that included the possibility of benefits or harms for both treatments, and when we detected the possibility of publication bias.
Retrograde lateral wires versus retrograde crossed wires (29 studies, 2068 children)
There was low‐certainty evidence of less nerve injury with retrograde lateral wires (RR 0.65, 95% CI 0.46 to 0.90; 28 studies, 1653 children). In a post hoc subgroup analysis, we noted a greater difference in the number of children with nerve injuries when lateral wires were compared to crossed wires inserted with a percutaneous medial wire technique (RR 0.41, 95% CI 0.20 to 0.81, favours lateral wires; 10 studies, 552 children), but little difference when an open technique was used (RR 0.91, 95% CI 0.59 to 1.40, favours lateral wires; 11 studies, 656 children). Although we noted a statistically significant difference between these subgroups from the interaction test (P = 0.05), we could not rule out the possibility that other factors could account for this difference.
We found little or no difference between the interventions in major complications, which were described as pin site infections in all studies (RR 1.08, 95% CI 0.65 to 1.79; 19 studies, 1126 children; low‐certainty evidence). For functional status (1 study, 35 children), treatment failure requiring re‐intervention (1 study, 60 children), and cosmetic deformity (2 studies, 95 children), there was very low‐certainty evidence showing no evidence of a difference between interventions.
Open reduction versus closed reduction (4 studies, 295 children)
Type of reduction method may make little or no difference to nerve injuries (RR 0.30, 95% CI 0.09 to 1.01, favours open reduction; 3 studies, 163 children). However, there may be fewer major complications (pin site infections) when closed reduction is used (RR 4.15, 95% CI 1.07 to 16.20; 4 studies, 253 children). The certainty of the evidence for these outcomes is low. No studies reported functional outcome, treatment failure requiring re‐intervention, or cosmetic deformity. The four studies in this comparison used direct visualisation during surgery. One additional study used a joystick technique for reduction, and we did not combine data from this study in analyses.
Surgical fixation using wires versus non‐surgical immobilisation using a cast (3 studies, 140 children)
There was very low‐certainty evidence showing little or no difference between interventions for treatment failure requiring re‐intervention (1 study, 60 children), nerve injury (3 studies, 140 children), major complications (3 studies, 126 children), and cosmetic deformity (2 studies, 80 children). No studies reported functional outcome.
Backslab versus sling (1 study, 50 children)
No nerve injuries or major complications were experienced by children in either group; this evidence is of very low certainty. Functional outcome, treatment failure, and cosmetic deformity were not reported.
Authors' conclusions
We found insufficient evidence for many treatments of supracondylar fractures. Fixation of displaced supracondylar fractures with retrograde lateral wires compared with crossed wires provided the most substantial body of evidence in this review, and our findings indicate that there may be a lower risk of nerve injury with retrograde lateral wires. In future trials of treatments, we would encourage the adoption of a core outcome set, which includes patient‐reported measures. Evaluation of the effectiveness of traction compared with surgical fixation would provide a valuable addition to this clinical field.
Plain language summary
What are the benefits and risks of different treatments for elbow fractures in children?
Key messages
‐ A child with a supracondylar elbow fracture (a broken bone in the upper elbow, approximately 5 cm above the elbow joint) may have a lower risk of nerve injury if two or more wires are inserted from the outside of the elbow rather than having one wire inserted from the inside of the elbow and one from the outside (crossed wires). The method used by the doctor to manually move the bones back into position may not increase or reduce the risk of nerve injury, but using a closed method may reduce the risk of an infection.
‐ Because we did not find enough studies about other treatments for these elbow fractures, their benefits and risks are unclear.
‐ More, well‐designed studies are needed to give better estimates of the benefits and harms of other treatments. These studies should focus on outcomes related to elbow movement, as well as quality of life and how upset the child is.
What are supracondylar elbow fractures?
This type of broken bone is in the upper arm bone, approximately 5 cm above the elbow joint. It is the most common broken bone in the elbow during childhood, and can affect a child's day‐to‐day function as well as their ability to play and do sport.
How are these broken bones treated?
Treatment varies according to whether the bone has moved out of position. If it has moved, the doctor may manually move it back into a normal position. Doctors do this using a 'closed reduction' (without opening up the skin) or 'open reduction' (after the skin has been opened up).
During surgery, metal wires are used to hold the bone in place whilst it heals. Doctors may use different types and numbers of wires, which are inserted from different angles.
If the bone has not moved, surgery may not be necessary. In which case, treatments to hold the bone in place whilst it heals include using a plaster cast, a sling, or using traction (with weights, ropes and pulleys).
What did we want to find out?
We wanted to find out:
‐ which types of treatments work best to heal the bone effectively; and
‐ whether these treatments are associated with any unwanted effects.
What did we do?
We searched for studies that compared a range of treatments. The most common treatments were:
‐ surgical treatments using different types of metal wires after the bone has been put back into position;
‐ open reduction or closed reduction;
‐ surgery or non‐surgical treatments; and
‐ different non‐surgical treatments.
We compared and summarised their results, and rated our confidence in the evidence, based on factors such as study methods and sizes.
What did we find?
We found 52 studies with 3594 children. Most children were about 5 to 8 years of age and most were boys. The studies were conducted in countries around the world; 33 studies were conducted in countries in South‐East Asia. Very few studies reported how they were funded.
Main results
‐ Wires inserted only from the outside of the elbow may reduce the risk of nerve injury compared with crossed wires (inserted from the outside and also from the inside of the elbow). However, there is probably little or no difference between these treatments in the number of children who develop an infection where the metal wires have been inserted (pin site infections). We do not know if either of these treatments affect elbow function, the risk of needing additional surgery, or any long‐term elbow deformity (where the elbow is no longer the normal shape).
‐ The initial method used to move the bone back into position may make little or no difference to the risk of nerve injury. However, children may have fewer pin site infections when closed reduction is used.
‐ There may be little or no difference between using metal wires to fix the bone or holding the bone in place with a cast in the need for additional surgery, nerve injury, pin site infections, or elbow deformity. But we are uncertain of these findings.
‐ We do not know if a plaster cast compared to a sling has any effect on the risk of nerve injury or pin site infections.
What are the limitations of the evidence?
Most of the studies were either not well‐designed or did not clearly report how they were conducted. This meant that we either had little confidence or no confidence in their findings. Our confidence was also reduced because there were not enough children in the studies to be certain about their findings. It is also possible that the studies that we found had exaggerated findings and that some studies with alternative results may be missing.
How up to date is this evidence?
The evidence is up to date to March 2021.
Summary of findings
Background
Description of the condition
A supracondylar fracture is a break in the humerus (upper arm bone) occurring up to two inches (5 cm) above the elbow joint. The condyles of the humerus are the two rounded prominences at the end of the bone that are part of the elbow joint. Supracondylar fractures are the most common form of elbow fracture in children, with an annual incidence between 60 and 177 per 100,000 children (Holt 2018; Houshian 2011); these fractures are rare in adults. Supracondylar elbow fractures can occur throughout childhood, but are most common in children between five and six years of age (Barr 2014; Holt 2018; Houshian 2011). Several epidemiological studies have reported little gender difference in the incidence of these injuries (Barr 2014; Farnsworth 1998; Holt 2018; Houshian 2011). However, a study of children in Hong Kong reported a higher incidence in boys, equating to a ratio of 17 boys to 10 girls (Cheng 1999).
The typical mechanism of injury is a fall onto an outstretched hand or a direct fall onto a flexed (bent) elbow (Farnsworth 1998). Only 1% of supracondylar fractures are open, with an associated wound providing a direct route for contamination of the bone ends; the remaining 99% are closed (with no open wound). Open fractures require urgent surgical treatment to reduce the risk of serious infection (Holt 2018).
Successful treatment of a supracondylar elbow fracture in a child leads to normal function with no lasting symptoms. However, complications of this injury can occur as a result of the initial injury or as a result of problems during surgery. The major blood vessels and nerves in the arm can be damaged during the injury, the operation, or as a result of compartment syndrome. The last mentioned is a very rare complication where there is excessive swelling in the arm, increasing pressure in the arm and compressing the blood vessels; this can cause permanent damage to the muscles and nerves if not identified and treated promptly. The other significant complication relates to the alignment of the arm. This can arise as a result of malunion (the bone healing up in the wrong position after injury), or as a result of altered growth around the elbow. Children may end up with an arm with cubitus varus (the forearm pointing towards the midline), cubitus valgus (excessive bending away from the midline), or rotational abnormalities. Cubitus varus is commonly unsightly and can be associated with reduced function of the limb.
To identify and try to prevent these serious complications, an adequate history and examination to identify nerve or blood vessel compromise are essential when assessing children with this fracture. Clinical findings along with fracture pattern and the level of displacement on X‐ray can be used to predict the risk of complications. In displaced supracondylar fractures, the reported rate of vascular injury ranges from 5% to 31% and nerve injury at presentation from 5% to 15% (Barr 2014; Del Valle‐Hernández 2017; Farnsworth 1998; Houshian 2011).
When describing the local vascular status of the arm of a child with a supracondylar fracture, a three‐class system is typically used, as described in Omid 2008.
A class 1 injury maintains palpable pulses and a pink hand and indicates normal vascular supply (pink, pulsed). The capillary refill time (how quickly the fingers return to a normal colour after squeezing the finger tip for 5 seconds) is rapid.
A class 2 injury is a pink, pulseless hand where the radial artery can no longer be felt but the capillary refill time after pressure is applied is still normal. This may indicate a compensated vascular insufficiency because of reduced blood flow.
A class 3 injury is the most worrying, with a white, pulseless hand with delayed capillary refill time indicating severe vascular compromise.
Ninety‐five per cent of supracondylar fractures are extension‐type injuries, where the elbow has been over‐extended and the elbow moves backwards in relation to the humerus. Five per cent are flexion type injuries, where the elbow has been over‐bent and the elbow has been pushed forwards in relation to the humerus; these are typically more unstable and more challenging to treat.
There are two commonly used classifications for describing the radiographic appearance of supracondylar fractures. The Gartland classification has been used extensively to describe these injuries and is useful for identification of type 1 (undisplaced) and type 3 (completely displaced) fractures (Gartland 1959). Type 2 fractures (intact posterior cortex) may rotate or have angulation and therefore represent a heterogeneous group of fractures. The Gartland classification has been modified to further describe type 2 fractures based on the presence of rotation (Wilkins 1996), and expanded to include fractures with no intact periosteum (Leitch 2006), as shown in Table 4.
1. Modifications to the Gartland classification system.
| Type | Original Gartland Classification (Gartland 1959) | Wilkins modification (Wilkins 1996) | Leitch et al expansion (Leitch 2006) |
| 1 | Undisplaced or minimally displaced fracture | ‐ | ‐ |
| 2 | Displaced distal fragment with an intact posterior cortex | ‐ | ‐ |
| 2a | ‐ | Displaced distal fragment with an intact posterior cortex with angulation of the distal fragment | ‐ |
| 2b | ‐ | Displaced distal fragment with an intact posterior cortex with angulation and rotation of the distal fragment | ‐ |
| 3 | Completely displaced with no cortical contact | ‐ | ‐ |
| 4 | ‐ | ‐ | Displaced distal fragment with no intact periosteum and multi‐directional instability |
Alternatively, the Arbeitsgemeinschaft für Osteosynthesefragen group (German for 'Association for the Study of Internal Fixation') or AO/ASIF classification offers improved clarity in describing the expected stability of these fractures (Figure 1; Slongo 2006).
1.
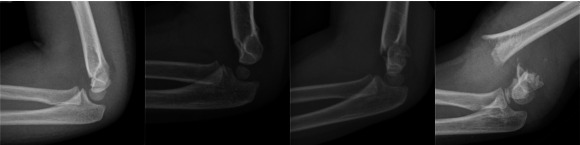
Supracondylar elbow fractures. Left to right AO/ASIF Classification 1, 2, 3, and 4
Type 1 fractures are incomplete and undisplaced.
Type 2 fractures are incomplete but displaced (hinged backwards).
Type 3 fractures are complete breaks with some contact between the bone fragments but with rotation or angulation of the elbow.
Type 4 fractures are off‐ended with no bony contact between the two fragments.
Types 1 and 2 fractures are usually stable with a very low complication rate. Typically, stable fractures require no realignment of the broken (fractured) bone. Types 3 and 4 are unstable injuries with a much higher incidence of malunion (not healing in the correct position). Type 4 injuries are highly associated with damage to blood vessels and nerves, along with the risk of compartment syndrome.
All these injuries usually require three to six weeks of immobilisation followed by a gentle, phased return to activity. It commonly takes two to three months for the recovery of normal function, although this can be much longer if complications occur.
Description of the intervention
There are several treatments available for supracondylar fractures, the choice of which will depend on factors including the severity of the injury, nerve and vascular status of the limb, and surgeon's preference. Recent studies have demonstrated divergence of surgeon preference practice from national guidelines, with unclear consequences for long‐term recovery (Tzatzairis 2021).
Non‐surgical treatments of undisplaced fractures involve immobilisation of the arm in a sling, splint, or cast for three to five weeks to keep the elbow in the correct position. A cast is typically constructed of plaster of Paris or a synthetic resin, and extends from above the elbow to the forearm or the hand. Minimally displaced fractures can be treated in a number of ways. Some displacement can be accepted, relying on the child’s ability to remodel (grow the bone out straight), or it can be reduced. Reduction (repositioning the broken bone back to the normal alignment) commonly involves bending the elbow up to bring the bone into the normal position and then treating it in a sling, cast, or splint as above. Some displaced fractures can be treated with traction. Traction can be applied as skin traction (where the traction is strapped to the child's arm) or skeletal traction (where the traction is applied directly to the bone using a screw, pin, or wire). The non‐surgical method of skin traction is more common than skeletal traction, which requires an operation to insert the screw, pin or wire. In both methods, the limb is kept straight whilst it heals using weights attached through pulleys. The arm can be positioned to the side of the child (Dunlop traction) or above the child (overhead traction). Treatment with traction requires a prolonged hospital stay.
Surgical management of supracondylar fractures in children is achieved following general anaesthesia, using a closed or open technique to reduce (realign) the fractured bone. Stabilisation of the fracture is most commonly performed using percutaneous wires: smooth metal Kirschner wires (K‐wires) are placed through tiny incisions in the skin. Two or more wires are inserted, depending on the stability of the break. A crossed K‐wire technique will have an entry point on either side of the elbow forming an 'X' on X‐ray, whereas a lateral wire divergent technique has the wires inserted only from the outside part of the elbow. Children treated in this manner are commonly treated in cast to protect the wires until wire removal three to four weeks after the fixation. Alternative but rarer stabilisation techniques include the use of a plate and screws; or an external fixator, where a frame is seen on the outside of the limb, which holds the bones together whilst they heal.
The timing of surgery is typically dictated by the vascular status of the limb. The presence of a class 3 injury (white, pulseless hand) is a surgical emergency as it represents a limb‐threatening injury. Class 1 and 2 injuries (pink hand with or without radial pulse) are treated as urgent cases with careful monitoring to ensure that they do not evolve into class 3. The optimal timing of surgery for class 1 and 2 injuries is unclear, although it is accepted that those without a palpable pulse are of higher urgency.
Minimally displaced fractures are routinely reduced in the hospital emergency department and immobilised. Very displaced fractures require a more formal reduction with significant sedation or a general anaesthetic. Most centres will perform this in theatre as fixation of the fracture can be performed at the same time. Some centres will attempt reduction with sedation in the hospital emergency department to reduce the delay to reduction. Most severely displaced fractures are unstable and so require fixation in theatre even after an initial reduction. This leads to two procedures instead of one. Waiting for theatre availability often adds a significant delay that can cause an increase in nerve or vascular damage.
How the intervention might work
The fracture pattern and the presence of neurological or vascular compromise will impact the treatment proposed for a child with a supracondylar elbow fracture. More stable fractures are often treated without surgery whereas complete fractures typically require surgery.
Non‐operative interventions, including traction, immobilise the arm for enough time to allow bone healing and avoid the risk of surgical complications. However, potential complications associated with the non‐operative treatment of these fractures include malunion (bone healing with a deformity if the bones are not reduced and fixed), compartment syndrome, and pressure injury from casts.
Surgical reduction and stabilisation results in a more anatomical restoration of bony anatomy and better stabilisation of the fracture, though a cast is still required if percutaneous fixation is performed. The most common form of surgical fixation is to use two or three smooth K‐wires. Crossed wires (one entering the bone from each side of the elbow in a cross formation) is the most stable pattern for the wires. The main risk with this procedure is damage from the wire to the ulnar nerve on the inside part of the elbow. Many methods have been employed to try to protect this nerve, including explorations, minimal open reduction, and digital pressure from the thumb over the bone to keep the nerve out of the way. Rates of nerve injury with these techniques vary from 0% to 14%. The alternative treatment is to use two laterally‐placed wires on the outside part of the elbow. This reduces the chance of causing iatrogenic injury of the nerve on the inside part of the elbow but does not remove the risk completely. It is technically more challenging to do and has less stability to rotation in comparison with crossed wires. This may lead to higher rates of malunion than crossed wires.
Following surgery, pins can migrate or cause damage to local structures, including nerves. Internal fixation involves making a more substantial scar and can also lead to nerve injury. Surgery also introduces risks, including infection which can be superficial (skin) or deep (involving the bones); and risks associated with receiving a general anaesthetic. External fixation is recognised to be technically challenging but offers a more rigid fixation compared with percutaneous wires (Horst 2011). Many surgical techniques leave pins, wires, or screws that need to be removed at a second operation or in clinic. When these are removed in theatre, there is need for an additional (i.e. repeat) anaesthetic and a second risk of injury to nerves as the scars are opened and the metalwork is removed.
There is also uncertainty regarding the optimum time to take a child to theatre with this injury. Many surgeons are guided by the perfusion of the hand and neurological status at time of presentation (the pink pulsed hand or the white pulseless hand). It remains unclear if children benefit from having surgery overnight, or if outcomes are improved by waiting for surgery on a daytime routine trauma list (Terpstra 2022).
The timing and location of the reduction of a supracondylar fracture may be an indicator of the severity of injury or the availability of theatre time and sedation protocols in the hospital emergency department. A reduction in theatre can be supplemented with internal fixation with percutaneous wires or other fixation technique, but introduces additional delays as the child is admitted to the hospital and prepared for an anaesthetic. This delay may result in additional complications but may improve the quality of reduction and fixation options.
Why it is important to do this review
There is little controversy regarding non‐surgical treatment for completely undisplaced supracondylar fractures in children. These should be treated in a cast or a sling to keep the elbow bent until the break heals. There is uncertainty, however, regarding the ideal treatment for minimally displaced breaks that are hinged backwards (AO Type 2 fractures). Many centres treat these in a sling or a cast, but some surgeons will take these children to theatre for reduction and surgical fixation. This requires two procedures with uncertain evidence of improved outcome.
For significantly displaced fractures (AO Type 4), the only clear agreement in the literature is that they require some form of reduction (Vaquero‐Picado 2018). Treatment options vary from traction to reduction and fixation. There is no agreement on the timing of surgical intervention. It is well established that the longer a fracture remains displaced, the greater the risk of complications. It is known that vascular compromise and nerve injuries are commonly seen in these injuries and, rarely, compartment syndrome can develop. There is still no consensus as to the reasonable timing of surgical intervention (Terpstra 2022).
For surgical fixation, fractures are most commonly fixed using two or three smooth K‐wires. There is controversy regarding the configuration of these wires to maximise the fracture stability whilst minimising the risks of complications (American Academy of Orthopaedic Surgeons 2011).
Two areas of further uncertainty and variation in practice are: what is the best treatment for these injuries when there is also a vascular injury; and how much displacement can be accepted, given the ability of children's bones to remodel and align over time (Griffin 2008; Hell 2021; Mangat 2009).
In the USA, national database analysis suggests that there has been a decrease in the rate of open reduction for displaced supracondylar fractures, with an associated increase in closed reductions (Holt 2017). There is relatively little long‐term follow‐up available in the literature. Using the Flynn criteria (Flynn 1974), Sinikumpu 2016 included a 12‐year follow‐up showing that only 75% of those treated for a supracondylar fracture in childhood reported good or excellent function, whereas 17/51 (33%) of those with displaced fractures reported an unsatisfactory outcome post injury.
The 2012 American Association of Orthopedic Surgeons (AAOS) guidelines for the management of paediatric supracondylar fractures identified several areas of care with limited or inconclusive evidence, including the use of lateral‐only wires or crossed wires, the time threshold for reduction of displaced fractures, or the need for an open surgical exploration of vascular structures in a class 2 vascular injury (pink pulseless hand) (American Academy of Orthopaedic Surgeons 2011). The 2016 National Institute for Health and Care Excellence (NICE) NG38 guideline was unable to deliver any recommendations for the management of childhood supracondylar fractures (NICE 2016).
This review will analyse the current evidence to inform evidence‐based practice.
Objectives
To assess the effects (benefits and harms) of interventions for treating supracondylar elbow fractures in children.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) and quasi‐RCTs (method of allocating participants to a treatment that is not strictly random, e.g. by hospital number) that assess interventions for treating supracondylar elbow fractures in children. We were not anticipating any cluster‐RCTs where the unit of randomisation was a ward or other grouping of individuals. We would have included these studies if they had been appropriately designed.
Types of participants
We included studies where children have sustained an acute (usually presenting for treatment within seven days) supracondylar elbow fracture. We included children of all ages; thus, from birth to skeletal maturity.
Studies with mixed populations (e.g. children with other elbow fractures) were eligible for inclusion, provided children meeting the above inclusion criteria were the predominant subgroup, or data relating to their outcomes could be extracted either from the study manuscript or through contacting study authors.
Types of interventions
We divided interventions into non‐surgical and surgical categories. We did not include trials that investigated the management of soft tissue complications, pharmacological analgesia regimens, or rehabilitation strategies.
We planned to make the following comparisons.
-
Different forms of surgical intervention. Our main comparisons were:
different fixation techniques (e.g. crossed K‐wires versus lateral only; K‐wires versus internal fixation; internal versus external fixation);
different reduction techniques (open reduction, where the pieces of broken bone are put back into place through a surgical approach, versus closed reduction, where the bone is put into place without making a surgical wound).
Surgical versus non‐surgical treatment.
-
Different forms of traction. Our main comparison was:
type of traction: skeletal traction (placing a wire, screw, or pin into the bone) versus skin traction (strapping the traction to the arm).
-
Different methods of non‐surgical intervention. Our main comparisons were:
form of immobilisation (cast and sling versus sling alone versus skin traction);
duration of prescribed immobilisation (either following surgery or as primary treatment).
-
Timing and location of treatment:
location of closed reduction (hospital emergency department versus operating theatre);
timing of surgery as emergent (within 6 hours) or urgent (within 24 hours).
Types of outcome measures
Critical outcomes
The critical outcomes for presentation in the summary of findings tables are as follows.
Functional outcome, at medium‐ or long‐term follow‐up, as rated by the child or their parent using a validated outcome measure, such as the Activity Scale for Kids performance version (ASK‐p; Young 2000), Paediatric Outcome Data Collection Instrument (PODCI; Daltroy 1998), Disabilities of the Arm, Shoulder and Hand Score (DASH or quickDASH; Beaton 2005; Hudak 1996), or ABILHAND‐Kids score (Arnould 2004).
Treatment failure at any follow‐up point, as indicated by a loss of reduction requiring a re‐intervention (e.g. re‐fixation) or a symptomatic malunion or unacceptable deformity requiring re‐intervention after the fracture has healed. We expressed this as the number of children who experienced a treatment failure.
Nerve injury at any follow‐up point: this may be transient or permanent and affecting the radial, ulnar, or median nerve, singly or in combination. We expressed this as the number of children who experienced a nerve injury.
Major complications, including compartment syndrome, vascular injury, and infection, at any follow‐up point, expressed as the number of children who experienced a complication.
Cosmetic deformity measured either objectively or subjectively at medium‐ and long‐term follow‐up. In order to account for patient variation, we extracted carrying angle from studies as a marker of cosmetic deformity when presented as a loss of carrying angle. We did not include absolute values for carrying angle, as the range of carrying angles measured in children without elbow disorders ranges from 0° to 29° and also changes with age (Golden 2007; Sharma 2013). We set a threshold of 10° change as clinically significant to be consistent with the widely‐used grading system proposed by Flynn (Flynn 1974), and because it exceeds the measurement error of 6.5° when using a goniometer to measure carrying angle (Chapleau 2011).
Other outcomes
We also collected the following outcomes that were not for presentation in the summary of findings tables:
functional outcome, as rated by the child or their parent using a validated outcome measure, at early follow‐up;
range of movement at the elbow (flexion and extension) at medium‐term and long‐term follow‐up;
pain as assessed by child or parent using a child‐appropriate scale, such as Faces Pain Scale (Bieri 1990), at early, medium‐, and long‐term follow‐up;
quality of life for children assessed using a patient‐ or parent/carer‐reported outcome tool, such as EQ‐5D‐Y (Wille 2010), at early, medium‐, and long‐term follow‐up;
child or parent satisfaction at early, medium‐, and long‐term follow‐up;
return to sport and normal activities;
radiographic deformity, including that measured through loss of Baumann's angle and humeral‐capitellar angle at medium‐ and long‐term follow‐up;
emotional distress of the child, as measured using a validated tool such as the Paediatric Index of Emotional Distress (PI‐ED; O'Connor 2016) or the Pediatric Emotional Distress Scale (PEDS; Saylor 1999), at early follow‐up.
We included measurements for range of motion and radiographic parameters (e.g. Baumann's angle) where a relative change of movement or angle was presented. This was to account for the individual patient variation, with a wide spectrum of normal values for these outcomes. Where possible, we dichotomised range of motion using a threshold of 10° loss of movement to be consistent with the widely‐used grading system proposed by Flynn (Flynn 1974), and to exceed the measurement error of 7° to 10° when using a goniometer to measure range of movement (Chapleau 2011).
We planned to analyse timing of outcomes as early (less than three months following injury), medium‐term (three months to less than six months following injury) and long‐term (six months or longer) outcomes.
Resource use
We also recorded resource use (e.g. number of outpatient visits and routine cast changes; duration of hospitalisation), other costs, and findings of included trials reporting cost‐effectiveness analysis.
Search methods for identification of studies
Electronic searches
We searched for all published and unpublished relevant RCTs, without restrictions on language or publication status and in consultation with the Bone, Joint and Muscle Trauma (BJMT) Group Information Specialist.
We identified published, unpublished, and ongoing studies by searching the following databases from their inception:
Cochrane Central Register of Controlled Trials (CENTRAL, 9 March 2021, Issue 3) in the CRS‐Web;
MEDLINE (Ovid MEDLINE and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Daily and Versions 1946 to 9 March 2021);
Embase Ovid (1974 to 9 March 2021);
ClinicalTrials.gov (to 21 April 2020);
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP);
relevant conference proceedings, including the Bone and Joint Journal (BJJ) Orthopaedic Proceedings, proceedings of the annual meetings of the Pediatric Orthopaedic Society of North America (POSNA), and the European Paediatric Orthopaedic Society (EPOS).
At the time of the search, CENTRAL was fully up to date with all records from the BJMT Group’s Specialised Register so it was not necessary to search this separately.
In MEDLINE, we combined the subject‐specific terms with the sensitivity‐maximizing version of the Cochrane Highly Sensitive Search Strategy for identifying randomised trials (Lefebvre 2019). Search strategies can be found in Appendix 1.
Searching other resources
We checked the bibliographies of included studies and any relevant systematic reviews we identified for further references to relevant trials. We contacted experts and organisations in the field to obtain additional information on relevant trials. We did not perform a separate search for adverse effects of interventions used for the treatment of supracondylar elbow fractures.
Data collection and analysis
Selection of studies
Two review authors (BAM and SC) independently screened search results for eligible studies. We sought full‐text articles for any study judged eligible by either review author. The same two review authors then performed independent study selection. We resolved any disagreements by discussion or, if necessary, we obtained adjudication by a third author (BJO). We did not mask the source and authorship of the trial citations or reports.
Data extraction and management
Two review authors (BAM and SC or AI) independently extracted qualitative and quantitative data from each included trial using a data extraction form, which was piloted on two trials. We collected information on study characteristics, such as the study design and setting, the intended and actual study population, the study interventions and other care provided, outcomes measurement, and results. We contacted trialists for further details as necessary. We resolved any differences or disagreements by checking trial reports, contacting trial authors, discussion between the two review authors (BAM and SC or AI), or adjudication by a third review author (BJO). Two review authors (BAM and SC) entered the data into Review Manager 5 (Review Manager 2014).
Assessment of risk of bias in included studies
Two review authors (BAM and SC or AI) independently assessed risk of bias using Cochrane's risk of bias tool, without masking of the source and authorship of the trial reports (Higgins 2011). We resolved any disagreement by involving the senior review author (BJO). We judged each study as ‘low risk’, ‘unclear’, or ‘high risk’ of bias for the following domains:
random sequence generation (selection bias);
allocation concealment (selection bias);
blinding of participants and personnel (performance bias);
blinding of outcome assessment (detection bias);
completeness of outcome data (attrition bias);
selective outcome reporting (reporting bias);
other potential sources of bias.
We considered subjectively‐ (parent or child) reported outcomes and objectively‐ (including clinician‐rated) reported outcomes separately in our assessment of blinding and completeness of outcome data.
Measures of treatment effect
To measure treatment effect, we calculated risk ratios (RR) for binary outcomes and mean differences (MD) for continuous outcomes. We planned to calculate standardised mean differences when pooling data from continuous outcomes based on different scoring schemes. We planned to present final scores in preference to change scores. For all outcomes, we presented 95% confidence intervals (95% CIs).
Unit of analysis issues
We anticipated that for individually randomised trials, the unit of analysis would be individual children randomised to treatments, as bilateral supracondylar fractures are very rare. Although cluster‐randomised trials were unlikely, we took appropriate measures to avoid unit‐of‐analysis issues as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Whilst we did not expect to identify any cross‐over trials, given their study design is inappropriate for the condition and interventions covered by this review, we planned to use data from the first phase only if such trials were included. Where study participants crossed from one treatment group to another, we planned to analyse the results according to their allocation group (intention‐to‐treat analysis). We were aware of potential issues with multiple measurements if studies reported repeated follow‐up or described a total number of complications without expressing how many children had multiple complications. When reporting a composite measure of all major complications, we confirmed with study authors that individual children did not experience more than one complication.
Dealing with missing data
We contacted study authors to obtain missing data and information. Where possible, we calculated missing standard deviations from other data (standard errors, 95% CIs, exact P values). We did not impute missing standard deviations or other data. We performed a sensitivity analysis to investigate the possible effects of missing data.
Assessment of heterogeneity
We performed assessment for heterogeneity through visual inspection of forest plots and calculation of the Chi² statistic. We calculated study inconsistency using the I² statistic. Interpretation of these statistics followed the Cochrane Handbook for Systematic Reviews of Interventions with a statistically significant level of 0.10 for the Chi² test. We interpreted I² statistic values using recommendations from the Cochrane Handbook for Systematic Reviews of Interventions: 0% to 40% might not be important; 30% to 60% may represent moderate heterogeneity; 50% to 90% may represent substantial heterogeneity; and 75% to 100% may represent very substantial heterogeneity (Higgins 2011).
Assessment of reporting biases
Where more than 10 studies contributed data to an analysis, we constructed a funnel plot to explore the potential for publication bias as well as for possible small‐study biases. We planned to assess the magnitude of publication bias first by visual inspection of the asymmetry of the funnel plot. If this appeared markedly asymmetric, we planned to consider performing formal statistical tests (Egger 1997).
Data synthesis
When we considered it appropriate, we pooled the results of comparable studies using both fixed‐effect and random‐effects models. Our choice of model was guided by careful consideration of the extent of heterogeneity and whether it could be explained, in addition to other factors, such as the number and size of included studies. We used 95% CIs throughout. We did not pool data where there was substantial heterogeneity (I² ≥ 75%) that could not be explained by the diversity of methodological or clinical features among studies. Where pooling data was inappropriate, we still presented study data in the analyses or tables for illustrative purposes and reported these in the text.
Subgroup analysis and investigation of heterogeneity
We planned to perform subgroup analysis for the following groups but found that insufficient studies reported study characteristics to allow for meaningful analysis.
Levels of displacement: e.g. using the AO/ASIF classification 1 and 2 versus 3 and 4.
Presence of vascular injury (pink pulsed versus pink pulseless versus white hand).
Timing of surgery as defined by emergent (within 6 hours) or urgent (within 24 hours).
Presence or not of nerve injury.
Age of child at time of injury: age 0 to 4 (preschool), 5 to 10 (primary school), 11+ (secondary school).
In addition to these planned subgroups, we performed a post hoc subgroup analysis for the nerve injury outcome for comparisons that involved inserting a K‐wire through the medial epicondyle: medial wire inserted with a percutaneous (through the skin) technique versus medial wire inserted using an open technique (where a cut in the skin was made to identify the bone before inserting the wire).
We investigated whether the results of subgroups were significantly different by inspecting the overlap of CIs and performing the test for subgroup differences available in Review Manager 5 (Review Manager 2014).
Sensitivity analysis
For critical outcomes reported in the summary of findings tables, we performed sensitivity analyses to detect the following:
influence of any small‐study effects by comparing the random‐effects model output to a fixed‐effect model calculated with the Mantel‐Haenszel method;
impact of inclusion and exclusion of studies published only as abstracts;
impact of including studies with high risk of bias; in particular, those identified with allocation bias or a lack of blinding for outcome assessors;
analysis of the result of any missing data on review conclusions.
Whilst conducting the search, we noted that some studies appeared to have duplicate publications with different author teams. We also noted that several studies were published in journals that were not indexed in PubMed. Because we could not rule out the possibility of compromised data integrity, we conducted two additional sensitivity analyses on the critical outcomes reported in the summary of findings tables:
impact of inclusion and exclusion of studies published in journals that were not indexed in PubMed;
impact of inclusion and exclusion of studies that had multiple publications from different author teams.
Summary of findings and assessment of the certainty of the evidence
Two review authors used the GRADE system to assess the certainty of the body of evidence associated with the five critical outcomes in the review (Schünemann 2019):
functional outcome at final study follow‐up;
treatment failure requiring re‐intervention at final study follow‐up;
nerve injury at final study follow‐up;
Major complications (all complications) during study follow‐up;
cosmetic deformity in the long term (from six months onwards).
Cosmetic deformity was measured using various criteria. For the summary of findings tables, we used the number of children with cubitus varus.
The GRADE approach assesses the certainty of a body of evidence based on the extent to which we can be confident that an estimate of effect or association reflects the item being assessed. Evaluation of the certainty of a body of evidence considers within‐study risk of bias (study limitations), directness of the evidence (indirectness), heterogeneity of the data (inconsistency), precision of the effect estimates (imprecision), and risk of publication bias. The certainty of the evidence could be high, moderate, low or very low, being downgraded by one or two levels depending on the presence and extent of concerns in each of the five GRADE domains. We used footnotes to describe reasons for downgrading the certainty of the evidence for each outcome, and we used these judgements when drawing conclusions in the review.
We did not construct summary of findings tables for all comparisons in this review. Instead, we selected comparisons which we judged to have the most clinical relevance and which also provided the most substantial body of evidence. We therefore constructed summary of findings tables for the following comparisons in this review, using the GRADE profiler software (GRADEpro GDT):
different forms of surgical intervention: retrograde lateral wires versus retrograde crossed wires;
different forms of surgical intervention: open versus closed reduction;
surgical versus non‐surgical intervention: surgical fixation versus immobilisation.
We also planned to construct a summary of findings table for backslab versus sling (from the comparison of different methods of non‐surgical intervention). However, only one study investigated this comparison and data were either not reported for the critical outcomes or included no events in either group; thus, we did not construct this table.
Results
Description of studies
Results of the search
After the removal of duplicates from the search results, we screened 1222 titles and abstracts, which included handsearching of conference proceedings, backward citation searches, and searches of clinical trials registers. We reviewed the full text of 130 records, and selected 52 studies (with 69 records) for inclusion in the review. We excluded 48 records, and report the details of 35 key studies from these excluded records. Ten studies are awaiting classification, and we identified three ongoing studies. See Figure 2.
2.
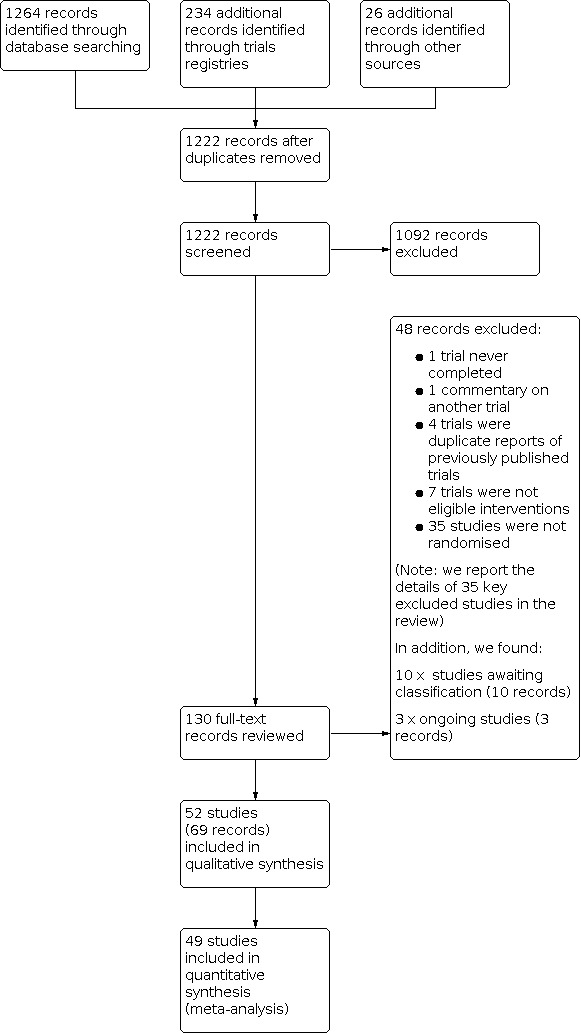
Study flow diagram
Included studies
Two studies were available as conference reports (Mulpuri 2016; Said 2015), and two studies were available as theses (Raj 2018; Vaidya 2009). The remaining studies were published as full reports in scientific journals. All reports were in English, aside from Dehghan 2012, the full text of which was written in Persian and for which we obtained a translation.
We requested additional information from study authors of all included trials. We received additional unpublished information for six trials (Abubeih 2019; Gaston 2010; Gopinathan 2018; Naik 2017; Oakley 2009; Subash 2020). We had insufficient detail to report outcome data for three included studies (Ahmad 2020; Das 2019; Rakha 2020). We report the details of the individual studies' designs, participants, interventions, outcomes, conflicts of interest, and funding in Characteristics of included studies.
Setting
A summary of study setting and characteristics is shown in Table 5. The publication dates of the included trials ranged from 2004 to 2021. The trials were completed in 16 different countries, with 33 studies from South‐East Asia (India: Aher 2018; Ajmera 2013; Arun 2018; Das 2019; Gholap 2020; Gopinathan 2018; Jain 2019; Kalia 2018; Kumar 2021; Maity 2012; Mandal 2018; Naik 2017; Naveen 2017; Raj 2018; Palange 2019; Patil 2017; Prashant 2016; Ray 2019; Sankar 2019; Shah 2017; Subash 2020, Pakistan: Ahmad 2020; Anwar 2011; Majeed 2020; Rakha 2020; Saeed 2020; Shafi‐Ur‐Rehman 2013, Nepal: Afaque 2020; Pandey 2008; Vaidya 2009, China: Zhu 2016, Malaysia: Foead 2004, Thailand: Kaewpornsawan 2001); four studies from North America (USA: Gaston 2010; Kocher 2007; Tripuraneni 2009, Canada: Mulpuri 2016); two studies from Europe (Croatia: Ducic 2016a, Italy: Pavone 2016); 11 studies from Middle Eastern countries (Egypt: Abdel Karim 2016; Abubeih 2019; Othman 2017; Rizk 2019; Sadek 2018; Said 2015; Shamma 2020, Turkey: Altay 2011; Ercin 2016, Iraq: Dawood 2011, Iran Dehghan 2012); and two studies from Oceania (Australia: Oakley 2009, Papua New Guinea: Kuzma 2014).
2. Summary characteristics of included studies.
| Study | Country | Number of participants (mean age in years, % male) | Fracture type (numbers of fractures) | Reduction technique | Medial wire technique |
| Retrograde lateral wires versus retrograde crossed wires | |||||
| Abdel Karim 2016 | Egypt | 60 children: mean age 5.1, 70% male | Gartland 2 (7) and Gartland 3 (52) | Closed | Open (1.5 cm to 3.0 cm incision) |
| Abubeih 2019 | Egypt | 91 children: mean age for children who completed follow‐up was 5.1, 67% male | Gartland 3 (91) | Closed | Open (1.5 cm to 3.0 cm incision) |
| Afaque 2020 | Nepal | 84 children: mean age for children who completed follow‐up was 7.0, 62% male | Gartland 2, 3 or 4 (84) | Closed | Percutaneous |
| Aher 2018 | India | 60 children: mean age 7, 90% male | Gartland 3 (60) | Closed | Percutaneous |
| Ahmad 2020 | Pakistan | 70 children: mean age and gender distribution not specified | Gartland 1 and 2 (70) | Closed | Percutaneous |
| Anwar 2011 | Pakistan | 50 children: mean age 7, 33% male | Gartland 2 and 3 supracondylar fractures (50) | Closed | Not specified |
| Arun 2018 | India | 68 children: mean age 8.4, 68.7% male | Gartland 3 (68) | Closed | Percutaneous |
| Das 2019 | India | 50 children; mean age not reported, 56% male | Gartland 3 and 4 (50) | Closed | Not specified |
| Foead 2004 | Malaysia | 66 children: mean age 5.8, gender split not specified | Gartland 2 or 3 fractures (66) | Closed | Not specified |
| Gaston 2010 | USA | 104 children: mean age 6, 51% male | Gartland 3 (104) | Closed | Percutaneous |
| Gholap 2020 | India | 30 children: mean age 6.8, 70% male | Gartland 3 (30) | Closed | Open (incision length not specified) |
| Kocher 2007 | USA | 52 children: mean age 5.9, 44.2% male | Gartland 3 (52) | Closed | Open (1.5 cm to 3.0 cm incision) |
| Maity 2012 | India | 160 children: mean age 6.2, 61.9% male | Gartland 2 (71) and Gartland 3 (89) | Closed | Open (1 cm incision) |
| Majeed 2020 | Pakistan | 180 children: mean age 6.5, 63.9% male | Fracture classification not specified | Closed | Not specified |
| Mandal 2018 | India | 60 children: mean age 8.1, 68.3% male | Gartland 2 (7) and 33 Gartland 3 (33) | Closed | Not specified |
| Mulpuri 2016 | Canada | 52 children: mean age and gender split not specified | Gartland 3 (52) | Closed | Not specified |
| Naik 2017 | India | 57 children: mean age 6.7, 61.2% male | Gartland 3a (46) and Gartland 3b (9) | Closed | Open (small incision) |
| Naveen 2017 | India | 46 children: mean age 7.5, 63% male | Gartland 2 (15) and Gartland 3 (25) | Closed | Percutaneous |
| Othman 2017a | Egypt | 47 children: mean age 5.5, gender split not reported | Gartland (22) and Gartland 3 (25) | Closed | Percutaneous |
| Palange 2019 | India | 30 children: mean age 7, 63.3% male | Gartland 3 (30) | Closed | Open (small incision) |
| Patil 2017 | India | 30 children: mean age 8, 63.3% male | Gartland 3 (30) | Closed | Percutaneous |
| Pavone 2016 | Italy | 35 children: mean age 6, 72.4% male | Gartland 3 (35) | Closed | Not specified |
| Prashant 2016 | India | 76 children: mean age for children who completed follow‐up was 8.4, 72.6% male | Gartland 3 (76) | Closed | Percutaneous |
| Raj 2018 | India | 20 children: mean age 8.4, 70% male | Gartland 3 (20) | Closed | Not specified |
| Said 2015 | Egypt | 44 children: mean age for children who completed follow‐up was 5.8, 70.0% male | Gartland 3 (44) | Closed | Percutaneous |
| Shafi‐Ur‐Rehman 2013 | Pakistan | 200 children: mean age 6.2, 79.9% male | Gartland 3 (200) | Closed | Not specified |
| Subash 2020 | India | 66 children: mean age for children who completed follow‐up was 6.1, 63.3% male | Fracture types not specified | Closed | Open |
| Tripuraneni 2009 | USA | 40 children: mean age 4.9, gender split not specified | Gartland 2 (5) and Gartland 3 (35) | Closed | Percutaneous |
| Vaidya 2009 | Nepal | 60 children: mean age 5.8, 66.3% male | Gartland 3 (60) | Closed | Open (2 cm to 3 cm incision) |
| Dawood 2011 | Iraq | 21 children: mean age 6.5, 61.9% male | Gartland 3 (21) | Open | Open |
| Sankar 2019 | India | 60 children: mean age 7.6, 70% male | Gartland 3 (60) | Open | Open |
| Lateral crossed (Dorgan) wires versus retrograde crossed wires | |||||
| Altay 2011 | Turkey | 29 children: mean age 7.8, 67.9% male | Gartland 3 (29) | Closed | Not specified |
| Ducic 2016a | Croatia | 138 children: mean age 6.5, 65.2% male | Gartland 2a (12), Gartland 2b (35) and Gartland 3 (91) | Closed | Not specified |
| Kalia 2018 | India | 60 children: mean age 6.7, 63.3% male | Gartland 2 (19) and Gartland 3 (41) | Closed | Open (stab incision and direct visualisation) |
| Rizk 2019 | Egypt | 50 children: mean age 6.5, 64% male | Gartland 3 (50) | Closed | Percutaneous |
| Lateral crossed (Dorgan) wires versus retrograde lateral wires | |||||
| Sadek 2018 | Egypt | 40 children: mean age 6.2, 72.5% male | Gartland 2 or 3 (40) | Closed | N/A |
| Posterior intrafocal wire versus retrograde crossed wires | |||||
| Jain 2019 | India | 168 children: mean age 6.8, 70.2% male | Gartland 2 (58) and Gartland 3 (110) | Closed | N/A |
| Retrograde lateral wires in parallel versus divergent configuration | |||||
| Gopinathan 2018 | India | 30 children: mean age 7.6, 77% male | Gartland 3 (30) | Closed | N/A |
| Shamma 2020 | Egypt | 30 children: mean age 5.1, 70% male | Gartland 2 (3) and Gartland 3 (27) | Closed | N/A |
| Mini‐open crossed wires versus percutaneous crossed wires | |||||
| Ercin 2016 | Turkey | 104 children: mean age 6.5, 60.6% male | Gartland 3 (104) | Closed | N/A |
| Buried versus non‐buried wires | |||||
| Saeed 2020 | Pakistan | 80 children: mean age 7.5, 56.3% male | Fracture types not specified | Open | Open |
| Open reduction versus closed reduction | |||||
| Ajmera 2013 | India | 87 children: mean age for children who completed follow‐up was 7, 52.3% male | Gartland 3 (87) | N/A | Percutaneous |
| Dehghan 2012 | Iran | 90 children: mean age 6.1, 55% male | Gartland 3 (90) | N/A | Not specified |
| Kaewpornsawan 2001c | Thailand | 28 children: mean age 7.6, 67.9% male | Gartland 3 (28) | N/A | Percutaneous |
| Rakha 2020 | Pakistan | 130 children; mean age 7.3, 77.7% male | Garland 3 (130) | N/A | Not specified |
| Ray 2019 | India | 70 children: mean age 7, 64% male | Gartland 3 (70) | N/A | Not specified |
| Zhu 2016b | China | 68 children: mean age 7.8, 54.4% male | Gartland 2 (39) and Gartland 3 (29) | N/A | N/A |
| Surgical fixation versus non‐surgical (immobilisation) | |||||
| Kumar 2021 | India | 60 children: mean age and gender split not specified | Fracture types not specified | Closed | Percutaneous |
| Pandey 2008 | Nepal | 60 children: mean age 7.7, 68.3% male | Gartland 2b or 3 supracondylar fractures (60) | Closed | Percutaneous |
| Shah 2017 | India | 20 children: mean age and gender split not specified | Gartland 3 (20) | Closed | Not specified |
| Skin traction versus olecranon skeletal traction | |||||
| Kuzma 2014 | Papua New Guinea | 133 children: mean age 7.5, 59.4% male | Gartland 2 (6) and Gartland 3 (127) | Closed | N/A |
| Backslab versus sling | |||||
| Oakley 2009 | Australia | 50 children: mean age 5.5, 62% male | Gartland 1 (50) | N/A | N/A |
aThree‐arm study (retrograde crossed wires, retrograde lateral wires and lateral crossed (Dorgan) wires). Data are for all included participants bOpen reduction achieved using an indirect (joystick) technique so not included in meta‐analysis as did not involve direct visualisation of the fracture cStudy compared open and closed reduction and retrograde crossed wires and lateral crossed (Dorgan) wires
N/A: not applicable
Participants
Sex and age
The 52 included trials recruited a total of 3594 children. Four studies did not report the mean age or the gender split of the included children (Ahmad 2020; Kumar 2021; Mulpuri 2016; Shah 2017), three studies did not report the gender split of their participants (Foead 2004; Othman 2017; Tripuraneni 2009), and one study did not report the mean age (Das 2019).
The majority of the participants in most studies were male. In four studies, more than 75% of participants were male (Aher 2018; Gopinathan 2018; Rakha 2020; Shafi‐Ur‐Rehman 2013). In 19 studies, 65% to 74.9% of the participants were male (Abdel Karim 2016; Abubeih 2019; Altay 2011; Anwar 2011; Arun 2018; Ducic 2016a; Gholap 2020; Jain 2019; Kaewpornsawan 2001; Mandal 2018; Raj 2018; Pandey 2008; Pavone 2016; Prashant 2016; Sadek 2018; Said 2015; Sankar 2019; Shamma 2020; Vaidya 2009). In 21 studies, 50% to 64.9% of the participants were male (Afaque 2020; Ajmera 2013; Das 2019; Dawood 2011; Dehghan 2012; Ercin 2016; Gaston 2010; Kalia 2018; Kuzma 2014; Maity 2012; Majeed 2020; Naik 2017; Naveen 2017; Oakley 2009; Palange 2019; Patil 2017; Ray 2019; Rizk 2019; Saeed 2020; Subash 2020; Zhu 2016). In one study, there were more females than males, with 44.2% male participants (Kocher 2007).
The upper age limit for inclusion in studies varied, with two studies using an upper limit of 18 years (Gholap 2020; Oakley 2009), one study using 16 years (Kuzma 2014), four studies using 15 years (Jain 2019; Majeed 2020; Raj 2018; Ray 2019), one study using 14 years (Shah 2017), four studies using 13 years (Naik 2017; Naveen 2017; Palange 2019; Patil 2017), 18 studies using 12 years (Afaque 2020; Aher 2018; Ahmad 2020; Ajmera 2013; Anwar 2011; Arun 2018; Foead 2004; Gopinathan 2018; Kaewpornsawan 2001; Kumar 2021; Maity 2012; Mandal 2018; Prashant 2016; Rakha 2020; Said 2015; Sankar 2019; Vaidya 2009; Zhu 2016), one study using 11 years (Pandey 2008), six studies using 10 years (Abdel Karim 2016; Dawood 2011; Dehghan 2012; Kocher 2007; Shafi‐Ur‐Rehman 2013; Shamma 2020), one study using nine years (Abubeih 2019), and one study using seven years (Mulpuri 2016). One study had an upper limit of skeletal maturity (Gaston 2010), and 12 studies did not report an upper age limit (Altay 2011; Das 2019; Ducic 2016a; Ercin 2016; Kalia 2018; Othman 2017; Pavone 2016; Rizk 2019; Sadek 2018; Saeed 2020; Subash 2020; Tripuraneni 2009).
The lower age limit for study inclusion also varied, with 12 studies recruiting children from birth (Abdel Karim 2016; Afaque 2020; Dehghan 2012; Jain 2019; Kuzma 2014; Majeed 2020; Naik 2017; Raj 2018; Oakley 2009; Palange 2019; Pandey 2008; Shamma 2020), five studies from one year (Ahmad 2020; Anwar 2011; Foead 2004; Kaewpornsawan 2001; Shah 2017), five studies from two years (Aher 2018; Gopinathan 2018; Maity 2012; Ray 2019; Said 2015), 11 studies from three years (Abubeih 2019; Ajmera 2013; Gholap 2020; Kocher 2007; Kumar 2021; Mulpuri 2016; Naveen 2017; Prashant 2016; Sankar 2019; Shafi‐Ur‐Rehman 2013; Vaidya 2009), three studies from four years (Arun 2018; Dawood 2011; Zhu 2016), and three studies from five years (Mandal 2018; Patil 2017; Rakha 2020). A lower age limit was not reported by 13 studies (Altay 2011; Das 2019; Ducic 2016a; Ercin 2016; Gaston 2010; Kalia 2018; Othman 2017; Pavone 2016; Rizk 2019; Sadek 2018; Saeed 2020; Subash 2020; Tripuraneni 2009).
Despite the variability in inclusion criteria, the mean age of participants in each study was more consistent. The mean age of the participants in studies ranged from 4.9 years (Tripuraneni 2009) to 8.4 years (Arun 2018; Raj 2018; Prashant 2016). The mean age was less than 5 years in one study (Tripuraneni 2009), between 5 to 5.9 years in nine studies (Abdel Karim 2016; Abubeih 2019; Foead 2004; Kocher 2007; Oakley 2009; Othman 2017; Said 2015; Shamma 2020; Vaidya 2009), between 6 to 6.9 in 16 studies (Dawood 2011; Ducic 2016a; Dehghan 2012; Ercin 2016; Gaston 2010; Gholap 2020; Jain 2019; Kalia 2018; Maity 2012; Majeed 2020; Naik 2017; Pavone 2016; Rizk 2019; Sadek 2018; Shafi‐Ur‐Rehman 2013; Subash 2020), between 7 to 7.9 years in 16 studies (Afaque 2020; Aher 2018; Ajmera 2013; Altay 2011; Anwar 2011; Gopinathan 2018; Kaewpornsawan 2001; Kuzma 2014; Naveen 2017; Palange 2019; Pandey 2008; Rakha 2020; Ray 2019; Saeed 2020; Sankar 2019; Zhu 2016), and between 8 to 8.4 years in five studies (Arun 2018; Mandal 2018; Raj 2018; Patil 2017; Prashant 2016). The mean age was not reported in Ahmad 2020
Fracture types
The Gartland classification was used almost universally to describe the included fracture types. Children with Gartland 3 fractures were included in 28 studies (Abubeih 2019; Aher 2018; Ajmera 2013; Altay 2011; Arun 2018; Dawood 2011; Dehghan 2012; Ercin 2016; Gaston 2010; Gholap 2020; Gopinathan 2018; Kaewpornsawan 2001; Kocher 2007; Mulpuri 2016; Naik 2017; Raj 2018; Rakha 2020; Palange 2019; Patil 2017; Pavone 2016; Prashant 2016; Ray 2019; Rizk 2019; Said 2015; Sankar 2019; Shafi‐Ur‐Rehman 2013; Shah 2017; Vaidya 2009), children with a combination of Gartland 2 and 3 fractures were included in 16 studies (Abdel Karim 2016; Anwar 2011; Ducic 2016a; Foead 2004; Jain 2019; Kalia 2018; Kuzma 2014; Maity 2012; Mandal 2018; Naveen 2017; Othman 2017; Pandey 2008; Sadek 2018; Shamma 2020; Tripuraneni 2009; Zhu 2016), children with a combination of Gartland 3 and 4 were included in one study (Das 2019), and children with a combination of Gartland 2, 3, and 4 in one study (Afaque 2020). One study exclusively included children with Gartland 1 fractures (Oakley 2009), and one study included Gartland 1 and 2 fractures (Ahmad 2020). Four studies did not specify the amount of displacement or Gartland classification for inclusion in the study (Kumar 2021; Majeed 2020; Saeed 2020; Subash 2020).
None of the studies undertook a subgroup analysis or presented results for different fracture types.
Comparisons
We grouped the trials according to their primary comparisons. The majority of the trials addressed different types of surgical intervention, particularly the use of crossed K‐wires compared with lateral entry wires. One study involving 47 children performed a three‐way comparison of retrograde crossed wires, retrograde lateral wires and lateral crossed (Dorgan) wires (Othman 2017); this study is included in three comparisons, below.
Different forms of surgical interventions
Forty‐one studies involving 2931 children compared different forms of surgical interventions. A summary of the different pinning techniques identified are summarised in Table 6.
3. Summary of wire configurations used in included studies.
| Group name | Typical wire entry points | Wire direction | Number of studies | Comments |
| Retrograde crossed wires | 1) Lateral condyle 2) Medial epicondyle |
All retrograde | 35 RCTs | Typically avoid crossing at the fracture site Can use open technique or percutaneous technique to protect the ulnar nerve from the medial wire Open technique allows direct visualisation of the bone and retraction of the nerve. Percutaneous techniques usually extend the elbow after inserting the lateral wire and roll the nerve back before inserting a medial wire anterior to the ulnar groove. |
| Retrograde lateral wires | 2 or 3 wires from lateral condyle | All retrograde | 32 RCTs | Can be placed in a parallel or divergent configuration Can be supplemented by an additional medial wire if there are intra‐operative concerns around stability |
| Lateral crossed (Dorgan) wires | 1) Lateral condyle 2) Lateral metaphysis |
1) Retrograde 2) Antegrade |
7 RCTs | Antegrade wire advanced into medial epicondyle but not through cortex to avoid injury to ulnar nerve |
| Posterior intrafocal wire | 1) Lateral condyle 2) Direct posterior |
1) Retrograde 2) Into fracture site (intrafocal) |
1 RCT | Posterior wire passed in the midline into the fracture site to aid reduction of the fracture |
Retrograde lateral wires versus retrograde crossed wires. Following reduction, the use of retrograde crossed wires with a medial and lateral entry point or retrograde lateral wires were compared in 31 studies involving a total of 2188 children. In 29 of these studies, the initial reduction was performed closed (Abdel Karim 2016; Abubeih 2019; Afaque 2020; Aher 2018; Ahmad 2020; Anwar 2011; Arun 2018; Das 2019; Foead 2004; Gaston 2010; Gholap 2020; Kocher 2007; Maity 2012; Majeed 2020; Mandal 2018; Mulpuri 2016; Naik 2017; Naveen 2017; Othman 2017; Palange 2019; Patil 2017; Pavone 2016; Prashant 2016; Raj 2018; Said 2015; Shafi‐Ur‐Rehman 2013; Subash 2020; Tripuraneni 2009; Vaidya 2009). In two studies, the initial reduction was performed with an open approach with direct visualisation of the fracture (Dawood 2011; Sankar 2019).
Lateral crossed (Dorgan) wires versus retrograde crossed wires. Five studies involving 310 children compared the use of lateral crossed (Dorgan) wires with retrograde crossed wires with a medial and lateral entry point (Altay 2011; Ducic 2016a; Kalia 2018; Othman 2017; Rizk 2019). All fixations were completed after a closed reduction.
Retrograde lateral wires versus lateral crossed (Dorgan) wires. Two studies involving 72 children compared the use of retrograde crossed wires with a medial and lateral entry point with lateral crossed (Dorgan) wires following a closed reduction (Othman 2017; Sadek 2018).
Retrograde crossed wires versus posterior intrafocal wires. One study including 168 children compared the use of retrograde crossed wires and a posterior intrafocal technique where lateral and posterior wires were used following closed reduction (Jain 2019).
Retrograde lateral wires in a parallel versus divergent configuration. Two studies involving a total of 60 children compared a parallel and divergent configuration for laterally placed wires (Gopinathan 2018; Shamma 2020)
Retrograde crossed wires using a mini‐open technique or inserted percutaneously. One study including 104 children compared the insertion of the medial wire in a retrograde crossed wire configuration using a mini‐open technique compared with a percutaneous technique (Ercin 2016).
Buried versus non‐buried wires. One study including 80 children compared buried with non‐buried wires following open reduction (Saeed 2020).
External fixation or internal fixation. We identified no studies that compared external fixation with internal fixation.
Open versus closed reduction of displaced fractures
Different reduction techniques were compared by six studies including 493 children (Ajmera 2013; Dehghan 2012; Kaewpornsawan 2001; Rakha 2020; Ray 2019; Zhu 2016). Four studies compared an open reduction using a posterior approach followed by retrograde crossed wires with a closed reduction followed by retrograde crossed wires (Ajmera 2013; Dehghan 2012; Rakha 2020; Ray 2019). One study compared a lateral approach open reduction followed by lateral crossed (Dorgan) wires with a closed reduction and retrograde crossed wires (Kaewpornsawan 2001). One study compared a joystick‐assisted reduction followed by retrograde crossed wires with a closed reduction followed by retrograde crossed wires (Zhu 2016).
Surgical versus non surgical treatment
Three studies involving 140 children compared application of posterior backslab with fixation with retrograde crossed K‐wires (Kumar 2021; Pandey 2008; Shah 2017).
Different forms of traction
One study including 133 children compared different forms of traction for Gartland 3 supracondylar fractures (Kuzma 2014). This study compared the use of skeletal traction with a pin in the olecranon to skin traction for two to three weeks. Skeletal traction was applied using a general anaesthetic with a pin inserted into the olecranon and overhead traction with the elbow in flexion and the forearm supported on a sling. Skin traction was applied under sedation and was held to the skin using elastic bandage. The elbow was maintained in extension with the shoulder in 90° to 100° abduction.
Different methods of non‐surgical intervention
One study including 100 children evaluated the use of a collar and cuff sling compared to a posterior fibreglass backslab, both of which were prescribed for an initial 12 to 16 days but could be extended for an additional 14 days if symptoms had not resolved (Oakley 2009). In this study, children had undisplaced or minimally displaced fractures.
No studies compared cast and sling or sling alone versus skin traction, and no studies compared different durations of immobilisation.
Timing and location of treatment
We identified no studies that compared different locations for closed reduction (i.e. the hospital emergency department versus the operating theatre) or the timing of surgery for displaced fractures.
Outcomes
We included three studies in the review for which there were no available outcome data (Ahmad 2020; Das 2019; Rakha 2020). For retrograde lateral wires versus retrograde crossed wires, Shafi‐Ur‐Rehman 2013 was the only study contributing data for other important outcomes and not for the critical review outcomes. All other studies reported data for at least one critical outcome. Data were mostly reported at medium term (three months to less than six months following injury) and long term (six months or longer).
Funding sources
Eleven studies stated that they had received no funding (Abubeih 2019; Afaque 2020; Altay 2011; Anwar 2011; Gopinathan 2018; Kumar 2021; Maity 2012; Naveen 2017; Rizk 2019; Sankar 2019; Zhu 2016). The remaining studies did not report sources of funding.
Excluded studies
Here, we report the details of 35 key excluded studies (see Characteristics of excluded studies).
We excluded seven studies because the interventions were not eligible for inclusion in the review. Three studies compared different approaches to reduction (Arif 2014; Ensafdaran 2005; Siddiq 2020), two studies compared prone versus supine positions for surgery (Rawoot 2014; Venkatadass 2015), and two studies compared different forms of elbow casting (Chen 2001; Silva 2018).
We excluded one clinical trials registration report as the study was withdrawn owing to a failure to recruit a sufficient number of participants (NCT00904137).
We excluded four studies which we believed were copies of other eligible studies; these copy study reports were published using different author names but with sufficiently similar study characteristics to another study. In the review, we used the study publication which we believed to be the original report and excluded the copy report. We therefore excluded El‐Ngehy 2018 (which we believed to be a copy of Naik 2017), Hegazy 2020 (which we believed to be a copy of Shamma 2020), Sadik 2015 (which we believed to be a copy of Maity 2012), and Shah 2013 (which we believed to be a copy of Shafi‐Ur‐Rehman 2013). We reported this information to the relevant journal editors for further investigation.
We excluded the remaining studies because they used a non‐randomised study design which was not apparent until we had viewed the full text.
Studies awaiting classification
We categorised 10 studies as awaiting classification. We identified five studies during title and abstract screening but we were unable to access the full texts of these study reports to confirm if they were RCTs (Afridi 2002; Andreasi 1985; Boparai 2006; Botchu 2006; Evans 1998). We identified two studies during backward citation searching of relevant systematic reviews (Bing 2017; Lu 2011), but we were unable to source the abstracts or full texts to confirm eligibility. He 2009 compares different forms of splint. However, we could not determine from a translated manuscript whether the study was randomised. Similarly, we could not determine whether Sapkota 2019 was randomised. Attempts to contact the study authors for these two studies were unsuccessful.
NCT04582123 compared fixation with two crossed pins versus three crossed pins. This study is listed in a clinical trials register as complete but the results are not reported and we await publication of the full text.
Ongoing studies
We identified three ongoing studies that are potentially eligible. ACTRN12612000480886 is a trial registration for an RCT of immobilisation position being completed in Australia. The study start date was in 2012 and we attempted to contact the study authors for a status update but the email address is no longer active. PACTR201702001960109 is a trial registration for an RCT of crossed wires versus lateral wires registered in 2016; attempts to contact the study authors have been unsuccessful. CTRI/2020/06/025504 is a trial registration for an RCT of two versus three lateral wires; this study was registered in June 2020.
Risk of bias in included studies
All studies had methodological or reporting limitations that prevented them from being rated as low risk of bias for all domains (Figure 3; Figure 4). We did not conduct risk of bias assessment for three studies because these studies did not report outcome data for this review (Ahmad 2020; Das 2019; Rakha 2020). The risk of bias figures therefore include blank spaces for these trials.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
4.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study. Blank spaces indicate that we did not conduct risk of bias assessment; we did not include outcome data for these studies.
Allocation
Twenty‐five studies used an adequate method for random sequence generation and we judged these studies to be at low risk of bias for this domain (Abdel Karim 2016; Abubeih 2019; Afaque 2020; Aher 2018; Anwar 2011; Dehghan 2012; Ducic 2016a; Foead 2004; Gopinathan 2018; Kaewpornsawan 2001; Kocher 2007; Kuzma 2014; Maity 2012; Mulpuri 2016; Naveen 2017; Oakley 2009; Palange 2019; Prashant 2016; Ray 2019; Said 2015; Sankar 2019; Shamma 2020; Subash 2020; Vaidya 2009; Zhu 2016). We judged five of these studies to also be at low risk of bias for allocation concealment (Abubeih 2019; Ducic 2016a; Maity 2012; Oakley 2009; Subash 2020).
Quasi‐randomised techniques were reported in five studies (Ercin 2016; Gaston 2010; Jain 2019; Naik 2017; Tripuraneni 2009). Three studies allocated participants to treatments based on the day of admission and the surgeon who was on call to perform the operation (Ercin 2016; Gaston 2010; Tripuraneni 2009). Jain 2019 allocated treatments to children on an alternating basis, and Naik 2017 used the child's inpatient number to allocate treatments. We judged these studies to be at high risk of bias. Opaque envelopes were used in the Gopinathan 2018, Kuzma 2014 and Zhu 2016 studies but these were not pre‐numbered and were drawn from a box with a limited number of envelopes. For these three studies, we judged the random sequence generation to be at low risk of bias and the allocation concealment to be at high risk.
The remaining studies were at unclear risk of selection bias because of insufficient reporting.
Blinding
Most studies were at high or unclear risk of blinding due to a lack of blinding or independence of personnel, participants, and outcome assessors.
As the majority of the comparisons are of surgical procedures, we judged it would not be possible to blind an operating surgeon or surgical team to allocated treatments in the same way that can be accomplished in blinded drug trials. Equally, it is near‐impossible to blind participants to their treatment allocation due to the presence of external devices, pins, or scars. We felt that this lack of personnel and participant blinding might impact performance if the practitioner providing the intervention was a part of the study team. This was confirmed to be the case in four studies; we therefore assigned high risk of performance bias to these studies (Abdel Karim 2016; Abubeih 2019; Foead 2004; Kaewpornsawan 2001).
We judged three studies to have low risk of performance bias because interventions were provided by practitioners who were independent of the study team (Gaston 2010; Kuzma 2014; Palange 2019). We judged risk of performance bias in the remaining studies to be unclear.
The use of non‐blinded assessors was explicit in nine studies and we judged these studies to be at high risk of detection bias (Abdel Karim 2016; Abubeih 2019; Ducic 2016a; Gaston 2010; Kocher 2007; Kuzma 2014; Maity 2012; Oakley 2009; Zhu 2016). However, risk of detection bias was unclear in the remaining studies.
Incomplete outcome data
We agreed that the minimum requirements for a study to be assigned a low risk of bias in this domain would be:
a follow‐up rate exceeding 70%;
evidence that any participants lost to follow‐up were missing at random (i.e. loss to follow‐up balanced between groups);
an intention‐to‐treat analysis provided in the study report.
Twenty‐five studies reported no losses (Abdel Karim 2016; Altay 2011; Anwar 2011; Arun 2018; Dawood 2011; Dehghan 2012; Ducic 2016a; Gholap 2020; Gopinathan 2018; Kaewpornsawan 2001; Kalia 2018; Kumar 2021; Majeed 2020; Naik 2017; Raj 2018; Palange 2019; Patil 2017; Pavone 2016; Ray 2019; Rizk 2019; Sadek 2018; Saeed 2020; Shafi‐Ur‐Rehman 2013; Shah 2017; Shamma 2020). Eight studies had a small loss to follow‐up which was balanced between groups (Abubeih 2019; Afaque 2020; Foead 2004; Jain 2019; Naveen 2017; Oakley 2009; Prashant 2016; Vaidya 2009). During the study by Tripuraneni 2009, seven children did not attend follow‐up following pin removal but it was judged that this was unlikely to impact the extracted outcome of nerve injury. We judged these 34 studies to have a low risk of attrition bias.
We deemed seven studies to be at high risk of attrition bias. Ercin 2016 excluded 15 children following allocation for having fixation with two rather than three wires. Kuzma 2014 and Maity 2012 had loss to follow‐up rates of 16% and 18%, respectively, but adjusted for missing data using a 'last result carried forward' technique with no presentation of unadjusted results. Two children with significant complications were excluded following randomisation and intervention in Mandal 2018. In the abstracts of the Mulpuri 2016 study, a per‐protocol analysis was performed, with a disproportionate exclusion of children with lateral wires who were more likely to receive an additional medial wire if there were intra‐operative concerns about stability. Pandey 2008 had an imbalance in loss to follow‐up of 27% and 20% with more children in the cast group absconding. This may be related to children in a cast with good outcomes not returning for follow‐up as it is easier to remove a cast at home than remove wires. Zhu 2016 had an imbalance of children excluded from analysis in the closed reduction group and presented a per‐protocol analysis.
We judged the remaining seven studies to have an unclear risk of attrition bias with insufficient information to assess the impact of loss to follow‐up on the intervention groups (Aher 2018; Ajmera 2013; Gaston 2010; Kocher 2007; Othman 2017; Sankar 2019; Subash 2020).
Selective reporting
A prospective trial registration or protocol was available for only one study (Mulpuri 2016). This identified long‐term radiographic outcomes that were not reported in the published abstracts and it is possible that this planned long‐term analysis will be presented in a full paper; we judged this study to have a high risk of selective reporting bias in this review. For all other studies, no prospective trial registration or protocol was available so we judged all the remaining studies to have an unclear risk of selective reporting bias.
Other potential sources of bias
One study had an unexplained difference in open reduction rates between intervention groups (80% versus 20%) which may have impacted on outcomes (Raj 2018), and we judged this study to be at high risk of bias.
We judged four studies to have an unclear risk of other bias (Maity 2012; Naik 2017; Shafi‐Ur‐Rehman 2013; Shamma 2020). We identified apparent copy publications of these studies (see Excluded studies), and although we assumed that these four studies were the original publications, we could not rule out the possibility that these studies were in fact conducted by other study author teams.
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings 1. Retrograde lateral wires compared with retrograde crossed wires for treatment of displaced supracondylar elbow fractures in children.
| Retrograde lateral wires compared with retrograde crossed wires for treatment of displaced supracondylar elbow fractures in children | ||||||
|
Population: children with displaced supracondylar elbow fractures Settings: inpatient management settings. Included studies were conducted in: Egypt, India, Iraq, Italy, Malaysia, Nepal, Pakistan, USA Intervention: retrograde lateral wires in a parallel or divergent configuration Comparison: retrograde crossed wires | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of children (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk with crossed wiresa | Corresponding risk with lateral wires | |||||
|
Functional outcome Measured by Mayo Elbow Performance Score (range 0 to 100, with higher scores indicating better function) Mean follow‐up: 42 to 44 months |
Mean score of 96 | Mean score 2 points higher | 35 children (1 study) |
⊕⊕⊝⊝ Very lowb |
Unable to calculate relative effect estimate because distribution values were unavailable. There is no formal MCID for the Mayo Elbow Performance Score in childhood fractures. However, a 2‐point difference is unlikely to be clinically significant. |
|
|
Treatment failure requiring re‐intervention Follow‐up: 6 months |
0 out of 30 | 1 out of 30c |
RR 3.00 (0.13 to 70.83) |
60 children (1 study) |
⊕⊝⊝⊝ Very lowb |
|
|
Nerve injury Follow‐up: range from 2 months to 67 months |
86 per 1000 | 56 per 1000 (40 to 78) |
RR 0.65 (0.46 to 0.90) |
1653 children (28 studies) |
⊕⊕⊝⊝ Lowd |
In post hoc subgroup analysis, we noted fewer injuries when lateral wires were used with a percutaneous technique (RR 0.41, 95% CI 0.20 to 0.81, favours lateral wires; 10 studies, 552 participants), but little difference in nerve injuries when an open technique was used (RR 0.91, 95% CI 0.59 to 1.40, favours lateral wires; 11 studies, 656 participants). In formal tests for subgroup interactions, we noted a possible difference between these subgroups (P = 0.05). |
|
Major complications In all studies, these were pin site infections. Follow‐up: range from 3 months to 67 months |
43 per 1000 | 47 per 1000 (28 to 78) |
RR 1.08 (0.65 to 1.79) |
1126 children (19 studies) |
⊕⊕⊝⊝ Lowe |
|
|
Cosmetic deformity Measured as number of children with cubitus varus Follow‐up: long‐term Time points in the included studies ranged from 6 months to 44 months |
Study population |
RR 3.13 (0.55 to 17.98) |
95 children (2 studies) |
⊕⊝⊝⊝ Very lowb |
||
| 19 per 1000 |
60 per 1000 (11 to 346) |
|||||
| *The basis for the assumed risk is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MCID: minimal clinically important difference; RR: risk ratio | ||||||
|
GRADE Working Group grades of evidence
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDerived from the data reported in the included studies for the crossed wires group, or from the pooled estimate of the crossed wires group when data is available from more than one study bDowngraded by one level for serious risk of bias owing to unclear risks of bias in at least one domain, and two levels for very serious imprecision because of the small sample size and small difference between mean scores cData reported in the included study in the lateral wires group dDowngraded by one level for serious risk of bias owing to unclear risks of bias in at least one domain, and one level because we detected possible publication bias (from visual inspection of a funnel plot, and from sensitivity analysis of studies published in journals that are not indexed in PubMed) eDowngraded by one level for serious risk of bias owing to unclear risks of bias in at least one domain, and one level for serious imprecision because the CI includes possible benefits and harms for both treatments
Summary of findings 2. Open reduction compared with closed reduction position for treatment of displaced supracondylar fractures in children.
| Open reduction compared with closed reduction position for treatment of displaced supracondylar elbow fractures in children | ||||||
|
Population: children with displaced supracondylar elbow fractures Settings: inpatient management. Included studies were conducted in India, Iran, and Thailand Intervention: fracture reduced with an open technique using direct visualisation Comparison: fracture reduced with a closed technique | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of children (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk with closed reductiona | Corresponding risk with open reduction | |||||
| Functional outcome | ‐ | ‐ | Not estimable | ‐ | No studies reported this outcome | |
| Treatment failure requiring re‐intervention | ‐ | ‐ | Not estimable | ‐ | No studies reported this outcome | |
|
Nerve injury Follow‐up: range from 3 to 12 months |
111 per 1000 | 33 per 1000 (10 to 112) |
RR 0.30 (0.09 to 1.01) |
163 children (3 studies) |
⊕⊕⊝⊝ Lowb | In addition, 1 study (68 participants) used a joystick technique for visualisation and reported no incidences of nerve injury. |
|
Major complications Follow‐up: range from 3 to 22 months All complications were pin site infections |
16 per 1000 | 66 per 1000 (17 to 257) |
RR 4.15 (1.07 to 16.20) |
253 children (4 studies) |
⊕⊕⊝⊝ Lowb | In addition, 1 study (59 participants) used a joystick technique for visualisation and reported little or no difference in pin site infections. |
|
Cosmetic deformity Measured as the presence of cubitus varus Follow‐up: long‐term |
‐ | ‐ | Not estimable | ‐ | ‐ | No studies using direct visualisation reported this outcome. 1 study (59 participants) used a joystick technique for visualisation and reported little or no difference in cubitus varus |
| *The basis for the assumed risk is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MCID: minimal clinically important difference; RCT: randomised controlled trial; RR: risk ratio | ||||||
|
GRADE Working Group grades of evidence
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aAssumed risk taken to be mean risk in control group bDowngraded one level for serious risk of bias because included studies had unclear risks of bias in at least one domain, and one level for serious imprecision because the CI included possible benefits and harms for both treatments
Summary of findings 3. Surgical fixation versus non‐surgical immobilisation for displaced fractures.
| Surgical fixation compared with non‐surgical immobilisation for displaced fractures | ||||||
|
Population: children with displaced fractures Settings: inpatient setting. Included studies conducted in India and Nepal Intervention: surgical fixation with wires Comparison: non‐surgical immobilisation with cast | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of children (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk with non‐surgical immobilisationa | Corresponding risk with surgical fixation | |||||
| Functional outcome | ‐ | ‐ | Not estimable | ‐ | ‐ | No studies reported this outcome |
|
Treatment failure requiring re‐intervention Follow‐up: 6 months |
33 per 1000 | 11 per 1000 (0 to 262) |
RR 0.33 (0.01 to 7.87) |
60 children (1 study) |
⊕⊝⊝⊝ Very lowb |
|
|
Nerve injury Follow‐up: 6 to 12 months |
14 per 1000 |
36 per 1000 (7 to 178) |
RR 2.50 (0.50 to 12.46) |
140 children (3 studies) |
⊕⊝⊝⊝
Very lowb |
4 postoperative ulnar nerve injuries that recovered by end of follow‐up in the surgical fixation groups. 2 preoperative (1 ulnar nerve and 1 radial nerve palsy) injuries in each group |
|
Major complications Pin site infections. Follow‐up: 6‐12 months |
0 out of 62 | 3 out of 64 |
RR 4.00 (0.47 to 34.11) |
126 children (3 studies) |
⊕⊝⊝⊝ Very lowb |
In addition, 1 study reported a single preoperative vascular injury in the non‐surgical treatment group |
|
Cosmetic deformity measured as presence of cubitus varus Follow‐up: long‐term (6 to 12 months) |
175 per 1000 |
35 per 1000 (7 to 191) |
RR 0.20 (0.04 to 1.09) |
80 children (2 studies) |
⊕⊝⊝⊝ Very lowb |
|
| *The basis for the assumed risk is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
|
GRADE Working Group grades of evidence
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aAssumed risk taken to be mean risk in control group bDowngraded by one level for serious risk of bias owing to unclear or high risks of bias in at least one domain, and two levels for very serious risk of bias imprecision because of the small sample size and wide CI cData as reported in the included studies
We report detailed data for critical outcomes for five comparisons; data for other important outcomes are summarised in the text and in additional tables. The first comparison (different forms of surgical interventions) included seven different comparisons of fixation techniques. Because most data were available for retrograde lateral versus retrograde crossed wires, and because this is the most clinically relevant comparison, we presented the findings for other types of fixation techniques in summary text with additional tables.
See the summary of findings tables for the critical outcomes for: retrograde lateral versus retrograde crossed wires (Table 1), open reduction versus closed reduction (Table 2), and surgical fixation versus non‐surgical immobilisation (Table 3).
1a. Different forms of surgical interventions: different fixation techniques
Retrograde lateral versus retrograde crossed wires
Twenty‐nine studies evaluated the use of retrograde crossed wires and retrograde lateral wires (see Table 5).
Critical outcomes
1. Functional outcome
Long‐term functional outcome was measured in one study (35 children) using the Mayo Elbow Performance Score at 42 to 44 months after surgery (Pavone 2016). This scoring system ranges from 0 to 100 with higher scores indicating better function; we note that this score is designed for use in adult elbow disorders (Morrey 1993), but has not been formally validated for use in childhood fractures (Marson 2020a). We were unable to calculate an effect estimate because study authors did not report distribution values or P values. Study authors reported a difference of two points between the lateral and crossed wires groups, with higher scores in the lateral wires group (98 points in the lateral wires group versus 96 points in the crossed wires group). We judged the certainty of this evidence to be very low. We downgraded by one level for serious risk of bias and two levels for very serious imprecision.
2. Treatment failure requiring intervention
Only one study (60 children) reported treatment failure requiring revision surgery (Mandal 2018). We found little or no difference according to the two interventions (risk ratio (RR) 3.00, 95% confidence interval (CI) 0.13 to 70.83, favours crossed wires; 1 study, 60 children; very low‐certainty evidence; Analysis 1.1). The single event for this outcome, a varus collapse of the fracture) occurred with lateral wires. We downgraded the evidence by one level for serious risk of bias and two levels for very serious imprecision.
1.1. Analysis.

Comparison 1: Different forms of surgical intervention: retrograde lateral wires versus retrograde crossed wires, Outcome 1: Treatment failure requiring a re‐intervention
3. Nerve injury
Of the 29 studies in this comparison, all except Shafi‐Ur‐Rehman 2013 reported nerve injury. We found fewer incidences of nerve injury when lateral wires were used (RR 0.65, 95% CI 0.46 to 0.90, favours lateral wires; I2 = 0%; 28 studies, 1653 children; low‐certainty evidence; Analysis 1.2; Figure 5). All nerve injuries were transient and resolved within 12 months of injury. We noted that two studies included ulnar nerve injuries in children allocated to retrograde lateral wires (Abdel Karim 2016; Gaston 2010); these injuries were the result of an additional medial wire being inserted due to a perceived lack of stability in the operating room. We generated a funnel plot for this analysis and, from visual inspection of this plot, we could not rule out the possibility of publication bias or small‐study effects (Figure 6); sensitivity analysis also indicated a possible bias from studies published in non‐indexed journals (see below). We downgraded the certainty of the evidence by one level owing to serious risks of bias and one level for publication bias.
1.2. Analysis.

Comparison 1: Different forms of surgical intervention: retrograde lateral wires versus retrograde crossed wires, Outcome 2: Nerve injury
5.

Forest plot of comparison 6: different forms of surgical intervention: retrograde crossed wires versus retrograde lateral wires, outcome 6.1 nerve injury
6.

Funnel plot for retrograde lateral wires compared with retrograde crossed wires: Analysis 1.2 Nerve injury
4. Major complications
Major complications were reported in 18 studies (Abdel Karim 2016; Abubeih 2019; Afaque 2020; Aher 2018; Foead 2004; Kocher 2007; Maity 2012; Mandal 2018; Naik 2017; Naveen 2017; Othman 2017; Patil 2017; Pavone 2016; Prashant 2016; Raj 2018; Said 2015; Sankar 2019; Vaidya 2009). The effect estimate was imprecise, indicating possible benefits as well as harms for both interventions (RR 1.08, 95% CI 0.65 to 1.79, favours crossed wires; I2 = 0%; 19 studies, 1126 children; Analysis 1.3); all complications were pin site infections. We judged the certainty of this evidence to be low, downgrading one level for serious risks of bias and one level for serious imprecision owing to the wide CI in the effect estimates. We did not detect possible publication bias from a funnel plot generated for this outcome.
1.3. Analysis.

Comparison 1: Different forms of surgical intervention: retrograde lateral wires versus retrograde crossed wires, Outcome 3: Major complications (pin site infections)
5. Cosmetic deformity
In the medium term:
Cubitus varus. One study reported children with this deformity measured three months after surgery (Abubeih 2019). The effect estimate was imprecise, indicating possible benefits and harms for both interventions in the medium term (RR 2.91, 95% CI 0.12 to 69.08, favours crossed wires; 1 study, 67 children; Analysis 1.4).
Loss of carrying angle of more than 10° (measured as number of events). One study reported children with a loss of carrying angle of more than 10° measured at four months (Dawood 2011). The effect estimate was imprecise, indicating possible benefits and harms for both interventions in the medium term (RR 1.10, 95% CI 0.08 to 15.36, favours crossed wires; 1 study, 21 children; Analysis 1.4).
Loss of carrying angle (measured as degrees of loss). Five studies reported the mean degree of loss of the carrying angle at three months (Afaque 2020; Kocher 2007; Maity 2012), and four months (Dawood 2011; Said 2015). We found little or no difference in degrees of loss in the medium term between the intervention groups (mean difference (MD) ‐0.10°, 95% CI ‐0.44º to 0.23°, favours lateral wires; I2 = 0%; 5 studies, 320 children; Analysis 1.5).
1.4. Analysis.

Comparison 1: Different forms of surgical intervention: retrograde lateral wires versus retrograde crossed wires, Outcome 4: Cosmetic deformity: loss of carrying angle > 10 degrees or elbow deformity
1.5. Analysis.

Comparison 1: Different forms of surgical intervention: retrograde lateral wires versus retrograde crossed wires, Outcome 5: Cosmetic deformity: loss of carrying angle (degrees of loss)
In the long term:
Cubitus varus. Two studies reported this outcome in the longer term, at six months (Mandal 2018), and at four years (Pavone 2016), and the effect estimate was also imprecise (RR 3.13, 95% CI 0.55 to 17.98, favours crossed wires; I2 = 41%; 2 studies, 95 children; Analysis 1.4). We downgraded the certainty of the evidence by one level for serious risk of bias and two levels for imprecision owing to the small sample size and wide CI.
Loss of carrying angle of more than 10° (measured as number of events). Six studies reported this outcome in the longer term at six months (Anwar 2011; Foead 2004; Mandal 2018), seven months (Othman 2017), two years (Naik 2017), and four years (Pavone 2016). The effect estimate was imprecise (RR 1.27, 95% CI 0.39 to 4.20, favours crossed wires; I2 = 0%; 6 studies, 288 children; Analysis 1.4); this analysis included three studies in which there were no events in either group (Anwar 2011; Mandal 2018; Pavone 2016).
Loss of carrying angle (measured as degrees of loss). Ten studies reported this outcome in the longer term at six months (Aher 2018; Anwar 2011; Foead 2004; Gholap 2020; Mandal 2018; Palange 2019; Prashant 2016; Subash 2020; Vaidya 2009), and at four years (Pavone 2016). We similarly found little or no difference (MD 0.14°, 95% CI ‐0.26° to 0.54°, favours crossed wires; I2 = 0%; 10 studies, 502 children). The MD in loss of carrying angle between groups is less than 1°, which is much lower than the measurement error of using a goniometer to measure carrying angle, and is thus highly unlikely to be clinically significant.
Other important outcomes
Studies reported range of movement, return to sport or normal activities, and radiographic deformity. We noted that fewer children had loss of reduction in the medium term when crossed wires were used (RR 2.00, 95% CI 1.21 to 3.30, favours crossed wires; I2 = 14%; 7 studies, 469 children; Analysis 1.10). However, the effect estimates at other time points and for all other measures of radiographic deformity, as well as effect estimates for the other clinical outcomes, were all imprecise and included the possibility of benefits and harms for both treatments. For resource use, we did not pool the data for operative time because of substantial statistical heterogeneity in the two included studies, and we found little or no difference between treatments in the length of hospital stay in one study. See Table 7. No studies reported functional outcome in the early follow‐up period, pain, quality of life, child or parent satisfaction, or emotional distress of the child.
1.10. Analysis.

Comparison 1: Different forms of surgical intervention: retrograde lateral wires versus retrograde crossed wires, Outcome 10: Radiographic deformity: loss of reduction
4. Other important outcomes: retrograde lateral wires versus retrograde crossed wires.
| Other important outcomes: retrograde lateral wires versus retrograde crossed wires | ||
| Outcome | Studies | Effect estimate |
|
Range of movement at the elbow: loss of flexion Follow‐up: medium term (range from 3 months to 4 months) |
Abdel Karim 2016; Abubeih 2019; Dawood 2011 | RR 1.24, 95% CI 0.37 to 4.16, favours crossed wires; I2 = 0%; 3 studies, 148 participants; Analysis 1.6 |
|
Range of movement at the elbow: loss of extension Follow‐up: medium term (4 months) |
Dawood 2011 | RR 0.73, 95% CI 0.15 to 3.53, favours lateral wires; 1 study, 21 participants; Analysis 1.6 |
|
Range of movement at the elbow: loss of flexion Follow‐up: long term (6 months) |
Anwar 2011; Foead 2004 | RR 1.41, 95% CI 0.84 to 2.35, favours crossed wires; I2 = 0%; 2 studies, 105 participants; Analysis 1.6 |
|
Range of movement at the elbow: loss of extension Follow‐up: long term (6 months) |
Anwar 2011; Foead 2004 | RR 0.85, 95% CI 0.40 to 1.80, favours lateral wires; I2 = 0%; 2 studies, 105 participants; Analysis 1.6 |
|
Range of movement at the elbow: loss of total range of movement Follow‐up: long term (range from 6 months to 2 years) |
Mandal 2018; Naik 2017; Othman 2017 | RR 1.26, 95% CI 0.56 to 2.82, favours crossed wires; I2 = 0%; 3 studies, 148 participants; Analysis 1.6 |
|
Range of movement at the elbow: degrees of loss of elbow flexion? Follow‐up: long term (6 months) |
Foead 2004 | MD 2.58, 95% CI ‐2.48 to 7.64, favours crossed wires; 1 study, 55 participants; Analysis 1.7 |
|
Range of movement at the elbow: degrees of loss of elbow extension? Follow‐up: long term (6 months) |
Foead 2004 | MD ‐0.03, 95% CI ‐5.35 to 5.29, favours elbow extension; 1 study, 55 participants; Analysis 1.7 |
|
Range of movement at the elbow: degrees of loss of total range of movement Follow‐up: long term (6 months) |
Aher 2018; Gholap 2020; Prashant 2016 | MD 0.71, 95% CI ‐0.52 to 1.95, favours crossed wires; 2 studies, 92 participants; Analysis 1.7. This analysis does not include Aher 2018, in which data were reported without distribution values (mean loss in lateral group was 3°, mean loss in crossed wires group was 2°). |
|
Return to sport or normal activities Follow‐up: medium term (range from 3 months to 4 months) |
Kocher 2007; Mandal 2018; Said 2015 | RR 1.23, 95% CI 0.31 to 4.83, favours crossed wires; I2 = 0%; 3 studies, 151 participants; Analysis 1.8 |
|
Return to sport or normal activities Follow‐up: long term (range from 6 months to 4 years) |
Pavone 2016; Vaidya 2009 | RR 0.54, 95% CI 0.08 to 3.53, favours lateral wires; I2 = 0%; 2 studies, 95 participants; Analysis 1.8 |
| Return to sport or normal activities: time taken to return to normal activities (in weeks) | Mandal 2018 | MD 0.50 weeks, 95% CI ‐0.12 to 1.12, favours crossed wires; 1 study, 58 participants; Analysis 1.9 |
|
Radiographic deformity: loss of reduction (measured as number of events) Defined in the majority of studies as a loss of Baumann's angle greater than 6° Follow‐up: early (3 weeks) |
Shafi‐Ur‐Rehman 2013 | RR 1.37, 95% CI 0.93 to 2.00, favours crossed wires; Analysis 1.10 |
|
Radiographic deformity: loss of reduction (measured as number of events) Defined in the majority of studies as a loss of Baumann's angle greater than 6° Follow‐up: medium term (range from 3 to 4 months) |
Abdel Karim 2016; Abubeih 2019; Aher 2018; Kocher 2007; Maity 2012; Said 2015; Vaidya 2009 | RR 2.00, 95% CI 1.21 to 3.30, favours crossed wires; I2 = 14%; 7 studies, 469 participants; Analysis 1.10 |
|
Radiographic deformity: loss of reduction (measured as number of events) Defined in the majority of studies as a loss of Baumann's angle greater than 6° Follow‐up: long‐term (6 months) |
Patil 2017; Prashant 2016 | RR 4.00, 95% CI 0.46 to 34.45, favours crossed wires; I2 = 0%; 2 studies, 92 participants; Analysis 1.10 |
|
Radiographic deformity: degrees of loss of Baumann's angle Follow‐up: medium term (range from 3 months to 4 months) |
Abubeih 2019; Afaque 2020; Dawood 2011; Kocher 2007; Maity 2012; Said 2015; Vaidya 2009 | MD 0.06, 95% CI ‐0.22 to 0.33, favours crossed wires; I2 = 0%; 7 studies, 447 participants; Analysis 1.11 |
|
Radiographic deformity: degrees of loss of lateral humeral‐capitellar angle Follow‐up: medium term (3 months) |
Vaidya 2009 | MD ‐0.20, 95% CI ‐2.86 to 2.46, favours lateral wires; 1 study, 60 participants; Analysis 1.11 |
|
Radiographic deformity: degrees of loss of Baumann's angle Follow‐up: long term (range from 6 months to 4 years) |
Anwar 2011; Foead 2004; Othman 2017; Palange 2019; Pavone 2016; Prashant 2016; Raj 2018 | MD 0.05, 95% CI ‐0.00 to 0.11, favours lateral wires; I2 = 0%; 7 studies, 283 participants; Analysis 1.11a In addition, Subash 2020 reported data without distribution values (mean in lateral wires group: 5.5°; mean in crossed wires group: 5.24°). |
|
Radiographic deformity: degrees of loss of lateral humeral‐capitellar angle Follow‐up: long term (range from 6 months to 7 months) |
Othman 2017 | MD 0.01, 95% CI‐0.06 to 0.08, favours crossed wires; 1 study, 31 participants; Analysis 1.11. In addition, Subash 2020 reported data without distribution values (mean in lateral wires group: 6.4°; mean in crossed wires group: 6.54°). |
|
Radiographic deformity: degrees of loss of humeral diaphyseal‐metaphysealangle Follow‐up: long term (6 months) |
Anwar 2011; Prashant 2016 | MD 0.10, 95% CI ‐0.18 to 0.39, favours crossed wires; I2 = 0%; 2 studies, 112 participants; Analysis 1.11 |
|
Radiographic deformity: degrees of loss of humeral medial epicondylar epiphyseal angle Follow‐up: long term (6 months) |
Foead 2004 | MD 0.86, 95% CI ‐2.27 to 3.99, favours crossed wires; 1 study, 55 participants; Analysis 1.11 |
| Resource use: operative time | Abubeih 2019; Naik 2017 | We did not pool the data owing to substantial statistical heterogeneity (I2 = 92%). Data from individual studies are reported in Analysis 1.12. |
| Resource use: length of hospital stay (days) | Prashant 2016 | MD ‐0.19, 95% CI ‐0.48 to 0.10, favours lateral wires; 1 study, 62 participants; Analysis 1.12 |
aWe noted that data in one of the included studies in this analysis was inconsistent with other studies, and we could not rule out the possibility of data error in the study report (Othman 2017). CI: confidence interval; MD: mean difference; RR: risk ratio
Subgroup analysis
We found that insufficient studies reported study characteristics to allow for meaningful subgroup analysis using our pre‐specified subgroups. We conducted a post hoc subgroup analysis for nerve injury according to the technique used to insert the medial wire. This analysis included only studies that reported whether wires were inserted using a percutaneous or open technique, and therefore does not include data for seven studies that did not describe this detail (Anwar 2011; Foead 2004; Majeed 2020; Mandal 2018; Mulpuri 2016; Pavone 2016; Raj 2018). The formal test for subgroup differences indicated a difference according to technique (P = 0.05), with fewer injuries when lateral wires were used with a percutaneous technique (RR 0.41, 95% CI 0.20 to 0.81, favours lateral wires; I2 = 0%; 10 studies, 552 children). There was little difference in nerve injuries when an open technique was used (RR 0.91, 95% CI 0.59 to 1.40, favours lateral wires; I2 = 0%; 11 studies, 656 children). We could not rule out the possibility that other factors could explain this difference in results for these subgroups.
Sensitivity analysis
Influence of any small‐study effects by comparing the random‐effects model output to a fixed‐effect model calculated with the Mantel‐Haenszel method. We recalculated all effect estimates for critical outcomes using the random‐effects model. For nerve injury, we noted a wider confidence interval (CI) with random‐effects model (RR 0.71, 95% CI 0.50 to 1.00, favours lateral wires). We noted no other important changes to the effect estimates.
Impact of inclusion and exclusion of studies published only as abstracts. Two studies were available only as abstracts (Mulpuri 2016; Said 2015). Exclusion of these studies made no difference to our interpretation of the effect estimates.
Impact of including studies with high risk of bias; in particular, those identified with allocation bias or lack of blinding for outcome assessors. We excluded two studies from relevant primary analyses owing to risk of bias concerns (Gaston 2010; Naik 2017). For nerve injury, we noted a wider CI when pooling the data without these studies (RR 0.69, 95% CI 0.48 to 1.00, favours lateral wires; 26 studies, 1492 children). However, we noted no other differences to our interpretation of the effect estimates.
Analysis of the result of any missing data on the review conclusions: we excluded studies that we judged to have high or unclear risk of attrition bias owing to participant loss. This did not alter our interpretation of the effect estimates.
Impact of studies published in journals that are not indexed in PubMed. We excluded 15 studies from the primary analysis of nerve injury (Abubeih 2019; Aher 2018; Anwar 2011; Arun 2018; Dawood 2011; Gholap 2020; Majeed 2020; Mandal 2018; Naveen 2017; Othman 2017; Palange 2019; Raj 2018; Sankar 2019; Subash 2020; Vaidya 2009). We noted that the effect estimate without these studies was imprecise and now included the possibility of benefit with crossed wires (RR 0.71, 95% CI 0.48 to 1.05; 13 studies, 817 children). For major complications, we excluded nine studies (Abubeih 2019; Aher 2018; Mandal 2018; Naveen 2017; Othman 2017; Patil 2017; Raj 2018; Sankar 2019; Vaidya 2009); this sensitivity analysis did not alter our interpretation of the effect estimate.
Impact of studies published in duplicate by different author teams. We excluded two studies from the primary analyses of nerve injury and major complications (Maity 2012; Naik 2017). This did not alter our interpretation of the effect estimates.
Other comparisons of different fixation techniques
We report the summary effects for those outcomes reported by study authors of other forms of surgical interventions in Table 8. This table includes data for the following comparisons:
5. Different forms of surgical interventions: effects of critical and other important outcomes for other comparison groups.
| Lateral crossed (Dorgan) wires versus retrograde crossed wires | ||
| Outcome | Studies | Effect estimate |
| Nerve injury | Altay 2011; Ducic 2016a; Kalia 2018; Othman 2017; Rizk 2019 | RR 0.26, 95% CI 0.09 to 0.76, favours Dorgan wires; I2 = 0%; 5 studies, 307 participants; Analysis 2.1 |
| Major complications: all complications | Altay 2011; Ducic 2016a; Kalia 2018; Othman 2017; Rizk 2019 | RR 1.94, 95% CI 0.88 to 4.30, favours retrograde crossed wires; I2 = 0%; 5 studies, 307 participants; Analysis 2.2 |
| Major complications: pin site infection | Altay 2011; Ducic 2016a; Kalia 2018; Othman 2017; Rizk 2019 | RR 2.51, 95% CI 0.92 to 6.82, favours retrograde crossed wires; I2 = 0%; 5 studies, 307 participants; Analysis 2.2 |
| Major complications: vascular injuries | Ducic 2016a | RR 1.06, 95% CI 0.28 to 4.07, favours retrograde crossed wires; 1 study, 138 participants; Analysis 2.2 |
| Cosmetic deformity: loss of carrying angle > 10° (long term) | Ducic 2016a; Othman 2017; Rizk 2019 | RR 1.35, 95% CI 0.37 to 4.85, favours retrograde crossed wires; I2 = 0%; 3 studies, 218 participants; Analysis 2.3 |
| Cosmetic deformity: loss of carrying angle (degrees of loss) | Rizk 2019 | MD 0.30°, 95% CI ‐2.20° to 2.80°, favours retrograde crossed wires; 1 study, 50 participants; Analysis 2.4 |
| Range of motion: loss of total range of motion > 10° (long term) | Ducic 2016a; Othman 2017; Rizk 2019 | RR 1.14, 95% CI 0.08 to 16.63, favours retrograde crossed wires; I2 = 0%; 3 studies, 218 participants; Analysis 2.5 |
| Radiographic deformity: loss of Baumann's angle (long term) | Othman 2017; Rizk 2019 | MD 0.04°, 95% CI ‐0.01° to 0.09°, favours retrograde crossed wires; 2 studies, 80 participants; Analysis 2.6 |
| Radiographic deformity: loss of lateral humeral‐capitellar angle (long term) | Othman 2017; Rizk 2019 | MD 0.08°, 95% CI 0.00° to 0.16°, favours retrograde crossed wires; I2 = 0%; 2 studies, 80 participants; Analysis 2.6 This difference is unlikely to be clinically important. |
| Resource use (operative time; minutes) | Ducic 2016a | MD 7.88, 95% CI 6.27 to 9.49; favours retrograde crossed wires; 1 study, 138 participants; Analysis 2.7 |
| Resource use (radiographic exposure time; minutes) | Ducic 2016a | MD 2.65, 95% CI 1.90 to 3.40, favours retrograde crossed wires; 1 study, 138 participants; Analysis 2.7 |
| Lateral crossed (Dorgan) wires versus retrograde lateral wires | ||
| Outcome | Studies | Effect estimate |
| Nerve injury | Othman 2017; Sadek 2018 | RR 1.07, 95% CI 0.07 to 15.54, favours retrograde lateral wires; I2 = 0%; 2 studies, 69 participants; Analysis 3.1 |
| Major complications: pin site infections | Othman 2017; Sadek 2018 | RR 2.06, 95% CI 0.56 to 7.56, favours retrograde lateral wires; I2 = 0%; 2 studies, 69 participants; Analysis 3.2 |
| Cosmetic deformity: loss of carrying angle > 10° (long‐term) | Othman 2017; Sadek 2018 | RR 0.68, 95% CI 0.21 to 2.15, favours Dorgan wires; I2 = 0%; 2 studies, 69 participants; Analysis 3.3 |
| Range of motion: loss of total range of motion > 10° (long term) | Othman 2017; Sadek 2018 | RR 0.72, 95% CI 0.26 to 2.00, favours Dorgan wires; I2 = 0%; 2 studies, 69 participants; Analysis 3.4 |
| Radiographic deformity: degrees of loss of Baumann's angle (long term) | Othman 2017 | MD ‐0.02°, 95% CI ‐0.08° to 0.04°, favours Dorgan wires; 1 study, 29 participants; Analysis 3.5 |
| Radiographic deformity: degrees of loss of lateral humeral‐capitellar angle ‐ long term | Othman 2017 | MD 0.07°, 95% CI ‐0.01° to 0.15°, favours Dorgan wires; 1 study, 29 participants; Analysis 3.6 |
| Posterior intrafocal wire versus retrograde crossed wires | ||
| Outcome | Studies | Effect estimate |
| Nerve injury | Jain 2019 | RR 0.33, 95% CI 0.07 to 1.60, favours intrafocal; 1 study, 168 participants; Analysis 4.1 |
| Major complications: pin site infections | Jain 2019 | RR 0.71, 95% CI 0.24 to 2.16, favours intrafocal; 1 study, 168 participants; Analysis 4.2 |
| Cosmetic deformity: loss of carrying angle (long term) | Jain 2019 | RR 1.88, 95% CI 0.84 to 4.19, favours retrograde crossed wires; 1 study, 168 participants; Analysis 4.3 |
| Cosmetic deformity: cubitus varus | Jain 2019 | RR 1.25, 95% CI 0.35 to 4.49, favours retrograde crossed wires; 1 study, 168 participants; Analysis 4.3 |
| Range of motion: loss of total range of motion > 10° (long term) | Jain 2019 | RR 1.33, 95% CI 0.73 to 2.42, favours retrograde crossed wires; 1 study, 168 participants; Analysis 4.4 |
| Radiographic deformity: loss of reduction (long term) | Jain 2019 | RR 1.79, 95% CI 1.28 to 2.52, favours retrograde crossed wires; 1 study, 168 participants; Analysis 4.5 |
| Retrograde lateral wires in a parallel versus divergent configuration | ||
| Outcome | Studies | Effect estimate |
| Nerve injury | Gopinathan 2018; Shamma 2020 | Not estimable (no events in either group) |
| Major complications: compartment syndrome | Gopinathan 2018 | RR 1.80, 95% CI 0.08 to 40.75, favours divergent wires; 1 study, 30 participants; Analysis 5.1 |
| Range of movement: loss of movement (medium term) | Shamma 2020 | MD ‐0.10, 95% CI ‐1.32 to 1.12, favours parallel wires; 1 study, 30 participants; Analysis 5.2 |
| Range of movement: loss of extension (medium term) | Shamma 2020 | MD 0.40, 95% CI ‐0.42 to 1.22, favours divergent wires; 1 study, 30 participants; Analysis 5.2 |
| Cosmetic deformity: loss of carrying angle > 10° (medium term) | Gopinathan 2018; Shamma 2020 | Not estimable (no events in either group) |
| Cosmetic deformity: degrees of loss of carrying angle (medium term) | Gopinathan 2018; Shamma 2020 | MD 0.02°, 95% CI ‐0.65° to 0.69°, favours divergent wires; 2 studies, 60 participants; Analysis 5.3 |
| Range of motion: loss of flexion (medium term) | Gopinathan 2018; Shamma 2020 | RR 0.87, 95% CI 0.17 to 4.42, favours parallel wires; 2 studies, 60 participants; Analysis 5.4 |
| Range of motion: loss of extension (medium term) | Gopinathan 2018; Shamma 2020 | RR 3.00, 95% CI 0.16 to 57.36, favours divergent wires; 2 studies, 60 participants; Analysis 5.4 |
| Radiographic deformity: degrees of loss of Baumann's angle (medium term) | Gopinathan 2018; Shamma 2020 | MD 0.87°, 95% CI ‐0.00° to 1.75°, favours divergent wires; 2 studies, 60 participants; Analysis 5.5 This difference is unlikely to be clinically important. |
| Mini‐open crossed wires versus percutaneous crossed wires | ||
| Outcome | Studies | Effect estimate |
| Nerve injury | Ercin 2016 | RR 2.05, 95% CI 0.48 to 8.69, favours percutaneous wires; 1 study, 104 participants; Analysis 6.1 |
| Major complications: pin site infections | Ercin 2016 | RR 1.02, 95% CI 0.18 to 5.87, favours percutaneous wires; 1 study, 104 participants; Analysis 6.2 |
| Resource use (length of hospital stay; days) | Ercin 2016 | MD 0.28, 95% CI ‐0.19 to 0.75, favours percutaneous wires; 1 study, 104 participants; Analysis 6.3 |
| Resource use (anaesthesia time) | Ercin 2016 | MD ‐5.85, 95% CI ‐12.82 to 1.12, favours mini‐open wires; 1 study, 104 participants; Analysis 6.3 |
| Buried versus non‐buried wires | ||
| Outcome | Studies | Effect estimate |
| Major complications: pin site infections | Saeed 2020 | RR 0.13, 95% CI 0.02 to 0.95, favours buried wires; 1 study, 80 participants; Analysis 7.1 |
CI: confidence interval; MD: mean difference; RR: risk ratio
lateral crossed (Dorgan) wires versus retrograde crossed wires;
lateral crossed (Dorgan) wires versus retrograde lateral wires;
posterior intrafocal wires versus retrograde crossed wires;
retrograde lateral wires in a parallel versus divergent configuration;
mini‐open crossed wires versus percutaneous crossed wires;
buried versus non‐buried wires.
Whilst most effect estimates were imprecise, indicating little or no difference between treatments, we noted an effect in favour of the following treatments:
lateral crossed (Dorgan wires) versus retrograde crossed wires: children treated with Dorgan wires had fewer nerve injuries (RR 0.26, 95% CI 0.09 to 0.76, favours Dorgan wires; I2 = 0%; 5 studies, 307 children; Analysis 2.1);
posterior intrafocal wires versus retrograde crossed wires: children treated with retrograde crossed wires were less likely to have a loss of reduction in the long term (RR 1.79, 95% CI 1.28 to 2.52, favours crossed wires; 1 study, 168 children; Analysis 4.5);
buried versus non‐buried wires: children treated with buried wires were less likely to have pin site infections (RR 0.13, 95% CI 0.02 to 0.95, favours buried wires; 1 study, 80 children; Analysis 7.1).
2.1. Analysis.

Comparison 2: Different forms of surgical intervention: lateral crossed (Dorgan) wires versus retrograde crossed wires, Outcome 1: Nerve injury
4.5. Analysis.

Comparison 4: Different forms of surgical intervention: posterior intrafocal wires versus retrograde crossed wires, Outcome 5: Radiographic deformity: loss of reduction at long‐term follow‐up
7.1. Analysis.

Comparison 7: Different forms of surgical intervention: buried versus non‐buried wires, Outcome 1: Major complications: pin site infections
1b. Different forms of surgical intervention: different reduction techniques
Open versus closed reduction for displaced fractures
Five studies with a total of 222 children compared open with closed techniques for the reduction of displaced supracondylar fractures (see Table 5). Ajmera 2013, Dehghan 2012, Kaewpornsawan 2001, and Ray 2019 used direct visualisation of the fracture as part of the open reduction, whereas Zhu 2016 used an open technique to position a joystick pin in the humeral shaft to facilitate reduction without exposing the fracture site. We analysed the data for this study separately to the direct visualisation techniques because it is clinically distinct from the direct visualisation technique. We did not conduct subgroup analysis for this comparison because there were insufficient studies.
Critical outcomes
1. Functional outcome
No studies reported this outcome.
2. Treatment failure requiring intervention
No studies reported this outcome.
3. Nerve injury
Four studies reported nerve injury (Ajmera 2013; Kaewpornsawan 2001; Ray 2019; Zhu 2016). For those studies in which direct visualisation was used, we found little or no difference between reduction techniques (RR 0.30, 95% CI 0.09 to 1.01, favours open reduction; I2 = 0%; 3 studies, 163 children; low‐certainty evidence; Analysis 8.1); we downgraded by one level for serious imprecision and one level for serious risks of bias.
8.1. Analysis.

Comparison 8: Different forms of surgical intervention: open versus closed reduction of displaced fractures, Outcome 1: Nerve injury
There were no incidences of nerve injury in either group in Zhu 2016.
4. Major complications
Five studies reported pin site infections (Ajmera 2013; Dehghan 2012; Kaewpornsawan 2001; Ray 2019; Zhu 2016). For those studies in which direct visualisation was used, we found fewer infections after closed reduction was used (RR 4.15, 95% CI 1.07 to 16.20; I2 = 0%; 4 studies, 253 children; low‐certainty evidence; Analysis 8.2); we downgraded by one level for serious imprecision and one level for serious risks of bias.
8.2. Analysis.

Comparison 8: Different forms of surgical intervention: open versus closed reduction of displaced fractures, Outcome 2: Major complications: pin site infections
The effect estimate was imprecise for this outcome when a joystick technique was used to support visualisation (RR 1.10, 95% CI 0.20 to 6.12, favours closed reduction; 1 study, 59 children; Analysis 8.2).
5. Cosmetic deformity
In the medium term:
Cubitus varus. At three months, Ajmera 2013 found little or no difference in the risk of development of cubitus varus (RR 0.19, 95% CI 0.01 to 3.89, favours open reduction; 1 study, 65 children; Analysis 8.3).
Loss of carrying angle of more than 10° (measured as number of events). At three months, Ajmera 2013 found little or no difference in the risk of developing a loss of carrying angle greater than 10° (RR 0.28, 95% CI 0.06 to1.23, favours open reduction; 1 study, 65 children; Analysis 8.3).
8.3. Analysis.

Comparison 8: Different forms of surgical intervention: open versus closed reduction of displaced fractures, Outcome 3: Cosmetic deformity: loss of carrying angle > 10 degrees or elbow deformity (medium‐ and long‐term)
In the long term:
Cubitus varus. Zhu 2016 found no difference in cubitus varus rates between joystick and open reduction when measured at 30 to 33 months follow‐up (RR 1.10, 95% CI 0.20 to 6.12, favours closed reduction; 1 study, 59 children; Analysis 8.3).
Loss of carrying angle (measured as degrees of loss). Ray 2019 reported an improved outcome (a reduced loss of carrying angle) at 12 months when closed reduction was used (MD ‐1.12°, 95% CI ‐2.11° to ‐0.13°, favours open reduction; Analysis 8.4).
8.4. Analysis.

Comparison 8: Different forms of surgical intervention: open versus closed reduction of displaced fractures, Outcome 4: Cosmetic deformity: loss of carrying angle in the long term (degrees of loss)
Other important outcomes
Studies reported range of movement at the elbow, patient satisfaction, and radiographic deformity. We found that fewer children had a loss of range of movement in the medium term after closed reduction (RR 3.20, 95% CI 1.05 to 9.77, favours closed reduction; 2 studies, 93 children; Analysis 8.5). There was less satisfaction with scar appearance following open reduction, when evaluated by children or parent (MD ‐0.60, 95% CI ‐1.09 to ‐0.11; 1 study, 28 children; Analysis 8.7), and by assessors blinded to treatment allocation (MD ‐0.50, 95% CI ‐0.87 to ‐0.13; 1 study, 28 children; Analysis 8.7). We also noted less deformity according to Baumann's angle at 12 months when open reduction was used (MD 1.14°, 95% CI 0.21° to 2.07°, favours open reduction; 1 study, 70 children; Analysis 8.8). Ray 2019 reported a shorter hospital stay after closed reduction (MD 1.30 days, 95% CI 0.99 to 1.61, favours closed reduction; 1 study, 70 children; Analysis 8.10). Zhu 2016 reported a much shorter operative time for the closed reduction group when a joystick technique was used (MD 17.70 minutes, 95% 11.41 to 23.99, favours closed reduction; 1 study, 68 children; Analysis 8.10). Effects estimates for other outcomes were imprecise and included the possibility of benefits and harms for both treatments. See Table 9. No studies reported functional outcome, pain, quality of life, return to sport and normal activities, and emotional distress of the child.
8.5. Analysis.

Comparison 8: Different forms of surgical intervention: open versus closed reduction of displaced fractures, Outcome 5: Range of movement: loss of total range of movement > 10 degrees at medium term
8.7. Analysis.

Comparison 8: Different forms of surgical intervention: open versus closed reduction of displaced fractures, Outcome 7: Patient satisfaction with scar appearance (higher scores indicates more satisfaction)
8.8. Analysis.

Comparison 8: Different forms of surgical intervention: open versus closed reduction of displaced fractures, Outcome 8: Radiographic deformity: difference in Baumann's angle (medium and long term)
8.10. Analysis.

Comparison 8: Different forms of surgical intervention: open versus closed reduction of displaced fractures, Outcome 10: Resource use
6. Other important outcomes: open reduction versus closed reduction.
| Other important outcomes: open reduction versus closed reduction | |||
| Outcome | Studies (using direct visualisation) | Effect estimate (using direct visualisation) | Effect estimate using joystick technique (in Zhu 2016 only) |
|
Range of movement at the elbow: loss of total range of movement > 10 degrees Follow‐up: medium term (range from 3 months to 5 months) |
Ajmera 2013; Kaewpornsawan 2001 | RR 3.20, 95% CI 1.05 to 9.77, favours closed reduction; 2 studies, 93 participants; Analysis 8.5 | Not estimable (no events in either group) |
|
Range of movement at the elbow: degrees of loss of carrying angle Follow‐up: long term (12 months) |
Ray 2019 | MD 0.94°, 95% CI ‐0.29° to 2.17°, favours closed reduction; 1 study, 60 participants; Analysis 8.6 | ‐ |
|
Patient satisfaction Assessed using non‐validated 10‐point scoring system to evaluate scar appearance (higher scores indicating higher levels of satisfaction) Follow‐up: 5 months |
Kaewpornsawan 2001 | Participant‐assessed: MD ‐0.60, 95% CI ‐1.09 to ‐0.11, favours closed reduction; 1 study, 28 participants; Analysis 8.7 Assessor blinded to treatment allocation: MD ‐0.50, 95% CI ‐0.87 to ‐0.13, favours closed reduction; 1 study, 28 participants; Analysis 8.7 |
‐ |
|
Radiographic deformity: loss of Baumann's angle Follow‐up: medium term (5 months) |
Kaewpornsawan 2001 | MD 0.12°, 95% CI ‐1.14° to 1.38°, favours closed reduction; 1 study, 28 participants; Analysis 8.8 | ‐ |
|
Radiographic deformity: loss of Baumann's angle Follow‐up: long term (12 months) |
Ray 2019 | MD 1.14°, 95% CI 0.21° to 2.07°, favours open reduction; 1 study, 70 participants; Analysis 8.8 | ‐ |
|
Radiographic deformity: capitulum lying posterior to the anterior humeral line Follow‐up: long term (30 to 33 months) |
‐ | ‐ | RR 0.37, 95% CI 0.04 to 3.83, favours open reduction; 1 study, 59 participants; Analysis 8.9 |
| Resource use (operative time in minutes) | Ray 2019 | MD ‐6.30, 95% CI ‐20.11 to 7.51, favours open reduction; 1 study, 70 participants; Analysis 8.10 | MD 17.7, 95% CI 11.41 to 23.99, favours closed reduction; 1 study, 68 participants; Analysis 8.10 |
| Resource use (length of hospital stay in days) | Ray 2019 | MD 1.30, 95% CI 0.99 to 1.61, favours closed reduction; 1 study, 70 participants; Analysis 8.10 | MD 0.20, 95% CI ‐0.47 to 0.87, favours closed reduction; 1 study, 68 participants; Analysis 8.10 |
CI: confidence interval; MD: mean difference; RR: risk ratio
Sensitivity analysis
Influence of any small‐study effects by comparing the random‐effects model output to a fixed‐effect model calculated with the Mantel‐Haenszel method. We recalculated all effect estimates for critical outcomes using the random‐effects model. For pin site infections, we noted that the effect estimate was imprecise when a random‐effects model was used (RR 3.53, 95% CI 0.87 to 14.43; 4 studies, 253 children). We noted no other important changes to the effect estimates.
We did not conduct other planned sensitivity analyses because studies in this comparison were all published with full study reports, were not at high risk of bias, or had no notable missing data. We did not conduct post hoc sensitivity analysis. We noted that three studies in this comparison were published in journals that are not indexed in PubMed (Ajmera 2013; Dehghan 2012; Ray 2019); this left only one study and thus sensitivity analysis was not meaningful. No studies in this comparison had duplicate publications and this post hoc sensitivity analysis was therefore not applicable to this comparison.
2. Surgical versus non‐surgical treatment
Surgical fixation versus non‐surgical immobilisation for displaced fractures
Three studies with 140 children compared surgical fixation with non‐surgical immobilisation for displaced fractures (see Table 5).
Critical outcomes
1. Functional outcome
No studies reported this outcome.
2. Treatment failure requiring intervention
Only Pandey 2008 reported this outcome, with only one child (in the non‐surgical group) experiencing treatment failure (RR 0.33, 95% CI 0.01 to 7.87, favours surgical treatment; 1 study, 60 children; Analysis 9.1). The child with treatment failure required open reduction one week after the index procedure. We downgraded the certainty of the evidence by three levels: one for serious risk of bias and two for very serious imprecision because of the small sample size and wide CI indicating possible benefits and harms for both treatments.
9.1. Analysis.

Comparison 9: Surgical versus non‐surgical treatment: surgical fixation versus non‐surgical immobilisation, Outcome 1: Treatment failure
3. Nerve injury
Three studies reported nerve injury (Kumar 2021; Pandey 2008; Shah 2017). We found little or no difference between treatments in nerve injury (RR 2.50, 95% CI 0.50 to 12.46, favours non‐surgical treatment; I2 = 0%; 3 studies, 140 children; very low‐certainty evidence; Analysis 9.2). In Pandey 2008, the nerve injury in the cast group was present pre‐intervention and an ulnar nerve injury was identified in the wire group following treatment. In Shah 2017, a radial nerve injury was identified pre‐procedure in the wire fixation group with no nerve injuries in the group treated without wires. Kumar 2021 reported that two children developed an ulnar nerve injury following percutaneous pinning with no post‐manipulation injuries in the group treated in above‐elbow cast. Two preoperative nerve injuries were reported (one radial and one median) but it was not specified to which group the children with these injuries were allocated. We downgraded the certainty of the evidence by three levels: one for serious risk of bias and two for very serious imprecision because of the small sample size and wide CI indicating possible benefits and harms for both treatments.
9.2. Analysis.

Comparison 9: Surgical versus non‐surgical treatment: surgical fixation versus non‐surgical immobilisation, Outcome 2: Nerve injury
4. Major complications
Major complications were reported in three studies (Kumar 2021; Pandey 2008; Shah 2017). We found little or no difference between treatments in pin site infections (RR 4.00, 95% CI 0.47 to 34.11, favours non‐surgical treatment; I2 = 0%; 3 studies, 126 children; very low‐certainty evidence; Analysis 9.3); we noted that there were no events in Pandey 2008. In Shah 2017, one child from the non‐surgical treatment group had a pre‐intervention vascular injury which we did not include in this analysis. We downgraded the certainty of the evidence by three levels: one for serious risk of bias and two for very serious imprecision because of the small sample size and wide CI indicating possible benefits and harms for both treatments.
9.3. Analysis.

Comparison 9: Surgical versus non‐surgical treatment: surgical fixation versus non‐surgical immobilisation, Outcome 3: Major complications: pin site infections
5. Cosmetic deformity
In all studies, long‐term cosmetic deformity was measured at 6 months (Kumar 2021; Pandey 2008), or 6 to 12 months (Shah 2017). No studies measured this outcome in the medium term.
Cubitus varus. Two studies reported this outcome (Kumar 2021; Shah 2017). The effect estimate was imprecise and we found little or no difference between treatments (RR 0.20, 95% CI 0.04 to 1.09, favours surgical fixation; I2 = 0%; 2 studies, 80 children; very low‐certainty evidence; Analysis 9.4). We downgraded the certainty of the evidence by three levels: one level for serious risk of bias and two levels for imprecision because of the small sample size and the wide CI indicating possible benefits and harms for both treatments.
Loss of carrying angle of more than 10° (measured as number of events). Two studies reported this outcome (Pandey 2008; Shah 2017). We found that the risk of loss was less likely after surgical treatment (RR 0.29, 95% CI 0.10 to 0.81, favours surgical fixation; I2 = 0%; 2 studies, 66 children; Analysis 9.4).
Cubitus valgus. Reported in only one study (Shah 2017), we found little or no difference between treatments for this outcome (RR 0.33, 95% CI 0.02 to 7.32, favours surgical fixation; 1 study, 20 children; Analysis 9.4).
Loss of carrying angle (degrees of loss). Two studies reported this outcome (Pandey 2008; Shah 2017). In Pandey 2008, we noted that there was a better outcome when surgical fixation was used (MD ‐3.20°, 95% CI ‐6.09° to ‐0.31°; 1 study, 46 children; Analysis 9.5). In Shah 2017, degrees of loss were reported without standard deviations (mean of 6.8° with internal fixation and a mean of 5.8° with non‐surgical immobilisation).
9.4. Analysis.

Comparison 9: Surgical versus non‐surgical treatment: surgical fixation versus non‐surgical immobilisation, Outcome 4: Cosmetic deformity: loss of carrying angle > 10 degrees or elbow deformity
9.5. Analysis.

Comparison 9: Surgical versus non‐surgical treatment: surgical fixation versus non‐surgical immobilisation, Outcome 5: Cosmetic deformity: degrees of loss of carrying angle at long‐term follow‐up
Other important outcomes
Studies reported range of movement (number of children with loss of movement > 10°, degrees of loss of flexion and extension, and loss of total range of movement). The effect estimates were all imprecise and included the possibility of benefits and harms for both treatments (Table 10). No studies reported functional outcome, pain, quality of life, child or parent satisfaction, return to sport and normal activities, or emotional distress of the child.
7. Other important outcomes: surgical fixation versus non‐surgical immobilisation.
| Other important outcomes: surgical fixation versus non‐surgical immobilisation | ||
| Outcome | Studies | Effect estimate |
|
Range of movement at the elbow: loss of movement > 10° Follow‐up: long term (6 to 12 months) |
Pandey 2008; Shah 2017 | RR 0.24, 95% CI 0.06 to 1.03, favours surgical fixation; I2 = 0%; 2 studies, 66 participants; Analysis 9.6 |
|
Range of movement at the elbow: degrees of loss of flexion Follow‐up: long term (6 months) |
Pandey 2008 | MD ‐0.80°, 95% CI ‐3.74° to 2.14°, favours surgical fixation; 1 study, 46 participants; Analysis 9.7 |
|
Range of movement at the elbow:degrees of loss of extension Follow‐up: long term (6 months) |
Pandey 2008 | MD ‐0.10°, 95% CI ‐1.93° to 1.73°, favours surgical fixation; 1 study, 46 participants; Analysis 9.7 |
|
Range of movement at the elbow:degrees of loss of total range of movement Follow‐up: long term (6 to 12 months) |
Shah 2017 | Surgical fixation group: mean 8.8° (reported without SD) Non‐surgical immobilisation group: mean 7.3° (reported without SD) |
CI: confidence interval; MD: mean difference; RR: risk ratio; SD: standard deviation
Sensitivity analysis
Influence of any small‐study effects by comparing the random‐effects model output to a fixed‐effect model calculated with the Mantel‐Haenszel method. We recalculated all effect estimates for critical outcomes using the random‐effects model which did not alter our interpretation of any effect estimates.
Analysis of the result of any missing data on the review outcomes. We reconsidered analysis of long‐term loss of carrying angle which had been reported by Shah 2017 without standard deviations (SDs). In a best case and worst case scenario, where the SDs were the same as the smallest or largest SD measured by Pandey 2008, there was no difference in the loss of carrying angle (MD ‐1.69, 95% CI ‐4.00 to 0.63; and MD ‐2.09, 95% CI ‐4.57 to 0.39).
We did not conduct sensitivity analysis for other pre‐defined criteria because all studies were published with full study reports and none were at high risk of allocation bias and detection bias. We note that all the studies in this comparison group were published in journals that were not indexed in PubMed, but none of these studies had duplicate publications; post hoc subgroup analysis was therefore not possible.
3. Different forms of traction
Skin traction versus olecranon skeletal traction for displaced fractures
Only one study compared the use of traction applied to the skin with elasticated bandage to overhead skeletal traction with a pin placed in the olecranon under general anaesthetic (Kuzma 2014). Skin traction was provided to 66 children and skeletal traction to 67 children (see Table 5).
Critical outcomes
1. Functional outcome
Kuzma 2014 did not report this outcome.
2. Treatment failure requiring intervention
Kuzma 2014 did not report this outcome.
3. Nerve injury
We found little difference in the number of children who experienced a nerve injury according to the type of traction (RR 1.69, 95% CI 0.42 to 6.80, favours skeletal traction; 1 study, 133 children; very low‐certainty evidence; Analysis 10.1). Five children treated in skin traction and three children treated in skeletal traction experienced nerve injury. We note that all nerve injuries were present before the treatment was applied. We downgraded the certainty of the evidence by one level for serious risk of bias and two levels for very serious imprecision because the evidence was from few participants and the CI indicated possible benefits and harms for both treatments.
10.1. Analysis.

Comparison 10: Different forms of traction: skin traction versus olecranon skeletal traction, Outcome 1: Nerve injury
4. Major complications
No children treated in skin traction had major complications in the form of pin site infection, whilst four children in skeletal traction experienced pin site infection (RR 0.11, 95% CI 0.01 to 2.05, favours skin traction; 1 study, 133 children; very low‐certainty evidence; Analysis 10.2). We downgraded the certainty of the evidence by one level for serious risk of bias and two levels for very serious imprecision because the evidence was from few participants and the CI indicated possible benefits and harms for both treatments.
10.2. Analysis.

Comparison 10: Different forms of traction: skin traction versus olecranon skeletal traction, Outcome 2: Major complications
5. Cosmetic deformity
Cosmetic deformity was assessed through the development of cubitus varus at 3 months. Cubitus varus was present in three children treated in skin traction and five children treated in skeletal traction (RR 0.61, 95% CI 0.15 to 2.45, favours skin traction; 1 study, 133 children; very low‐certainty evidence; Analysis 10.3). We downgraded the certainty of the evidence by one level for serious risk of bias and two levels for very serious imprecision because the evidence was from few participants and the CI indicated possible benefits and harms for both treatments.
10.3. Analysis.

Comparison 10: Different forms of traction: skin traction versus olecranon skeletal traction, Outcome 3: Cosmetic deformity: cubitus varus
Other important outcomes
Kuzma 2014 reported range of movement at the elbow (loss of > 10° of flexion or > 5° of hyperextension). Loss of flexion was experienced by 33 children treated in skin traction and 42 children treated in skeletal traction (RR 0.80, 95% CI 0.59 to 1.08, favours skin traction; 1 study, 133 children; Analysis 10.4), and hyperextension was found in five children treated in skin traction and one child treated in skeletal traction (RR 5.08, 95% CI 0.61 to 42.28, favours skeletal traction; 1 study, 133 children; Analysis 10.4). For resource use, we noted a shorter duration of traction when skin traction was used (MD‐2.7 days, 95% CI ‐3.95 to ‐1.45, favours skin traction; 1 study, 133 children; Analysis 10.5).
10.4. Analysis.

Comparison 10: Different forms of traction: skin traction versus olecranon skeletal traction, Outcome 4: Range of movement (medium term)
10.5. Analysis.

Comparison 10: Different forms of traction: skin traction versus olecranon skeletal traction, Outcome 5: Resource use (duration of traction)
This study did not report data for functional outcome, pain, quality of life, child or parent satisfaction, return to sport and normal activities, and emotional distress of the child.
4. Different forms of non‐surgical intervention
Backslab versus sling for undisplaced fractures
The treatment of undisplaced, Gartland 1 supracondylar fractures was evaluated in one study with 50 children (Oakley 2009). See Table 5.
Critical outcomes
Oakley 2009 did not report functional outcome, treatment failure, and cosmetic deformity. No nerve injuries or major complications were experienced by children in either group; we judged the certainty of this evidence to be very low owing to serious risks of imprecision in the small sample size and risks of bias in the included study.
Other important outcomes
Oakley 2009 also reported pain (intensity of pain, duration of pain, and duration of analgesia use), parental satisfaction, and ability to return to normal activities (number of children returning to normal activities within two weeks, and the time to resume normal activities). Study authors reported data for pain and time to resume normal activities using median (interquartile range (IQR)) values, and we did not calculate effect estimates for these data because they may have been skewed. Study authors noted that children resumed normal activities sooner when a backslab was used (P = 0.01). However, the effects for other reported outcomes showed little or no difference between treatments. See Table 11. This study did not report functional outcome, range of movement, quality of life, radiographic deformity, or emotional distress of the child.
8. Other important outcomes: backslab versus sling.
| Other important outcomes: backslab versus sling | ||
| Outcome | Studies | Data as reported by study authors |
|
Pain Assessed with 100‐point VAS (lower scores indicate less pain) |
Oakley 2009 | Backslab group, median (IQR): 28 (20 to 40) Sling group, median (IQR): 33 (25 to 53) P = 0.21; 1 study, 48 participants |
|
Pain Assessed as number of days experiencing pain |
Oakley 2009 | Backslab group, median (IQR): (1 to 8) days Sling group, median (IQR): 6 (5 to 9.5) days P = 0.07; 1 study, 48 participants |
|
Pain Assessed as days requiring analgesia |
Oakley 2009 | Backslab group, median (IQR): 2 (1 to 5) days Sling group, median (IQR): 3 (2 to 4.5) days P value not reported; 1 study, 48 participants |
|
Parent satisfaction Assessed by asking if parents would be prepared to use the same immobilisation device Follow‐up: at time of removal |
Oakley 2009 | RR 0.21, 95% CI 0.03 to 1.77, favours backslab; 1 study, 50 participants; Analysis 11.1 |
|
Return to normal activities Follow‐up: at 2 weeks |
Oakley 2009 | RR 1.41, 95% CI 0.49 to 4.02, favours backslab; 1 study, 50 participants; Analysis 11.2 |
| Time to return to normal activities | Oakley 2009 | Backslab group, median (IQR): 2 (1 to 3) days Sling group, median (IQR): 7 (3 to 13) days P = 0.01; 1 study, 48 participants |
CI: confidence interval; IQR: interquartile range; MD: mean difference; RR: risk ratio; VAS: visual analogue scale
Discussion
Summary of main results
We included 52 studies (47 RCTs and five quasi‐RCTs) with 3594 children who had supracondylar elbow fractures. We also identified three ongoing studies.
We found evidence for seven different surgical fixation techniques. We also found evidence for open compared with closed reduction, surgical fixation compared with non‐surgical immobilisation, different forms of traction, and different forms of non‐surgical intervention. No studies compared different timings of the intervention or different locations. We report below the main findings of four of these comparisons, representing the most clinically relevant and the most substantial bodies of evidence in the review.
Retrograde lateral wires versus retrograde crossed wires (29 studies, 2068 children)
We found low‐certainty evidence of less nerve injury with retrograde lateral wires. We conducted a post hoc subgroup analysis of this data, using only the studies that reported whether an open or closed technique was used, and we noted fewer injuries when lateral wires were used with a percutaneous technique, but little difference in nerve injuries when an open technique was used. In formal tests for subgroup interactions, we noted a difference between these subgroups (P = 0.05), but could not rule out the possibility that other factors could explain the difference between these groups.
We also found little or no difference between the intervention in major complications, which were described as pin site infections in all studies (low‐certainty evidence). For functional status, treatment failure requiring re‐intervention, and cosmetic deformity, there was very low‐certainty evidence showing no evidence of a difference between interventions; this evidence was derived from only one or two studies.
Open reduction versus closed reduction (4 studies, 295 children)
We found little or no difference between open and closed reduction techniques in the risk of nerve injuries (low‐certainty evidence). However, there may be fewer major complications (pin site infections) when closed reduction is used. No studies reported functional outcome, treatment failure requiring re‐intervention, or cosmetic deformity. The four studies in this comparison used direct visualisation during surgery. One additional study used a joystick technique for visualisation and we did not combine data from this study in analyses.
Surgical fixation using wires versus non‐surgical immobilisation using a cast (3 studies, 140 children)
We found very low‐certainty evidence showing little or no difference between interventions for treatment failure requiring re‐intervention, nerve injury, major complications, and cosmetic deformity. No studies reported functional outcome.
Backslab versus sling (1 study, 50 children)
In this single study, no nerve injuries or major complications were experienced by children in either group; this evidence is of very low certainty. Functional outcome, treatment failure, and cosmetic deformity were not reported.
Overall completeness and applicability of evidence
The availability of evidence for each of the comparisons completed in this review varied greatly. The comparison of retrograde lateral and retrograde crossed wires included the most studies, with outcome data for nerve injury and major complications. Whilst only one study reported a functional outcome using an elbow‐specific score, many studies provided a functional grading through presentation of a Flynn score (Flynn 1974). In its original version, this score is a composite of loss of total range of motion and carrying angle, and has also been modified to include loss of flexion and extension. Many of the studies used this grading system in some form, and where studies presented domains separately, it was possible to include those data in this review. For other outcomes, there was a lack of consistency in reporting, with several different parameters, particularly in the assessment of range of motion and radiographic deformity. Disappointingly, none of the included studies reported a quality of life score for children or emotional distress for children, which we identified as important outcomes in the development of the review protocol. For comparisons besides retrograde lateral versus retrograde crossed wires, there was limited evidence from relatively few RCTs to evaluate the impact of different interventions on the outcomes for the children.
The overall study population in this review had a male predominance with a maximum mean age of 8.4 years; this is in keeping with the baseline characteristics of this injury. Undisplaced fractures were universally excluded from the studies of different surgical interventions. Some of the studies of surgical interventions included fractures that were not completely displaced, though results for this group were not presented separately in any study, preventing the subgroup analysis of this group. Equally, no studies presented results of different age groups separately, which may have an impact on outcomes related to remodelling and rehabilitation.
The majority of the interventions identified in this review are relevant to international practice.
None of the studies presented data for all the critical outcomes in the review. This suggests that the follow‐up periods of the trials may be insufficient for children to become sufficiently symptomatic following a supracondylar malunion to require further corrective surgery. Major complications were typically reported as pin site infections only, with variable reporting of compartment syndrome and vascular injury. For nerve injuries, we could not easily account for the number of injuries that were evident prior to the intervention. However, because the studies were all randomised, we could infer that size of the difference between interventions groups was associated with the intervention rather than pre‐intervention injuries.
Quality of the evidence
For all comparisons and outcomes, we judged that the certainty of the available evidence was very low or low. We downgraded all outcomes at least one level for risk of bias, which was usually for unclear reporting of randomisation technique or allocation concealment. We downgraded many outcomes for imprecision as a consequence of small sample sizes, low event rates, and accompanying wide confidence intervals. For nerve injury, we also could not rule out the possibility of publication bias evident in our visual inspection of a funnel plot. This outcome also included a large number of studies published in journals that were not indexed in PubMed, which appeared to influence the data more strongly in favour of retrograde lateral wires.
We did not need to downgrade any outcomes for inconsistency, as heterogeneity was low for the majority of outcomes, and the included studies provided direct evidence.
Potential biases in the review process
This review has been conducted in line with the previously published protocol (Marson 2020b). The searches have been performed using a robust search strategy, and we are confident that all relevant trials in the conventional medical literature have been included.
Searches of bibliographic databases and trials registries led to the identification of several trials not identified in the initial search that had been published in the grey literature in journals that were not indexed. We used sensitivity analysis to explore whether these studies influenced the data, and we could not rule out the possibility that these studies influenced the effect estimate for nerve injury. We also conducted a post hoc sensitivity analysis to investigate concerns related to a small number of studies that were published by different author teams; these studies did not appear to influence the finding of the critical outcomes in the review.
Many of the additional trials identified from non‐indexed journals had unclear reporting of the randomisation technique and allocation concealment, although all studies indicated that a random factor was used to allocate children to groups. Although we attempted to contact study authors, we received few responses. We could not rule out the possibility that some of these studies may be quasi‐randomised in design, and we have been mindful of this when drawing our conclusions.
From discussion with our clinical authors, we agreed to conduct a subgroup analysis of nerve injury data of retrograde lateral wires versus retrograde crossed wires according to whether an open or closed technique was used. We note that this was a post hoc decision, and because technique was not reported in all studies, the subgroup analysis included fewer studies than the primary analysis. Whilst we believed it was clinically important to report the findings from this subgroup analysis, we cannot be certain whether the result of the formal tests for subgroup interaction is truly indicative of a difference in technique, as other factors may be also be responsible for this result. In order to advise caution with this finding, we have stated this uncertainty throughout the relevant sections of the review.
Agreements and disagreements with other studies or reviews
We provide a detailed review of the 21 systematic reviews identified as part of searching, in Appendix 2. Whilst our findings are broadly consistent with previous reviews, we have been cautious in our interpretation of our findings, given the limitations present in the literature. Whilst we would intuitively agree with the American Academy of Orthopaedic Surgeons' (AAOS) guidelines that surgical management is not indicated for undisplaced fractures (American Academy of Orthopaedic Surgeons 2011), there is a lack of direct RCT evidence to support this, and the two RCTs that compare different non‐surgical treatments have limited medium‐ and long‐term follow‐up (Oakley 2009; Silva 2018). The quality of evidence to confirm or refute the findings of the Kurer 1990 and Yeomans 2018 reviews is very low, with one RCT comparing different forms of cast following reduction of displaced fractures, one RCT comparing different forms of traction, and two RCTs comparing surgical and non‐surgical fixation. We are concerned about the high redisplacement rate described in Pandey 2008, following application of a plaster cast in flexion with high rates of cosmetic deformity, similar to the rates identified in the Kurer 1990 review.
Authors' conclusions
Implications for practice.
For children with supracondylar elbow fractures, retrograde lateral wires may result in fewer nerve injuries than retrograde crossed wires.
Currently, there is insufficient evidence to determine the effectiveness of other treatments, and all findings in the review had low‐ to very low‐certainty evidence.
Implications for research.
There are several key directions for future research uncovered by this review. There was considerable heterogeneity of outcomes presented by different trials, which has limited our ability to combine all identified trials into the meta‐analyses. There is an urgent need to agree and harmonise outcomes, and the tools to measure them, to ensure all trials measure and report key results that can then be pooled. This can be achieved with the adoption of a core outcome set to encourage harmonisation of outcome reporting (Marson 2020c). Additional preparatory work in the anticipation of future trials would be to undertake validation of functional, emotional, and quality of life scores for children with supracondylar fractures to ensure validity, reliability, and interpretability of patient‐reported scores. We need to establish the maximum acceptable loss of reduction, range of motion, and cosmetic deformity that have clinical implications for children and their parents, being mindful that there may be variations based on cultural and social differences in populations.
Whilst we have been unable to answer if displaced fractures should be treated with reduction and casting or by other techniques (e.g. traction or surgical fixation), we would suggest that this is not a key research priority, given the American Academy of Orthopaedic Surgeons (AAOS) and British Orthopaedic Association/British Society for Children's Orthopaedic Surgery (BOA/BSCOS) guideline and the high treatment failure rates when casting has been used. A comparison of traction and surgical fixation would be of greater benefit, particularly for application in resource‐poor environments around the world or for settings where access to anaesthesia is limited. Any further comparison of retrograde crossed and retrograde lateral wires should be high quality and capture outcomes using validated patient‐reported scores with adequate follow‐up. As the key uncertainty is if the increased loss of reduction has any clinical significance, such a trial would need to be large in scale.
Throughout this review, we have acknowledged that blinding of participants and outcome assessors is challenging, particularly as treatments can be viewed as they are applied or removed. We have assessed studies as low risk of bias for blinding when there is some evidence of separation between the core study team and those undertaking the majority of the interventions or performing the assessments. Children and their parents are in an ideal position to provide insight into their experience and function following an intervention, and this is unlikely to be clouded by preconceived ideas of superiority of one intervention over another. There is scope for improvements in study reporting, with prospective trial registration and publication of trial protocols to improve study transparency and confidence surrounding the quality of evidence.
Other key uncertainties that remain unanswered following this review are the following.
Should children with undisplaced supracondylar fractures be immobilised in a plaster cast or sling?
Should children with displaced fractures undergo reduction in the hospital emergency department prior to theatre?
Should children with displaced fractures have emergency surgery (planned within 12 hours, including overnight) or urgent surgery (surgery within 24 hours, but not overnight)?
What duration of immobilisation should children be prescribed following an undisplaced fracture or surgical fixation for a displaced fracture?
History
Protocol first published: Issue 5, 2020
Acknowledgements
We are grateful to Joanne Elliott for editorial support on the review, and Maria Clarke for her help with the search. We thank Toby Lasserson for his comments and help at editorial review. We also thank Gregory Firth (clinical referee) for his helpful feedback.
Ben Marson is funded by a National Institute for Health Research (NIHR) Doctoral Fellowship NIHR300240, and this project was supported by the National Institute for Health Research via Cochrane Infrastructure funding to the Cochrane Bone, Joint and Muscle Trauma (BJMT) Group. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service or the Department of Health and Social Care.
Editorial and peer‐reviewer contributions
Cochrane BJMT supported the authors in the development of this review. Sharon Lewis is a members of the Cochrane BJMT editorial base, but was not involved in the editorial process or decision making for this review.
The following people conducted the editorial process for this article:
Sign‐off Editor (final editorial decision): Xavier Griffin, Co‐ordinating Editor, Cochrane BJMT;
Managing Editor (selected peer reviewers, collated peer‐reviewer comments, provided editorial guidance to authors, edited the article): Joanne Elliott, Managing Editor, Cochrane Bone, Joint and Muscle Trauma Group;
Methodological Editor (advised on methodology and review content): Toby Lasserson, Deputy Editor in Chief, Cochrane;
Copy Editor (copy‐editing and production): Faith Armitage, Copy Edit Support Team;
Peer‐reviewers (provided comments and recommended an editorial decision): Gregory Firth, Consultant Orthopaedic Surgeon, Barts Health NHS Trust.
Appendices
Appendix 1. Search strategies
CENTRAL (CRS‐WEB)
1 MESH DESCRIPTOR Humeral fractures AND CENTRAL:TARGET (151) 2 ((humer* or elbow* or supracondylar) NEAR3 fractur*):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET (771) 3 #1 OR #2 (771) 4 MESH DESCRIPTOR Infant EXPLODE ALL AND CENTRAL:TARGET (31895) 5 MESH DESCRIPTOR child EXPLODE ALL AND CENTRAL:TARGET (55482) 6. MESH DESCRIPTOR Adolescent AND CENTRAL:TARGET (103537) 7. MESH DESCRIPTOR Minors AND CENTRAL:TARGET (8) 8 MESH DESCRIPTOR Puberty EXPLODE ALL AND CENTRAL:TARGET (361) 9. MESH DESCRIPTOR Pediatrics EXPLODE ALL AND CENTRAL:TARGET (697) 10 MESH DESCRIPTOR Schools AND CENTRAL:TARGET (1896) 11 MESH DESCRIPTOR Schools, Nursery AND CENTRAL:TARGET (38) 12 (Infant* or Infancy or Newborn* or New born* or Baby* or Babies or Neonat* or Preterm* or Prematur* or Postmatur* or Child* or Schoolchild* or School age* or Preschool* or pre school* or Kid or kids or Toddler* or Adoles* or Teen* or Youth or Boy* or Girl* or Minors* or Pubert* or Pubescen* or Prepubescen* or Paediatric* or Paediatric* or Peadiatric* or Nursery school* or Kindergar* or Primary school* or Secondary school* or Elementary school* or High school* or Highschool*):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET (290823) 13 #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 (290961) 14 #3 AND #13 (173)
Medline (Ovid)
1 Humeral fractures/ (7480) 2 ((humer* or elbow* or supracondylar) adj3 fractur*).tw. (9450) 3 1 or 2 (12760) 4 exp infant/ (1128327) 5 exp child/ (1889823) 6 Adolescent/ (2004518) 7 Minors/ (2563) 8 exp puberty/ (17542) 9 exp Pediatrics/ (57450) 10 schools/ or schools, nursery/ (38732) 11 (Infant* or Infancy or Newborn* or New born* or Baby* or Babies or Neonat* or Preterm* or Prematur* or Postmatur* or Child* or Schoolchild* or School age* or Preschool* or pre school* or Kid or kids or Toddler* or Adoles* or Teen* or Youth or Boy* or Girl* or Minors* or Pubert* or Pubescen* or Prepubescen* or Paediatric* or Paediatric* or Peadiatric* or Nursery school* or Kindergar* or Primary school* or Secondary school* or Elementary school* or High school* or Highschool*).tw. (2371790) 12 or/4‐11 (4301396) 13 3 and 12 (4856) 14 randomized controlled trial.pt. (504064) 15 controlled clinical trial.pt. (93621) 16 randomized.ab. (476541) 17 placebo.ab. (206951) 18 drug therapy.fs. (2196015) 19 randomly.ab. (331424) 20 trial.ab. (502119) 21 groups.ab. (2035564) 22 or/14‐21 (4682772) 23 exp animals/ not humans.sh. (4691338) 24 22 not 23 (4059881) 25 13 and 24 (490)
Embase (Ovid)
1 humerus fracture/ (9134) 2 ((humer* or elbow* or supracondylar) adj3 fractur*).tw. (10525) 3 1 or 2 (14657) 4 exp infant/ (970229) 5 exp child/ (2571123) 6 adolescent/ (1497450) 7 "minor (person)"/ (608) 8 exp puberty/ (40664) 9 exp pediatrics/ (104698) 10 school/ (61164) 11 nursery school/ or nursery/ (4319) 12 (Infant* or Infancy or Newborn* or New born* or Baby* or Babies or Neonat* or Preterm* or Prematur* or Postmatur* or Child* or Schoolchild* or School age* or Preschool* or pre school* or Kid or kids or Toddler* or Adoles* or Teen* or Youth or Boy* or Girl* or Minors* or Pubert* or Pubescen* or Prepubescen* or Paediatric* or Paediatric* or Peadiatric* or Nursery school* or Kindergar* or Primary school* or Secondary school* or Elementary school* or High school* or Highschool*).tw. (2902301) 13 or/4‐12 (4267623) 14 3 and 13 (4501) 15 Randomized controlled trial/ (598975) 16 Controlled clinical study/ (464022) 17 Random*.ti,ab. (1522606) 18 randomization/ (86540) 19 intermethod comparison/ (258958) 20 placebo.ti,ab. (304061) 21 (compare or compared or comparison).ti. (505574) 22 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. (2087932) 23 (open adj label).ti,ab. (78418) 24 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. (230369) 25 double blind procedure/ (171471) 26 parallel group*1.ti,ab. (25256) 27 (crossover or cross over).ti,ab. (104213) 28 ((assign* or match or matched or allocation) adj5 (alternate or group*1 or intervention*1 or patient*1 or subject*1 or participant*1)).ti,ab. (326475) 29 (assigned or allocated).ti,ab. (384325) 30 (controlled adj7 (study or design or trial)).ti,ab. (344446) 31 (volunteer or volunteers).ti,ab. (244931) 32 trial.ti. (296562) 33 or/15‐32 (4626136) 34 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) (6376913) 35 33 not 34 (3993501) 36 14 and 35 (593)
ClinicalTrials.gov
Humerus Fracture (139) Supracondylar Fracture (22)
Appendix 2. Review of previous systematic reviews
Our searches identified 21 relevant previous systematic reviews and two national treatment guidelines that have assessed elements of this comparison. The earliest review was published in 1990 (Kurer 1990), with eight reviews published between 1991 and 2011 (Babal 2010; Brauer 2007; Loizou 2009; Pretell 2010; Pretell‐Mazzini 2010a; Pretell‐Mazzini 2010b; Slobogean 2010; White 2010). The American Academy of Orthopaedic Surgeons (AAOS) published its guideline in 2011 (American Academy of Orthopaedic Surgeons 2011), and the British Orthopaedic Association/British Society for Children's Orthopaedic Surgery (BOA/BSCOS) guideline was published in 2014 (British Orthopaedic Association 2014). The remaining 12 reviews were published between 2011 and 2021 (Carrazzone 2021; Catena 2019; Dekker 2016; Delniotis 2019; De Pellegrin 2018; Farrow 2018; Lin‐Guo 2018; Na 2018; Patriota 2017; Woratanarat 2012; Yeomans 2018; Yousri 2012).
The searches for the AAOS guideline included studies from January 1966 to July 2010. The guideline proposes four recommendations based on the literature and two consensus‐based recommendations. They identified moderate‐level evidence for non‐surgical immobilisation of undisplaced fractures, and closed reduction and surgical fixation of displaced fractures. They found limited‐quality evidence for the use of lateral pins to prevent harm to the patient, and for the use of open reduction for a fracture mal‐reduced after closed reduction. The guideline included many prospective, non‐randomised studies, and used the indirect evidence of Oakley 2009 to infer that simple immobilisation is suitable for undisplaced fractures. The guideline included two RCTs to recommend the use of surgical fixation for displaced fractures (Kaewpornsawan 2001; Pandey 2008), augmented with nine other studies. The group found no difference in cosmetic deformity or radiographic loss of reduction between surgical fixation and non‐surgical immobilisation, with one study demonstrating loss of range of movement with non‐surgical immobilisation. The guideline did not use the GRADE approach, and rated the quality of evidence higher than we would have. We also did not find a significant difference in cosmetic deformity or loss of reduction, and also did not find an improvement in range of motion when surgery was performed. The limited recommendation to use lateral wires is based on three trials (Foead 2004; Kocher 2007; Tripuraneni 2009), and 12 other studies. A pooled re‐operation odds ratio of 1.3 (95% CI 0.5 to 3.4) was found in favour of crossed wires with an odds ratio for ulnar nerve injury of 0.27 (95% CI 0.13 to 0.59). This is consistent with our findings, with a greater effect size driven predominantly by the included non‐randomised studies. The guideline did not find any RCTs to support the limited recommendation to perform an open reduction following varus malposition of a fracture following closed reduction.
The BOA/BSCOS guideline presents a professional consensus that children with displaced supracondylar fractures should have surgery on the day of admission, or the next morning if presenting overnight. Crossed wires are identified to have lower rates of loss of reduction whilst lateral wires reduce risk of nerve injury. A technique should be used to protect the ulnar nerve if a medial wire is used, though a specific technique is not recommended. These findings on wire technique correspond to the findings of this review, though we have only found one RCT that evaluated ulnar nerve protection techniques and assessed it to be of low quality. The guideline recommends the use of 2 mm wires to improve stability; a recommendation for which we have been unable to find supporting RCT evidence.
Yeomans 2018 reviewed the literature for comparisons of non‐surgical management of displaced supracondylar fractures, with a focus on resource‐poor settings. The review identified 46 studies, of which none were RCTs. The authors concluded that the current evidence regarding the non‐surgical management of supracondylar fractures (casting or traction) is inconclusive. Kurer 1990 identified 27 observational studies evaluating closed reduction and casting or traction for displaced fractures, nine studies relating to closed reduction with wire fixation, and eight studies on open reduction and wire fixation. This review found casting to be associated with a 60% loss of reduction rate with good results from traction and closed or open reduction and fixation. This review concludes that reduction and cast immobilisation is inadequate for displaced fractures.
Nine previous reviews have compared the use of retrograde lateral and retrograde crossed wires; these are summarised in Appendix 3. In the most recent review, Carrazzone 2021 concluded that lateral wires are safer than crossed wires but are more prone to a loss of reduction. The Carrazzone 2021 review defined loss of reduction as a loss of Baumann's angle > 6°, loss of > 10° of carrying angle, or loss of > 10° humeral‐capitellar angle, and it was not clear how unit of analysis issues were resolved in this composite outcome. The authors used the Cochrane risk of bias tool and assigned the studies the same risk of bias for selection bias, were more likely to rate studies as at high risk of bias for blinding, and more conservative in rating other biases than our review. The pooled analysis identified a relative risk of 0.45 (95% CI 0.21 to 0.99) for nerve injury when lateral wires were used, in keeping with our findings. An increased rate of loss of reduction was also demonstrated, with a RR of 1.39 (95% CI 1.04 to 1.85).
Na 2018 performed a systematic review of RCTs and observational studies for lateral entry and crossed wires that identified 24 studies, including nine RCTs. This total included two trials identified as awaiting classification due to the unavailability of the original manuscripts (Bing 2017; Vaidya 2013a). Na 2018 did not perform an evaluation of study quality. The pooled analysis of randomised trials showed no difference in the rates of children graded as 'excellent' using the Flynn score (RR 1.02, 95% CI 0.87 to 0.99). A reduction in the rate of nerve injury was demonstrated for lateral entry wires (RR 0.29, 95% CI 0.10 to 0.79). The rates of loss of reduction were not significant, though this included randomised and observational studies (RR 1.37, 95% CI 0.91 to 2.07). The authors concluded a wiring strategy of three divergent wires offered best stability, functional outcome, and nerve injury reduction, though this was not explicitly demonstrated from the data presented.
In contrast, Patriota 2017 suggested a differential approach to fixation based on Gartland classification, based on a subgroup analysis that suggests Gartland 3‐4 fractures are more likely to experience a loss of reduction (P = 0.04), and analysis of one RCT with 60 children showing a mini‐open technique had no change in ulnar nerve injury rate compared to lateral wires (RR 0.14, 95% CI 0.01 to 2.79). Dekker 2016 did not identify convincing evidence from seven RCTs and six observational studies that either approach was superior, with no significant difference in loss of reduction or rates of achieving a Flynn grade of excellent. The pooled ulnar nerve injury rate was a RR of 3.04 (95% CI 1.16 to 7.99). Yousri 2012 undertook a systematic review and narrative synthesis of four RCTs, identifying limitations in study design for studies published before 2011; they could not draw any firm conclusions. Woratanarat 2012 suggested using lateral wires for displaced fractures, based on pooling results from two RCTs and six observational studies, with a higher risk of nerve injury following crossed wires (RR 4.5, 95% CI 2.1 to 9.7) and lower risk of loss of reduction (RR 0.6, 95% CI 0.4 to 1.0). Slobogean 2010 found a higher risk of ulnar nerve injury following crossed wires, but did not evaluate any other outcomes (pooled risk difference 0.035, 95% CI 0.014 to 0.045). In a review predominantly of observational studies, Babal 2010 found a pooled nerve injury rate of 4.1% following retrograde crossed wires and 3.9% following lateral wires; and Brauer 2007 found a modest difference in nerve injury rates between these two groups with a RR of 1.84 (95% CI 1.01 to 3.36).
Three systematic reviews have reviewed the use of an open or closed reduction (Lin‐Guo 2018; Pretell‐Mazzini 2010a; Pretell‐Mazzini 2010b; see Appendix 4). Lin‐Guo 2018 identified two RCTs (Kaewpornsawan 2001; Lu 2011), of which one RCT was graded as low risk of bias for all domains (Lu 2011). Unfortunately, we have not been able to source this trial from the reference provided. The review authors concluded that stability and outcomes are similar between open and closed reduction, but the findings were limited by sample size and further study is necessary. A pair of reviews were published in 2010: evaluating the use of closed or open reduction (Pretell‐Mazzini 2010a), and the surgical approach when undertaking an open reduction (Pretell‐Mazzini 2010b), respectively. In these reviews, a single RCT was identified. The authors concluded that one to two attempts at closed reduction should be performed before progressing to an anterior‐medial open reduction. The quality of the evidence supporting these conclusions is limited.
Two reviews of management of vascular injuries following supracondylar fracture have been published (Delniotis 2019; White 2010; see Appendix 5). In a review of grade 2 and 3 vascular injuries, Delniotis 2019 identified 16 studies, none of which were randomised trials. The authors concluded that children with pulseless hands should have their fractures reduced urgently and fixed with percutaneous wires. Following reduction, if the hand becomes pink and perfused, the authors propose that observation is sufficient, with exploration of the artery only indicated if the hand remains cold and white. White 2010 performed a systematic review and expert survey, and concluded that the rate of vascular pathology following a grade 2 injury is 82% and therefore surgical exploration should be considered. This review was limited due to a lack of RCTs.
The timing of surgery for displaced fractures has been assessed through meta‐analysis of retrospective cohorts, as no RCTs were identified (Appendix 6). The Farrow 2018 review identified 12 cohorts. When these heterogeneous studies were pooled, there was no difference in the need for open reduction (RR 0.01, 95% CI ‐0.03 to 06; I2 = 75%), nerve injury, major complications, cosmetic deformity, or functional outcome (a Flynn grade of poor). The authors found the cutoff for early surgery to be variable: they included one study where the cutoff for early surgery was six hours; four studies with a cutoff of eight hours; five studies with a cutoff of 12 hours; and two studies where the cutoff was 24 hours. They concluded that surgery could be safely delayed overnight, but acknowledged that the strength of evidence was low to very low. In an earlier review, Loizou 2009 found a higher rate of open reduction in delayed surgery from five observational studies (odds ratio (OR) 0.37, 95% CI 0.21 to 0.66). This review included three studies where the cutoff for early intervention was eight hours and two where the cutoff was 12 hours.
De Pellegrin 2018 reviewed the role of patient positioning and the impact on nerve injury. Searches included studies published up to 31 December 2017 and identified two RCTs (Tripuraneni 2009; Venkatadass 2015). The original objective of this review was to identify comparative studies of prone and supine closed reduction of fractures, but the authors were unable to identify sufficient numbers of studies to perform this comparison. Instead, the authors extracted nerve injury rates from observational studies in different populations, and concluded that nerve injuries only occurred following reduction in a supine position. However, a lack of comparative studies limits the strength of this finding. A similar review was published as Catena 2019 with the same search date and included studies, and we presumed it to be a duplicate publication.
Appendix 3. Summary of systematic reviews comparing retrograde lateral wires with retrograde crossed wires
| Review | Included studies | Main outcomes | Author conclusions |
| Carrazzone 2021 | 12 RCTs (Abdel Karim 2016; Afaque 2020; Anwar 2011; Foead 2004; Gaston 2010; Kocher 2007; Maity 2012; Naveen 2017; Prashant 2016; Shafi‐Ur‐Rehman 2013; Vaidya 2009; Venkatadass 2015) | Loss of reduction Nerve injury Functional outcome (Flynn grade satisfactory) |
Low‐quality evidence to show lateral wires are safer with fewer nerve injuries but crossed wires are more stable |
| Na 2018 | 9 RCTs (Anwar 2011; Foead 2004; Gaston 2010; Kocher 2007; Maity 2012; Prashant 2016; Tripuraneni 2009; Vaidya 2009; Bing 2017) 13 observational studies |
Loss of reduction Nerve injury Functional outcome (Flynn grade excellent) |
A wiring strategy of three divergent wires offered best stability, functional outcome, and nerve injury reduction |
| Patriota 2017, | 8 RCTs (Anwar 2011; Foead 2004; Gaston 2010; Kocher 2007; Maity 2012; Mazda 2001*; Tripuraneni 2009; Vaidya 2013a) | Ulnar nerve injury Loss of reduction Baumann's angle Carrying angle Loss of carrying angle |
Gartland 2 fractures be fixed with lateral wires and Gartland 3‐4 are fixed with crossed wires inserted with a mini‐open technique |
| Dekker 2016 | 7 RCTs (Anwar 2011; Foead 2004; Gaston 2010; Kocher 2007; Maity 2012; Tripuraneni 2009; Vaidya 2009) 6 observational studies |
Functional outcome (Flynn grade excellent) Loss of reduction Ulnar nerve injury |
The available evidence does not support the use of either technique |
| Yousri 2012 | 4 RCTs (Foead 2004; Gaston 2010; Kocher 2007; Tripuraneni 2009) | Ulnar nerve injury Loss of reduction Loss of carrying angle Radiographic measures |
Cannot draw any firm conclusions based on the identified evidence |
| Woratanarat 2012 | 2 RCTs (Foead 2004; Kocher 2007) 16 observational studies |
Ulnar nerve injury Loss of reduction Late deformity Flynn grade poor or fair |
Fixation with crossed wires reduces the risk of loss of reduction but has higher nerve injuries. Fixation with lateral wires is the favoured procedure |
| Slobogean 2010 | 2 RCTs (Foead 2004; Kocher 2007) 30 observational studies |
Ulnar nerve injury | Crossed wires have a higher risk of nerve injury |
| Babal 2010 | 2 RCTs (Foead 2004; Kocher 2007) 53 observational studies |
Nerve injury | Crossed wires have a higher risk of nerve injury, but lateral wires also have a risk of ulnar nerve injury |
| Brauer 2007 | 2 RCTs (Foead 2004; Kaewpornsawan 2001) 33 observational studies |
Loss of reduction Nerve injury |
Crossed wires seem to be the more stable construct |
*Mazda 2001 was screened for inclusion in this Cochrane review but found to be a non‐randomised study
Appendix 4. Summary of systematic reviews comparing closed with open reduction of displaced fractures
| Review | Included studies | Main outcomes | Conclusions |
| Lin‐Guo 2018 | 2 RCTs (Kaewpornsawan 2001; Lu 2011) | Ulnar nerve injury Major complications Flynn grade |
Similar outcomes from closed and open reduction |
| Pretell‐Mazzini 2010b | 1 RCT (Kaewpornsawan 2001) 6 observational studies |
Flynn grade excellent, good, fair or poor Cosmetic outcome Non‐union Nerve injury Major complications |
Anterior‐medial approach may be associated with best functional and cosmetic outcomes |
| Pretell‐Mazzini 2010a | 1 RCT (Kaewpornsawan 2001) 2 observational studies |
Flynn grade excellent, good, fair or poor Cosmetic outcome Baumann's angle Time to union Major complications |
Suggest starting with 1 or 2 attempts at closed reduction and proceed to open reduction if closed reduction fails |
Appendix 5. Summary of systematic reviews comparing interventions for vascular injuries
| Review | Included studies | Main outcomes | Conclusions |
| Delniotis 2019 | 0 RCTs 16 observational studies |
Functional outcomes Major complications Vascular occlusion |
Children with pulseless hands should have their fractures reduced urgently and fixed with percutaneous wires |
| White 2010 | 0 RCTs 17 observational studies 1 survey |
Perfusion following reduction Major complications Vascular occlusion |
Suggest low threshold for vascular exploration in grade 2 vascular injuries (pink, pulseless hands) |
Appendix 6. Summary of systematic reviews comparing early and delayed surgery for displaced fractures
| Review | Included studies | Main outcomes | Conclusions |
| Farrow 2018 | 0 RCTs 12 observational studies |
Open reduction Nerve injury Major complications Functional outcome (Flynn grade of poor) |
Surgery could be safely delayed overnight |
| Loizou 2009 | 0 RCTs 5 observational studies |
Open reduction |
Surgery should be performed within 12 hours of injury |
Data and analyses
Comparison 1. Different forms of surgical intervention: retrograde lateral wires versus retrograde crossed wires.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Treatment failure requiring a re‐intervention | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.2 Nerve injury | 28 | 1653 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.46, 0.90] |
| 1.3 Major complications (pin site infections) | 18 | 1022 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.65, 1.79] |
| 1.4 Cosmetic deformity: loss of carrying angle > 10 degrees or elbow deformity | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.4.1 Medium‐term cubitus varus | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.91 [0.12, 69.08] |
| 1.4.2 Medium‐term loss of carrying angle > 10° | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.08, 15.36] |
| 1.4.3 Long‐term cubitus varus | 2 | 95 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.13 [0.55, 17.98] |
| 1.4.4 Long‐term loss of carrying angle > 10° | 6 | 288 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.39, 4.20] |
| 1.5 Cosmetic deformity: loss of carrying angle (degrees of loss) | 15 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.5.1 Medium‐term loss of carrying angle | 5 | 320 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.44, 0.23] |
| 1.5.2 Long‐term loss of carrying angle | 10 | 502 | Mean Difference (IV, Fixed, 95% CI) | 0.14 [‐0.26, 0.54] |
| 1.6 Cosmetic deformity: degrees of loss of carrying angle at long‐term follow‐up | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.6.1 Medium‐term loss of flexion | 3 | 148 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.37, 4.16] |
| 1.6.2 Medium‐term loss of extension | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.15, 3.53] |
| 1.6.3 Long‐term loss of flexion | 2 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.84, 2.35] |
| 1.6.4 Long‐term loss of extension | 2 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.40, 1.80] |
| 1.6.5 Long‐term loss of total range of movement | 3 | 148 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.56, 2.82] |
| 1.7 Range of motion: loss of movement | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.7.1 Long‐term loss of elbow flexion | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | 2.58 [‐2.48, 7.64] |
| 1.7.2 Long‐term loss of elbow extension | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐5.35, 5.29] |
| 1.7.3 Long‐term loss of total range of motion | 2 | 92 | Mean Difference (IV, Fixed, 95% CI) | 0.71 [‐0.52, 1.95] |
| 1.8 Unable to return to sport and normal activities | 5 | 246 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.31, 2.69] |
| 1.8.1 Medium term | 3 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.31, 4.83] |
| 1.8.2 Long term | 2 | 95 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.08, 3.53] |
| 1.9 Return to sport and normal activities: time to return to normal activities | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.10 Radiographic deformity: loss of reduction | 10 | 761 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [1.23, 2.26] |
| 1.10.1 Early | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.93, 2.00] |
| 1.10.2 Medium term | 7 | 469 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.00 [1.21, 3.30] |
| 1.10.3 Long term | 2 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.00 [0.46, 34.45] |
| 1.11 Radiographic deformity: loss of radiographic angle | 14 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.11.1 Medium‐term loss of Baumann's angle | 7 | 447 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐0.22, 0.33] |
| 1.11.2 Long‐term loss of Baumann's angle | 7 | 283 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.00, 0.11] |
| 1.11.3 Medium‐term loss of lateral humeral‐capitellar angle | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐2.86, 2.46] |
| 1.11.4 Long‐term loss of lateral humeral‐capitellar angle | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.06, 0.08] |
| 1.11.5 Long‐term loss of humeral diaphyseal‐metaphyseal angle | 2 | 112 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.18, 0.39] |
| 1.11.6 Long‐term loss of humeral medial epicondylar epiphyseal angle | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | 0.86 [‐2.27, 3.99] |
| 1.12 Resource use | 3 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.12.1 Operative time (minutes) | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.12.2 Length of stay (days) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.13 Nerve injury: subgrouped according to technique for insertion of medial wire | 21 | 1208 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.49, 1.02] |
| 1.13.1 Percutaneous crossed wires technique | 10 | 552 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.20, 0.81] |
| 1.13.2 Open crossed wires technique | 11 | 656 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.59, 1.40] |
1.6. Analysis.

Comparison 1: Different forms of surgical intervention: retrograde lateral wires versus retrograde crossed wires, Outcome 6: Cosmetic deformity: degrees of loss of carrying angle at long‐term follow‐up
1.7. Analysis.

Comparison 1: Different forms of surgical intervention: retrograde lateral wires versus retrograde crossed wires, Outcome 7: Range of motion: loss of movement
1.8. Analysis.

Comparison 1: Different forms of surgical intervention: retrograde lateral wires versus retrograde crossed wires, Outcome 8: Unable to return to sport and normal activities
1.9. Analysis.

Comparison 1: Different forms of surgical intervention: retrograde lateral wires versus retrograde crossed wires, Outcome 9: Return to sport and normal activities: time to return to normal activities
1.11. Analysis.

Comparison 1: Different forms of surgical intervention: retrograde lateral wires versus retrograde crossed wires, Outcome 11: Radiographic deformity: loss of radiographic angle
1.12. Analysis.

Comparison 1: Different forms of surgical intervention: retrograde lateral wires versus retrograde crossed wires, Outcome 12: Resource use
1.13. Analysis.

Comparison 1: Different forms of surgical intervention: retrograde lateral wires versus retrograde crossed wires, Outcome 13: Nerve injury: subgrouped according to technique for insertion of medial wire
Comparison 2. Different forms of surgical intervention: lateral crossed (Dorgan) wires versus retrograde crossed wires.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Nerve injury | 5 | 307 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.09, 0.76] |
| 2.2 Major complications | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.2.1 All complications | 5 | 307 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.94 [0.88, 4.30] |
| 2.2.2 Pin site infections | 5 | 307 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.51 [0.92, 6.82] |
| 2.2.3 Vascular injuries | 1 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.28, 4.07] |
| 2.3 Cosmetic deformity: loss of carrying angle > 10 degrees | 3 | 218 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.37, 4.85] |
| 2.4 Cosmetic deformity: loss of carrying angle (degrees of loss) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.5 Range of motion: long‐term loss of total range of motion > 10 degrees | 3 | 218 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.08, 16.63] |
| 2.6 Radiographic deformity: long‐term loss of radiographic angle | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.6.1 Long‐term loss of Baumann's angle | 2 | 80 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.01, 0.09] |
| 2.6.2 Long‐term loss of lateral humeral‐capitellar angle | 2 | 80 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [0.00, 0.16] |
| 2.7 Resource use | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.7.1 Operative time (minutes) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.7.2 Radiographic exposure time (minutes) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
2.2. Analysis.

Comparison 2: Different forms of surgical intervention: lateral crossed (Dorgan) wires versus retrograde crossed wires, Outcome 2: Major complications
2.3. Analysis.

Comparison 2: Different forms of surgical intervention: lateral crossed (Dorgan) wires versus retrograde crossed wires, Outcome 3: Cosmetic deformity: loss of carrying angle > 10 degrees
2.4. Analysis.

Comparison 2: Different forms of surgical intervention: lateral crossed (Dorgan) wires versus retrograde crossed wires, Outcome 4: Cosmetic deformity: loss of carrying angle (degrees of loss)
2.5. Analysis.

Comparison 2: Different forms of surgical intervention: lateral crossed (Dorgan) wires versus retrograde crossed wires, Outcome 5: Range of motion: long‐term loss of total range of motion > 10 degrees
2.6. Analysis.

Comparison 2: Different forms of surgical intervention: lateral crossed (Dorgan) wires versus retrograde crossed wires, Outcome 6: Radiographic deformity: long‐term loss of radiographic angle
2.7. Analysis.

Comparison 2: Different forms of surgical intervention: lateral crossed (Dorgan) wires versus retrograde crossed wires, Outcome 7: Resource use
Comparison 3. Different forms of surgical intervention: lateral crossed (Dorgan) wires versus retrograde lateral wires.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Nerve injury | 2 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.07, 15.54] |
| 3.2 Major complications: pin site infections | 2 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.06 [0.56, 7.56] |
| 3.3 Cosmetic deformity: long‐term loss of carrying angle > 10 degrees | 2 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.21, 2.15] |
| 3.4 Range of motion: long‐term loss of total range of motion > 10 degrees | 2 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.26, 2.00] |
| 3.5 Radiographic deformity: long‐term loss of Baumann's angle | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.6 Radiographic deformity: long‐term loss of humeral‐capitellar angle | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
3.1. Analysis.

Comparison 3: Different forms of surgical intervention: lateral crossed (Dorgan) wires versus retrograde lateral wires, Outcome 1: Nerve injury
3.2. Analysis.

Comparison 3: Different forms of surgical intervention: lateral crossed (Dorgan) wires versus retrograde lateral wires, Outcome 2: Major complications: pin site infections
3.3. Analysis.

Comparison 3: Different forms of surgical intervention: lateral crossed (Dorgan) wires versus retrograde lateral wires, Outcome 3: Cosmetic deformity: long‐term loss of carrying angle > 10 degrees
3.4. Analysis.

Comparison 3: Different forms of surgical intervention: lateral crossed (Dorgan) wires versus retrograde lateral wires, Outcome 4: Range of motion: long‐term loss of total range of motion > 10 degrees
3.5. Analysis.

Comparison 3: Different forms of surgical intervention: lateral crossed (Dorgan) wires versus retrograde lateral wires, Outcome 5: Radiographic deformity: long‐term loss of Baumann's angle
3.6. Analysis.

Comparison 3: Different forms of surgical intervention: lateral crossed (Dorgan) wires versus retrograde lateral wires, Outcome 6: Radiographic deformity: long‐term loss of humeral‐capitellar angle
Comparison 4. Different forms of surgical intervention: posterior intrafocal wires versus retrograde crossed wires.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Nerve injury | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.2 Major complications (pin site infection) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.3 Cosmetic deformity: loss of carrying angle > 10 degrees or elbow deformity | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.3.1 Long‐term loss of carrying angle | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.3.2 Long‐term cubitus varus | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.4 Range of motion: loss of range of motion > 10 degrees at long‐term follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.5 Radiographic deformity: loss of reduction at long‐term follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
4.1. Analysis.

Comparison 4: Different forms of surgical intervention: posterior intrafocal wires versus retrograde crossed wires, Outcome 1: Nerve injury
4.2. Analysis.

Comparison 4: Different forms of surgical intervention: posterior intrafocal wires versus retrograde crossed wires, Outcome 2: Major complications (pin site infection)
4.3. Analysis.

Comparison 4: Different forms of surgical intervention: posterior intrafocal wires versus retrograde crossed wires, Outcome 3: Cosmetic deformity: loss of carrying angle > 10 degrees or elbow deformity
4.4. Analysis.

Comparison 4: Different forms of surgical intervention: posterior intrafocal wires versus retrograde crossed wires, Outcome 4: Range of motion: loss of range of motion > 10 degrees at long‐term follow‐up
Comparison 5. Different forms of surgical intervention: retrograde lateral wires in a parallel versus divergent configuration.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Major complications | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1.1 Compartment syndrome | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.2 Range of movement: loss of movement | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.2.1 Medium‐term loss of flexion | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.2.2 Medium‐term loss of extension | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.3 Cosmetic deformity: medium‐term loss of carrying angle | 2 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.65, 0.69] |
| 5.4 Range of motion: medium‐term loss of flexion and extension | 2 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.32, 5.20] |
| 5.4.1 Medium‐term loss of flexion | 2 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.17, 4.42] |
| 5.4.2 Medium‐term loss of extension | 2 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.00 [0.16, 57.36] |
| 5.5 Radiographic deformity: medium‐term loss of Baumann's angle | 2 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.87 [‐0.00, 1.75] |
5.1. Analysis.

Comparison 5: Different forms of surgical intervention: retrograde lateral wires in a parallel versus divergent configuration, Outcome 1: Major complications
5.2. Analysis.

Comparison 5: Different forms of surgical intervention: retrograde lateral wires in a parallel versus divergent configuration, Outcome 2: Range of movement: loss of movement
5.3. Analysis.

Comparison 5: Different forms of surgical intervention: retrograde lateral wires in a parallel versus divergent configuration, Outcome 3: Cosmetic deformity: medium‐term loss of carrying angle
5.4. Analysis.

Comparison 5: Different forms of surgical intervention: retrograde lateral wires in a parallel versus divergent configuration, Outcome 4: Range of motion: medium‐term loss of flexion and extension
5.5. Analysis.

Comparison 5: Different forms of surgical intervention: retrograde lateral wires in a parallel versus divergent configuration, Outcome 5: Radiographic deformity: medium‐term loss of Baumann's angle
Comparison 6. Different forms of surgical intervention: mini‐open crossed wires versus percutaneous crossed wires.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Nerve injury | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.2 Major complications | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.3 Resource use | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.3.1 Length of hospital stay (days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.3.2 Anaesthesia time | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
6.1. Analysis.

Comparison 6: Different forms of surgical intervention: mini‐open crossed wires versus percutaneous crossed wires, Outcome 1: Nerve injury
6.2. Analysis.

Comparison 6: Different forms of surgical intervention: mini‐open crossed wires versus percutaneous crossed wires, Outcome 2: Major complications
6.3. Analysis.

Comparison 6: Different forms of surgical intervention: mini‐open crossed wires versus percutaneous crossed wires, Outcome 3: Resource use
Comparison 7. Different forms of surgical intervention: buried versus non‐buried wires.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Major complications: pin site infections | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 8. Different forms of surgical intervention: open versus closed reduction of displaced fractures.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Nerve injury | 3 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.09, 1.01] |
| 8.2 Major complications: pin site infections | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.2.1 Direct visualisation | 4 | 253 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.15 [1.07, 16.20] |
| 8.2.2 Joystick | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.20, 6.12] |
| 8.3 Cosmetic deformity: loss of carrying angle > 10 degrees or elbow deformity (medium‐ and long‐term) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.3.1 Medium‐term cubitus varus | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.3.2 Medium‐term loss of carrying angle | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.3.3 Long‐term cubitus varus | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.4 Cosmetic deformity: loss of carrying angle in the long term (degrees of loss) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.5 Range of movement: loss of total range of movement > 10 degrees at medium term | 2 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.20 [1.05, 9.77] |
| 8.6 Range of movement: loss of range of movement at long‐term follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.7 Patient satisfaction with scar appearance (higher scores indicates more satisfaction) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.7.1 Patient reported score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.7.2 Blinded assessor score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.8 Radiographic deformity: difference in Baumann's angle (medium and long term) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.8.1 Medium‐term loss of Baumann's angle | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.8.2 Long‐term loss of Baumann's angle | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.9 Radiographic deformity: capitellum posterior to anterior humeral line at late follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.10 Resource use | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.10.1 Operative time (minutes); direct visualisation | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.10.2 Length of hospital stay (days); direct visualisation | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.10.3 Operative time (minutes); joystick technique | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.10.4 Length of hospital stay (days); joystick technique | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
8.6. Analysis.

Comparison 8: Different forms of surgical intervention: open versus closed reduction of displaced fractures, Outcome 6: Range of movement: loss of range of movement at long‐term follow‐up
8.9. Analysis.

Comparison 8: Different forms of surgical intervention: open versus closed reduction of displaced fractures, Outcome 9: Radiographic deformity: capitellum posterior to anterior humeral line at late follow‐up
Comparison 9. Surgical versus non‐surgical treatment: surgical fixation versus non‐surgical immobilisation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Treatment failure | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9.2 Nerve injury | 3 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.50 [0.50, 12.46] |
| 9.3 Major complications: pin site infections | 3 | 126 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.00 [0.47, 34.11] |
| 9.4 Cosmetic deformity: loss of carrying angle > 10 degrees or elbow deformity | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.4.1 Long‐term cubitus varus | 2 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.04, 1.09] |
| 9.4.2 Long‐term loss of carrying angle | 2 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.10, 0.81] |
| 9.4.3 Long‐term cubitus valgus | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 7.32] |
| 9.5 Cosmetic deformity: degrees of loss of carrying angle at long‐term follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.6 Range of movement: loss of > 10 degrees total movement at long‐term follow‐up | 2 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.06, 1.03] |
| 9.7 Range of movement: degrees of loss of movement at long‐term follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.7.1 Long‐term loss of flexion | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.7.2 Long‐term loss of extension | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
9.6. Analysis.

Comparison 9: Surgical versus non‐surgical treatment: surgical fixation versus non‐surgical immobilisation, Outcome 6: Range of movement: loss of > 10 degrees total movement at long‐term follow‐up
9.7. Analysis.

Comparison 9: Surgical versus non‐surgical treatment: surgical fixation versus non‐surgical immobilisation, Outcome 7: Range of movement: degrees of loss of movement at long‐term follow‐up
Comparison 10. Different forms of traction: skin traction versus olecranon skeletal traction.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 10.1 Nerve injury | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10.2 Major complications | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10.3 Cosmetic deformity: cubitus varus | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10.4 Range of movement (medium term) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10.4.1 > 10° loss of flexion | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10.4.2 > 5° of hyperextension | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10.5 Resource use (duration of traction) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 11. Different forms of non‐surgical intervention: backslab versus sling for undisplaced fractures.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 11.1 Parental satisfaction (unsure or unwilling to use same device again) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11.2 Return to normal activities at 2 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
11.1. Analysis.

Comparison 11: Different forms of non‐surgical intervention: backslab versus sling for undisplaced fractures, Outcome 1: Parental satisfaction (unsure or unwilling to use same device again)
11.2. Analysis.

Comparison 11: Different forms of non‐surgical intervention: backslab versus sling for undisplaced fractures, Outcome 2: Return to normal activities at 2 weeks
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abdel Karim 2016.
| Study characteristics | ||
| Methods |
Design: randomised trial Study duration: December 2011 to February 2013 Power calculation: no prospective calculation of sample size |
|
| Participants |
Setting: Department of Orthopaedics and Traumatology, Cairo University Hospitals, Egypt Sample size: 60 children Inclusion criteria: children aged 0 to 10 with Gartland 2‐3 supracondylar fracture Exclusion criteria: open fractures, fractures with vascular injury, fractures with compartment syndrome, fractures with preoperative ulnar nerve injury, bilateral fractures |
|
| Interventions |
Group 1 (30 children): manipulation of fracture in theatre with fixation using retrograde wires inserted from the lateral side Group 2 (30 children): manipulation of fracture in theatre with fixation using retrograde wires inserted from the medial and lateral side using an open technique (1.5 cm to 3.0cm incision) for the medial wire |
|
| Outcomes | 1) Nerve injury 2) Major complications (pin site infections) 3) Range of movement (loss of movement > 10° at 3 months) 4) Radiographic deformity (loss of reduction as defined by translation, angulation, or rotation at the fracture site at 3 months) |
|
| Notes |
Loss to follow‐up: none reported Funding source: not stated Declarations: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The patients were randomized to be managed with either crossed pins or lateral pins configuration through the use of opaque sealed envelopes” |
| Allocation concealment (selection bias) | Unclear risk | Quote: “The patients were randomized to be managed with either crossed pins or lateral pins configuration through the use of opaque sealed envelopes” Comment: unclear if the sealed envelopes were sequentially numbered |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “All surgeries were performed by junior trainees in their first 3 years of training. The main surgeon was either a second‐ or third‐year resident (in a 3‐year residency program), and the assistant was a first‐, second‐, or third‐year resident. They were supervised by an attending surgeon, who was present in the operative premises and who reviewed the intraoperative images on the image intensifier before the procedure was completed”… “Follow‐up assessment of each patient was performed by the same team throughout the trial (a third‐year resident together with an attending). Both the surgeons and the patients were not blinded of the treatment received throughout the trial”. Comment: the non‐blinded surgical team completed the study, surgery and outcome assessment. |
| Blinding of outcome assessment (detection bias) | High risk | Quote: “All surgeries were performed by junior trainees in their first 3 years of training. The main surgeon was either a second‐ or third‐year resident (in a 3‐year residency program), and the assistant was a first‐, second‐, or third‐year resident. They were supervised by an attending surgeon, who was present in the operative premises and who reviewed the intraoperative images on the image intensifier before the procedure was completed”… “Follow‐up assessment of each patient was performed by the same team throughout the trial (a third‐year resident together with an attending). Both the surgeons and the patients were not blinded of the treatment received throughout the trial”. Comment: the non‐blinded surgical team completed the study, surgery and outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All patients returned for both clinical and radiographic evaluations at 1 week, 3–4 weeks, 6 weeks and 3 months." |
| Selective reporting (reporting bias) | Unclear risk | Comment: no prospective registration or trial protocol. Some participants had longer follow‐up based on the development of complications. The reporting of these participants is not explicit. |
| Other bias | Low risk | None identified |
Abubeih 2019.
| Study characteristics | ||
| Methods |
Design: randomised trial Study duration: June 2013 to October 2015 Power calculation: no prospective calculation of sample size |
|
| Participants |
Setting: Department of Orthopaedics, Assiut University, Assiut, Egypt Sample size: sample selected as the 91 children who met inclusion criteria in study period. Inclusion criteria: children aged 3 to 9 with extension type Gartland 3 supracondylar fracture Exclusion criteria: associated injury in the same limb, previous fracture in the same limb, open fracture, fracture requiring open reduction, and associated neurovascular injury requiring surgical exploration |
|
| Interventions |
Group 1 (46 children): manipulation of fracture in theatre with fixation using retrograde wires inserted from the lateral side Group 2 (45 children): manipulation of fracture in theatre with fixation using retrograde wires inserted from the medial and lateral side using an open technique (1.5 cm to 3.0cm incision) for the medial wire |
|
| Outcomes | 1) Nerve injury 2) Major complications (pin site infection) 3) Cosmetic deformity (cubitus varus at 12 weeks) 4) Range of movement (loss of flexion at 12 weeks) 5) Radiographic deformity (loss of reduction as loss of Baumann's angle > 6° at 12 weeks) |
|
| Notes |
Loss to follow‐up: Group 1 12/46 (26.1%), Group 2 12/45 (26.7%) Funding source: none Declarations: none Additional information about the study received by e‐mail from Dr Hossam M.A. Abubeih: "As regard Your questions about my published paper on Paediatric SCFH 1. Do you have the cosmetic factors and functional factors that contribute to your two group’s Flynn classification? Yes, Cosmetic loss of carrying angle is an essential part of the Flynn Criteria (table 1) 2. Were sequentially numbered envelopes used to in your study? Yes, in our study randomization done by concealed envelope technique using Sequentially numbered, opaque, sealed envelopes. 3. Were your outcome assessors blinded to treatment allocation? No the assessors were two of the authors participating in the study as we said in the article Two authors examined all patients” |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The patients were randomized into two groups by the concealed envelope technique” |
| Allocation concealment (selection bias) | Low risk | Quote: "in our study randomization done by concealed envelope technique using Sequentially numbered, opaque, sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Prof Wael El‐Adly and Dr Hatem Bakr performed the surgical procedures." Comment: surgical procedures were performed by study authors without blinding |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "Measurements of angles and analysis of results were done by two authors: Dr Hossam M.A. Abubeih and Prof Kamal El‐Gaafary."... "the assessors were two of the authors participating in the study as we said in the article Two authors examined all patients” Comment: outcome assessment performed by two study authors. Correspondence confirmed no blinding used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 12 participants in each group lost to follow‐up (26%). Loss to follow‐up appears balanced |
| Selective reporting (reporting bias) | Unclear risk | Comment: no prospective registration or trial protocol |
| Other bias | Low risk | None identified |
Afaque 2020.
| Study characteristics | ||
| Methods |
Design: randomised trial Study duration: March 2014 to May 2015 Power calculation: no prospective calculation of sample size |
|
| Participants |
Setting: Department of Orthopaedics, B.P. Koirala Institute of Health Sciences, Dharan, Nepal Sample size: 84 children Inclusion criteria: children aged 0 to 12 with Gartland 2, 3 or 4 supracondylar fracture Exclusion criteria: open fracture (Gustilo Grade II and III), neurovascular deficit and delayed presentation greater than 7 days |
|
| Interventions |
Group 1 (41 children): manipulation of fracture in theatre with fixation using retrograde wires inserted from the lateral side Group 2 (43 children): manipulation of fracture in theatre with fixation using retrograde wires inserted from the medial and lateral side using a percutaneous technique |
|
| Outcomes | 1) Postoperative nerve injury 2) Major complications (pin site infections, compartment syndrome, and vascular injury) 3) Cosmetic deformity (carrying angle at 3 months) 4) Range of motion at 3 months 5) Radiographic (Baumann's angle at 3 months) |
|
| Notes |
Loss to follow‐up: Group 1 5/41 (9.8%) including 2 children who had open reduction, Group 2 2/43 (7.0%) including 2 who had open reduction Funding source: none Declarations: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was done using excel random generation technique" Comment: random element to treatment allocation |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient detail on allocation concealment to assess |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: insufficient evidence to assess blinding of personnel |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: insufficient description provided of outcome assessor to evaluate outcome blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 8% missing data – including one extra child excluded following an open reduction in group II. Appears low and balanced between groups |
| Selective reporting (reporting bias) | Unclear risk | Comment: no published protocol or prospective trial registration identified. Clinical review at several time points that were not reported and limited postoperative radiographic parameters. Baumann’s angle measured twice but directly reported once |
| Other bias | Low risk | None identified |
Aher 2018.
| Study characteristics | ||
| Methods |
Design: randomised trial Study duration: January 2015 to June 2017 Power calculation: no prospective calculation of sample size. |
|
| Participants |
Setting: Department of Orthopedics, Gandhi Medical College, Bhopal Madhya Pradesh, India Sample size: 60 children Inclusion criteria: children aged 2 to 12 with Gartland 3 supracondylar fracture Exclusion criteria: open fractures, floating elbow injuries, previous fractures in the same elbow, delayed presentation > 7 days |
|
| Interventions |
Group 1 (30 children): manipulation of fracture in theatre with fixation using retrograde wires inserted from the lateral side Group 2 (30 children): manipulation of fracture in theatre with fixation using retrograde wires inserted from the medial and lateral side. A percutaneous technique was used for the medial wire. |
|
| Outcomes | 1) Nerve injury 2) Major complications (pin site infection) 3) Cosmetic deformity (loss of carrying angle at 4 to 12 months) 4) Range of movement (loss of total range of movement at 4 to 12 months) 5) Radiographic deformity (loss of reduction of Baumann's angle > 5° at 4 to 12 months) |
|
| Notes |
Loss to follow‐up: not formally specified but intended follow‐up was 12 months. Average follow‐up was reported as 6 months and minimum follow‐up as 4 months Funding source: not stated Declarations: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “This was a prospective, randomized study”… “Patients were allocated to one of the groups (Crossed pins Vs Two Lateral Pins) with the help of computer‐generated random table” |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient detail on allocation concealment to assess |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: it is not clear who performed the interventions and their relationship to the study team |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: it is not clear who performed the outcomes assessment and their relationship to the study team |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "the patient was followed up at 1‐month, 2month, 4 month, 6 months and final followup at 12 months"..."The average follow‐up duration for patients was of 6 months". Comment: there is a discrepancy between the planned follow‐up and actual mean follow‐up, suggesting that some participants did not complete the 12‐month follow‐up. No further information is specified. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no published protocol or prospective trial registration was identified |
| Other bias | Low risk | None identified |
Ahmad 2020.
| Study characteristics | ||
| Methods |
Design: randomised study Study duration: February 2017 to February 2018 Power calculation: no prospective calculation of sample size |
|
| Participants |
Setting: Nishter Hospital, Multan, Pakistan Inclusion criteria: aged 1 to 12 with displaced supracondylar fracture (however this was stated in text to be Gartland 1 or 2) Exclusion criteria: delayed presentation > 3 days, previous supracondylar fracture |
|
| Interventions |
Group 1: (35 children): manipulation of fracture in theatre with fixation using retrograde wires inserted from the lateral side Group 2: (35 children): manipulation of fracture in theatre with fixation using retrograde wires inserted from the medial and lateral side In both groups, children were admitted to the ward and immobilised in skin traction with the elbow in full extension. Following surgery, children were immobilised in an above‐elbow plaster cast for three weeks. |
|
| Outcomes | 1) Cosmetic deformity measured as carrying angle loss – reference and time point not specified 2) Range of motion as measured as loss of extension and loss of flexion – reference and time point not specified 3) Radiographic deformity measured as loss of Baumann's angle and loss of medial epicondylar epiphyseal angle ‐ reference and time point not specified |
|
| Notes | We were unable to include outcome data in the review. Confirmation required of the time points for observations reported in the manuscript. We therefore did not conduct risk of bias assessment for this study. Funding source: not stated Declarations: not stated |
|
Ajmera 2013.
| Study characteristics | ||
| Methods |
Design: randomised trial Study duration: June 2010 to December 2012 Power calculation: no prospective calculation of sample size |
|
| Participants |
Setting: Department of Orthopaedics, M.Y. Hospital & M.G.M. Medical College, India Sample size: 87 children Inclusion criteria: children aged 3 to 12 with Gartland 3 supracondylar fracture Exclusion criteria: delayed presentation > 7 days, nerve injuries, previous injury or surgery to the elbow and open fractures |
|
| Interventions |
Group 1 (unclear how many children allocated to this group): closed manipulation of fracture in theatre with fixation using crossed wires inserted from medial and lateral side. A percutaneous technique was used for the medial wire. Group 2 (unclear how many children allocated to this group): posterior approach open reduction of fracture in theatre with fixation using crossed wires inserted from the medial and lateral side |
|
| Outcomes | 1) Nerve injury 2) Major complications (pin site infection and vascular injuries) 3) Cosmetic deformity (loss of carrying angle and cubitus varus at 3 months) 4) Range of movement (loss of total range of motion at 3 months) |
|
| Notes |
Loss to follow‐up: not specified but final group size for group 1 of 32 and group 2 of 33 (22 lost to follow‐up) Funding source: not stated Declarations: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “The patients were randomly assigned to the following two groups” ... "patients were randomly divided" Comment: unclear how randomisation was performed |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient detail on allocation concealment to assess |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: it is not clear who performed the interventions and their relationship to the study team |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: it is not clear who performed the outcomes assessment and their relationship to the study team |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: “Total 87 cases were included in the study out of which 22 were lost during follow up.” Comment: no indication of the original allocation of participants so unclear if imbalance in rates of loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Comment: no published protocol or prospective trial registration was identified |
| Other bias | Low risk | None identified |
Altay 2011.
| Study characteristics | ||
| Methods |
Design: randomised trial Study duration: not stated Power calculation: no prospective calculation of sample size |
|
| Participants |
Setting: Department of Orthopaedic Surgery, Faculty of Medicine, Harran University, Turkey Sample size: 29 children Inclusion criteria: children (age not specified in inclusion criteria) with Gartland 3 supracondylar fractures Exclusion criteria: associated injuries, nerve lesions, infection, non/malunion, open fractures, bilateral injuries |
|
| Interventions |
Group 1 (14 children): manipulation of fracture in theatre with fixation using one retrograde wire and one antegrade wire inserted from the lateral side (Dorgan wires) Group 2 (15 children): manipulation of fracture in theatre with fixation using retrograde wires inserted from the medial and lateral side. A percutaneous technique was used for the medial wire. |
|
| Outcomes | 1) Nerve injury 2) Major complications (pin site infections and compartment syndrome) |
|
| Notes |
Loss to follow‐up: none reported Cosmesis and range of movement recorded but not presented in sufficient detail to extract Funding source: none Declarations: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method not stated |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Randomization was achieved in a double‐blinded fashion using an envelope containing group assignments.” Comment: unclear if the envelopes were opaque, sealed, or ordered sequentially |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: “Randomization was achieved in a double‐blinded fashion using an envelope containing group assignments.” Comment: no further details regarding blinding of participants or personnel |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: “Randomization was achieved in a double‐blinded fashion using an envelope containing group assignments” Comment: it is unclear who performed the outcome assessment for clinical nerve dysfunction, cosmesis, and range of motion, and if they were blinded to the treatment provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no reported loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Comment: no published protocol or prospective trial registration was identified. Cosmetic deformity and range of movements not reported in published abstract or full text |
| Other bias | Low risk | None identified |
Anwar 2011.
| Study characteristics | ||
| Methods |
Design: randomised trial Study duration: January 2008 to July 2009 Power calculation: no prospective calculation of sample size. |
|
| Participants |
Setting: Department of Orthopedics, Hayatabad Medical Complex, Peshawar, Pakistan Sample size: 50 children Inclusion criteria: children aged 1 to 12 with a displaced (Gartland 2‐3) supracondylar elbow fracture Exclusion criteria: neurovascular complications, open injuries, and patients for whom closed reduction was not possible |
|
| Interventions |
Group 1: (25 children): manipulation of fracture in theatre with fixation using retrograde wires inserted from the lateral side Group 2: (25 children): manipulation of fracture in theatre with fixation using retrograde wires inserted from the medial and lateral side |
|
| Outcomes | 1) Nerve injury 2) Cosmetic deformity (change in carrying angle at 6 months and modified Flynn criteria for carrying angle) 3) Range of motion (reduction of flexion and extension at 6 months and modified Flynn criteria for flexion and extension) 4) Radiographic (Baumann's angle and diaphyseal‐metaphyseal angle at 3 months) |
|
| Notes |
Loss to follow‐up: none reported Funding source: none Declarations: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients were randomly allocated in two groups by lottery method" |
| Allocation concealment (selection bias) | Unclear risk | Comment: no detail on lottery technique and if it would be possible for study staff to predict next allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: it is not clear who performed the interventions and their relationship to the study team |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: it is not clear who performed the outcomes assessment and their relationship to the study team |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no reported loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Comment: no published protocol or prospective trial registration was identified |
| Other bias | Low risk | None identified |
Arun 2018.
| Study characteristics | ||
| Methods |
Design: Randomised study Study duration: August 2016 to July 2018 Power calculation: no prospective calculation of sample size. |
|
| Participants |
Setting: Department of Orthopaedics, Navodaya Medical College Hospital and Research Centre, Raichur, Karnataka, India Sample size: 68 children Inclusion criteria: children aged 4 to 12 with extension type Gartland 3 supracondylar fracture Exclusion criteria: open fracture, neurovascular injury, delayed presentation > 4 days, associated ipsilateral limb fracture and previous ipsilateral elbow injury |
|
| Interventions |
Group 1 (38 children): manipulation of fracture in theatre with fixation using retrograde wires inserted from the lateral side Group 2 (30 children): manipulation of fracture in theatre with fixation using retrograde wires inserted from the medial and lateral side. A percutaneous technique was used for the medial wire. |
|
| Outcomes | 1) Nerve injury 2) Major complications 3) Cosmetic deformity at 6 months* 4) Range of motion at 6 months* 5) Radiographic deformity at 6 months* |
|
| Notes | * Outcomes 3 & 4 were combined as a single outcome in the Flynn classification. Outcome 5 was not reported in the manuscript. Loss to follow‐up: none reported Funding source: not stated Declerations: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: [Abstract, methods and results] "A prospective, single blinded, randomized control trial"..."Patients were selected for Lateral entry or Medial‐Lateral entry using a Randomization table."..."38 were treated with lateral pinning and 30 were treated with cross pinning technique based on randomization". [Discussion] "The limitations of this study is the lack of randomization regarding the selection of pinning technique as this was decided by the operating surgeon at the time of surgery" Comment: there is inconsistency regarding the description of the allocation in this study. |
| Allocation concealment (selection bias) | Unclear risk | Comment: it is unclear if the randomisation table was concealed to study staff. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: it is not clear who performed the interventions and their relationship to the study team. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: it is not clear who performed the outcomes assessment and their relationship to the study team. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no reported loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Comment: no published protocol or prospective trial registration was identified. Radiographic outcomes identified in methods but not reported |
| Other bias | Low risk | None identified |
Das 2019.
| Study characteristics | ||
| Methods |
Design: described as randomised study. However, we note uneven distribution of participants to each group and expect that this is a quasi‐randomised trial. Study duration: December 2017 to June 2018 Power calculation: no prospective calculation of sample size |
|
| Participants |
Setting: NRS Medical College and Hospital, Kolkata, India Inclusion criteria: children (age not specified in inclusion criteria) with Gartland 3‐4 supracondylar fracture Exclusion criteria: delayed presentation > 5 days, previous supracondylar fracture neurovascular injury |
|
| Interventions |
Group 1: (28 children): manipulation of fracture in theatre with fixation using retrograde wires inserted from the lateral side Group 2: (14 children): manipulation of fracture in theatre with fixation using retrograde wires inserted from the medial and lateral side Group 3: (8 children): manipulation of fracture in theatre with fixation using retrograde wires inserted from the lateral side The preoperative treatment was not specified in the report. Following surgery, children were immobilised in a posterior plaster cast for 3 to 4 weeks. |
|
| Outcomes | 1) Major complications measured as pin site infections – the groups where the infections occurred was not specified. 2) Nerve injury – the groups in which the nerve injuries occurred was not specified. 3) Range of motion as measured as range of motion of 10° to 110° ‐ the groups in which the elbow stiffness occurred was not specified. |
|
| Notes | We were unable to include outcome data in the review because data were reported overall, rather than by group. Funding source: not stated Declarations: not stated |
|
Dawood 2011.
| Study characteristics | ||
| Methods |
Design: randomised trial Study duration: February 2010 to January 2011 Power calculation: no prospective calculation of sample size |
|
| Participants |
Setting: Department of Surgery, College of Medicine, Tikrit University, Tikrit, Iraq Sample size: 21 children Inclusion criteria: children aged 4 to 10 with Gartland type 3 supracondylar fractures Exclusion criteria: previous fracture in either elbow, delayed presentation > 2 days |
|
| Interventions |
Group 1 (10 children): posterior approach open reduction of fracture in theatre with fixation using retrograde wires inserted from the lateral side Group 2 (11 children): posterior approach open reduction of fracture in theatre with fixation using retrograde wires inserted from the medial and lateral side |
|
| Outcomes | 1) Nerve injury 2) Cosmetic deformity (carrying angle and carrying angle loss > 10 degrees at 4 months) 3) Range of motion (flexion and extension loss at 4 months) 4) Radiographic deformity (Baumann's angle loss at 4 months) |
|
| Notes |
Loss to follow‐up: no loss to follow‐up reported Funding source: not stated Declarations: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Two groups of patients were selected randomly” Comment: unclear how randomisation was performed |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient detail on allocation concealment to assess |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: it is not clear who performed the interventions and their relationship to the study team. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: it is not clear who performed the outcomes assessment and their relationship to the study team. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no reported loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Comment: no published protocol or prospective trial registration was identified |
| Other bias | Low risk | None identified |
Dehghan 2012.
| Study characteristics | ||
| Methods |
Design: randomised trial Study duration: 2010 Power calculation: no prospective calculation of sample size |
|
| Participants |
Setting: Ayatollah Kashani hospital, Iran Sample size: 90 children Inclusion criteria: children aged 2 to 10 with Gartland 3 supracondylar fractures Exclusion criteria: delayed presentation more than 5 days, previous arm fracture, head injury, underlying disease, unavailability to attend follow‐up, referral to other centres due to complications |
|
| Interventions |
Group 1 (45 children): closed manipulation of fracture in theatre with fixation using wires Group 2 (45 children): posterior approach open reduction of fracture in theatre with fixation using wires |
|
| Outcomes | 1) Major complications (pin site infection) | |
| Notes |
Loss to follow‐up: no loss to follow‐up reported Funding source: not stated Declarations: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "through Random Number Table allocated in two groups" |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient detail on allocation concealment to assess |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: it is not clear who performed the interventions and their relationship to the study team. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: it is not clear who performed the outcomes assessment and their relationship to the study team. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no reported loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Comment: no published protocol or prospective trial registration was identified. The trial was registered after recruitment was complete. |
| Other bias | Low risk | None identified |
Ducic 2016a.
| Study characteristics | ||
| Methods |
Design: randomised trial Study duration: February 2010 to April 2014 Power calculation: no prospective calculation of sample size |
|
| Participants |
Setting: University Children’s Hospital, Belgrade, Serbia Sample size: 138 children Inclusion criteria: children (age not specified in inclusion criteria) with extension type (Gartland 2 or 3) supracondylar fractures Exclusion criteria: open fractures, serious neurovascular complications and children with fractures requiring an open reduction |
|
| Interventions |
Group 1 (67 children): manipulation of fracture in theatre with fixation using one retrograde wire and one antegrade wire inserted from the lateral side (Dorgan wires) Group 2 (71 children): manipulation of fracture in theatre with fixation using retrograde wires inserted from the medial and lateral side. The medial wire technique was not specified. |
|
| Outcomes | 1) Nerve injury 2) Major complications (pin site infections) 3) Cosmesis (change in carrying angle at 9 to 14 months) 4) Range of movement (loss of total range of movement at 9 to 14 months) |
|
| Notes |
Loss to follow‐up: none reported Funding source: not stated Declarations: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "They were randomized by random number generator using R software environment where odd numbers were assigned to Dorgan’s method of fixation" |
| Allocation concealment (selection bias) | Low risk | Comment: no allocation sequence to conceal therefore study staff unable to predict next allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: it is not clear who performed the interventions and their relationship to the study team. |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "A prospective randomised non‐blinded comparison of conventional and Dorgan's crossed pins" Comment: no blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no reported loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Comment: no published protocol or prospective trial registration was identified |
| Other bias | Low risk | None identified |
Ercin 2016.
| Study characteristics | ||
| Methods |
Design: quasi‐randomised study based on day‐of‐week allocation Study duration: 2011 to 2013 Power calculation: power calculation: no prospective calculation of sample size. Sample of 104 children selected but not justified. |
|
| Participants |
Setting: Department of Orthopaedics and Traumatology, Dr. Sadi Konuk Training and Research Hospital, Istanbul, Turkey Sample size: 104 children Inclusion criteria: children (age unspecified in inclusion criteria) with Gartland 3 supracondylar fractures fixed with 2 lateral and one medial K‐wire Exclusion criteria: flexion‐type fractures, neurovascular injuries, open fractures, previous ipsilateral elbow fractures, children who did not attend final follow‐up or fixation with two rather than three wires |
|
| Interventions |
Group 1 (63 children): manipulation of fracture in theatre with fixation using retrograde wires inserted from the medial and lateral side using a percutaneous technique Group 2 (41 children): manipulation of fracture in theatre with fixation using retrograde wires inserted from the medial and lateral side using a mini‐open technique for the medial wire |
|
| Outcomes | 1) Nerve injury 2) Major complications (pin site infections) 3) Radiographic deformity* |
|
| Notes |
Loss to follow‐up: no reported loss to follow‐up *Mean follow‐up of 8.93 months (range 3.13 to 14.73 months), with discharge when full elbow range of motion achieved. Radiographic deformity not described in sufficient detail in paper for further analysis Funding source: not stated Declarations: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: “Children were randomized based on which orthopedic surgeon was on trauma call” |
| Allocation concealment (selection bias) | High risk | Comment: as treatment allocated by surgeon on take, it would be obvious what treatment the presenting child would have |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: it is not clear who performed the interventions and their relationship to the study team. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: it is not clear who performed the outcome assessment and their relationship to the study team. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 17 participants lost from allocation to analysis (15 fixed with 2 wires) |
| Selective reporting (reporting bias) | Unclear risk | Comment: no published protocol or prospective trial registration was identified. |
| Other bias | Low risk | None identified |
Foead 2004.
| Study characteristics | ||
| Methods |
Design: randomised trial Study duration: May 2000 to December 2001 Power calculation: no prospective calculation of sample size |
|
| Participants |
Setting: Department of Orthopaedic Surgery, University Malaya Medical Center, Kuala Lumpur, Malaysia Sample size: 66 children Inclusion criteria: children aged 1 to 12 with displaced supracondylar fractures (Gartland 2 or 3) Exclusion criteria: late presentation (> 3 days since injury), previous elbow fracture, simultaneous fracture in the same arm |
|
| Interventions |
Group 1 (32 children): manipulation of fracture in theatre with fixation using retrograde wires inserted from the lateral side Group 2 (34 children): manipulation of fracture in theatre with fixation using retrograde wires inserted from the medial and lateral side |
|
| Outcomes | 1) Nerve injury 2) Major complications (pin site infections, compartment syndrome, vascular deficit requiring exploration) 3) Cosmetic outcome (loss of carrying angle at 3 to 15 months) 4) Range of movement (loss of flexion and extension at 3 to 15 months) 5) Radiographic deformity (loss of Baumann's angle and medial epicondylar epiphyseal angle at 3 to 15 months) |
|
| Notes |
Loss to follow‐up: Group 1 5/32 (15.6%) and Group 2 6/34 (17.6%) Mean follow‐up: 8.9 months Funding source: not stated Declarations: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The main investigator would perform the randomisation by drawing lots—odd numbers signify medial‐lateral pin fixation, while even numbers would be treated by 2 lateral pin fixation". |
| Allocation concealment (selection bias) | Unclear risk | Comment: unclear if there was a fixed number of lots that could be drawn that would allow study staff to predict likelihood of next treatment allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Surgery was performed by senior orthopaedic trainees supervised by one of the authors". Comment: no apparent blinding or separation of persons performing the intervention |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: “All the patients were followed up at the paediatric orthopaedic out‐patient clinic and reviewed by one of the authors”. Comment: unclear if any blinding was used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "11 of the 66 patients defaulted follow‐up after the plaster casts were removed". Comment: participants followed up until full range of movement achieved, then outcomes assumed to have been maintained. Loss to follow‐up balanced between the groups |
| Selective reporting (reporting bias) | Unclear risk | Comment: no published protocol or prospective trial registration was identified |
| Other bias | Low risk | None identified |
Gaston 2010.
| Study characteristics | ||
| Methods |
Design: quasi‐randomised study Study duration: March 2005 to July 2006 Power calculation: no prospective calculation of sample size |
|
| Participants |
Setting: OrthoCarolina, Charlotte, North Carolina, USA Sample size: 104 children Inclusion criteria: children (skeletally immature) with Gartland 3 extension type supracondylar fractures Exclusion criteria: delayed presentation (> 24 hours), open fractures, inadequate perioperative radiographs, inadequate follow‐up |
|
| Interventions |
Group 1 (47 children): manipulation of fracture in theatre with fixation using retrograde wires inserted from the lateral side Group 2 (57 children): manipulation of fracture in theatre with fixation using retrograde wires inserted from the medial and lateral side using a percutaneous technique |
|
| Outcomes | 1) Nerve injury 2) Major complications (compartment syndrome) |
|
| Notes | Loss of reduction also reported at time of pin removal so not included in meta‐analysis Loss to follow‐up: 16 participants excluded following treatment allocation for inadequate radiographs or incomplete follow‐up. No information available regarding which groups these children were in. Funding source: not stated Declarations: nil Additional information received from Glenn Gaston:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Patients were then randomized in a blocked‐randomization manner based on day of presentation to the emergency room and the on‐call physician that day" |
| Allocation concealment (selection bias) | High risk | Comment: day of the week allocation means that children could be deferred to another day and therefore another surgeon |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: surgeons performed their default preferred technique independent of the study |
| Blinding of outcome assessment (detection bias) | High risk | Quote [full report]: "All measurements were made by a panel consisting of an attending pediatric orthopaedist, a chief orthopaedic resident, and a junior orthopaedic resident" Quote [unpublished]: "Were the surgeons who assessed post‐operative nerve injury blinded to the treatment provided? ‐ No"..."Was the panel who performed the radiographic measurements blinded to treatment allocation?‐ No" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: 16 participants were excluded from the study as they did not complete follow‐up. It is not specified to which group these participants were allocated. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no published protocol or prospective trial registration was identified. |
| Other bias | Low risk | None identified |
Gholap 2020.
| Study characteristics | ||
| Methods |
Design: randomised trial Study duration: 2‐year period Power calculation: no prospective calculation of sample size. |
|
| Participants |
Setting: Department of Orthopedics. Dr. D.Y. Patil Medical College, Hospital and Research Center, Pimpri, Maharashtra, India Sample size: 30 children Inclusion criteria: children aged 3 to 18 with Gartland 3 supracondylar fracture Exclusion criteria: neurovascular injury, open fractures |
|
| Interventions |
Group 1 (15 children): manipulation of fracture in theatre with fixation using retrograde wires inserted from the lateral side Group 2 (15 children): manipulation of fracture in theatre with fixation using retrograde wires inserted from the medial and lateral side using a mini‐open technique |
|
| Outcomes | 1) Nerve injury 2) Major complications 3) Cosmetic deformity (loss of carrying angle at 6 months) 4) Range of movement (loss of total range of movement at 6 months) |
|
| Notes |
Loss to follow‐up: no reported loss to follow‐up Funding source: not stated Declarations: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The study was a single center, prospective, randomized controlled clinical trial” Comment: technique for delivering random sequence was not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: unclear if any concealment was performed of the allocation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: unclear who provided the treatments and if they were involved as part of the study |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: unclear who assessed the outcomes and if they were blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no reported loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Comment: no published protocol or prospective trial registration was identified. Mayo elbow score mentioned in abstract but not in body of report. Insufficient information to combine complication outcomes in analysis |
| Other bias | Low risk | None identified |
Gopinathan 2018.
| Study characteristics | ||
| Methods |
Design: randomised trial Study duration: April 2014 to September 2015 Power calculation: no prospective calculation of sample size |
|
| Participants |
Setting: Department of Orthopaedics, AIIMS, Bhopal, Madhya Pradesh, India Sample size: 30 children Inclusion criteria: children aged 2 to 12 with Gartland 3 supracondylar fractures Exclusion criteria: delayed presentation > 7 days, vascular injuries, open fractures, transphyseal injuries, skin blisters, established compartment syndrome, children requiring open reduction |
|
| Interventions |
Group 1 (19 children): manipulation of fracture in theatre with fixation using retrograde wires inserted from the lateral side in a parallel configuration Group 2 (11 children): manipulation of fracture in theatre with fixation using retrograde wires inserted from the lateral side using a divergent configuration |
|
| Outcomes | 1) Nerve injury (iatrogenic) 2) Cosmetic deformity (change in carrying angle at 3 months) 3) Range of movement (loss of flexion or extension at 3 months) 4) Radiographic deformity (loss of reduction and change in Baumann's angle at 3 months) |
|
| Notes |
Loss to follow‐up: no reported loss to follow‐up Funding source: none Declarations: none Additional information from Prateek Behera by email: "1. the child who developed compartment syndrome had received parallel [sic] pins for his fracture. 2 and 3. At the centre where the investigation was performed (I have moved now) around 25‐35 cases of displaced SCH fractures are operated every year. Based on this and considering a maximum of 40 patients. 80 identical envelopes were taken. Half of them had a slip with parallel written and half had a slip with divergent written on them. All the 80 were sealed and were kept in a box, from which one fixed investigator used to pick up one envelope once a child was enrolled. No specific allocation sequence was considered. This was a simple method that depended on the chance of an envelope getting picked up" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The children were randomized into two groups using the opaque sealed envelope technique" |
| Allocation concealment (selection bias) | High risk | Quote: "80 identical envelopes were taken. Half of them had a slip with parallel written and the other half had a slip with divergent written on them. All the 80 were sealed and were kept in a box, from which one fixed investigator used to pick up one envelope once a child was enrolled" Comment: it would be possible for the study team to begin to predict allocation as the study progressed and number of envelopes decreased. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: unclear if the operating surgeons were involved as part of the study team |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: unclear if the outcome assessors were blinded to trial allocations |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no reported loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Comment: no published protocol or prospective trial registration was identified |
| Other bias | Low risk | None identified |
Jain 2019.
| Study characteristics | ||
| Methods |
Design: quasi‐randomised study Study duration: February 2012 to November 2013 Power calculation: no prospective calculation of sample size |
|
| Participants |
Setting: Department of Orthopaedic, SMS Medical College & Hospital, Jaipur, India Sample size: 168 children Inclusion criteria: children aged 0 to 15 with extension type Gartland 2 or 3 supracondylar fractures Exclusion criteria: vascular injuries, comminuted fractures with intra‐articular extension, flexion type fracture |
|
| Interventions |
Group 1 (84 children): manipulation of fracture in theatre with fixation using one retrograde wire from the lateral side and one posterior intrafocal wire Group 2 (84 children): manipulation of fracture in theatre with fixation using retrograde wires inserted from the medial and lateral side. A percutaneous technique was used for the medial wire. |
|
| Outcomes | 1) Nerve injury 2) Major complications (pin site infection) 3) Cosmetic deformity (cubitus varus and loss of carrying angle > 10 degrees at 6 months) 4) Range of movement (loss of total range of movement at 6 months) 5) Radiographic deformity (loss of reduction with change in Baumann's angle > 6 degrees at 6 months) |
|
| Notes |
Loss to follow‐up: Group 1 6/84 (7.1%) and Group 2 4/84 (4.8%) Funding source: not stated Declarations: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: “System random sample technique: all odd number of cases allotted to CPF group and all even number of patient allotted to PILPF group” Comment: sequence generated through alternation |
| Allocation concealment (selection bias) | High risk | Comment: sequence generated through alternation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: it is not clear who performed the interventions and their relationship to the study team. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: it is not clear who performed the outcomes assessment and their relationship to the study team. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: small loss to follow‐up for radiographic outcomes (6.0%) that seem to be balanced between groups. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no published protocol or prospective trial registration was identified. |
| Other bias | Low risk | None identified |
Kaewpornsawan 2001.
| Study characteristics | ||
| Methods |
Design: randomised trial Study duration: June 1996 to April 1999 Power calculation: to detect a difference in Baumann's angle of 5 degrees at 80% power was 14 cases per group |
|
| Participants |
Setting: Faculty of Medicine, Siriraj Hospital, Bangkok, Thailand Sample size: 28 children Inclusion criteria: children aged 1 to 12 with totally displaced supracondylar fractures Exclusion criteria: open fracture, delayed presentation > 7 days, ipsilateral forearm fracture, vascular injury, compartment syndrome, abnormal growth and development |
|
| Interventions |
Group 1 (14 children): closed manipulation of fracture in theatre with fixation using two or three retrograde wires Group 2 (14 children): lateral approach open reduction of fracture in theatre with fixation using crossed wires inserted from the lateral side |
|
| Outcomes | 1) Nerve injury 2) Major complications 3) Cosmetic deformity (patient satisfaction with scar and appearance at 14 to 30 weeks) 4) Range of motion (loss of total range of motion > 10 degrees at 14 to 30 weeks) 5) Radiographic deformity (loss of Baumann's angle, loss of reduction at 14 to 30 weeks) |
|
| Notes |
Loss to follow‐up: no reported loss to follow‐up Mean follow‐up 20 weeks Funding source: not stated Declarations: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Children randomly divided into two groups by block randomization" |
| Allocation concealment (selection bias) | Unclear risk | Comment: method of concealment is not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The operation in both groups was performed by the author" Comment: all procedures performed by a single surgeon who was not blinded to allocation and part of the study team |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "Blinded assessor provided an assessment of scar and appearance" Comment: for most outcomes, it was unclear if the outcome assessors were blinded to trial allocations. For the assessment of scar and appearance, we judged detection bias to be high risk. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no reported loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Comment: no published protocol or prospective trial registration was identified. |
| Other bias | Low risk | None identified |
Kalia 2018.
| Study characteristics | ||
| Methods |
Design: randomised study Study duration: 2017 to 2018 Power calculation: no prospective calculation of sample size |
|
| Participants |
Setting: Maharishi Markandeshwar Medical College and Hospital, Kumarhatti, Solan, India Sample size: 60 children Inclusion criteria: children (age not specified in inclusion criteria) with extension type Gartland 2 or 3 supracondylar fracture Exclusion criteria: open fractures, fractures with vascular injury, and fractures requiring open reduction |
|
| Interventions |
Group 1 (30 children): manipulation of fracture in theatre with fixation using one retrograde wire and one antegrade wire inserted from the lateral side Group 2 (30 children): manipulation of fracture in theatre with fixation using retrograde wires inserted from the medial and lateral side. A mini‐open technique was used for the medial wire. |
|
| Outcomes | 1) Nerve injury 2) Major complications (pin site infections) 3) Cosmetic deformity (loss of carrying angle > 10 degrees at 2 to 12 months)* 4) Range of movement (loss of total range of movement > 10 degrees at 2 to 12 months)* |
|
| Notes | *Outcomes 3 and 4 unable to be combined in analysis as unclear how many participants had outcomes measured at the different time points Loss to follow‐up: none reported Funding source: not stated Declerations: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “In the first method 30 patients selected at random underwent standard cross‐k wiring” Comment: unclear how randomisation was performed |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient detail on allocation concealment to assess |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: it is not clear who performed the interventions and their relationship to the study team. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: it is not clear who performed the outcomes assessment and their relationship to the study team. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no reported loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Comment: no published protocol or prospective trial registration was identified. |
| Other bias | Low risk | None identified |
Kocher 2007.
| Study characteristics | ||
| Methods |
Design: randomised trial Study duration: May 2003 to January 2005 Power calculation: prospective calculation completed – 80% power to detect a 10% difference in loss of reduction required 52 participants |
|
| Participants |
Setting: Children’s Hospital Boston, Massachusetts, USA Sample size: 66 children Inclusion criteria: children aged 3 to 10 years with a Gartland 3 extension type fracture Exclusion criteria: delayed presentation > 48 hours, open fractures, fracture requiring neurovascular exploration, floating elbow injury, bilateral fractures, previous ipsilateral fracture, or an inability to perform a preoperative neurovascular examination |
|
| Interventions |
Group 1 (28 children): manipulation of fracture in theatre with fixation using retrograde wires inserted from the lateral side Group 2 (24 children): manipulation of fracture in theatre with fixation using retrograde wires inserted from the medial and lateral side using a mini‐open technique |
|
| Outcomes | 1) Nerve injury 2) Major complications 3) Cosmetic deformity (carrying angle at 3 months) 4) Return to normal activities at 3 months 5) Range of motion (total range of motion loss greater than 10 degrees at 3 months) 5) Radiographic deformity (loss of Baumann's angle > 6 degrees, change in Baumann's angle and change in humeral‐capitellar angle at 3 months) |
|
| Notes |
Loss to follow‐up: 13 children excluded following allocation for open reduction, deviation from prescribed pin position, not meeting inclusion criteria, and inadequate fixation on review Funding source: not stated Declarations: authors received funding from implant companies |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomized to be treated either with lateral entry pin fixation or with medial and lateral entry pin fixation according to an assignment, produced by a random number generator, in a sealed envelope" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "A permuted block (block size, 4) randomization design was used. The envelopes were opened in the operating room, after closed reduction and at the time of percutaneous pin fixation" Comment: unclear if the envelopes were numbered and opened sequentially |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: unclear if the ten operating surgeons were involved in the study or if they may have had influence on the study findings |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "Clinical evaluation was performed by attending pediatric orthopaedic surgeons. The surgeons and the patients were not blinded to the type of pin construct" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Of the sixty‐six patients, five were excluded from the study because they had undergone open reduction; four, because the surgeon had added pins to the randomized pin configuration; two, because the fracture was deemed to be type II on review; two, because the pin configuration was not acceptable according to the protocol; and one, because the preoperative neurovascular examination was inadequate" Comment: it seems that 14 participants (21.2%) were excluded from analysis after randomisation and it is unclear to which groups these children were allocated. No intention‐to treat‐analysis presented following exclusions |
| Selective reporting (reporting bias) | Unclear risk | Comment: no published protocol or prospective trial registration was identified |
| Other bias | Low risk | None identified |
Kumar 2021.
| Study characteristics | ||
| Methods |
Design: randomised study Study duration: April 2019 to March 2020 Power calculation: no prospective calculation of sample size |
|
| Participants |
Setting: Department of Orthopaedics, Maharaja Suheldev Autonomous State Medical College, Baharaich, India Inclusion criteria: children (aged 3 to 12) with displaced supracondylar fractures Exclusion criteria: delayed presentation > 7 days, open fractures, previous fractures to the elbow, fracture requiring open reduction |
|
| Interventions |
Group 1: (30 children): manipulation of fracture in theatre and immobilisation in above‐elbow plaster of Paris backslab Group 2: (30 children): closed reduction of fracture in theatre with fixation using retrograde wires inserted from the medial and lateral side. A percutaneous technique was used for the medial wire. The preoperative immobilisation was not described. Both groups were immobilised in an above‐elbow plaster backslab and followed up at 1, 3, 12 and 24 weeks. It was not specified when the wires or plaster were removed. |
|
| Outcomes |
Outcomes included in this review: 1) Nerve injury 2) Major complications measured as pin tract infections Outcomes not included in this review: 1) Cosmetic deformity as measured as loss of carrying angle at 6 months ‐ outcome was reported as composite outcome with range of motion as Flynn grade 2) Range of motion measured as loss of extension and loss of flexion – outcome was reported as composite outcome with cosmetic deformity as Flynn grade 3) Malunion – outcome was not specified as clinical or radiological or what definition was used for evaluation. |
|
| Notes |
Funding source: none Declarations: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “This prospective randomized controlled study” Comment: no further information about randomisation technique |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to assess allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: it is not clear who performed the interventions and their relationship to the study team. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: it is not clear who performed the outcomes assessment and their relationship to the study team. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no reported loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Comment: no published protocol or prospective trial registration was identified. |
| Other bias | Low risk | None identified |
Kuzma 2014.
| Study characteristics | ||
| Methods |
Design: randomised study Study duration: 2009 to 2012 Sample size: calculation based on a presumed effect size of 0.5, though is was unclear what the primary outcome was for this study or if the primary outcome was a continuous variable for which this sample size calculation would be appropriate. The authors report the sample size was identified to be 64 per group to achieve 80% power. |
|
| Participants |
Setting: Modilon General Hospital, Madang, Papua New Guinea Sample size: 133 children Inclusion criteria: children aged 0 to 16 with Gartland 2 or 3 supracondylar fractures Exclusion criteria: open fractures, flexion type fractures, intra‐articular fractures, additional same‐limb fractures, visceral or head injuries, previous treatment at another hospital for the same injury |
|
| Interventions |
Group 1 (66 children): skin traction applied under sedation for 2 to 3 weeks Group 2 (67 children): manipulation under anaesthesia in theatre and insertion of an olecranon K‐wire or Steinmann pin and application of traction for 2 to 3 weeks followed by removal of metalwork under anaesthesia in theatre |
|
| Outcomes | 1) Nerve injuries 2) Major complications (compartment syndrome and pin site infection) 3) Cosmetic deformity at 3 to 6 month follow‐up (cubitus varus) 4) Range of movement at 3 to 6 month follow‐up (loss of flexion > 10 degrees, hyperextension > 5 degrees) |
|
| Notes |
Loss to follow‐up: Group 1 11/66 (16.7%), Group 2 10/67 (14.9%) Funding source: not stated Declarations: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "After taking the informed consent, the surgeon randomly allocated participants to an overhead skeletal traction or straight‐arm skin traction by hand drawing from a box of sealed opaque envelopes" |
| Allocation concealment (selection bias) | High risk | Quote: "there were 70 envelopes in the 1st box and same amount in the 2nd box" Comment: there was a fixed number of envelopes of both treatments so study staff may have been able to predict allocation for children later in the study. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Skeletal traction was performed both by the consultant and registrars in orthopaedic ward, they were not formally part of the study team." Comment: interventions performed by personnel not directly involved in trial so unlikely to impact outcomes |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "Outcome measures were assessed by the researcher... [B]linding the outcome assessors was deemed not possible because... the skeletal traction... left [a] small scar." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 15.8% loss to follow‐up balanced between groups. Adjustment for loss to follow‐up using last result carried forward. Unadjusted analysis unavailable due to computer failure |
| Selective reporting (reporting bias) | Unclear risk | Comment: no published protocol or prospective trial registration was identified. |
| Other bias | Low risk | None identified |
Maity 2012.
| Study characteristics | ||
| Methods |
Design: randomised trial Study duration: October 2007 to September 2010 Power calculation: sample of 160 to detect a 15% difference in the rate of major loss of reduction at 80% power with a 25% loss to follow‐up |
|
| Participants |
Setting: Department of Orthopaedics, Burdwan Medical College, Burdwan, West Bengal, India Sample size: 160 children Inclusion criteria: children aged 2 to 12 with displaced Gartland 2 or 3 extension type supracondylar fractures Exclusion criteria: bilateral fractures, delayed presentation > 72 hours, additional injury in the same limb, previous fracture in the same limb, need for open reduction, floating elbow, failure to perform an adequate preoperative neurovascular examination, neurovascular injury requiring exploration |
|
| Interventions |
Group 1 (80 children): manipulation of fracture in theatre with fixation using retrograde wires inserted from the lateral side Group 2 (80 children): manipulation of fracture in theatre with fixation using retrograde wires inserted from the medial and lateral side using a mini‐open technique |
|
| Outcomes | 1) Nerve injury 2) Major complications 3) Cosmetic deformity (carrying angle at 3 months) 4) Range of movement (passive flexion, extension and total range of movement at 3 months) 5) Radiographic deformity (Baumann's angle and change in Baumann's angle at 3 months) |
|
| Notes | Identical report published in Iraqi Journal of Medical Sciences (Sadik 2015). This publication preceded the duplicate by three years and is assumed to be the initial study report. Loss to follow‐up: Group 1 14/80 (17.5%), Group 2 16/80 (20.0%) Funding source: none Declarations: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The random assignment scheme was created from a table of random numbers" |
| Allocation concealment (selection bias) | Low risk | Quote: "Opaque prenumbered sealed envelopes containing random assignments were maintained by the hospital pharmacist." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: unclear who performed the interventions and their relationship to the study team |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "Follow‐up assessment of each patient was done by the same doctor throughout the trial. Both the surgeons and the patients were not blinded of the treatment received throughout the trial". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "All statistical analysis was based on an “intention to treat” principle; therefore patients who withdrew from the study, the data at the time of withdrawal were carried forward to all subsequent evaluations" Comment: 30 (18.8%) participants lost to follow‐up with no presentation of unadjusted scores. Last result carried forward to adjust for missing data, and loss to follow‐up reasonably balanced between groups. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no published protocol or prospective trial registration was identified |
| Other bias | Unclear risk | During our search, we identified an identical version of this report, but with a different author team (Sadik 2015). We assumed that this report was the original publication because it preceded Sadik 2015 by three years. However, we could not rule out the possibility that this publication was conducted by other study authors. |
Majeed 2020.
| Study characteristics | ||
| Methods |
Design: randomised study Study duration: January 2018 to June 2018 Power calculation: no prospective calculation of sample size. |
|
| Participants |
Setting: Department of Orthopaedics Surgery, Services Hospital, Lahore, Pakistan Sample size: 180 children Inclusion criteria: children aged 0 to 15 with supracondylar fracture Exclusion criteria: children with ‘complications related to fracture’ |
|
| Interventions |
Group 1 (90 children): manipulation of fracture in theatre with fixation using retrograde wires inserted from the lateral side Group 2 (90 children): manipulation of fracture in theatre with fixation using retrograde wires inserted from the medial and lateral side. The technique for the medial wire was not specified. |
|
| Outcomes | 1) Nerve injury 2) Cosmetic deformity* 3) Range of motion* |
|
| Notes | *Outcomes 2 and 3 were combined in a Flynn classification score so could not be used in the analysis Loss to follow‐up: none stated Funding source: not stated Declarations: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Study Design: Randomized controlled trial”… “Total patients were divided into two groups (A and B)” Comment: unclear how randomisation was performed |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient detail on allocation concealment to assess |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: it is not clear who performed the interventions and their relationship to the study team. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: it is not clear who performed the outcomes assessment and their relationship to the study team. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no reported loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Comment: no published protocol or prospective trial registration was identified. |
| Other bias | Low risk | None identified |
Mandal 2018.
| Study characteristics | ||
| Methods |
Design: randomised study Study duration: March 2015 to September 2016 Power calculation: no prospective calculation of sample size. |
|
| Participants |
Setting: Medical College and Hospital, Kolkata, India Sample size: sample of 60 children selected but not justified. Inclusion criteria: children aged 5 to 15 with Gartland 2 or 3 supracondylar fractures who had failed attempted closed reduction in the emergency department Exclusion criteria: open fractures, delayed presentation > 5 days, patients unfit for surgery, neurovascular injury, compartment syndrome |
|
| Interventions |
Group 1 (30 children): manipulation of fracture in theatre with fixation using three retrograde wires inserted from the lateral side Group 2 (30 children): manipulation of fracture in theatre with fixation using two retrograde wires inserted from the medial and lateral side. It was not specified if an open or percutaneous technique was used for the medial wire. |
|
| Outcomes | 1) Treatment failure requiring re‐fixation 2) Nerve injury 3) Major complications 4) Cosmetic deformity at 6‐month follow‐up 5) Range of movement at 6‐month follow‐up 6) Return to sport and normal activities 7) Radiographic deformity at 6‐month follow‐up |
|
| Notes |
Loss to follow‐up: Group 1 1/30 (3.3%), Group 2 1/30 (3.3%) Funding source: not stated Declarations: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Each of the patients from the study sample was selected randomly for crossed medial and lateral pin fixation and lateral entry three divergent wires“ Comment: unclear how the randomisation was performed |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to evaluate allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: unclear who performed the interventions and their relationship to the study team |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: insufficient information to evaluate blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 2 participants excluded following complications that may have had a significant impact on results |
| Selective reporting (reporting bias) | Unclear risk | Comment: no published protocol or prospective trial registration was identified |
| Other bias | Low risk | None identified |
Mulpuri 2016.
| Study characteristics | ||
| Methods |
'sDesign: non‐inferiority randomised trial Study duration: not stated Power calculation: no prospective calculation of sample size provided |
|
| Participants |
Setting: British Columbia Children’s Hospital, Vancouver, British Columbia, Canada Sample size: 55 children Inclusion criteria: children aged 3 to 7 with a Gartland 3 supracondylar fracture Exclusion criteria: open fracture, preoperative ulnar nerve injury, compartment syndrome or vascular injury |
|
| Interventions |
Group 1 (23 children): manipulation of fracture in theatre with fixation using retrograde wires inserted from the lateral side Group 2 (29 children): manipulation of fracture in theatre with fixation using retrograde wires inserted from the medial and lateral side. It was not specified if an open or percutaneous technique was used for the medial wire. |
|
| Outcomes | 1) Nerve injuries 2) Radiographic deformity (change in Baumann's angle at 3 weeks) 3) Cosmetic deformity (carrying angle at 3 years)* 4) Range of movement at 3 years* 5) Radiographic deformity (humeral‐capitellar angle)* |
|
| Notes | * Outcomes 3 to 5 not presented in either available conference abstract Loss to follow‐up: Group 1 1/23 (4.3%), Group 2 6/29 (20.7%) Funding source: not stated Declarations: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Consenting patients were block randomized into one of two groups" |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to evaluate allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: insufficient information to evaluate blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: insufficient information to evaluate blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: per‐protocol analysis presented with patients excluded if an additional wire was required. This impacts the crossed wire group more than the lateral wire group. |
| Selective reporting (reporting bias) | High risk | Comment: prospective registration identifies Flynn scores at three years and humeral‐capitellar angle which are not reported in the presented abstracts |
| Other bias | Low risk | None identified |
Naik 2017.
| Study characteristics | ||
| Methods |
Design: quasi‐randomised study Study duration: May 2013 to May 2015 Power calculation: no prospective calculation of sample size provided |
|
| Participants |
Setting: Medical College and Hospital, Kolkata, India Sample size: 60 children Inclusion criteria: children age 0 to 13 with Gartland 3 supracondylar fractures Exclusion criteria: flexion type injuries, open fractures |
|
| Interventions |
Group 1 (30 children): manipulation of fracture in theatre with fixation using retrograde wires inserted from the lateral side Group 2 (30 children): manipulation of fracture in theatre with fixation using retrograde wires inserted from the medial and lateral side. A mini‐open technique was used for the medial wire. |
|
| Outcomes | 1) Nerve injury 2) Major complications 3) Cosmetic deformity (carrying angle at 6 months) 4) Range of movement (loss of total range of motion at 6 months) |
|
| Notes |
Loss to follow‐up: Group 1 1/30, Group 2 1/30 but both with significant complications (varus collapse requiring revision and nerve injury) Funding source: not stated Declarations: not stated An identical report was published in Zagazig University Medical Journal with different authors (El‐Ngehy 2018). This publication preceded the duplicate by one year and is assumed to be the initial study report. Additional information received through communication with study authors: Functional (range of motion) scores according to Flynn criteria for both groups at 2 years: Group A (lateral wires) Excellent 4, Good 17, Fair 7, Poor 0 Group B (crossed wires) Excellent 3, Good 21, Fair 5, Poor 0 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "The randomization process was done by the odd and even number technique in which the patients with even inpatient numbers were assigned in Group A while the odd inpatient number patients were allotted in Group B." |
| Allocation concealment (selection bias) | High risk | Comment: the randomisation technique was open so could be predicted by study staff deciding if a child should be approached for the study |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: insufficient information to evaluate blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: insufficient information to evaluate blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no reported loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Comment: no published protocol or prospective trial registration was identified |
| Other bias | Unclear risk | During our search, we identified an identical version of this report, but with a different author team (El‐Ngehy 2018). We assumed that this report was the original publication because it preceded El‐Ngehy 2018 by 12 months. However, we could not rule out the possibility that this publication was conducted by other study authors. |
Naveen 2017.
| Study characteristics | ||
| Methods |
Design: randomised trial Study duration: May 2016 to January 2017 Power calculation: no prospective calculation of sample size provided |
|
| Participants |
Setting: Department of Orthopedics, Shivamogga, India Sample size: 42 children Inclusion criteria: children aged 3 to 13 with Gartland 2 or 3 extension type supracondylar fracture Exclusion criteria: open fractures or neurovascular involvement |
|
| Interventions |
Group 1 (21 children): manipulation of fracture in theatre with fixation using retrograde wires inserted from the lateral side Group 2 (21 children): manipulation of fracture in theatre with fixation using retrograde wires inserted from the medial and lateral side. A percutaneous technique was used for the medial wire. |
|
| Outcomes | 1) Nerve injury 2) Major complications |
|
| Notes |
Loss to follow‐up: 1 child (4.8%) in each group Funding source: none Declarations: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Lateral only pinning or crossed medio lateral pinning group was done according to the random assignment scheme” |
| Allocation concealment (selection bias) | Unclear risk | Comment: unclear how allocation sequence was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: insufficient information to assess blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: insufficient information to assess blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Commment: low loss to follow‐up with one child lost to follow‐up in each group (4.8%) |
| Selective reporting (reporting bias) | Unclear risk | Comment: no published protocol or prospective trial registration was identified |
| Other bias | Low risk | None identified |
Oakley 2009.
| Study characteristics | ||
| Methods |
Design: randomised trial Study duration: September 2003 to March 2005 Power calculation: prospective calculation to detect a 20 mm difference in pain intensity with 80% power required 24 per group |
|
| Participants |
Setting: Department of Emergency Medicine, Royal Children’s Hospital, Melbourne, Australia Sample size: 50 children Inclusion criteria: children 0 to 18 with a Gartland 1 supracondylar fracture Exclusion criteria: additional injuries to the upper limb, other serious injuries, nerve or vascular injury, inability to complete a diary in English |
|
| Interventions |
Group 1 (27 children): fracture immobilised in the hospital emergency department with a fibreglass above‐elbow backslab and broad arm sling for 12 to 16 days Group 2 (23 children): fracture immobilised in the hospital emergency department with a collar and cuff sling for 12 to 16 days |
|
| Outcomes | 1) Nerve injury 2) Pain (pain intensity and duration) 3) Parental satisfaction at 2 to 4 weeks 4) Return to normal activities (time to return to normal activities) |
|
| Notes |
Loss to follow‐up: one diary not completed but all returned for clinical review Additional information received through communication with study authors (20 September 2020): Allocation sequence: used sequentially‐numbered, opaque envelopes for randomisation Resumption of normal activity was measured through use of diary and face‐to‐face visit Duration of pain was defined as time before two consecutive days of no pain were reported The pain assessment on a daily basis was a two‐part question ‐ has your child reported pain today. If “yes” then the pain was scored – (on the VAS). No pain of zero was recorded when recordings were written so the mean was calculated on the pain scores recorded. Funding source: not stated Declarations: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was by opaque‐sealed envelope, with a computer‐generated sequence in blocks of 6." |
| Allocation concealment (selection bias) | Low risk | Quote: "We used sequentially numbered opaque envelopes for randomisation" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: unclear who provided the treatments and if they were involved as part of the study |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "Reviewers were not blinded to the method of immobilization of the patient; however, standardized questionnaires and examination were used to limit bias. Interviews were conducted by the managing clinician (not researchers) to limit bias on reported satisfaction." Comment: most outcomes assessed by parents using a visual analogue score recorded in a diary and confirmed at face‐to‐face review. Parents not blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: one participant lost to follow‐up (incomplete diary) in both groups. Questionnaires filled in by all participants |
| Selective reporting (reporting bias) | Unclear risk | Comment: no published protocol or prospective trial registration was identified |
| Other bias | Low risk | None identified |
Othman 2017.
| Study characteristics | ||
| Methods |
Design: randomised trial Study duration: 2013 to 2015 Power calculation: no prospective calculation of sample size provided |
|
| Participants |
Setting: Department of Orthopedics, Faculty of Medicine, Zagazig University, Egypt Sample size: 47 children Inclusion criteria: children (age not specified in inclusion criteria) with Gartland 2 or 3 supracondylar fracture Exclusion criteria: open fractures, fractures that required open reduction, vascular injuries, previous ipsilateral elbow fracture, delayed presentation > 3 days |
|
| Interventions |
Group 1 (16 children): manipulation of fracture in theatre with fixation using retrograde wires inserted from the lateral side Group 2 (17 children): manipulation of fracture in theatre with fixation using retrograde wires inserted from the medial and lateral side. A percutaneous technique was used for the medial wire. Group 3 (16 children): manipulation of fracture in theatre with fixation using one retrograde wire and one antegrade wire inserted from the lateral side |
|
| Outcomes | 1) Nerve injury 2) Major complications 3) Cosmetic deformity (carrying angle loss > 10 degrees at 6 to 10 months) 4) Range of motion (loss of range of motion > 10 degrees at 6 to 10 months) 5) Radiographic deformity (loss of Baumann's angle and humeral‐capitellar angle at 6 to 10 months) |
|
| Notes |
Loss to follow‐up: 2 children; the allocation of these two children was not specified Funding source: not stated Declarations: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “The patients were randomised to three different methods of pinning” Comment: unclear how randomisation was performed |
| Allocation concealment (selection bias) | Unclear risk | Comment: technique for allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: unclear who provided the treatments and if they were involved as part of the study |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: unclear who assessed the outcomes and if they were blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: 2 participants lost to follow‐up (4.3%). It was not specified to which groups these children were allocated. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no published protocol or prospective trial registration was identified. |
| Other bias | Low risk | None identified |
Palange 2019.
| Study characteristics | ||
| Methods |
Design: randomised trial Study duration: September 2016 to September 2017 Power calculation: no prospective calculation of sample size provided |
|
| Participants |
Setting: Grant Government Medical College & Sir JJ Group of Hospitals, Mumbai, Maharashtra, India Sample size: 30 children Inclusion criteria: children aged 0 to 13 with extension type Gartland 3 supracondylar fractures Exclusion criteria: open injuries, those with associated injuries to ipsilateral elbow |
|
| Interventions |
Group 1 (15 children): manipulation of fracture in theatre with fixation using retrograde wires inserted from the lateral side Group 2 (15 children): manipulation of fracture in theatre with fixation using retrograde wires inserted from the medial and lateral side. A mini‐open technique was used for the medial wire. |
|
| Outcomes | 1) Nerve injury 2) Cosmetic deformity at 6 months* 3) Range of movement at 6 months* 4) Radiographic deformity (loss of Baumann's angle at 6 months) |
|
| Notes | * Outcomes 2 and 3 were combined and reported as part of a Flynn classification so could not be combined in analysis Loss to follow‐up: none reported Funding source: not stated Declarations: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The randomization was done by the chit‐pulling system, where a staff nurse in the operating room, who was completely unassociate[d] with the study had to draw a chit from a bowl of concealed chits which were marked either 'A' or 'B'" |
| Allocation concealment (selection bias) | Unclear risk | Comment: unclear if there was a fixed number of chits that decreased during the study making it possible to predict the likelihood of the next allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “The operating surgeon was not shown the pre‐operative x rays of the patients before this randomization procedure to avoid bias” … “All surgeries were carried out by a single senior surgeon (Dr. SMK)” Comment: the operating surgeon is not listed as a study author and seems to be independent from the study team. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: unclear who assessed the outcomes and if they were blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no reported loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Comment: no published protocol or prospective trial registration was identified |
| Other bias | Low risk | None identified |
Pandey 2008.
| Study characteristics | ||
| Methods |
Design: prospective randomised Study duration: January 2004 to December 2004 Power calculation: no prospective calculation of sample size provided |
|
| Participants |
Setting: B.P. Koirala Institute of Health sciences, Dharan, Nepal Sample size: 60 children Inclusion criteria: children aged 0 to 11 years with extension type Gartland 2b or 3 supracondylar fractures Exclusion criteria: open fractures, delayed presentation > 7 days |
|
| Interventions |
Group 1 (30 children): manipulation of fracture in theatre and immobilisation in above‐elbow plaster of Paris backslab Group 2 (30 children): manipulation of fracture in theatre with fixation using retrograde wires inserted from the medial and lateral side. A percutaneous technique was used for the medial wire. |
|
| Outcomes | 1) Treatment failure requiring revision 2) Nerve injury 3) Major complications 4) Cosmetic deformity (carrying angle at 6 months) 5) Range of motion (loss of total range of motion > 10 degrees and loss of elbow flexion or extension at 6 months) |
|
| Notes |
Loss to follow‐up: Group 1 8/30 (26.7%), Group 2 6/30 (20%) Funding source: not stated Declarations: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: technique for delivering random sequence was not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: technique for allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: unclear who provided the treatments and if they were involved as part of the study |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: unclear who assessed the outcomes and if they were blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: loss to follow‐up of 8 (26.7%) in plaster backslab group and 6 (20%) in K‐wire group. Loss to follow‐up may be related to outcome (as children with good outcomes may not return to clinic). |
| Selective reporting (reporting bias) | Unclear risk | Comment: no published protocol or prospective trial registration was identified. |
| Other bias | Low risk | None identified |
Patil 2017.
| Study characteristics | ||
| Methods |
Design: randomised trial Study duration: dates not specified Power calculation: no prospective calculation of sample size provided |
|
| Participants |
Setting: Department of Orthopaedics, Krishna Institute of Medical Sciences, Karad, India Sample size: 30 children Inclusion criteria: children aged 5 to 13 with Gartland 3 supracondylar fracture Exclusion criteria: delayed presentation > 4 days, inability to take part in postoperative rehabilitation, open fractures, medical contraindications to surgery, fracture requiring open reduction or neurovascular exploration, previous ipsilateral elbow fracture, and floating elbow injury |
|
| Interventions |
Group 1 (15 children): manipulation of fracture in theatre with fixation using retrograde wires inserted from the lateral side Group 2 (15 children): manipulation of fracture in theatre with fixation using retrograde wires inserted from the medial and lateral side. A percutaneous technique was used for the medial wire. |
|
| Outcomes | 1) Nerve injury 2) Major complications 3) Cosmetic deformity at 6 months* 4) Range of motion at 6 months* 5) Radiographic deformity at 6 months* |
|
| Notes |
Loss to follow‐up: none reported *insufficient information to combine results in meta‐analysis Funding source: not stated Declarations: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "prospective comparative randomized controlled trial" Comment: technique for delivering random sequence was not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: unclear if any concealment was performed of the allocation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Surgery was performed by a senior orthopedic surgeon who was well trained in this technique" Comment: unclear who provided the treatments and if they were involved as part of the study |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: unclear who assessed the outcomes and if they were blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no reported loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Comment: no published protocol or prospective trial registration was identified. Mayo elbow score mentioned in abstract but not in body of report. Insufficient information to combine functional and radiographic outcomes in analysis |
| Other bias | Low risk | None identified |
Pavone 2016.
| Study characteristics | ||
| Methods |
Design: randomised trial Study duration: May 2005 to December 2012 Power calculation: no prospective calculation of sample size |
|
| Participants |
Setting: Orthopedic Clinic of the University of Catania, Catania, Italy Sample size: 35 children Inclusion criteria: children (age not specified in inclusion criteria) with displaced supracondylar fractures Exclusion criteria: open fractures, adolescents, multiple bone fractures, admission to intensive care |
|
| Interventions |
Group 1 (13 children): manipulation of fracture in theatre with fixation using retrograde wires inserted from the lateral side Group 2 (22 children): manipulation of fracture in theatre with fixation using retrograde wires inserted from the medial and lateral side. The technique for the medial wire was not specified. |
|
| Outcomes | 1) Functional outcome (Mayo Elbow Performance Score at 13 to 67 months) 2) Nerve injury 3) Major complications 4) Cosmetic deformity (loss of carrying angle and cubitus varus at 13 to 67 months) 5) Return to sport and normal activities at 13 to 67 months 6) Radiographic deformity (loss of Baumann's angle at 13 to 67 months) |
|
| Notes |
Loss to follow‐up: none reported Funding source: not stated Declarations: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “The children, belonging to this study, were randomly submitted to two different wires techniques “…“Twenty‐nine patients with displaced (Gartland type III) extension type supracondylar fractures of the humerus randomly treated by closed reduction and percutaneous fixation with crossed medial‐lateral pins or two lateral pins” Comment: unclear how randomisation was performed |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient detail on allocation concealment to assess |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: it is not clear who performed the interventions and their relationship to the study team. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: it is not clear who performed the outcomes assessment and their relationship to the study team. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no reported loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Comment: no published protocol or prospective trial registration was identified. |
| Other bias | Low risk | None identified |
Prashant 2016.
| Study characteristics | ||
| Methods |
Design: randomised trial Study duration: not stated Power calculation: no prospective calculation of sample size |
|
| Participants |
Setting: Department of Orthopaedics, Gauhati Medical College and Hospital, Guwahati, Assam, India Sample size: 76 children Inclusion criteria: children aged 3 to 12 with a Gartland 3 supracondylar fracture Exclusion criteria: open fractures, delayed presentation > 4 days, neurovascular injury, contraindications to surgery, fracture requiring open reduction, previous ipsilateral elbow fracture, and floating elbow injury |
|
| Interventions |
Group 1 (original number of children not stated): manipulation of fracture in theatre with fixation using retrograde wires inserted from the lateral side Group 2 (original number of children not stated): manipulation of fracture in theatre with fixation using retrograde wires inserted from the medial and lateral side. A percutaneous technique was used for the medial wire. |
|
| Outcomes | 1) Nerve injury 2) Major complications (pin site infection) 3) Cosmetic deformity (loss of carrying angle at a minimum of 6 months) 4) Range of motion (loss of total range of motion at a minimum of 6 months) 5) Radiographic deformity (loss of Baumann's angle, loss of Baumann's angle > 6 degrees loss of metaphyseal‐diaphyseal angle) |
|
| Notes |
Loss to follow‐up: 14 children excluded following randomisation. It is unclear to which groups these children were initially assigned but the groups appeared balanced. Funding source: not stated Declarations: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The method of patient selection for lateral entry or medial‐lateral entry was random, using a computer generated randomization table" |
| Allocation concealment (selection bias) | Unclear risk | Comment: unclear if any concealment of the allocation sequence was performed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: unclear who provided the treatments and if they were involved as part of the study |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "We conducted a prospective, single‐blinded randomized control trial" Comment: unclear who assessed the outcomes and if they were blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 14 (18.4%) loss to follow‐up that seem to be balanced between the groups |
| Selective reporting (reporting bias) | Unclear risk | Comment: no published protocol or prospective trial registration was identified |
| Other bias | Low risk | None identified |
Raj 2018.
| Study characteristics | ||
| Methods |
Design: randomised trial Study duration: May 2015 to September 2017 Power calculation: no prospective calculation of sample size |
|
| Participants |
Setting: Rajiv Gandhi Government General Hospital, Chennai, India Sample size: 20 children Inclusion criteria: children aged 0 to 15 with displaced Gartland 3 supracondylar fracture Exclusion criteria: neurovascular injuries, previous elbow injury |
|
| Interventions |
Group 1 (10 children): manipulation of fracture in theatre with fixation using retrograde wires inserted from the lateral side Group 2 (10 children): manipulation of fracture in theatre with fixation using retrograde wires inserted from the medial and lateral side. The medial wire technique was not specified. |
|
| Outcomes | 1) Nerve injury 2) Major complication (pin site infections) 3) Cosmetic deformity (carrying angle loss at 6 months) 4) Range of motion (extension, flexion, and total range of motion at 6 months) 5) Radiographic deformity (loss of reduction and change in Baumann's angle at 6 months) |
|
| Notes | Report available as thesis only. No peer‐reviewed paper identified Loss to follow‐up: none reported Funding source: not stated Declarations: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Technique of Kirschner wire fixation of the fracture was allocated to the patients randomly” Comment: unclear how randomisation was performed |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient detail on allocation concealment to assess |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: it is not clear who performed the interventions and their relationship to the study team. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: it is not clear who performed the outcomes assessment and their relationship to the study team. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no reported loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Comment: no published protocol or prospective trial registration was identified. |
| Other bias | High risk | Comment: rate of open reduction 90% in crossed wires group and 20% in lateral wires group. This difference in open reduction rates may have an impact on outcomes. |
Rakha 2020.
| Study characteristics | ||
| Methods |
Design: randomised trial Study duration: March 2018 to September 2018 Power calculation: power of study at 80% and level of significance of 5% |
|
| Participants |
Setting: Department of Orthopaedics, Allied Hospital, Faisalabad, Pakistan Sample size: non‐probability consecutive sampling 130 participants Inclusion criteria: either gender ranging from 5 to 12 years with supracondylar Gartland type III fracture diagnosed on X‐ray within one week of fracture Exclusion criteria: open or closed Gartland type III fracture associated with neurovascular injury, previous fracture in the same elbow or associated ipsilateral fractures |
|
| Interventions |
Group 1 (65 children): open reduction and fixation with K‐wires in crossed pattern Group 2 (65 children): closed reduction using image intensifier and fixation with K‐wires in crossed pattern |
|
| Outcomes | 1) Assessment using Flynn's criteria | |
| Notes |
Loss to follow‐up: not applicable as the study reported no review outcomes Funding source: not reported Declarations: study authors declare no conflicts of interest Notes: we did not conduct risk of bias assessment because we were unable to include outcome data in the review |
|
Ray 2019.
| Study characteristics | ||
| Methods |
Design: randomised trial Study duration: August 2016 to January 2018 Power calculation: no prospective calculation of sample size provided. |
|
| Participants |
Setting: Department of Orthopaedics, Regional Institute of Medical Sciences (RIMS), Imphal, India Sample size: 70 children Inclusion criteria: children aged 2 to 15 with extension type Gartland 3 supracondylar fracture Exclusion criteria: delayed presentation > 14 days, open fractures, associated neurovascular impairment following fracture, ipsilateral fracture of distal humerus or elbow joint |
|
| Interventions |
Group 1 (35 children): closed manipulation of fracture in theatre with fixation using crossed wires inserted from medial and lateral side. A percutaneous technique was used for the medial wire. Group 2 (35 children): medial approach open reduction of fracture in theatre with fixation using crossed wires inserted from the medial and lateral side |
|
| Outcomes | 1) Nerve injury 2) Major complications (pin site infection) 3) Cosmetic deformity (loss of carrying angle at 12 months) 4) Range of movement (loss of total range of movement at 6 and 12 months) 5) Radiographic deformity (loss of Baumann's angle at 12 months) |
|
| Notes |
Loss to follow‐up: none reported Funding source: not stated Declarations: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients fulfilling the eligibility criteria are included in the study. Restricted randomization is done using block size of 4. Each eligible patient in a block fulfilling recruitment criteria is assigned to either Group 1 or Group 2 using lottery method" Comment: unclear exactly how randomisation was performed but there seems to be a random component to allocation |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient detail on allocation concealment to assess |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: it is not clear who performed the interventions and their relationship to the study team |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: it is not clear who performed the outcomes assessment and their relationship to the study team |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no reported loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Comment: no published protocol or prospective trial registration was identified |
| Other bias | Low risk | None identified |
Rizk 2019.
| Study characteristics | ||
| Methods |
Design: randomised trial Study duration: February 2014 to October 2017 Power calculation: no prospective calculation of sample size provided. Sample of 50 children selected but not justified |
|
| Participants |
Setting: Department of Orthopaedics and Traumatology, Faculty of Medicine, Benha University, Benha, Egypt Sample size: 50 children Inclusion criteria: children (age not specified in inclusion criteria) with Gartland 3 supracondylar fractures Exclusion criteria: open fractures, patients with associated ipsilateral arm or forearm fractures, patients who required open reduction, and neurovascular injury |
|
| Interventions |
Group 1 (25 children): manipulation of fracture in theatre with fixation using two retrograde wires and two antegrade wires inserted from the lateral side Group 2 (25 children): manipulation of fracture in theatre with fixation using two retrograde wires inserted from the medial and lateral side using a percutaneous technique for the medial wire |
|
| Outcomes | 1) Nerve injury 2) Major complications 3) Cosmesis (carrying angle at 12 to 40 months) 4) Range of motion at 12 to 40 months' follow‐up 5) Radiographic deformity (loss of Baumann's angle and humeral‐capitellar angle at 12 to 40 months' follow‐up) |
|
| Notes |
Loss to follow‐up: none reported Funding source: none Declarations: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “This is a prospective randomized control study” Comment: no further information on randomisation technique provided |
| Allocation concealment (selection bias) | Unclear risk | Quote: “This is a prospective randomized control study” Comment: no further information on allocation concealment technique provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: unclear who provided the treatments and if they were involved as part of the study |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: unclear who assessed the outcomes and if they were blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no reported loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Comment: no published protocol or prospective trial registration was identified |
| Other bias | Low risk | None identified |
Sadek 2018.
| Study characteristics | ||
| Methods |
Design: prospective randomised study Study duration: June 2015 to January 2016 Power calculation: no prospective calculation of sample size. |
|
| Participants |
Setting: Orthopaedic Department, Sohag University Hospital, Egypt Sample size: 40 children Inclusion criteria: children (age not specified in inclusion criteria) with Gartland 2 or 3 supracondylar fracture Exclusion criteria: previous ipsilateral fracture, polytrauma, or neurovascular injury |
|
| Interventions |
Group 1 (20 children): manipulation of fracture in theatre with fixation using one retrograde wire and one antegrade wire inserted from the lateral side (Dorgan wires) Group 2 (20 children): manipulation of fracture in theatre with fixation using retrograde wires inserted from the lateral side |
|
| Outcomes | 1) Nerve injuries 2) Major complications (pin site infections) 3) Cosmetic deformity (loss of carrying angle at 23 to 29 weeks) 4) Range of movement (loss of range of movement at 23 to 29 weeks) 5) Radiographic deformity (absolute Baumann's angle, humeral‐capitellar angle and metaphyseal‐diaphyseal angle at 23 to 29 weeks)* |
|
| Notes | *Outcome 5 not reported in sufficient detail to include in analysis Loss to follow‐up: none reported Funding source: not stated Declarations: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “40 children were treated for type II and type III supracondylar fracture in humerus. [C]hildren divided randomly into two groups, group A and B” Comment: unclear how randomisation was performed |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient detail on allocation concealment to assess |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: it is not clear who performed the interventions and their relationship to the study team |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: it is not clear who performed the outcomes assessment and their relationship to the study team |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no reported loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Comment: no published protocol or prospective trial registration was identified |
| Other bias | Low risk | None identified |
Saeed 2020.
| Study characteristics | ||
| Methods |
Design: randomised study Study duration: June 2014 to June 2017 Power calculation: to detect a 17.42% decrease in rates of pin site infections with 80% power, a sample size of 40 per group was identified |
|
| Participants |
Setting: Allied Hospital/Faisalabad Medical University, Faisalabad, Pakistan Inclusion criteria: children with supracondylar fracture Exclusion criteria: none specified in manuscript |
|
| Interventions |
Group 1: (40 children): open reduction of fracture through a medial approach followed by retrograde wires inserted from medial and lateral sides in a non‐buried configuration Group 2: (40 children): open reduction of fracture through a medial approach followed by retrograde wires inserted from medial and lateral sides in a buried configuration Both groups were immobilised for 2 weeks in a posterior above‐elbow backslab with removal of wires at 4 weeks. It was not specified if wires were removed in clinic or if they were removed following a second anaesthetic in theatre. |
|
| Outcomes |
Outcomes included in this review: 1) Major complications: pin site infection by week 4 |
|
| Notes |
Loss to follow‐up: none reported Funding source: not stated Declarations: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “All the patients were randomly divided into 2 groups by using computer generated random number” |
| Allocation concealment (selection bias) | Unclear risk | Comment: unclear how randomisation was performed and what techniques were used to provide allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: unclear who provided the treatments and if they were involved as part of the study |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: it is not clear who performed the outcomes assessment and their relationship to the study team. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no reported loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Comment: no published protocol or prospective trial registration was identified |
| Other bias | Low risk | None identified |
Said 2015.
| Study characteristics | ||
| Methods |
Design: randomised trial Study duration: June 2012 to May 2013 Power calculation: no prospective calculation of sample size |
|
| Participants |
Setting: Department of Orthopedics surgery, Qena University Hospital, Qena, Egypt Sample size: 44 children Inclusion criteria: children aged 2 to 12 with extension type Gartland supracondylar fracture Exclusion criteria: bilateral fracture, ipsilateral or previous limb injury, open fracture, failed closed reduction, neurovascular injury |
|
| Interventions |
Group 1 (not specified how many children were allocated to this group): manipulation of fracture in theatre with fixation using retrograde wires inserted from the lateral side Group 2 (not specified how many children were allocated to this group): manipulation of fracture in theatre with fixation using retrograde wires inserted from the medial and lateral side. A percutaneous technique was used for the medial wire. |
|
| Outcomes | 1) Nerve injury 2) Major complications (pin site infections) 3) Cosmetic deformity (loss of carrying angle at 3 months) 4) Range of motion (flexion and extension loss and total range of movement at 3 months) 5) Return to sport and normal activities at 3 months 6) Radiographic deformity (loss of reduction and loss of Baumann's angle at 3 months) |
|
| Notes |
Loss to follow‐up: 4 (9.1%) children were lost. It is not specified to which groups these children were originally allocated but the final groups appear balanced. Funding source: not stated Declarations: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Patients were randomly selected by drawing lots with even numbers included in‐group A (two parallel lateral wires) and odd numbers in group B (medial and lateral wires).” |
| Allocation concealment (selection bias) | Unclear risk | Comment: unclear if there was a fixed number of lots available to draw from which may have been predicted by study staff |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: it is not clear who performed the interventions and their relationship to the study team. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: it is not clear who performed the outcomes assessment and their relationship to the study team. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “44 children were treated for completely displaced type III supracondylar fracture humerus. 4 children were excluded from the study due to loss for follow up” Comment: unclear to which groups the four children lost to follow‐up had been allocated but the final groups appear balanced |
| Selective reporting (reporting bias) | Unclear risk | Comment: no published protocol or prospective trial registration was identified |
| Other bias | Low risk | None identified |
Sankar 2019.
| Study characteristics | ||
| Methods |
Design: randomised trial Study duration: July 2011 to October 2012 Power calculation: no prospective calculation of sample size |
|
| Participants |
Setting: Department of Orthopaedics, Government Medical College, Kottayam, India Sample size: 66 children Inclusion criteria: children aged 3 to 12 with Gartland 3 supracondylar fracture Exclusion criteria: delayed presentation > 4 days, previous elbow fracture, inability to perform neurological evaluation, floating elbow fracture |
|
| Interventions |
Group 1 (not specified how many children were allocated to this group): posterior approach open reduction of fracture in theatre with fixation using retrograde wires inserted from the lateral side Group 2 (not specified how many children were allocated to this group): posterior approach open reduction of fracture in theatre with fixation using retrograde wires inserted from the medial and lateral side. |
|
| Outcomes | 1) Nerve injury 2) Major complications (wound infection and vascular injuries) 3) Cosmetic deformity (loss of carrying angle at 6 months)* 4) Range of movement (total range of movement at 3 and 6 months and loss of flexion and extension at 6 months)* |
|
| Notes | * Outcomes 3 and 4 combined to form Flynn classification and not reported separately in study Loss to follow‐up: 6 (9.1%) children were lost. It is not specified to which groups these children were originally allocated but the final groups appear balanced. Funding source: none Declarations: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Patients were randomly selected by drawing lots with even numbers included in Group A (lateral entry) and odd number in Group B (medial and lateral entry)” |
| Allocation concealment (selection bias) | Unclear risk | Comment: unclear if there was a fixed number of lots that could be drawn that would allow study staff to predict likelihood of next treatment allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: it is not clear who performed the interventions and their relationship to the study team. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: it is not clear who performed the outcomes assessment and their relationship to the study team. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: “During the study period 66 patients were treated for completely displaced supracondylar fractures of humerus in children. Six patients were excluded from the study due to loss of follow up” Comment: unclear to which groups the 6 children lost to follow‐up belonged |
| Selective reporting (reporting bias) | Unclear risk | Comment: no published protocol or prospective trial registration was identified |
| Other bias | Low risk | None identified |
Shafi‐Ur‐Rehman 2013.
| Study characteristics | ||
| Methods |
Design: randomised trial Study duration: June 2011 to Febuary 2012 Power calculation: no prospective calculation of sample size provided |
|
| Participants |
Setting: Department of Orthopedic Surgery, Shaikh Zayed Hospital, Postgraduate Medical Institute (PGMI), Lahore, Pakistan Sample size: 200 children Inclusion criteria: children aged 3 to 10 with Gartland 2 or 3 supracondylar fractures Exclusion criteria: delayed presentation > 7 days, neurovascular injury, open fractures, ipsilateral forearm fractures |
|
| Interventions |
Group 1 (100 children): manipulation of fracture in theatre with fixation using retrograde wires inserted from the lateral side Group 2 (100 children): manipulation of fracture in theatre with fixation using retrograde wires inserted from the medial and lateral side. It was not specified if an open or percutaneous technique was used for the medial wire. |
|
| Outcomes | 1) Range of movement at 3 months' follow‐up 2) Radiographic deformity (loss of reduction) |
|
| Notes | Radiographic deformity measured at 3 weeks Range of movement expressed as excellent (0 to 5 degrees loss of total range of movement) or not excellent Loss to follow‐up: none reported Funding source: not stated Declarations: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: technique for delivering random sequence was not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: unclear if any concealment of the allocation sequence was performed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: unclear who provided the treatments and if they were involved as part of the study |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: unclear who undertook the outcome assessment and if they were blinded to treatment allocations |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no reported loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Comment: no published protocol or prospective trial registration was identified |
| Other bias | Unclear risk | During our search, we identified that a second study has also been published with identical group sizes, demographics, and outcomes, but from different hospitals and inclusion periods (Shah 2013). Although we believed that Shafi‐Ur‐Rehman 2013 was the original study, we could not rule out the possibility that it was in fact conducted by different study authors. |
Shah 2017.
| Study characteristics | ||
| Methods |
Design: randomised trial Study duration: August 2011 to July 2013 Power calculation: no prospective calculation of sample size |
|
| Participants |
Setting: Department of Orthopaedics, Khaja Bandanawaz Institute of Medical Science (KBNIMS), Kalaburagi, Karnataka, India Sample size: 20 children Inclusion criteria: children aged 1 to 14 with Gartland 3 supracondylar fractures Exclusion criteria: nil |
|
| Interventions |
Group 1 (10 children): manipulation of fracture in theatre and immobilisation in above‐elbow plaster of Paris backslab Group 2 (10 children): open reduction of fracture in theatre with fixation using retrograde wires inserted from the medial and lateral side. A percutaneous technique was used for the medial wire. |
|
| Outcomes | 1) Nerve injuries 2) Major complications (vascular injury and pin site infections) 3) Cosmetic deformity (loss of carrying angle, cubitus varus and cubitus valgus at 6 to 12 months) 4) Range of movement (loss of total range of movement at 6 to 12 months) |
|
| Notes |
Loss to follow‐up: none reported Funding source: not stated Declarations: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “20 type‐III fractures were taken for comparative study. In a random manner, two equal groups were drawn” Comment: unclear how randomisation was performed |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient detail on allocation concealment to assess |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: it is not clear who performed the interventions and their relationship to the study team. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: it is not clear who performed the outcomes assessment and their relationship to the study team. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no reported loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Comment: no published protocol or prospective trial registration was identified. |
| Other bias | Low risk | None identified |
Shamma 2020.
| Study characteristics | ||
| Methods |
Design: randomised trial Study duration: March 2019 to November 2019 Power calculation: no prospective calculation of sample size provided |
|
| Participants |
Setting: Department of Orthopedic Surgery, Faculty of Medicine, Al‐Azhar University, Cairo, Egypt Sample size: 60 children Inclusion criteria: children aged 0 to 10 with Gartland 2 or 3 supracondylar fracture Exclusion criteria: open fractures, fractures with neurovascular injury, fractures with compartmental syndrome, |
|
| Interventions |
Group 1 (15 children): manipulation of fracture in theatre with fixation using retrograde wires inserted from the lateral side in a parallel configuration Group 2 (15 children): manipulation of fracture in theatre with fixation using retrograde wires inserted from the lateral side using a divergent configuration |
|
| Outcomes | 1) Nerve injury 2) Major complications (pin site infections, vascular injuries and compartment syndrome) 3) Cosmetic deformity (loss of carrying angle at 3 months) 4) Range of motion (loss of flexion and extension at 3 months) 5) Radiographic deformity (loss of Baumann's angle at 3 months) |
|
| Notes |
Loss to follow‐up: none reported Funding source: not stated Declarations: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Surgery was planned and technique was selected according to random number generated by computer” |
| Allocation concealment (selection bias) | Unclear risk | Quote: the treatment was “enveloped securely so as to be opened at surgery time” Comment: unclear if envelopes were sequentially labelled to prevent study staff predicting next allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: it is not clear who performed the interventions and their relationship to the study team. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: it is not clear who performed the outcomes assessment and their relationship to the study team. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no reported loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Comment: no published protocol or prospective trial registration was identified. |
| Other bias | Unclear risk | During our search, we identified an identical version of this report, but with a different author team (Hegazy 2020). We assumed that Shamma 2020 was the original publication. However, we could not rule out the possibility that it was in fact conducted by other study authors. |
Subash 2020.
| Study characteristics | ||
| Methods |
Design: randomised study Study duration: January 2012 to December 2015 Power calculation: no prospective calculation of sample size |
|
| Participants |
Setting: Department of Orthopaedics, Saveetha Medical College and Hospital, Thandalam, Chennai, Tamil Nadu, India Inclusion criteria: children with supracondylar fractures Exclusion criteria: open fractures, fractures requiring open reduction, multiple fractures |
|
| Interventions |
Group 1 (unclear how many children allocated to this group): manipulation of fracture in theatre with fixation using retrograde wires inserted from the lateral side Group 2 (unclear how many children allocated to this group): manipulation of fracture in theatre with fixation using retrograde wires inserted from the medial and lateral side. A mini‐open approach was made to protect the ulnar nerve. Children in both groups were immobilised in an arm sling prior to surgical intervention. Following surgery, both groups were immobilised in an above‐elbow plaster backslab with the elbow flexed to 70‐80°. The wires and plaster were removed at 3 weeks. |
|
| Outcomes |
Outcomes included in this review: 1) Nerve injuries 2) Cosmetic deformity measured as loss of carrying angle at 6 months 3) Range of movement measured through total range of motion at 6 months 4) Radiographic deformity measures as loss of humeral‐capitellar angle and Baumann's angle at 6 months Outcomes not included in this review: 1) Minor limitations in function at 6 months – insufficiently‐defined outcome |
|
| Notes | 6 participants lost to follow‐up. The groups for these losses to follow‐up were not specified but the final group size was 29 for group 1 and 31 for group 2. Funding source: not stated Declarations: not stated Additional information received on 25 March 2021 from the corresponding author regarding nerve injuries: “There were no preoperative nerve injuries in the cross pinning group Regards Dr yeshwanth Subash” |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The children were then allocated into 2 groups by flipping a coin method” |
| Allocation concealment (selection bias) | Low risk | Comment: there was no pre‐determined allocation sequence to conceal |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: unclear who provided the treatments and if they were involved as part of the study |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: unclear who undertook the outcome assessment and if they were blinded to treatment allocations |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 6 participants lost to follow‐up. It was not specified to which groups these children were originally allocated. Final groups have some imbalance |
| Selective reporting (reporting bias) | Unclear risk | Comment: no published protocol or prospective trial registration was identified. |
| Other bias | Low risk | None identified |
Tripuraneni 2009.
| Study characteristics | ||
| Methods |
Design: surgeon randomised study Study duration: March 2004 to August 2006 Power calculation: no prospective calculation of sample size provided |
|
| Participants |
Setting: Department of Orthopaedic Surgery, Carrie Tingley Hospital, Albuquerque, New Mexico, USA Sample size: 40 children Inclusion criteria: children (age not specified in inclusion criteria) with a Gartland type 2 or 3 supracondylar fracture Exclusion criteria: revision fixation cases |
|
| Interventions |
Group 1 (20 children): manipulation of fracture in theatre with fixation using retrograde wires inserted from the lateral side Group 2 (20 children): manipulation of fracture in theatre with fixation using retrograde wires inserted from the medial and lateral side. A percutaneous technique was used for the medial wire. |
|
| Outcomes | 1) Nerve injury | |
| Notes | Radiographic deformity and range of motion assessed at 4 and 8 weeks only (early follow‐up) Loss to follow‐up: 7 (17.5%) children did not attend final follow‐up. It is not specified from which groups these 7 children were lost. Funding source: not stated Declarations: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Children were randomized based on which pediatric fellowship‐trained orthopedic surgeon was on trauma call" |
| Allocation concealment (selection bias) | High risk | Comment: day of the week allocation means that study staff could predict allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: unclear who provided the treatments and if they were involved as part of the study |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "All postoperative visits, including radiographic evaluations, were supervised by the attending surgeons" Comment: unclear who undertook the outcome assessment and if they were blinded to treatment allocations |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 7 participants (17.5%) were lost to final follow‐up following pin removal. However, the only extracted outcome was nerve injury, which is unlikely to be impacted by this loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no published protocol or prospective trial registration was identified. |
| Other bias | Low risk | None identified |
Vaidya 2009.
| Study characteristics | ||
| Methods |
Design: randomised trial Study duration: January 2006 to December 2008 Power calculation: no prospective calculation of sample size |
|
| Participants |
Setting: Department of Orthopaedics, Asha Hospital, Bharatpur, Nepal Sample size: 66 children Inclusion criteria: children aged 3 to 12 with Gartland 3 supracondylar fracture Exclusion criteria: delayed presentation > 4 days, previous fracture in the same limb, open fractures, fractures requiring open reduction, floating elbow, and an inability to perform an adequate neurovascular exam |
|
| Interventions |
Group 1 (unclear how many children were initially allocated to this group): manipulation of fracture in theatre with fixation using retrograde wires inserted from the lateral side Group 2 (unclear how many children were initially allocated to this group): manipulation of fracture in theatre with fixation using retrograde wires inserted from the medial and lateral side. A mini‐open technique was used for the medial wire. |
|
| Outcomes | 1) Nerve injury 2) Major complications 3) Cosmetic deformity measured as loss of carrying angle at 6‐month follow‐up 4) Return to function at 6‐month follow‐up 5) Radiographic deformity: loss of Baumann's angle and loss of reduction at 3 months |
|
| Notes |
Loss to follow‐up: 6 children lost from randomisation to follow‐up. It is unclear to which groups these children belonged. Funding source: not stated Declarations: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly selected by drawing lots with even number included in group A and odd number in group B" |
| Allocation concealment (selection bias) | Unclear risk | Comment: unclear if there was a fixed number of lots that could be drawn that would allow study staff to predict likelihood of next treatment allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "surgery was performed by senior orthopaedic surgeon" Comment: unclear who provided the treatments and if they were involved as part of the study |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "Clinical evaluation was performed by senior orthopaedic surgeon" Comment: unclear who undertook the outcome assessment and if they were blinded to treatment allocations |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6 children lost from randomisation to follow‐up. It is unclear to which groups these children belonged. Final groups appear balanced |
| Selective reporting (reporting bias) | Unclear risk | Comment: no published protocol or prospective trial registration was identified. |
| Other bias | Low risk | None identified |
Zhu 2016.
| Study characteristics | ||
| Methods |
Design: randomised trial Study duration: February 2009 to December 2012 Power calculation: no prospective calculation of sample size provided. Sample of 68 children selected but not justified |
|
| Participants |
Setting: Department of Orthopaedic Surgery, Second Affiliated Hospital of Wenzhou Medical College, Wenzhou, China Sample size: 68 children Inclusion criteria: children aged 4 to 12 with unstable Gartland 2 or 3 supracondylar fractures that were displaced > 2 mm in coronal and/or sagittal plane Exclusion criteria: open fractures, neurovascular injuries, fractures and/or dislocation of other parts of the ipsilateral limb, congenital malformation of the ipsilateral elbow joint, additional vital organs damage |
|
| Interventions |
Group 1 (34 children): closed manipulation of fracture in theatre with fixation using two or three retrograde wires Group 2 (34 children): joystick‐assisted (mini‐open) manipulation of fracture in theatre with fixation using two or three retrograde wires |
|
| Outcomes | 1) Nerve injury 2) Major complications 3) Radiographic deformity (varus malunion at a mean of 30 to 33 months' follow‐up) |
|
| Notes |
Loss to follow‐up: no reported loss to follow‐up. However, 5 children who required joystick‐assisted manipulation following attempted closed reduction (group 1) were excluded from the analysis, leaving a per‐protocol analysis only. Funding source: none Declarations: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "type of treatment was chosen at random by drawing from the box containing an equal number of envelopes with either of the two fracture reduction methods" |
| Allocation concealment (selection bias) | High risk | Comment: there was a fixed number of envelopes of both treatments so study staff may have been able to predict allocation for children later in the study. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: unclear who provided the treatments and if they were involved as part of the study |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "Both radiographic and clinical evaluations at last follow‐up were performed by one of the authors (WH) who were not involved in the care of the patients."..."it was impossible to perform blindness to both the surgeon and patients, which might influence the results." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: no reported loss to follow‐up. However, 5 children who required joystick‐assisted manipulation following attempted closed reduction (group 1) were excluded from the analysis, leaving a per‐protocol analysis only. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no published protocol or prospective trial registration was identified. |
| Other bias | Low risk | None identified |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| AboHasan 2020 | Non‐randomised study comparing lateral wires with crossed wires in supracondylar elbow fractures |
| Aktekin 2008 | Closed and open reduction via posterior approach with percutaneous pinning of posteromedial displaced supracondylar humerus fractures in children. We excluded this study because it was a retrospective study with no randomisation. |
| Arif 2014 | RCT, children aged 2 to 11 years with a Gartland 3 supracondylar fracture. We excluded this study because the interventions were not eligible for this review (open reduction through a medial approach with retrograde crossed wire fixation of fracture versus open reduction through a lateral approach with retrograde crossed wire fixation of fracture). |
| Arif 2017 | Non‐randomised study evaluating open reduction and fixation with K‐wires |
| Ariyawatkul 2016 | Retrospective, non‐randomised study to evaluate closed pinning and conservative treatment in supracondylar elbow fractures |
| Arnala 1991 | Non‐randomised study evaluating surgical fixation, traction, and reduction followed by plaster cast |
| Balanescu 2013 | Non‐randomised study evaluating reduction methods in supracondylar fractures |
| Bales 2010 | Non‐randomised study evaluating the effects of treatment (closed reduction and percutaneous pinning) within 21 hours or later than 21 hours after presentation to the urgent treatment centre |
| Bulbul 2011 | Non‐randomised, case series in which all participants were treated with closed reduction and lateral pinning with some additional participants also treated with medial pinning |
| Chen 2001 | RCT, 95 children with Gartland 3 supracondylar fractures. We excluded this study because the study compared two forms of elbow casting (posterior plaster of Paris backslab with the elbow in at least 90° flexion or a posterior and anterior plaster of Paris backslab with the elbow in full extension and supination). |
| Diri 2003 | Non‐randomised study comparing three treatments: closed reduction and cast immobilisation; skeletal traction and cast immobilisation; open reduction and internal fixation with K‐wires |
| Ducic 2016b | Non‐randomised study comparing three treatments: closed reduction with percutaneous pinning; open reduction with K‐wires fixation; closed reduction with cast immobilisation |
| El‐Ngehy 2018 | We excluded this study because it appears to be a duplicate study of Naik 2017 with identical sample size, patient demographics, and outcomes, but from different authors and institution. |
| Ensafdaran 2005 | RCT comparing lateral versus posterior approach for reduction and fixation using two crossed pins. We excluded this study because the comparison group was not eligible for this review. |
| Eren 2005 | Non‐randomised study comparing lateral and medial approaches to pin fixation |
| Fahmy 2009 | Non‐randomised study comparing a single posterior wire with two wires for fixation of supracondylar fractures |
| Hegazy 2020 | We excluded this study because it appears to be a duplicate study of Shamma 2020 with identical sample size, patient demographics, sample x‐rays, and outcomes, but from different authors and institution. |
| Keskin 2014 | Non‐randomised study comparing open reduction with closed reduction and percutaneous pinning in displaced supracondylar fractures |
| Khan 2007 | Non‐randomised study in which supracondylar elbow fractures were treated by closed reduction and percutaneous pinning, either from lateral side only or also from medial side |
| Lee 2008 | Non‐randomised study evaluating efficacy of lateral or parallel pins using 3 K‐wires or Steinmann pins for fixation of supracondylar fractures |
| Li 2013 | No description of randomisation; we assumed that it was most likely to be a non‐randomised study. Compared elbow brace with external fixation |
| Li 2017 | Non‐randomised study comparing bioabsorbable PDLLA pin fixation after open reduction with lateral external fixation after closed reduction |
| Marashi 2013 | Non‐randomised study comparing supination with pronation after cast immobilisation in children with supracondylar fractures |
| NCT00904137 | Trial registration only: full trial failed to recruit sufficient participants and the results of this trial have not been published |
| Pescatori 2012 | Non‐randomised study comparing supine with prone position during percutaneous pinning with K‐wires |
| Rawoot 2014 | RCT, children (age not specified in inclusion criteria) with Gartland 2 or 3 supracondylar fracture. We excluded this study because the interventions were not eligible for this review (reduction and fixation of the fracture using K‐wires in a supine position versus reduction and fixation of the fracture using K‐wires in a prone position). |
| Roy 2019 | Non‐randomised study comparing percutaneous pinning with K‐wires from lateral side and both sides |
| Sadik 2015 | We excluded this study because it appears to be a duplicate study of Maity 2012 with identical sample size, patient demographics, and outcomes, but from different authors and institution. |
| Shah 2013 | We excluded this study because it appears to be a duplicate study of Shafi‐Ur‐Rehman 2013 with identical sample size, patient demographics, and outcomes. |
| Shoaib 2003 | Non‐randomised study; all children with supracondylar fractures were treated with closed reduction and casting |
| Siddiq 2020 | RCT, children aged 2 to 14 years with a Gartland 2 or 3 supracondylar fracture. We excluded this study because the interventions were not eligible for this review (open reduction through a medial approach with retrograde crossed wire fixation of fracture versus open reduction through a lateral approach with retrograde crossed wire fixation of fracture). |
| Silva 2018 | RCT with 100 children with Gartland 1 supracondylar fracture. We excluded this study because it compared two different casting materials which was not an eligible comparison for this review (hard fibreglass cast (3M “Scotchcast” Plus Casting Tape) or a soft fibreglass cast (3M “Scotchcast” Soft Cast Casting Tape)). |
| Sutton 1992 | Non‐randomised study comparing traction with percutaneous pinning in supracondylar fractures |
| Venkatadass 2015 | RCT, children aged 3 to 14 years with Gartland type 3 fractures. We excluded this study because the interventions were not eligible for this review (reduction and fixation of the fracture using K‐wires in a supine position versus reduction and fixation of the fracture using K‐wires in a prone position). |
| Young 2012 | Non‐randomised study comparing crossed wires fixation with skeletal traction |
PDLLA: poly‐D, L‐lactic acid; K‐wires: Kirschner wires; RCT: randomised controlled trial
Characteristics of studies awaiting classification [ordered by study ID]
Afridi 2002.
| Methods | Prospective randomised study |
| Participants | Children with supracondylar fractures of the humerus |
| Interventions | Unknown |
| Outcomes | Unknown |
| Notes | We were unable to source the abstract and full text for this review, and assessed possible eligibility only from the title |
Andreasi 1985.
| Methods | Unknown |
| Participants | Children with supracondylar fractures of the humerus |
| Interventions | Two methods of percutaneous osteosynthesis with Kirschner wire |
| Outcomes | Unknown |
| Notes | We were unable to source the abstract and full text for this review, and assessed possible eligibility only from the title |
Bing 2017.
| Methods | Unknown |
| Participants | Unknown |
| Interventions | Unknown; believed to be 24 children with lateral entry wires |
| Outcomes | Unknown; believed to be 17 children with crossed wires |
| Notes | Identified in 2018 systematic review. We were unable to source the abstract and full text for this review, and assessed possible eligibility only from the title |
Boparai 2006.
| Methods | Unknown |
| Participants | Children with supracondylar fractures |
| Interventions | Closed reduction versus open reduction |
| Outcomes | Unknown |
| Notes | We were unable to source the abstract and full text for this review, and assessed possible eligibility only from the title |
Botchu 2006.
| Methods | Unknown |
| Participants | Children with displaced supracondylar fractures of the humerus |
| Interventions | Unknown |
| Outcomes | Unknown; possibly surgical versus non‐surgical management of displaced fractures |
| Notes | We were unable to source the abstract and full text for this review, and assessed possible eligibility only from the title |
Evans 1998.
| Methods | Randomised clinical trial |
| Participants | Children with supracondylar fractures of the humerus |
| Interventions | Unknown |
| Outcomes | Unknown |
| Notes | We were unable to source the abstract and full text for this review, and assessed possible eligibility only from the title |
He 2009.
| Methods | Possibly randomised |
| Participants | Children with displaced fractures |
| Interventions | Two different forms of external immobilisation |
| Outcomes | Cosmetic deformity, range of motion |
| Notes | We could not determine from the translated manuscript whether the study is randomised. Attempts to contact the study authors for clarification were unsuccessful |
Lu 2011.
| Methods | Unknown |
| Participants | Unknown |
| Interventions | Unknown; believed to be children with lateral entry wires |
| Outcomes | Unknown; believed to be children with crossed wires |
| Notes | Identified in 2018 systematic review. We were unable to source the abstract and full text for this review, and assessed possible eligibility only from the title |
NCT04582123.
| Methods | Randomised controlled trial |
| Participants | Estimated enrolment: 45 Inclusion criteria: children with closed extension Type III of supracondylar fractures of humerus Exclusion criteria: Type I and Type II injuries, flexion type injuries, open fractures, > 12 years of age |
| Interventions | Fixation of supracondylar humerus fractures with 2 crossed pins and 3 crossed pins |
| Outcomes | Comparison of Flynn's grade |
| Notes | Study is listed as complete in the clinical trials register; however, results are not included in the register. We await publication of the full text. |
Sapkota 2019.
| Methods | Possibly randomised |
| Participants | Children with Gartland 3 fractures |
| Interventions | Retrograde lateral wires versus retrograde crossed wires |
| Outcomes | Nerve injury, cosmetic deformity, range of motion, radiographic deformity |
| Notes | We could not determine whether the study was randomised. We attempted to contact the study authors but this was unsuccessful. |
Characteristics of ongoing studies [ordered by study ID]
ACTRN12612000480886.
| Study name | Study of treatment methods for undisplaced supracondylar humeral (elbow) fractures in children |
| Methods | Randomised trial |
| Participants | 180 children aged 3 to 8 years with Gartland 1 supracondylar fractures |
| Interventions | Collar and cuff at 120° versus collar and cuff at 90° versus posterior plaster slab at 90° |
| Outcomes | i) Pain ii) Functional outcome using Activity Scale for Kids performance version (ASK‐P) score |
| Starting date | 1 August 2012 |
| Contact information | Jemma Bates‐Smith: Jemma.Bates‐Smith@health.wa.gov.au |
| Notes |
CTRI/2020/06/025504.
| Study name | Comparison of short term functional and radiological outcomes between two versus three lateral pin fixation techniques in fractures of lower part on arm bone in children |
| Methods | Randomised trial |
| Participants | Children with Gartland 3 supracondylar fractures |
| Interventions | Fixation with two or three lateral wires |
| Outcomes | i) Flynn grade |
| Starting date | Not specified |
| Contact information | Not specified |
| Notes |
PACTR201702001960109.
| Study name | Comparison between crossing wires versus two lateral wires in the management of displaced supracondylar humerus in children |
| Methods | Prospective RCT using coin toss for allocation |
| Participants | Inclusion criteria: children aged 2 to 9 years with Gartland 2 to 3 supracondylar fracture Exclusion criteria: open fractures, failed closed reduction, floating elbow, failure to perform preoperative neurovascular assessment, neurovascular injury requiring surgical exploration |
| Interventions | Group I: manipulation of fracture in theatre with fixation using retrograde wires inserted from the lateral side Group 2: manipulation of fracture in theatre with fixation using retrograde wires inserted from the medial and lateral side. |
| Outcomes | Radiological outcomes; grading of the clinical outcomes No additional details |
| Starting date | 6 January 2016 |
| Contact information | Hesham Abdelsadek, Professor in Orthopedic Department, Suez Canal University Hospital heshamsadeq58@yahoo.com |
| Notes |
RCT: randomised controlled trial
Differences between protocol and review
Types of interventions: we changed the order of these interventions. During the review process, we noted a higher research interest in types of surgical interventions and judged that this should be given a higher priority in the review.
Types of outcomes: we had not explicitly specified measurements for cosmetic deformity (when measured as carrying angle), range of motion, and radiographic deformity through the measurement of angles. We included change scores for these three measurements rather than absolute values. This is because these three outcomes have a range of normal values that demonstrate considerable personal variation. We judged that differences in the loss of carrying angle, loss of range of motion, and loss of radiographic angle would be more valid than differences in mean absolute values. We also set a threshold for the dichotomising of loss of carrying angle and range of motion. For this, we set a threshold of 10° as a value that could be reliably measured using a goniometer and may have a bearing on outcome for children. We could not find any literature to identify a minimal clinically important difference for these outcomes in children, and would suggest clinical correlation of these outcomes is a priority for research. In addition, we reordered the 'other outcomes' according to interventions which we believed had more clinical relevance.
Subgroup analysis and investigation of heterogeneity: we conducted an additional subgroup analysis for the use of retrograde wires. We found a clinical heterogeneity in the technique used for the placement of the medial wire in a crossed K‐wire configuration which, we felt, was important to explore and analyse as a potential contributor to nerve injury and rates of major complications.
Sensitivity analysis: we conducted two additional sensitivity analyses because we could not rule out the possibility of compromised data integrity: we found a large number of eligible studies that were published in journals not indexed in PubMed, and we found some studies that were published in duplicate by other author teams. We also clarified that sensitivity analyses were limited to the critical outcomes reported in the summary of findings tables.
Summary of findings and assessment of the certainty of the evidence: we provided additional information in this section of the Methods, including a decision to limit the summary of findings tables only to those that we judged to be most clinically relevant.
Contributions of authors
Ben Marson, Simon Craxford, Kathryn Price, and Benjamin Ollivere all conceived the review and designed the protocol. Ben Marson prepared the first draft. Ben Marson, Simon Craxford, and Adeel Ikram performed data extraction and risk of bias assessments. Benjamin Ollivere is the guarantor of the protocol.
Sources of support
Internal sources
-
The University of Nottingham, UK
Host institution for Ben Marson, Simon Craxford and Benjamin Ollivere
-
Nottingham University Hospitals NHS Trust, UK
Host institution for Kathryn Price
External sources
-
National Institute for Health Research (NIHR), UK
Ben Marson is funded by a National Institute for Health Research (NIHR), Doctoral Fellowship NIHR300240
Declarations of interest
Ben Marson: none known Adeel Ikram: none known Simon Craxford: none known Kathryn Price: none known Benjamin Ollivere: none known
New
References
References to studies included in this review
Abdel Karim 2016 {published data only (unpublished sought but not used)}
- Abdel Karim M, Hosny A, Nasef Abdelatif NM, Hegazy MM, Awadallah WR, Khaled SA, et al. Crossed wires versus 2 lateral wires in management of supracondylar fracture of the humerus in children in the hands of junior trainees. Journal of Orthopaedic Trauma 2016;30(4):e123-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Hosney A, Abdel Karim M, Mohamadi H. Crossed wires versus two lateral wires in management of supracondylar fracture of the humerus in children. In: American Academy of Orthopaedic Surgeons Annual Meeting: 2014 Mar 11-15; New Orleans (LA). 2014.
Abubeih 2019 {published and unpublished data}
- Abubeih HM, El-Adly W, El-Gaafary K, Bakr H. Percutaneous cross-pinning versus two lateral entry pinning in Gartland type III pediatric supracondylar humerus fractures. Egyptian Orthopaedic Journal 2019;54:52-61. [Google Scholar]
- Marson BA. [personal communication]. Email to: HM Abdullah 28 November 2020.
Afaque 2020 {published data only}
- Afaque SF, Singh A, Maharjan R, Ranjan R, Panda AK, Mishra A. Comparison of clinic-radiological outcome of cross pinning versus lateral pinning for displaced supracondylar fracture of humerus in children: a randomized controlled trial. Journal of Clinical Orthopaedics and Trauma 2020;11(2):259-63. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Aher 2018 {published data only}
- Aher D, Mishra RK, Gohiya A. Comparative study of two techniques of percutaneous pinning of displaced supracondylar humerus fracture. Orthopaedic Journal of M.P. Chapter 2018;24(1):3-7. [Google Scholar]
Ahmad 2020 {published data only}
- Ahmad MM, Hussain HU, Raza MM, Ahmad S, Iqbal AM, Iqbal U. Comparison of two methods of percutaneous pin fixation in displaced supracondylar fractures of the humerus in children. Medical Journal of South Punjab 2020;1(1):31-5. [Google Scholar]
Ajmera 2013 {published data only}
- Ajmera A, Mukherjee A, Bhargava P, Katare BS, Singh A. Comparison of the percutaneous versus open reduction for displased supracondylar humerus fractures in children. Orthopaedic Journal of MP Chapter 2013;19(2):29-35. [Google Scholar]
Altay 2011 {published data only}
- Altay MA, Erturk C, Altay M, Belhan O, Isikan UE. Ultrasonographic examination of the radial and ulnar nerves after percutaneous cross-wiring of supracondylar humerus fractures in children: a prospective, randomized controlled study. European Journal of Trauma and Emergency Surgery 2011;37(Suppl 1):S101. [DOI] [PubMed] [Google Scholar]
- Altay MA, Erturk C, Altay M, Belhan O, Isikan UE. Ultrasonographic examination of the radial and ulnar nerves after percutaneous cross-wiring of supracondylar humerus fractures in children: a prospective, randomized controlled study. Journal of Pediatric Orthopaedics. Part B 2011;20(5):334-40. [PMID: ] [DOI] [PubMed] [Google Scholar]
Anwar 2011 {published data only}
- Anwar W, Rahman N, Iqbal MJ, Khan MA. Comparison of the two methods of percutaneous K-wire fixation in displaced supracondylar fracture of humerus in children. Journal of Postgraduate Medical Institute 2011;25(4):356-61. [Google Scholar]
Arun 2018 {published data only}
- Arun KN, Ramachandra K, Veerabhadra J, Suman NV, Paila SP, Nagadurga N. A prospective study of crossed versus lateral pinning for displaced extension-type supracondylar fractures of humerus. International Journal of Orthopaedics Sciences 2018;4(4):737-40. [Google Scholar]
Das 2019 {published data only}
- Das S, Santra S, Rahman M, Kar R. The clinical and radiological outcome of percutaneous pinning, in displaced supracondylar fracture of the humerus of children. Journal of the Indian Medical Association 2019;117(10):36-7. [Google Scholar]
Dawood 2011 {published data only}
- Dawood WF. Medial-lateral internal pin fixation compared to 2-lateral internal pin fixation in treatment of completely displaced supracondylar fractures of the humerus in children. Medical Journal of Tikrit University 2011;2(172):61-6. [Google Scholar]
Dehghan 2012 {published data only (unpublished sought but not used)}
- Dehghan M, Bahmani MT. The comparison of two surgical methods of close fixation with pin and open fixation for treatment of supracondylar fracture in under 10 years old patients. Yafte 2012;14(3):67-74. [Google Scholar]
- IRCT201105146480N1. The comparison of two surgery methods for treatment of supracondylar fracture in children under 10 years old [The comparison of two surgery methods for treatment of supracondylar fracture in children under 10 years old in Ayatollah Kashani hospital]. en.irct.ir/trial/6916 (first received 6 November 2011).
Ducic 2016a {published data only}
- Ducic S, Radlovic V, Bukva B, Radojicic Z, Vrgoc G, Brkic I, et al. A prospective randomised non-blinded comparison of conventional and Dorgan's crossed pins for paediatric supracondylar humeral fractures. Injury 2016;47(11):2479-83. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ercin 2016 {published data only}
- Ercin E, Bilgili MG, Baca E, Basaran SH, Bayrak A, Kural C, et al. Medial mini-open versus percutaneous pin fixation for type III supracondylar fractures in children. Turkish Journal of Trauma and Emergency Surgery 2016;22(4):350-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Foead 2004 {published data only}
- Foead A, Penafort R, Saw A, Sengupta S. Comparison of two methods of percutaneous pin fixation in displaced supracondylar fractures of the humerus in children. Journal of Orthopaedic Surgery (Hong Kong) 2004;12(1):76-82. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gaston 2010 {published and unpublished data}
- Gaston RG, Cates TB, Blanco JC. Crossed versus lateral-entry pin fixation of type 3 supracondylar fractures in children. In: American Academy of Orthopaedic Surgeons Annual Meeting; 2007 Feb 14-18; San Diego (CA). 2007.
- Gaston RG, Cates TB, Devito D, Schmitz M, Schrader T, Busch M, et al. Medial and lateral pin versus lateral-entry pin fixation for type 3 supracondylar fractures in children: a prospective, surgeon-randomized study. Journal of Pediatric Orthopaedics 2010;30(8):799-806. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Marson BA. [personal communication]. Email to: G Gaston 26 November 2020.
Gholap 2020 {published data only}
- Gholap A, Wokhlu A, Gholap P. Gartlands type III supracondylar humerus fractures - cross k-wiring versus lateral k-wiring: a preliminary study. International Journal of Orthopaedics Sciences 2020;6(1):1259-62. [Google Scholar]
Gopinathan 2018 {published and unpublished data}
- Gopinathan NR, Sajid M, Sudesh P, Behera P. Outcome analysis of lateral pinning for displaced supracondylar fractures in children using three Kirschner wires in parallel and divergent configuration. Indian Journal of Orthopaedics 2018;52(5):554-60. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marson BA. [personal communication]. Email to: P Behera 18 September 2020.
Jain 2019 {published data only}
- Jain S, Agrawal S, Banshiwal RC. Comparative study of posterior intrafocal with lateral pinning versus cross pinning for extension type supracondylar fracture humerus in children. National Journal of Clinical Orthopaedics 2019;3(1):134-9. [Google Scholar]
Kaewpornsawan 2001 {published data only}
- Kaewpornsawan K. Comparison between closed reduction with percutaneous pinning and open reduction with pinning in children with closed totally displaced supracondylar humeral fractures: a randomized controlled trial. Journal of Pediatric Orthopaedics. Part B 2001;10(2):131-7. [PMID: ] [PubMed] [Google Scholar]
Kalia 2018 {published data only}
- Kalia G, Singh N. Prospective randomised comparison study of 60 cases of type 2 and type 3 supracondylar fractures of humerus in children using conventional cross K wire fixation vs Dorgan's lateral fixation. Indian Journal of Applied Research 2018;8(7):70-1. [Google Scholar]
Kocher 2007 {published data only}
- Kocher MS, Kasser JR, Waters PM, Bae D, Snyder BD, Hresko MT, et al. Lateral entry compared with medial and lateral entry pin fixation for completely displaced supracondylar humeral fractures in children: a randomized clinical trial. Journal of Bone and Joint Surgery - Series A 2007;89(4):706-12. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Mininder SK, Waters PM, Kasser JR, Hresko MT, Bae DS, Hedequist DJ, et al. Lateral vs medial/lateral pinning of type 3 supracondylar humerus fractures in children: an RCT. In: American Academy of Orthopaedic Surgeons Annual Meeting; 2006 Mar 22-26; Chicago (IL). 2006.
- Yen YM, Kocher MS. Lateral entry compared with medial and lateral entry pin fixation for completely displaced supracondylar humeral fractures in children. Journal of Bone and Joint Surgery - Series A 2008;90(Suppl 2 (part 1)):20-30. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kumar 2021 {published data only}
- Kumar M, Singh JP. Comparative evaluation of result of conservative versus percuteneous K-wire fixation in type III supracondylar fracture humerus in children. International Journal of Health and Clinical Research 2021;4(5):145-8. [Google Scholar]
Kuzma 2014 {published and unpublished data}
- ACTRN12610000026022. Randomized clinical trial to compare straight arm skin traction and olecranon skeletal traction for displaced supracondylar fractures of the humerus in children [Randomised clinical trial to compare the effect of straight arm skin traction versus olecranon skeletal traction on cubitus varus deformity and range of movement in children with displaced supracondylar fracture of the humerus]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=320763&isReview=true (first received 11 January 2010).
- Kuzma J. A comparison of skin vs. skeletal traction in the management of childhood humeral supracondylar fractures: randomized clinical trial. Internet Journal of Orthopedic Surgery 2014;22(1):1-7. [Google Scholar]
Maity 2012 {published data only}
- Maity A, Saha D, Roy D. A prospective randomised, controlled clinical trial comparing medial and lateral entry pinning with lateral entry pinning for percutaneous fixation of displaced extension type supracondylar fractures of the humerus in children. Journal of Orthopaedic Surgery and Research 2012;7:6-14. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Majeed 2020 {published data only}
- Majeed F, Saddique M, Nasir H, Shams A. Comparison of closed reduction and percutaneous cross verses parallel k wire fixation in supracondylar fractures of the humerus in children. Professional Medical Journal 2020;27(03):476-80. [Google Scholar]
Mandal 2018 {published data only}
- Mandal J, Sarkar P, Singh A, Banka PK, Bindumadhavan S. Study of clinical and radiological outcomes in paediatric supracondylar humerus fractures treated with 3 lateral pins versus crossed pins. International Journal of Orthopaedics Sciences 2018;4(2):745-9. [Google Scholar]
Mulpuri 2016 {published data only}
- Mulpuri K, Dobbe A, Schaeffer E, Miyanji F, Alvarez C, Cooper A, et al. Management of displaced supracondylar fractures of the humerus using lateral versus cross k wires: a prospective randomised trial. In: Pediatric Orthopaedic Society of North America (POSNA) Annual Meeting; 2016 Apr 27-30; Indianapolis (IN). 2016:e-Poster #8.
- Mulpuri K, Dobbe A, Schaeffer E, Miyanji F, Alvarez C, Cooper A, et al. Management of displaced supracondylar fractures of the humerus using lateral versus cross k wires: a prospective randomised trial. Orthopaedic Proceedings. The British Editorial Society of Bone & Joint Surgery 2016;98-B(Suppl 21):93. [Google Scholar]
- NCT00358787. Management of displaced supracondylar fractures of the humerus using lateral vs. crossed K-wires [Management of displaced supracondylar fractures of the humerus using lateral versus cross K wires: a prospective randomized clinical trial]. clinicaltrials.gov/ct2/show/record/NCT00358787 (first received 1 August 2006).
Naik 2017 {published and unpublished data}
- Marson BA. [personal communication]. Email to: GM Sharma 29 September 2020.
- Naik LG, Sharma GM, Badgire KS, Qureshi F, Waghchoure C, Jain V. Cross pinning versus lateral pinning in the management of type III supracondylar humerus fractures in children. Journal of Clinical and Diagnostic Research 2017;11(8):RC01-03. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Naveen 2017 {published data only}
- Naveen PR, Chaitanya PR. A prospective study of crossed versus lateral only pinning in the treatment of displaced supracondylar fractures of the humerus in children. International Journal of Orthopaedics Sciences 2017;3(3):400-4. [Google Scholar]
Oakley 2009 {published and unpublished data}
- ACTRN12607000047493. A randomised controlled trial of two methods of immobilising supracondylar fractures of the humerus [Is immobilisation with collar and cuff or backslab and sling associated with less pain and better parent satisfaction in children with minimally displaced supracondylar fractures of the humerus?]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=81800&isReview=true (first received 12 January 2007).
- Marson BA. [personal communication]. Email to: E Oakley 13 November 2020.
- Oakley E, Barnett P, Babl FE. Backslab versus nonbackslab for immobilization of undisplaced supracondylar fractures: a randomized trial. Pediatric Emergency Care 2009;25(7):452-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Othman 2017 {published data only}
- Othman M, Nahla A, El-Malt A. A comparative study of three percutaneous pinning techniques for paediatric supracondylar humeral fractures. ARC Journal of Othopedics 2017;2(2):11-9.
Palange 2019 {published data only}
- Palange ND, Prasannakumar GS, Mane A, Pawar E. A comparison between percutaneous cross k wire and lateral k wires fixation in management of Type III Gartland paediatric supracondylar fractures. International Journal of Orthopaedics Sciences 2019;5(2):119-22. [Google Scholar]
Pandey 2008 {published data only}
- Pandey S, Shrestha D, Gorg M, Singh GK, Singh MP. Treatment of supracondylar fracture of the humerus (type IIB and III) in children: a prospective randomized controlled trial comparing two methods. Kathmandu University Medical Journal 2008;6(23):310-8. [DOI] [PubMed] [Google Scholar]
Patil 2017 {published data only}
- Patil SP, Gaonkar N, Pandey P, Kumar S, Shah R, Garud A, et al. A comparative study of two percutaneous pinning techniques (Cross K wire vs Lateral K wire) for Gartland type III pediatric supracondylar fracture of the humerus. International Journal of Orthopaedics Sciences 2017;3(4):665-8. [Google Scholar]
Pavone 2016 {published data only}
- Pavone V, Riccioli M, Testa G, Lucenti L, De Cristo C, Condorelli G, et al. Surgical treatment of displaced supracondylar pediatric humerus fractures: comparison of two pinning techniques. Journal of Functional Morphology and Kinesiology 2016;1(1):39-47. [Google Scholar]
Prashant 2016 {published data only}
- Prashant K, Lakhotia D, Bhattacharyya TD, Mahanta AK, Ravoof A. A comparative study of two percutaneous pinning techniques (lateral vs medial-lateral) for Gartland type III pediatric supracondylar fracture of the humerus. Journal of Orthopaedics and Traumatology 2016;17(3):223-9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Raj 2018 {unpublished data only}
- Raj KN. Comparision of Functional Outcome Between Traditional and Lateral Crossed Pinning in Supra condylar Humerus Fractures of Children [Masters thesis]. Chennai: Madras Medical College, 2018. [Google Scholar]
Rakha 2020 {published data only}
- Rahka A, Khan RD, Arshad A, Khan ZAli, Ahmad S, Mahmood S. Comparison of efficacy between open and close reduction in supracondylar fracture of humerus in children using Flynn’s criteria. Annals of Punjab Medical College (APMC) 2020;14(1):32-6. [Google Scholar]
Ray 2019 {published data only}
- Ray B, Waikhom S. A comparative study of closed reduction and percutaneous crossed pinning vs open reduction and crossed pinning in Gartland Type III supracondylar fracture of humerus in children. International Journal of Scientific Research 2019;8(3):15-9. [Google Scholar]
Rizk 2019 {published data only}
- Rizk AS, Kandil MI. Conventional versus lateral cross-pinning (Dorgan’s technique) for fixation of displaced pediatric supracondylar humeral fractures: a randomized comparative study. Egyptian Orthopaedic Journal 2019;53:348-58. [Google Scholar]
Sadek 2018 {published data only}
- Sadek AA, Ibrahim MI, Elazab HE, Abdel Rahman HH. Comparison of fixation of supracondylar humeral fractures in children by lateral cross-wiring technique versus traditional lateral pinning. Sohag Medical Journal 2018;22(1):265-71. [Google Scholar]
Saeed 2020 {published data only}
- Saeed UB, Waseem M, Hassan AR, Khan ZA, Gill D, Ahmad S. Supracondylar fracture, buried vs non buried K wires. Professional Medical Journal 2020;27(03):467-71. [Google Scholar]
Said 2015 {unpublished data only}
- Said EA. Crossed-pin configuration versus lateral pins in treatment of supracondylar humeral fracture in children. In: 36th Société Internationale de Chirurgie Orthopédique et de Traumatologie'(SICOT) Orthopaedic World Congress; 2015 Sep 17-19; Guangzhou (China). 2015.
Sankar 2019 {published data only}
- Sankar S, D’Souza JJ, Debuka E, Vyas T, Jagani N. The functional outcome of displaced supracondylar fracture humerus in children treated by open reduction and internal fixation by lateral pins compared to medial and lateral pins: a prospective study. National Journal of Clinical Orthopaedics 2019;3(2):78-84. [Google Scholar]
Shafi‐Ur‐Rehman 2013 {published data only}
- Shafi-Ur-Rehman, Hayat K, Bajwa A, Yousaf M, Rehman A, Shafaq SA. To compare the outcome of patients with supracondylar fractures of humerus treated by cross K-wires and lateral entry K-wires in children. Pakistan Journal of Medical and Health Sciences 2013;7(2):500-5. [Google Scholar]
- Shah ZA, Arif U. Displaced supracondylar humeral fractures; treatment among children: crossed versus lateral pinning. Professional Medical Journal 2013;20(5):818-24. [Google Scholar]
Shah 2017 {published data only}
- Shah SA, Asimuddin M. Management of supracondylar fractures of the humerus in children: conservative versus operative. International Journal of Orthopaedics 2017;3(1):14-20. [Google Scholar]
- Shah SA, Asimuddin M. Management of supracondylar fractures of the humerus in children: conservative versus operative. Scholars Journal of Applied Medical Sciences 2016;4(6B):1960-9. [Google Scholar]
Shamma 2020 {published data only}
- Shamma AE, Moawad Abd El-Motalb M, Abd El-Hamed ME, Tash E. Lateral divergent pinning versus lateral parallel pinning in management of supracondylar fractures of the humerus in children. Al-Azhar Medical Journal 2020;49(2):513-24. [Google Scholar]
Subash 2020 {published data only}
- Subash Y, Natarajan S, Lydia M. A study comparing two different pinning techniques in supracondylar fractures of the humerus. International Journal of Research in Pharmaceutical Sciences 2020;11(Special Issue 2):48-53. [Google Scholar]
Tripuraneni 2009 {published data only}
- Tripuraneni KR, Bosch PP, Schwend RM, Yaste JJ. Prospective, surgeon-randomized evaluation of crossed pins versus lateral pins for unstable supracondylar humerus fractures in children. Journal of Pediatric Orthopaedics. Part B 2009;18(2):93-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Vaidya 2009 {published data only}
- Vaidya SM. Percutaneous fixation of displaced supracondylar fracture in children comparing lateral with medial and lateral pin [Masters thesis]. Mahé, Seychelles: University of Seychelles, 2009. [Google Scholar]
Zhu 2016 {published data only}
- Zhu YL, Hu W, Yu XB, Wu YS, Sun LJ. A comparative study of two closed reduction methods for pediatric supracondylar humeral fractures. Journal of Orthopaedic Science 2016;21(5):609-13. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
AboHasan 2020 {published data only}
- AboHasan MA, Idrees K, Fahmy MH. Closed reduction and lateral percutaneous pin fixation for displaced supracondylar humerus fractures in children. Zagazig University Medical Journal 2020;26(2):233-8. [Google Scholar]
Aktekin 2008 {published data only}
- Aktekin CN, Toprak A, Ozturk AM, Altay M, Ozkurt B, Tabak AY. Open reduction via posterior triceps sparing approach in comparison with closed treatment of posteromedial displaced Gartland type III supracondylar humerus fractures. Journal of Pediatric Orthopaedics. Part B 2008;17(4):171-8. [DOI] [PubMed] [Google Scholar]
Arif 2014 {published data only}
- Arif M, Azeem M, Khurshid J. Surgical outcome of medial and lateral approach in treatment of paediatriac humeral supracondylar fracture. Journal of Sheikh Zayed Medical College 2014;5(3):644-7. [Google Scholar]
Arif 2017 {published data only}
- Arif M, Akram R, Zaman AU, Ahmad I, Aziz A. Outcome of lateral approach for displaced supracondylar humerus fractures in children. Pakistan Journal of Medical and Health Sciences 2017;11(3):825-8. [Google Scholar]
Ariyawatkul 2016 {published data only}
- Ariyawatkul T, Eamsobhana P, Kaewpornsawan K. The necessity of fixation in Gartland type 2 supracondylar fracture of the distal humerus in children (modified Gartland type 2A and 2B). Journal of Pediatric Orthopaedics. Part B 2016;25(2):159-64. [DOI] [PubMed] [Google Scholar]
Arnala 1991 {published data only}
- Arnala I, Paananen H, Lindell-Iwan L. Supracondylar fractures of the humerus in children. European Journal of Pediatric Surgery 1991;1(1):27-9. [DOI] [PubMed] [Google Scholar]
Balanescu 2013 {published data only}
- Balanescu R, Ulici A, Rosca D, Topor L, Barbu M. Neurovascular abnormalities in Gartland III supracondylar fractures in children. Chirurgia (Bucuresti) 2013;108(2):241-4. [PubMed] [Google Scholar]
Bales 2010 {published data only}
- Bales JG, Spencer HT, Wong MA, Fong YJ, Zionts LE, Silva M. The effects of surgical delay on the outcome of pediatric supracondylar humeral fractures. Journal of Pediatric Orthopedics 2010;30(8):785-91. [DOI] [PubMed] [Google Scholar]
Bulbul 2011 {published data only}
- Bulbul M, Ayanoglu S, Imren Y, Kahraman S, Esenyel CZ, Gurbuz H. Does two parallel lateral-only pin configuration provide stable osteosynthesis for pediatric supracondylar humerus fractures? Nobel Medicus 2011;7(3):36-40. [Google Scholar]
Chen 2001 {published data only}
- Chen RS, Liu XS, Lin XM, Feng M, Zhu JM, Ye FQ. Supracondylar extension fracture of the humerus in children. Journal of Bone and Joint Surgery. British Volume 2001;83-B(6):883-7. [DOI] [PubMed] [Google Scholar]
Diri 2003 {published data only}
- Diri B, Tomak Y, Karaismailoglu TN. The treatment of displaced supracondylar fractures of the humerus in children (an evaluation of three different treatment methods). Turkish Journal of Trauma & Emergency Surgery 2003;9(1):62-9. [PubMed] [Google Scholar]
Ducic 2016b {published data only}
- Ducic S, Bumbasirevic M, Radlovic V, Nikic P, Bukumiric Z, Brdar R, et al. Displaced supracondylar humeral fractures in children: comparison of three treatment approaches. Srpski Arhiv Za Celokupno Lekarstvo 2016;144(1-2):46-51. [DOI] [PubMed] [Google Scholar]
El‐Ngehy 2018 {published data only}
- El-Ngehy Alaa AM, Eladawy Amr M, El-Sharkawi Walid F, Naeem Muhammed MG. Crossed pinning versus two lateral wires in the management of displaced supracondylar humerus fractures in children. Zagazig University Medical Journal 2018;24(6):467-72. [Google Scholar]
Ensafdaran 2005 {published data only}
- Ensafdaran A, Emami MJ, Borghei M. A comparative study of lateral approach versus posterior approach for the surgical treatment of supracondylar fractures of the humerus in children. Medical Journal of The Islamic Republic of Iran (MJIRI) 2005;19(3):213-7. [Google Scholar]
Eren 2005 {published data only}
- Eren A, Ozkut AT, Altintas F, Guven M. Comparison between the lateral and medial approaches in terms of functional and cosmetic results in the surgical treatment of type III supracondylar humeral fractures in children. Acta Orthopaedica et Traumatologica Turcica 2005;39(3):199-204. [PubMed] [Google Scholar]
Fahmy 2009 {published data only}
- Fahmy MA, Hatata MZ, Al-Seesi H. Posterior intrafocal pinning for extension-type supracondylar fractures of the humerus in children. Journal of Bone and Joint Surgery - Series B 2009;91(9):1232-6. [DOI] [PubMed] [Google Scholar]
Hegazy 2020 {published data only}
- Hegazy MO, Meselhy MA, EL-Nabasy AA. Lateral divergent pinning versus lateral parallel pinning in management of supracondylar fractures of the humerus in children. Benha Journal of Applied Sciences 2020;5(6 (part 2)):1-6. [Google Scholar]
Keskin 2014 {published data only}
- Keskin D, Sen H. The comparative evaluation of treatment outcomes in pediatric displaced supracondylar humerus fractures managed with either open or closed reduction and percutaneous pinning. Acta Chirurgiae Orthopaedicae et Traumatologiae Cechoslovaca 2014;81(6):380-6. [PubMed] [Google Scholar]
Khan 2007 {published data only}
- Khan AQ, Goel S, Abbas M, Sherwani MK. Percutaneous K-wiring for Gartland type III supracondylar humerus fractures in children. Saudi Medical Journal 2007;28(4):603-6. [PubMed] [Google Scholar]
Lee 2008 {published data only}
- Lee YH, Lee SK, Kim BS, Chung MS, Baek GH, Gong HS, et al. Three lateral divergent or parallel pin fixations for the treatment of displaced supracondylar humerus fractures in children. Journal of Pediatric Orthopaedics 2008;28(4):417-22. [DOI] [PubMed] [Google Scholar]
Li 2013 {published data only}
- Li WQ, Guo YM, He J, Lu S. Dynamic external fixation for stabilizing Baumman angle during midanaphase in supracondylar fracture of humerus in children. China Journal of Orthopaedics and Traumatology 2013;26(8):656-8. [PubMed] [Google Scholar]
Li 2017 {published data only}
- Li J, Fu D, Yu C, Wang S, Ze R, Tang X. Surgical management of delayed irreducible Gartland III supracondylar fractures in children: open reduction and internal fixation versus external fixation. Journal of Shoulder and Elbow Surgery 2017;26(2):299-304. [DOI] [PubMed] [Google Scholar]
Marashi 2013 {published data only}
- Marashi Nejad SA, Mehdi Nasab S, Baianfar M. Effect of supination versus pronation in the non-operative treatment of pediatric supracondylar humerus fractures. Archives of Trauma Research 2013;2(1):26-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00904137 {published data only}
- NCT00904137. Treatment of type I supracondylar fractures of the humerus. clinicaltrials.gov/ct2/show/NCT00904137 (first received 19 May 2009).
Pescatori 2012 {published data only}
- Pescatori E, Memeo A, Brivio A, Trapletti A, Camurri S, Pedretti L, et al. Supracondylar humerus fractures in children: a comparison of experiences. Journal of Pediatric Orthopaedics. Part B 2012;21(6):505-13. [DOI] [PubMed] [Google Scholar]
Rawoot 2014 {unpublished data only}
- Rawoot A, du Toit J, Ikram A. The treatment of Gartland 2 and 3 supracondylar humerus fractures in children in either a prone or supine position: practical implications for future treatment. British Editorial Society of Bone & Joint Surgery 2014;96:11. [Google Scholar]
Roy 2019 {published data only}
- Roy MK, Alam MT, Rahman MW, Islam MS, Sayeed KA, Kamal MZ, et al. Comparative study of stabilization of humerus supracondylar fracture in children by percutaneous pinning from lateral side and both sides. Mymensingh Medical Journal: MMJ 2019;28(1):15-22. [PubMed] [Google Scholar]
Sadik 2015 {published data only}
- Sadik Diaa G. Medial and lateral percutaneous fixation versus lateral fixation for treatment of Gartland type ii, iii supracondylar fracture of humerus in children. Iraqi Journal of Medical Sciences 2015;13(2):183-90. [Google Scholar]
Shah 2013 {published data only}
- Shah ZA, Arif U. Displaced supracondylar humeral fractures; treatment among children: crossed versus lateral pinning. Professional Medical Journal 2013;20(5):818-24. [Google Scholar]
Shoaib 2003 {published data only}
- Shoaib M, Hussain A, Kamran H, Ali J. Outcome of closed reduction and casting in displaced supracondylar fracture of humerus in children. Journal of Ayub Medical College, Abbottabad : JAMC 2003;15(4):23-5. [PubMed] [Google Scholar]
Siddiq 2020 {published data only}
- Siddiq K, ullah Mehmood A, Hameed MH, Chishti MK, Ali MN, Hussain N. Comparison of outcome with medial versus lateral approach for operative fixation of superacondylar humeral fractures in paediatric patients. Professional Medical Journal 2020;27(10):2045-9. [Google Scholar]
Silva 2018 {published data only}
- Silva M, Sadlik G, Avoian T, Ebramzadeh E. A removable long-arm soft cast to treat nondisplaced pediatric elbow fractures: a randomized, controlled trial. Journal of Pediatric Orthopaedics 2018;38(4):223-9. [DOI] [PubMed] [Google Scholar]
Sutton 1992 {published data only}
- Sutton WR, Greene WB, Georgopoulos G, Dameron TB Jr. Displaced supracondylar humeral fractures in children. A comparison of results and costs in patients treated by skeletal traction versus percutaneous pinning. Clinical Orthopaedics & Related Research 1992;278:81-7. [PubMed] [Google Scholar]
Venkatadass 2015 {published data only}
- Venkatadass K, Balachandar G, Rajasekaran S. Is prone position Ideal for manipulation and pinning of displaced pediatric extension-type supracondylar fractures of humerus? A randomized control trial. Journal of Pediatric Orthopedics 2015;35(7):672-6. [DOI] [PubMed] [Google Scholar]
Young 2012 {published data only}
- Young S, Fevang JM, Gullaksen G, Nilsen PT, Engesaeter LB. Parent and patient satisfaction after treatment for supracondylar humerus fractures in 139 children: no difference between skeletal traction and crossed pin fixation at long-term followup. Advances in Orthopaedics 2012;2012:[6 p.]. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Afridi 2002 {published data only}
- Afridi HD, Ansar AH. Prospective randomised study of the management of supracondylar (humerus) fractures in children. Medical Channel Journal 2002;8(1):41-3. [Google Scholar]
Andreasi 1985 {published data only}
- Andreasi A. Comparison of two methods of percutaneous osteosynthesis with Kirschner wires in diastatic supracondylar fractures of the humerus in children. Minerva Ortopedica 1985;36(5):295-300. [Google Scholar]
Bing 2017 {published data only}
- Bing C, Zhenglin L, Yanjun G. Clinical observation of early closed reduction and flexible lateral Kirschner wire external fixation for supracondylar fracture of humerus in children. Journal of Mathematical Medicine 2017;30:1288–90. [Google Scholar]
Boparai 2006 {published data only}
- Boparai RS, Diwan D. Supracondylar fractures in children - closed reduction vs open reduction. Indian Journal of Orthopaedics 2006;40(2):103-7. [Google Scholar]
Botchu 2006 {published data only}
- Botchu R, Shetty S, Shetty V, Shetty AC. Displaced supracondylar fractures of humerus in children - to pin or not to? European Journal of Trauma 2006;32(Suppl 1):162. [Google Scholar]
Evans 1998 {published data only}
- Evans SC, Hamilton P, Godette G, Torode IP, Dickens DR, Broughton NS, et al. Supracondylar fractures of the humerus in children: a randomised clinical trial [abstract]. Journal of Bone and Joint Surgery: British Volume 1998;80(Suppl 2):140-1. [Google Scholar]
He 2009 {published data only}
- He BX, Zhang B, Tan YJ. Comparison of clinical effects of various external fixation for the treatment of humeral supracondylar fracture. China Journal of Orthopaedics and Traumatology 2009;22(3):190-2. [PubMed] [Google Scholar]
Lu 2011 {published data only}
- Lu X, Luo B, Li K. Different methods for treatment of patients with supracondylar fractures of the humerus: a randomized study. China Modern Medicine 2011;18:74–5. [Google Scholar]
NCT04582123 {published data only}
- NCT04582123. Comparison of cross pin configurations in supracondylar humerus fracture treatment: 2 pins versus 3 pins. clinicaltrials.gov/show/NCT04582123 (first received 9 October 2020).
Sapkota 2019 {published data only}
- Sapkota K, Wahegaonkar K, Ranjeet N, Thapa U, Onta P. Comparison of cross pinning versus lateral three pins in type three supracondylar fracture of distal humerus in children. Asian Journal of Medical Sciences 2019;10:58-61. [Google Scholar]
References to ongoing studies
ACTRN12612000480886 {published data only}
- ACTRN12612000480886. Study of treatment methods for undisplaced supracondylar humeral (elbow) fractures in children [The effect of three different types of external immobilisation methods comparing pain during application and functional outcome for children with undisplaced supracondylar humeral fractures]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=362446&isReview=true (first received 2 June 2012).
CTRI/2020/06/025504 {published data only}
- CTRI/2020/06/025504. Comparison of short term functional and radiological outcomes between two versus three lateral pin fixation techniques in fractures of lower part on arm bone in children [Comparison of short term functional and radiological outcomes between two versus three lateral pinning techniques in Gartland type III supracondylar humerus fractures in children - a randomized control trial]. www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2020/06/025504 (first received 1 June 2020).
PACTR201702001960109 {published data only}
- PACTR201702001960109. Treatment of supracondylar humerus fractures in children [Comparison between crossing wires versus two lateral wires in the management of displaced supracondylar humerus in children]. trialsearch.who.int/?TrialID=PACTR201702001960109 (first received 3 January 2017).
Additional references
American Academy of Orthopaedic Surgeons 2011
- American Academy of Orthopaedic Surgeons. AAOS Guideline of the treatment of pediatric supracondylar humerus fractures; September 2011. www.aaos.org/research/guidelines/SupracondylarFracture/Summary_of_Recs.pdf (accessed 5 August 2019).
Arnould 2004
- Arnould C, Penta M, Renders A, Thonnard J-L. ABILHAND-Kids: a measure of manual ability in children with cerebral palsy. Neurology 2004;63(6):1045-52. [DOI] [PubMed] [Google Scholar]
Babal 2010
- Babal JC, Mehlman CT, Klein G. Nerve injuries associated with pediatric supracondylar humeral fractures: a meta-analysis. Journal of Pediatric Orthopaedics 2010;30(3):253-63. [DOI] [PubMed] [Google Scholar]
Barr 2014
- Barr LV. Paediatric supracondylar humeral fractures: epidemiology, mechanisms and incidence during school holidays. Journal of Children's Orthopaedics 2014;8(2):167-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Beaton 2005
- Beaton DE, Wright JG, Katz JN, The Upper Extremity Collaborative Group. Development of the QuickDASH: comparison of three item-reduction approaches. Journal of Bone and Joint Surgery 2005;87A(5):1038-46. [DOI] [PubMed] [Google Scholar]
Bieri 1990
- Bieri D, Reeve RA, Champion GD, Addicoat L, Ziegler JB. The Faces Pain Scale for the self-assessment of the severity of pain experienced by children: development, initial validation, and preliminary investigation for ratio scale properties. Pain 1990;41(2):139-50. [DOI] [PubMed] [Google Scholar]
Brauer 2007
- Brauer CA, Lee BM, Bae DS, Waters PM, Kocher MS. A systematic review of medial and lateral entry pinning versus lateral entry pinning for supracondylar fractures of the humerus. Journal of Pediatric Orthopaedics 2007;27(2):181-6. [DOI] [PubMed] [Google Scholar]
British Orthopaedic Association 2014
- British Orthopaedic Association, British Society for Children's Orthopaedic Surgery. Supracondylar Fractures of the Humerus in Children. London (UK): British Orthopaedic Association, 2014. [Google Scholar]
Carrazzone 2021
- Carrazzone OL, Barbachan Mansur NS, Matsunaga FT, Matsumoto MH, Faloppa F, Belloti JC, et al. Crossed versus lateral K-wire fixation of supracondylar fractures of the humerus in children: a meta-analysis of randomized controlled trials. Journal of Shoulder and Elbow Surgery 2021;30(2):439-48. [DOI] [PubMed] [Google Scholar]
Catena 2019
- Catena N, Calevo MG, Fracassetti D, Moharamzadeh D, Origo C, De Pellegrin M. Risk of ulnar nerve injury during cross-pinning in supine and prone position for supracondylar humeral fractures in children: a recent literature review. European Journal of Orthopaedic Surgery & Traumatology 2019;29(6):1169-75. [DOI] [PubMed] [Google Scholar]
Chapleau 2011
- Chapleau J, Canet F, Petit Y, Laflamme GY, Rouleau DM. Validity of goniometric elbow measurements: comparative study with a radiographic method. Clinical Orthopaedics and Related Research 2011;469(11):3134-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cheng 1999
- Cheng JC, Ng BK, Ying SY, Lam PK. A 10-year study of the changes in the pattern and treatment of 6,493 fractures. Journal of Pediatric Orthopedics 1999;19(3):344-50. [PubMed] [Google Scholar]
Daltroy 1998
- Daltroy LH, Liang MH, Fossel AH, Goldberg MJ. The POSNA pediatric musculoskeletal functional health questionnaire: report on reliability, validity, and sensitivity to change. Pediatric Outcomes Instrument Development Group. Pediatric Orthopaedic Society of North America. Journal of Pediatric Orthopedics 1998;18(5):561-71. [DOI] [PubMed] [Google Scholar]
Dekker 2016
- Dekker AE, Krijnen P, Schipper IB. Results of crossed versus lateral entry K-wire fixation of displaced pediatric supracondylar humeral fractures: a systematic review and meta-analysis. Injury 2016;47(11):2391-8. [DOI] [PubMed] [Google Scholar]
Delniotis 2019
- Delniotis I, Delniotis A, Saloupis P, Gavriilidou A, Galanis N, Kyriakou A, et al. Management of the pediatric pulseless supracondylar humeral fracture: a systematic review and comparison study of "Watchful Expectancy Strategy" versus Surgical Exploration of the Brachial Artery. Annals of Vascular Surgery 2019;55:260-71. [DOI] [PubMed] [Google Scholar]
Del Valle‐Hernández 2017
- Del Valle-Hernández E, Marrero-Barrera PA, Beaton D, Bravo D, Santiago S, Guzmán-Pérez H, et al. Complications associated with pediatric supracondylar humeral fractures. Puerto Rico Health Sciences Journal 2017;36(1):37-40. [PubMed] [Google Scholar]
De Pellegrin 2018
- De Pellegrin M, Fracassetti D, Moharamzadeh D, Origo C, Catena N. Advantages and disadvantages of the prone position in the surgical treatment of supracondylar humerus fractures in children. A literature review. Injury 2018;49 Suppl 3:S37-42. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Farnsworth 1998
- Farnsworth CL, Silva PD, Mubarak SJ. Etiology of supracondylar humerus fractures. Journal of Pediatric Orthopedics 1998;18(1):38-42. [PubMed] [Google Scholar]
Farrow 2018
- Farrow L, Ablett AD, Mills L, Barker S. Early versus delayed surgery for paediatric supracondylar humeral fractures in the absence of vascular compromise: a systematic review and meta-analysis. Bone & Joint Journal 2018;100-b(12):1535-41. [DOI] [PubMed] [Google Scholar]
Flynn 1974
- Flynn JC, Matthews JG, Benoit RL. Blind pinning of displaced supracondylar fractures of the humerus in children. Sixteen years' experience with long-term follow-up. Journal of Bone and Joint Surgery 1974;56(2):263-72. [PubMed] [Google Scholar]
Gartland 1959
- Gartland JJ. Management of supracondylar fractures of the humerus in children. Surgery, Gynecology & Obstetrics 1959;109(2):145-54. [PubMed] [Google Scholar]
Golden 2007
- Golden DW, Jhee JT, Gilpin SP, Sawyer JR. Elbow range of motion and clinical carrying angle in a healthy pediatric population. Journal of Pediatric Orthopaedics B 2007;16(2):144-9. [DOI] [PubMed] [Google Scholar]
Griffin 2008
- Griffin KJ, Walsh SR, Tang TY, Boyle JR, Hayes PD. The pink pulseless hand: a review of the literature regarding management of vascular complications of supracondylar humeral fractures in children. European Journal of Vascular and Endovascular Surgery 2008;36(6):697-702. [DOI] [PubMed] [Google Scholar]
Hell 2021
- Hell AK, Gadomski C, Braunschweid L. Spontaneous humeral torsion deformity correction after displaced supracondylar fractures in children. BMC Muscloeskeletal Disorders 2021;22:1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Holt 2017
- Holt JB, Glass NA, Bedard NA, Weinstein SL, Shah AS. Emerging U.S. national trends in the treatment of pediatric supracondylar humeral fractures. Journal of Bone and Joint Surgery 2017;99(8):681-7. [DOI] [PubMed] [Google Scholar]
Holt 2018
- Holt JB, Glass NA, Shah AS. Understanding the epidemiology of pediatric supracondylar humeral fractures in the United States. Journal of Pediatric Orthopedics 2018;38(5):e245-51. [DOI] [PubMed] [Google Scholar]
Horst 2011
- Horst M, Altermatt S, Weber DM, Weil R, Ramseier LE. Pitfalls of lateral external fixation for supracondylar humeral fractures in children. European Journal of Trauma and Emergency Surgery 2011;37(4):405-10. [DOI] [PubMed] [Google Scholar]
Houshian 2011
- Houshian S, Mehdi B, Larsen MS. The epidemiology of elbow fracture in children: analysis of 355 fractures, with special reference to supracondylar humerus fractures. Journal of Orthopaedic Science 2001;6(4):312-15. [DOI] [PubMed] [Google Scholar]
Hudak 1996
- Hudak PL, Amadio PC, Bombardier C. Development of an upper extremity outcome measure: the DASH (Disabilities of the Arm, Shoulder and Hand) [Corrected]. The Upper Extremity Collaborative Group (UECG). American Journal of Industrial Medicine 1996;29(6):602-8. [DOI] [PubMed] [Google Scholar]
Kurer 1990
- Kurer MH, Regan MW. Completely displaced supracondylar fracture of the humerus in children: a review of 1708 comparable cases. Clinical Orthopaedics and Related Research 1990;256:205-14. [PubMed] [Google Scholar]
Lefebvre 2019
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf MI, et al. Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from: www.training.cochrane.org/handbook.
Leitch 2006
- Leitch KK, Kay RM, Femino JD, Tolo VT, Storer SK, Skaggs DL. Treatment of multidirectionally unstable supracondylar humeral fractures in children: a modified Gartland type-IV fracture. Journal of Bone and Joint Surgery 2006;88(5):980-5. [DOI] [PubMed] [Google Scholar]
Lin‐Guo 2018
- Lin-Guo, Zhang XN, Yang JP, Wang Z, Qi Y, Shan-Zhu, et al. A systematic review and meta-analysis of two different managements for supracondylar humeral fractures in children. Journal of Orthopaedic Surgery and Research 2018;13(1):141. [DOI] [PMC free article] [PubMed] [Google Scholar]
Loizou 2009
- Loizou CL, Simillis C, Hutchinson JR. A systematic review of early versus delayed treatment for type III supracondylar humeral fractures in children. Injury 2009;40(3):245-8. [DOI] [PubMed] [Google Scholar]
Mangat 2009
- Mangat KS, Martin AG, Bache CE. The ‘pulseless pink’ hand after supracondylar fracture of the humerus in children. Journal of Bone and Joint Surgery. British Volume 2009;91-B(11):1521-5. [DOI] [PubMed] [Google Scholar]
Marson 2020a
- Marson BA, Craxford S, Deshmukh SR, Grindlay DJ, Manning JC, Ollivere BJ. Quality of patient-reported outcomes used for quality of life, physical function, and functional capacity in trials of childhood fractures. Bone and Joint Journal 2020;102-B(12):1599–1607. [DOI] [PubMed] [Google Scholar]
Marson 2020c
- Marson BA, Manning JC, James M, Craxford S, Deshmukh SR, Ollivere BJ, CORE-Kids group. CORE-Kids: a protocol for the development of a core outcome set for childhood fractures. BMJ Open 2020;10(2):e036224. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morrey 1993
- Morrey BF. Functional evaluation of the elbow. In: Morrey BF, editors(s). The Elbow and Its Disorders. Philadelphia (PA): Saunders, 1993:86. [Google Scholar]
Na 2018
- Na Y, Bai R, Zhao Z, Han C, Kong L, Ren Y, et al. Comparison of lateral entry with crossed entry pinning for pediatric supracondylar humeral fractures: a meta-analysis. Journal of Orthopaedic Surgery and Research 2018;13(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2016
- National Institute for Health and Care Excellent (NICE). Fractures (non-complex): assessment and management. NICE guideline [NG38]; February 2016. www.nice.org.uk/guidance/ng38/chapter/recommendations (accessed 11 February 2021).
O'Connor 2016
- O'Connor S, Ferguson E, Carney T, House E, O'Connor RC. The development and evaluation of the paediatric index of emotional distress (PI-ED). Social Psychiatry and Psychiatric Epidemiology 2016;51(1):15-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Omid 2008
- Omid R, Choi PD, Skaggs DL. Supracondylar humeral fractures in children. Journal of Bone and Joint Surgery. American Volume 2008;90(5):1121-32. [DOI] [PubMed] [Google Scholar]
Patriota 2017
- Patriota GS, Assunção FC, Assunção CA. What is the best fixation technique for the treatment of supracondylar humerus fractures in children? Revista Brasileira de Ortopedia (English Edition) 2017;52(4):428-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pretell 2010
- Pretell Mazzini J, Rodriguez Martin J, Andres Esteban EM. Surgical approaches for open reduction and pinning in severely displaced supracondylar humerus fractures in children: a systematic review. Journal of Children's Orthopaedics 2010;4(2):143-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pretell‐Mazzini 2010a
- Pretell-Mazzini J, Rodriguez-Martin J, Andres-Esteban EM. Does open reduction and pinning affect outcome in severely displaced supracondylar humeral fractures in children? A systematic review. Strategies in Trauma and Limb Reconstruction 2010;5(2):57-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pretell‐Mazzini 2010b
- Pretell-Mazzini J, Rodriguez-Martin J, Andres-Esteban EM. Surgical approaches for open reduction and pinning in severely displaced supracondylar humerus fractures in children: a systematic review. Journal of Children's Orthopaedics 2010;4:143-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Saylor 1999
- Saylor CF, Swenson CC, Reynolds SS, Taylor M. The Pediatric Emotional Distress Scale: a brief screening measure for young children exposed to traumatic events. Journal of Clinical Child Psychology 1999;28(1):70-1. [DOI] [PubMed] [Google Scholar]
Schünemann 2019
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. Draft version (29 January 2019) for inclusion in: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions, Version 6.1 (updated September 2020), Cochrane, 2020. Available from handbook.cochrane.org.
Sharma 2013
- Sharma K, Mansur DI, Khanal K, Haque MK. Variation of carrying angle with age, sex, height and special reference to side. Kathmandu University Medical Journal (KUMJ) 2013;11(44):315-8. [DOI] [PubMed] [Google Scholar]
Sinikumpu 2016
- Sinikumpu JJ, Victorzon S, Pokka T, Lindholm EL, Peljo T, Serlo W. The long-term outcome of childhood supracondylar humeral fractures: a population-based follow up study with a minimum follow up of ten years and normal matched comparisons. Bone & Joint Journal 2016;98-B(10):1410-7. [DOI] [PubMed] [Google Scholar]
Slobogean 2010
- Slobogean BL, Jackman H, Tennant S, Slobogean GP, Mulpuri K. Iatrogenic ulnar nerve injury after the surgical treatment of displaced supracondylar fractures of the humerus: number needed to harm, a systematic review. Journal of Children's Orthopaedics 2010;30(5):430-6. [DOI] [PubMed] [Google Scholar]
Slongo 2006
- Slongo T, Audigé L, Schlickewei W, Clavert JM, Hunter J. Development and validation of the AO pediatric comprehensive classification of long bone fractures by the Pediatric Expert Group of the AO Foundation in collaboration with AO Clinical Investigation and Documentation and the International Association for Pediatric Traumatology. Journal of Pediatric Orthopedics 2006;26(1):43-9. [DOI] [PubMed] [Google Scholar]
Terpstra 2022
- Terpstra SE, Burgers PT, Van der Heide HJ, Witte PV. Pediatric supracondylar humerus fractures: should we avoid surgery during after-hours? Children 2022;9(2):189. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tzatzairis 2021
- Tzatzairis T, Firth G, Loke WJ, Serlis A, Ramachandran M. Does compliance with BOAST guidelines matter for displaced supracondylar fractures in children?: the experience of a tertiary referral major trauma centre over a 3.5-year period. Journal of Pediatric Orthopaedics. Part B 2021;1(30):154-60. [DOI] [PubMed] [Google Scholar]
Vaquero‐Picado 2018
- Vaquero-Picado A, González-Morán G, Moraleda L. Management of supracondylar fractures of the humerus in children. EFORT Open Reviews 2018;3(10):526-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
White 2010
- White L, Mehlman CT, Crawford AH. Perfused, pulseless, and puzzling: a systematic review of vascular injuries in pediatric supracondylar humerus fractures and results of a POSNA questionnaire. Journal of Pediatric Orthopaedics 2010;30(4):328-35. [DOI] [PubMed] [Google Scholar]
Wilkins 1996
- Wilkins KE. Fractures and dislocations of the elbow region. In: Rookwood CA Jr, Wilkins KE, King RE, editors(s). Fractures in Children. 4th edition. Vol. 3. Philadelphia (PA): Lippincott-Raven, 1996. [Google Scholar]
Wille 2010
- Wille N, Badia X, Bansel G, Burstrom K, Cavrini G, Devlin N, et al. Development of the EQ-5D-Y: a child-friendly version of the EQ-5D. Quality of Life Research 2010;19(6):875-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Woratanarat 2012
- Woratanarat P, Angsanuntsukh C, Rattanasiri S, Attia J, Woratanarat T, Thakkinstian A. Meta-analysis of pinning in supracondylar fracture of the humerus in children. Journal of Orthopaedic Trauma 2012;26(1):48-53. [DOI] [PubMed] [Google Scholar]
Yeomans 2018
- Yeomans D, Graham SM, Mkandawire NC, Harrison WJ, Perry DC. Conservative management of displaced paediatric supracondylar fractures: a systematic review. Tropical Doctor 2018;48(4):359-65. [DOI] [PubMed] [Google Scholar]
Young 2000
- Young NL, Williams JI, Yoshida KK, Wright JG. Measurement properties of the activities scale for kids. Journal of Clinical Epidemiology 2000;52(2):125-37. [DOI] [PubMed] [Google Scholar]
Yousri 2012
- Yousri T, Tarassoli P, Whitehouse M, Monsell F, Khan WS. Systematic review of randomized controlled trials comparing efficacy of crossed versus lateral K-wire fixation in extension type Gartland type III supracondylar fractures of the humerus in children. Ortopedia, Traumatologia, Rehabilitacja 2012;14(5):397-405. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Marson 2020b
- Marson BA, Craxford S, Price KR, Ollivere BJ. Interventions for treating supracondylar elbow fractures in children. Cochrane Database of Systematic Reviews 2020, Issue 5. Art. No: CD013609. [DOI: 10.1002/14651858.CD013609] [DOI] [PMC free article] [PubMed] [Google Scholar]


