Abstract
Background
Surgical repair of partial anomalous pulmonary venous return (PAPVR) to the superior vena cava (SVC) using the Warden procedure has favorable outcomes. However, there remain some concerns after the Warden procedure, such as sinoatrial nodal dysfunction and systemic or pulmonary venous stenosis. We investigated the outcomes of the Warden procedure for repair of PAPVR to the SVC.
Methods
This retrospective study included 22 consecutive patients who underwent the Warden procedure for PAPVR between 2002 and 2018. The median age and body weight at operation were 27.5 months (interquartile range [IQR], 5.0–56.8 months) and 13.2 kg (IQR, 6.5–16.0 kg), respectively. The median follow-up duration was 6.2 years (IQR, 3.5–11.6 years).
Results
There were no cases of early or late mortality. No patients had postoperative heart rhythm problems, except 1 patient who showed transient sinoatrial nodal dysfunction in the immediate postoperative period. Procedure-related complications requiring reintervention occurred in 5 patients, including 3 of 4 SVC stenosis cases and 2 pulmonary venous stenosis cases during follow-up. The rate of freedom from reintervention related to the Warden procedure was 75.9% at 10 years.
Conclusion
In cases requiring extension or creation of an atrial septal defect to achieve a sufficient venous pathway, or interposition of an entire circumferential conduit between the SVC and right atrium due to the shortness of the SVC in the Warden procedure, stenotic complications of the venous pathway occurred. Careful observation of changes in the pressure gradient or anatomical stenosis is required in such patients.
Keywords: Congenital heart disease, Pulmonary veins, Atrial heart septal defects
Introduction
The most common anatomical form of partial anomalous pulmonary venous return (PAPVR) is an abnormal connection of the right pulmonary vein to the superior vena cava (SVC) [1,2]. Several surgical procedures can be performed to correct this type of PAPVR. Warden et al. [3] introduced a surgical technique, known as the Warden procedure, in which the SVC is transected from the right atrium (RA) and re-anastomosed to the RA appendage. Thus, the procedure uses an intra-cardiac baffle between the abnormal pulmonary vein opening and atrial septal defect (ASD) without interfering with the SVC inflow [3].
Recent studies have reported favorable short-and mid-term outcomes of the Warden procedure [4-6]. However, although the prevalence is relatively low, there remain some concerns about complications after the Warden procedure, such as sinoatrial nodal dysfunction and SVC or pulmonary venous stenosis at the anastomosis site [7,8]. We reviewed our experience with the Warden procedure for PAPVR to the SVC at Seoul National University Children’s Hospital over the past 17 years.
Methods
Ethics statement
The institutional review board of Seoul National University Hospital Biomedical Research Institute reviewed the study protocol and approved the study as a minimal-risk retrospective study that did not require informed consent (approval no., H-2009-135-1159).
Study population
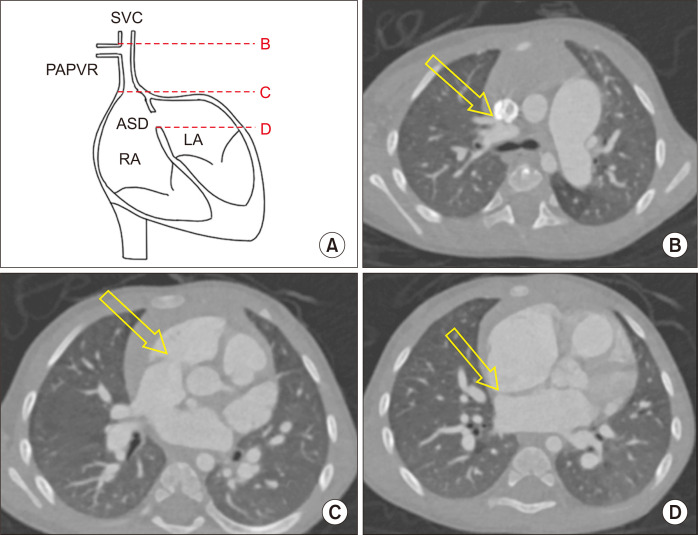
All data were obtained retrospectively. Twenty-two patients who consecutively underwent the Warden procedure for PAPVR to the SVC from January 2002 to December 2018 were enrolled. Diagnoses were performed using transthoracic echocardiography or computed tomography (CT). The preoperative characteristics of the enrolled patients are summarized in Table 1. The median age and body weight at the time of operation were 27.5 months (interquartile range [IQR], 5.0–56.8 months) and 13.2 kg (IQR, 6.5–16.0 kg), respectively. The size of the SVC at distal level of the uppermost PAPVR and the distances from the site of uppermost PAPVR to the RA-SVC junction and from the RA- SVC junction to the lower margin of the ASD were measured on preoperative CT to identify any anatomical factors that could contribute to postoperative complications. Preoperative CT was performed in 17 patients (77.3%), and the aforementioned distances were measured indirectly in axial images by multiplying the thickness of an axial image by the number of images because the coronal view was available for only 1 patient (Fig. 1). Half (n=11) of the patients had anomalous pulmonary venous connections solely involving the right upper pulmonary vein, while the other half (n=11) simultaneously had abnormal pulmonary venous connections other than the right upper pulmonary vein, such as an anomalous right middle pulmonary vein or left upper pulmonary vein. The types of accompanying ASD were sinus venosus (combined with other types or isolated; n=14, 63.6%), isolated secundum (without sinus venosus type; n=5, 22.7%), and patent foramen ovale (PFO; n=2, 9.1%). There was 1 patient (4.5%) with an intact atrial septum. Other associated cardiac anomalies included patent ductus arteriosus (n=3), ventricular septal defect (n=2), complete atrioventricular septal defect (n=1), coarctation of the aorta (n=1), total anomalous pulmonary venous return (n=1), pulmonary atresia with ventricular septal defect (n=1), and Ebstein anomaly (n=1). Preoperative electrocardiography (ECG) showed a normal sinus rhythm in all enrolled patients.
Table 1.
Preoperative characteristics (N=22)
| Characteristic | Value |
|---|---|
| Age (mo) | 27.5 (5.0–56.8) |
| Body weight (kg) | 13.2 (6.5–16.0) |
| Male | 9 (40.9) |
| Anatomical characteristics | |
| SVC diameter (mm) | 12.31 (9.29–12.89) |
| Distance of the anomalous PV drainage site from the RA-SVC junction (mm) | 17.5 (11.2–22.4) |
| Distance of ASD from the RA-SVC junction | 14.4 (3.6–21.0) |
| Anomalous PV | |
| RUPV only | 11 (50.0) |
| More than RUPV | 11 (50.0) |
| RMPV to SVC | 5 (22.7) |
| RMPV to RA | 3 (13.6) |
| All RPV to SVC | 1 (4.5) |
| LUPV to innominate vein | 1 (4.5) |
| TAPVR to RA-SVC junction | 1 (4.5) |
| Atrial septal defect | |
| Sinus venosus | 14 (63.7) |
| Isolated secundum | 5 (22.7) |
| Patent foramen ovale | 2 (9.1) |
| Intact atrial septum | 1 (4.5) |
| Associated anomaly | |
| PDA | 3 (13.6) |
| VSD | 2 (9.1) |
| Complete AVSD | 1 (4.5) |
| Coarctation of the aorta | 1 (4.5) |
| TAPVR | 1 (4.5) |
| Pulmonary atresia with VSD | 1 (4.5) |
| Ebstein anomaly | 1 (4.5) |
Values are presented as median (interquartile range) or number (%).
SVC, superior vena cava; PV, pulmonary vein; RA, right atrium; ASD, atrial septal defect; RUPV, right upper pulmonary vein; RMPV, right middle pulmonary vein; RPV, right pulmonary vein; LUPV, left upper pulmonary vein; TAPVR, total anomalous pulmonary venous return; PDA, patent ductus arteriosus; VSD, ventricular septal defect; AVSD, atrioventricular septal defect.
Fig. 1.
(A) Diagram of preoperative computed tomography measurements. (B) Distal level of the uppermost partial anomalous pulmonary venous return (PAPVR). (C) Level of the superior vena cava (SVC)-right atrium (RA) junction. (D) Level of the lower margin of the atrial septal defect (ASD). Each structure is marked with yellow arrow. The distances from (B) to (C) and from (C) to (D) were measured indirectly in an axial view by multiplying the thickness of the axial image by the number of images. LA, left atrium.
The follow-up data of these patients were collected until December 2021. Therefore, the median follow-up duration was 6.2 years (IQR, 3.5–11.6 years). Follow-up ECG and echocardiography data were available for all enrolled patients.
Operative strategy
After median sternotomy, the pericardium was harvested to create a baffle. The SVC and the innominate vein were dissected extensively to prevent stenosis by tension, and the azygos vein was divided. For venous drainage during cardiopulmonary bypass (CPB) support, we usually used an innominate vein with the inferior vena cava, or a single venous cannula within the RA with cardiotomy suckers to allow inflow drainage from the SVC during anastomosis. We applied intermittent clamping of the SVC to avoid possible postoperative stenosis from purse-string sutures for cannulation. Direct cannulation into the SVC was avoided in all cases.
Surgery was performed under standard CPB with mild hypothermia (32°C), together with antegrade cardioplegia. Surgical correction of any other concomitant anomaly was performed before the Warden procedure. The SVC was divided above the site of the abnormal pulmonary vein connection. The proximal stump of the SVC was closed directly or with an autologous pericardial patch. To reconstruct the pulmonary venous pathway, a baffle was made between the ASD and SVC orifices using various materials (Table 2). The ASD was extended in 8 patients (36.4%; n=4 in the secundum type; n=2 in the sinus venosus type; n=2 in PFO), and atrial septectomy was performed in 1 patient (4.5%) with an intact atrial septum. Before anastomosis of the SVC to the RA appendage, trabeculations within the RA appendage were trimmed thoroughly to prevent possible stenosis or thrombosis under a low-pressure system. Direct anastomosis of the SVC to the RA appendage was performed to reconstruct the systemic venous pathway in most patients (16 patients, 72.7%). In the other patients, various techniques were used to reduce tension at the anastomosis site, consisting of patch augmentation of the anterior wall (n=3, 13.6%), application of an autologous pericardial roll between the SVC and the RA appendage (n=2, 9.1%), and a pedicled flap of the right atrial wall (n=1, 4.5%) (Table 2). The median CPB time and aortic cross-clamp time were 168 minutes (IQR, 120–188 minutes) and 68 minutes (IQR, 58–106 minutes), respectively.
Table 2.
Procedural characteristics (N=22)
| Structure | Procedure | No. (%) |
|---|---|---|
| Atrial septal defect | Left untouched | 13 (59.1) |
| Extension | 8 (36.4) | |
| Creation | 1 (4.5) | |
| Pulmonary venous pathway | Autologous pericardium | 13 (59.1) |
| Polytetrafluoroethylene patch | 8 (36.4) | |
| Bovine pericardium | 1 (4.5) | |
| Systemic venous pathway | Direct implantation | 16 (72.7) |
| Patch augmentation of anterior wall | 3 (13.6) | |
| Autologous pericardial roll | 2 (9.1) | |
| Right atrial wall pedicled flap | 1 (4.5) |
Statistical analysis
Continuous variables are expressed as median with IQR, and categorical variables are presented as frequencies (%). Differences in baseline characteristics between the groups were evaluated using the Mann-Whitney U test and the Fisher exact test for continuous and categorical variables, respectively. The survival and event-free survival rates were estimated using the Kaplan-Meier method. The statistical analyses were performed using IBM SPSS statistical software ver. 25.0 (IBM Corp., Armonk, NY, USA). All p-values were 2-tailed, and a p-value of <0.05 was considered to indicate statistical significance.
Results
Overall outcomes
There were no cases of early or late mortality after surgery. Transient sinoatrial nodal dysfunction with accelerated junctional rhythm occurred in only 1 patient immediately after the Warden procedure. Atrial pacing was performed until postoperative day 7. Follow-up ECG of the patient on postoperative day 13 showed a normal sinus rhythm. There were no other problems related to heart rhythm during the follow-up period in any patients. Five patients required reinterventions associated with the Warden pathways; 2 in pulmonary venous pathways and 3 in the systemic venous pathway (Table 3). The median diameter of the SVC was 12.3 mm (IQR, 9.3–12.9 mm). The median distance from the PAPVR to the RA-SVC junction was 17.5 mm (IQR, 11.2–22.4 mm) and that from the RA-SVC junction to the lower margin of the ASD was 14.4 mm (IQR, 3.6–21 mm), respectively.
Table 3.
Details of reinterventions
| No. | Age | Body weight (kg) | Concomitant anomaly | ASD type | Patch for baffling | SVC anastomosis | Complication | Reintervention | Interval (Warden–reintervention) (mo) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 10 years | 23.3 | None | Secundum | Bovine pericardium | Autologous pericardial roll | SVC stenosis | SVC stent insertion | 6 |
| 2 | 3 months | 5.8 | Ebstein anomaly | Secundum | GA-fixed autopericardium | Direct anastomosis | SVC stenosis | SVC ballooning | 17 |
| 3 | 28 months | 14.1 | None | Sinus venosus, PFO | PTFE | Direct anastomosis | SVC stenosis | SVC ballooning | 21 |
| 4 | 16 days | 2.9 | CoA, PDA | PFO | PTFE | Direct anastomosis | PV stenosis | Atrial septectomy | 12 |
| 5 | 11 months | 8.7 | PA with VSD, PDA | Intact atrial septum | PTFE | Autologous pericardial roll | PV stenosis, SVC stenosis | Atrial septectomy | 34 |
ASD, atrial septal defect; SVC, superior vena cava; GA, glutaraldehyde; PFO, patent foramen ovale; PTFE, polytetrafluoroethylene; CoA, coarctation of the aorta; PDA, patent ductus arteriosus; PV, pulmonary vein; PA, pulmonary atresia; VSD, ventricular septal defect.
Superior vena cava-right atrium pathway stenosis (n=4)
Three patients required an additional procedure for SVC stenosis that occurred postoperatively. One patient required stent insertion to the SVC 6 months after the Warden procedure, in which an autologous pericardial roll about 10 mm in diameter was used as a conduit between the SVC and the right atrial appendage because of a long distance between the RA appendage and PAPVR at the time of corrective surgery, at the age of 10 years old. The distance between PAPVR and the RA-SVC junction in the preoperative CT of this patient was 31.2 mm, which was the longest among all the enrolled patients. Facial edema developed after 5 months after the Warden procedure, and the mean pressure gradient through the SVC to RA anastomosis site was 13 mm Hg on echocardiography and 21 mm Hg in a cardiac catheterization study. After a 10-mm SVC stent was inserted, the SVC flow was maintained well with a mean pressure gradient of 0.82 mm Hg for a period of 60 months.
Another patient, who required SVC stent insertion, was 28 months old with a body weight of 14.1 kg at the time of the Warden procedure. The preoperative SVC diameter and distance between PAPVR and RA-SVC junction of this patient were 12.6 mm and 16.8 mm, respectively, on preoperative CT. The SVC was directly anastomosed to the RA appendage, and postoperative echocardiography and CT revealed no problems. However, the mean pressure gradient at the SVC-RA anastomosis site reached 18 mm Hg 9 months after the Warden procedure; thus, balloon angioplasty was performed. Although the balloon angioplasty appeared effective immediate after the procedure, a pressure gradient developed again up to 16 mm Hg (catheterization study), and stent (10 mm) insertion was ultimately performed 5 months after ballooning. The pressure gradient through the systemic venous pathway was maintained well with a pressure gradient of 1.2 mm Hg after the second percutaneous intervention.
The last patient required SVC balloon angioplasty 17 months after the Warden procedure. The age and the body weight of the patient were 3 months and 5.8 kg, respectively, at the time of operation. On preoperative CT, the size of the SVC and the distance from PAPVR to the RA-SVC junction were 7.8 mm and 11.2 mm, respectively. The SVC and the RA appendage were directly anastomosed and there were no problems on postoperative echocardiography and CT. However, the pressure gradient was 17.7 mm Hg on echocardiography and 18 mm Hg in a cardiac catheterization study 16 months after the operation, and balloon angioplasty was performed. Although the mean pressure gradient improved after reintervention, it had increased again in the last follow-up echocardiography 25 months after intervention.
We have 1 more patient whose SVC-RA anastomosis site is stenotic and plan to perform a percutaneous intervention. This patient is described in the next section, focusing on pulmonary venous pathway stenosis, because interventions for pulmonary venous pathway stenosis were also performed in this patient.
Pulmonary venous pathway stenosis
Two patients required atrial septectomy after the Warden procedure due to pulmonary venous drainage obstruction resulting from spontaneous stenosis of the original ASD. One of these patients received extension of the PFO and required reintervention 12 months after the Warden procedure. This patient was the youngest (16 days) and smallest (2.9 kg) at the time of the operation in this study. The patient’s PFO was widely opened at the time of the initial Warden procedure, and the baffle was made with a polytetrafluoroethylene (PTFE) patch. Three months after the initial operation, the change in the right upper pulmonary vein inflow was not significant on echocardiography, but engorgement of the right pulmonary veins and congestion of the right lung parenchyma were observed on CT, suggesting pulmonary venous drainage pathway stenosis. Twelve months after surgery, the patient was admitted due to fever and respiratory difficulty. Despite intensive care, the patient experienced cardiac arrest accompanied by pulmonary hemorrhage. CT scans revealed an obstruction in the previous atrial septectomy site, and the patient underwent emergency atrial septectomy. The mean pressure gradient immediately after surgery was 5 mm Hg, which decreased to 1.7–2.2 mm Hg before discharge and was maintained well until the last follow-up. We are using aspirin for this patient.
The second patient had an intact atrial septum and underwent atrial septectomy. This patient also underwent concomitant right ventricle to pulmonary artery (RV-PA) conduit interposition and pulmonary artery angioplasty because of pulmonary atresia. An intra-cardiac baffle for the pulmonary venous pathway was made with a PTFE patch, and a connection between the SVC and RA was made with a pericardial roll. The patient had no specific signs or symptoms after the initial operation. However, pulmonary venous pathway restriction due to atrial septal tissue ingrowth gradually worsened and the RV-PA conduit also became stenotic on follow-up echocardiography. Eventually, the patient underwent additional atrial septectomy and RV-PA conduit change at 34 months after the Warden procedure. The patient underwent another RV-PA conduit change during follow-up, and a percutaneous intervention is planned for stenosis of the pericardial roll conduit between the SVC and RA soon, which has not been managed since the initial operation.
Risk factors for reinterventions
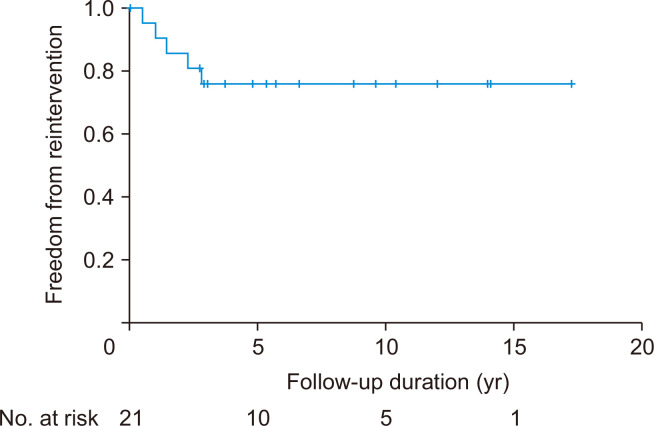
The rate of freedom from reintervention related to the Warden procedure was 75.9% at 5 and 10 years, respectively (Fig. 2). In the risk factor analysis, relationship between the use of autologous pericardial roll and reintervention was statistically significant in the Fisher exact test (odds ratio, 1.667; 95% confidence interval [CI], 0.815–3.409; p= 0.043). The distances from the PAPVR to the SVC-RA junction and from the SVC-RA junction to the lower margin of the ASD were not significantly related to reintervention after surgery (p=0.955 and p=0.734, respectively). Four of the 5 patients with complications requiring reintervention underwent concomitant procedures on the atrial septum, including ASD extension and atrial septectomy. Nevertheless, this tendency did not reach statistical significance in the Fisher exact test (odds ratio, 9.6; 95% CI, 0.848–108.717; p=0.116).
Fig. 2.
Kaplan-Meier curve of freedom from reintervention after the Warden procedure.
We suspected that young age at the time of the Warden procedure could be a risk factor for reintervention because 3 out of 7 infants (age <1 year) presented complications. However, this factor did not show statistical significance (odds ratio, 4.875; 95% CI, 0.590–40.258; p=0.274). In terms of ASD types, we confirmed that the patients with sinus venosus type of ASD showed significantly better outcomes in terms of reintervention after the Warden procedure (odds ratio, 0.77; 95% CI, 0.007–0.901; p=0.039). However, in the multivariable analysis, we could not identify any parameters meaningfully associated with reintervention (Table 4).
Table 4.
Univariate analysis of potential risk factors for reintervention during follow-up
| Variable | Odds ratio (95% confidence interval) | p-value |
|---|---|---|
| Age <1 yr | 4.875 (0.590–40.258) | 0.274 |
| Body weight | - | 0.493 |
| Male sex | 1.050 (0.137–8.021) | 1.000 |
| Preoperative SVC size | - | 0.497 |
| Preoperative distance (PAPVR to RA-SVC junction) | - | 0.955 |
| Preoperative distance (RA-SVC junction to ASD) | - | 0.734 |
| Abnormal pulmonary vein other than RUPV | 1.688 (0.222–12.809) | 1.000 |
| Concomitant ASD procedure (extension, atrial septectomy) | 9.6 (0.848–108.717) | 0.116 |
| Other concomitant procedures | 2.75 (0.355–21.3) | 0.609 |
| Sinus venosus type ASD | 0.77 (0.007–0.901) | 0.039 |
| Use of autologous pericardial roll | 1.667 (0.815–3.409) | 0.043 |
SVC, superior vena cava; PAPVR, partial anomalous pulmonary venous return; RA, right atrium; ASD, atrial septal defect; RUPV, right upper pulmonary vein.
Discussion
Several surgical options have been introduced for the correction of PAPVR, such as the single-patch technique, the double-patch technique, and the Warden procedure. However, it is very challenging to perform successful surgical correction with single-patch intra-cardiac baffling if the abnormal pulmonary vein is connected to the distal SVC. In such cases, the double-patch technique or Warden procedure can be considered [2]. Some researchers have recently reported favorable early and midterm postoperative outcomes of the Warden procedure [4,5,9]. In this study, there were no cases of early or late mortality. The rate of freedom from reintervention at 10 years after surgery was 86.5%. The results of this study are consistent with those of previous reports regarding the surgical outcomes of the Warden procedure.
Heart rhythm problems
Attenhofer Jost et al. [10] reviewed 131 patients who underwent surgical repair of sinus venosus-type ASD and reported that there were several patients with postoperative arrhythmias, consisting of 7 patients with sinus node dysfunction or permanent pacemaker and 15 patients with atrial fibrillation at a follow-up of 144±99 months. They suggested that the potential mechanism of rhythm complications after surgery for sinus venosus-type ASD and PAPVR include anatomical anomalies of the sinus node or surgical trauma to the sinus node, the internodal tract, and the blood supply to the sinus node. Zubritskiy et al. [6] compared the postoperative outcomes of the double patch technique and the Warden procedure in 40 patients with a median follow-up duration of 22 months. Both groups showed no early mortality and similar postoperative outcomes; however, the incidence of sinoatrial nodal dysfunction was significantly higher in the double patch group [6]. Stewart et al. [8] also reported similar results, in that 55% of the patients who underwent the double-patch technique developed rhythm complications, while none of the patients in the Warden procedure group did so. According to them, this is because the Warden procedure does not include an incision near the sinoatrial node or the sinoatrial artery. Correspondingly, only 1 patient in our report revealed postoperative sinoatrial nodal dysfunction, which was transient and recovered spontaneously before discharge. This patient had a large secundum-type ASD, and the Warden procedure was performed without atrial manipulation.
Superior vena cava-right atrium pathway stenosis
SVC obstruction occurs infrequently, but it is still an important complication after the Warden procedure. Federspiel et al. [11] retrospectively reviewed the medical records of patients treated surgically for anomalous right upper pulmonary veins at a single center. Among the 9 patients who underwent the Warden procedure, 2 required early surgical revision of the anastomosis site due to SVC obstruction [11]. Park et al. [12] demonstrated that 3 patients had systemic venous pathway obstruction out of 30 patients who underwent the Warden procedure, and 2 of them needed reoperation. In their study, these 3 patients had common features, including low body weight (<7 kg) and younger age (<2 years). They reported that SVC obstruction is related to technical issues and can therefore occur in situations where tension- free anastomosis is not guaranteed. The authors argued that patch augmentation in the anastomosis of the SVC and RA appendage might reduce the frequency of SVC obstruction in situations where tension-free anastomosis is not guaranteed. Similarly, Kim et al. [13] reported that they adopted a RA appendage flap in SVC-RA anastomosis in 10 patients during the Warden procedure, and SVC obstruction did not occur during a median follow-up of 29.5 months. In our study, 3 patients underwent reintervention for SVC stenosis and 1 patient is a potential candidate for reintervention (patient number 5 in Table 3, who received a pericardial roll). Both patients who received an autologous pericardial roll conduit between the SVC and RA showed progression of SVC inflow stenosis. One of them had the longest distance from PAPVR to the RA-SVC junction (31.2 mm on preoperative CT), while the median value of this distance in all patients was 17.5 mm. The relationship between the distance from PAPVR to the SVC-RA junction and reintervention was not statistically significant, probably because of the small number of patients. However, it seems clear that surgical strategies and techniques guaranteeing tension-free anastomosis and maintaining the growth potential of the SVC might be essential to prevent SVC stenosis, especially in patients with an anomalous pulmonary vein with a high connection to the SVC.
Pulmonary venous pathway stenosis
Zhu et al. [14] reported the outcomes of the Warden procedure with an ASD enlargement procedure, which was indicated when an intact atrial septum was present or the size of the ASD was smaller than the orifice of the abnormal pulmonary vein. Pulmonary venous tract stenosis requiring surgical reintervention occurred in 2 cases in their report [14]. In their study, degenerative thickening of the pericardial baffle patch was the main mechanism of pulmonary venous tract stenosis. The authors speculated that a long distance between the pulmonary vein orifice and ASD may contribute to baffle obstruction. In our patients, 1 patient with an intact atrial septum underwent concomitant atrial septectomy, and another patient underwent a concomitant ASD widening procedure for PFO. Both patients required additional atrial septectomy at 12 and 34 months after the Warden procedure. The presence of the sinus venosus type ASD was significantly related to reintervention after the surgery, with an odds ratio of 0.77 and a p-value of 0.039. However, a concomitant ASD widening procedure was not revealed as a risk factor for stenosis of the pulmonary venous pathway, with a p-value of 0.116. In some previous reports on the natural course of ASD, sinus venosus-type ASD usually did not decrease in size [15]; however, secundum ASD is spontaneously closed or decreased in size to less than 3 mm in 62% of the patients, and the age at diagnosis was also associated with the incidence of spontaneous ASD closure [16]. In patients who require procedures such as the creation of an atrial septum or extension of the native atrial septum, or need additional material for connection between SVC and RA, especially in cases where the entire conduit is used, we should closely observe changes in the pressure gradient through the pulmonary venous or systemic venous pathway over time via echocardiography or CT, as well as patient’s symptoms or signs related to venous stenosis.
This study has several limitations. First, this was a retrospective study conducted at a single center. The number of enrolled patients was relatively small to draw conclusions for risk factor analysis. Furthermore, the median follow-up duration of this study is less than 10 years; therefore, further research on long-term outcomes is required.
In conclusion, the Warden procedure is a safe and effective surgical option for treating PAPVR to the SVC. While performing the SVC to the RA appendage anastomosis, tension-free anastomosis should be guaranteed, and surgical techniques that do not interfere with growth potential should be selected. In cases that require a concomitant ASD widening procedure to achieve a sufficient venous pathway or interposition of a conduit between the SVC and RA due to the shortness of the SVC in the Warden procedure, stenotic complications of the venous pathway may occur. Careful observation of changes in the pressure gradient or anatomical stenosis is required in such patients.
Footnotes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
References
- 1.Healey JE., Jr An anatomic survey of anomalous pulmonary veins: their clinical significance. J Thorac Surg. 1952;23:433–44. doi: 10.1016/S0096-5588(20)31117-X. [DOI] [PubMed] [Google Scholar]
- 2.Alsoufi B, Cai S, Van Arsdell GS, Williams WG, Caldarone CA, Coles JG. Outcomes after surgical treatment of children with partial anomalous pulmonary venous connection. Ann Thorac Surg. 2007;84:2020–6. doi: 10.1016/j.athoracsur.2007.05.046. [DOI] [PubMed] [Google Scholar]
- 3.Warden HE, Gustafson RA, Tarnay TJ, Neal WA. An alternative method for repair of partial anomalous pulmonary venous connection to the superior vena cava. Ann Thorac Surg. 1984;38:601–5. doi: 10.1016/S0003-4975(10)62317-X. [DOI] [PubMed] [Google Scholar]
- 4.Kottayil BP, Dharan BS, Menon S, et al. Anomalous pulmonary venous connection to superior vena cava: Warden technique. Eur J Cardiothorac Surg. 2011;39:388–91. doi: 10.1016/j.ejcts.2010.06.036. [DOI] [PubMed] [Google Scholar]
- 5.Yong MS, Griffiths S, Robertson T, et al. Outcomes of the Warden procedure for partial anomalous pulmonary venous drainage in children. Interact Cardiovasc Thorac Surg. 2018;27:422–6. doi: 10.1093/icvts/ivy097. [DOI] [PubMed] [Google Scholar]
- 6.Zubritskiy A, Naberukhin Y, Arkhipov A, et al. Outcomes of double-patch and Warden techniques in patients with supracardiac partial anomalous pulmonary venous connection. Heart Lung Circ. 2020;29:156–61. doi: 10.1016/j.hlc.2018.11.020. [DOI] [PubMed] [Google Scholar]
- 7.Gustafson RA, Warden HE, Murray GF. Partial anomalous pulmonary venous connection to the superior vena cava. Ann Thorac Surg. 1995;60(6 Suppl):S614–7. doi: 10.1016/S0003-4975(21)01211-X. [DOI] [PubMed] [Google Scholar]
- 8.Stewart RD, Bailliard F, Kelle AM, Backer CL, Young L, Mavroudis C. Evolving surgical strategy for sinus venosus atrial septal defect: effect on sinus node function and late venous obstruction. Ann Thorac Surg. 2007;84:1651–5. doi: 10.1016/j.athoracsur.2007.04.130. [DOI] [PubMed] [Google Scholar]
- 9.Pavy C, Gavira N, Maminirina P, Baron O. Right partial anomalous pulmonary venous connection to the superior vena cava following the Warden procedure. J Card Surg. 2018;33:565–9. doi: 10.1111/jocs.13782. [DOI] [PubMed] [Google Scholar]
- 10.Attenhofer Jost CH, Connolly HM, Danielson GK, et al. Sinus venosus atrial septal defect: long-term postoperative outcome for 115 patients. Circulation. 2005;112:1953–8. doi: 10.1161/CIRCULATIONAHA.104.493775. [DOI] [PubMed] [Google Scholar]
- 11.Federspiel JM, Das De S, Lilley S, et al. Superior vena cava inflow following repair for anomalous right pulmonary venous drainage in children. Pediatr Cardiol. 2019;40:1275–83. doi: 10.1007/s00246-019-02148-6. [DOI] [PubMed] [Google Scholar]
- 12.Park CS, Kwak JG, Lee C, et al. Partial anomalous pulmonary venous connection to the superior vena cava: the outcome after the Warden procedure. Eur J Cardiothorac Surg. 2012;41:261–5. doi: 10.1016/j.ejcts.2011.05.043. [DOI] [PubMed] [Google Scholar]
- 13.Kim C, Cho YH, Lee M, et al. Surgery for partial anomalous pulmonary venous connections: modification of the Warden procedure with a right atrial appendage flap. Korean J Thorac Cardiovasc Surg. 2014;47:94–9. doi: 10.5090/kjtcs.2014.47.2.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu J, Kotani Y, Chetan D, et al. Is restrictive atrial septal defect a risk in partial anomalous pulmonary venous drainage repair? Ann Thorac Surg. 2014;97:1664–70. doi: 10.1016/j.athoracsur.2014.01.051. [DOI] [PubMed] [Google Scholar]
- 15.Geva T, Martins JD, Wald RM. Atrial septal defects. Lancet. 2014;383:1921–32. doi: 10.1016/S0140-6736(13)62145-5. [DOI] [PubMed] [Google Scholar]
- 16.Hanslik A, Pospisil U, Salzer-Muhar U, Greber-Platzer S, Male C. Predictors of spontaneous closure of isolated secundum atrial septal defect in children: a longitudinal study. Pediatrics. 2006;118:1560–5. doi: 10.1542/peds.2005-3037. [DOI] [PubMed] [Google Scholar]