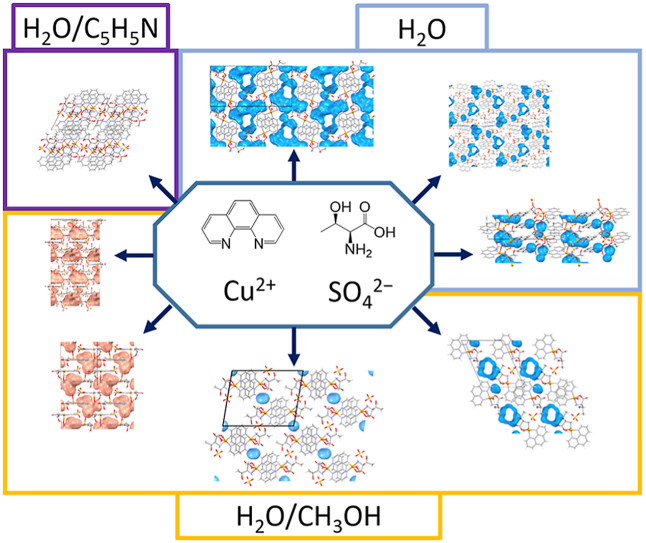
Abstract
Solution-based and solid-state reactions of copper(ii) compounds, 1,10-phenanthroline and l-threonine were investigated. Eight new ternary coordination compounds were obtained:
[Cu(l-Thr)(H2O)(phen)]2SO4∙10H2O (1a∙10H2O), [Cu(l-Thr)(H2O) (phen)]2SO4∙4.3H2O (1a∙4.3H2O), {[Cu(μ-l-Thr)(phen)]2SO4∙3.5H2O}n (1b∙3.5H2O), [Cu(l-Thr)(H2O)(phen)][Cu(l-Thr)(CH3OH)(phen)]SO4∙2H2O∙CH3OH (1c∙2H2O∙CH3OH), [Cu(l-Thr)(H2O)(phen)][Cu(l-Thr)(CH3OH)(phen)]SO4∙4H2O (1c∙4H2O), [Cu(l-Thr)(CH3OH)(phen)]2SO4∙H2O (1d∙H2O), [Cu(l-Thr)(H2O)(phen)][Cu(l-Thr)(CH3OH)(phen)][Cu(SO4)(l-Thr)(phen)]HSO4∙H2O∙3CH3OH (1e·H2O·3CH3OH), [Cu(l-Thr)(H2O)(phen)][Cu(l-Thr)(phen)(py)]SO4 (1f) (phen = 1,10-phenanthroline, l-Thr = l-threoninate, py = pyridine). X-ray crystal structure analysis of the prepared ternary coordination compounds revealed extensive hydrogen bonding and π-interactions that link complex species, anions and solvent molecules into 3D architectures. The water/methanol solvent molecules are found in pockets and/or channels in seven solvates. ESR spectra of different types of compounds were also investigated. In all measured compounds the unpaired electron of the copper(II) ion is located in the dx2-y2 orbital which is in agreement with elongated square-pyramidal geometry. Compound 1a∙10H2O showed substantial cytotoxic activity toward human hepatocellular carcinoma (HepG2) and acute monocytic leukaemia (THP-1) cell lines.
Keywords: Ternary coordination compounds; Solvatomorphs; Crystal structures; ESR spectra; Solution-based synthesis; Mechanochemical synthesis; Copper complexes; l-threonine; 1,10-Phenanthroline
Graphical abstract
Ternary coordination compounds; Solvatomorphs; Crystal structures; ESR spectra; Solution-based synthesis; Mechanochemical synthesis; Copper complexes; Lthreonine; 1,10-Phenanthroline.
1. Introduction
Investigation of ternary coordination compounds of essential metals with amino acids and heterocyclic bases have been in focus for many years. Previous studies of such coordination compounds can be divided into three main study directions: biological activity, crystal structures and porosity. Most studies of these systems include copper as an essential metal. Copper is important for the function of several enzymes and proteins (cytochrome oxidase, superoxide dismutase, ascorbate oxidase, tyrosinase). Its deficiency or overload is associated with Menkes and Wilson disease, respectively [1].
Several Cu coordination compounds with amino acids and heterocyclic ligands have been prepared and some of them have been identified as potential drugs [2, 3, 4, 5, 6, 7] or functional porous materials [8, 9, 10]. Ruiz-Azuara has invented a process to prepare ternary coordination compounds of the type [Cu(N–N)(N–O)]NO3, with the N–N ligand corresponding to 4,7-diphenyl-1,10-phenanthroline and the N–O ligand preferably being one of the aminocarboxylates (glycinate, alaninate, isoleucinate, leucinate, serinate and valinate) to be used as anticancerogenic agents for treatment of cancerogenic tumors [11, 12]. Furthermore, Ruiz-Azuara et al. have investigated antineoplastic properties of a class of coordination compounds, called Casiopeinas® of the general formula [Cu(N–N)(α-l-aminocarboxylate)]2+/+ (N–N ligands are substituted 2,2′-bipyridines or 1,10-phenanthrolines, α-l-aminocarboxylates are glycinate, alaninate, isoleucinate, leucinate, serinate or valinate). It was shown that the presence of a N–N ligand is crucial for preservation of antiproliferative activity, whereas the α-l-aminocarboxylate withdraws electrons and enhances π-interactions with adenine. Also, an aminocarboxylate as a ligand with various binding possibilities contributes to other properties such as plasticity of the coordination sphere, prevention of oxidation and biocompatibility of the metal ion environment. Some of these complexes can induce cell apoptosis in vitro and also exhibit excellent in vivo antitumor activity [13].
Since amino acids possess different side chains, their coordination compounds can create structures of higher dimensionalities (1D, 2D and 3D) through different non-covalent interactions (hydrogen bonds, π-stacking). In most of the ternary copper coordination compounds with amino acids and 1,10-phenanthroline the copper(ii) atom is either five- or six-coordinated [14]. Depending on the amino acid and the presence of a heterocyclic base, different architectures can be formed and, among others, porous structures into which can be incorporated guest molecules such as solvents to produce different solvatomorphs [5, 15]. In CSD there are 98 data sets of ternary copper coordination compounds involving aminoacidates and 1,10-phenanthroline. These 98 data sets being published in 80 different articles show the lack of systematic exploration of the crystal structures and structure-property relationship of such copper(ii) ternary coordination compounds. Only in two research articles different solvates of these ternary coordination compounds were investigated – monohydrate and unhydrated alaninato complexes [16] and six serinato solvates involving water and/or methanol [5]. Both described alaninato compounds are coordination polymers, where carboxylic group acts as bridge between two copper(II) atoms. In serinato compounds, all six compounds contain monomeric complex cations of general formula [Cu(ser) (L)(phen)]+ (ser = l-serinato; L = H2O or CH3OH; phen = 1,10-phenanthroline). Three of those compounds contain only water as solvent, two of them contain only methanol, while one compound contains mixture of water and methanol molecules (coordinated and crystallization molecules).
In the early papers published in the 1980s coordination of the copper atom was the main concern of researchers [17, 18, 19], while in later papers intermolecular interactions were explored [20, 21]. In the recent years, as mentioned earlier, such ternary coordination compounds are mostly investigated for their antiproliferative activity [3, 13, 22, 23]. In contrast to solid-state, these complexes are well explored in solution. Binding modes at different pH-values of ternary coordination compounds were investigated with different amino acids and heterocyclic bases, including l-threonine and 1,10-phenanthroline [24, 25]. It was shown that ternary coordination species [Cu(aa)(N–N)]+ (aa = aminoacidate; N–N = 2,2′-bipyridine or 1,10-phenanthroline) are more stable than either [Cu(aa)2] or [Cu(N–N)2]2+ binary compounds [25, 26, 27, 28].
As a part of our ongoing research on ternary copper(ii) coordination compounds with amino acids and heterocyclic bases [5, 29, 30, 31], in this work we explored the versatility of intermolecular interactions in copper ternary coordination compounds with l-threonine and 1,10-phenanthroline for the purpose of crystal engineering and investigation of structure-property relationships, such as magnetic property (ESR spectroscopy) or biological activity. We have performed the reactions of copper (ii) compounds with l-threonine and 1,10-phenanthroline by solution methods and solid-state reactions. These two ligands were chosen having in mind a polar ligand and a nonpolar ligand, and their ability to form different types of intermolecular contacts. The effects of solvent (water, methanol, pyridine (py) or their binary mixtures) on crystallization and crystal structures was investigated. The following new compounds were obtained: [Cu(l-Thr)(H2O)(phen)]2SO4∙10H2O (1a∙10H2O), [Cu(l-Thr)(H2O)(phen)]2SO4∙4.3H2O (1a∙4.3H2O), {[Cu(μ-l-Thr)(phen)]2SO4∙3.5H2O}n (1b∙3.5H2O), [Cu(l-Thr)(H2O)(phen)][Cu(l-Thr)(CH3OH)(phen)]SO4∙2H2O∙CH3OH (1c∙2H2O∙CH3OH), [Cu(l-Thr)(H2O)(phen)][Cu(l-Thr) (CH3OH)(phen)]SO4∙4H2O (1c∙4H2O), [Cu(l-Thr)(CH3OH)(phen)]2SO4∙H2O (1d∙H2O), [Cu(l-Thr)(H2O)(phen)][Cu(l-Thr)(CH3OH)(phen)][Cu(SO4)(l-Thr)(phen)]HSO4∙H2O∙3CH3OH (1e·H2O·3CH3OH), [Cu(l-Thr)(H2O)(phen)][Cu(l-Thr)(phen)(py)]SO4 (1f).
2. Materials and methods
Copper(ii) sulfate pentahydrate (Gram-Mol), anhydrous copper (ii) sulfate (Acros), 1,10-phenanthroline monohydrate (Merck), l-threonine (Fischer Scientific), methanol (Alkaloid), DMEM/F12 medium (Sigma), FBS (Sigma), DMSO (Sigma) and staurosporine (Sigma) were obtained from commercial sources and used without further purification. Copper(ii) hydroxide was prepared by the method of Agte [32, 33]. Copper(ii) sulfate trihydrate was prepared by heating copper(ii) sulfate pentahydrate to 100 °C and copper(ii) sulfate monohydrate by heating copper(ii) sulfate pentahydrate to 150 °C. Anhydrous 1,10-phenanthroline was prepared by heating 1,10-phenanthroline monohydrate to 130 °C. Attenuated total reflectance infrared (ATR-IR) spectra were measured on a Thermo Scientific™ Nicolet™ iS50 FTIR Spectrometer equipped with an ATR module in the spectral range 4000–400 cm−1 with a resolution of 4 cm−1. Powder X-ray diffraction (PXRD) was performed on a Malvern Panalytical Aeris diffractometer in a Bragg-Brentano geometry with CuKα radiation (λ = 1.54184 Å) at room temperature. The samples were contained on a silicon holder and the diffractograms were measured in 2θ range 5–40° with a step size of 0.022° and 15.0 s of measurement time per step. PXRD data were analyzed using HighScore Plus program [34]. Mechanochemical syntheses were performed on a Retch MM200 grinder operating at frequency of 25 Hz for 15 min. Teflon jars (volume 14 mL) and stainless steel balls (diameter 8 mm) were used for grinding.
2.1. Solution-based syntheses
2.1.1. Synthesis and crystallization of [Cu(l-Thr)(H2O)(phen)]2SO4·10H2O (1a·10H2O) and [Cu(l-Thr)(H2O)(phen)]2SO4·4.3H2O (1a·4.3H2O)
Copper(ii) sulphate pentahydrate (64.1 mg, 0.25 mmol), copper(ii) hydroxide (25.5 mg, 0.25 mmol), 1,10-phenanthroline monohydrate (101.1 mg, 0.5 mmol) and l-threonine (60.2 mg, 0.5 mmol) were dissolved in water (10 mL). Solution was heated at 100 °C for 45 min. The solution was filtered and slowly evaporated for a week. Eventually, dark blue needles of either 1a·10H2O and/or 1a·4.3H2O crystallized. Crystals of 1a·10H2O were suitable for single-crystal X-ray diffraction analysis. Crystals of 1a·4.3H2O diffracted poorly so synchrotron radiation was used for data collection. Both crystals of 1a·10H2O and 1a·4.3H2O decompose when taken out of the solution. IR (ATR) for 1a·10H2O: /cm−1 = 3600–2900 (m), 3341 (s), 3255 (s), 3120 (s), 3060 (m), 2976 (m), 2935(m), 2806 (w), 1658 (s), 1603 (s), 1519 (m), 1494 (w), 1430 (m), 1402 (m), 1367 (m), 1345 (w), 1298 (w), 1262 (w), 1224 (w), 1185 (m), 1142 (m), 1099 (m), 1059 (s), 1035 (s), 1005 (m), 976 (m), 911 (m), 874 (m), 856 (s), 813 (w), 783 (w), 736 (w), 722 (s), 681 (w), 645 (w), 605 (w), 576(w), 556 (w), 474 (w), 428 (m).
2.1.2. Synthesis and crystallization of [Cu(l-Thr)(H2O)(phen)]2SO4·10H2O (1a·10H2O)
Copper(ii) sulphate pentahydrate (64.6 mg, 0.25 mmol), copper(ii) hydroxide (25.2 mg, 0.25 mmol), 1,10-phenanthroline monohydrate (100.3 mg, 0.5 mmol) and l-threonine (61.3 mg, 0.5 mmol) were dissolved in methanol/water mixture (10 mL; v/v = 7:3 or 1:9) and heated for 45 min at 80 °C. The dark blue solution was filtered and after few days dark blue needles of 1a·10H2O were formed. Crystals were suitable for single-crystal X-ray diffraction analysis.
2.1.3. Synthesis and crystallization of [Cu(l-Thr)(H2O)(phen)]2SO4·10H2O (1a·10H2O), [Cu(l-Thr)(H2O)(phen)][Cu(l-Thr)(CH3OH)(phen)]SO4∙2H2O∙CH3OH (1c∙2H2O∙CH3OH) and [Cu(l-Thr)(phen) (CH3OH)]2SO4·H2O (1d·H2O)
Copper(ii) sulphate pentahydrate (64.1 mg, 0.25 mmol), copper(ii) hydroxide (24.7 mg, 0.25 mmol), 1,10-phenanthroline monohydrate (99.2 mg, 0.5 mmol) and l-threonine (59.3 mg, 0.5 mmol) were dissolved in methanol (10 mL). The solution was heated at 80 °C for 45 min and filtered. Dark blue solution was left to evaporate and after few days dark blue needles (1a·10H2O) and/or prisms (1c∙2H2O∙CH3OH and/or 1d·H2O) suitable for single-crystal X-ray analysis were formed. Crystals of 1c∙2H2O∙CH3OH slowly decompose outside of solution while those of 1d·H2O are stable at room temperature. IR (ATR) for 1c∙2H2O∙CH3OH: /cm−1 = 3600–2900 (m), 3206 (s), 3114 (s), 2978 (m), 2935(m), 2906 (m), 2832 (m), 1630 (s), 1610 (s), 1518 (m), 1496 (w), 1429 (m), 1386 (m), 1361 (m), 1347 (m), 1260 (w), 1228 (w), 1196 (m), 1140 (m), 1084 (m), 1054 (s), 1044 (s), 1027 (s), 1002 (s), 968 (m), 902 (m), 867 (m), 853 (s), 808 (w), 783 (w), 741 (m), 722 (s), 686 (w), 650 (w), 611 (w), 556 (s), 490 (w), 466 (w), 431 (m).
2.1.4. Synthesis and crystallization of [Cu(l-Thr)(H2O)(phen)][Cu(l-Thr) (CH3OH) (phen)]SO4∙4H2O (1c∙4H2O) and [Cu(l-Thr)(H2O)(phen)][Cu(l-Thr)(CH3OH)(phen)][Cu(SO4) (l-Thr) (phen)]HSO4·H2O·3CH3OH (1e·H2O·3CH3OH)
Copper(ii) sulphate monohydrate (46.8 mg, 0.25 mmol), copper(ii) hydroxide (24.9 mg, 0.25 mmol), anhydrous 1,10-phenanthroline (90.9 mg, 0.5 mmol), and l-threonine (62.3 mg, 0.5 mmol) were dissolved in methanol (10 mL). The solution was heated at 80 °C for 45 min, filtered and left to evaporate. Eventually, dark blue prisms (1c∙4H2O) and/or needles (1e·H2O·3CH3OH) formed after a few weeks. Crystals of 1e·H2O·3CH3OH are highly unstable outside solution and decompose in few seconds. Crystals of 1c∙4H2O were suitable for single-crystal X-ray diffraction, while crystals of 1e·H2O·3CH3OH diffracted poorly and were analysed by synchrotron irradiation.
2.1.5. Synthesis and crystallization of [Cu(l-Thr) (phen)(CH3OH)]2SO4·H2O (1d·H2O) and [Cu(l-Thr)(H2O)(phen)][Cu(l-Thr)(CH3OH)(phen)][Cu(SO4)(l-Thr) (phen)]HSO4∙H2O∙3CH3OH (1e·H2O·3CH3OH)
Anhydrous copper(ii) sulphate (40.0 mg, 0.25 mmol), copper(ii) hydroxide (24.3 mg, 0.25 mmol), anhydrous 1,10-phenanthroline (90.1 mg, 0.5 mmol) and l-threonine (60.3 mg, 0.5 mmol) were dissolved in methanol (10 mL). The solution was heated at 80 °C for 45 min, filtered and left to evaporate. After a few days blue prisms (1d·H2O), needles (1e·H2O·3CH3OH) and/or light blue prisms ([Cu(SO4)(phen)2]·CH3OH (CSD refcode MUNHIO [35]) were formed.
2.1.6. Synthesis and crystallization of {[Cu(μ-l-Thr)(phen)]2SO4·3.5H2O}n (1b·3.5H2O)
Copper(ii) sulphate pentahydrate (66.0 mg, 0.25 mmol), copper(ii) hydroxide (24.5 mg, 0.25 mmol), 1,10-phenanthroline monohydrate (101.2 mg, 0.5 mmol), l-threonine (61.5 mg, 0.5 mmol) were dissolved in a water/pyridine mixture (10 mL; v/v = 1:9) and heated at 80 °C for 45 min, filtered and left to evaporate. After a few days blue needles of 1b·3.5H2O were formed. Crystals of 1b·3.5H2O are unstable outside of solution, but were suitable for single-crystal X-ray diffraction analysis. IR (ATR) for 1b·3.5H2O: /cm−1 = 3600–2900 (m), 3294 (m), 3089 (s), 3062 (m), 3044 (m), 3023 (m), 2972 (w), 2933 (m), 2830 (w), 1623 (s), 1603 (s), 1519 (m), 1492 (m), 1448 (m), 1430 (m), 1400 (m), 1387 (m), 1349 (w), 1315 (w), 1267 (w), 1260 (w), 1224 (w), 1162 (m), 1144 (m), 1072 (s), 1050 (s), 1043 (s), 1013 (m), 903 (m), 873 (m), 851 (s), 807 (w), 779 (m), 761 (m), 739 (m), 719 sw), 700 (s), 637 (w), 616 (w), 599 (m), 558 (w), 540 (w), 534 (w), 523 (w), 510 (w), 500 (w), 490 (w), 482 (w), 466 (w), 446 (m), 430 (m), 416 (m).
2.1.7. Synthesis and crystallization of [Cu(l-Thr)(phen)(CH3OH)]2SO4·2H2O (1d·H2O)
Copper(ii) sulphate pentahydrate (66.1 mg, 0.25 mmol), copper(ii) hydroxide (24.8 mg, 0.25 mmol), 1,10-phenanthroline monohydrate (99.5 mg, 0.5 mmol), l-threonine (62.3 mg, 0.5 mmol) were dissolved in methanol/pyridine mixture (10 mL; v/v = 9:1) and heated at 80 °C for 45 min, filtered and left to evaporate. After a few weeks blue prisms of 1d·H2O were formed.
2.1.8. Synthesis and crystallization of {[Cu(μ-l-Thr)(phen)]2SO4·3.5H2O}n (1b·3.5H2O) and [Cu(l-Thr)(phen)(CH3OH)]2SO4·2H2O (1d·H2O)
Copper(ii) sulphate pentahydrate (63.0 mg, 0.25 mmol), copper(ii) hydroxide (24.5 mg, 0.25 mmol), 1,10-phenanthroline monohydrate (99.8 mg, 0.5 mmol), and l-threonine (59.6 mg, 0.5 mmol) were dissolved in methanol/pyridine mixture (10 mL; v/v = 7:3) and heated at 80 °C for 45 min, filtered and left to evaporate. After a few days blue needles of 1b·3.5H2O and/or prisms of 1d·H2O were formed.
2.1.9. Synthesis and crystallization of [Cu(l-Thr)(H2O)(phen)][Cu(l-Thr)(phen)(py)]SO4 (1f)
Copper(ii) sulphate pentahydrate (63–64 mg, 0.25 mmol), copper(ii) hydroxide (24 mg, 0.25 mmol), 1,10-phenanthroline monohydrate (100–106 mg, 0.5 mmol) and l-threonine (62–66 mg, 0.5 mmol) were dissolved in a methanol/pyridine mixture (10 mL; v/v = 3:7 or 1:9) and heated at 80 °C for 45 min, filtered and left to evaporate. After several weeks few prisms of 1f were formed. IR (ATR) for 1f: /cm−1 = 3550–2900 (m), 3226 (s), 3154 (m), 3058 (s), 3008 (m), 2965 (m), 2930 (m), 2873 (w), 1630 (s), 1600 (s), 1586 (s), 1520 (m), 1492 (w), 1432 (m), 1416 (w), 1371 (m), 1348 (m), 1325 (m), 1277 (w), 1221 (m), 1145 (m), 1095 (m), 1064 (m), 1032 (s), 1009 (m), 958 (m), 938 (m), 921 (w), 909 (w), 873 (m), 863 (s), 847 (s), 780 (m), 761 (m), 739 (m), 722 (s), 706 (s), 648 (m), 623 (w), 600 (s), 582 (m), 562 (w), 509 (w), 499 (w), 490 (w), 460 (w), 445 (m), 429 (m), 417 (m), 408 (m).
2.2. Mechanochemical syntheses
2.2.1. Synthesis of [Cu(l-Thr)(H2O)(phen)]2SO4·10H2O (1a·10H2O)
Copper(ii) sulphate pentahydrate (62.5 mg, 0.25 mmol), copper(ii) hydroxide (24.4 mg, 0.25 mmol), anhydrous 1,10-phenanthroline (90.2 mg, 0.5 mmol) and l-threonine (59.5 mg, 0.5 mmol) were mixed in a Teflon jar (V = 14 mL) and 24 μL of water was added (η = 0.1 μL mg−1) [36]. Grinding was performed using one steel ball (8 mm) at a vibration frequency 25 Hz for 15 min. The product was analysed by powder X-ray diffraction. Powder diffraction pattern was consistent with powder pattern calculated from crystal structure of 1a·10H2O (Fig. S1 in Supplementary information). Another PXRD experiments were performed on the same sample after 2 and 5 min and it was confirmed that the decomposition of compound began (Fig. S1 in Supplementary information).
2.2.2. Synthesis of [Cu(l-Thr)(H2O)(phen)]2SO4·10H2O (1a·10H2O) and[Cu(l-Thr)(H2O)(phen)][Cu(l-Thr)(CH3OH)(phen)]SO4·2H2O·CH3OH (1c·2H2O·CH3OH)
Copper(ii) sulphate pentahydrate (62.4 mg, 0.25 mmol), copper(ii) hydroxide (24.7 mg, 0.25 mmol), anhydrous 1,10-phenanthroline (90.1 mg, 0.5 mmol) and l-threonine (59.9 mg, 0.5 mmol) were mixed in a Teflon jar (V = 14 mL) and 47 μL of methanol was added (η = 0.2 μL mg−1). Grinding was perfomed using one steel ball (8 mm) at vibration frequency 25 Hz for 15 min. The product was analysed by powder X-ray diffraction. The powder diffraction pattern was consistent with the powder pattern calculated from crystal structures of 1a·10H2O and 1c·2H2O·CH3OH (Fig. S1 in Supplementary information).
2.2.3. Synthesis of [Cu(l-Thr)(H2O)(phen)][Cu(l-Thr)(CH3OH) (phen)]SO4·2H2O·CH3OH (1c·2H2O·CH3OH)
Copper(ii) sulphate trihydrate (53.5 mg, 0.25 mmol), copper(ii) hydroxide (24.3 mg, 0.25 mmol), anhydrous 1,10-phenanthroline (90.5 mg, 0.5 mmol) and l-threonine (59.5 mg, 0.5 mmol) were mixed in a Teflon jar (V = 14 mL) and 46 μL of methanol was added (η = 0.2 μL mg−1). Grinding was perfomed using one steel ball (8 mm) at vibration frequency 25 Hz for 15 min. The product was analysed by powder X-ray diffraction. The powder diffraction pattern was consistent with the powder pattern calculated from crystal structure of 1c·2H2O·CH3OH (Fig. S1 in Supplementary information).
2.2.4. Synthesis of [Cu(l-Thr)(CH3OH)(phen)]2SO4·H2O (1d·H2O)
Anhydrous copper(ii) sulphate (39.9 mg, 0.5 mmol), copper(ii) hydroxide (24.7 mg, 0.25 mmol), anhydrous 1,10-phenanthroline (90.1 mg, 0.5 mmol) and l-threonine (59.5 mg, 0.5 mmol) were mixed in a Teflon jar (V = 14 mL) and 43 μL of methanol was added (η = 0.2 μL mg−1). Grinding was perfomed using one steel ball (8 mm) at vibration frequency 25 Hz for 15 min. The product was analysed by powder X-ray diffraction. Most diffraction maxima were consistent with the powder pattern calculated from the crystal structure of 1d·H2O, but a small amount of unknown phase was also detected (Fig. S1 in Supplementary information).
2.3. Single-crystal X-ray diffraction
Single-crystal X-ray diffraction data of 1a∙10H2O, 1b∙3.5H2O, 1c∙2H2O∙CH3OH, 1c∙4H2O and 1f were measured on a Rigaku XtaLAB Synergy diffractometer using CuKα radiation (λ = 1.54184 Å) with HyPix6000HE detector at 170 (1b∙3.5H2O, 1c∙2H2O∙CH3OH, 1c∙4H2O and 1f) or 180 K (1a∙10H2O). Diffraction data of 1d∙H2O were collected on an Oxford Diffraction Xcallibur3 CCD diffractometer using MoKα radiation (λ = 0.71073 Å) at room temperature. X-ray diffraction data of 1a∙4.3H2O and 1e·H2O·3CH3OH were collected at Elettra Sincrotrone Trieste using synchrotron radiation (λ = 0.70000 Å) at XRD1 beamline equipped with Dectris Pilatus 2M detector at 100 K. Data reduction for all data sets was performed using the CrysAlis software package [37]. All crystal structures except 1f were solved by SHELXS [38] and 1f was solved by SHELXT [39] program. Full-matrix least-squares refinements based on F2 against all reflections were carried out by a SHELXL-2017/1 program [40]. Structures were visualized using the Mercury program [41]. Geometrical parameters were calculated with PLATON [42]. All nonhydrogen atoms were refined anisotropically. Most of the hydrogen atoms were refined at calculated positions. Hydrogen atoms belonging to the water or methanol molecules and hydrogensulfate ions were found in the Fourier difference maps and restrained to positions according to the idealized geometry of the respective group. In 1a∙4.3H2O hydrogen atoms belonging to water molecules were constrained to positions found in the Fourier difference map according to the idealized geometry. One of the symmetrically independent l-threoninate residue in 1a∙4.3H2O is disordered over two sites with occupancies of 0.52 and 0.48. In the part with the higher occupancy factor one water molecule is also present (with the same occupancy factor), while in the other part there is no additional water molecule, so the average number of crystallization water molecules per formula unit is 4.3. In 1b∙3.5H2O one of the symmetrically independent sulfate ion is disordered over two positions around one of the S–O axis, so that three oxygen atoms are split in two parts with individual occupancy factors 0.67 and 0.33. After visualization of the crystal structure of 1a∙10H2O it was noticed that two unusually large voids are present in the structure (volume of each void ≈20 Å3, calculated with PLATON), however, there is no significant residual electron density inside voids.
2.4. ESR spectroscopy
The ESR measurements were performed on a Bruker Elexsys 580 FT/CW spectrometer from the room down to the liquid nitrogen temperature. The microwave frequency was around 9.7 GHz with the magnetic field modulation amplitude of 0.5 mT and modulation frequency of 100 kHz. The investigated compounds 1a·10H2O and 1b∙3.5H2O were unstable outside of solution so the samples were kept in respective mother liquors till measurements.
2.5. In vitro cytotoxic activity
Cytotoxicity experiments were performed at the School of Medicine, University of Zagreb. Since all prepared compounds contain similar complex species which are biologically active, we chose 1a·10H2O as the representative compound. All prepared compounds are highly soluble in water and moderately soluble in methanol and DMSO. The in vivo cytotoxicity of the 1a∙10H2O was assessed on the hepatocellular carcinoma (HepG2, ATCC HB−8065) and human acute monocytic leukaemia (THP-1, ATCC TIB−202) cell lines. CellTiter 96® AQueous One Solution Cell Proliferation Assay (MTS) was used. It is a tetrazolium-based cell viability assay that measures the metabolic capacity of cells in culture [43]. Serial dilutions of the tested compound in 96-well microtiter plates were prepared in 100–0.05 μM concentration range in an appropriate cell medium (50 μL). Cells were added to plates in an appropriate number per well (50 μL). Control wells consisted of media only (blank) or cells with 1% DMSO added (control). Plates were incubated overnight at 37 °C in a 5% CO2 atmosphere. For determining inhibition of cellular proliferation or inducement of cytotoxic effect CellTiter 96 Aqueous One Solution Cell Proliferation Assay (Promega, G3580) was used. 10 μL of MTS reagent was dispensed per well. Plates were incubated for 2 h at 37 °C in a 5% CO2 atmosphere and the absorbance was recorded at 490 nm using a 96-well Spectramax i3x plate reader. Results were analyzed in GraphPad Prism software.
3. Results and discussion
3.1. Synthesis and crystallization
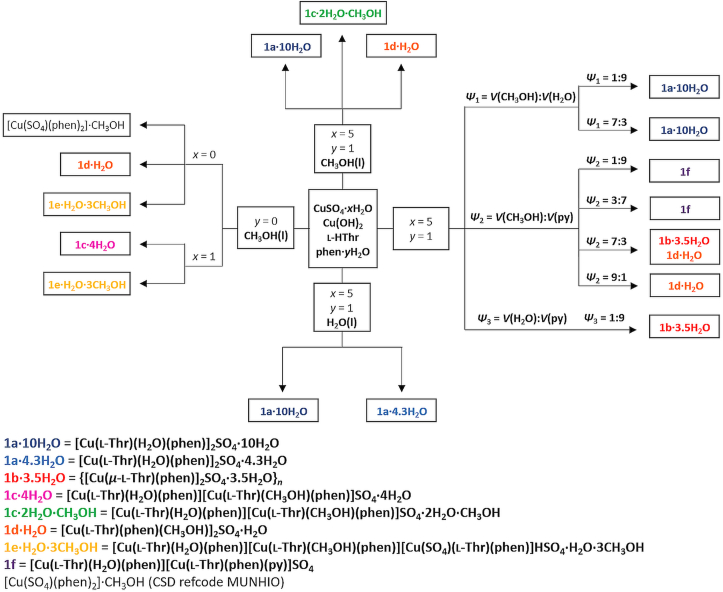
Overview of the solution-based synthetic procedures of all eight coordination compounds is given in Scheme 1. All monomeric compounds except 1e·H2O·3CH3OH contain two complex cations, [Cu(l-Thr)(L)(phen)]+ (L = H2O, CH3OH and/or py), and a sulfate counterion while 1e·H2O·3CH3OH contains three complex units, [Cu(l-Thr)(H2O)(phen)]+, [Cu(l-Thr)(CH3OH)(phen)]+ and [Cu(SO4) (l-Thr)(phen)]− and a hydrogen sulfate counterion. Compound 1b·3.5H2O contains polymeric chains {[Cu(μ-l-Thr)(phen)]+}n and sulfate counter ions. As seen in Scheme 1, many synthetic conditions yielded two or three different products. If fully hydrated reactants (copper(ii) sulfate pentahydrate, 1,10-phenanthroline monohydrate) in pure water or high ratio of water is used, 1a·10H2O crystallizes in most cases, and in some cases 1a·4.3H2O or their mixture. If fully hydrated reactants and pure methanol as solvent are used three different products crystallized: 1a·10H2O, 1c·2H2O·CH3OH and/or 1d·H2O. If less hydrated reactants (anhydrous or monohydrate of copper(ii) sulfate, anhydrous 1,10-phenanthroline) and pure methanol were used 1c·4H2O, 1d·H2O and/or 1e·H2O·3CH3OH crystallized from the solution. If we look at the formula units of 1c·4H2O, 1c·2H2O·CH3OH, 1d·H2O and 1e·H2O·3CH3OH it is seen that all these compounds contain both water and methanol molecules (coordinated and/or as crystallization molecules) so it is possible that at high methanol to water ratio, there is small difference in their stability and there is a possibility for any of them to crystallize. Small variances of conditions, such as room temperature, evaporation rate or size and shape of the crystallization dish, may influence the final crystal product. 1b∙3.5H2O crystallized from the water/pyridine mixture (v/v = 1:9). The same product crystallizes from the methanol/pyridine solution (v/v = 7:3), but in a mixture with 1d·H2O. It seems pyridine has an effect as a tailor-made additive on the formation of polymeric 1b∙3.5H2O. At higher pyridine to methanol ratio 1f crystallizes but with an extremely low yield, due to low solubility of reactants. Although in some of the synthetic conditions there is a very low water content, water is present in all products (coordinated and/or as crystallization molecules). The ability of water molecules to act as donors and acceptors of hydrogen bonds may be crucial for the stability of crystal structures in these ternary coordination compounds.
Scheme 1.
Solution-based syntheses of all eight copper(ii) coordination compounds.
Similar to the solution-based syntheses, small differences in water content influenced the final product of mechanochemical syntheses so we tried to use different hydrates of copper(ii) sulfate and 1,10-phenanthroline. Three compounds were obtained from mechanochemical syntheses: 1a·10H2O, 1c·2H2O·CH3OH and 1d·H2O. Compound 1a·10H2O was obtained when copper(ii) sulphate pentahydrate, copper(ii) hydroxide, anhydrous 1,10-phenanthroline and small amount of water were used for LAG synthesis. The same product, but in a mixture with 1c·2H2O·CH3OH, was obtained when water was replaced by a small amount of methanol. If copper(II) sulfate pentahydrate was replaced by copper(II) sulfate trihydrate and small amount of methanol was used for LAG synthesis, pure 1c·2H2O·CH3OH was obtained. When anhydrous reactants (copper(ii) sulfate and 1,10-phenanthroline) and small amount of methanol were used 1d·H2O was obtained, but a small amount of an unknown phase was also present.
3.2. Infrared spectroscopy
IR (ATR) spectra were measured for 1a∙10H2O, 1b∙3.5H2O, 1c∙2H2O∙CH3OH and 1f. IR spectra of all analysed compounds are very similar to each other due to similarity in the structure and coordination of the ligands. A broad band in the range 3600–2900 cm−1 (3550–2900 cm−1 for 1f) corresponding to O–H stretching suggests extensive hydrogen bonding in the crystal structures. Since there are two or more symmetrically independent l-threoninate ligands in all compounds, several bands corresponding to asymmetric stretching of the carboxylate groups are overlapped. In all cases, at least two bands can be distinguished one at higher wavenumbers in the range 1658–1623 cm−1 (uncoordinated or bridging oxygen atom) and one at lower wavenumbers in the range 1610–1600 cm−1 (coordinated oxygen atom).
3.3. Crystal structures
All compounds crystallize in noncentrosymmetric space groups in either monoclinic or triclinic crystal system. Crystallographic data are given in Tables S1 and S2 in Supplementary information. The asymmetric units of 1a∙10H2O, 1a∙4.3H2O, 1b∙3.5H2O, 1c∙2H2O∙CH3OH, 1c∙4H2O, 1d∙H2O, 1e·H2O·3CH3OH and 1f are shown in Figs. S2 and S3 in Supplementary information.
3.3.1. Coordination of copper(ii) ion
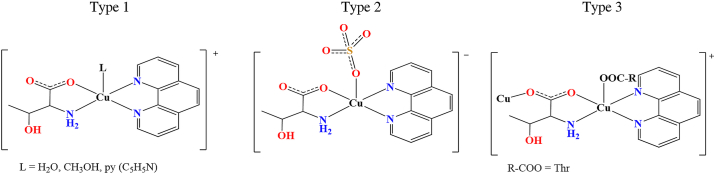
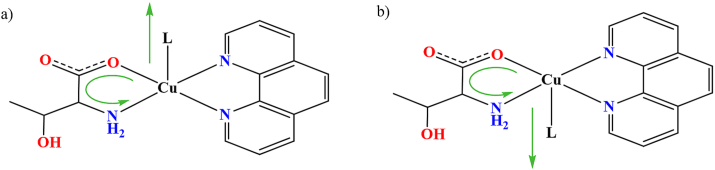
All compounds are composed of complex species of three different types (type 1, type 2 and/or type 3), considering a molecule or ion coordinated to copper(ii) ion in the apical position (Figure 1), with sulfate or hydrogensulfate counterions (Fig. S2 and S3 in Supplementary information).
Figure 1.
Three types of complex species based on copper(ii) coordination in the apical position.
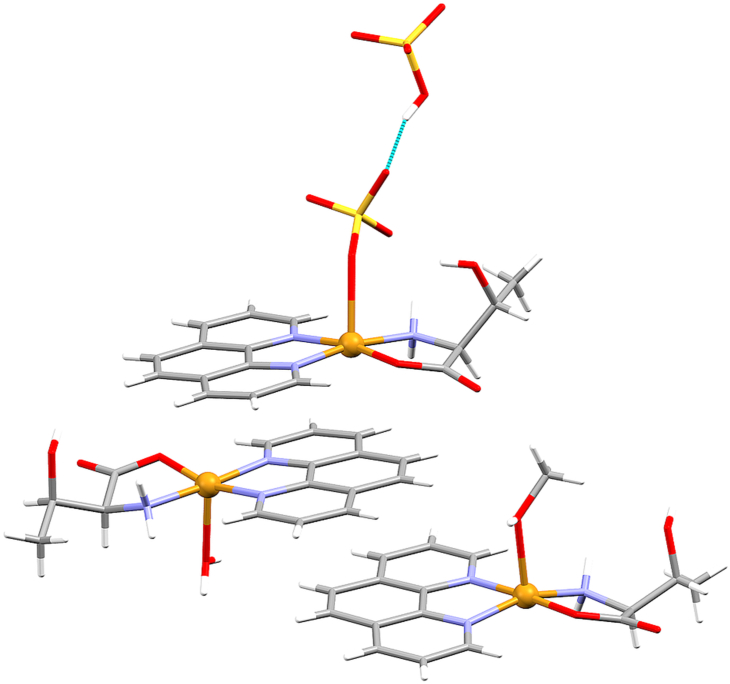
In complex cations of type 1, solvent molecule (water, methanol or pyridine) is coordinated in the apical position, forming complex cations [Cu(l-Thr)(L)(phen)]+ (L = H2O, CH3OH or py). All compounds except 1b·3.5H2O contain type 1 complex cations. We have obtained two different hydrates with two cations of type 1 with apically coordinated water molecule, 1a∙10H2O and 1a∙4.3H2O; one hydrate with two cations of type 1 with apically coordinated methanol molecule, 1d∙H2O; two different solvates with one cation of type 1 with apically coordinated water and one with a methanol molecule, 1c∙2H2O∙CH3OH and 1c∙4H2O; as well as compound 1f with one cation of type 1 with apically coordinated water and one with coordinated pyridine molecule. Type 2 complex anion has coordinated sulfate anion in the apical position forming complex anion [Cu(SO4)(l-Thr)(phen)]−. Only compound 1e·H2O·3CH3OH contains type 2 complex anion together with two complex cations of type 1 (one cation with an apically coordinated water and one with a methanol molecule) (Figure 2). Complex unit of type 3 is quite different since it contains carboxylate of adjacent complex cation coordinated to copper(ii) ion in the apical position forming a polymeric chain {[Cu(μ-l-Thr)(phen)]+}n. 1b·3.5H2O is the only compound containing a type 3 complex unit.
Figure 2.
Type 1 ([Cu(l-Thr)(H2O)(phen)]+ and [Cu(l-Thr)(CH3OH)(phen)]+) and type 2 ([Cu(SO4)(l-Thr)(phen)]−) complex species in 1e·H2O·3CH3OH. Hydrogen bond between hydrogen sulfate ion and coordinated sulfate is presented with blue dotted line.
All eight ternary coordination compounds contain copper(ii) square-pyramidal pentacoordinated complex species, with elongated apical Cu–X bond (X = O or N), due to the Jahn-Teller effect. In all types of the investigated compounds, 1,10-phenanthroline and l-threoninate are coordinated to the copper(ii) atom in the basal plane. Bond length and angles in the basal plane (Cu–N bond lengths are in the range 1.971(2)–2.039(6) Å, and Cu–O lengths are in the range 1.899(16)–1.974(14) Å) correspond to those in similar compounds found in the literature [7, 44] (Table S3 in Supplementary information). Most of the Cu–Owater apical bonds (2.181(4)–2.257(6) Å) are somewhat shorter than other apical Cu–O bonds (d(Cu–Omethanol) = 2.244(7)–2.347(3) Å; d(Cu–Ocarboxylate) = 2.282(7)–2.338(6) Å and d(Cu–Osulfate) = 2.382 (6) Å) (Table S3 in Supplementary information). The apical Cu–Npyridine bond (2.175 (9) Å) in 1f is shorter than the apical Cu–O bonds (Table S3 in Supplementary information).
3.3.2. Non-covalent interactions and structural isomerism
All structures contain numerous potential hydrogen bond donors and acceptors, such as amino-, hydroxyl- and carboxylate groups of l-threoninate, solvent molecules, sulfate or hydrogensulfate ions, so most of the compounds contain complex hydrogen-bonded frameworks. Some hydrogen bond motifs are conserved in all eight compounds. At least one amino- and one hydroxyl group of l-threoninate ligand in each compound are involved in N–H···Osulfate and O–H···Osulfate hydrogen bonds, which act as a bridge between two adjacent complex species (in 1e∙H2O∙3CH3OH one N–H···Osulfate and O–H···Osulfate hydrogen bond is intramolecular) (Table S4 and S5 in Supplementary information).
In 1e∙H2O∙3CH3OH a rare hydrogen sulfate···sulfate hydrogen bond is found (Figure 2). In a CSD search in which close contacts of hydrogen sulfate and sulfate anions were defined as an intermolecular contact with H⋯O distance shorter than the sum of van der Waals radii, 298 data sets were found. In another search, the same query was defined but limited to the structures containing any transitional metal. 51 data sets were found, which is only 1.5% of all data sets found in CSD containing any transitional metal and sulfate ions. In 1e∙H2O∙3CH3OH the sulfate ion is coordinated to the copper atom in one of the symmetrically independent complex species and acts as an acceptor for the hydrogen bond with the hydrogen sulfate ion (d(O–H⋯O) = 2.574 (8) Å; Fig. S4 in Supplementary information). S–O bond distances in the coordinated sulfate are in the range 1.463(7)–1.481(5) Å, and the unprotonated S–O groups of the hydrogen sulfate ion S–O bond distances are in the range 1.435(6)–1.472(7) Å. In the protonated S–O group, the S–O bond is significantly longer with a distance of 1.568 (6) Å, which is in accordance with literature data [45, 46].
Due to asymmetrically coordinated copper in the basal plane, two optical isomers may be distinguished based on the position of the apically coordinated atom. “Up”, ↑, and “down”, ↓, positions of apically coordinated atom are defined using the right-hand rule shown in Figure 3.
Figure 3.
Right-hand rule for defining a) “up”, ↑ and b) “down”, ↓ optical isomers in ternary copper(ii) coordination compounds. Rotation is chosen from heavier towards lighter atoms in l-threoninate, and the straight arrow shows: “up”, ↑, or “down”, ↓, direction.
All compounds contain both ↑ and ↓ isomers, however, since all structures are noncentrosymmetric, not all structures contain mixtures of those isomers. In 1a∙10H2O, 1a∙4.3H2O, 1b∙3.5H2O, and 1d∙H2O only one type of the apical ligand is present, and all those structures contain mixtures of ↑ and ↓ isomers. In 1c∙2H2O∙CH3OH, 1c∙4H2O, 1e∙H2O∙3CH3OH, and 1f, all complex cations with apically coordinated water are ↓ isomers, while complexes with apically coordinated methanol, pyridine and sulfate ions are all ↑ isomers.
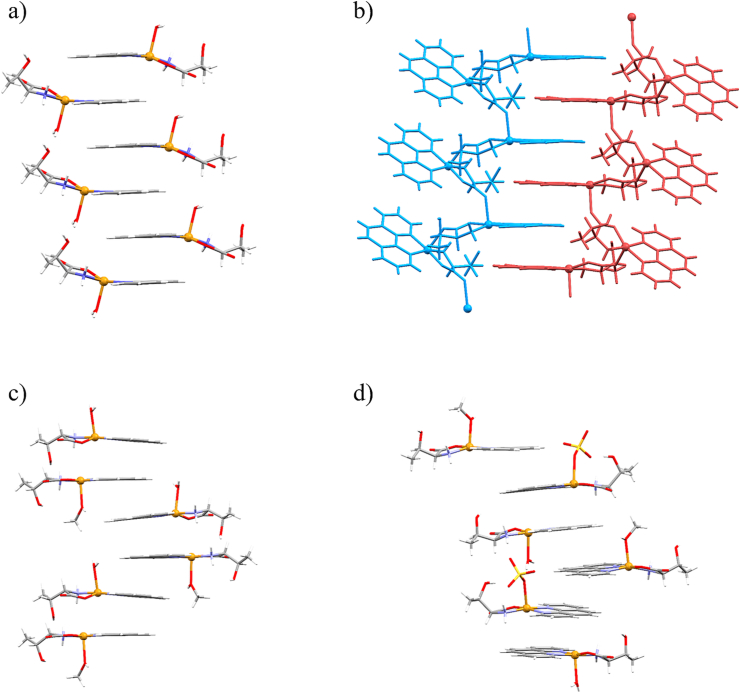
1,10-phenanthroline ligands tend to form supramolecular 1D pillars through π-interactions, which are formed in all eight compounds. 1,10-phenanthroline ligands of the adjacent complexes within the supramolecular chain are almost coplanar in all compounds. Depending on the type of the ligands are coordinated in the apical position, different types of chains are formed. In compounds 1a·10H2O, 1a·4.3H2O, 1b·3.5H2O, 1c·4H2O and 1d·H2O, complex species are stacked through π-interactions with ↑ and ↓ complexes alternating so that ↑ complexes are on one side of the π-stacked chain, while ↓ complexes are on the other side of the chain (Figure 4a).
Figure 4.
Different types of stacking of the complex species: a) alternating ↑H2O and ↓H2O complex cations in 1a∙10H2O; b) interlocked polymeric chains in 1b∙3.5H2O; c) ↑H2O and ↓MeOH complex cations dimers connected in zig-zag fashion in 1c∙H2O∙2CH3OH; d) 1D pillars formed by stacking of all three complex species alternating in order ↑MeOH ↑SO4 ↓H2O in 1e∙H2O∙3CH3OH.
In 1b·3.5H2O polymeric chains are interlocked by π-interactions forming supramolecular 2D sheets (Figure 4b.). In 1c·H2O·2CH3OH and 1f ↑ and ↓ complex cations form sandwich-like dimers which are alternating in a zig-zag pattern (Figure 4c). Finally, 1e∙H2O∙3CH3OH contains three different complex species, having apically coordinated water or methanol molecules or sulfate ions. In this compound 1D pillars are formed by stacking all three complex species alternating in order ↑MeOH ↑SO4 ↓H2O (Figure 4d).
3.3.3. Crystallization solvent molecules
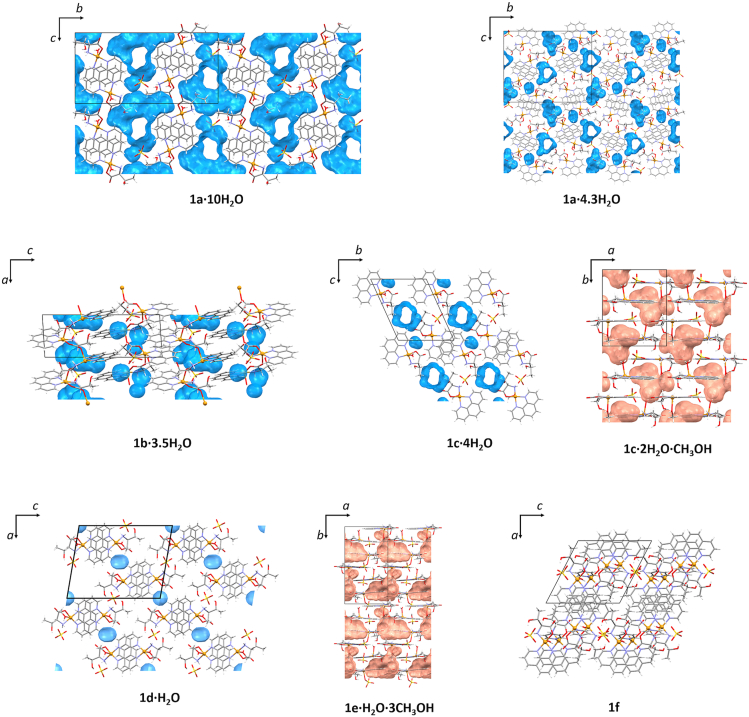
Specific structure of investigated copper(ii) ternary complex species, with half of the complex being hydrophobic, and the other half hydrophilic, has a consequence that hydrophobic phenanthroline ligands are oriented towards the inner side of π-stacked 1D pillars, while the hydrophilic parts of the complex species are oriented towards the outside of 1D pillars. This feature of the complex species is probably the reason why so many different solvates may be formed from very similar synthetic conditions. Between the pillars, crystallization solvent molecules form either 1D infinite chains (1a∙10H2O), pockets and 1D chains (1a∙4.3H2O, 1c·4H2O) or pockets (1b∙3.5H2O, 1c∙H2O∙2CH3OH, 1d∙H2O, 1e∙H2O∙3CH3OH), as shown in Table 1 and Figure 5. It seems that not only the type and number of crystallization solvent molecules but also the type of coordinated species in the apical position has an influence on the formation of 1D chains. In 1a∙10H2O crystallization water molecules occupy 25% of the unit cell volume, and this is the only compound having only 1D chains. 1a∙4.3H2O, 1b∙3.5H2O, 1c·4H2O, 1c∙H2O∙2CH3OH and 1e∙H2O∙3CH3OH have comparable ratio of crystallization water molecules (Table 1), but only 1a∙4.3H2O and 1c·4H2O form 1D chains, along with pockets, of water molecules.
Table 1.
Types of packing of crystallization solvent molecules and volume ratio of solvent molecules in all investigated compounds.
| Compound | Type of packing | V (solvent)/V (unit cell) |
|---|---|---|
| 1a∙10H2O | 1D channels | 25.7% |
| 1a∙4.3H2O | 1D channels and pockets | 12.1% |
| 1b∙3.5H2O | pockets | 14.1% |
| 1c·4H2O | 1D channels and pockets | 11.3% |
| 1c∙H2O∙2CH3OH | pockets | 11.2% |
| 1d∙H2O | pockets | 5.2% |
| 1e∙H2O∙3CH3OH | pockets | 14.1% |
| 1f | / | 0% |
Figure 5.
Crystal packing of all eight compounds: 1a∙10H2O, 1a∙4.3H2O, 1b∙3.5H2O, 1c∙2H2O∙CH3OH, 1c∙4H2O, 1d∙H2O, 1e·H2O·3CH3OH and 1f. Blue surfaces represent contact surface of water molecules, and orange surfaces represent contact surface of water and methanol molecules. Disordered water molecule in 1a∙4.3H2O is not represented with contact surface.
Influence of the apically coordinated molecule on the formation of pockets or channels of solvent molecules is also observed in the crystal structure of 1f. In this structure, one of the symmetrically independent complex cations has coordinated pyridine, while the other has a coordinated water molecule. Coordinated pyridine does not have a free acceptor or strong hydrogen bond donor atoms, and the structure does not contain crystallization solvent molecules.
3.4. ESR spectroscopy
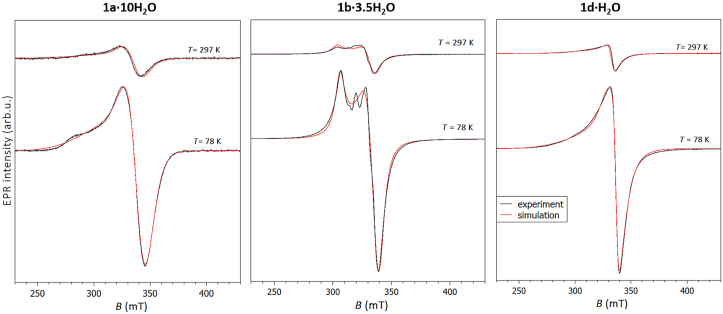
Three polycrystalline ternary copper(ii) complexes 1a∙10H2O, 1b∙3.5H2O and 1d∙H2O were investigated by X-band electron spin resonance (ESR) spectroscopy. The representative spectra, obtained at room temperature (297 K) and liquid nitrogen temperature (78 K), are shown in Figure 6.
Figure 6.
Experimental (black lines) and simulated (red lines) ESR spectra of polycrystalline samples of the investigated complexes 1a∙10H2O (left), 1b∙3.5H2O (middle) and 1d∙H2O (right). The ESR intensities of the spectra at different temperatures are presented in the real ratios.
The spectral simulations were performed by EasySpin software [47] using the following form of the spin-Hamiltonian for copper (ii) ions [48, 49]:
| H = μBB ⋅ g ⋅ S + S ⋅ A ⋅ I. | (1) |
In Eq. (1) μB is Bohr magneton, g is the g-tensor, B is the magnetic field, S and I are the electron and nuclear spin operator, respectively while hyperfine tensor A describes interaction between electron and nuclear spins. For all simulations, the second term in Eq. (1) was omitted because hyperfine interaction was not experimentally observed.
The spectra were simulated using the same values of g-tensor at both temperatures, allowing linewidth of assumed Lorentzian lineshape to change with temperature. The spin-Hamiltonian values, obtained from the simulations, are given in Table 2. Small variations in the local geometry of the Cu(ii) coordination can cause distribution of gx, gy and gz-values around some average values [50]. This effect described by gstrain parameters is also considered in the simulations with the values presented in Table 2. The obtained g-values gx ≈ gy < gz are expected for the elongated octahedral, square pyramidal or square planar copper geometry [51], and are in agreement with structurally determined distorted square pyramidal geometries in these compounds. Although all investigated complexes contain more than one copper(ii) atom per unit cell, only one ESR line is observed in the powder spectra due to similar geometries and g-parameters. The distances between two nearest copper atoms are 6.584 Å and 6.389 Å, for 1a∙10H2O and 1d∙H2O, respectively, while 1b∙3.5H2O is an one-dimensional zig-zag polymer with the distance between two nearest copper atoms of 5.487 Å. Therefore, the Cu–Cu distances are large enough to observe significant exchange interactions in these compounds. 1b∙3.5H2O is unstable outside of solution and a sample was poorly grinded into powder, resulting in observation of additional crystalline peaks in the powder spectrum.
Table 2.
The principal g-values obtained from the ESR spectral simulations, together with the parameter used for the simulations: gstrain and linewidths lw.
| Compound | g = [gxgygz] | gstrain | lw (mT) | T (K) |
|---|---|---|---|---|
| 1a∙10H2O | [2.05 2.05 2.30] | [0.08 0.13 0.44] [0.10 0.09 0.52] |
0.23 0.23 |
78 297 |
| 1b∙3.5H2O | [2.05 2.09 2.27] | [0.02 0.04 0.00] [0.01 0.00 0.00] |
5.68 6.60 |
78 297 |
| 1d∙H2O | [2.06 2.07 2.25] | [0.02 0.1 0.45] [0.0 0.00 0.31] |
0.23 4.51 |
78 297 |
3.5. Cytotoxicity
The results of the cell viability assay with IC50 values are presented in Table 3. 1a∙10H2O showed substantial cytotoxic activity toward human hepatocellular carcinoma (HepG2) and acute monocytic leukaemia (THP-1) cell lines. Moreover, it exhibited somewhat lowered cytotoxicity against THP-1 cells. Similar ternary copper complexes, investigated by different research groups, showed that mode of their action against tumor cells was DNA cleavage via reactive oxygen species (ROS) generation, mitochondrical toxicity or direct interaction with DNA after their administration to tumor cells [13, 52, 53, 54, 55]. Quantitative structure-activity relationship (QSAR) studies on similar ternary copper compounds indicated that the presence of phenanthroline was necessary to preserve the antiproliferative activity and that the nature of the O,N coligand had a poor influence on biological activity [56]. We believe that the square pyramidal structure of compound 1a∙10H2O with planar phenathroline ligand, l-Thr coligand and apically coordinated water molecule provides an optimal geometry for interaction with DNA strands through π-stacking interaction (phenathroline with DNA bases) and hydrogen bonds (l-Thr and apically coordinated water molecule with DNA bases) [3, 57]. However, comprehensive research on mode of action of 1a∙10H2O is needed to compare antitumor activities on different cell lines.
Table 3.
In vitro cytotoxicity (IC50 valuesa) of compound 1a∙10H2O.
| Compound |
IC50/μmol L−1 |
|
|---|---|---|
| HepG2 | THP1 | |
| 1a∙10H2O | 60.6 | 3.64 |
| staurosporine | 34.7 | 0.2 |
Concentration that causes 50% inhibition of the cell growth.
4. Conclusions
Eight new ternary copper(ii) coordination compounds with 1,10-phenanthroline and l-threonine were obtained by solution-based syntheses (1a∙10H2O, 1a∙4.3H2O, 1b∙3.5H2O, 1c∙2H2O∙CH3OH, 1c∙4H2O, 1d∙H2O, 1e·H2O·3CH3OH, 1f) and three of them also by mechanochemical syntheses (1a∙10H2O, 1c∙2H2O∙CH3OH and 1d∙H2O). We have found that different synthetic approach can control porous and non-porous structures in a controlled manner. It was shown that small differences in water content in synthetic mixtures (and possibly atmospheric conditions) have a high influence on the final product in both solution-based and mechanochemical syntheses, so in some cases two or three different products were obtained. All compounds consist of three different types of complex unit (named type 1, type2 and type 3). In all complex units the copper(ii) ion is pentacoordinated by a l-threoninate and a phenanthroline ligand in the equatorial plane, and apically by a solvent molecule (either a water, methanol or pyridine molecule) in the type 1 complex cation, by sulphate in the type 2 complex anion and by carboxylate of a neighbouring complex cation in the type 3 complex unit. Two types of diastereomers are found for the complex unit regarding the position of the apically coordinated species in relation to the chiral l-threoninato ligand (named up and down). All compounds except 1f are different solvates with solvent molecules situated either in the endless 1D channels and/or pockets. In all crystal structures of the solvates, the complex units, sulfate or hydrogensulfate anions and solvent molecules are linked by hydrogen bonds and π-interactions into 3D supramolecular frameworks. Compounds described in this study form some predictive intermolecular interactions such as π-interactions or certain hydrogen bond motifs (N–H···Osulfate and O–H···Osulfate). Knowledge of intermolecular interactions might help in the design of new compounds for potential drug design and/or functional material in the separation industry. ESR analysis of the different types of compounds showed agreement of the obtained g-values of the copper unpaired electron with the elongated square-pyramidal geometries of copper ions. Compound 1a∙10H2O showed substantial cytotoxic activity toward human HepG2 and THP-1 cell lines. Found biologycal activity provides an incentive to further study in different tumor cells.
Associated content
Supporting Information. The supporting material contains crystallographic data, ORTEP drawings of crystal structures, selected hydrogen bond distances and angles, powder X-ray diffraction patterns.
Declarations
Author contribution statement
Darko Vušak, Jurica Jurec: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Katarina Ležaić: Performed the experiments; Analyzed and interpreted the data.
Dijana Žilić, Biserka Prugovečki: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by the Croatian Government and the European Union through the European Regional Development Fund-Competitiveness and Cohesion Operational Programme (Grant KK.01.1.1.02.0016), and The Croatian Science Foundation (grant no IP-2018-01-3168).
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Footnotes
This article is a part of the "Coordination compounds" Special issue
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Linder M.C. Vol. 10. Springer Science & Business Media; 2013. (Biochemistry of Copper). [Google Scholar]
- 2.Yodoshi M., Odoko M., Okabe N. Structures and DNA-binding and cleavage properties of ternary copper(II) complexes of Glycine with phenanthroline, bipyridine, and bipyridylamine. Chem. Pharm. Bull. 2007;55(6):853–860. doi: 10.1248/cpb.55.853. [DOI] [PubMed] [Google Scholar]
- 3.García-Ramos J.C., Galindo-Murillo R., Tovar-Tovar A., Alonso-Saenz A.L., Gómez-Vidales V., Flores-Álamo M., Ortiz-Frade L., Cortes-Guzmán F., Moreno-Esparza R., Campero A., Ruiz-Azuara L. The π-back-bonding modulation and its impact in the electronic properties of CuII antineoplastic compounds: an experimental and theoretical study. Chem. Eur J. 2014;20:13730–13741. doi: 10.1002/chem.201402775. [DOI] [PubMed] [Google Scholar]
- 4.Seng H.-L., Wang W.-S., Kong S.-M., Ong H.-K.A., Win Y.-F., Rahman R.N.Z.R.A., Chikira M., Leong W.-F., Ahmad M., Khoo A.-S.-B., Ng C.-H. Biological and cytoselective anticancer properties of copper(II)-polypyridyl complexes modulated by auxiliary methylated glycine ligand. Biometals. 2012;25:1061–1081. doi: 10.1007/s10534-012-9572-4. [DOI] [PubMed] [Google Scholar]
- 5.Vušak D., Prugovečki B., Milić D., Marković M., Petković I., Kralj M., Matković-Čalogović D. Synthesis and crystal structure of solvated complexes of copper (II) with serine and phenanthroline and their solid-state-to-solid-state transformation into one stable solvate. Cryst. Growth Des. 2017;17:6049–6061. [Google Scholar]
- 6.Ng P.Y., Chye S.M., Tiong Y.L., Chan C.W., Tan K.W., Ooi I.H., Ng C.H. Enantiomeric pairs of copper(II) polypyridyl-alanine complex salts: anticancer studies. Transit. Met. Chem. 2018;43:479–496. [Google Scholar]
- 7.Zhang S., Zhu Y., Tu C., Wei H., Yang Z., Lin L., Ding J., Zhang J., Guo Z. A novel cytotoxic ternary copper(II) complex of 1,10-phenanthroline and L-threonine with DNA nuclease activity. J. Inorg. Biochem. 2004;98:2099–2106. doi: 10.1016/j.jinorgbio.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 8.Ren C.-.X., Ji M., Yao Q.-X., Cai L.-X., Tan B., Zhang J. Targeted functionalization of porous materials for separation of alcohol/water mixtures by modular assembly. Chem. Eur. J. 2014;20:14846–14852. doi: 10.1002/chem.201403822. [DOI] [PubMed] [Google Scholar]
- 9.Lou B.-Y., Jiang F.-L., Wu B.-L., Yuan D.-Q., Hong M.-C. From helical array to porous architecture: exploring the use of side chains of amino acids to engineer 1D infinite coordination polymeric chain into porous frameworks. Cryst. Growth Des. 2006;6(4):989–993. [Google Scholar]
- 10.Lou B.-Y., Wang R.-H., Yuan D.-Q., Wu B.-L., Jiang F.-L., Hong M.-C. Two homochiral 3D supramolecular architectures assembled from 4,4'-bipyridine-bridged copper(II) amino acid helical chains. Inorg. Chem. Commun. 2005;8:971–974. [Google Scholar]
- 11.Ruiz-Azuara L. Vol. 5. 1996. Copper Amino Acidate Diimine Nitrate Compounds and Their Methyl Derivatives and a Process for Preparing Them; p. 576. U.S. Patent. 326, November 19. [Google Scholar]
- 12.Ruiz-Azuara L. Process to obtain new mixed copper aminoacidate complexes from phenylatephenanthroline to be used as anticancerigenic agents. U.S. Patent RE. 1997;35:458. Febrary 18. [Google Scholar]
- 13.Ruiz-Azuara L., Bravo-Gómez M.E. Copper compounds in cancer Chemotherapy. Curr. Med. Chem. 2010;17:3606–3615. doi: 10.2174/092986710793213751. [DOI] [PubMed] [Google Scholar]
- 14.Groom C.R., Bruno I.J., Lightfoot M.P., Ward S.C. The Cambridge structural database. Acta Crystallogr. 2016;B72:171–179. doi: 10.1107/S2052520616003954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Low M.L., Chan C.W., Ng P.Y., Ooi I.H., Maah M.J., Chye S.M., Tan K.W., Ng S.W. Ternary and binary copper(II) complexes: synthesis, characterization, ROS-inductive, proteasome inhibitory, and anticancer properties. J. Coord. Chem. 2017;70(2):223–241. [Google Scholar]
- 16.Zhang W.C., Tang X., Lu X. One-dimensional chiral copper(II) complexes with novel nano-structures and superior antitumor activity. J. Inorg. Biochem. 2016;156:105–112. doi: 10.1016/j.jinorgbio.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Antolini L., Battaglia L.P., Bonamartini Corradi A., Marcotrigiano G., Menabue L., Pellacani G.C., Saladini M., Sola M. Tridentate facially coordinated L-aspartate ion complexation with the copper(II) ion: spectroscopic and structural properties of aqua(L-aspartato)(1,10-phenanthroline)copper(II) tetrahydrate. Inorg. Chem. 1986;25:2901–2904. [Google Scholar]
- 18.Battaglia L.P., Bonamartini Corradi A., Antolini L., Marcotrigiano G., Menabue L., Pellacani G.C. Coordination behavior of L-aspartic acid: ternary nickel(II) complexes with imidazoles. Crystal and molecular structure of (L-aspartate)tris(imidazole)nickel(II) J. Am. Chem. Soc. 1982;104:2407–2411. [Google Scholar]
- 19.Antolini L., Marcotrigiano G., Menabue L., Pellacani G.C. Coordination behavior of L-aspartic acid: thermal, spectroscopic, magnetic, and structural properties of aqua(L-aspartato)(2,2'-bipyridine)copper(II) trihydrate. Inorg. Chem. 1983;22:141–145. [Google Scholar]
- 20.Wojciechowska A., Rojek T., Malik−Gajewska M., Jerzykiewicz M., Wysokiński R., Gągor A., Rytlewski P., Staszak Z., Duczmal M. Crystal and molecular structure stabilized by weak interaction in unique 3,5-diiodo-L-tyrosinato copper(II) complex – synthesis, experimental and theoretical studies. Mater. Sci. Eng. B. 2020;262 [Google Scholar]
- 21.Su C.-C., Tai T.-Y., Wu S.-P., Wang S.-L., Liao F.-L. Spectroscopic and electronic properties of mixed ligand aminoacidatocopper(II) complexes. Polyhedron. 1999;18:2361–2368. [Google Scholar]
- 22.İnci D., Aydın R., Huriyet H., Zorlu Y., Çinkılıç N. Newly synthesized Cu(II) pyrazino[2,3-f][1,10]phenanthroline complexes as potential anticancer candidates. Appl. Organomet. Chem. 2018;32:e4309. [Google Scholar]
- 23.İnci D., Aydın R., Sevgi T., Zorlu Y., Demirkan E. Synthesis, crystal structure, stability studies, DNA/albumin interactions, and antimicrobial activities of two Cu(II) complexes with amino acids and 5-nitro-1,10-phenanthroline. J. Coord. Chem. 2017;70:512–543. [Google Scholar]
- 24.Chen R.-T., Zhang Q.-Y., Li Y.-J. Kinetics and mechanisms of ternary complex formation between (5-X-1,10-phenanthroline)copper(II) and threonine. Acta Chim. Sin. 1989;47:342–348. [Google Scholar]
- 25.Letter J.E., Bauman J.E. A thermodynamic study of the complexation reactions for a series of amino acids related to serine with copper(II) and nickel(II) J. Am. Chem. Soc. 1970;92:437–442. [Google Scholar]
- 26.Gasque L., Moreno-Esparza R., Ruiz-Ramírez L. Stability of ternary copper and nickel complexes with 1,10-phenanthroline. J. Inorg. Biochem. 1992;48:121–127. [Google Scholar]
- 27.İnci D., Aydın R. Stabilities of the ternary complexes of copper(II) with substituted 1,10-phenanthrolines and some amino acids in aqueous solution. J. Solut. Chem. 2014;43:711–726. [Google Scholar]
- 28.Türkel N. Çiğdem Şahin, Stability of binary and ternary copper(II) complexes with 1,10-phenanthroline, 2,2'-bipyridyl and some α-amino acids in aqueous medium. Chem. Pharm. Bull. 2009;57:694–699. doi: 10.1248/cpb.57.694. [DOI] [PubMed] [Google Scholar]
- 29.Vušak D., Prugovečki B., Matković- Čalogović D. Synthesis, structure and chemical properties of copper(II) complexes with 2,2‘-bipyridine and L-serine: porous materials and polymorphism. Acta Crystallogr. 2018;A74:E337. [Google Scholar]
- 30.Vušak D., Ležaić K., Prugovečki B., Matković-Čalogović D. Coordination polymers and solvatomorphs-copper complexes with amino acids and 2,2'-bipyridine. Acta Crystallogr. 2019;A74:E530. [Google Scholar]
- 31.Prugovečki B., Vušak D., Ležaić K., Jurković M. Ternary coordination compounds of copper with amino acids and 1,10-phenanthroline–structural insight and biological activity. Acta Crystallogr. 2021;A77:C975. [Google Scholar]
- 32.Brauer G., editor. Handbook of Preparative Inorganic Chemistry. second ed. Academic Press; New York: 1965. [Google Scholar]
- 33.Agte A.N., Golynko N.S. Production of chemically-pure Cu(OH)2 and Cu(OAc)2. Determination of the solubility of Cu(OAc)2 in water. Production of technical Cu(OAc)2. Trudy Leningr. Khim.-Tekh. Inst. 1940;8:140–149. [Google Scholar]
- 34.Degen T., Sadki M., Bron E., Nénert U. König i G. The HighScore suite. Powder Diffr. 2014;29:S13–S18. [Google Scholar]
- 35.Melnic E., Coropceanu E.B., Kulikova O.V., Siminel A.V., Anderson D., Rivera-Jacquez H.J., Masunov A.E., Fonari M.S., Kravtsov V. Ch. Robust packing patterns and luminescence quenching in mononuclear [Cu(II)(phen)2] sulfates. J. Phys. Chem. C. 2014;118:30087–30100. [Google Scholar]
- 36.J.-L. Do i T. Friščić Mechanochemistry: a force of synthesis. ACS Cent. Sci. 2017;3:13–19. doi: 10.1021/acscentsci.6b00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.CrysAlisPRO Software System . Oxford Diffraction / Agilent Technologies UK Ltd; Yarnton, England: 2018. [Google Scholar]
- 38.Sheldrick G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008;64:112–122. doi: 10.1107/S0108767307043930. [DOI] [PubMed] [Google Scholar]
- 39.Sheldrick G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015;71:3–8. doi: 10.1107/S2053229614024218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sheldrick G.M. SHELXT – Integrated space-group and crystalstructure determination. Acta Crystallogr. Sect. A Found. Adv. 2015;71:3–8. doi: 10.1107/S2053273314026370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Macrae C.F., Bruno I.J., Chisholm J.A., Edgington P.R., McCabe P., Pidcock E., Rodriguez-Monge L., Taylor R., van de Streek i J., Wood P.A. Mercury CSD 2.0 – new features for thevisualization and investigation of crystal structures. J. Appl. Crystallogr. 2008;41:466–470. [Google Scholar]
- 42.Spek A.L. Structure validation in chemical crystallography. Acta Crystallogr. Sect. D Biol. Crystallogr. 2009;65:148–155. doi: 10.1107/S090744490804362X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65(1–2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 44.Tav Y.-H., Teoh S.-G., Sek K.-L., Loh W.-S., Fun H.-K. Aqua(1,10-phenanthroline-κ2N,N')(DL-threoninato-κ2N,O1)copper(II) chloride dihydrate. Acta Crystallogr. E. 2010;66:m595–m596. doi: 10.1107/S1600536810015278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kole G.K., Tan G.K., Vittal J.J. Anion-controlled stereoselective synthesis of Cyclobutane derivatives by solid-state [2 + 2] Cycloaddition reaction of the salts of trans-3-(4-Pyridyl) acrylic acid. Org. Lett. 2010;12(1):128–131. doi: 10.1021/ol9025233. [DOI] [PubMed] [Google Scholar]
- 46.Tong M.-L., Zheng S.-L., Chen X.-M. Self-assembly of two- and three-dimensional coordination networks with hexamethylenetetramine and different silver(I) salts. Chem. Eur J. 2000;6(No. 20):3729–3738. doi: 10.1002/1521-3765(20001016)6:20<3729::aid-chem3729>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 47.Stoll S., Schweiger A. EasySpin, a comprehensive software package for spectral simulation and analysis in EPR. J. Magn. Reson. 2006;178:42. doi: 10.1016/j.jmr.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 48.Kahn O. Wiley-VCH Inc.; 1993. Molecular Magnetism. [Google Scholar]
- 49.Carrington A., MAD . Harper & Row; New York: 1967. Introduction to Magnetic Resonance. [Google Scholar]
- 50.Szymańska B., Skrzypek D., Kovala-Demertzi D., Staninska M., Demertzis M.A. Synthesis and spectroscopic study of copper(II) and manganese(II) complexes with pipemidic acid. Spectrochim. Acta. 2006;63:518–523. doi: 10.1016/j.saa.2005.05.038. [DOI] [PubMed] [Google Scholar]
- 51.Garribba E., Micera G. The determination of the geometry of Cu(II) complexes: an EPR spectroscopy experiment. J. Chem. Educ. 2006;83:1229–1232. [Google Scholar]
- 52.Santini C., Pellei M., Gandin V., Porchia M., Tisato F., Marzano C. Advances in copper complexes as anticancer agents. Chem. Rev. 2014;114:815–862. doi: 10.1021/cr400135x. [DOI] [PubMed] [Google Scholar]
- 53.Valencia-Cruz A.I., Uribe-Figueroa L.I., Galindo-Murillo R., López K.B., Gutiérrez A.G., Vázquez-Aguirre A., Ruiz-Azuara L., Hernández-Lemus E., Mejía C C. Whole genome gene expression analysis reveals casiopeina-induced apoptosis pathways. PLoS One. 2013;8(1) doi: 10.1371/journal.pone.0054664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gutiérrez A.G., Vázquez-Aguirre a A., García-Ramos J.C., Flores-Alamo M., Hernández-Lemus E., Ruiz-Azuara L., Mejía C. Copper(II) mixed chelate compounds induce apoptosis through reactive oxygen species in neuroblastoma cell line CHP-212. J. Inorg. Biochem. 2013;126:17–25. doi: 10.1016/j.jinorgbio.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 55.Becco L., García-Ramos J.C., Ruiz Azuara L., Gambino D., Garat B. Analysis of the DNA interaction of copper compounds belonging to the Casiopeínas® antitumoral series. Biol. Trace Elem. Res. 2014;161:210–215. doi: 10.1007/s12011-014-0098-1. [DOI] [PubMed] [Google Scholar]
- 56.Bravo-Gomez M.E., Garcia-Ramos J.C., Gracia-Mora I., Ruiz-Azuara L.J. Antiproliferative activity and QSAR study of copper(II) mixed chelate [Cu(N–N)(acetylacetonato)]NO3 and [Cu(N–N)(glycinato)]NO3 complexes,(Casiopeínas®) Inorg. Biochemistry. 2009;103:299–309. doi: 10.1016/j.jinorgbio.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 57.Galindo-Murillo R., Hernandez-Lima J., Gonzalez-Rendon M., Cortes-Guzman F., Ruiz-Azuara L., Moreno-Esparza R. π-Stacking between Casiopeínas® and DNA bases. Phys. Chem. Chem. Phys. 2011;13:14510–14515. doi: 10.1039/c1cp20183b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supplementary material/referenced in article.