Abstract
Background
Sarcopenia was thought to be associated with adverse outcomes and will cause lots of health expenditure. But the relationship between sarcopenia and catastrophic health expenditure (CHE) had been little explored. Here, we examined the distribution of sarcopenia in relation to medical and payment burdens.
Methods
We used data from three waves of China Health and Retirement Longitudinal Study including 14 130 participants from 9077 households aged over 50 years old. Sarcopenia was operationalized according to the Asian Working Group for Sarcopenia 2019. Medical expenditure was obtained by self‐reported data, and CHE was identified by WHO definitions. We used the negative binomial regression model and logistic mixed‐effects models to examine the associations between sarcopenia and medical and CHE.
Results
A total of 14 130 participants [52.2% female, aged 60.8 (SD 9.3)] from 9077 households were included in this study. The prevalence of sarcopenia was 19.8%, 11.9% for moderate sarcopenia, and 7.9% for severe sarcopenia, respectively. We identified 1416 household CHE events in all three waves. Severe sarcopenia was associated with an increase in the number of inpatient visits [incidence rate ratio 1.31, 95% confidence interval (CI): 1.03–1.66, P = 0.03] and the risk of CHE (odds ratio: 1.04, 95% CI: 1.01–1.07, P < 0.01). We saw similar effects in health service use of sarcopenia in different socio‐economic groups. Moderate sarcopenia increased the risk of CHE in the lowest socio‐economic group (odds ratio 1.03, 95% CI: 1.01–1.06, P = 0.03) and had no statistical significance in other groups. The association between severe sarcopenia and CHE did not attenuate after the adjustment of disease factors.
Conclusions
Severe sarcopenia may increase the risk of CHE. Timely and effective intervention on moderate sarcopenia from severe sarcopenia will contribute to reduce the health burden.
Keywords: Sarcopenia, Health burden, Health expenditure, Financial risk protection
Background
Sarcopenia is a syndrome characterized by loss of muscle mass and function in the elderly that reduces mobility and raises the likelihood of adverse outcomes such as falls, fractures, and death. 1 It has been recognized as a muscle disease with an ICD‐10‐MC Diagnosis Code in some countries. 2 With the aggravation of aging in the world, the prevalence of sarcopenia will increase rapidly. It is reported that the prevalence of sarcopenia is 1–29% in community‐dwelling populations by different definitions and 14–33% in residents requiring long‐term care. 3
The Asian Working Group for Sarcopenia 2019 consensus (AWGS 2019) defined the sarcopenia as ‘age‐related loss of muscle mass, plus low muscle strength, and/or low physical performance’ and specified cut‐offs for each diagnostic component. 4 The presence of sarcopenia increases the risk for hospitalization and the costs of care during hospitalization. 5
Adverse outcomes of sarcopenia require results in health services and may increase medical costs and families' burden. 6 Accessing these services can lead to individuals having to pay catastrophic proportions of their available income and push many households into poverty. 7 Catastrophic health expenditure (CHE) is defined as health spending that exceeds a predefined percentage or threshold of a household's ability to pay for health care, 8 reflecting whether families have fallen into financially disastrous due to health care costs.
The economic impact of non‐communicable diseases and their implications on financial stress has been an emerging area of research for the past 10 years. 9 , 10 Some previous researches have studied the relationship between adverse outcomes of sarcopenia and health expenditure. 11 , 12 These evidence suggest that falls and hip fractures impose high economic costs on individuals and households. However, little attention has been directly given to sarcopenia's implications for individuals, households, health systems, and the economy. Therefore, it is necessary to measure the potential risks of sarcopenia and CHE.
The Chinese Government has implemented the Healthy China 2030 Plan and a series of health insurance reform policies to improve the health level of the residents and minimize the financial burden. 13 The China New Health System Reform has expanded social health insurance coverage that 95.7% of the Chinese population were covered in 2011. 14 Blueprint for integrating the urban and rural resident schemes was announced in 2016 to improve fairness in health insurance coverage. 15 However, low levels of service coverage for some beneficiaries and high levels of patient cost‐sharing (from out‐of‐pocket fees for health insurance plans) have raised concerns about the lack of adequate financial protection for patients. 16
Health and diseases also play an important role in overall poverty alleviation by alleviating low‐income families' ‘sickness poor’ and ‘poverty due to illness’ problems in China. 17 , 18 Preventing catastrophic health spending among households of poverty and already out of poverty is particularly important. Therefore, identifying risk factors and early indications of CHE is necessary. We used nationally representative panel survey data to examine sarcopenia and its association with CHE.
Methods
Study design and population
The data were obtained from the China Health and Retirement Longitudinal Study (CHARLS), designed to collect a nationally representative sample of Chinese aged residents. The baseline survey was conducted in 2011, and follow‐up surveys were carried out every 2 years. 19 Compared with the Chinese population census of 2010, CHARLS was quite similar to the Chinese national population. 20
CHARLS used multistage stratified probability‐proportionate‐to‐size sampling and collected high‐quality data via one‐to‐one interviews with a structured questionnaire. At the individual sampling level, if the selected households had more than one individual, CHARLS randomly selected one of them as the main interviewee and interviewed his or her spouse at the same time. A detailed description of the objectives and methods of CHARLS has been reported elsewhere. 19 The Biomedical Ethics Review Committee of Peking University approved CHARLS, and the ethical approval number was IRB00001052‐11015. All participants signed written informed consent.
Of the 24 954 participants in CHARLS, we identified 14 130 participants from 9077 households aged over 50 without loss data for inpatient visits analysis. Health expenditure was calculated on a household basis and some households had more than one participant in CHARLS. Multiple participants in one household may affect the accuracy of the final results because this research was an analysis based on household data. We processed the data based on the household level to avoid the effect. In each round of follow‐up, we randomly selected one participant from all household participants as the main participants for this research if the household had multiple participants. After sampling, one individual per household per survey was included in the health expenditure analytical sample. The data used for CHE analyses included 11 387 participants from 9077 households. Meanwhile, we identified 4509 households with 1 male and 1 female (Figure 1).
Figure 1.
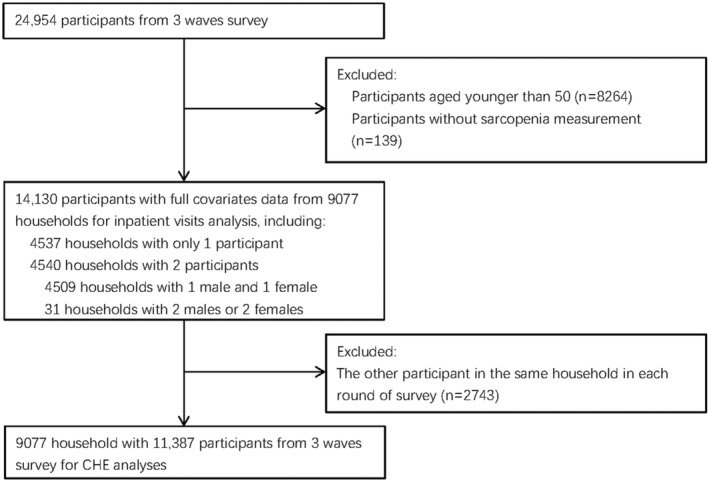
Flowchart on the sample selection and exclusion. CHE, catastrophic health expenditure.
Sarcopenia
The AWGS 2019 algorithm for identifying and diagnosing older adults was used in this study. 21 In AWGS 2019, sarcopenia was diagnosed when low muscle mass with low muscle strength or low physical performance, and severe sarcopenia was diagnosed if individuals with low muscle mass, low muscle strength, and low physical performance. 4 In this research, we defined low muscle mass with low muscle strength or low physical performance (sarcopenia in AWGS 2019) as moderate sarcopenia for distinction.
We used SARC‐F questionnaires for case finding recommended by AWGS 2019. 21 A SARC‐F score ≥4 was considered as positive.
Muscle strength
Skeletal muscle strength in this study was measured by handgrip strength. We took the average of maximum available values with two hands. If participants were unable to perform grip strength measurement in both hands, the value of the available hand was used. According to AWGS 2019, the cut‐off points for low handgrip strength were <18 kg in women and <28 kg in men.
Physical performance
Gait speed and 5‐time chair stand test were used to assess physical performance. The cut‐off points were 6 m walk <1.0 m/s for gait speed and time ≥12 s for 5‐time chair stand test. Participants who could not pass either of the tests were considered to have low physical performance.
Skeletal muscle mass measurement
The muscle mass was estimated by the appendicular skeletal muscle mass (ASM) using a previously validated equation in a Chinese population 22 :
The body weight, height, and age were measured in kilograms, centimetres, and years, respectively. For sex, the value 1 was for men, and the value 2 for women. Using dual‐energy X‐ray absorptiometry (DEXA) as the gold standard, this model can well predict Chinese ASM. 22
After estimating the ASM values, the muscle mass index (SMI) was calculated using the ASM divided by the square of the height in metres (SMI = ASM/height2). In accordance with previous studies, 23 , 24 , 25 the cut‐off of the SMI used in this study was based on the 20% lowest percentile of the study population; therefore, a low muscle mass was classified if SMI was <7.13 kg/m2 in men or <5.46 kg/m2 in women.
In this study, we use the classification method recommended by AWGS 2019. 4 Participants were considered having sarcopenia if they had low ASM and low muscle strength and/or low physical performance. Participants were recognized as moderate sarcopenia if they had low ASM and low muscle strength or low physical performance and were considered for severe sarcopenia if they had low ASM, low muscle strength, and low physical performance.
Catastrophic health expenditure
In CHARLS, participants were asked about their use of medical resources and their medical expenditure. Participants' self‐reported information on how much they paid in total and how much they paid out of pocket (deducting the reimbursed expenses) were collected. Outpatient expenses were collected during the past month and inpatient expenses were collected during the past year.
We multiplied the monthly outpatient spending by 12 to calculate the annual spending for each participant as outpatient care charge. Then we summed out‐of‐pocket expenses incurred by inpatients and outpatients as household annual out‐of‐pocket payments, which was used as the numerator for calculating the incidence of catastrophic health spending.
The household's ability to pay defined the ability to pay for health care as total household expenditure minus an amount corresponding to spending on basic needs. In this study, we defined it as household total expenditure minus actual food spending and used it as the denominator. 8
We created a binary variable to indicate whether the participant's family had catastrophic medical expenses or not. CHE was defined as if the out‐of‐pocket spending on health reached or exceeded 40% of a household's capacity to pay.
Covariates
This study included household and individual covariates; the latter included socio‐demographic and health status covariates. All covariates were obtained by questionnaire conducted during three‐wave surveys except physical anthropometry. Household covariates included the area of residence (rural and urban) and socio‐economic status. Responders' socio‐demographic characteristics included gender, education level (illiterate, can read and write, primary school, junior school, and high school and above), marital status (married, divorced or widowed, and never married), and health insurance [no insurance, urban employee basic medical insurance (UEBMI), urban–rural resident medical insurance (URRMI), govern insurance, and other insurances].
We used annual per‐capita household consumption spending as a proxy for socio‐economic status and used four socio‐economic groups based on quartiles of per‐capita household consumption expenditure.
We defined four socio‐economic groups according to the quartile of per‐capita household consumption expenditure (Quartile 1, <¥2650 per person per year; Quartile 2, ¥2650 to <5730 per person per year; Quartile 3, ¥5730 to <12 020 per person per year; and Quartile 4, ≥¥12 020 per person per year).
Health and lifestyle covariates in this study included age, body mass index (BMI), current smoking status (current smokers and non‐current smokers), current drinking status (drink more than once a week and non‐drinker), and self‐reported diagnosed diseases (hypertension, dyslipidemia, diabetes, cancer, chronic lung disease, liver disease, heart disease, stroke, kidney disease, digestive system disease, psychiatric disease, memory‐related disease, rheumatic disease, and asthma). BMI was calculated from height and weight measured during surveys.
Statistical analysis
Characteristics of the participants were summarized by the sarcopenia group before and after sampling. In order to examine the consistency of the taken‐out sample with the original sample to ensure the representative, t‐tests were applied to examine continuous variables while χ 2 test was applied to examine categorical variables.
We used negative binomial regression models to estimate the incidence rate ratio and 95% confidence interval (CI) for the association between the sarcopenia group and the number of inpatient hospital visits. Poisson regression was not applicable for the analysis because of the overdispersion of inpatient visits. Logistic mixed‐effects models were used to evaluate the odds ratio (OR) and 95% CI for the associations between sarcopenia and CHE in consideration of the nested structure of the data (repeated measurements of CHE for example). All analyses were adjusted for age, gender, education, marital status, residence, BMI, socio‐economic status, health insurance, current smoking status, and current drinking status.
We did subgroup analyses to explore the differential effect in population and household individual number groups. Participants were stratified by socio‐economic status, and the associations were examined by the same regression model but with the socio‐economic status variable removed. We also examined the effect of different individuals in households with one male and one female by using the same regression with separated individual variables.
Logistic mixed‐effects model was used to evaluate the association between other diseases and sarcopenia. In order to examine the potential roles of other diseases in driving participants into CHE, we assessed the effects of other 14 kinds of diseases by adding one disease at a time to the main analysis. We also included an additional model examining all kinds of diseases. Further analyses included examinations of the association among detailed diseases subgroups by stratifying participants into subgroups by their self‐reported disease status.
We performed a series of sensitivity analyses. Firstly, we included all individuals in all households for analysis. Then we excluded participants with more than 4 diseases and more than 2 from 14 types of diseases to avoid the interference caused by multimorbidity. Finally, we used the WHO and World Bank's definitions of CHE at various thresholds: 10% and 25% of the total household consumption expenditure by WHO definitions and 25% and 40% of the non‐food household consumption expenditure by World Bank definitions.
All statistical analyses were conducted by the R 4.1.0 and were based on two‐sided significance of P value < 0.05.
Results
Participants' characteristics
We identified 14 130 participants without missing data from 9077 households, among which 4537 households had only one participant and 4540 households had one main participant and his or her spouse. We randomly selected one participant from these households in each wave in this study. Participants' socio‐economic and socio‐demographic characteristics before and after sampling were shown in Table 1. The median age of participants before sampling was 60.8 years. A total of 6756 (47.8%) participants were male, 7374 (52.2%) were female, and 11 727 (83.0%) were married. A total of 7650 (64.7%) were residing in rural areas and 13 192 (93.4%) had at least one kind of health insurance. The prevalence of sarcopenia was 19.8%, among which 11.9% for moderate sarcopenia and 7.9% for severe sarcopenia, categorized by AWGS 2019. Statistical test results of participants' characteristics from multiple participant households before and after sampling were shown in Supporting Information, Table S1.
Table 1.
Characteristics of the subjects before sampling
| Variable | Before sampling | After sampling | ||||||
|---|---|---|---|---|---|---|---|---|
|
Overall N = 14 130 |
Non‐sarcopenia N = 11 336 |
Moderate sarcopenia N = 1682 |
Severe sarcopenia N = 1112 |
Overall N = 11 387 |
Non‐sarcopenia N = 9096 |
Moderate sarcopenia N = 1407 |
Severe sarcopenia N = 884 |
|
| Age, years (SD) | 60.8 (9.3) | 58.7 (8.4) | 67.0 (7.8) | 72.4 (8.0) | 60.6 (9.5) | 58.5 (8.5) | 67.1 (7.8) | 72.7 (8.0) |
| Gender | ||||||||
| Male | 6756 | 5399 | 813 | 544 | 5330 | 4218 | 685 | 427 |
| Female | 7374 | 5937 | 869 | 568 | 6057 | 4878 | 722 | 457 |
| Education | ||||||||
| Illiterate | 3667 | 2524 | 612 | 531 | 3014 | 2054 | 526 | 434 |
| Can read and write | 2597 | 1963 | 386 | 248 | 2107 | 1602 | 308 | 197 |
| Primary school | 3201 | 2568 | 412 | 221 | 2566 | 2054 | 347 | 165 |
| Junior middle school | 2978 | 2706 | 193 | 79 | 2385 | 2164 | 159 | 62 |
| High school and above | 1687 | 1575 | 79 | 33 | 1315 | 1222 | 67 | 26 |
| Marital status | ||||||||
| Married | 11 727 | 9677 | 1285 | 765 | 8985 | 7429 | 1016 | 540 |
| Divorced | 742 | 631 | 67 | 44 | 741 | 639 | 61 | 41 |
| Widowed | 1560 | 967 | 311 | 282 | 1560 | 967 | 311 | 282 |
| Never married | 101 | 61 | 19 | 21 | 101 | 61 | 19 | 21 |
| Residence | ||||||||
| Urban | 8824 | 6738 | 1238 | 848 | 7190 | 5484 | 1032 | 674 |
| Rural | 5306 | 4598 | 444 | 264 | 4197 | 3612 | 375 | 210 |
| BMI | ||||||||
| 18.5–23.9 | 7016 | 4916 | 1327 | 773 | 5707 | 3985 | 1097 | 625 |
| <18.5 | 874 | 199 | 346 | 329 | 718 | 165 | 302 | 251 |
| 24–27.9 | 4502 | 4483 | 9 | 10 | 3563 | 3547 | 8 | 8 |
| ≥28 | 1738 | 1738 | 0 | 0 | 1399 | 1399 | 0 | 0 |
| Socio‐economic group | ||||||||
| Quartile 1 (lowest) | 3272 | 2312 | 538 | 422 | 2585 | 1783 | 468 | 334 |
| Quartile 2 | 3386 | 2696 | 427 | 263 | 2596 | 2063 | 342 | 191 |
| Quartile 3 | 3513 | 2952 | 358 | 203 | 2841 | 2382 | 291 | 168 |
| Quartile 4 | 3959 | 3376 | 359 | 224 | 3365 | 2868 | 306 | 191 |
| Health insurance | ||||||||
| No insurance | 938 | 738 | 103 | 97 | 747 | 585 | 90 | 72 |
| UEBMI | 1537 | 1372 | 111 | 54 | 1190 | 1050 | 95 | 45 |
| URRMI | 11 188 | 8844 | 1412 | 932 | 9076 | 7160 | 1173 | 743 |
| Govern insurance | 299 | 245 | 38 | 16 | 242 | 199 | 31 | 12 |
| Other insurances | 168 | 137 | 18 | 13 | 132 | 102 | 18 | 12 |
| Smoking | 6187 | 4850 | 814 | 523 | 4878 | 3791 | 680 | 407 |
| Drinking | 3719 | 3012 | 449 | 258 | 2957 | 2370 | 392 | 195 |
BMI, body mass index; SD, standard deviation; UEBMI, urban employee basic medical insurance; URRMI, urban–rural resident medical insurance.
Association between sarcopenia and annual inpatient visits
The number of inpatient visits was increased with age and influenced by socio‐demographic factors like socio‐economic and health insurance. The average number of annual hospitalizations visit was 0.134 in all groups, 0.143 in the sarcopenia group, and 0.185 in the severe group. Severe sarcopenia was associated with an increase in the number of inpatient visits (incidence rate ratio 1.31, 95% CI: 1.03–1.66) after being adjusted by all of the covariates (Table 2).
Table 2.
Association between sarcopenia and inpatient visits
| Variable | Incidence rate ratio (95% CI) | P value |
|---|---|---|
| Age, per 10 years | 1.44 (1.33–1.55) | <0.01 |
| Gender | ||
| Male | 1 (ref) | |
| Female | 0.86 (0.72–1.03) | 0.11 |
| Sarcopenia | ||
| Non‐sarcopenia | 1 (ref) | |
| Moderate sarcopenia | 1.17 (0.95–1.43) | 0.13 |
| Severe sarcopenia | 1.31 (1.03–1.66) | 0.03 |
| Socio‐economic group | ||
| Quartile 1 (lowest) | 1 (ref) | |
| Quartile 2 | 1.81 (1.41–2.32) | <0.01 |
| Quartile 3 | 2.11 (1.55–2.88) | <0.01 |
| Quartile 4 | 2.15 (1.47–3.17) | <0.01 |
| Education | ||
| Illiterate | 1 (ref) | |
| Can read and write | 1.02 (0.85–1.23) | 0.80 |
| Primary school | 1.03 (0.86–1.23) | 0.73 |
| Junior school | 0.96 (0.79–1.16) | 0.66 |
| High school and above | 0.84 (0.66–1.06) | 0.15 |
| Marital status | ||
| Married | 1 (ref) | |
| Divorced | 0.69 (0.5–0.94) | 0.02 |
| Widowed | 0.99 (0.81–1.21) | 0.90 |
| Never married | 0.48 (0.14–1.3) | 0.19 |
| Living area | ||
| Rural | 1 (ref) | |
| Urban | 0.86 (0.75–0.98) | 0.02 |
| BMI | ||
| <18.5 | 1 (ref) | |
| 18.5–23.9 | 0.82 (0.63–1.06) | 0.13 |
| 24–27.9 | 1.01 (0.87–1.17) | 0.88 |
| ≥28 | 1.42 (1.18–1.71) | <0.01 |
| Smoking | 1.02 (0.86–1.21) | 0.81 |
| Drinking | 0.55 (0.47–0.64) | <0.01 |
| Health insurances | ||
| No insurance | 1 (ref) | |
| UEBMI | 1.99 (1.42–2.8) | <0.01 |
| URRMI | 1.88 (1.41–2.54) | <0.01 |
| Govern insurance | 2.18 (1.37–3.46) | <0.01 |
| Other insurances | 1.26 (0.63–2.4) | 0.49 |
BMI, body mass index; CI, confidence interval; UEBMI, urban employee basic medical insurance; URRMI, urban–rural resident medical insurance.
Association between sarcopenia and catastrophic health expenditure
In three‐wave survey of 9077 households, a total of 1416 cases of CHE were identified. At household level, households with one sarcopenia individual (OR 1.02, 95% CI: 1.00–1.04) and two sarcopenia individuals (OR 1.08, 95% CI: 1.04–1.11) had higher risk of CHE than households without sarcopenia individual(s) (Table 3).
Table 3.
Household factors and catastrophic health expenditure
| Variable | Odds ratio (95% CI) | P value |
|---|---|---|
| Living area | ||
| Rural | 1 (ref) | |
| Urban | 0.97 (0.96–0.98) | <0.01 |
| Socio‐economic group | ||
| Quartile 1 (lowest) | 1 (ref) | |
| Quartile 2 | 0.98 (0.97–1.00) | 0.04 |
| Quartile 3 | 0.97 (0.95–0.98) | <0.01 |
| Quartile 4 | 0.96 (0.94–0.97) | <0.01 |
| Household size | ||
| 1 participant | 1 (ref) | |
| 2 participants | 0.99 (0.98–1.00) | 0.29 |
| Number of member(s) with sarcopenia | ||
| 0 participant | 1 (ref) | |
| 1 participant | 1.02 (1.00–1.04) | <0.01 |
| 2 participants | 1.08 (1.04–1.11) | <0.01 |
CI, confidence interval.
After sampling, severe sarcopenia was associated with a higher increase in CHEs (OR 1.04, 95% CI: 1.01–1.07, Table S2). The effect of sarcopenia on CHEs was quite different after being stratified by socio‐economic status. Sarcopenia was associated with the risk of health expenditures in the lowest and second lowest socio‐economic groups and severe sarcopenia was associated with the risk of health in the first three groups. However, the effect of both sarcopenia and severe sarcopenia on CHEs did not persist in the highest socio‐economic quartile (Figure 2, Table S3).
Figure 2.
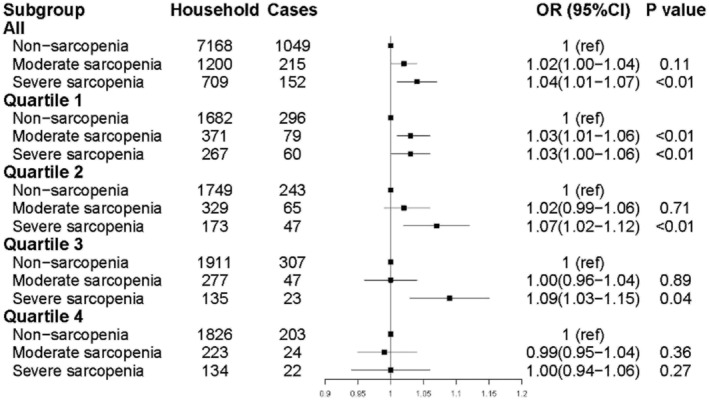
Association between sarcopenia and risk of CHE by socio‐economic groups. CHE, catastrophic health expenditure; CI, confidence interval; OR, odds ratio.
When we performed the subgroup analysis of households with one male and one female, female sarcopenia individuals contributed more to the CHEs (OR 1.06, 95% CI: 1.02–1.10) in these households while male sarcopenia individuals showed no statistically significance (Table S4).
Association between sarcopenia, chronic diseases, and catastrophic health expenditure
Prevalence and association between other 14 kinds of disease and sarcopenia were shown in Tables S5 and S6. When the roles of other chronic diseases were assessed, the association between sarcopenia and CHE did not attenuate (Table S7). Table S8 showed the risk of CHE in different disease subgroups. The associations between sarcopenia and CHE only exist in cancer and chronic lung disease subgroups, possibly because of the low prevalence of the diseases.
Sensitivity analyses
We did several sensitivity analyses. First, we examined this association in all individuals (without sampling), and the association consisted of our main analyses (Table S9). We excluded participants with physical multimorbidity to avoid interference caused by other diseases. We found that the risk of sarcopenia to CHE was not affected by multiple disease factors. Then we repeated our CHE analysis using the WHO and World Bank's definitions at various household expenditure thresholds. We found the associations between sarcopenia and CHEs were in line with the findings in the main analyses at different thresholds (Tables S9 and S10).
Discussion
We exploited data from CHARLS to estimate the sarcopenia on the risk of CHE, which is an analysis of a nationally representative longitudinal survey of middle‐aged and older Chinese people. We found that the prevalence of sarcopenia was 19.8%, and severe sarcopenia was associated with a higher risk in inpatient visits and CHE.
Previous studies have studied the association between sarcopenia and adverse health outcomes risks 26 , 27 and the association between these adverse outcomes and economic burden. 28 , 29 , 30 , 31 Our study provides new evidence on the association between sarcopenia and the use of health services across socio‐economic groups.
We found that severe sarcopenia was associated with the risk of inpatient visits and CHE while moderate sarcopenia showed no significant effect on them, suggesting that the medical demand and the risk of CHE increase with the severity of sarcopenia. Interestedly, this association appeared to be similar between the lowest and the second highest economic groups. Moderate sarcopenia was associated with CHE in the lowest economic group whereas no significant statistical significance in all other three groups. This might be because higher income brackets have the higher economic capacity and need to have greater absolute levels of health expenditures to trigger CHE. These results suggest that people in the lower economic group are more vulnerable to overspending on health care and suffered a greater economic burden than the richest, which means interventions on CHE related to sarcopenia may be more beneficial to the poor. For households with couples, female individuals with sarcopenia were associated with higher risk of CHE, suggesting that interventions for females in couples may achieve greater benefits.
Recently, comprehensive intervention for geriatrics, including sarcopenia, has gradually become a research hotspot. 32 , 33 , 34 Lots of intervention programmes have been completed to help prevent sarcopenia in elderly people living in the community, 32 , 35 showing that sarcopenia can be improved and its progress can be delayed. Our evidence suggests that preventing sarcopenia from becoming severe sarcopenia may reduce the occurrence of adverse events and economic burden, especially for the poor. Based on those progress, our results provided a basis for the additional economic potential of these interventions and a potential strategy for preventing CHE by the early identification and intervention of sarcopenia.
Health care insurance had a limited impact on out‐of‐pocket health care expenditure in this study, consisted with previous studies on other diseases in China. 36 , 37 Some evidence suggested that when additional population groups acquired coverage in formal insurance schemes, the incidence of catastrophic payments did not always change. 9 Our population has a high rate of insurance and the coverage rate was 93.4%. Besides, uninsured people may tend to be more confident about their health, and this may underestimate the effectiveness of health insurance.
We found that inpatients' visit was increased in participants with health insurance. People who have insurance might have more intensive health care use, or the actual health care needs of the uninsured people may be underestimated. Different types of health insurance have different effects. UEBMI and govern insurance reduce the risk of CHE while other insurance schemes in China showed no significance.
Sarcopenia is also often accompanied by other diseases and multimorbidity. 38 , 39 The latter is also associated with CHE. 37 We included the diseases into the model and the association did not attenuate. In stratified analyses, however, for most of other chronic disease patients (12 of the 14 kinds of diseases), neither moderate sarcopenia nor severe sarcopenia had statistical significance on CHE. This might be because of the small number of the patients and the small number of CHE cases among these patients. Unexpectedly, we found some diseases (cancer, heart diseases, and digestive system diseases) had no statistical significance with sarcopenia. This might be because our data were obtained from community‐dwelling people and patients with severe diseases or nursing needs were more likely to live in nursing houses or similar institutions, which may cause fewer participants with severe diseases in our study. We also excluded people with more than four diseases or people with more than two diseases in sensitivity analysis and the result remained the same. However, the interaction between sarcopenia, multimorbidity, and CHE needs further study in the future.
Our study has several important strengths. First, we used repeatedly measured data from a large representative sample of middle and older Chinese adults. The sample achieve a nationally representative coverage and the data were collected through face‐to‐face interviews, resulting in a high response rate and high quality. Secondly, the definition of CHE means it would inevitably interfere from other members of the family. We transform data to household‐based panel data by sampling and used a random effect model to neutralize these biases. Finally, with the development of aging, there will be more people with sarcopenia and other aging diseases. Our findings provide new evidence to inform the development of targeted policies and interventions for the intervention of sarcopenia.
Our study had several limitations. Firstly, the muscle mass was estimated by a previously validated equation in a Chinese population instead of physical measurement. Secondly, the economic status data of households and health expenditure amount were self‐reported by participants, and some of them might be inaccurate. Finally, we could not know the specific amount of expenditure caused by specific diseases. Although we included other common chronic conditions in analyses, the specific contribution of sarcopenia to health expenditure is still unknown. These limitations need to be taken into consideration in further studies.
Conclusions
We studied the association between sarcopenia and health burden in CHARLS. Severe sarcopenia was associated with more inpatient visits and the risk of CHE. These findings are important as they suggest that the prevention and treatment of sarcopenia could be targeted for reducing the growing financial burden of health expenditure.
Conflict of interests
We declare that we have no conflicts of interest.
Supporting information
Table S1. Characteristics of the subjects from multiple subject households
Table S2 sarcopenia and catastrophic health expenditure
Table S3 sarcopenia and catastrophic health expenditure by socio‐demographic characteristics
Table S4 sarcopenia and catastrophic health expenditure in the household with 1 male and 1 female
Table S5 self‐reported diseases prevalence among participants
Table S6 association between other diseases and sarcopenia
Table S7 association between sarcopenia group and risk of CHE, further adjusted for different diseases
Table S8 association between sarcopenia group and risk of CHE by subgroups of different diseases
Table S9 sensitivity analyses on association between sarcopenia and risk of CHE
Table S10 sensitivity analyses of the association between sarcopenia and risk of catastrophic health expenditure
Acknowledgements
This research uses data from China Health and Retirement Longitudinal Study (CHARLS). We thank the CHARLS team for providing data and the students who participated in the survey for their cooperation. We thank all participants and staff involved in this research. The Biomedical Ethics Review Committee of Peking University approved CHARLS. The ethical approval number was IRB00001052‐11015.
The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. 40
Ye C., Zheng X., Aihemaitijiang S., Wang R., Halimulati M., Huang X., and Zhang Z. (2022) Sarcopenia and catastrophic health expenditure by socio‐economic groups in China: an analysis of household‐based panel data, Journal of Cachexia, Sarcopenia and Muscle, 13, 1938–1947, 10.1002/jcsm.12997
References
- 1. Larsson L, Degens H, Li M, Salviati L, Lee YI, Thompson W, et al. Sarcopenia: aging‐related loss of muscle mass and function. Physiol Rev 2019;99:427–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vellas B, Fielding RA, Bens C, Bernabei R, Cawthon PM, Cederholm T, et al. Implications of ICD‐10 for sarcopenia clinical practice and clinical trials: report by the International Conference on Frailty and Sarcopenia Research Task Force. J Frailty Aging 2018;7:2–9. [DOI] [PubMed] [Google Scholar]
- 3. Dennison EM, Sayer AA, Cooper C. Epidemiology of sarcopenia and insight into possible therapeutic targets. Nat Rev Rheumatol 2017;13:340–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc 2020;21:300–7 e2. [DOI] [PubMed] [Google Scholar]
- 5. Cawthon PM, Lui LY, Taylor BC, McCulloch CE, Cauley JA, Lapidus J, et al. Clinical definitions of sarcopenia and risk of hospitalization in community‐dwelling older men: the Osteoporotic Fractures in Men Study. J Gerontol A Biol Sci Med Sci 2017;72:1383–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mills KM, Sadler S, Peterson K, Pang L. An economic evaluation of preventing falls using a new exercise program in institutionalized elderly. J Phys Act Health 2018;15:397–402. [DOI] [PubMed] [Google Scholar]
- 7. Xu K, Evans DB, Kawabata K, Zeramdini R, Klavus J, Murray CJ. Household catastrophic health expenditure: a multicountry analysis. Lancet 2003;362:111–117. [DOI] [PubMed] [Google Scholar]
- 8. Cylus J, Thomson S, Evetovits T. Catastrophic health spending in Europe: equity and policy implications of different calculation methods. Bull World Health Organ 2018;96:599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wagstaff A, Flores G, Hsu J, Smitz MF, Chepynoga K, Buisman LR, et al. Progress on catastrophic health spending in 133 countries: a retrospective observational study. Lancet Glob Health 2018;6:e169–e179. [DOI] [PubMed] [Google Scholar]
- 10. Palladino R, Tayu Lee J, Ashworth M, Triassi M, Millett C. Associations between multimorbidity, healthcare utilisation and health status: evidence from 16 European countries. Age Ageing 2016;45:431–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Florence CS, Bergen G, Atherly A, Burns E, Stevens J, Drake C. Medical costs of fatal and nonfatal falls in older adults. J Am Geriatr Soc 2018;66:693–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Su FY, Fu ML, Zhao QH, Huang HH, Luo D, Xiao MZ. Analysis of hospitalization costs related to fall injuries in elderly patients. World J Clin Cases 2021;9:1271–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tan X, Liu X, Shao H. Healthy China 2030: a vision for health care. Value Health Reg Issues 2017;12:112–114. [DOI] [PubMed] [Google Scholar]
- 14. Meng Q, Xu L, Zhang Y, Qian J, Cai M, Xin Y, et al. Trends in access to health services and financial protection in China between 2003 and 2011: a cross‐sectional study. Lancet 2012;379:805–814. [DOI] [PubMed] [Google Scholar]
- 15. He AJ, Wu S. Towards universal health coverage via social health insurance in China: systemic fragmentation, reform imperatives, and policy alternatives. Appl Health Econ Health Policy 2017;15:707–716. [DOI] [PubMed] [Google Scholar]
- 16. Meng Q, Fang H, Liu X, Yuan B, Xu J. Consolidating the social health insurance schemes in China: towards an equitable and efficient health system. Lancet 2015;386:1484–1492. [DOI] [PubMed] [Google Scholar]
- 17. Chen C, Pan J. The effect of the health poverty alleviation project on financial risk protection for rural residents: evidence from Chishui City, China. Int J Equity Health 2019;18:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li Y, Wu Q, Liu C, Kang Z, Xie X, Yin H, et al. Catastrophic health expenditure and rural household impoverishment in China: what role does the new cooperative health insurance scheme play? PLoS One 2014;9:e93253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhao Y, Strauss J, Yang G, Giles J, Hu P, Hu Y, et al. China Health and Retirement Longitudinal Study: 2011–2012 National Baseline User's Guide. National School of Development, Peking University; 2013. [Google Scholar]
- 20. Zhao Y, Hu Y, Smith JP, Strauss J, Yang G. Cohort profile: the China Health and Retirement Longitudinal Study (CHARLS). Int J Epidemiol 2014;43:61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Malmstrom TK, Morley JE. SARC‐F: a simple questionnaire to rapidly diagnose sarcopenia. J Am Med Dir Assoc 2013;14:531–532. [DOI] [PubMed] [Google Scholar]
- 22. Wen X, Wang M, Jiang CM, Zhang YM. Anthropometric equation for estimation of appendicular skeletal muscle mass in Chinese adults. Asia Pac J Clin Nutr 2011;20:551–556. [PubMed] [Google Scholar]
- 23. Delmonico MJ, Harris TB, Lee JS, Visser M, Nevitt M, Kritchevsky SB, et al. Alternative definitions of sarcopenia, lower extremity performance, and functional impairment with aging in older men and women. J Am Geriatr Soc 2007;55:769–774. [DOI] [PubMed] [Google Scholar]
- 24. Alexandre Tda S, Duarte YA, Santos JL, Wong R, Lebrao ML. Sarcopenia according to the European Working Group on Sarcopenia in Older People (EWGSOP) versus dynapenia as a risk factor for mortality in the elderly. J Nutr Health Aging 2014;18:751–756. [DOI] [PubMed] [Google Scholar]
- 25. Newman AB, Kupelian V, Visser M, Simonsick E, Goodpaster B, Nevitt M, et al. Sarcopenia: alternative definitions and associations with lower extremity function. J Am Geriatr Soc 2003;51:1602–1609. [DOI] [PubMed] [Google Scholar]
- 26. Yeung SSY, Reijnierse EM, Pham VK, Trappenburg MC, Lim WK, Meskers CGM, et al. Sarcopenia and its association with falls and fractures in older adults: a systematic review and meta‐analysis. J Cachexia Sarcopenia Muscle 2019;10:485–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bianchi L, Ferrucci L, Cherubini A, Maggio M, Bandinelli S, Savino E, et al. The predictive value of the EWGSOP definition of sarcopenia: results from the InCHIANTI study. J Gerontol A Biol Sci Med Sci 2016;71:259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Peng K, Tian M, Andersen M, Zhang J, Liu Y, Wang Q, et al. Incidence, risk factors and economic burden of fall‐related injuries in older Chinese people: a systematic review. Inj Prev 2019;25:4–12. [DOI] [PubMed] [Google Scholar]
- 29. Weidlich D, Andersson FL, Oelke M, Drake MJ, Jonasson AF, Guest JF. Annual direct and indirect costs attributable to nocturia in Germany, Sweden, and the UK. Eur J Health Econ 2017;18:761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Herberholz C, Phuntsho S. Medical, transportation and spiritual out‐of‐pocket health expenditure on outpatient and inpatient visits in Bhutan. Soc Sci Med 2021;273:113780. [DOI] [PubMed] [Google Scholar]
- 31. Morioka N, Moriwaki M, Tomio J, Kashiwagi M, Fushimi K, Ogata Y. Structure and process of dementia care and patient outcomes after hip surgery in elderly people with dementia: a retrospective observational study in Japan. Int J Nurs Stud 2020;102:103470. [DOI] [PubMed] [Google Scholar]
- 32. Watanabe Y, Yamada Y, Yoshida T, Yokoyama K, Miyake M, Yamagata E, et al. Comprehensive geriatric intervention in community‐dwelling older adults: a cluster‐randomized controlled trial. J Cachexia Sarcopenia Muscle 2020;11:26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Argiles JM, Busquets S, Stemmler B, Lopez‐Soriano FJ. Cachexia and sarcopenia: mechanisms and potential targets for intervention. Curr Opin Pharmacol 2015;22:100–106. [DOI] [PubMed] [Google Scholar]
- 34. Cruz‐Jentoft AJ, Landi F, Schneider SM, Zuniga C, Arai H, Boirie Y, et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 2014;43:748–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Beckwee D, Delaere A, Aelbrecht S, Baert V, Beaudart C, Bruyere O, et al. Exercise interventions for the prevention and treatment of sarcopenia. A systematic umbrella review. J Nutr Health Aging 2019;23:494–502. [DOI] [PubMed] [Google Scholar]
- 36. Leng A, Jing J, Nicholas S, Wang J. Catastrophic health expenditure of cancer patients at the end‐of‐life: a retrospective observational study in China. BMC Palliat Care 2019;18:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhao Y, Atun R, Oldenburg B, McPake B, Tang S, Mercer SW, et al. Physical multimorbidity, health service use, and catastrophic health expenditure by socioeconomic groups in China: an analysis of population‐based panel data. Lancet Glob Health 2020;8:e840–e849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dodds RM, Granic A, Robinson SM, Sayer AA. Sarcopenia, long‐term conditions, and multimorbidity: findings from UK Biobank participants. J Cachexia Sarcopenia Muscle 2020;11:62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bernabeu‐Wittel M, Gomez‐Diaz R, Gonzalez‐Molina A, Vidal‐Serrano S, Diez‐Manglano J, Salgado F, et al. Oxidative stress, telomere shortening, and apoptosis associated to sarcopenia and frailty in patients with multimorbidity. J Clin Med 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2021. J Cachexia Sarcopenia Muscle 2021;12:2259–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Characteristics of the subjects from multiple subject households
Table S2 sarcopenia and catastrophic health expenditure
Table S3 sarcopenia and catastrophic health expenditure by socio‐demographic characteristics
Table S4 sarcopenia and catastrophic health expenditure in the household with 1 male and 1 female
Table S5 self‐reported diseases prevalence among participants
Table S6 association between other diseases and sarcopenia
Table S7 association between sarcopenia group and risk of CHE, further adjusted for different diseases
Table S8 association between sarcopenia group and risk of CHE by subgroups of different diseases
Table S9 sensitivity analyses on association between sarcopenia and risk of CHE
Table S10 sensitivity analyses of the association between sarcopenia and risk of catastrophic health expenditure


