Abstract
Statin intolerance is a clinical syndrome whereby adverse effects (AEs) associated with statin therapy [most commonly statin‐associated muscle symptoms (SAMS)] result in the discontinuation of therapy and consequently increase the risk of adverse cardiovascular outcomes. However, complete statin intolerance occurs in only a small minority of treated patients (estimated prevalence of only 3–5%). Many perceived AEs are misattributed (e.g. physical musculoskeletal injury and inflammatory myopathies), and subjective symptoms occur as a result of the fact that patients expect them to do so when taking medicines (the nocebo/drucebo effect)—what might be truth even for over 50% of all patients with muscle weakness/pain. Clear guidance is necessary to enable the optimal management of plasma in real‐world clinical practice in patients who experience subjective AEs. In this Position Paper of the International Lipid Expert Panel (ILEP), we present a step‐by‐step patient‐centred approach to the identification and management of SAMS with a particular focus on strategies to prevent and manage the nocebo/drucebo effect and to improve long‐term compliance with lipid‐lowering therapy.
Keywords: Drucebo effect, Nocebo effect, SAMS, Statin intolerance
Introduction
Lipid‐lowering and cardiovascular disease reduction
Reduction of the circulating concentrations of apolipoprotein B (ApoB) containing lipoproteins, notably low‐density lipoprotein cholesterol (LDL‐C), has been consistently demonstrated to be effective in the primary and secondary prevention of cardiovascular (CV) disease (CVD) across a wide range of clinical study designs. 1 , 2 Statins reduce the endogenous production of cholesterol by inhibiting 3‐hydroxy‐3‐methyl‐glutaryl‐coenzyme A reductase (HMGCR), upregulate hepatic LDL‐receptors and reduce the risk of major adverse CV events (MACE). 3 Analysis of data from multiple randomized controlled trials (RCTs) suggests that each 1 mmol/L reduction in LDL‐C with statin therapy produces a relative reduction of about 25% in the rate of major vascular events during each year of treatment. 3 Although statin therapy is generally well‐tolerated, in some patients, it is associated with adverse effects (AEs). 4 , 5 Evidence from RCTs has causally linked statin therapy with myopathy [diagnosed by the combination of muscle weakness or pain and elevated circulating levels of creatine kinase (CK), rhabdomyolysis, 3 , 6 increased incidence of new‐onset diabetes (NOD), 3 , 7 temporary elevations of alanine aminotransferase (ALT)] 8 ; there are also some suggestions of an association between statins (and low and extremely low levels of LDL‐C) and haemorrhagic stroke; however, recent evidence has not confirmed this link. 3 Observational studies and case reports have also shown associations between statin therapy and additional AEs, including serious liver injury, memory loss, cataract, and kidney injury. 3 , 7 However, for these symptoms, causality has not been demonstrated. 9
Statin‐associated AEs are sometimes sufficiently severe to lead to the discontinuation of treatment, 9 , 10 , 11 with statin‐associated muscle symptoms (SAMS) being cited as the most frequent reason for stopping therapy 12 and the incidence of SAMS increasing with treatment intensity. 13 However, muscle pain has many potential causes and is a common symptom in older adults who are likely to be eligible for statin therapy. 13 Therefore, misattribution of effects not caused by statins is likely to occur.
The phenomenon of ‘statin intolerance’ and the associated cessation of therapy (or reduction in dose) is associated with increased risk of myocardial infarction and coronary heart disease 14 and a composite outcome of myocardial infarction, stroke, or death. 15 A recent meta‐analysis by the Lipid and Blood Pressure Meta‐analysis collaboration (LBPMC) Group, and the International Lipid Expert Panel (ILEP) has demonstrated that intolerance occurs worldwide in between 5.9 and 7.0% (depending on the diagnostic criteria used) of statin‐treated patients worldwide. 16 It is therefore imperative that practitioners have clear guidance about how to manage muscle symptoms in individuals on statin therapy. The clear aim of the guidance should be to enable patient‐centred discussions about the benefits and risks of treatment to ensure that lipid‐lowering therapy (LLT) is not stopped inappropriately and that effective CV risk‐reduction strategies are made available for individuals who cannot tolerate statin therapy at the necessary intensity to ameliorate their CVD risk.
The nocebo/drucebo effect on SAMS
Definitions
In addition to misattribution of aches and pains, a substantial proportion of SAMS result from the action of taking medicines and the expectation that medicines cause side effects. It has frequently been observed in clinical trials that more AEs are reported when open‐label statins are used than when patients are blinded to their treatment. 17 A systematic review of trials that included both open‐label and blinded phases estimated that between 38% and 78% of SAMS‐related statin intolerance could be attributed to expectation alone. 17
It is clear that neurobiological mechanisms can contribute to percived AEs. 18 However, the definition and name of this phenomenon requires clarification. The term ‘placebo effect’ describes a beneficial action that results from the expectation that an inert substance will do good. Conversely, the term ‘nocebo effect’ refers to harm resulting from an inert substance as a result of expectation. These terms are commonly applied to the beneficial and AEs of drugs (e.g. muscle pain on statin therapy is often attributed to the ‘nocebo effect’). However, this application is problematic because drugs are not inert substances; by definition, they have pharmacological actions. Placebo/nocebo effects can only be truly quantified in clinical trials, which include an arm, in which participants receive no treatment (in addition to a ‘placebo’ and an active comparator), and this is a rare situation. 17 , 19
To overcome this difficulty, in 2018 the ILEP have introduced the concept of the drucebo (DRUg + plaCEBO) effect, which compares symptom intensity when using a drug under blinded and open‐label conditions and gives quantitative insight into the extent to which symptoms may result from expectation alone. 17 Beneficial effects caused by expectation, rather than the pharmacological action of the drug (analogous to placebo), are termed ‘positive drucebo effect’, whereas AEs (analogous to nocebo) are termed ‘negative drucebo effect’. These terms are better suited to dealing with drugs than ‘placebo’ and ‘nocebo’ and can often be estimated using existing trial data 17 , 19 (Figure 1). However, in recognition of the widespread use of the term ‘nocebo’ in relation to drug therapy, we will use the term nocebo/drucebo throughout this paper. Strictly, the terms are not synonymous, but in the context of preventing and managing AEs (the focus of this paper), it is not essential to differentiate between them. Prevention and management of the nocebo/drucebo effect is essential to achieving optimal LLT and management of CV risk in a large number of patients.
Figure 1.
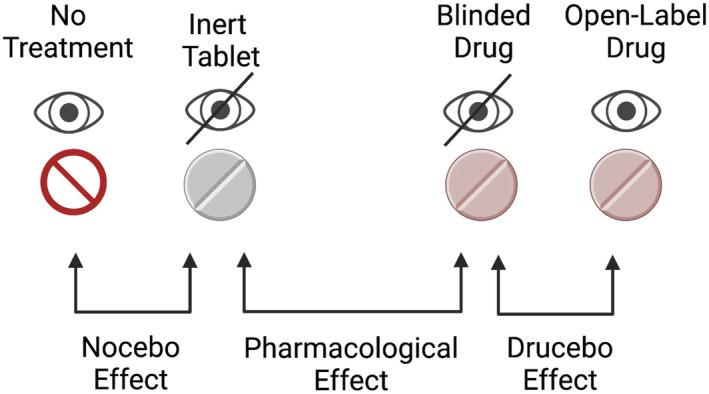
Nocebo, drucebo, and pharmacological effects explained. 17 The nocebo effect refers to adverse effects experienced when taking an inert substance (i.e. the difference in symptom intensity between no treatment, and an inert tablet), and is analogous to the placebo effect (albeit with adverse rather than desired symptoms). The drucebo effect is defined as the difference in the frequency or intensity of symptoms between blinded and open‐label use of a drug. The difference between symptoms experienced with an inert tablet and an apparently identically drug‐containing tablet represents the true pharmacological effect of the drug. Image created using Biorender.com and originally published in European Heart Journal 19 (reused with permission—Licence No. 5203820225699).
Available data
The recent Self‐Assessment Method for Statin Side‐effects Or Nocebo (SAMSON) Trial employed a novel and inventive approach to quantifying the nocebo/drucebo effect in individual patients. 20 The study recruited patients (n = 60) who had recently ceased taking statins as a result of AEs (predominantly, but not exclusively, SAMS). Over the course of 12 months, participants alternated in random order between 1 month periods of ‘no treatment’, placebo tablets, and statin‐containing tablets and reported the intensity of their symptoms each day using a smartphone app. 20 The inclusion of a period of ‘no treatment’ in SAMSON allowed a true estimation of the ‘nocebo effect’, that is, the difference in symptom intensity between taking nothing and taking an inert ‘placebo’ tablet. 19 This study design, in which patients' crossover between treatments and act as their own ‘control’, is often referred to as an ‘n‐of‐one’ trial. 20 The authors noticed that symptom severity was similar in the periods of statin use and when patients were taking ‘placebo’ tablets. However, symptoms were substantially lower in the periods of ‘no treatment’. This clearly demonstrates that real symptoms result from the action of taking tablets but that the symptoms are not caused by the pharmacological agent (the statin) in medicines. 20 In addition to the important demonstration of the nocebo/drucebo effect, the SAMSON trial also demonstrated a useful approach to advance patient care. Graphical presentations of patient's scores during the different study periods were shared with them at the end of the trial. These were used to help inform patient‐centred decision making. Six months after the trial was completed, participants were followed up, and over half had restarted statin therapy or planned to do so. 20 Despite some limitations in the trial (time of and way of statin intolerance diagnosis, the number of patients included, and their characteristics), we believe it is a very useful approach (however difficult in clinical practice) to confirm the nocebo/drucebo approach. 21
A similar study, statinWISE, enrolled 200 patients who had stopped or were considering stopping statin therapy. 22 Unlike SAMSON, statinWISE did not include a ‘no‐treatment’ arm. Participants were randomized to six 2 month periods of atorvastatin 20 mg daily or placebo. No difference was observed between the severity of AEs during periods of statin therapy or placebo. Two‐thirds of participants were able to resume statin therapy. 22
Statin intolerance—definition and consequences
To ensure that statin therapy is only ceased when there is credible evidence of a causal relationship between statin therapy and symptoms, various organizations have developed definitions and diagnostic criteria for statin intolerance. In 2015, ILEP published a position paper, in which a unified definition of statin intolerance was proposed. 23 This definition characterized statin intolerance based upon four criteria: (i) the inability of the patient to tolerate at least two different statins at the lowest available dose, (ii) intolerance associated with confirmed statin‐related AEs or significant biomarker abnormalities (e.g. elevated CK), (iii) improvement of symptoms or resolution of upon dose decrease or discontinuation of statins, and (iv) the exclusion of predisposing factors such as drug–drug interactions, thyroid disorders, vitamin D deficiency, and pre‐existing neuromuscular disorders. 23 The position paper extensively reviewed the risk factors for statin intolerance and differentiated between complete statin intolerance (intolerance to any statin at any dose) and partial statin intolerance (intolerance to some statins at some doses). 23 , 24 Definitions of statin intolerance have also been suggested by the National Lipid Association (NLA), 25 the European Atherosclerosis Society (EAS), 6 the Luso‐Latin American Consortium, 26 and the Canadian Consensus Working Group. 27 Importantly, this latter definition identifies that statin intolerance occurs when AEs lead ‘to failure of maintenance of therapeutic goals, as defined by national guidelines’ 27 (Table 1).
Table 1.
Available definitions of statin intolerance
| Author | Year | Definition | Reference |
|---|---|---|---|
| National Lipid Association | 2014 | Inability to tolerate at least two statins: one statin at the lowest starting daily dose and another statin at any daily dose, due to either objectionable symptoms (real or perceived) or abnormal laboratory determinations, which are temporally related to statin treatment and reversible upon statin discontinuation | 25 |
| International Lipid Expert Panel | 2015 |
(1) The inability to tolerate at least two different statins – one statin at the lowest starting average daily dose and the other statin at any dose. (2) Intolerance associated with confirmed, intolerable statin‐related adverse effect(s) or significant biomarker abnormalities. (3) Symptom or biomarker changes resolution or significant improvement upon dose decrease or discontinuation. (4) Symptoms or biomarker changes not attributable to established predispositions such as drug–drug interactions and recognized conditions increasing the risk of statin intolerance |
23 , 24 |
| Canadian Consensus Working Group | 2016 | A clinical syndrome, not caused by drug interactions or risk factors for untreated intolerance and characterized by significant symptoms and/or biomarker abnormalities that prevent the long‐term use and adherence to statins documented by challenge /dechallenge/rechallenge, where appropriate, using at least two statins, including atorvastatin and rosuvastatin, and that leads to failure of maintenance of therapeutic goals, as defined by national guidelines | 27 |
| European Atherosclerosis Society | 2015 | The assessment of SAMS includes the nature of muscle symptoms, increased creatine kinase levels and their temporal association with initiation of therapy with statin, and statin therapy suspension and rechallenge | 6 |
| Luso‐Latin American Consortium | 2017 |
(I) Pharmacologic (Ia) inability to tolerate at least two statins at any dose, OR (Ib) inability to tolerate doses higher than 5 mg of rosuvastatin; 10 mg atorvastatin; 20 mg of simvastatin; 20 mg of pravastatin; 20 mg of lovastatin; 40 mg of fluvastatin; or 2 mg of pitavastatin, AND (Ic) symptoms or CK changes NOT attributable to established drug–drug interactions and recognized conditions increasing the risk of statin intolerance (II) Symptomatic (IIa) intolerable muscle symptoms (muscle pain, weakness or cramps, even with normal or mildly changed CK) OR (IIb) severe myopathy (SAMS 4) (III) Etiologic (IIIa) plausible time relationship (0–12 weeks) with the introduction of statin, dose increase or introduction of a drug competing for the same metabolic pathway, AND/OR (IIIb) resolution or improvement of symptoms after discontinuation of statin (usually in 2–4 weeks), AND (IIIc) with worsening in less than 4 weeks after the new exposure (rechallenge) |
It should be noted that all current definitions depend upon a range of clinical observations and characteristics. Although CK is commonly used as an objective marker of myopathy, it cannot be considered as a specific marker for statin intolerance, and indeed no specific and selective biomarker tests for statin intolerance exist. Elevated CK can occur as a result of exercise, genetic variants, and deficiencies in coenzyme Q10 and vitamin D. 8 A large number of alternative biomarkers have been suggested, including lactate dehydrogenase, fatty acid‐binding protein 3 (FABP3), myosin light chain 1 (MLC1), myosin light chain 3 (MYL3), and skeletal muscle troponin I (sTnI). However, these have not yet resulted in clinically useful tests. 28
The need for a patient‐centred approach
The ‘n‐of‐1’ approach used in the SAMSON trial (refer to The nocebo/drucebo effect on SAMS section) provides an extremely useful demonstration of the influence of the nocebo/drucebo effect on individuals. 20 However, the protocol used in the study takes a year to administer and may be hard to implement in clinical practice owing to the logistics of placebo use, randomization and unblinding. Additionally, allocating high‐risk patients to periods of placebo or no treatment is undesirable and would be expected to increase their CV risk by increasing exposure to LDL‐C.
Statin‐intolerance guidelines (Statin intolerance—definition and consequences section) provide objective means to identify patients with true, complete statin intolerance and to differentiate these individuals from those who might indeed be able to tolerate statin treatment. However, in clinical practice, it may take several months to achieve a tolerable regimen of statin and/or combination therapy. In patients at very high and extremely high risk of CVD, this delay might significantly increase the risk of CVD events. 29
However, a formal diagnosis of either statin intolerance or the nocebo/drucebo effect does not guarantee good patient care. The SAMSON 20 and statinWISE 22 trials have demonstrated that sharing symptom data with patients allows between half and two‐thirds of patients to resume statin therapy, although even these approaches leave a substantial proportion of patients without life‐saving LLT. More generally, the long‐term persistence of statin therapy is very poor. A holistic and patient‐centred approach is therefore required to enable patients to use LLT to reduce their life‐long exposure to LDL‐C, particularly in patients with subjective symptoms in the absence of abnormal biomarkers, when the nocebo/drucebo effect is the likely cause.
Any approach to CV risk‐reduction must be based on the premise that ‘lower (and earlier) is better for longer’ with respect to LDL‐C30 but must also recognize that not all patients may be willing to take guideline‐recommended doses of statins, at least initially and that ‘any is better than none’ when considering LLT. Furthermore, current understanding around the nature of SAMS should be exploited proactively to prevent the emergence of symptoms, rather than implementing an approach that reacts to symptoms when they occur.
Objectives and organization of this International Lipid Expert Panel position paper
In this Position Paper of the ILEP, we present a step‐by‐step patient‐centred approach to the identification and management of SAMS (the most common reason for statin discontinuation 12 , 31 with a particular focus on strategies to prevent and manage the nocebo/drucebo effect and to improve long‐term compliance with LLT.
Where appropriate, the level of evidence and the strength of recommendations are categorized accordingly (Tables 2 and 3). While working on this position paper, we strictly followed the ILEP scientific policy on the preparation of the recommendations. Briefly, (i) the idea on this paper was suggested by Prof. Maciej Banach (M.B.), and Dr Peter Penson (P.P.), which was formally sent to the Steering Committee of the ILEP (refer to www.ilep.eu for details) for approval. Next, (ii) official e‐mail to all ILEP members were sent, inviting them to be a part of the Writing Committee (WC) of this paper, in which we also presented the concrete tasks to be performed and the detailed schedule on how to work with the paper. After establishment of the WC, (iii) M.B. & P.P. started to work on the main content and scientific assumption of the paper, which were next presented to the members of the WC (due to pandemic time both using online platforms and via e‐mails). Next, (iv) together with selected members of the WC, we worked on the draft version of the recommendations, which were next extensively discussed with all the WC members, putting specially emphasis on the management figures and tables with recommendations. In case of disagreement, each recommendation was voted. In the next step (v), the final draft of recommendations was sent to all ILEP members for the internal review process and approval. Each comment and suggestion from the ILEP members was responded and discussed.
Table 2.
Classes of recommendation

Table 3.
Level of evidence

Rather than using an n‐of‐1 approach to definitively diagnose the drucebo/nocebo effect, the guidelines recommend strategies that will reduce the likelihood of symptoms in all patients. When symptoms do occur, objective strategies are used to distinguish between patients with serious adverse events requiring statin discontinuation and those with subjective symptoms and normal biomarkers. Appropriate strategies are proposed for the management of each group. Our approaches aim to achieve the optimal reduction of CV risk, either through continued statin therapy or by the use of combination therapy or alternative drugs and management strategies. The overall approach is presented as an algorithm (Figure 2), each stage of which is elaborated below.
Figure 2.
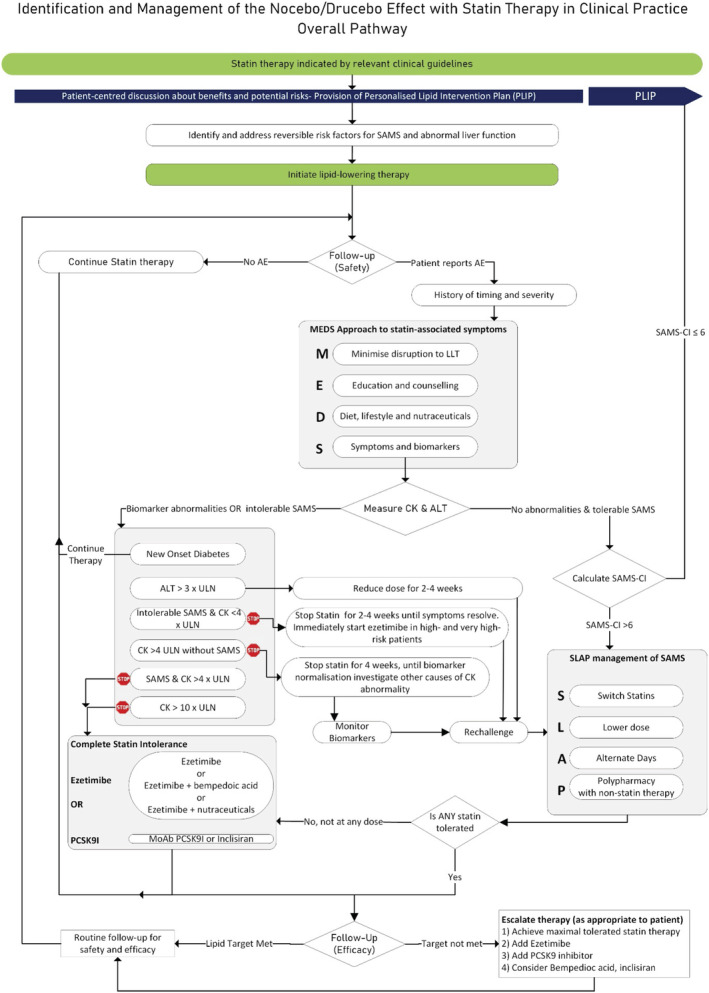
International Lipid Expert Panel (ILEP) algorithm for the management of the nocebo/drucebo effect in statin‐intolerant patients. Note that SLAP proposes a range of options to be considered in a patient‐centred manner, rather than a set of actions which should be enacted in a particular order. Abbreviations: AE, adverse effects; ALT alanine aminotransferase; CK, creatine kinase; PCSK9I, proprotein convertase subtilisin kexin type 9 inhibitors; SAMS, statin‐associated muscle symptoms; MoAb, monoclonal antibody; SAMS‐CI, statin‐associated muscle symptoms–clinical index; ULN, upper limit of normal.
A step‐by‐step approach to avoiding and managing the nocebo/drucebo effect with statin treatment
The following recommendations address the prevention and management of the nocebo/drucebo effect and serious objective AEs during the initiation of statin therapy and thorough follow‐up.
Initiation of statin therapy
In general, the management of treatment‐associated AEs is typically reactive. However, the extensive data available relating to statin therapy permits a more proactive approach, including steps to prevent AEs from occurring and to reducing the likelihood of misattribution of unrelated symptoms. Therefore, prescribers should consider the nocebo/drucebo effect at the point of initiating therapy. Furthermore, patients should be given sufficient information about the rationale and benefits of therapy to allow them to make informed decisions about their future care, should symptoms occur. We propose the use of a personalized lipid intervention plan (PLIP) to aid in this process. 32
Personalized lipid intervention plan
The PLIP is a one‐page document designed to provide important information in an accessible manner (Figure 3). It aims to promote patient‐centred decision making by informing patients about the benefits of lipid‐lowering treatment. This is accomplished quantitatively by using a locally validated risk calculator to estimate the individual's current 10 year risk of CVD with and without statin therapy (however, it is important to recognize that secondary prevention patients are not included in many prediction tools 33 and can be at extremely high risk of recurrent events 29 , 34 ). The PLIP also provides a candid explanation of the likelihood of AEs, including the important information that muscle symptoms are common but are rarely caused by statins. 35 The PLIP also contains details of the patient's specific dose of statin therapy and their personal LDL‐C target to act as an aide‐memoire, and to assist the patient in follow‐up conversations, thereby potentially improving long‐term compliance. Generic lifestyle advice is provided based upon the American Heart Association (AHA) 7‐item ‘Life's Simple 7’ tool. 36 Motivational interviewing has been demonstrated to improve long‐term compliance with statin therapy, 37 and the provision of information in the PLIP will aid motivational interviewing conversations with patients.
Figure 3.

Proposed template for the personal lipid intervention plan (PLIP).
Reversible risk factors for SAMS
Bearing in mind the fact that any AEs that occur during treatment (causal or otherwise) may limit adherence and compliance, every effort should be made to avoid muscle symptoms and the misattribution of symptoms to statin therapy. Where known reversible factors that predispose to SAMS exist, these should be corrected or discussed with the patient prior to commencing statin therapy. 38 A comprehensive overview of reversible risk factors has been provided elsewhere, 39 and these are briefly summarized below in this section.
Exercise is commonly undertaken as a result of lifestyle advice given at the time of onset of statin therapy and might be associated with muscle pain and elevated CK. 39 , 40 Muscle symptoms resulting from exercise can easily be misattributed to statin therapy. Exercise should clearly be encouraged during any consultation regarding the management of CV risk. However, patients should be made aware of the likely muscle symptoms and potentially be provided with a personalized exercise schedule to minimize the likelihood of injury through inappropriate exertion, especially if they have previously been very sedentary.
Thyroid disorders, and in particular hypothyroidism, predispose patients to SAMS 23 , 24 , 39 ; therefore, if a thyroid disorder is suspected, based upon the patients' clinical history, this should be investigated and managed appropriately before commencing statin therapy.
Several non‐randomized studies 41 , 42 , 43 , 44 have associated SAMS with vitamin D deficiency. A recent RCT comparing vitamin D3 and placebo did not show improved adherence to statin therapy over the 36 month follow‐up in vitamin D3‐treated patients but showed improvement in secondary endpoints, suggesting that vitamin D3 supplementation may be beneficial, improving the persistence of statin therapy over a 24 month period in older adults on long‐term statin therapy, especially for those on simvastatin. 39 , 45 Although inconclusive, these data should be borne in mind when commencing potentially vitamin D deficient patients on statin therapy.
Polypharmacy is very common in patients treated with statins 46 and the risk for drug–drug interactions is high. Particular care should be taken with some antifungal medicines, macrolide antibiotics, human immunodeficiency virus (HIV) protease inhibitors, sildenafil, nefazodone, calcium channel blockers, cyclosporine, danazol, amiodarone, or ranolazine. 39 It may be necessary to switch to alternative long‐term medicines or to complete short courses of drugs before commencing statin therapy. Given that AEs arising at any point during therapy may limit adherence, great care should be taken in all statin‐treated patients to avoid drug–drug interactions throughout the duration of treatment. This is especially important now, during the coronavirus pandemic, as some drugs used for coronavirus disease (COVID‐19) may increase the risk of drug–drug interactions with statins (including antiretroviral drugs lopinavir/ritonavir, macrolides, and tocilizumab), as we described in detail in previous ILEP recommendations. 47
A family history of AEs of statins may indicate a genetic susceptibility to SAMS. 39 Multiple mechanisms may underlie such effects. Polymorphisms of CYP450 enzymes (the main pathway for the metabolism of some statins) may be responsible, 48 in which case the patient may be able to tolerate alternative statins. In most of the cases of genetic predisposition to statin intolerance, patients may also be intolerant to other drugs used for CVD. 49 , 50 , 51 Routine testing for polymorphisms is not warranted at present (although it may feature increasingly in the future to aid personalized medicine). However, any known polymorphisms should be taken into account when prescribing statins, with prescribers seeking advice from specialized medicines information services, if necessary.
Routine follow‐up of statin therapy for safety and efficacy
Patients receiving LLT should be routinely followed up to evaluate the efficacy and safety of their treatment. Safety follow‐up involves asking about the history of AEs. Treatment‐emergent effects and their management are considered later (Managing adverse effects section). Efficacy follow‐up should ensure that lipid targets are met, if necessary, by escalating therapy by achieving a maximally tolerated statin dose and by using additional evidence‐based drugs (Evidence‐based lipid‐lowering formulary section), including ezetimibe, monoclonal antibody inhibitors of PCSK9, bempedoic acid, and inclisiran (Figure 2).
Managing adverse effects
We propose the MEDS (Table 4) approach to the initial management of all patients with suspected AEs on statin therapy. 19 , 32 This relies on Minimizing disruption to therapy; Educating the patient regarding the benefits of statin therapy, using Diet and nutraceuticals to complement pharmaceutical lipid‐lowering, and monitoring Symptoms and biomarkers. The approach is designed both to enable the practitioner to gain an appreciation of the likely cause of the symptoms and to facilitate patient‐centred care.
Table 4.
MEDS approach to treating all patients with statin intolerance
| Step | Brief description | Rationale | |
|---|---|---|---|
| M | Minimize | Minimize disruption to lipid‐lowering therapy | Cessation of therapy is associated with increased incidence of adverse CV events |
| E | Educate | Ensure the patient has sufficient knowledge about the proven benefits of statin therapy | To enable the patient to make an informed decision about continuation of therapy |
| D | Diet/nutraceuticals | Offer advice about dietary and nutraceutical approaches to lipid modification | To provide additive or synergistic reduction in LDL‐C, and potentially to prevent dose escalation |
| S | Symptoms/biomarkers | Monitor symptoms and relevant biomarkers | To enable effective symptomatic management and early identification of severe adverse effects |
Minimize unnecessary disruption to lipid‐lowering therapy (MEDS Step 1)
Cessation of statin therapy is associated with poor clinical outcomes. 14 , 15 Even short‐term cessation of therapy from 4 to 6 weeks has been associated with rebound inflammation 52 and instability in atherosclerotic plaques. 53 The approach of stopping statin therapy for 4–6 weeks (dechallenge) followed by a return to statin therapy (rechallenge) is very helpful when determining the causality of potential AEs of statins and in order to prevent harm to the patient if severe AEs are suspected (however it does not exclude the nocebo/drucebo effect). It has been demonstrated that dechallenge/rechallenge after SAMS allows approximately 2/3 of patients to resume statin therapy. 54 However, clinical practice shows that when dechallenge with dose adjustment results in the relief of symptoms, rechallenge rarely occurs within 4–6 months and often not for 3–6 months or longer (partly as a result of infrequent visits of the patient to their treating physician). 55 Therefore, after discontinuation of statins, non‐statin LLT should be initiated immediately. 29 Particularly in high‐risk patients, dechallenge may increase the risk of CV events (due to LDL‐C visit to visit variability and instability of atherosclerotic plaques 56 ). Therefore, when possible, statin therapy should be continued (even at the lowest doses or by employing alternate‐day administration). Consideration should be given as to whether reversible risk factors associated with SAMS, such as drug–drug interactions (Reversible risk factors for SAMS section), have emerged since the initiation of statin therapy, and if so, these should be addressed.
Educate the patient about the benefits of statin therapy (MEDS Step 2)
When patients present with symptoms suggesting statin intolerance, the opportunity to re‐emphasize the proven benefits of statin therapy should be taken. An appropriate and succinct form of words is suggested in the National Institute for Health and Care Excellence (NICE) guidelines for the reduction of CVD risk, which state: ‘Tell the person that any statin at any dose reduces cardiovascular disease CVD risk’. 57 The calculation of the individual's heart age, and demonstration how this would be altered by addressing modifiable risk factors (including cholesterol), might also be an effective option. 58 Furthermore, the PLIP should be used as a focus of discussion to provide the patient with clear information about the benefits and risks of therapy.
Diet and nutraceuticals (MEDS Step 3)
When optimal lipid‐lowering cannot be achieved because of statin intolerance, diet and nutraceuticals may be helpful in additionally lowering LDL‐C. In fact, lifestyle changes might affect LDL‐C in an number of ways. However, a suitable well‐balanced diet seems to be the most effective approach (and may reduce LDL‐C by >10%; in contrast, regular exercises reduce LDL‐C by 5–7% and weight loss by 8–10%). 59 Unfortunately, only about 20% of our patients are adherent to lifestyle changes. 59
A wide range of nutraceuticals have demonstrated lipid‐lowering effects and have been proposed for use in statin intolerance. 60 They include red yeast rice (RYR), bergamot, berberine, artichoke, soluble fibres, garlic, soy derivatives, and plant sterols and stanols. 60 , 61 It should be noted that the extent of lipid‐lowering achieved with nutraceutical therapy is generally modest (usually up to 25%) in comparison to statins, and, except for RYR, omega‐3 fatty acids and phytosterols, CV outcomes trials have not been conducted. 62 However, many nutraceuticals have additional pleiotropic effects, which would be expected to be of benefit in the prevention of CV disease. These include anti‐inflammatory 63 and antioxidant effects and beneficial effects on arterial stiffness and endothelial function. 60 Thus, nutraceuticals (especially in combination) are likely to be a useful additional means of achieving lipid targets in statin‐intolerant patients. Readers are directed to recent comprehensive reviews on this topic for further guidance. 60 , 61 , 62 , 64 , 65
Symptoms and biomarkers (MEDS Step 4)
A detailed history of symptoms should be taken, and biomarkers (including ALT and CK) should be measured. A multi‐step approach to symptoms in statin intolerance 39 , 66 has been presented previously, and is adapted here. By considering the timing, history, and severity of symptoms, the clinician can ensure patient safety by identifying rare cases of serious AEs requiring further investigation or statin discontinuation. Furthermore, the information gathered aids the management of alternative causes of AEs initially attributed to statin therapy. Each of the steps is elaborated in further detail later.
Timing
The temporal relationship between treatment administration and effect provides important information about the likelihood of a causal relationship between statin therapy and symptoms. This focus on timing addresses the ‘temporality’ aspect of Bradford Hill's criteria for causation. 67 The following facts should be established:
When dose‐limiting symptoms were first experienced by the patient.
When statin therapy was initiated, and if (and when) any increases in dose have occurred.
If alternative causes of symptoms are suspected, the timing of possible secondary causes should be established. 39
It has been estimated that over 75% of SAMS appear within the first 12 weeks of treatment and that 90% occur within 6 months. 39 Therefore, symptoms emerging after a longer duration of therapy are unlikely to be caused by statins unless they are precipitated by a drug–drug interaction or some other change in circumstances (e.g. worsening thyroid, renal and/or liver function). 27 Because of the latency of onset and cessation of drug effects, AEs that occur immediately upon starting statin therapy or resolve immediately upon withdrawing therapy are unlikely to be caused by statins. 10 , 27 , 68 A rigorous approach to studying the temporal relationship between statin therapy and muscle pain can be achieved by dechallenging (stopping or reducing statin therapy for a period of time) and rechallenging (restarting therapy with the same or a different statin) as part of the calculation of the SAMS‐Clinical Index (Managing patients with no biomarker abnormalities and tolerable SAMS section). 69
The timing of symptom onset should also be considered in determining the likelihood of causality in the case of non‐muscle‐related AEs of statins. In a registry study conducted in the USA, the onset of elevated liver enzymes (alanine aminotransferase (ALT) or aspartate transaminase (AST) >5× upper limit of normal (ULN) and/or alkaline phosphatase (ALP) >2 ULN) did not occur before 1 month of therapy, and the median latency of onset was around 5 months. 70 , 71
History
A full clinical and family history is necessary in order to identify conditions and circumstances that predispose to statin intolerance. In many cases, these are modifiable (refer to Reversible risk factors for SAMS section) and should be addressed where possible. Based on the history, we might also learn whether there is any family history of statin intolerance and therefore whether we might suspect a genetic predisposition. Consideration should also be given to alternative causes of muscle pain, in particular peripheral arterial disease, which can be investigated by measuring the ankle‐brachial index.
Severity
An estimation of the severity of the symptoms resulting in statin intolerance is very important. This enables the physician to exclude the possibility of the symptoms resulting from rare serious illnesses. Furthermore, it supports a person‐centred approach, allowing the patient to balance the potential benefits of treatment with any associated discomfort.
Patients should be asked about the tolerability of their symptoms. At the same time, the beneficial effects of treatment should be emphasized [refer to Educate the patient about the benefits of statin therapy (MEDS Step 2) section], and in case of symptom tolerability, to motivate the patients to continue statin therapy (in most cases myalgia disappears after few weeks of therapy). 10 , 39 Where SAMS is suspected, CK should be monitored, although it should be appreciated that this biomarker quantifies muscle damage and is not specific to SAMS.
The diagnostic information collected in the step‐by‐step approach can be used to categorize patients according to the nature and severity of their statin intolerance. This categorization then suggests the optimal approach to the management of the patient's condition.
Managing patients with no biomarker abnormalities and tolerable SAMS
When biomarker abnormalities are absent, it is likely to be safe to continue with statin therapy in most of the cases (with reduced dose, alternative day therapy, or combination therapy). 39 Further diagnostic information can be collected to aid future management. The SAMS clinical index (SAMS‐CI) is a validated tool for assessing the likelihood that SAMS in an individual patient are caused or worsened by statin use. 69 The SAMS‐CI is easy to apply and has good inter‐rater reliability. The SAMS‐CI considers the location and patterns of the muscle symptoms and the timing of symptoms relative to starting, stopping statin therapy. A low SAMS‐CI score indicates that it is unlikely that the symptoms are caused by statins. 10 , 69 , 72 In such cases, the symptoms are likely to be explained by the nocebo/drucebo effect and/or obviously other causes of muscle pain. The SAMS‐CI should be calculated in this patient group (this involves dechallenging and rechallenging statin therapy). The results should be used in the context of patient‐centred care, and strict recommendations are probably not appropriate. However, where SAMS‐CI is ≤6, and there is a very low likelihood of a causal effect of statins on the patient's symptoms; after excluding other reasons, the revisiting patient education with the PLIP is recommended. When there is an increasing likelihood of causality (SAMS‐CI > 6), then the SLAP (Switch drugs, Lower dose, Alternate‐day dosing, Polypharmacy) management algorithm (SLAP technique for the patient‐centred management of SAMS section) may be most appropriate.
Managing patients with biomarker abnormalities and/or intolerable SAMS
In patients with intolerable SAMS or clearly abnormal biomarkers, dose reduction, cessation of statin therapy and further investigation may be necessary to ensure patient safety. In the following sections, we provide guidance for specific groups of patients.
Patients with new‐onset diabetes (NOD)
While patients are less likely to present with NOD than SAMS as a side effect, tests performed as part of biomarker investigations may reveal NOD. In this case, there is no reason to stop statin therapy. In the Justification for the Use of Statins in Primary Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) trial, the average time from randomization to diagnosis of type 2 diabetes (T2DM) was only 5.4 weeks shorter in the rosuvastatin group than the control group (84.3 vs. 89.7 weeks). 73 However, the role of statins in causing NOD is largely irrelevant to the management of these patients, as the JUPITER investigators concluded that the CV and mortality benefits of statin therapy by far exceed the diabetes hazard, including among those at higher risk for developing diabetes. 73 The meta‐analysis by Preiss et al.74 showed that intense statin treatment is beneficial since it significantly outweighs the risks of NOD—there was one additional case of T2DM for every 498 patients treated for 1 year compared with one fewer patient experiencing a CV event for every 155 patients treated for 1 year. 74 , 75 These results confirmed the previous observations that the harm associated with possible NOD is 5.4× less than the benefits associated with the prevention of coronary deaths or myocardial infarctions for each mmol/L reduction in LDL‐C. 75 Thus, continued statin therapy is recommended in patients with NOD. 76 , 77 Consideration should be given to informing patients at high risk of NOD (particularly those with impaired fasting glucose, but also metabolic syndrome, obesity, family history of type 2 diabetes) of the risk at the time of statin initiation and monitoring plasma glucose (Table 5).
Table 5.
ILEP recommendations on the management with new‐onset diabetes (NOD)

Patients with elevated ALT (>3 times ULN)
The incidence of ALT elevation on statin therapy is low and temporary and can usually be resolved by reducing the dose of statin without the need to stop treatment. After 2–4 weeks, it is usually possible to return to the original dose. 39
Discontinuation of statin therapy due to ALT elevation is one of the most commonly observed reasons for therapeutic inertia. 23 Based on data from the most recent meta‐analysis (22 studies with 195 602 subjects were included) the authors showed that in patients with chronic liver hepatitis, the levels of ALT and AST were reduced slightly following statin therapy, however this reduction was not significant. 78
The prescriber should consider the use of the SLAP algorithm to maximize long‐term adherence to LLT (SLAP technique for the patient‐centred management of SAMS section) (Table 6).
Table 6.
ILEP recommendations on the management with elevated level of ALT

ALT, alanine aminotransferase.
Patients with SAMS and CK < 4 ULN and intolerable muscle pain
In this situation (which might result from extreme nocebo/drucebo effects) intolerable muscle pain requires cessation of statin therapy, even in the absence of objective markers of muscle damage, and irrespectively of causality confirmation with statin therapy or not. Nevertheless, efforts must be made to restore LLT as soon as possible to avoid unnecessarily elevated CV risk. In high‐risk patients, ezetimibe should be started immediately to reduce exposure to LDL‐C 47 (Table 7).
Table 7.
ILEP recommendations on the management with patients with intolerable SAMS and CK < 4 ULN

ALT, alanine aminotransferase; CK, creatine kinase; SAMS, statin‐associated muscle symptoms.
Patients with CK > 4 ULN without SAMS
In patients with biomarker abnormalities (CK > 4 x ULN) without SAMS, statin therapy should be stopped for at least four weeks, after which biomarkers should be re‐investigated. If biomarkers have normalized, statin rechallenge should occur at a lower dose (and in combination therapy with ezetimibe based on the CVD risk), and all elements of the SLAP algorithm (SLAP technique for the patient‐centred management of SAMS section) can be considered (Table 8). It is always very important to differentiate significant CK elevations as an effect of statin therapy compared with other possible reasons (e.g. intensive exercise, drugs, viral infections, alcoholism, muscle damage, hypothyroidism, connective tissue and/or rheumatological disorders, acute coronary syndromes); we cannot also exclude so‐called idiopathic hyper‐CK‐aemia 79 (Table 9).
Table 8.
ILEP recommendations on the management with patients without SAMS and CK > 4 ULN

ALT, alanine aminotransferase; CK, creatine kinase; ILEP, International Lipid Expert Panel; SAMS, statin‐associated muscle symptoms.
Table 9.
The most common causes of CK elevation
| Chronic diseases | Medications | Toxins | Metabolic disturbances | Muscle trauma/disorders | Others |
|---|---|---|---|---|---|
|
Endocrine disorders Hyperthyroidism Hypothyroidism Hypoparathyroidism Acromegaly Cushing syndrome Connective tissue disorders Rheumatological diseases Cardiac disease (heart failure, valvular, tachycardia, myocarditis, ACS) Acute kidney disease Viral illnesses Celiac disease |
Statins Fibrates Antiretrovirals Beta‐blockers Clozapine Angiotensin receptor blocking agents Hydroxychloroquine Isotretinoin Colchicine Steroids |
Ethanol Cocaine Heroin Amphetamine |
Hyponatraemia Hypokalaemia Hypophosphataemia |
Muscle dystrophies Metabolic and mitochondrial disorders of muscle Inflammatory myopathies Others Familial elevated CK Sarcoid myopathy Motor neuron diseases Charcot–Marie–Tooth disease Other congenital diseases Intramuscular injections Needle electromyography Seizures |
Ethnicity (black Americans may have elevated baseline CK levels) Intensive exercise Surgery Malignancy MacroCK Ssevere chills Predisposition to malignant hyperthermia Idiopathic hyperCKaemia |
Patients with SAMS and CK > 4 ULN & with CK > 10 x ULN
Patients with extremely elevated CK levels (>4 and especially those with >10x ULN) are likely to be completely intolerant to statin therapy. Statin therapy should be immediately stopped, and management strategies for individuals with complete statin intolerance (Management strategies in patients with complete statin intolerance section) should be employed. These patients should always be carefully examined to determine the cause of extremely elevated CK levels (Table 10).
Table 10.
ILEP recommendations on the management with SAMS with CK > 4 ULN

CK, creatine kinase; ILEP, International Lipid Expert Panel.
Management strategies in patients with complete statin intolerance
When patients are unable to tolerate any statin at any dose (about 3–5% of patients with statin intolerance) because of intolerable AEs, 39 non‐statin drugs from the Evidence‐Based Lipid‐Lowering Formulary should be considered (Evidence‐based lipid‐lowering formulary section). In patients with a family history of statin intolerance, and those at high risk of statin intolerance, taking into account recognized risk factors of statin intolerance (e.g. elderly patients, liver/kidney impairment, and polypharmacy), starting with the combination therapy of lower statin dose and ezetimibe—with the doses recommended for the patient's risk—might be considered. The specific choice of drug will depend upon patient characteristics, CVD risk, comorbidities and concomitant medications, in addition to considerations of drug availability, reimbursement and cost. However, ezetimibe (Ezetimibe section) should form the basis of the initial stages of therapy. Ezetimibe can be used as monotherapy, or in combination with bempedoic acid (Bempedoic acid section), or nutraceuticals (Nutraceuticals section), as well as with PCSK9 inhibition‐based therapies—monoclonal antibody PCSK9 inhibitors (Monoclonal antibody inhibitors of PCSK9 section) and inclisiran (Inclisiran section) may be used (Table 11, Figure 2).
Table 11.
Summary of the ILEP recommendations on the management with SAMS

CVD, cardiovascular disease; ILEP, International Lipid Expert Panel; SAMS, statin‐associated muscle symptoms.
SLAP technique for the patient‐centred management of SAMS
We propose the acronym SLAP (Table 12) to summarize potential approaches to the management of partial statin intolerance (a group comprising 95% of patients with statin intolerance). 39 It is not necessary to follow these steps in that order, but the most appropriate approaches should be chosen based on patient characteristics and comorbidities.
Table 12.
SLAP approach to managing partial statin intolerance
| Step | Brief description | Rationale | |
|---|---|---|---|
| S | Switch statin | Rechallenge patient with a different statin. Consider using a drug with alternative partitioning chemistry (hydrophilic vs. lipophilic) or metabolic pathway to drug which caused intolerance |
Some adverse effects may be drug‐ rather than class‐specific. Patient may be unwilling to be rechallenged with a drug they associate with adverse effects |
| L | Lower dose | Reduce daily dose of statin |
Adverse effects are dose‐dependent. Adequate LDL‐C reduction may be possible with a lower dose |
| A | Alternate‐day dosing | Consider alternate‐day dosing |
Adverse effects are dose‐dependent. Adequate LDL‐C reduction may be possible with alternate‐day dosing |
| P | Polypharmacy | Add another lipid‐lowering drug with proven efficacy on hard outcomes | If adequate LDL‐C reduction cannot be achieved with monotherapy, polypharmacy is appropriate |
LDL‐C, low‐density lipoprotein cholesterol.
While being strongly convinced that ‘lower is better for longer’ and ‘the earlier on target, the better’ 30 , 59 , 80 , 81 with respect to LDL‐C, our recommendations are patient‐centred and recognize that not all patients may be willing to take guideline‐recommended doses of statins and that ‘any is better than none’ when considering LLT. Where initial dosing strategies do not meet LDL‐C targets, then consideration must be given to escalating therapy over time to optimize therapy.
In response to correspondence on this topic, 17 the SAMSON lead investigators have advised against strategies such as dose reduction on the basis that they reinforce the idea of causality in the patient's mind, especially as they are often applied when symptoms are most intense (and therefore likely to decline regardless of subsequent management). 82 However, we believe that these have to be balanced against the necessity of patient‐centred care, and if a patient is willing to continue LLT (albeit of suboptimal intensity), this is a better outcome than them taking no treatment at all. It should also be noted that in SAMSON, the investigators had very strong evidence of the likely causality of the symptoms, owing to their rigorous and innovative 12 month randomized assessment of symptoms under different conditions. These data are not available in routine practice, and therefore, practitioners cannot be as confident that reported symptoms are not caused by the drug in any way. Thus, dose reduction (and similar) strategies are prudent.
Switch between statins
While statin intolerance may sometimes occur as a class effect; it may also manifest as a response to a particular drug. In such situations, switching from one statin to another may be sufficient to resolve the symptoms of intolerance. 23 , 39 Statins are metabolized by a range of mechanisms (Table 13). Changing from one statin to another may resolve symptoms that result from individual variations in metabolism or drug–drug interactions. Statins vary in their physicochemical properties. Atorvastatin, simvastatin, fluvastatin, lovastatin, and pitavastatin are lipophilic, whereas pravastatin and rosuvastatin are hydrophilic. Switching from a hydrophilic to a lipophilic drug, or vice versa, may be useful in patients with SAMS; however, it cannot be treated as a rule, as it might happen that switching from one lipophilic statin to another might cause relief of the symptoms. 6 , 8 , 23 , 24 Dechallenge of statins and rechallenge (including with a different statin) in the PROSISA (Prevalence Of Statin‐Associated Muscle Symptoms In Italy) study allowed 2/3 of participants to resume statin therapy after initially reporting AEs. 54
Table 13.
Pharmacokinetic and chemical properties of statins
| Drug | Lipophilicity | Metabolism |
|---|---|---|
| Atorvastatin | Lipophilic | Hydroxylation, oxidation, CYP3A4 |
| Fluvastatin | Lipophilic | CYP2C9 > CYP 2C8, CYP 3A4 |
| Lovastatin | Lipophilic | CYP3A4 |
| Pitavastatin | Lipophilic | Glucuronidation, UGT1A3, UGT 2B7 > CYP2C8, CYP 2C9 |
| Pravastatin | Hydrophilic | Sulfation, hydroxylation, oxidation |
| Rosuvastatin | Hydrophilic | Biliary excretion, CYP2C9, CYP2C19 |
| Simvastatin | Lipophilic | CYP3A4 |
Modified from Rosenson et al.10
Lower dose
Dose reduction is a common approach to the management of SAMS 10 , 77 and can provide valuable insight as to whether an AE is dose‐dependent (pharmacological) or idiosyncratic. It is critically important to remember that even the lowest dose of statin might be important in the prevention of CVD events; therefore, we should do our best to avoid statin discontinuation. Equally important is that when we need to reduce the dose due to confirmed SAMS, we should always consider adding ezetimibe (and/or other non‐statin LLT), to allow LDL‐C target achievement, especially in high‐risk to extremely high‐risk patients. 10 , 29 , 39 Furthermore, if low statin doses are tolerated, the dose can be slowly escalated.
Alternate‐day dosing
Similar to the approach of dose reduction is the strategy of the use of alternate‐day‐dosing of statins, rather than the more usual once daily dosing. Several RCTs have investigated this approach, and the results have been combined in a meta‐analysis conducted by the LBPMC Group. 83 Overall, 13 studies (1023 patients) were included in the analysis, and there was no statistically significant differences in lowering of LDL‐C or triglycerides between daily and alternate‐day dosing of atorvastatin and rosuvastatin. Both regimens were well tolerated. 83 While there is no outcomes data to support this approach, it may be a reasonable strategy to improve the persistence of statin therapy in patients with mild SAMS and in need of a statin.
Clinical practice with statin intolerance patients sometimes forces the physicians to use statins with a long elimination half‐life (for rosuvastatin it is approximately 19 h, for atorvastatin 14 h, and for pitavastatin 12 h), even every third day (twice a week) at the lowest doses in order to maintain treatment. 84
Polypharmacy: combination therapy drugs from evidence‐based lipid‐lowering formulary
When patients can tolerate a reduced (or alternate‐day) statin dose, but therapeutic targets are not met, add‐on therapy with non‐statin lipid‐lowering agents may be appropriate, 39 in line with clinical evidence and guidance. While the term ‘polypharmacy’ often implies unnecessary use of multiple medicines, in the contest of LLT, there is strong evidence that additional lipid‐lowering targets make the attainment of targets more likely and, when used appropriately, result in better clinical outcomes. In the evidence‐based lipid formulary (Evidence‐based lipid‐lowering formulary section), we briefly summarize the evidence for the use of ezetimibe (Ezetimibe section), monoclonal antibodies of PCSK9 (Monoclonal antibody inhibitors of PCSK9 section), inclisiran (Inclisiran section), bempedoic acid (Bempedoic acid section), and nutraceuticals (Nutraceuticals section). The choice of a particular drug from this list will likely depend on both patient‐specific factors and local cost and reimbursement policies, especially for PCSK9 inhibitors and inclisiran. Physicians may consider the use of lipid‐modifying nutraceuticals [Diet and nutraceuticals (MEDS Step 3) section] and should remember that patients may be self‐medicating with these agents in any event.
Evidence‐based lipid‐lowering formulary
This section summarizes the evidence for non‐statin lipid‐lowering drugs, which might be used in combinations with low‐dose statins in the case of partial statin intolerance and may replace statins in the case of complete intolerance.
Ezetimibe
Ezetimibe reduces the intestinal absorption of cholesterol by blocking the Niemann–Pick C1‐like 1 protein on epithelial cells. In the IMPROVE‐IT trial, ezetimibe (10 mg/day) was demonstrated to result in a further reduction in CV events in combination with statin therapy in 18 144 patients with ACS. 85 The primary endpoint was a composite of CV death, nonfatal myocardial infarction, unstable angina requiring rehospitalization, coronary revascularisation (≥30 days after randomization), or nonfatal stroke, and median follow‐up was 6 years. The hazard ratio (HR) was 0.936 [95% confidence interval (CI) 0.89–0.99, P = 0.016], 85 and the benefits of the therapy were higher with the higher baseline risk of patients (e.g. with diabetes). 86 A recent secondary analysis of IMPROVE‐IT data focusing on patients >75 years of age, demonstrated that this group had the greatest absolute benefit from adding ezetimibe to statin therapy. 87 A combination of statin and ezetimibe may be particularly helpful in this population as older adults are among those most at risk of AEs of high‐dose statins. 23 , 25 , 27
Thus, ezetimibe is an excellent choice as an add‐on when treatment targets cannot be met with statin therapy. Ezetimibe may also be useful as monotherapy when a patient suffers complete statin intolerance (it might be applied immediately after discontinuation, especially in very high‐risk patients), and in combination therapy (as indicated, based on the risk and LDL‐C goal) in all those with partial statin intolerance. As monotherapy, ezetimibe effectively lowers LDL‐C (by 15–20%) 88 and Lp(a) by 7% (there is still some inconsistency in the available data on this context), 89 although outcomes data for monotherapy are lacking. 90 The recommendations on how to use ezetimibe in statin‐intolerant patients are presented in Table 11.
Monoclonal antibody inhibitors of PCSK9
Proprotein convertase subtilisin/kexin type 9 (PCSK9) is a regulatory protein that binds to LDL‐receptors on hepatocytes and promotes their inactivation by internalization into the cytoplasm of the cell. 91 Inhibition of PCSK9 increases available LDL‐receptors on hepatocytes, which results in more extensive removal of circulating LDL particles. PCSK9 has been targeted by the use of monoclonal antibodies (described below) 92 and by siRNA in the case of inclisiran (Inclisiran section). 93
Monoclonal antibody inhibitors of PCSK9 (of which alirocumab and evolocumab are currently licensed) substantially reduce circulating concentrations of LDL‐C (by about 60% in monotherapy), and they represent a substantial advance in the management of dyslipidaemias. 92 They are injected subcutaneously every 2–4 weeks. Particularly encouraging results have been observed in individuals with statin intolerance. 91 , 94 , 95 The GAUSS‐3 trial recruited participants with confirmed statin intolerance, in whom 24 weeks of treatment with evolocumab was associated with a 53% reduction in LDL‐C. It is encouraging that muscle‐related symptoms were reported in only 21% of participants treated with evolocumab. 96 Similarly, the ODYSSEY‐ALTERNATIVE trial demonstrated that 24 weeks of treatment with alirocumab reduced mean LDL‐C by 45%. 97 Although these lipid‐lowering actions are not equivalent to outcomes data and therefore should be interpreted cautiously in clinical‐decision making, it should be noted that PCSK9 inhibition has been demonstrated to significantly reduce CV events against a background of statin therapy in the FOURIER 98 and ODYSSEY‐Outcomes 99 trials with evolocumab and alirocumab, respectively. PCSK9 inhibitors opened a new era in the management of statin‐intolerant patients, who often have high CV risk owing to highly elevated level of LDL‐C at baseline (even >180 mg/dL as it was observed in both trials with PCSK9 inhibitors 96 , 97 ), which is associated with a very high risk of CV events. 14
Unfortunately, PCSK9 inhibitors are very costly, and availability and reimbursement are challenging in many parts of the world. However, their remarkable effectiveness in LDL‐C lowering, impressive safety and emerging profile of outcomes reduction makes these agents a critical part of statin intolerance management. That is why, based on the approach ‘the lower, the better’, but especially ‘the earlier the better’, especially for statin intolerant patients after acute coronary syndrome (ACS), there is a recommendation not to delay rechallenge (such a delay often lasts for several months in clinical practice and may increase the risk of atheroma plaque instability and recurrent CV events), but to start combinationtherapy (ezetimibe and PCSK9 inhibitors) immediately after statin discontinuation (if reimbursement criteria allow) 29 (Table 11).
Inclisiran
In contrast to the monoclonal antibody inhibitors of PCSK9 (Monoclonal antibody inhibitors of PCSK9 section), inclisiran is a synthetic small interfering RNA (siRNA) that binds to the mRNA for PCSK9 and thus acts as an inhibitor of translation, reducing PCSK9 production and thereby improving clearance of LDL‐C. 100 Inclisiran was evaluated in 501 patients in the ORION‐1 RCT, which was conducted in patients with elevated LDL‐C and a high risk of CVD. 100 Participants received either a single dose of inclisiran (200, 300, or 500 mg) or two doses, 90 days apart (100, 200, or 300 mg). 100 Following 180 days of treatment, LDL‐C was significantly reduced from 27.9% to 41.9% in patients receiving the single‐dose and 35.5% to 52.6% in patients receiving the two‐dose regimen. 100 Follow‐up studies (pooled analysis of the data from ORION 9‐11 trials) have demonstrated that the LDL‐C lowering effect of two doses of inclisiran persists for over 18 months and reduces LDL‐C by as much as 55%/70 mg/dL. 101 The ORION development program (ORION 1‐18 studies) includes patients with very high CV risk with atherosclerotuc cardiovascular disease (ASCVD) and/or heterozygous familial hyperchoesterolaemia (HeFH) similarly to PCSK9 inhibitors studies, subjects with risk equivalents of ASCVD, those with homozygous hypercholesterolaemia (HoFH) (both adults and adolescents) as well as patients with hepatic impairment, renal failure (including patients with chronic kidney disease and eGFR between 15 and 30 mL/min), and healthy volunteers. 84 Interestingly, there are no specific studies dedicated to statin intolerant patients, however the way the drug is administered (2 doses/year) makes it extremely useful both for patients with statin intolerance, statin nonadherence, as well as those that are not willing to use statins 93 , 102 (Table 11). We obviously need to wait for the results of the ORION‐4 CV outcomes trial (primary estimated completion date is December 2024) to see whether this significant LDL‐C reduction, as well as improved adherence, will be associated with the significant reduction of CV events. 93 , 102 Inclisiran was approved for use in the European Union in December 2020, but has not yet been approved by the FDA.
Bempedoic acid
Bempedoic acid (ETC‐1002) is a novel lipid‐lowering agent inhibiting adenosine triphosphate‐citrate lyase (ACLY), an enzyme involved in cholesterol biosynthesis two steps upstream of 3‐hydroxy‐3‐methylglutaryl‐coenzyme A reductase. 103 It is a prodrug converted into the active compound (bempedoic acid‐coenzyme A) by long‐chain acyl‐CoA synthetase‐1 (ASCV1L) in the liver. Due to the high first‐pass effect, the systemic exposure to bempedoic acid is low, which could therefore explain less frequently observed muscle‐related symptoms. 104 Early studies with hypercholesterolemic patients showed LDL‐C reduction LDL‐C reduction by 26.6% in doses up to 120 mg daily. 105 It was also well‐tolerated as monotherapy in patients with statin intolerance and decreased LDL‐C by 28.7% using doses up to 240 mg a day. 106
When added to ongoing low‐ to moderate‐intensity statin therapy, 120 and 180 mg of bempedoic acid once daily produced incremental LDL‐C lowering (by 17.3% and 24.3%, respectively) compared with placebo (−4.2%) in a phase IIb multicentre, double‐blind, randomized study of 12 week duration. 107 Importantly, AEs did not differ among the three groups, including muscle‐related events, and in fact, the latter were less common in the active treatment group. Of note, 10% of patients had a history of statin discontinuation due to muscle‐related symptoms before inclusion. 107 Bempedoic acid 120 or 180 mg alone reduced LDL‐C by 27 to 30% in statin tolerant and intolerant patients in another Phase 2b study, and it was significantly greater effect compared with ezetimibe monotherapy. 108 The combination of both agents reached 43% and 48% LDL‐C reduction, respectively. The tolerability profile was similar in all treatment groups. 108
In the Evaluation of the Efficacy and Safety of Bempedoic Acid (ETC‐1002) as Add‐on to Ezetimibe Therapy in Patients With Elevated LDL‐C (CLEAR Tranquility) Phase 3, multicentre, double‐blind, placebo‐controlled study, 269 patients were randomized to bempedoic acid 180 mg or placebo (2:1) for 12 weeks after a 4‐week run‐in of ezetimibe 10 mg/day. 109 All patients had a history of statin‐associated symptoms. Concomitant lipid‐lowering drugs were used by 44.8%, and about one‐third were on low‐dose or very low‐dose statins (higher than low‐dose statin was an exclusion criterion). Bempedoic acid as an add‐on to ezetimibe reduced LDL‐C by 28.5% more than placebo (+5.0%). Subgroup analyses suggested greater LDL‐C reduction in statin non‐users than users (−34.7% vs. −20.5%, respectively). The study drug was safe and well‐tolerated with a similarly low rate of muscle symptoms in both groups. 109
The effectiveness at LDL‐C reduction and tolerability of bempedoic acid as an add‐on to maximally tolerated statin therapy was further confirmed in the CLEAR Harmony Trial. 110 Patients randomized to receive bempedoic acid 180 mg once daily (n = 1488) or placebo (n = 742) had similar rates of AEs and serious AEs during a 52 week period irrespective of the intensity of statin therapy. Importantly, the rate of muscle disorders was not significantly increased (13.1% vs. 10.1%), although the incidence of AEs leading to discontinuation of the study drug was slightly higher in the bempedoic acid group (10.9% vs. 7.1%). There was a higher incidence of gout (1.2% vs. 0.3%) and a lower incidence of new‐onset or worsening diabetes (1.2% vs. 0.3%). The mean LDL‐C level was reduced by 16.5% at Week 12. 110 All these positive results were confirmed in the Phase 3 trials pooled analysis of 3623 patients, including 614 patients with statin intolerance. 111 Patients with statin intolerance had a mean (SD) baseline LDL‐C level of 144.4 (38.8) mg/dL and the percentage changes in LDL‐C levels at week 12 were −23.0% in the bempedoic acid group and 1.5% in the placebo group (difference −24.5%). The decrease in LDL‐C levels with bempedoic acid was sustained during long‐term follow‐up with difference of −22.2% at Week 24. 111 All studies also showed significant improvements in TC, non‐HDL‐C and ApoB levels, and no significant changes in TG and HDL‐C levels. Notably, reduction of high‐sensitivity C reactive protein (hs‐CRP) up to −42% was statistically significant in the vast majority of the studies, and no worsening of glycaemic control has been observed in patients with diabetes. 112
In summary, for patients unable to use optimal intensity statin therapy, bempedoic acid has a good potential of further lipid‐lowering in various clinical settings, including as an add‐on to very low‐dose, low‐dose, and moderate‐dose statins, in combination with ezetimibe or as a monotherapy. Whether LDL‐C reduction with bempedoic acid translates into improved clinical outcomes remains to be demonstrated by ongoing clinical outcome study CLEAR Outcomes (the first CV outcomes trial with statin intolerant patients only) expected to be completed in 2022. Results of a large Mendelian randomization study showed associations between genetic variants mimicking ACLY inhibitors with a lower risk of CVD. 113 Bempedoic acid has been demonstrated to be safe and to effectively lower LDL‐C in combination therapy with ezetimibe. 114 Both bempedoic acid, and fixed combination with ezetimibe, were approved by the United States Food and Drug Administration (FDA) (February 2020) and the European Medicines Agency (EMA) (April 2020), and soon should be an important part of the therapy of patients with statin intolerance (Table 11).
Nutraceuticals
If not considered previously, nutraceuticals may be considered as part of the approach to lipid‐lowering [Diet and nutraceuticals (MEDS Step 3) section], especially in patients with statin intolerance. Here in these guidelines, we adopted (with some modifications) the 2018 ILEP recommendations on the use of nutraceuticals in statin‐intolerant patients 60 , 115 , 116 (Table 14).
Table 14.
Summary of the ILEP recommendations on the application of nutraceuticals in statin intolerant patients

ILEP, International Lipid Expert Panel; LDL‐C, low‐density lipoprotein cholesterols.
Maximum recommended doses as dietary supplement recommended by the draft (2021) recommendations by the European Food Safety Authority (EFSA).
Limitations and cautions
With the exception of the use of PCSK9 inhibitors in statin‐intolerant patients, there is relatively little evidence from RCTs measuring clinically relevant outcomes in statin‐intolerant patients. Until long‐term outcomes trials results are available to provide answers to all the questions addressed in these recommendations, expert consensus, based upon existing evidence, is probably the best approach to make treatment decisions. The purpose of this consensus document is to provide such recommendations. Medical professionals are encouraged to consider our recommendations when making decisions regarding the treatment of patients with lipid disorders and statin intolerance. However, the position paper does not override in any way the individual responsibility of healthcare professionals to make appropriate, accurate and patient‐centred decisions, considering the patient's medical history, and in consultation with the patient and/or, where appropriate, their guardian or caretaker. It is also the responsibility of health professionals to verify the doses, rules and regulations applicable to drugs, and devices at the time of their prescription or use. The authors of this position paper are aware that the use of recommendations depends on several judgement calls that take account of the values and preferences of the patient.
Conclusions
Statins are usually very well tolerated; however, in common with all medicines, statins, may cause AEs in some patients. Statin intolerance occurs when side effects attributable to statin therapy lead to discontinuation or suboptimal use of these drugs. However, many cases of subjective AEs are misattributed to statins or occur as a result of the nocebo/drucebo effect. To overcome these barriers to effective CV risk reduction, this position paper has presented a step‐by‐step approach to the management of the nocebo/drucebo effect, with a particular focus on the prevention and management of subjective symptoms such as SAMS.
Conflict of interest
Peter E. Penson has received honoraria and/or travel reimbursement for events sponsored by AKCEA, Amgen, AMRYT, Link Medical, Mylan, Napp, Sanofi; Eric Bruckert: speakers bureau: Servier, Mylan, Sanofi, Amgen, Akcea; consultant to Amgen, MSD, Sanofi, Novartis, Danone, Aegerion, Ionis Pharmaceuticals, Amarin, Akcea, Servier, Mylan, Silence Therpautic; Zeljko Reiner: speakers bureau: Sanofi, Novartis; Gani Bajraktari: speakers bureau: KRKA; Manfredi Rizzo: speakers bureau: Amgen, Astra Zeneca, Boehringer Ingelheim, Eli Lilly, Meda, Mylan, Merck Sharp & Dohme, Novo Nordisk, Roche Diagnostics, Sanofi, and Servier; consultant to Amgen, Astra Zeneca, Boehringer Ingelheim, Eli Lilly, Meda, Mylan, Merck Sharp & Dohme, Novo Nordisk, Roche Diagnostics, Sanofi, and Servier; Medical and Scientific Advisor, Europe East and South at Novo Nordisk; Dimitri P. Mikhailidis has given talks, acted as a consultant or attended conferences sponsored by Amgen and Novo Nordisk; Gustavs Latkovskis: speakers bureau: Abbott Laboratories, Amgen, Astra Zeneca, Bayer, Boehringer Ingelheim, Grindex, Medtronic, Mylan, Novartis, Novo Nordisk, Pfizer, Roche, Sanofi, Servier, Siemens Laboratories, Zentiva; consultant to Amgen, Bayer, Boehringer Ingelheim, Grindex, Novartis, Novo Nordisk, Sanofi, Servier; Peter P. Toth: speakers bureau: Amgen, Esperion, Kowa, Merck, Novo‐Nordisk; consultant to Amarin, bio89, Kowa, Merck, Resverlogix, Theravance; Daniel Pella: received honoraria for events sponsored by Amgen, Jamieson, Novartis, MSD, Pfizer, Servier; Fahad Alnouri is in advisory board and giving lectures supported by Amgen, AMRYT Pharma and Novartis; Stephan von Haehling: has been a paid consultant for and/or received honoraria payments from AstraZeneca, Bayer, Boehringer Ingelheim, BRAHMS, Chugai, Grünenthal, Helsinn, Hexal, Novartis, Pharmacosmos, Respicardia, Roche, Servier, Sorin, and Vifor. SvH reports research support from Amgen, Boehringer Ingelheim, IMI, and the German Center for Cardiovascular Research (DZHK); Maciej Banach: speakers bureau: Amgen, Herbapol, Kogen, KRKA, Polpharma, Mylan/Viatris, Novartis, Novo‐Nordisk, Sanofi‐Aventis, Teva, Zentiva; consultant to Amgen, Daichii Sankyo, Esperion, Freia Pharmaceuticals, Novartis, Novo‐Nordisk, Polfarmex, Sanofi‐Aventis; Grants from Amgen, Mylan/Viatris, Sanofi and Valeant; CMO at Nomi Biotech Corporation Ltd; all other authors have no conflict of interest.
†International Lipid Expert Panel Experts (alphabetically)
Julio Acosta (Cátedra de Cardiología Clínica de la Escuela Médica Razetti de la Universidad Central de Venezuela, Caracas, Venezuela); Mutaz Al‐Khnifsawi (Al‐Qadisiyah University, Faculty of Medicine, Department of Internal Medicine, Diwaniya City, Iraq); Fahad Alnouri (Cardiovascular Prevention Unit, Adult Cardiology Department. Prince Sultan Cardiac Centre Riyadh, Saudi Arabia); Fahma Amar (Unit of Diabetes & Metabolism, Alexandria University, Alexandria, Egypt); Atanas G. Atanasov (Institute of Genetics and Animal Biotechnology of the Polish Academy of Sciences, Jastrzebiec, Poland; Department of Pharmacognosy, University of Vienna, Vienna, Austria; Ludwig Boltzmann Institute for Digital Health and Patient Safety, Medical University of Vienna, Vienna, Austria); Gani Bajraktari (Institute of Public Health and Clinical Medicine, Umeå University, Umeå, Sweden; Clinic of Cardiology, University Clinical Centre of Kosovo, Prishtina, Kosovo; Medical Faculty, University of Prishtina, Prishtina, Kosovo); Maciej Banach (Department of Hypertension, Medical University of Lodz, Poland; Cardiovascular Research Centre, University of Zielona‐Gora, Zielona‐Gora, Poland); Sonu Bhaskar (Department of Neurology & Neurophysiology, Liverpool Hospital and South Western Sydney Local Health District, Sydney, NSW, Australia); Ibadete Bytyçi (Department of Public Health and Clinical Medicine, Umeå University, Umeå, Sweden & Clinic of Cardiology, University Clinical Centre of Kosova, Prishtina, Kosovo); Bojko Bjelakovic (Clinic of Pediatrics, Clinical Center, Nis, Faculty of Medicine, University of Nis, Serbia); Eric Bruckert (Pitié‐Salpetrière Hospital and Sorbonne University, Cardio metabolic Institute, Paris, France); Alberto Cafferata (Council of Epidemiology and Cardiovascular Prevention "Dr. Mario Ciruzzi", Argentine Society of Cardiology, Buenos Aires, Argentina); Richard Ceska (Third Department of Medicine ‐ Department of Endocrinology and Metabolism of the First Faculty of Medicine, Charles University and General University Hospital, Prague, Czech Republic); Arrigo F.G. Cicero (IRCCS Policlinico S.Orsola‐Malpighi, University of Bologna, Italy); Xavier Collet (Institute of Metabolic and Cardiovascular Diseases, Inserm, Toulouse, France); Magdalena Daccord (FH Europe); Olivier Descamps (Department of Internal Medicine, Centres Hospitaliers Jolimont, Haine Saint‐Paul, Belgium; Department of Cardiology, Cliniques Universitaires Saint‐Luc, Bruxells, Belgium); Dragan Djuric (Institute of Medical Physiology "Richard Burian" Faculty of Medicine, University of Belgrade, Belgrade, Serbia); Ronen Durst (Cardiology Department, Hadassah Hebrew University Medical Center, Ein Kerem, Jerusalem, Israel); Marat V. Ezhov (National Medical Research Center of Cardiology, Moscow, Russia); Zlatko Fras (Preventive Cardiology Unit, Department of Vascular Medicine, Division of Medicine, University Medical Centre Ljubljana, Slovenia; Faculty of Medicine, University of Ljubljana, Ljubljana, Slovenia); Dan Gaita (Institutul de Boli Cardiovasculare, Universitatea de Medicina si Farmacie Victor Babes din Timisoara, Romania); Adrian V. Hernandez (Health Outcomes, Policy, and Evidence Synthesis (HOPES) Group, University of Connecticut School of Pharmacy, Storrs, CT, USA; Vicerrectorado de Investigación, Universidad San Ignacio de Loyola (USIL), Lima, Peru); Steven R. Jones (the Johns Hopkins Ciccarone Center for the Prevention of Heart Disease, Baltimore, MD, USA); Jacek Jozwiak (Department of Family Medicine and Public Health Faculty of Medicine University of Opole, Opole, Poland); Nona Kakauridze (Department of Internal Medicine, Faculty of Medicine, Tbilisi State Medical University, Tbilisi, Georgia); Amani Kallel (University of Tunis El Manar, Faculty of Medicine of Tunis, Tunis, Tunisia); Niki Katsiki (Diabetes Center, Division of Endocrinology and Metabolism, First Department of Internal Medicine, AHEPA University Hospital, Aristotle University of Thessaloniki, Thessaloniki, Greece); Amit Khera (Department of Cardiology, UT Southwestern Medical Center, Dallas, TX, USA); Karam Kostner (Mater Hospital, University of Queensland, St Lucia, QLD, Australia); Raimondas Kubilius (Department of Rehabilitation, Medical Academy, Lithuanian University of Health Sciences, Kaunas, Lithuania); Gustavs Latkovskis (Institute of Cardiology and Regenerative Medicine, Faculty of Medicine, University of Latvia, Riga, Latvia; Pauls Stradins Clinical University Hospital, Riga, Latvia); G.B. John Mancini (Department of Medicine, Division of Cardiology, University of British Columbia, Vancouver, British Columbia, Canada); A. David Marais (Chemical Pathology Division of the Department of Pathology, University of Cape Town Health Science Faculty, Cape Town, South Africa); Seth S. Martin (Ciccarone Center for Prevention of Heart Disease, Division of Cardiology, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, MD, USA); Julio Acosta Martinez (Medico Cardiologo de la Policlinica Metropolitana, Carcass, Venezuela); Mohsen Mazidi (Department of Twin Research and Genetic Epidemiology, King's College London, St Thomas' Hospital, Strand, London, UK); Dimitri P. Mikhailidis (Department of Clinical Biochemistry, Royal Free Campus, University College London Medical School, University College London (UCL), London, UK); Erkin Mirrakhimov (Kyrgyz State Medical Academy, Bishkek, Kyrgyzstan); Andre R. Miserez (diagene Research Institute, Reinach, Switzerland; University of Basel, Basel, Switzerland); Olena Mitchenko (Dyslipidaemia Department, Institute of Cardiology AMS of Ukraine, Ukraine); Natalya P. Mitkovskaya (Belarusian State Medical Univer‐ sity, Minsk, Republic of Belarus); Patrick M. Moriarty (Division of Clinical Pharmacology, Division of Internal Medicine, University of Kansas Medical Center, Kansas City, Kansas, USA); Seyed Mohammad Nabavi (Applied Biotechnology Research Center, Baqiyatallah University of Medical Sciences, Tehran, Iran); Devaki Nair (Department of Clinical Biochemistry, the Royal Free London NHS Foundation Trust, Pond Street, London, UK); Demosthenes B. Panagiotakos (School of Health Science and Education, Department of Nutrition and Dietetics, Harokopio University of Athens, Athens, Greece); György Paragh (Department of Internal Medicine, Faculty of Medicine, University of Debrecen, Debrecen, Hungary); Daniel Pella (1st Department of Internal Medicine, Faculty of Medicine, Pavol Jozef Safarik University, Košice, Slovakia); Peter E. Penson (School of Pharmacy and Biomolecular Sciences, Liverpool John Moores University, Liverpool, UK); Zaneta Petrulioniene (Vilnius University Faculty of Medicine, Vilnius, Lithuania; Vilnius University Hospital Santaros Klinikos, Vilnius, Lithuania); Matteo Pirro (Department of Medicine, University of Perugia, Perugia, Italy); Arman Postadzhiyan (Bulgarian Society of Cardiology, Medical University of Sofia, Sofia, Bulgaria); Raman Puri (I P Apollo Hospital, New Delhi, India); Ashraf Reda (Menoufia University, President of EAVA); Željko Reiner (University Hospital Center Zagreb, Department of Internal Medicine, School of Medicine, University of Zagreb, Zagreb, Croatia); Dina Radenkovic (Health Longevity Performance Optimisation Institute, Cambridge, UK); Michał Rakowski (International Lipid Expert Panel, Poland; The Bio‐Med‐Chem Doctoral School of the University of Lodz and Lodz Institutes of the Polish Academy of Sciences/University of Lodz, Faculty of Biology and Environmental Protection, Department of Molecular Biophysics, University of Lodz, Lodz, Poland); Jemaa Riadh (Laboratory of Biochemistry, Faculty of Medicine of Tunis, Rabta Hospital, University of Tunis El Manar, Tunis, Tunisia); Dimitri Richter (Cardiac Department, Euroclinic, Athens, Greece); Manfredi Rizzo (Biomedical Department of Internal Medicine and Medical Specialties, University of Palermo, Palermo, Italy); Massimiliano Ruscica (Department of Pharmacological and Biomolecular Sciences, University of Milan, Milan, Italy); Amirhossein Sahebkar (Biotechnology Research Center, Pharmaceutical Technology Institute, Mashhad University of Medical Sciences, Mashhad, Iran); Naveed Sattar (Institute of Cardiovascular and Medical Sciences, University of Glasgow, Glasgow, UK); Maria‐Corina Serban (Department of Functional Sciences, Discipline of Pathophysiology, "Victor Babes" University of Medicine and Pharmacy, Timisoara, Romania); Abdullah M. A Shehab (Medical Education Department, United Arab Emirates University, Al Ain, United Arab Emirates); Aleksandr B. Shek (Department of Ischemic Heart Disease and Atherosclerosis, Republican Specialised Center of Cardiology, Tashkent, Uzbekistan); Cesare R. Sirtori (Dipartimento di Scienze Farmacologiche e Biomolecolari, Università di Milano Centro Dislipidemie, Grande Ospedale Metropolitano, Niguarda Ca'Granda President, Fondazione Carlo Sirtori); Claudia Stefanutti (Department of Molecular Medicine, Sapienza University of Rome, Rome, Italy); Tomasz Tomasik (Department of Family Medicine, Jagiellonian University Medical College, Krakow, Poland); Peter P. Toth (The Johns Hopkins Ciccarone Center for the Prevention of Heart Disease, Baltimore, MD, USA); Margus Viigimaa (Tallinn University of Technology, North Estonia Medical Centre, Tallinn, Estonia); Pedro Valdivielso (Catedrático de Medicina, Departamento de Medicina y Dermatología, Universidad de Málaga, España); Dragos Vinereanu (Cardiology Department, University and Emergency Hospital, Bucharest, Romania, University of Medicine and Pharmacy Carol Davila, Bucharest, Romania); Branislav Vohnout (Institute of Nutrition, Faculty of Nursing and Health Professional Studies and Coordination Centre for Familial Hyperlipoproteinemias, Slovak Medical University in Bratislava, Bratislava, Slovakia; Institute of Epidemiology, School of Medicine, Comenius University, Bratislava, Slovakia); Stephan von Haehling (Department of Cardiology and Pneumology, Heart Center Göttingen, University of Göttingen Medical Center, Georg‐August‐University, Göttingen, Germany); Michal Vrablik (1st Faculty of Medicine, Charles University and General University Hospital, Prague, Czech Republic); Nathan D. Wong (Department of Medicine, School of Medicine University of California, Irvine, CA, USA; Heart Disease Prevention Program, Division of Cardiology, University of California, Irvine, California, USA); Hung‐I Yeh (Department of Medicine, Mackay Medical College, Taipei, Taiwan; Cardiovascular Division, Department of Internal Medicine, MacKay Memorial Hospital, Taipei, Taiwan); Jiang Zhisheng (Institute of Cardiovascular Disease, University of South China, Hengyang, Hunan, China); Andreas Zirlik (University Heart Centre Freiburg University, Department of Cardiology and Angiology I, Faculty of Medicine, University of Freiburg, Freiburg, Germany).
Acknowledgements
This position paper was written independently; no company or institution supported it financially. No professional writer was involved in the preparation of this position paper.
Penson P. E., Bruckert E., Marais D., Reiner Ž., Pirro M., Sahebkar A., Bajraktari G., Mirrakhimov E., Rizzo M., Mikhailidis D. P., Sachinidis A., Gaita D., Latkovskis G., Mazidi M., Toth P. P., Pella D., Alnouri F., Postadzhiyan A., Yeh H.‐I., Mancini G. B. J., von Haehling S., Banach M., and International Lipid Expert Panel (ILEP) (2022) Step‐by‐step diagnosis and management of the nocebo/drucebo effect in statin‐associated muscle symptoms patients: a position paper from the International Lipid Expert Panel (ILEP), Journal of Cachexia, Sarcopenia and Muscle, 13, 1596–1622, 10.1002/jcsm.12960
References
- 1. Ference BA, Cannon CP, Landmesser U, Luscher TF, Catapano AL, Ray KK. Reduction of low density lipoprotein‐cholesterol and cardiovascular events with proprotein convertase subtilisin‐kexin type 9 (PCSK9) inhibitors and statins: an analysis of FOURIER, SPIRE, and the Cholesterol Treatment Trialists Collaboration. Eur Heart J 2018;39:2540–2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Reiner Z. Statins in the primary prevention of cardiovascular disease. Nat Rev Cardiol 2013;10:453–464. [DOI] [PubMed] [Google Scholar]
- 3. Collins R, Reith C, Emberson J, Armitage J, Baigent C, Blackwell L, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet 2016;388:2532–2561. [DOI] [PubMed] [Google Scholar]
- 4. Thompson PD, Panza G, Zaleski A, Taylor B. Statin‐sssociated side effects. J Am Coll Cardiol 2016;67:2395–2410. [DOI] [PubMed] [Google Scholar]
- 5. Simic I, Reiner Z. Adverse effects of statins—myths and reality. Curr Pharm Des 2015;21:1220–1226. [DOI] [PubMed] [Google Scholar]
- 6. Stroes ES, Thompson PD, Corsini A, Vladutiu GD, Raal FJ, Ray KK, et al. Statin‐associated muscle symptoms: impact on statin therapy‐European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J 2015;36:1012–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mach F, Ray KK, Wiklund O, Corsini A, Catapano AL, Bruckert E, et al. Adverse effects of statin therapy: perception vs. the evidence—focus on glucose homeostasis, cognitive, renal and hepatic function, haemorrhagic stroke and cataract. Eur Heart J 2018;39:2526–2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Toth PP, Patti AM, Giglio RV, Nikolic D, Castellino G, Rizzo M, et al. Management of statin intolerance in 2018: still more questions than answers. Am J Cardiovasc Drugs 2018;18:157–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Banach M, Stulc T, Dent R, Toth PP. Statin non‐adherence and residual cardiovascular risk: there is need for substantial improvement. Int J Cardiol 2016;225:184–196. [DOI] [PubMed] [Google Scholar]
- 10. Rosenson RS, Baker S, Banach M, Borow KM, Braun LT, Bruckert E, et al. Optimizing cholesterol treatment in patients with muscle complaints. J Am Coll Cardiol 2017;70:1290–1301. [DOI] [PubMed] [Google Scholar]
- 11. Banach M, Serban MC. Discussion around statin discontinuation in older adults and patients with wasting diseases. J Cachexia Sarcopenia Muscle 2016;7:396–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cohen JD, Brinton EA, Ito MK, Jacobson TA. Understanding Statin Use in America and Gaps in Patient Education (USAGE): an internet‐based survey of 10,138 current and former statin users. J Clin Lipidol 2012;6:208–215. [DOI] [PubMed] [Google Scholar]
- 13. Davis JW, Weller SC. Intensity of statin therapy and muscle symptoms: a network meta‐analysis of 153 000 patients. BMJ Open 2021;11:e043714. 10.1136/bmjopen-2020-043714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Serban MC, Colantonio LD, Manthripragada AD, Monda KL, Bittner VA, Banach M, et al. Statin intolerance and risk of coronary heart events and all‐cause mortality following myocardial infarction. J Am Coll Cardiol 2017;69:1386–1395. [DOI] [PubMed] [Google Scholar]
- 15. Zhang H, Plutzky J, Shubina M, Turchin A. Continued statin prescriptions after adverse reactions and patient outcomes: a cohort study. Ann Intern Med 2017;167:221–227. [DOI] [PubMed] [Google Scholar]
- 16. Bytyçi I, Penson PE, Mikhailidis DP, Wong ND, Hernandez AV, Sahebkar A, et al. Prevalence of statin intolerance: a meta‐analysis. Eur Heart J 2022. 10.1093/eurheartj/ehac015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Penson PE, Mancini GBJ, Toth PP, Martin SS, Watts GF, Sahebkar A, et al. Introducing the ‘Drucebo’ effect in statin therapy: a systematic review of studies comparing reported rates of statin‐associated muscle symptoms, under blinded and open‐label conditions. J Cachexia Sarcopenia Muscle 2018;9:1023–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Smith LE, Webster RK, Rubin GJ. A systematic review of factors associated with side‐effect expectations from medical interventions. Health Expect 2020;23:731–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Penson PE, Banach M. Nocebo/drucebo effect in statin intolerant patients—an attempt at recommendations. Eur Heart J 2021;42:4787–4788. [DOI] [PubMed] [Google Scholar]
- 20. Wood FA, Howard JP, Finegold JA, Nowbar AN, Thompson DM, Arnold AD, et al. N‐of‐1 trial of a statin, placebo, or no treatment to assess side effects. N Engl J Med 2020;383:2182–2184. [DOI] [PubMed] [Google Scholar]
- 21. Banach M, Penson PE. Drucebo effect—the challenge we should all definitely face! Arch Med Sci 2021;17:542–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Herrett E, Williamson E, Brack K, Beaumont D, Perkins A, Thayne A, et al. Statin treatment and muscle symptoms: series of randomised, placebo controlled n‐of‐1 trials. BMJ 2021;372:372:n135. 10.1136/bmj.n135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Banach M, Rizzo M, Toth PP, Farnier M, Davidson MH, Al‐Rasadi K, et al. Statin intolerance—an attempt at a unified definition. Position paper from an International Lipid Expert Panel. Expert Opin Drug Saf 2015;14:935–955. [DOI] [PubMed] [Google Scholar]
- 24. Banach M, Rizzo M, Toth PP, Farnier M, Davidson MH, Al‐Rasadi K, et al. Statin intolerance—an attempt at a unified definition. Position paper from an International Lipid Expert Panel. Arch Med Sci 2015;11:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jacobson TA, Ito MK, Maki KC, Orringer CE, Bays HE, Jones PH, et al. National Lipid Association recommendations for patient‐centered management of dyslipidemia: part 1—executive summary. J Clin Lipidol 2014;8:473–488. [DOI] [PubMed] [Google Scholar]
- 26. Sposito AC, Faria Neto JR, Carvalho LS, Lorenzatti A, Cafferata A, Elikir G, et al. Statin‐associated muscle symptoms: position paper from the Luso‐Latin American Consortium. Curr Med Res Opin 2017;33:239–251. [DOI] [PubMed] [Google Scholar]
- 27. Mancini GB, Baker S, Bergeron J, Fitchett D, Frohlich J, Genest J, et al. Diagnosis, prevention, and management of statin adverse effects and intolerance: Canadian Consensus Working Group Update (2016). Can J Cardiol 2016;32:S35–S65. [DOI] [PubMed] [Google Scholar]
- 28. Muntean DM, Thompson PD, Catapano AL, Stasiolek M, Fabis J, Muntner P, et al. Statin‐associated myopathy and the quest for biomarkers: can we effectively predict statin‐associated muscle symptoms? Drug Discov Today 2017;22:85–96. [DOI] [PubMed] [Google Scholar]
- 29. Banach M, Penson PE, Vrablik M, Bunc M, Dyrbus K, Fedacko J, et al. Optimal use of lipid‐lowering therapy after acute coronary syndromes: a position paper endorsed by the International Lipid Expert Panel (ILEP). Pharmacol Res 2021;166:105499. 10.1016/j.phrs.2021.105499 [DOI] [PubMed] [Google Scholar]
- 30. Banach M, Penson PE. Statins and LDL‐C in secondary prevention—so much progress, so far to go. JAMA Netw Open 2020;3:e2025675. 10.1001/jamanetworkopen.2020.25675 [DOI] [PubMed] [Google Scholar]
- 31. Reiner Z. Resistance and intolerance to statins. Nutr Metab Cardiovasc Dis 2014;24:1057–1066. [DOI] [PubMed] [Google Scholar]
- 32. Penson P, Toth P, Mikhailidis D, Ezhov M, Fras Z, Mitchenko O, et al. Step by step diagnosis and management of statin intolerance: position paper from an international lipid expert panel. Eur Heart J 2019;40:P705. 10.1093/eurheartj/ehz747.0310 [DOI] [Google Scholar]
- 33. Wilson PW, D'Agostino R Sr, Bhatt DL, Eagle K, Pencina MJ, Smith SC, et al. An international model to predict recurrent cardiovascular disease. Am J Med 2012;125:695, e1–703. [DOI] [PubMed] [Google Scholar]
- 34. Dyrbuś K, Gąsior M, Desperak P, Trzeciak P, Nowak J, Penson PE, et al. Risk‐factors associated with extremely high cardiovascular risk of mid‐ and long‐term mortality following myocardial infarction: analysis of the hyperlipidaemia Therapy in tERtiary Cardiological cEnTer (TERCET) registry. Atherosclerosis 2021;333:16–23. [DOI] [PubMed] [Google Scholar]
- 35. Robinson JG. The neuropsychology of statin intolerance. Nat Rev Cardiol 2021;18:153–154. [DOI] [PubMed] [Google Scholar]
- 36. Lloyd‐Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic Impact Goal through 2020 and beyond. Circulation 2010;121:586–613. [DOI] [PubMed] [Google Scholar]
- 37. Abughosh SM, Vadhariya A, Johnson ML, Essien EJ, Esse TW, Serna O, et al. Enhancing statin adherence using a motivational interviewing intervention and past adherence trajectories in patients with suboptimal adherence. J Manag Care Spec Pharm 2019;25:1053–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pulipati VP, Davidson MH. How I treat statin‐associated side effects in an outpatient setting. Future Cardiol 2021;17:1249–1260. [DOI] [PubMed] [Google Scholar]
- 39. Banach M, Mikhailidis DP. Statin intolerance: some practical hints. Cardiol Clin 2018;36:225–231. [DOI] [PubMed] [Google Scholar]
- 40. Thompson PD, Clarkson PM, Rosenson RS. National Lipid Association Statin Safety Task Force Muscle Safety Expert Panel. An assessment of statin safety by muscle experts. Am J Cardiol 2006;97:69C–76C. [DOI] [PubMed] [Google Scholar]
- 41. Michalska‐Kasiczak M, Sahebkar A, Mikhailidis DP, Rysz J, Muntner P, Toth PP, et al. Analysis of vitamin D levels in patients with and without statin‐associated myalgia—a systematic review and meta‐analysis of 7 studies with 2420 patients. Int J Cardiol 2015;178:111–116. [DOI] [PubMed] [Google Scholar]
- 42. Mergenhagen K, Ott M, Heckman K, Rubin LM, Kellick K. Low vitamin D as a risk factor for the development of myalgia in patients taking high‐dose simvastatin: a retrospective review. Clin Ther 2014;36:770–777. [DOI] [PubMed] [Google Scholar]
- 43. Morioka TY, Lee AJ, Bertisch S, Buettner C. Vitamin D status modifies the association between statin use and musculoskeletal pain: a population based study. Atherosclerosis 2015;238:77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Khayznikov M, Hemachrandra K, Pandit R, Kumar A, Wang P, Glueck CJ. Statin intolerance because of myalgia, myositis, myopathy, or myonecrosis can in most cases be safely resolved by vitamin D supplementation. N Am J Med Sci 2015;7:86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wu Z, Camargo CA Jr, Khaw KT, Waayer D, Lawes CM, Toop L, et al. Effects of vitamin D supplementation on adherence to and persistence with long‐term statin therapy: secondary analysis from the randomized, double‐blind, placebo‐controlled ViDA study. Atherosclerosis 2018;273:59–66. [DOI] [PubMed] [Google Scholar]
- 46. Bakhai A, Rigney U, Hollis S, Emmas C. Co‐administration of statins with cytochrome P450 3A4 inhibitors in a UK primary care population. Pharmacoepidemiol Drug Saf 2012;21:485–493. [DOI] [PubMed] [Google Scholar]
- 47. Banach M, Penson PE, Fras Z, Vrablik M, Pella D, Reiner Ž, et al. Brief recommendations on the management of adult patients with familial hypercholesterolemia during the COVID‐19 pandemic. Pharmacol Res 2020;158:104891. 10.1016/j.phrs.2020.104891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mombelli G. Statin muscle toxicity and genetic risk factors. International Journal of Genomic Medicine 2013;01. 02;01. 10.4172/2332-0672.1000111 [DOI] [Google Scholar]
- 49. Patel J, Superko HR, Martin SS, Blumenthal RS, Christopher‐Stine L. Genetic and immunologic susceptibility to statin‐related myopathy. Atherosclerosis 2015;240:260–271. [DOI] [PubMed] [Google Scholar]
- 50. Gluba‐Brzozka A, Franczyk B, Toth PP, Rysz J, Banach M. Molecular mechanisms of statin intolerance. Arch Med Sci 2016;12:645–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rong S, McDonald JG, Engelking LJ. Cholesterol auxotrophy and intolerance to ezetimibe in mice with SREBP‐2 deficiency in the intestine. J Lipid Res 2017;58:1988–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sposito AC, Carvalho LS, Cintra RM, Araújo AL, Ono AH, Andrade JM, et al. Rebound inflammatory response during the acute phase of myocardial infarction after simvastatin withdrawal. Atherosclerosis 2009;207:191–194. [DOI] [PubMed] [Google Scholar]
- 53. Banach M, Serban C, Sahebkar A, Mikhailidis DP, Ursoniu S, Ray KK. Impact of statin therapy on coronary plaque composition: a systematic review and meta‐analysis of virtual histology intravascular ultrasound studies. BMC Med 2015;13:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Casula M, Gazzotti M, Bonaiti F, OImastroni E, Arca M, Averna M, et al. Reported muscle symptoms during statin treatment amongst Italian dyslipidaemic patients in the real‐life setting: the PROSISA Study. J Intern Med 2021;290:116–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhang H, Plutzky J, Skentzos S, Olmastroni E, Arca M, Averna M, et al. Discontinuation of statins in routine care settings: a cohort study. Ann Intern Med 2013;158:526–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bangalore S, Breazna A, DeMicco DA, Wun CC, Messerli FH, TNT Steering Committee and Investigators . Visit‐to‐visit low‐density lipoprotein cholesterol variability and risk of cardiovascular outcomes: insights from the TNT trial. J Am Coll Cardiol 2015;65:1539–1548. [DOI] [PubMed] [Google Scholar]
- 57. National Institute for Health and Care Excellence (NICE) . CG181 cardiovascular disease: risk assessment and reduction, including lipid modification. 2014. Updated 2016.
- 58. Bonner C, Bell K, Jansen J, Glasziou P, Irwig L, Doust J, et al. Should heart age calculators be used alongside absolute cardiovascular disease risk assessment? BMC Cardiovasc Disord 2018;18:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Penson PE, Pirro M, Banach M. LDL‐C: lower is better for longer‐even at low risk. BMC Med 2020;18:320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Banach M, Patti AM, Giglio RV, Cicero AF, Atanasov AG, Bajraktari G, et al. The role of nutraceuticals in statin intolerant patients. J Am Coll Cardiol 2018;72:96–118. [DOI] [PubMed] [Google Scholar]
- 61. Cicero AFG, Colletti A, Bajraktari G, Descamps O, Djuric DM, Ezhov M, et al. Lipid lowering nutraceuticals in clinical practice: position paper from an International Lipid Expert Panel. Arch Med Sci 2017;13:965–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Penson PE, Banach M. Natural compounds as anti‐atherogenic agents: clinical evidence for improved cardiovascular outcomes. Atherosclerosis 2021;316:58–65. [DOI] [PubMed] [Google Scholar]
- 63. Ruscica M, Penson PE, Ferri N, Sirtori CR, Pirro M, Mancini GJ, et al. Impact of nutraceuticals on markers of systemic inflammation: potential relevance to cardiovascular diseases—a position paper from the International Lipid Expert Panel (ILEP). Prog Cardiovasc Dis 2021;67:40–52. [DOI] [PubMed] [Google Scholar]
- 64. Penson PE, Banach M. The role of nutraceuticals in the optimization of lipid‐lowering therapy in high‐risk patients with dyslipidaemia. Curr Atheroscler Rep 2020;22:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sosnowska B, Penson P, Banach M. The role of nutraceuticals in the prevention of cardiovascular disease. Cardiovasc Diagn Ther 2017;7:S21–S31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Moşteoru S, Gaiţă D, Banach M. An update on PCSK9 inhibitors‐ pharmacokinetics, drug interactions, and toxicity. Expert Opin Drug Metab Toxicol. 2020;16:1199–1205. [DOI] [PubMed] [Google Scholar]
- 67. Hill AB. The environment and disease: association or causation? Proc R Soc Med 1965;58:295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Parker BA, Capizzi JA, Grimaldi AS, Clarkson PM, Cole SM, Keadle J, et al. Effect of statins on skeletal muscle function. Circulation 2013;127:96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rosenson RS, Miller K, Bayliss M, Sanchez RJ, Baccara‐Dinet MT, Chibedi‐De‐Roche D, et al. The Statin‐Associated Muscle Symptom Clinical Index (SAMS‐CI): revision for clinical use, content validation, and inter‐rater reliability. Cardiovasc Drugs Ther 2017;31:179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bjornsson ES. Hepatotoxicity of statins and other lipid‐lowering agents. Liver Int 2017;37:173–178. [DOI] [PubMed] [Google Scholar]
- 71. Russo MW, Hoofnagle JH, Gu J, Fontana RJ, Barnhart H, Kleiner DE, et al. Spectrum of statin hepatotoxicity: experience of the drug‐induced liver injury network. Hepatology 2014;60:679–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Rosenson RS, Baker SK, Jacobson TA, Kopecky SL, Parker BA. The National Lipid Association's Muscle Safety Expert Panel. An assessment by the Statin Muscle Safety Task Force: 2014 update. J Clin Lipidol 2014;8:S58–S71. [DOI] [PubMed] [Google Scholar]
- 73. Ridker PM, Pradhan A, MacFadyen JG, Libby P, Glynn RJ. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: an analysis from the JUPITER trial. Lancet 2012;380:565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Preiss D, Seshasai SR, Welsh P, Murphy SA, Ho JE, Waters DD, et al. Risk of incident diabetes with intensive‐dose compared with moderate‐dose statin therapy: a meta‐analysis. JAMA 2011;305:2556–2564. [DOI] [PubMed] [Google Scholar]
- 75. Banach M, Mikhailidis DP. Statin therapy and new‐onset diabetes: an attempt at recommendations. Expert Rev Endocrinol Metab 2013;8:213–216. [DOI] [PubMed] [Google Scholar]
- 76. Banach M, Jankowski P, Jozwiak J, Cybulska B, Windak A, Guzik T, et al. PoLA/CFPiP/PCS guidelines for the management of dyslipidaemias for family physicians 2016. Arch Med Sci 2017;13:1–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, et al. ESC/EAS guidelines for the management of dyslipidaemias: the Task Force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) developed with the special contribution of the European Assocciation for Cardiovascular Prevention & Rehabilitation (EACPR). Atherosclerosis 2016;253:281–344. [DOI] [PubMed] [Google Scholar]
- 78. Vahedian‐Azimi A, Shojaie S, Banach M, Heidari F, Cicero AF, Khoshfetrat M, et al. Statin therapy in chronic viral hepatitis: a systematic review and meta‐analysis of nine studies with 195,602 participants. Ann Med 2021;53:1227–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kaushik P, Gonuguntla A. Idiopathic benign hyper‐CK‐emia. Int J Biomed Sci 2009;5:79–80. [PMC free article] [PubMed] [Google Scholar]
- 80. Banach M, Penson PE. Lipid‐lowering therapies: better together. Atherosclerosis 2021;320:86–88. [DOI] [PubMed] [Google Scholar]
- 81. Cybulska B, Klosiewicz‐Latoszek L, Penson PE, Nabavi SM, Lavie CJ, Banach M. How much should LDL cholesterol be lowered in secondary prevention? Clinical efficacy and safety in the era of PCSK9 inhibitors. Prog Cardiovasc Dis 2020;67:65–74. [DOI] [PubMed] [Google Scholar]
- 82. Howard JP, Francis DP. Response to “Drucebo effect—the challenge we should all definitely face!”. Arch Med Sci 2021;17:544–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Awad K, Mikhailidis DP, Toth PP, Jones SR, Moriarty P, Lip GY, et al. Efficacy and safety of alternate‐day versus daily dosing of statins: a systematic review and meta‐analysis. Cardiovasc Drugs Ther 2017;31:419–431. [DOI] [PubMed] [Google Scholar]
- 84. Gadarla M, Kearns AK, Thompson PD. Efficacy of rosuvastatin (5 mg and 10 mg) twice a week in patients intolerant to daily statins. Am J Cardiol 2008;101:1747–1748. [DOI] [PubMed] [Google Scholar]
- 85. Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015;372:2387–2397. [DOI] [PubMed] [Google Scholar]
- 86. Banach M, Nikolic D, Rizzo M, Toth PP. IMPROVE‐IT: what have we learned? Curr Opin Cardiol 2016;31:426–433. [DOI] [PubMed] [Google Scholar]
- 87. Bach RG, Cannon CP, Giugliano RP, White JA, Lokhnygina Y, Bohula EA, et al. Effect of simvastatin‐ezetimibe compared with simvastatin monotherapy after acute coronary syndrome among patients 75 years or older: a secondary analysis of a randomized clinical trial. JAMA Cardiol 2019;4:846–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Pandor A, Ara RM, Tumur I, Wilkinson AJ, Paisley S, Duenas A, et al. Ezetimibe monotherapy for cholesterol lowering in 2,722 people: systematic review and meta‐analysis of randomized controlled trials. J Intern Med 2009;265:568–580. [DOI] [PubMed] [Google Scholar]
- 89. Awad K, Mikhailidis DP, Katsiki N, Muntner P, Banach M, Lipid and Blood Pressure Meta‐analysis Collaboration Group . Effect of ezetimibe monotherapy on plasma lipoprotein(a) concentrations in patients with primary hypercholesterolemia: a systematic review and meta‐analysis of randomized controlled trials. Drugs 2018;78:453–462. [DOI] [PubMed] [Google Scholar]
- 90. Zhan S, Tang M, Liu F, Xia P, Shu M, Wu X. Ezetimibe for the prevention of cardiovascular disease and all‐cause mortality events. Cochrane Database Syst Rev 2018;11:CD012502. 10.1002/14651858.CD012502.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Dadu RT, Ballantyne CM. Lipid lowering with PCSK9 inhibitors. Nat Rev Cardiol 2014;11:563–575. [DOI] [PubMed] [Google Scholar]
- 92. Banach M, Penson PE. What have we learned about lipids and cardiovascular risk from PCSK9 inhibitor outcome trials: ODYSSEY and FOURIER? Cardiovasc Res 2019;115:e26–e31. [DOI] [PubMed] [Google Scholar]
- 93. Dyrbus K, Gasior M, Penson P, Ray KK, Banach M. Inclisiran—new hope in the management of lipid disorders? J Clin Lipidol 2020;14:16–27. [DOI] [PubMed] [Google Scholar]
- 94. Sattar N, Preiss D, Robinson JG, Djedjos CS, Elliott M, Somaratne R, et al. Lipid‐lowering efficacy of the PCSK9 inhibitor evolocumab (AMG 145) in patients with type 2 diabetes: a meta‐analysis of individual patient data. Lancet Diabetes Endocrinol 2016;4:403–410. [DOI] [PubMed] [Google Scholar]
- 95. Stein EA, Giugliano RP, Koren MJ, Raal FJ, Roth EM, Weiss R, et al. Efficacy and safety of evolocumab (AMG 145), a fully human monoclonal antibody to PCSK9, in hyperlipidaemic patients on various background lipid therapies: pooled analysis of 1359 patients in four phase 2 trials. Eur Heart J 2014;35:2249–2259. [DOI] [PubMed] [Google Scholar]
- 96. Nissen SE, Stroes E, Dent‐Acosta RE, Rosenson RS, Lehman SJ, Sattar N, et al. Efficacy and tolerability of evolocumab vs ezetimibe in patients with muscle‐related statin intolerance: the GAUSS‐3 randomized clinical trial. JAMA 2016;315:1580–1590. [DOI] [PubMed] [Google Scholar]
- 97. Moriarty PM, Thompson PD, Cannon CP, Guyton JR, Bergeron J, Zieve FJ, et al. Efficacy and safety of alirocumab vs ezetimibe in statin‐intolerant patients, with a statin rechallenge arm: the ODYSSEY ALTERNATIVE randomized trial. J Clin Lipidol 2015;9:758–769. [DOI] [PubMed] [Google Scholar]
- 98. Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, et al. Evolocumab and Clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017;376:1713–1722. [DOI] [PubMed] [Google Scholar]
- 99. Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med 2018;379:2097–2107. [DOI] [PubMed] [Google Scholar]
- 100. Ray KK, Landmesser U, Leiter LA, Kallend D, Dufour R, Karakas M, et al. Inclisiran in patients at high cardiovascular risk with elevated LDL cholesterol. N Engl J Med 2017;376:1430–1440. [DOI] [PubMed] [Google Scholar]
- 101. Wright RS, Ray KK, Raal FJ, Kallend DG, Jaros M, Koenig W, et al. Pooled patient‐level analysis of inclisiran trials in patients with familial hypercholesterolemia or atherosclerosis. J Am Coll Cardiol 2021;77:1182–1193. [DOI] [PubMed] [Google Scholar]
- 102. Giglio RV, Pantea Stoian A, Al‐Rasadi K, Banach M, Patti AM, Ciaccio M, et al. Novel therapeutical approaches to managing atherosclerotic risk. Int J Mol Sci 2021;22:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Penson P, McGowan M, Banach M. Evaluating bempedoic acid for the treatment of hyperlipidaemia. Expert Opin Investig Drugs 2017;26:251–259. [DOI] [PubMed] [Google Scholar]
- 104. Pinkosky SL, Newton RS, Day EA, Ford RJ, Lhotak S, Austin RC, et al. Liver‐specific ATP‐citrate lyase inhibition by bempedoic acid decreases LDL‐C and attenuates atherosclerosis. Nat Commun 2016;7:13457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Ballantyne CM, Davidson MH, Macdougall DE, Bays HE, DiCarlo LA, Rosenberg NL, et al. Efficacy and safety of a novel dual modulator of adenosine triphosphate‐citrate lyase and adenosine monophosphate‐activated protein kinase in patients with hypercholesterolemia: results of a multicenter, randomized, double‐blind, placebo‐controlled, parallel‐group trial. J Am Coll Cardiol 2013;62:1154–1162. [DOI] [PubMed] [Google Scholar]
- 106. Thompson PD, Rubino J, Janik MJ, MacDougall DE, McBride SJ, Margulies JR, et al. Use of ETC‐1002 to treat hypercholesterolemia in patients with statin intolerance. J Clin Lipidol 2015;9:295–304. [DOI] [PubMed] [Google Scholar]
- 107. Ballantyne CM, McKenney JM, MacDougall DE, Margulies JR, Robinson PL, Hanselman JC, et al. Effect of ETC‐1002 on serum low‐density lipoprotein cholesterol in hypercholesterolemic patients receiving statin therapy. Am J Cardiol 2016;117:1928–1933. [DOI] [PubMed] [Google Scholar]
- 108. Thompson PD, MacDougall DE, Newton RS, Margulies JR, Hanselman JC, Orloff DG, et al. Treatment with ETC‐1002 alone and in combination with ezetimibe lowers LDL cholesterol in hypercholesterolemic patients with or without statin intolerance. J Clin Lipidol 2016;10:556–567. [DOI] [PubMed] [Google Scholar]
- 109. Ballantyne CM, Banach M, Mancini GBJ, Lepor NE, Hanselman JC, Zhao X, et al. Efficacy and safety of bempedoic acid added to ezetimibe in statin‐intolerant patients with hypercholesterolemia: a randomized, placebo‐controlled study. Atherosclerosis 2018;277:195–203. [DOI] [PubMed] [Google Scholar]
- 110. Ray KK, Bays HE, Catapano AL, Lalwani ND, Bloedon LT, Sterling LR, et al. Safety and efficacy of bempedoic acid to reduce LDL cholesterol. N Engl J Med 2019;380:1022–1032. [DOI] [PubMed] [Google Scholar]
- 111. Banach M, Duell PB, Gotto AM Jr, Laufs U, Leiter LA, Mancini GJ, et al. Association of bempedoic acid administration with atherogenic lipid levels in phase 3 randomized clinical trials of patients with hypercholesterolemia. JAMA Cardiol 2020;5:1124–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Gutierrez MJ, Rosenberg NL, Macdougall DE, Hanselman JC, Margulies JR, Strange P, et al. Efficacy and safety of ETC‐1002, a novel investigational low‐density lipoprotein‐cholesterol‐lowering therapy for the treatment of patients with hypercholesterolemia and type 2 diabetes mellitus. Arterioscler Thromb Vasc Biol 2014;34:676–683. [DOI] [PubMed] [Google Scholar]
- 113. Ference BA, Ray KK, Catapano AL, Ference TB, Burgess S, Neff DR, et al. Mendelian randomization study of ACLY and cardiovascular disease. N Engl J Med 2019;380:1033–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Ballantyne CM, Laufs U, Ray KK, Leiter LA, Bays HE, Goldberg AC, et al. Bempedoic acid plus ezetimibe fixed‐dose combination in patients with hypercholesterolemia and high CVD risk treated with maximally tolerated statin therapy. Eur J Prev Cardiol 2020;27:593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Banach M, Burchardt P, Chlebus K, Dobrowolski P, Dudek D, Dyrbuś K, et al. PoLA/CFPiP/PCS/PSLD/PSD/PSH guidelines on diagnosis and therapy of lipid disorders in Poland 2021. Arch Med Sci 2021;17:1447–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Gencer B, Djousse L, Al‐Ramady OT, Cook NR, Manson JE, Albert CM. Effect of long‐term marine ɷ‐3 fatty acids supplementation on the risk of atrial fibrillation in randomized controlled trials of cardiovascular outcomes: a systematic review and meta‐analysis. Circulation 2021;144:1981–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]


