Abstract
Von Economo neurons (VENs) have been mentioned in the medical literature since the second half of the 19th century; however, it was not until the second decade of the 20th century that their cytomorphology was described in detail. To date, VENs have been found in limbic sectors of the frontal, temporal and insular lobes. In humans, their density seems to decrease in the caudo‐rostral and ventro‐dorsal direction; that is, from the anterior regions of the cingulate and insular cortices towards the frontal pole and the superior frontal gyrus. Several studies have provided similar descriptions of the shape of the VEN soma, but the size of the soma varies from one cortical region to another. There is consensus among different authors about the selective vulnerability of VENs in certain pathologies, in which a deterioration of the capacities involved in social behaviour is observed. In this review, we propose that the restriction of VENs towards the sectors linked to limbic information processing in Homo sapiens gives them a possible functional role in relation to the structures in which they are located. However, given the divergence in characteristics such as location, density, size and biochemical profile among VENs of different cortical sectors, the activities in which they participate could allow them to partake in a wide spectrum of neurological functions, including autonomic responses and executive functions.
Keywords: anterior cingulate cortex, insula, limbic system, medial prefrontal cortex, von Economo neurons
VENs are present in the limbic sectors of the human frontal and insular lobes. VENs density decreases in the caudo‐rostral and ventro‐dorsal directions. VENs could participate in autonomous responses and executive functions.
1. INTRODUCTION
Nearly a century after the first cytomorphological characterization of von Economo neurons (VENs) in humans (von Economo 1926), many questions remain regarding their synaptic targets and functional role(s). VENs have been linked, among other roles, with intuitive assessment and the generation of rapid responses in the midst of changing social contexts, based on their morphological characteristics and their location in specific cortical regions (Allman et al. 2005).
Evidence of the vulnerability of VENs in pathologies such as amyotrophic lateral sclerosis (ALS), alcoholism, autism, psychosis and frontotemporal dementia, conditions where a deterioration in social function is a preponderant feature, gives support to this hypothesis (Allman et al. 2005; Braak & Del Tredici 2018; Brüne et al. 2010; Kim et al. 2012; Krause et al. 2017; Santillo et al. 2013; Santos et al. 2011; Senatorov et al. 2015; Simms et al. 2009; Uppal et al. 2014).
It has been reported that VENs are ubiquitous in the cerebral cortex of non‐primate mammals (Butti et al. 2009, 2014; Butti & Hof 2010; Hakeem et al. 2009; Hof & Van Der Gucht 2007; Raghanti et al. 2015, 2019), leading some authors to postulate that rather than belonging to a particular cell type, these cells correspond to pyramidal neurons that have been modified in response to functional demands, including the mechanical pressure created by cortical expansion and gyrification (Raghanti et al. 2015). Their location appears to be more restricted in primates (Allman et al. 2011; Evrard 2018). More specifically, they have been found in the anterior insular cortex, the anterior cingulate cortex, the temporal lobe and the medial portions of prefrontal areas BA9 and BA10 in humans (Allman et al. 2002; Evrard 2019; Fajardo et al. 2008; González‐Acosta et al. 2018; Nimchinsky 1999; Nimchinsky et al. 1995; Raghanti et al. 2015). VENs have been found in the deep portion of cortical layer V or sublayer Vb in all studies on Homo sapiens. Other sectors, such as the dorsolateral prefrontal cortex and the occipital pole, have been explored; however, the presence of VENs has not been confirmed there (Fajardo et al. 2008; González‐Acosta et al. 2018; Raghanti et al. 2015). The density and size of their soma differ from one cortical region to another, as does their biochemical profile (Cobos & Seeley 2015; González‐Acosta et al. 2018; Stimpson et al. 2011). Based on our literature review, we consider that the circumscribed location of VENs in human limbic cortical regions suggests that they could play a broad functional role, ranging from the generation of autonomic responses to emerging cortical functions of greater phylogenetic derivation such as social cognition and mind theory.
The following scientific literature databases were used to search for bibliographic material: PubMed, Scopus, Lilacs and Google Scholar. The search terms used were ‘von Economo Neurons’, ‘VENs’, ‘spindle cells’, ‘fusiform cells’ and ‘corkscrew cell’. The search was not limited to a specific time period; we included all articles published on the subject, from the first article reporting on these cells in 1926 (von Economo 1926) to the most recent article published in 2021. We found a total of 45 articles that fill inclusion criteria (see Table 1) and discarded 158 documents:15 reviews, 2 theses, 130 duplicates, 4 perspectives or opinions and 7 articles that did not refer to morphological findings on these neurons (see Figure 1).
TABLE 1.
Original articles on von Economo neurons
| Authors | Analysis type | Methods | Studied in | Cortical region | Cortical layer | Functional asymmetry | Structural asymmetry | |
|---|---|---|---|---|---|---|---|---|
| 1 | (von Economo 1926) | Morphological, pathological, cytoarchitectonic | Silver staining | Humans | BA24, Insula | V | No | NO |
| 2 | (Ngowyang 1936) | Histopathological | Cresyl violet and Silver staining | Humans and Non‐human animals | Insula, Hippocampus | V | NO | NO |
| 3 | (Nimchinsky et al. 1995) | Cytoarchitectonic, morphological, pathological | Immunohistochemistry | Humans | BA24 | V | NO | NO |
| 4 | (Seeley et al. 2006) | Morphological | Immunohistochemistry | Humans | BA24 | V | NO | NO |
| 5 | (Watson et al. 2006) | Morphological | Cresyl violet and Golgi staining | Humans | BA24, Insula | V | NO | NO |
| 6 | (Hof & Van Der Gucht 2007) | Histological, morphological | Histochemistry | Non‐human animals | BA24, Insula, frontopolar cortex | V | NO | NO |
| 7 | (Kaufman et al. 2008) | Histological | Cresyl violet staining | Humans | BA24, Insula | NO | YES | |
| 8 | (Fajardo et al. 2008) | Histological | Immunohistochemistry | Humans | BA9 | V | NO | NO |
| 9 | (Simms et al. 2009) | Pathological, cytoarchitectonic, morphological | Cresyl violet staining | Humans | BA24 | III | NO | NO |
| 10 | (Butti et al. 2009) | Histological | Cresyl violet staining | Non‐human animals | BA24, Insula, frontopolar cortex | V | NO | NO |
| 11 | (Hakeem et al. 2009) | Histological | Histochemistry, Immunohistochemistry | Non‐human animals | BA24, Insula, prefrontal and dorsolateral cortex | V | NO | YES |
| 12 | (Butti & Hof 2010) | Cytoarchitectonic, comparative among species | Cresyl violet staining | Non‐human animals | Insula | V | NO | NO |
| 13 | (Allman et al. 2010) | Histological | Histochemistry, Immunohistochemistry | Humans and Non‐human animals | BA24, Insula | III and V | NO | YES |
| 14 | (Brüne et al. 2010) | Histological, histopathological | Cresyl violet staining | Humans | BA24 | YES | YES | |
| 15 | (Stimpson et al. 2011) | Biochemical | Immunohistochemistry | BA24 | V | NO | NO | |
| 16 | (Brüne et al. 2011) | Morphological, histopathological | Cresyl violet staining | Humans | BA24 | V | NO | YES |
| 17 | (Santos et al. 2011) | Morphological | Immunohistochemistry | Humans | Insula | V | NO | NO |
| 18 | (Kim et al. 2012) | Histological, histopathological, morphological | Cresyl violet staining | Humans | BA24, Insula | V | YES | YES |
| 19 | (Evrard et al. 2012) | Cytoarchitectonic | Immunohistochemistry | Non‐human animals | BA24 | V | NO | YES |
| 20 | (Morel et al. 2013) | Histological, cytoarchitectonic, anatomical | Histochemistry, Immunohistochemistry, MRI | Humans | Insula | NO | NO | |
| 21 | (Santillo & Englund 2014) | Histological, histopathological | Cresyl violet and Luxol Fast Blue staining | Humans | BA24 | V | NO | NO |
| 22 | (Uppal et al. 2014) | Histological, histopathological | Cresyl violet staining | Humans | BA24 | V | NO | NO |
| 23 | (Senatorov et al. 2015) | Pathological | Histochemistry | Humans | Insula | V | NO | NO |
| 24 | (Butti et al. 2014) | Cytoarchitectonic | Immunohistochemistry | Non‐human animals | BA24, Insula, frontopolar and dorsolateral prefrontal cortex | V | NO | NO |
| 25 | (Raghanti et al. 2015) | Histological, morphological | Cresyl violet staining | Non‐human animals | BA24, Insula, frontal and occipital poles | V | NO | NO |
| 26 | (Cobos & Seeley 2015) | Biochemical | Histochemistry, Immunohistochemistry, and in situ hybridization | Humans | BA24, Insula | V | NO | NO |
| 27 | (Krause et al. 2017) | Histological, morphological, subcellular | Electronic microscopy | Humans | BA24 | V | NO | NO |
| 28 | (Yang et al. 2017) | Morphological, pathological | Cresyl violet staining | Humans | BA24 | V | NO | NO |
| 29 | (Braak & Del Tredici 2018) | Histopathological | Immunohistochemistry | Humans | BA24 | V | NO | NO |
| 30 | (Dijkstra et al. 2018) | Biochemical | Immunohistochemistry, in situ hybridization | Humans | BA24, Insula | V | NO | NO |
| 31 | (González‐Acosta et al. 2018) | Histological | Immunohistochemistry | Humans | Medial frontopolar cortex | V | YES | YES |
| 32 | (Gefen et al. 2018) | Histological, histopathological | Cresyl violet staining, Immunohistochemistry | Humans | BA24, BA25 | NO | NO | |
| 33 | (Yang et al. 2019) | Biochemical, histological | Transcriptomic | Humans | BA24 | V | NO | NO |
| 34 | (Raghanti et al. 2019) | Histological, morphological | Cresyl violet staining and MRI | Non‐human animals | Frontal and occipital poles, BA24, Insula, BA4, BA3‐1‐2, BA41‐42, BA17 | V | NO | NO |
| 35 | (Nana et al. 2019) | Histopathological | Immunohistochemistry | Humans | Insula | V | NO | |
| 36 | (Lin et al. 2019) | Histopathological | Histochemistry and Immunohistochemistry | Humans | BA24, Insula | V | NO | NO |
| 37 | (Yang et al. 2019) | Biochemical, histological | In situ hybridization, I Immunohistochemistry | Humans | BA24 | V | NO | NO |
| 38 | (Gami‐Patel et al. 2019) | Histological, histopathological | Histochemistry and Immunohistochemistry | Humans | BA24 | V | NO | NO |
| 39 | (Banovac et al. 2019) | Morphological | Golgi staining and Immunohistochemistry | Humans | BA24 | V | NO | NO |
| 40 | (Tan et al. 2019) | Histopathological | Histochemistry and Immunohistochemistry | Humans | BA24 | V | NO | NO |
| 41 | (Horn 2020) | Histological | Histochemistry and Immunohistochemistry | Non‐human animals | Insula | V | NO | NO |
| 42 | (Correa‐Júnior et al. 2020) | Histology, morphological | Thionine and Golgi staining | Humans | BA24 | V | NO | NO |
| 43 | (Cabeen et al. 2020) | Histological | Cresyl violet and Gallyas staining | Non‐human animals | Insula | NO | YES | |
| 44 | (Hodge et al. 2020) | Biochemical, electrophysiological | Transcriptomic, in situ hybridization and electrophysiology | Humans | Insula | V | NO | NO |
| 45 | (Jacot‐Descombes et al. 2020) | Histological, pathological, morphological | Cresyl violet staining | Humans | BA24, Insula | V | NO | NO |
Note: The chronological organization of the original articles reviewed that analyse histological, morphological and pathological aspects of VENs in humans and other non‐human animals.
FIGURE 1.
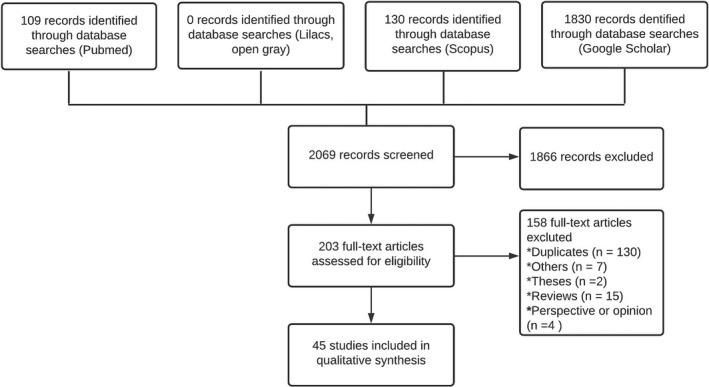
PRISMA flowchart displaying the literature research
2. DISCOVERY AND REDISCOVERY OF VON ECONOMO NEURONS
Although ‘fusiform cells’ were described in the second decade of the 20th century by Constantin von Economo (von Economo 1926), an Austrian psychiatrist and neurologist, and currently bear this name in his honour (Allman et al. 2005), VENs had already been observed by other neuroanatomists. For example, Betz noticed their presence in the cingulate gyrus and emphasized their large size, compared with nerve cells in the same cortical layer (Betz 1881). In the first decade of the 20th century, Ramón y Cajal highlighted their prevalence in the infragranular layers of the cingulate gyrus and the insula (Ramón y Cajal, 1901). Recent reviews of the evolution of this neuronal type reported that Hammarberg observed them in 1895 in the ‘Gyrus Centralis Anterior’, a region that corresponds in contemporary neuroanatomic nomenclature to the precentral gyrus (Butti et al. 2013; Hammarberg 1895). Ngowyang reported the presence of VENs in the subiculum and entorhinal cortex in 1936 (Ngowyang 1936). However, there was a long silent period during which VENs seemed to fall outside the focus of interest of neuroscientific studies. It was not until the second half of the last decade of the 20th century that Nimchinsky et al. provided a detailed description of VENs with novel qualitative and quantitative data (Nimchinsky 1999; Nimchinsky et al. 1995). From this rediscovery of VENs (Nimchinsky et al. 1995) until the present, they have been found in two additional cortical locations in humans (Fajardo et al. 2008; González‐Acosta et al. 2018), and other studies have described their biochemical profile (Cobos & Seeley 2015; Stimpson et al. 2011) and vulnerability to certain pathologies (Brüne et al. 2010; Kaufman et al. 2008; Santillo & Englund 2014; Santos et al. 2011).
3. CYTOMORPHOLOGICAL AND HISTOLOGICAL CHARACTERISTICS THAT DEFINE VENs
There is currently a relative consensus on the cytomorphological characteristics of VENs, based on descriptions by various authors. VENs have a large fusiform cell body (always larger than that of the pyramidal neurons with which they share a laminar location). Single broad and low‐branched dendritic processes extend from the apical and basal poles of the cell body (Allman et al. 2002; Nimchinsky 1999). The diameter of the initial segment of the VEN dendritic process is usually similar to that of its cell body, giving the neuron a cylindrical appearance in which it is difficult to distinguish the end of the soma from the beginning of the process. The apical and basal VEN dendritic processes extend perpendicular to the pial surface and the subcortical white matter, respectively (Allman et al. 2002; Fajardo et al. 2008). Apparently, it is not the topology of the apical dendrite that morphologically distinguishes VENs from other neurons. In general, the basal part of the body axis is helical, and the perisomatic characteristics that distinguish them are the rather thick origin of the apical stem and the gradual narrowing of the stem (Banovac et al. 2019). These cytomorphological characteristics, and their variations, have resulted in various appellations including fusiform, spindle, corkscrew and rod cells (Figure 2) (Allman et al. 2002; Allman et al. 2011; Butti et al. 2013; von Economo 1926).
FIGURE 2.
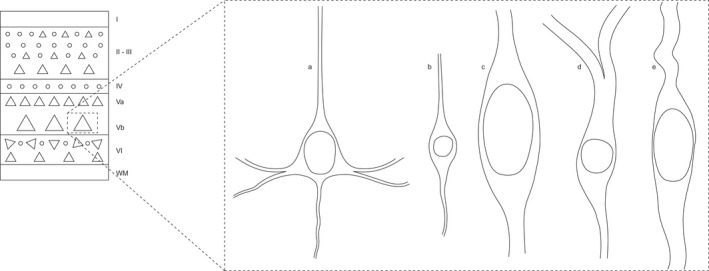
Laminar location and morphological characteristics of human VENs. The diagram on the left shows the location of VENs in the deep part of layer V. The images on the right show the morphology of a pyramidal neuron (a), a fusiform neuron in layer VI (b) and three morphological variants of VENs (c, d and e). The size of the soma of these illustrated neurons maintains the relative proportions found in cortical tissue
Watson et al. compared in detail the dendritic arborization of VENs with that of the pyramidal neurons located in the same cortical layer of the human frontoinsular cortex and anterior cingulate cortex. They reported that the dendritic arborization of VENs was radial, long and narrow, with the apical and basal processes homogeneous in terms of branching, length and number of intersections. They found no significant differences in the number of dendritic spines between apical and basal processes, with a maximum spine density at 190–240 μm from the soma. The length of VEN dendrites was 2.5 times shorter than that of pyramidal neurons (Watson et al. 2006).
However, Banovac et al. analysed sections of BA24 stained with rapid Golgi and Golgi‐Cox in brain tissue of five adult human specimens and found a high dendritic spine density on all side branches and on the branches arising from the basal stem ending. The described dendritic spines had a mushroom shape and were relatively long, and no mushroom‐shaped dendritic spines were found on the VEN soma (Banovac et al. 2019). It has been reported, for this same area, that spine density varies according to its proximal and distal location, the latter varying between 0.85 and 4.2 (Correa‐Júnior et al. 2020).
The large size of the VEN soma has always been a predominant feature of their morphology. However, this is not an invariant feature in the different cortical regions where they are present. A study of layer V of the human anterior cingulate cortex (BA24) showed that VENs have a soma average volume of 20,822 μm3, reaching a size 4.6 times larger than that of pyramidal soma in the same layer (Nimchinsky 1999). In contrast, a more recent study proposed labelling these cells as VEN 1, VEN 2 and VEN 3, according to their morphology in BA24, with axial lengths of 37.5 μm, 44.1 μm and 43.8 μm, respectively (Correa‐Júnior et al. 2020).
A comparison of the soma area of VENs in the dorsomedial portion of BA9 and BA24 indicated that VENs are smaller in BA9 (536 μm2 ± 161.5) than in BA24 (627.5 μm2 ± 96.92) (González‐Acosta et al. 2018). We recently found that VENs are also located on the medial surface of the human frontopolar cortex. In our study, the VEN soma area was measured on an average of 369.29 μm2 in this region, as opposed to 257.11 μm2 for pyramidal cells in the same layer; that is, the VEN:pyramidal cell size ratio was approximately 1.5 (González‐Acosta et al. 2018). These data suggest that the size of the VEN cell body varies according to the cortical region where these cells are located and that they are smaller in the frontal pole. Despite presenting regional variations, VENs are always larger than the pyramidal neurons with which they share a laminar location (Figure 2).
In the human cerebral cortex, fusiform morphology is not exclusive to a neuronal subtype. In fact, different subtypes of cortical interneurons with a spindle‐shaped soma, such as double bouquet cells and bipolar cells, have also been described (Markram et al. 2004). Fusiform cells have been observed in lamina VI that appeared to be simply smaller than VENs. Studies that have compared the size of VEN soma in layer V with fusiform cell soma in layer VI have confirmed that in the anterior cingulate cortex, VEN can reach up to 12 times the size of fusiform cells (Nimchinsky 1999). In the frontal pole, the tendency of VENs to be larger than the fusiform cells of lamina VI remained constant; however, the size difference was only approximately 1.9 times (Figure 2) (González‐Acosta et al. 2018).
Among the diverse studies undertaken in humans, there are significant variations in the reported data regarding the number of VENs, both within and between regions of the cerebral cortex (Butti et al. 2014; Fajardo et al. 2008; González‐Acosta et al. 2018). For example, it has been reported that the proportion of VENs ranged between 0.56% and 1.38% of total neurons in the anterior cingulate cortex (Allman et al. 2011). We found that VENs corresponded to 3% of the total neuronal population of sublayer Vb in BA24 (Fajardo et al. 2008). The maximum value reported for this cortical region was 13% (Raghanti et al. 2015). Allman et al. reported that VENs corresponded to 1.2% of neurons in the frontoinsular region, whereas Raghanti et al. found that VENs corresponded to 11% of neurons in layer V, this being the maximum percentage of VENs reported for this cortical region to date (Allman et al. 2011; Raghanti et al. 2015). The percentage of VENs reported in BA9 was 0.5% (Fajardo et al. 2008). In the medial region of BA10, we found that the percentage of VENs with respect to pyramidal neurons in sublayer Vb varied significantly between the cerebral hemispheres, ranging from 0.27% to 0.34% in the left hemisphere and from 0.62% to 1.54% in the right hemisphere (González‐Acosta et al. 2018). These are the lowest reported amounts of VENs to date. In conclusion, these data suggest that VEN density decreases on a gradient towards the frontal pole, away from the cingulate gyrus. In addition, the distribution of VENs presents an interhemispheric asymmetry (see Table 1), with greater density towards the right hemisphere (González‐Acosta et al. 2018), a trend that has also been observed in other species (Evrard et al. 2012). Another interesting recently described trend is that of a greater abundance of VENs in the crests of the gyri rather than in the walls of the sulci (González‐Acosta et al. 2018; Raghanti et al. 2015).
4. HUMAN CORTICAL REGIONS WITH VENs
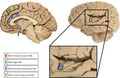
There are descriptions of VENs from the temporal, frontal and insular lobes of humans. Other anatomists mentioned these cells prior to von Economo, referring to a neuronal type with characteristics similar to those of VENs (including morphology, cortical and laminar location) found in the anterior regions of the cingulate and the insula (Figure 3), predominantly in layer V (Betz 1881; Hammarberg 1895; Ramón y Cajal, S. 1901). After 1926, von Economo conducted an exhaustive analysis at the cortical level, confirming the presence of VENs exclusively in the deep layers of the same cortical regions (von Economo & Koskinas 1925). Subsequently, other anatomists also made brief mention of cells with the typical morphology of VENs, always in the aforementioned cortical sectors (Ramón y Cajal, S. 1901). Recently, the presence of VENs was confirmed in cortical areas BA24 (Nimchinsky et al. 1995) and in the anterior region of the insula also known as agranular insula or frontoinsular cortex according to Brodman’s nomenclature and other cytoarchitectonic maps. Some authors reported that VENs were located in a specific cytoarchitectonic area of the insula called the ‘lateral agranular area’, whereas other authors stated that according to their findings, VENs could reach sectors characterized as disgranular (Allman et al. 2010; Evrard et al. 2012; Horn 2020; Morel et al. 2013). In all previous studies, the location of these neurons was restricted to the deep portion of layer V or sublayer Vb (Nimchinsky 1999). In 2015, a search for VENs in broad sectors of the cortex of several animal species was undertaken; VENs were found in numerous locations, including the polar regions of the frontal and occipital lobes. Until that time VENs had not been observed in these sectors of the human cerebral cortex (Raghanti et al. 2015).
FIGURE 3.
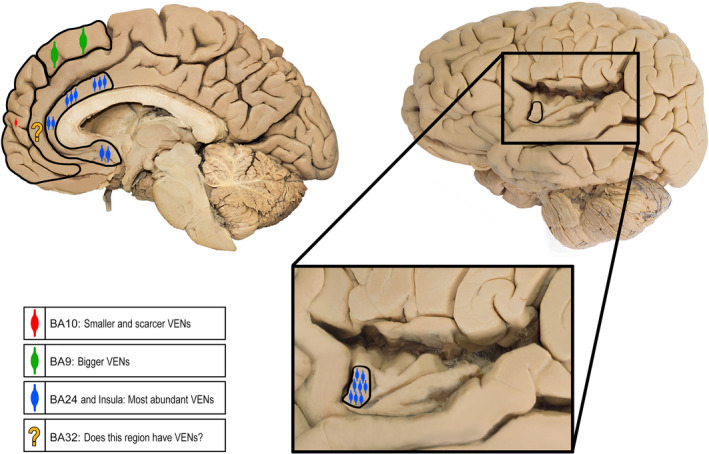
Cortical areas with confirmed presence of VENs. The image on the left shows the location of VENs on the medial surface of the cerebral hemispheres, including BA9, BA10 and BA24. We wondered about the possibility that other areas of the medial surface of the frontal lobe, for example BA32, also have VENs. The image on the right shows the insula along with a magnification of it to indicate the location of VENs in the frontoinsular region. Each illustrated cortical area shows variations in the quantity and size of the soma of VENs
Although a large number of studies coincide with a circumscribed location of VENs in the anterior regions of the cingulate and insular cortex, a review published by Butti et al. revealed that in 1936, Ngowyang reported the existence of these cells in the subiculum and entorhinal cortex, which constitutes a great change in the pattern of location previously described (Ngowyang 1936). According to that review, VENs are not neurons confined to the insula and anterior cingulate cortex but could also be located in the basal and medial portions of the temporal lobe (Butti et al. 2013).
It is now known that these neurons are also located on the medial surface of the human frontal lobe (Figure 3). VENs were identified in 2008 on the medial surface of the superior frontal gyrus (BA9), expressing neuronal nuclei (NeuN) and microtubule‐associated protein 2 (MAP2) (Fajardo et al. 2008). In the same study, the presence of VENs in other areas of the lateral surface of the prefrontal cortex, such as BA46, BA45 and BA10, as well as in the occipital pole (BA17) was ruled out. Until 2014, several studies were carried out on the human frontal pole searching for these neurons with negative results (Fajardo et al. 2008; Raghanti et al. 2015); nevertheless, we carried out a new exploration focused on the medial surface of the frontal pole because we considered it a plausible location is given anatomical, evolutionary and connectivity criteria. In this new search, the presence of VENs in the polar region of medial BA10 was evidenced (Figure 3). VENs of the medial region of the human frontal pole have the same morphological features and laminar location described previously, with the exception that their size and density were considerably smaller than those of other cortical regions (González‐Acosta et al. 2018).
According to our literature review on the morphological characteristics of VENs, in 33 out of 45 articles, human brain tissue was analysed exclusively; in 10 of these articles, non‐human animal brain tissue was studied, and in the two remainings, both brain tissue from human and non‐human animals. Only 2 of the 45 articles consider VEN in layer III. Most of the original studies (34 of 45) of VENs have been carried out in the anterior cingulate gyrus (BA24); and in the insula (24 of 45), six have been carried out in the frontopolar cortex (including BA10), and seven have considered other regions of the cerebral cortex (BA9, BA25, dorsolateral prefrontal cortex, hippocampus, motor cortex, sensory cortex, visual cortex and auditory cortex). Nine original studies on VENs have evaluated possible structural asymmetries in their distribution (see Table 1).
5. BIOCHEMICAL PROFILE OF VON ECONOMO NEURONS
The biochemical characteristics of VENs were first indicated by their intense immunostaining for non‐phosphorylated neurofilament proteins; this constituted indirect evidence of a robust axon that could participate in the transfer of information to distant sectors of the cortical grey matter (Nimchinsky et al. 1995; Stimpson et al. 2011). Subsequently, a study found projection neurons in layer V of the frontoinsular cortex labelled intensely by the bombesin peptides neuromedin B (NMB) and gastrin‐releasing peptide (GRP) including VENs and fork cells (Allman et al. 2011). The peptides NMB and GRP participate peripherally in the release of digestive enzymes, muscle contraction in peristalsis and the organization of immunological mechanisms in response to the intake of potentially harmful substances (Jensen et al. 2008). It has been suggested that in the central nervous system, in structures such as the insula, these peptides could be involved in visceral interoceptive integration with higher mental functions such as motivation and decision‐making (Allman et al. 2011). The same study found that the protein encoded by the gene DISC1 resulted in strong staining on the soma and dendritic processes of VENs. In some specimens, about 90% of VENs were DISC1‐positive, while other types of neurons located in the different cortical layers only reached 36% (Allman et al. 2011). DISC1 is a gene that participates in the regulation of phenomena such as cell proliferation, differentiation and migration as well as in the definition of dendritic architecture. The dendritic organization of neurons is mediated by the suppression of the growth of secondary and tertiary branches, and therefore, DISC1 could be decisive for one of the morphotypic characteristics of VEN: a dendritic process derived from each pole of the soma with few ramifications (Allman et al. 2011; Cobos & Seeley 2015; Duan et al. 2007).
A study analysed the expression of the proteins cAMP‐dependent activating‐transcription factor 3 (ATF3), interleukin 4 receptor (IL4Rα) and NMB in the anterior cingulate cortex of 21 hominids (including Homo sapiens) (Stimpson et al. 2011). As previously described, the layer V cells showed the highest immunoreactivity (IL4Rα 83%, ATF3 81%, NMB 82%), with VENs showing the highest expression for each of these three markers compared with other neural types in the same layer. Labelling for ATF3 and IL4Rα was significantly higher in Homo sapiens than in the other hominids, reaching a percentage of 31% and 66% of the total VEN population, respectively (Stimpson et al. 2011). ATF3 is a member of the family of mammalian activation transcription factors (CREB), characterized by its response to cAMP. This factor has been involved in functions such as spinal cord pain modulation, and it is also known to participate in the body’s reactions to stressful events (Chen et al. 1996; Latrémoliere et al. 2008). Since the activity of the anterior cingulate cortex has been related to the unpleasant perception of pain, the presence of ATF3 in this cortical region could be part of the elements necessary for such a function (Rainville et al. 1997; Stimpson et al. 2011). IL4Rα is known to be a type of cytokine I receptor to which interleukin‐4 and ‐13 are linked to the regulation of immunoglobulin E, helping as a mediator of allergic responses in the immune system, particularly asthma, responses to which the anterior cingulate cortex has also been linked (Rosenkranz & Davidson 2009; Wenzel et al. 2007). In the central nervous system, it has been linked to inflammatory reactions and some studies associate it with schizophrenia (Nawa et al. 2000; Nawa & Takei 2006).
Cobos and Seeley, using in situ hybridization and immunohistochemistry for NeuN in five human subjects of 3 months, 6 and 65 years of age, examined the expression of seven transcription factors (SOX5, TBR1, FEZF2, CTIP2, LMO4, FOXP1 and SATB2) in the anterior cingulate cortex and the anterior insula to obtain evidence of the possible axonal projection targets of VENs, taking into account that the manifestation of these markers may be specifically associated with intrahemispheric (Cabeen et al. 2020) and commissural cortico‐cortical, cortico‐thalamic and other non‐thalamic cortico‐subcortical efferents (Cobos & Seeley 2015). According to the results of that study, although VENs responded predominantly to transcription factors expressed in neurons whose axons extend distally (in the direction of non‐thalamic subcortical structures), they could also do so more proximally, including axons that pass through the corpus callosum (Cobos & Seeley 2015).
Recently, an analysis of the gene expression of layer V neurons in the frontoinsular cortex and anterior cingulate cortex through sequencing of nuclear RNA added to evidence that supports the hypothesis that the VEN axons mainly reach extra‐telencephalic structures (Hodge et al. 2020; Yang et al. 2019). Despite these biochemical, cytomorphological and anatomical profiles, to date, it has not been possible to identify the target of the axons of VENs or the characteristics of their firing. Electrophysiological and biochemical techniques to obtain this valuable information require experimentation with live specimens, and no animal species that possess VENs and are susceptible to experimentation have yet been found. Moreover, it has been suggested that VENs could possess a new and uncharacterized cortical monoaminergic function that sets them apart from most other neurons in mammalian layer V (Dijkstra et al. 2018). To date, only six original studies where they have attempted a biochemical characterization of VENs, through techniques such as transcriptomics, in situ hybridization, RNA sequencing and immunohistochemistry have been found (see Table 1).
6. VEN VULNERABILITY IN NEUROPATHOLOGICAL ENTITIES
A particular vulnerability of VENs has been observed in several types of neuropsychiatric disorders, such as the case of autism, for which experimental evidence indicates that there are changes in the quantity, location and morphology of VENs. In some studies, subjects who had been diagnosed with autism had lower overall cell density and almost total absence of VENs (Uppal et al. 2014), whereas, in other studies, there was an increase in the proportion of VENs compared with the number of pyramidal cells (Santos et al. 2011; Simms et al. 2009). In subjects with autism, there may be an atypical location of VENs in addition to morphological changes such as wider soma (Santos et al. 2011; Simms et al. 2009; Uppal et al. 2014). On the other hand, atypical localization of VENs in the agenesis of the corpus callosum and an important reduction in the number of these cells in the frontoinsular region and the anterior cingulate cortex in these same subjects has also been found (Kaufman et al. 2008). Another study showed a reduction of VENs in the anterior cingulate cortex in cases of early onset schizophrenia (Brüne et al. 2010). This study found that patients who committed suicide had a pattern of greater grouping of VENs compared with psychotic patients who died from natural causes (Brüne et al. 2011).
For example, regarding mental disorders, significant macroscopic anatomical changes were found in the amygdala and anterior insula of alcoholic individuals as well as a decrease in VENs of approximately 60% (Senatorov et al. 2015). A significant increase in lysosomal aggregations in VENs compared with controls was also described in patients diagnosed with schizophrenia and bipolar disorder (Krause et al. 2017).
Seeley et al. were the first to report a specific involvement of VENs in the anterior cingulum and frontoinsular cortex of patients with frontotemporal dementia (FTD), compared with patients with Alzheimer’s disease (AD) and control subjects (Seeley et al. 2006). Their findings indicated a selective loss of this neuronal type, with a decrease of up to 74%, and also changes in morphology. Other studies confirmed the selective vulnerability of VENs in FTD using a larger sample; they reported a neuronal degeneration of 53% and 41% compared with control subjects and patients with AD, respectively (Kim et al. 2012; Santillo et al. 2013). On the other hand, Gefen et al. showed an increase in VENs in SuperAgers compared with control subjects and/or a significant decrease in neuronal loss compared with the two pathologies (Gefen et al. 2018). It is worth noting that the study by Tan et al. did not report significant VENs loss in any of the AD cases with associated frontotemporal dementia (Tan et al. 2019).
The same study pointed to the specific and predominant cellular fragility of VENs, compared with neurons in layers II and III (Santillo & Englund 2014). A predominant alteration of VENs was also found recently in a behavioural variant of frontotemporal dementia with C9orf72 expansion (C9‐bvFTD), in which there was a 57% reduction in VENs (Gami‐Patel et al. 2019). Another study analysed specifically this same variant (C9‐bvFTD) but did not find significant differences in VEN density compared with ALS cases and controls (Yang et al. 2017). A disproportionate aggregation of the tau protein was also recently reported in the V337 M, A152T and IVS10 + 16 variants (Lin et al. 2019). There was an early and disproportionate aggregation of TDP‐43 in the VENs and hairpin cells of this type of patient; this was correlated with the anatomical and clinical seriousness of the case, including the loss of emotional empathy (Nana et al. 2019).
In a study of patients with familial dysautonomia, an altered distribution of VENs compared with controls was observed. VENs were found in the orbitofrontal and inferior frontal cortex; they were located in layer Va and were organized in small clusters near the blood vessels (Jacot‐Descombes et al. 2020).
7. DISCUSSION
Our literature review showed that VENs have been observed in limbic sectors of the human temporal, frontal and insular lobes. There is a consensus regarding the presence of VENs predominantly in the anterior portions of the cingulum and the insula in humans. VENs found in the temporal lobe have been located in the entorhinal cortex and the subiculum; however, we do not know details of their morphology, density, laminar location or biochemical profile. In the case of the frontal lobe, in addition to the anterior cingulate cortex, VENs have been characterized on the medial surface, specifically in the superior frontal gyrus (BA9) and the pole (BA10) (Figure 3) (Fajardo et al. 2008; González‐Acosta et al. 2018).
The review by Butti et al. (Butti et al. 2013) stated that Hammarberg reported the presence of VENs on the lateral surface of the frontal lobe as early as 1895 (in the ‘Gyrus Centralis Anterior’). However, we interpret that report differently. We believe that what Hammarberg reported was the presence of giant neurons that extended to the layer of the fusiform cells. In this sense, the fusiform cell layer refers to layer VI, and the so‐called ‘Gyrus Centralis Anterior’ corresponds to the precentral gyrus, that is the primary motor cortex or BA4. Therefore, it is likely that the giant cells that Hammarberg observed correspond to Betz motor neurons, which may eventually reach the superficial part of layer VI, and not to VENs (Hammarberg 1895).
Human non‐limbic areas of the frontal (BA4, BA44/45, BA46 and lateral BA10) and occipital lobes have been searched, but no VENs have yet been found in those areas (Fajardo et al. 2008; Raghanti et al. 2015). As for the density of these neurons, we find that the data described by different authors are heterogeneous, even for the same cortical sector (Fajardo et al. 2008; González‐Acosta et al. 2018; Raghanti et al. 2015). Regarding the differences observed in the number of VENs found in different studies that have focused on the same regions of the cerebral cortex, that is the anterior cingulate gyrus and the rostral insula, it is not possible to estimate whether such differences are due to anatomical variants of the cell population studied, or if they are due to the methodologies used to mark and quantify them. However, the data available to date suggest that the density of VENs increases in the vicinity of the ‘limbic lobe’ (anterior cingulate and frontoinsular cortices) and decreases in the rostral and dorsal direction within the medial surface of the frontal lobe, with a lower density in BA9 and a minimum value at the frontal pole (Figure 3) (Fajardo et al. 2008; González‐Acosta et al. 2018; Raghanti et al. 2015). Similarly, there are reports of VENs being distributed mainly on the crest of the gyri in the human cerebral cortex as well as a marked asymmetry in density in favour of the right hemisphere (González‐Acosta et al. 2018; Raghanti et al. 2015).
There is greater consensus regarding the morphological features of VENs, specifically the fusiform aspect of the soma and the gradual narrowing of the proximal dendritic tree, which extends almost imperceptibly from the cell body (Fajardo et al. 2008; González‐Acosta et al. 2018; Markram et al. 2004; Triarhou 2013). Regarding size, it has always been observed that VENs are larger than pyramidal neurons of layer V, and they seem to be larger in the cortical sectors where they are more abundant. There even seems to be a correspondence between the number of neurons and their size, with larger neurons at the locations where they are most abundant (the anterior regions of the cingulum and the insula), whereas those in BA9 are less frequent and smaller, and those in BA10 are the smallest and most infrequent (Figure 3) (Fajardo et al. 2008; González‐Acosta et al. 2018; Raghanti et al. 2015).
Based on the data compiled in this review, we propose that VENs can be considered a particular cell type or a modification of pyramidal neurons that could fulfil a functional role associated with the limbic regions where they have been found in humans. Although it has been reported that VENs are ubiquitous in other species, in Homo sapiens, they have a restricted and specific location. Initially, it was proposed that VENs could correspond to a neuronal archetype that supports functionally the mental abilities of superior primates (and mammals with highly complex social behaviour), such as interoception, theory of mind and the generation of rapid responses when faced with changing social contexts. Some findings in humans that supported this idea are the location in the anterior portions of the cingulum and the insula, the connections and functions associated with these cortical regions, the asymmetry favouring the right hemisphere (preferably associated with sympathetic activity) and their selective vulnerability in pathologies in which there is a deterioration of the faculties involved in social interaction. However, it was found that these neurons have a ubiquitous cortical distribution in a wide number of species, much less derived in evolutionary terms than primates and cetaceans, and it has been suggested that VENs represent a specialization of pyramidal neurons in response to functional demands. It has also been suggested that the morphology of VENs arises in response to the mechanical pressure resulting from the extension and gyrification of the cerebral cortex. A finding that would support this last idea is the fact that VENs are mostly distributed on the crests of the gyri; however, it cannot explain why VENs are also found in some lissencephalic species (Raghanti et al. 2015).
As previously mentioned, whether VENs correspond to a modification of pyramidal cells or a specific neuronal type, we consider that VENs may fulfil a specific functional role associated with limbic cortical structures because they are restricted to those areas in humans. This is supported by evidence that has shown that VENs predominantly make connections with subcortical limbic and autonomic structures (Ibegbu et al. 2015).
Although they predominantly express transcription factors associated with cells whose axon projects to subcortical structures, VENs may also establish interhemispheric and even intrahemispheric cortical–cortical connections mainly located on the medial surface of areas BA9 and BA10. An interesting fact that points in the same direction are that the soma of VENs is smaller in the frontal pole, so they would support an axon that would not extend over long distances. The cortical structure and the relative distribution of projection neurons in medial areas BA9 and BA10 (Barbas & Rempel‐Clower 1997) allow us to suggest that VENs establish connections with cortical areas of greater hierarchical order such as those of the dorsolateral prefrontal cortex. Functionally, this would lead us to think that VENs are important in triggering not only autonomic responses but also responses associated with emerging higher cognitive functions such as the theory of mind and metacognition. In effect, this connectivity pattern would constitute a bridge between purely limbic contents and executive functions of the highest level of complexity. This idea is consistent with the selective vulnerability of VENs in the neuropsychiatric pathologies mentioned in this review. To corroborate this hypothesis, it would be necessary to perform new studies on VENs in other areas where they would be expected to exist (e.g. medial surface of the frontal lobe) (Figure 3) as well as in other clearly non‐limbic structures to confirm their absence.
AUTHOR CONTRIBUTIONS
CG and EB had the idea for the article, CG and EB carried out the literature search, CG, MC, DO, LB and EB analysed the information obtained from the literature review, CG wrote the first draft and MC, DO, LB and EB critically reviewed the manuscript.
CONFLICTS OF INTEREST
The authors declare that they have no conflict of interest.
ETHICS APPROVAL
Not applicable.
CONSENT TO PARTICIPATE
Not applicable.
CONSENT FOR PUBLICATION
Not applicable.
CODE AVAILABILITY
Not applicabsle.
González‐Acosta, CA , Ortiz‐Muñoz, D , Becerra‐Hernández, LV , Casanova, MF , Buriticá, E. (2022) Von Economo neurons: Cellular specialization of human limbic cortices? Journal of Anatomy, 241,20–32. 10.1111/joa.13642
Funding informationThis study was funded by the Vicerrectoría de Investigaciones of the Universidad del Valle through project CI 1902 of 2021.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- Allman, J. , Hakeem, A. & Watson, K. (2002) Two phylogenetic specializations in the human brain. The Neuroscientist, 8(4), 335–346. 10.1177/107385840200800409 [DOI] [PubMed] [Google Scholar]
- Allman J.M., Watson K.K., Tetreault N.A. & Hakeem A.Y. (2005) Intuition and autism: a possible role for Von Economo neurons. Trends in Cognitive Sciences, 9(8), 367–373. 10.1016/j.tics.2005.06.008 [DOI] [PubMed] [Google Scholar]
- Allman J.M., Tetreault N.A., Hakeem A.Y., Manaye K.F., Semendeferi K., Erwin J.M. et al. (2010) The von Economo neurons in frontoinsular and anterior cingulate cortex in great apes and humans. Brain Structure and Function, 214(5‐6), 495–517. 10.1007/s00429-010-0254-0 [DOI] [PubMed] [Google Scholar]
- Allman J.M., Tetreault N.A., Hakeem A.Y., Manaye K.F., Semendeferi K., Erwin J.M. et al. (2011) The von Economo neurons in the frontoinsular and anterior cingulate cortex. Annals of the New York Academy of Sciences, 1225(1), 59–71. 10.1111/j.1749-6632.2011.06011.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banovac I., Sedmak D., Džaja D., Jalšovec D., Jovanov Milošević N., Rašin M.R. et al. (2019) Somato‐dendritic morphology and axon origin site specify von Economo neurons as a subclass of modified pyramidal neurons in the human anterior cingulate cortex. Journal of Anatomy, 235(3), 651–669. 10.1111/joa.13068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas, H. & Rempel‐Clower, N. (1997) Cortical structure predicts the pattern of corticocortical connections. Cerebral Cortex, 7(7), 635–646. 10.1093/cercor/7.7.635 [DOI] [PubMed] [Google Scholar]
- Betz, W. (1881) Ueber die feiner Structur der Gehirnrinde des Menschen. Zentralbl Med Wiss, 19, 231–234. [Google Scholar]
- Braak, H. , Del Tredici, K. , Braak H. & Del Tredici K. (2018) Anterior cingulate cortex TDP‐43 pathology in sporadic amyotrophic lateral sclerosis. Journal of Neuropathology & Experimental Neurology, 77(1), 74–83. 10.1093/jnen/nlx104 [DOI] [PubMed] [Google Scholar]
- Brüne M., Schöbel A., Karau R., Benali A., Faustmann P.M., Juckel G. et al. (2010) Von Economo neuron density in the anterior cingulate cortex is reduced in early onset schizophrenia. Acta Neuropathologica, 119(6), 771–778. 10.1007/s00401-010-0673-2 [DOI] [PubMed] [Google Scholar]
- Brüne M., Schöbel A., Karau R., Faustmann P.M., Dermietzel R., Juckel G. et al. (2011) Neuroanatomical correlates of suicide in psychosis: the possible role of von Economo neurons. PLoS ONE, 6(6), e20936. 10.1371/journal.pone.0020936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butti C., Sherwood C.C., Hakeem A.Y., Allman J.M. & Hof P.R. (2009) Total number and volume of Von Economo neurons in the cerebral cortex of cetaceans. The Journal of Comparative Neurology, 515(2), 243–259. 10.1002/cne.22055 [DOI] [PubMed] [Google Scholar]
- Butti C., Santos M., Uppal N. & Hof P.R. (2013) Von Economo neurons: clinical and evolutionary perspectives. Cortex, 49(1), 312–326. 10.1016/j.cortex.2011.10.004 [DOI] [PubMed] [Google Scholar]
- Butti C., Ewan fordyce R., Ann Raghanti M., Gu X., Bonar C.J., Wicinski B.A. et al. (2014) The cerebral cortex of the pygmy hippopotamus, hexaprotodon liberiensis (cetartiodactyla, hippopotamidae): MRI, cytoarchitecture, and neuronal morphology. The Anatomical Record, 297(4), 670–700. 10.1002/ar.22875 [DOI] [PubMed] [Google Scholar]
- Butti, C. and Hof, P. R. (2010) The insular cortex: a comparative perspective, 477–493. doi: 10.1007/s00429-010-0264-y. [DOI] [PubMed]
- Cabeen R.P., Glass L., Erwin J.M., Hof P.R., Toga A.W. & Allman J.M. (2020) The connections of the insular VEN area in great apes: A histologically‐guided ex vivo diffusion tractography study. Progress in Neurobiology, 195, 101941. 10.1016/j.pneurobio.2020.101941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B.P., Wolfgang C.D. & Hai T. (1996) Analysis of ATF3, a transcription factor induced by physiological stresses and modulated by gadd153/Chop10. Molecular and Cellular Biology, 16(3), 1157–1168. 10.1128/mcb.16.3.1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobos, I. & Seeley, W.W. (2015) Human von Economo neurons express transcription factors associated with layer V subcerebral projection neurons. Cerebral Cortex, 25, 213–220. 10.1093/cercor/bht219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa‐Júnior N.D., Renner J., Fuentealba‐Villarroel F., Hilbig A. & Rasia‐Filho A.A. (2020) Dendritic and spine heterogeneity of von Economo neurons in the human cingulate cortex. Frontiers in Synaptic Neuroscience, 12. 10.3389/fnsyn.2020.00025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkstra, A. A. , Dijkstra A.A., Lin L‐C., Nana A.L., Gaus S.E. & Seeley W.W. (2018) Von Economo neurons and fork cells: a neurochemical signature linked to monoaminergic function. Cerebral Cortex, 28(1), 131–144. 10.1093/cercor/bhw358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan X., Chang J.H., Ge S., Faulkner R.L., Kim J.Y., Kitabatake Y. et al. (2007) Disrupted‐in‐schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell, 130(6), 1146–1158. 10.1016/j.cell.2007.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Economo, C. (1926) Eine neue Art Spezialzellen des Lobus cinguli und Lobus insulae. Zeitschrift für die gesamte Neurologie und Psychiatrie, 100(1), 706–712. [Google Scholar]
- von Economo C & Koskinas GN (1925) ‘Die cytoarchitectonic der hirnrinde des erwachsenen menschen’, J Springer.
- Evrard H.C. (2018) Von Economo and fork neurons in the monkey insula, implications for evolution of cognition. Current Opinion in Behavioral Sciences, 21, 182–190. 10.1016/j.cobeha.2018.05.006 [DOI] [Google Scholar]
- Evrard, H.C. (2019) The organization of the primate insular cortex. Frontiers in Neuroanatomy, 13, 1–21. 10.3389/fnana.2019.00043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evrard, H.C. , Forro, T. & Logothetis, N.K. (2012) Von Economo neurons in the anterior insula of the macaque monkey. Neuron, 74(3), 482–489. 10.1016/j.neuron.2012.03.003 [DOI] [PubMed] [Google Scholar]
- Fajardo C., Escobar M.I., Buriticá E., Arteaga G., Umbarila J., Casanova M.F. et al. (2008) Von Economo neurons are present in the dorsolateral (dysgranular) prefrontal cortex of humans. Neuroscience Letters, 435(3), 215–218. 10.1016/j.neulet.2008.02.048 [DOI] [PubMed] [Google Scholar]
- Gami‐Patel P., Dijken I., Swieten J.C., Pijnenburg Y.A.L., Rozemuller A.J.M., Hoozemans J.J.M. et al. (2019) Von Economo neurons are part of a larger neuronal population that are selectively vulnerable in C9orf72 frontotemporal dementia. Neuropathology and Applied Neurobiology, 45(7), 671–680. 10.1111/nan.12558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gefen T., Papastefan S.T., Rezvanian A., Bigio E.H., Weintraub S. et al. (2018) Von Economo neurons of the anterior cingulate across the lifespan and in Alzheimer's disease. Cortex, 99, 69–77. 10.1016/j.cortex.2017.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González‐Acosta C.A., Escobar M.I., Casanova M.F., Pimienta H.J. & Buriticá E. (2018) Von Economo neurons in the human medial frontopolar cortex. Frontiers in Neuroanatomy, 12. 10.3389/fnana.2018.00064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakeem A.Y., Sherwood C.C., Bonar C.J., Butti C., Hof P.R. & Allman J.M. (2009) Von Economo neurons in the elephant brain. The Anatomical Record: Advances in Integrative Anatomy and Evolutionary Biology, 292(2), 242–248. 10.1002/ar.20829 [DOI] [PubMed] [Google Scholar]
- Hammarberg, C. (1895) Studien über Klinik und Pathologie der Idiotie, nebst Untersuchungen über die normale Anatomie der Hirnrinde. Uppsala: Berling. [Google Scholar]
- Hodge R.D., Miller J.A., Novotny M., Kalmbach B.E., Ting J.T., Bakken T.E. et al. (2020) Transcriptomic evidence that von Economo neurons are regionally specialized extratelencephalic‐projecting excitatory neurons. Nature Communications, 11(1). 10.1038/s41467-020-14952-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hof, P.R. & Van Der Gucht, E. (2007) Structure of the cerebral cortex of the humpback whale, Megaptera novaeangliae (Cetacea, Mysticeti, Balaenopteridae). Anatomical Record, 290(1), 1–31. 10.1002/ar.20407 [DOI] [PubMed] [Google Scholar]
- Horn, F.M. (2020) Elemental localization of the von Economo neuron and fork neuron in the insular cortex in humans and macaque monkeys. Universität Tübingen. [Google Scholar]
- Ibegbu A.O., Umana U.E., Hamman O.W. & Adamu S.A. (2015) Von Economo neurons: a review of the anatomy and functions. Journal of Experimental and Clinical Anatomy, 14(2), 126. 10.4103/1596-2393.177023 [DOI] [Google Scholar]
- Jacot‐Descombes S., Keshav N., Brosch C.M.S., Wicinski B., Warda T., Norcliffe‐Kaufmann L. et al. (2020) Von economo neuron pathology in familial dysautonomia: quantitative assessment and possible implications. Journal of Neuropathology & Experimental Neurology, 79(10), 1072–1083. 10.1093/jnen/nlaa095 [DOI] [PubMed] [Google Scholar]
- Jensen R.T., Battey J.F., Spindel E.R. & Benya R.V. (2008) International Union of Pharmacology. LXVIII. Mammalian bombesin receptors: nomenclature, distribution, pharmacology, signaling, and functions in normal and disease states. Pharmacological Reviews, 60(1), 1–42. 10.1124/pr.107.07108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J.A., Paul L.K., Manaye K.F., Granstedt A.E., Hof P.R., Hakeem A.Y. et al. (2008) Selective reduction of Von Economo neuron number in agenesis of the corpus callosum. Acta Neuropathologica, 116(5), 479–489. 10.1007/s00401-008-0434-7 [DOI] [PubMed] [Google Scholar]
- Kim E‐J., Sidhu M., Gaus S.E., Huang E.J., Hof P.R., Miller B.L. et al. (2012) Selective frontoinsular Von Economo neuron and fork cell loss in early behavioral variant frontotemporal dementia. Cerebral Cortex, 22(2), 251–259. 10.1093/cercor/bhr004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause M., Theiss C. & Brüne M. (2017) Ultrastructural alterations of Von Economo neurons in the anterior cingulate cortex in schizophrenia. The Anatomical Record, 300(11), 2017–2024. 10.1002/ar.23635 [DOI] [PubMed] [Google Scholar]
- Latremoliere A., Mauborgne A., Masson J., Bourgoin S., Kayser V., Hamon M. et al. (2008) Differential implication of proinflammatory cytokine interleukin‐6 in the development of cephalic versus extracephalic neuropathic pain in rats. Journal of Neuroscience, 28(34), 8489–8501. 10.1523/jneurosci.2552-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L‐C., Nana A.L., Hepker M., Hwang J‐H.L., Gaus S.E., Spina S. et al. (2019) Preferential tau aggregation in von Economo neurons and fork cells in frontotemporal lobar degeneration with specific MAPT variants. Acta Neuropathologica Communications, 7(1). 10.1186/s40478-019-0809-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H., Toledo‐Rodriguez M., Wang Y., Gupta A., Silberberg G. & Wu C. (2004) Interneurons of the neocortical inhibitory system. Nature Reviews Neuroscience, 5(10), 793–807. 10.1038/nrn1519 [DOI] [PubMed] [Google Scholar]
- Morel A., Gallay M.N., Baechler A., Wyss M. & Gallay D.S. (2013) The human insula: architectonic organization and postmortem MRI registration. Neuroscience, 236, 117–135. 10.1016/j.neuroscience.2012.12.076 [DOI] [PubMed] [Google Scholar]
- Nana A.L., Sidhu M., Gaus S.E., Hwang J‐H.L., Li L., Park Y. et al. (2019) Neurons selectively targeted in frontotemporal dementia reveal early stage TDP‐43 pathobiology. Acta Neuropathologica, 137(1), 27–46. 10.1007/s00401-018-1942-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawa, H. , Takahashi, M. & Patterson, P.H. (2000) Cytokine and growth factor involvement in schizophrenia — support for the developmental model. Molecular Psychiatry, 5, 594–603. [DOI] [PubMed] [Google Scholar]
- Nawa, H. & Takei, N. (2006) Recent progress in animal modeling of immune inflammatory processes in schizophrenia: implication of specific cytokines. Neuroscience Research, 56(1), 2–13. 10.1016/j.neures.2006.06.002 [DOI] [PubMed] [Google Scholar]
- Ngowyang, G. (1936) Neuere Befunde über die Gabelzellen. Zeitschrift für Zellforschung und Mikroskopische Anatomie, 25(2), 236–239. [Google Scholar]
- Nimchinsky E.A., Vogt B.A., Morrison J.H. & Hof P.R. (1995) Spindle neurons of the human anterior cingul. Ate cortex. The Journal of Comparative Neurology, 355(1), 27–37. 10.1002/cne.903550106 [DOI] [PubMed] [Google Scholar]
- Nimchinsky E.A., Gilissen E., Allman J.M., Perl D.P., Erwin J.M. & Hof P.R. (1999) A neuronal morphologic type unique to humans and great apes. Proceedings of the National Academy of Sciences, 96(9), 5268–5273. 10.1073/pnas.96.9.5268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghanti M.A., Spurlock L.B., Robert Treichler F., Weigel S.E., Stimmelmayr R. et al. (2015) An analysis of von Economo neurons in the cerebral cortex of cetaceans, artiodactyls, and perissodactyls. Brain Structure and Function, 220(4), 2303–2314. 10.1007/s00429-014-0792-y [DOI] [PubMed] [Google Scholar]
- Raghanti M.A., Wicinski B., Meierovich R., Warda T, Dickstein D.L., Reidenberg J.S. et al. (2019) A comparison of the cortical structure of the bowhead whale (Balaena mysticetus), a basal mysticete, with other cetaceans. The Anatomical Record, 302(5), 745–760. 10.1002/ar.23991 [DOI] [PubMed] [Google Scholar]
- Rainville P., Duncan G.H., Price D.D., Carrier B. & Bushnell M.C. (1997) Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science, 277(5328), 968–971. 10.1126/science.277.5328.968 [DOI] [PubMed] [Google Scholar]
- Ramón y Cajal S. (1901) Studies on the human cerebral cortex IV: structure of the olfactory cerebral cortex of man and mammals. Trab. Lab. Invest. Biol. Univ. Madrid, 1, 1–140. [Google Scholar]
- Rosenkranz, M.A. & Davidson, R.J. (2009) Affective neural circuitry and mind‐body influences in asthma. NeuroImage, 47(3), 972–980. 10.1016/j.neuroimage.2009.05.042.Affective [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santillo, A.F. & Englund, E. (2014) Greater loss of von Economo neurons than loss of layer II and III neurons in behavioral variant frontotemporal dementia. American Journal of Neurodegenerative Disease, 3(2), 64–71. [PMC free article] [PubMed] [Google Scholar]
- Santillo, A. , Nilsson, C. & Englund, E. (2013) von Economo neurones are selectively targeted in frontotemporal dementia. Neuropathology and Applied Neurobiology, 39, 572–579. 10.1111/nan.12021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos M., Uppal N., Butti C., Wicinski B., Schmeidler J., Giannakopoulos P. et al. (2011) von Economo neurons in autism: a stereologic study of the frontoinsular cortex in children. Brain Research, 1380, 206–217. 10.1016/j.brainres.2010.08.067 [DOI] [PubMed] [Google Scholar]
- Seeley W.W., Carlin D.A., Allman J.M., Macedo M.N., Bush C., Miller B.L. et al. (2006) Early frontotemporal dementia targets neurons unique to apes and humans. Annals of Neurology, 60(6), 660–667. 10.1002/ana.21055 [DOI] [PubMed] [Google Scholar]
- Senatorov V.V., Damadzic R., Mann C.L., Schwandt M.L., George D.T. et al. (2015) Reduced anterior insula, enlarged amygdala in alcoholism and associated depleted von Economo neurons. Brain, 138(1), 69–79. 10.1093/brain/awu305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms M.L., Kemper T.L., Timbie C.M., Bauman M.L. & Blatt G.J. (2009) The anterior cingulate cortex in autism: heterogeneity of qualitative and quantitative cytoarchitectonic features suggests possible subgroups. Acta Neuropathologica, 118(5), 673–684. 10.1007/s00401-009-0568-2 [DOI] [PubMed] [Google Scholar]
- Stimpson C.D., Tetreault N.A., Allman J.M., Jacobs B., Butti C., Hof P.R. et al. (2011) Biochemical specificity of von economo neurons in hominoids. American Journal of Human Biology, 23(1), 22–28. 10.1002/ajhb.21135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan R.H., Yang Y., McCann H., Shepherd C. & Halliday G.M. (2019) Von Economo neurons in behavioral variant frontotemporal dementia with underlying alzheimer’s disease. Journal of Alzheimer's Disease, 69(4), 963–967. 10.3233/jad-180900 [DOI] [PubMed] [Google Scholar]
- Triarhou, L.C. (2013) The cytoarchitectonic map of Constantin von Economo and Georg N. Koskinas. In: Microstructural parcellation of the human cerebral cortex. Berlin: Springer, pp. 33–54. 10.1007/978-3-642-37824-9 [DOI] [Google Scholar]
- Uppal N., Wicinski B., Buxbaum J.D., Heinsen H., Schmitz C. & Hof P.R. (2014) Neuropathology of the anterior midcingulate cortex in young children with autism. Journal of Neuropathology & Experimental Neurology, 73(9), 891–902. 10.1097/nen.0000000000000108 [DOI] [PubMed] [Google Scholar]
- Watson, K.K. , Jones, T.K. & Allman, J.M. (2006) Dendritic architecture of the von Economo neurons. Neuroscience, 141(3), 1107–1112. 10.1016/j.neuroscience.2006.04.084 [DOI] [PubMed] [Google Scholar]
- Wenzel S.E., Balzar S., Ampleford E., Hawkins G.A., Busse W.W., Calhoun W.J. et al. (2007) IL4Rα mutations are associated with asthma exacerbations and mast cell/IgE expression. American Journal of Respiratory and Critical Care Medicine, 175(6), 570–576. 10.1164/rccm.200607-909oc [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Yang Y, Yuan J., Sun Y., Dai J. & Su B. (2019) Transcriptomic landscape of von Economo neurons in human anterior cingulate cortex revealed by microdissected‐cell RNA sequencing. Cerebral Cortex, 29(2), 838–851. 10.1093/cercor/bhy286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Halliday G.M., Hodges J.R. & Tan R.H. (2017) von Economo neuron density and thalamus volumes in behavioral deficits in frontotemporal dementia cases with and without a C9ORF72 repeat expansion. Journal of Alzheimer's Disease, 58(3), 701–709. 10.3233/jad-170002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


