Abstract
Background
A higher protein intake has been associated with a higher muscle mass and lower mortality rates in the general population, but data about protein intake and survival in patients with heart failure (HF) are lacking.
Methods
We studied the prevalence, predictors, and clinical outcome of estimated protein intake in 2516 patients from the BIOlogy Study to TAilored Treatment in Chronic Heart Failure (BIOSTAT‐CHF) index cohort. Protein intake was calculated in spot urine samples using a validated formula [13.9 + 0.907 * body mass index (BMI) (kg/m2) + 0.0305 * urinary urea nitrogen level (mg/dL)]. Association with mortality was assessed using multivariable Cox regression models. All findings were validated in an independent cohort.
Results
We included 2282 HF patients (mean age 68 ± 12 years and 27% female). Lower estimated protein intake in HF patients was associated with a lower BMI, but with more signs of congestion. Mortality rate in the lowest quartile was 32%, compared with 18% in the highest quartile (P < 0.001). In a multivariable model, lower estimated protein intake was associated with a higher risk of death compared with the highest quartile [hazard ratio (HR) 1.50; 95% confidence interval (CI) 1.03–2.18, P = 0.036 for the lowest quartile and HR 1.46; 95% CI 1.00–2.18, P = 0.049 for the second quartile].
Conclusions
An estimated lower protein intake was associated with a lower BMI, but signs of congestion were more prevalent. A lower estimated protein intake was independently associated with a higher mortality risk.
Keywords: Heart failure, Obesity, Body mass index, Protein, Mortality
Introduction
Malnourishment and frailty are common in patients with heart failure (HF) and are associated with a poor prognosis. 1 , 2 , 3 Dietary proteins are essential in mammals in forming all amino acids, and adequate protein intake is therefore pivotal. In the general population, the minimum recommended dietary allowance (RDA) for protein is 0.8 g/kg of body weight, for all ages and regardless of sex. 4 However, it could be anticipated that patients with HF may benefit from a higher protein intake, because they have a higher protein requirement due to anabolic resistance and decreased muscle perfusion. Nevertheless, in contrast, they often have a lower protein intake due to physical disabilities, socio‐economic conditions, and comorbidities. 5 This imbalance in need and supply might further impair the clinical outcome of patients with HF. Although there is some evidence addressing the importance of dietary factors in HF progression and outcomes, not much is known about protein intake in patients with HF and guidelines do not provide recommendations regarding protein intake. 6 Assessment of protein intake could therefore be of pivotal essence and could lead to possible dietary interventions and subsequent adequate monitoring, aiming to optimize protein intake in HF patients. We therefore investigated the clinical correlates and outcomes associated with estimated protein intake in a patient population at large with HF.
Methods
Study population
For the current analysis, we used data from BIOSTAT‐CHF (A systems BIOlogy Study to Tailored Treatment in Chronic Heart Failure). BIOSTAT‐CHF is a multicentre, prospective observational study in two independent cohorts of patients with HF. 7 , 8 , 9 , 10 For this study, the BIOSTAT‐CHF index cohort (n = 2516) was used for the primary analysis, and the results were validated in the Scottish validation cohort (n = 1738). Main inclusion criteria for the index cohort were a diagnosis of worsening HF in patients with either a left ventricular ejection fraction (LVEF) < 40% or plasma N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) of >2000 pg/mL who had to be treated with at least 40 mg of furosemide or equivalent and were on sub‐optimal dose of angiotensin‐converting enzyme (ACE) inhibitors and/or angiotensin receptor blockers (ARBs). Main inclusion criteria for the validation cohort were documented HF and patients had to be treated with at least 20 mg of furosemide or equivalent per day and were anticipated to be uptitrated with ACE inhibitors, ARBs, and/or beta‐blockers. The complete list of inclusion and exclusion criteria and main outcome data has been previously published elsewhere. 7 , 8 , 11 The study complied with the Declaration of Helsinki, local ethics committee has approved the research protocol, and all patients signed informed consent. On the present analysis, HF with reduced ejection fraction was defined as an LVEF < 40%, HF with mid‐range ejection fraction (HFmrEF) as an LVEF between 40% and 50%, and HF with preserved ejection fraction (HFpEF) as an LVEF equal or above 50%, according to the most recent European Society of Cardiology HF guidelines. 6
Urinary analysis
Urine samples were available in 2282 patients from the index cohort and 1424 patients from the validation cohort. Baseline spot sample urine measurements were stored at −80°C. Urinary measurements were performed in the laboratory of the University Medical Center Groningen, using routine clinical chemistry measurement on a Roche Cobas® analyser. Protein intake in 24 h urine was calculated by the Maroni formula. Because we used spot samples, we used the adjusted Maroni formula as previously published. 12 , 13 The formula used for protein intake in gram per day was as follows: 13.9 + 0.907 * body mass index (BMI) (kg/m2) + 0.0305 * urinary urea nitrogen level (mg/dL). Because the adjusted formula was validated in a cohort with renal function comparable with our cohort, but it was not validated in an HF population, we performed additional analyses using data from the Additive renin Inhibition with Aliskiren on renal blood flow and Neurohormonal Activation in patients with Chronic Heart Failure and Renal Dysfunction cohort (ARIANA‐CHF‐RD). 14 We calculated protein intake in 24 h urine according to the Maroni formula, performed the same analysis with the currently used adjusted formula for spot urine, and found a good correlation between both (Supporting Information, Figure S1). We also constructed a Bland–Altman plot, showing similar results between the use of estimating protein intake using 24 h urine and the adjusted formula (Figure S2). To assess the robustness of our findings, we replicated the analyses with spot urine urea nitrogen/creatinine ratio and gave similar results. All analyses on protein intake and associations with outcome were validated in the Scottish BIOSTAT‐CHF cohort.
Statistical analysis
Estimated protein intake in gram per day was divided into sex‐specific quartiles. Normally distributed data are shown as means and standard deviation, whereas not normally distributed data as medians and 25th until 75th percentile and categorical variables as percentages and frequencies. Differences between subgroups of estimated protein intake were tested using one‐way ANOVA for normally distributed data; skewed data were tested using the χ 2 test or the Kruskal–Wallis test when appropriate. Partial correlations were assessed to determine associates with estimated protein intake. Univariable significant variables (P < 0.1) were entered in a multivariable model by backward selection. The final model consisted of demographics, clinical variables, and laboratory measurements. Cox proportional hazard analysis was performed to determine hazard ratios (HRs) for the different groups. The models were not corrected for variables already in the initial formula (e.g. BMI/weight/height and urea nitrogen). Restricted cubic splines were assessed to explore the functional association between estimated protein intake and mortality. Non‐normally distributed variables were transformed accordingly. Results were summarized by adjusted HRs of the linear model, depicted as a solid line, and 95% confidence intervals (CIs) of the more functional model by using restricted cubic splines, depicted as dotted lines. To assess an independent contribution, all multivariable models were adjusted for a previously published prognostic model within BIOSTAT‐CHF, in addition to common confounders such as estimated glomerular filtration rate, in‐hospital inclusion of the patient, and HF severity. 11 All analyses were performed using IBM SPSS Statistics Version 23 and R: A Language and Environment for Statistical Computing, Version 3.4.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline characteristics
In total, 2282 patients with available measurements were included, of which 1676 were men (73%) and 606 were women (27%). Mean estimated protein intake was 55 ± 11 g/day. In the total population, 75% of the HF patients did not meet the RDA of 0.8 g/kg of bodyweight/day and actually had less estimated protein intake than this minimum recommended intake. The baseline characteristics are depicted in Table 1.
Table 1.
Baseline characteristics
| Estimated protein intake (g/day) | 1st quartile | 2nd quartile | 3rd quartile | 4th quartile | P for trend |
|---|---|---|---|---|---|
| 570 | 571 | 571 | 570 | ||
| Protein intake (g/day) | 43 [40–45] | 50 [48–52] | 57 [55–59] | 69 [65–74] | <0.001 |
| Age (years) | 70 ± 13 | 70 ± 11 | 68 ± 12 | 65 ± 12 | <0.001 |
| Male (%) | 419 (74) | 419 (73) | 419 (73) | 419 (74) | 1.000 |
| Clinical profile | |||||
| LVEF (%) | 30 ± 11 | 32 ± 11 | 31 ± 10 | 31 ± 10 | 0.126 |
| HFrEF (%) | 457 (89) | 444 (87) | 454 (89) | 483 (93) | 0.043 |
| HFmrEF (%) | 21 (4) | 25 (5) | 27 (5) | 12 (2) | |
| HFpEF (%) | 34 (7) | 39 (8) | 29 (6) | 22 (5) | |
| NT‐proBNP (ng/L) | 5652 [2887–10 755] | 4375 [2526–9278] | 3693 [2095–7163] | 3161 [1602–5917] | <0.001 |
| Height (cm) | 171 ± 9 | 170 ± 9 | 171 ± 9 | 171 ± 9 | 0.476 |
| Weight (kg) | 70.9 ± 13.1 | 80.3 ± 14.3 | 85.0 ± 17.2 | 90.5 ± 21.0 | <0.001 |
| Body mass index (kg/m2) | 24.2 [22.1–26.2] | 27.5 [24.6–29.9] | 28.1 [24.9–32.6] | 30.0 [26.3–34.1] | <0.001 |
| RDA a (%) | 33 (6) | 54 (10) | 120 (21) | 282 (50) | <0.001 |
| In‐hospital inclusion (%) | 397 (70) | 405 (71) | 400 (70) | 320 (56) | <0.001 |
| Oedema present (%) | 290 (62) | 300 (64) | 280 (57) | 257 (54) | 0.008 |
| Rales (%) | 320 (58) | 307 (56) | 290 (52) | 254 (46) | <0.001 |
| Hepatomegaly (%) | 107 (19) | 86 (15) | 62 (11) | 64 (11) | <0.001 |
| ASAT (U/L) | 26.0 [19.0–38.0] | 25.3 [18.0–35.0] | 25.0 [19.0–32.0] | 25.0 [20.0–35.0] | 0.321 |
| ALAT (U/L) | 25.0 [16.0–41.0] | 24.0 [16.0–34.1] | 24.0 [18.0–41.0] | 27.0 [18.0–41.0] | 0.029 |
| Gamma‐GT (U/L) | 61.0 [30.0–128.0] | 63.0 [29.3–118.8] | 46.0 [26.8–87.3] | 44.0 [25.0–90.0] | <0.001 |
| Alkaline phosphatase (μg/L) | 92.0 [71.0–126.5] | 86.0 [65.0–119.0] | 82.0 [63.0–111.5] | 79.5 [63.0–115.0] | 0.004 |
| eGFR (mL/min/1.73 m2) | 58.6 ± 23.4 | 56.9 ± 22.6 | 61.1 ± 22.5 | 66.9 ± 21.2 | <0.001 |
| Creatinine, serum (μmol/L) | 105.2 [85.8–139.5] | 109.0 [88.0–141.4] | 103.0 [84.0–132.4] | 96.6 [81.0–114.9] | <0.001 |
| Urea, serum (mmol/L) | 12.9 [7.8–21.7] | 12.0 [8.1–18.9] | 11.1 [7.4–17.1] | 9.6 [7.1–15.4] | <0.001 |
| Medical history | |||||
| Hypertension (%) | 325 (57) | 372 (65) | 366 (64) | 371 (65) | 0.011 |
| Myocardial infarction (%) | 225 (40) | 214 (38) | 216 (38) | 211 (37) | 0.842 |
| PCI (%) | 134 (24) | 119 (21) | 136 (24) | 107 (19) | 0.126 |
| CABG (%) | 104 (18) | 110 (19) | 94 (17) | 80 (14) | 0.095 |
| Diabetes mellitus (%) | 163 (29) | 192 (34) | 200 (35) | 200 (35) | 0.064 |
| Stroke (%) | 48 (8) | 68 (12) | 44 (8) | 45 (8) | 0.043 |
| Atrial fibrillation (%) | 253 (44) | 273 (48) | 266 (47) | 230 (40) | 0.059 |
| COPD (%) | 100 (18) | 113 (20) | 108 (19) | 78 (14) | 0.035 |
| Peripheral arterial disease (%) | 62 (11) | 73 (13) | 68 (12) | 49 (9) | 0.128 |
| NYHA class | 0.039 | ||||
| 1 | 62 (11) | 35 (6) | 54 (10) | 53 (9) | |
| 2 | 253 (44) | 273 (48) | 254 (45) | 274 (48) | |
| 3 | 183 (32) | 180 (32) | 159 (28) | 146 (26) | |
| 4 | 16 (3) | 16 (3) | 20 (4) | 19 (3) | |
| Beta‐blocker use (%) | 462 (81) | 471 (83) | 477 (84) | 489 (86) | 0.181 |
| MRA use (%) | 286 (50) | 308 (54) | 308 (54) | 324 (57) | 0.162 |
| Diuretics use (%) | 570 (100) | 571 (100) | 570 (100) | 569 (100) | 0.572 |
| ACE‐I/ARB use (%) | 395 (69) | 395 (69) | 418 (73) | 438 (77) | 0.010 |
| Malnutrition markers | |||||
| Albumin (g/L) | 32.0 ± 9.2 | 31.7 ± 8.7 | 32.1 ± 8.7 | 33.5 ± 7.8 | 0.003 |
| Haemoglobin (g/dL) | 12.9 ± 2.0 | 13.1 ± 1.9 | 13.1 ± 1.8 | 13.7 ± 1.8 | <0.001 |
| Total cholesterol (mmol/L) | 3.80 [3.13–4.70] | 4.00 [3.30–4.85] | 4.11 [3.40–5.01] | 4.41 [3.50–5.30] | <0.001 |
| CRP (mg/L) | 13.0 [5.0–24.8] | 14.2 [6.4–28.0] | 13.0 [5.9–27.5] | 12.2 [5.4–26.2] | 0.089 |
| Outcome | |||||
| All‐cause mortality (%) | 180 (32) | 163 (29) | 139 (24) | 100 (18) | <0.001 |
| Days of follow‐up | 589 [429–826] | 605 [466–806] | 623 [467–823] | 655 [518–823] | |
ACE‐I, angiotensin‐converting enzyme inhibitor; ALAT, alanine transaminase; ARB, angiotensin receptor blocker; ASAT, aspartate transaminase; CABG, coronary artery bypass graft; COPD, chronic obstructive pulmonary disease; CRP, C‐reactive protein; eGFR, estimated glomerular filtration rate; gamma‐GT, gamma‐glutamyltransferase; HFmrEF, heart failure with mid‐range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; RDA, recommended dietary allowance.
Values are given as means ± standard deviation, median (25th to 75th percentiles), or percentage and frequency.
Recommended dietary allowance for protein is a minimum of 0.8 g/kg of body weight.
Patients in the lowest quartile were older, with a mean age of 70 ± 13 years, and had a lower BMI and higher levels of NT‐proBNP (all P < 0.001).
Despite a lower BMI in the lowest quartile, they had significantly more peripheral oedema (62% vs. 54% in the highest quartile, P = 0.008), more rales (58% vs. 46% in the highest quartile, P < 0.001), and more hepatomegaly (19% vs. 11% in the highest quartile, P < 0.001).
Furthermore, serum albumin, haemoglobin, and total cholesterol were significantly lower in the lowest quartile (P = 0.003, P < 0.001, and P < 0.001, respectively), while serum creatinine levels were higher in the lowest quartile (P < 0.001).
The partial correlations with estimated protein intake on a continuous scale adjusted for age are shown in Table 2. Decreased estimated protein intake was associated with a lower BMI (r = 0.443, P < 0.001), higher levels of NT‐proBNP (r = −0.257, P < 0.001), lower haemoglobin levels (r = 0.132, P < 0.001), and higher levels of markers of liver dysfunction such as gamma‐glutamyltransferase and alkaline phosphatase (r = −0.119, P < 0.001 and r = −0.101, P = 0.001, respectively). We also found that a lower estimated protein intake was correlated with more severe signs of congestion, such as hepatomegaly (r = −0.086, P < 0.001) and the presence of rales (r = −0.077, P < 0.001).
Table 2.
Partial correlation with estimated protein intake adjusted for age
| Estimated protein intake (g/day) | ||
|---|---|---|
| Variable | r | P‐value |
| Urinary urea | 0.900 | <0.001 |
| BMI | 0.443 | <0.001 |
| NT‐proBNP | −0.257 | <0.001 |
| Haemoglobin | 0.132 | <0.001 |
| Gamma‐GT | −0.119 | <0.001 |
| Alkaline phosphatase | −0.101 | 0.001 |
| eGFR | 0.099 | <0.001 |
| Total cholesterol | 0.094 | 0.001 |
| Hepatomegaly | −0.086 | <0.001 |
| Rales | −0.077 | <0.001 |
| Albumin | 0.056 | 0.008 |
| Peripheral oedema | −0.044 | 0.067 |
BMI, body mass index; eGFR, estimated glomerular filtration rate; gamma‐GT, gamma‐glutamyltransferase; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide.
Outcome
During a median follow‐up of 21 months, 26% of the patients had died, ranging from 32% in the lowest quartile to 18% in the highest estimated protein intake quartile (P < 0.001).
The main results of the Kaplan–Meier showed that patients in the lowest quartile of estimated protein intake had a significantly higher mortality rate compared with patients who had a higher daily estimated protein intake, log rank P‐value < 0.001 (Figure 1). Similar results were obtained in the validation cohort (Figure S3) where patients with a lower estimated protein intake had the highest mortality rates (log rank P‐value = 0.017). We found no significant interaction between LVEF and estimated protein intake (P = 0.126).
Figure 1.
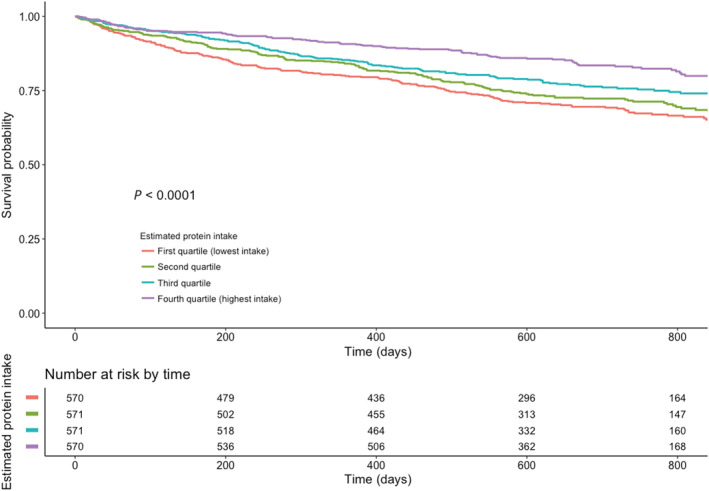
Kaplan–Meier curve for quartiles of estimated protein intake per day.
The adjusted HR for all‐cause mortality on a continuous scale for estimated protein intake is shown in Figure 2. For mortality, a higher estimated protein intake was associated with significantly lower mortality risks. When assessing this in a multivariable Cox model for estimated protein intake on a continuous level per log decrease, we found an HR 1.97, 95% CI 1.01–3.84, P = 0.048 (Table 3). For the comparison between quartiles, we used the highest estimated protein intake quartile as a reference category.
Figure 2.
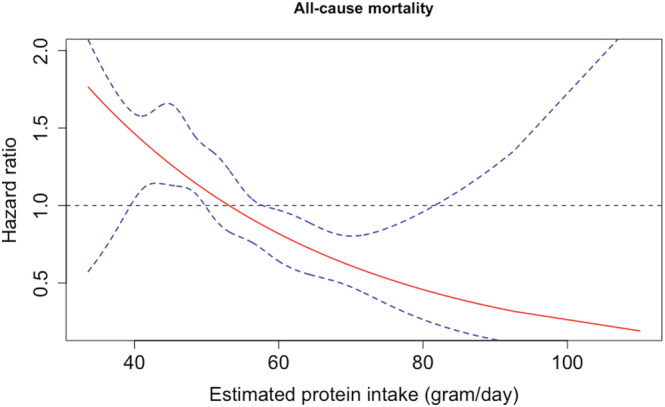
Adjusted effect of estimated protein intake on all‐cause mortality. Solid line shows the estimated linear relation, while the dotted lines represent the 95% confidence intervals using restricted cubic splines.
Table 3.
Cox regression model
|
All‐cause mortality Estimated protein intake (g/day) |
HR [95% CI] | P‐value | HR a [95% CI] | P‐value | HR b [95% CI] | P‐value |
|---|---|---|---|---|---|---|
| Protein intake (g/day) per log decrease | 3.86 [2.54–5.89] | <0.001 | 2.96 [1.92–4.57] | <0.001 | 1.97 [1.01–3.84] | 0.048 |
|
All‐cause mortality Estimated protein intake (g/day) |
HR [95% CI] | P‐value | HR a [95% CI] | P‐value | HR b [95% CI] | P‐value |
|---|---|---|---|---|---|---|
| 4th quartile (highest) | Ref | Ref | Ref | |||
| 3rd quartile | 1.45 [1.12–1.88] | 0.004 | 1.35 [1.04–1.75] | 0.023 | 1.03 [0.68–1.56] | 0.881 |
| 2nd quartile | 1.75 [1.36–2.24] | <0.001 | 1.55 [1.20–1.99] | 0.001 | 1.46 [1.00–2.18] | 0.049 |
| 1st quartile (lowest) | 1.99 [1.56–2.54] | <0.001 | 1.75 [1.37–2.24] | <0.001 | 1.50 [1.03–2.18] | 0.036 |
CI, confidence interval; HR, hazard ratio.
Corrected for age.
Corrected for age, haemoglobin, log N‐terminal pro‐brain natriuretic peptide, estimated glomerular filtration rate, New York Heart Association class, history of diabetes, in‐hospital inclusion, use of beta‐blocker, and use of angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker.
In a univariable model, all quartiles differed significantly compared with the highest quartile (HR 1.45; 95% CI 1.12–1.88, P = 0.004 for the third quartile; for the second quartile, HR 1.75; 95% CI 1.36–2.24, P < 0.001; and for the quartile with the lowest estimated protein intake, HR 1.99; 95% CI 1.56–2.54, P < 0.001). In the multivariable adjusted model, these HRs remained significantly higher for the second quartile and the quartile with the lowest estimated protein intake compared with patients in the highest quartile (HR 1.46; 95% CI 1.00–2.18, P = 0.049 and HR 1.50; 95% CI 1.03–2.18, P = 0.036, respectively).
The findings were validated in the validation cohort. There was substantial overlap in patient characteristics, and the findings observed in the validation cohort were fairly consistent with the findings in the index cohort (Tables S1 and S2 and Figures S3 and S4).
Discussion
The main findings of the present study were that in a large HF cohort, we showed that a lower estimated protein intake in patients with HF was associated with a lower BMI and more signs of congestion, and a lower estimated protein intake was independently strongly associated with an increased mortality risk. These findings were validated and confirmed in an independently selected cohort.
Although intuitively this may seem logical, this had not been shown before in an HF population.
Malnutrition
A common finding in chronic HF patients is malnutrition or cachexia, with up to 50% of the patients being malnourished, and is often associated with worse outcome. 3 Because HF is often accompanied by an inflammatory component, the term cachexia is more often used; however, these are often interchangeable. 15 This might eventually evolve into cardiac cachexia, which is associated with an extremely poor prognosis and is typically accompanied by muscle wasting. 16 While serum creatinine is a good measurement for muscle wasting, where low levels are associated with more muscle wasting, we found in our cohort higher levels in patients in the lowest quartile of protein intake. Therefore, it is less likely that muscle wasting plays a role in our cohort.
In the absence of food intake questionnaires, malnourishment can be assessed by studying biomarkers. One of the most studied biomarkers in malnutrition is serum albumin, where lower levels are found in malnourished patients. Other biomarkers that are associated with malnourished patients are lower haemoglobin levels and total cholesterol. 17 , 18 Consistent with these findings, we found lower total cholesterol, lower haemoglobin levels, and lower serum albumin levels in patients with lower protein intake, suggesting a more malnourished state in patients in the lowest quartile of protein intake. Consistent with these findings, we found that 75% of the HF patients in our cohort did not meet the RDA of 0.8 g/kg of body weight and actually had less protein intake than the minimum daily recommended intake.
An important finding of our study was that HF patients with a lower estimated protein intake showed more signs of fluid overload such as peripheral oedema, rales, and more hepatomegaly. Despite more congestion, their BMI was lower. These findings imply that, although these patients had a lower BMI, they actually had more severe signs of HF. Besides hepatomegaly, a lower estimated protein intake was also associated with higher levels of markers of liver dysfunction, possibly related to more venous congestion and leading to gastrointestinal congestion. Splanchnic veins are highly compliant and therefore act often as a venous reservoir, because in HF the body is often in a state of neurohormonal activation. 19 The neurohormonal activation on itself triggers sodium and fluid retention, which causes among others intestinal congestion. This is often accompanied by a variety of symptoms such as nausea, abdominal bloating/complaints, and weight loss. 20 One of the reasons for the lower estimated protein intake in our group could possibly be due to one of these digestive disorders and/or gastrointestinal symptoms and therefore losing appetite, because these patients had more severe signs of HF. Another factor might be that due to intestinal congestion, there is an increased permeability and altered absorption of essential nutrients in the intestines. 21 One of these essential nutrients is protein, found in a variety of foods such as red meats, milk, cheese, fish, nuts, and egg. Dietary protein is essential for forming all amino acids, because humans are unable to form all amino acids themselves and need dietary protein. 22 Furthermore, proteins are essential for building up muscle mass, whereas in the elderly, muscle mass is lost due to aging and chronic illnesses such as HF. 23 , 24 Due to the fact that elderly HF patients may have a higher protein need due to anabolic resistance and a lower muscle perfusion, one can even hypothesize that HF patients need a higher amount of proteins to maintain muscle mass.
Protein intake and outcome
Prior studies have assessed the association of protein intake and the benefit on quality of life; however, data on protein intake and mortality are scarce. 25 , 26 A previous randomized double‐blind pilot study has shown benefit on quality of life and tumour necrosis factor alpha levels after 18 weeks with a high caloric–high protein diet in 29 HF patients. 25 This study demonstrated the possible use of proteins as an intervention for improving quality of life; however, the aim was to increase caloric intake in cachectic HF patients. A previous observational study has shown that both plant and animal proteins could provide a substantial health benefit in the general population, and in a recently published large epidemiological cohort, protein intake was inversely associated with mortality risk and non‐cardiovascular disease mortality. 27 , 28 However, the vast majority of the studies performed with protein intake were observational and conducted in the general population. Data in the HF population are lacking. Loss in body weight is known to be associated with mortality in HF patients, and because proteins might help maintain muscle mass in these patients, a high protein diet might be beneficial; however, this warrants further research. Although this study shows a strong association between protein intake and mortality, a causal relationship could not be established by the present study, and thus, further research by conducting a randomized controlled trial is warranted. We found a strong association between lower protein intake and mortality. For both of our multivariable models, we corrected for inpatient or outpatient inclusion, because in‐hospital patients might benefit from regular nutrient meals. These patients could benefit by building up higher muscle mass and therefore create a larger reserve, as seen in lower mortality rates by treating inpatients with higher protein diets. 29 , 30
Study limitations
Firstly, protein intake was estimated using a formula, and not directly measured. Secondly, we used spot urine samples instead of 24 h urine samples and therefore used an altered formula to calculate protein intake. Although a good correlation between the two formulae was found, the altered formula tended to underestimate protein intake at higher levels. Therefore, we used the lower ranges as a reference category, and despite the underestimation in the higher regions, HRs between the groups were still significant. Thirdly, food intake questionnaires were not performed and could therefore not be assessed.
Conclusions
A lower estimated protein intake in HF patients is independently associated with higher mortality risks. Although these findings suggest that HF patients could possibly benefit from a high protein diet, conclusive evidence of the potential benefit of a high protein diet in patients with HF by prospective controlled clinical studies is needed.
Conflict of interests
M.M. has received consulting honoraria from Amgen, Astra Zeneca, Novartis, Relypsa, and Servier and speaker's fees from Abbott Vascular and Servier. S.D.A. reports receiving fees from Abbott, Actimed, Bayer, Boehringer Ingelheim, Cardiac Dimension, Cordio, Impulse Dynamics, Novartis, Occlutech, Servier, and Vifor Pharma, and grant support from Abbott and Vifor Pharma. C.C.L. received consultancy fees and/or research grants from Amgen, Astra Zeneca, MSD, Novartis, Boehringher Ingelhiem, Vifor Pharma and Servier. A.A.V. reports consultancy fees and/or research grants from Amgen, Bayer, Boehringer Ingelheim, Merck/MSD, Novartis, Roche Diagnostics, Servier, Trevena, and Vifor. All other authors declare no conflict of interest.
The study complied with the Declaration of Helsinki, local ethics committee has approved the research protocol, and all patients signed informed consent. The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. 31
Funding
This work was supported by the Netherlands Cardiovascular Research Initiative: an initiative with support of the Dutch Heart Foundation (CVON2014‐11 RECONNECT) and a grant from the European Commission (FP7‐242209‐BIOSTAT‐CHF).
Supporting information
Table S1. Baseline characteristics validation cohort
Table S2. Cox‐regression model Validation cohort
Figure S1. Estimated protein intake in 24‐hour urine according to the Maroni formula (Y‐axis) plotted against adjusted Maroni formula (X‐axis) when used to estimate protein intake in the 24‐hour urine in ARIANA‐CHF‐RD. Pearson's correlation coefficient = 0.70
Figure S2. Bland–Altman plot of estimating protein intake using Maroni formula with 24 hour urine and using adjusted Maroni formula
Figure S3. Kaplan–Meier curve for quartiles of estimated protein intake per day ‐ Validation cohort
Figure S4. Kaplan–Meier curve for quartiles of estimated protein intake per day using urine urea nitrogen/urine creatinine ratio in the index cohort
Streng K. W., Hillege H. L., ter Maaten J. M., van Veldhuisen D. J., Dickstein K., Ng L. L., Samani N. J., Metra M., Ponikowski P., Cleland J. G., Anker S. D., Romaine S. P. R., Damman K., van der Meer P., Lang C. C., and Voors A. A. (2022) Clinical implications of low estimated protein intake in patients with heart failure, Journal of Cachexia, Sarcopenia and Muscle, 13, 1762–1770, 10.1002/jcsm.12973
References
- 1. Yoshihisa A, Kanno Y, Watanabe S, Yokokawa T, Abe S, Miyata M, et al. Impact of nutritional indices on mortality in patients with heart failure. Open Heart 2018;5:e000730. 10.1136/openhrt-2017-000730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Denfeld QE, Winters‐Stone K, Mudd JO, Gelow JM, Kurdi S, Lee CS. The prevalence of frailty in heart failure: a systematic review and meta‐analysis. Int J Cardiol 2017;236:283–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sze S, Pellicori P, Kazmi S, Rigby A, Cleland JGF, Wong K, et al. Prevalence and prognostic significance of malnutrition using 3 scoring systems among outpatients with heart failure: a comparison with body mass index, Vol. 6. JACC Heart Fail; 2018. p 476–486. [DOI] [PubMed] [Google Scholar]
- 4. Joint WHO/FAO/UNU Expert Consultation . Protein and amino acid requirements in human nutrition. World Health Organ Tech Rep Ser 2007;935:1–265, back cover. [PubMed] [Google Scholar]
- 5. Deutz NE, Bauer JM, Barazzoni R, Biolo G, Boirie Y, Bosy‐Westphal A, et al. Protein intake and exercise for optimal muscle function with aging: recommendations from the ESPEN Expert Group. Clin Nutr 2014;33:929–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
- 7. Voors AA, Anker SD, Cleland JG, Dickstein K, Filippatos G, van der Harst P, et al. A systems BIOlogy Study to TAilored Treatment in Chronic Heart Failure: rationale, design, and baseline characteristics of BIOSTAT‐CHF. Eur J Heart Fail 2016;18:716–726. [DOI] [PubMed] [Google Scholar]
- 8. Ouwerkerk W, Voors AA, Anker SD, Cleland JG, Dickstein K, Filippatos G, et al. Determinants and clinical outcome of uptitration of ACE‐inhibitors and beta‐blockers in patients with heart failure: a prospective European study. Eur Heart J 2017;38:1883–1890. [DOI] [PubMed] [Google Scholar]
- 9. Ferreira JP, Rossignol P, Machu JL, Sharma A, Girerd N, Anker SD, et al. Mineralocorticoid receptor antagonist pattern of use in heart failure with reduced ejection fraction: findings from BIOSTAT‐CHF. Eur J Heart Fail 2017;19:1284–1293. [DOI] [PubMed] [Google Scholar]
- 10. Ouwerkerk W, Zwinderman AH, Ng LL, Demissei B, Hillege HL, Zannad F, et al. Biomarker‐guided versus guideline‐based treatment of patients with heart failure: results from BIOSTAT‐CHF. J Am Coll Cardiol 2018;71:386–398. [DOI] [PubMed] [Google Scholar]
- 11. Voors AA, Ouwerkerk W, Zannad F, van Veldhuisen DJ, Samani NJ, Ponikowski P, et al. Development and validation of multivariable models to predict mortality and hospitalization in patients with heart failure. Eur J Heart Fail 2017;19:627–634. [DOI] [PubMed] [Google Scholar]
- 12. Kanno H, Kanda E, Sato A, Sakamoto K, Kanno Y. Estimation of daily protein intake based on spot urine urea nitrogen concentration in chronic kidney disease patients. Clin Exp Nephrol 2016;20:258–264. [DOI] [PubMed] [Google Scholar]
- 13. Maroni BJ, Steinman TI, Mitch WE. A method for estimating nitrogen intake of patients with chronic renal failure. Kidney Int 1985;27:58–65. [DOI] [PubMed] [Google Scholar]
- 14. Schroten NF, Damman K, Hemmelder MH, Voors AA, Navis G, Gaillard CA, et al. Effect of additive renin inhibition with aliskiren on renal blood flow in patients with Chronic Heart Failure and Renal Dysfunction (Additive Renin Inhibition with Aliskiren on renal blood flow and Neurohormonal Activation in patients with Chronic Heart Failure and Renal Dysfunction). Am Heart J 2015;169:693–701.e3. [DOI] [PubMed] [Google Scholar]
- 15. von Haehling S, Ebner N, Dos Santos MR, Springer J, Anker SD. Muscle wasting and cachexia in heart failure: mechanisms and therapies. Nat Rev Cardiol 2017;14:323–341. [DOI] [PubMed] [Google Scholar]
- 16. Springer J, Springer JI, Anker SD. Muscle wasting and sarcopenia in heart failure and beyond: update 2017. ESC Heart Fail 2017;4:492–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang Z, Pereira SL, Luo M, Matheson EM. Evaluation of blood biomarkers associated with risk of malnutrition in older adults: a systematic review and meta‐analysis. Nutrients 2017;9. 10.3390/nu9080829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Amare H, Hamza L, Asefa H. Malnutrition and associated factors among heart failure patients on follow up at Jimma University specialized hospital, Ethiopia. BMC Cardiovasc Disord 2015;15:128–015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fudim M, Hernandez AF, Felker GM. Role of volume redistribution in the congestion of heart failure. J Am Heart Assoc 2017;6:e006817. 10.1161/JAHA.117.006817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Valentova M, von Haehling S, Bauditz J, Doehner W, Ebner N, Bekfani T, et al. Intestinal congestion and right ventricular dysfunction: a link with appetite loss, inflammation, and cachexia in chronic heart failure. Eur Heart J 2016;37:1684–1691. [DOI] [PubMed] [Google Scholar]
- 21. Sundaram V, Fang JC. Gastrointestinal and liver issues in heart failure. Circulation 2016;133:1696–1703. [DOI] [PubMed] [Google Scholar]
- 22. Bihuniak JD, Insogna KL. The effects of dietary protein and amino acids on skeletal metabolism. Mol Cell Endocrinol 2015;410:78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tieland M, Borgonjen‐Van den Berg KJ, van Loon LJ, de Groot LC. Dietary protein intake in community‐dwelling, frail, and institutionalized elderly people: scope for improvement. Eur J Nutr 2012;51:173–179. [DOI] [PubMed] [Google Scholar]
- 24. Bauer J, Biolo G, Cederholm T, Cesari M, Cruz‐Jentoft AJ, Morley JE, et al. Evidence‐based recommendations for optimal dietary protein intake in older people: a position paper from the PROT‐AGE Study Group. J Am Med Dir Assoc 2013;14:542–559. [DOI] [PubMed] [Google Scholar]
- 25. Rozentryt P, von Haehling S, Lainscak M, Nowak JU, Kalantar‐Zadeh K, Polonski L, et al. The effects of a high‐caloric protein‐rich oral nutritional supplement in patients with chronic heart failure and cachexia on quality of life, body composition, and inflammation markers: a randomized, double‐blind pilot study. J Cachexia Sarcopenia Muscle 2010;1:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Evangelista LS, Heber D, Li Z, Bowerman S, Hamilton MA, Fonarow GC. Reduced body weight and adiposity with a high‐protein diet improves functional status, lipid profiles, glycemic control, and quality of life in patients with heart failure: a feasibility study. J Cardiovasc Nurs 2009;24:207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dehghan M, Mente A, Zhang X, Swaminathan S, Li W, Mohan V, et al. Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): a prospective cohort study. Lancet 2017;390:2050–2062. [DOI] [PubMed] [Google Scholar]
- 28. Song M, Fung TT, Hu FB, Willett WC, Longo VD, Chan AT, et al. Association of animal and plant protein intake with all‐cause and cause‐specific mortality. JAMA Intern Med 2016;176:1453–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Deutz NE, Matheson EM, Matarese LE, Luo M, Baggs GE, Nelson JL, et al. Readmission and mortality in malnourished, older, hospitalized adults treated with a specialized oral nutritional supplement: a randomized clinical trial. Clin Nutr 2016;35:18–26. [DOI] [PubMed] [Google Scholar]
- 30. Landi F, Calvani R, Tosato M, Martone AM, Ortolani E, Savera G, et al. Protein intake and muscle health in old age: from biological plausibility to clinical evidence. Nutrients 2016;8:295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2021. J Cachexia Sarcopenia Muscle 2021;12:2259–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline characteristics validation cohort
Table S2. Cox‐regression model Validation cohort
Figure S1. Estimated protein intake in 24‐hour urine according to the Maroni formula (Y‐axis) plotted against adjusted Maroni formula (X‐axis) when used to estimate protein intake in the 24‐hour urine in ARIANA‐CHF‐RD. Pearson's correlation coefficient = 0.70
Figure S2. Bland–Altman plot of estimating protein intake using Maroni formula with 24 hour urine and using adjusted Maroni formula
Figure S3. Kaplan–Meier curve for quartiles of estimated protein intake per day ‐ Validation cohort
Figure S4. Kaplan–Meier curve for quartiles of estimated protein intake per day using urine urea nitrogen/urine creatinine ratio in the index cohort


