Abstract
Background
Chemotherapy‐induced toxicities frequently occur in non‐small cell lung cancer (NSCLC) patients treated with platinum‐based chemotherapy. Low skeletal muscle mass (SMM) has been associated with a higher incidence of toxicities for several types of cancers and cytostatics. The aim of this study was to evaluate the association between skeletal muscle measures and chemotherapy‐induced toxicity in a large cohort of NSCLC patients.
Methods
A multicentre prospective follow‐up study (PGxLUNG, NTR number NL5373610015) in NSCLC patients was conducted. Included were patients diagnosed with NSCLC (stage II–IV) treated with first‐line platinum‐based (cisplatin or carboplatin) chemotherapy of whom pretreatment imaging was available. Skeletal muscle area (SMA) segmentation was performed on abdominal imaging at the level of the third lumbar vertebra (L3). SMA at the level of L3 was corrected for squared height (m2) to yield the lumbar skeletal muscle mass index (LSMI). Skeletal muscle density (SMD) was calculated as the mean Hounsfield Unit (HU) of the segmented SMA. SMM and SMD were categorized as low, intermediate, and high, based on LSMI and mean HU tertiles, respectively. Chemotherapy‐induced toxicity was scored using CTCAE v4.03 and categorized into haematological (anaemia, leukocytopenia, neutropenia, and thrombocytopenia), non‐haematological (nephrotoxicity, neurotoxicity, and esophagitis), and dose‐limiting toxicity (DLT) (treatment switch, delay, de‐escalation, discontinuation, or hospitalization). The relationship between SMM, SMD, and toxicities was assessed with logistic regression modelling taking into account potential confounders like gender and body mass index (BMI).
Results
In total, 297 patients (male n = 167, median age 64 years) were included. Haematological toxicity grade 3/4 was experienced in 36.6% (n = 108) of the patients, 24.6% (n = 73) experienced any non‐haematological toxicity grade ≥2, and 55.6% (n = 165) any DLT. Multivariate logistic regression analysis showed that low SMM (ORadj 2.41, 95% CI 1.31–4.45, P = 0.005) and age at diagnosis >65 years (ORadj 1.76, 95% CI 1.07–2.90, P = 0.025) were statistically significantly associated with overall haematological toxicity grade 3/4. No statistically significant associations were found between low SMM or low SMD and non‐haematological toxicities. Low SMM (ORadj 2.23, 95% CI 1.23–4.04, P = 0.008) and high SMD (ORadj 0.41, 95% CI 0.23–0.74, P = 0.003) were statistically significantly associated with a higher respectively lower risk of DLT.
Conclusions
Non‐small cell lung cancer patients with pretreatment low SMM are at significant higher risk for haematological toxicities grade 3/4 and DLT. NSCLC patients with high SMD are at significant lower risk for DLT. Further studies should be aimed to investigate whether platinum dosing based on skeletal muscle measurements and/or improvement of pretreatment SMM/SMD could reduce the risk of toxicity without compromising efficacy.
Keywords: Non‐small cell lung cancer, Body composition, Skeletal muscle mass, Platinum‐based chemotherapy, Chemotherapy‐induced toxicity
Introduction
Lung cancer is worldwide the leading cause of cancer‐related deaths. 1 Although immune therapy changed the therapeutic landscape, platinum‐based chemotherapy (including cisplatin or carboplatin) is still considered as the standard first‐line therapy for the vast majority of patients. Nevertheless, the degree and impact of the efficacy and toxicity of chemotherapy differ remarkably among patients. 2 Although platinum‐based therapy can be effective in treating lung cancer, chemotherapy‐induced toxicity is common and can lead to treatment discontinuation or hospitalization. In addition, dose‐limiting toxicity (DLT) may influence disease progression because patients receive suboptimal treatment (i.e. in terms of therapeutic regimen, timing, and dose), which may negatively impact both prognosis and quality of life.
Over the past years, a relationship has been observed between low skeletal muscle mass (SMM) and poor treatment outcomes in lung cancer. 3 , 4 , 5 , 6 , 7 Besides, several studies in different types of cancers have shown that low SMM leads to significant risk for chemotherapy‐related toxicities and DLTs. 8 , 9 , 10 , 11 , 12 An explanation for the relationship between low SMM and toxicity might be altered pharmacokinetics because hydrophilic drugs, such as platinum agents, mainly distribute in the lean body mass (LBM) of which SMM is the largest contributor. 13 Consequently, it can be hypothesized that patients with low SMM will have higher blood levels of chemotherapeutic agents, resulting in an increased risk of chemotherapy‐induced toxicity. In addition, pretreatment low SMM was demonstrated to be independently associated with frailty in multiple studies in patients with head and neck cancer. 14 , 15 Given the potential association between SMM and the occurrence of chemotherapy‐induced toxicities, 12 information about SMM values of individual patients can possibly help physicians identify patients at risk for poor treatment tolerability. 16 For lung cancer patients, recently, Halvorson et al. performed a study in patients with limited small cell lung cancer (SCLC) and found that patients who received a high dose of cisplatin per kilogram LBM had more often haematological toxicity and neutropenic infections. 17 In a study performed by Srdic et al. in non‐small cell lung cancer (NSCLC) patients treated with platinum‐based chemotherapy, no association was found between skeletal muscle measurements and chemotherapy‐induced toxicity. 18 However, only 55 patients met the inclusion criteria for muscle mass measurements. This low number of included patients may have contributed to the fact that in this study no association was found between skeletal muscle measurements and chemotherapy‐induced toxicity. Therefore, the present study aimed to evaluate the association between SMM, SMD and chemotherapy‐induced toxicity in a multicentre prospective follow‐up study of a large cohort of NSCLC patients receiving first‐line platinum‐based chemotherapy.
Materials and methods
Setting, study design, and study population
This study was performed as part of the PGxLUNG study, in which 350 patients were included. The study design of the PGxLUNG study has been published previously. 19 In brief, patients of the PGxLUNG study were recruited from an academic hospital (University Medical Center Utrecht), two teaching hospitals (St. Antonius Hospital Nieuwegein/Utrecht and Meander Medical Center Amersfoort), and three general hospitals (Diakonessenhuis Utrecht/Zeist, Groene Hart Ziekenhuis Gouda, and Ziekenhuis Rivierenland Tiel), all in the Netherlands, between February 2016 and August 2019. The inclusion criteria for this multicentre prospective follow‐up study were as follows: (i) ≥18 years of age, (ii) radiologically confirmed stage II‐IV NSCLC, (iii) planned or initiated first‐line treatment with platinum‐based (cisplatin or carboplatin) chemotherapy or chemoradiotherapy (according to the contemporary ESMO Clinical Practice Guidelines), and (iv) previously platinum‐based chemotherapy‐naïve. Patients of the PGxLUNG cohort of whom a pretreatment abdominal imaging was available were included for the present study.
Ethical considerations
The study was approved by the accredited Medical Research Ethics Committee in Nieuwegein (MEC‐U, number R15.056), and the study procedures were implemented in accordance with the Declaration of Helsinki (64th WMA General Assembly, Fortaleza, Brazil, October 2013). The PGxLUNG study was registered on the Netherlands National Trial Register (NTR) on 26 April 2016 (NTR number NL5373610015). All patients provided written informed consent.
Image analysis and anthropometric measurements
The skeletal muscle area at the level of the third lumbar vertebra (L3) has shown excellent correlation with whole body skeletal muscle mass as measured with abdominal imaging (considered as the golden standard). 20 Segmentation of SMM was manually performed using Slice‐o‐matic version 5.0 (Tomovision, Canada), using a muscle‐specific Hounsfield Unit (HU) range between −29 and +150. SMM was measured on pretreatment abdominal computed tomography (CT) imaging [as part of whole body positron emission tomography‐CT imaging], which were routinely acquired for diagnostic workup. At the level of L3 on a single axial‐slice, the area of the psoas, erector spinae, quadratus lumborum, transversus abdominis, external and internal obliques, and rectus abdominis muscles were segmented, and this yielded the total skeletal muscle area (SMA) (Figure 1). SMA was divided by squared height (m2) to obtain the lumbar skeletal muscle mass index (LSMI). The mean HU of the segmented SMA was retrieved and represents the skeletal muscle density (SMD) as surrogate measure of muscle quality. 21 Because contrast may influence the mean HU (higher HU), SMD was not calculated for patients who received pretreatment contrast enhanced CT. All scans were assessed by one trained individual (N. C.).
Figure 1.
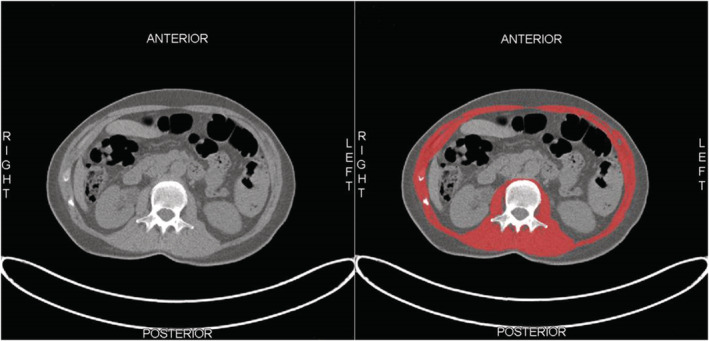
Example of segmentation of skeletal muscle tissue at the level of the third lumbar vertebra (L3). (A) Unsegmented skeletal muscle tissue. (B) Segmented skeletal muscle tissue (red).
Chemotherapy‐induced toxicity
Registration of chemotherapy‐induced toxicities [using the Common Terminology Criteria for Adverse Events (CTCAE, version 4.03) or predefined definitions] took place throughout all cycles of platinum‐based chemotherapy, and at 3, 6, and 12 months after treatment initiation. Endpoints were chemotherapy‐induced toxicities, defined as haematological, non‐haematological, and/or dose‐limiting toxicities. Haematological toxicities, including anaemia (haemoglobin <7.0 mmol), leukocytopenia (leukocytes <4.0·109/L), neutropenia (neutrophils <1.6·109/L), and thrombocytopenia (thrombocytes <150·109/L), were assessed by recording the nadir blood counts. Blood counts were performed at prespecified timepoints; prior to each cycle and at 3, 6, and 12 months after treatment initiation. Additional counts between follow‐up moments were performed at the discretion of the treating physician. Blood counts were scored according to the CTCAE version 4.03. Haematological toxicities CTCAE grade 3/4 were considered as severe toxicities. Non‐haematological toxicities comprised nephrotoxicity, neurotoxicity, and esophagitis, assessed by lung oncologists using CTCAE version 4.03. Non‐haematological toxicities CTCAE grade 2 or higher were considered as severe toxicities. DLT was defined as ‘switching treatment’ (cisplatin to carboplatin), ‘treatment delay’ (≥7 days from initially planned), ‘treatment de‐escalation’ (dose reduction ≥25% of platinum agent), early treatment termination, and hospitalization ≥1 day, all due to chemotherapy‐induced side effects.
Registration of chemotherapy‐induced toxicities and blood counts took place throughout all cycles of platinum‐based chemotherapy. The follow‐up period of haematological toxicities and DLT was 3 months after chemotherapy initiation, as these toxicities are expected to occur soon after and are related to chemotherapy administration. For non‐haematological toxicities and treatment‐related hospitalization, the follow‐up period was 12 months after chemotherapy initiation, as these toxicities may also occur after a longer period of time after end of treatment.
Potential confounders and/or effect modifiers
The following parameters were considered to be potentially confounding and/or effect modifying variables for chemotherapy‐induced toxicities: gender, age (≤65 years vs. >65 years), weight, body surface area (BSA) (Dubois method), 22 co‐morbidities (Charlson co‐morbidity index score, 23 2–3 vs. 4–5 vs. ≥6), Eastern Cooperative Oncology Group (ECOG) performance status 24 (ECOG PS 0–1 vs. ≥2), absolute dose of platinum agent [carboplatin (mg), cisplatin (mg/BSA)], renal function (eGFR using CKD‐EPI formula, 25 <60 mL/min/1.73 m2 vs. ≥60 mL/min/1.73 m2), serum albumin level (<37.5 g/L vs. ≥37.5 g/L), 18 and body mass index (BMI) (<18.5 kg/m2 vs. 18.5 to <25 kg/m2 vs. 25 to <30 kg/m2 vs. ≥30 kg/m2). 26
Data analysis
All data were extracted from the hospitals' electronic information system which contain patients' medical records and managed using REDCap electronic data capture tools. 27
Standard summary statistics were used to describe the sample data set by using SPSS version 26.0 (IBM SPPS Statistics) and visualized using GraphPad Prism version 8.3. The strength of the association between skeletal muscle measures (SMM and SMD) and chemotherapy‐induced toxicity was assessed in univariate and multivariate setting with logistic regression modelling and expressed as odds ratios (OR) with corresponding 95% confidence intervals (95% CI). Covariates used in the multivariate analysis were those aforementioned potential confounders and/or effect modifiers with statistical significance (P‐value <0.10) in univariate logistic regression analysis or with clinical significance based on previous studies. In the multivariate analysis, a P‐value <0.05 (two‐sided) was considered statistically significant. Because cut‐off values for skeletal muscle measures are lacking, patients were stratified into three equal groups by SMM and SMD status. Patients were categorized into low SMM, intermediate SMM, and high SMM for the first, second, and third tertiles of LSMI, respectively. For SMD, patients were categorized into low SMD, intermediate SMD, and high SMD for the first, second, and third tertiles of the mean HU, respectively. Sarcopenic obesity was defined as the presence of both low SMM and obesity (≥30 kg/m2).
Results
Population characteristics
In total, 297 patients of the PGxLUNG cohort (n = 350) with previously untreated NSCLC, receiving at least one cycle of platinum‐based chemotherapy between April 2011 and July 2019, were included. Data on SMM/SMD were not available for 51 patients (pretreatment abdominal imaging not available), and two patients died before the first clinical evaluation. In addition, 13 patients underwent contrast‐enhanced pretreatment imaging; consequently, HU values of these patients could not be used to quantify muscle quality (SMD). The clinical characteristics of the patients are summarized in Table 1. Median time between the pretreatment imaging and start of the first cycle of platinum‐based chemotherapy was 41 days (IQR 27–69). The median number of cycles of platinum‐based chemotherapy was three (IQR 2–4). Median sum (IQR) of cisplatin dose/BSA for low, intermediate, and high SMM was 225 mg/m2 (80–276), 225 mg/m2 (152–279), and 223 mg/m2 (154–279); median sum (IQR) of cisplatin dose/BSA for low, intermediate, and high SMD was 208 mg/m2 (149–277), 219 mg/m2 (94–278), and 226 mg/m2 (171–292), respectively. Median sum (IQR) of absolute carboplatin dose for low, intermediate, and high SMM was 1650 mg (1082–2190), 1868 mg (1025–2410), and 1850 mg (1460–2410), for low, intermediate, and high SMD it was 1738 mg (1086–2250), 1750 mg (1120–2210), and 2063 mg (1450–2600), respectively.
Table 1.
Demographic and clinical characteristics (n = 297)
| Characteristics | N (%) |
|---|---|
| Male, n (%) | 167 (56.2) |
| Age at diagnosis in years, mean ± SD | 64.3 ± 9.5 |
| >65 years, n (%) | 155 (52.2) |
| Charlson co‐morbidity index # , n (%) | |
| 2–3 | 100 (33.7) |
| 4–5 | 98 (33.0) |
| ≥ 6 | 99 (33.3) |
| Performance status, n (%) | |
| ECOG 0 | 115 (38.7) |
| ECOG 1 | 133 (44.8) |
| ECOG ≥2 | 8 (2.7) |
| Unknown | 41 (13.8) |
| Disease stage, n (%) | |
| IIA | 6 (2.0) |
| IIB | 27 (9.1) |
| IIIA | 58 (19.5) |
| IIIB | 72 (24.2) |
| IV | 134 (45.1) |
| Tumour histology, n (%) | |
| Squamous | 72 (24.2) |
| Non‐squamous | 186 (62.6) |
| Large cell | 6 (2.0) |
| Combined or unspecified | 33 (11.1) |
| Smoking status, n (%) | |
| Never | 14 (4.7) |
| Current/former | 270 (90.9) |
| Unknown | 13 (4.4) |
| Treatment intention, n (%) | |
| Curative/adjuvant | 152 (51.2) |
| Palliative | 145 (48.8) |
| Radiotherapy (RT) regimen, n (%) | |
| No thoracic RT | 162 (54.5) |
| Concurrent thoracic RT | 50 (16.8) |
| Sequential thoracic RT | 85 (28.7) |
| Carboplatin‐based chemotherapy, n (%) | 205 (69.0) |
| Number of cycles, median (IQR) | 3 (2–4) |
| Cumulative dose (mg), median (IQR) | 1780 (1125–2280) |
| Cisplatin‐based chemotherapy, n (%) | 133 (44.8) |
| Number of cycles, median (IQR) | 3 (2–4) |
| Cumulative dose (mg/m2), median (IQR) | 225 (150–277) |
| Renal function, eGFR, CKD‐EPI | |
| Median mL/min/1.73 m2, (IQR) | 89 (76–90) |
| <60 mL/min/1.73 m2, n (%) | 24 (8.1) |
| Serum albumin (g/L), median (IQR) | 40.1 (36.7–42.5) |
| <37.5 (g/L), n (%) | 81 (27.3) |
| ≥37.5 (g/L), n (%) | 190 (64.0) |
| Unknown | 26 (8.7) |
Charlson co‐morbidity index score provides a simple means to quantify the effect of co‐morbid illnesses, including cardiovascular diseases, chronic obstructive pulmonary disease, liver disease, and diabetes mellitus, among others and accounts for the aggregate effect if multiple concurrent diseases. A higher score indicates more co‐morbidities.
ECOG, Eastern Cooperative Oncology Group; eGFR, estimated glomerular filtration rate; IQR, interquartile range; RT, radiotherapy; SD, standard deviation.
Image analysis and anthropometric measurements
Mean weight was 77 kg (IQR 65–88 kg). The majority of patients had normal weight (40.1%) or overweight (39.1%), as indicated by a body mass index (BMI) of 18.5 to <25 kg/m2 and 25 to <30 kg/m2, respectively. A median BSA of 1.91 m2 (IQR 1.75–2.05 m2) was found. In total, nine patients (3%) suffered from sarcopenic obesity. Female patients were overrepresented in the low SMM group [73 women (73.7%) vs. 26 men (26.3%), P < 0.001].
Skeletal muscle mass/skeletal muscle density status and chemotherapy‐induced toxicity
In the Supporting Information, Table S1, chemotherapy‐induced toxicities stratified by the SMM status (Table S1A) and SMD status (Table S1B) are shown. Haematological toxicities during platinum‐based chemotherapy were very common, as 90.2% of the patients developed any kind of haematological toxicity grade ≥1. As shown in Figure 2A, overall haematological toxicity grade 3/4 occurred significantly more often in patients with low SMM (48.5%) compared with patients with intermediate (28.6%) or high SMM (32.7%). Besides, patients with low SMM had a statistically significant lower Hb nadir (5.7 mmol/L, IQR 5.2–6.5) compared with patients with intermediate (6.0 mmol/L, IQR 5.4–6.8) or high (6.5 mmol/L, IQR 5.8–7.3) SMM (Table S1A). In addition, low SMD status was associated with statistically significant lower Hb, leukocytes, and thrombocytes nadirs, as shown in Table S1B. No significant associations were found between SMM or SMD status and overall non‐haematological toxicity (Figure 2B, E). The distribution by severity of chemotherapy‐induced (non‐)haematological toxicities (as scored by the CTCAE), stratified by SMM and SMD, is available in Figures S1 and S2.
Figure 2.
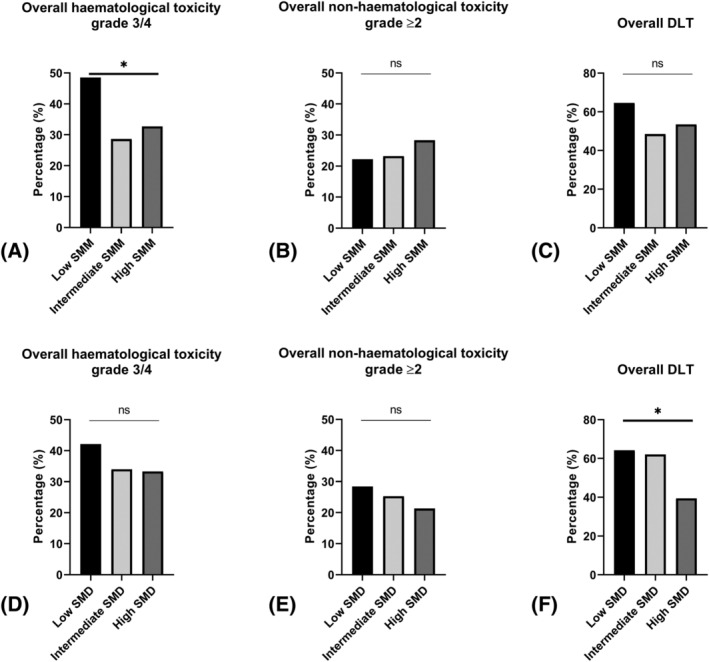
Chemotherapy‐induced toxicity stratified by SMM and SMD status. Percentage of chemotherapy‐induced toxicity stratified by low, intermediate, and high SMM and SMD status using the Pierson χ 2 test or Fisher's exact test (in case the cell count in any of the tables was <5). *P < 0.05. Composite endpoints: Overall haematological toxicity grade 3/4 scored using CTCAE: Anaemia OR leukocytopenia OR neutropenia OR thrombocytopenia; overall non‐haematological toxicity CTCAE grade ≥2 scored using CTCAE: Nephrotoxicity OR neurotoxicity OR esophagitis; overall dose‐limiting toxicity: Switching treatment (cisplatin to carboplatin) OR treatment delay (≥7 days) OR treatment de‐escalation (≥25%) OR treatment termination OR treatment‐related hospitalization. (A) Overall haematological toxicity stratified by SMM status. (B) Overall non‐haematological toxicity stratified by SMM status. (C) Overall DLT stratified by SMM status. (D) Overall haematological toxicity stratified by SMD status. (E) Overall non‐haematological toxicity stratified by SMD status. (F) Overall DLT stratified by SMD status. CTCAE, common terminology criteria for adverse events; DLT, dose‐limiting toxicity; ns, not statistically significant; SMD, skeletal muscle density; SMM, skeletal muscle mass.
In total, 55.6% of the patients developed any DLT, and for 32.7% of the patients, unplanned treatment‐related hospitalization was necessary. Patients with low SMM tended to develop DLT (64.6%) more frequently compared with patients with intermediate (48.5%) or high (53.5%) SMM (Figure 2C). SMD was found statistically significant associated with treatment‐related hospitalization (Table S2B, low SMD 44.2%, intermediate SMD 33.7% vs. high SMD 21.3%, respectively) as well as with overall DLT (low SMD 64.2%, intermediate SMD 62.1% vs. high SMD 39.4%, respectively, Figure 2F). No statistically significant associations were found between sarcopenic obesity status and chemotherapy‐induced toxicities.
Multivariate analysis
Table 2 shows the univariate and multivariate logistic regression analysis for the association with overall haematological toxicity grade 3/4. In univariate analysis, low SMM status (OR 2.35, 95% CI 1.31–4.24; P = 0.004) and age at diagnosis >65 years (OR 1.73, 95% CI 1.07–2.80; P = 0.026) were statistically significantly associated with increased risk of haematological toxicity grade 3/4. Although BMI was not statistically associated with haematological toxicities in univariate analysis, BMI was added in the multivariate analysis based on the well‐known correlation with SMM. As shown in Table 2, low SMM status (ORadj 2.41, 95% CI 1.31–4.45; P = 0.005) and age at diagnosis >65 years (ORadj 1.76, 95% CI 1.07–2.90; P = 0.025) were confirmed in multivariate logistic regression analysis to be significantly associated with chemotherapy‐induced overall haematological toxicity grade 3/4, while BMI status was not significantly associated. Low SMM (ORadj 2.23, 95% CI 1.23–4.04; P = 0.008) and high SMD (ORadj 0.41, 95% CI 0.23–0.74; P = 0.003) were significantly associated with overall DLT (Table 3).
Table 2.
Univariate and multivariate analysis of overall haematological toxicity grade 3/4
| Univariate analysis a | Multivariate analysis b | Multivariate analysis b | ||
|---|---|---|---|---|
| SMM | SMD | |||
| Characteristics | N = 295 | Crude OR (95% CI) | Adjusted OR c (95% CI) | Adjusted OR d (95% CI) |
| SMM status | ||||
| Intermediate | 99 | Ref. | Ref. | Ref. |
| Low | 98 | 2.35 (1.31–4.24) | 2.41 (1.31–4.45)* | 2.38 (1.25–4.50)* |
| High | 98 | 1.21 (0.66–2.23) | 1.18 (0.63–2.18) | 1.19 (0.63–2.25) |
| SMD status | ||||
| Intermediate | 94 | Ref. | Ref. | |
| Low | 95 | 1.41 (0.78–2.54) | 1.16 (0.61–2.18) | |
| High | 93 | 0.97 (0.53–1.78) | 1.16 (0.61–2.20) | |
| Gender | ||||
| Male | 165 | Ref. | ||
| Female | 130 | 1.46 (0.91–2.35) | ||
| Age at diagnosis in years | ||||
| ≤65 years | 140 | Ref. | Ref. | Ref. |
| >65 years | 155 | 1.73 (1.07–2.80) | 1.76 (1.07–2.90)* | 1.73 (1.02–2.94)* |
| ECOG PS | ||||
| 0 | 115 | Ref. | ||
| 1 | 133 | 1.40 (0.82–2.40) | ||
| ≥2 | 8 | 2.49 (0.59–10.53) | ||
| BMI (kg/m2) | ||||
| 18.5 to <25 | 118 | Ref. | Ref. | Ref. |
| <18.5 | 11 | 0.57 (0.14–2.25) | 0.38 (0.09–1.56) | 0.54 (0.12–2.36) |
| 25 to <30 | 116 | 0.80 (0.47–1.35) | 0.86 (0.49–1.52) | 0.81 (0.46–1.48) |
| ≥30 | 50 | 0.85 (0.43–1.69) | 0.96 (0.46–1.99) | 0.97 (0.45–2.10) |
| Charlson co‐morbidity index # | ||||
| 2–3 | 99 | Ref. | ||
| 4–5 | 97 | 0.99 (0.56–1.76) | ||
| >6 | 99 | 0.70 (0.39–1.26) | ||
| Renal function (mL/min/1.73 m2) | ||||
| ≥60 | 273 | Ref. | ||
| <60 | 22 | 0.80 (0.31–2.02) | ||
| Serum albumin (g/L) | ||||
| ≥37.5 | 190 | Ref. | ||
| <37.5 | 81 | 1.46 (0.85–2.49) | ||
| BSA (m2) | 295 | 0.47 (0.16–1.41) | ||
| Weight (kg) | 295 | 0.99 (0.98–1.01) |
Univariate logistic regression analysis.
Multivariate logistic regression analysis (backward: Wald).
Adjusted odds ratio: adjusted for SMM status, age at diagnosis and BMI in multivariate logistic regression analysis.
Adjusted odds ratio: adjusted for SMD status, SMM status, age at diagnosis and BMI in multivariate logistic regression analysis.
P‐value < 0.05.
Charlson co‐morbidity index score provides a simple means to quantify the effect of co‐morbid illnesses, including cardiovascular diseases, chronic obstructive pulmonary disease, liver disease, and diabetes mellitus, among others and accounts for the aggregate effect if multiple concurrent diseases. A higher score indicates more co‐morbidities.
BMI, body mass index; BSA, body surface area; ECOG PS, Eastern Cooperative Oncology Group Performance Status; OR, odds ratio; SD, standard deviation; SMD, skeletal muscle density; SMM, skeletal muscle mass.
Table 3.
Univariate and multivariate analysis of overall dose‐limiting toxicity
| Univariate analysis a | Multivariate analysis b | Multivariate analysis b | ||
|---|---|---|---|---|
| SMM | SMD | |||
| Characteristics | N = 297 | Crude OR (95% CI) | Adjusted OR c (95% CI) | Adjusted OR d (95% CI) |
| SMM status | ||||
| Intermediate | 99 | Ref. | Ref. | Ref. |
| Low | 99 | 1.94 (1.10–3.44) | 2.23 (1.23–4.04)* | 2.11 (1.12–3.98)* |
| High | 99 | 1.22 (0.70–2.14) | 1.16 (0.66–2.03) | 1.25 (0.69–2.25) |
| SMD status | ||||
| Intermediate | 95 | Ref. | Ref. | |
| Low | 95 | 1.10 (0.61–1.97) | 0.94 (0.50–1.75) | |
| High | 94 | 0.40 (0.22–0.71) | 0.41 (0.23–0.74)* | |
| Gender | ||||
| Male | 167 | Ref. | ||
| Female | 130 | 1.17 (0.74–1.85) | ||
| Age at diagnosis in years | ||||
| ≤65 years | 142 | Ref. | ||
| >65 years | 155 | 1.11 (0.70–1.75) | ||
| ECOG PS | ||||
| 0 | 115 | Ref. | ||
| 1 | 133 | 1.22 (0.74–2.01) | ||
| ≥2 | 8 | 1.48 (0.34–6.47) | ||
| 1.03 (0.50–2.10) | ||||
| BMI (kg/m2) | ||||
| 18.5 to <25 | 119 | Ref. | Ref. | Ref. |
| <18.5 | 11 | 0.74 (0.21–2.56) | 0.56 (0.16–2.01) | 0.56 (0.13–2.32) |
| 25 to <30 | 116 | 1.17 (0.70–1.96) | 1.36 (0.79–2.32) | 1.25 (0.71–2.19) |
| ≥30 | 51 | 1.38 (0.71–2.69) | 1.66 (0.83–3.33) | 1.44 (0.68–3.06) |
| Charlson co‐morbidity index # | ||||
| 2–3 | 100 | Ref. | ||
| 4–5 | 98 | 1.23 (0.70–2.16) | ||
| >6 | 99 | 1.25 (0.72–2.19) | ||
| Renal function (mL/min/1.73 m2) | ||||
| ≥60 | 273 | Ref. | ||
| <60 | 24 | 2.05 (0.82–5.12) | ||
| Serum albumin (g/L) | ||||
| ≥37.5 | 190 | Ref. | ||
| <37.5 | 81 | 1.48 (0.87–2.52) | ||
| BSA (m2) | 297 | 1.16 (0.41–3.30) | ||
| Weight (kg) | 297 | 1.00 (0.99–1.02) |
Univariate logistic regression analysis.
Multivariate logistic regression analysis (Backward: Wald).
Adjusted odds ratio: adjusted for SMM status and BMI in multivariate logistic regression analysis.
Adjusted odds ratio: adjusted for SMD status, SMM status, and BMI in multivariate logistic regression analysis.
P‐value < 0.05.
Charlson co‐morbidity index score provides a simple means to quantify the effect of co‐morbid illnesses, including cardiovascular diseases, chronic obstructive pulmonary disease, liver disease, and diabetes mellitus, among others and accounts for the aggregate effect if multiple concurrent diseases. A higher score indicates more co‐morbidities.
BMI, body mass index; BSA, body surface area; ECOG PS, Eastern Cooperative Oncology Group Performance Status; OR, odds ratio; SD, standard deviation; SMD, skeletal muscle density; SMM, skeletal muscle mass.
Discussion
Main findings
Chemotherapy‐induced toxicity frequently occurs in NSCLC patients treated with platinum‐based chemotherapy. Previous studies have shown that low SMM is associated with chemotherapy‐induced toxicity, across chemotherapeutic regimens and cancer types. 12 Although some studies 16 , 28 have described the prognostic value of body composition in patients with NSCLC treated with chemotherapy, data on treatment tolerability [in terms of (non‐)haematological and dose‐limiting toxicity] and the association with skeletal muscle measures in large cohorts are lacking. To the best of our knowledge, this is the largest clinical study that evaluates the association between pretreatment skeletal muscle measurements and chemotherapy‐induced toxicity in NSCLC patients treated with platinum‐based chemotherapy. The present prospective follow‐up study demonstrated that low SMM increased the risk of severe haematological toxicity nearly 2.5‐fold. In addition, low SMM and high SMD were significantly associated with a 2‐fold higher and 2.5‐fold lower risk of DLT, respectively.
The differences in incidence of chemotherapy‐induced toxicity among patients with various skeletal muscle status may be explained by the correlation between SMM and anthropometric measurements (such as BMI, weight, and BSA) which might predict the pharmacokinetics of platinum‐agents. However, in our cohort, no correlation between chemotherapy‐induced toxicity and BMI, weight, and/or BSA was found, while SMM and SMD were associated with severe haematological and dose‐limiting toxicity. In addition, patients with low SMM were generally more likely to receive a lower cumulative cisplatin and carboplatin dose compared with patients with intermediate or high SMM, which is a potential validation of the need for dose reduction or different treatment regimens in patients with low SMM compared with patients with intermediate/high SMM. In a recent study among 151 patients with solid tumours treated with capecitabine (a hydrophilic chemotherapeutic agent), no alterations in pharmacokinetics of capecitabine and the active and toxic metabolite 5‐FU were observed in patients with low SMM. 29 The previously identified increased toxicity and decreased survival in patients with low SMM could therefore not be explained by changes in pharmacokinetic characteristics of capecitabine and its metabolites. 29 In addition, according to a pharmacokinetic study, in 184 oesophageal cancer patients treated with paclitaxel, skeletal muscle measures were not superior to BSA in predicting pharmacokinetics of paclitaxel and did not have any added value to BSA. 30
An additional explanation for a higher incidence of chemotherapy‐induced toxicity in patients with low SMM is the correlation between low SMM and frailty, which has been observed in multiple studies performed in patients with head and neck cancer. 14 , 15 In addition, Portal et al. also described low L3 skeletal muscle measures as surrogate marker for frailty, which can support the prognostication process of NSCLC patients. 31 In clinical practice, a frailty assessment is based on clinical characteristics like overall performance status and co‐morbidity indices. However, it has been shown that clinicians tend to overestimate a patient's physical fitness. 32 , 33 Moreover, the present study could not establish an association between ECOG performance status or the Charlson co‐morbidity‐index score and chemotherapy‐induced toxicity. So it seems plausible that objective skeletal muscle measures may support predicting treatment tolerability and clinical decision making. Besides the role of SMM in chemotherapy‐induced toxicity, a chemotherapeutic agent itself, like cisplatin, can cause muscle wasting by activating a wide range of mechanisms, like inducing nuclear factor‐kB signalling. 34 , 35 Consequently, SMM and SMD may further decrease during treatment, thereby negatively affecting chemotherapy tolerability leading to suboptimal treatment. The muscle wasting effect may be further increased due to the combination of different chemotherapeutic agents, which represent the standard treatment regimen for NSCLC patients. Hence, the effect of these different combinations on muscle wasting should be further elucidated.
Strengths and limitations
The present study has several strengths. First, to the best of our knowledge, this is the largest prospective follow‐up study exploring the association between skeletal muscle measures and chemotherapy‐induced toxicity of NSCLC patients receiving first‐line platinum‐based chemotherapy. Second, the variables collected in our cohort were based on real‐world clinical data. Therefore, the results of this study reflect the actual clinical setting, which strengthens the possibility of extrapolating our findings.
The present analysis has some limitations. First, because population specific cutoff values for skeletal muscle measures in NSCLC patients are lacking, patients were stratified into three equal groups by SMM and SMD status. Consequently, comparing our results with studies using different cutoff values is complicated. However, a strong association between skeletal muscle measurements and chemotherapy‐induced toxicity was found in our cohort. Second, in the present study, changes in body composition during chemotherapy were not taken into account, because repeated measures were lacking. Because early loss of SMM during first‐line chemotherapy may be a poor prognostic factor in stage IV NSCLC patients, 16 muscle wasting during chemotherapy may also act as an effect modifier for chemotherapy‐induced toxicity. Third, no data were available regarding recent weight loss before start of chemotherapy, COPD, and cardiovascular disease status, which may all act as confounders or effect modifiers for SMM/SMD status. Nevertheless, surrogate markers for nutritional status (serum albumin level) and co‐morbidity (Charlson co‐morbidity index score) were used. In addition, because the number of available blood counts in between follow‐up moments differs among patients, the nadir values may be lower than reported for our study patients. This might be an explanation for the fact that no association was found between low skeletal muscle measures and neutropenia.
Potential clinical relevance
In clinical practice, chemotherapy‐induced toxicity will frequently result into clinical interventions such as delaying chemotherapy, dose adjustment, or treatment switch, all affecting treatment effectiveness adversely. The present results indicate an association between low SMM and the incidence of chemotherapy‐induced toxicity in NSCLC patients treated with first‐line platinum‐based chemotherapy. Therefore, pretreatment skeletal muscle measurements may be useful to select patients at higher risk for chemotherapy‐induced toxicity. In addition, dose‐adjustments based on image analysis could result in better treatment tolerance in patients with low SMM, which is especially relevant in a palliative setting. In contrast, patients with high SMM or high SMD may benefit from a higher dose of chemotherapy, thereby improving treatment effectiveness. Hence, pretreatment evaluation of SMM and SMD, as well as repeated measures during treatment, may provide opportunities for tailored therapy and could have a significant impact on clinical care.
Future research
Based on our results, future studies should focus on finding the optimal cutoff values to differentiate NSCLC patients with and without low SMM and low SMD. SMD represents the muscle lipid content and is a marker of muscle quality, whereas SMM represents muscle quantity. In literature, SMM is investigated more often in patients with cancer than SMD due to current technological possibilities of muscle segmentation. For SMM segmentation, there is not any confounding effect of scanning with or without contrast enhancement. However, for SMD, it is still a debate whether scanning with contrast enhancement may influence the measurement of muscle lipid content. Because SMD is measured based on the mean HU, the HU makes up the grayscale in medical CT imaging, which may be influenced by contrast application. 36 Nevertheless, it is likely that SMD has the potential to be a better marker of muscle fitness than SMM because it describes the muscle quality rather than the quantity, and quality may be better related to functional status than quantity. Indeed, Williams et al. found that SMD was better related to frailty than SMM in older patients with cancer. 37 Further research is needed to investigate a robust measurement of SMD in patients with cancer. Consequently, it will be possible to select patients who are at a higher risk for chemotherapy‐induced toxicity. Therefore, a clinical study that investigates chemotherapy doses based on skeletal muscle measurements would be an important next step. To determine accurately whether patients with low SMM and low SMD will benefit more from dose reduction, ideally a randomized controlled trial should be performed. In such a phase 3 trial, dose adjustments based on skeletal muscle measures (e.g. a starting dose of cisplatin in a range of 60–90 mg/m2) should be compared with a fixed cisplatin starting dose of 75 mg/m2. To ensure that in patients with dose adjustments based on low SMM status treatment effectiveness is not reduced, endpoints should be focused on both toxicity and treatment response (in terms of radiological response, progression free survival, and/or overall survival).
In addition, future research should be focused on the quantification of pretreatment L3 skeletal muscle mass in patients diagnosed with NSCLC and its association with frailty. Subsequently, impact analysis of the implementation of routine skeletal muscle measurements on clinical decision‐making should be of special interest. Currently, manual segmentation of skeletal muscle mass requires multiple steps and is time‐consuming, which may limit its use in routine clinical practice. However, an automated method for accurate and reproducible segmentation of skeletal muscle area at L3, as recently described by Amarasinghe et al., radically increases the prospect of implementation routine determination of skeletal muscle measures in clinical practice. 38
Besides, research should indicate whether patients will profit from improved physical fitness and higher SMM status (prehabilitation) before chemotherapy, in line with preoperative physical exercise interventions. 39
Conclusions
In conclusion, this prospective follow‐up study suggests that NSCLC patients with pretreatment low SMM are at a significantly higher risk to develop chemotherapy‐induced severe haematological toxicity and DLT. NSCLC patients with high SMD are at significant lower risk for DLT. Future studies have to reveal whether skeletal muscle measurements have a higher correlation with the pharmacokinetics of chemotherapeutics than the current dosing strategies based on weight or BSA and to reveal the association with frailty. Such results may provide an explanation for increased toxicity in patients with low SMM. Moreover, research should be focused on whether chemotherapy dosing based on SMM could reduce the risk of chemotherapy‐induced toxicity without compromising effectiveness. Future studies should also investigate whether improvement of pretreatment SMM/SMD could reduce the risk of chemotherapy induced toxicity.
Conflict of interest
The authors have nothing to declare. The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. 40
Funding
The PGxLUNG study was funded by the St. Antonius Onderzoeksfonds and patient funding. Financial support for the genotyping (data not used in this study) was provided by Roche Nederland B.V. The funding sources had no role in study design, data collection, data analysis, data interpretation, or the writing of the report.
Supporting information
Table S1A. Chemotherapy‐induced toxicity stratified by SMM status
Table S1B. Chemotherapy‐induced toxicity stratified by SMD status
Figure S1. Chemotherapy‐induced haematological toxicity stratified by SMM and SMD status
Figure S2. Chemotherapy‐induced non‐haematological toxicity stratified by SMM and SMD status
Acknowledgements
The authors would like to thank Dr. J. Lavalaye (Department of Nuclear Medicine, St. Antonius Hospital, Nieuwegein, the Netherlands), Dr. P. J. Hagen (Department of Nuclear Medicine, Diakonessen Hospital, Utrecht, the Netherlands), Drs. J. P. Esser (Department of Nuclear Medicine, Meander Medical Center, the Netherlands), and Drs. H. Adams (Department of Nuclear Medicine, Groene Hart Hospital, Gouda, the Netherlands) for providing the CT scans.
de Jong C., Chargi N., Herder G. J. M., van Haarlem S. W. A., van der Meer F., van Lindert A. S. R., ten Heuvel A., Brouwer J., de Jong P. A., Devriese L. A., Huitema A. D. R., Egberts T. C. G., de Bree R., and Deneer V. H. M. (2022) The association between skeletal muscle measures and chemotherapy‐induced toxicity in non‐small cell lung cancer patients, Journal of Cachexia, Sarcopenia and Muscle, 13, 1554–1564, 10.1002/jcsm.12967
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2. Spira A, Ettinger DS. Multidisciplinary management of lung cancer. Wood AJJ, ed. N Engl J Med 2004;350:379–392. [DOI] [PubMed] [Google Scholar]
- 3. Deng HY, Zha P, Hou L, Huang KL. Does sarcopenia have any impact on survival of patients with surgically treated non‐small‐cell lung cancer? Interact Cardiovasc Thorac Surg 2019;29:144–147. [DOI] [PubMed] [Google Scholar]
- 4. Kawaguchi Y, Hanaoka J, Ohshio Y, Okamoto K, Kaku R, Hayashi K, et al. Sarcopenia predicts poor postoperative outcome in elderly patients with lung cancer. Gen Thorac Cardiovasc Surg 2019;67:949–954. [DOI] [PubMed] [Google Scholar]
- 5. Mallet R, Decazes P, Modzelewski R, Lequesne J, Vera P, Dubray B, et al. Prognostic value of low skeletal muscle mass in patient treated by exclusive curative radiochemotherapy for a NSCLC. Sci Rep 2021;11:10628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Buentzel J, Heinz J, Bleckmann A, Buentzel J, Heinz J, Bleckmann A, et al. Sarcopenia as prognostic factor in lung cancer patients: A systematic review and meta‐analysis. Anticancer Res 2019;39:4603–4612. [DOI] [PubMed] [Google Scholar]
- 7. Yang M, Shen Y, Tan L, Li W. Prognostic value of sarcopenia in lung cancer: a systematic review and meta‐analysis. Chest 2019;156:101–111. [DOI] [PubMed] [Google Scholar]
- 8. Kurk S, Peeters P, Stellato R, Dorresteijn B, Jong P, Jourdan M, et al. Skeletal muscle mass loss and dose‐limiting toxicities in metastatic colorectal cancer patients. J Cachexia Sarcopenia Muscle 2019;10:803–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ali R, Baracos VE, Sawyer MB, Bianchi L, Roberts S, Assenat E, et al. Lean body mass as an independent determinant of dose‐limiting toxicity and neuropathy in patients with colon cancer treated with FOLFOX regimens. Cancer Med 2016;5:607–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sjøblom B, Benth JŠ, Grønberg BH, Baracos VE, Sawyer MB, Fløtten Ø, et al. Drug dose per kilogram lean body mass predicts hematologic toxicity from carboplatin‐doublet chemotherapy in advanced non–small‐cell lung cancer. Clin Lung Cancer 2017;18:e129–e136. [DOI] [PubMed] [Google Scholar]
- 11. Willemsen ACH, Hoeben A, Lalisang RI, van Helvoort A, Wesseling FWR, Hoebers F, et al. Disease‐induced and treatment‐induced alterations in body composition in locally advanced head and neck squamous cell carcinoma. J Cachexia Sarcopenia Muscle 2020;11:145–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huiskamp LFJ, Chargi N, Devriese LA, May AM, Huitema ADR, de Bree R. The predictive value of low skeletal muscle mass assessed on cross‐sectional imaging for anti‐cancer drug toxicity: a systematic review and meta‐analysis. J Clin Med 2020;9:3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population‐based study. Lancet Oncol 2008;9:629–635. [DOI] [PubMed] [Google Scholar]
- 14. Zwart AT, van der Hoorn A, van Ooijen PMA, Steenbakkers RJHM, de Bock GH, Halmos GB. CT‐measured skeletal muscle mass used to assess frailty in patients with head and neck cancer. J Cachexia Sarcopenia Muscle 2019;10:1060–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Meerkerk CDA, Chargi N, de Jong PA, van den Bos F, de Bree R. Low skeletal muscle mass predicts frailty in elderly head and neck cancer patients. Eur Arch Otorhinolaryngol 2021;279:967–977. 10.1007/s00405-021-06835-0 Epub ahead of print. PMID: 33956205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Degens JHRJ, Sanders KJC, de Jong EEC, Groen HJM, Smit EF, Aerts JG, et al. The prognostic value of early onset, CT derived loss of muscle and adipose tissue during chemotherapy in metastatic non‐small cell lung cancer. Lung Cancer 2019;133–135. [DOI] [PubMed] [Google Scholar]
- 17. Halvorsen TO, Valan CD, Slaaen M, Grønberg BH. Associations between muscle measures, survival, and toxicity in patients with limited stage small cell lung cancer. J Cachexia Sarcopenia Muscle 2020;11:1283–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Srdic D, Plestina S, Sverko‐Peternac A, Nikolac N, Simundic A‐M, Samarzija M. Cancer cachexia, sarcopenia and biochemical markers in patients with advanced non‐small cell lung cancer—chemotherapy toxicity and prognostic value. Support Care Cancer 2016;24:4495–4502. [DOI] [PubMed] [Google Scholar]
- 19. de Jong C, Herder GJM, Deneer VHM. Genetic variants as predictors of toxicity and response in patients with non‐small cell lung cancer undergoing first‐line platinum‐based chemotherapy: design of the multicenter PGxLUNG study. Thoracic Cancer 2020;11:3634–3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shen W, Punyanitya M, Wang Z, Gallagher D, St.‐Onge MP, Albu J, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross‐sectional image. J Appl Physiol 2004;97:2333–2338. [DOI] [PubMed] [Google Scholar]
- 21. Aubrey J, Esfandiari N, Baracos VE, Buteau FA, Frenette J, Putman CT, et al. Measurement of skeletal muscle radiation attenuation and basis of its biological variation. Acta Physiologica 2014;210:489–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. du Bois D, du Bois EF. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition (Burbank, Los Angeles County, Calif) 1989;5:303–311, discussion 312. Accessed April 10, 2021. https://europepmc.org/article/med/2520314 [PubMed] [Google Scholar]
- 23. Charlson M, Pompei PL, Ales K, Mackenzie C, Charlson ME, Pompei P, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 24. Oken MM, Creech RH, Davis TE. Toxicology and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649–656. [PubMed] [Google Scholar]
- 25. Levey AS, Stevens LA. Estimating GFR using the CKD epidemiology collaboration (CKD‐EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis 2010;55:622–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. WHO definition BMI . Weblocation: body mass index‐for‐age (BMI‐for‐age) (who.int). Accessed 16 july 2021.
- 27. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim EY, Kim YJ, Kim YS, Kim KW, Jeon JY, Kim KG. Prognostic significance of radiodensity‐based skeletal muscle quantification using preoperative CT in resected non‐small cell lung cancer. J Thorac Dis 2021;Feb;13:754–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Molenaar‐Kuijsten L, Jacobs BAW, Kurk SA, May AM, Dorlo TPC, Beijnen JH, et al. Worse capecitabine treatment outcome in patients with a low skeletal muscle mass is not explained by altered pharmacokinetics. Cancer Med 2021;4781–4789. 10.1002/cam4.4038. Epub ahead of print. PMID: 34121365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van Doorn L, Crombag MBS, Rier HN, van Vugt JLA, van Kesteren C, Bins S, et al. The influence of body composition on the systemic exposure of paclitaxel in esophageal cancer patients. Pharmaceuticals (Basel) 2021;14:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Portal D, Hofstetter L, Eshed I, Dan Lantsman C, Sella T, Urban D, et al. L3 skeletal muscle index (L3SMI) is a surrogate marker of sarcopenia and frailty in non‐small cell lung cancer patients. Cancer Manag Res 2019;11:2579–2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ando M, Ando Y, Hasegawa Y, Shimokata K, Minami H, Wakai K, et al. Prognostic value of performance status assessed by patients themselves, nurses, and oncologists in advanced non‐small cell lung cancer. Br J Cancer 2001;85:1634–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Blagden SP, Charman SC, Sharples LD, Magee LR, Gilligan D. Performance status score: do patients and their oncologists agree? Br J Cancer 2003;89:1022–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Conte E, Bresciani E, Rizzi L, Cappellari O, de Luca A, Torsello A, et al. Cisplatin‐induced skeletal muscle dysfunction: mechanisms and counteracting therapeutic strategies. Int J Mol Sci 2020;21:1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sakai H, Sagara A, Arakawa K, Sugiyama R, Hirosaki A, Takase K, et al. Mechanisms of cisplatin‐induced muscle atrophy. Toxicol Appl Pharmacol 2014;278:190–199. [DOI] [PubMed] [Google Scholar]
- 36. van Vugt JLA, Coebergh van den Braak RRJ, Schippers HJW, Veen KM, Levolger S, de Bruin RWF, et al. Contrast‐enhancement influences skeletal muscle density, but not skeletal muscle mass, measurements on computed tomography. Clin Nutr 2018;37:1707–1714. [DOI] [PubMed] [Google Scholar]
- 37. Williams GR, Deal AM, Muss HB, Weinberg MS, Sanoff HK, Guerard EJ, et al. Frailty and skeletal muscle in older adults with cancer. J Geriatr Oncol 2018;9:68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Amarasinghe KC, Lopes J, Beraldo J, Kiss N, Bucknell N, Everitt S, et al. A deep learning model to automate skeletal muscle area measurement on computed tomography images. Front Oncol 2021;11:580806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Avancini A, Sartori G, Gkountakos A, Casali M, Trestini I, Tregnago D, et al. Physical activity and exercise in lung cancer care: will promises be fulfilled? Oncologist 2020;25:e555–e569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. von Haehling S, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2021. J Cachexia Sarcopenia Muscle 2021;2259–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1A. Chemotherapy‐induced toxicity stratified by SMM status
Table S1B. Chemotherapy‐induced toxicity stratified by SMD status
Figure S1. Chemotherapy‐induced haematological toxicity stratified by SMM and SMD status
Figure S2. Chemotherapy‐induced non‐haematological toxicity stratified by SMM and SMD status


