Key Points
Question
Do pulse oximetry discrepancies, hidden hypoxemia, and clinical outcomes differ among racial and ethnic subgroups?
Findings
In this cross-sectional study of 5 databases with 87 971 patients, significant disparities in pulse oximetry accuracy across racial and ethnic subgroups (ie, Asian, Black, Hispanic, and White individuals) were found, with higher rates of hidden hypoxemia associated with mortality, future organ dysfunction, and abnormal laboratory test results.
Meaning
In this study, discrepancies in pulse oximetry accuracy among racial and ethnic subgroups were associated with higher rates of hidden hypoxemia, mortality, and organ dysfunction.
This cross-sectional study examines racial and ethnic discrepancies between oxygen saturation measured by pulse oximetry and arterial blood gas and their associations with clinical outcomes.
Abstract
Importance
Discrepancies in oxygen saturation measured by pulse oximetry (Spo2), when compared with arterial oxygen saturation (Sao2) measured by arterial blood gas (ABG), may differentially affect patients according to race and ethnicity. However, the association of these disparities with health outcomes is unknown.
Objective
To examine racial and ethnic discrepancies between Sao2 and Spo2 measures and their associations with clinical outcomes.
Design, Setting, and Participants
This multicenter, retrospective, cross-sectional study included 3 publicly available electronic health record (EHR) databases (ie, the Electronic Intensive Care Unit–Clinical Research Database and Medical Information Mart for Intensive Care III and IV) as well as Emory Healthcare (2014-2021) and Grady Memorial (2014-2020) databases, spanning 215 hospitals and 382 ICUs. From 141 600 hospital encounters with recorded ABG measurements, 87 971 participants with first ABG measurements and an Spo2 of at least 88% within 5 minutes before the ABG test were included.
Exposures
Patients with hidden hypoxemia (ie, Spo2≥88% but Sao2 <88%).
Main Outcomes and Measures
Outcomes, stratified by race and ethnicity, were Sao2 for each Spo2, hidden hypoxemia prevalence, initial demographic characteristics (age, sex), clinical outcomes (in-hospital mortality, length of stay), organ dysfunction by scores (Sequential Organ Failure Assessment [SOFA]), and laboratory values (lactate and creatinine levels) before and 24 hours after the ABG measurement.
Results
The first Spo2-Sao2 pairs from 87 971 patient encounters (27 713 [42.9%] women; mean [SE] age, 62.2 [17.0] years; 1919 [2.3%] Asian patients; 26 032 [29.6%] Black patients; 2397 [2.7%] Hispanic patients, and 57 632 [65.5%] White patients) were analyzed, with 4859 (5.5%) having hidden hypoxemia. Hidden hypoxemia was observed in all subgroups with varying incidence (Black: 1785 [6.8%]; Hispanic: 160 [6.0%]; Asian: 92 [4.8%]; White: 2822 [4.9%]) and was associated with greater organ dysfunction 24 hours after the ABG measurement, as evidenced by higher mean (SE) SOFA scores (7.2 [0.1] vs 6.29 [0.02]) and higher in-hospital mortality (eg, among Black patients: 369 [21.1%] vs 3557 [15.0%]; P < .001). Furthermore, patients with hidden hypoxemia had higher mean (SE) lactate levels before (3.15 [0.09] mg/dL vs 2.66 [0.02] mg/dL) and 24 hours after (2.83 [0.14] mg/dL vs 2.27 [0.02] mg/dL) the ABG test, with less lactate clearance (−0.54 [0.12] mg/dL vs −0.79 [0.03] mg/dL).
Conclusions and Relevance
In this study, there was greater variability in oxygen saturation levels for a given Spo2 level in patients who self-identified as Black, followed by Hispanic, Asian, and White. Patients with and without hidden hypoxemia were demographically and clinically similar at baseline ABG measurement by SOFA scores, but those with hidden hypoxemia subsequently experienced higher organ dysfunction scores and higher in-hospital mortality.
Introduction
Recently, reports of systemic racial bias in which oxygen saturation measured by pulse oximeter (Spo2) overestimates the true arterial oxygen saturation (Sao2) in patients with darkly pigmented skin has raised concerns about the clinical accuracy of pulse oximetry.1 Sjoding et al1 used race listed in the EHR as a proxy for skin color to retrospectively analyze the accuracy of pulse oximetry during hypoxemia in 2 high-acuity adult cohorts. They described occult hypoxemia as an Sao2 of less than 88% when the Spo2 was between 92% and 96%. In both cohorts, the incidence of hidden hypoxemia was almost 3 times higher among patients self-reported as Black vs White.1
Pulse oximetry is a useful tool to monitor blood oxygen saturation without obtaining an invasive arterial blood gas (ABG) measurement. The US Food and Drug Administration (FDA) requires root mean square accuracy within 2% for values between 70% and 100%, implying that an adequate pulse oximeter returns an Spo2 value within 2% to 3% of the Sao2 value (ie, a range of 4%-6%) only two-thirds of the time.2
Studies have highlighted the inaccuracy of pulse oximetry in critically ill patients; however, smaller sample sizes hindered in-depth analysis of race, ethnicity, and outcomes (eTable 1 in the Supplement).3,4,5,6,7 This study used 5 large EHR data sets of critically ill patients to further evaluate the incidence and clinical outcomes of hidden hypoxemia across racial and ethnic groups.
Methods
This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.8 Data in Medical Information Mart for Intensive Care III (MIMIC-III), MIMIC-IV, and Electronic Intensive Care Unit–Clinical Research Database (eICU-CRD) had been previously deidentified and did not require a waiver for informed consent. The Medical Information Mart for Intensive Care (MIMIC) database is a collaboration between the Beth Israel Deaconess Medical Center and the Laboratory for Computational Physiology at the Massachusetts Institute of Technology. The database contains granular, deidentified ICU data from the Beth Israel Deaconess Medical Center. The data have been generated from more than 70 intensive care unit beds with medical, surgical, cardiac, and neurological patients. We used the latest data version, MIMIC-III (version 1.4), which contains deidentified data associated with 53 423 ICU admissions.9 Physionet approved the use of MIMIC for this study. Emory University approved the use of the Emory and Grady databases for research, with a complete HIPAA and informed consent waiver.
Data Sources
Open Source PhysioNet Databases
The eICU-CRD (comprising 335 ICUs across 208 hospitals) and MIMIC databases (comprising 6 ICUs in 1 hospital) were used. The Sequential Organ Failure Assessment (SOFA) scores (eTable 2 in the Supplement), including the cardiovascular SOFA (CVSOFA) and respiratory SOFA (RSOFA) scores, were provided by MIT Laboratory of Computational Physiology.9,10,11,12,13
Emory Healthcare and Grady Memorial
Data were collected from all units in Emory Healthcare (277 units, including 26 ICUs, across 4 hospitals) and Grady Memorial Hospital (73 units, including 9 ICUs, in 1 hospital). Emory Healthcare and Grady patient data spanned 2014 to 2021 and 2014 to 2020, respectively. SOFA scores and its components were not available for the Emory and Grady databases.
Data Extraction
Data analysis was conducted with R version 3.6.3 (R Project for Statistical Computing) and Python version 3.6 (Python Software Foundation). All ABG and Spo2 values were extracted from the EHR.
Inclusion and Exclusion Criteria
An Spo2 range of 88% to 100% was selected as an interval in which patients may have hypoxemia but falsely be considered as having arterial blood oxygenation in the reference range according to Sao2. Each ABG-measured Sao2 was matched with the closest Spo2 value recorded within the previous 5 minutes. To eliminate repeated measurements and limit confounding, only the first ABG measurement from each hospital encounter was used. Spo2 measurements of less than 88% were not examined because of low prevalence in the EHR.
Race and Ethnicity
In each EHR data set, race and ethnicity were defined using self-reported demographic data, including administrative entries with additional identifiers. All patients with race and ethnicity information who could not be classified as Asian, Black, Hispanic, or White were excluded. Patients were stratified by age, sex, race and ethnicity, and CVSOFA score. If any of these characteristics were missing, a patient was excluded from the corresponding subgroup analysis but included in the overall analysis.
Statistical Analysis
For each racial and ethnic group, the frequency of ABG measurement was characterized using 2 complementary analyses. First, encounters with ABG measurements were compared with encounters without ABGs measurements to determine the likelihood of receiving an ABG measurement during an encounter by race and ethnicity. Second, to characterize the rate of ABG collection across encounters with at least 1 ABG, the total number of ABGs normalized by the length of stay (in days) was calculated. Given possible confounding by illness severity, the second estimates were stratified by CVSOFA score at the time of ABG.
Differences in Spo2-Sao2 pairs were characterized by modified Bland-Altman plots. We used χ2 tests to compare the distribution of categorical variables (eg, sex) between any 2 groups, while Mann-Whitney nonparametric tests were used for continuous and ordinal variables (eg, age, SOFA score). Notably, differences in the distribution of numeric clinical end points (eg, SOFA score) were evaluated via bootstrapping (100 iterations), followed by a Mann-Whitney nonparametric test. Differences between stratified odds differences (eg, risk of hidden hypoxemia by race and ethnicity) were tested with the Breslow-Day test.
Hidden hypoxemia was defined as an Spo2of88% or greater despite an Sao2 of less than 88%. Patients with and without hidden hypoxemia were compared at the time of ABG measurement by baseline demographic characteristics (age, sex) and by organ failure scores (SOFA, RSOFA, CVSOFA). The long-term association of hidden hypoxemia with clinical outcomes was analyzed by estimating differences in length of stay and in-hospital mortality. The short-term association of hidden hypoxemia with organ dysfunction was examined using SOFA and CVSOFA scores measured 24 hours after the baseline ABG measurement. Associations between hidden hypoxemia, RSOFA, and in-hospital mortality were also examined. The consequences of hidden hypoxemia were also evaluated by comparing the last serum lactate and serum creatinine levels in a 7-day window before the ABG measurement with the first value in a 7-day window starting 24 hours after the ABG measurement. Values were compared at each time in addition to the difference between the before and after values.
Multivariate logistic regression was used for assessing binary end points (eg, in-hospital mortality), multivariate ordinal regression for numerical end points (eg, CVSOFA and RSOFA scores), and multivariate linear models for continuous end points (eg, creatinine lactate levels), using analysis of variance to test for the impact of hidden hypoxemia while adjusting for other covariates (eg, age, sex, SOFA score at time of ABG measurement) (eAppendix 1 in the Supplement). Missing data for any covariates were flagged with a separate binary variable.
Statistical analysis was conducted with R version 3.6.3 (R Project for Statistical Computing) and Python version 3.6 (Python Software Foundation). Statistical significance was set at P < .05, and all tests were 2-tailed.
Results
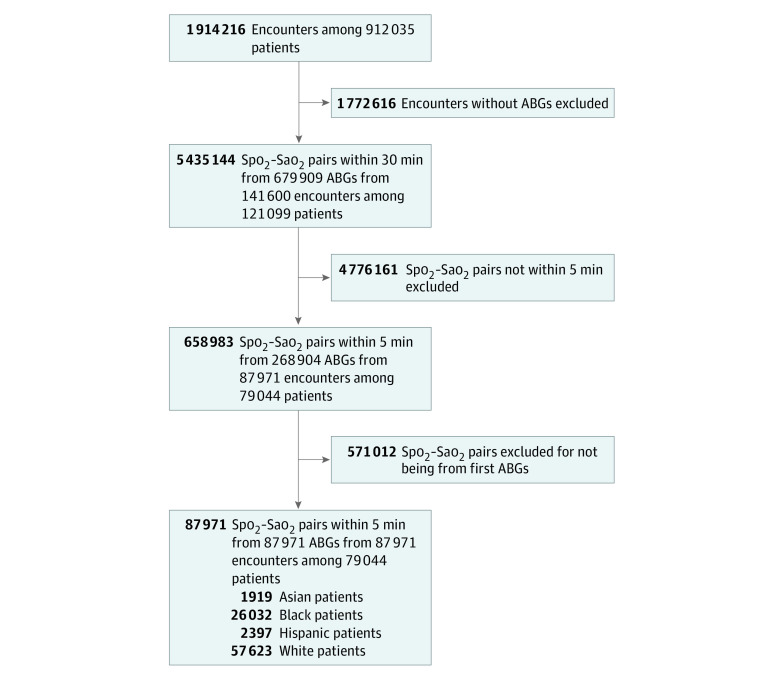
The first Spo2-Sao2 pairs from 87 971 patient encounters (27 713 [42.9%] women; mean [SE] age, 62.2 [17.0] years) were analyzed among 4 race/ethnicity subgroups (Asian, 1919 patients [2.3%]; Black, 26 032 [29.6%]; Hispanic, 2397 patients [2.7%]; White, 57 623 patients [65.5%]), with 4859 (5.5%) having hidden hypoxemia. In total, 141 600 patients, with 679 909 ABGs and 5 435 144 Spo2-Sao2 pairs within 30 minutes of each other were identified. Patient characteristics are presented in Table 1 and Figure 1. Restricting to Spo2 measurements to those within the 5 minutes preceding the ABG measurement resulted in 268 904 Spo2-Sao2 pairs; further selecting the first ABG in an encounter led to 87 971 Spo2-Sao2 pairs (eAppendix 2 in the Supplement). Sample sizes were considerably smaller for Asian and Hispanic subgroups.
Table 1. Characteristics of Patients with Spo2 of at Least 88% 5 Minutes Before First ABG Measurementa.
| Characteristic | Patients, No. (%) |
|---|---|
| Total, No. | |
| Patients | 79 044 |
| Hospital encounters | 87 971 |
| ABG | 87 971 |
| Spo2-Sao2 pairs | 87 971 |
| Sex | |
| Female | 37 713 (42.9) |
| Male | 50 258 (57.1) |
| Age, mean (SE), y | 62.18 (16.97) |
| Sao2, mean (SE), % | 95.33 (0.03) |
| Spo2, mean (SE), % | 97.12 (0.01) |
| Location | |
| ICU | 75 397 (85.7) |
| ED | 6071 (6.9) |
| Stepdown | 570 (0.6) |
| Floor | 3193 (3.6) |
| PACU | 542 (0.6) |
| OR | 1112 (1.3) |
| Other or unknown | 1086 (1.2) |
| Race and ethnicity | |
| Asian | 1919 (2.2) |
| Black | 26 032 (29.6) |
| Hispanic | 2397 (2.7) |
| White | 57 623 (65.5) |
| Database | |
| eICU-CRD | 38 693 (44.0) |
| MIMIC-III | 2017 (2.3) |
| MIMIC-IV | 4353 (5.0) |
| Emory | 33 157 (37.7) |
| Grady | 9751 (11.1) |
| Cardiovascular SOFA score, mean (SE)b | 0.67 (0.006) |
Abbreviations: ABG, arterial blood gas; ED, emergency department; eICU-CRD, Electronic Intensive Care Unit–Clinical Research Database; ICU, intensive care unit; MIMIC, Medical Information Mart for Intensive Care; OR, operating room; PACU, postanesthesia care unit; Sao2, arterial oxygen saturation; SOFA, Sequential Organ Failure Assessment; Spo2, oxygen saturation by pulse oximeter.
Table presents patient characteristics for all ABGs examined, which represents characteristics of patients with an Spo2 within the 5 minutes preceding the ABG, based on the first ABG measurement of their hospital encounter. When applicable, SEs are provided. They were obtained using simple bootstrap with 100 iterations.
Cardiovascular SOFA scores available only for patients in eICU-CRD, MIMIC-III, and MIMIC-IV databases.
Figure 1. Flow Diagram.
Aggregate flow diagram for all data sets. ABG indicates arterial blood gas; Sao2, arterial oxygen saturation; Spo2, oxygen saturation by pulse oximetry.
Missingness
Missing data on length of stay and in-hospital mortality was 0.1% (96 encounters) and 0.4% (369 encounters), respectively. Given that there were no SOFA score data from Emory and Grady, RSOFA, CVSOFA, and SOFA scores had 52.7% (46 381 encounters), 52.9% (46 538 encounters), and 52.7% (46 381 encounters) missingness, respectively. Missingness for Spo2 variability and other variables are described in eTable 4 and eTable 5 in the Supplement.
ABG Measurement Frequency by Race and Ethnicity
There were differences in the likelihood of receiving an ABG measurement during a hospital encounter that varied by race and ethnicity, with the White subgroup most likely to receive an initial ABG measurement despite similar RSOFA and CVSOFA scores: White patients, 85 872 of 1 532 492 encounters (5.6%); Asian patients, 3249 of 95 813 (3.4%); Black patients, 49 053 of 1 781 868 (2.8%); and Hispanic patients, 3426 of 179 617 (1.9%) (eTable 6 in the Supplement). Although following the first ABG, subsequent ABG frequencies were similar across racial and ethnic subgroups (eTable 7 in the Supplement). A table comparing patients with and without ABG measurements across races and ethnicities appears in eTable 3 in the Supplement.
Comparison of Spo2 With Sao2 by Race and Ethnicity
The White subgroup was used as a reference because it had the highest prevalence in the data set. At most Spo2 values, there were statistically significant differences in true oxygen saturation levels between White patients compared with those in other racial and ethnic subgroups, although these differences were small in magnitude (evaluated using means and medians) (eTable 5 in the Supplement). Similar results were obtained when using all ABGs measured during a patient’s hospitalization (268 904) instead of the first ABG measurement only (87 971) (eFigure 1 in the Supplement). This finding was robust to adjustment for sex, age, and RSOFA and CVSOFA scores (eFigures 2-5 in the Supplement).
Hidden Hypoxemia by Race and Ethnicity
Hidden hypoxemia occurred across all racial and ethnic subgroups, assessed using the first ABG measurement with an Spo2 level greater than 88%. Patients self-identified as Black had higher Sao2 variability for any given Spo2 value, as evidenced by a larger IQR (eg, median [IQR] Sao2 at Spo2 of 88%, Black patients: 90.10% [10.13]; White patients, 90.00% [9.10]). There was a varying incidence of hidden hypoxemia among racial and ethnic group in descending order: Black, 1785 [6.8%]; Hispanic, 160 [6.0%]; Asian, 92 [4.8%]; White, 2822 [4.9%] (P < .001) (Figure 2; eTable 8 and eTable 9 in the Supplement).
Figure 2. Modified Bland-Altman Plots by Race and Ethnicity.

On each plot, the bold horizontal lines represent the mean bias (defined as the difference between the oxygen saturation measured by pulse oximetry [Spo2] and arterial blood gas value [Sao2]) for each of the 2 Spo2 groups. The blue lines, with dashed blue lines indicating 95% CIs, are for the group with Spo2 of 88% to 92%, and the orange lines, with dashed orange lines indicating 95% CIs, for the group with Spo2 of 93% to 96%. The solid black line indicates the absence of bias (ie, Spo2 − Sao2 = 0). When the mean bias is above the black line, there is positive bias in the pulse oximetry measurement (ie, the observed Spo2 is greater than the true Sao2). In contrast, when the mean bias is below the black line, there is negative bias in the pulse oximetry measurement (ie, the observed Spo2 is below the true Sao2).
Organ Dysfunction at Time of First ABG Measurement and 24 Hours Later
Across all racial and ethnic groups statistically significant, although clinically small, differences in baseline organ dysfunction (SOFA, CVSOFA, RSOFA) were present at the time of the first ABG measurements between patients with and without hidden hypoxemia (Table 2). Furthermore, across all racial and ethnic groups, patients with hidden hypoxemia subsequently experienced greater organ dysfunction than patients without hidden hypoxemia, as evidenced by higher CVSOFA and SOFA scores measured 24 hours after the ABG measurement was drawn (mean [SE] CVSOFA: 1.48 [0.03] vs 1.25 [0.01]; mean [SE] SOFA: 7.2 [0.1] vs 6.29 [0.02]) (Table 2). This difference persisted even when adjusted for age, sex, and SOFA score (data not shown).
Table 2. Descriptive Statistics for Patients With Hidden Hypoxemia vs Patients Without Hypoxemiaa.
| Characteristic | Asian patients, mean (SE) | P value | Black patients, mean (SE) | P value | Hispanic patients, mean (SE) | P value | White patients, mean (SE) | P value | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hidden hypoxemia | No hypoxemia | Hidden hypoxemia | No hypoxemia | Hidden hypoxemia | No hypoxemia | Hidden hypoxemia | No hypoxemia | |||||
| No. (%) | 94 (4.9) | 1825 (95.1) | NA | 1789 (6.9) | 24 243 (93.1) | NA | 145 (6.0) | 2252 (94.0) | NA | 2831 (4.9) | 54 792 (95.1) | NA |
| Age, y | 63.93 (2.46) | 62.34 (0.51) | <.001 | 58.26 (0.51) | 57.76 (0.15) | <.001 | 57.48 (2.05) | 59.53 (0.52) | <.001 | 64.03 (0.41) | 64.29 (0.1) | <.001 |
| Sex, No. (%) | ||||||||||||
| Female | 36 (38.3) | 724 (39.7) | .89 | 862 (48.2) | 11 243 (46.4) | .11 | 75 (51.7) | 1028 (45.6) | .15 | 1241 (43.8) | 22 504 (41.1) | .01 |
| Male | 58 (61.7) | 1101 (60.3) | 927 (51.8) | 13 000 (53.6) | 70 (48.3) | 1224 (54.4) | 1590 (56.2) | 32 288 (58.9) | ||||
| Serum creatinine levels, mg/dL | ||||||||||||
| Before ABG | 1.73 (0.24) | 1.59 (0.07) | <.001 | 2.37 (0.09) | 2.27 (0.03) | <.001 | 1.79 (0.19) | 1.66 (0.04) | <.001 | 1.73 (0.04) | 1.46 (0.01) | <.001 |
| After ABG | 1.79 (0.31) | 1.44 (0.06) | <.001 | 2.2 (0.08) | 2.07 (0.02) | <.001 | 2.01 (0.33) | 1.6 (0.07) | <.001 | 1.53 (0.06) | 1.38 (0.01) | <.001 |
| Difference | 0.05 (0.19) | −0.1 (0.04) | <.001 | −0.21 (0.06) | −0.2 (0.02) | .08 | −0.15 (0.19) | −0.17 (0.04) | .33 | −0.17 (0.05) | −0.07 (0.01) | <.001 |
| Serum lactate levels, mg/dL | ||||||||||||
| Before ABG | 4.37 (0.82) | 2.87 (0.11) | <.001 | 3.4 (0.13) | 2.85 (0.03) | <.001 | 2.96 (0.45) | 2.87 (0.09) | .26 | 2.99 (0.1) | 2.56 (0.02) | <.001 |
| After ABG | 2.97 (0.65) | 2.32 (0.15) | <.001 | 3.27 (0.22) | 2.5 (0.04) | <.001 | 3.4 (0.8) | 2.24 (0.13) | <.001 | 2.51 (0.14) | 2.15 (0.03) | <.001 |
| Difference, | −1.61 (0.86) | −1.14 (0.2) | <.001 | −0.34 (0.25) | −0.82 (0.05) | <.001 | −0.41 (0.79) | −1.14 (0.18) | <.001 | −0.68 (0.2) | −0.74 (0.04) | .00 |
| Long-term clinical outcomes | ||||||||||||
| Hospital LOS, db | 13.63 (1.81) | 13.18 (0.5) | .02 | 13.51 (0.58) | 16.48 (0.18) | .00 | 14.01 (2.11) | 13.18 (0.56) | .00 | 10.67 (0.35) | 11.19 (0.07) | .00 |
| In-hospital death, No. (%) | 20 (21.3) | 286 (15.7) | .13 | 369 (20.6) | 3557 (14.7) | <.001 | 35 (24.1) | 439 (19.5) | .06 | 738 (26.1) | 8238 (15.0) | <.001 |
| SOFA scorec | ||||||||||||
| At the time of ABG | ||||||||||||
| CVSOFA | 0.61 (0.2) | 0.73 (0.06) | <.001 | 0.98 (0.07) | 0.85 (0.02) | <.001 | 1.05 (0.15) | 1.03 (0.03) | .14 | 1.13 (0.03) | 0.99 (0.01) | <.001 |
| RSOFA | 1.56 (0.32) | 1.26 (0.06) | <.001 | 1.87 (0.12) | 1.4 (0.03) | <.001 | 2.02 (0.17) | 1.44 (0.04) | <.001 | 1.81 (0.04) | 1.38 (0.01) | <.001 |
| SOFA | 6.03 (0.75) | 5.82 (0.15) | <.001 | 4.98 (0.25) | 5.26 (0.06) | <.001 | 5.09 (0.54) | 5.37 (0.09) | <.001 | 5.27 (0.1) | 5.22 (0.03) | <.001 |
| 24 h After ABG | ||||||||||||
| CVSOFA | 1.52 (0.2) | 1.36 (0.05) | <.001 | 7.02 (0.29) | 6.33 (0.07) | <.001 | 1.56 (0.15) | 1.32 (0.03) | <.001 | 1.48 (0.03) | 1.26 (0.01) | <.001 |
| RSOFA | 1.58 (0.28) | 1.27 (0.07) | <.001 | 1.87 (0.1) | 1.4 (0.03) | <.001 | 2.01 (0.18) | 1.45 (0.04) | <.001 | 1.79 (0.04) | 1.37 (0.01) | <.001 |
| SOFA | 7.17 (0.77) | 5.62 (0.17) | <.001 | 8.17 (0.27) | 7.26 (0.08) | <.001 | 7.49 (0.54) | 6.3 (0.1) | <.001 | 7.2 (0.1) | 6.3 (0.02) | <.001 |
Abbreviations: ABG, arterial blood gas; CVSOFA, cardiovascular Sequential Organ Failure Assessment; LOS, length of stay; NA, not applicable; RSOFA, respiratory Sequential Organ Failure Assessment; SOFA, Sequential Organ Failure Assessment.
SI conversion factors: To convert creatinine to micromoles per liter, multiply by 88.4; lactate to millimoles per liter, multiply by 0.111.
The table presents baseline patient demographic (age, sex) and clinical characteristics (SOFA, CVSOFA) at the time of ABG, stratified by race and ethnicity. When applicable, SEs are provided. These were obtained using simple bootstrap with 100 iterations.
Length of stay computed for survivors only.
SOFA scores available only for patients in the Electronic Intensive Care Unit–Clinical Research Database and Medical Information Mart for Intensive Care databases.
Clinical Outcomes
Across all racial and ethnic groups, patients with hidden hypoxemia had higher in-hospital mortality than patients without hidden hypoxemia (Table 2). The difference was significant for Black and White patients (eg, Black patients: 369 [21.1%] vs 3557 [15.0%]; P < .001). This association persisted even when adjusted for age, sex, SOFA score. However, there was no differences in length of stay for patients with and without hidden hypoxemia when considering survivors only (Table 2).
Across all racial and ethnic subgroups, patients with hidden hypoxemia had a significantly higher mean (SE) serum creatinine level (1.96 [0.04] mg/dL vs 1.69 [0.01] mg/dL [to convert to micromoles per liter, multiply by 88.4]; P < .001) and serum lactate level (3.15 [0.09] mg/dL vs 2.66 [0.02] mg/dL [to convert to millimoles per liter, multiply by 0.111]; P < .001) before the ABG measurement than patients without hypoxemia. Patients with hidden hypoxemia continued to maintain significantly higher mean (SE) serum creatinine (1.86 [0.05] mg/dL vs 1.63 [0.01] mg/dL; P < .001) and lactate (2.83 [0.14] mg/dL vs 2.83 [0.14] mg/dL; P < .001) values after the ABG measurements. When comparing values before and after the ABG, serum lactate demonstrated a smaller mean (SE) decrease among patients with hidden hypoxemia overall (−0.54 [0.12] vs −0.79 [0.03]; P < .001) and in all racial and ethnic groups except Asian patients. However, the difference in serum creatinine levels before and after the ABG measurement was not consistent across race and ethnicity and hidden hypoxemia status.
Hidden Hypoxemia and RSOFA Score
The likelihood of hidden hypoxemia increased with higher RSOFA values and was highest among patients with the highest RSOFA scores at baseline (ie, at the time of ABG) (eTable 10 in the Supplement). The presence of hidden hypoxemia, irrespective of RSOFA score, was associated with higher risk of mortality (eTable 11 in the Supplement).
Risk of Hidden Hypoxemia by Race and Ethnicity
Hidden hypoxemia occurred across racial and ethnic groups, but the risk differed among subgroups when calculated with a risk-threshold of 5%. White patients had a 5% risk of hidden hypoxemia at an Spo2 of 94%, whereas this 5% risk occurred at a higher Spo2 for Black (97%), Hispanic (97%), and Asian (95%) patients. The risk of hidden hypoxemia at an SpO2 of 93% to 96% is 6.5% in White patients, increasing to 6.6% for Hispanic patients and up to 10.9% for Black and Asian patients (a 68% higher relative risk). In conjunction with eTable 8 in the Supplement, which shows more granular data, a clinician could select their threshold for an acceptable risk of hidden hypoxemia by Spo2 target by selecting the highest Spo2 with a fixed risk of hidden hypoxemia. To ensure a risk of hidden hypoxemia of less than 10%, Spo2 should be greater than 93% among Asian patients (mean [SE] risk, 11.0% [5.0]), 96% among Black patients (mean [SE] risk, 8.2% [1.0]); 92% among Hispanic patients (mean [SE] risk, 18.4% [6.0]), and 93% among White patients (mean [SE] risk, 10.6% [1.0]) (Table 3).
Table 3. Risk of Hidden Hypoxemia by Race and Ethnicitya.
| Spo2 group, % | Asian patients | Black patients | Hispanic patients | White patients | ||||
|---|---|---|---|---|---|---|---|---|
| Mean (SE) % | No. | Mean (SE) % | No. | Mean (SE) % | No. | Mean (SE) % | No. | |
| 88-92 | 24.2 (5.0) | 150 | 26.1 (1.0) | 1993 | 28.3 (4.0) | 182 | 22.8 (1.0) | 5304 |
| 93-96 | 6.8 (2.0) | 412 | 10.9 (1.0) | 5454 | 6.6 (1.0) | 594 | 6.5 (0.0) | 15 893 |
| 97-100 | 2.6 (1.0) | 1357 | 4.3 (0.0) | 18 585 | 4.5 (1.0) | 1621 | 2.7 (0.0) | 36 426 |
Abbreviations: Sao2, oxygen saturation; Spo2, pulse oximeter.
The risk of hidden hypoxemia (ie, Spo2 ≥88%; Sao2 <88%) characterized by race and ethnicity. Each cell in the table represents the percentage of patients with hidden hypoxemia in the considered racial and ethnic subgroup for a given pulse oximetry grouping. The corresponding bootstrapped SE of the mean for 100 iterations is also provided. The number indicates the total number of patients in each subgroup at that given pulse oximetry grouping (with and without hidden hypoxemia).
Discussion
To our knowledge, this study is the first to characterize the prevalence of hidden hypoxemia (Sao2 <88%, but Spo2 ≥88%) by race and ethnicity in hospitalized patients’ first ABG measurements in their hospitalization across 5 large US databases. Racial and ethnic disparities in the incidence of hidden hypoxemia in the hospital are worrying because low oxygen saturation levels, when undetected, can lead to complications in the short and long term.
This analysis demonstrates that all racial and ethnic groups experienced discrepancies in pulse oximetry resulting in hidden hypoxemia; however, it was more prevalent in Asian, Black, and Hispanic patients than White patients. There is a risk of hidden hypoxemia at all Spo2 values, and this risk increases as Spo2 approaches 88%. When hidden hypoxemia occurs, despite similar organ dysfunction scores at the time of the ABG measurement, it was associated with increased in-hospital mortality, increased short-term future organ dysfunction (ie, SOFA, RSOFA, and CVSOFA scores), and increased laboratory findings (ie, lactate and creatinine levels), even when adjusting for covariates, such as age, sex, and SOFA score. These continue to hold true when restricted to ABGs with carboxyhemoglobin and methemoglobin levels less than 2% (eTable 12 in the Supplement). Furthermore, the presence of hidden hypoxemia was independently associated with increased mortality for all RSOFA values, suggesting that hidden hypoxemia and RSOFA are complementary. Finally, to maintain a risk of hidden hypoxemia less than 10%, each race and ethnicity would have a different Spo2 threshold: Asian, 93%; Black, 95%; Hispanic, 92%, White, 93%) (Table 3; eTable 9 in the Supplement).
As this analysis was restricted to the first ABG measurement in a hospitalization, the clinician could not have been aware of hidden hypoxemia prior to the ABG test. It is therefore unknowable how long a patient was truly hypoxemic before their ABG measurement, although the clinician would be aware of hypoxemia once they had the ABG results. Despite similar organ dysfunction scores at the time of the ABG measurement, patients with hidden hypoxemia had greater laboratory abnormalities (ie, for lactate and creatinine levels) before the ABG measurement that persisted for at least 24 hours, suggesting that these patients may have more severe illness. This study was not designed to assess causality; it is both plausible that the patient’s illness could be causative of hidden hypoxemia (and thus be a marker of dysfunction) and that hidden hypoxemia for an unknown (perhaps prolonged) duration resulted in worse organ dysfunction.
The effects of hypoxia can be organized by the duration a patient experiences hypoxia. Brief, acute episodes of hypoxia have been associated with electrocardiogram changes14 and, in healthy patients, brief cognitive impairment without sustained long-term cognitive changes.15 However, hypoxia has been associated with increased oxidative stress, reactive-oxygen species, angiogenesis, hypoxia-inducible factors, and systemic and vascular inflammation with endothelial dysfunction.16,17,18 Critically ill patients may have impaired tolerance of these changes,17 and hypoxia can result in kidney injury and lactic acidosis.19,20,21
Hidden hypoxemia was prevalent across all racial and ethnic subgroups, but it disproportionately affected certain groups because pulse oximeters are not tested or calibrated on an adequate number of individuals with varying skin pigmentation. Since 2013, the FDA has required that the test sample for pulse oximeters include at least 15% people with diverse skin pigmentation, including 2 individuals with darkly pigmented skin.22 However, this sampling does not reflect the United States 2010 census of Asian (5.9% of population), Black (13.4%), Hispanic (18.5%), and White (60.1%) individuals.23,24 Going forward, population differences will only increase in relevance as the United States becomes more racially and ethnically diverse.25 Furthermore, although older studies questioned the accuracy of pulse oximetry in critical illness, sample sizes were too small to examine the issue of skin color and race and ethnicity in meaningful detail (eFigure 6 in the Supplement).3,4,5,6,7
The results of this study highlight several societal and medical issues. First, pulse oximeters are inadequately tested or calibrated in hospitalized patients, despite often being the intended population. Second, a 2% accuracy range (4% total) is too wide at low blood oxygenation levels, and third, pulse oximeters are insufficiently tested across different racial and ethnic groups prior to approval by the FDA. As the results of this study demonstrate, this combination has unintended negative health outcomes. By providing a data-driven approach to identify hidden hypoxemia using pulse oximetry, this study is a step toward greater health care equity.
While a short-term solution to hidden hypoxemia may be to more frequently sample ABG values, such a strategy is invasive and inefficient.26 If anything, greater ABG sampling is merely a stopgap to cover the use of imperfect medical technology. The important message is that health care devices, like predictive algorithms and medications, must be designed more inclusively to achieve comparable measurement accuracy irrespective of race and ethnicity. As noted in previous studies,1,27,28 pulse oximetry devices are not reliably accurate and do not capture blood oxygenation readings equally across different skin colors. In the meantime, prudent clinicians should note the Spo2 reading at the time the ABG is drawn to accurately identify any Spo2-to-Sao2 discrepancy once the ABG result is reported.
It is important to be cognizant of the patient population in which pulse oximeters used in critical care are validated. There is a need for more transparency in the labeling of all patient care devices, including the detailed characteristics of groups on which they were evaluated. To further achieve more equitable health outcomes, we call for reinforced testing and recalibration of health care devices—across all target patient populations.
Limitations
This retrospective EHR analysis has inherent limitations that were systematically addressed. First, Sao2-Spo2 pairs combine measurements that are not always collected simultaneously. Analysis was thus restricted to Spo2 values recorded in the 5 minutes preceding the ABG test. Shock and critical illness, in conjunction with other comorbid conditions (eg, peripheral arterial disease, diabetes), may further affect pulse oximetry accuracy and need further characterization for proper adjustment for confounding beyond CVSOFA score.
There was high missingness, especially in SOFA scores and laboratory values, that was accounted for with missing flags during regression analysis; SOFA scores were only calculated for eICU-CRD, MIMIC-III, and MIMIC-IV data and were not calculated for Emory or Grady data. Multiple imputation methods could improve robustness. Additionally, the EHR data did not record Spo2 signal quality or pulse oximeter brand, leading to unclear knowledge of Spo2 accuracy and homogeneity.
There may be a selection bias in acquiring ABG measurements. Given similar SOFA scores at the time of testing, White patients were significantly more likely to receive the criterion-standard test. It is plausible that there was selection bias with underdetection of hidden hypoxemia among Asian, Black, and Hispanic patients. The disparities in clinical outcomes may be underestimated if more ABG tests were performed in these racial and ethnic subgroups. Additionally, these retrospective analyses reflect associations; future studies should be designed to assess causality.
Conclusions
In this study, all racial and ethnic subgroups experienced high variability in arterial oxygen saturation for fixed pulse oximetry levels, with a greater discrepancy in patients self-reporting as Asian, Black, and Hispanic than White. Small but statistically significant differences in the bias of Spo2 measurements (vs true Sao2 measurements) were associated with increased incidence of hidden hypoxemia (Sao2<88% despite Spo2 ≥88%). Although demographically and clinically similar to patients without hypoxemia at baseline ABG measurement, those with hidden hypoxemia had higher rates of organ dysfunction 24 hours later and higher in-hospital mortality. Validation of all health technologies, including pulse oximetry, must be performed across a wider range of patient populations to avoid perpetuating harm from miscalibration.22
eAppendix 1. Supplementary Methods
eAppendix 2. Supplementary Results
eFigure 1. Spo2-Sao2 by Race and Ethnicity, for All ABG Measurements Taken Throughout 268 904 Hospital Encounters
eFigure 2. Spo2-Sao2 by Race and Ethnicity, for the First ABG Measurement per 87 971 Hospital Encounters
eFigure 3. Spo2-Sao2 by Race and Ethnicity, for the First ABG Measurement per 87 971 Hospital Encounters, Stratified by Age Group
eFigure 4. Spo2-Sao2 by Race and Ethnicity, for the First ABG Measurement per 87 971 Hospital Encounters, Stratified by Sex
eFigure 5. Spo2-Sao2 by Race and Ethnicity, for the First ABG Measurement per 87 971 Hospital Encounter, Stratified by Cardiovascular SOFA Score
eFigure 6. Directed Acyclic Graph for Race and Ethnicity, Hidden Hypoxemia, Organ Dysfunction, and Mortality
eTable 1. Literature Review
eTable 2. SOFA Score Components, as per Vincent et al, 1996
eTable 3. Other Patient Characteristics
eTable 4. Characterization of Variable Missingness Across the 5 EHR Data Sets
eTable 5. Spo2 Variability for All Spo2 Values Within the 5 Minutes Preceding the ABG Measurement
eTable 6. Percentage of Encounters With Arterial Blood Gases, by Race and Ethnicity
eTable 7. Rate of ABG Measurements Obtained Throughout a Hospital Encounter by Race and Ethnicity, Stratified by Cardiovascular SOFA Score
eTable 8. Descriptive Statistics for All Sao2-Spo2 Pairs With Spo2 of at least 88%
eTable 9. Total Number of Patients per Spo2 Level and Characterization of Hidden Hypoxemia Incidence, Stratified by Race and Ethnicity
eTable 10. Characterization of the Distribution of Hidden Hypoxemia by Respiratory SOFA Score
eTable 11. Distribution of In-Hospital Mortality, Stratified by Respiratory SOFA Score and Presence of Hidden Hypoxemia
eTable 12. Descriptive Statistics for Patients With Hidden Hypoxemia vs Patients Without Hypoxemia, Carboxyhemoglobin Less than 2 and Methemoglobin Less Than 2
References
- 1.Sjoding MW, Dickson RP, Iwashyna TJ, Gay SE, Valley TS. Racial bias in pulse oximetry measurement. N Engl J Med. 2020;383(25):2477-2478. doi: 10.1056/NEJMc2029240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.US Food and Drug Administration . Pulse oximeter accuracy and limitations: FDA safety communication. February 19, 2021. Accessed March 22, 2021. https://www.fda.gov/medical-devices/safety-communications/pulse-oximeter-accuracy-and-limitations-fda-safety-communication
- 3.Seguin P, Le Rouzo A, Tanguy M, Guillou YM, Feuillu A, Mallédant Y. Evidence for the need of bedside accuracy of pulse oximetry in an intensive care unit. Crit Care Med. 2000;28(3):703-706. doi: 10.1097/00003246-200003000-00017 [DOI] [PubMed] [Google Scholar]
- 4.Van de Louw A, Cracco C, Cerf C, et al. Accuracy of pulse oximetry in the intensive care unit. Intensive Care Med. 2001;27(10):1606-1613. doi: 10.1007/s001340101064 [DOI] [PubMed] [Google Scholar]
- 5.Perkins GD, McAuley DF, Giles S, Routledge H, Gao F. Do changes in pulse oximeter oxygen saturation predict equivalent changes in arterial oxygen saturation? Crit Care. 2003;7(4):R67. doi: 10.1186/cc2339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilson BJ, Cowan HJ, Lord JA, Zuege DJ, Zygun DA. The accuracy of pulse oximetry in emergency department patients with severe sepsis and septic shock: a retrospective cohort study. BMC Emerg Med. 2010;10:9. doi: 10.1186/1471-227X-10-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh AK, Sahi MS, Mahawar B, Rajpurohit S. Comparative evaluation of accuracy of pulse oximeters and factors affecting their performance in a tertiary intensive care unit. J Clin Diagn Res. 2017;11(6):OC05-OC08. doi: 10.1183/1393003.congress-2017.PA2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vandenbroucke JP, Von Elm E, Altman DG, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration; translation to Russian. Digital Diagnostics. 2021;2(2):1-10. doi: 10.17816/dd70821 [DOI] [Google Scholar]
- 9.Pollard T, Johnson A, Raffa J, Celi LA, Badawi O, Mark R. eICU Collaborative Research Database. PhysioNet. April 15, 2019. Accessed February 15, 2020. https://physionet.org/content/eicu-crd/2.0/
- 10.Pollard TJ, Johnson AEW, Raffa JD, Celi LA, Mark RG, Badawi O. The eICU Collaborative Research Database, a freely available multi-center database for critical care research. Sci Data. 2018;5:180178. doi: 10.1038/sdata.2018.178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldberger AL, Amaral LAN, Glass L, et al. PhysioBank, PhysioToolkit, and PhysioNet: components of a new research resource for complex physiologic signals. Circulation. 2000;101(23):E215-E220. doi: 10.1161/01.cir.101.23.e215 [DOI] [PubMed] [Google Scholar]
- 12.Johnson AEW, Pollard TJ, Shen L, et al. MIMIC-III, a freely accessible critical care database. Sci Data. 2016;3:160035. doi: 10.1038/sdata.2016.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vincent J-L, Moreno R, Takala J, et al. ; the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine . The SOFA (Sepsis-Related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22(7):707-710. doi: 10.1007/s001340050156 [DOI] [PubMed] [Google Scholar]
- 14.Coustet B, Lhuissier FJ, Vincent R, Richalet JP. Electrocardiographic changes during exercise in acute hypoxia and susceptibility to severe high-altitude illnesses. Circulation. 2015;131(9):786-794. doi: 10.1161/CIRCULATIONAHA.114.013144 [DOI] [PubMed] [Google Scholar]
- 15.Bickler PE, Feiner JR, Lipnick MS, Batchelder P, MacLeod DB, Severinghaus JW. Effects of acute, profound hypoxia on healthy humans: implications for safety of tests evaluating pulse oximetry or tissue oximetry performance. Anesth Analg. 2017;124(1):146-153. doi: 10.1213/ANE.0000000000001421 [DOI] [PubMed] [Google Scholar]
- 16.Dewan NA, Nieto FJ, Somers VK. Intermittent hypoxemia and OSA: implications for comorbidities. Chest. 2015;147(1):266-274. doi: 10.1378/chest.14-0500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacIntyre NR. Tissue hypoxia: implications for the respiratory clinician. Respir Care. 2014;59(10):1590-1596. doi: 10.4187/respcare.03357 [DOI] [PubMed] [Google Scholar]
- 18.Colgan SP, Campbell EL, Kominsky DJ. Hypoxia and mucosal inflammation. Annu Rev Pathol. 2016;11:77-100. doi: 10.1146/annurev-pathol-012615-044231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jansen TC, van Bommel J, Schoonderbeek FJ, et al. ; LACTATE study group . Early lactate-guided therapy in intensive care unit patients: a multicenter, open-label, randomized controlled trial. Am J Respir Crit Care Med. 2010;182(6):752-761. doi: 10.1164/rccm.200912-1918OC [DOI] [PubMed] [Google Scholar]
- 20.Kruse O, Grunnet N, Barfod C. Blood lactate as a predictor for in-hospital mortality in patients admitted acutely to hospital: a systematic review. Scand J Trauma Resusc Emerg Med. 2011;19:74. doi: 10.1186/1757-7241-19-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scholz H, Boivin FJ, Schmidt-Ott KM, et al. Kidney physiology and susceptibility to acute kidney injury: implications for renoprotection. Nat Rev Nephrol. 2021;17(5):335-349. doi: 10.1038/s41581-021-00394-7 [DOI] [PubMed] [Google Scholar]
- 22.US Food and Drug Administration . Pulse oximeters—premarket notification submissions [510(k)s]: guidance for industry and Food and Drug Administration staff. March 2013. Accessed April 2, 2021. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/pulse-oximeters-premarket-notification-submissions-510ks-guidance-industry-and-food-and-drug
- 23.US Census Bureau . 2017 National population projections tables: main series. Accessed April 2, 2021. https://www.census.gov/data/tables/2017/demo/popproj/2017-summary-tables.html
- 24.US Census Bureau . QuickFacts: United States. Accessed April 4, 2021. https://www.census.gov/quickfacts/fact/table/US/PST045219
- 25.Ghosh I. Visualizing the US population by race. December 28, 2020. Accessed April 2, 2021. https://www.visualcapitalist.com/visualizing-u-s-population-by-race/
- 26.Blum FE, Lund ET, Hall HA, Tachauer AD, Chedrawy EG, Zilberstein J. Reevaluation of the utilization of arterial blood gas analysis in the intensive care unit: effects on patient safety and patient outcome. J Crit Care. 2015;30(2):438.e1-438.e5. doi: 10.1016/j.jcrc.2014.10.025 [DOI] [PubMed] [Google Scholar]
- 27.Feiner JR, Severinghaus JW, Bickler PE. Dark skin decreases the accuracy of pulse oximeters at low oxygen saturation: the effects of oximeter probe type and gender. Anesth Analg. 2007;105(6)(suppl):S18-S23. doi: 10.1213/01.ane.0000285988.35174.d9 [DOI] [PubMed] [Google Scholar]
- 28.Bickler PE, Feiner JR, Severinghaus JW. Effects of skin pigmentation on pulse oximeter accuracy at low saturation. Anesthesiology. 2005;102(4):715-719. doi: 10.1097/00000542-200504000-00004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Supplementary Methods
eAppendix 2. Supplementary Results
eFigure 1. Spo2-Sao2 by Race and Ethnicity, for All ABG Measurements Taken Throughout 268 904 Hospital Encounters
eFigure 2. Spo2-Sao2 by Race and Ethnicity, for the First ABG Measurement per 87 971 Hospital Encounters
eFigure 3. Spo2-Sao2 by Race and Ethnicity, for the First ABG Measurement per 87 971 Hospital Encounters, Stratified by Age Group
eFigure 4. Spo2-Sao2 by Race and Ethnicity, for the First ABG Measurement per 87 971 Hospital Encounters, Stratified by Sex
eFigure 5. Spo2-Sao2 by Race and Ethnicity, for the First ABG Measurement per 87 971 Hospital Encounter, Stratified by Cardiovascular SOFA Score
eFigure 6. Directed Acyclic Graph for Race and Ethnicity, Hidden Hypoxemia, Organ Dysfunction, and Mortality
eTable 1. Literature Review
eTable 2. SOFA Score Components, as per Vincent et al, 1996
eTable 3. Other Patient Characteristics
eTable 4. Characterization of Variable Missingness Across the 5 EHR Data Sets
eTable 5. Spo2 Variability for All Spo2 Values Within the 5 Minutes Preceding the ABG Measurement
eTable 6. Percentage of Encounters With Arterial Blood Gases, by Race and Ethnicity
eTable 7. Rate of ABG Measurements Obtained Throughout a Hospital Encounter by Race and Ethnicity, Stratified by Cardiovascular SOFA Score
eTable 8. Descriptive Statistics for All Sao2-Spo2 Pairs With Spo2 of at least 88%
eTable 9. Total Number of Patients per Spo2 Level and Characterization of Hidden Hypoxemia Incidence, Stratified by Race and Ethnicity
eTable 10. Characterization of the Distribution of Hidden Hypoxemia by Respiratory SOFA Score
eTable 11. Distribution of In-Hospital Mortality, Stratified by Respiratory SOFA Score and Presence of Hidden Hypoxemia
eTable 12. Descriptive Statistics for Patients With Hidden Hypoxemia vs Patients Without Hypoxemia, Carboxyhemoglobin Less than 2 and Methemoglobin Less Than 2