Highlights
-
•
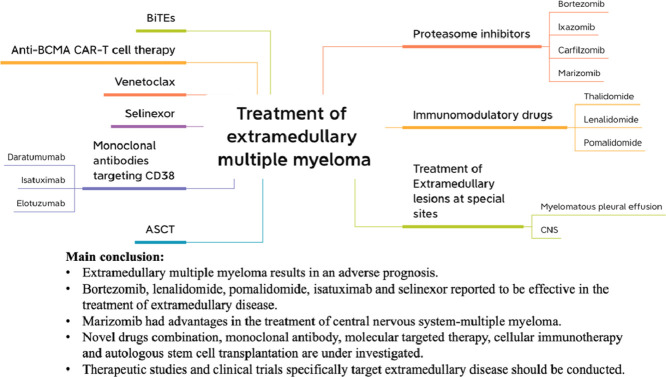
Extramedullary multiple myeloma results in an adverse prognosis.
-
•
Novel agents such as bortezomib, lenalidomide, pomalidomide, isatuximab and selinexor showed efficacy and were recommended to treat EMD.
-
•
For EMD at special sites, marizomib has advantages in the treatment of CNS-MM; Daratumumab combining with intrapleural bortezomib administration is active in treating myelomatous pleural effusion.
-
•
Based on treatment experience of EMD in our department, we summarized treatment approach for EMD.
Keywords: Extramedullary disease, Multiple myeloma, Treatment
Abstract
Extramedullary disease (EMD) is characterized by plasma cells outside of bone marrow in multiple myeloma (MM) patients, which results in an adverse prognosis. The cornerstone of treatment consists of combination therapy including proteasome inhibitors, immunomodulatory agents, steroids, followed by consolidative autologous hematopoietic stem cell transplantation in eligible patients. This review summarized the recent advances in the treatment of EMD. Bortezomib based therapy showed efficacy and was recommended to treat EMD. Marizomib had advantages in the treatment of central nervous system-multiple myeloma (CNS-MM) because of its good central nervous system penetrability. Immunomodulatory drugs such as lenalidomide and pomalidomide have been reported to be effective. Isatuximab and selinexor were also active. Based on the treatment experience of EMD in our department, we summarized treatment approach for EMD. However, the benefits of patients with EMD from the new era of novel drugs were limited. Novel drugs combination, monoclonal antibody, molecular targeted therapy, cellular immunotherapy and autologous stem cell transplantation (ASCT) are under investigation. Therapeutic studies and clinical trials specifically target EMD should be conducted. Hopefully, these treatment options for EMD will be demonstrated efficacy in the future.
Graphical abstract
Introduction
Multiple myeloma (MM) is characterized by the proliferation of malignant clonal plasma cells (PCs) accumulating in the bone marrow (BM) [1]. Extramedullary disease (EMD) is defined by the presence of PCs outside the BM, such as soft tissue and organs, especially late in the disease course [2,3]. Clinically, there is a lack of consensus on the classification of EMD. Kumar et al. [4] identified six types of EMD: a)Solitary plasmacytoma: evidence of clonal PCs in bone or soft tissue lesions confirmed by biopsy with no clonal PCs in BM, no imaging abnormality and absence of CRAB criteria; b)Solitary plasmacytoma with minimal marrow involvement (<10% clonal PCs in BM); c)EM-B(bone-related): soft tissue masses associated with osteolytic bone lesions; d)EM-S(soft tissue-related): soft tissue infiltration of PCs not contiguous with bone lesions; e)Organ or central nervous system (CNS) infiltrating EMD; f)Plasma cell leukemia (PCL). EMD can be present throughout the course of the disease [5]. There are few data focusing on the incidence of EMD. According to the literature, approximately 7%-17% MM patients found EMD at the time of diagnosis, and 6%-20% MM patients found EMD during the course of the disease, depending on the studies [6], [7], [8].
A considerable number of studies have confirmed the presence of EMD, either at diagnosis of MM or at relapse , was a poor prognostic factor. In a recent retrospective study, Lee et al. reported 275 newly diagnosed MM patients, 54 of whom had EMD at diagnosis of MM. After a median follow-up of 24.6 months, median overall survival (OS) and median progress-free survival (PFS) in patients with EMD were shorter than those patients with non-EMD (median OS in EMD patients was 44.3 months, p=0.006; median PFS was 20.3 months and 29.1 months, p=0.035) [9]. A study on relapsed MM patients showed patients without EMD had significantly longer OS than patients with EMD (109 vs. 38 months; P<0.001) [5]. Table 1 summarized outcomes of EMD in various studies. The mechanisms of EMD are only partially known and the best treatment strategies so far are inconclusive [10]. This review presented a summary of the current treatment strategies for MM patients with EMD.
Table 1.
Outcomes of EMD in various studies
| Author | Patient group | Type of EMD | Survival analysis | Reference | |
|---|---|---|---|---|---|
| PFS | OS | ||||
| Lee et al | Newly diagnosed MM with EMD(54/275) | EM-B(47),EM-S(7) | 20.3 months in EMD vs. 29.1 in non-EMD(P = 0.035) | 44.3 months in EMD vs. N/A in non-EMD(P = 0.006) | [9] |
| Pour et al | EMD at relapse(55/226) | EM-B(23),EM-S(32) | N/A | 45 months in EM-B vs 30 months in EM-S(p=0.022);38 months in EMD vs.109 months in non-EMD(P<0.001) | [5] |
| Mangiacavalli et al | EMD at relapse(93/329) | EM-B(34),EM-S(48),both EM-B and EM-S(11) | N/A | 28.8 months in EM-B vs 19.2 months in EM-S(p=0.006);24 months in EMD vs.132 months in non-EMD(P <0.001) | [93] |
| Beksac et al | EMD at diagnosis(130) | EM-B(38),EM-S(92) | 51.7 months in EM-B vs 38.9 months in EM-S(p=0.034) | 46.5 months in EM-B vs not reached in EM-S(p=0.002) | [94] |
| EMD at relapse(96) | EM-B(12),EM-S(84) | 20.9 months in EM-B vs 9.1 months in EM-S(p=0.249) | 11.4 months in EM-B vs 39.8 months in EM-S(p=0.093) | ||
| Montefusco et al | Newly diagnosed MM with EMD(267/2332) | EM-B(243),EM-S(12) | 25.3 months in EMD vs. 25.2 in non-EMD | 63.5 months in EMD vs.79.9 in non-EMD(P = 0.01) | [95] |
| Kumar et al | Newly diagnosed MM with EMD(44/271) | EM-B(30),EM-S(8),both EM-B and EM-S(6) | 18 months in EMD vs. 44 in non-EMD(P <0.001) | 32 months in EMD vs.100 in non-EMD(P < 0.001) | [65] |
Proteasome inhibitors (PIs)
Bortezomib
Bortezomib, with extensive tissue penetration [11], is commonly used for EMD treatment currently, as recommended by Mayo Clinic in 2017 [12]. Laura et al. [13] reported EMD disappeared after bortezomib therapy in three of four relapsed or refractory multiple myeloma (RRMM) patients. In the study by Landau et al. [14], 14 patients with EMD received sequential bortezomib, liposomal doxorubicin and dexamethasone (BDD) followed by thalidomide and dexamethasone with/without bortezomib (BTD or TD), and the overall survival rate was 86% (12/14). Lakshman et al. [15] studied 141 RRMM patients who had been treated with VDT-PACE (dexamethasone/thalidomide/cisplatin/doxorubicin/cyclophosphamide/etoposide/bortezomib). The overall response rate (ORR) did not differ between patients with EMD versus those without EMD at the initiation of VDT-PACE treatment (57.1% vs. 52.9%; P = 0.631). This suggests that bortezomib-containing regimen may alleviate the poor outcome of EMD in RRMM.
Ixazomib
The study of ixazomib-based regimens in EMD patients was limited. Ixazomib in combination with lenalidomide and dexamethasone (IRD) has been proved to have a significant improvement in PFS for RRMM patients based on the results of the phase 3 TOURMALINE-MM1 study [16]. However, Minarik et al. found patients with EMD did not benefit from IRD treatment. The PFS of patients in the IRD cohort was similar to patients in the RD (lenalidomide, dexamethasone) cohort (6.5 vs 10.9 months, HR 1.24 [0.54–2.86]) [17]. The efficacy of ixazomib in the EMD needs to be confirmed in the future study.
Carfilzomib
In relapsed MM patients, adding carfilzomib to lenalidomide and dexamethasone significantly improved PFS [18]. In the study of Zhou et al., 45 RRMM patients with EMD received carfilzomib-based therapy. Thirty-three of the 45 patients had imaging follow-up data that could be used to determine the best response of EMD. They observed serological and extramedullary ORR of 59% and 27% with nine patients partial response (PR), nine stable disease (SD), nine progressive disease (PD) and six mixed response of EMD lesions. The median PFS and OS were five (95% CI, 3.5–6.5) and ten (95% CI, 7.5–12.5) months, respectively [19]. Muchtar et al. reported EMD patients who received carfilzomib containing regimens had a shorter duration of response (DOR) compared with non-EMD patients (3.9 months vs. 9.3 months, p=0.06). The ORR did not reach a statistical difference between the two entities (40% vs. 49%, p=0.39), however, the clinical benefit response (CBR) rate in patients with EMD was significantly lower than in those without EMD, suggesting a higher rate of true non-responders in the presence of EMD [20]. Therefore, carfilzomib has a limited effect on EMD.
Marizomib
Marizomib is a novel and irreversible PI for the treatment of RRMM patients and is able to cross the blood-brain barrier [21, 22]. However, limited clinical studies on marizomib, compared with other PIs, have been conducted especially for the EMD management. Intriguingly, marizomib had shown central nervous system-multiple myeloma (CNS-MM) efficacy in case reports [23].
Immunomodulatory drugs (IMiDs)
Thalidomide
Thalidomide is the first-generation immunomodulatory drug for the treatment of MM. Laura reported none of 11 patients with EMD responded to thalidomide treatment as a single agent [24]. Meanwhile, in the study by Bladé et al. [25], none of five patients with EMD responded to thalidomide. Avigdor et al. [26] and Anagnostopoulos et al. [27] reported MM patients treated with thalidomide developed EMD despite a good response in BM. The lack of efficacy of thalidomide on EMD has been reported by others [28]. Consequently, thalidomide has been proved to be ineffective in the treatment of EMD.
Lenalidomide
Lenalidomide was recommended as a first-line treatment for newly diagnosed MM. Calvo-Villas et al. [29] analyzed 18 EMD patients treated with lenalidomide in combination with dexamethasone. After a median treatment period of seven, 61.1% (eleven patients) showed response in EMD including complete disappearance in 44.4% (eight patients) and reduction in size in 16.6% (three patients). The response rate of this study was similar to previously reported with bortezomib [11,[30], [31], [32]] and lenalidomide [33]. The median OS and PFS were 14.6 and 9.8 months, respectively. They also confirmed response rate of EMD after lenalidomide treatment was higher than thalidomide in their study series [24,25].
Pomalidomide
Pomalidomide is approved for the treatment of RRMM, especially for those who are refractory to lenalidomide [34]. Short et al. [35] reported 13 patients with EMD and all of them had received novel agents with 100% patients exposed to thalidomide or lenalidomide and 78% exposed to bortezomib. After pomalidomide plus low-dose dexamethasone treatment, two patients achieved CR (with complete disappearance of EMD), two patients had PR (≥ 50% reduction in EMD) and two SD. All of the patients with EMD had significantly shorter OS compared with those without EMD. In the study by Jiménez-Segura et al. [36], only two EM-B patients of 21 had extramedullary response to pomalidomide-dexamethasone therapy. The median PFS from the start of treatment with pomalidomide-dexamethasone was 1.7 months and the median OS of 4.5 months. This study showed pomalidomide-dexamethasone therapy has limited efficacy in patients with EMD, especially EM-S. In our experience [37], we conducted an observational study of six patients with EMD whose prior multi-lines of therapies were four (range 2-5). After extramedullary relapse, they received pomalidomide-based treatment (bortezomib/dexamethasone/pomalidomide, pomalidomide/adriamycin/dexamethasone, daratumumab/pomalidomide/dexamethasone,dexamethasone/pomalidomide/cisplatin/adriamycin/cyclophosphamide/etoposide, pomalidomide/ixazomib/dexamethasone), 83% of patients had extramedullary response with 50% CR and 33% PR, and one patient experienced extramedullary progression. For serological response, one patient got CR, 4 got PR, and 1 got PD. The median PFS was 5 months and the median OS was 8 months from diagnosis of EMD. Therefore, the efficacy of pomalidomide on EMD needs further investigation, in combination with other novel agents might be considered.
Monoclonal antibodies targeting CD38
Daratumumab
Daratumumab showed limited efficacy in EMD and this phenomenon can be explained by decreased CD38 expression on extramedullary PCs [38]. A real-world retrospective study revealed modest efficacy of single-agent daratumumab in 41 RRMM patients, including 32% of patients with EMD. The ORR was 24.4% and CBR was 39%. The median OS and median PFS were 1.9 months and 6.5 months, respectively [39]. In the SIRIUS trial, 106 patients received daratumumab 16 mg/kg, 14 of whom had EMD and only three patients responded [40]. Pick et al. reported only two of nine heavily pretreated patients with EMD who were given daratumumab achieved PR, but both of them got PD in 50 and 85 days [41].
Isatuximab
Isatuximab is a monoclonal antibody that binds to a specific epitope on the human cell CD38 receptor [42]. The ICARIA-MM trial has demonstrated the benefit of combining isatuximab with pomalidomide and dexamethasone in RRMM. In this trial, the median PFS was 11.5 months (95% CI, 8.9-13.9) in the isatuximab-pomalidomide-dexamethasone (Isa-Pd) group versus 6.5 months (95% CI, 4.5-8.3) in the pomalidomide-dexamethasone (Pd) group [43]. At this trial entry, 24 patients present EMD with 14 patients in the Isa-Pd group and 10 in the Pd group. The PFS was prolonged in Isa-Pd group (4.57 months) versus Pd group (1.56 months). The ORR was also improved: 50% (7/14) and 10% (1/10) in the Isa-Pd and Pd arms, respectively. The extramedullary lesions of two patients showed CR at Cycle 3 and significant reduction at Cycle 4 [44]. Accordingly, Isa-Pd showed promising efficacy in RRMM patients with EMD.
Elotuzumab
Elotuzumab, the first-in-class humanized immunoglobulin G1 against SLAMF7 (also named CS1) monoclonal antibody, exerts a dual effect to kill MM cells by directly mediating anti-body-dependent cell-mediated cytotoxicity (ADCC) through the CD16 pathway and activating NK cells [45,46]. Elotuzumab in combination with lenalidomide or pomalidomide plus low-dose dexamethasone for RRMM patients reported promising results in the ELOQUENT-2 trial [47,48]. A case report showed EMD disappeared after eight cycles of elotuzumab/lenalidomide/dexamethasone treatment in one RRMM patient [49]. Danhof et al. [50] reported 15 EMD patients (eight had EM-B, three EM-S, four both EM-B and EM-S) received elotuzumab-based therapy. The ORR was 40% with one patient VGPR and five PR, which in comparison was lower than the 79% and 53% in ELOQUENT-2 and ELOQUENT-3 clinical trials, respectively. For extramedullary lesions, regression or SD was noted in four patients, and six patients got PD. They also reported lower SLAMF7 expression on plasma cells at extramedullary sites compared with BMPCs in a patient with EMD in the pleural, which may explain the relatively poor outcomes in EMD patients treated with elotuzumab [51]. Currently, the efficacy of elotuzumab in EMD is under investigation and SLAMF7-directed immunotherapies such as antibody-drug conjugates and CAR-T cells are expected further development.
Selinexor
Nuclear export protein XPO1 is overexpressed in MM, allowing cancer cells to evade apoptotic and antiproliferative signals [52]. Selinexor is an inhibitor of XPO1-mediated nuclear export and has been approved in the USA in July 2019 for the treatment of RRMM patients who have received at least four prior therapies and whose disease are refractory to at least two PIs/IMiDs, and anti-CD38 monoclonal antibody [53]. In the STROM study, Yee et al. analyzed 27 patients with EMD who were treated with selinexor in combination with low-dose dexamethasone (Sel-dex) and 16 of them were available to assess EMD. They found that 5 patients got objective responses, 2 got minimal response (MR), 4 got SD and 5 got objective PD. These observations support the finding that Sel-dex is active in patients with EMD [54].
Venetoclax
High expression of anti-apoptotic protein BCL-2 in the BM microenvironment promotes survival of multiple myeloma cells [55]. Venetoclax is a BCL-2 inhibitor, inducing apoptosis in MM cells [56]. The survival of MM cells is highly dependent on BCL-2 protein. MM cells with t(11:14) translocation are particularly sensitive to venetoclax [57]. Venatoclax could be considered for patients with soft-tissue EMD, but there is still a lack of reports on venetoclax for the treatment of EMD [58].
Anti-BCMA chimeric antigen receptor (CAR) T cell therapy
Anti-BCMA CAR-T cell therapy has shown efficacy in RRMM [59]. Xu et al. [60] reported 17 MM patients received anti-BCMA CAR-T cell therapy and the results showed 88% of patients responded. After a follow-up of 11 months, 47.1% of patients remained in ongoing response status, which suggested anti-BCMA CAR-T cell therapy had demonstrated very good efficacy in the treatment of MM. Notably, four of five patients with EMD in this study had a poor prognosis, all of whom relapsed within one year, and all of the relapsed extramedullary lesions appeared in a new location with medullary progression. In Deng et al’ s report [61], twenty RRMM patients were enrolled in the study in which seven patients had EMD. Although the grades of cytokine release syndrome (CRS) and immune effector cell-associated neurotoxic syndrome (ICANS) were much higher in patients with EMD, there was no difference in the ORR between patients with and without EMD (71.43% versus 80%, p = 0.876). There was no difference in the PFS rate and OS rate between the two groups at 180 days (PFS rate: 42.9% versus 84.6%, p=0.068; OS rate: 71.5% versus 92.3%, p= 0.220). However, both PFS rate and OS rate were lower in patients with EMD than that of patients without EMD at 360 days (PFS rate: 28.6% versus 72.5%, p= 0.037; OS rate: 28.6% versus 81.0%, p= 0.030). The results suggest that anti-BCMA CAR-T cell therapy can provide a short remission for EMD patients responded. Whether its efficacy can be strengthened by bridging hematopoietic stem cell transplantation and radiotherapy needs to be further explored.
Bispecific T-cell engagers (BiTEs)
Limited data are available on BiTEs for EMD patients. REGN5458 is an anti-BCMA and anti-CD3 bispecific antibody [62]. In the trial NCT03761108, an 81-year-old male with EMD was treated at the initial dose level of 3 mg REGN5458 and after 12 weeks, he had a resolution of EMD [63].
Autologous stem cell transplantation (ASCT)
There is no unified conclusion on whether ASCT can overcome the adverse prognosis. ASCT was more effective in the EM-S group than in the EM-B group [64]. Lee et al. [9] reported 275 MM patients, 54 of whom had EMD at diagnosis. In 154 transplant-eligible patients, there was no difference in OS and PFS in 27 patients with EMD and 127 patients without EMD (two-year OS: 82.9% versus 84.7%, p=0.487; two-year PFS: 54.2% versus 58.7%, p=0.339). Meanwhile, in 121 transplant-ineligible patients, outcomes were different between 27 patients presence EMD and 94 patients absence of EMD (two-year OS: 44.2% versus 80.8%, p=0.007; two-year PFS: 39.6% versus 48.2%, p=0.054). This study demonstrated ASCT can overcome the adverse prognostic effects caused by EMD and EMD was a poor prognostic factor for transplant-ineligible patients. However, in Kumar et al's study, 16 of 44 patients achieved CR after ASCT compared to 142 among those without EMD (36.4% versus 89.9%, P<0.002). As their statement that CR rate after ASCT was the most important predictor for OS and PFS, median OS and median PFS were shorter in patients with EMD than without EMD (median OS: 32 versus 100 months, P<0.001; median PFS: 18 versus 44 months, P<0.001). The lower response rate to ASCT and inferior PFS and OS in patients with EMD may be explained by most patients were ISS stage III and Durie Salmon stage IIIB [65]. The choice of transplant regimens remains controversial. A retrospective study from EMBT concluded that tandem ASCT and single ASCT had the same PFS and OS [66]. Gagelmann et al. showed that tandem autologous transplant could significantly improve survival in EMD with high-risk cytogenetics [67].
Treatment of extramedullary lesions at special sites
EM-B has a better prognosis than EM-S [68]. The median OS was 12 months for patients with EM-B and five months for patients with EM-S [5]. However, most studies of treatment for EMD did not distinguish between EM-B and EM-S.
Myelomatous pleural effusion (MPE) is caused by direct infiltration of PCs and has an incidence of 1% [69]. No consensus exists regarding the standard treatment option of MPE. Literature reported that bortezomib plus dexamethasone can lead to pleural effusion absorption or even CR in some MPE patients [70, 71], but the median OS from diagnosis of MPE was only 4 months [72]. Intrapleural administrations of interferon-alpha [73], doxorubicin [74] and bortezomib have been tried in isolated cases, and the results from intrapleural bortezomib were encouraging [75]. Lannitto et al. [75] reported an MPE patient had a reduction of pleural effusion after four courses of intrapleural bortezomib administration. After a median follow-up of 12 months, his serum M-component remained no progression without significant side effects. A 57-yer-old female developed MPE after diagnosis of MM in our department. After daratumumab combining with intrapleural bortezomib administration, the abnormal PCs in the pleural effusion disappeared, accompanied with serum free light chain decreased significantly.
Multiple myeloma involving the central nervous system (CNS) is a rare complication and is associated with poor prognosis. In 4060 MM patients evaluated at Mayo Clinic from 1998 to 2014, 0.7% had myelomatous involvement of the CNS. OS was significantly shorter in the CNS-MM group than in patients without CNS disease (40 months versus 93 months) [76]. Although survival of CNS-MM patients is still unsatisfactory compared with general MM patients and optimal therapy for CNS-MM is unclear, surprising positive results seem to emerge with the use of novel agents [77]. Craniospinal radiation, triple intrathecal chemotherapy (glucocorticoids, methotrexate and cytarabine) and systemic IMiD-based therapy were recommended [78]. For patients with CNS involvement, difficulty in treatment is the poor penetrability of novel agents across the blood-brain barrier (BBB). Pomalidomide (CSF concentration 39%) and thalidomide (CSF concentration 30%-60%) are more permeable to the BBB than lenalidomide (CSF concentration 11%) [79], [80], [81], [82]. Marizomib has a CSF penetration of 30% [21], but other PIs drugs have a lower penetration (bortezomib, CSF concentration 5% [83]). The BBB can become more permeable in disease states, raising the possibility of daratumumab being able to cross the BBB. Elhassadi et al. presented a case of CNS plasmacytoma achieved a durable CR treated with radiotherapy, intrathecal chemotherapy and daratumumab [84]. Most current enrollment criteria for CAR-T therapy excluded patients with central invasion, so the efficacy in the treatment of CNS-MM was unknown. Comparison of CSF/plasma ratio of drugs were summarized in Table 2.
Table 2.
Comparison of CSF/plasma ratio of drugs.
| Drug | CSF/Plasma ratio % | Model | Reference |
|---|---|---|---|
| Thalidomide | 30%-60% | A 63-year-old female diagnosed with IgG-κ MM | [80] |
| Thalidomide | 42% | Rhesus monkeys | [82] |
| Lenalidomide | 11% | ||
| Lenalidomide | 5% | Murine CNS lymphoma model | [79] |
| Pomalidomide | 39% | ||
| Bortezomib | 5% | Male Sprague-Dawley rats | [83] |
| Marizomib | 30% | Cynomolgus monkeys and rats | [21] |
| Carfilzomib | 0% | Male Sprague-Dawley rats and female BALB/c and BNX mice | [96] |
Treatment approach
To the best of our knowledge, there are no consensus or guidelines regarding EMD treatment. In this article, we reviewed published series and attempted to provide effective treatment for EMD patients by combining the clinical experience of our department.
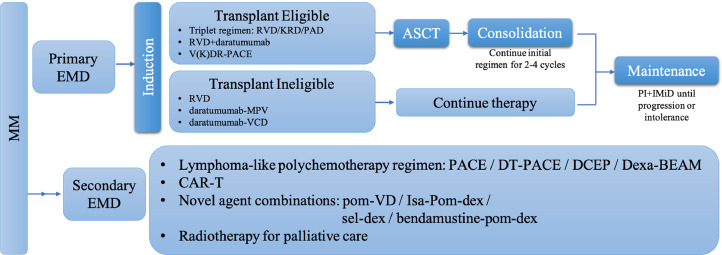
In patients with confirmed MM, biopsy of soft-tissue lesion and sensitive imaging especially positron emission tomography/computed tomography (PET/CT) and magnetic resonance imaging (MRI) are currently considered the most accurate technique for detecting and monitoring the extent of extramedullary sites [85], [86], [87]. Treatment regimens for EMD patients are established on the basis of their age, fitness and condition of the extramedullary lesions, with acceptable patients receiving intensive induction therapy followed by ASCT and then consolidation and maintenance treatment, while transplant-ineligible patients receiving several cycles of induction therapy and maintenance. For primary EMD (at the time of MM diagnosis) patients, triplet regimens such as bortezomib-based therapy RVD (lenalidomide/bortezomib/dexamethasone), KRD (carfilzomib/lenalidomide/dexamethasone) and PAD (bortezomib/doxorubicin/dexamethasone) constitute the best preparative induction regimens for transplant-eligible patients. Considering that daratumumab improves the efficacy of RVD, the addition of daratumumab would be rational in front-line therapy [86]. In younger and fitness patients with proliferative EMD, more intensive treatment therapy such as RVD or KRD plus PACE (cisplatin/doxorubicin/cyclophosphamide/etoposide) should be considered to reduce disease burden rapidly [88]. RVD, daratumumab-MPV (melphalan/prednisone/dexamethasone), daratumumab-VCD (bortezomib/cyclophosphamide/dexamethasone) seem to be the choice of induction treatment therapy for transplant-ineligible patients [86,88]. As for patients who have extramedullary relapse and are heavily pretreated, lymphoma-like polychemotherapy regimens such as PACE, DCEP (dexamethasone/cyclophosphamide/etoposide/cisplatin) and Dexa-BEAM (dexamethasone/carmustine/etoposide/doxorubicin/melphalan) should be considered. Concerning more novel agents, pomalidomide-dexamethasone therapy in combination with bortezomib or PACE have been proved efficacy for EMD patients in our department. Bendamustine/pomalidomide/dexamethasone showed promising response rate for RRMM patients with EMD in a phase II study [89]. Isa-Pd (isatuximab/pomalidomide/dexamethasone) was reported to be effective in ICARIA-MM trial [90]. Selinexor in associated with dexamethasone was active according to the STROM study [54]. Anti-BCMA CAR-T cell therapy can provide a rapid and deep remission with disappearance of extensive extramedullary lesions and whether its efficacy can be strengthened by bridging ASCT needs further studied [91,92]. Our management approaches for EMD patients are shown in Fig. 1.
Fig. 1.
Treatment approach for EMD. MM: multiple myeloma; EMD: extramedullary disease; RVD: lenalidomide-bortezomib-dexamethasone; KRD: carfilzomib-lenalidomide-dexamethasone; PAD: bortezomib-doxorubicin-dexamethasone; V(K)DR: bortezomib(carfilzomib)-dexamethasone-lenalidomide; PACE: cisplatin-doxorubicin-cyclophosphamide-etoposide; ASCT: autologous stem cell transplantation; PI: proteasome inhibitor; IMiD: immunomodulatory drug; MVP: melphalan-prednisone-bortezomib; VCD: cyclophosphamide-bortezomib-dexamethasone; DT: dexamethasone-thalidomide; DCEP: dexamethasone-cyclophosphamide-etoposide-cisplatin; Dexa-BEAM:dexamethasone-carmustine-etoposide-doxorubicin-melphalan; CAR-T: chimeric antigen receptor T cell therapy; pom-VD: pomalidomide-bortezomib-dexamethasone; Isa-Pom-dex: isatuximab-pomalidomide-dexamethasone; Sel-dex: selinexor-dexamethasone; Bendamustine-pom-dex: bendamustine-pomalidomide-dexamethasone.
Conclusions
EMD suggests a poor prognosis and should be considered as high-risk MM. Currently, no specific treatment options have been made for EMD. In this review, we summarized the previous literature, including researches, clinical trials and case reports, and listed the effects of several therapeutic strategies for EMD in the era of new drugs. This knowledge will direct the therapeutic regimens that target EMD. Meanwhile, there is an urgent demand to identify treatment strategies for patients with EMD.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Palumbo A, Anderson K. Multiple myeloma. N. Engl. J. Med. 2011;364(11):1046–1060. doi: 10.1056/NEJMra1011442. [DOI] [PubMed] [Google Scholar]
- 2.Touzeau C, Moreau P. How I treat extramedullary myeloma. Blood. 2016;127(8):971–976. doi: 10.1182/blood-2015-07-635383. [DOI] [PubMed] [Google Scholar]
- 3.Kumar SK, Rajkumar V, Kyle RA, et al. Multiple myeloma. Nat. Rev. Dis. Primers. 2017;3:17046. doi: 10.1038/nrdp.2017.46. [DOI] [PubMed] [Google Scholar]
- 4.Bansal R, Rakshit S, Kumar S. Extramedullary disease in multiple myeloma. Blood Cancer J. 2021;11(9):161. doi: 10.1038/s41408-021-00527-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pour L, Sevcikova S, Greslikova H, et al. Soft-tissue extramedullary multiple myeloma prognosis is significantly worse in comparison to bone-related extramedullary relapse. Haematologica. 2014;99(2):360–364. doi: 10.3324/haematol.2013.094409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Varettoni M, Corso A, Pica G, et al. Incidence, presenting features and outcome of extramedullary disease in multiple myeloma: a longitudinal study on 1003 consecutive patients. Ann. Oncol. 2010;21(2):325–330. doi: 10.1093/annonc/mdp329. [DOI] [PubMed] [Google Scholar]
- 7.Cerny J, Fadare O, Hutchinson L, et al. Clinicopathological features of extramedullary recurrence/relapse of multiple myeloma. Eur. J. Haematol. 2008;81(1):65–69. doi: 10.1111/j.1600-0609.2008.01087.x. [DOI] [PubMed] [Google Scholar]
- 8.Wu P, Davies FE, Boyd K, et al. The impact of extramedullary disease at presentation on the outcome of myeloma. Leuk. Lymphoma. 2009;50(2):230–235. doi: 10.1080/10428190802657751. [DOI] [PubMed] [Google Scholar]
- 9.Lee SE, Kim JH, Jeon YW, et al. Impact of extramedullary plasmacytomas on outcomes according to treatment approach in newly diagnosed symptomatic multiple myeloma. Ann. Hematol. 2015;94(3):445–452. doi: 10.1007/s00277-014-2216-8. [DOI] [PubMed] [Google Scholar]
- 10.Bladé J, Fernández de Larrea C, Rosiñol L, et al. Soft-tissue plasmacytomas in multiple myeloma: incidence, mechanisms of extramedullary spread, and treatment approach. J. Clin. Oncol. 2011;29(28):3805–3812. doi: 10.1200/JCO.2011.34.9290. [DOI] [PubMed] [Google Scholar]
- 11.Patriarca F, Prosdocimo S, Tomadini V, et al. Efficacy of bortezomib therapy for extramedullary relapse of myeloma after autologous and non-myeloablative allogeneic transplantation. Haematologica. 2005;90(2):278–279. [PubMed] [Google Scholar]
- 12.Dingli D, Ailawadhi S, Bergsagel PL, et al. Therapy for Relapsed Multiple Myeloma: Guidelines From the Mayo Stratification for Myeloma and Risk-Adapted Therapy. Mayo Clin. Proc. 2017;92(4):578–598. doi: 10.1016/j.mayocp.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laura R, Cibeira MT, Uriburu C, et al. Bortezomib: an effective agent in extramedullary disease in multiple myeloma. Eur. J. Haematol. 2006;76(5):405–408. doi: 10.1111/j.0902-4441.2005.t01-1-EJH2462.x. [DOI] [PubMed] [Google Scholar]
- 14.Landau H, Pandit-Taskar N, Hassoun H, et al. Bortezomib, liposomal doxorubicin and dexamethasone followed by thalidomide and dexamethasone is an effective treatment for patients with newly diagnosed multiple myeloma with Internatinal Staging System stage II or III, or extramedullary disease. Leuk. Lymphoma. 2012;53(2):275–281. doi: 10.3109/10428194.2011.606943. [DOI] [PubMed] [Google Scholar]
- 15.Lakshman A, Singh PP, Rajkumar SV, et al. Efficacy of VDT PACE-like regimens in treatment of relapsed/refractory multiple myeloma. Am. J. Hematol. 2018;93(2):179–186. doi: 10.1002/ajh.24954. [DOI] [PubMed] [Google Scholar]
- 16.Moreau P, Masszi T, Grzasko N, et al. Oral Ixazomib, Lenalidomide, and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2016;374(17):1621–1634. doi: 10.1056/NEJMoa1516282. [DOI] [PubMed] [Google Scholar]
- 17.Minarik J, Pika T, Radocha J, et al. Survival benefit of ixazomib, lenalidomide and dexamethasone (IRD) over lenalidomide and dexamethasone (Rd) in relapsed and refractory multiple myeloma patients in routine clinical practice. BMC Cancer. 2021;21(1):73. doi: 10.1186/s12885-020-07732-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stewart AK, Rajkumar SV, Dimopoulos MA, et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N. Engl. J. Med. 2015;372(2):142–152. doi: 10.1056/NEJMoa1411321. [DOI] [PubMed] [Google Scholar]
- 19.Zhou X, Fluchter P, Nickel K, et al. Carfilzomib based treatment strategies in the management of relapsed/refractory multiple myeloma with extramedullary disease. Cancers (Basel) 2020;12(4) doi: 10.3390/cancers12041035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muchtar E, Gatt ME, Rouvio O, et al. Efficacy and safety of salvage therapy using Carfilzomib for relapsed or refractory multiple myeloma patients: a multicentre retrospective observational study. Br. J. Haematol. 2016;172(1):89–96. doi: 10.1111/bjh.13799. [DOI] [PubMed] [Google Scholar]
- 21.Di K, Lloyd GK, Abraham V, et al. Marizomib activity as a single agent in malignant gliomas: ability to cross the blood-brain barrier. Neuro Oncol. 2016;18(6):840–848. doi: 10.1093/neuonc/nov299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richardson PG, Zimmerman TM, Hofmeister CC, et al. Phase 1 study of marizomib in relapsed or relapsed and refractory multiple myeloma: NPI-0052-101 Part 1. Blood. 2016;127(22):2693–2700. doi: 10.1182/blood-2015-12-686378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Badros A, Singh Z, Dhakal B, et al. Marizomib for central nervous system-multiple myeloma. Br. J. Haematol. 2017;177(2):221–225. doi: 10.1111/bjh.14498. [DOI] [PubMed] [Google Scholar]
- 24.Rosiñol L, Cibeira MT, Bladé J, et al. Extramedullary multiple myeloma escapes the effect of thalidomide. Haematologica. 2004;89(7):832–836. [PubMed] [Google Scholar]
- 25.Bladé J, Perales M, Rosiñol L, et al. Thalidomide in multiple myeloma: lack of response of soft-tissue plasmacytomas. Br. J. Haematol. 2001;113(2):422–424. doi: 10.1046/j.1365-2141.2001.02765.x. [DOI] [PubMed] [Google Scholar]
- 26.Avigdor A, Raanani P, Levi I, et al. Extramedullary progression despite a good response in the bone marrow in patients treated with thalidomide for multiple myeloma. Leuk. Lymphoma. 2001;42(4):683–687. doi: 10.3109/10428190109099330. [DOI] [PubMed] [Google Scholar]
- 27.Anagnostopoulos A, Hamilos G, Zorzou MP, et al. Discordant response or progression in patients with myeloma treated with thalidomide-based regimens. Leuk. Lymphoma. 2004;45(1):113–116. doi: 10.1080/1042819031000151860. [DOI] [PubMed] [Google Scholar]
- 28.Myers B, Grimley C, Crouch D, et al. Lack of response to thalidomide in plasmacytomas. Br. J. Haematol. 2001;115(1):234. [PubMed] [Google Scholar]
- 29.Calvo-Villas JM, Alegre A, Calle C, et al. Lenalidomide is effective for extramedullary disease in relapsed or refractory multiple myeloma. Eur. J. Haematol. 2011;87(3):281–284. doi: 10.1111/j.1600-0609.2011.01644.x. [DOI] [PubMed] [Google Scholar]
- 30.Laura R, Cibeira MT, Uriburu C, et al. Bortezomib: an effective agent in extramedullary disease in multiple myeloma. Eur. J. Haematol. 2006;76(5):405–408. doi: 10.1111/j.0902-4441.2005.t01-1-EJH2462.x. [DOI] [PubMed] [Google Scholar]
- 31.Paubelle E, Coppo P, Garderet L, et al. Complete remission with bortezomib on plasmocytomas in an end-stage patient with refractory multiple myeloma who failed all other therapies including hematopoietic stem cell transplantation: possible enhancement of graft-vs-tumor effect. Leukemia. 2005;19(9):1702–1704. doi: 10.1038/sj.leu.2403855. [DOI] [PubMed] [Google Scholar]
- 32.Krauth MT, Bankier A, Valent P, et al. Sustained remission including marked regression of a paravertebral plasmacytoma in a patient with heavily pretreated, relapsed multiple myeloma after treatment with bortezomib. Leuk. Res. 2005;29(12):1473–1477. doi: 10.1016/j.leukres.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 33.Alegre A, Aguado B, Giraldo P, et al. Lenalidomide is effective as salvage therapy in refractory or relapsed multiple myeloma: analysis of the Spanish Compassionate Use Registry in advanced patients. Int. J. Hematol. 2011;93(3):351–360. doi: 10.1007/s12185-011-0785-z. [DOI] [PubMed] [Google Scholar]
- 34.Shah N, Chari A, Scott E, et al. B-cell maturation antigen (BCMA) in multiple myeloma: rationale for targeting and current therapeutic approaches. Leukemia. 2020;34(4):985–1005. doi: 10.1038/s41375-020-0734-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Short KD, Rajkumar SV, Larson D, et al. Incidence of extramedullary disease in patients with multiple myeloma in the era of novel therapy, and the activity of pomalidomide on extramedullary myeloma. Leukemia. 2011;25(6):906–908. doi: 10.1038/leu.2011.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiménez-Segura R, Granell M, Gironella M, et al. Pomalidomide-dexamethasone for treatment of soft-tissue plasmacytomas in patients with relapsed /refractory multiple myeloma. Eur. J. Haematol. 2019;102(5):389–394. doi: 10.1111/ejh.13217. [DOI] [PubMed] [Google Scholar]
- 37.Li Y, Ji J, Lu H, et al. Pomalidomide-based therapy for extramedullary multiple myeloma. Hematology. 2022;27(1):88–94. doi: 10.1080/16078454.2021.2019364. [DOI] [PubMed] [Google Scholar]
- 38.Jelinek T, Sevcikova T, Zihala D, et al. Limited efficacy of daratumumab in multiple myeloma with extramedullary disease. Leukemia. 2021 doi: 10.1038/s41375-021-01343-w. [DOI] [PubMed] [Google Scholar]
- 39.Jullien M, Trudel S, Tessoulin B, et al. Single-agent daratumumab in very advanced relapsed and refractory multiple myeloma patients: a real-life single-center retrospective study. Ann. Hematol. 2019;98(6):1435–1440. doi: 10.1007/s00277-019-03655-5. [DOI] [PubMed] [Google Scholar]
- 40.Lonial S, Weiss BM, Usmani SZ, et al. Daratumumab monotherapy in patients with treatment-refractory multiple myeloma (SIRIUS): an open-label, randomised, phase 2 trial. Lancet. 2016;387(10027):1551–1560. doi: 10.1016/S0140-6736(15)01120-4. [DOI] [PubMed] [Google Scholar]
- 41.Pick M, Vainstein V, Goldschmidt N, et al. Daratumumab resistance is frequent in advanced-stage multiple myeloma patients irrespective of CD38 expression and is related to dismal prognosis. Eur. J. Haematol. 2018;100(5):494–501. doi: 10.1111/ejh.13046. [DOI] [PubMed] [Google Scholar]
- 42.Dimopoulos M, Bringhen S, Anttila P, et al. Isatuximab as monotherapy and combined with dexamethasone in patients with relapsed/refractory multiple myeloma. Blood. 2021;137(9):1154–1165. doi: 10.1182/blood.2020008209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Attal M, Richardson PG, Rajkumar SV, et al. Isatuximab plus pomalidomide and low-dose dexamethasone versus pomalidomide and low-dose dexamethasone in patients with relapsed and refractory multiple myeloma (ICARIA-MM): a randomised, multicentre, open-label, phase 3 study. Lancet. 2019;394(10214):2096–2107. doi: 10.1016/S0140-6736(19)32556-5. [DOI] [PubMed] [Google Scholar]
- 44.Beksac M RP, Unal A, Corradini P, DeLimpasi S, Gulbas Z. Isatuximab plus pomalidomide and dexamethasone in patients with relapsed/refractory multiple myeloma and soft-tissue plasmacytomas: ICARIA-MM subgroup analysis. EHA library 06/12/20;294895;EP978. https://library.ehaweb.org/eha/2020/eha25th/294895/meral.beksac.isatuximab.plus.pomalidomide.and.dexamethasone.in.patients.with.htmlf=listing%3D0%2Abrowseby%3D8%2Asortby%3D1%2Asearch%3Dicaria. Accessed January 2021.
- 45.Lonial S, Dimopoulos M, Palumbo A, et al. Elotuzumab Therapy for Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2015;373(7):621–631. doi: 10.1056/NEJMoa1505654. [DOI] [PubMed] [Google Scholar]
- 46.Cho SF, Xing L, Anderson KC, et al. Promising Antigens for the New Frontier of Targeted Immunotherapy in Multiple Myeloma. Cancers (Basel) 2021;13(23) doi: 10.3390/cancers13236136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dimopoulos MA, Lonial S, White D, et al. Elotuzumab, lenalidomide, and dexamethasone in RRMM: final overall survival results from the phase 3 randomized ELOQUENT-2 study. Blood Cancer J. 2020;10(9):91. doi: 10.1038/s41408-020-00357-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dimopoulos MA, Dytfeld D, Grosicki S, et al. Elotuzumab plus Pomalidomide and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2018;379(19):1811–1822. doi: 10.1056/NEJMoa1805762. [DOI] [PubMed] [Google Scholar]
- 49.Kashima E, Fujieda A, Nato Y, et al. [Successful treatment with a combination of elotuzumab, lenalidomide and dexamethasone of extramedullary disease in a patient with refractory multiple myeloma] Rinsho Ketsueki. 2020;61(3):223–227. doi: 10.11406/rinketsu.61.223. [DOI] [PubMed] [Google Scholar]
- 50.Danhof S, Rasche L, Mottok A, et al. Elotuzumab for the treatment of extramedullary myeloma: a retrospective analysis of clinical efficacy and SLAMF7 expression patterns. Ann. Hematol. 2021;100(6):1537–1546. doi: 10.1007/s00277-021-04447-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Danhof S, Strifler S, Hose D, et al. Clinical and biological characteristics of myeloma patients influence response to elotuzumab combination therapy. J. Cancer Res. Clin. Oncol. 2019;145(3):561–571. doi: 10.1007/s00432-018-2807-1. [DOI] [PubMed] [Google Scholar]
- 52.Bahlis NJ, Sutherland H, White D, et al. Selinexor plus low-dose bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma. Blood. 2018;132(24):2546–2554. doi: 10.1182/blood-2018-06-858852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.FDA grants accelerated approval to selinexor for multiple myeloma. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-selinexor-multiple-myeloma.
- 54.Yee AJ, Huff CA, Chari A, et al. Response to Therapy and the Effectiveness of Treatment with Selinexor and Dexamethasone in Patients with Penta-Exposed Triple-Class Refractory Myeloma Who Had Plasmacytomas. Blood. 2019;134(Supplement_1):3140. [Google Scholar]
- 55.Kumar SK, Harrison SJ, Cavo M, et al. Venetoclax or placebo in combination with bortezomib and dexamethasone in patients with relapsed or refractory multiple myeloma (BELLINI): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2020;21(12):1630–1642. doi: 10.1016/S1470-2045(20)30525-8. [DOI] [PubMed] [Google Scholar]
- 56.Chauhan D, Velankar M, Brahmandam M, et al. A novel Bcl-2/Bcl-X(L)/Bcl-w inhibitor ABT-737 as therapy in multiple myeloma. Oncogene. 2007;26(16):2374–2380. doi: 10.1038/sj.onc.1210028. [DOI] [PubMed] [Google Scholar]
- 57.Touzeau C, Dousset C, Le Gouill S, et al. The Bcl-2 specific BH3 mimetic ABT-199: a promising targeted therapy for t(11;14) multiple myeloma. Leukemia. 2014;28(1):210–212. doi: 10.1038/leu.2013.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rosinol L, Beksac M, Zamagni E, et al. Expert review on soft-tissue plasmacytomas in multiple myeloma: definition, disease assessment and treatment considerations. Br. J. Haematol. 2021;194(3):496–507. doi: 10.1111/bjh.17338. [DOI] [PubMed] [Google Scholar]
- 59.Ali SA, Shi V, Maric I, et al. T cells expressing an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of multiple myeloma. Blood. 2016;128(13):1688–1700. doi: 10.1182/blood-2016-04-711903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu J, Chen LJ, Yang SS, et al. Exploratory trial of a biepitopic CAR T-targeting B cell maturation antigen in relapsed/refractory multiple myeloma. Proc. Natl. Acad. Sci. U. S. A. 2019;116(19):9543–9551. doi: 10.1073/pnas.1819745116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Deng H, Liu M, Yuan T, et al. Efficacy of Humanized Anti-BCMA CAR T Cell Therapy in Relapsed/Refractory Multiple Myeloma Patients With and Without Extramedullary Disease. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.720571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.DiLillo DJ, Olson K, Mohrs K, et al. A BCMAxCD3 bispecific T cell-engaging antibody demonstrates robust antitumor efficacy similar to that of anti-BCMA CAR T cells. Blood Adv. 2021;5(5):1291–1304. doi: 10.1182/bloodadvances.2020002736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cooper D, Madduri D, Lentzsch S, et al. Safety and Preliminary Clinical Activity of REGN5458, an Anti-Bcma x Anti-CD3 Bispecific Antibody, in Patients with Relapsed/Refractory Multiple Myeloma. Blood. 2019;134(Supplement_1):3176. [Google Scholar]
- 64.Li J, Shen KN, Huang WR, et al. Autologous stem cell transplant can overcome poor prognosis in patients with multiple myeloma with extramedullary plasmacytoma. Leuk. Lymphoma. 2014;55(7):1687–1690. doi: 10.3109/10428194.2013.853296. [DOI] [PubMed] [Google Scholar]
- 65.Kumar L, Gogi R, Patel AK, et al. Multiple myeloma with extramedullary disease: impact of autologous stem cell transplantation on outcome. Bone Marrow Transplant. 2017;52(10):1473–1475. doi: 10.1038/bmt.2017.165. [DOI] [PubMed] [Google Scholar]
- 66.Gagelmann N, Eikema DJ, Iacobelli S, et al. Impact of extramedullary disease in patients with newly diagnosed multiple myeloma undergoing autologous stem cell transplantation: a study from the Chronic Malignancies Working Party of the EBMT. Haematologica. 2018;103(5):890–897. doi: 10.3324/haematol.2017.178434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gagelmann N, Eikema DJ, Koster L, et al. Tandem autologous stem cell transplantation improves outcomes in newly diagnosed multiple myeloma with extramedullary disease and high-risk cytogenetics: a study from the Chronic Malignancies Working Party of the European society for blood and marrow transplantation. Biol. Blood Marrow Transplant. 2019;25(11):2134–2142. doi: 10.1016/j.bbmt.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 68.Li G, Song YP, Lv Y, et al. Clinical characteristics and prognostic analysis of multiple myeloma with extramedullary disease: a seer-based study. J. Oncol. 2021;2021 doi: 10.1155/2021/6681521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Martinez-Iribarren A, Fernandez-Prendes C. A case of myelomatous pleural effusion: an unusual onset of multiple myeloma. Blood. 2020;135(2):153. doi: 10.1182/blood.2019002471. [DOI] [PubMed] [Google Scholar]
- 70.Sekiguchi Y, Shirane S, Imai H, et al. Response to low-dose bortezomib in plasma cell leukemia patients with malignant pleural effusion and ascites: a case report and a review of the literature. Intern. Med. 2012;51(11):1393–1398. doi: 10.2169/internalmedicine.51.7061. [DOI] [PubMed] [Google Scholar]
- 71.Yanamandra U, Deo P, Sahu KK, et al. Clinicopathological profile of myelomatous pleural effusion: single-center real-world experience and review of literature. Clin. Lymphoma Myeloma Leuk. 2019;19(3):183–189. doi: 10.1016/j.clml.2018.12.003. e1. [DOI] [PubMed] [Google Scholar]
- 72.Schlie K, Westerback A, DeVorkin L, et al. Survival of effector CD8+ T cells during influenza infection is dependent on autophagy. J. Immunol. 2015;194(9):4277–4286. doi: 10.4049/jimmunol.1402571. [DOI] [PubMed] [Google Scholar]
- 73.Makino S, Yamahara S, Nagake Y, et al. Bence-Jones myeloma with pleural effusion: response to alpha-interferon and combined chemotherapy. Intern. Med. 1992;31(5):617–621. doi: 10.2169/internalmedicine.31.617. [DOI] [PubMed] [Google Scholar]
- 74.Iannitto E, Scaglione R, Musso M, et al. Intrapleural adriamycin in treatment of myelomatous pleural effusion: a case report. Haematologica. 1988;73(4):325–326. [PubMed] [Google Scholar]
- 75.Iannitto E, Minardi V, Tripodo C. Use of intrapleural bortezomib in myelomatous pleural effusion. Br. J. Haematol. 2007;139(4):621–622. doi: 10.1111/j.1365-2141.2007.06735.x. [DOI] [PubMed] [Google Scholar]
- 76.Paludo J, Painuly U, Kumar S, et al. Myelomatous Involvement of the Central Nervous System. Clin. Lymphoma Myeloma Leuk. 2016;16(11):644–654. doi: 10.1016/j.clml.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 77.Gozzetti A, Cerase A, Lotti F, et al. Extramedullary intracranial localization of multiple myeloma and treatment with novel agents: a retrospective survey of 50 patients. Cancer. 2012;118(6):1574–1584. doi: 10.1002/cncr.26447. [DOI] [PubMed] [Google Scholar]
- 78.Chen CI, Masih-Khan E, Jiang H, et al. Central nervous system involvement with multiple myeloma: long term survival can be achieved with radiation, intrathecal chemotherapy, and immunomodulatory agents. Br. J. Haematol. 2013;162(4):483–488. doi: 10.1111/bjh.12414. [DOI] [PubMed] [Google Scholar]
- 79.Li Z, Qiu Y, Personett D, et al. Pomalidomide shows significant therapeutic activity against CNS lymphoma with a major impact on the tumor microenvironment in murine models. PLoS One. 2013;8(8):e71754. doi: 10.1371/journal.pone.0071754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yutaka H, Mariko Y, Shinichiro O, et al. Thalidomide for the treatment of leptomeningeal multiple myeloma. Eur. J. Haematol. 2006;76(4):358–359. doi: 10.1111/j.1600-0609.2005.00591.x. [DOI] [PubMed] [Google Scholar]
- 81.Gozzetti A, Cerase A. Novel agents in CNS myeloma treatment. Cent. Nerv. Syst. Agents Med. Chem. 2014;14(1):23–27. doi: 10.2174/1871524914999140818111514. [DOI] [PubMed] [Google Scholar]
- 82.Muscal JA, Sun Y, Nuchtern JG, et al. Plasma and cerebrospinal fluid pharmacokinetics of thalidomide and lenalidomide in nonhuman primates. Cancer Chemother. Pharmacol. 2012;69(4):943–947. doi: 10.1007/s00280-011-1781-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hemeryck A, Geerts R, Monbaliu J, et al. Tissue distribution and depletion kinetics of bortezomib and bortezomib-related radioactivity in male rats after single and repeated intravenous injection of 14 C-bortezomib. Cancer Chemother. Pharmacol. 2007;60(6):777–787. doi: 10.1007/s00280-007-0424-9. [DOI] [PubMed] [Google Scholar]
- 84.Elhassadi E, Murphy M, Hacking D, et al. Durable treatment response of relapsing CNS plasmacytoma using intrathecal chemotherapy, radiotherapy, and Daratumumab. Clin. Case Rep. 2018;6(4):723–728. doi: 10.1002/ccr3.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cavo M, Terpos E, Nanni C, et al. Role of (18)F-FDG PET/CT in the diagnosis and management of multiple myeloma and other plasma cell disorders: a consensus statement by the International Myeloma Working Group. Lancet Oncol. 2017;18(4) doi: 10.1016/S1470-2045(17)30189-4. e206-e17. [DOI] [PubMed] [Google Scholar]
- 86.Rosiñol L, Beksac M, Zamagni E, et al. Expert review on soft-tissue plasmacytomas in multiple myeloma: definition, disease assessment and treatment considerations. Br. J. Haematol. 2021;194(3):496–507. doi: 10.1111/bjh.17338. [DOI] [PubMed] [Google Scholar]
- 87.Zamagni E, Nanni C, Dozza L, et al. Standardization of (18)F-FDG-PET/CT According to Deauville Criteria for Metabolic Complete Response Definition in Newly Diagnosed Multiple Myeloma. J. Clin. Oncol. 2021;39(2):116–125. doi: 10.1200/JCO.20.00386. [DOI] [PubMed] [Google Scholar]
- 88.Bhutani M, Foureau DM, Atrash S, et al. Extramedullary multiple myeloma. Leukemia. 2020;34(1):1–20. doi: 10.1038/s41375-019-0660-0. [DOI] [PubMed] [Google Scholar]
- 89.Kumar S, Sharma A, Malik PS, et al. Bendamustine in combination with pomalidomide and dexamethasone in relapsed/refractory multiple myeloma: a phase II trial. Br. J. Haematol. 2022 doi: 10.1111/bjh.18200. [DOI] [PubMed] [Google Scholar]
- 90.Attal M, Richardson PG, Rajkumar SV, et al. Isatuximab plus pomalidomide and low-dose dexamethasone versus pomalidomide and low-dose dexamethasone in patients with relapsed and refractory multiple myeloma (ICARIA-MM): a randomised, multicentre, open-label, phase 3 study. Lancet. 2019;394(10214):2096–2107. doi: 10.1016/S0140-6736(19)32556-5. [DOI] [PubMed] [Google Scholar]
- 91.Brudno JN, Maric I, Hartman SD, et al. T cells genetically modified to express an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of poor-prognosis relapsed multiple myeloma. J. Clin. Oncol. 2018;36(22):2267–2280. doi: 10.1200/JCO.2018.77.8084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Smith EL, Mailankody S, Staehr M, et al. BCMA-targeted CAR T-cell therapy plus radiotherapy for the treatment of refractory myeloma reveals potential synergy. Cancer Immunol. Res. 2019;7(7):1047–1053. doi: 10.1158/2326-6066.CIR-18-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mangiacavalli S, Pompa A, Ferretti V, et al. The possible role of burden of therapy on the risk of myeloma extramedullary spread. Ann. Hematol. 2017;96(1):73–80. doi: 10.1007/s00277-016-2847-z. [DOI] [PubMed] [Google Scholar]
- 94.Beksac M, Seval GC, Kanellias N, et al. A real world multicenter retrospective study on extramedullary disease from Balkan Myeloma Study Group and Barcelona University: analysis of parameters that improve outcome. Haematologica. 2020;105(1):201–208. doi: 10.3324/haematol.2019.219295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Montefusco V, Gay F, Spada S, et al. Outcome of paraosseous extra-medullary disease in newly diagnosed multiple myeloma patients treated with new drugs. Haematologica. 2020;105(1):193–200. doi: 10.3324/haematol.2019.219139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Demo SD, Kirk CJ, Aujay MA, et al. Antitumor activity of PR-171, a novel irreversible inhibitor of the proteasome. Cancer Res. 2007;67(13):6383–6391. doi: 10.1158/0008-5472.CAN-06-4086. [DOI] [PubMed] [Google Scholar]