Abstract
Ischemic injury to the heart induces mitochondrial dysfunction due to increasing oxidative stress. MG53, also known as TRIM72, is highly expressed in striated muscle, is secreted as a myokine after exercise, and is essential for repairing damaged plasma membrane of many tissues by interacting with the membrane lipid phosphatidylserine (PS). We hypothesized MG53 could preserve mitochondrial integrity after an ischemic event by binding to the mitochondrial-specific lipid, cardiolipin (CL), for mitochondria protection to prevent mitophagy. Fluorescent imaging and Western blotting experiments showed recombinant human MG53 (rhMG53) translocated to the mitochondria after ischemic injury in vivo and in vitro. Fluorescent imaging indicated rhMG53 treatment reduced superoxide generation in ex vivo and in vitro models. Lipid-binding assay indicated MG53 binds to CL. Transfecting cardiomyocytes with the mitochondria-targeted mt-mKeima showed inhibition of mitophagy after MG53 treatment. Overall, we show that rhMG53 treatment may preserve cardiac function by preserving mitochondria in cardiomyocytes. These findings suggest MG53's interactions with mitochondria could be an attractive avenue for developing MG53 as a targeted protein therapy for cardioprotection.
Keywords: Cardioprotection, Mitophagy, Cell membrane repair, TRIM72, Myocardial infarction, Cardiolipin
Graphical abstract
Highlights
-
•
Recombinant MG53 treatment before myocardial infarction prevents reactive oxygen species in cardiomyocytes.
-
•
MG53 moves to the mitochondria after ischemic stress.
-
•
MG53 treatment prevents parkin associated mitophagy after oxidative stress.
1. Introduction
Ischemic heart disease remains one of the top causes of mortality worldwide [1]. Ischemic/reperfusion injury like acute myocardial infarction (MI) causes a loss of healthy mitochondria from reactive oxygen species (ROS) release, inhibiting energy production and resulting in cardiomyocyte death [2,3]. Therapeutic approaches that preserve mitochondria integrity would be invaluable for improving survival after ischemic injury.
MG53, also known as TRIM72, is highly expressed in skeletal muscle and modestly in other tissues. It is essential for repairing damage to plasma membrane [4] and can be secreted from skeletal muscle, where it acts as a myokine to repair other damaged tissues [5]. MG53's membrane repair function is associated with changes in oxidative state inside the cell [4,6], and extracellular MG53 can target the exposed phosphatidylserine (PS) to facilitate the repair of injured tissues [7]. We have shown that mg53−/− mice are more susceptible to injury [4,[8], [9], [10]], and treatment with exogenous recombinant human MG53 (rhMG53) reduces damage and restores functions in tissues like the kidney [10], lung [9], and heart [11].
Previously, we have shown that exogenous [11] and endogenous [12] MG53 can preserve cardiac tissue morphology, ejection fraction, and fractional shortening after an ischemic event. However, these earlier studies focused on cardiac function and did not delve into the mechanisms protecting the cardiac tissue, such as maintaining mitochondria that are susceptible to ischemic/reperfusion injury. MG53's link to mitochondrial protection after such an event is unclear. We demonstrated that endogenous MG53 co-localized with mitochondria in skeletal muscles derived from mice fed with a high-fat diet [13]. Additionally, Chung et al. [14] found enrichment of endogenous TRIM72 (MG53) in the mitochondria fraction of mouse hearts subjected to ischemic preconditioning, an experimental technique to induce resistance to a temporary lack of oxygen. While these studies show MG53 is essential for cell survival and a potential interaction of MG53 with the mitochondria, the mechanism behind MG53's role in facilitating mitochondrial protection and how this may protect the heart after an ischemic event like MI remains unknown.
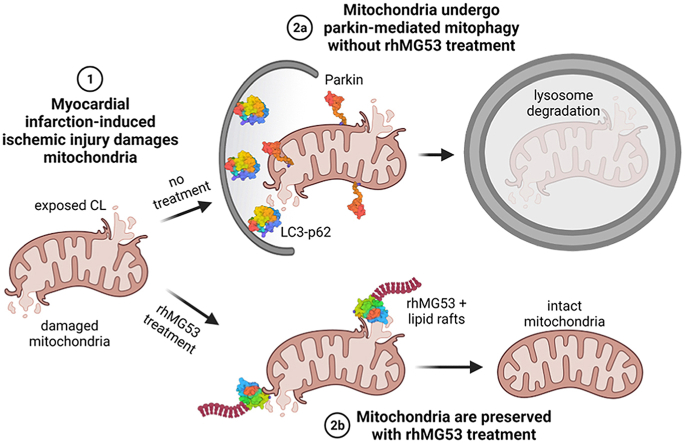
In this study, we test whether exogenous rhMG53 can either directly prevent oxidative stress-induced injury to mitochondria or indirectly preserve the pool of healthy mitochondria through the modulation of mitophagy. We found that MG53 can bind to cardiolipin (CL), a mitochondria-specific phospholipid, to prevent injury to the mitochondria. Furthermore, rhMG53 treatment mitigates the engulfment of mitochondria by the lysosome and reduces parkin localization to the mitochondria.
2. Methods
2.1. Porcine model of angioplasty-induced myocardial infarction
Chinese experimental miniature swine were provided by Beijing Experimental Animal Reproduction and Regulation Center (Grade II, Certificate No. Jing-030). All porcine experiments in this study were performed in accordance with China Academy of Chinese Medical Sciences Guide for Laboratory Animals that conforms to the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health. According to established methods, experimental pigs underwent balloon inflation of the left anterior descending (LAD) coronary artery [11]. rhMG53 was administered at a 1 mg/kg dose immediately after experimental intervention through the jugular vein. Infarct size and location for histology samples were previously reported in Liu et al. [11].
2.2. Murine model of acute myocardial infarction
All murine experiments in this study were performed following The Ohio State University IACUC-approved protocols. The mg53−/− and wild-type littermate mice were used. Saline or 1 mg/kg rhMG53 dissolved in saline was injected via the tail vein 10 min prior to induction of MI via LAD coronary artery ligation as previously described [15]. Ischemia was maintained for 60 min and then released. After ischemia, the mouse recovered for 6 h before cardiac tissue collection for histology and immunofluorescent staining.
2.3. Neonatal cardiomyocyte isolation
Neonatal cardiomyocytes were isolated and pooled from six to ten 1–2 day old C57BL/6 mice. Animals were decapitated, hearts removed and washed into PBS (without Ca2+, Mg2+) with 20 mM BDM, atria excised, ventricles transferred to 250 μL isolation solution (HBSS (without Ca2+, Mg2+) with 0.0125% trypsin, 20 mM BDM) drop and then minced. After mincing hearts into small pieces (approximately 0.5–1 mm3 or smaller), minced hearts were transferred into a 50 mL conical tube containing 10 mL of the isolation solution (on ice) and incubated with gentle agitation at 4 °C for 1–2 h. After allowing the tissue fragments to sink to the bottom of the tube, the supernatant was removed, and 5 mL prewarmed digestion solution (L-15 medium with 20 mM BDM, 1 mg/mL Worthington collagenase type II) was added for a 5 min digestion with gentle agitation in 37 °C. The supernatant was then transferred to a new tube with an additional 4% FBS to stop the digestion. The heart fragment digestion was repeated 5 times. After collecting all the supernatant into 50 mL conical tube, it was passed through a 75 μm cell strainer and centrifuged at 1,000 rpm for 4 min. The resulting cell pellet was resuspended in 10 mL plating medium (65% DMEM, 19% M-199, 10% horse serum, 5% FBS, 1% penicillin/streptomycin) and added to a 10 cm cell culture dish for 1–3 h to remove highly adhesive fibroblasts and endothelial cells. After incubation, non-adherent cardiomyocytes from the 10 cm culture dish were collected and transferred to a sterile 15 mL conical tube. Approximately 1.5 × 105 cells/cm2 were then added to a Geltrex (Thermo Fisher, A1413301)-coated glass-bottom dish. The cells were left undisturbed in a cell culture incubator for 16–24 h to adhere and spread to the bottom. One day after plating, the plating medium was replaced with a maintenance medium (78% DMEM, 17% M-199, 4% horse serum, 1% penicillin/streptomycin) and cultured for additional 2–5 days.
2.4. Oxidative damage to neonatal cardiomyocytes
Neonatal cardiomyocytes were preincubated for 1 h with/without 10 μg/mL BSA or rhMG53, treated with 50 μM H2O2 in HBSS (in mM, 140 NaCl, 5 KCl, 0.5 MgCl2, 0.4 MgSO4, 1 CaCl2, 0.3 Na2HPO4, 0.4 KH2PO4, 6 Glucose, 20 HEPES, pH 7.2). TMRE (50 nM) was applied to the cells and images were collected for up to 60 min using a Nikon A1R confocal microscope with a 20x objective at 37 °C using a 561 nm excitation and 595 nm emission setting.
2.5. Oxidative damage to HL-1 cardiomyocytes
To generate a hypoxia/reoxygenation model of oxidative stress, we adapted the protocol for hypoxia, energy depletion, and acidosis (HEDA) from Åström-Olsson et al. [18]. HL-1 cardiomyocytes (Sigma, SCC065) were cultured at low passages (7–20) in supplemented Claycomb media according to established protocols [16,17]. Briefly, HL-1 cells in PBS pH 6.7 without glucose, Mg2+, or Ca2+ were placed in a sealed chamber gassed with 5% CO2, 1% O2, and 94% N2 to establish the HEDA environment for 24 h. Cells were reoxygenated in BSS (in mM, 140 NaCl, 2.8 KCl, 2 MgCl2, 1 CaCl2, 12 Glucose, 10 HEPES, pH 7.4) at 37 °C for 1 h. Control cells were incubated in BSS at 5% CO2 and 37 °C. For both conditions, cells were incubated with either 10 μg/mL BSA or rhMG53.
2.6. Confocal microscopy
Porcine and mouse tissue samples were embedded in paraffin, a cross-section of the heart was taken (5 μm thickness), and stained with antibodies against MG53 [4] and COX IV (Cell Signaling Technologies, 11967S). MG53 (633 nm), COX IV (546 nm), and DAPI (405 nm) stained slides were imaged on a Zeiss 780 Confocal microscope using Zen 2012 software (Zeiss). Co-localization of MG53 and COX IV was captured by fluorescence within the infarct area in the left ventricle and quantified using FIJI (ImageJ) [16].
HL-1 cells cultured on 35 mm glass-bottom dishes were incubated with 5 μM MitoSOX Red (Thermo Fisher, M36008) for 15 min at 37 °C, protected from light. Cells were rinsed and imaged in BSS. Images were collected with a Zeiss 780 confocal microscope with a 40x objective. The fluorescent integrated density for each cell was measured for analysis using FIJI.
2.7. Mitochondrial FLASH event measurement in isolated mouse cardiomyocytes
Transgenic mice expressing mt-cpYFP provided an assessment of the dynamic changes in superoxide signals from the mitochondria [[17], [18], [19], [20], [21], [22]]. Cardiomyocytes were isolated from the mt-cpYFP transgenic mice following the protocol of Wang et al. [19]. The isolated cardiomyocytes were subjected to 3 h of hypoxia and 2 h of reoxygenation. Live cell imaging of the dynamic changes of the mt-cpYFP fluorescence was conducted on a Zeiss 510 confocal microscope at 40x at 1 frame/second by alternating excitation at 488 nm and collecting emission at 561 nm [19]. Flash events were analyzed using FIJI (ImageJ) [16].
2.8. Lipid binding assays
ELISA Snoopers® (Avanti Polar Lipids) lipid strips assessed rhMG53 binding capacity to PS and 18:1 CL. 8-well strips pre-coated with either lipid were incubated with increasing doses of rhMG53 (0, 0.3125, 0.625, 1.25, 2.5, 5, and 10 μg/mL). ELISAs were performed according to the manufacturer's protocol. Signal was developed using Streptavidin-HRP detection antibody and TMB Substrate Reagent (BD OptEIA, 555214) and quantified using a Flex Station III plate reader. Monoclonal MG53 antibody (Clone 5259, generated by Dr. Hiroshi Takeshima, Kyoto, Japan) [4] conjugated to biotin was used for detection [4].
2.9. Mitophagy assay
pCHAC-mt-mKeima plasmid (Addgene plasmid #72342) [23] was packaged into lentivirus and infected HL-1 cells. Cells were imaged on a Nikon microscope in a 37 °C heated and humidified chamber with 5% CO2 in HL-1 media. Movies were taken using a Nikon A1R with 488 or 561 nm excitation and 560–720 nm emission for 2 h after 1 mM H2O2 treatment. Images were collected before treatment and 2 h later. Images were analyzed for fluorescent intensity for the 488-excitation channel (green) and the 561-excitation channel (red) using FIJI (ImageJ) [16].
2.10. Western blotting
Mitochondria fraction of HL-1 cells were isolated using the Mitochondria Isolation Kit for Cultured Cells (Thermo Fisher, 89874) following the manufacturer's protocol. Briefly, HL-1 cells that underwent BSS or HEDA treatment with 10 μg/mL BSA or rhMG53 were trypsinized with TrypLE (Thermo Fisher, 12604021) and resuspended in 2 mL Claycomb media. Cells were counted, and 8 million cells were collected for fractionation. The remaining cells were lysed with RIPA buffer (10 mM Tris-HCl, pH 7.2, 150 mM NaCl, 1% NP-40, 0.5% SDS, and 0.5% deoxycholate) and supplemented with a cocktail of protease inhibitors (Sigma, S8820). The 8 million cells were centrifuged to collect the pellet and gently lysed using kit reagents. The supernatant cytosolic fraction was retained from the fractionation. The resulting mitochondrial pellet was lysed with 2% CHAPS in tris-buffered saline.
Protein membranes were developed by chemiluminescence using SuperSignal west femto maximum sensitivity substrate (Thermo Scientific, 34096) on a ChemiDoc MP Imaging System (Bio-Rad). Lysates were separated on a 10% SDS-PAGE acrylamide gel and transferred to polyvinylidene fluoride membranes (PVDF) (Millipore). The blots were washed with Tris-buffered saline Tween-20 (TBS-T), blocked with 5% milk in TBS-T for 1 h. Blots were probed with antibodies against MG53 (Clone 914, generated by Dr. Hiroshi Takeshima, Kyoto, Japan), GAPDH (CST, 2118S), COX IV (CST, 11967S), p62/SQSTM1 (CST, 39749), and Parkin (CST, 4211). COX IV was used as a marker for mitochondria enrichment, while GAPDH marked the total and cytosolic fractions. Protein membranes were developed by chemiluminescence using SuperSignal west femto maximum sensitivity substrate (Thermo Scientific, 34096) on a ChemiDoc MP Imaging System (Bio-Rad). Bio-Rad Image Lab™Version 6.0 software was used to calculate the band intensity for each western blot. Adjusted integrated density for each band was normalized to the appropriate loading control (COX IV for mitochondria fraction and GAPDH for total/cytosolic fractions). The fold difference was calculated by dividing the normalized density of each condition to the control condition to compare between trials.
2.11. Statistical analysis
Data were analyzed by several statistical methods (e.g., paired or unpaired t-tests, ANOVA, etc.) using commercial Prism software (GraphPad Prism 9). Data are presented as means ± SD (unless otherwise noted). To compare multiple groups, one-way or two-way multifactorial analysis of variance (ANOVA) was used to determine statistical significance. Bartlett's test was used to determine if the standard deviations were significantly different, necessitating the use of the Brown-Forsythe or Welch's tests. Multiple testing corrections such as Dunnett's T3 multiple comparisons test or Šídák's multiple comparisons test were used. For 2-way ANOVAs, normality was tested using the Anderson-Darling and Kolmogorov-Smirnov tests. For all statistical tests: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
3. Results
3.1. rhMG53 targets mitochondria in mouse and pig models of myocardial infarction
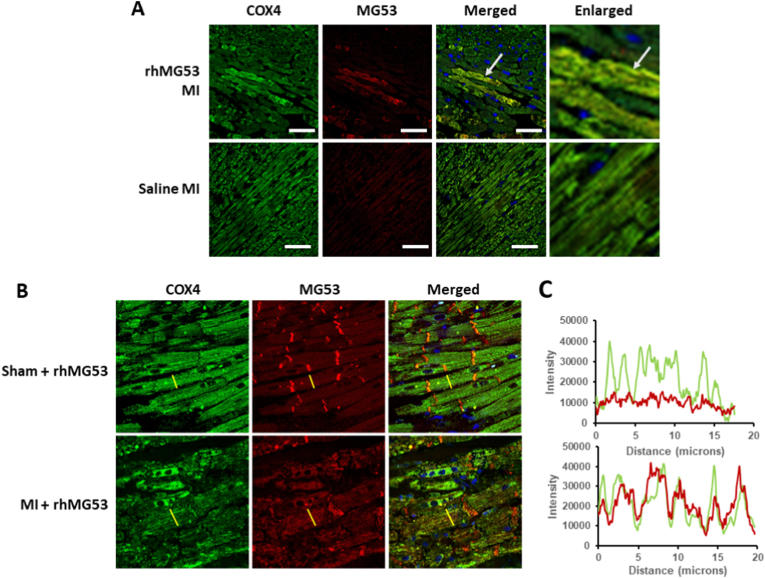
MG53 predominately travels to the damaged cell membrane following skeletal muscle cell membrane damage. In healthy striated muscle, MG53 typically localizes to the t-tubules and cytosol. After an acute MI injury in mg53−/− mice treated with rhMG53, immunofluorescent staining revealed rhMG53 localization to the plasma membrane and intercalated discs (red), as expected (Fig. 1A). RhMG53 also co-localized with the mitochondria, indicated by an alignment of the red MG53 signal and the green COX IV signal (Fig. 1A).
Fig. 1.
rhMG53 localizes to the intercalated disc and mitochondria in mouse and pig cardiomyocytes after MI (A) Immunofluorescence (IF) of mg53 −/− mouse hearts after MI injury showed no MG53 signal in the saline treatment. Treatment with 2 mg/kg rhMG53 and staining of the left ventricle for MG53 (red), COX IV (green), and DAPI (blue) reveals co-localization of MG53 with mitochondria. White arrow indicates area of MG53 and COX4 co-localization. (B) IF of pig hearts treated with 2 mg/kg rhMG53 with MI injury highlights MG53 localization at the intercalated discs with a translocation to the mitochondria after MI injury. (C) Intensity analysis along the indicated grey line in part C shows an over-lapping striated pattern of MG53 and COX IV in pig heart with MI injury. White scale bar = 50 μm. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
We confirmed the dual plasma membrane and mitochondria localization in a porcine model of MI, which more closely mimics human heart damage. MG53 localizes mainly to the intercalated disks (Fig. 1B, top). However, MG53 shifts from the intercalated discs to localization with mitochondria after MI challenge (Fig. 1B, bottom). This shift to localization is highlighted in the cross-sectional fluorescent intensity analysis in Fig. 1C, where MG53's changes from low intensity and no clear pattern in the sham treatment to a higher intensity that mimics the striated mitochondrial pattern after MI.
This localization is consistent with earlier studies in the heart by Chung et al. [14], who found enrichment of TRIM72/MG53 in mitochondria fraction derived from ischemia-reperfusion injured mouse heart. Moreover, the mitochondria localization pattern of rhMG53 in the porcine heart is consistent with our previous observation with skeletal muscle from high-fat diet treated mice [13].
3.2. Mitochondrial membrane integrity is maintained by rhMG53 after oxidative stress
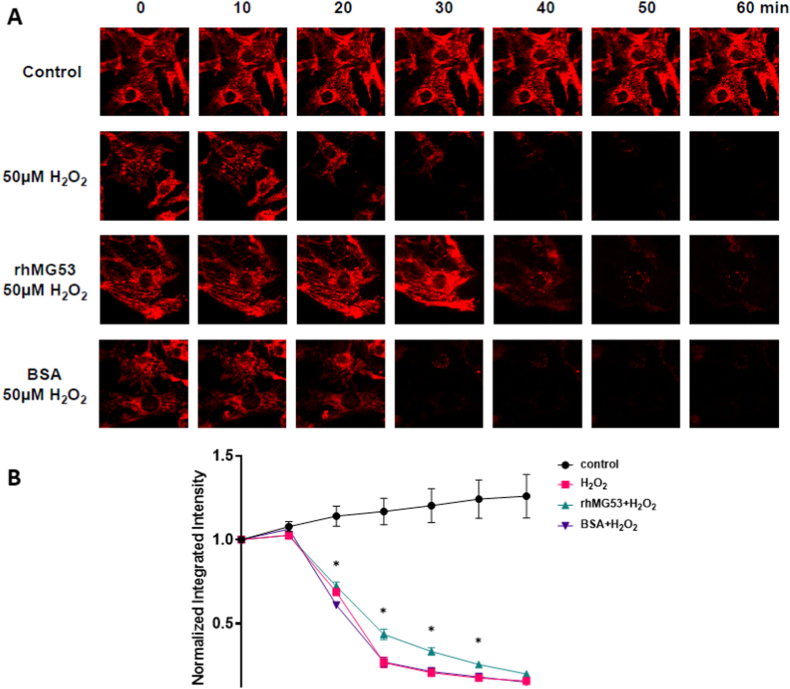
Earlier studies on the protective effects of MG53 on the heart have shown that the presence of MG53 prevents the loss of the mitochondrial membrane potential [12]. We verified this in isolated neonatal cardiomyocytes treated with 50 μM H2O2 using TMRE as a readout for mitochondrial membrane potential (Fig. 2). rhMG53 treated preserved mitochondrial membrane integrity longer than BSA or no treatment.
Fig. 2.
Exogenous rhMG53 maintains mitochondrial membrane integrity in neonatal cardiomyocytes Neonatal cardiomyocytes treated with either 10 μg/mL rhMG53 or BSA and (A) representative images of TMRE fluorescence when cells were treated with 50 μM H2O2 (B) Quantification of TMRE normalized to baseline (time = 0 min). Data are presented as mean ± SEM and significance was determined by unpaired t-test. N = 10.
3.3. Superoxide release in isolated cardiomyocytes after hypoxia/reoxygenation is inhibited by rhMG53
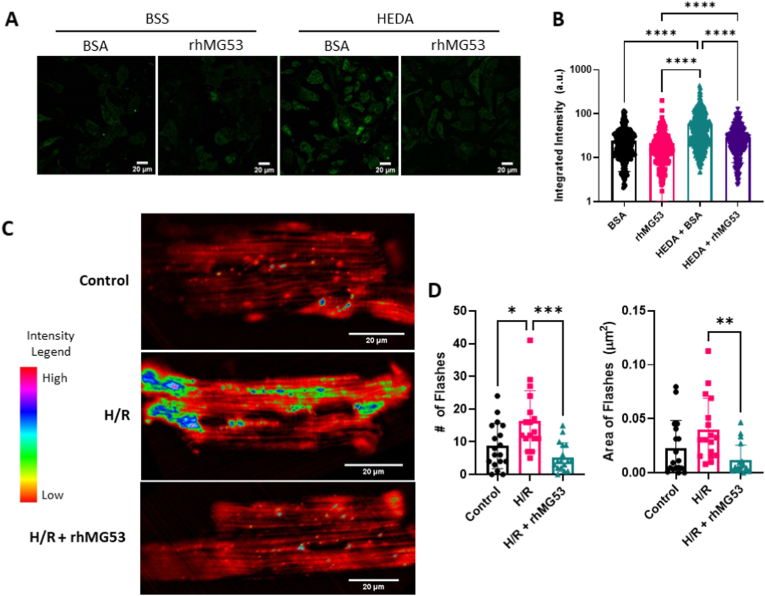
Therefore, we wanted to take this finding further and determine whether that also prevents the accumulation of mitochondrial-generated ROS after ischemic damage. We used the HL-1 cardiac muscle cell line derived from mice with the MitoSOX Red fluorescent indicator to measure rhMG53's effect on mitochondria after oxidative damage. As shown in Fig. 3A, rhMG53 treatment under normoxic conditions did not alter ROS release from the mitochondria. However, we observed a significant increase in MitoSOX Red fluorescent intensity in cells that underwent hypoxia, energy depletion, and acidosis (HEDA) in the presence of BSA, indicating high concentrations of mitochondrial ROS in the cytoplasm that results from damaged mitochondria. Treating cells with rhMG53 during HEDA injury resulted in a significantly reduced MitoSOX Red fluorescent intensity like the control conditions (Fig. 3B). This suggests rhMG53 treatment prevents the generation of mitochondrial ROS in response to oxidative injury.
Fig. 3.
Exogenous rhMG53 reduces hypoxia/reoxygenation-induced release of reactive oxygen species. (A) HEDA oxidative stress induced release of ROS from the mitochondria, as indicated with an increase in MitoSOX intensity after HEDA treatment. (B) MitoSOX intensity was quantified as the integrated density for each cell. N = 281 BSS BSA, N = 191 BSS rhMG53, N = 440 HEDA BSA, and N = 252 HEDA rhMG53 (3 biological replicates). HL-1 cells were treated with MitoSOX Red after hypoxia/reoxygenation using the HEDA protocol. Significance determined by Krustal-Wallis test with Dunn's multiple comparison test. (C) Surface plots of the amplitude and distribution of mitoflashes in cardiomyocytes isolated from mt-cpYFP transgenic mice, under normoxia (control) condition, after hypoxia/reoxygenation (H/R), or hypoxia/reoxygenation +10 μg/mL rhMG53 (H/R+rhMG53). (D) Hypoxia/reoxygenation increased the number and size of mitoflashes, while treatment with MG53 significantly reduced mitoflash number and size. N = 18 Control, N = 17 H/R, and N = 17 H/R rhMG53. Significance determined by Brown-Forsythe test with Dunnett's T3 multiple comparisons test. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
To ensure MG53's prevention of mito-ROS accumulation after ischemic condition also occurs in true cardiomyocytes, we used an ex-vivo mouse model of mito-ROS analysis. Wang et al. [24] developed a novel indicator targeted to the mitochondrial matrix called circularly permuted yellow fluorescent protein (mt-cpYFP) that selectively fluoresce in the presence of O2, the main ROS created by the electron transport chain in the mitochondria and is only released into the cytoplasm after damage to the mitochondria. We have recently used this tool to study mitochondrial ROS generation in the mouse model of amyotrophic lateral sclerosis (ALS), termed “mitoFlash” [17,[20], [21], [22]]. In cardiomyocytes derived from the mt-cpYFP mice, we monitored mitochondrial superoxide generation after hypoxia/reoxygenation following our previous protocol [19]. Under basal conditions, mitoflash events are rare (Fig. 3C and D). However, subjecting the cardiomyocytes to hypoxia and reoxygenation significantly increases the frequency and area covered by superoxide flashes (Fig. 3C and D). When cardiomyocytes are treated with 2 μg/mL rhMG53 before hypoxia/reoxygenation, significantly fewer superoxide flashes occur covering a smaller area of the cardiomyocyte. This suggests rhMG53 treatment reduces superoxide production in the mitochondria after oxidative injury.
3.4. rhMG53 binds to cardiolipin to preserve mitochondria integrity under oxidative stress
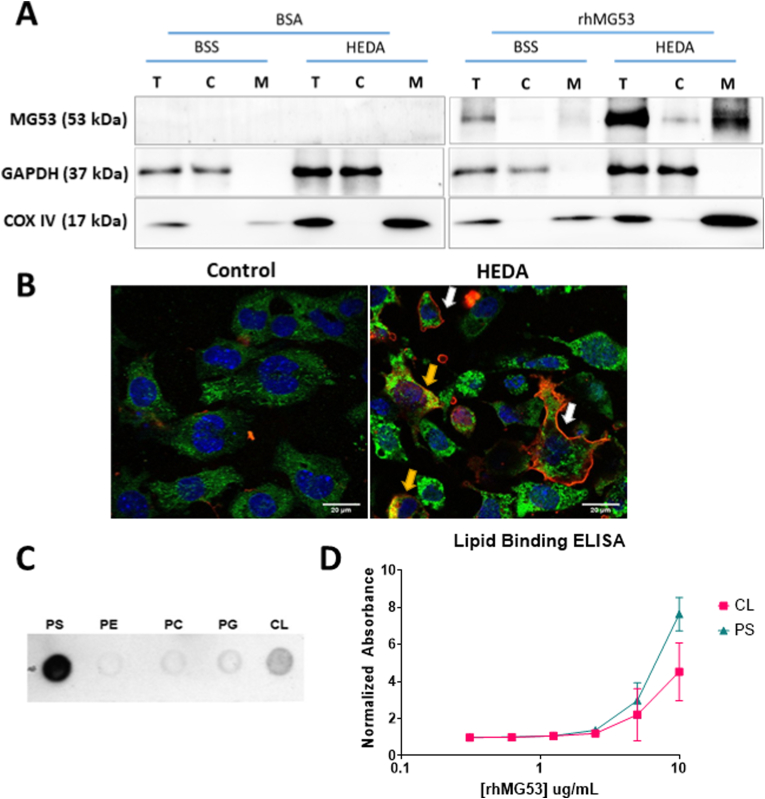
To probe into how treatment of rhMG53 is preventing mitochondrial ROS generation, we performed subcellular fractionation of HL-1 cells with or without HEDA treatment to separate the cytosolic proteins from proteins associated with the mitochondria. As shown in Fig. 4A, MG53 was enriched in the mitochondria fraction after HEDA damage compared to the mitochondria fraction under normal conditions. Only GAPDH in the cytosolic lanes and COX IV in the mitochondrial lanes of the western blot indicate clear separation of the subcellular fractions.
Fig. 4.
Oxidative stress leads to enrichment of rhMG53 at mitochondria in HL-1 cells by binding to the mitochondria lipid cardiolipin. (A) Representative western blots of 3 separate experiments of HL-1 cells treated with either 10 μg/mL BSA or rhMG53 underwent normoxia (BSS) or HEDA treatment and fractionated showing total protein (T), cytoplasmic protein (C), and mitochondrial protein (M). Purity of fractionation indicated with GAPDH and COX IV blotting. (B) rhMG53 tagged with Alexa647 binds to both the plasma membrane (white arrow) and localize to mitochondria (yellow arrow) of HL-1 after HEDA treatment. (C) rhMG53 binds to both phosphatidylserine (PS) and cardiolipin (CL). MG53 exhibits little or no binding to phosphatidylethanolamine (PE), phosphatidylcholine (PC), or phosphatidylglycerol (PG). (D) Quantitative lipid-based ELISA revealed dose-dependent binding of rhMG53 to PS and CL. N = 4. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Confocal imaging of cells that underwent normal or HEDA conditions and treated with rhMG53 confirmed the subcellular fractionation results (Fig. 4B). Cells that were not damaged did not have any rhMG53 entry into the cell. Conversely, damaged cells exhibited rhMG53 signal at both the plasma membrane (white arrows) and inside the cell surrounding the mitochondrial COX IV signal (yellow arrows).
We have previously shown that rhMG53 directly interacts with the phosphatidylserine (PS) on the plasma membrane to facilitate protection against injury to tissues by bringing lipid rafts to patch the damaged membrane [4]. However, unlike the plasma membrane, the mitochondrial membrane does not have PS. Thus, we hypothesized that MG53 might also interact with the phospholipid cardiolipin (CL) present on the mitochondria after damage to facilitate mitochondria membrane repair injury during oxidative stress. We used a lipid dot-blot assay to test whether rhMG53 can bind to CL. Fig. 4C demonstrates that MG53 binds to PS and CL, but not other typical membrane phospholipids. Quantitative lipid-based ELISA verified a dose-dependent binding of rhMG53 to PS and CL (Fig. 4D).
3.5. rhMG53 prevents engulfment of mitochondria by lysosome after HEDA
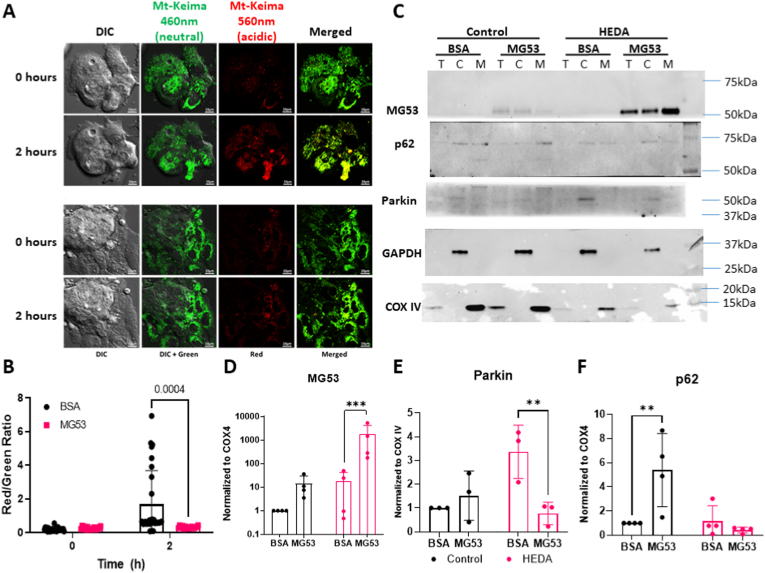
Cells undergo mitophagy to degrade damaged mitochondria to preserve a pool of healthy mitochondria. HL-1 cells were infected with lentivirus carrying a mitochondrial-tagged keima fluorescent protein, mt-keima, to determine the how MG53 might be involved in mitophagy. This tag has a fluorescent emission shift when pH changes from 3 to 8; fluorescing green at a neutral pH like the cytoplasm, shifting to red under acidic pH, similar to that found in the lysosome [23].
Fig. 5A and B reveals HL-1 cells pre-treated with BSA, which underwent oxidative damage, showed a substantial shift towards red fluorescence, an indicator of mitochondria in the acidic lysosome compartments. Treatment with rhMG53 prevents that shift, with mitochondria maintaining their bright green fluorescence. This suggests that treating cells with rhMG53 reduces mitochondria encasement by the lysosome for degradation. We verified this by performing another cell fractionation and probed for parkin and p62. We found that treating cells with rhMG53 during HEDA reduced the amount of parkin within the mitochondrial fraction compared to the HEDA group treated with BSA (p = 0.0082) (Fig. 5C and D). Staining for p62 revealed rhMG53 treatment under control conditions increased p62 expression compared to BSA treatment (p = 0.0055). However, we saw no difference in p62 localization to the mitochondrial fraction after HEDA-induced oxidative stress. Upregulation of p62 in the total lysate regardless of BSA or rhMG53 treatment indicated HEDA induced the autophagic processes.
Fig. 5.
rhMG53 reduces lysosome-engulfment of mitochondria after H2O2treatment in HL-1 cells. (A) HL-1 cells expressing mt-Keima underwent hypoxia/reoxygenation treatment after a 1hr pre-treatment of 10 μg/mL BSA or rhMG53. Imaging revealed a strong shift towards red fluorescence indicating acidic pH in cells treated with BSA while cells treated with rhMG53 retained the green fluorescence of neutral pH. (B) Fluorescent intensity density for each cell was quantified and a ratio of red to green was calculated to determine degree of mitophagy present. Data was anlayzed via 2-way ANOVA with Šídák's multiple comparisons test. N = 28 BSA, and N = 16 rhMG53 (C) Western blot of HL-1 cells that underwent normoxia or hypoxia and then fractionated to show total protein (T), cytoplasmic protein (C), and mitochondrial protein (M). (D) Quantification of MG53 on a log scale, (E) parkin, and (F) p62 in the mitochondrial fraction normalized to COX IV signal. Signal density for each band was analyzed by 2-way ANOVA with Šídák's multiple comparisons test. N = 3–5 biological replicates. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
Discovering tissue preservation methods after ischemic events like a heart attack is essential to improving survival, and maintaining a healthy mitochondria population after ischemic insult is essential for cell and tissue function. We have previously described how MG53, either endogenous or exogenous, can protect cells and tissue from ischemic injury by fixing damage to the plasma membrane [[9], [10], [11], [12],25,26]. Additionally, Chung et al. showed that endogenous MG53 was elevated in the mitochondria fraction of mouse hearts after ischemia in cardiomyocytes [14]. This study shows that exogenous rhMG53 can improve cardiomyocyte survival by preserving mitochondrial health after ischemic injury.
During ischemic/reperfusion injury, mitochondria in cardiomyocytes are severely damaged from elevated oxidative stress, resulting in excessive mitochondrial ROS production and mitophagy [27,28]. Mitophagy is a specialized form of autophagy that targets damaged mitochondria through increased Parkin signal on the outer mitochondrial membrane to recruit p62/LC3 complexes [29]. Through several models of oxidative stress including hypoxia/reoxygenation and hydrogen peroxide treatments in vitro and an acute MI model in vivo, we showed exogenous rhMG53 co-localized to the mitochondria to preserve mitochondria integrity. rhMG53 treatment maintained the mitochondrial membrane potential and reduced mitochondrial-associated ROS leak into the cytoplasm in cultured HL-1 cells and primary adult mouse cardiomyocytes. This resulted in reduced parkin localization at the mitochondria, indicating a reduction in mitophagy after oxidative stress.
Previous studies have shown that the human heart contains a lower level of endogenous MG53 than rodent hearts, suggesting the need for the exogenous application of the protein for cardioprotection [30]. When a cell is damaged, PS, which is on the plasma membrane's inner leaflet, is exposed [31], attracting cell membrane repair proteins like Annexin 5 and MG53 to the injury site [4]. These proteins attach to lipid rafts to form a membrane repair patch. MG53 can also bind to CL, a mitochondrial-specific lipid flipped from the inner mitochondrial matrix to the outer mitochondrial matrix after oxidative stress [32,33]. MG53 likely preserves mitochondria integrity after oxidative stress similar to plasma membrane repair by binding to CL for mitochondrial membrane repair using lipid rafts, reducing mitochondrial ROS accumulation and mitophagy.
Several recent studies have questioned the safety of rhGM53 application as a therapeutic agent [34,35]. We recently showed that sustained overexpression of MG53 does not alter metabolism while increasing regenerative capacity [5,36]. In this study, exogenous rhMG53 to cells does not alter mitochondrial ROS production under basal conditions, further supporting the safety of rhMG53 treatment. However, there was increased p62 expression when rhMG53 was added under basal conditions, suggesting there may be an increase in autophagy. Future studies will be required to determine whether this increase is a clinical concern when attempting to use rhMG53 to preserve cardiomyocytes after ischemic/reperfusion injury.
Overall, this study provides a novel mechanism behind how rhMG53 treatment may be a clinically relevant strategy for reducing cardiomyocyte injury and maintaining cardiac function in patients after ischemic injury. Although we show preservation of mitochondrial membrane potential, reduction in mitochondrial ROS production, and a decrease in mitophagy in cardiomyocytes using several methods of oxidative stress, this study is limited by a lack of mitochondrial function, which would further elucidate MG53's effects preserving mitochondria after ischemic/reperfusion injury. Additionally, further studies on the timing of rhMG53 application for preserving tissue function will be required. Understanding MG53's interactions with mitochondria could be an attractive avenue for developing MG53 as a targeted protein therapy for cardioprotection and potentially other regenerative medicine applications.
Sources of funding
This work was supported by NIH grants awarded to JM, PL and JZ, by American Heart Association Grant #19TPA34850169 to HZ, and partially supported by American Heart Association Grant #18PRE34030430 and the National Center for Advancing Translational Sciences TL-1 fellowship TL1TR002735 awarded to KGF.
Declaration of competing interest
JM is a founder of TRIM-edicine, Inc., a university spin-off biotechnology company that develops MG53 for regenerative medicine application. Intellectual properties related to MG53 were maintained by Rutgers University and the Ohio State University.
Contributor Information
Hua Zhu, Email: Hua.zhu@osumc.edu.
Jianjie Ma, Email: Jianjie.ma@osumc.edu.
References
- 1.Nowbar A.N., et al. Mortality from ischemic heart disease. Circ. Cardiovasc. Qual. Outcomes. 2019;12(6) doi: 10.1161/CIRCOUTCOMES.118.005375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sucher R., et al. Intracellular signaling pathways control mitochondrial events associated with the development of ischemia/reperfusion-associated damage. Transpl. Int. 2009;22(9):922–930. doi: 10.1111/j.1432-2277.2009.00883.x. [DOI] [PubMed] [Google Scholar]
- 3.Ramachandra C.J.A., et al. Mitochondria in acute myocardial infarction and cardioprotection. EBioMedicine. 2020;57 doi: 10.1016/j.ebiom.2020.102884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cai C., et al. MG53 nucleates assembly of cell membrane repair machinery. Nat. Cell Biol. 2009;11(1):56–64. doi: 10.1038/ncb1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bian Z., et al. Sustained elevation of MG53 in the bloodstream increases tissue regenerative capacity without compromising metabolic function. Nat. Commun. 2019;10(1):4659. doi: 10.1038/s41467-019-12483-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hwang M., et al. Redox-dependent oligomerization through a leucine zipper motif is essential for MG53-mediated cell membrane repair. Am. J. Physiol. Cell Physiol. 2011;301(1):C106–C114. doi: 10.1152/ajpcell.00382.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weisleder N., et al. Recombinant MG53 protein modulates therapeutic cell membrane repair in treatment of muscular dystrophy. Sci. Transl. Med. 2012;4(139) doi: 10.1126/scitranslmed.3003921. 139ra85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y., et al. MG53 participates in ischaemic postconditioning through the RISK signalling pathway. Cardiovasc. Res. 2011;91(1):108–115. doi: 10.1093/cvr/cvr029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jia Y., et al. Treatment of acute lung injury by targeting MG53-mediated cell membrane repair. Nat. Commun. 2014;5:4387. doi: 10.1038/ncomms5387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duann P., et al. MG53-mediated cell membrane repair protects against acute kidney injury. Sci. Transl. Med. 2015;7(279) doi: 10.1126/scitranslmed.3010755. 279ra36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu J., et al. Cardioprotection of recombinant human MG53 protein in a porcine model of ischemia and reperfusion injury. J. Mol. Cell. Cardiol. 2015;80:10–19. doi: 10.1016/j.yjmcc.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X., et al. Cardioprotection of ischemia/reperfusion injury by cholesterol-dependent MG53-mediated membrane repair. Circ. Res. 2010;107(1):76–83. doi: 10.1161/CIRCRESAHA.109.215822. [DOI] [PubMed] [Google Scholar]
- 13.Ma H., et al. Effect of metabolic syndrome on mitsugumin 53 expression and function. PLoS One. 2015;10(5) doi: 10.1371/journal.pone.0124128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chung Y.W., et al. Targeted disruption of PDE3B, but not PDE3A, protects murine heart from ischemia/reperfusion injury. Proc. Natl. Acad. Sci. U. S. A. 2015;112(17):E2253–E2262. doi: 10.1073/pnas.1416230112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu H., et al. Amelioration of ischemia-reperfusion-induced muscle injury by the recombinant human MG53 protein. Muscle Nerve. 2015;52(5):852–858. doi: 10.1002/mus.24619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schindelin J., et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012;9(7):676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fang H., et al. Imaging superoxide flash and metabolism-coupled mitochondrial permeability transition in living animals. Cell Res. 2011;21(9):1295–1304. doi: 10.1038/cr.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding Y., et al. Mitoflash altered by metabolic stress in insulin-resistant skeletal muscle. J. Mol. Med. (Berl.) 2015;93(10):1119–1130. doi: 10.1007/s00109-015-1278-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Z., et al. Irisin protects heart against ischemia-reperfusion injury through a SOD2-dependent mitochondria mechanism. J. Cardiovasc. Pharmacol. 2018;72(6):259–269. doi: 10.1097/FJC.0000000000000608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiao Y., et al. ROS-related mitochondrial dysfunction in skeletal muscle of an ALS mouse model during the disease progression. Pharmacol. Res. 2018;138:25–36. doi: 10.1016/j.phrs.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang H., et al. ALS-associated mutation SOD1(G93A) leads to abnormal mitochondrial dynamics in osteocytes. Bone. 2018;106:126–138. doi: 10.1016/j.bone.2017.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou J., et al. Dysregulated mitochondrial Ca(2+) and ROS signaling in skeletal muscle of ALS mouse model. Arch. Biochem. Biophys. 2019;663:249–258. doi: 10.1016/j.abb.2019.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun N., et al. A fluorescence-based imaging method to measure in vitro and in vivo mitophagy using mt-Keima. Nat. Protoc. 2017;12(8):1576–1587. doi: 10.1038/nprot.2017.060. [DOI] [PubMed] [Google Scholar]
- 24.Wang W., et al. Superoxide flashes in single mitochondria. Cell. 2008;134(2):279–290. doi: 10.1016/j.cell.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cao C.M., et al. MG53 constitutes a primary determinant of cardiac ischemic preconditioning. Circulation. 2010;121(23):2565–2574. doi: 10.1161/CIRCULATIONAHA.110.954628. [DOI] [PubMed] [Google Scholar]
- 26.Yao Y., et al. MG53 permeates through blood-brain barrier to protect ischemic brain injury. Oncotarget. 2016;7(16):22474–22485. doi: 10.18632/oncotarget.7965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bartz R.R., Suliman H.B., Piantadosi C.A. Redox mechanisms of cardiomyocyte mitochondrial protection. Front. Physiol. 2015;6:291. doi: 10.3389/fphys.2015.00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luan Y., et al. Emerging role of mitophagy in the heart: therapeutic potentials to modulate mitophagy in cardiac diseases. Oxid. Med. Cell. Longev. 2021;2021 doi: 10.1155/2021/3259963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Narendra D., et al. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J. Cell Biol. 2008;183(5):795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lemckert F.A., et al. Lack of MG53 in human heart precludes utility as a biomarker of myocardial injury or endogenous cardioprotective factor. Cardiovasc. Res. 2016;110(2):178–187. doi: 10.1093/cvr/cvw017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagata S., et al. Exposure of phosphatidylserine on the cell surface. Cell Death Differ. 2016;23(6):952–961. doi: 10.1038/cdd.2016.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paradies G., et al. Role of cardiolipin in mitochondrial function and dynamics in health and disease: molecular and pharmacological aspects. Cells. 2019;8(7) doi: 10.3390/cells8070728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paradies G., et al. Role of cardiolipin peroxidation and Ca2+ in mitochondrial dysfunction and disease. Cell Calcium. 2009;45(6):643–650. doi: 10.1016/j.ceca.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 34.Feng H., et al. MG53 E3 ligase-dead mutant protects diabetic hearts from acute ischemic/reperfusion injury and ameliorates diet-induced cardiometabolic damage. Diabetes. 2022;71(2):298–314. doi: 10.2337/db21-0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yi J.S., et al. MG53-induced IRS-1 ubiquitination negatively regulates skeletal myogenesis and insulin signalling. Nat. Commun. 2013;4:2354. doi: 10.1038/ncomms3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Q., et al. MG53 does not manifest the development of diabetes in db/db mice. Diabetes. 2020;69(5):1052–1064. doi: 10.2337/db19-0807. [DOI] [PMC free article] [PubMed] [Google Scholar]