This case-control study examines data from NSQIP, the SEER program, and the National Cancer Database to evaluate trends in lumpectomy and mastectomy rates in the treatment of breast cancer.
Key Points
Question
How have the trends in the surgical treatment of breast cancer changed over time?
Findings
In this case-control study, lumpectomy rates significantly increased and mastectomy rates significantly decreased beginning in 2013. Unilateral mastectomy rates, while previously declining, have stabilized beginning in 2013 with a concurrent stabilization in contralateral prophylactic mastectomy rates since 2013.
Meaning
Increasing rates of lumpectomy compared with mastectomy, and stabilized yet increased rates of unilateral mastectomy compared with contralateral prophylactic mastectomy, indicate a movement toward less invasive surgical practices throughout the study period examined.
Abstract
Importance
Rates of lumpectomy for breast cancer management in the United States previously declined in favor of more aggressive surgical options, such as mastectomy and contralateral prophylactic mastectomy (CPM).
Objective
To evaluate longitudinal trends in the rates of lumpectomy and mastectomy, including unilateral mastectomy vs CPM rates, and to determine characteristics associated with current surgical practice using 3 national data sets.
Design and Setting
Data from the National Surgical Quality Improvement Program (NSQIP), Surveillance, Epidemiology, and End Results (SEER) program, and National Cancer Database (NCDB) were examined to evaluate trends in lumpectomy and mastectomy rates from 2005 through 2017. Mastectomy rates were also evaluated with a focus on CPM. Longitudinal trends were analyzed using the Cochran-Armitage test for trend. Multivariate logistic regression models were performed on the NCDB data set to identify predictors of lumpectomy and CPM.
Results
A study sample of 3 467 645 female surgical breast cancer patients was analyzed. Lumpectomy rates reached a nadir between 2010 and 2013, with a significant increase thereafter. Conversely, in comparison with lumpectomy rates, overall mastectomy rates declined significantly starting in 2013. Cochran-Armitage trend tests demonstrated an annual decrease in lumpectomy rates of 1.31% (95% CI, 1.30%-1.32%), 0.07% (95% CI, 0.01%-0.12%), and 0.15% (95% CI, 0.15%-0.16%) for NSQIP, SEER, and NCDB, respectively, from 2005 to 2013 (P < .001, P = .01, and P < .001, respectively). From 2013 to 2017, the annual increase in lumpectomy rates was 0.96% (95% CI, 0.95%-0.98%), 1.60% (95% CI, 1.59%-1.62%), and 1.66% (95% CI, 1.65%-1.67%) for NSQIP, SEER, and NCDB, respectively (all P < .001). Comparisons of specific mastectomy types showed that unilateral mastectomy and CPM rates stabilized after 2013, with unilateral mastectomy rates remaining higher than CPM rates throughout the entire time period.
Conclusions
This observational longitudinal analysis indicated a trend reversal with an increase in lumpectomy rates since 2013 and an associated decline in mastectomies. The steady increase in CPM rates from 2005 to 2013 has since stabilized. The reasons for the recent reversal in trends are likely multifactorial. Further qualitative and quantitative research is required to understand the factors driving these recent practice changes and their associations with patient-reported outcomes.
Introduction
The surgical treatment of breast cancer has evolved considerably.1 Large-scale clinical trials have prompted breast cancer treatment to move away from radical mastectomy in favor of less invasive approaches such as simple mastectomy and breast-conserving therapy (BCT).2,3,4,5,6 Similar treatment de-escalation has occurred in the management of the axilla, with sentinel lymph node biopsy replacing axillary lymph node dissection for staging patients as clinically node negative and for treatment of those with limited nodal disease.6,7,8,9,10 Simultaneously, the widespread use of adjuvant systemic therapy for early breast cancers has led to a decline in rates of ipsilateral breast tumor recurrences and development of contralateral primary cancers.
In 2006, both unilateral mastectomy and lumpectomy rates began to decline with an unanticipated increase in contralateral prophylactic mastectomy (CPM), despite the lack of a survival advantage for the bilateral surgery.11,12,13,14,15 More recent trends in the surgical management of breast cancer are unknown, limiting our ability to understand the effect of research initiatives to reduce less invasive surgery.
The aim of our study was to examine modern trends in the surgical management of breast cancer. We used 3 large national databases to measure trends in rates of lumpectomy vs mastectomy and unilateral mastectomy vs CPM over a 13-year-period from 2005 through 2017. In addition, we sought to determine predictors associated with the receipt of lumpectomy vs mastectomy and CPM vs unilateral mastectomy.
Methods
Study Design
Using the National Surgical Quality Improvement Program (NSQIP), Surveillance, Epidemiology, and End Results (SEER) program, and National Cancer Database (NCDB), we performed an observational longitudinal analysis of female patients who underwent lumpectomy or mastectomy from 2005 through 2017. Surgical procedure was determined by Current Procedural Terminology codes (NSQIP) and site-specific surgery codes (SEER and NCDB) (eTables 1 and 2 in the Supplement). Among patients who underwent mastectomy, trends in unilateral mastectomy and CPM were examined. When we studied trends using SEER and NCDB, codes labeled “laterality not specified” could not be placed into unilateral mastectomy or CPM cohorts and were excluded.
Databases
NSQIP is an outcomes-based database that aims to assess quality of care for surgical patients by reporting 30-day morbidity and mortality outcomes for all major inpatient and outpatient surgical procedures.16 SEER is an oncology database that captures approximately 30% of all newly diagnosed cancers in the United States by collecting data from strategically selected regions for accurate population-based cancer reports.17,18 NCDB, the largest clinical cancer registry in the world, collects data from more than 1500 Commission on Cancer–accredited facilities, capturing more than 70% of newly diagnosed cancer cases nationwide.19
Variables of Interest
Patient-specific variables were compared between lumpectomy and mastectomy cohorts and unilateral mastectomy and CPM cohorts. Age, race, and ethnicity data were provided in all 3 databases. NSQIP-specific variables included inpatient vs outpatient status and American Society of Anesthesiologists classification. SEER and NCDB variables included area population density, median income quartile, tumor behavior, grade, histology, hormonal status, and TNM and overall cancer staging. NCDB-specific variables included facility location and type, insurance/payor, Charlson-Deyo comorbidity index, and neoadjuvant radiation and chemotherapy status.
Data Analysis
Annual proportions of lumpectomy vs mastectomy were assessed from 2005 through 2017. On determining any notable changes in rates, we compared the period before the change with that after the change to determine if the difference was significant. Lumpectomy trends were examined using the Cochran-Armitage trend test, and P < .05 was considered a significant rate change. Annual rates of unilateral mastectomy and CPM per 1000 mastectomies were assessed using Poisson regression; incidence rate ratios with P < .05 were considered a significant rate change. Unadjusted comparisons of patient characteristics for lumpectomy vs mastectomy cohorts and unilateral mastectomy vs CPM cohorts were performed using Fisher exact test and reported as odds ratios with 95% CIs.
On determining a notable reversal in lumpectomy rates in 2013, we compared NCDB patients who underwent lumpectomy just before (2011) and after (2017) this change to assess whether changes in patient-, cancer-, or facility-specific variables had a potential association with changes in practice patterns and rates of lumpectomy vs mastectomy. These years were chosen as the most recent year of data examined (2017) and 2 years before the noted change (2011). Multivariable logistic regression analyses of predictors for lumpectomy vs mastectomy and CPM vs unilateral mastectomy were performed using NCDB to analyze associations between surgery type and patient-specific variables. We chose NCDB for these more in-depth analyses because it is the largest of the 3 databases and provides the most patient-, cancer-, and facility-specific details. Statistical analyses were performed using R statistical software (version 4.0.3, tidyverse package).
Results
Lumpectomy vs Mastectomy
Data for a total of 3 467 152 female patients who underwent surgical treatment for breast cancer were analyzed (1 912 771 lumpectomy; 1 554 381 mastectomy). NCDB had the largest cohort with 2 306 879 patients (66.5%), followed by SEER with 790 627 patients (22.8%) and NSQIP with 369 646 patients (10.7%). Basic patient demographics by data set for lumpectomy and mastectomy patients are shown in eTable 3 in the Supplement. The SEER and NCDB cohorts were skewed toward a slightly older population than NSQIP. The majority of patients were non-Hispanic and White. Additional comparisons between the cohorts can be found in eTables 7-9 in the Supplement.
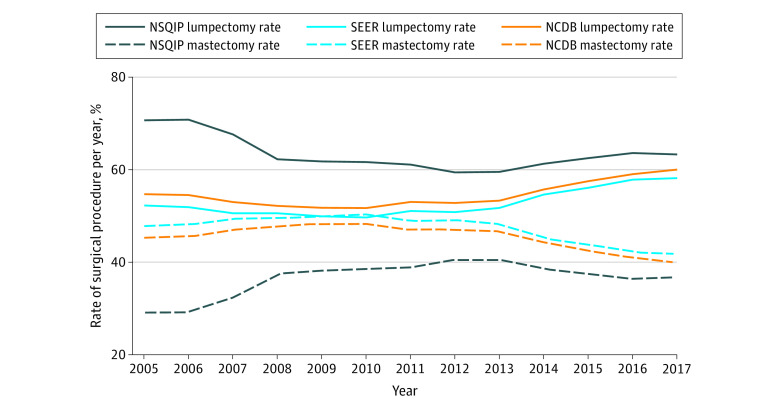
Figure 1 and Table 1 present yearly rates of lumpectomy and mastectomy, demonstrating an overall decrease in lumpectomy rates from 2005 to 2013; the trend reversal, or an increase in lumpectomy rates, begins in 2013 and continues through 2017. This 2013 time point was used as the inflection point for cohort analyses given the noted changes. NSQIP, SEER, and NCDB demonstrated an overall 6.37%, 12.5%, and 12.5% increase in lumpectomy rates comparing 2013 with 2017, respectively, which was associated with a corresponding decrease in mastectomy rates (Table 1). Cochran-Armitage trend tests demonstrated an annual decrease in lumpectomy rates of 1.31% (95% CI, 1.30%-1.32%), 0.07% (95% CI, 0.01%-0.12%), and 0.15% (95% CI, 0.15%-0.16%) per year for NSQIP, SEER, and NCDB, respectively, from 2005 to 2013 (P < .001, P = .01, P < .001, respectively). From 2013 to 2017, the annual increase in lumpectomy rates was 0.96% (95% CI, 0.95%-0.98%), 1.60% (95% CI, 1.59%-1.62%), and 1.66% (95% CI, 1.65%-1.67%) for NSQIP, SEER, and NCDB, respectively (all P < .001).
Figure 1. Trends in Lumpectomy and Mastectomy Rates (2005-2017).
NCDB indicates National Cancer Database; NSQIP, National Surgical Quality Improvement Program; SEER, Surveillance, Epidemiology, and End Results program.
Table 1. Lumpectomy and Mastectomy Rates by Data Set.
| Year | NSQIP | SEER | NCDB | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total No. of surgical breast procedures | Rate, % | Total No. of surgical breast procedures | Rate, % | Total No. of surgical breast procedures | Rate, % | ||||
| Lumpectomy | Mastectomy | Lumpectomy | Mastectomy | Lumpectomy | Mastectomy | ||||
| 2005 | 3321 | 70.7 | 29.3 | 51 495 | 52.3 | 47.7 | 136 119 | 54.7 | 45.3 |
| 2006 | 11 117 | 70.8 | 29.2 | 53 300 | 51.9 | 48.1 | 143 766 | 54.5 | 45.5 |
| 2007 | 19 575 | 67.6 | 32.4 | 55 826 | 50.6 | 49.4 | 152 507 | 53.0 | 47.0 |
| 2008 | 21 551 | 62.3 | 37.7 | 58 186 | 50.6 | 49.4 | 160 430 | 52.2 | 47.8 |
| 2009 | 25 865 | 61.8 | 38.2 | 59 764 | 49.9 | 50.1 | 169 842 | 51.8 | 48.2 |
| 2010 | 25 336 | 61.7 | 38.3 | 58 709 | 49.7 | 50.3 | 168 795 | 51.7 | 48.3 |
| 2011 | 25 746 | 61.1 | 38.9 | 60 963 | 51.1 | 48.9 | 178 536 | 53.0 | 47.0 |
| 2012 | 30 346 | 59.4 | 40.6 | 62 323 | 50.8 | 49.2 | 185 209 | 52.8 | 47.2 |
| 2013a | 35 596 | 59.5 | 40.5 | 63 993 | 51.7 | 48.3 | 194 560 | 53.3 | 46.7 |
| 2014 | 38 190 | 61.3 | 38.7 | 65 564 | 54.6 | 45.4 | 201 938 | 55.7 | 44.3 |
| 2015 | 41 967 | 62.5 | 37.5 | 66 969 | 56.1 | 43.9 | 205 954 | 57.5 | 42.5 |
| 2016 | 45 463 | 63.6 | 36.4 | 66 583 | 57.9 | 42.1 | 205 463 | 59.0 | 41.0 |
| 2017a | 45 573 | 63.3 | 36.7 | 67 094 | 58.2 | 41.8 | 204 111 | 60.0 | 40.0 |
Abbreviations: NCDB, National Cancer Database; NSQIP, National Surgical Quality Improvement Program; SEER, Surveillance, Epidemiology, and End Results program.
Cochran-Armitage trend tests demonstrated an annual decrease in lumpectomy rates of 1.31% (95% CI, 1.30%-1.32%), 0.07% (95% CI, 0.01%-0.12%), and 0.15% (95% CI, 0.15%-0.16%) for NSQIP, SEER, and NCDB, respectively, from 2005 to 2013 (P < .001, P = .01, and P < .001, respectively). From 2013 to 2017, the annual increase in lumpectomy rates was 0.96% (95% CI, 0.95%-0.98%), 1.60% (95% CI, 1.59%-1.62%), and 1.66% (95% CI, 1.65%-1.67%) for NSQIP, SEER, and NCDB, respectively (all P < .001).
Based on the trend reversal in lumpectomy rates in 2013, we compared patient characteristics among NCDB patients undergoing lumpectomy who were treated before and after this time point (2011 vs 2017) (eTable 4 in the Supplement). From 2011 to 2017, the proportion of patients aged 40 to 59 years decreased, and the proportion of patients aged 60 to 79 years increased with a concurrent increase in the proportion of patients with Medicare (39.6% to 44.7%; P < .001). Minimal differences were noted in clinical T, N, and M staging. The proportion of patients treated at an academic research program increased slightly (29.3% to 32.2%; P < .001). No clinically relevant differences were noted in distributions of facility location, race, ethnicity, population density, median income quartiles, Charlson-Deyo score, tumor grade, and tumor histology.
Multivariable logistic regression analysis was performed on the NCDB data set to further assess factors associated with receipt of lumpectomy (Table 2). The strongest predictors of lumpectomy were older age, Black race, treatment at a community center, and clinical N0 disease. Odds ratios for lumpectomy increased with older age and decreased as the clinical T stage increased from 1 to 4 and as the overall clinical cancer stage increased from 1 to 3. The odds of having a lumpectomy decreased overall from 2005 to 2010 and increased from 2012 to 2017.
Table 2. Multivariable Analysis of Predictors for Lumpectomy vs Mastectomy (National Cancer Database).
| Variable | Odds ratio (95% CI)a | P value |
|---|---|---|
| Age, y | ||
| <40 | 1 [Reference] | |
| 40-49 | 1.879 (1.846-1.913) | <.001 |
| 50-59 | 2.779 (2.731-2.828) | <.001 |
| 60-69 | 3.408 (3.347-3.469) | <.001 |
| 70-79 | 3.674 (3.605-3.745) | <.001 |
| ≥80 | 3.749 (3.674-3.827) | <.001 |
| Raceb | ||
| American Indian, Aleutian, or Eskimo | 0.988 (0.937-1.042) | .62 |
| Black | 1.207 (1.195-1.218) | <.001 |
| Asian or Pacific Islander | 0.864 (0.851-0.877) | <.001 |
| White | 1 [Reference] | |
| Other race or race unknown | 1.134 (1.11-1.158) | <.001 |
| Ethnicityb | ||
| Hispanic/Spanish | 1.063 (1.05-1.077) | <.001 |
| Non-Hispanic/Spanish | 1 [Reference] | |
| Facility type | ||
| Academic/research program | 0.904 (0.895-0.914) | <.001 |
| Community center | 1 [Reference] | |
| Comprehensive Community Cancer Program | 0.902 (0.893-0.912) | <.001 |
| Integrated Network Cancer Programs | 0.893 (0.883-0.904) | <.001 |
| Insurance/payor | ||
| Private insurance/managed care | 1 [Reference] | |
| Medicaid | 1.008 (0.996-1.021) | .19 |
| Medicare | 0.977 (0.969-0.985) | <.001 |
| Other government | 0.922 (0.897-0.948) | <.001 |
| Not insured | 1.055 (1.032-1.078) | <.001 |
| Median income quartile, $ | ||
| <38 000 | 1 [Reference] | |
| 38 000-47 999 | 1.060 (1.049-1.071) | <.001 |
| 48 000-62 999 | 1.116 (1.106-1.127) | <.001 |
| >63 000 | 1.181 (1.17-1.192) | <.001 |
| Charlson-Deyo comorbidity index | ||
| 0 | 1 [Reference] | |
| 1 | 0.805 (0.799-0.812) | <.001 |
| 2 | 0.745 (0.733-0.759) | <.001 |
| ≥3 | 0.728 (0.707-0.749) | <.001 |
| Neoadjuvant radiation | ||
| No | 1 [Reference] | |
| Yes | 1.633 (1.553-1.718) | <.001 |
| Neoadjuvant chemotherapy | ||
| No | 1 [Reference] | |
| Yes | 1.001 (0.987-1.015) | .93 |
| Clinical T stage | ||
| 0 | 1 [Reference] | |
| In situ | 1.427 (1.365-1.493) | <.001 |
| 1 | 1.918 (1.833-2.006) | .70 |
| 2 | 1.294 (1.237-1.355) | <.001 |
| 3 | 0.432 (0.411-0.454) | <.001 |
| 4 | 0.268 (0.254-0.284) | <.001 |
| Clinical N stage | ||
| 0 | 1 [Reference] | |
| Micrometastases | 0.411 (0.143-1.177) | .10 |
| 1 | 0.684 (0.675-0.693) | <.001 |
| 2 | 0.579 (0.559-0.599) | <.001 |
| 3 | 0.512 (0.49-0.535) | <.001 |
| Clinical M stage | ||
| No metastases | 1 [Reference] | |
| Micrometastases | 0.848 (0.637-1.129) | .26 |
| Macrometastases | 1.105 (0.997-1.225) | .06 |
| Overall clinical cancer stage | ||
| 0 | 1 [Reference] | |
| 1 | 0.810 (0.786-0.834) | <.001 |
| 2 | 0.579 (0.562-0.597) | <.001 |
| 3 | 0.504 (0.485-0.525) | <.001 |
| 4 | 0.534 (0.48-0.594) | <.001 |
| Year | ||
| 2005 | 1 [Reference] | |
| 2006 | 0.987 (0.971-1.002) | .09 |
| 2007 | 0.894 (0.88-0.908) | <.001 |
| 2008 | 0.788 (0.776-0.801) | <.001 |
| 2009 | 0.747 (0.735-0.759) | <.001 |
| 2010 | 0.728 (0.717-0.74) | <.001 |
| 2011 | 0.758 (0.746-0.77) | <.001 |
| 2012 | 0.738 (0.726-0.749) | <.001 |
| 2013 | 0.747 (0.736-0.759) | <.001 |
| 2014 | 0.827 (0.814-0.839) | <.001 |
| 2015 | 0.892 (0.878-0.906) | <.001 |
| 2016 | 0.946 (0.931-0.961) | <.001 |
| 2017 | 0.981 (0.966-0.996) | .02 |
Abbreviations: M, metastasis; N, node; T, tumor.
An odds ratio of greater than 1.00 indicates a higher likelihood for lumpectomy.
Race and ethnicity data were provided by the National Cancer Database.
Unilateral Mastectomy vs CPM
From 2005 through 2017, data for 922 816 unilateral mastectomy patients and 417 587 CPM patients were examined. Demographic data for patients undergoing unilateral mastectomy and CPM are shown in eTable 5 in the Supplement. In all 3 databases, the majority (>70%) of patients who had CPM were younger than 60 years. A higher percentage of patients in the CPM cohorts were White compared with the unilateral mastectomy cohorts, whereas the percentages of Asian and Black patients in the unilateral mastectomy cohorts were higher compared with the CPM cohorts. Additional comparisons can be found in eTables 10-12 in the Supplement.
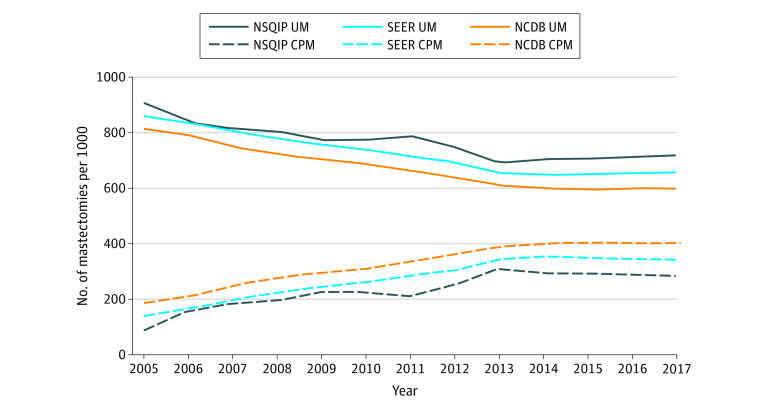
Rates of mastectomy type are presented in Figure 2 and eTable 6 in the Supplement. An initial increase in CPM rates was noted from 2005 to 2013 with a subsequent stabilization from 2013 to 2017. Rates of CPM increased in NSQIP, SEER, and NCDB by 8.72% (95% CI, 6.35%-11.15%), 10.73% (95% CI, 9.43%-12.05%), and 8.74% (95% CI, 7.55%-9.94%) annually from 2005 to 2013 (P < .001). The increase in CPM rate stabilized after 2013, with trends showing CPM rate changes of −1.97% (95% CI, −2.48% to −1.48%), −0.25% (95% CI, −0.92% to 0.43%), and 0.64% (95% CI, 0.02% to 1.26%) annually from 2013 to 2017 (P < .001; P = .47; P = .04). Despite recent CPM rate stabilization, the use of CPM increased from 2005 through 2017 overall, demonstrating a 205.6%, 145.1%, and 116.2% increase in NSQIP, SEER, and NCDB, respectively.
Figure 2. Unilateral Mastectomy (UM) and Contralateral Prophylactic Mastectomy (CPM) Rates (2005-2017).
NCDB indicates National Cancer Database; NSQIP, National Surgical Quality Improvement Program; SEER, Surveillance, Epidemiology, and End Results program.
Multivariable logistic regression analysis was performed to assess factors associated with receipt of CPM (Table 3). The strongest predictors of CPM were younger age, White race, treatment at an Integrated Network Cancer Program, and residence in a zip code with a higher median income. Odds ratios for CPM decreased with increasing age. The odds of having CPM increased over time when examining year of surgery but began to stabilize in 2013 and 2014, with an odds ratio of 2.57 (95% CI, 2.50-2.64) for CPM in 2017 (P < .001) with the year 2005 as a reference.
Table 3. Multivariable Analysis of Predictors for Contralateral Prophylactic Mastectomy vs Unilateral Mastectomy (National Cancer Database).
| Variable | Odds ratio (95% CI)a | P value |
|---|---|---|
| Age, y | ||
| <40 | 1 [Reference] | |
| 40-49 | 0.431 (0.422-0.441) | <.001 |
| 50-59 | 0.244 (0.239-0.249) | <.001 |
| 60-69 | 0.153 (0.15-0.157) | <.001 |
| 70-79 | 0.084 (0.081-0.086) | <.001 |
| ≥80 | 0.031 (0.03-0.032) | <.001 |
| Raceb | ||
| American Indian, Aleutian, or Eskimo | 0.771 (0.713-0.834) | <.001 |
| Black | 0.536 (0.528-0.544) | <.001 |
| Asian or Pacific Islander | 0.505 (0.493-0.518) | <.001 |
| White | 1 [Reference] | |
| Other race or race unknown | 0.77 (0.746-0.795) | <.001 |
| Ethnicityb | ||
| Hispanic/Spanish | 0.647 (0.634-0.659) | <.001 |
| Non-Hispanic/Spanish | 1 [Reference] | |
| Facility type | ||
| Academic/research program | 1.244 (1.221-1.266) | <.001 |
| Community center | 1 [Reference] | |
| Comprehensive Community Cancer Program | 1.371 (1.347-1.395) | <.001 |
| Integrated Network Cancer Programs | 1.45 (1.422-1.478) | <.001 |
| Insurance/payor | ||
| Private insurance/managed care | 1 [Reference] | |
| Medicaid | 0.675 (0.663-0.687) | <.001 |
| Medicare | 0.802 (0.791-0.813) | <.001 |
| Other government | 1.038 (1.001-1.076) | .046 |
| Not insured | 0.553 (0.535-0.571) | <.001 |
| Median income quartile, $ | ||
| <38 000 | 1 [Reference] | |
| 38 000-47 999 | 1.066 (1.049-1.083) | <.001 |
| 48 000-62 999 | 1.094 (1.078-1.111) | <.001 |
| >63 000 | 1.115 (1.099-1.131) | <.001 |
| Charlson-Deyo comorbidity index | ||
| 0 | 1 [Reference] | |
| 1 | 1.097 (1.083-1.112) | <.001 |
| 2 | 1.045 (1.013-1.078) | .005 |
| ≥3 | 1.045 (1.013-1.078) | .04 |
| Neoadjuvant radiation | ||
| No | 1 [Reference] | |
| Yes | 0.644 (0.598-0.694) | <.001 |
| Neoadjuvant chemotherapy | ||
| No | 1 [Reference] | |
| Yes | 1.224 (1.205-1.245) | <.001 |
| Clinical T stage | ||
| 0 | 1 [Reference] | |
| In situ | 0.922 (0.865-0.983) | .01 |
| 1 | 0.806 (0.756-0.858) | <.001 |
| 2 | 0.867 (0.814-0.923) | .001 |
| 3 | 1.067 (0.999-1.139) | .06 |
| 4 | 0.988 (0.921-1.059) | .73 |
| Clinical N stage | ||
| 0 | 1 [Reference] | |
| Micrometastases | 1.890 (0.631-5.665) | <.001 |
| 1 | 1.157 (1.138-1.177) | <.001 |
| 2 | 1.143 (1.101-1.186) | <.001 |
| 3 | 1.181 (1.131-1.232) | <.001 |
| Clinical M stage | ||
| No metastases | 1 [Reference] | |
| Micrometastases | 1.392 (0.974-1.99) | .07 |
| Macrometastases | 0.856 (0.743-0.987) | .03 |
| Overall clinical cancer stage | ||
| 0 | 1 [Reference] | |
| 1 | 1.114 (1.064-1.167) | <.001 |
| 2 | 1.368 (1.305-1.433) | <.001 |
| 3 | 1.4 (1.325-1.478) | <.001 |
| 4 | 1.158 (0.998-1.344) | .05 |
| Year | ||
| 2005 | 1 [Reference] | |
| 2006 | 1.139 (1.106-1.173) | <.001 |
| 2007 | 1.513 (1.471-1.556) | <.001 |
| 2008 | 1.867 (1.816-1.919) | <.001 |
| 2009 | 2.07 (2.014-2.127) | <.001 |
| 2010 | 2.248 (2.187-2.31) | <.001 |
| 2011 | 2.434 (2.369-2.501) | <.001 |
| 2012 | 2.724 (2.652-2.798) | <.001 |
| 2013 | 3.005 (2.926-3.086) | <.001 |
| 2014 | 2.894 (2.818-2.973) | <.001 |
| 2015 | 2.818 (2.744-2.895) | <.001 |
| 2016 | 2.628 (2.558-2.7) | <.001 |
| 2017 | 2.572 (2.504-2.643) | <.001 |
Abbreviations: T, tumor; N, node; M, metastasis.
An odds ratio of greater than 1.00 indicates a higher likelihood for contralateral prophylactic mastectomy.
Race and ethnicity data were provided by the National Cancer Database.
Discussion
In this multidatabase longitudinal epidemiologic examination of nationwide trends in breast cancer management, we have, for the first time, demonstrated a de-escalation in surgical treatments in recent years. This represents a trend reversal in 2 aspects of surgical oncology. Lumpectomy rates reached a nadir in 2013 but have increased again significantly in the years that followed. During the same period, CPM rates, which had increased rapidly from 2005 to 2013, stabilized. Reasons for these clinical practice changes are likely multifactorial and underscore the preference-sensitive nature of breast cancer surgical management.
Trends in Lumpectomy vs Mastectomy Rates
After the National Institutes of Health (NIH) 1990 Consensus Statement and the publication of studies supporting the equivalent safety and efficacy of BCT compared with mastectomy, there was an increase in the number of lumpectomies being performed.3,5,20,21,22,23 The subsequent decrease in lumpectomy rates from 2005 to 2013 documented herein and elsewhere was unexpected given the safety and less invasive nature of lumpectomy when compared with mastectomy.24,25,26,27,28,29,30 Potential reasons for increasing mastectomy rates included fear of local recurrence, disinterest in radiation treatment (due to fear of adverse effects or inability to regularly travel to a radiation center), and lack of patient understanding of the equivalent efficacy and survival from BCT vs mastectomy in patients who were eligible for BCT.31,32 Another explanation for the previous increase in mastectomy rates was the increasing use of bilateral mastectomy for unilateral disease (CPM) in the mid- to late 2000s as a result of increased awareness of genetic predispositions to breast cancer and increased publicity surrounding celebrities undergoing mastectomy.30,33,34,35,36 Various studies noting this increase in mastectomy rates have called for physician reassessment of patient education on indications for mastectomy to improve care for patients.26,27,28,30,37
While the 1990 NIH Consensus Statement was believed to largely influence the increase in lumpectomy rates throughout the 1990s and early 2000s,24,25,27,28 the cause of the rise after 2013 appears to be multifactorial and likely involves a preference for less invasive surgical practices.24,27,28 When comparing the patient populations for lumpectomy in 2011 with those from 2017, few differences were noted in oncologic characteristics, suggesting that it was not eligibility for BCT that was changing but rather overall practice patterns.
Physician involvement in decision-making is directly associated with a patient’s likelihood of receiving a lumpectomy rather than mastectomy.38,39 Increased mastectomy rates have also been linked to a lack of communication by the physician leading to inadequate patient education/understanding of their options.31,40 The importance of appropriate patient education cannot be overlooked. Lee et al39 used a survey to assess the decision-making process for patients undergoing surgical treatment for breast cancer between 2008 and 2011; only approximately half of the patients reported awareness that BCT and mastectomy result in equivalent survival. Increased patient education and improved communication of surgical options by physicians may be associated with the recent reversal in lumpectomy trends.31,37,39
The comparison of patients who underwent lumpectomy from 2011 vs 2017 suggests that the proportion of older patients (most notably 70-79 years of age) undergoing lumpectomy has become more prominent than before, supported by a concurrent increase in the proportion of patients undergoing lumpectomy with Medicare. Older patients had a higher likelihood of undergoing lumpectomy than mastectomy (patients 70-79 years of age were 3.67 times more likely and patients ≥80 years of age were 3.75 times more likely to receive lumpectomy than patients <40 years). As the average life expectancy continued to increase in the United States, more elderly patients may have been diagnosed with breast cancer, influencing the increase in lumpectomy rates in more recent years as well.41
The ACOSOG Z0011 trial showed safety in sentinel lymph node biopsy (rather than axillary dissection) in patients with clinical T1N0M0 or T2N0M0 breast cancer with 1 or 2 pathologically positive sentinel nodes undergoing lumpectomy with plans for whole breast irradiation.9 This landmark trial changed practice by decreasing rates of axillary lymph node dissection.6,10,42,43,44 However, it may have also influenced the subsequent increase in lumpectomy rates, because patients with clinical T1N0M0 or T2N0M0 disease who meet criteria for lumpectomy may be encouraged to undergo lumpectomy with radiation in order to avoid the potential need for axillary lymph node dissection; whereas, if these patients underwent mastectomy and were found to have 1 or 2 pathologically positive nodes, they would not meet the Z0011 trial criteria and would have an axillary dissection and the risks associated with it.
Furthermore, improvements in radiation therapy, chemotherapy, and other systemic therapies have led to a reduction in local recurrence rates, encouraging patients to seek BCT with increased reassurance from surgeons.45,46 Radiation centers have grown in number and have become more accessible, potentially further motivating patients to choose lumpectomy with radiation over mastectomy.31,47 Another potential influence on the recent increase in lumpectomy rates is the growth in options and rates of oncoplastic procedures associated with lumpectomy.48
Trends in CPM vs Unilateral Mastectomy
Our study provides the most updated analysis of CPM trends to date. Previous studies using national databases examined CPM rates up to 2012, demonstrating an overall increase in CPM utilization.15,29,30,34,49,50,51,52,53,54,55,56,57,58,59,60 Our study demonstrates that after 2012, CPM rates have stabilized, and patients who are younger and White and have a higher socioeconomic status show an increased likelihood for undergoing CPM, which is largely consistent with previous literature.34,49,57,60,61 From 2005 to 2013, later year of treatment increased the likelihood for undergoing CPM, while the likelihood of undergoing CPM each year after 2013 was similar.
The majority of bilateral mastectomies are performed in women who are not at a significantly increased risk for contralateral breast cancer development.30,33,34 While national databases do not provide information regarding the primary decision-maker (surgeon, oncologist, patient), many studies have suggested that the decision is multifactorial.32,34,35,60,62,63,64 Han et al32 used a questionnaire to assess rationale for choice in CPM, with the most commonly reported reasons being fear of developing a contralateral breast cancer, anxiety about future tests/screening, younger age at diagnosis, and desire for symmetry, followed by encouragement by health care professionals and family or friends. Other literature discussing the previous increase in CPM rates reinforced that concern for future contralateral breast cancer, fear of death (despite the lack of reduction in mortality with CPM), and desire for symmetry were commonly reported reasons.30,34,36
Overestimation of contralateral breast cancer risk, misconceptions of mortality, and lack of appreciation for increased complication risks associated with a bilateral procedure are due to insufficient education by physicians and gaps in understanding by patients.30,32,34,36,65,66,67,68,69 When surveying various stakeholders’ concerns regarding CPM, Shamsunder et al36 concluded that while preferences differed among stakeholders, shared decision-making and improved patient education are paramount. In addition, patients have reported combating the vulnerability associated with their cancer diagnosis by choosing CPM as a method of taking control over their diagnosis.62,63 Reported increases in CPM rates were consistent in the literature and provoked controversy regarding optimal patient care and safety.28,32
Increased use of CPM raised concerns regarding its overuse, possibly influencing the subsequent stabilization after 2013. In 2016, as part of the Choosing Wisely campaign, the American Society of Breast Surgeons stated that surgeons should not routinely perform CPM in patients with unilateral breast cancer until they have been provided with sufficient information regarding their low risk of developing contralateral breast cancer and negligible effect on life expectancy.11,12 According to our data, it appears this sentiment was already well understood by breast surgeons before the 2016 formal recommendation, as the stabilization of CPM rates began in 2013. Our study suggests there has been a change in practice patterns over time toward less invasive approaches that conserve the uninvolved breast. This was likely associated with improved patient education and more comprehensive conversations with patients about risks and benefits. The recent trends indicate that multidisciplinary, evidence-based, and more informative approaches have been put into effect.
Lastly, previous literature has demonstrated that patients undergoing lumpectomy with positive margins requiring return to the operating room for re-excision are more likely to undergo conversion to mastectomy and even CPM because of aesthetic concerns if they are unable to receive a sufficient oncoplastic procedure, disinterest in potential additional surgery in the future, and patient fear of cancer recurrence.70,71,72,73 However, a multidisciplinary consensus panel that included contributors from the Society of Surgical Oncology and the American Society for Radiation Oncology performed a meta-analysis examining the optimal margin for invasive stage I and II breast cancer in patients undergoing BCT (lumpectomy with whole-breast irradiation) and determined that using the definition of a negative margin as “no ink on tumor” was not only associated with low rates of ipsilateral breast cancer recurrence but also had the potential to decrease rates of re-excision.72 Therefore, acceptance of the “no ink on tumor” definition of a negative margin may be associated with the decrease in mastectomy rates (due to a decrease in conversion to mastectomy) and stabilization of CPM rates demonstrated in our study.
Limitations
The limitations of the study are inherent to large national databases, including errors in information collection and large sample sizes causing small numeric differences of questionable clinical significance to be highly statistically significant. Clinical significance was acknowledged in this study in addition to statistical significance. None of the databases include information regarding ratio of tumor size to breast size, multicentricity of cancers, previous chest/mantle radiation, and other contraindications to BCT. NSQIP includes patients who have breast surgery for both oncologic and benign reasons, which skews this data set toward a younger population. In addition, as information regarding BRCA status is unavailable, when evaluating unilateral mastectomy vs CPM rates in SEER and NCDB, it is unclear what proportion of patients who underwent CPM had a clinical indication for bilateral mastectomy. We chose 2013 as the inflection point in the lumpectomy trend after the examination of all 3 data sets. The inflection point is likely between 2010 and 2013 in the oncologic data sets, with a clear increase in lumpectomy rates occurring after 2013. However, despite the various patient populations and limitations of the data sets, we observed similar trends in the surgical treatment of breast cancer over the 13-year period, indicating that such observations may represent true changes over time.
Conclusions
This epidemiologic evaluation of the surgical management of breast cancer using 3 nationwide data sets demonstrates a trend reversal in lumpectomy rates since reaching a nadir in 2013 with an associated decline in mastectomies. An increase in the number of older patients may be associated with this change in trend. The steady growth in rates of CPM from 2005 to 2013 has also stabilized. Further qualitative and quantitative research is required to understand the factors driving these changes as well as their association with patient-reported outcomes.
eTable 1. CPT Codes
eTable 2. Site-Specific Surgery Codes
eTable 3. Demographics for Lumpectomy and Mastectomy Patients (2005–2017)
eTable 4. Demographics for Lumpectomy Patients in 2011 vs 2017 (NCDB)
eTable 5. Demographics for Unilateral Mastectomy and Contralateral Mastectomy Patients (2005–2017)
eTable 6. Unilateral Mastectomy and Contralateral Prophylactic Mastectomy Rates
eTable 7. NSQIP Demographics for Lumpectomy and Mastectomy Patients (2005–2017)
eTable 8. SEER Demographics for Lumpectomy and Mastectomy Patients (2005–2017)
eTable 9. NCDB Demographics for Lumpectomy and Mastectomy Patients (2005–2017)
eTable 10. NSQIP Demographics for Unilateral Mastectomy and Contralateral Mastectomy Patients (2005–2017)
eTable 11. SEER Demographics for Unilateral Mastectomy and Contralateral Mastectomy Patients (2005–2017)
eTable 12. NCDB Demographics for Unilateral Mastectomy and Contralateral Mastectomy Patients (2005–2017)
References
- 1.Halsted WS. The results of operations for the cure of cancer of the breast performed at the Johns Hopkins Hospital from June, 1889, to January, 1894. Ann Surg. 1894;20(5):497-555. doi: 10.1097/00000658-189407000-00075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fisher B, Montague E, Redmond C, et al. Comparison of radical mastectomy with alternative treatments for primary breast cancer: a first report of results from a prospective randomized clinical trial. Cancer. 1977;39(6)(suppl):2827-2839. doi: [DOI] [PubMed] [Google Scholar]
- 3.Fisher B, Bauer M, Margolese R, et al. Five-year results of a randomized clinical trial comparing total mastectomy and segmental mastectomy with or without radiation in the treatment of breast cancer. N Engl J Med. 1985;312(11):665-673. doi: 10.1056/NEJM198503143121101 [DOI] [PubMed] [Google Scholar]
- 4.Fisher B, Anderson S; National Surgical Adjuvant Breast and Bowel Project . Conservative surgery for the management of invasive and noninvasive carcinoma of the breast: NSABP trials. World J Surg. 1994;18(1):63-69. doi: 10.1007/BF00348193 [DOI] [PubMed] [Google Scholar]
- 5.Fisher B, Jeong JH, Anderson S, Bryant J, Fisher ER, Wolmark N. Twenty-five-year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. N Engl J Med. 2002;347(8):567-575. doi: 10.1056/NEJMoa020128 [DOI] [PubMed] [Google Scholar]
- 6.Black DM, Mittendorf EA. Landmark trials affecting the surgical management of invasive breast cancer. Surg Clin North Am. 2013;93(2):501-518. doi: 10.1016/j.suc.2012.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krag DN, Anderson SJ, Julian TB, et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol. 2010;11(10):927-933. doi: 10.1016/S1470-2045(10)70207-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Veronesi U, Viale G, Paganelli G, et al. Sentinel lymph node biopsy in breast cancer: ten-year results of a randomized controlled study. Ann Surg. 2010;251(4):595-600. doi: 10.1097/SLA.0b013e3181c0e92a [DOI] [PubMed] [Google Scholar]
- 9.Giuliano AE, McCall L, Beitsch P, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: the American College of Surgeons Oncology Group Z0011 randomized trial. Ann Surg. 2010;252(3):426-432. doi: 10.1097/SLA.0b013e3181f08f32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011;305(6):569-575. doi: 10.1001/jama.2011.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Society of Breast Surgeons . Choosing wisely: five things physicians and patients should question. Published June 27, 2016. https://www.breastsurgeons.org/docs/resources/2016_asbrs_cw_recommendations.pdf
- 12.Landercasper J, Bailey L, Berry TS, et al. Measures of appropriateness and value for breast surgeons and their patients: the American Society of Breast Surgeons Choosing Wisely initiative. Ann Surg Oncol. 2016;23(10):3112-3118. doi: 10.1245/s10434-016-5327-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Basu NN, Ross GL, Evans DG, Barr L. The Manchester guidelines for contralateral risk-reducing mastectomy. World J Surg Oncol. 2015;13:237. doi: 10.1186/s12957-015-0638-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carbine NE, Lostumbo L, Wallace J, Ko H. Risk-reducing mastectomy for the prevention of primary breast cancer. Cochrane Database Syst Rev. 2018;(4):CD002748. doi: 10.1002/14651858.CD002748.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong SM, Freedman RA, Sagara Y, Aydogan F, Barry WT, Golshan M. Growing use of contralateral prophylactic mastectomy despite no improvement in long-term survival for invasive breast cancer. Ann Surg. 2017;265(3):581-589. doi: 10.1097/SLA.0000000000001698 [DOI] [PubMed] [Google Scholar]
- 16.American College of Surgeons . ACS National Surgical Quality Improvement Program. Accessed July 21, 2021. https://www.facs.org/quality-programs/acs-nsqip
- 17.National Cancer Institute . SEER incidence data, 1975-2018. Accessed July 21, 2021. https://seer.cancer.gov/data/
- 18.National Cancer Institute . Overview of the SEER program. Accessed July 21, 2021. https://seer.cancer.gov/about/overview.html
- 19.American College of Surgeons . National Cancer Database. Accessed July 21, 2021. https://www.facs.org/quality-programs/cancer/ncdb
- 20.Jacobson JA, Danforth DN, Cowan KH, et al. Ten-year results of a comparison of conservation with mastectomy in the treatment of stage I and II breast cancer. N Engl J Med. 1995;332(14):907-911. doi: 10.1056/NEJM199504063321402 [DOI] [PubMed] [Google Scholar]
- 21.Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347(16):1227-1232. doi: 10.1056/NEJMoa020989 [DOI] [PubMed] [Google Scholar]
- 22.Poggi MM, Danforth DN, Sciuto LC, et al. Eighteen-year results in the treatment of early breast carcinoma with mastectomy versus breast conservation therapy: the National Cancer Institute Randomized Trial. Cancer. 2003;98(4):697-702. doi: 10.1002/cncr.11580 [DOI] [PubMed] [Google Scholar]
- 23.NIH consensus conference . Treatment of early-stage breast cancer. JAMA. 1991;265(3):391-395. doi: 10.1001/jama.1991.03460030097037 [DOI] [PubMed] [Google Scholar]
- 24.Lazovich D, Solomon CC, Thomas DB, Moe RE, White E. Breast conservation therapy in the United States following the 1990 National Institutes of Health Consensus Development Conference on the treatment of patients with early stage invasive breast carcinoma. Cancer. 1999;86(4):628-637. doi: [DOI] [PubMed] [Google Scholar]
- 25.Du X, Freeman DH Jr, Syblik DA. What drove changes in the use of breast conserving surgery since the early 1980s? the role of the clinical trial, celebrity action and an NIH consensus statement. Breast Cancer Res Treat. 2000;62(1):71-79. doi: 10.1023/A:1006414122201 [DOI] [PubMed] [Google Scholar]
- 26.McGuire KP, Santillan AA, Kaur P, et al. Are mastectomies on the rise? a 13-year trend analysis of the selection of mastectomy versus breast conservation therapy in 5865 patients. Ann Surg Oncol. 2009;16(10):2682-2690. doi: 10.1245/s10434-009-0635-x [DOI] [PubMed] [Google Scholar]
- 27.Katipamula R, Degnim AC, Hoskin T, et al. Trends in mastectomy rates at the Mayo Clinic Rochester: effect of surgical year and preoperative magnetic resonance imaging. J Clin Oncol. 2009;27(25):4082-4088. doi: 10.1200/JCO.2008.19.4225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tuttle TM, Rueth NM, Abbott A, Virnig BA. Trends in the local treatment of breast cancer: should we be worried? J Surg Oncol. 2011;103(4):313-316. doi: 10.1002/jso.21699 [DOI] [PubMed] [Google Scholar]
- 29.Habermann EB, Abbott A, Parsons HM, Virnig BA, Al-Refaie WB, Tuttle TM. Are mastectomy rates really increasing in the United States? J Clin Oncol. 2010;28(21):3437-3441. doi: 10.1200/JCO.2009.27.6774 [DOI] [PubMed] [Google Scholar]
- 30.Kummerow KL, Du L, Penson DF, Shyr Y, Hooks MA. Nationwide trends in mastectomy for early-stage breast cancer. JAMA Surg. 2015;150(1):9-16. doi: 10.1001/jamasurg.2014.2895 [DOI] [PubMed] [Google Scholar]
- 31.McCahill LE, Privette AR, Hart MR, James TA. Are mastectomy rates a reasonable quality measure of breast cancer surgery? Am J Surg. 2009;197(2):216-221. doi: 10.1016/j.amjsurg.2007.12.056 [DOI] [PubMed] [Google Scholar]
- 32.Han E, Johnson N, Glissmeyer M, et al. Increasing incidence of bilateral mastectomies: the patient perspective. Am J Surg. 2011;201(5):615-618. doi: 10.1016/j.amjsurg.2011.01.018 [DOI] [PubMed] [Google Scholar]
- 33.Hawley ST, Jagsi R, Morrow M, et al. Social and clinical determinants of contralateral prophylactic mastectomy. JAMA Surg. 2014;149(6):582-589. doi: 10.1001/jamasurg.2013.5689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lim DW, Metcalfe KA, Narod SA. Bilateral mastectomy in women with unilateral breast cancer: a review. JAMA Surg. 2021;156(6):569-576. doi: 10.1001/jamasurg.2020.6664 [DOI] [PubMed] [Google Scholar]
- 35.Venetis MK, MacGeorge EL, Baptiste DF, et al. Social network, surgeon, and media influence on the decision to undergo contralateral prophylactic mastectomy. Am J Clin Oncol. 2018;41(6):519-525. doi: 10.1097/COC.0000000000000321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shamsunder MG, Panchal H, Pilewskie M, Lee C, Razdan SN, Matros E. Understanding stakeholder preference for contralateral prophylactic mastectomy: a conjoint analysis. J Am Coll Surg. 2021;233(5):606-618.e1. doi: 10.1016/j.jamcollsurg.2021.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morrow M, Harris JR. More mastectomies: is this what patients really want? J Clin Oncol. 2009;27(25):4038-4040. doi: 10.1200/JCO.2009.23.0078 [DOI] [PubMed] [Google Scholar]
- 38.Katz SJ, Lantz PM, Janz NK, et al. Patterns and correlates of local therapy for women with ductal carcinoma-in-situ. J Clin Oncol. 2005;23(13):3001-3007. doi: 10.1200/JCO.2005.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee CN, Chang Y, Adimorah N, et al. Decision making about surgery for early-stage breast cancer. J Am Coll Surg. 2012;214(1):1-10. doi: 10.1016/j.jamcollsurg.2011.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Katz SJ, Lantz PM, Zemencuk JK. Correlates of surgical treatment type for women with noninvasive and invasive breast cancer. J Womens Health Gend Based Med. 2001;10(7):659-670. doi: 10.1089/15246090152563533 [DOI] [PubMed] [Google Scholar]
- 41.Arias E, Tejada-Vera B, Ahmad F. Provisional life expectancy estimates for January through June, 2020. Vital Statistics Rapid Release. Accessed July 21, 2021. https://www.cdc.gov/nchs/data/vsrr/VSRR10-508.pdf
- 42.Caudle AS, Hunt KK, Tucker SL, et al. American College of Surgeons Oncology Group (ACOSOG) Z0011: impact on surgeon practice patterns. Ann Surg Oncol. 2012;19(10):3144-3151. doi: 10.1245/s10434-012-2531-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gainer SM, Hunt KK, Beitsch P, Caudle AS, Mittendorf EA, Lucci A. Changing behavior in clinical practice in response to the ACOSOG Z0011 trial: a survey of the American Society of Breast Surgeons. Ann Surg Oncol. 2012;19(10):3152-3158. doi: 10.1245/s10434-012-2523-z [DOI] [PubMed] [Google Scholar]
- 44.Wright GP, Mater ME, Sobel HL, et al. Measuring the impact of the American College of Surgeons Oncology Group Z0011 trial on breast cancer surgery in a community health system. Am J Surg. 2015;209(2):240-245. doi: 10.1016/j.amjsurg.2014.07.001 [DOI] [PubMed] [Google Scholar]
- 45.Neuman H, Schumacher J, Hanlon B, et al. Local recurrence rates after breast-conserving therapy in patients receiving modern era therapy. Abstract presented at: American Society of Breast Surgeons Annual Meeting; May 4, 2018. Abstract 403956 [Google Scholar]
- 46.Samson K. Post-lumpectomy recurrence rates down sharply with treatment advances. Oncol Times. 2018;40(13):50. doi: 10.1097/01.COT.0000542461.00053.b6 [DOI] [Google Scholar]
- 47.Maroongroge S, Wallington DG, Taylor PA, et al. Geographic access to radiation therapy in the United States. Int J Radiat Oncol. 2020;108(3):S97-S98. doi: 10.1016/j.ijrobp.2020.07.2270 [DOI] [PubMed] [Google Scholar]
- 48.Panchal H, Shukla D, Razdan SN, El-Tamer M, Matros E, Henderson PW. American trends in oncoplastic breast surgery for 2006-2015: a retrospective analysis of NSQIP database. J Plast Reconstr Aesthet Surg. 2021;74(3):644-710. doi: 10.1016/j.bjps.2020.08.028 [DOI] [PubMed] [Google Scholar]
- 49.Tuttle TM, Habermann EB, Grund EH, Morris TJ, Virnig BA. Increasing use of contralateral prophylactic mastectomy for breast cancer patients: a trend toward more aggressive surgical treatment. J Clin Oncol. 2007;25(33):5203-5209. doi: 10.1200/JCO.2007.12.3141 [DOI] [PubMed] [Google Scholar]
- 50.Tuttle TM, Jarosek S, Habermann EB, et al. Increasing rates of contralateral prophylactic mastectomy among patients with ductal carcinoma in situ. J Clin Oncol. 2009;27(9):1362-1367. doi: 10.1200/JCO.2008.20.1681 [DOI] [PubMed] [Google Scholar]
- 51.Yao K, Stewart AK, Winchester DJ, Winchester DP. Trends in contralateral prophylactic mastectomy for unilateral cancer: a report from the National Cancer Data Base, 1998-2007. Ann Surg Oncol. 2010;17(10):2554-2562. doi: 10.1245/s10434-010-1091-3 [DOI] [PubMed] [Google Scholar]
- 52.Albornoz CR, Bach PB, Mehrara BJ, et al. A paradigm shift in U.S. breast reconstruction: increasing implant rates. Plast Reconstr Surg. 2013;131(1):15-23. doi: 10.1097/PRS.0b013e3182729cde [DOI] [PubMed] [Google Scholar]
- 53.Cemal Y, Albornoz CR, Disa JJ, et al. A paradigm shift in U.S. breast reconstruction: part 2. the influence of changing mastectomy patterns on reconstructive rate and method. Plast Reconstr Surg. 2013;131(3):320e-326e. doi: 10.1097/PRS.0b013e31827cf576 [DOI] [PubMed] [Google Scholar]
- 54.Pesce CE, Liederbach E, Czechura T, Winchester DJ, Yao K. Changing surgical trends in young patients with early stage breast cancer, 2003 to 2010: a report from the National Cancer Database. J Am Coll Surg. 2014;219(1):19-28. doi: 10.1016/j.jamcollsurg.2014.03.043 [DOI] [PubMed] [Google Scholar]
- 55.Agarwal S, Kidwell KM, Kraft CT, et al. Defining the relationship between patient decisions to undergo breast reconstruction and contralateral prophylactic mastectomy. Plast Reconstr Surg. 2015;135(3):661-670. doi: 10.1097/PRS.0000000000001044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Albornoz CR, Matros E, Lee CN, et al. Bilateral mastectomy versus breast-conserving surgery for early-stage breast cancer: the role of breast reconstruction. Plast Reconstr Surg. 2015;135(6):1518-1526. doi: 10.1097/PRS.0000000000001276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grimmer L, Liederbach E, Velasco J, Pesce C, Wang CH, Yao K. Variation in contralateral prophylactic mastectomy rates according to racial groups in young women with breast cancer, 1998 to 2011: a report from the National Cancer Database. J Am Coll Surg. 2015;221(1):187-196. doi: 10.1016/j.jamcollsurg.2015.03.033 [DOI] [PubMed] [Google Scholar]
- 58.Vaz-Luis I, Hughes ME, Cronin A, et al. Trends in the use of mastectomy in women with small node-negative breast cancer treated at US academic centers. Breast Cancer Res Treat. 2016;155(3):569-578. doi: 10.1007/s10549-016-3707-1 [DOI] [PubMed] [Google Scholar]
- 59.Nash R, Goodman M, Lin CC, et al. State variation in the receipt of a contralateral prophylactic mastectomy among women who received a diagnosis of invasive unilateral early-stage breast cancer in the United States, 2004-2012. JAMA Surg. 2017;152(7):648-657. doi: 10.1001/jamasurg.2017.0115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang T, Baskin AS, Dossett LA. Deimplementation of the Choosing Wisely recommendations for low-value breast cancer surgery: a systematic review. JAMA Surg. 2020;155(8):759-770. doi: 10.1001/jamasurg.2020.0322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arrington AK, Jarosek SL, Virnig BA, Habermann EB, Tuttle TM. Patient and surgeon characteristics associated with increased use of contralateral prophylactic mastectomy in patients with breast cancer. Ann Surg Oncol. 2009;16(10):2697-2704. doi: 10.1245/s10434-009-0641-z [DOI] [PubMed] [Google Scholar]
- 62.Rosenberg SM, Greaney ML, Patenaude AF, Sepucha KR, Meyer ME, Partridge AH. “I don’t want to take chances”: a qualitative exploration of surgical decision making in young breast cancer survivors. Psychooncology. 2018;27(6):1524-1529. doi: 10.1002/pon.4683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Covelli AM, Baxter NN, Fitch MI, McCready DR, Wright FC. ‘Taking control of cancer’: understanding women’s choice for mastectomy. Ann Surg Oncol. 2015;22(2):383-391. doi: 10.1245/s10434-014-4033-7 [DOI] [PubMed] [Google Scholar]
- 64.Lopez CD, Bluebond-Langner R, Houssock CA, Slezak SS, Bellavance E. Plastic and reconstructive surgeons’ knowledge and comfort of contralateral prophylactic mastectomy: a survey of the American Society of Plastic Surgeons. Front Oncol. 2019;8:647. doi: 10.3389/fonc.2018.00647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hamilton JG, Genoff MC, Salerno M, et al. Psychosocial factors associated with the uptake of contralateral prophylactic mastectomy among BRCA1/2 mutation noncarriers with newly diagnosed breast cancer. Breast Cancer Res Treat. 2017;162(2):297-306. doi: 10.1007/s10549-017-4123-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kaiser K, Cameron KA, Beaumont J, et al. What does risk of future cancer mean to breast cancer patients? Breast Cancer Res Treat. 2019;175(3):579-584. doi: 10.1007/s10549-019-05182-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rosenberg SM, Tracy MS, Meyer ME, et al. Perceptions, knowledge, and satisfaction with contralateral prophylactic mastectomy among young women with breast cancer: a cross-sectional survey. Ann Intern Med. 2013;159(6):373-381. doi: 10.7326/0003-4819-159-6-201309170-00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hunt KK, Euhus DM, Boughey JC, et al. Society of Surgical Oncology Breast Disease Working Group Statement on Prophylactic (Risk-Reducing) Mastectomy. Ann Surg Oncol. 2017;24(2):375-397. doi: 10.1245/s10434-016-5688-z [DOI] [PubMed] [Google Scholar]
- 69.Goldflam K, Hunt KK, Gershenwald JE, et al. Contralateral prophylactic mastectomy: predictors of significant histologic findings. Cancer. 2004;101(9):1977-1986. doi: 10.1002/cncr.20617 [DOI] [PubMed] [Google Scholar]
- 70.Morrow M, Jagsi R, Alderman AK, et al. Surgeon recommendations and receipt of mastectomy for treatment of breast cancer. JAMA. 2009;302(14):1551-1556. doi: 10.1001/jama.2009.1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.King TA, Sakr R, Patil S, et al. Clinical management factors contribute to the decision for contralateral prophylactic mastectomy. J Clin Oncol. 2011;29(16):2158-2164. doi: 10.1200/JCO.2010.29.4041 [DOI] [PubMed] [Google Scholar]
- 72.Moran MS, Schnitt SJ, Giuliano AE, et al. ; Society of Surgical Oncology; American Society for Radiation Oncology . Society of Surgical Oncology-American Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. J Clin Oncol. 2014;32(14):1507-1515. doi: 10.1200/JCO.2013.53.3935 [DOI] [PubMed] [Google Scholar]
- 73.Heeg E, Jensen MB, Hölmich LR, et al. Rates of re-excision and conversion to mastectomy after breast-conserving surgery with or without oncoplastic surgery: a nationwide population-based study. Br J Surg. 2020;107(13):1762-1772. doi: 10.1002/bjs.11838 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. CPT Codes
eTable 2. Site-Specific Surgery Codes
eTable 3. Demographics for Lumpectomy and Mastectomy Patients (2005–2017)
eTable 4. Demographics for Lumpectomy Patients in 2011 vs 2017 (NCDB)
eTable 5. Demographics for Unilateral Mastectomy and Contralateral Mastectomy Patients (2005–2017)
eTable 6. Unilateral Mastectomy and Contralateral Prophylactic Mastectomy Rates
eTable 7. NSQIP Demographics for Lumpectomy and Mastectomy Patients (2005–2017)
eTable 8. SEER Demographics for Lumpectomy and Mastectomy Patients (2005–2017)
eTable 9. NCDB Demographics for Lumpectomy and Mastectomy Patients (2005–2017)
eTable 10. NSQIP Demographics for Unilateral Mastectomy and Contralateral Mastectomy Patients (2005–2017)
eTable 11. SEER Demographics for Unilateral Mastectomy and Contralateral Mastectomy Patients (2005–2017)
eTable 12. NCDB Demographics for Unilateral Mastectomy and Contralateral Mastectomy Patients (2005–2017)