This randomized clinical trial investigates the efficacy and safety of an external saphenous vein graft support device by determining the progression of intimal hyperplasia in patients undergoing a coronary bypass graft procedure.
Key Points
Question
Does an external support device for saphenous vein grafts reduce progression of intimal hyperplasia 1 year after coronary artery bypass surgery?
Findings
In this randomized clinical trial that enrolled 224 patients, the estimated mean intimal hyperplasia area was 5.11 mm2 in supported grafts and 5.79 mm2 in unsupported grafts (P = .07) after imputation of data missing due to graft occlusion or severe disease. Five patients died, and 16 patients experienced a major cardiac or cerebrovascular event.
Meaning
The difference in intimal hyperplasia area between supported and unsupported grafts at 12 months did not achieve statistical significance, after accounting for early graft occlusions.
Abstract
Importance
Intimal hyperplasia and subsequent saphenous vein graft failure may have significant adverse clinical effects in patients undergoing coronary artery bypass surgery. External support of saphenous vein grafts has the potential to prevent vein graft dilation and hence slow the rate of intimal hyperplasia and increase long-term vein patency.
Objective
To determine efficacy, as measured by intimal hyperplasia, and safety of an external saphenous vein graft support device in patients undergoing a coronary bypass graft procedure.
Design, Setting, and Participants
This within-patient randomized, open-label, multicenter study was conducted at 17 Cardiothoracic Surgical Trials Network centers in North America. Between January 2018 and February 2019, 224 patients with multivessel coronary artery disease undergoing isolated bypass surgery were enrolled. For each patient, 1 of 2 vein grafts was randomized to receive external support or no support.
Interventions
External vein graft support or no support.
Main Outcomes and Measures
The primary efficacy end point was intimal hyperplasia area assessed by intravascular ultrasound at 12 months postrandomization for each study graft. Secondary confirmatory end points were lumen diameter uniformity assessed by angiography and graft failure (≥50% stenosis) by quantitative coronary angiography. Major cardiac and cerebrovascular events were collected through month 12.
Results
Among 224 patients (mean [SD] age, 65.8 [8.3] years; 178 [79.5%] male), 203 (90.6%) were eligible for intravascular ultrasound, of which 85 (41.9%) had at least 1 study graft occluded or severely diseased at 12 months (55 supported, 56 unsupported). After imputation of data missing because of graft occlusion or severe disease, the estimated mean (SE) intimal hyperplasia area was 5.11 (0.16) mm2 in supported grafts and 5.79 (0.20) mm2 in unsupported grafts (P = .07). In a sensitivity analysis of 113 patients with both grafts imaged, the mean intimal hyperplasia area was 4.58 (0.18) mm2 and 5.12 (0.23) mm2 in supported and unsupported grafts, respectively (P = .04). By 12 months, 5 patients (2.2%) died and 16 patients (7.1%) experienced a major cardiac or cerebrovascular event.
Conclusions and Relevance
The 12-month difference in intimal hyperplasia area between supported and unsupported grafts did not achieve statistical significance. Cumulative mortality and major cardiac or cerebrovascular events rates were similar to those in other randomized coronary artery bypass trials. Further investigation to assess the effect of external graft support devices on long-term graft patency and clinical outcomes is warranted.
Trial Registration
ClinicalTrials.gov Identifier: NCT03209609
Introduction
Coronary artery bypass graft (CABG) surgery, the most commonly performed cardiac operation, has been shown to improve survival in appropriately selected patients with complex left main and/or multivessel coronary artery disease.1 Major limitations of CABG surgery include graft failure, particularly with the use of saphenous veins, and progression of underlying native atherosclerotic disease. Despite the demonstrated benefits, arterial grafts, other than the left internal mammary artery, have not been widely used for the treatment of non–left anterior descending (LAD) disease; autologous saphenous vein grafts (SVGs) continue to be the most widely used bypass conduits.2
Progressive SVG disease after CABG is associated with poor long-term clinical outcomes.3,4 Several factors have been implicated in the short- and long-term failure of SVGs, including the method of conduit harvest, graft manipulation at surgery, poor graft quality, target artery size and quality, and progression of disease in both bypass graft and native coronary vessels.5,6 Approximately 10% to 15% of SVGs fail within the first month after surgery because of trauma from harvesting techniques, preexisting vein abnormalities, and surgery-associated factors such as insufficient graft flow, size mismatch between the graft and the target coronary artery, and poor outflow due to diffuse and more distal coronary artery disease.7,8
Long-term patency of SVGs is known to be adversely affected by the development of diffuse intimal hyperplasia (IH) and accelerated atherosclerosis. Saphenous vein graft disease results in part from exposure to arterial pressures, turbulent flow patterns, enhanced pulsatility, shear stress, and increased wall tension.9,10,11 These factors stimulate vascular smooth muscle cell proliferation and accelerate the progression of IH and atherosclerosis, ultimately leading to SVG stenosis and occlusion.12,13,14,15,16 The patency of SVGs at 10 years after CABG is approximately 50% to 60%; moreover, only half of patent grafts are disease free.17
An external support device that wraps around the SVG was developed to prevent conduit dilation, attenuate the higher arterial pressure, mitigate against any associated increase in wall tension, enhance lumen uniformity, and reduce smooth muscle cell proliferation, IH, and atherosclerosis. Although this external support device would not be expected to reduce the risk of short-term SVG occlusion caused by intraoperative technical factors, it may increase SVG intermediate to long-term patency by limiting graft IH. Recent small trials of this device using intravascular ultrasound (IVUS) have demonstrated a reduction in IH and graft irregularity, markers of early graft disease.18,19,20,21 Based on this preliminary evidence, we conducted a randomized clinical trial to assess the effect of the device in reducing IH and SVG irregularity in patients undergoing CABG for multivessel coronary artery disease.
Methods
Trial Design and Oversight
This is a multicenter, randomized, within-patient clinical trial, in which for each patient, 1 SVG was randomized to be supported by an external support device while another SVG was not supported and served as control. The SVGs were randomized electronically using stratified, block randomization. They were constructed to non-LAD vessels. The trial was designed to enroll 224 patients and was conducted at 17 centers in North America.
The trial was overseen by a Coordinating Center, an independent adjudication committee, and an independent data and safety monitoring board. An investigational device exemption was approved by the US Food and Drug Administration. Participating institutional review boards approved the protocol; all patients gave informed consent. The Cardiothoracic Surgical Trials Network investigators designed the trial, in collaboration with the device manufacturer (Vascular Graft Solutions), and network investigators collected and analyzed the data. This study followed the Consolidated Standards of Reporting Trials (CONSORT) guidelines.
Patients and Interventions
This trial enrolled adult patients with multivessel atherosclerotic coronary artery disease scheduled to undergo CABG. Race and ethnicity information was self-reported by the patients and categorized as Asian, Black or African American, Native Hawaiian or Other Pacific Islander, White, or more than 1 race and Hispanic or Latino or not Hispanic or Latino, respectively. Race and ethnicity data were collected because including participants from racial and ethnic minority groups in clinical trials is critical for scientific, ethical, and social reasons and for the generalizability of the trial results.
Key inclusion criteria were age 21 years or older and planned on-pump isolated CABG, with use of an internal mammary artery graft to the LAD and 2 or more SVGs to other native vessels having at least 75% proximal stenosis and comparable angiographic size and runoff. Exclusion criteria included prior CABG surgery, concomitant non-CABG cardiac procedures, and emergency surgery. Patients presenting with severe SVG varicosity as assessed after vein harvesting and before randomization were excluded. The trial protocol in Supplement 2 describes all eligibility criteria.
The external support device used in this trial is a tubular structure consisting of a combination of deformable cobalt chromium wires. The device has a flexible structure that allows it to be placed over the autologous SVG with adjustment of length and diameter to achieve optimal graft coverage. After standardized vein graft harvest and preparation during surgery, the segments of available vein conduit were committed to the required target coronary arteries and then randomly assigned to receive the external support or no support.
End Points
The trial was designed with 1 primary and 2 secondary confirmatory end points. The primary efficacy end point was the area of IH (plaque + media, in millimeters squared) assessed by IVUS at 12 months postrandomization for each study graft (device-supported and unsupported). Secondary confirmatory end points were SVG lumen diameter uniformity assessed by invasive angiography for all patent SVGs and reported by the Fitzgibbon patency scale and SVG failure defined as 50% or greater graft stenosis by quantitative coronary angiography (QCA).
Additional secondary efficacy end points measured at 12 months postrandomization included IH thickness (plaque + media, in millimeters), lumen diameter uniformity by coefficient of variation, graft failure separately for right and left coronary artery territories, and the ratio of SVG lumen diameter to target coronary artery lumen diameter assessed by QCA. Thrombolysis in Myocardial Infarction (TIMI) flow grade was assessed visually.
Clinical events were assessed during the first 12 months and will be assessed annually up to 60 months; these included readmissions and major adverse coronary and cerebrovascular events (MACCE), which were death of any cause, stroke, myocardial infarction, and ischemia-driven target vessel revascularization of the supported SVG or target coronary artery. Serious adverse events were assessed during the first 12 months. The incidence of repeat revascularization of supported and unsupported vein grafts (or bypassed target coronary artery) will be assessed over 60 months postrandomization.
Statistical Analysis
A sample size of 224 patients was calculated to achieve 90% power to detect a difference in IH area of 0.4 mm2 between the supported and unsupported SVGs. We anticipated that 13% of patients would not receive IVUS at 12 months because of SVG occlusion or severe stenosis and that 15% would drop out during the trial. Sample size estimates were based on the assumptions that IH area is normally distributed with an SD of 1.7 mm2 in both supported and unsupported SVGs, and the intraclass correlation of grafts within the same patient is 0.5.
A multiple imputation approach that penalized occluded or severely diseased grafts with higher values of IH was used to impute missing values of grafts not imaged by IVUS because of occlusion or severe disease. (Higher values were generated by adding a penalty to the imputed values drawn from a normal distribution with mean [shift parameter] = 1.70 and SD 0.25.) A modified form of the Wilcoxon signed rank test was used for the analysis of the primary outcome. Specifically, if the within-patient randomized SVGs were both occluded/severely diseased, they were assigned an absolute value of 0 for the difference in IH area irrespective of the imputed values. Pairs with a value of 0 were excluded from the computation of the test statistic as is common for the Wilcoxon signed rank test. If only 1 of the 2 within-patient randomized grafts was occluded/severely diseased, then the absolute difference between the observed and the imputed score was assigned. However, the sign associated with the rank for this difference favored the nonoccluded/non–severely diseased graft. If neither graft was occluded/severely diseased, they were treated as usual in the computation of the Wilcoxon signed rank test. Sensitivity analyses were conducted to test the validity of the imputation model.
For the secondary confirmatory end point, a proportional odds model for clustered data was planned for the analysis of the Fitzgibbon classification. A McNemar test for paired binary observations was planned for the comparison of graft failure in supported and unsupported grafts. Event rates for MACCE, serious adverse events, and readmissions were calculated as the ratio of the total number of events over 12 months divided by total patient-years at risk for the specific event from randomization. A Poisson model with robust variance estimation was used in the computation of the 95% CIs for these rates.
All tests were carried out at the 2-sided .05 significance level using SAS version 9.4 (SAS Institute). To maintain the type I error at its nominal level, a sequential test procedure was used for the analysis of the primary and the 2 secondary confirmatory end points, whereby the latter would be tested only if the null hypothesis was rejected on the primary end point at the .05 level.
The primary end point was evaluated in the full analysis set, which includes all randomized grafts for which the study procedure was initiated according to the intention-to-treat principle (statistical analysis plan in Supplement 3). The Fitzgibbon classification was evaluated in the analysis population for which the outcome could be ascertained in either the supported and/or unsupported grafts at month 12, and graft failure was evaluated in the population for which it could be ascertained for both the supported and unsupported grafts at month 12. Clinical events were analyzed in all randomized patients.
Results
Patients
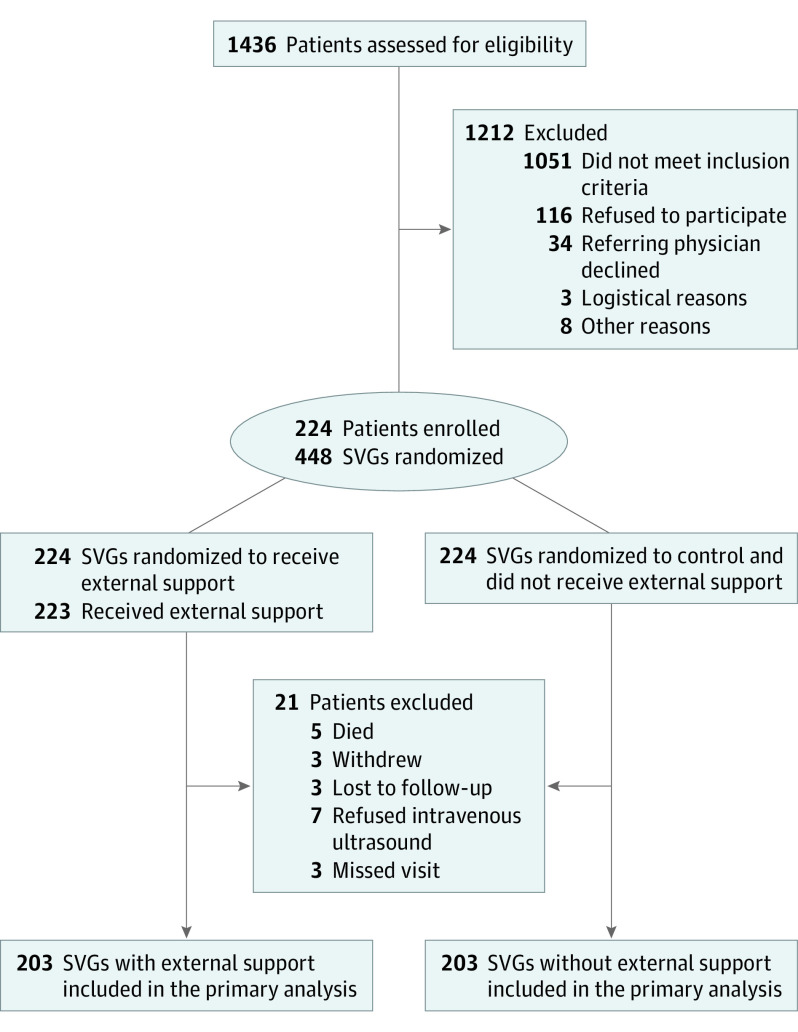
Between January 2018 and February 2019, 1436 patients were screened, 385 were eligible, and 224 were enrolled (Figure 1). Mean (SD) age was 65.8 (8.3) years, 46 (20.5%) were women, 199 of 216 patients (92.1%) were White, and 20 of 220 (9.1%) were Hispanic. Diabetes was present in 114 patients (50.9%), 93 (41.5%) experienced a prior myocardial infarction, and 55 (24.6%) had a prior percutaneous coronary intervention (Table 1). Of 221 patients, 64 (29.0%) did not report angina, whereas 82 patients (37.1%) had Canadian Cardiovascular Society class III or IV angina. Median left ventricular ejection fraction was 55% (IQR, 50%-60%). The vast majority of SVGs (169 [75.4%]) were harvested endoscopically. Table 2 shows the characteristics of vein grafts randomized to receive device support or no support.
Figure 1. CONSORT Diagram.
The 3 most common reasons for not meeting inclusion criteria were not having 2 or more vein grafts to native vessels with at least 75% stenosis and comparable runoff, having a concomitant non-CABG cardiac surgical procedure, and having documented or suspected untreated diffuse peripheral vascular disease. At 1 year, 203 patients were eligible for intravascular ultrasound (IVUS) imaging. Among the 203 saphenous vein grafts (SVGs) with external support, 143 SVGs had the intimal hyperplasia area assessed by IVUS; 55 SVGs were occluded/severely diseased, which precluded the IVUS procedure; and intimal hyperplasia area was not measurable in 5 SVGs for technical reasons. Among the 203 SVGs without external support, 142 SVGs had the intimal hyperplasia area assessed by IVUS; 56 SVGs were occluded/severely diseased, which precluded the IVUS procedure; and intimal hyperplasia area was not measurable in 5 SVGs for technical reasons. CABG indicates coronary artery bypass graft procedure.
Table 1. Baseline Patient and Operative Characteristics.
| All patients (n = 224) | |
|---|---|
| Age, mean (SD), y | 65.8 (8.3) |
| Sex | |
| Female | 46 (20.5) |
| Male | 178 (79.5) |
| Race, No./total No. (%)a | |
| Asian | 1/216 (0.5) |
| Black or African American | 14/216 (6.5) |
| Native Hawaiian or Other Pacific Islander | 1/216 (0.5) |
| White | 199/216 (92.1) |
| More than 1 race | 1/216 (0.5) |
| Ethnicity, No./total No. (%)b | |
| Hispanic or Latino | 20/220 (9.1) |
| Not Hispanic or Latino | 200/220 (90.9) |
| BMI, mean (SD)c | 30.2 (5.6) |
| Medical history | |
| Current smoker or ex-smoker | 123 (54.9) |
| Diabetes | 114 (50.9) |
| Hypertension | 191 (85.3) |
| Hyperlipidemia | 188 (83.9) |
| Peripheral arterial disease | 10 (4.5) |
| COPD | 16 (7.1) |
| Prior stroke in past year | 2 (0.9) |
| Prior myocardial infarction | 93 (41.5) |
| Prior PCI | 55 (24.6) |
| Cardiac measures and laboratory results | |
| NYHA class, No./total No. (%)a | |
| No heart failure | 34/215 (15.8) |
| Class I/II | 155/215 (72.1) |
| Class III/IV | 26/215 (12.1) |
| CCS class, No./total No. (%)b | |
| No angina | 64/221 (29.0) |
| Grade I/II | 75/221 (33.9) |
| Grade III/IV | 82/221 (37.1) |
| LVEF, median (IQR), % | 55.0 (50.0-60.0) |
| Creatinine, median (IQR), mg/dL | 1.0 (0.8-1.1) |
| Operative characteristics | |
| Surgery time, mean (SD), min | 268.5 (68.5) |
| Clamp time, mean (SD), min | 83.0 (25.8) |
| Bypass time, mean (SD), min | 110.5 (35.1) |
| SVG harvesting technique | |
| Direct | 41 (18.3) |
| Endoscopic | 169 (75.4) |
| Bridge | 14 (6.3) |
| Vein origin | |
| Above the knee | 36 (16.1) |
| Below the knee | 17 (7.6) |
| Both above and below the knee | 171 (76.3) |
| Vein preservation | |
| Saline/heparinized saline | 143 (63.8) |
| pH-buffered/heparinized pH-buffered | 56 (25.0) |
| Autologous blood | 4 (1.8) |
| >1 Method | 21 (9.4) |
| Vein varicosity, No./total No. (%) | |
| None | 154/223 (69.1) |
| Mild/moderate | 69/223 (30.9) |
| Grafts per patient | |
| 3 | 137 (61.2) |
| 4 | 72 (32.1) |
| 5 | 14 (6.3) |
| 7 | 1 (0.4) |
Abbreviations: BMI, body mass index; CCS, Canadian Cardiovascular Society; COPD, chronic obstructive pulmonary disease; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; SVG, saphenous vein grafts.
SI conversion factor: To convert creatinine from mg/dL to μmol/L, multiply by 88.4.
Race was self-reported by the patients.
Ethnicity was self-reported by the patients.
Calculated as weight in kilograms divided by height in meters squared.
Table 2. Study Graft Characteristics.
| No. (%) | ||
|---|---|---|
| Device supported (n = 224) | Device unsupported (n = 224) | |
| Native artery % stenosis | ||
| 50%-74% Stenosis | 1 (0.4) | 0 |
| 75%-99% Occlusion | 196 (87.5) | 187 (83.5) |
| 100% Occlusion | 27 (12.1) | 37 (16.5) |
| Coronary artery diameter, median (IQR), mm | 1.6 (1.5-2.0) | 1.8 (1.5-2.0) |
| Graft length, median (IQR), cm | 14.8 (12.0-16.0) | 15.0 (12.0-17.0) |
| Systolic pressure at TTFM, median (IQR), mm Hg | 102.0 (80.0-113.0) | 102.0 (80.0-115.0) |
| Final TTFM flow, median (IQR), mL/min | 42.0 (28.0-59.0) | 39.5 (25.0-61.0) |
| Final TTFM pulsatility index, median (IQR) | 2.2 (1.7-3.1) | 2.5 (1.8-3.7) |
| Coronary target vessels | ||
| Diagonal 1 | 25 (11.2) | 25 (11.2) |
| Diagonal 2 | 8 (3.6) | 4 (1.8) |
| CRX | 1 (0.4) | 5 (2.2) |
| OM1 | 50 (22.3) | 43 (19.2) |
| OM2 | 25 (11.2) | 24 (10.7) |
| OM3 | 3 (1.3) | 4 (1.8) |
| RI | 10 (4.5) | 8 (3.6) |
| RCA | 27 (12.1) | 16 (7.1) |
| PDA | 68 (30.4) | 89 (39.7) |
| PLB | 7 (3.1) | 6 (2.7) |
Abbreviations: CRX, circumflex; OM, obtuse marginal, RCA, right coronary artery; PDA, posterior descending artery; PLB, posterior lateral branch; RI, ramus intermediate; TTFM, transit time flow measurement.
Intimal Hyperplasia Area
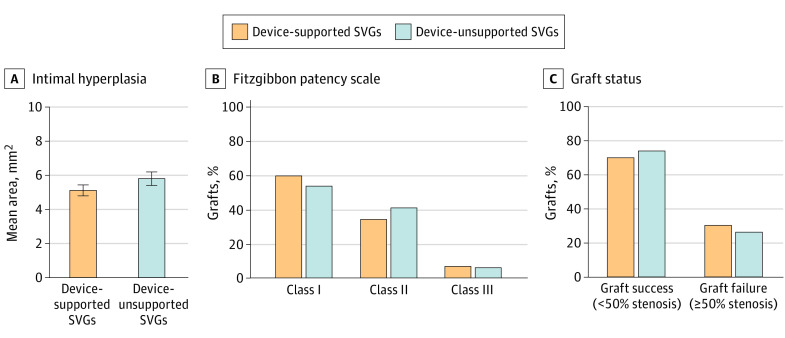
At 1 year, 21 patients (9.4%) were not eligible for IVUS because of death (n = 5), refusal (n = 7), withdrawal or loss to follow-up (n = 6), or missed visit (n = 3). Of the 203 patients (with 406 study grafts randomized) who were eligible for IVUS imaging, 85 patients (41.9%) had 1 or more SVGs occluded or severely diseased (eTable 1 in Supplement 1), which precluded the IVUS procedure (26 patients had both randomized grafts occluded or severely diseased, 29 had only the supported graft occluded or severely diseased, and 30 had the control graft occluded or severely diseased). As such, 295 of 406 SVGs (72.7%; 148 supported and 147 control grafts) were evaluated with IVUS at 12 months. Of these, IH area was not measurable in 10 SVGs (5 in each group) for technical reasons. The analysis of the primary end point incorporates both observed and imputed data, the latter because of missing values due to occlusion, severe SVG disease, or technical issues (eTable 2 in Supplement 1). In this analysis, the estimated mean (SE) IH area was 5.11 (0.16) mm2 in supported SVGs and 5.79 (0.20) mm2 in unsupported SVGs (P = .07) (Figure 2). In a sensitivity analysis of patients who had both grafts imaged by IVUS (n = 113), the observed mean (SE) IH area was 4.58 (0.18) mm2 in supported SVGs and 5.12 (0.23) mm2 in the unsupported SVGs (P = .04) (eFigure in Supplement 1).
Figure 2. Primary and Secondary Confirmatory End Points at 12 Months for Supported and Unsupported Saphenous Vein Grafts (SVGs).
Estimated mean intimal hyperplasia area and variance were based on 30 imputations with estimates combined using the Rubin rule for device-supported and unsupported grafts. Error bars indicate 95% CI. In the Fitzgibbon patency scale, class I indicates no intimal irregularity; class II, irregularity of less than 50% of estimated intimal surface; and class III, irregularity of 50% or more of estimated intimal surface.
In another set of sensitivity analyses, we varied the shift parameter so that smaller values reflected lower penalty on imputed IH area of occluded/severely diseased grafts and larger values a higher penalty. The results of these sensitivity analyses were consistent with the analysis of the primary end point (eTable 5 in Supplement 1). Additional sensitivity analyses are found in the eMethods and eTable 4 in Supplement 1; eTables 6 and 7 in Supplement 1 list additional characteristics of occluded/severely diseased grafts.
Consistent with the findings regarding IH area, mean (SD) IH thickness was lower in the supported than the unsupported grafts (0.38 [0.14] mm vs 0.43 [0.16] mm; 95% CI for difference, −0.075 to −0.013) (eTable 3 in Supplement 1).
Fitzgibbon Classification and Graft Failure
Lumen diameter uniformity was categorized according to the Fitzgibbon patency scale. Among 153 supported SVGs imaged by angiography, 91 (59.5%) were in class I (no intimal irregularity), 52 (34.0%) were in class II (irregularity of <50% of estimated intimal surface), and 10 (6.5%) were in class III (irregularity of >50% of estimated intimal surface). In comparison, among the 157 unsupported SVGs, 84 (53.5%) were in class I, 64 (40.8%) were in class II, and 9 (5.7%) were in class III (Figure 2). In patients with both grafts imaged within 1 year (n = 202), graft failure (≥50% stenosis) occurred in 30.2% (61/202) of supported SVGs compared with 26.2% (53/202) of controls. Other imaging outcomes were similar between supported and unsupported grafts (eTable 3 in Supplement 1). Of note, the patency of left internal mammary artery grafts as assessed at the time of the primary end point was 98%.
Mortality, MACCE, and Readmissions
Sixteen patients (7.1%) experienced a MACCE, and the median time from randomization to first event was 1.9 months (Table 3). The rate of MACCE at 12 months was 0.1/patient-year (95% CI, 0.06-0.17). Five patients (2.2%) died by 12 months, 6 patients (2.7%) had a stroke, and 7 (3.1%) a myocardial infarction. Three patients (1.3%) underwent ischemic-driven target vessel revascularization of the supported graft or associated target coronary artery. At 12 months, 62 patients (27.7%) had been readmitted; the main causes of readmission were major infection, localized infections, myocardial infarction, stroke, and pulmonary embolism.
Table 3. Safety Data at 12 Months.
| No. (%) | Event rate per patient-year (95% CI) | ||
|---|---|---|---|
| Events | Patientsa | ||
| MACCE | |||
| Mortality | 5 (22.7) | 5 (2.2) | 0.023 (0.009-0.055) |
| Stroke | 7 (31.8) | 6 (2.7) | 0.032 (0.014-0.074) |
| Myocardial infarction | 7 (31.8) | 7 (3.1) | 0.032 (0.015-0.067) |
| Ischemic-driven target vessel revascularization of supported graft or associated target coronary artery | 3 (13.6) | 3 (1.3) | 0.014 (0.004-0.042) |
| Composite MACCE | 22 (100) | 16 (7.1) | 0.101 (0.061-0.168) |
| Non-MACCE | |||
| Non-MACCE revascularizationb | 10 (6.8) | 10 (4.5) | 0.046 (0.025-0.084) |
| Bleeding | 2 (1.4) | 2 (0.9) | 0.009 (0.002-0.036) |
| Cardiac arrest | 2 (1.4) | 2 (0.9) | 0.009 (0.002-0.037) |
| Sustained ventricular arrhythmia requiring defibrillation or cardioversion | 3 (2.1) | 3 (1.3) | 0.014 (0.004-0.042) |
| Sustained supraventricular arrhythmia requiring drug treatment or cardioversion | 14 (9.6) | 13 (5.8) | 0.064 (0.037-0.111) |
| Cardiac conduction abnormalities or sustained bradycardia requiring permanent pacemaker placement | 3 (2.1) | 3 (1.3) | 0.014 (0.004-0.042) |
| Pericardial fluid collection | 2 (1.4) | 2 (0.9) | 0.009 (0.002-0.037) |
| Pleural effusion | 7 (4.8) | 7 (3.1) | 0.032 (0.015-0.067) |
| Pneumothorax | 2 (1.4) | 2 (0.9) | 0.009 (0.002-0.036) |
| Hepatic dysfunction | 1 (0.7) | 1 (0.4) | 0.005 (0.001-0.033) |
| Localized infection | 23 (15.8) | 18 (8.0) | 0.105 (0.064-0.174) |
| Sepsis | 4 (2.7) | 4 (1.8) | 0.018 (0.007-0.049) |
| Kidney failure | 1 (0.7) | 1 (0.4) | 0.005 (0.001-0.033) |
| Respiratory failure | 4 (2.7) | 4 (1.8) | 0.018 (0.007-0.049) |
| Heart failure | 7 (4.8) | 6 (2.7) | 0.032 (0.014-0.074) |
| Arterial non-CNS thromboembolism | 1 (0.7) | 1 (0.4) | 0.005 (0.001-0.032) |
| Venous thromboembolic event | 8 (5.5) | 7 (3.1) | 0.037 (0.017-0.079) |
| Wound dehiscence | 1 (0.7) | 1 (0.4) | 0.005 (0.001-0.033) |
| Other adverse event | 51 (34.9) | 37 (16.5) | 0.234 (0.162-0.337) |
| All serious AEs and non-MACCE revascularizations | 146 (100) | 85 (37.9) | 0.670 (0.528-0.849) |
| Hospitalizations, all causes | 89 | 62 (27.7) | 0.421 (0.315-0.561) |
Abbreviations: AE, adverse event; CNS, central nervous system; MACCE, major adverse coronary and cerebrovascular events.
The denominator is 224 patients.
Non-MAACE revascularizations include non–ischemic-driven target vessel revascularization of supported graft or associated target coronary artery (n = 1), ischemic or nonischemic target vessel revascularization of unsupported graft or associated target coronary artery (n = 5), or revascularization of nonstudy vein grafts/vessels (n = 4).
Discussion
Failure of SVGs remains the major limitation to the long-term success of CABG, exposing patients to the risks of postoperative angina, myocardial infarction, repeat revascularization, heart failure, arrhythmia, and death. In this randomized within-patient trial, we evaluated the use of an external SVG scaffolding designed to reduce IH and promote SVG integrity. Although SVGs supported with the device showed lesser degrees of IH at 12 months compared with unsupported SVGs, the difference did not reach statistical significance (IH area 5.11 [0.16] mm2 in supported vs 5.79 [0.20] mm2 in unsupported grafts; P = .07). Of note, the primary analysis required a higher than expected amount of imputation for missing data related to early graft occlusion or severe SVG disease. Among patients who had both grafts angiographically imaged by 12 months, equally high rates of graft failure (≥50% stenosis) were observed in supported and unsupported grafts. Lumen uniformity was also similar in supported and unsupported SVGs at 12 months. The incidences of mortality and MACCE over 12 months were consistent with previous reports.22,23,24 Because each patient had both supported and unsupported grafts, attribution of clinical events to the device is challenging. Nonetheless, we did not find evidence to suggest that the device affected overall adverse event rates or the tendency to early graft occlusion.
While mechanical factors and endothelial damage leading to thrombotic occlusion are responsible for early SVG failure, IH and subsequent atherosclerosis are thought to account for graft failure beyond the first year after CABG. The development of IH in SVGs sets in motion pathophysiologic sequelae that lead to luminal remodeling, graft atherosclerosis, and ultimately graft failure. Hence, efforts to mitigate IH at the outset may reduce this cascade of events that can lead to adverse clinical outcomes. Although antiplatelet and lipid-lowering agents have had an effect on early graft patency, little evidence exists to support their effect, if any, on IH and long-term graft failure.
A novel approach to attempt to abrogate IH has been the application of external stents to the SVG at the time of CABG surgery. The rationale for this intervention is manifold and includes mitigation of the wall stretch induced by abrupt conduit arterialization when transposing it from a low-pressure venous system to a high-pressure arterial circuit, enhancement of lumen uniformity and conduit-to-target size matching, and induction of adventitial neovascularization.11 Early trials of this technology have yielded promising results.19,20,21
In the first small European trial of this scaffold, there was a significant reduction in IH area in supported compared with unsupported grafts at 12 months after surgery (n = 29/30), similar in magnitude to the effect we found.19 Longer-term follow-up (4.5 years) in 21 patients found that SVG failure rates, defined as greater than 50% stenosis, were not different between supported and unsupported grafts. However, IH area and intimal thickness were significantly lower, and Fitzgibbon class I patency score was significantly higher in supported grafts.21 In a larger European, multicenter, within-patient randomized study (n = 184), the primary end point was the Fitzgibbon patency scale in supported and unsupported grafts at 2 years.20 Angiography was completed in only 128 patients, and graft failure rates, defined as above, were similar between supported and unsupported SVGs, with significant improvement in Fitzgibbon class I for device-supported grafts. In a subgroup of 51 patients undergoing IVUS at 2 years, supported SVGs again showed a significant 22.5% reduction in mean IH area.
A shortcoming of these prior studies is that their analyses were restricted to IVUS-accessible SVGs. By contrast, our analysis was designed to account for missing data due to graft occlusion or severe disease by incorporating imputation into the primary end point analysis. While IH is generally felt to affect long-term SVG patency, our conservative analytical approach took into consideration the possibility of an association of IH, however small, with early graft occlusion. This analytical approach, given the higher than estimated incidence of 12-month SVG occlusion or severe disease (42% observed vs 13% estimated), may have affected our ability to observe a statistically significant difference in IH area, because it substantially increased the variability of the measurements. While the SVG occlusion rate observed in our study is higher than that reported in some previous trials, it is comparable with the 42% rate of per-patient graft occlusions at 12 months seen in the PREVENT-IV trial,25 which assessed the ability of the E2F transcription factor decoy edifoligide to prevent vein graft failure in patients undergoing CABG surgery.
The cause of SVG failure has been an area of active research. As observed in prior studies, the majority of patients with angiographic vein graft failure do not have a MACCE between surgery and documentation of graft failure.26 This observation suggests that some vein grafts may fail because they are not needed to provide blood flow rather than because they are defective. Factors associated with vein graft failure include the duration of surgery, target artery quality and runoff, antiplatelet therapy use, and endoscopic vein graft harvesting.27,28 We observed higher rates of graft failure in this trial than in prior studies with this scaffold, which were conducted in Europe with lower use of endoscopic vein graft harvesting. It is also possible that patient selection bias played a role in the relatively high rates of SVG failure if surgeons preferentially enrolled patients with diffuse coronary disease or non-LAD coronary arteries with poor runoff into this trial, believing these patients to be poor candidates for multiple- or all-arterial graft procedures.
Limitations
This trial has several limitations. The trial population was not sufficiently diverse, with enrollment of 20.5% women and 6.5% Black and 9.1% Hispanic patients. A recent article based on the Society of Thoracic Surgeons database, which captures more than 95% of all cardiac surgical procedures in the United States, observed that only 33% of patients receiving isolated CABG between 2011 and 2019 were women, around 7.5% were Black, and 7% Hispanic.29 Efforts to understand the underrepresentation of women and minorities in the surgical population and clinical trial arena, and how to overcome these limitations, have become a priority for the Cardiothoracic Surgical Trials Network. The within-patient randomized study design, which was chosen to obviate individual biological differences that could account for dissimilar magnitudes of IH, limits our ability to attribute MACCE directly to the device. Nonetheless, the individual component and overall MACCE rates are similar to those described in major CABG randomized trials.22,23,24 In addition, while the primary end point was the difference in IH area between device-supported and unsupported SVGs at 12 months, whether different degrees of postoperative IH will affect longer-term graft patency and clinical outcomes will require additional follow-up. In this trial, patients will be followed up for 5 years postrandomization to assess long-term MACCE. A strength of this trial is that our results are based on a very high percentage of patients (89%) who returned at 12 months for IVUS and angiography, and thus, our study population comprises the largest cohort of patients undergoing SVG imaging by IVUS after CABG surgery.
Conclusions
In this trial, external scaffolding of SVGs did not achieve the primary end point of reducing IH area compared with unsupported grafts. Ongoing, longer-term follow-up may demonstrate a differential effect of IH progression on clinical outcomes. Further investigation of external graft support devices intended to improve long-term graft patency and clinical outcomes is warranted, in conjunction with novel measures to mitigate early graft occlusion.
eTable 1. Angiography results
eTable 2. IVUS results
eFigure. Within-patient differences in IH area
eTable 3. Secondary endpoints
eMethods. Sensitivity analysis of primary endpoints
eTable 4. Different analysis populations and missing data assumption
eTable 5. Varying penalty for occluded/severely diseased grafts in primary endpoint
eTable 6. Characteristics of patients with occluded/severely diseased graft(s)
eTable 7. Characteristics of occluded/severely diseased grafts by randomization arm
Trial protocol
Statistical analysis plan
Data sharing statement
References
- 1.Mack MJ, Squiers JJ, Lytle BW, DiMaio JM, Mohr FW. Myocardial revascularization surgery: JACC historical breakthroughs in perspective. J Am Coll Cardiol. 2021;78(4):365-383. doi: 10.1016/j.jacc.2021.04.099 [DOI] [PubMed] [Google Scholar]
- 2.Caliskan E, de Souza DR, Böning A, et al. Saphenous vein grafts in contemporary coronary artery bypass graft surgery. Nat Rev Cardiol. 2020;17(3):155-169. doi: 10.1038/s41569-019-0249-3 [DOI] [PubMed] [Google Scholar]
- 3.Motwani JG, Topol EJ. Aortocoronary saphenous vein graft disease: pathogenesis, predisposition, and prevention. Circulation. 1998;97(9):916-931. doi: 10.1161/01.CIR.97.9.916 [DOI] [PubMed] [Google Scholar]
- 4.Cox JL, Chiasson DA, Gotlieb AI. Stranger in a strange land: the pathogenesis of saphenous vein graft stenosis with emphasis on structural and functional differences between veins and arteries. Prog Cardiovasc Dis. 1991;34(1):45-68. doi: 10.1016/0033-0620(91)90019-I [DOI] [PubMed] [Google Scholar]
- 5.Harskamp RE, Lopes RD, Baisden CE, de Winter RJ, Alexander JH. Saphenous vein graft failure after coronary artery bypass surgery: pathophysiology, management, and future directions. Ann Surg. 2013;257(5):824-833. doi: 10.1097/SLA.0b013e318288c38d [DOI] [PubMed] [Google Scholar]
- 6.Gaudino M, Antoniades C, Benedetto U, et al. ; ATLANTIC (Arterial Grafting International Consortium) Alliance . Mechanisms, consequences, and prevention of coronary graft failure. Circulation. 2017;136(18):1749-1764. doi: 10.1161/CIRCULATIONAHA.117.027597 [DOI] [PubMed] [Google Scholar]
- 7.Zientara A, Rings L, Bruijnen H, et al. Early silent graft failure in off-pump coronary artery bypass grafting: a computed tomography analysis. Eur J Cardiothorac Surg. 2019;56(5):919-925. doi: 10.1093/ejcts/ezz112 [DOI] [PubMed] [Google Scholar]
- 8.Kim KB, Choi JW, Oh SJ, et al. Twenty-year experience with off-pump coronary artery bypass grafting and early postoperative angiography. Ann Thorac Surg. 2020;109(4):1112-1119. doi: 10.1016/j.athoracsur.2019.07.053 [DOI] [PubMed] [Google Scholar]
- 9.Verrier ED, Boyle EM Jr. Endothelial cell injury in cardiovascular surgery. Ann Thorac Surg. 1996;62(3):915-922. doi: 10.1016/S0003-4975(96)00528-0 [DOI] [PubMed] [Google Scholar]
- 10.Nachman RL, Silverstein R. Hypercoagulable states. Ann Intern Med. 1993;119(8):819-827. doi: 10.7326/0003-4819-119-8-199310150-00008 [DOI] [PubMed] [Google Scholar]
- 11.Hu J, Wan S. External support in preventing vein graft failure. Asian Cardiovasc Thorac Ann. 2012;20(5):615-622. doi: 10.1177/0218492312456980 [DOI] [PubMed] [Google Scholar]
- 12.Barboriak JJ, Pintar K, Van Horn DL, Batayias GE, Korns ME. Pathologic findings in the aortocoronary vein grafts: a scanning electron microscope study. Atherosclerosis. 1978;29(1):69-80. doi: 10.1016/0021-9150(78)90095-3 [DOI] [PubMed] [Google Scholar]
- 13.Domanski MJ, Borkowf CB, Campeau L, et al. ; Post-CABG Trial Investigators . Prognostic factors for atherosclerosis progression in saphenous vein grafts: the postcoronary artery bypass graft (Post-CABG) trial. J Am Coll Cardiol. 2000;36(6):1877-1883. doi: 10.1016/S0735-1097(00)00973-6 [DOI] [PubMed] [Google Scholar]
- 14.Liu SQ. Prevention of focal intimal hyperplasia in rat vein grafts by using a tissue engineering approach. Atherosclerosis. 1998;140(2):365-377. doi: 10.1016/S0021-9150(98)00143-9 [DOI] [PubMed] [Google Scholar]
- 15.Mehta D, Izzat MB, Bryan AJ, Angelini GD. Towards the prevention of vein graft failure. Int J Cardiol. 1997;62(suppl 1):S55-S63. doi: 10.1016/S0167-5273(97)00214-3 [DOI] [PubMed] [Google Scholar]
- 16.Ratliff NB, Myles JL. Rapidly progressive atherosclerosis in aortocoronary saphenous vein grafts: possible immune-mediated disease. Arch Pathol Lab Med. 1989;113(7):772-776. [PubMed] [Google Scholar]
- 17.Hillis LD, Smith PK, Anderson JL, et al. 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;124(23):e652-e735. doi: 10.1161/CIR.0b013e31823c074e [DOI] [PubMed] [Google Scholar]
- 18.Taggart DP, Amin S, Djordjevic J, et al. A prospective study of external stenting of saphenous vein grafts to the right coronary artery: the VEST II study. Eur J Cardiothorac Surg. 2017;51(5):952-958. doi: 10.1093/ejcts/ezw438 [DOI] [PubMed] [Google Scholar]
- 19.Taggart DP, Ben Gal Y, Lees B, et al. A randomized trial of external stenting for saphenous vein grafts in coronary artery bypass grafting. Ann Thorac Surg. 2015;99(6):2039-2045. doi: 10.1016/j.athoracsur.2015.01.060 [DOI] [PubMed] [Google Scholar]
- 20.Taggart DP, Gavrilov Y, Krasopoulos G, et al. ; InVESTigators . External stenting and disease progression in saphenous vein grafts two years after coronary artery bypass grafting: a multicenter randomized trial. J Thorac Cardiovasc Surg. 2021;S0022-5223(21)00723-6. doi: 10.1016/j.jtcvs.2021.03.120 [DOI] [PubMed] [Google Scholar]
- 21.Taggart DP, Webb CM, Desouza A, et al. Long-term performance of an external stent for saphenous vein grafts: the VEST IV trial. J Cardiothorac Surg. 2018;13(1):117. doi: 10.1186/s13019-018-0803-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farkouh ME, Domanski M, Sleeper LA, et al. ; FREEDOM Trial Investigators . Strategies for multivessel revascularization in patients with diabetes. N Engl J Med. 2012;367(25):2375-2384. doi: 10.1056/NEJMoa1211585 [DOI] [PubMed] [Google Scholar]
- 23.Mohr FW, Morice MC, Kappetein AP, et al. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet. 2013;381(9867):629-638. doi: 10.1016/S0140-6736(13)60141-5 [DOI] [PubMed] [Google Scholar]
- 24.Park SJ, Kim YH, Park DW, et al. Randomized trial of stents versus bypass surgery for left main coronary artery disease. N Engl J Med. 2011;364(18):1718-1727. doi: 10.1056/NEJMoa1100452 [DOI] [PubMed] [Google Scholar]
- 25.Alexander JH, Hafley G, Harrington RA, et al. ; PREVENT IV Investigators . Efficacy and safety of edifoligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: a randomized controlled trial. JAMA. 2005;294(19):2446-2454. doi: 10.1001/jama.294.19.2446 [DOI] [PubMed] [Google Scholar]
- 26.Lopes RD, Mehta RH, Hafley GE, et al. ; Project of Ex Vivo Vein Graft Engineering via Transfection IV (PREVENT IV) Investigators . Relationship between vein graft failure and subsequent clinical outcomes after coronary artery bypass surgery. Circulation. 2012;125(6):749-756. doi: 10.1161/CIRCULATIONAHA.111.040311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hess CN, Lopes RD, Gibson CM, et al. Saphenous vein graft failure after coronary artery bypass surgery: insights from PREVENT IV. Circulation. 2014;130(17):1445-1451. doi: 10.1161/CIRCULATIONAHA.113.008193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tian M, Wang X, Sun H, et al. No-touch versus conventional vein harvesting techniques at 12 months after coronary artery bypass grafting surgery: multicenter randomized, controlled trial. Circulation. 2021;144(14):1120-1129. doi: 10.1161/CIRCULATIONAHA.121.055525 [DOI] [PubMed] [Google Scholar]
- 29.Jawitz OK, Lawton JS, Thibault D, et al. Sex differences in coronary artery bypass grafting techniques: a Society of Thoracic Surgeons database analysis. Ann Thorac Surg. 2021;S0003-4975(21)01250-9. doi: 10.1016/j.athoracsur.2021.06.039 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Angiography results
eTable 2. IVUS results
eFigure. Within-patient differences in IH area
eTable 3. Secondary endpoints
eMethods. Sensitivity analysis of primary endpoints
eTable 4. Different analysis populations and missing data assumption
eTable 5. Varying penalty for occluded/severely diseased grafts in primary endpoint
eTable 6. Characteristics of patients with occluded/severely diseased graft(s)
eTable 7. Characteristics of occluded/severely diseased grafts by randomization arm
Trial protocol
Statistical analysis plan
Data sharing statement