Abstract
Cadmium (Cd), a toxic environmental contaminant, induces neurodegenerative disorders. Resveratrol, a natural product, has been found to exert neuroprotective effects. However, little is known regarding the effect of resveratrol on Cd-evoked neurotoxicity. Here, we show that resveratrol effectively reversed Cd-elicited cell viability reduction, morphological change, nuclear fragmentation and condensation, as well as activation of caspase-3 in neuronal cells, implying neuroprotection against Cd-poisoning by resveratrol. Further research revealed that both c-Jun N-terminal kinase (JNK) and extracellular signal-regulated kinases 1/2 (Erk1/2) were involved in the inhibitory effect of resveratrol on Cd-induced cell death, as selective inhibitors of Erk1/2 (U0126) and JNK (SP600125), or over-expression of dominant negative mitogen-activated protein kinase kinase 1 (MKK1) or dominant negative c-Jun potentiated resveratrol’s prevention of Cd-induced phosphorylation of JNK and Erk1/2, as well as cell death in neuronal cells. Interestingly, resveratrol potently rescued the cells from Cd-induced suppression of protein phosphatases 2A (PP2A) and 5 (PP5) activity. Over-expression of PP2A or PP5 strengthened the inhibitory effects of resveratrol on Cd-induced activation of Erk1/2 and/or JNK, as well as cell death. The results indicate that resveratrol prevents Cd-induced activation of Erk1/2 and JNK pathways and neuronal cell death in part via activating PP2A and PP5. Our findings strongly support the notion that resveratrol may serve as a potential therapeutic agent in the prevention of Cd-induced neurodegenerative diseases.
Keywords: cadmium, mitogen-activated protein kinase, neuronal cells, protein phosphatase 2A, protein phosphatase 5, resveratrol
Cadmium (Cd) is one of the most toxic pollutants in the environment (water, air, and soil), because of its release from the smelting, burning of fossil fuels and municipal wastes, refining of metals, and cigarette smoking (Wang and Du 2013). Clinical and epidemiological evidence has demonstrated that Cd, because of its long biological half-life (15–20 years), exhibits toxic effects on many organs/systems such as kidney (Johri et al. 2010), liver (Jomova and Valko 2011), lung (Jiang et al. 2008), and testis (Thompson and Bannigan 2008) and the CNS (Okuda et al. 1997; Lopez et al. 2003; Mendez-Armenta and Rios 2007). Cd can pass through the blood–brain barrier and accumulate in the brain, causing the dysfunction of the nervous system, with symptoms such as headache and vertigo, slowing of vasomotor functioning, peripheral neuropathy, decreased equilibrium, psychomotor speed, learning disabilities, and neurobehavioral defects in attention (Pihl and Parkes 1977; Wright et al. 2006; Wang and Du 2013). The neurotoxicity of Cd has been proposed as a possible etiological factor for neurodegenerative diseases, including Parkinson’s disease (PD), Alzheimer’s disease (AD), and amyotrophic lateral sclerosis (ALS) (Okuda et al. 1997; Panayi et al. 2002; Mendez-Armenta and Rios 2007; Goncalves et al. 2010).
MAPKs belong to dual (Tyr and Ser/Thr) protein kinases, and play a crucial role in signal transduction and regulate numerous cellular events such as cell growth and survival (Kyriakis and Avruch 2012). There are at least three distinct subfamilies of MAPKs, including the extracellular signal-regulated kinases (ERK)1/2, ERK3/4, ERK5, ERK7/8, the Jun N-terminal kinases (JNK)1/2/3, and the p38 MAPKs p38α/β/γ/δ in mammalian cells (Kyriakis and Avruch 2012). It has been demonstrated that persistent activation of Erk1/2, JNK and/or p38 MAPK contributes to Cd-induced apoptosis in numerous types of cells, including neuronal cells (Rockwell et al. 2004; Kim et al. 2005). Our previous studies have shown that all the three MAPK members can be activated by Cd in neuronal cells, but Cd-induced neuronal apoptosis is only partially related to activation of Erk1/2 and JNK, but not p38 (Chen et al. 2008b, 2011b). It is well known that the phosphorylation of MAPKs is balanced by specific MAPK kinases and phosphatases (Kyriakis and Avruch 2001; Kim and Choi 2010). The cross-talk between parallel pathways of the MAPK cascade depends on the activity and expression of protein phosphatases, such as MAPK phosphatase 1 (MKP-1), serine/threonine protein phosphatase 2A (PP2A), and protein phosphatase 5 (PP5), which have been identified to directly dephosphorylate and inactivate Erk1/2, JNK or p38 (Franklin and Kraft 1997; Huang et al. 2004; Chen et al. 2008a, 2009; Han et al. 2012). Increasing findings have implicated MKP-1 and PP2A as the major phosphatases that negatively regulate Erk1/2, JNK and/or p38, whereas PP5 negatively regulates JNK/p38 pathway, involved in stress response (Franklin and Kraft 1997; Huang et al. 2004; Chen et al. 2008a, 2009; Han et al. 2012). Recently, this group has observed that Cd activates Erk1/2 and JNK pathways leading to apoptosis by inhibition of PP2A and PP5 in neuronal cells (Chen et al. 2008a). Hence, we postulated that a compound that can regulate PP2A/PP5-dependent Erk1/2/JNK signaling pathways might be useful to prevent Cd-poisoning.
Resveratrol (3,4′,5-trihydroxystilbene) is a pharmacologically active natural polyphenol from red grapes, peanuts, and red wine (Pervaiz and Holme 2009; Liu et al. 2010b; Wu et al. 2011). Resveratrol has been shown to possess numerous biological and pharmacological effects, including antioxidant, anti-inflammatory, anti-carcinogenic, and cardioprotective properties (Pervaiz and Holme 2009; Shakibaei et al. 2009; Gurusamy et al. 2010; Liu et al. 2010b; Wu et al. 2011). Resveratrol inhibits growth and induces apoptotic or autophagic cell death by activating JNK or p38 MAPK, and/or by suppressing phosphoinositide 3′-kinase (PI3K)/Akt and mammalian target of rapamycin signaling pathways in a variety of human tumor cells, such as human T24 bladder cancer cells, chronic myelogenous leukemia cells, human malignant B cells, human U251 glioma cells, human breast cancer lines MDA-MB-231, MCF-7, and BT-549 (Shimizu et al. 2006; Jiang et al. 2009; Bai et al. 2010; Puissant et al. 2010; He et al. 2011; Wu and Liu 2013). Interestingly, resveratrol also has neuroprotective roles in the models of neurodegenerative disorders in vitro and in vivo, such as PD, AD, and ALS (Jang et al. 2007; Lee et al. 2007; Jin et al. 2008; Liu et al. 2011; Wu et al. 2011; Lin et al. 2014a,b; Zhang et al. 2014; Zhou et al. 2014). However, whether and how resveratrol prevents Cd-induced neurotoxicity remains largely unknown. Here, for the first time, we show that resveratrol prevented Cd-induced activation of Erk1/2 and JNK pathways and neuronal cell death via activating PP2A and PP5. Our results suggest that resveratrol may be exploited for prevention of Cd-induced neurodegenerative diseases.
Materials and methods
Materials
Cadmium chloride, poly-d-lysine (PDL), 4′,6-diamidino-2-phenylindole (DAPI), and protease inhibitor cocktail were purchased from Sigma (St Louis, MO, USA). Resveratrol, SP600125, U0126, and PD169136 (Sigma) were dissolved in dimethylsulfoxide to prepare 100 mM resveratrol, 20 mM SP600125, 5 mM U0126, and 20 mM PD169136 as stock solutions, and stored at −20°C. Dulbecco’s modified Eagle’s medium, 0.05% Trypsin-EDTA, NEUROBASAL™ Media, and B27 Supplement were purchased from Invitrogen (Grand Island, NY, USA). Horse serum and fetal bovine serum were supplied by Hyclone (Logan, UT, USA). Enhanced chemiluminescence reagent was from Millipore (Billerica, MA, USA). The following antibodies were used: PP2ACα (BD Biosciences, San Jose, CA, USA), PP2A-A subunit, PP2A-B subunit (Millipore), JNK1, phospho-JNK (Thr183/Tyr185), c-Jun, phospho-c-Jun (Ser63), phospho-Erk1/2 (Thr202/Tyr204), p38, phospho-p38 (Thr180/Tyr182), cleaved-caspase-3 (Cell Signaling Technology, Beverly, MA, USA), Erk2, demethylated-PP2A, PP5, MKP-1 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), phospho-PP2A (Epitomics, Burlingame, CA, USA), FLAG, hemagglutinin (HA), β-tubulin (Sigma), goat anti-rabbit IgG-horseradish peroxidase (HRP), goat anti-mouse IgG-HRP, and rabbit anti-goat IgG-HRP (Pierce, Rockford, IL, USA). Other chemicals were purchased from local commercial sources and were of analytical grade.
Cell culture
Rat pheochromocytoma (PC12) cell line was from American Type Culture Collection (ATCC) (Manassas, VA, USA), which was seeded in a 6-well or 96-well plate coated with 0.2 μg/mL PDL and incubated as described (Chen et al. 2014). Primary murine neurons were isolated from fetal mouse cerebral cortexes of 16–18 days of gestation in female ICR mice (being pregnant), and then seeded in a 6-well or 96-well plate coated with 10 μg/mL PDL for experiments after 6 days of culture as described (Chen et al. 2010, 2014).
Recombinant adenoviral constructs and infection of cells
The recombinant adenoviruses expressing HA-tagged wild-type (wt) human PP5 (Ad-PP5), FLAG-tagged wt rat PP2ACα (Ad-PP2A), FLAG-tagged dominant negative c-Jun (Ad-dn-c-Jun), FLAG-tagged dominant negative MKK1 (Ad-MKK1-K97M), and the control virus expressing the green fluorescent protein (GFP) (Ad-GFP) were described previously (Huang et al. 2003, 2004; Chen et al. 2009; Liu et al. 2010a). For experiments, PC12 cells were grown in the growth medium and infected with the individual adenovirus for 24 h at 5 of multiplicity of infection (MOI = 5). Subsequently, cells were used for experiments. Ad-GFP served as a control. Expression of FLAG-tagged PP2A, dn-c-Jun or MKK1, and HA-tagged PP5 were determined by western blotting with antibodies to FLAG and HA, respectively.
LDH release assay
PC12 cells, seeded in a PDL-coated 96-well plate (1 × 104 cells/well), were treated with resveratrol (0–400 μM) for 24 h. Dimethylsulfoxide was used as normal control without resveratrol. Lactate dehydrogenase (LDH) activity in culture medium was determined using LDH Assay Kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China), following the instructions of the supplier.
Analysis for cell viability and morphology
PC12 cells and/or primary neurons were seeded in a PDL-coated 96-well plate (1 × 104 cells/well) or 6-well plate (5 × 105 cells/well). The next day, cells were treated with different concentrations of resveratrol (0–400 μM) for 24 h, or pre-treated with/without resveratrol (100 μM) for 1 h and then exposed to Cd (10 and 20 μM) for 24 h with five replicates of each treatment. Afterwards, cell viability was detected by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay as described (Chen et al. 2011b). The morphological images were captured under a Nikon Eclipse TE2000-U inverted phase-contrast microscope (Nikon, Tokyo, Japan) (200×) equipped with a digital camera.
Live cell detection by trypan blue exclusion
PC12 cells were seeded at a density of 5 × 105 cells/well in a PDL-coated 6-well plate. The next day, cells were exposed to Cd (10 and 20 μM) for 24 h following pre-incubation with/without resveratrol (25, 50, and 100 μM) for 1 h with five replicates of each treatment. In some cases, after infected with Ad-PP2A, Ad-PP5, Ad-dn-c-Jun, Ad-MKK1-K97M, or Ad-GFP, the cells were pre-treated with resveratrol (100 μM) for 1 h and then exposed to Cd (10 μM) for 24 h. Subsequently, live cells were monitored by counting viable cells using trypan blue exclusion.
DAPI staining
PC12 cells and/or primary neurons, seeded at a density of 5 × 105 cells/well in a 6-well plate containing a PDL-coated glass coverslip per well, were treated with/without Cd (10 and 20 μM) for 24 h following pre-incubation with/without resveratrol (100 μM) for 1 h, or with/without Cd (10 μM) for 24 h following pre-incubation with/without resveratrol (100 μM) in the presence or absence of SP600125 (20 μM), U0126 (5 μM), or PD169136 (20 μM) for 1 h with five replicates of each treatment. In some cases, after infected with Ad-PP2A, Ad-PP5, Ad-dn-c-Jun, Ad-MKK1-K97M, or Ad-GFP, the cells were pre-treated with/without resveratrol (100 μM) for 1 h and then exposed to Cd (10 μM) for 24 h. Subsequently, the apoptotic cells were evaluated using DAPI staining as described (Chen et al. 2008b). Photographs were captured under a fluorescence microscope (Nikon 80i) equipped with a digital camera.
TUNEL staining
PC12 cells and primary neurons, seeded at a density of 5 × 105 cells/well in a 6-well plate containing a PDL-coated glass coverslip per well, were treated with/without Cd (10 and 20 μM) for 24 h following pre-incubation with/without resveratrol (100 μM) for 1 h with five replicates of each treatment. Afterward, cells were fixed with 4% paraformaldehyde prepared in phosphate-buffered saline for 2 h at 4°C, followed by the terminal deoxynucleotidyl transferase (TdT)-mediated deoxyuridine triphosphate (dUTP) nick-end labeling (TUNEL) staining, according to the manufacturer’s instructions of In Situ Cell Death Detection Kit® (Roche, Mannheim, Germany). Finally, fluorescent imaging for all stained samples was captured under a fluorescence microscope (Nikon 80i) equipped with digital camera, followed by quantitative analysis using the integral optical density (IOD) by Image-Pro Plus 6.0 software (Media Cybernetics Inc., Newburyport, MA, USA).
Western blot analysis
After treatments, cells were briefly washed with cold phosphate-buffered saline, and then on ice, lysed in the radioimmunoprecipitation assay buffer. Subsequently, western blotting was performed, and the blots for detected protein were semi-quantified using NIH Image J software (National Institutes of Health, Bethesda, MD, USA) as described previously (Chen et al. 2010, 2014).
Statistical analysis
Results were expressed as mean ± SE. Student’s t-test for non-paired replicates was used to identify statistically significant differences between treatment means. Group variability and interaction were compared using either one-way or two-way anova followed by Bonferroni’s post-tests to compare replicate means. Significance was accepted at p < 0.05.
Results
Resveratrol attenuates Cd-induced cell viability reduction and morphological change in neuronal cells
To find a proper concentration of resveratrol for this research, PC12 cells were treated with different concentrations of resveratrol (0–400 μM) for 24 h. We showed that at low concentrations (5–100 μM), resveratrol did not significantly affect cell viability (Fig. 1a). However, at high concentrations (> 150 μM), resveratrol markedly caused cell viability reduction in a concentration-dependent manner (Fig. 1a). This is in agreement with the finding that resveratrol elicits cytotoxicity when its concentration exceeds cell toleration (Tseng et al. 2004). Consistently, significant cytotoxic effects triggered by resveratrol appeared at high concentrations (150–400 μM), but not at low concentrations (5–100 μM) (Fig. 1b), as detected by LDH release assay. Therefore, our data reveal that 5–100 μM resveratrol is not toxic to PC12 cells, which can be employed to investigate the protective effect of resveratrol on Cd neurotoxicity.
Fig. 1.
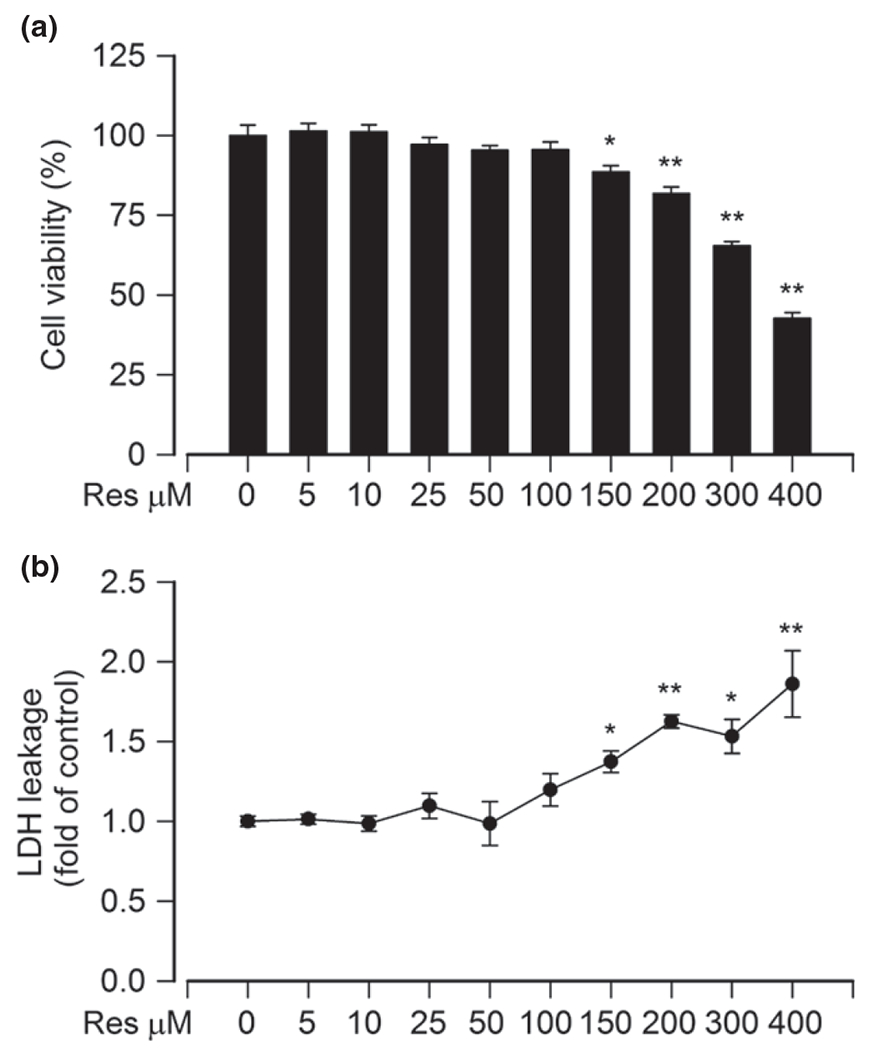
Low concentrations of resveratrol do not display cytotoxic effects on PC12 cells. PC12 cells were treated with resveratrol (Res, 0–400 μM) for 24 h. (a) Cell viability was evaluated using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, and (b) lactate dehydrogenase (LDH) activity in culture medium was determined by LDH release assay, showing that 5–100 μM Res had no cytotoxic effects on PC12 cells in culture. Results are presented as mean ± SE, n = 5. Using one-way anova, *p < 0.05, **p < 0.01, difference with control group.
To determine resveratrol’s protection against Cd-induced neuronal cell death, PC12 cells and/or primary neurons were pre-treated with resveratrol (0–100 μM) for 1 h and then exposed to Cd (10 and 20 μM) for 24 h. We observed that the number of live cells in the Cd/resveratrol group was significantly higher than that in the Cd alone group, and a concentration-dependent resveratrol prevention of Cd-reduced viability occurred in PC12 cells (Fig. 2a), as determined by trypan blue exclusion. By morphological analysis, resveratrol alone did not obviously alter cell shape. Many PC12 cells because of Cd became round or shrunken, with a great loss of cell integrity, which was strikingly reversed by resveratrol (Fig. 2b). Similar results were seen in primary neurons (Fig. 2b). The results imply that resveratrol may ameliorate Cd-evoked neuronal cell death.
Fig. 2.
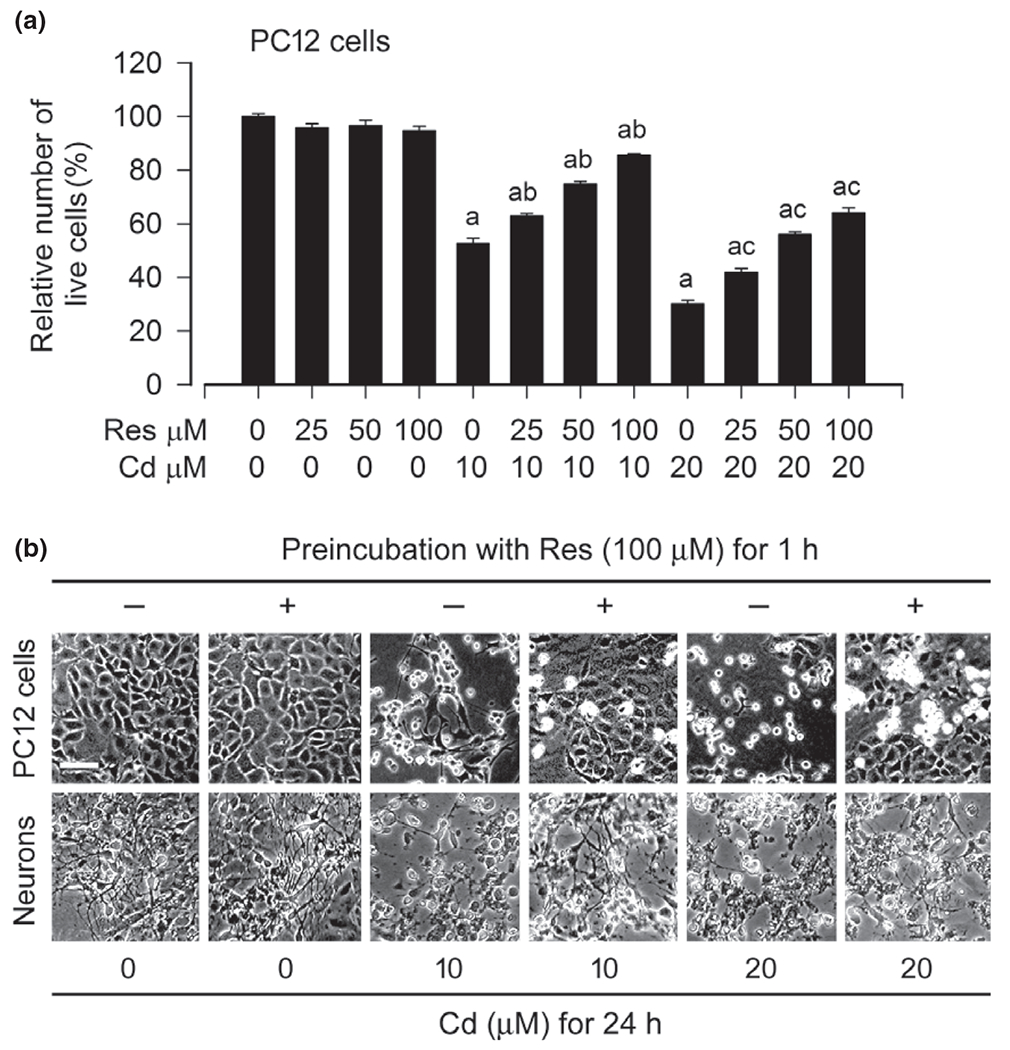
Resveratrol prevents Cd from reducing cell viability and altering cell morphology in neuronal cells. PC12 cells and/or primary neurons were pre-treated with resveratrol (Res, 0–100 μM for 1 h, and then exposed to Cd (10 and 20 μM for 24 h. (a) Live cells were detected by counting viable cells using trypan blue exclusion, showing that Res rescued PC12 cells from Cd-induced reduction of cell viability concentration-dependently. (b) Morphology of PC12 cells and primary neurons was visualized under a Nikon Eclipse TE2000-U inverted phase-contrast microscope (200×) equipped with a digital camera. Scale bar: 100 μm. Results are presented as mean ± SE, n = 5. Using one-way anova, ap < 0.05, difference with control group; bp < 0.05, difference with 10 μM Cd group; cp < 0.05, difference with 20 μM Cd group.
Resveratrol prevents Cd-induced apoptosis in neuronal cells
Numerous studies have consistently shown that Cd-induced neurotoxicity is attributed to its induction of neuronal apoptosis (Lopez et al. 2003; Mendez-Armenta and Rios 2007; Chen et al. 2008b, 2011b; Xu et al. 2011; Chen et al. 2014; Jiang et al. 2014). To elucidate the role of resveratrol in intervening Cd-induced neuronal apoptosis, PC12 cells, and primary neurons were exposed to Cd (10 and 20 μM) for 24 h following pre-treatment with resveratrol (100 μM) for 1 h. Subsequently, DAPI and TUNEL staining were employed to assess the status of apoptotic cell death. As expected, pre-treatment with resveratrol significantly attenuated the percentages of cells with nuclear fragmentation and condensation (arrows), a hallmark of apoptosis (Hao et al. 2013), and concurrently the number of TUNEL-positive cells with fragmented DNA (in green) in PC12 cells and primary neurons triggered by Cd exposure (Fig. 3a–c). Furthermore, we also examined proteolytic cleavages of caspase-3 in PC12 cells and primary neurons. Our western blot results revealed that treatment with Cd for 4 h elicited robust cleavages of caspase-3 in the cells (Fig. 3d). However, resveratrol potently blocked the event, consistent with the results from DAPI and TUNEL staining. Taken together, our results strongly support the notion that resveratrol, as a pharmacologically active compound, has an obvious protective effect on Cd-induced neuronal apoptosis.
Fig. 3.
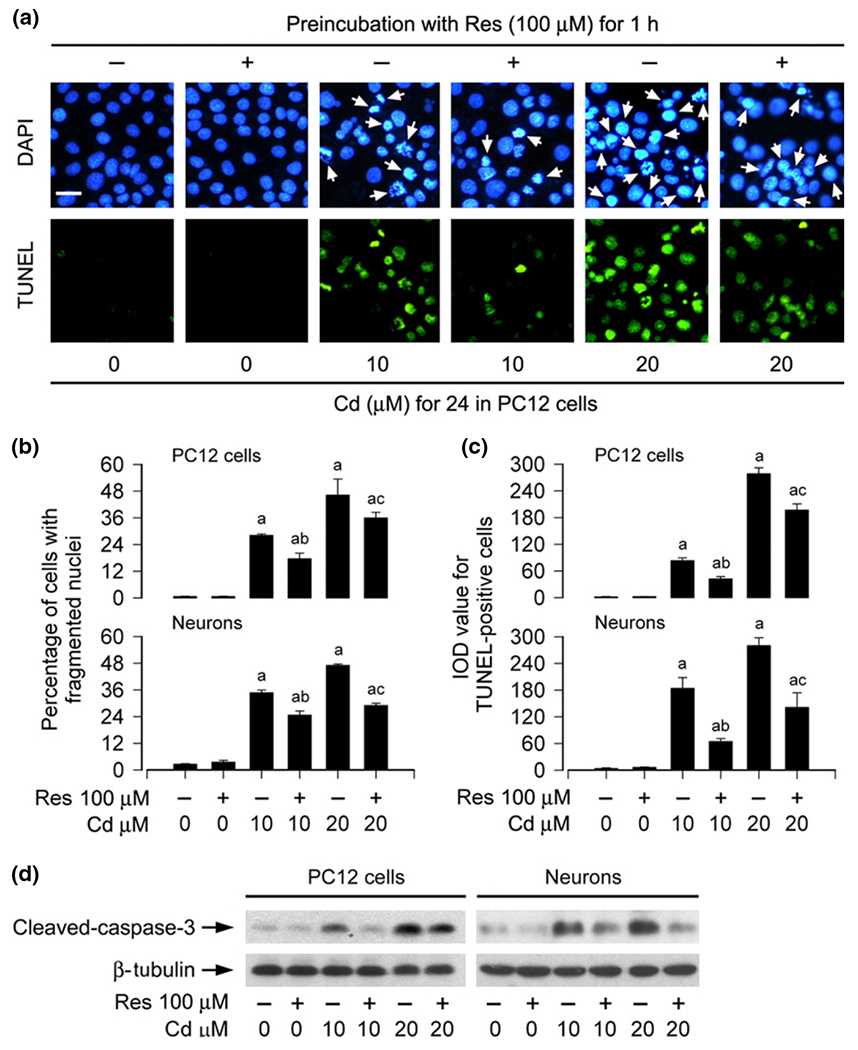
Resveratrol prevents Cd-induced apoptosis of neuronal cells. PC12 cells and primary neurons were pre-treated with/without resveratrol (Res, 100 μM for 1 h, and then exposed to Cd (10 and 20 μM for 4 h (for western blotting) or 24 h (for 4′ ,6-diamidino-2-phenylindole, DAPI and TUNEL staining). (a) Apoptosis in PC12 cells was evaluated by nuclear fragmentation and condensation (arrows) using DAPI staining (upper panel) and concurrently by in situ detection of fragmented DNA (in green) using TUNEL staining (lower panel). Scale bar: 20 μm. (b and c) The percentages of cells with fragmented nuclei and the fluorescence staining of TUNEL-positive cells were quantified, showing that resveratrol markedly attenuated Cd-induced apoptosis in PC12 cells and primary neurons. (d) Indicated cell lysates were subjected to western blot analysis using antibodies to cleaved-caspase-3. The blots were probed for β-tubulin as a loading control. Similar results were observed in at least three independent experiments. Results are presented as mean ± SE, n = 3–5. Using one-way anova, ap < 0.05, difference with control group; bp < 0.05, difference with 10 μM Cd group; cp < 0.05, difference with 20 μM Cd group.
Resveratrol inhibits Cd-induced neuronal cell death by blocking JNK and Erk1/2 pathways
A number of studies have documented that resveratrol may alter the activity of MAPKs including JNK, Erk1/2 and/or p38 under various conditions, thus affecting cell proliferation, survival, and apoptosis (Woo et al. 2004; von Kriegsheim et al. 2006; Shimizu et al. 2006; Huang et al. 2008; El-Mowafy et al. 2009; Puissant et al. 2010). Our previous studies have identified that Cd activates JNK, Erk1/2, and p38, but only JNK and Erk1/2 participate in Cd-induced apoptosis in neuronal cells (Chen et al. 2008b). Therefore, we reasoned that resveratrol might attenuate Cd-induced neuronal apoptosis by suppressing MAPK pathway. To this end, PC12 cells and primary neurons were pre-treated with resveratrol (0–100 μM) for 1 h, and then exposed to Cd (10 and 20 μM) for 4 h, followed by western blot analysis. We found that resveratrol slightly decreased the basal levels of phosphorylation of JNK, Erk1/2, and p38 in PC12 cells and primary neurons (Fig. 4a and b). Of importance, resveratrol concentration-dependently suppressed Cd-induced phosphorylation of JNK, particularly protein expression and phosphorylation of c-Jun, a substrate of JNK in the cells (Fig. 4a and b). Consistently, Cd-induced phosphorylation of Erk1/2 and p38 was also dramatically diminished by resveratrol (Fig. 4a and b).
Fig. 4.
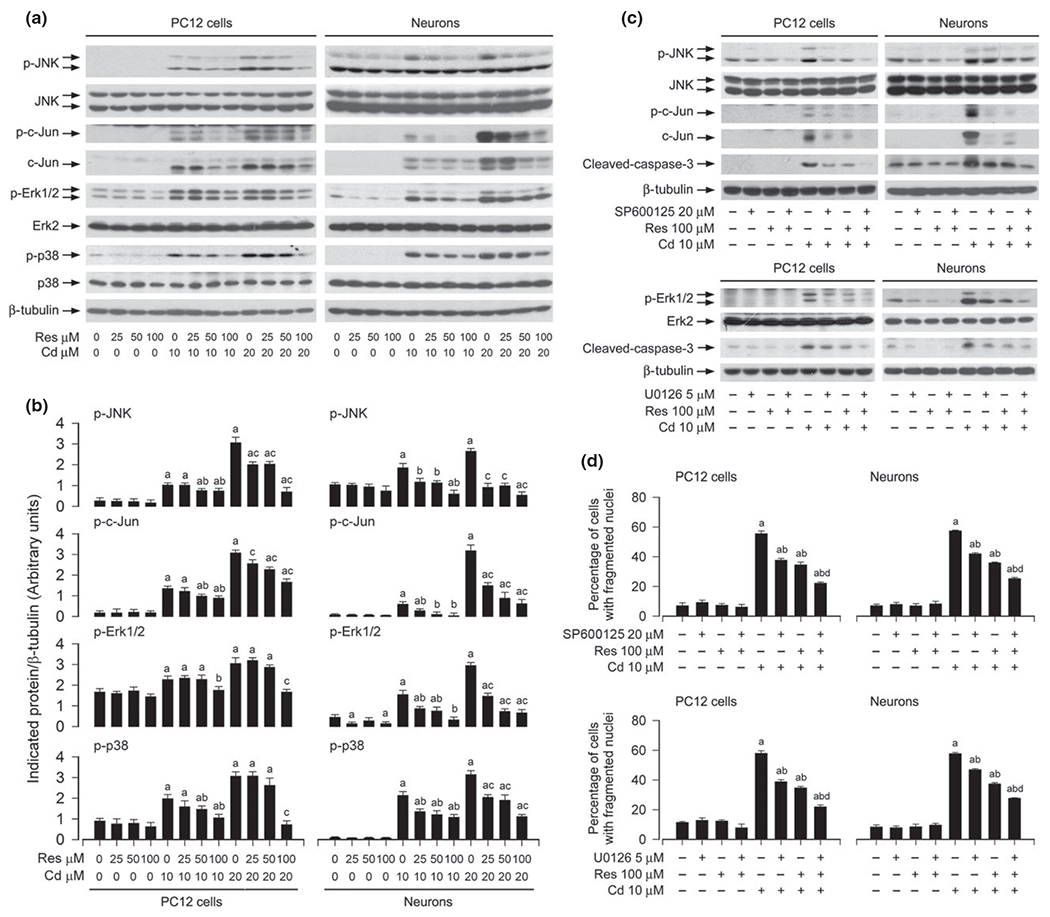
Resveratrol inhibits Cd-induced neuronal cell death by blocking JNK and extracellular signal-regulated kinases 1/2 (Erk1/2) pathways. PC12 cells and primary neurons were pre-treated with resveratrol (Res, 0–100 μM for 1 h, or with/without Res (100 μM in the presence or absence of SP600125 (20 μM or U0126 (5 μM for 1 h, and then exposed to Cd (10 and/or 20 μM for 4 h (for western blotting) or 24 h (for 4′,6-diamidino-2-phenylindole, DAPI staining). (a–c) Indicated cell lysates were subjected to western blot analysis using indicated antibodies, showing that resveratrol partially inhibited Cd-induced phosphorylation of JNK/c-Jun, Erk1/2 and p38 in the cells (a and b), and inhibitors of Erk1/2 (U0126) and JNK (SP600125) strengthened the inhibitory activity of resveratrol (c). The blots were probed for β-tubulin as a loading control. Similar results were observed in at least three independent experiments (a and c), and blots for p-JNK, p-c-Jun, p-Erk1/2, p-p38 were semi-quantified (b). (d) The percentages of apoptotic cells with fragmented nuclei were quantified by DAPI staining, showing that pharmacological inhibition of JNK and Erk1/2 enhanced resveratrol prevention of Cd-induced neuronal cell death. Results are presented as mean ± SE, n = 5. Using one-way or two-way anova, ap < 0.05, difference with control group; bp < 0.05, difference with 10 μM Cd group; cp < 0.05, difference with 20 μM Cd group; dp < 0.05, difference with Cd/SP600125 group, Cd/U0126 group or Cd/Res group.
Fig. 5.
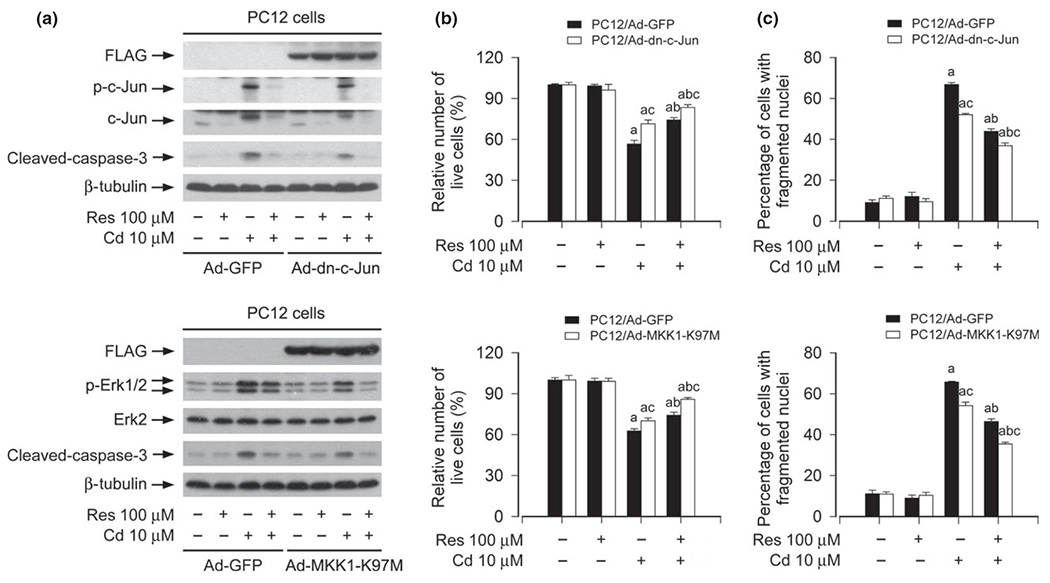
Expression of dominant negative c-Jun or dominant negative MKK1 potentiates resveratrol inhibition of Cd-induced neuronal cell death. PC12 cells, infected with Ad-dn-c-Jun, Ad-MKK1-K97M and Ad-GFP (as control), respectively, were pre-treated with/without resveratrol (Res, 100 μM for 1 h, and then exposed to Cd (10 μM for 4 h (for western blotting) or 24 h (for live cell analysis, 4′,6-diamidino-2-phenylindole, DAPI staining). (a) Indicated cell lysates were subjected to western blot analysis using indicated antibodies. The blots were probed for β-tubulin as a loading control. Similar results were observed in at least three independent experiments. (b) Live cells were detected by counting viable cells using trypan blue exclusion. (c) The percentages of apoptotic cells with fragmented nuclei were quantified by DAPI staining. Results are presented as mean ± SE, n = 5. Using one-way anova or Student’s t-test, ap < 0.05, difference with control group; bp < 0.05, difference with 10 μM Cd group; cp < 0.05, Ad-dn-c-Jun group or Ad-MKK1-K97M group versus Ad-GFP group.
To address how JNK, Erk1/2, and p38 are implicated in resveratrol prevention of neuronal cell death caused by Cd, we used SP600125 (a specific JNK inhibitor), U0126 (a specific inhibitor of MKK1/2, upstream kinases of Erk1/2), and PD169136 (a specific p38 inhibitor). PC12 cells were treated with resveratrol (100 μM), SP600125 (20 μM), U0126 (5 μM), or PD169136 (20 μM) alone, or co-treated with resveratrol (100 μM)/SP600125 (20 μM, U0126 (5 μM, or PD169136 (20 μM for 1 h, and then exposed to Cd (10 μM) for 4 h or 24 h. The results showed that SP600125 or U0126 alone substantially attenuated Cd-induced activation of JNK/c-Jun or Erk1/2, and caspase-3 in PC12 cells and primary neurons, respectively (Fig. 4c). Furthermore, co-treatment with resveratrol/SP600125 or resveratrol/U0126 exhibited a stronger inhibitory effect on Cd-induced p-JNK or p-Erk1/2, and cleaved-caspase-3 (Fig. 4c). In line with this, co-treatment with resveratrol/SP600125 or resveratrol/U0126 also rescued cells from Cd-induced apoptosis more potently than resveratrol, SP600125, or U0126 alone, as determined by morphological analysis (data not shown), DAPI staining (Fig. 4d), respectively. In addition, although PD169136 (20 μM) blocked Cd-induced phosphorylation of p38, co-treatment with resveratrol/PD169136 failed to strengthen the inhibitory effect of resveratrol on Cd-induced cytotoxicity (data not shown). Taken together, our results support that resveratrol may prevent Cd-induced neuronal cell death partially by blocking JNK and Erk1/2 pathways.
Expression of dominant negative c-Jun or MKK1 potentiates resveratrol inhibition of Cd-induced neuronal cell death
To further validate the role of resveratrol in suppressing Cd-induced activation of JNK and Erk1/2 cascades contributing to neuronal cell death, genetic approaches were taken. Ectopic expression of dn-c-Jun and MKK1-K97M, but not GFP, substantially blocked Cd-induced phosphorylation of c-Jun and Erk1/2 (Fig. 5a), respectively. Consistently, Cd-induced cleavage of caspase-3 was obviously diminished by expression of dn-c-Jun or MKK1-K97M (Fig. 5a). Furthermore, our assay for live and apoptotic cells showed that expression of dn-c-Jun or MKK1-K97M also partially rescued PC12 cells from apoptosis induced by Cd (Fig. 5b and c). Of importance, addition of resveratrol exhibited more inhibitory effect on Cd-induced activation of c-Jun and Erk1/2, as well as apoptosis in Ad-dn-c-Jun- or Ad-MKK1-K97M group than in Ad-GFP group (Fig. 5a–c). In addition, infection of PC12 cells with lentiviral shRNA to p38 silenced expression of p38 protein by ~ 90%, yet silencing p38 failed to strengthen resveratrol’s prevention of Cd-induced neuronal apoptosis (data not shown). Thus, our results demonstrate that resveratrol inhibits Cd-induced cell death in neuronal cells, at least in part, through blocking JNK and Erk1/2 cascades.
Resveratrol prevents Cd from reducing PP2A and PP5 activity in neuronal cells
Increasing data have shown that MKP-1 and PP2A negatively regulate Erk1/2, JNK and/or p38, and PP5 negatively regulates JNK/p38, involved in stress response (Franklin and Kraft 1997; Huang et al. 2004; Chen et al. 2008a, 2009; Han et al. 2012). Our previous research has demonstrated that Cd activates Erk1/2 and JNK pathways leading to apoptosis by inhibition of PP2A and PP5 in neuronal cells (Chen et al. 2008a), and this study has found that resveratrol inhibited Cd activation of Erk1/2 and JNK pathways contributing to neuronal cell death (Figs 4 and 5). Therefore, we postulated that resveratrol inhibits Cd activation of JNK, Erk1/2, and p38 pathways by preventing Cd from inhibiting PP2A and PP5. To this end, PC12 cells and primary neurons were pre-treated with/without resveratrol (100 μM) for 1 h, and then exposed to Cd (10 and 20 μM for 4 h, followed by western blot analysis. As shown in Fig. 6, Cd and/or resveratrol did not apparently alter cellular protein levels of MKP-1 and PP2Ac. However, pre-treatment with resveratrol dramatically reversed Cd-increased expression of demethylated- and phospho-PP2A (Fig. 6), two events that are related to decreased activity of PP2A (Janssens and Goris 2001), and Cd-reduced protein level of PP5 (Fig. 6). Sequentially, we also examined whether resveratrol affects expression of PP2A-A or PP2A-B in response to Cd. Consistent with our previous finding (Chen et al. 2008a), Cd reduced the protein level of PP2A-A, but not PP2A-B (Fig. 6). Interestingly, resveratrol markedly relieved the decrease in PP2A-A expression in PC12 cells and primary neurons induced by Cd (Fig. 6). Together, our results indicate that resveratrol prevents Cd-inhibited PP2A activity, by rescuing protein level of PP2A-A, and attenuating demethylation and phosphorylation of PP2Ac; and resveratrol prevents Cd-inhibited PP5, by rescuing protein levels of PP5 and PP2A-A. The findings implicate that resveratrol may suppress Cd activation of JNK, Erk1/2, and p38 pathways by preventing Cd from inhibiting PP2A and PP5.
Fig. 6.
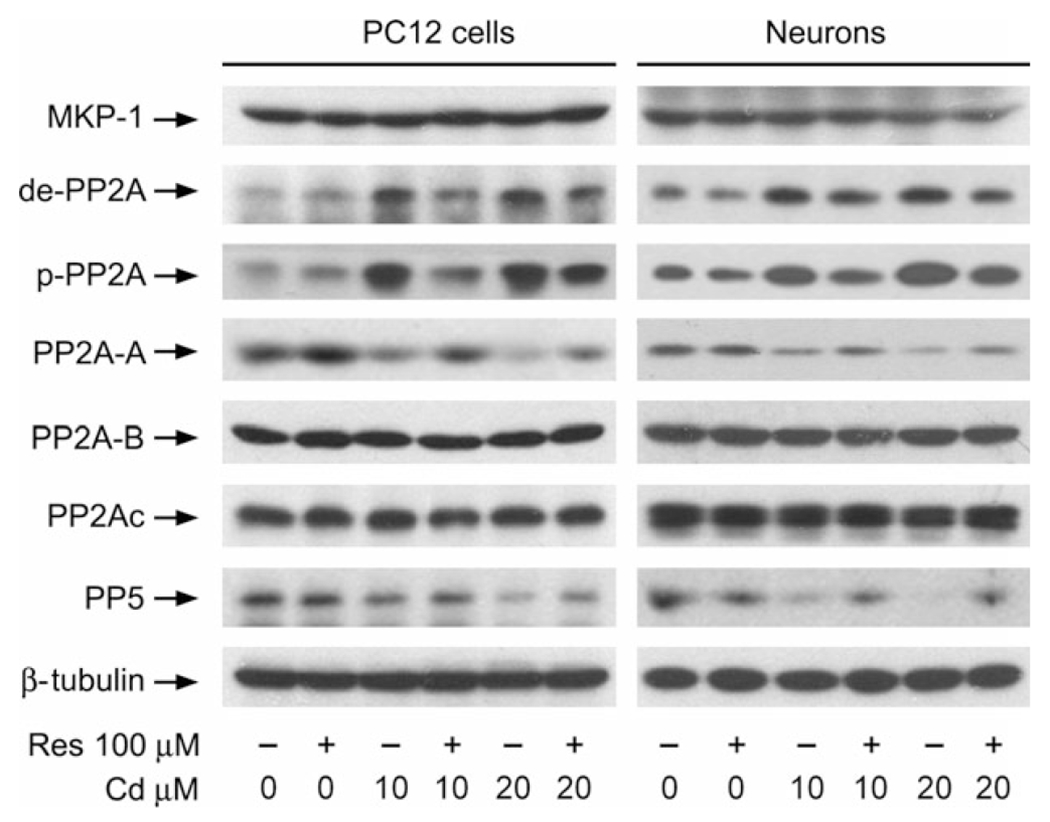
Resveratrol prevents Cd from reducing PP2A and PP5 activity in neuronal cells. PC12 cells and primary neurons were pre-treated with/without resveratrol (Res, 100 μM for 1 h, and then exposed to Cd (10 and 20 μM for 4 h, followed by western blot analysis using indicated antibodies, showing that resveratrol significantly inhibited Cd-induced reduction of PP2A and PP5 activity in the cells. The blots were probed for β-tubulin as a loading control. Similar results were observed in at least three independent experiments.
Over-expression of PP2A or PP5 strengthens resveratrol inhibition of Cd-induced activation of JNK, Erk1/2, and p38, as well as neuronal cell death
To substantiate the roles of PP2A and PP5 in resveratrol inhibition of Cd-induced activation of MAPKs and neuronal cell death, PC12 cells, infected, respectively, with Ad-PP2A, Ad-PP5, and Ad-GFP (as control), were pre-treated with/without resveratrol (100 μM) for 1 h, and then exposed to Cd (10 and 20 μM) for 4 h or 24 h. By western blot analysis, we observed that over-expression of FLAG-tagged PP2A partially prevented Cd-induced activation of Erk1/2, JNK, p38 and caspase-3, and potentiated the inhibitory effect of resveratrol on the events (Fig. 7a and b). Using morphological analysis, trypan blue exclusion, and DAPI staining, we found that over-expression of PP2A alone partially prevented Cd-induced morphological alteration (data not shown), live cell reduction (Fig. 7c), and apoptosis (Fig. 7d) in PC12 cells. However, addition of resveratrol rendered more significant resistance to Cd-induced cell death in Ad-PP2A-infected group than in Ad-GFP-infected group (Fig. 7c and d). It has been known that PP5 acts as a physiological inhibitor of ASK1-JNK/p38 pathways by negative feedback (Morita et al. 2001). PP5 also suppress Raf-MEK-ERK pathway (von Kriegsheim et al. 2006). Our group has recently revealed that PP5 activity is involved in activation of JNK, Erk1/2, and p38 cascades leading to death in neuronal cells induced by hydrogen peroxide (Chen et al. 2009), whereas PP5 is only related to activation of JNK pathway contributing to cell death, but not Erk1/2, in Cd-induced neuronal cells (Chen et al. 2008a). Consistent with the previous findings (Morita et al. 2001; Chen et al. 2008a), in this study, we found that over-expression of PP5 in part blocked Cd-induced activation of JNK and p38, but not Erk1/2, as well as cell death, and strengthened the inhibitory activity of resveratrol (Fig. 7e–h). Taken together, our data strongly suggest that resveratrol prevents Cd-induced neuronal cell death by rescuing PP2A/PP5, thus blocking activation of Erk1/2 and JNK cascade.
Fig. 7.
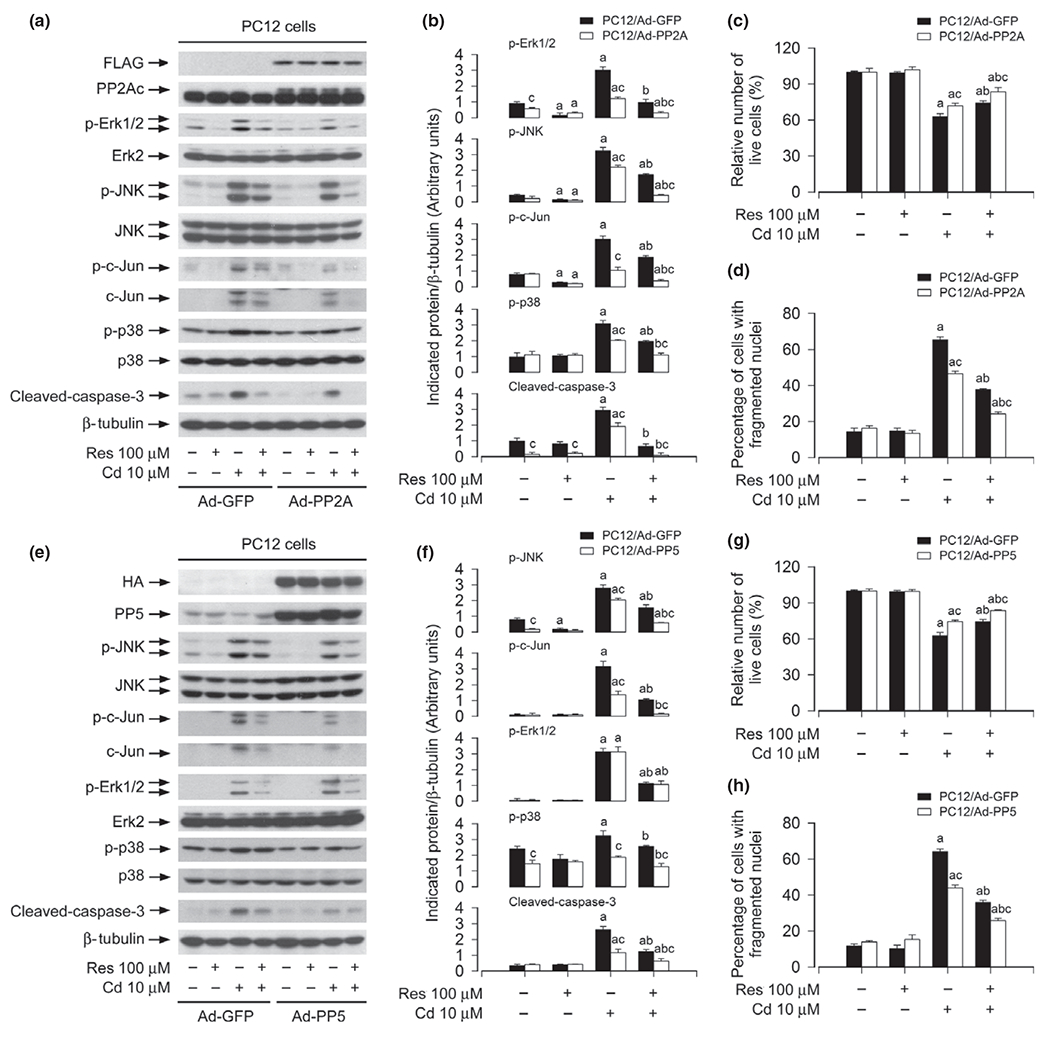
Over-expression of PP2A and PP5 strengthens resveratrol inhibition of Cd-induced activation of extracellular signal-regulated kinases 1/2 (Erk1/2), JNK and/or p38, as well as cell death. PC12 cells, infected with Ad-PP2A, Ad-PP5, or Ad-GFP (as control), were pre-treated with/without resveratrol (Res, 100 μM for 1 h, and then exposed to Cd (10 μM for 4 h (for western blotting) or 24 h (for live cell analysis, 4′,6-diamidino-2-phenylindole, DAPI staining). (a and e) Cell lysates were subjected to western blot analysis using indicated antibodies. The blots were probed for β-tubulin as a loading control. Similar results were observed in at least three independent experiments (a and e), and blots for p-JNK, p-c-Jun, p-Erk1/2, p-p38, cleaved-caspase-3 were semi-quantified (b and f). (c and g) Live cells were detected by counting viable cells using trypan blue exclusion. (d and h) The percentages of apoptotic cells with fragmented nuclei were quantified by DAPI staining. Results are presented as mean ± SE, n = 5. Using one-way anova or Student’s t-test, ap < 0.05, difference with control group; bp < 0.05, difference with 10 μM Cd group; cp < 0.05, Ad-PP2A group or Ad-PP5 group versus Ad-GFP group.
Discussion
Cadmium, a toxic environmental contaminant, can penetrate the blood–brain barrier, thereby causing CNS neurotoxicity following either acute or chronic exposure (Pihl and Parkes 1977; Wright et al. 2006; Wang and Du 2013). A growing number of studies have shown Cd’s neurotoxicity as an etiological factor of neurodegenerative diseases including PD, AD, and ALS (Okuda et al. 1997; Panayi et al. 2002; Mendez-Armenta and Rios 2007; Goncalves et al. 2010). Therefore, finding a novel therapeutic strategy to prevent Cd-induced neurodegenerative diseases is needed. Resveratrol, a nature-derived compound, has various biological activities in a variety of species and cell lines, and especially possesses neuroprotective activity in the models of neurodegenerative disorders in vitro and in vivo, such as PD, AD, and ALS (Jang et al. 2007; Lee et al. 2007; Jin et al. 2008; Liu et al. 2011; Wu et al. 2011; Lin et al. 2014a,b; Zhang et al. 2014; Zhou et al. 2014). For instance, resveratrol extends the lifespan of diverse species including Saccharomyces cerevisiae, Caenorhabditis elegans, and Drosophila melanogaster (Baur et al. 2006). Resveratrol shifts the physiology of middle-aged mice on a high-calorie diet toward that of mice on a standard diet and significantly increases their survival (Baur et al. 2006). Resveratrol prevents cortical neurons from β-amyloid-evoked damages (Zhang et al. 2014). Resveratrol also protects astrocytes against traumatic brain injury through inhibiting apoptotic and autophagic cell death (Lin et al. 2014a,b). It has been shown that resveratrol protects against Cd-induced oxidative damage in mice (Eybl et al. 2006). However, the molecular mechanisms by which resveratrol exerts the protective action against Cd-induced neurotoxicity is poorly understood. Here, we present evidence that resveratrol effectively reversed Cd-elicited cell viability reduction, morphological change, nuclear fragmentation and condensation, as well as activation of caspase-3 in neuronal cells. These results are in line with the recent studies on the effects of other natural products on Cd’s toxicity. For example, curcumin attenuates Cd-induced oxidative damage (Eybl et al. 2006). Epigallocatechin 3-gallate (EGCG) protects against Cd-induced rat brain mitochondrial dysfunction (Abib et al. 2011). Celastrol prevents Cd-induced neuronal cell death (Chen et al. 2014). Our present finding implies resveratrol’s neuroprotection against Cd-poisoning as well.
It has been shown that resveratrol may alter the activity of MAPKs including JNK, Erk1/2 and/or p38 under various conditions, and thus affecting cell proliferation, survival, and apoptosis (Woo et al. 2004; von Kriegsheim et al. 2006; Shimizu et al. 2006; Huang et al. 2008; El-Mowafy et al. 2009; Puissant et al. 2010). For example, in human Caski cells, resveratrol inhibits phorbol myristate acetate-mediated activation of JNK involved in controlling growth and invasiveness of tumors (Woo et al. 2004). In 3T3 fibroblasts, resveratrol protects against 4-hydroxynonenal-induced apoptosis by blocking JNK and c-Jun/AP-1 signaling (von Kriegsheim et al. 2006). In human coronary smooth muscle cells, resveratrol exerts more prominent inhibition of endothelin-1-evoked cell proliferation and Erk1/2 activation (El-Mowafy et al. 2009). However, in contrast, in human malignant B cells, resveratrol induces activation of p38 pathway contributing to the B-cell apoptosis (Shimizu et al. 2006). In chronic myelogenous leukemia cells, resveratrol triggers autophagic cell death via JNK-mediated p62 over-expression (Puissant et al. 2010). Of importance, in PC12 cells, several groups have found that treatment of PC12 cells with β-amyloid or gallic acid causes activation of JNK pathway and cell death, which is effectively blocked by resveratrol (Jang and Surh 2003; Kang et al. 2009). In addition, pre-treatment with resveratrol inhibits H2O2-induced PC12 cell death by up-regulation of heme oxygenase-1 (HO-1), which is via activation of Erk1/2 and Akt signaling (Chen et al. 2005). In this study, we found that resveratrol inhibited Cd-induced phosphorylation of JNK, Erk1/2, and p38, including protein expression and phosphorylation of c-Jun, the substrate of JNK, in PC12 cells and primary neurons. Our previous studies have demonstrated that Cd activates JNK, Erk1/2, and p38, but only JNK and Erk1/2 participate in neuronal apoptosis caused by Cd (Chen et al. 2008b). Similarly, recent data have also demonstrated that intraperitoneal administration of Cd (1 mg/kg daily) for 2 weeks in mice results in a strong phosphorylation of JNK, Erk1/2, and p38 in the cerebral cortex and hippocampus, and activation of JNK and Erk1/2 participates in cell death (Wang et al. 2014). To elucidate whether resveratrol prevents Cd-induced neuronal cell death by inhibition of Cd-induced activation of JNK/Jun, Erk1/2, and p38 pathways, pharmacological JNK inhibitor SP600125, Erk1/2 inhibitor U0126 and p38 inhibitor PD169136, or genetic manipulation of c-Jun, MKK1, or p38 activity, was utilized. Interestingly, we found that SP600125 or U0126 (Fig. 4d), but not PD169136 (data not shown), enhanced the inhibitory effect of resveratrol on Cd-induced apoptosis, as determined by DAPI staining. Furthermore, ectopic expression of dn-c-Jun or MKK1-K97M (Fig. 5a–c), but not down-regulation of p38 (data not shown), also strengthened resveratrol prevention of Cd-induced neuronal apoptosis. It is worth mentioning that p38 inhibitor PD169136 and silencing p38 had no effect on resveratrol prevention of Cd-induced apoptosis in neuronal cells, indicating that resveratrol exerts its protection against neuronal apoptosis independently of p38, although resveratrol also suppressed Cd-evoked activation of p38 pathway. This is in consistence with the reports that resveratrol has protective effect in the models of β-amyloid- or gallic acid-induced PC12 cells through blocking p-JNK (Jang and Surh 2003; Kang et al. 2009), and Erk1/2 pathway inhibitor U0126, but not p38 inhibitor SB203580, mimicks the inhibitory action of resveratrol on glutamate-induced neuronal cell death (Cho et al. 2014). Taken together, our data support the notion that resveratrol protects against Cd-induced neuronal apoptosis partially by blocking JNK and Erk1/2 pathways.
In this study, we also found that resveratrol prevented Cd from inhibiting PP2A and PP5 activity in PC12 cells and primary neurons. It appeared that the patterns of resveratrol in rescuing PP2A and PP5 were different. Resveratrol did not affect cellular protein expression of PP2Ac, but suppressed Cd-induced demethylation and phosphorylation of PP2Ac (Tyr307) (Fig. 6), two events related to inactivation of PP2A (Janssens and Goris 2001). In contrast, resveratrol prevented Cd from down-regulating cellular protein expression of PP5. Furthermore, resveratrol prevented Cd from reducing protein expression of PP2A-A, a regulatory subunit for both PP2A and PP5 (Janssens and Goris 2001). In this study, we also observed the manifestation of PP2A-B, another regulatory subunit for both PP2A and PP5 (Janssens and Goris 2001), showing that Cd and/or resveratrol did not alter protein levels of PP2A-B. It is known that rapamycin inhibits PP5 by dissociating PP2A-B from PP5 (Huang et al. 2004). Whether Cd inhibits PP5 by dissociating PP2A-A or PP2A-B from PP5, and resveratrol ameliorates the events remains to be determined. It should be mentioned in this study, we did not notice an apparent effect of Cd and/or resveratrol on the cellular protein level of MKP-1 (Fig. 6), a phosphatase that dephosphorylates Erk1/2, JNK, and p38. However, we could not exclude the possibility that Cd might actually inhibit the phosphatase activity of MKP-1, which is relieved by resveratrol, by other mechanisms.
Numerous studies have shown that PP2A and PP5 negatively regulate MAPKs including Erk1/2, JNK, and/or p38 cascade (Franklin and Kraft 1997; Huang et al. 2004; Chen et al. 2008a, 2009; Han et al. 2012). Here, we identified that resveratrol inhibited Cd-induced activation of the MAPK cascade through preventing Cd from inhibition of PP2A and PP5. This is supported by the findings that over-expression of PP2A or PP5 partially prevented Cd-induced activation of JNK, caspase-3 and/or Erk1/2, p38, as well as cell death, and strengthened the inhibitory activity of resveratrol for Cd-elicited the events (Fig. 7). Based on the entire data from our observations and other literatures, to our knowledge, this is the first report showing that resveratrol protects against Cd-induced neuronal cell death by rescuing PP2A/PP5-dependent inhibition of Erk1/2 and JNK cascade.
A new question that arises from the current work is how resveratrol attenuates Cd-induced PP2A/PP5 reduction and consequential activation of Erk1/2 and JNK pathways contributing to apoptosis in neuronal cells. It is well known that resveratrol is a natural compound of flavonoids with three phenolic hydroxyl groups. Accumulated studies have revealed that resveratrol can competitively combine to free radicals, interfere with the overproduction of free radicals, and thus reducing their content in vivo (Liu et al. 2011). Resveratrol prevents Cd-induced lipid peroxidation and ameliorating the adverse effect of Cd on antioxidant status in mice (Eybl et al. 2006). In response to oxidative stress, JNK, Erk1/2 and/or p38 can be activated (Chen et al. 2008a, 2009; Choi et al. 2011; Yang et al. 2013), and PP2A and PP5 can be down-regulated or inactivated (Chen et al. 2008a, 2009). Currently, we have no idea whether Cd affects oxidation of PP2A and PP5. However, recent studies have reported that resveratrol diminishes reactive oxygen species (ROS)-mediated oxidative functional damages by inhibiting activation of NADPH oxidases (NOXs), including NOX1, NOX2, and NOX4 in oxidized low-density lipoproteins (oxLDL)- or high glucose-induced vascular endothelial cells (Chow et al. 2007; Chen et al. 2013), and in hypoxia-induced rat model of pulmonary arterial hypertension (Liu et al. 2014). Our group has demonstrated that Cd activates JNK, Erk1/2, and p38 pathways via induction of ROS (Chen et al. 2008a), and identified that Cd induces ROS production by up-regulating the expression of NOX2 and its regulatory proteins (p22phox, p67phox, p40phox, p47phox, and Rac1) in PC12 and SH-SY5Y cells (Chen et al. 2011a). Therefore, we deduce that resveratrol is likely to act by mechanisms that counteract Cd-induced oxidative stress, thereby not only preventing Cd-induced activation of JNK, Erk1/2, and p38, but also blocking Cd-induced inactivation of PP2A and PP5. Undoubtedly, more studies are needed to address this issue.
In conclusion, we have shown that resveratrol is an attractive molecule for preventing Cd-induced neurotoxicity because of its ability to intervene at different levels in PP2A and PP5 pathways. By rescuing PP2A and PP5, resveratrol prevents Cd-induced activation of Erk1/2 and JNK pathways, thereby exerting a potent neuroprotective effect on Cd-induced apoptotic cell death in neuronal cells. Hence, we propose that this compound may have important therapeutic applications in the prevention of Cd-induced neurodegenerative diseases.
Acknowledgments and conflict of interest disclosure
This study was supported in part by the grants from National Natural Science Foundation of China (30971486, 81271416; L.C.), Project for the Priority Academic Program Development and Natural Science Foundation of Jiangsu Higher Education Institutions of China (10KJA180027; L.C.), NIH (CA115414; S.H.), American Cancer Society (RSG-08-135-01-CNE; S.H.), Louisiana Board of Regents (NSF-2009-PFUND-144; S.H.), and NSFC for Talents Training in Basic Science (J1103507, J1210025; C.S., L.C.). The authors declare no conflicts of interest.
All experiments were conducted in compliance with the ARRIVE guidelines.
Abbreviations used:
- AD
Alzheimer’s disease
- ALS
amyotrophic lateral sclerosis
- Erk1/2
extracellular signal-regulated kinases 1/2
- PDL
poly-d-lysine
- PD
Parkinson’s disease
- PP2A
protein phosphatase 2A
References
- Abib RT, Peres KC, Barbosa AM et al. (2011) Epigallocatechin-3-gallate protects rat brain mitochondria against cadmium-induced damage. Food Chem. Toxicol 49, 2618–2623. [DOI] [PubMed] [Google Scholar]
- Bai Y, Mao QQ, Qin J, Zheng XY, Wang YB, Yang K, Shen HF and Xie LP (2010) Resveratrol induces apoptosis and cell cycle arrest of human T24 bladder cancer cells in vitro and inhibits tumor growth in vivo. Cancer Sci. 101, 488–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL et al. (2006) Resveratrol improves health and survival of mice on a high-calorie diet. Nature 444, 337–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Jang JH, Li MH and Surh YJ (2005) Resveratrol upregulates heme oxygenase-1 expression via activation of NF-E2-related factor 2 in PC12 cells. Biochem. Biophys. Res. Commun 331, 993–1000. [DOI] [PubMed] [Google Scholar]
- Chen L, Liu L and Huang S (2008a) Cadmium activates the mitogen-activated protein kinase (MAPK) pathway via induction of reactive oxygen species and inhibition of protein phosphatases 2A and 5. Free Radic. Biol. Med 45, 1035–1044. [DOI] [PubMed] [Google Scholar]
- Chen L, Liu L, Luo Y and Huang S (2008b) MAPK and mTOR pathways are involved in cadmium-induced neuronal apoptosis. J. Neurochem 105, 251–261. [DOI] [PubMed] [Google Scholar]
- Chen L, Liu L, Yin J, Luo Y and Huang S (2009) Hydrogen peroxide-induced neuronal apoptosis is associated with inhibition of protein phosphatase 2A and 5, leading to activation of MAPK pathway. Int. J. Biochem. Cell Biol 41, 1284–1295. [DOI] [PubMed] [Google Scholar]
- Chen L, Xu B, Liu L, Luo Y, Yin J, Zhou H, Chen W, Shen T, Han X and Huang S (2010) Hydrogen peroxide inhibits mTOR signaling by activation of AMPKα leading to apoptosis of neuronal cells. Lab. Invest 90, 762–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Xu B, Liu L, Luo Y, Zhou H, Chen W, Shen T, Han X, Kontos CD and Huang S (2011a) Cadmium induction of reactive oxygen species activates the mTOR pathway, leading to neuronal cell death. Free Radic. Biol. Med 50, 624–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Xu Y, Xu B et al. (2011b) CaMKII is involved in cadmium activation of MAPK and mTOR pathways leading to neuronal cell death. J. Neurochem 119, 1108–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Qian LH, Deng B, Liu ZM, Zhao Y and Le YY (2013) Resveratrol protects vascular endothelial cells from high glucose-induced apoptosis through inhibition of NADPH oxidase activation-driven oxidative stress. CNS Neurosci. Ther 19, 675–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Gu C, Xu C, Zhang J, Xu Y, Ren Q, Guo M, Huang S and Chen L (2014) Celastrol prevents cadmium-induced neuronal cell death via targeting JNK and PTEN-Akt/mTOR network. J. Neurochem 128, 256–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho KS, Lee EJ, Kwon KJ et al. (2014) Resveratrol downregulates a glutamate-induced tissue plasminogen activator via Erk and AMPK/mTOR pathways in rat primary cortical neurons. Food Funct. 5, 951–960. [DOI] [PubMed] [Google Scholar]
- Choi HJ, Kang KS, Fukui M and Zhu BT (2011) Critical role of the JNK-p53-GADD45α apoptotic cascade in mediating oxidative cytotoxicity in hippocampal neurons. Br. J. Pharmacol 162,175–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow SE, Hshu YC, Wang JS and Chen JK (2007) Resveratrol attenuates oxLDL-stimulated NADPH oxidase activity and protects endothelial cells from oxidative functional damages. J. Appl. Physiol 102, 1520–1527. [DOI] [PubMed] [Google Scholar]
- El-Mowafy AM, Alkhalaf M and Nassar NN (2009) Resveratrol reverses ET-1-evoked mitogenic effects in human coronary arterial cells by activating the kinase-G to inhibit ERK-enzymes. Int. J. Cardiol 136, 263–269. [DOI] [PubMed] [Google Scholar]
- Eybl V, Kotyzova D and Koutensky J (2006) Comparative study of natural antioxidants - curcumin, resveratrol and melatonin - in cadmium-induced oxidative damage in mice. Toxicology 225, 150–156. [DOI] [PubMed] [Google Scholar]
- Franklin CC and Kraft AS (1997) Conditional expression of the mitogen-activated protein kinase (MAPK) phosphatase MKP-1 preferentially inhibits p38 MAPK and stress-activated protein kinase in U937 cells. J. Biol. Chem 272, 16917–16923. [DOI] [PubMed] [Google Scholar]
- Goncalves JF, Fiorenza AM, Spanevello RM et al. (2010) N-acetylcysteine prevents memory deficits, the decrease in acetylcholinesterase activity and oxidative stress in rats exposed to cadmium. Chem. Biol. Interact 186, 53–60. [DOI] [PubMed] [Google Scholar]
- Gurusamy N, Lekli I, Mukherjee S, Ray D, Ahsan MK, Gherghiceanu M, Popescu LM and Das DK (2010) Cardioprotection by resveratrol: a novel mechanism via autophagy involving the mTORC2 pathway. Cardiovasc. Res 86, 103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Xu B, Beevers CS et al. (2012) Curcumin inhibits protein phosphatases 2A and 5, leading to activation of mitogen-activated proteinkinases and death in tumor cells. Carcinogenesis 33,868–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao B, Cheng S, Clancy CJ and Nguyen MH (2013) Caspofungin kills Candida albicans by causing both cellular apoptosis and necrosis. Antimicrob. Agents Chemother 57, 326–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Wang Y, Zhu J, Orloff M and Eng C (2011) Resveratrol enhances the anti-tumor activity of the mTOR inhibitor rapamycin in multiple breast cancer cell lines mainly by suppressing rapamycin-induced AKT signaling. Cancer Lett. 301, 168–176. [DOI] [PubMed] [Google Scholar]
- Huang S, Shu L, Dilling M, Easton J, Harwood F, Ichijo H and Houghton P (2003) Sustained activation of the JNK cascade and rapamycin-induced apoptosis are suppressed by p53/p21(Cip1). Mol. Cell 11, 1491–1501. [DOI] [PubMed] [Google Scholar]
- Huang S, Shu L, Easton J, Harwood FC, Germain GS, Ichijo H and Houghton PJ (2004) Inhibition of mammalian target of rapamycin activates apoptosis signal-regulating kinase 1 signaling by suppressing protein phosphatase 5 activity. J. Biol. Chem 279, 36490–36496. [DOI] [PubMed] [Google Scholar]
- Huang Z, Wang C, Wei L, Wang J, Fan Y, Wang L, Wang Y and Chen T (2008) Resveratrol inhibits EMMPRIN expression via P38 and ERK1/2 pathways in PMA-induced THP-1 cells. Biochem. Biophys. Res. Commun 374, 517–521. [DOI] [PubMed] [Google Scholar]
- Jang JH and Surh YJ (2003) Protective effect of resveratrol on beta-amyloid-induced oxidative PC12 cell death. Free Radic. Biol. Med 34, 1100–1110. [DOI] [PubMed] [Google Scholar]
- Jang MH, Piao XL, Kim HY, Cho EJ, Baek SH, Kwon SW and Park JH (2007) Resveratrol oligomers from Vitis amurensis attenuate beta-amyloid-induced oxidative stress in PC12 cells. Biol. Pharm. Bull 30, 1130–1134. [DOI] [PubMed] [Google Scholar]
- Janssens V and Goris J (2001) Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem. J 3539, 417–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang G, Xu L, Song S, Zhu C, Wu Q, Zhang L and Wu L (2008) Effects of long-term low-dose cadmium exposure on genomic DNA methylation in human embryo lung fibroblast cells. Toxicology 244, 49–55. [DOI] [PubMed] [Google Scholar]
- Jiang H, Shang X, Wu H, Gautam SC, Al-Holou S, Li C, Kuo J, Zhang L and Chopp M (2009) Resveratrol downregulates PI3K/Akt/mTOR signaling pathways in human U251 glioma cells. J. Exp. Ther. Oncol 8, 25–33. [PMC free article] [PubMed] [Google Scholar]
- Jiang C, Yuan Y, Hu F, Wang Q, Zhang K, Wang Y, Gu J, Liu X, Bian J and Liu Z (2014) Cadmium induces PC12 cells apoptosis via an extracellular signal-regulated kinase and c-Jun N-terminal kinase-mediated mitochondrial apoptotic pathway. Biol. Trace Elem. Res 158, 249–258. [DOI] [PubMed] [Google Scholar]
- Jin F, Wu Q, Lu YF, Gong QH and Shi JS (2008) Neuroprotective effect of resveratrol on 6-OHDA-induced Parkinson’s disease in rats. Eur. J. Pharmacol 600, 78–82. [DOI] [PubMed] [Google Scholar]
- Johri N, Jacquillet G and Unwin R (2010) Heavy metal poisoning: the effects of cadmium on the kidney. Biometals 23, 783–792. [DOI] [PubMed] [Google Scholar]
- Jomova K and Valko M (2011) Advances in metal-induced oxidative stress and human disease. Toxicology 283, 65–87. [DOI] [PubMed] [Google Scholar]
- Kang MK, Kang NJ, Jang YJ, Lee KW and Lee HJ (2009) Gallic acid induces neuronal cell death through activation of c-Jun N-terminal kinase and downregulation of Bcl-2. Ann. N. Y. Acad. Sci 1171, 514–520. [DOI] [PubMed] [Google Scholar]
- Kim EK and Choi EJ (2010) Pathological roles of MAPK signaling pathways in human diseases. Biochim. Biophys. Acta 1802,396–405. [DOI] [PubMed] [Google Scholar]
- Kim SD, Moon CK, Eun SY, Ryu PD and Jo SA (2005) Identification of ASK1, MKK4, JNK, c-Jun, and caspase-3 as a signaling cascade involved in cadmium-induced neuronal cell apoptosis. Biochem. Biophys. Res. Commun 328, 326–334. [DOI] [PubMed] [Google Scholar]
- von Kriegsheim A, Pitt A, Grindlay GJ, Kolch W and Dhillon AS (2006) Regulation of the Raf-MEK-ERK pathway by protein phosphatase 5. Nat. Cell Biol 8, 1011–1016. [DOI] [PubMed] [Google Scholar]
- Kutuk O, Poli G and Basaga H (2006) Resveratrol protects against 4-hydroxynonenal-induced apoptosis by blocking JNK and c-JUN/AP-1 signaling. Toxicol. Sci 90, 120–132. [DOI] [PubMed] [Google Scholar]
- Kyriakis JM and Avruch J (2001) Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev 81, 807–869. [DOI] [PubMed] [Google Scholar]
- Kyriakis JM and Avruch J (2012) Mammalian MAPK signal transduction pathways activated by stress and inflammation: a 10-year update. Physiol. Rev 92, 689–737. [DOI] [PubMed] [Google Scholar]
- Lee MK, Kang SJ, Poncz M, Song KJ and Park KS (2007) Resveratrol protects SH-SY5Y neuroblastoma cells from apoptosis induced by dopamine. Exp. Mol. Med 39, 376–384. [DOI] [PubMed] [Google Scholar]
- Lin CJ, Chen TH, Yang LY and Shih CM (2014a) Resveratrol protects astrocytes against traumatic brain injury through inhibiting apoptotic and autophagic cell death. Cell Death Dis. 5, e1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin TK, Chen SD, Chuang YC et al. (2014b) Resveratrol partially prevents rotenone-induced neurotoxicity in dopaminergic SH-SY5Y cells through induction of heme oxygenase-1 dependent autophagy. Int. J. Mol. Sci 15, 1625–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Chen L, Luo Y, Chen W, Zhou H, Xu B, Han X, Shen T and Huang S (2010a) Rapamycin inhibits IGF-1 stimulated cell motility through PP2A pathway. PLoS ONE 5, e10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Wilk SA, Wang A, Zhou L, Wang RH, Ogawa W, Deng C, Dong LQ and Liu F (2010b) Resveratrol inhibits mTOR signaling by promoting the interaction between mTOR and DEPTOR. J. Biol. Chem 285, 36387–36394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Shi Z, Fan L, Zhang C, Wang K and Wang B (2011) Resveratrol improves neuron protection and functional recovery in rat model of spinal cord injury. Brain Res. 1374, 100–109. [DOI] [PubMed] [Google Scholar]
- Liu B, Luo XJ, Yang ZB, Zhang JJ, Li TB, Zhang XJ, Ma QL, Zhang GG, Hu CP and Peng J (2014) Inhibition of NOX/VPO1 pathway and inflammatory reaction by trimethoxystilbene in prevention of cardiovascular remodeling in hypoxia-induced pulmonary hypertensive rats. J. Cardiovasc. Pharmacol 63, 567–576. [DOI] [PubMed] [Google Scholar]
- Lopez E, Figueroa S, Oset-Gasque MJ and Gonzalez MP (2003) Apoptosis and necrosis: two distinct events induced by cadmium in cortical neurons in culture. Br. J. Pharmacol 138, 901–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez-Armenta M and Rios C (2007) Cadmium neurotoxicity. Environ. Toxicol. Pharmacol 23, 350–358. [DOI] [PubMed] [Google Scholar]
- Morita K, Saitoh M, Tobiume K, Matsuura H, Enomoto S, Nishitoh H and Ichijo H (2001) Negative feedback regulation of ASK1 by protein phosphatase 5 (PP5) in response to oxidative stress. EMBO J. 20, 6028–6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda B, Iwamoto Y, Tachibana H and Sugita M (1997) Parkinsonism after acute cadmium poisoning. Clin. Neurol. Neurosurg 99, 263–265. [DOI] [PubMed] [Google Scholar]
- Panayi AE, Spyrou NM, Iversen BS, White MA and Part P (2002) Determination of cadmium and zinc in Alzheimer’s brain tissue using inductively coupled plasma mass spectrometry. J. Neurol. Sci 195, 1–10. [DOI] [PubMed] [Google Scholar]
- Pervaiz S and Holme AL (2009) Resveratrol: its biologic targets and functional activity. Antioxid. Redox Signal 11, 2851–2897. [DOI] [PubMed] [Google Scholar]
- Pihl RO and Parkes M (1977) Hair element content in learning disabled children. Science 198, 204–206. [DOI] [PubMed] [Google Scholar]
- Puissant A, Robert G, Fenouille N, Luciano F, Cassuto JP, Raynaud S and Auberger P (2010) Resveratrol promotes autophagic cell death in chronic myelogenous leukemia cells via JNK-mediated p62/SQSTM1 expression and AMPK activation. Cancer Res. 70, 1042–1052. [DOI] [PubMed] [Google Scholar]
- Rockwell P, Martinez J, Papa L and Gomes E (2004) Redox regulates COX-2 upregulation and cell death in the neuronal response to cadmium. Cell. Signal 16, 343–353. [DOI] [PubMed] [Google Scholar]
- Shakibaei M, Harikumar KB and Aggarwal BB (2009) Resveratrol addiction: to die or not to die. Mol. Nutr. Food Res 53, 115–128. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Nakazato T, Xian MJ, Sagawa M, Ikeda Y and Kizaki M (2006) Resveratrol induces apoptosis of human malignant B cells by activation of caspase-3 and p38 MAP kinase pathways. Biochem. Pharmacol 71, 742–750. [DOI] [PubMed] [Google Scholar]
- Thompson J and Bannigan J (2008) Cadmium: toxic effects on the reproductive system and the embryo. Reprod. Toxicol 25, 304–315. [DOI] [PubMed] [Google Scholar]
- Tseng SH, Lin SM, Chen JC, Su YH, Huang HY, Chen CK, Lin PY and Chen Y (2004) Resveratrol suppresses the angiogenesis and tumor growth of gliomas in rats. Clin. Cancer Res 10, 2190–2202. [DOI] [PubMed] [Google Scholar]
- Wang B and Du Y (2013) Cadmium and its neurotoxic effects. Oxid. Med. Cell. Longev 2013, 898034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Xiao JL, Ling YH,Meng XJ,Wu B, Yang XY and Zou F. (2014) BNIP3 upregulation by ERK and JNK mediates cadmium-induced necrosis in neuronal cells. Toxicol. Sci 140, 393–402. [DOI] [PubMed] [Google Scholar]
- Woo JH, Lim JH, Kim YH, Suh SI, Min DS, Chang JS, Lee YH, Park JW and Kwon TK (2004) Resveratrol inhibits phorbol myristate acetate-induced matrix metalloproteinase-9 expression by inhibiting JNK and PKC delta signal transduction. Oncogene 23, 1845–1853. [DOI] [PubMed] [Google Scholar]
- Wright RO, Amarasiriwardena C, Woolf AD, Jim R and Bellinger DC (2006) Neuropsychological correlates of hair arsenic, manganese, and cadmium levels in school-age children residing near a hazardous waste site. Neurotoxicology 27, 210–216. [DOI] [PubMed] [Google Scholar]
- Wu Y and Liu F (2013) Targeting mTOR: evaluating the therapeutic potential of resveratrol for cancer treatment. Anticancer Agents Med. Chem 13, 1032–1038. [DOI] [PubMed] [Google Scholar]
- Wu Y, Li X, Zhu JX, Xie W, Le W, Fan Z, Jankovic J and Pan T (2011) Resveratrol-activated AMPK/SIRT1/autophagy in cellular models of Parkinson’ s disease. Neurosignals 19, 163–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Chen S, Luo Y et al. (2011) Calcium signaling is involved in cadmium-induced neuronal apoptosis via induction of reactive oxygen species and activation of MAPK/mTOR network. PLoS ONE 6, e19052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Hou L, Li Y, Ni J and Liu L (2013) Neuronal necrosis and spreading death in a Drosophila genetic model. Cell Death Dis. 4, e723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Feng X, Wu J et al. (2014) Neuroprotective effects of resveratrol on damages of mouse cortical neurons induced by beta-amyloid through activation of SIRT1/Akt1 pathway. BioFactors 40, 258–267. [DOI] [PubMed] [Google Scholar]
- Zhou XM, Zhou ML, Zhang XS, Zhuang Z, Li T, Shi JX and Zhang X (2014) Resveratrol prevents neuronal apoptosis in an early brain injury model. J. Surg. Res 189, 159–165. [DOI] [PubMed] [Google Scholar]


