Abstract
Pancreatic ductal adenocarcinoma (PDAC) is a complex disease with significant intra-tumoral heterogeneity (ITH). Currently, no reliable PDAC tumor model is available that can present ITH profiles in a controlled manner. We develop an in vitro microfluidic tumor model mimicking the heterogeneous accumulation of key driver mutations of human PDAC using cancer cells derived from genetically engineered mouse models. These murine pancreatic cancer cell lines have KPC (Kras and Trp53 mutations) and KIC genotypes (Kras mutation and Cdkn2a deletion). Also, the KIC genotypes have two distinct phenotypes – mesenchymal or epithelial. The tumor model mimics the ITH of human PDAC to study the effects of ITH on the gemcitabine response. The results show gemcitabine resistance induced by ITH. Remarkably, it shows that cancer cell–cell interactions induce the gemcitabine resistance potentially through epithelial–mesenchymal-transition. The tumor model can provide a useful testbed to study interaction mechanisms between heterogeneous cancer cell subpopulations.
Introduction
Cancer is a progressive disease that evolves into the highly complex and heterogeneous tumor microenvironment (TME). The dynamic nature of the complex TME impedes the clinical success of anticancer therapy by manipulating pathological tumor responses. Indeed, pancreatic ductal adenocarcinoma (PDAC) poses a significant clinical challenge with a dismal 8% five year survival rate.1 The low survival rate is associated with the late clinical presentation with advanced disease, multiple gene perturbations, and desmoplastic stroma.2 The desmoplastic stroma plays a critical role in escalating therapeutic resistance through reciprocal interactions with tumor cells.3,4 Specifically, PDAC tumors display various genomic and phenotypic diversity, known as intra-tumoral heterogeneity (ITH).5-12 ITH has been recognized as a crucial cause of clinical failure in effective treatments for PDAC patients.9,10 Recent studies have demonstrated that chemoresistance can be induced in cancer cells by ITH through selective pressure on the drug-resistant cancer cell clonal populations.13-15 Although the clinical challenges associated with ITH have been widely recognized, the mechanisms of the chemoresistance by ITH are still poorly understood.10,11 To discover effective therapeutic strategies, new and innovative tumor models are highly desirable to mimic the complex and heterogeneous features in TME.
ITH represents the presence of the tumor subpopulations identified by genomic mutations or molecular subtypes.2,16 The key oncogenic mutations of human PDAC include KRAS, CDKN2A (encoding P16INK4a and P19ARF), TP53 (encoding P53 protein), and SMAD4.2,17 These mutations are accumulated over PDAC initiation and progression. Starting from the activation of the KRAS oncogene, mutations of tumor suppressor genes occur in turn of inactivation of CDKN2A (P16) and then inactivation of TP53 (P53). This prolonged accumulation of mutations leads to highly heterogenous PDAC. Thus, intratumoral heterogeneity arises in both spatial and temporal manners. The key driver mutations play critical roles in inducing diverse pathological processes during PDAC progression, including cell metabolism, DNA damage repair, hypoxia response, desmoplastic stroma, and aberrant molecular signaling pathways.16-18 In addition to the genomic diversity, cancer subpopulations express distinctive phenotypes, yielding a central aspect of ITH dynamics.8,18,19 The cancer phenotypes are altered through reciprocal interactions between the tumor cells with desmoplastic stroma, evolving ITH in PDAC.3,4,20 The phenotype transitions are instrumental processes involving tumor progression with perturbed molecular signaling such as TGF-β. Indeed, the epithelial–mesenchymal transition (EMT) implements tumor progression and enhances the intrinsic therapeutic resistance of pancreatic cancer cells (PCCs).21-23
Over a decade, in vitro tumor models have been developed to recapitulate the complex TME.13,24,25 Beyond the 2D monolayer-culture testbed, potential anti-cancer compounds are commonly screened using the cell spheroid models for 3D culture conditions.13,26 Still, the desmoplastic stroma is not faithfully reconstituted in either 2D or spheroid models, which poses multiple transport barriers.27 These barriers include dense packing of stromal cells, the extracellular matrix proteins, and extremely high interstitial fluid pressure (IFP). These collectively impede drug delivery.28-33 To address this limitation, 3D microfluidic in vitro models have been developed.24,34-38 These microfluidic tumor models recapitulate the transport dynamics, providing appropriate environmental conditions of TME such as leaky vasculature, condensed stroma components, and elevated IFP.35 Despite of the progress, there is still a lack of tumor models mimicking the intra-tumoral heterogeneity, which refers to tumor cell sub-populations with diverse genotypic and phenotypic profiles within a given tumor.16,18,39
Most of the current tumor models, however, have been developed using cells possessing uniform molecular and phenotypic profiles.26,37,38,40,41 Recently, the engineered microenvironments controlled in microfluidics have widely contributed to drug discovery for various diseases.42-47 Specifically, the practical approaches for the co-culture system mimic the heterogeneous TME in the tumor models.44,47-51 The co-culture models of tumor–stroma cells exhibit the effect of cancer–stroma cell interaction, such as cancer-associated fibroblasts (CAFs) in inducing tumor invasion and diminishing drug efficacy.47,50 Furthermore, the tumor-endothelium co-culture in microfluidic platform recapitulate angiogenesis in TME.42,48,52 Nguyen et al.48 showed the invasive PDAC could induce the endothelial ablation in the in vitro co-culture platform comprising of two adjacent structures with a biomimetic blood vessel and a pancreatic cancer duct. The engineered tumor vascular networks in vitro were developed to provide the effective drug screening platform.49 Nevertheless, there is still a lack of tumor models mimicking ITH.
The cause and effect of ITH are important in tumor pathobiology.53,54 The genetic landscape altered by accumulating the oncogenic mutations over the PDAC stages, and the acquisition of phenotype change with perturbed signaling pathways derive ITH. However, it is difficult to study due to a lack of a reliable model allowing control of the ITH characteristics. In vitro multi-cellular spheroid models were evolved to evaluate the therapeutic response of the heterogeneous cancer cell compositions.55,56 But the mixture of the cell types does not assure the control of oncogenic mutations. The complex diversity of the subtypes displaying ITH includes distinct driver mutations and phenotype descriptions depending on the tumor stages and TME conditions, which is the critical gap in developing ITH tumor models. Genetically engineered models (GEMMs) can recapitulate many histologic and genetic features of human PDAC. These include key driver mutations, epithelial duct structures, and the surrounding stromal tissue. GEMMs are also used to predict drug treatment response.31,57 In GEMMs, one or two of driver mutations are induced, and subsequent interactions between heterogeneous cancer cell subgroups are difficult to study. Patient-derived xenograft (PDXs) model is better in mimicking ITH and predicting drug treatment response.13 Although PDXs may carry the ITH of tumors from a given patient, their ITH characteristics are challenging to control. Alternatively, Lee et al.58 developed a patient-driven organoids in vitro model as well as PDX to recapitulate ITH. Despite recent advances,58,59 the presentation of tumors with heterogenous gene mutations and molecular diversity is rare in vitro tumor models. Thus, new models that carry heterogeneous genetic and phenotypic features of human PDAC are highly desired.
This study aims to develop a new in vitro model of PDAC with a controlled ITH to elucidate the effects of ITH on gemcitabine response. The new model adopts GEMM-driven murine pancreatic cancer cell lines exhibiting the key driver mutations. Moreover, these cell types are mono- and co-cultured to systematically study the ITH. The novel co-culture system to engineer ITH is developed in a microfluidic platform where the cells are cultured within the perfused 3D extracellular matrix to mimic drug transport and action in the PDAC TME. Two PCC genotypes, derived from PDACs of GEMM (KPC – with Kras and Trp53 mutations, and KIC – with Kras mutation and Cdkn2a deletion), are employed to develop the ITH-mimicking composition. These genotypes represent key driver mutations of human PDAC. Additionally, two phenotypes of KIC tumor cells are used to mimic phenotype heterogeneity – epithelial phenotype KIC (eKIC) and mesenchymal phenotype KIC (mKIC). These cells are mono- or co-cultured on the microfluidic platform to reconstitute tumors with ITH. Using the model, the genotype- and the phenotype-specific responses to gemcitabine are assessed. The results are further discussed to interpret the drug resistance mechanisms of PDAC. The new model faithfully recapitulates the disease with controlled ITH and TME conditions for systematic study and accelerated screening of promising therapeutics.
Materials and methods
Cells and reagents
Murine pancreatic cancer cell lines (KPC2, eKIC, and mKIC) were isolated from PDAC tumors from two different genotypes of GEMMs. KPC2 cells were isolated from PDAC tissues of KPC mice (LSL-KrasG12D/+, LSL-Trp53R172H/+, Elastasepr-CreER alleles). The KIC cell lines, eKIC and mKIC, were provided by Dr. Murray Korc at the Indiana University. The cell lines were established from KIC mice in which Kras mutation was combined with the deletion of the Ink4a locus (Ink4a/ArfL/L) to generate the Pdx1-Cre; LSL-KrasG12D/+; Ink4a/Arf−/−GEMM.60,61 Two distinct phenotypes of the KIC cells were identified by transcription levels of E-cadherin (Cdh1), snail, MMP9, and fibronectin (FN1) reported in a previous study.62 The murine PCCs were maintained in RPMI 1640 with 2.05 mM l-glutamine (GE Healthcare Bio-Sciences Corp., MA, USA) supplemented by 5% v/v fetal bovine serum (FBS) and 100 μg ml−1 penicillin/streptomycin (P/S). The cells were regularly harvested by 0.05% trypsin and 0.53 mM EDTA (Life Technologies, CA, USA) when grown to ~80% confluency in 75 cm2 T-flasks and incubated at 37 °C with 5% CO2. Harvested cells were used for experiments by culturing on 2D and iT-MOC, or sub-cultured while maintaining them below 15th passage.
qPCR
To validate the genotypes of cell lines, KPC2, eKIC, and mKIC, the genomic DNA was isolated with Genomic Mini Kit (Viogene), and total RNA was isolated with Trizol reagent (Invitrogen) following manufacturer's instruction. 1 μg of total RNA was subjected to cDNA synthesis with iScript kit (Bio-Rad). Genomic DNA and cDNA were referred to PCR with high-fidelity PrimeStar GXL DNA Polymerase (TaKaRa). PCR products were gel purified and sequenced by genomics sequencing lab, Purdue University. Forward and reverse primer sequences used for PCR are as following:
Kras (GAGGCCTGCTGAAAATGACTGAG and GGCAAATACACAAAGAAAGCCCTCC), p53 (CCTGCAGTCTGGGACAGCCAAG and CACATGTACTTGTAGTGGATGGTGG), and p16 (GTGATGATGATGGGCAACGTTC and GGCGTGCTTGAGCTGAAGCTA)
Fabrication of interstitial tumor-microenvironment-on-chip (iT-MOC)
The microfluidic model, iT-MOC, is a 3D culture device with two layers separated with a porous membrane. Both layers feature microchannels that have thicknesses of 100 μm. The bottom layer is composed of a 1 mm-wide interstitial channel for cell culture connected to two 300 μm-wide side channels that enable lymphatic drainage. The top layer includes a 300 μm-wide capillary channel positioned above the interstitial channel. It is separated from the bottom by a transparent porous polycarbonate (PC) membrane with a pore diameter of 1 μm (Whatman Cyclopore, 7091-4710). The capillary channel is perfused with culture media and allows the exchange of fluid, nutrients, and drugs with the interstitial channel through the membrane interface. Device construction involves two micro-patterned polydimethylsiloxane (PDMS) stamps fabricated by soft lithography.34,63,64 Briefly, a photoresist (Microchem SU-8 2075) was deposited on a clean silicon dioxide wafer and exposed to UV light through a mask with desired channel features. The photoresist exposed part was cured, resulting in extruded channel features, which were later transferred to PDMS stamps by replica molding. The PDMS stamps were blanked through by a 3 mm biopsy punch to produce the inlet–outlet ports and bonded together with the membrane by oxygen plasma treatment. For bonding, the PC membrane was treated by 5% v/v (3-aminopropyl)triethoxysilane (APTES) in distilled H2O at 80 °C for 20 minutes and air-dried thoroughly before the assembly. Finally, the devices were perfused by distilled water filtered through a 0.2 μm polyethersulfone (PES) filter (Corning, NY, USA) for 12 hours and heat-dried to remove any residues from the fabrication process.
Preparation of cell–collagen mixture
After fabrication, a mixture of the cells and collagen solution was loaded through the interstitial channel. In the cell–matrix mixture, cells were bathed with 6 mg ml−1 of type I collagen (Corning Inc., NY, USA), 10× PBS, NaOH, HEPE solution, FBS, Glu, P/S, and cell-culture grade distilled water. The cells were either used alone in the iT-MOC as the mono-culture group or were co-cultured at a 1 : 1 initial cell density ratio to investigate interactions between PCCs. The initial cell density of the mixture when the cells were loaded in iT-MOC was 2 × 106 cells per mL for mono-culture models and 4 × 106 cells per mL for co-culture models. The cells were loaded into the microfluidic devices by applying suction pressure through the interstitial channel with a syringe while blocking lymphatic and capillary channels. After loading the cell mixture into the iT-MOC, the devices were incubated at 37 °C for 1 hour for gel formation of the collagen. Afterward, the devices were perfused by culture medium through culture medium reservoirs connected to the capillary and lymphatic channels. The devices were kept in an incubator with 37 °C and 5% CO2 conditions throughout the experiments.
Immunofluorescence
After culturing the cells in the iT-MOC for three days, cells were fixed with 10% formaldehyde solution. A day later, the cells were permeabilized with 0.02% Triton-X solution in PBS and blocked with M.O.M blocking reagent with avidin solution in PBS for an hour. After washing three times, the samples were indirectly stained with anti-E-cadherin (E-cad) and anti-type IV collagen using biotin-conjugated immunochemistry. The confocal immunofluorescence was captured by structured illumination (OptiGrid, Qioptiq imaging) augmented IX71 and further processed using ImageJ. The multi-z step immunofluorescent images were projected in an image stack. The expression levels of E-cad and type IV collagen were quantified by image processing. The maximum and minimum intensity of images of each group were consistently matched. The process was performed separately between E-cad and type IV collagen.
Flow cytometry
Fluorescence-activated cell sorting (FACS) technique was used to compare the phenotype changes induced by cell–cell interactions. Mono- and co-culture of all the cancer cell types were prepared as a 2D monolayer in T75 flasks. To distinguish the cell lines in co-culture cases, KPC2 and eKIC were stained with CFSE (Life Technologies, CA, USA) before plating on the flask. Briefly, the supernatant of each cell suspension was replaced with a CFSE solution (12.5 μg ml−1 in the culture medium, RPMI RPMI 1640 with 2.05 mM l-glutamine supplemented by 5% v/v FBS and 100 μg ml−1 P/S) and incubated for 20 minutes. After washing three times with the CFSE-free medium by replacing the supernatant, the cells were plated in the flasks. CFSE-stained KPC2 and eKIC were prepared for both mono-culture and co-culture cases. The cells were maintained in the incubator for 3 days and harvested using enzyme-free cell dissociation buffer (Life Technologies, CA, USA) to avoid any damage of cell adhesion molecules.
Drug sensitivity assay
The sensitivity of PCCs to gemcitabine was assessed with or without mKIC using conventional microwell plates as 2D monolayers and, separately, in 3D culture using the iT-MOC. For drug testing on 2D culture, 4000 cells per well were plated on the 24-well plate and allowed to form monolayers for 2 days. The cells were then incubated for 24 hours with gemcitabine at three different concentrations, 0 (control), 0.2, and 20 μM. Cells were then washed with the normal culture medium, and incubation was continued for an additional 24 hours to provide sufficient time to capture the latent effects of drug action on the cells. Similarly, cells were pre-cultured for 2 days in the iT-MOC and then perfused through the capillary channel in the absence (control) or presence of 0.2 μM or 20 μM gemcitabine. The pressure difference between the capillary channel (20 mm H2O) and the lymphatic channels (5 mm H2O) was applied to allow the drug to penetrate through the porous membrane. The cells in the interstitial channel were exposed to the drug for 24 hours. Capillary and lymphatic channels were then washed with the culture medium, and all cells were incubated for 48 hours in the absence of gemcitabine.
Drug sensitivity from both 2D monolayer and the iT-MOC assay was analyzed by evaluating cell viability at the end of the incubations. Viable cell growth was assessed by staining nuclei acid using Hoechst 33342 (Sigma-Aldrich, St. Louis, MO). The nuclei of dead cells were considered as minimized by giving the 48 hour post culture period.65 Nuclear fluorescence was determined with an inverted microscope (Olympus IX71, Japan) using a DAPI filter. Cell survival was calculated by normalizing the treatment group's nuclear area to control group (0 μM of gemcitabine) as follows:
| (1) |
The cell survival indicated cell response to the drug by comparing viable cell growth of the treatment group with respect to control growth. In co-culture models, cells were distinguished using red fluorescent protein (RFP) transfected mKIC detected using a TRITC filter.
Statistical analysis
All experiments were repeated at least 3 times for each treatment group. The data was reported in the form of mean ± standard estimated error (S.E.M). Data points in the drug sensitivity assay were statistically analyzed using a one-way analysis of variance (ANOVA) or student t-test. The comparison between 2D and iT-MOC was analyzed in each treatment group by using Tukey post hoc multiple comparisons test provided in ANOVA. Drug sensitivity differences between mono-culture and co-culture in iT-MOC were analyzed for 0.2 μM gemcitabine treatment groups. Cell survivals of each cell line in 0.2 μM gemcitabine groups of mono-culture and co-culture were tested with student t-test. In addition, the E-cad expression transition from mono-culture to co-culture was statistically analyzed by using Student's t-test and Kolmogorov–Smirnov test. Mean fluorescent intensities (MFIs) of each cell line from mono-culture and co-culture were compared by student t-test, and the Kolmogorov–Smirnov test analyzed the data distribution tendency. The differences were recognized as statistically significant when p-value < 0.05.
Results
Characteristics of KPC2, eKIC, and mKIC
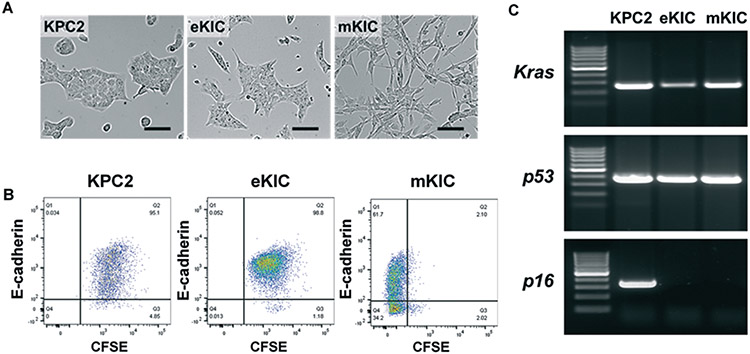
In developing the ITH model, the diversity in genetic mutations and phenotypes is constituted by GEMM-driven cell lines, two KIC lines (eKIC and mKIC) and a KPC line (KPC2). The cell lines are characterized and presented in Fig. 1. The morphology of the KPC2 and eKIC mono-culture in 2D shows the cobblestone shape of epithelial cells. In contrast, mKIC cells have spindle-shaped mesenchymal morphology, as shown in Fig. 1A. To further assess the phenotype characteristics of these cells, expressions of E-cadherin (E-cad) are analyzed by the FACS technique. As shown in Fig. 1B, an epithelial marker E-cad, is highly expressed in KPC2 and eKIC. On the other hand, mKIC shows significantly lower E-cad expression compared to the other two cell lines. Although a sub-population of mKIC cells expresses E-cad, the E-cad high mKIC population (63.8%) is distinctively lower than those of eKIC (98.5%) and KPC2 (95.1%). The difference is statistically significant showing p < 0.001 in both Student's t-test for MFI and Kolmogorov–Smirnov test for distribution comparisons. The results confirm that KPC2 and eKIC have epithelial phenotypes, whereas mKIC does mesenchymal-like as identified in our previous study.62 The KPC2 and eKIC cells expressed significantly higher transcription levels of E-cad (epithelial marker) than mKIC, while snail, MMP9, and fibronectin (FN1) (mesenchymal marker) were present at higher levels in mKIC. In addition, each cell line's genomic mutations are investigated by qPCR and genomic sequencing. The results confirm that the KPC2 line has mutations of Kras and p53 (Trp53 alias) with intact p16 (Cdkn2a alias), whereas both KIC lines contain mutations in Kras and the deletion of p16. The sequence of Kras is confirmed, showing that all three cell lines contain the G12D mutation. KPC2 cells have the mutation in R172H of Trp53, whereas eKIC and mKIC show wild type Trp53 (presented in ESI† Fig. S1). Both genomic DNA and cDNA of KIC lines are confirmed with the deletion of p16 exon2, whereas KPC2 has WT p16.
Fig. 1.
Characteristics of KPC2, eKIC, and mKIC in 2D monolayer. (A) Bright-field micrograph of 2D monolayer of KPC2, eKIC, and mKIC. (B) Fluorescence-activated cell sorting (FACS) for E-cadherin (E-cad) and CFSE expressed in the cells. KPC2 and eKIC were stained by CFSE to distinguish from mKIC in co-cultures. A majority of the KPC2 and eKIC cells expressed E-cad whereas mKIC cells had a large negative population. (C) qPCR for Kras, p53, and p16 mutations. Scale bars indicate 100 μm.
iT-MOC mimics the intra-tumoral heterogeneity of PDAC by GEMM-driven cancer cell lines
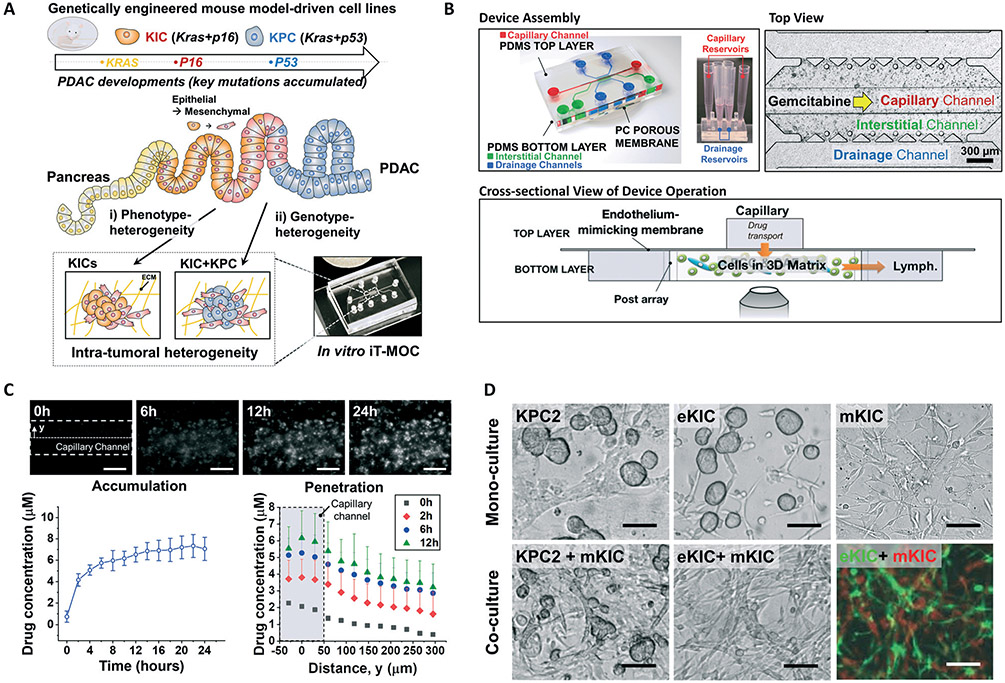
By facilitating the murine PDAC cell types in our iT-MOC, an in vitro tumor model with controlled ITH is established to assess the drug transport and response of these pancreatic cell lines. An overview of the device design, construction, and application is illustrated in Fig. 2. It has been recognized that the tumor cells in PDAC compose of diverse subpopulations with mutations of KRAS, CDNK2A, and TP53.2 Genetic alteration during PDAC development starts with activation of the KRAS oncogene, inactivation of CDKN2A (P16 mutation), and finally, inactivation of the tumor suppressor gene TP53 (P53 mutation). These alterations are attributed to the sequential transition from precursor lesions such as pancreatic intraepithelial neoplasia (PanIN) to invasive carcinoma PDAC.17,66 Here, we use GEMM-driven PCCs with different driver mutations either mono- or co-cultured in the microfluidic system. The KIC or KPC cells, and their co-culture are used to mimic different stages of PDAC tumors – early stage of PDAC, where Kras and p16 mutations occur, and later stage, where Kras, p16 and p53 mutations do (Fig. 2A). The i) phenotypic heterogeneity is achieved by co-culturing the KIC cells with different phenotypic characteristics, eKIC and mKIC. The two cell lines showed distinctly different phenotype showing different expression level of E-cad, snail, MMP9, and FN1,62 although those contain same driver mutations of Kras and p16. Also, the ii) genotype-heterogeneity is established by co-culturing the KPC2 cells with mKIC. The genotype heterogeneity creates a distinction from p16 mutation of mKIC and p53 mutation of KPC2 while both cell lines contain Kras mutations.
Fig. 2.
Design, construction, and functional components of iT-MOC. (A) Schematic illustration of functional model of intra-tumoral heterogeneous features in vitro composited by genetically engineered mouse model-driven cell lines to capture different PDAC progression stages. (B) The assembled iT-MOC device schematic, top view, and cross-sectional view. iT-MOC has two PDMS layers separated with a porous membrane. Close-up at the cell culture chamber during a drug sensitivity assay where chemotherapy drug gemcitabine is perfused through the capillary channel. (C) Doxorubicin transport and accumulation to eKIC in iT-MOC. Doxorubicin is accumulated through cellular-uptake as shown in the time-lapse fluorescence micrograph. Temporal drug accumulation and spatial drug diffusion show doxorubicin transport featured in iT-MOC mimicking in vivo transport dynamics. (D) Bright-field micrograph of KPC2, eKIC, and mKIC in iT-MOC in mono-culture and co-culture models. In co-culture models, recognized morphology transition of eKIC with mKIC was observed, where cells were distinguishable by using the transfected cells of GFP-eKIC and RFP-mKIC. Scale bars indicate 100 μm.
In addition to the ITH features, the model mimics the transport steps at the PDAC TME. The drug transport in the human PDAC TME includes extravasation, interstitial transport, cellular uptake, and lymphatic drainage.17,34 The transport scheme is recapitulated in the iT-MOC. Transport characteristics of iT-MOC were extensively characterized in our earlier works.34,63 The iT-MOC device, developed by PDMS microfluidic structures, comprises of two layers with a porous membrane between the layers. The top layer contains a capillary channel where the drug is introduced. In the bottom layer, the drug particles perfused through the porous membrane accumulate within the interstitial channel that cells embedded within an ECM composed of type I collagen, and finally flushed through the drainage channels on the side of the interstitial channel. (Fig. 2B) Perfusion flow conditions within the iT-MOC device are controlled by differences in hydrostatic pressure applied to the capillary, interstitial, and lymphatic channels. This allows the interstitial fluid pressure gradient and flow rates to be controlled, as described previously.35,63,64 In this study, a hydrostatic pressure difference of 20 mm H2O is used, where the higher pressure is applied to the capillary channels. This pressure difference scaled down from the physiological interstitial fluid pressure of 20 mm Hg for mammary carcinoma was found appropriate for establishing an interstitial flow rate on device within the range typically observed in TME.67,68 In addition, for a given pressure difference, the transvascular flux of drug particles is determined by the permeability of the porous membrane which is estimated based on our earlier work,63 and provided in the ESI† for different conditions.
Doxorubicin transport dynamics in iT-MOC
The drug transport characteristics of the iT-MOC platform are confirmed in terms of temporal accumulation and spatial penetration to tumors using fluorescent doxorubicin of a model drug. The doxorubicin is introduced along the capillary channel at 2 μM for 24 h and then replaced with a drug-free medium mimicking in vivo pharmacokinetics as described previously.35 As shown in Fig. 2C, the drug is rapidly accumulated during the first 6 hours, and the rate of accumulation is significantly slowed at later time points. The spatial concentrations of doxorubicin show that the drug penetrates through the interstitial channel from the capillary channel. These spatiotemporal features of the drug transport in the iT-MOC platform mimic those in TME in vivo.
Significant morphology transition of eKIC interacting with mKIC
Micrographs of cells cultured on the iT-MOC platforms are presented in Fig. 2D. Mono-cultured KPC2 and eKIC cells, surrounded by the collagen matrices, have formed spheroid-like clusters. Typically, more than 20 cells are aggregated in a cluster for both cell types. Contrary to the KPC2 and eKIC cells, mono-cultured mKIC cells have a mesenchymal-like morphology. Interestingly, when these cells are co-cultured, the morphology of eKIC cells with mKIC is drastically transformed into mesenchymal morphology, whereas KPC2 cells with mKIC retain their spheroid-like morphology. The eKIC cells are intertwined with mKIC cells, and hardly distinguishable in the brightfield micrograph. In the observations, the significant morphology transitions occurred only in the co-culture groups, indicating that each cell line's spontaneous transition is negligible. Each cell type's potential to evolve to other phenotypes warrants future research. The observations suggest that interactions between these two cancer cell types induce multiple changes of the eKIC cells, including morphological changes and loss of epithelial characteristics, which may cause increased resistance to gemcitabine.21-23
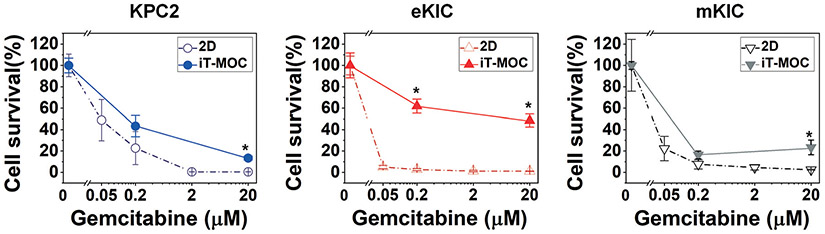
ITH induces gemcitabine resistance
The response of these cancer cells to gemcitabine, the first-line chemotherapeutic drug for PDAC, is characterized and presented in Fig. 3. When these cells are cultured in 2D configuration, all three cells are sensitive to low dosages of gemcitabine, while the KPC2 line is slightly more resistant than the other two lines. The EC 50 value of eKIC is significantly smaller than 0.05 μM and the EC 50 value of mKIC is around 0.014 ± 0.005 μM, whereas the EC 50 value of KPC2 is closer to 0.05 μM as 0.046 ± 0.008 μM. When the cells are cultured on the iT-MOC platform, all cells become more resistant to gemcitabine, as noted in Fig. 3. The eKIC line in iT-MOC shows the most significant increase in drug resistance. Approximately 50–60% of cells survived in both 0.2 μM and 20 μM gemcitabine treatments. In 2D culture, the eKIC line is very responsive to these dosages. On the other hand, the drug response of KPC2 in iTMOC is comparable to that in 2D culture, although cell survival slightly increased at 0.2 μM gemcitabine treatment without statistical significance. The mKIC line in iT-MOC also shows responsive results to gemcitabine consistent with the results from 2D culture. The differences in the eKIC response between 2D and iT-MOC cultures imply the impact of the 3D microenvironment. These observations confirm that the 3D TME poses physical barriers to drug transport and induces drug resistance by cell–matrix interactions.
Fig. 3.
Cellular response to gemcitabine in mono-cultured KPC2 (blue), eKIC (red), and mKIC (grey) on 2D (dash lines) and iT-MOC (solid lines). The cytotoxicity on murine pancreatic cancer cell lines isolated from PDAC arising in KPC GEMM and the KIC GEMM, respectively, was evaluated to investigate gemcitabine responses. Mono-cultured PCCs were assayed using 2D monolayer and iT-MOC; n ≥ 3. Dot; mean ± S.E.M. Statistical analysis is for the comparison of iT-MOC with 2D monolayer counterparts. *: p < 0.05 (Tukey post hoc multiple comparisons test).
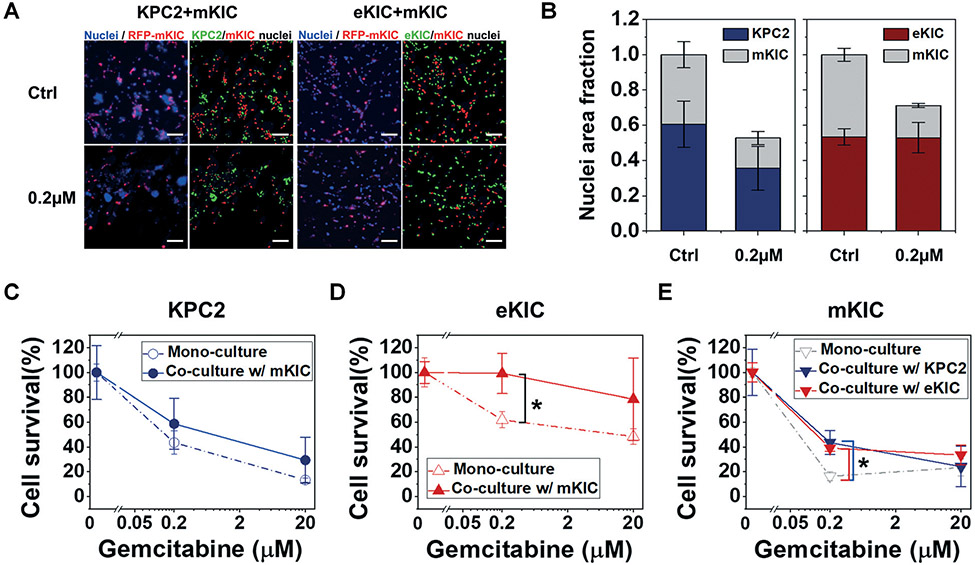
The cancer cells are co-cultured to mimic genotypic ITH (i.e., KPC2–mKIC co-culture) and phenotype ITH i.e., eKIC–mKIC), and their response to gemcitabine is assessed. In these co-culture groups, cancer cells are mixed at a 1 : 1 cell number ratio and loaded in iT-MOC platforms. Each cell in co-cultured iT-MOC is distinguished, as shown in Fig. 4A. In control groups shown in Fig. 4A and B, the growth of KPC2 is similar to mKIC in the co-culture after a 5 day culture. eKIC growth is also similar to mKIC growth. Thus, no cell lines have a notable selective advantage of faster cell proliferation. After the gemcitabine treatments, the eKIC–mKIC co-culture group shows relatively higher collective cell survival than the KPC2–mKIC group. The survival of each subpopulation is presented in Fig. 4C-E. Overall, gemcitabine resistance of all cancer cell types increases compared to their mono-cultures. The most significant increase in the gemcitabine resistance is observed in eKIC cells co-cultured with mKIC. Cell survival of eKIC is almost 100% in the 0.2 μM gemcitabine treatment group of the co-culture model. This increased resistance alludes to the interaction between eKIC and mKIC, which has a critical impact on drug resistance. The other cell lines of KPC2 and mKIC in co-culture also show an increase in gemcitabine resistance. This suggests that increased drug resistance is caused by ITH-derived cancer cell–cell interactions.
Fig. 4.
Response of KPC2 and eKIC co-cultured with mKIC to gemcitabine. Initial co-culture ratio in both KPC2–mKIC and eKIC–mKIC models were 1 : 1. (A) Fluorescent micrograph of co-cultured KPC2–mKIC and eKIC–mKIC of control (Ctrl), 0.2 μM, and 20 μM gemcitabine treatment groups. In (A), nuclei (blue) of each cell are distinguished in green (KPC2 and eKIC) and red (mKIC). (B) Nuclei area fraction of each cell line was defined over the total nuclear area of ctrl, representing the cell growth in co-culture condition in control (Ctrl) and 0.2 μM gemcitabine treatment groups. Cell survival of each cell line in 0.2 μM and 20 μM gemcitabine treatment groups of (C) KPC2, (D) eKIC, and (E) mKIC in mono-culture (dash line) and co-culture (solid line) were presented. In results, eKIC was significantly resistant to the 0.2 μM gemcitabine. To determine each cell line, RFP-mKIC was used. The co-cultured PCCs were studied in iT-MOC; n = 3. Bar: mean ± S.E.M. Statistical analysis was done for comparisons of co-culture and mono-culture of 0.2 μM and 20 μM gemcitabine treatment groups. *: p < 0.05 (Student's t-test). Scale bar indicates 100 μm.
E-cad of epithelial cells reduces by interactions with mesenchymal cells
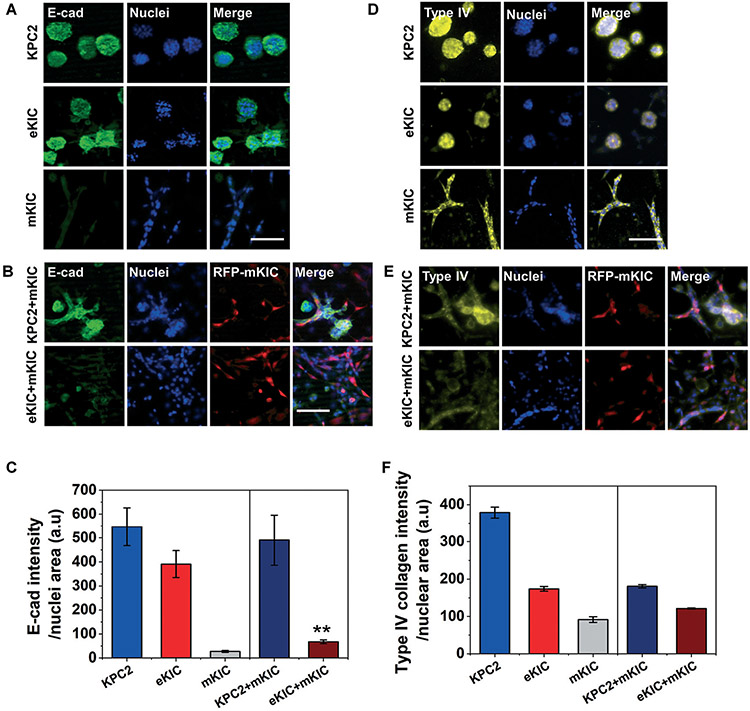
We found significant morphological changes in eKIC co-cultured with mKIC (Fig. 2D), as well as the enhanced gemcitabine resistance in co-culture models (Fig. 4D and E). Based on the observations, we hypothesize that the drug resistance is induced by the cancer–cancer cell interactions, potentially through phenotype transition of the epithelial PCCs caused by interactions with mesenchymal-like PCCs. It has been well reported that chemoresistance could be induced by EMT, which also plays a crucial role in cancer metastasis.69-74 To test the hypothesis, we assess the change of E-cad expression, loss of which is considered to be an early indicator of EMT.75,76 Immunofluorescent micrographs of E-cad are presented in Fig. 5A. In mono-cultured KPC2 and eKIC, E-cad is highly expressed and shows apparent cell–cell adhesions in a cell cluster-like morphology. In contrast, mKIC barely expresses E-cad in the iT-MOC. When co-culturing cells (KPC2–mKIC or eKIC–mKIC) in iT-MOC, E-cad expression in eKIC is significantly reduced, particularly where the eKIC cells are interfaced with mKIC. For co-cultured KPC2, the E-cad expression is also reduced, but some KPC2 cells still express E-cad considerably and maintain the spheroid-like morphology. The results imply that the interactions between heterogeneous cancer cells may induce the phenotype transition of epithelial cancer cell types. Nonetheless, the extent of the interaction shows subtype-dependence. In order to confirm the EMT, additional molecular studies showing the induction of several transcription factors such as snail and twist can be further researched. In addition, the expression of type IV collagen is assessed, as shown in Fig. 5D. All cancer cells express the type IV collagen, although the expression level shows cell-type dependence (KPC2 > eKIC > mKIC). The expression of type IV collagen indicates that the PCCs could synthesize a new ECM protein, type IV collagen. When co-cultured, a decrease of collagen IV is noted in the KPC2–mKIC group. It indicates the loss of epithelial features by cancer cell–cancer cell interactions, which could lead to the EMT. However, it warrants further research on whether cells gain mesenchymal features by mesenchymal markers, including vimentin, snail, and N-cadherin.74,76
Fig. 5.
Immunostaining micrographs of (A–C) E-cad (green) and (D–F) type IV collagen (yellow) in iT-MOC models. Immunofluorescent levels of E-cad in (A) mono-cultured KPC2, eKIC, and mKIC and (B) co-cultured KPC2 and eKIC with the RFP-mKIC (red) in iT-MOC were quantified in (C) E-cad intensity per nuclear area. E-cad expression of eKIC decreased significantly by interacting mKIC. Type IV collagen expression in (D) mono-cultured KPC2, eKIC, and mKIC and (E) co-cultured KPC2 and eKIC with the RFP-mKIC (red) was quanfied in (F). Type IV collagen expressed in all cells. A slight decrease of type IV collagen was noted in the KPC2–mKIC co-culture group. n ≥ 3; bar: mean ± S.E.M. Statistical analysis was done for comparisons of co-culture and mono-culture by assuming the E-cad level of mKIC is negligible in co-culture. **: p < 0.01 (Student's t-test). Scale bar indicates 100 μm.
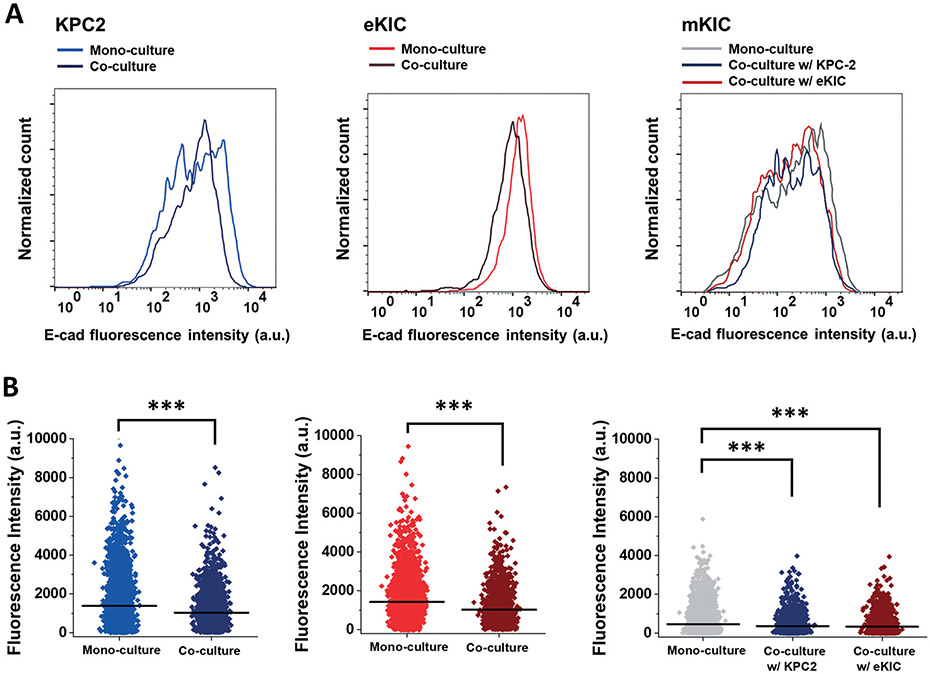
The E-cad expression is further analyzed at the individual cell level using flow cytometry, as presented in Fig. 6. The single-cell analysis aims to evaluate the cell number variation expressing E-cad. The immunofluorescent results exhibit the intensity variation of the E-cad expression level. Still, the question remains whether the cell populations expressing E-cad were changed or the intensity level of each cell was changed. By using the sensitive measurement with single cell-based flow cytometry, the cell populations expressing E-cad are closely assessed in response to the cell–cell interactions in co-culture groups. To minimize any other extrinsic stimuli to confirm the effect of just cell–cell interaction via contact, the cells are co-cultured as a 2D monolayer. Consequently, the distribution of E-cad expression shows that a number of the cells lose the E-cad expression by interacting with other cells during co-culture (Fig. 6). These results are further analyzed using mean fluorescent intensity (MFI). The MFI is recognized as the average E-cad fluorescence intensity at the single-cell level. The scattered plots confirm that the MFI of all three cells in the co-culture groups decrease significantly (p < 0.001). These results indicate that the cancer cell–cancer cell interactions achieved by co-culture could induce reduction of the E-cad expression in significant cell populations. The FACS results show that significant populations of KPC2 and mKIC cells also lose the E-cad expression in interacting with the other cell types, whereas the cells did not show the significant changes in immunofluorescence micrographs.
Fig. 6.
E-cad expression in FACS. Expression change of E-cad on CFSE–KPC2 and CFSE–eKIC by presenting RFP-mKIC (red) was evaluated by FASC in mono-cultured KPC2, eKIC, and mKIC and co-cultured KPC2 and eKIC with mKIC. In iT-MOC co-culture, cell lines were distinguished by CFSE staining. (A) Histogram of E-cad distribution (B) E-cad fluorescence scatter plots. Dot: Fluorescence intensity of E-cad from a single cell. Line: Mean fluorescence intensity (MFI) of the distribution. ***: p < 0.001 (both Student's t-test and Kolmogorov–Smirnov test).
Discussion
This study demonstrates a new tumor model of PDAC reconstituting intra-tumoral heterogeneity in a controlled manner. Using GEMM-derived cell lines, the present model reconstitutes intra-tumoral heterogeneity of driver mutations to mimic two different stages of PDAC progression – an early stage with Kras and p16 mutations, and a late stage where Kras, p16 and p53 mutations present, as illustrated in Fig. 2A.2,17,77,78 This is achieved by mono- or co-culture of cancer cells derived from GEMM with two of key driver mutations. This approach is based on the rationale that ITH is induced by prolonged accumulation of oncogenic mutations and phenotypic changes in cancer cell populations, rather than by a random mixture of cancer cells. In human PDAC, these mutations are accumulated throughout PDAC initiation and progression.17 At the PDAC stages, activating mutations in KRAS are present in nearly 100% of tumors. Inactivating mutations of CDKN2A and TP53 occur in 90% and 75% of pancreatic cancers, respectively.2,77,78 The heterogeneity arises in a distinct manner depending on the disease stages. In this sense, it has been recognized that the two aspects of ITH—phenotype and genotype heterogeneity—complicate the development of effective therapeutics.79 The present iT-MOC model has demonstrated a new design strategy for in vitro reconstitution of PDAC tumors at different stages of disease progression.
Moreover, the present results show the effects of PCC–ECM interactions on the efficacy of drugs. Indeed, all cell types in iT-MOC became more resistant to the drug than their 2D counterparts. These observations confirm that the 3D TME poses physical barriers to drug transport and induces drug resistance by cell–matrix interactions.
The present model shows that drug resistance can be induced by cancer cell–cancer-cell interaction between different subgroups. This is a distinctively new finding since the drug resistance in heterogeneous tumors has been thought to be due to selective survival of resistant subgroups of cancer cells.13-15 The present gemcitabine efficacy results of the co-culture iT-MOC models shows increased gemcitabine resistance of the cells. The phenotype-transition and drug response in the ITH model shows the most drastic response in KIC cells. By interacting with mKIC, the epithelial morphology of eKIC has been transmitted to mesenchymal-like morphology, and drug resistance increased most significantly. At a glance, the results seem to carry the genotype-specific response. Although it requires further research, it may be relevant to p16 mutation-specific signaling pathways. Our previous study for the ITH effect in PDAC invasion with the corresponding cells has shown that eKIC was highly responsive to transforming growth factor-β (TGF-β), which is known to induce EMT and cancer invasion compared to KPC2.62 Accordingly, the phenotype transition represented as EMT is a crucial key to reveal the impact of the ITH in PDAC presented in the drug resistance induced by the cell–cell interactions. Recent studies report that an intrinsic mechanism to correlate EMT with gemcitabine resistance in PCCs.69,71,72,74,80,81 EMT regulators such as Zeb-1 is highly linked with the drug resistance in human PCCs.69 EMT inducing gene expression such as twist and snail are reported as an essential correlation to gemcitabine resistance by GEMM studies.74 Similarly, the activation of Notch signaling was associated with EMT in gemcitabine resistance cells.81 Besides, the presented co-culture results show that cell–cell interactions between the cancer cells could increase gemcitabine resistance. The cell types acquiring the resistance change their morphology from epithelial to mesenchymal one and lose an EMT marker, E-cadherin. To confirm the resistance is directly related to the EMT, further research is warranted. This is a new observation since drug resistance by the ITH has been thought as evolutional survival of drug resistant subpopulations. Moreover, this acquired resistance is different from one by cancer–stromal cell interactions. Our results show that even a drug sensitive subpopulation acquires drug resistance after cancer cell–cancer cell interaction in the tumor model mimicking ITH.
In this study, we used devices with standalone membranes, i.e., without any cells lining the surface of the membrane. In the absence of endothelial cells, it became possible to study cell–cell interactions and drug responses of cancer cells without the confounding effects of other cell types within the culture. While porous membranes can have permeabilities over the physiological regime presented by the tumor vasculature, the net effect of excess permeability on the transport of fluids could be compensated by applying hydrostatic pressure levels scaled down from physiological values across different compartments of the device as described earlier. While our main focus was to recapitulate ITH in a microfluidic cancer model, an in-depth understanding of the transport environments realized within microfluidic tissue culture platforms by functional scaling is also important and requires future research.
Conclusions
This study demonstrates a novel 3D tumor model of PDAC reconstituting ITH by adopting GEMM-driven cell lines. The model establishes a novel testbed with ITH control for key driver mutations of PDAC by taking advantage of GEMM. The model is capable of considering genotype and phenotype heterogeneity with engineered subpopulations of PDAC to capture different PDAC progression stages. Furthermore, it provides a reliable 3D environment mimicking the transport dynamics of TME. We show that the model can be a useful tool to investigate targetted features of TME with controls of ECM compartments as well as the driver mutations. Although the present iT-MOC model successfully recapitulates the intratumor heterogeneity in a controlled manner, incorporation of stromal cells, including CAFs and immune cells, in the model warrants further investigation. CAFs play a central role in PDAC progression, drug resistance through cancer cell–CAF interaction, and drug transport barriers.20,82 iT-MOC could be further applied for patient-specific therapeutic strategies by capturing and recapitulating targeted features of TME.
Supplementary Material
Acknowledgements
This work is partially supported by grants from National Institutes of Health (U01 HL143403, UL1 TR002529 to BH, and R01 CA211098, R01 CA124586 to SFK), a Challenge Award from the Purdue University Center for Cancer Research (P30 CA023168), and the Walther Embedding Program in Physical Sciences in Oncology. The authors thank for the assistance from Gene Editing Core directed by Dr. Wen-Hung Wang, Bindley Biosciences, Purdue University. The authors are also very grateful to Dr. Murray Korc for providing two KIC cell lines.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
Electronic supplementary information (ESI) available. See DOI: 10.1039/d0lc00707b
Notes and references
- 1.Siegel RL, Miller KD and Jemal A, Ca-Cancer J. Clin, 2017, 67, 7–30. [DOI] [PubMed] [Google Scholar]
- 2.Kleeff J, Korc M, Apte M, La Vecchia C, Johnson CD, Biankin AV, Neale RE, Tempero M, Tuveson DA, Hruban RH and Neoptolemos JP, Nat. Rev. Dis. Primers, 2016, 2, 16022. [DOI] [PubMed] [Google Scholar]
- 3.Erkan M, Hausmann S, Michalski CW, Fingerle AA, Dobritz M, Kleeff J and Friess H, Nat. Rev. Gastroenterol. Hepatol, 2012, 9, 454–467. [DOI] [PubMed] [Google Scholar]
- 4.Mahadevan D and Von Hoff DD, Mol. Cancer Ther, 2007, 6, 1186–1197. [DOI] [PubMed] [Google Scholar]
- 5.Samuel N and Hudson TJ, Nat. Rev. Gastroenterol. Hepatol, 2012, 9, 77. [DOI] [PubMed] [Google Scholar]
- 6.Sipos B, Möser S, Kalthoff H, Török V, Löhr M and Klöppel G, Virchows Arch., 2003, 442, 444–452. [DOI] [PubMed] [Google Scholar]
- 7.Campbell PJ, Yachida S, Mudie LJ, Stephens PJ, Pleasance ED, Stebbings LA, Morsberger LA, Latimer C, McLaren S and Lin M-L, Nature, 2010, 467, 1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Almendro V, Marusyk A and Polyak K, Annu. Rev. Pathol.: Mech. Dis, 2013, 8, 277–302. [DOI] [PubMed] [Google Scholar]
- 9.Burrell RA, McGranahan N, Bartek J and Swanton C, Nature, 2013, 501, 338. [DOI] [PubMed] [Google Scholar]
- 10.Fisher R, Pusztai L and Swanton C, Br. J. Cancer, 2013, 108, 479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marusyk A, Almendro V and Polyak K, Nat. Rev. Cancer, 2012, 12, 323. [DOI] [PubMed] [Google Scholar]
- 12.Patel AP, Tirosh I, Trombetta JJ, Shalek AK, Gillespie SM, Wakimoto H, Cahill DP, Nahed BV, Curry WT and Martuza RL, Science, 2014, 344, 1396–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fong EL, Harrington DA, Farach-Carson MC and Yu H, Biomaterials, 2016, 108, 197–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turner NC and Reis-Filho JS, Lancet Oncol., 2012, 13, e178–e185. [DOI] [PubMed] [Google Scholar]
- 15.Burrell RA and Swanton C, Mol. Oncol, 2014, 8, 1095–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bailey P, Chang DK, Nones K, Johns AL, Patch A-M, Gingras M-C, Miller DK, Christ AN, Bruxner TJC, Quinn MC, Nourse C, Murtaugh LC, Harliwong I, Idrisoglu S, Manning S, Nourbakhsh E, Wani S, Fink L, Holmes O, Chin V, Anderson MJ, Kazakoff S, Leonard C, Newell F, Waddell N, Wood S, Xu Q, Wilson PJ, Cloonan N, Kassahn KS, Taylor D, Quek K, Robertson A, Pantano L, Mincarelli L, Sanchez LN, Evers L, Wu J, Pinese M, Cowley MJ, Jones MD, Colvin EK, Nagrial AM, Humphrey ES, Chantrill LA, Mawson A, Humphris J, Chou A, Pajic M, Scarlett CJ, Pinho AV, Giry-Laterriere M, Rooman I, Samra JS, Kench JG, Lovell JA, Merrett ND, Toon CW, Epari K, Nguyen NQ, Barbour A, Zeps N, Moran-Jones K, Jamieson NB, Graham JS, Duthie F, Oien K, Hair J, Grützmann R, Maitra A, Iacobuzio-Donahue CA, Wolfgang CL, Morgan RA, Lawlor RT, Corbo V, Bassi C, Rusev B, Capelli P, Salvia R, Tortora G, Mukhopadhyay D, Petersen GM, Munzy DM, Fisher WE, Karim SA, Eshleman JR, Hruban RH, Pilarsky C, Morton JP, Sansom OJ, Scarpa A, Musgrove EA, Bailey U-MH, Hofmann O, Sutherland RL, Wheeler DA, Gill AJ, Gibbs RA, Pearson JV, Waddell N, Biankin AV and Grimmond SM, Nature, 2016, 531, 47–52. [DOI] [PubMed] [Google Scholar]
- 17.Han B, Qu C, Park K, Konieczny SF and Korc M, Cancer Lett., 2016, 380, 319–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan-Seng-Yue M, Kim JC, Wilson GW, Ng K, Figueroa EF, O'Kane GM, Connor AA, Denroche RE, Grant RC and McLeod J, Nat. Genet, 2020, 52, 231–240. [DOI] [PubMed] [Google Scholar]
- 19.Monteiro L, Da Silva L, Lipinski B, Fauvet F, Vigneron A, Puisieux A and Martinez P, iScience, 2020, 23, 101061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ligorio M, Sil S, Malagon-Lopez J, Nieman LT, Misale S, Di Pilato M, Ebright RY, Karabacak MN, Kulkarni AS, Liu A, Vincent Jordan N, Franses JW, Philipp J, Kreuzer J, Desai N, Arora KS, Rajurkar M, Horwitz E, Neyaz A, Tai E, Magnus NKC, Vo KD, Yashaswini CN, Marangoni F, Boukhali M, Fatherree JP, Damon LJ, Xega K, Desai R, Choz M, Bersani F, Langenbucher A, Thapar V, Morris R, Wellner UF, Schilling O, Lawrence MS, Liss AS, Rivera MN, Deshpande V, Benes CH, Maheswaran S, Haber DA, Fernandez-Del-Castillo C, Ferrone CR, Haas W, Aryee MJ and Ting DT, Cell, 2019, 178, 160–175.e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Queiroz KCS, Shi K, Duitman J, Aberson HL, Wilmink JW, van Noesel CJM, Richel DJ and Spek CA, Int.J. Cancer, 2014, 135, 2294–2304. [DOI] [PubMed] [Google Scholar]
- 22.Schober M, Jesenofsky R, Faissner R, Weidenauer C, Hagmann W, Michl P, Heuchel RL, Haas SL and Löhr JM, Cancers, 2014, 6, 2137–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Makohon-Moore A and Iacobuzio-Donahue CA, Nat. Rev. Cancer, 2016, 16, 553–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim HN, Habbit NL, Su C-Y, Choi N, Ahn EH, Lipke EA and Kim D-H, Adv. Funct. Mater, 2019, 29, 1807553. [Google Scholar]
- 25.Moon H.-r. and Han B, in Biomaterials for Cancer Therapeutics, ed. Park K, Woodhead Publishing, 2nd edn, 2020, pp. 423–443. [Google Scholar]
- 26.Pampaloni F, Reynaud EG and Stelzer EHK, Nat. Rev. Mol. Cell Biol, 2007, 8, 839–845. [DOI] [PubMed] [Google Scholar]
- 27.Han B, Qu C, Park K, Konieczny SF and Korc M, Cancer Lett., 2016, 380, 319–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Christopher CD, Kathleen ED and Sunil RH, Gastroenterology, 2016, 150, 1545–1557.e1542.27072672 [Google Scholar]
- 29.Grantab R, Sivananthan S and Tannock IF, Cancer Res., 2006, 66, 1033–1039. [DOI] [PubMed] [Google Scholar]
- 30.Kern SE, Med. Clin. North Am, 2000, 84, 691–695, xi. [DOI] [PubMed] [Google Scholar]
- 31.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D, Frese KK, Denicola G, Feig C, Combs C, Winter SP, Ireland-Zecchini H, Reichelt S, Howat WJ, Chang A, Dhara M, Wang L, Ruckert F, Grutzmann R, Pilarsky C, Izeradjene K, Hingorani SR, Huang P, Davies SE, Plunkett W, Egorin M, Hruban RH, Whitebread N, McGovern K, Adams J, Iacobuzio-Donahue C, Griffiths J and Tuveson DA, Science, 2009, 324, 1457–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Provenzano PP and Hingorani SR, Br. J. Cancer, 2013, 108, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stromnes IM, DelGiorno KE, Greenberg PD and Hingorani SR, Carcinogenesis, 2014, 35, 1451–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ozcelikkale A, Moon H.-r., Linnes M and Han B, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol, 2017, 9, e1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ozcelikkale A, Shin K, Noe-Kim V, Elzey BD, Dong Z, Zhang J-T, Kim K, Kwon IC, Park K and Han B, J. Controlled Release, 2017, 266, 129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cao X, Ashfaq R, Cheng F, Maharjan S, Li J, Ying G, Hassan S, Xiao H, Yue K and Zhang YS, Adv. Funct. Mater, 2019, 29, 1807173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loessner D, Stok KS, Lutolf MP, Hutmacher DW, Clements JA and Rizzi SC, Biomaterials, 2010, 31, 8494–8506. [DOI] [PubMed] [Google Scholar]
- 38.Weigelt B and Bissell MJ, Semin. Cancer Biol, 2008, 18, 311–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jamal-Hanjani M, Quezada SA, Larkin J and Swanton C, Clin. Cancer Res, 2015, 21, 1258–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deer EL, Gonzalez-Hernandez J, Coursen JD, Shea JE, Ngatia J, Scaife CL, Firpo MA and Mulvihill SJ, Pancreas, 2010, 39, 425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mertz DR, Ahmed T and Takayama S, Lab Chip, 2018, 18, 2378–2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rothbauer M, Zirath H and Ertl P, Lab Chip, 2018, 18, 249–270. [DOI] [PubMed] [Google Scholar]
- 43.Song C, Zhang Y, Li C, Chen G, Kang X and Wang Q, Adv. Funct. Mater, 2016, 26, 4192–4200. [Google Scholar]
- 44.Moraes C, Mehta G, Lesher-Perez SC and Takayama S, Ann. Biomed. Eng, 2012, 40, 1211–1227. [DOI] [PubMed] [Google Scholar]
- 45.Chen L-J, Ito S, Kai H, Nagamine K, Nagai N, Nishizawa M, Abe T and Kaji H, Sci. Rep, 2017, 7, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim HJ, Lee J, Choi J-H, Bahinski A and Ingber DE, J. Visualized Exp, 2016, e54344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen Y-C, Jung S, Zhang Z, Wicha MS and Yoon E, Integr. Biol, 2019, 11, 353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nguyen D-HT, Lee E, Alimperti S, Norgard RJ, Wong A, Lee JJ-K, Eyckmans J, Stanger BZ and Chen CS, Sci. Adv, 2019, 5, eaav6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sobrino A, Phan DT, Datta R, Wang X, Hachey SJ, Romero-Löpez M, Gratton E, Lee AP, George SC and Hughes CC, Sci. Rep, 2016, 6, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ying L, Zhu Z, Xu Z, He T, Li E, Guo Z, Liu F, Jiang C and Wang Q, PLoS One, 2015, 10, e0129593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sontheimer-Phelps A, Hassell BA and Ingber DE, Nat. Rev. Cancer, 2019, 19, 65–81. [DOI] [PubMed] [Google Scholar]
- 52.Ko J, Ahn J, Kim S, Lee Y, Lee J, Park D and Jeon NL, Lab Chip, 2019, 19, 2822–2833. [DOI] [PubMed] [Google Scholar]
- 53.Rübben A and Araujo A, J. Transl. Med, 2017, 15, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Felsenstein M, Hruban RH and Wood LD, Adv. Anat. Pathol, 2018, 25, 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vamvakidou AP, Mondrinos MJ, Petushi SP, Garcia FU, Lelkes PI and Tozeren A, Biomol J. Screening, 2007, 12, 13–20. [DOI] [PubMed] [Google Scholar]
- 56.Charoen KM, Fallica B, Colson YL, Zaman MH and Grinstaff MW, Biomaterials, 2014, 35, 2264–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Singh M, Lima A, Molina R, Hamilton P, Clermont AC, Devasthali V, Thompson JD, Cheng JH, Bou Reslan H, Ho CCK, Cao TC, Lee CV, Nannini MA, Fuh G, Carano RAD, Koeppen H, Yu RX, Forrest WF, Plowman GD and Johnson L, Nat. Biotechnol, 2010, 28, 585–593. [DOI] [PubMed] [Google Scholar]
- 58.Lee SH, Hu W, Matulay JT, Silva MV, Owczarek TB, Kim K, Chua CW, Barlow LJ, Kandoth C and Williams AB, Cell, 2018, 173, 515–528.e517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gudbergsson JM, Kostrikov S, Johnsen KB, Fliedner FP, Stolberg CB, Humle N, Hansen AE, Kristensen BW, Christiansen G and Kjær A, Exp. Cell Res, 2019, 379, 73–82. [DOI] [PubMed] [Google Scholar]
- 60.Sempere LF, Gunn JR and Korc M, Cancer Biol. Ther, 2011, 12, 198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Whipple CA, Young AL and Korc M, Oncogene, 2011, 31, 2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bradney MJ, Venis SM, Yang Y, Konieczny SF and Han B, Small, 2020, 1905500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kwak B, Ozcelikkale A, Shin CS, Park K and Han B, J. Controlled Release, 2014, 194, 157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shin K, Klosterhoff BS and Han B, Mol. Pharmaceutics, 2016, 13, 2214–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kihlmark M, Imreh G and Hallberg E, J. Cell Sci, 2001, 114, 3643–3653. [DOI] [PubMed] [Google Scholar]
- 66.Jones S, Zhang X, Parsons DW, Lin JCH, Leary RJ, Angenendt P, Mankoo P, Carter H, Kamiyama H, Jimeno A, Hong SM, Fu B, Lin MT, Calhoun ES, Kamiyama M, Walter K, Nikolskaya T, Nikolsky Y, Hartigan J, Smith DR, Hidalgo M, Leach SD, Klein AP, Jaffee EM, Goggins M, Maitra A, Iacobuzio-Donahue C, Eshleman JR, Kern SE, Hruban RH, Karchin R, Papadopoulos N, Parmigiani G, Vogelstein B, Velculescu VE and Kinzler KW, Science, 2008, 321, 1801–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Heldin C-H, Rubin K, Pietras K and Östman A, Nat. Rev. Cancer, 2004, 4, 806–813. [DOI] [PubMed] [Google Scholar]
- 68.Wiig H and Swartz MA, Physiol. Rev, 2012, 92, 1005–1060. [DOI] [PubMed] [Google Scholar]
- 69.Arumugam T, Ramachandran V, Fournier KF, Wang H, Marquis L, Abbruzzese JL, Gallick GE, Logsdon CD, McConkey DJ and Choi W, Cancer Res., 2009, 69, 5820–5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li Y, VandenBoom TG, Kong D, Wang Z, Ali S, Philip PA and Sarkar FH, Cancer Res., 2009, 69, 6704–6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Smith BN and Bhowmick NA, J. Clin. Med, 2016, 5, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sarkar FH, Li Y, Wang Z and Kong D, Minerva Chir., 2009, 64, 489–500. [PMC free article] [PubMed] [Google Scholar]
- 73.Elaskalani O, Razak NBA, Falasca M and Metharom P, World J. Gastrointest. Oncol, 2017, 9, 37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zheng X, Carstens JL, Kim J, Scheible M, Kaye J, Sugimoto H, Wu C-C, LeBleu VS and Kalluri R, Nature, 2015, 527, 525–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jeanes A, Gottardi CJ and Yap AS, Oncogene, 2008, 27, 6920–6929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zeisberg M and Neilson EG, J. Clin. Invest, 2009, 119, 1429–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Waters AM and Der CJ, Cold Spring Harbor Perspect. Med, 2018, 8, a031435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bailey J, Hendley A, Lafaro K, Pruski M, Jones N, Alsina J, Younes M, Maitra A, McAllister F and Iacobuzio-Donahue C, Oncogene, 2016, 35, 4282–4288. [DOI] [PubMed] [Google Scholar]
- 79.Dagogo-Jack I and Shaw AT, Nat. Rev. Clin. Oncol, 2018, 15, 81. [DOI] [PubMed] [Google Scholar]
- 80.Porter RL, Magnus NKC, Thapar V, Morris R, Szabolcs A, Neyaz A, Kulkarni AS, Tai E, Chougule A, Hillis A, Golczer G, Guo H, Yamada T, Kurokawa T, Yashaswini C, Ligorio M, Vo KD, Nieman L, Liss AS, Deshpande V, Lawrence MS, Maheswaran S, Fernandez-Del Castillo C, Hong TS, Ryan DP, O'Dwyer PJ, Drebin JA, Ferrone CR, Haber DA and Ting DT, Proc. Natl. Acad. Sci. U. S. A, 2019, 116, 26835–26845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang Z, Li Y, Kong D, Banerjee S, Ahmad A, Azmi AS, Ali S, Abbruzzese JL, Gallick GE and Sarkar FH, Cancer Res., 2009, 69, 2400–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Öhlund D, Elyada E and Tuveson D, J. Exp. Med, 2014, 211, 1503–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.