Abstract
The management of hyperglycemia in patients admitted to hospital is mainly based on insulin therapy. However, the positive and rapid effects of sodium-glucose cotransporter 2 inhibitors (SGLT2i) on cardiorenal outcomes raises the possibility that they might confer benefits to hospitalized patients. In recent, well designed, randomized trials (SOLOIST-WHF and EMPULSE) recruiting inpatients with heart failure (HF), SGLT2i demonstrated the potential to improve survival and quality of life and reduce the number of HF events, time to first HF event, hospitalizations, and urgent visits for HF compared with placebo. They were also well tolerated, whereas incidence of diabetic ketoacidosis was low. In EMBODY, empagliflozin was shown to be protective against the deleterious effects of cardiac injury in patients with acute myocardial infarction. In DARE-19, the administration of dapagliflozin to inpatients with cardiometabolic risk factors and COVID-19 was based on the hypothesis that the anti-inflammatory properties of SGLT2i could alleviate organ damage. Although the findings did not reach statistical significance, the efficacy and safety profiles of the drug were encouraging. These promising findings in the field of cardiometabolic medicine set the stage for future research to explore whether the benefits of gliflozins can expand to inpatients with non-cardiometabolic disorders, including sepsis, cirrhotic ascites, and malignancies. The concept of inpatient use of SGLT2i has evolved greatly over the past few years. The latest evidence suggests that SGLT2i may be effective and safe in the hospital setting, provided patients are carefully selected and closely monitored. Real-world data will prove whether present hope about inpatient use of gliflozins will transform into future confidence.
Key Points
| High-quality evidence suggests that sodium-glucose cotransporter 2 inhibitors (SGLT2i) can improve outcomes in hospitalized individuals with heart failure. |
| Proper selection and close follow-up of patients are needed to mitigate risks. |
| The inpatient use of SGLT2i in non-cardiometabolic disorders deserves further evaluation. |
Introduction
Back in 2020, we speculated on the risk–benefit ratio of administering sodium-glucose cotransporter 2 inhibitors (SGLT2i) to hospitalized patients with diabetes mellitus and concluded that “potential benefits deriving from the use of SGLT2i in the inpatient setting cannot mitigate possible risks, at least until robust evidence on their efficacy in hospitalized individuals become available” [1].
In general, the management of hyperglycemia in patients admitted to hospital is based on insulin therapy. Oral agents are withheld during hospitalization due to lower hypoglycemic potency compared with insulin, potential interactions with concomitantly administered drugs, and several safety concerns related to altered pharmacokinetics in cases of renal or liver dysfunction [2]. However, gliflozins constitute a unique paradigm of antidiabetic agents. Although initially developed as drugs for the management of hyperglycemia, they soon became known to significantly improve outcomes related to heart failure (HF) and chronic kidney disease (CKD), leading to their licensed use in patients with both entities, regardless of diabetes status. In this context, the wide spectrum of the off-target effects of SGLT2i prompted their evaluation as potential therapeutic options for nonmetabolic disorders, including infections, cancer, and cognitive impairment [3, 4].
The rationale behind the use of SGLT2i in hospitals is based on the practical dosing scheme, the low risk of hypoglycemia (if given as antihyperglycemic agents), but mainly to their potential to alleviate the deleterious effects of cardiorenal injury. On the other hand, safety issues with respect to the risk of diabetic ketoacidosis (DKA), hypovolemia, and genital infections need careful consideration. At the time of our previous review, relevant data on the administration of gliflozins in the acute phase of any disease entity were exclusively from animal studies. This article aims to discuss updated evidence on the use of SGLT2i in the inpatient setting, in light of recently published human studies.
Recent Evidence
Sodium-Glucose Cotransporter 2 Inhibitors (SGLT2i) in Hospitalized Patients with COVID-19
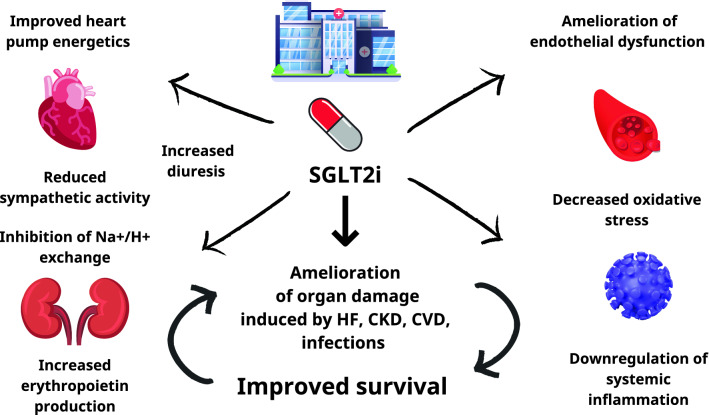
Important extra-glycemic properties of SGLT2i that justify their use in acute disease include attenuation of endothelial dysfunction, improved heart pump energetics, inhibition of Na+/H+ exchanger in the kidney and the heart, downregulation of sympathetic activity and oxidative stress, and increased erythropoietin production (Fig. 1) [5, 6]. Some of the mechanisms involved in organ failure during infection with SARS-CoV-2 have been suggested to be common with those implicated in the development of diabetic complications [7]. SGLT2i exert systemic anti-inflammatory actions that could prove useful in cases of overactivation of the immune system in the context of viral infections, thus mitigating organ damage. DARE-19 (Dapagliflozin in Respiratory Failure in Patients With COVID-19) was a trial of 1250 hospitalized patients with COVID-19 and cardiometabolic risk factors (i.e., hypertension, type 2 diabetes [T2D], atherosclerotic cardiovascular disease [CVD], HF, and CKD), who were randomized 1:1 to either placebo or dapagliflozin [8]. Compared with placebo, numerically fewer participants in the dapagliflozin arm experienced the composite primary outcome of time to new or worsened organ dysfunction or death (13.8 vs 11.2%, hazard ratio [HR] 0.80, 95% confidence interval [CI] 0.58–1.10; p = 0.17). Although the findings failed to reach statistical significance, it should be considered that the trial was short (30 days) and took place during an evolving pandemic, while numbers recruited were relatively small. Moreover, the continuous improvement in the care of patients with COVID-19 led to a respective falling trend of mortality rates, which made the prediction of the number of events required for statistical certainty challenging [9].
Fig. 1.
Potential mechanisms that mediate the benefits of SGLT2i in hospitalized patients. CKD chronic kidney disease, CVD cardiovascular disease, HF heart failure, SGLT2i sodium glucose co transporter 2 inhibitors
Prior to DARE-19, the risk of DKA was a major argument against the use of SGLT2i in the acute setting. Indeed, a shift in substrate utilization from carbohydrate to fat oxidation along with an increase in glucagon levels, dehydration and insulinopenia can lead patients treated with these agents to develop euglycemic DKA, especially in the context of precipitating factors that are often seen in inpatients, including severe infections, prolonged starvation, or surgical interventions [1, 10, 11]. In DARE-19, monitoring of acid–base balance and renal function was required daily as per the study’s protocol, whereas history of DKA and critical illness were key exclusion criteria. Among the 625 patients randomized to the dapagliflozin group, there were only two cases of DKA. Both occurred in people with diabetes and resolved quickly with appropriate treatment. Therefore, a strong message of this trial is that SGLT2i can be used safely in the hospital, provided candidate recipients are carefully selected and patients are closely monitored [9].
SGLT2i in Hospitalized Patients with Heart Failure
SGLT2i can affect the natural history of HF by reducing hospitalization rates, CVD, and all-cause mortality and by improving renal outcomes and quality of life [12, 13]. While some of the effects of the class seem to be consistent across the full spectrum of the disease, SGLT2i have been shown to reduce the risk of cardiac death only in people with HF with reduced ejection fraction (HFrEF) [14]. In contrast, among patients with HF with preserved ejection fraction, the benefits are mainly driven by a reduction in rehospitalization rates.
In the SOLOIST-WHF (Effect of Sotagliflozin on Cardiovascular Events in Patients With Type 2 Diabetes Post Worsening Heart Failure) trial, 1222 patients with T2D requiring hospitalization due to exacerbation of HF were randomly assigned to either sotagliflozin or placebo [15]. This was the first study in which a SGLT2i was initiated before hospital discharge in a significant percentage of study participants (48.8%). After a median follow-up of 9 months, the use of sotagliflozin resulted in a significant reduction in the composite primary outcome of cardiovascular death and hospitalizations and urgent visits for HF (number of events per 100 patients-years was 51.0 and 76.3 for sotagliflozin and placebo, respectively; HR 0.67, 95% CI 0.52–0.85; p < 0.001). The importance of starting SGLT2i during hospitalization is highlighted by the fact that the risk reduction appeared evident 28 days after first administration of the drug [16]. These data encourage an ‘earlier, better’ approach to the introduction of gliflozins into the pharmaceutical armory against HF, which clearly represents a paradigm shift in the management of the disorder [17].
In the early years of the use of SGLT2i in daily practice, there were several concerns over the safety of their co-administration with conventional diuretics, which are considered the cornerstone of the management of inpatients with HF. In SOLOIST-WHF, the vast majority of participants (95.4% in the sotagliflozin group) received loop diuretics and a large percentage of them (66.3%) were treated with mineralocorticoid receptor agonists (MRA). However, the incidence of hypotension and acute renal injury was similar when comparing the intervention and placebo groups (6.0 vs 4.6% and 4.1 vs 4.4%, respectively). Mordi et al. have recently reported that empagliflozin significantly increased 24-h urine volume without amplifying urinary sodium excretion when used in parallel with loop diuretics [18]. These data suggest that the diuretic effects of SGLT2i are primarily driven by fluid clearance from the interstitial space, rather than the circulating volume, which explains the low risk of dehydration with SGLT2i therapy [19]. Furthermore, Shirakabe et al. showed that among people with diabetes and compensated HF, co-administration of loop diuretics and empagliflozin led to a decrease in the dose of the latter [20]. Also, empagliflozin improved the production of erythropoietin, which represents a potential protective mechanism against the development of renal injury induced by hypovolemia and dehydration.
The administration of MRA in patients with acute decompensated HF is often restricted because of the risk of hyperkalemia. A meta-analysis incorporating data from 24,246 individuals with T2D showed a 28% lower risk of hyperkalemia in patients receiving SGLT2i compared with those receiving placebo [21]. These results were recently replicated by Neuen et al. [22] showing that the use of SGLT2i among 49,875 people with T2D and established CVD or at high cardiovascular risk was associated with a lower risk of serious hyperkalemia (HR 0.84; 95% CI 0.76–0.93), without increasing the risk of hypokalemia. In the EMPEROR-preserved (Empagliflozin Outcome Trial in Patients with Chronic Heart Failure with Preserved Ejection Fraction) population, empagliflozin reduced the primary outcome and hyperkalemia and these benefits were consistent regardless of background treatment with MRA [23]. Additionally, combined therapy of SGLT2i with the MRA finerenone appears to result in greater reductions in urine albumin excretion among patients with diabetic kidney disease compared with those achieved with finerenone alone [24]. Collectively, these data suggest an additive effect of SGLT2i and diuretics with an acceptable safety profile.
Another key study assessing inpatient initiation of SGLT2i is EMPULSE. In this trial, 530 patients hospitalized with acute HF (47% had diabetes) were randomized to receive empagliflozin 10 mg or placebo in addition to standard treatment [25]. During 90 days of follow-up, more patients in the active treatment group experienced clinical benefit (defined as a hierarchical composite of death, number of HF events and time to first HF event, or change from baseline in the Kansas City Cardiomyopathy Questionnaire Total Symptom Score) compared with placebo (stratified win ratio 1.36, 95% CI 1.09–1.68; p = 0.0054). Comparing placebo and SGLT2i groups, renal complications were numerically higher in the former group, (acute kidney injury [7.2 vs 3.8%], renal impairment [4.2 vs 3.5%], and urinary tract infections [6.4 vs 4.2%]). Interestingly, no cases of DKA were reported in either study arm, although it should be noted that less than half of the study participants had diabetes.
It is important to emphasize that the magnitude of glycosuria promoted by SGLT2i depends on plasma glucose concentrations [26]. Therefore, genital mycotic infections, which are the most common adverse event of the class, are not expected to be an issue in individuals without diabetes. A recent meta-analysis that pooled data from five high-quality randomized controlled trials (RCTs) reassuringly demonstrated a similar risk of urinary tract infections (including severe ones, such as urosepsis) among patients with HF assigned to SGLT2i compared with those assigned to placebo (relative risk 1.09, 95% CI 0.94–1.26; p = 0.24) [27]. From a pathophysiological perspective, the development of DKA requires the presence of absolute or relative insulinopenia. Thus, it is unlikely that patients without diabetes, and having an adequate endogenous insulin production, develop this worrying complication of SGLT2i therapy.
SGLT2i in Hospitalized Patients with Acute Cardiovascular Episodes
Several animal studies indicate that SGLT2i can attenuate the harmful effects of acute myocardial ischemia [28–31]. However, relevant human studies were not available until recently. In the EMBODY trial, 105 patients with acute myocardial infarction (MI) were randomized to receive empagliflozin or placebo [32]. Treatment with SGLT2i resulted in a significant improvement in heart rate turbulence, suggesting a protective effect of the class against cardiac sympathetic and parasympathetic abnormalities induced by acute myocardial injury. EMBODY was another study in which empagliflozin was well tolerated and no safety concerns emerged during the trial, but it should be noted that this was a small study. These findings are in line with a post hoc analysis of DAPA-HF (Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure), showing that dapagliflozin significantly reduced the risk of the composite outcome of sudden death, resuscitated cardiac arrest, and incidence of ventricular arrhythmias in a high-risk population with HF [33]. Together, these data indicate that SGLT2i might have a place in the management of acute cardiovascular episodes with potential to reduce mortality and improve outcomes [34]. The results of the ongoing trial DAPA-MI (Dapagliflozin Effects on Cardiovascular Events in Patients With an Acute Heart Attack) are expected to shed more light on this issue. In this multicenter, parallel group, event-driven, registry-based RCT that recruits patients without diabetes who present with MI, the effect of dapagliflozin versus placebo in terms of preventing hospitalization for HF or cardiovascular death will be evaluated [35].
The Clinician’s Perspective
Despite the encouraging results from the world of clinical trials, translating trial data into daily practice remains a challenge. SGLT2i have been tested as adjunct therapy in patients with inadequately controlled type 1 diabetes (T1D) and proved to be effective in reducing glycated hemoglobin, body weight, and insulin dose [36, 37]. In these studies, the safety profile supported a favorable risk–benefit balance. However, real-world evidence suggested that DKA remains a concern with the use of SGLT2i in autoimmune diabetes [38]. The recent withdrawal of dapagliflozin as an adjunctive therapy in T1D added to the concerns, although this was a commercial decision and not done for safety reasons [39].
There are always concerns that RCTs do not necessarily represent real-world practice given the selection process and the closer monitoring. In this context, EMBODY was conducted only in the Japanese population, and the percentage of Black participants in EMPULSE was relatively low (7.9% in the empagliflozin group). This could be important considering available data showing that African Americans with T2D are more likely to develop DKA compared with other ethnic groups [40]. DKA is a potentially fatal complication that can be experienced by a certain number of patients, especially when SGLT2i are introduced during hospitalization. Thus, physicians should ensure that blood pH, ketones, and dietary intake are monitored regularly. In EMPULSE, the requirement for patient stabilization might have excluded older and frailer patients [25]. Furthermore, many RCTs exclude patients who are older than 75 years, have a body mass index < 20 kg/m2 and an estimated glomerular filtration rate (eGFR) < 20 mL/min/1.73m2, which are all characteristics that describe the patient population that is difficult to treat with SGLT2i. However, it can be argued that good clinical practice dictates that selection of patients for various therapies is done with care, while monitoring needs to be intensified in the presence of known side effects, particularly at the initiation phase, to ensure a high benefit/risk ratio.
Another important aspect to consider is the chronology of the inpatient phases in various clinical entities. In the case of people with COVID-19 and diabetes, a more severe metabolic decompensation is observed during the first days of hospital stay, when the risk of DKA may be more pronounced [41]. According to the protocol of the studies that recruited patients with HF, SGLT2i was started after stabilization, which means that people with severe disease may not have been adequately studied. Together, these data indicate that the use of SGLT2i should probably be considered in the latter, safer phases of an inpatient stay.
It could be said that the renal effects of the inpatient use of SGLT2i should be studied in more detail, considering that so far most studies have focused on safety rather than potential benefits. In DARE-19 and EMPULSE, a composite renal endpoint consisting of a decrease in the eGFR and the initiation of renal replacement therapy (with the addition of all-cause death in DARE-19) was a secondary outcome. In both trials, the start of SGLT2i in the hospital proved to be safe in terms of renal function. However, it has been suggested that stricter criteria, including biomarkers of glomerular and tubular injury, should be implemented in upcoming studies to better define renal risk [42]. For example, the definition of kidney damage in DARE-19 (doubling of serum creatinine compared with baseline during index hospitalization or serious adverse event, with the preferred term of acute kidney injury, after discharge, initiation of renal replacement therapy, or death from any cause over 30 days) excludes stage 1 kidney injury or even worse stages that could only present as oliguria. Taking into account the hemodynamically driven reduction in eGFR and, by extension, in glomerular perfusion observed during the first weeks of therapy with gliflozins, starting such an agent in hydrated or hypovolemic patients could have adverse consequences on renal function and survival.
Previous research has recognized the magnitude of symptoms, low systolic blood pressure, impaired renal function, and elevated troponin levels at admission as factors correlating with mortality rates among HF inpatients [43]. Specific patient features have been related to a greater risk for the development of DKA during hospitalization, including race and age [44]. In patients with diabetes and COVID-19, insulin treatment prior to admission, duration of diabetes, and obesity are important predictors of mortality during hospitalization [45]. On the other hand, recurrent hospitalizations for HF increase cardiovascular mortality by 2.65–5.95 times (depending on the number of admissions) [46], suggesting that there is still room for improvement in the treatment of these patients. Although high-risk individuals may be prone to adverse drug effects, they may also be more likely to benefit from modern treatments, especially where conventional therapies fail or prove insufficient. In this context, it has recently been proposed that initiation of the four basic classes of drugs (SGLT2i, MRA, angiotensin-converting enzyme inhibitors, and angiotensin receptor-neprilysin inhibitors) before discharge in people with HFrEF could tackle clinical inertia and address multiple pathophysiological abnormalities early in the course of the disease, leading to better survival rates in the long run [47]. However, in an era where precision medicine concepts are gaining ground, it is important to identify patients with specific characteristics who could benefit the most from the in-hospital use of gliflozins and other drugs for HF.
Conclusion
During the past 2 years, the concept of the in-hospital use of SGLT2i evolved through evidence from well-designed randomized trials demonstrating that these drugs might be both effective and well tolerated to start in the inpatient setting, provided that patients are properly selected and closely monitored. The promising findings in the field of cardiometabolic medicine set the stage for future research to explore whether the benefits of gliflozins can expand to inpatients with non-cardiometabolic disorders. Considering encouraging preclinical data [48, 49] and the wealth of mechanisms of action of these drugs, these could include, but are not restricted to, sepsis, cirrhotic ascites, and malignancies.
Additional trials are needed to fully characterize hospitalized patients who would benefit the most from such therapies while keeping risk of complications to a minimum. It should also be noted that the management and follow-up of patients in RCTs may differ from the daily clinical setting. Therefore, real-world data are much needed to prove whether the present hope about the inpatient use of gliflozins will transform into future confidence.
Declarations
Conflict of interest
TK has received honoraria for lectures from AstraZeneca, Boehringer Ingelheim, Pharmaserve Lilly, and Novo Nordisk, for advisory boards from Novo Nordisk, and has participated in sponsored studies by Eli-Lilly and Novo Nordisk; OGM has received speaker honoraria and educational grants from Sanofi, Eli Lilly, Boehringer Ingelheim and Novo Nordisk; RAA has received institutional research grants from Abbott, Bayer, Eli Lilly, NovoNordisk and Roche, and honoraria/education support/consultant from Abbott, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Menarini Pharmaceuticals, Merck Sharp & Dohme, NovoNordisk, and Takeda; XG-M has received honoraria for lectures/advisory boards from Astra-Zeneca, Boehringer-Ingelheim, Esteve, Lilly, Merck, Novo-Nordisk, and Sanofi; GD has received honoraria for lectures and financial support for research (through the University of Athens Medical School) from Abbott, Astra Zeneca, VIANEX, Pharmaserve Lilly, ELPEN, Galenica, MSD, Novartis, Novo Nordisk, and Sanofi; KK has received honoraria for lectures/advisory boards and research support from Astra Zeneca, Boehringer Ingelheim, Pharmaserve Lilly, Sanofi-Aventis, ELPEN, MSD, and Novo Nordisk; PZ declares no potential conflicts of interest that might be relevant to this work.
Funding
No external funds were used in the preparation of this manuscript.
Author contributions
TK reviewed the literature and drafted the first version of the manuscript. OGM, RAA, XG-M, PZ, GD, and KK reviewed the literature and edited the manuscript. All authors have read and approved the final version of the manuscript.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
References
- 1.Koufakis T, Mustafa OG, Ajjan RA, et al. The use of sodium-glucose co-transporter 2 inhibitors in the inpatient setting: is the risk worth taking? J Clin Pharm Ther. 2020;45:883–891. doi: 10.1111/jcpt.13107. [DOI] [PubMed] [Google Scholar]
- 2.Koufakis T, Mustafa OG, Zebekakis P, et al. Oral antidiabetes agents for the management of inpatient hyperglycaemia: so far, yet so close. Diabet Med. 2020;37:1418–1426. doi: 10.1111/dme.14329. [DOI] [PubMed] [Google Scholar]
- 3.Bonora BM, Avogaro A, Fadini GP. Extraglycemic effects of SGLT2 inhibitors: a review of the evidence. Diabetes Metab Syndr Obes. 2020;13:161–174. doi: 10.2147/DMSO.S233538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mone P, Lombardi A, Gambardella J, et al. Empagliflozin improves cognitive impairment in frail older adults with type 2 diabetes and heart failure with preserved ejection fraction. Diabetes Care. 2022 doi: 10.2337/dc21-2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koufakis T, Vas P, Maltese G, et al. Antiatherosclerotic effects of sodium-glucose cotransporter 2 inhibitors: an underrecognized piece of the big puzzle? J Clin Endocrinol Metab. 2022 doi: 10.1210/clinem/dgac116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marathias KP, Lambadiari VA, Markakis KP, et al. Competing effects of renin angiotensin system blockade and sodium-glucose cotransporter-2 inhibitors on erythropoietin secretion in diabetes. Am J Nephrol. 2020;51:349–356. doi: 10.1159/000507272. [DOI] [PubMed] [Google Scholar]
- 7.Koufakis T, Pavlidis AN, Metallidis S, et al. Sodium-glucose co-transporter 2 inhibitors in COVID-19: meeting at the crossroads between heart, diabetes and infectious diseases. Int J Clin Pharm. 2021;43:764–767. doi: 10.1007/s11096-021-01256-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kosiborod MN, Esterline R, Furtado RHM, et al. Dapagliflozin in patients with cardiometabolic risk factors hospitalised with COVID-19 (DARE-19): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2021;9:586–594. doi: 10.1016/S2213-8587(21)00180-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koufakis T, Maltese G, Metallidis S, et al. Looking deeper into the findings of DARE-19: failure or an open door to future success? Pharmacol Res. 2021;173:105872. doi: 10.1016/j.phrs.2021.105872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor SI, Blau JE, Rother KI. SGLT2 inhibitors may predispose to ketoacidosis. J Clin Endocrinol Metab. 2015;100:2849–2852. doi: 10.1210/jc.2015-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perry RJ, Rabin-Court A, Song JD, et al. Dehydration and insulinopenia are necessary and sufficient for euglycemic ketoacidosis in SGLT2 inhibitor-treated rats. Nat Commun. 2019;10:548. doi: 10.1038/s41467-019-08466-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zannad F, Ferreira JP, Pocock SJ, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet. 2020;396:819–829. doi: 10.1016/S0140-6736(20)31824-9. [DOI] [PubMed] [Google Scholar]
- 13.He Z, Yang L, Nie Y, et al. Effects of SGLT-2 inhibitors on health-related quality of life and exercise capacity in heart failure patients with reduced ejection fraction: a systematic review and meta-analysis. Int J Cardiol. 2021;345:83–88. doi: 10.1016/j.ijcard.2021.10.008. [DOI] [PubMed] [Google Scholar]
- 14.Zhao L, Guo W, Huang W, Wang L, Huang S. Benefit of sodium-glucose cotransporter-2 inhibitors on survival outcome is related to the type of heart failure: a meta-analysis. Diabetes Res Clin Pract. 2022;187:109871. doi: 10.1016/j.diabres.2022.109871. [DOI] [PubMed] [Google Scholar]
- 15.Bhatt DL, Szarek M, Steg PG, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. 2021;384:117–128. doi: 10.1056/NEJMoa2030183. [DOI] [PubMed] [Google Scholar]
- 16.Bhatt DL. Sotagliflozin in diabetes patients with recent worsening heart failure—SOLOIST-WHF. In: Presented at the American Heart Association 2020 virtual scientific sessions; November 13–17, 2020.
- 17.Koufakis T, Mustafa OG, Tsimihodimos V, et al. Insights into the results of sotagliflozin cardiovascular outcome trials: is dual inhibition the cherry on the cake of cardiorenal protection? Drugs. 2021;81:1365–1371. doi: 10.1007/s40265-021-01559-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mordi NA, Mordi IR, Singh JS, et al. Renal and cardiovascular effects of SGLT2 inhibition in combination with loop diuretics in patients with type 2 diabetes and chronic heart failure: the RECEDE-CHF trial. Circulation. 2020;142:1713–1724. doi: 10.1161/CIRCULATIONAHA.120.048739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bashier A, Khalifa AA, Abdelgadir EI, et al. Safety of sodium-glucose cotransporter 2 inhibitors (SGLT2-I) during the month of ramadan in muslim patients with type 2 diabetes. Oman Med J. 2018;33:104–110. doi: 10.5001/omj.2018.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shirakabe A, Matsushita M, Kiuchi K, et al. Empagliflozin administration can decrease the dose of loop diuretics and prevent the exacerbation of renal tubular injury in patients with compensated heart failure complicated by diabetes. Circ Rep. 2020;2:565–575. doi: 10.1253/circrep.CR-20-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Charlwood C, Chudasama J, Darling A, et al. Sodium glucose co-transporter 2 inhibitors do not increase the risk of hyperkaleamia in type 2 diabetes: a systematic review and meta-analysis. Diabetologia. 2021;64:S228. [Google Scholar]
- 22.Neuen BL, Oshima M, Agarwal R, et al. Sodium-glucose cotransporter 2 inhibitors and risk of hyperkalemia in people with type 2 diabetes: a meta-analysis of individual participant data from randomized controlled trials. Circulation. 2022 doi: 10.1161/circulationaha.121.057736. [DOI] [PubMed] [Google Scholar]
- 23.Ferreira JP, Butler J, Zannad F, et al. Mineralocorticoid receptor antagonists and empagliflozin in patients with heart failure and preserved ejection fraction. J Am Coll Cardiol. 2022;79:1129–1137. doi: 10.1016/j.jacc.2022.01.029. [DOI] [PubMed] [Google Scholar]
- 24.Bakris GL, Agarwal R, Anker SD, et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med. 2020;383:2219–2229. doi: 10.1056/NEJMoa2025845. [DOI] [PubMed] [Google Scholar]
- 25.Voors AA, Angermann CE, Teerlink JR, et al. The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: a multinational randomized trial. Nat Med. 2022;28:568–574. doi: 10.1038/s41591-021-01659-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.List JF, Whaley JM. Glucose dynamics and mechanistic implications of SGLT2 inhibitors in animals and humans. Kidney Int Suppl. 2011;79:S20–S27. doi: 10.1038/ki.2010.512. [DOI] [PubMed] [Google Scholar]
- 27.Borovac JA, Kurir T, Mustapic I, et al. SGLT2 inhibitors and the risk of urinary tract infections in patients with heart failure: a pooled analysis examining safety endpoints. Kardiol Pol. 2022;80:198–201. doi: 10.33963/KP.a2021.0172. [DOI] [PubMed] [Google Scholar]
- 28.Sayour AA, Korkmaz-Icöz S, Loganathan S, et al. Acute canagliflozin treatment protects against in vivo myocardial ischemia-reperfusion injury in non-diabetic male rats and enhances endothelium-dependent vasorelaxation. J Transl Med. 2019;17:127. doi: 10.1186/s12967-019-1881-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mizuno M, Kuno A, Yano T, et al. Empagliflozin normalizes the size and number of mitochondria and prevents reduction in mitochondrial size after myocardial infarction in diabetic hearts. Physiol Rep. 2018;6:e13741. doi: 10.14814/phy2.13741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee TM, Chang NC, Lin SZ. Dapagliflozin, a selective SGLT2 Inhibitor, attenuated cardiac fibrosis by regulating the macrophage polarization via STAT3 signaling in infarcted rat hearts. Free Radic Biol Med. 2017;104:298–310. doi: 10.1016/j.freeradbiomed.2017.01.035. [DOI] [PubMed] [Google Scholar]
- 31.Oshima H, Miki T, Kuno A, et al. Empagliflozin, an SGLT2 inhibitor, reduced the mortality rate after acute myocardial infarction with modification of cardiac metabolomes and antioxidants in diabetic rats. J Pharmacol Exp Ther. 2019;368:524–534. doi: 10.1124/jpet.118.253666. [DOI] [PubMed] [Google Scholar]
- 32.Shimizu W, Kubota Y, Hoshika Y, et al. Effects of empagliflozin versus placebo on cardiac sympathetic activity in acute myocardial infarction patients with type 2 diabetes mellitus: the EMBODY trial. Cardiovasc Diabetol. 2020;19(1):148. doi: 10.1186/s12933-020-01127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Curtain JP, Docherty KF, Jhund PS, et al. Effect of dapagliflozin on ventricular arrhythmias, resuscitated cardiac arrest, or sudden death in DAPA-HF. Eur Heart J. 2021;42:3727–3738. doi: 10.1093/eurheartj/ehab560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koufakis T, Giannakoulas G, Zebekakis P, et al. The effect of dapagliflozin on ventricular arrhythmias, cardiac arrest, or sudden death in people with heart failure: a tick in another box for sodium-glucose cotransporter 2 inhibitors. Expert Opin Pharmacother. 2022;23:321–325. doi: 10.1080/14656566.2021.2003329. [DOI] [PubMed] [Google Scholar]
- 35.Dapagliflozin Effects on Cardiovascular Events in Patients With an Acute Heart Attack (DAPA-MI). https://clinicaltrials.gov/ct2/show/NCT04564742?term=NCT04564742&draw=2&rank=1. Accessed 8 May 2022.
- 36.Mathieu C, Dandona P, Gillard P, et al. Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes (the DEPICT-2 Study): 24-week results from a randomized controlled trial. Diabetes Care. 2018;41:1938–1946. doi: 10.2337/dc18-0623. [DOI] [PubMed] [Google Scholar]
- 37.Dandona P, Mathieu C, Phillip M, et al. Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes (DEPICT-1): 24 week results from a multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol. 2017;5:864–876. doi: 10.1016/S2213-8587(17)30308-X. [DOI] [PubMed] [Google Scholar]
- 38.Palanca A, van Nes F, Pardo F, et al. Real-world evidence of efficacy and safety of SGLT2 inhibitors as adjunctive therapy in adults with type 1 diabetes: a european two-center experience. Diabetes Care. 2022;45:650–658. doi: 10.2337/dc21-1584. [DOI] [PubMed] [Google Scholar]
- 39.Mahase E. Type 1 diabetes drug was withdrawn because of a “commercial conflict of interest,” charity argues. BMJ. 2022;376:o373. doi: 10.1136/bmj.o373. [DOI] [PubMed] [Google Scholar]
- 40.Nyenwe E, Loganathan R, Blum S, et al. Admissions for diabetic ketoacidosis in ethnic minority groups in a city hospital. Metabolism. 2007;56:172–178. doi: 10.1016/j.metabol.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 41.Bornstein SR, Rubino F, Khunti K, et al. Practical recommendations for the management of diabetes in patients with COVID-19. Lancet Diabetes Endocrinol. 2020;8:546–550. doi: 10.1016/S2213-8587(20)30152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reis T, Ostermann M, Zarbock A, et al. Dapagliflozin in patients with COVID-19: mind the kidneys. Lancet Diabetes Endocrinol. 2022;10:97–98. doi: 10.1016/S2213-8587(21)00329-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carubelli V, Cotter G, Davison B, et al. In-hospital worsening heart failure in patients admitted for acute heart failure. Int J Cardiol. 2016;225:353–361. doi: 10.1016/j.ijcard.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 44.Ranasinghe U, Acharya S. Hospital-Acquired diabetic ketoacidosis (HADKA)—an analysis comparing two major Australian hospitals. Int J Diabetes Clin Res. 2021;8:140. [Google Scholar]
- 45.Agarwal S, Schechter C, Southern W, et al. Preadmission diabetes-specific risk factors for mortality in hospitalized patients with diabetes and coronavirus disease 2019. Diabetes Care. 2020;43:2339–2344. doi: 10.2337/dc20-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lahoz R, Fagan A, McSharry M, et al. Recurrent heart failure hospitalizations are associated with increased cardiovascular mortality in patients with heart failure in Clinical Practice Research Datalink. ESC Heart Fail. 2020;7:1688–1699. doi: 10.1002/ehf2.12727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Borovac JA. Early in-hospital initiation and optimization of comprehensive disease-modifying pharmacotherapy in patients with heart failure with reduced ejection fraction: a time for the paradigm shift. Expert Rev Cardiovasc Ther. 2022;20:91–94. doi: 10.1080/14779072.2022.2039626. [DOI] [PubMed] [Google Scholar]
- 48.Gao Y, Wei L, Zhang DD, et al. SGLT2 inhibitors: a new dawn for recurrent/refractory cirrhotic ascites. J Clin Transl Hepatol. 2021;9:795–797. doi: 10.14218/JCTH.2021.00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Du J, Gu J, Deng J, et al. The expression and survival significance of sodium glucose transporters in pancreatic cancer. BMC Cancer. 2022;22:116. doi: 10.1186/s12885-021-09060-4. [DOI] [PMC free article] [PubMed] [Google Scholar]