Abstract
The brain has many connections with various organs. Recent advances have demonstrated the existence of a bidirectional central nervous system (CNS) and intestinal tract, that is, the brain-gut axis. Although studies have suggested that the brain and lung can communicate with each other through many pathways, whether there is a brain–lung axis remains still unknown. Based on previous findings, we put forward a hypothesis: there is a cross-talk between the central nervous system and the lung via neuroanatomical pathway, endocrine pathway, immune pathway, metabolites and microorganism pathway, gas pathway, that is, the brain–lung axis. Beyond the regulation of the physiological state in the body, bi-directional communication between the lung and the brain is associated with a variety of disease states, including lung diseases and CNS diseases. Exploring the brain–lung axis not only helps us to understand the development of the disease from different aspects, but also provides an important target for treatment strategies.
Keywords: Brain, Lung, Brain–lung axis, Crosstalk
Introduction
The central nervous system (CNS) broadly communicates with other systems, such as intestines, lungs, kidneys and other organs. The brain-gut axis is a typical example, which refers to the two-way pathway communicated between the CNS and the intestinal nervous system, involving nerve, endocrine and immunity system (Cryan and Dinan 2012). Intestinal microorganisms play an essential role in the brain-gut axis. The products of various intestinal microorganisms can affect the development, maturation, disease and other aspects of CNS (Cryan and Dinan 2012; Needham et al. 2020).
Lung is a vital organ for humans. The blood flow of lung is abundant, which is essential for gas exchange. Brain is the most oxygen-consuming organ in human body. Therefore, diseases in lung can easily influence brain. For example, pneumonia aggravated brain injury after stroke (Johnston et al. 1998). Besides, diseases of brain can affect lung. In the first 36 h after admission, 15.6% of stroke patients developed acute lung injury (ALI), 7.8% developed pneumonia or bronchitis during hospitalization (Bai et al. 2017). Compared with healthy controls, forced expiratory volume in one second (FEV-1), forced vital capacity (FVC), peak expiratory flow (PEF) and chest offset were significantly decreased in stroke patients (Corlateanu et al. 2018).
Additionally, related clinical studies have demonstrated that chronic obstructive pulmonary disease (COPD) is associated with cerebrovascular disease by increasing white matter lesions (Lahousse et al. 2015). A Rotterdam study also indicated that patients with COPD had a higher risk of ischemic stroke and hemorrhagic stroke (Portegies et al. 2016). The above studies suggest a communication network between CNS and lung. Therefore, similarly to the brain-gut axis, we propose a conception of “brain–lung axis”. The followings are the evidences illustrating the possible pathway of brain–lung axis, which is helpful to deepen the understanding of pathophysiology between CNS and lung.
Main Text
The brain–lung axis consists of the following components: CNS, autonomic nervous system, hypothalamus–pituitary–adrenal (HPA) axis, immune system, metabolites, bacterial microbiota and gas.
Neuroanatomical Pathway
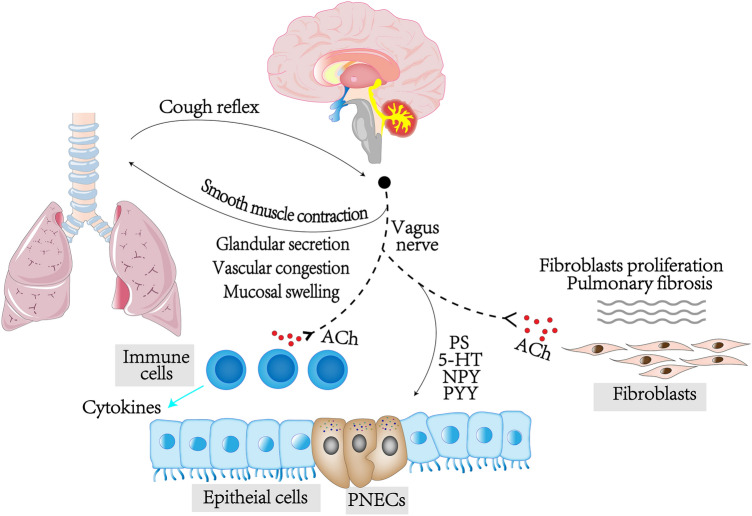
The bi-directional communication neuroanatomical pathway of the brain–lung axis is autonomic nervous system. It is mainly innervated by the parasympathetic nerve (vagus nerve) and the sympathetic nerve of the upper thoracic segment of the spinal cord, in which the vagus nerve plays a dominant role. The vagus nerve consists of 80% afferent fibers and 20% efferent fibers. It transmits information to the gastrointestinal tract, respiratory system and cardiovascular system (top–down) and receives feedback from the internal organs (bottom-up). When the vagus nerve is stimulated, it can release neurotransmitters acting on the receptors, resulting in bronchial smooth muscle contraction, glandular secretion, vascular congestion, and mucosal swelling. On the contrary, the activation of sympathetic nerve can relax bronchial smooth muscle, inhibit glandular secretion, contract small blood vessels, and subside mucosal swelling. The innervation of autonomic nerves is essential for the physiological activities of the lung.
The sensory nerve fibers of the vagus nerve conduct the feedback of lung, which in turn regulates the activity of the respiratory system. For example, the cough reflex receptor sends impulses from the nerve endings to the cough center of the medulla oblongata along the afferent fibers of the vagus nerve, which in turn, removes the secretion or foreign matter such as bacteria through coughing to clean the respiratory tract (Nonomura et al. 2017). The respiratory system also has other receptors, such as stimulation receptors that receive a variety of physical and chemical stimuli and tension receptors that sense alveolar dilation or edema. These receptors transmit stimuli to the CNS through the vagus nerve.
Owing to the different fibers of vagus nerve, it can transmit the information from CNS to lung, and conduct the feedback signal of lung to CNS, which is an important neural pathway of the brain–lung axis. Under pathological condition, the vagus nerve also participates in the bi-directional communication pathway between brain and lung. Vagus nerve is related to allergic diseases such as asthma, rhinitis and COPD, leading to symptoms such as sneezing, coughing, excessive mucus secretion, and bronchoconstriction (De Virgiliis and Di Giovanni 2020). Traumatic brain injury (TBI) or stroke can directly damage the vagus nerve. The vagus nerve participates in physiological functions such as swallowing and cough reflex, so damage to the vagus nerve will result in the weakening or loss of these functions. For instance, dysphagia increases the incidence of aspiration, and the weakness of cough reflex decreases respiratory secretion as well as excretion of bacteria, which promotes pulmonary infection (Liu et al. 2018). Similar to brain-gut axis, the destruction of brain tissue caused gastrointestinal motility disorders in mice, increased intestinal permeability, and promoted the migration of harmful intestinal microbes (Durgan et al. 2019).
In addition, the vagus nerve involves in cholinergic anti-inflammatory pathway (CAP), which plays a neuroimmune role (Pereira and Leite 2016). The nerve endings of the vagus nerve distribute in various organs and perceive physiological changes of the target organs through different types of receptors. The inflammatory cytokines activate the vagus nerve afferent fibers, and then the signal is transmitted through the vagus nerve to the solitary nucleus of medulla oblongata. The signal in solitary nucleus projects to the dorsal motor nucleus of vagus nerve and is transmitted to the nerve endings through the efferent nerves (Tracey 2009). Near the immune cells located in reticuloendothelial tissue, the efferent nerve endings release ACh which binds to macrophages and other special surface ACh receptors such as α7nAChR, then transmits signal into the cells to regulate the production of anti-inflammatory cytokines (Pereira and Leite 2016). The nerve endings of vagus nerve widely distribute in the lung. Hence, vagus nerve can act on lung diseases through CAP. Ida A J Giebelen et al. discovered that selective α7nAChR activation decreased the release of TNF-α in lipopolysaccharide (LPS) challenged lung tissue of mice (Giebelen et al. 2007). Vagotomy or deficiency of α7nAChR aggravated pulmonary infection, inflammation and injury, and increased the level of proinflammatory cytokines in blood, suggesting that pulmonary parasympathetic inflammatory reflex may locally limit the degree of pulmonary infection and inflammation (Huang et al. 2019). These phenomena can be observed in the brain-gut axis, in which targeting CAP can relieve intestinal diseases. Enzenilin, a partial agonist of α7nAChR, has recently been reported to alleviate colitis induced by trinitrobenzene sulfonic acid (TNBS) and dextran sodium sulfate (DSS) (Salaga et al. 2016). Vagus nerve stimulation therapy also effectively improves TNBS-induced colitis in mice, indicating that the intervention of CAP of vagus nerve might be a potential treatment for lung-related diseases (Bonaz 2007).
Interestingly, the dysfunction of CAP of vagus nerve contributes to the development of lung disease. The ACh secreted by the vagus nerve may bind to α7nAChR on fibroblasts and lead to the proliferation of fibroblasts and promote the progression of pulmonary fibrosis (Pieper et al. 2007). In the bleomycin-induced pulmonary fibrosis mice model, unilateral vagotomy alleviated collagen deposition and pulmonary fibrosis by reducing fibrotic cells and cytokines (TGF-β and IL-4) (Song et al. 2015). In addition, the incidence of brain injury and stroke-associated pneumonia (SAP) display an α7nAChR-dependent manner, indicating that the activation of CAP may be beneficial by reducing systemic inflammation, yet it may also induce immunosuppression in lung, which increases the incidence of bacterial pneumonia. Cynthia S. Samary et al. found that focal ischemic stroke decreased the phagocytic ability of the alveolar macrophages in rats, which was closely related to SAP (Samary et al. 2018). In short, the CAP of vagus nerve is a double-edged sword in the brain–lung axis. Proper activation promotes the regression of inflammation, but overreaction may aggravate infection and even promote the occurrence of lung disease. Therefore, it is necessary to clarify the specific molecular mechanisms of CAP of vagus nerve to make a precise regulation.
Neuropeptides are considered to be the critical mediators of communication between neurons and effector cells. Previous studies have suggested that neuropeptides are mainly secreted by neurons, but now it has been found that certain immune cells could secret neuropeptides, and these neuropeptides are thought to be powerful regulators of immune response (Snoek et al. 2010). Neuropeptides participate in brain-gut axis communication, from neuropeptides and neurotransmitters secreted by the brain to intestinal peptides secreted by gastrointestinal endocrine cells. Neuropeptides and peptide hormones are vital in the bi-directional communication in the brain-gut axis (Holzer and Farzi 2014). Nevertheless, the role of neuropeptides in the brain–lung axis needs to be further illustrated.
Pulmonary neuroendocrine cells (PNECs), the only innervated respiratory epithelial cells, as a portion of the airway epithelial system in lung, scatter in the airway epithelium (Boers et al. 1996). PNCs contain a significant number of secretory granules loading a variety of hormone-like peptides. The typical neuropeptides in the lungs are bombesin/gastrin-releasing hormone, calcitonin/calcitonin gene-related peptide, endothelin, substance P, cholecystokinin, etc., which may be significant mediators of the brain–lung axis. Studies have demonstrated that the number of PNECs increases in many lung diseases (e.g., COPD, bronchial asthma, small cell lung cancer [SCLC]), suggesting that it may be relevant to the pathophysiological mechanism of lung diseases (Chen et al. 2019a, b; Gu et al. 2014; Sui et al. 2018). Gu et al. found that the distribution of serotonin and neuropeptide receptors changed in the lungs of COPD patients, suggesting that increased PNEC-dependent chemotactic response may be one of the reasons for the change in sensitivity to volatile stimuli in COPD patients (Gu et al. 2014). By quantifying the size of PNECs and PNECs clusters in total PNECs, proximal bronchioles or distal respiratory bronchioles, it was found that the number of PNECs in asthma samples was higher than that in healthy controls, suggesting that the increase of PNECs, especially the PNECs expressing calcitonin gene-related peptide, may cause allergic asthma (Sui et al. 2018). In addition, PNECs act as accurate airway sensors that elicit immune responses through neuropeptides. Under the action of lung injury factors, PNECs proliferate massively and secrete various neuropeptides, coordinating with other epithelial cells. This alteration influences the chemotaxis of inflammatory cells and the secretory function of inflammatory cells. Allergen or ozone activates the sensory neurons in lung to release neuropeptides (e.g., tachykinin), which dilate blood vessels, increase the permeability of post-capillary venules, induce the adhesion of neutrophils, and promote neurogenic inflammation (O'Connor et al. 2004). Tachykinin directly or indirectly induces bronchus contraction by activating postganglionic cholinergic nerves and mast cells, which may be relevant to the hyperresponsiveness of the respiratory tract. (O'Connor et al. 2004) However, after nociceptive stimulation (e.g., TBI), vagal afferent neurons may secret substance P that mediates pulmonary vasodilation, vascular permeability, neutrophil initiation and migration (Yang et al. 2014). Conversely, blocking substance P receptor, the NK-1 receptor, inhibits the increase of neutrophils, reduces the bacterial clearance and promotes the occurrence of bacterial pneumonia (Douglas and Leeman 2011). In the ischemia–reperfusion model, denervated capsaicin-sensitive c fibers may aggravate pulmonary inflammation by depleting the calcitonin gene-related peptide (CGRP) of capsaicin-sensitive c fibers, suggesting that neuropeptides may protect the lungs from inflammation and injury (Ji et al. 2013). Those results indicated that neuropeptides perform different roles in different diseases, but the specific regulatory mechanism of neuropeptides remains to be further studied.
What’s more, neuropeptides such as neuropeptide Y (NPY) and peptide YY (PYY), are involved adjusting intestinal inflammation in the gut-brain axis. Trinitrobenzenesulfonic acid-induced colitis increased the level of NPY in brain and plasma, while gastrointestinal inflammation enhanced anxiety and depression-related behavior, yet these changes were reversed by deletion of NPY and/or PYY (Holzer and Farzi 2014). It is suggested that lung diseases may act on CNS through neuropeptide feedback (Fig. 1).
Fig. 1.
Vagus nerve could regulate bronchial smooth muscle contraction, glandular secretion, vascular congestion, and mucosal swelling in lung, while it could accept the stimulation from lung. Furthermore, vagus nerve releases acetylcholine (ACh), acting on ACh receptors, α7nAChR, of immune cells, which is the CAP. Pulmonary neuroendocrine cells, which are controlled by vagus nerve, secret neuroendocrine factors that regulate the physiological function of lung. 5-HT 5-hydroxytryptamine, NPY neuropeptide Y, PNECs pulmonary neuroendocrine cells, PS substance P, PYY peptide YY
The regulation of sympathetic nerve system (SNS) in lung mainly involved in the stress state, secreting catecholamines through the sympathetic adrenomedullary system (SAS), which will be discussed in the endocrine system below.
Endocrine Pathway
HPA axis and SAS are the central neuroendocrine regulatory systems responding to stress. HPA axis is composed of hypothalamus, pituitary and adrenal glands, communicating through a feedback interaction pathway. As the main part of the neuroendocrine system, it participates in regulating physiological activities and stress response. Stimulation of HPA axis is precipitated by release of corticotropin-releasing hormone (CRH) from the hypothalamus onto the pituitary gland to allow adrenocorticotrophic hormone (ACTH) to enter systemic circulation. Circulation ACTH acts quickly and briefly to stimulate the adrenal cortex to rapidly synthesize and secrete corticosteroids such as glucocorticoid (GC). During fetal development, GC released by the HPA axis is indispensable to the maturation of the fetal lung. The HPA axis is activated and releases GC under stressor. The acute response may inhibit immune response in airway by immunosuppression and anti-inflammation which involves in mobilizing circulating lymphocytes and granulocytes, inhibiting the activity of macrophages, the proliferation of T cells, and the response of Th1 cells (Abelson et al. 2010). Mental illness also influences the function of the lung through the HPA axis. Cohen S et al. found that chronic, persistent stress, for example, prolonged restraint stress, could induce immunosuppression and lead to a tilt towards an anti-inflammatory immune cell phenotype that increases susceptibility to diseases (e.g., upper respiratory tract infection) (Cohen et al. 2012); while stress-relieving factors (e.g., social support) can reverse the suppression of immune function (Kang et al. 1998). In addition, GC can directly induce the apoptosis of epithelial cells and weaken epithelial cells layer in the airway (Dorscheid et al. 2001). For instance, psychological stress influences the function of mucociliary clearance in the lung (Trueba and Ritz 2013). Studies have shown that repeated stress could change mucus properties, thus reducing the clearance of mucociliary, and increase the incidence of asthma, respiratory tract infection and other diseases (Trueba and Ritz 2013). This is consistent with the brain-gut axis, in which the activation of the HPA axis weakens the mucosal barrier in the intestinal tract and impacts the composition of intestinal microflora (Farzi et al. 2018).
Conversely, the respiratory system affects the HPA axis. Long-term hypercapnia (e.g., COPD) increases cortisol by stimulating the HPA axis, which may be mediated by the activation of paraventricular nucleus of hypothalamus through the projection from CO2-sensitive brainstem nuclei such as ventrolateral medulla and locus coeruleus (Abelson et al. 2010). Previous studies have suggested that healthy lung is sterile, but NGS sequencing has confirmed that there are complex and diverse bacterial communities in the mucosa of the lower respiratory tract (Wypych et al. 2019). In brain-gut axis, some studies showed that owing to the increased permeability of the intestinal barrier and microbial-driven proinflammatory state, intestinal microorganisms could activate the HPA axis, indicating that microorganisms in the lung may be able to activate the HPA axis through a mechanism similar to the brain-gut axis (Carabotti et al. 2015). However, more reliable and direct evidence is needed to demonstrate this mechanism. Furthermore, neuroendocrine tumors, for instance, SCLC, can release ACTH, increasing the level of GC, which results in clinical manifestations such as full moon, acne, hypertension, and hypokalemia (Gandhi and Johnson 2006). Beyond ACTH, these neuroendocrine tumors release other endocrine hormones such as antidiuretic hormone and growth hormone, which participate in the brain–lung axis. Interestingly, the HPA axis is not just a “bad guy”, it senses the homeostasis of lung, and then regulates the physiological balance of lung by accepting feedback on the HPA axis through multiple complex mechanisms. Pulmonary infection itself is a kind of stress. When the HPA axis responds to stress improperly, the loss function of inhibitory effect will contribute to the development and maintenance of respiratory diseases under airway inflammation state.
SAS is mainly relevant to excitement and vigilance during stress. The intense excitement of this system is involved in acutely responding to stress in body, mediating a series of metabolic and cardiovascular compensatory mechanisms to overcome the threat under stressors or the disruption of internal environment. After activation, the level of catecholamines in plasma elevates, including epinephrine, norepinephrine (NE), and dopamine. NE increases the mobility and proliferation of bacteria, elevates the expression of bacterial toxins, and enhances the pathogenicity of bacteria under stress, resulting in the increased incidence of pulmonary infection, which is consistent with NE and gut (Trueba and Ritz 2013). NE increases the proliferation of Gram-negative bacteria in intestinal mucosa and promotes the translocation of bacteria (Diard et al. 2009). What’s more, NE changes protein and metabolism state of bacteria in the respiratory tract of pigs, in which bacteria become more proliferative and cause infection (Oneal et al. 2008). In addition, NE can enhance the toxicity of specific pathogens and opportunistic bacteria, which ultimately promotes the occurrence of infection and exacerbates asthma symptoms (Trueba and Ritz 2013). Besides, the immune system is regulated by NE, another mechanism in the brain–lung axis. NE inhibits the maturation and activation of Th1 cells, and suppresses immune response, which ultimately increases the risk of infection. When the SNS is activated after stroke, the release of catecholamines may reduce the level of tumor necrosis factor-α (TNF-α) and increase the level of interleukin-10 (IL-10) through β-adrenergic receptors on immune cells. Blocking SNS with β-adrenergic receptor antagonist can reduce infectious complications (e.g., pneumonia) and mortality, indicating the importance of catecholamines in immunosuppression after stroke (Prass et al. 2003). Furthermore, catecholamines are significant in tumor-related diseases. Yun Xia et al. found that catecholamines could reshape the phenotype of tumor-associated macrophages, which promoted the polarization of macrophages to tumor-supported M2 phenotype, increased the level of proangiogenic factors and immunosuppressive cytokines, and inhibited the expression of proinflammatory cytokines (Xia et al. 2019). The above changes facilitate the immune escape of tumor cells which may promote the development of lung cancer. It hints that the inhibition of SAS may be the target of lung cancer by altering the function of macrophages, preventing angiogenesis, and reshaping the immunosuppressive microenvironment that supports the growth of invasive lung cancer.
Overall, the intimate connection and functional integration between respiratory system and endocrine system may be necessary for lung development and maintaining steady-state defense response, but excessive activity of endocrine system could accelerate lung injury (Fig. 2).
Fig. 2.
The endocrine system in brain–lung axis. The CRH is release from the hypothalamus onto the pituitary gland to allow ACTH to enter systemic circulation. Circulation ACTH acts quickly and briefly to stimulate the adrenal cortex to synthesize and secrete corticosteroids such as GC. GC plays an immunosuppression role in immune cells, induces the apoptosis of epithelial cells and reduces the ability of mucociliary clearance. Interestingly, lung cancer, for instance, SCLC, could secret ACTH which negatively regulates the HPA axis. Besides, the increase of catecholamine is induced by the activation of SAS after stress. NE increases the mobility and proliferation of bacteria, elevates the expression of bacterial toxins, and enhances the pathogenicity of bacteria. Furthermore, NE inhibits the maturation and activation of Th1 cells, and suppresses immune response, which ultimately increases the risk of infection
Immune Pathway
The immune system is composed of immune organs, immune cells, and immune molecules, which coordinate with various systems to maintain the stability of internal environment, suggesting that CNS and the lung can be indirectly connected through the immune system. It is now convinced that various CNS diseases directly lead to lung lesions through the immune system. Shan Wu et al. found that inflammatory cytokines were released into blood through blood–brain barrier (BBB) after intracerebral hemorrhage, which increased the infiltration of neutrophils from the peripheral circulation into lungs, destroying alveolar and secondary lung injury (Wu et al. 2006).
Due to the destruction of BBB after ischemic stroke, some necrotic substances (e.g., damage-associated molecular patterns [DAMPs]) are released into the blood (Kim et al. 2006). These substances may inhibit the peripheral immune response, for example, immune failure triggered by high mobility group box 1 (HMGB1)—receptor for advanced glycation end (RAGE) signal pathway, which increases the risk of post-stroke-associated pneumonia (Kim et al. 2018). Besides, the CNS indirectly communicates with lung through the autonomic nervous system and endocrine system, which regulate the immune system. As mentioned above, the CAP is related to controlling the immune function of macrophages through Ach released from nerve endings and nAChRα7 (Pereira and Leite 2016). For the endocrine system, activation of the HPA axis or SAS is bound to multiple endocrine substances, e.g., GC and catecholamines. GC can mobilize lymphocytes and granulocytes in circulation, inhibit the activity of macrophages, reduce the proliferation of T cells, restrain the immune response of Th1 cells immunity, and decrease the expression of proinflammatory factors and mediators (e.g., IL-1, IL-2, IL-6, TNF- α) (Abelson et al. 2010). Catecholamines regulate immune cells through β-adrenergic receptors (Prass et al. 2003). Overall, those alterations of endocrine hormones may increase the risk of pulmonary infection.
For lung diseases, such as lung infections, the body initiates an immune response to remove invasive pathogens. Nevertheless, over-activated immune systems could damage CNS. Ji Chen et al. demonstrated that circulating inflammatory markers, e.g., matrix metalloproteinases (MMP-9) and TNF- α, could invade CNS through different mechanisms, activating glial cells and exacerbating nerve cells death in rats with chronic pulmonary infection induced by LPS (Chen et al. 2019a, b). Another research has shown that the level of systemic cytokines and neutrophils increased after ALI, leading to dysfunction of other organs, including the brain (Bickenbach et al. 2009). Furthermore, cytokine storm may be one of the primary inducements of CNS complications following the infection of novel coronavirus (De Virgiliis and Di Giovanni 2020). Paraneoplastic neurological syndrome refers to the abnormal production of antibodies against neuronal antigens expressed in tumors, which could damage neuronal tissue far from the tumor (Gandhi and Johnson 2006). The common primary tumor is SCLC, which promotes the production of multiple antibodies such as Anti-CRMP/Anti-CV2 antibody, Anti-Hu antibody, Anti-amphiphysin antibody, etc. These antibodies would attack the brain regions that express these antigens in CNS, thus leading to various pathological changes of CNS, e.g., encephalomyelitis, limbic encephalitis, subacute cerebellar degeneration, and other autoimmune encephalitis (Kazarian and Laird-Offringa 2011). Recently, the intestinal environment has attracted much attention as a potential site for initiating self-reactive T cells during spontaneous experimental autoimmune encephalomyelitis (Berer et al. 2011). However, Francesca Odoardi et al. confirmed that the lung is the site where autoreactive T cells reactivate (Odoardi et al. 2012). Following local stimulation of the lung, autoreactive T cells proliferate strongly and enter the CNS to induce autoimmune diseases after assuming the characteristics of migration, which may be the mechanism of recurrence of multiple sclerosis induced by respiratory tract inflammation. Furthermore, Hosang et al. found that intratracheal infusion of neomycin disturbed the bacterial community of lung, resulting in the reduced reactivity of microglia, the reduced recruitment of inflammatory cells, and blocked the development of EAE (Hosang et al. 2022). The article demonstrated that the imbalance of pulmonary microorganisms changes the susceptibility of CNS to autoimmune diseases by changing the immunoreactivity of microglia. In short, CNS and lung can communicate directly or indirectly through the immune system, indicating immune system as a significant part in the communication of brain–lung axis (Fig. 3).
Fig. 3.
The Immune pathway in brain–lung axis. The releasing of DAMPs after brain injury could regulate immune system, which relates to the increased risk of pneumonia and ALI. Besides, the brain regulates lung by immune system through the CAP, HPA axis and SAS. Conversely, the over-activated immune systems after pneumonia could damage CNS. Furthermore, SCLC, which promotes the production of multiple autoantibodies, would attack the brain regions that express these antigens in CNS, thus leading to a variety of pathological changes in CNS, e.g., encephalomyelitis, limbic encephalitis, subacute cerebellar degeneration, and other autoimmune encephalitis. BBB blood–brain barrier, HMGB1 high mobility group box 1, MMP-9 matrix metalloproteinases, RAGE receptor for advanced glycation end
Metabolites and Microorganism Pathway
Except for neuropeptides, the metabolites of CNS or lung in various diseases are essential regulators for the interconnection between brain and lung axis. After acute brain injury (e.g., ischemic stroke or traumatic brain injury), necrotic neurons can release a series of substances (e.g., HMGB1, HSP, ATP, S100B protein, etc.) that are secreted into the blood through the damaged BBB (Santos et al. 2016). These products may be important mediators of the brain–lung axis, promoting proinflammatory responses, and resulting in lung injury. HMGB1 was detected in peripheral blood after 3 h in MCAO mice (Kim et al. 2006). Ruiting Li et al. found that HMGB1 inhibited the activity and function of Treg cells, regulated the polarization of macrophages and promoted inflammation, and then reduced LPS-induced ALI, suggesting that increasing HMGB1 in circulation after ischemic stroke may induce pulmonary edema by regulating pulmonary immune cells. However, the direct evidence still needs further research (Li et al. 2020). HMGB1 not only regulates lung function in ischemic stroke, and vice versa. Injection of LPS after 24 h in MCAO mice to simulate post-stroke infection, Il-Doo Kim et al. found that the expression of HMGB1 in peripheral blood increased significantly (Kim et al. 2015). HMGB1 aggravated the cerebral infarction volume and neurological function by coordination with LPS. Although little evidence demonstrated that the up-regulated HMBG1 originates from the peripheral or CNS, it suggests that pulmonary infection after stroke could deteriorate neurological function through HMGB1-LPS interaction. The mechanism remains to be further studied. Absolutely, the interaction of these metabolites is not specific to the brain–lung axis, but also exists in the brain-gut axis. For example, Diego F Niño et al. found that in mice with necrotizing enterocolitis, the activation of TLR4 signal pathway in intestinal epithelial cells increased HMGB1, which in turn promoted the activation of microglia and neurological dysfunction in the brain (Niño et al. 2018).
The metabolites of CNS may communicate between CNS and lung through carrier, the exosome. Exosome is a kind of extracellular vesicle with lipid bilayer membrane, rich in protein, lipid, and nucleic acid. It can mediate cell–cell signal transmission. It has been found that exosomes can transmit signals between CNS and peripheral nerve cells through cerebrospinal fluid, and connect peripheral circulation with CNS through BBB, suggesting that exosome and their contents may also transmit messages between brain–lung axis (Bátiz et al. 2015). EVs increase in circulation after TBI, related to ALI. These vesicles carrying proinflammatory cytokines can be absorbed by pulmonary endothelial cells, which promote the release of IL-1 β and IL-18 by mediating the activation of inflammasome, and eventually lead to apoptosis of pulmonary endothelial cells (Kerr et al. 2020). It was also reported that the circulating exosomes in patients with ischemic stroke could reflect the proinflammatory status and could activate macrophages, demonstrating that exosomes may also involve in ALI (Couch et al. 2017). Conversely, exosomes in lungs could pass through the BBB to transmit information into CNS. Exosome participates in the metastasis of lung cancer into CNS. Dong-Xue Gan et al. confirmed that exosomes derived from lung cancer cells were absorbed by brain vascular endothelial cells which transmit inhibitory signals to microglia, resulting in the decrease of M1 phenotypic microglia and the increase of M2 phenotypic microglia (Gan et al. 2020). And the shift of microglia phenotypes contributes to the brain metastasis of lung cancer cells. However, most of the research about exosomes are vitro, and the direct evidence that they communicate with each other in the brain–lung axis is insufficient. Furthermore, since exosomes in biological fluids are derived from various types of cells in the body, it is necessary to seek organ and/or cell type-specific exosome markers to distinguish their sources.
Bacteria and their products could act as direct mediators in brain–lung axis. Cryptococcus neoformans is a fungal pathogen, which enters the body mainly through inhalation. It can cause pneumonia and spread to CNS inducing meningoencephalitis (Miyazato 2016). Cryptococcus can infect CNS not only directly through transcellular pathway, but also through infected macrophages that enter CNS (Miyazato 2016). Other indirect evidences showed that lung microbiota could lead to CNS disease. COPD changes respiratory microflora and increases the risk of Parkinson’s disease (PD) and Alzheimer’s disease (AD) (Bell et al. 2019). Bordetella pertussis infection in mice is associated with inflammation and β deposition, while respiratory pathogen Klebsiella pneumoniae is significantly associated with AD (Bell et al. 2019). Interestingly, in multiple sclerosis, pertussis may alleviate EAE through an IL-10-dependent mechanism (Edwards et al. 2015). The outcome of CNS injury caused by different pulmonary pathogenic microorganisms may involve complex immune mechanisms. In addition, the virus is a small molecular pathogenic microorganism that can directly infect neurons by reverse axonal transport, and finally influence neurological function. For instance, virus that infects lung can enter CNS directly through the vagus nerve and reach the breath center in the brain, which in turn aggravates respiratory symptoms (e.g., respiratory distress) (De Virgiliis and Di Giovanni 2020).
LPS is a product of gram-negative bacteria. Study showed that after alveolar perfusion of LPS, it could be detected in blood, suggesting that the product of bacteria after pulmonary infection may directly enter the circulation (Sze et al. 2014). Other studies confirmed that in ALI caused by inhalation of LPS, similar microflora was detected in the lungs in bronchoalveolar lavage (BAL) and the blood, indicating that LPS could facilitate the migration of bacteria from the lungs into the blood system. After ischemic stroke, LPS damages the mitochondrial function of endothelial cells, aggravates the damage of BBB, and promotes the recruitment of neutrophils to infarct site (Doll et al. 2015). What is more, LPS is a recognized pathogenic factor of AD, which is used to construct the animal model of AD. Peripheral injection of LPS can activate astrocytes and microglia, and promote the expression of cyclooxygenase-2, inducible nitric oxide synthase, and proinflammatory cytokines in the brain (Catorce and Gevorkian 2016). The accumulation of amyloid precursor protein, amyloid β-peptide and hyperphosphorylated τ protein and the aggravation of memory impairment were observed in LPS-treated APP transgenic mice (Kitazawa et al. 2005; Sheng et al. 2003). Yuhai Zhao et al. reported that E. coli-rich LPS, extracted from the neocortex and hippocampus of AD brain might induce AD through the mechanism of neuroinflammation, indicating that respiratory tract-derived LPS may be involved in AD (Zhao et al. 2017). Interestingly, using LPS to simulate pulmonary infection delays the occurrence of EAE by stalling Th17 cells in lung, which may involve different immunomodulatory mechanisms (Kanayama et al. 2016).
Bacterial products can also enter the peripheral blood in the form of vesicles. Outer-membrane vesicles (OMVs) are a kind of vesicles derived from the outer membrane of Gram-negative bacteria (Schwechheimer and Kuehn 2015). They are similar to EVs in size, structure, and biological function, which are produced by mammalian cells (Yu et al. 2018). OMVs carry LPS which is the most abundant immunostimulatory component. PAMP, consisting of outer membrane porin, flagellin, and peptidoglycan, is another carrier that can stimulate immunity (Bauman and Kuehn 2006; Renelli et al. 2004). Microbial-derived OMVs transfer a wide range of materials, including bioactive proteins, lipids, nucleic acids, and virulence factors, to neighboring bacteria or host cells (epithelial cells, endothelial cells, immune cells) (Zhao et al. 2021). This biological information transmission plays a vital role in both intracellular (bacterial-bacterial) interactions and inter-kingdom (bacterial-host) communication. The peripheral bacterial OMVs directly or indirectly induce central neuropathy. Before OMVs reach the brain, they must cross the barrier between lung and brain. Some studies suggested that bacterial OMVs could pass through BBB directly. Intracardiac administration of actinomycetes OMVs could go through BBB and increase the expression of TNF-α through TLR8 and NF-κB signaling pathways (Han et al. 2019). The increase of TNF-α in CNS may induce inflammatory CNS diseases e.g., AD. The Aggregatibacter actinomycetemcomitans OMVs could successfully transfer extracellular RNA to monocytes/microglia in CNS and induce neuroinflammation related to the up-regulation of IL-6 through NF-κB signal pathway (Ha et al. 2020). In addition, OMVs from the circulatory system could come across the meninges and activate microglia (Han et al. 2019). These studies hint that OMVs enter CNS, followed by activating immune cells (e.g., astrocytes and microglia) through immune receptors, TLRs, triggering proinflammatory cytokines and causing neuronal damage. Interestingly, it was reported that oral fluorescein OMVs could be detected in the hippocampus of mice, while vagotomy blocked the transport of fluorescence-bound OMVs, suggesting that bacterial EVs could cross the BBB through the vagus nerve (Lee et al. 2020). These OMVs in CNS will lead to diseases in brain. It is generally believed that Gram-positive bacteria cannot produce OMVs, but recent studies have isolated lipid bilayer vesicles from Gram-positive bacteria (Cuesta et al. 2021). These vesicles are similar in shape to Gram-negative bacteria. Differently, the vesicles do not contain LPS, but contain cytoplasmic components (e.g., peptidoglycans and proteins), suggesting that these vesicles and contents may be mediators in the brain–lung axis90. However, there is still a lack of evidence that OMVs directly enter CNS from lung. Hence, further research is needed (Fig. 4).
Fig. 4.
Metabolites and microorganism pathway in brain–lung axis. The metabolites of CNS or lung in various diseases are essential regulators for the interconnection between brain and lung axis. Necrotic substances which are released after brain or lung injury could damage each other through immune system. What is more, exosomes may be communicating messages between brain and lung. Bacteria and their products [e.g., outer membrane vesicles (OMVs) and LPS] may be another communicating message. The bacteria or LPS after lung infection could directly invade brain, resulting in CNS infection. Besides, OMVs carrying bacteria toxins could damage BBB, activate glial cells and promote neuroinflammation. AD Alzheimer’s disease
Gas Pathway
The lung is the main organ for gas exchange, in which the external gas could influence other organs through the respiratory tract. Multiple evidences showed that CNS diseases were closely related to air pollution. If living in conditions with a high level of urban air pollution, the cognitive function of the elderly decreases, the risk of autism, AD and PD, and the incidence of stroke increases (Mumaw et al. 2016). The common air pollutant is ozone, which changes brainstem neurons, leading to memory impairment, sleep pattern interruption, cognitive decline, social behavior changes, and motor activity deficits (Gackière et al. 2011). Ozone changes CNS function via various mechanisms, which may eventually activate microglia and induce the proinflammatory environment in the brain. Acute ozone inhalation increases circulating stress hormones by activating the SAS and HPA axis. Adrenalectomy reduces the lung response induced by ozone (Henriquez et al. 2019). Overall, air pollution may not directly reach the brain to adjust its function, but through the immune system, endocrine system and other mechanisms.
In addition, a variety of lung diseases cause hypoxemia and hypercapnia, e.g., COPD, ARDS, interstitial pneumonia, and severe pulmonary infection, which are closely related to CNS disease. In the physiological state, when the blood oxygen content decreases, the cerebral blood flow will increase to maintain the brain oxygen demand. However, the periventricular white matter is located at the watershed of the cerebral artery, and patients with COPD are in a state of chronic hypoxemia, which causes low perfusion of white matter (Qin et al. 2020). This may be the potential mechanism that COPD is more likely to induce white matter hyperintensity (WMH). Chronic hypoxia is considered to be related to multiple pathological changes of AD. Hypoxia increases the production of Aβ, enhances the phosphorylation of τ, induces neuroinflammation, increases the production of reactive oxygen species, and promotes abnormal mitochondrial function (Zhang et al. 2019). What is more, hypercapnia aggravates cognitive impairment caused by hypoxemia (Liu et al. 2020). Interestingly, mild hypercapnia alleviates brain injury, and this protective effect is involved in regulating apoptosis regulatory proteins. Moderate hypercapnia also improves neurological dysfunction, yet severe hypercapnia aggravates brain edema and injury (Deng et al. 2020). The above discoveries show that hypoxemia or hypercapnia play different roles in different diseases of CNS, which may involve different regulatory pathways. The detailed regulatory mechanism needs to be further studied.
Conclusion
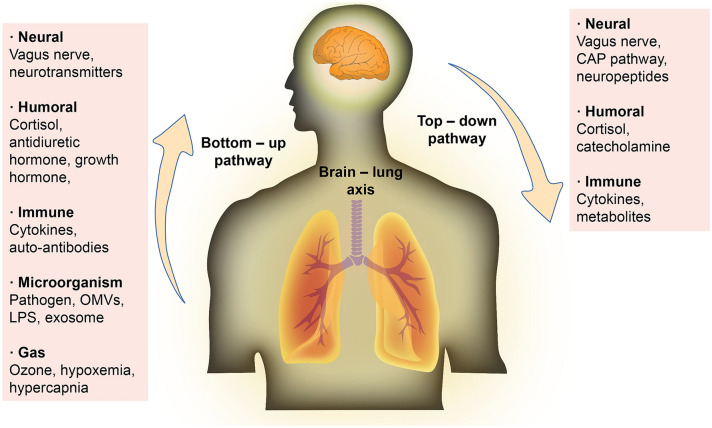
In this article, we review the intercommunication between the brain and lung, and propose the concept of “brain–lung axis”. We discuss the possible mechanisms involving nerves, endocrine, immunity, neuropeptides, microorganism and metabolites, gases and other pathways that participate in the brain–lung axis. Besides, these communication channels are not isolated, but closely related. For instance, the vagus nerve is not only the neural communication pathway between the brain–lung axis, but is also indirectly involved in regulating the immune system and neuropeptides. Nevertheless, the functions of the above pathways are similar in other organs. But the lungs have their own characteristics. Exploring the brain–lung axis not only helps us to understand the development of the disease from different aspects, but also provides an important target for treatment strategies (Fig. 5).
Fig. 5.
The total bidirectional pathways in brain–lung axis. Overall, the brain regulates the lung via neuroanatomical, humoral, immune and metabolic pathways. The lung influences the brain primarily via humoral, immune, pathogenic microorganisms and metabolic pathways. CAP cholinergic anti-inflammatory pathway, LPS lipopolysaccharide, OMVs outer membrane vesicles
Acknowledgements
Not applicable.
Author Contributions
All authors contributed to the study conception and design. LZ and WC: Conceptualization. CL and FL: Writing—original draft preparation. PW: Figure editing. GL and WL: Writing—reviewing and editing. CL, WC and FL: Contribute equal to this manuscript. All authors read and approved the final manuscript.
Funding
This work was funded by National Natural Science Funds of China (Grant Nos. 81971098 and 82104144).
Data Availability
All data and material are available.
Declarations
Conflict of interest
The authors confirm that they have no competing interests.
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publications
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chunyi Li, Wenli Chen and Feng Lin have contributed equally to this manuscript.
Contributor Information
Wenli Chen, Email: chenwenl@mail3.sysu.edu.cn.
Lei Zhang, Email: zhangl92@sysu.edu.cn.
References
- Abelson JL, Khan S, Giardino N (2010) Hpa axis, respiration and the airways in stress–a review in search of intersections. Biol Psychol 84(1):57–65. 10.1016/j.biopsycho.2010.01.021 [DOI] [PubMed] [Google Scholar]
- Bai W, Li W, Ning YL, Li P, Zhao Y, Yang N, Jiang YL, Liang ZP, Jiang DP, Wang Y, Zhang M, Zhou YG (2017) Blood glutamate levels are closely related to acute lung injury and prognosis after stroke. Front Neurol 8:755. 10.3389/fneur.2017.00755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bátiz LF, Castro MA, Burgos PV, Velásquez ZD, Muñoz RI, Lafourcade CA, Troncoso-Escudero P, Wyneken U (2015) Exosomes as novel regulators of adult neurogenic niches. Front Cell Neurosci 9:501. 10.3389/fncel.2015.00501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman SJ, Kuehn MJ (2006) Purification of outer membrane vesicles from pseudomonas aeruginosa and their activation of an il-8 response. Microbes Infect 8(9–10):2400–2408. 10.1016/j.micinf.2006.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell JS, Spencer JI, Yates RL, Yee SA, Jacobs BM, Deluca GC (2019) Invited review: from nose to gut—the role of the microbiome in neurological disease. Neuropathol Appl Neurobiol 45(3):195–215. 10.1111/nan.12520 [DOI] [PubMed] [Google Scholar]
- Berer K, Mues M, Koutrolos M, Rasbi ZA, Boziki M, Johner C, Wekerle H, Krishnamoorthy G (2011) Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 479(7374):538–541. 10.1038/nature10554 [DOI] [PubMed] [Google Scholar]
- Bickenbach J, Zoremba N, Fries M, Dembinski R, Doering R, Ogawa E, Rossaint R, Kuhlen R (2009) Low tidal volume ventilation in a porcine model of acute lung injury improves cerebral tissue oxygenation. Anesth Analg 109(3):847–855. 10.1213/ane.0b013e3181ad5769 [DOI] [PubMed] [Google Scholar]
- Boers JE, den Brok JL, Koudstaal J, Arends JW, Thunnissen FB (1996) Number and proliferation of neuroendocrine cells in normal human airway epithelium. Am J Respir Crit Care Med 154(3 Pt 1):758–763. 10.1164/ajrccm.154.3.8810616 [DOI] [PubMed] [Google Scholar]
- Bonaz B (2007) The cholinergic anti-inflammatory pathway and the gastrointestinal tract. Gastroenterology 133(4):1370–1373. 10.1053/j.gastro.2007.08.061 [DOI] [PubMed] [Google Scholar]
- Carabotti M, Scirocco A, Maselli MA, Severi C (2015) The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol 28(2):203–209 [PMC free article] [PubMed] [Google Scholar]
- Catorce MN, Gevorkian G (2016) Lps-induced murine neuroinflammation model: main features and suitability for pre-clinical assessment of nutraceuticals. Curr Neuropharmacol 14(2):155–164. 10.2174/1570159x14666151204122017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HJ, Poran A, Unni AM, Huang SX, Elemento O, Snoeck HW, Varmus H (2019a) Generation of pulmonary neuroendocrine cells and sclc-like tumors from human embryonic stem cells. J Exp Med 216(3):674–687. 10.1084/jem.20181155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Yan Y, Yuan F, Cao J, Li S, Eickhoff SB, Zhang J (2019b) Brain grey matter volume reduction and anxiety-like behavior in lipopolysaccharide-induced chronic pulmonary inflammation rats: a structural mri study with histological validation. Brain Behav Immun 76:182–197. 10.1016/j.bbi.2018.11.020 [DOI] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D, Doyle WJ, Miller GE, Frank E, Rabin BS, Turner RB (2012) Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc Natl Acad Sci USA 109(16):5995–5999. 10.1073/pnas.1118355109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corlateanu A, Covantev S, Mathioudakis AG, Botnaru V, Cazzola M, Siafakas N (2018) Chronic obstructive pulmonary disease and stroke. COPD 15(4):405–413. 10.1080/15412555.2018.1464551 [DOI] [PubMed] [Google Scholar]
- Couch Y, Akbar N, Davis S, Fischer R, Dickens AM, Neuhaus AA, Burgess AI, Rothwell PM, Buchan AM (2017) Inflammatory stroke extracellular vesicles induce macrophage activation. Stroke 48(8):2292–2296. 10.1161/STROKEAHA.117.017236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Dinan TG (2012) Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci 13(10):701–712. 10.1038/nrn3346 [DOI] [PubMed] [Google Scholar]
- Cuesta CM, Guerri C, Ureña J, Pascual M (2021) Role of microbiota-derived extracellular vesicles in gut-brain communication. Int J Mol Sci. 10.3390/ijms22084235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Virgiliis F, Di Giovanni S (2020) Lung innervation in the eye of a cytokine storm: neuroimmune interactions and covid-19. Nat Rev Neurol 16(11):645–652. 10.1038/s41582-020-0402-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng RM, Liu YC, Li JQ, Xu JG, Chen G (2020) The role of carbon dioxide in acute brain injury. Med Gas Res 10(2):81–84. 10.4103/2045-9912.285561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diard S, Liévin-Le MV, Toribio AL, Boum Y, Vigier F, Servin AL, Bouvet O (2009) Norepinephrine-dependently released dr fimbriae of diffusely adhering escherichia coli strain ih11128 promotes a mitogen-activated protein kinase erk1/2-dependent production of pro-inflammatory cytokine, il-8 in human intestinal caco-2/tc7 cells. Microbes Infect 11(10–11):886–894. 10.1016/j.micinf.2009.05.010 [DOI] [PubMed] [Google Scholar]
- Doll DN, Hu H, Sun J, Lewis SE, Simpkins JW, Ren X (2015) Mitochondrial crisis in cerebrovascular endothelial cells opens the blood-brain barrier. Stroke 46(6):1681–1689. 10.1161/STROKEAHA.115.009099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorscheid DR, Wojcik KR, Sun S, Marroquin B, White SR (2001) Apoptosis of airway epithelial cells induced by corticosteroids. Am J Respir Crit Care Med 164(10 Pt 1):1939–1947. 10.1164/ajrccm.164.10.2103013 [DOI] [PubMed] [Google Scholar]
- Douglas SD, Leeman SE (2011) Neurokinin-1 receptor: functional significance in the immune system in reference to selected infections and inflammation. Ann N Y Acad Sci 1217:83–95. 10.1111/j.1749-6632.2010.05826.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durgan DJ, Lee J, Mccullough LD, Bryan RJ (2019) Examining the role of the microbiota-gut-brain axis in stroke. Stroke 50(8):2270–2277. 10.1161/STROKEAHA.119.025140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards SC, Higgins SC, Mills K (2015) Respiratory infection with a bacterial pathogen attenuates CNS autoimmunity through il-10 induction. Brain Behav Immun 50:41–46. 10.1016/j.bbi.2015.06.009 [DOI] [PubMed] [Google Scholar]
- Farzi A, Fröhlich EE, Holzer P (2018) Gut microbiota and the neuroendocrine system. Neurotherapeutics 15(1):5–22. 10.1007/s13311-017-0600-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gackière F, Saliba L, Baude A, Bosler O, Strube C (2011) Ozone inhalation activates stress-responsive regions of the cns. J Neurochem 117(6):961–972. 10.1111/j.1471-4159.2011.07267.x [DOI] [PubMed] [Google Scholar]
- Gan DX, Wang YB, He MY, Chen ZY, Qin XX, Miao ZW, Chen YH, Li B (2020) Lung cancer cells-controlled dkk-1 production in brain metastatic cascade drive microglia to acquire a pro-tumorigenic phenotype. Front Cell Dev Biol 8:591405. 10.3389/fcell.2020.591405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi L, Johnson BE (2006) Paraneoplastic syndromes associated with small cell lung cancer. J Natl Compr Canc Netw 4(6):631–638. 10.6004/jnccn.2006.0052 [DOI] [PubMed] [Google Scholar]
- Giebelen IA, van Westerloo DJ, Larosa GJ, de Vos AF, van der Poll T (2007) Local stimulation of alpha7 cholinergic receptors inhibits lps-induced tnf-alpha release in the mouse lung. Shock 28(6):700–703. 10.1097/shk.0b013e318054dd89 [DOI] [PubMed] [Google Scholar]
- Gu X, Karp PH, Brody SL, Pierce RA, Welsh MJ, Holtzman MJ, Ben-Shahar Y (2014) Chemosensory functions for pulmonary neuroendocrine cells. Am J Respir Cell Mol Biol 50(3):637–646. 10.1165/rcmb.2013-0199OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha JY, Choi SY, Lee JH, Hong SH, Lee HJ (2020) Delivery of periodontopathogenic extracellular vesicles to brain monocytes and microglial il-6 promotion by RNA cargo. Front Mol Biosci 7:596366. 10.3389/fmolb.2020.596366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han EC, Choi SY, Lee Y, Park JW, Hong SH, Lee HJ (2019) Extracellular rnas in periodontopathogenic outer membrane vesicles promote tnf-α production in human macrophages and cross the blood-brain barrier in mice. FASEB J 33(12):13412–13422. 10.1096/fj.201901575R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriquez AR, House JS, Snow SJ, Miller CN, Schladweiler MC, Fisher A, Ren H, Valdez M, Kodavanti PR, Kodavanti UP (2019) Ozone-induced dysregulation of neuroendocrine axes requires adrenal-derived stress hormones. Toxicol Sci. 10.1093/toxsci/kfz182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer P, Farzi A (2014) Neuropeptides and the microbiota-gut-brain axis. Adv Exp Med Biol 817:195–219. 10.1007/978-1-4939-0897-4_9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosang L, Canals RC, van der Flier FJ, Hollensteiner J, Daniel R, Flügel A, Odoardi F (2022) The lung microbiome regulates brain autoimmunity. Nature 603(7899):138–144. 10.1038/s41586-022-04427-4 [DOI] [PubMed] [Google Scholar]
- Huang Y, Zhao C, Su X (2019) Neuroimmune regulation of lung infection and inflammation. QJM 112(7):483–487. 10.1093/qjmed/hcy154 [DOI] [PubMed] [Google Scholar]
- Ji P, Jiang T, Wang M, Wang R, Zhang L, Li Y (2013) Denervation of capsaicin-sensitive c fibers increases pulmonary inflammation induced by ischemia-reperfusion in rabbits. J Surg Res 184(2):782–789. 10.1016/j.jss.2012.12.011 [DOI] [PubMed] [Google Scholar]
- Johnston KC, Li JY, Lyden PD, Hanson SK, Feasby TE, Adams RJ, Faught RJ, Haley EJ (1998) Medical and neurological complications of ischemic stroke: experience from the ranttas trial. Ranttas Investig Stroke 29(2):447–453. 10.1161/01.str.29.2.447 [DOI] [PubMed] [Google Scholar]
- Kanayama M, Danzaki K, He YW, Shinohara ML (2016) Lung inflammation stalls th17-cell migration en route to the central nervous system during the development of experimental autoimmune encephalomyelitis. Int Immunol 28(9):463–469. 10.1093/intimm/dxw013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang DH, Coe CL, Karaszewski J, Mccarthy DO (1998) Relationship of social support to stress responses and immune function in healthy and asthmatic adolescents. Res Nurs Health 21(2):117–128. 10.1002/(sici)1098-240x(199804)21:2%3c117::aid-nur3%3e3.0.co;2-m [DOI] [PubMed] [Google Scholar]
- Kazarian M, Laird-Offringa IA (2011) Small-cell lung cancer-associated autoantibodies: potential applications to cancer diagnosis, early detection, and therapy. Mol Cancer 10:33. 10.1186/1476-4598-10-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr N, de Rivero VJ, Dietrich WD, Keane RW (2020) Neural-respiratory inflammasome axis in traumatic brain injury. Exp Neurol 323:113080. 10.1016/j.expneurol.2019.113080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JB, Sig CJ, Yu YM, Nam K, Piao CS, Kim SW, Lee MH, Han PL, Park JS, Lee JK (2006) Hmgb1, a novel cytokine-like mediator linking acute neuronal death and delayed neuroinflammation in the postischemic brain. J Neurosci 26(24):6413–6421. 10.1523/JNEUROSCI.3815-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim ID, Luo L, Lee HB, Lee HK, Lee JK (2015) Hmgb1-binding heptamer suppresses the synergistic effect of hmgb1 and lps by interacting directly with hmgb1. Neurosci Lett 593:40–44. 10.1016/j.neulet.2015.03.012 [DOI] [PubMed] [Google Scholar]
- Kim ID, Lee H, Kim SW, Lee HK, Choi J, Han PL, Lee JK (2018) Alarmin hmgb1 induces systemic and brain inflammatory exacerbation in post-stroke infection rat model. Cell Death Dis 9(4):426. 10.1038/s41419-018-0438-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitazawa M, Oddo S, Yamasaki TR, Green KN, Laferla FM (2005) Lipopolysaccharide-induced inflammation exacerbates tau pathology by a cyclin-dependent kinase 5-mediated pathway in a transgenic model of Alzheimer’s disease. J Neurosci 25(39):8843–8853. 10.1523/JNEUROSCI.2868-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahousse L, Tiemeier H, Ikram MA, Brusselle GG (2015) Chronic obstructive pulmonary disease and cerebrovascular disease: a comprehensive review. Respir Med 109(11):1371–1380. 10.1016/j.rmed.2015.07.014 [DOI] [PubMed] [Google Scholar]
- Lee KE, Kim JK, Han SK, Lee DY, Lee HJ, Yim SV, Kim DH (2020) The extracellular vesicle of gut microbial paenalcaligenes hominis is a risk factor for vagus nerve-mediated cognitive impairment. Microbiome 8(1):107. 10.1186/s40168-020-00881-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Zhang J, Pan S, Yuan Y, Qi H, Shu H, Hu Y, Ren L, Jiang Y, Yuan S (2020) Hmgb1 aggravates lipopolysaccharide-induced acute lung injury through suppressing the activity and function of tregs. Cell Immunol 356:104192. 10.1016/j.cellimm.2020.104192 [DOI] [PubMed] [Google Scholar]
- Liu DD, Chu SF, Chen C, Yang PF, Chen NH, He X (2018) Research progress in stroke-induced immunodepression syndrome (sids) and stroke-associated pneumonia (sap). Neurochem Int 114:42–54. 10.1016/j.neuint.2018.01.002 [DOI] [PubMed] [Google Scholar]
- Liu X, Ding H, Li X, Deng Y, Liu X, Wang K, Wen M, Chen S, Jiang W, Zeng H (2020) Hypercapnia exacerbates the blood-brain barrier disruption via promoting hif-1a nuclear translocation in the astrocytes of the hippocampus: implication in further cognitive impairment in hypoxemic adult rats. Neurochem Res 45(7):1674–1689. 10.1007/s11064-020-03038-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazato A (2016) mechanism of cryptococcus meningoencephalitis. Med Mycol J 57(1):J27–J32. 10.3314/mmj.57.J27 [DOI] [PubMed] [Google Scholar]
- Mumaw CL, Levesque S, Mcgraw C, Robertson S, Lucas S, Stafflinger JE, Campen MJ, Hall P, Norenberg JP, Anderson T, Lund AK, Mcdonald JD, Ottens AK, Block ML (2016) Microglial priming through the lung-brain axis: the role of air pollution-induced circulating factors. FASEB J 30(5):1880–1891. 10.1096/fj.201500047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham BD, Kaddurah-Daouk R, Mazmanian SK (2020) Gut microbial molecules in behavioural and neurodegenerative conditions. Nat Rev Neurosci 21(12):717–731. 10.1038/s41583-020-00381-0 [DOI] [PubMed] [Google Scholar]
- Niño DF, Zhou Q, Yamaguchi Y, Martin LY, Wang S, Fulton WB, Jia H, Lu P, Prindle TJ, Zhang F, Crawford J, Hou Z, Mori S, Chen LL, Guajardo A, Fatemi A, Pletnikov M, Kannan RM, Kannan S, Sodhi CP, Hackam DJ (2018) Cognitive impairments induced by necrotizing enterocolitis can be prevented by inhibiting microglial activation in mouse brain. Sci Transl Med. 10.1126/scitranslmed.aan0237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonomura K, Woo SH, Chang RB, Gillich A, Qiu Z, Francisco AG, Ranade SS, Liberles SD, Patapoutian A (2017) Piezo2 senses airway stretch and mediates lung inflation-induced apnoea. Nature 541(7636):176–181. 10.1038/nature20793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor TM, O’Connell J, O’Brien DI, Goode T, Bredin CP, Shanahan F (2004) The role of substance p in inflammatory disease. J Cell Physiol 201(2):167–180. 10.1002/jcp.20061 [DOI] [PubMed] [Google Scholar]
- Odoardi F, Sie C, Streyl K, Ulaganathan VK, Schläger C, Lodygin D, Heckelsmiller K, Nietfeld W, Ellwart J, Klinkert WE, Lottaz C, Nosov M, Brinkmann V, Spang R, Lehrach H, Vingron M, Wekerle H, Flügel-Koch C, Flügel A (2012) T cells become licensed in the lung to enter the central nervous system. Nature 488(7413):675–679. 10.1038/nature11337 [DOI] [PubMed] [Google Scholar]
- Oneal MJ, Schafer ER, Madsen ML, Minion FC (2008) Global transcriptional analysis of mycoplasma hyopneumoniae following exposure to norepinephrine. Microbiology (reading) 154(Pt 9):2581–2588. 10.1099/mic.0.2008/020230-0 [DOI] [PubMed] [Google Scholar]
- Pereira MR, Leite PE (2016) The involvement of parasympathetic and sympathetic nerve in the inflammatory reflex. J Cell Physiol 231(9):1862–1869. 10.1002/jcp.25307 [DOI] [PubMed] [Google Scholar]
- Pieper MP, Chaudhary NI, Park JE (2007) Acetylcholine-induced proliferation of fibroblasts and myofibroblasts in vitro is inhibited by tiotropium bromide. Life Sci 80(24–25):2270–2273. 10.1016/j.lfs.2007.02.034 [DOI] [PubMed] [Google Scholar]
- Portegies ML, Lahousse L, Joos GF, Hofman A, Koudstaal PJ, Stricker BH, Brusselle GG, Ikram MA (2016) Chronic obstructive pulmonary disease and the risk of stroke. The Rotterdam study. Am J Respir Crit Care Med 193(3):251–258. 10.1164/rccm.201505-0962OC [DOI] [PubMed] [Google Scholar]
- Prass K, Meisel C, Höflich C, Braun J, Halle E, Wolf T, Ruscher K, Victorov IV, Priller J, Dirnagl U, Volk HD, Meisel A (2003) Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke t helper cell type 1-like immunostimulation. J Exp Med 198(5):725–736. 10.1084/jem.20021098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin W, Yin J, Yang L, Yang S, Li Y, Li X, Hu W (2020) The relationship between chronic obstructive pulmonary disease and cerebral small vessel disease assessed by magnetic resonance imaging: a case-control study from a single center in Beijing, China. Med Sci Monit 26:e925703. 10.12659/MSM.925703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renelli M, Matias V, Lo RY, Beveridge TJ (2004) Dna-containing membrane vesicles of pseudomonas aeruginosa pao1 and their genetic transformation potential. Microbiology (reading) 150(Pt 7):2161–2169. 10.1099/mic.0.26841-0 [DOI] [PubMed] [Google Scholar]
- Salaga M, Blomster LV, Piechota-Polańczyk A, Zielińska M, Jacenik D, Cygankiewicz AI, Krajewska WM, Mikkelsen JD, Fichna J (2016) Encenicline, an α7 nicotinic acetylcholine receptor partial agonist, reduces immune cell infiltration in the colon and improves experimental colitis in mice. J Pharmacol Exp Ther 356(1):157–169. 10.1124/jpet.115.228205 [DOI] [PubMed] [Google Scholar]
- Samary CS, Ramos AB, Maia LA, Rocha NN, Santos CL, Magalhães RF, Clevelario AL, Pimentel-Coelho PM, Mendez-Otero R, Cruz FF, Capelozzi VL, Ferreira T, Koch T, de Abreu MG, Dos SC, Pelosi P, Silva PL, Rocco P (2018) Focal ischemic stroke leads to lung injury and reduces alveolar macrophage phagocytic capability in rats. Crit Care 22(1):249. 10.1186/s13054-018-2164-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos SC, Pelosi P, Leme SP, Rieken MRP (2016) Immunomodulation after ischemic stroke: potential mechanisms and implications for therapy. Crit Care 20(1):391. 10.1186/s13054-016-1573-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwechheimer C, Kuehn MJ (2015) Outer-membrane vesicles from gram-negative bacteria: biogenesis and functions. Nat Rev Microbiol 13(10):605–619. 10.1038/nrmicro3525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng JG, Bora SH, Xu G, Borchelt DR, Price DL, Koliatsos VE (2003) Lipopolysaccharide-induced-neuroinflammation increases intracellular accumulation of amyloid precursor protein and amyloid beta peptide in appswe transgenic mice. Neurobiol Dis 14(1):133–145. 10.1016/s0969-9961(03)00069-x [DOI] [PubMed] [Google Scholar]
- Snoek SA, Borensztajn KS, van den Wijngaard RM, de Jonge WJ (2010) Neuropeptide receptors in intestinal disease: physiology and therapeutic potential. Curr Pharm Des 16(9):1091–1105. 10.2174/138161210790963814 [DOI] [PubMed] [Google Scholar]
- Song N, Liu J, Shaheen S, Du L, Proctor M, Roman J, Yu J (2015) Vagotomy attenuates bleomycin-induced pulmonary fibrosis in mice. Sci Rep 5:13419. 10.1038/srep13419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui P, Wiesner DL, Xu J, Zhang Y, Lee J, Van Dyken S, Lashua A, Yu C, Klein BS, Locksley RM, Deutsch G, Sun X (2018) Pulmonary neuroendocrine cells amplify allergic asthma responses. Science. 10.1126/science.aan8546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze MA, Tsuruta M, Yang SW, Oh Y, Man SF, Hogg JC, Sin DD (2014) Changes in the bacterial microbiota in gut, blood, and lungs following acute lps instillation into mice lungs. PLoS ONE 9(10):e111228. 10.1371/journal.pone.0111228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey KJ (2009) Reflex control of immunity. Nat Rev Immunol 9(6):418–428. 10.1038/nri2566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trueba AF, Ritz T (2013) Stress, asthma, and respiratory infections: pathways involving airway immunology and microbial endocrinology. Brain Behav Immun 29:11–27. 10.1016/j.bbi.2012.09.012 [DOI] [PubMed] [Google Scholar]
- Wu S, Fang CX, Kim J, Ren J (2006) Enhanced pulmonary inflammation following experimental intracerebral hemorrhage. Exp Neurol 200(1):245–249. 10.1016/j.expneurol.2006.01.027 [DOI] [PubMed] [Google Scholar]
- Wypych TP, Wickramasinghe LC, Marsland BJ (2019) The influence of the microbiome on respiratory health. Nat Immunol 20(10):1279–1290. 10.1038/s41590-019-0451-9 [DOI] [PubMed] [Google Scholar]
- Xia Y, Wei Y, Li ZY, Cai XY, Zhang LL, Dong XR, Zhang S, Zhang RG, Meng R, Zhu F, Wu G (2019) Catecholamines contribute to the neovascularization of lung cancer via tumor-associated macrophages. Brain Behav Immun 81:111–121. 10.1016/j.bbi.2019.06.004 [DOI] [PubMed] [Google Scholar]
- Yang S, Stepien D, Hanseman D, Robinson B, Goodman MD, Pritts TA, Caldwell CC, Remick DG, Lentsch AB (2014) Substance p mediates reduced pneumonia rates after traumatic brain injury. Crit Care Med 42(9):2092–2100. 10.1097/CCM.0000000000000486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu YJ, Wang XH, Fan GC (2018) Versatile effects of bacterium-released membrane vesicles on mammalian cells and infectious/inflammatory diseases. Acta Pharmacol Sin 39(4):514–533. 10.1038/aps.2017.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Niu L, Li S, Le W (2019) Pathological impacts of chronic hypoxia on Alzheimer’s disease. ACS Chem Neurosci 10(2):902–909. 10.1021/acschemneuro.8b00442 [DOI] [PubMed] [Google Scholar]
- Zhao Y, Jaber V, Lukiw WJ (2017) Secretory products of the human gi tract microbiome and their potential impact on Alzheimer’s disease (ad): detection of lipopolysaccharide (lps) in ad hippocampus. Front Cell Infect Microbiol 7:318. 10.3389/fcimb.2017.00318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Ye Y, Gu L, Jian Z, Stary CM, Xiong X (2021) Extracellular vesicle-derived mirna as a novel regulatory system for bi-directional communication in gut-brain-microbiota axis. J Transl Med 19(1):202. 10.1186/s12967-021-02861-y [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and material are available.