Abstract

Cerebral endothelial H2S protects against cerebral ischemia-reperfusion injury through vasodilation, but its cerebral vasodilation mechanism and regulation of production are poorly understood. The RhoA-ROCK pathway plays important roles in vascular function. In this study, the roles of this pathway in the endothelial H2S production and vasodilation in rat cerebral arteries were investigated. Acetylcholine significantly increased H2S-generating enzyme cystathionine-γ-lyase (CSE) and 3-mercaptopyruvate sulfurtransferase (3-MST) protein expressions and H2S production in rat cerebrovascular endothelial cells (ECs), but the increases were markedly decreased by the M receptor blocker atropine or the CSE inhibitor dl-propargylglycine. Pretreatment with dl-propargylglycine or the 3-MST inhibitor l-aspartic acid markedly reduced the acetylcholine-increased H2S; CSE protein expression and H2S levels in the ECs were obviously attenuated by the RhoA agonist U46619 but increased by the RhoA inhibitor C3 transferase. U46619 also reduced 3-MST protein expression; Acetylcholine markedly inhibited RhoA protein expression and activity, but the inhibition was obviously reversed by atropine, dl-propargylglycine, and l-aspartic acid, respectively; Acetylcholine-induced endothelium-dependent vasodilation in rat cerebral basilar artery was significantly attenuated by pretreatment with dl-propargylglycine or l-aspartic acid or RhoA inhibitor CCG-1423 or ROCK inhibitor KD025, and was further decreased by co-pretreatment with dl-propargylglycine (or l-aspartic acid) and CCG-1423 (or KD025); NaHS significantly relaxed rat cerebral basilar artery vascular smooth muscle cells and inhibited ROCK1/2 activities, phosphorylated myosin light chain (MLC) protein expression, and KCl-increased [Ca2+]i, but these relaxation and inhibitions were markedly attenuated by pretreatment with C3 transferase or ROCK inhibitor Y27632. Our results demonstrated that endothelial H2S production is promoted by activation of the M receptor but inhibited by the RhoA-ROCK pathway in rat cerebral arteries; the endothelial H2S induces cerebral vasodilation by inhibiting this pathway to reduce phosphorylation of MLC and [Ca2+]i in vascular smooth muscle cells.
Introduction
Hydrogen sulfide (H2S) has been considered a pollutant and hazardous toxic gas for a long time. However, it is newly recognized as an important gaseous signal transmitter in mammals and has been implicated in diverse physiological functions and pathological processes, including hippocampal memory formation, regulation of vascular tone and blood pressure, cell angiogenesis, and inflammation.1,2 Endogenous H2S is mainly synthesized from l-cysteine by cystathionine β-synthase (CBS) and/or cystathionine-γ-lyase (CSE) in many types of mammalian cells.3 CBS is primarily located in nerve cells and liver cells, while CSE is present mostly in the cardiovascular system. In addition, endogenous H2S is also generated from 3-mercaptopyruvate by 3-mercaptopyruvate sulfurtransferase (3-MST), which is located in both the cytoplasm and mitochondria.
Endogenous and exogenous H2S produce vasodilation in various blood vessels.4−6 In the vasculature, the CSE protein is predominantly localized in the endothelium,7 indicating that vascular CSE-produced H2S is mainly generated in the endothelium. This finding agrees with the fact that H2S plays an important role in endothelium-dependent vasorelaxation.8 A previous study demonstrated that endothelial H2S protects against cerebral ischemia-reperfusion injury in rats.9 However, the regulatory mechanism of H2S generation in vascular endothelial cells (ECs) is poorly understood.
Endothelial H2S causes vasodilation in rat cerebral arteries by acting on Ca2+-activated K+ (KCa) channels in vascular smooth muscle cells (VSMCs).10 However, as a vascular relaxing factor, endothelial H2S may have more than one mechanism to relax cerebral vessels. For example, nitric oxide (NO), as a vascular relaxing factor, can initiate vasodilation not only by activating soluble guanylate cyclase to produce cyclic guanosine phosphate in VSMCs11 but also through inhibition of Rho-kinase signaling. Thus, in addition to promoting KCa channel opening, there may be other mechanisms involved in cerebral vasodilation of endothelial H2S.
Rho-kinase, also called rho-associated coiled coil-forming kinase (ROCK), is a direct and main downstream effector of RhoA, a small G protein. The RhoA-ROCK signaling pathway has critical roles in various cellular functions, such as cell proliferation, migration, contraction, and actin organization.12 In blood vessels, the RhoA-ROCK signaling pathway participates in the regulation of endothelial function and vascular tension.13−15 It is well known that the RhoA-ROCK signaling pathway is involved in endothelial NO synthase (eNOS) expression and NO production. Previous studies indicated that ROCK2 deletion increases eNOS expression and NO production in mice,14 but activation of the RhoA-ROCK signaling pathway inhibits the expression and activation of eNOS as well as NO production.16 Like NO, endothelial H2S is also an important endothelium-derived relaxing factor. However, it remains unclear whether the RhoA-ROCK signaling pathway is also involved in endothelial H2S production. Our recent study revealed that both exogenous and endothelial H2S could promote the phosphorylation of RhoA at Ser188 and inhibit its activation in neurons.17 And it is well known that the RhoA-ROCK signaling pathway plays a distinct role in the contraction of VSMCs.18,19 However, it is not clear whether the RhoA-ROCK signaling pathway participates in the regulation of endothelial H2S production and vasodilation in cerebrovascular vessels. Consequently, the present study was designed to investigate the role of the RhoA-ROCK signaling pathway in the endothelial H2S production and vasodilation in rat cerebral arteries.
Results and Discussion
H2S is an important vascular relaxing factor that results in vasodilation in various blood vessels, including cerebral arteries. Endothelial H2S may act as a backup for NO under ischemia-reperfusion20 and protect cerebral ECs21 and neurons.22 However, the regulation of its production and mechanisms of cerebral vasodilation need to be explored.
Role of the M Receptor in H2S-Generating Enzyme Expression and H2S Production
Factor VIII is a specific antigen of ECs. Using anti-factor VIII antibody, immunofluorescence staining examination showed that distinct green fluorescence appeared in the primary cultured rat cerebrovascular cells (Figure 1a), demonstrating that cultured rat cerebrovascular cells were ECs.
Figure 1.
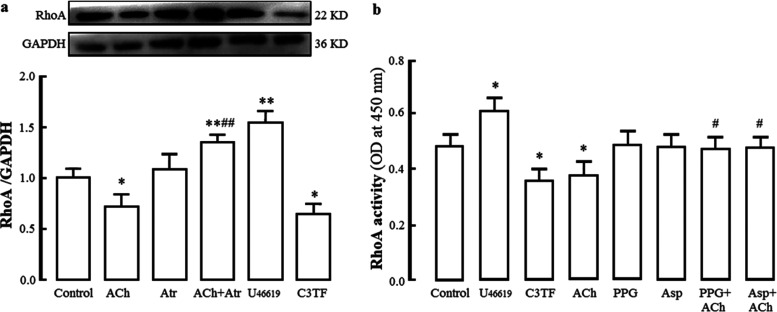
Roles of the M receptor and RhoA in H2S-generating enzyme CSE and 3-MST protein expression and H2S production in rat cerebrovascular endothelial cells. (a) Identification of primary cultured rat cerebrovascular endothelial cells. Immunofluorescence staining with anti-factor VIII antibody. Factor VIII exhibited green fluorescence in the cytoplasm, and the nucleus stained by 4′,6-diamidino-2-phenylindole (DAPI) presented blue fluorescence. (b) Effect of the M receptor agonist ACh and blocker Atr as well as the RhoA agonist U46619 and inhibitor C3TF on CSE and 3-MST protein expression (mean ± SD, n = 3). (c) Effects of the CSE inhibitor PPG and the 3-MST inhibitor Asp on basic and ACh-increased H2S production (mean ± SD, n = 6). (d) Effects of U46619 and C3TF on basic and ACh-increased H2S production (mean ± SD, n = 6). ACh: 1 μmol/L, Atr: 1 μmol/L; PPG: 100 μmol/L, Asp: 10 μmol/L, U46619: 100 nmol/L, C3TF: 1.0 μg/mL. *P < 0.05, **P < 0.01 vs the control group; #P < 0.05, ##P < 0.01 vs the ACh group.
It is well known that the M receptor agonist acetylcholine (ACh) can induce H2S production and H2S-mediated relaxation in rat cerebral arteries.10,20 As shown in Figure 1b, ACh (1 μmol/L) significantly increased H2S-generating enzyme CSE and 3-MST protein expression in rat cerebrovascular ECs compared with those in the control group, but the increases were markedly decreased by the M receptor-specific blocker atropine (Atr, 1 μmol/L); Figure 1c shows that ACh markedly increased but the CSE inhibitor dl-propargylglycine (PPG, 100 μmol/L) obviously reduced H2S content in rat cerebrovascular ECs compared with that in the control group. Pretreatment with PPG markedly reduced the ACh-increased H2S content. The results demonstrated that ACh activates the M receptor to increase CSE expression and the subsequent production of H2S in rat cerebrovascular ECs.
It is well known that 3-MST generates H2S from 3-mercaptopyruvate, which is produced from cysteine in the presence of α-ketoglutarate.23Figure 1b also demonstrates that ACh caused an obvious increase in the expression of 3-MST protein in ECs, and the increase was markedly decreased by Atr. Pretreatment with l-aspartic acid (Asp, 10 μmol/L), a 3-MST inhibitor, also significantly reduced the ACh-increased H2S content, but it had no notable effect on the H2S level in the basic state (Figure 1c). Because the cysteine concentration is much lower in the cytoplasm than in the mitochondria, 3-MST-produced H2S mainly occurs in the mitochondria, although 3-MST is located in both the cytoplasm and mitochondria.24 In the basic state, the H2S produced by 3-MST may be relatively low. This might account for the above-mentioned finding that Asp has no notable effect on the basic H2S level in ECs. Together with these data, our results suggested that activation of the M receptor also results in an increase in 3-MST-produced H2S in the mitochondria of rat cerebrovascular ECs.
Role of RhoA in CSE and 3-MST Protein Expression as well as H2S Production
It is very important to clarify the interactions among NO, H2S, and the RhoA-ROCK signaling pathway. NO is also an important endothelium-derived relaxing factor. H2S increases the eNOS phosphorylation and NO production in mouse aortic ECs.25 Our previous study indicated that during hypoxic injury of rat cerebrovascular ECs, the decrease of H2S occurred first, followed by a decrease of NO,26 suggesting that H2S might be beneficial for the NO production. On the other hand, NO donor sodium nitroprusside was found to increase the CSE expression and H2S production in rat vascular tissue.27 These data indicated that both NO and H2S promote each other in their biosynthesis. RhoA is a small GTPase protein. It and its downstream effector protein ROCK form the RhoA-ROCK signaling pathway in cells, which participates in the regulation of a variety of cellular functions, including growth, migration, differentiation, development, and contraction.28−30 A previous study indicated that the RhoA-ROCK signaling pathway inhibits the eNOS expression and NO production.16 The deletion of ROCK2 led to an increase of eNOS expression and NO production in mice,14 while activating the RhoA-ROCK signaling pathway induced a decrease of eNOS expression and activity as well as dephosphorylation of eNOS and inhibition of NO production;13,31 on the other hand, NO phosphorylates RhoA at Ser188 site and inhibits activation of RhoA, resulting in an inhibition of the RhoA-ROCK signaling pathway. These studies demonstrated there is a mutual inhibition between the RhoA-ROCK signaling pathway and NO generation. However, the interaction of the RhoA-ROCK signaling pathway with H2S is unclear in rat cerebral arteries.
As shown in Figure 1b, compared to the control group, CSE and 3-MST protein expression in rat cerebrovascular ECs was obviously reduced by the RhoA agonist U4661932 (100 nmol/L), and CSE but not 3-MST protein expression was significantly increased by the RhoA inhibitor C3 transferase33 (C3TF, 1.0 μg/mL). U46619 remarkably reduced but C3TF increased basic or ACh-increased H2S content in rat cerebrovascular ECs compared with that in the control group or the ACh group (Figure 1d). The results suggested that activation of RhoA downregulates but inhibition of RhoA upregulates CSE and 3-MST expression and H2S production in rat cerebrovascular ECs.
Inhibition of Endothelial H2S on RhoA Protein Expression and Activity
On the other hand, the effect of endothelial H2S on the RhoA-ROCK signaling pathway needs to be explored. Previous studies indicated that NO could lead to the inactivation of RhoA.34,35 Our recent study demonstrated that H2S protects rat hippocampal neurons from hypoxia-reoxygenation injury by reducing RhoA activity.17 However, there is still little information concerning the effect of endothelial H2S on RhoA in cerebrovascular ECs. As shown in Figure 2, compared to the control group, the RhoA agonist U46619 significantly increased RhoA protein expression and activity in rat cerebrovascular ECs, but the RhoA inhibitor C3TF notably decreased the protein expression and activity. Figure 2a shows that ACh markedly decreased RhoA protein expression in the ECs, and the decrease was blocked by Atr, a specific M receptor blocker. Figure 2b shows that ACh significantly inhibited RhoA activity in ECs, and the inhibition was reversed by PPG or Asp. Combined with the above-mentioned finding that activation of the M receptor promotes H2S generation in ECs, our results suggested that endothelial H2S inhibits RhoA expression and activity in rat cerebrovascular ECs. However, the direct interaction between the M receptor and RhoA in rat cerebrovascular ECs was still unclear and needs to be investigated in our next study.
Figure 2.
Inhibition of the M receptor agonist ACh on RhoA protein expression and activity in rat cerebrovascular ECs and the effects of the M receptor blocker Atr and the H2S-producing enzyme inhibitor PPG or Asp on the inhibition (mean ± SD, n = 3). (a) Protein expression. (b) RhoA activity. ACh: 1 μmol/L, Atr: 1 μmol/L, U46619: 0.1 μmol/L, C3TF: 1.0 μg/mL. *P < 0.05, **P < 0.01 vs the control group; #P < 0.05, ##P < 0.01 vs the ACh group.
Endothelial H2S-Mediated Cerebral Vasodilation in Rat
Increasing evidence demonstrates that exogenous and CES-produced H2S could induce cerebral vasorelaxation.9,20,36 As shown in Figure 3a, in the range of 1 × 10–7–1 × 10–5 mol/L, ACh induced a significant dilation in U46619-precontracted rat cerebral basilar artery (CBA) in a concentration-dependent manner (the PSS+ACh/+Endo group vs the PSS+vehicle/+Endo group), which was markedly abolished by denudation of endothelium (the PSS+ACh/–Endo group). The results indicated that ACh-induced vasodilation in rat CBA is endothelium-dependent.
Figure 3.
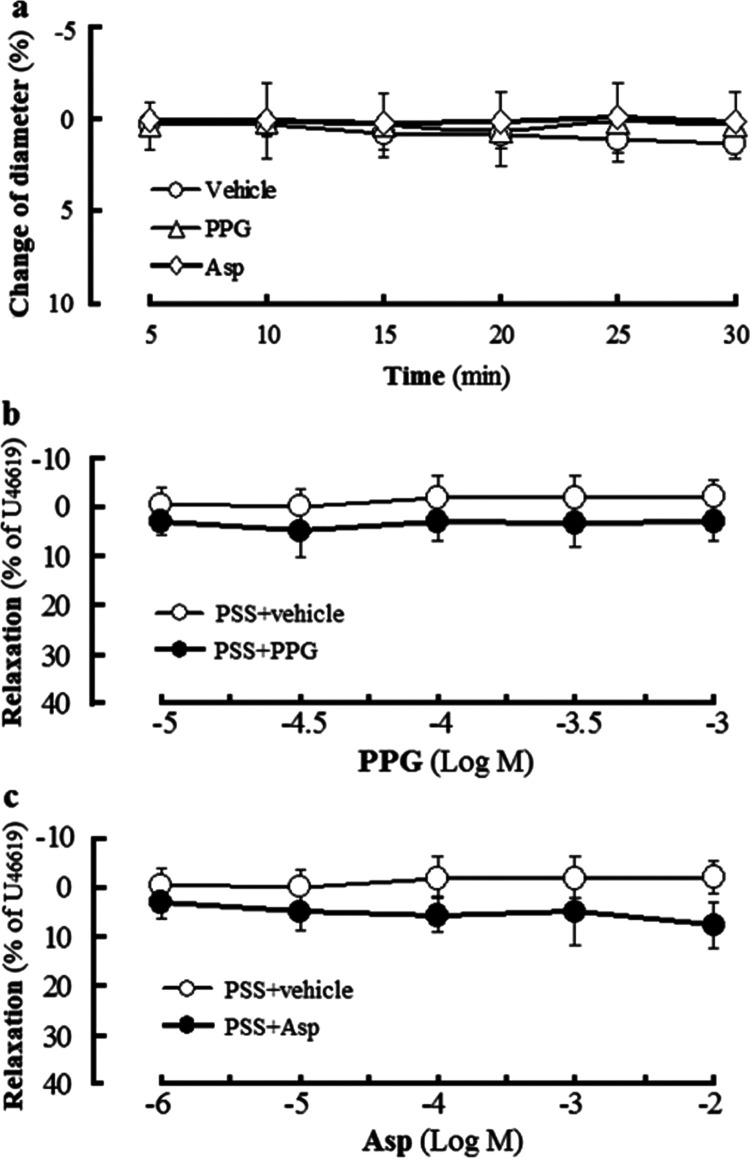
Effects of endothelium and pretreatment with H2S-producing enzyme inhibitor on ACh-induced vasodilation in rat cerebral basilar arteries (mean ± SD, n = 6). (a) Role of the endothelium in ACh-induced dilation in U46619-preconstricted arteries. (b) Effects of pretreatment with the CSE inhibitor PPG and the 3-MST inhibitor Asp on dilation of ACh in U46619-preconstricted arteries with endothelium. (c) Effect of PPG or Asp pretreatment on ACh-induced dilation in KCl-preconstricted arteries with endothelium. +Endo: artery ring with endothelium, −Endo: endothelium-denuded artery ring, PPG: 100 μmol/L, Asp: 10 μmol/L. **P < 0.01 vs the PPS+vehicle/+Endo group or the PPS+vehicle group; #P < 0.05, ##P < 0.01 vs the PPS+ACh/+Endo group or the PPS+ACh group; ΔΔP < 0.01 vs the PPG+ACh group.
Figure 4 shows that the H2S-producing enzyme CSE inhibitor PPG or the 3-MST inhibitor Asp had no obvious effect on either resting or U46619-precontracted rat CBA. However, Figure 3b,c shows that in U46619- or KCl-preconstricted rat CBA with endothelium, ACh-induced vasodilation was significantly attenuated by pretreatment with PPG or Asp, and the attenuation of vasodilation caused by Asp pretreatment was obviously less than that caused by pretreatment with PPG (the Asp+ACh group vs the PPG+ACh group), but further attenuation was not observed by combined pretreatment with PPG and Asp in U46619-precontracted rat CBA. The results indicated that both CSE-produced H2S and 3-MST-produced H2S mediate ACh-induced endothelium-dependent vasorelaxation in rat CBA, but CES-produced H2S plays a more considerable role in the vasorelaxation than 3-MST-produced H2S does.
Figure 4.
Effect of H2S-producing enzyme CSE inhibitor PPG or 3-MST inhibitor Asp in rat cerebral basilar artery with endothelium (mean ± SD, n = 6). (a) Effect of PPG or Asp in the resting artery. (b) effect of PPG in the U46619-precontracted artery. (c) effect of Asp in the U46619-precontracted artery. PPG: 100 μmol/L, Asp: 10 μmol/L.
Effects of RhoA and ROCK Inhibitors on Endothelial H2S-Mediated Vasodilation in Rat CBA
A previous study suggested that endothelial CSE-produced H2S inhibits RhoA activation and ROCK protein expression in mouse CBA VSMCs,36 suggesting that the RhoA-ROCK signaling pathway may be involved in endothelial H2S-induced cerebral vasodilation. However, this needs to be ascertained by further investigation.
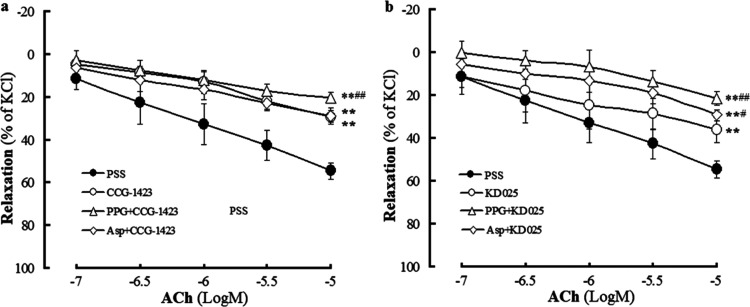
Figure 5 shows that both the RhoA inhibitor CCG-142337 and the ROCK inhibitor KD02538 induced significant vasodilation in KCl-precontracted rat CBA compared to that in the vehicle group, suggesting that inhibition of the RhoA-ROCK signaling pathway could produce vasodilation in rat CBA. Figure 5 also shows that CCG-1423 or KD025 at 1 × 10–6.5 mol/L neither had an effect on resting rat CBA nor relaxed KCl-precontracted rat CBA. However, Figure 6 shows that pretreatment with CCG-1423 or KD025 at this concentration significantly attenuated ACh-induced vasodilation in KCl-precontracted rat CBA (the CCG-1423 group or the KD025 group vs the PSS group), indicating that both RhoA and ROCK mediate the vasodilation of rat CBA to ACh. Together with the aforementioned endothelial H2S-mediated ACh-induced dilation in rat CBA, our results demonstrate that the RhoA-ROCK signaling pathway participates in endothelial H2S-mediated vasodilation in rat CBA. This conclusion is corroborated by the results that ACh-induced cerebral dilation in rat CBA was further attenuated by co-pretreatment with CCG-1423 and PPG or by co-pretreatment with KD025 and PPG (or Asp) (Figure 6).
Figure 5.
Effects of the RhoA inhibitor CCG-1423 and ROCK inhibitor KD025 in rat cerebral basilar artery with endothelium (mean ± SD, n = 6). (a) Effect of 1 × 10–6.5 mol/L CCG-1423 or KD025 in the resting artery and (b) vasodilation induced by CCG-1423 and KD025 in the KCl-precontracted artery. **P < 0.01 vs the vehicle group.
Figure 6.
Effect induced by pretreatment with the RhoA inhibitor CCG-1423 or the ROCK inhibitor KD025 alone and combined with the H2S-producing enzyme inhibitor on ACh-induced dilation in KCl-preconstricted rat cerebral basilar arteries with endothelium (mean ± SD, n = 6). (a) Pretreatment with CCG-1423 alone and combined with the CSE inhibitor PPG or the 3-MST inhibitor Asp. (b) Pretreatment with KD025 alone and combined with PPG or Asp. CCG-1423: 1 × 10–6 mol/L, KD025: 1 × 10–6 mol/L, PPG: 100 μmol/L, Asp: 10 μmol/L. **P < 0.01 vs PSS; #P < 0.05, ##P < 0.01 vs the CCG-1423 group or the KD025 group.
Roles of RhoA and ROCK in H2S-Induced Relaxation in Rat CBA VSMCs
Vasodilation of blood vessels depends on the relaxation of VSMCs. The relaxant effect of H2S in VSMCs is mostly obtained through vasodilation experiments. The present study directly observed the relaxation of H2S in rat CBA VSMCs. As shown in Figure 7a, primary cultured rat CBA VSMCs were identified by immunofluorescence staining examination using an anti-α smooth muscle actin (anti-α-SMA) antibody.
Figure 7.
Effect of pretreatment with the RhoA inhibitor C3TF or the ROCK inhibitor Y27632 on H2S-induced relaxation of rat cerebral basilar artery vascular smooth muscle cells (VSMCs) (mean ± SD, n = 6). (a) Identification of primary cultured rat cerebral basilar artery VSMCs. Immunofluorescence staining with anti-α-SMA antibody and phosphate buffer solution (PBS). α-SMA exhibited green fluorescence in the cytoplasm, and nuclei stained with 4′,6-diamidino-2-phenylindole (DAPI) presented blue fluorescence. (b) Relaxation of resting VSMCs. (c) Relaxation of KCl-precontracted VSMCs. C3TF: 1.0 μg/mL, Y27632: 10 μmol/L. **P < 0.01 vs the vehicle group; #P < 0.05, ##P < 0.01 vs the PSS pretreatment group.
As shown in Figure 7b,c, in the range of 12.5–200 μmol/L, the H2S donor NaHS significantly and concentration-dependently produced relaxation in both resting and KCl-precontracted rat CBA VSMCs. However, pretreatment with the RhoA inhibitor C3TF or the ROCK inhibitor Y27632 (10 μmol/L) significantly reduced NaHS-induced relaxation. Emax decreased from 20.3 ± 1.9 to 14.7 ± 2.4 or 16.2 ± 1.9% in resting VSMCs and from 91.4 ± 11.6 to 67.7 ± 4.7 or 78.8 ± 4.8% in KCl-precontracted VSMCs, respectively. Figure 8a,b shows that similar to C3TF, NaHS (100 μmol/L) markedly inhibited ROCK1 and ROCK2 activities in rat CBA VSMCs. Figure 8a,b also indicated that NaHS still inhibited ROCK1 and ROCK2 activities in rat CBA VSMCs pretreated with C3TF, suggesting that inhibition of H2S on ROCK1 and ROCK2 activities may be independent of RhoA. Together with the relaxation of VSMCs, it can be concluded that H2S induces the relaxation of rat CBA VSMCs by inhibiting the RhoA-ROCK signaling pathway.
Figure 8.
Inhibition of NaHS on ROCK activity, p-MLC protein expression and intracellular free Ca2+ concentration ([Ca2+]i) in rat cerebral basilar artery vascular smooth muscle cells and the effect of C3TF or Y27632 pretreatment on the inhibition. (a, b) ROCK1 and ROCK2 activities (mean ± SD, n = 5); (c) p-MLC protein expression (mean ± SD, n = 3); and (d) inhibition of the KCl-increased [Ca2+]i fluorescence intensity (FI) ratio (mean ± SD, n = 3). NaHS: 100 μmol/L, C3TF: 1.0 μg/mL, Y27632: 10 μmol/L. PBS: phosphate buffer solution. *P < 0.05, **P < 0.01 vs the PBS+vehicle group; #P < 0.05 vs the PBS+C3TF group; ΔP < 0.05 vs the PBS+NaHS group.
H2S-Inhibited Myosin Light Chain (MLC) Phosphorylation and Intracellular Free Ca2+ Concentration ([Ca2+]i) Increase and Role of RhoA or ROCK in the Inhibition
The contraction of VSMCs is primarily promoted by phosphorylation of MLC, which increases actin-activated myosin ATPase and consequent initiation contraction.39,40 Inhibition of MLC phosphorylation leads to VSMC relaxation. As a downstream target of the RhoA-ROCK signaling pathway, MLC is known to be directly phosphorylated by ROCK in vitro.41 It was reported that thiazovivin, a ROCK inhibitor, reversed angiotensin II-induced phosphorylation of MLC in cultured human VSMCs.42 [Ca2+]i plays a critical role in regulating the contraction of VSMCs. The increase in [Ca2+]i and then the formation of the Ca2+–calmodulin complex cause activation of MLC kinase, which leads to phosphorylation of MLC and subsequent VSMC contraction. It has been demonstrated that upregulation of the RhoA-ROCK signaling pathway increases cytosolic Ca2+ sensitization in smooth muscles, which increases vascular tone.43 In the rat aorta and mesenteric artery, ROCK causes ROCK-sensitive Ca2+ entry, which is distinct from voltage- and store-operated Ca2+ channels.44
As shown in Figure 8c,d, NaHS (100 μmol/L) significantly inhibited phosphorylated MLC (p-MLC) protein expression and 30 mmol/L KCl-increased [Ca2+]i fluorescence intensity in rat CBA VSMCs. However, in C3TF-pretreated VSMCs, the inhibitory effects were markedly reduced, and Y27632 pretreatment also significantly reduced NaHS-inhibited p-MLC protein expression. The results indicated that H2S could inhibit p-MLC protein expression and [Ca2+]i increase in rat CBA VSMCs, and RhoA or ROCK may be involved in the inhibition. Taken together with NaHS-induced relaxation and inhibition of ROCK activity, these data revealed that H2S relaxes rat CBA VSMCs by inhibiting the RhoA-ROCK signaling pathway to reduce MLC phosphorylation and [Ca2+]i increase.
In conclusion, the present study is the first to demonstrate the roles of the RhoA-ROCK signaling pathway in the endothelial H2S production and vasodilation in rat cerebral arteries. The main findings include the following (Figure 9): (1) in rat cerebrovascular ECs, endothelial H2S is primarily produced by CSE and 3-MST, and its production is promoted by the activation of the M receptor but inhibited by the RhoA-ROCK signaling pathway; (2) there was an interaction of reciprocal inhibition between activation of RhoA and production of H2S in the EC; and (3) endothelial H2S inhibited the RhoA-ROCK signaling pathway to reduce MLC phosphorylation and [Ca2+]i increase in VSMCs and to subsequently initiate vasodilation in rat CBA. These findings are very useful to illustrate the protective role of endothelial H2S in cerebral ischemia-reperfusion injury.
Figure 9.

Schematic diagram of the proposed role of the RhoA-ROCK signaling pathway in the endothelial H2S production and vasodilation in rat cerebral arteries. Acetylcholine (ACh) acting on the M receptor in the endothelial cell results in increases in CSE and 3-MST protein expression and H2S production. There is an interaction of reciprocal inhibition between the H2S and the RhoA. The H2S released from endothelial cell inhibits the RhoA-ROCK signaling pathway in the vascular smooth muscle cell, and the inhibition reduces phosphorylation of MLC (p-MLC) and intracellular free Ca2+ concentration ([Ca2+]i) and leads to a subsequent relaxation of the vascular smooth muscle cell.
Methods and Materials
Reagents
NaHS, ACh, Atr, C3TF, PPG, and Y27632 were purchased from Sigma Chemical (St. Louis); Asp was purchased from Solarbio (Beijing, China); KD025 was purchased from Merck (Darmstadt, Germany); CCG-1423 was purchased from ApexBio (Houston); fura-2-acetoxymethyl ester (Fura-2 AM) was purchased from Dojindo (Kumamoto, Japan); anti-α-SMA antibody, anti-β-actin antibody, and anti-p-MLC antibody were purchased from Affinity Biosciences (Changzhou, China); anti-CSE antibody and anti-RhoA antibody were purchased from Abcam (San Francisco); anti-3-MST antibody was purchased from Santa Cruz Biotechnology (Santa Cruz); anti-factor VIII antibody was purchased from Shanghai Fushen Biotechnology Co., Ltd. (Shanghai, China); H2S assay kit was purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China); RhoA activity assay kit, ROCK1 activity assay kit, and ROCK2 activity assay kit were purchased from Jiangsu Meimian Industrial Co., Ltd. (Yancheng, China); Dulbecco’s modified Eagle’s medium (DMEM) was purchased from HyClone (Beijing, China).
Animals
Adult Sprague–Dawley rats weighing 230–280 g of body weight aged 5–7 weeks (female to male = 1:1) were provided by the Experimental Animal Center of Anhui Medical University. The rats were housed in standardized cages (4–5 rats per cage, temperature: 22 ± 2 °C, relative humidity: 54 ± 3%) in a 12 h light/dark cycle and were given free access to food and water. The study and experimental procedures were approved by the Ethics Review Committee of the University, which follows the protocols outlined in the guide for the care and use of laboratory animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 2011).
Primary Cell Culture
Primary rat cerebrovascular endothelial cells (ECs) were prepared as previously described.17,18 Briefly, rats were anesthetized with isoflurane, and the brain was quickly harvested under sterile conditions. Cerebral arteries were isolated from the brain and minced into ∼1 mm3 pieces. The minced pieces were digested with 0.2% type II collagenase in DMEM at 37 °C for 30 min. The digested mixtures were centrifuged at 600g for 5 min to remove the collagenase solution. The precipitated cells were resuspended in DMEM/F12 medium supplemented with 5% fetal bovine serum (FBS), 1% basic fibroblast growth factor, 100 μg/mL heparin, and 1% penicillin–streptomycin and then cultured at 37 °C in a 5% CO2 incubator. The ECs were identified by immunofluorescence staining of factor VIII.
Primary cerebral VSMCs were obtained from rat CBA by trypsin enzymatic digestion as previously described.18 Rat CBA cells were cultured in DMEM supplemented with 20% FBS at 37 °C in a humidified incubator containing 5% CO2. The cultured cells were passaged by trypsin digestion when they reached 80–90% confluency. VSMCs were identified by immunofluorescence staining of α-SMA.
H2S Measurement
H2S was detected at 450 nm using H2S assay kits based on the formation of methylene blue as previously described10 according to the manufacturer’s instructions.
Western Blotting Assay
Western blotting assays were performed as previously described.21,36 Briefly, total proteins were extracted from primary rat cerebrovascular ECs or rat CBA VSMCs and quantified by BCA assay. Total protein (20 μg) was separated on 10% SDS-polyacrylamide gels and then transferred to polyvinylidene difluoride membranes. After being blocked with 5% skim milk, the membranes were incubated with the primary antibody overnight at 4 °C, followed by incubation with the appropriate secondary antibody at 37 °C for 1 h. The blot of each protein was developed with an enhanced chemiluminescence kit, and densitometry was used to determine the relative intensity of the blots.
Immunofluorescence Staining
Immunofluorescent identification of rat cerebrovascular ECs or rat CBA VSMCs was performed as previously reported.21 Briefly, after being fixed with 4% paraformaldehyde, the cells were permeabilized with 0.1% Triton X-100, blocked with 10% normal sheep serum (for ECs) or 1% bovine serum albumin (for VSMCs), and incubated with 1:200 primary antibody (anti-factor VIII antibody was used for ECs, and anti-α-SMA antibody was used for VSMCs) overnight at 4 °C. Then, the cells were incubated with a fluorescently labeled secondary antibody. The cell nucleus was stained with 4′,6-diamidino-2-phenylindole (DAPI). Immunofluorescent staining was detected under a laser scanning confocal microscope (TCS SP5, Mannheim, Germany).
Measurement of RhoA, ROCK1, and ROCK2 Activities
As described previously,17,45,46 the absorbance-based G-LISA activation assay Biochem kitTM was used to measure RhoA activity at 450 nm using a microplate spectrophotometer; ROCK1 and ROCK2 activities were measured at 450 nm using a kinase activity assay kit according to the manufacturer’s instructions.
Pressure Myography
Vasodilation was determined using a pressure myography system as previously described.20,46 Briefly, rats were sacrificed under anesthesia, and the brains were rapidly harvested and placed in precooled physiological salt dissolution (PSS, composition in mmol/L: NaCl 118, KCl 4.7, CaCl2 1.6, KH2PO4 1.2, MgSO4 1.2, NaHCO3 25, EDTA 0.026, glucose 5.5, pH 7.4) bubbled with 95% O2 + 5% CO2. CBA was carefully isolated from the brain and cut into an unbranched artery segment of 3 mm in length. The artery segment was inserted with glass micropipettes at both ends and fixed in a perfusion chamber of a DMT-114P Pressure Myograph System (Aarhus, Denmark), which was filled with PSS aerated with 95% O2 + 5% CO2 at 37 °C. The lumen of the segment was perfused with the same aerated PPS. After 60 min of equilibrium, 100 nmol/L U46619 or 30 mmol/L KCl was added to the luminal superfusate to induce stable vasocontraction. Vasodilation was subsequently caused by cumulatively adding ACh or NaHS. The diameter of the artery segment was continually measured by Pressure Myograph System software. Vasodilation was expressed as the percentage of the maximum diameter using the following formula
where Dmax is the initial diameter of the artery segment at equilibration for 60 min, Dmin is the stable diameter after adding KCl or U46619, and D is the diameter after adding ACh or NaHS.
VSMCs Relaxation Assay
As described previously,47 the prepared rat CBA VSMCs were plated into six-well plates (5 × 103 cells/well). The VSMCs were equilibrated and pretreated with PSS or C3TF or Y27632 for 30 min. KCl (30 mmol/L) was then added to precontract the VSMCs until a stable contraction was obtained, followed by the addition of NaHS to induce relaxation. Under an inverted microscope, changes in the long-axis length of the same cell were continually measured using Image-Pro Pro Plus 6.0 software. Relaxation of VSMCs was expressed as the percentage of the NaHS-increased long-axis length to KCl-shortened long-axis length using the following equation
where Lmax is the initial long-axis length at equilibration for 30 min, Lmin is the long-axis length after the addition of KCl, and L is the long-axis length after administration of NaHS.
Relaxation of NaHS in resting VSMCs was calculated by measuring the change in the long-axis length of KCl-untreated VSMCs.
Ca2+ Fluorescence Measurement
A fluorescence assay was used to measure [Ca2+]i according to previous reports.22,48 Briefly, primary cultured rat CBA VSMCs were pretreated with C3TF (1.0 μg/mL) or Y27632 (10 μmol/L) or phosphate buffer solution (PBS, composition in mmol/L: NaCl 137, KCl 2.7, Na2HPO4 10, KH2PO4 2, pH 7.4) for 6 h. The pretreated VSMCs were then incubated with DMEM/F12 and loaded with Fura-2 AM at a final concentration of 3 μmol/L at 37 °C for 30 min. After washing twice with Ca2+-free PSS (composition in mmol/L: NaCl 137, KCl 5.6, MgCl2 1.0, Na2HPO4 0.42, NaH2PO4 0.44, NaHCO3 4.2, glucose 10, HEPES 10, pH 7.4), the VSMCs were perfused with PSS containing 2 mmol/L CaCl2 at room temperature for 5 min. Ca2+ fluorescence was detected at 340/380 nm excitation and 505 nm emission wavelengths. [Ca2+]i was expressed as the fluorescence intensity (FI) ratio at 340/380 nm (F340/F380). The [Ca2+]i FI ratios of the VSMCs were measured at the resting state, with the addition of 30 mmol/L KCl and subsequent administration of 100 μmol/L NaHS. The inhibition of NaHS on the KCl-elevated [Ca2+]i was calculated according to the following formula
Statistical Analysis
Data are expressed as mean ± SD. Statistical analyses were performed by one-way ANOVA followed by the Duncan test to determine the difference between groups. A value of P < 0.05 was considered statistically significant.
Acknowledgments
The authors thank the National Natural Science Foundation of China (Nos. 81973510 and 81374002) and the Nature Science Research Project for Colleges and Universities in Anhui Province (No. KJ2021A1227) for financial support.
Author Contributions
† S.C., F.G., and X.L. contributed equally to this work. S.C., F.G., X.L., J.W., L.D., and Z.C. participated in research design and experiments. J.X. and M.X. contributed new reagents or analytical tools. S.C., F.G., and X.L. performed data analysis. S.C., Y.G., L.D., and Z.C. contributed to the writing of the manuscript.
The authors declare no competing financial interest.
References
- Mun J.; Kang H. M.; Jung J.; Park C. Role of hydrogen sulfide in cerebrovascular alteration during aging. Arch. Pharm. Res. 2019, 42, 446–454. 10.1007/s12272-019-01135-y. [DOI] [PubMed] [Google Scholar]
- Wen Y. D.; Wang H.; Zhu Y. Z. The drug developments of hydrogen sulfide on cardiovascular disease. Oxid. Med. Cell. Longevity 2018, 2018, 4010395 10.1155/2018/4010395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilek N.; Papapetropoulos A.; Toliver-Kinsky T.; Szabo C. Hydrogen sulfide: An endogenous regulator of the immune system. Pharmacol. Res. 2020, 161, 105119 10.1016/j.phrs.2020.105119. [DOI] [PubMed] [Google Scholar]
- Hart J. L. Vasorelaxation elicited by endogenous and exogenous hydrogen sulfide in mouse mesenteric arteries. Naunyn Schmiedeberg’s Arch. Pharmacol. 2020, 393, 551–564. 10.1007/s00210-019-01752-w. [DOI] [PubMed] [Google Scholar]
- Al-Magableh M. R.; Hart J. L. Mechanism of vasorelaxation and role of endogenous hydrogen sulfide production in mouse aorta. Naunyn Schmiedeberg’s Arch. Pharmacol. 2011, 383, 403–413. 10.1007/s00210-011-0608-z. [DOI] [PubMed] [Google Scholar]
- Streeter E.; Hart J.; Badoer E. An investigation of the mechanisms of hydrogen sulfide-induced vasorelaxation in rat middle cerebral arteries. Naunyn Schmiedeberg’s Arch. Pharmacol. 2012, 385, 991–1002. 10.1007/s00210-012-0779-2. [DOI] [PubMed] [Google Scholar]
- Yang G.; Wu L.; Jiang B.; Yang W.; Qi J.; Cao K.; Meng Q.; Mustafa A. K.; Mu W.; Zhang S.; Snyder S. H.; Wang R. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science 2008, 322, 587–590. 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bełtowski J.; Jamroz-Wiśniewska J. A. Hydrogen sulfide and endothelium-dependent vasorelaxation. Molecules 2014, 19, 21183–21199. 10.3390/molecules191221183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen J. Y.; Wang M.; Li Y. N.; Jiang H. H.; Sun X. J.; Chen Z. W. Vascular Protection of Hydrogen Sulfide on Cerebral Ischemia/Reperfusion Injury in Rats. Front. Neurol. 2018, 9, 779 10.3389/fneur.2018.00779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M.; Hu Y.; Fan Y.; Guo Y.; Chen F.; Chen S.; Li Q.; Chen Z. Involvement of Hydrogen Sulfide in Endothelium-Derived Relaxing Factor-Mediated Responses in Rat Cerebral Arteries. J. Vasc. Res. 2016, 53, 172–185. 10.1159/000448712. [DOI] [PubMed] [Google Scholar]
- Vanhoutte P. M.; Shimokawa H.; Feletou M.; Tang E. H. Endothelial dysfunction and vascular disease-a 30th anniversary update. Acta Physiol. 2017, 219, 22–96. 10.1111/apha.12646. [DOI] [PubMed] [Google Scholar]
- Tang L.; Dai F.; Liu Y.; Yu X.; Huang C.; Wang Y.; Yao W. RhoA/ROCK signaling regulates smooth muscle phenotypic modulation and vascular remodeling via the JNK pathway and vimentin cytoskeleton. Pharmacol. Res. 2018, 133, 201–212. 10.1016/j.phrs.2018.05.011. [DOI] [PubMed] [Google Scholar]
- Hassona M. D. H.; Abouelnaga Z. A.; Elnakish M. T.; Awad M. M.; Alhaj M.; Goldschmidt-Clermont P. J.; Hassanain H. Vascular hypertrophy-associated hypertension of profilin1 transgenic mouse model leads to functional remodeling of peripheral arteries. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H2112–H2120. 10.1152/ajpheart.00016.2010. [DOI] [PubMed] [Google Scholar]
- Hiroi Y.; Noma K.; Kim H. H.; Sladojevic N.; Tabit C. E.; Li Y.; Soydan G.; Salomone S.; Moskowitz M. A.; Liao J. K. Neuroprotection Mediated by Upregulation of Endothelial Nitric Oxide Synthase in Rho-Associated, Coiled-Coil-Containing Kinase 2 Deficient Mice. Circ. J. 2018, 82, 1195–1204. 10.1253/circj.CJ-17-0732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G.; Peng X.; Wu Y.; Li T.; Liu L. Involvement of connexin 43 phosphorylation and gap junctional communication between smooth muscle cells in vasopressin-induced ROCK-dependent vasoconstriction after hemorrhagic shock. Am. J. Physiol. Cell Physiol. 2017, 313, C362–C370. 10.1152/ajpcell.00258.2016. [DOI] [PubMed] [Google Scholar]
- Ming X. F.; Viswambharan H.; Barandier C.; Ruffieux J.; Kaibuchi K.; Rusconi S.; Yang Z. Rho GTPase/Rho kinase negatively regulates endothelial nitric oxide synthase phosphorylation through the inhibition of protein kinase B/Akt in human endothelial cells. Mol. Cell Biol. 2002, 22, 8467–8477. 10.1128/MCB.22.24.8467-8477.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.; Wen J.; Chen Z. H2S protects hippocampal neurons against hypoxia-reoxygenation injury by promoting RhoA phosphorylation at Ser188. Cell Death Discovery 2021, 7, 132 10.1038/s41420-021-00514-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.; Zheng X. R.; Riddick N.; Bryden M.; Baur W.; Zhang X.; Surks H. K. ROCK isoform regulation of myosin phosphatase and contractility in vascular smooth muscle cells. Circ. Res. 2009, 104, 531–540. 10.1161/CIRCRESAHA.108.188524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitaley K.; Webb R. C. Nitric oxide induces dilation of rat aorta via inhibition of rho-kinase signaling. Hypertension 2002, 39, 438–442. 10.1161/hy02t2.102960. [DOI] [PubMed] [Google Scholar]
- Han J.; Chen Z. W.; He G. W. Acetylcholine- and sodium hydrosulfide-induced endothelium-dependent relaxation and hyperpolarization in cerebral vessels of global cerebral ischemia–reperfusion rat. J. Pharmacol. Sci. 2013, 121, 318–326. 10.1254/jphs.12277FP. [DOI] [PubMed] [Google Scholar]
- Zhang F.; Chen S.; Wen J. Y.; Chen Z. W. 3-Mercaptopyruvate sulfurtransferase/hydrogen sulfide protects cerebral endothelial cells against oxygen-glucose deprivation/reoxygenation-induced injury via mitoprotection and inhibition of the RhoA/ROCK pathway. Am. J. Physiol. Cell Physiol. 2020, 319, C720–C733. 10.1152/ajpcell.00014.2020. [DOI] [PubMed] [Google Scholar]
- Wen J. Y.; Zhang J.; Chen S.; Chen Y.; Zhang Y.; Ma Z. Y.; Zhang F.; Xie W. M.; Fan Y. F.; Duan J. S.; Chen Z. W. Endothelium-derived hydrogen sulfide acts as a hyperpolarizing factor and exerts neuroprotective effects via activation of large-conductance Ca2+-activated K+ channels. Br. J. Pharmacol. 2021, 178, 4155–4175. 10.1111/bph.15607. [DOI] [PubMed] [Google Scholar]
- Wu D.; Hu Q.; Zhu D. An Update on Hydrogen Sulfide and Nitric Oxide Interactions in the Cardiovascular System. Oxid. Med. Cell. Longevity 2018, 2018, 4579140 10.1155/2018/4579140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. W.; Moore P. K. H2S Synthesizing Enzymes: Biochemistry and Molecular Aspects. Handb. Exp. Pharmacol. 2015, 230, 3–25. 10.1007/978-3-319-18144-8_1. [DOI] [PubMed] [Google Scholar]
- Altaany Z.; Moccia F.; Munaron L.; Mancardi D.; Wang R. Hydrogen sulfide and endothelial dysfunction: relationship with nitric oxide. Curr. Med. Chem. 2014, 21, 3646–3661. 10.2174/0929867321666140706142930. [DOI] [PubMed] [Google Scholar]
- Wang L. F.; Chen Z. W. Anoxic injury down-regulates hydrogen sulfide in rat cerebrovascular endothelial cells and H2S-mediated activation of RhoA-ROCK pathway. Acta Univ. Med. Anhui 2019, 54, 50–55. [Google Scholar]
- Nagpure B. V.; Bian J. S. Interaction of Hydrogen Sulfide with Nitric Oxide in the Cardiovascular System. Oxid. Med. Cell. Longevity 2016, 2016, 6904327 10.1155/2016/6904327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Z.; Jia Y.; Liu H.; He M.; Yang Y.; Xiao W.; Li Y. RhoA/ROCK pathway: implication in osteoarthritis and therapeutic targets. Am. J. Transl. Res. 2019, 11, 5324–5331. [PMC free article] [PubMed] [Google Scholar]
- Wirth A. Rho kinase and hypertension. Biochim. Biophys. Acta 2010, 1802, 1276–1284. 10.1016/j.bbadis.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Mulherkar S.; Tolias K. F. RhoA-ROCK signaling as a therapeutic target in traumatic brain injury. Cells 2020, 9, 245 10.3390/cells9010245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruhashi T.; Noma K.; Iwamoto Y.; Iwamoto A.; Oda N.; Kajikawa M.; Matsumoto T.; Hidaka T.; Kihara Y.; Chayama K.; Nakashima A.; Goto C.; Liao J. K.; Higashi Y. Critical role of exogenous nitric oxide in ROCK activity in vascular smooth muscle cells. PLoS One 2014, 9, e109017 10.1371/journal.pone.0109017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Y. X.; Zhai J. Y.; Du H. B.; Jiang L. N.; Zhang L. M.; Wang C.; Zhao Z. A.; Zhang C. H.; Zhao Z. G. Estrogen enhances the microvascular reactivity through RhoA-ROCK pathway in female mice during hemorrhagic shock. Shock 2021, 56, 611–620. 10.1097/SHK.0000000000001776. [DOI] [PubMed] [Google Scholar]
- Wang F.; Zhan R.; Chen L.; Dai X.; Wang W.; Guo R.; Li X.; Li Z.; Wang L.; Huang S.; Shen J.; Li S.; Cao C. RhoA promotes epidermal stem cell proliferation via PKN1-cyclin D1 signaling. PLoS One 2017, 12, e0172613 10.1371/journal.pone.0172613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauzeau V.; Le Jeune H.; Cario-Toumaniantz C.; Smolenski A.; Lohmann S. M.; Bertoglio J.; Chardin P.; Pacaud P.; Loirand G. Cyclic GMP-dependent protein kinase signaling pathway inhibits RhoA-induced Ca2+ sensitization of contraction in vascular smooth muscle. J. Biol. Chem. 2000, 275, 21722–21729. 10.1074/jbc.M000753200. [DOI] [PubMed] [Google Scholar]
- Sawada N.; Itoh H.; Yamashita J.; Doi K.; Inoue M.; Masatsugu K.; Fukunaga Y.; Sakaguchi S.; Sone M.; Yamahara K.; Yurugi T.; Nakao K. cGMP-dependent protein kinase phosphorylates and inactivates RhoA. Biochem. Biophys. Res. Commun. 2001, 280, 798–805. 10.1006/bbrc.2000.4194. [DOI] [PubMed] [Google Scholar]
- Wen J. Y.; Gao S. S.; Chen F. L.; Chen S.; Wang M.; Chen Z. W. Role of CSE-produced H2S on cerebrovascular relaxation via RhoA-ROCK inhibition and cerebral ischemia–reperfusion injury in mice. ACS Chem. Neurosci. 2019, 10, 1565–1574. 10.1021/acschemneuro.8b00533. [DOI] [PubMed] [Google Scholar]
- Liu G.; Yan T.; Li X.; Sun J.; Zhang B.; Wang H.; Zhu Y. Daam1 activates RhoA to regulate Wnt5a-induced glioblastoma cell invasion. Oncol. Rep. 2018, 39, 465–472. 10.3892/or.2017.6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. Y.; Stevens R. P.; Kash M.; Zhou C.; Koloteva A.; Renema P.; Paudel S. S.; Stevens T. KD025 shifts pulmonary endothelial cell bioenergetics and decreases baseline lung permeability. Am. J. Respir. Cell Mol. Biol. 2020, 63, 519–530. 10.1165/rcmb.2019-0435OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M.; Ma L.; Hall J. E.; Liu X.; Ying Z. Dual regulation of tumor necrosis factor-α on myosin light chain phosphorylation in vascular smooth muscle. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, H398–H406. 10.1152/ajpheart.00691.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodsome T. P.; Polzin A.; Kitazawa K.; Eto M.; Kitazawa T. Agonist- and depolarization-induced signals for myosin light chain phosphorylation and force generation of cultured vascular smooth muscle cells. J. Cell Sci. 2006, 119, 1769–1780. 10.1242/jcs.02805. [DOI] [PubMed] [Google Scholar]
- Totsukawa G.; Yamakita Y.; Yamashiro S.; Hartshorne D. J.; Sasaki Y.; Matsumura F. Distinct roles of ROCK (Rho-kinase) and MLCK in spatial regulation of MLC phosphorylation for assembly of stress fibers and focal adhesions in 3T3 fibroblasts. J. Cell Biol. 2000, 150, 797–806. 10.1083/jcb.150.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X.; Luo T.; Luo X.; Tang Z. Resveratrol prevents AngII-induced hypertension via AMPK activation and RhoA/ROCK suppression in mice. Hypertens. Res. 2014, 37, 803–810. 10.1038/hr.2014.90. [DOI] [PubMed] [Google Scholar]
- Ren J.; Fang C. X. Small guanine nucleotide-binding protein Rho and myocardial function. Acta Pharmacol. Sin. 2005, 26, 279–285. 10.1111/j.1745-7254.2005.00059.x. [DOI] [PubMed] [Google Scholar]
- Martinsen A.; Baeyens N.; Yerna X.; Morel N. Rho kinase regulation of vasopressin-induced calcium entry in vascular smooth muscle cell: comparison between rat isolated aorta and cultured aortic cells. Cell Calcium 2012, 52, 413–421. 10.1016/j.ceca.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Sun Z.; Wu X.; Li W.; Peng H.; Shen X.; Ma L.; Liu H.; Li H. RhoA/rock signaling mediates peroxynitrite-induced functional impairment of Rat coronary vessels. BMC Cardiovasc. Disord. 2016, 16, 193 10.1186/s12872-016-0372-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.; Chen Z. W.; Guo Y. Study on relationship between H2S induced vasodilation of rat cerebral basilar artery and RhoA as well as ROCK. Chin. Pharmacol. Bull. 2020, 6, 952–956. [Google Scholar]
- Chen J.; Chen Z. Effect of hydrogen sulfide on migration, proliferation and relaxation of rat basal artery vascular smooth muscle cells. Acta Univ. Med. Anhui 2020, 55, 1510–1514. [Google Scholar]
- Gutiérrez-Martín Y.; Martín-Romero F. J.; Henao F.; Gutiérrez-Merino C. Alteration of cytosolic free calcium homeostasis by SIN-1: high sensitivity of L-type Ca2+ channels to extracellular oxidative/nitrosative stress in cerebellar granule cells. J. Neurochem. 2005, 92, 973–989. 10.1111/j.1471-4159.2004.02964.x. [DOI] [PubMed] [Google Scholar]