Abstract
目的
探讨脯氨酸4-羟化酶Ⅱ(P4HA2)在肝癌细胞发生发展中的作用及相关机制。
方法
利用GEPIA、Human Protein Atlas数据库预测P4HA2在肝癌中的表达情况,利用K-M plotter在线数据库分析P4HA2的表达情况与肝癌预后的关系,采用qRT-PCR和Western blot检测肝癌细胞和正常肝细胞中P4HA2的表达。构建慢病毒载体,用携带P4HA2 shRNA和Con shRNA的慢病毒载体分别转染肝癌SNU-449和Hep-3B细胞系,建立沉默表达P4HA2的细胞株(shP4HA2组)和对照组细胞株(NC组)。采用CCK-8、集落形成试验、划痕实验和Transwell实验分别检测细胞增殖、迁移和侵袭能力。采用Western blot实验检测上皮-间质转化和PI3K/Akt/mTOR信号通路相关蛋白表达情况。
结果
在线数据库分析结果显示,肝癌组织中P4HA2表达高于正常肝组织(P < 0.05)。同时,肝癌细胞系中P4HA2 mRNA和蛋白表达水平也高于正常肝细胞(P < 0.01)。慢病毒干扰后,与NC组相比,shP4HA2组中mRNA和蛋白表达水平下降(P < 0.05)。P4HA2基因表达沉默后,细胞的增殖、迁移和侵袭受到抑制。Western blot显示,相对于NC组,shP4HA2组的E-cadherin蛋白表达上升,N-cadherin、Snail蛋白表达下降(P < 0.05),在PI3K/ AKT/mTOR通路中,磷酸化的PI3K(P-PI3K)、AKT(P-AKT)和mTOR(P-mTOR)显示出较低的水平(P < 0.05)。
结论
P4HA2通过激活PI3K/Akt/mTOR信号通路影响肝癌细胞的增殖、迁移、侵袭,促进肝癌的发生发展。
Keywords: 肝癌, 脯氨酸4-羟化酶Ⅱ, 迁移侵袭, PI3K/AKT/mTOR
Abstract
Objective
To investigate the role of proline 4-hydroxylase Ⅱ (P4HA2) in the occurrence and progression of liver cancer.
Methods
GEPIA and Human Protein Atlas database were used to predict the expression of P4HA2 in hepatocellular carcinoma (HCC), and K-M plotter online database was used to analyze the relationship between P4HA2 expression and the prognosis of HCC. We also examined the expressions of P4HA2 in HCC cells and normal hepatocytes using qRT-PCR and Western blotting. With lentivirus-mediated RNA interference, P4HA2 expression was knocked down in hepatoma SNU-449 and Hep-3B cells, and the changes in cell proliferation, migration and invasion were assessed using cell counting kit-8 (CCK-8) assay, colony formation test, scratch test and Transwell assay. The changes in the expressions of epithelial-mesenchymal transition (EMT) and PI3K/Akt/mTOR signal pathway-related proteins were detected using Western blotting.
Results
Online database analysis showed that the expression of P4HA2 was significantly higher in HCC tissues than in normal liver tissues (P < 0.05). The expression levels of P4HA2 mRNA and protein were also significantly higher in HCC cell lines than in normal hepatocytes (P < 0.01). Lentivirus-mediated RNA interference of P4HA2 significantly lowered the expression levels of P4HA2 mRNA and protein in the hepatoma cells (P < 0.05) and caused obvious inhibition of cell proliferation, migration and invasion. P4HA2 knockdown significantly increased the expression of E-cadherin protein, lowered the expressions of N-cadherin and Snail, and obviously decreased the expressions of phosphorylated PI3K, AKT and mTOR (P < 0.05).
Conclusion
P4HA2 enhances the proliferation, migration, invasion, and EMT of hepatoma cells by activating the PI3K/Akt/mTOR signaling pathway to promote the occurrence and progression of liver cancer.
Keywords: hepatocellular carcinoma, prolyl-4-hydroxylase alpha polypeptide Ⅱ, invasion and migration, PI3K/AKT/mTOR pathway
肝细胞癌(HCC)近几十年来发病率上升,虽然在临床和实验性癌症治疗方面取得了很大进展,但由于术后肿瘤复发和转移率高,HCC患者的总体预后较差[1-3]。肝癌的发生发展可能是一个多因素、多步骤的过程[3],但目前关于其具体的分子机制尚不清楚。因此,更好地了解HCC发生发展的分子机制对肝癌靶向治疗具有重要意义。细胞外基质(ECM)由多种大分子组成,包括胶原蛋白、纤维连接蛋白、弹性蛋白、层粘连蛋白、透明质酸和蛋白多糖[4]。ECM作为肿瘤微环境中含量最丰富的成分,可以调控肿瘤细胞行为和肿瘤进展,胶原蛋白是ECM的主要成分,具有促进肿瘤发展的作用,例如I型胶原是主要的纤维胶原,在多种癌症中高表达,通过促进肿瘤细胞增殖、迁移、侵袭、上皮间质转化(EMT)和化疗耐药促进癌症的进展[5-7]。Ⅳ型胶原是基底膜形成所需的非纤维性胶原,它促进肿瘤细胞粘附和迁移[8]。
脯氨酸4-羟化酶Ⅱ(P4HA2)是胶原蛋白形成的关键酶,可以催化胶原蛋白中的脯氨酸羟化,形成羟脯氨酸,促进胶原蛋白形成正确的空间结构[9]。P4HA2可以通过增加胶原的生成来调节癌细胞的行为,P4HA2与缺氧、胶原蛋白的沉积、糖酵解以及癌细胞的迁移侵袭有很大的相关性[10-12],并且P4HA2在许多肿瘤中表达增加,如乳腺癌、前列腺癌和宫颈癌[13-15]。有研究表明阿司匹林通过抑制NF-κB和LMCD1-AS1/let-7g轴靶向调控P4HA2来抑制肝癌的进展[16],但现有研究未进一步阐明P4HA2与肝癌之间的分子机制。
本研究利用数据库对P4HA2的表达及预后进行预测,之后构建P4HA2沉默模型,通过功能缺失性实验来观察P4HA2在肝癌细胞系中的增殖、迁移侵袭作用,旨在阐明P4HA2通过激活PI3K/AKT/mTOR通路来促进肝癌细胞的发生发展,为肝癌的诊断及治疗寻求新的肿瘤标志物及肿瘤治疗的新靶点。
1. 材料和方法
1.1. 材料
人肝癌细胞系SNU-449、Hep-3B(武汉普诺赛公司)。sh-P4HA2慢病毒构建由武汉汉恒生物公司构建完成,胎牛血清(clark);DMEM、1640培养基(Gibco);CCK-8试剂盒(碧云天)。Tranwell小室(Corning),P4HA2、E-cadherin、N-cadherin、snail、β-actin、p-Akt抗体(Proteintech);p-PI3K(ABcam);p-mTOR(Cell Signaling Technology)。
1.2. 方法
1.2.1. 生物信息学分析
通过GEPIA(<a href="http://gepia.cancer-pku.cn/" target="_blank">http://gepia.cancer-pku.cn/</a>)<sup>[<xref ref-type="bibr" rid="b17">17</xref>]</sup>、Human Protein Atlas(<a href="https://www.proteinatlas.org/" target="_blank">https://www.proteinatlas.org/</a>)<sup>[<xref ref-type="bibr" rid="b18">18</xref>]</sup>分析P4HA2的表达及相关指标,KM plotter(<a href="http://kmplot.com/analysis/" target="_blank">http://kmplot.com/analysis/</a>)<sup>[<xref ref-type="bibr" rid="b19">19</xref>]</sup>数据库评估P4HA2的预后作用。
1.2.2. 细胞培养
人肝癌细胞系(SMMC-7721、HepG2、HUH7、SNU-449和Hep3B)和人正常肝细胞(L-02)分别在含10%胎牛血清、1%的青霉素-链霉素的RPMI 1640或DMEM培养基中培养,放置于37 ℃,含5% CO2的细胞培养箱中,1~2 d换液,待细胞长到90% 以上传代或继续其他实验。
1.2.3. 慢病毒转染
以携带特异性针对人P4HA2基因的shRNA重组慢病毒载体(shP4HA2-1、shP4HA2-2、shP4HA2-3)感染Hep3B和SNU449细胞,构建P4HA2低表达的SNU-449和Hep3B细胞,以shRNA-阴性对照为对照组(NC),转染后用嘌呤霉素筛选。干扰序列见表 1。
1.
干扰序列
Interference sequence
| Gene name | Top strand | Bottom strand |
| NC | GATCCGTTCTCCGAACGTGTCACGTAAT TCAAGAGATTACGTGACACGTTCGGAGA ATTTTTTC |
AATTGAAAAAATTCTCCGAACGTGTCAC GTAATCTCTTGAATTACGTGACACGTTC GGAGAACG |
| shP4HA2-1 | GATCCGCAGCTGTGTTCTGGTACAACCT CTTCTCGAGAAGAGGTTGTACCAGAACA CAGCTGTTTTTTG |
AATTCAAAAAACAGCTGTGTTCTGGTAC AACCTCTTCTCGAGAAGAGGTTGTACCA GAACACAGCTGCG |
| shP4HA2-2 | GATCCGGCTGCAAGTGGGTCTCCAATAA GTCTCGAGACTTATTGGAGACCCACTTG CAGCCTTTTTTG |
AATTCAAAAAAGGCTGCAAGTGGGTCT CCAATAAGTCTCGAGACTTATTGGAGAC CCACTTGCAGCCG |
| shP4HA2-3 | GATCCGGAGTTCTTGAGACCTTGTGGAT CACTCGAGTGATCCACAAGGTCTCAAGA ACTCCTTTTTTG |
AATTCAAAAAAGGAGTTCTTGAGACCTT GTGGATCACTCGAGTGATCCACAAGGTC TCAAGAACTCCG |
1.2.4. qRT-PCR
细胞经慢病毒转染后使用Trizol提取总RNA,并逆转录成cDNA,以β-actin为内参进行PCR扩增。引物序列如下:
P4HA2-Forward:
5'-GGCCTGGTTTGGTGTCCTG3',
P4HA2-Reverse:
5'-GCCCAGCTCTTAATCTTGGAAAG3'。
β-Actin-Forward:
5'-GTGGACATCCGCAAAGAC-3',
β-Actin -Reverse:
5'-AAAGGGTGTAACGCAACTA-3'。
1.2.5. CCK-8实验
在Hep-3B细胞中,将shP4HA2组和NC组按2000/孔接种到96孔板中,SNU-449细胞3000/孔。复孔5个/组,分别于24、48、72、96 h加入10 μL CCK-8试剂,37 ℃培养4 h后,检测吸光度值A450 nm。
1.2.6. 创伤愈合实验
细胞接种于六孔板中,24 h后用10 μL短枪头划痕,PBS洗涤后加入完全培养基,观察0 h、24 h后迁移情况,用显微镜拍摄细胞间隙,用ImageJ软件分析。
1.2.7. Transwell检测细胞迁移和侵袭
用胰酶消化各组细胞并计数,在无血清培养基中制备细胞悬液,密度为5×104/孔。对于细胞侵袭,Matrigel解冻后用基础培养基稀释10倍,然后加到上层小室,放入37 ℃培养箱中1~2 h。之后将细胞接种于涂有Matrigel的上室,下室加入800 µL完全培养基,37 ℃培养24 h。对于细胞迁移,使用无Matrigel的小室,同样方法培养24 h后,用4%多聚甲醛固定30 min,0.1%结晶紫染色20 min,然后用纯水洗涤漂浮色,棉签拭去未穿过的细胞并在光学显微镜下观察拍照。
1.2.8. 克隆形成实验
将shP4HA2组和NC组按500/孔接种到六孔板中,培养5 d左右,待长出可见的细胞团即可固定染色,拍照计数。
1.2.9. 荧光克隆实验
将两组细胞分别接种到96孔板中(2000/孔),并在温箱中孵育5 d,每天用荧光显微镜拍照测定。
1.2.10. Western blot
收取shP4HA2组和NC组细胞,加入适量RIPA、PMSF冰上裂解,BCA法蛋白定量。SDSPAGE电泳分离后,然后转至PVDF膜上。室温下快速封闭液封闭15 min,随后一抗(1∶1000)在4 ℃下摇床过夜,次日用二抗孵育2 h,TBST洗膜3次之后ECL显影,检测蛋白条带。
1.3. 统计学分析
实验数据采用SPSS16.0、Graph Pad软件进行统计分析。所有实验均重复3次,定量数据采用均数±标准差表示,两组间比较采取独立样本t检验,多组间比较采用方差分析。P < 0.05为差异具有统计学意义。
2. 结果
2.1. P4HA2在肝癌细胞中的表达情况与预后关系
GEPIA数据库结果显示,与癌旁组织相比,P4HA2在肝癌组织表达上调(160例为癌旁组织,369例为肝癌组织)(P < 0.05,图 1A)。HPA数据库P4HA2在蛋白水平表达上也显示升高(图 1B)。在线数据库GEPIA、Kaplan-Meier Plotter评估P4HA2在HCC患者中的预后, 结果显示,P4HA2的表达越高,HCC患者的总生存率和疾病特异性生存期越短(P < 0.05,图 1C、D)。
1.
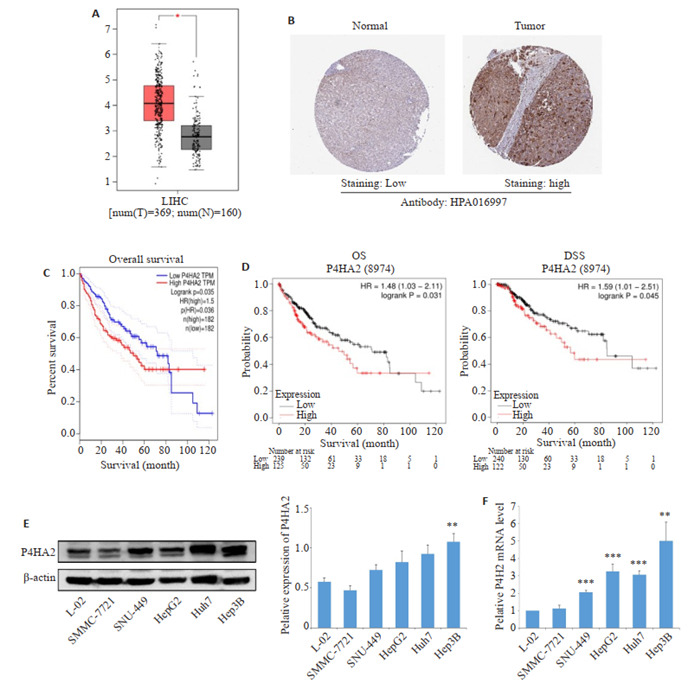
P4HA2在肝癌中的表达情况及其预后
Expression of P4HA2 in hepatocellular carcinoma (HCC) and its association with prognosis. A, C: P4HA2 expression and overall survival in HCC (GEPIA database). B: Expression of P4HA2 protein in HCC (Human Protein Atlas database). D: Kaplan-Meier survival analysis of the relationship between P4HA2 expression and prognosis of HCC patients. E, F: P4HA2 expression was up-regulated in HCC cells. The expression of P4HA2 was analyzed in 5 human hepatoma cell lines SMMC-7721, SNU-449, HepG2, Huh7, Hep3B and normal hepatocyte line L-02 by qRT-PCR and Western blotting. **P < 0.01, ***P < 0.001 vs L-02.
采用qRT-PCR和Western blot法检测肝癌细胞株和正常肝细胞中P4HA2的表达,结果显示,与正常肝细胞L-02相比,P4HA2在肝癌细胞株SNU-449、HepG2、Huh7、Hep3B中的表达均增加,差异有统计学意义,其中Hep3B中的表达增强最为明显(P < 0.01,图 1E、F)。因此选择Hep3B和SNU-449细胞系进行后续实验。
2.2. 构建P4HA2沉默模型
携带P4HA2 shRNA的慢病毒转染肝癌细胞,qRTPCR和Western blot检测在Hep3B和SNU-449细胞系中shP4HA2组的P4HA2 mRNA和蛋白表达效果,其中shP4HA2-3组的沉默效果最好(P < 0.05,图 2)。因此选择shP4HA2-3进行后续试验。
2.
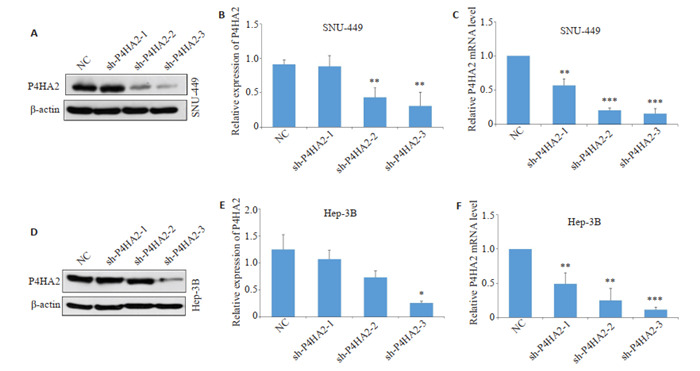
构建P4HA2慢病毒沉默肝癌细胞模型
Construction of hepatoma cell models with lentivirus-mediated P4HA2 silencing. Western blotting (A, B, D, E) and qRTPCR (C, F) were used to examine the silencing efficiency of 3 P4HA2 shRNAs in Hep3B and SNU-449 cells. *P < 0.05, **P < 0.01, ***P < 0.001 vs NC.
2.3. 沉默P4HA2抑制Hep3B和SNU-449细胞的增殖
荧光克隆实验结果显示,shP4HA2组细胞较NC组细胞增殖能力下降(P < 0.05,图 3A);CCK-8实验结果显示,与对照组相比,沉默P4HA2基因抑制Hep3B和SNU-449细胞系的增殖活力(P < 0.05,图 3B);细胞克隆形成实验显示,shP4HA2组细胞的集落数目较NC组明显减少,集落数目差异有统计学意义(P < 0.01,图 3C)。
3.
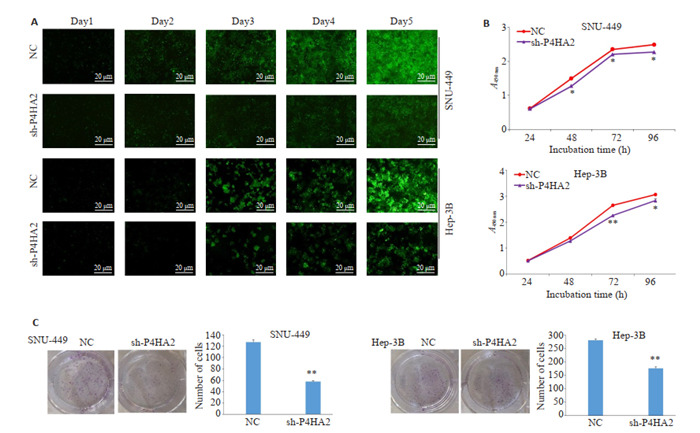
沉默P4HA2对Hep3B和SNU-449细胞增殖的影响
Effect of P4HA2 silencing on the proliferation of Hep3B and SNU-449 cells. A: Cells proliferation detected by fluorescence cloning experiment for five days. B: CCK8 test for assessing cell proliferation ability in shP4HA2 group and NC group. C: Clone formation test. Silencing P4HA2 significantly reduces the number of colonies in SNU-449 and Hep3B cells. *P < 0.05, **P < 0.01 vs NC.
2.4. 沉默P4HA2抑制Hep3B和SNU-449细胞的迁移和侵袭能力
创伤愈合实验显示,与NC组相比,shP4HA2组24 h划痕宽度较宽,沉默P4HA2降低了Hep3B和SNU- 449细胞的创伤愈合能力(P < 0.01,图 4)。Transwell小室实验结果显示,shP4HA2组与NC组相比,24 h穿过小室膜的迁移和侵袭细胞数目明显减少(P < 0.01,图 5)。
4.
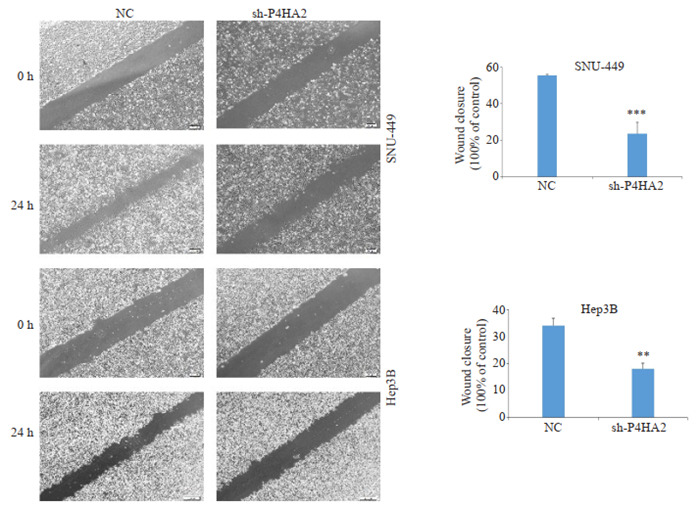
细胞划痕实验检测沉默P4HA2对肝癌细胞迁移的影响
Effect of P4HA2 silencing on the migration of hepatoma cells detected by cell scratch test (Scale bar=200 μm). Lentivirus-infected Hep3B and SNU-449 cells were photographed at 24 h to examine the healing rate. Data are presented as percentages of the 0-h and 24-h gap distance and displayed in histograms. **P < 0.01, ***P < 0.001.
5.
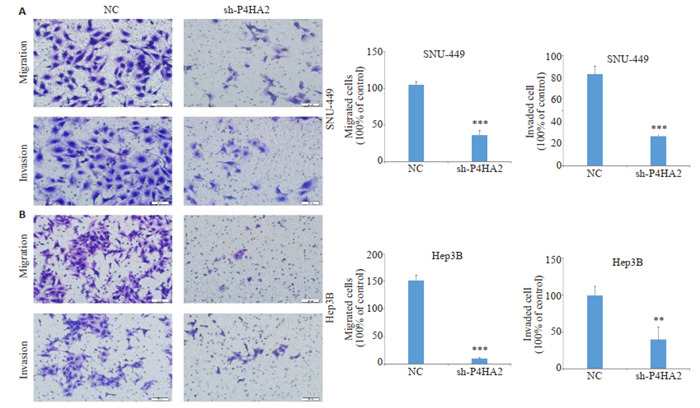
Transwell实验检测沉默肝癌细胞P4HA2对迁移侵袭的影响
Transwell assay for detecting the effect of P4HA2 silencing on cell migration and invasion (Scale bar=50 μm). A: Images of migration and invasion of SNU-449 cell lines (left) and statistical analysis of the cell numbers (right). B: Images of migration and invasion of Hep3B cell lines (left) and statistical analysis of cell numbers (right). **P < 0.01, ***P < 0.001.
2.5. 沉默P4HA2阻碍肝癌细胞系上皮-间质转化
Western blot结果显示,shP4HA2组E-cadherin的蛋白表达水平较NC组增加,而N-cadherin和Snail的表达下降(P < 0.05,图 6)。
6.
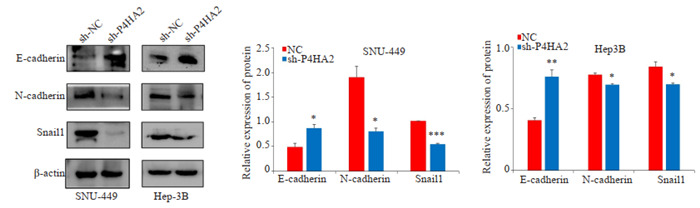
沉默P4HA2后肝癌细胞EMT相关标志物的变化
Western blotting for detecting changes in EMT-related markers (E-cadherin, N-cadherin, and Snail-1) in hepatoma cells after silencing P4HA2. *P < 0.05, **P < 0.01, ***P < 0.001 vs NC.
2.6. 沉默P4HA2抑制Hep3B和SNU-449细胞中PI3K/ AKT/mTOR信号通路的激活
Western blot结果显示,与NC组相比,sh-P4HA2组抑制了Hep3B和SNU-449细胞中p-PI3K、p-Akt和pmTOR蛋白的表达(P < 0.05,图 7)。
7.
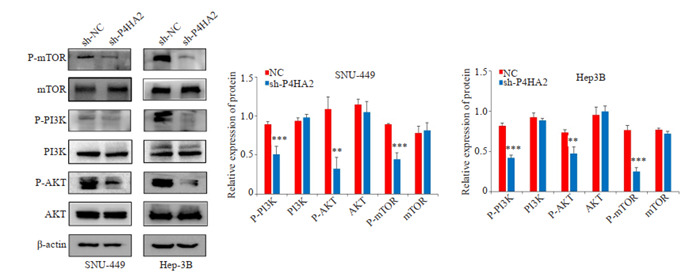
沉默P4HA2抑制肝癌细胞PI3K/AKT通路
Western blotting shows that silencing P4HA2 inhibits PI3K/Akt pathway in Hep3B and SNU-449 cells. **P < 0.01, ***P < 0.001 vs NC.
3. 讨论
肝癌的发展经常与炎症和纤维化相关[20, 21]。其中,细胞外基质的沉积在纤维化过程中起着重要作用[22-24],例如它可促进细胞生长、存活、迁移、粘附、血管生成,进而导致癌症进展。胶原作为细胞外基质中含量最丰富的成分,通过提高组织的硬度和抗拉强度[25],来维持细胞外结构。P4HA2是胶原合成的关键蛋白,在促进肿瘤的进展中起着重要作用。它参与了多个生物学过程,如胶原成熟、上皮间质转化、肿瘤进展中ECM的异常构建以及阿尔兹海默症中淀粉样蛋白β的沉积[16, 26-28]。P4HA2与肝纤维化相关性肝癌的发生有关[16],因此研究P4HA2在肝癌中的表达具有重要意义。本研究通过GEPIA、Human Protein Atlas数据库显示,与癌旁组织相比,P4HA2在肝癌组织中表达增高。Kaplan-Meier Plotter数据库分析P4HA2与肿瘤预后的关系,结果显示P4HA2表达越高,预后越差,总生存率、疾病特异性生存期均显著缩短。Western blot和QRT-PCR结果也显示,与正常肝细胞L-02相比,P4HA2在其他肝癌细胞中表达增加,与生物信息学分析结果一致。
P4HA2在多种肿瘤中表达增加,其过表达与肿瘤的增殖、转移、预后有密切的相关性。有研究发现在口腔鳞状细胞癌中,P4HA2的表达上调与转移密切相关[29],P4HA2可通过诱导胶原沉积促进乳腺癌转移[30]。本研究为了验证P4HA2与肝癌细胞的功能,用携带P4HA2 shRNA的慢病毒载体转染肝癌细胞系Hep-3B、SNU- 449,构建沉默表达模型。CCK-8、克隆斑、划痕、transwell等实验结果显示沉默P4HA2后肝癌细胞的增殖、迁移和侵袭能力降低,提示P4HA2在肝癌的发展进程中发挥着重要的作用。EMT常发生于肿瘤细胞侵袭和转移的早期阶段,细胞经历从上皮极化到间充质成纤维细胞的形态转化[31],参与侵袭、血管生成和转移相关的化疗耐药[32]。已有文献报道胶原蛋白的生物合成和沉积与癌症进展中的EMT过程密切相关[33]。在胶质瘤细胞中,P4HA2通过胶原依赖的PI3K/AKT通路激活EMT促进癌症的进展[11]。本研究发现在肝癌细胞系Hep3B和SNU-449中沉默P4HA2后,E-cadherin的表达增加,N-cadherin、Snail的表达下降,从而抑制肿瘤细胞EMT的过程,这些发现揭示了P4HA2通过EMT过程抑制HCC细胞迁移和侵袭。
PI3K/Akt/mTOR信号通路调控多种细胞功能,如增殖、存活、迁移和代谢;并且与多种肿瘤的发生发展密切相关[34, 35]。在非小细胞肺癌中,PI3K/AKT/mTOR通路与肿瘤发生和疾病进展密切相关。在乳腺癌中,PI3K/AKT/mTOR与细胞转化、肿瘤发生、癌症进展和耐药性相关。本研究利用Western blot检测PI3K/AKT/ mTOR通路相关蛋白的表达,实验结果显示,P4HA2基因被敲除后,p-PI3K、p-AKT和p-mTOR均受到抑制,而总蛋白却没有发生明显改变,表明P4HA2可能通过影响PI3K/AKT/mTOR途径而调控肝癌细胞的增殖、迁移、侵袭,参与肝癌的发生发展。本实验对P4HA2和HCC之间做了初步的体外实验,并且进行了部分凋亡方面的预实验,但实验结果总是容易反复,需进一步进行探索。所以要彻底了解P4HA2其内在机制,需要进行包括体内实验在内的一系列深入研究。
综上所述,本研究结果表明P4HA2在肝癌细胞中上调,可能通过PI3K/AKT/mTOR通路促进肝癌细胞的发生和发展,为肝癌的诊断及治疗寻求新的肿瘤标志物及肿瘤治疗的新靶点提供理论依据。
Biography
商玲,在读硕士研究生,E-mail: shanglingo@163.com
Funding Statement
安徽省教育厅自然科学研究项目(KJ2017A234);蚌埠医学院科技项目(2020byzd023)
Contributor Information
商 玲 (Ling SHANG), Email: shanglingo@163.com.
武 文娟 (Wenjuan WU), Email: wuwj_2012@126.com.
References
- 1.Waly Raphael S, Zhang YD, Chen YX. Hepatocellular carcinoma: focus on different aspects of management. ISRN Oncol. 2012;136:421673–9. doi: 10.5402/2012/421673. [Waly Raphael S, Zhang YD, Chen YX. Hepatocellular carcinoma: focus on different aspects of management[J]. ISRN Oncol, 2012, 136: 421673-9.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allemani C, Weir HK, Carreira H, et al. Global surveillance of cancer survival 1995-2009: analysis of individual data for 25, 676, 887 patients from 279 population-based registries in 67 countries (CONCORD-2. Lancet. 2015;385(9972):977–1010. doi: 10.1016/S0140-6736(14)62038-9. [Allemani C, Weir HK, Carreira H, et al. Global surveillance of cancer survival 1995-2009: analysis of individual data for 25, 676, 887 patients from 279 population-based registries in 67 countries (CONCORD-2[) J]. Lancet, 2015, 385(9972): 977-1010.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Serper M, Taddei TH, Mehta R, et al. Association of provider specialty and multidisciplinary care with hepatocellular carcinoma treatment and mortality. Gastroenterology. 2017;152(8):1954–64. doi: 10.1053/j.gastro.2017.02.040. [Serper M, Taddei TH, Mehta R, et al. Association of provider specialty and multidisciplinary care with hepatocellular carcinoma treatment and mortality[J]. Gastroenterology, 2017, 152(8): 1954-64.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gorres KL, Raines RT. Prolyl 4-hydroxylase. Crit Rev Biochem Mol Biol. 2010;45(2):106–24. doi: 10.3109/10409231003627991. [Gorres KL, Raines RT. Prolyl 4-hydroxylase[J]. Crit Rev Biochem Mol Biol, 2010, 45(2): 106-24.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng XL, Liu WY, Xiang JX, et al. Collagen I promotes hepatocellular carcinoma cell proliferation by regulating integrin β1/ FAK signaling pathway in nonalcoholic fatty liver. Oncotarget. 2017;8(56):95586–95. doi: 10.18632/oncotarget.21525. [Zheng XL, Liu WY, Xiang JX, et al. Collagen I promotes hepatocellular carcinoma cell proliferation by regulating integrin β1/ FAK signaling pathway in nonalcoholic fatty liver[J]. Oncotarget, 2017, 8(56): 95586-95.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamazaki S, Higuchi Y, Ishibashi M, et al. Collagen type Ⅰ induces EGFR-TKI resistance in EGFR-mutated cancer cells by mTOR activation through Akt-independent pathway. Cancer Sci. 2018;109(6):2063–73. doi: 10.1111/cas.13624. [Yamazaki S, Higuchi Y, Ishibashi M, et al. Collagen type Ⅰ induces EGFR-TKI resistance in EGFR-mutated cancer cells by mTOR activation through Akt-independent pathway[J]. Cancer Sci, 2018, 109(6): 2063-73.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang MC, Wang CJ, Liao PC, et al. Hepatic stellate cells secretes type Ⅰ collagen to trigger epithelial mesenchymal transition of hepatoma cells. Am J Cancer Res. 2014;4(6):751–63. [Yang MC, Wang CJ, Liao PC, et al. Hepatic stellate cells secretes type Ⅰ collagen to trigger epithelial mesenchymal transition of hepatoma cells[J]. Am J Cancer Res, 2014, 4(6): 751-63.] [PMC free article] [PubMed] [Google Scholar]
- 8.Öhlund D, Franklin O, Lundberg E, et al. Type Ⅳ collagen stimulates pancreatic cancer cell proliferation, migration, and inhibits apoptosis through an autocrine loop. BMC Cancer. 2013;13:154–62. doi: 10.1186/1471-2407-13-154. [Öhlund D, Franklin O, Lundberg E, et al. Type Ⅳ collagen stimulates pancreatic cancer cell proliferation, migration, and inhibits apoptosis through an autocrine loop[J]. BMC Cancer, 2013, 13: 154-62.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Myllyharju J. Prolyl 4-hydroxylases, the key enzymes of collagen biosynthesis. Matrix Biol. 2003;22(1):15–24. doi: 10.1016/S0945-053X(03)00006-4. [Myllyharju J. Prolyl 4-hydroxylases, the key enzymes of collagen biosynthesis[J]. Matrix Biol, 2003, 22(1): 15-24.] [DOI] [PubMed] [Google Scholar]
- 10.Kaluz S, Zhang Q, Kuranaga Y, et al. Targeting HIF-activated collagen prolyl 4-hydroxylase expression disrupts collagen deposition and blocks primary and metastatic uveal melanoma growth. Oncogene. 2021;40(33):5182–91. doi: 10.1038/s41388-021-01919-x. [Kaluz S, Zhang Q, Kuranaga Y, et al. Targeting HIF-activated collagen prolyl 4-hydroxylase expression disrupts collagen deposition and blocks primary and metastatic uveal melanoma growth[J]. Oncogene, 2021, 40(33): 5182-91.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin J, Jiang L, Wang XG, et al. P4HA2 promotes epithelial-tomesenchymal transition and glioma malignancy through the collagen-dependent PI3K/AKT pathway. J Oncol. 2021;131:1406853–60. doi: 10.1155/2021/1406853. [Lin J, Jiang L, Wang XG, et al. P4HA2 promotes epithelial-tomesenchymal transition and glioma malignancy through the collagen-dependent PI3K/AKT pathway[J]. J Oncol, 2021, 131: 1406853-60.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li QX, Wang QY, Zhang QY, et al. Collagen prolyl 4-hydroxylase 2 predicts worse prognosis and promotes glycolysis in cervical cancer. Am J Transl Res. 2019;11(11):6938–51. [Li QX, Wang QY, Zhang QY, et al. Collagen prolyl 4-hydroxylase 2 predicts worse prognosis and promotes glycolysis in cervical cancer [J]. Am J Transl Res, 2019, 11(11): 6938-51.] [PMC free article] [PubMed] [Google Scholar]
- 13.Toss MS, Miligy IM, Gorringe KL, et al. Prolyl-4-hydroxylase Α subunit 2 (P4HA2) expression is a predictor of poor outcome in breast ductal carcinoma in situ (DCIS) Br J Cancer. 2018;119(12):1518–26. doi: 10.1038/s41416-018-0337-x. [Toss MS, Miligy IM, Gorringe KL, et al. Prolyl-4-hydroxylase Α subunit 2 (P4HA2) expression is a predictor of poor outcome in breast ductal carcinoma in situ (DCIS)[J]. Br J Cancer, 2018, 119 (12): 1518-26.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu M, Peng RQ, Liang X, et al. P4HA2-induced prolyl hydroxylation suppresses YAP1-mediated prostate cancer cell migration, invasion, and metastasis. Oncogene. 2021;40(41):6049–56. doi: 10.1038/s41388-021-02000-3. [Zhu M, Peng RQ, Liang X, et al. P4HA2-induced prolyl hydroxylation suppresses YAP1-mediated prostate cancer cell migration, invasion, and metastasis[J]. Oncogene, 2021, 40(41): 6049-56.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu YY, Zhang XR, Wang J, et al. P4HA2 promotes cell proliferation and migration in glioblastoma. Oncol Lett. 2021;22(2):601–8. doi: 10.3892/ol.2021.12862. [Wu YY, Zhang XR, Wang J, et al. P4HA2 promotes cell proliferation and migration in glioblastoma[J]. Oncol Lett, 2021, 22(2): 601-8.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang TJ, Fu XL, Jin TZ, et al. Aspirin targets P4HA2 through inhibiting NF-κB and LMCD1-AS1/let-7g to inhibit tumour growth and collagen deposition in hepatocellular carcinoma. EBioMedicine. 2019;45:168–80. doi: 10.1016/j.ebiom.2019.06.048. [Wang TJ, Fu XL, Jin TZ, et al. Aspirin targets P4HA2 through inhibiting NF-κB and LMCD1-AS1/let-7g to inhibit tumour growth and collagen deposition in hepatocellular carcinoma[J]. EBioMedicine, 2019, 45: 168-80.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li CW, Tang ZF, Zhang WJ, et al. GEPIA2021: integrating multiple deconvolution-based analysis into GEPIA. Nucleic Acids Res. 2021;49(W1):W242–6. doi: 10.1093/nar/gkab418. [Li CW, Tang ZF, Zhang WJ, et al. GEPIA2021: integrating multiple deconvolution-based analysis into GEPIA[J]. Nucleic Acids Res, 2021, 49(W1): W242-6.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asplund A, Edqvist PHD, Schwenk JM, et al. Antibodies for profiling the human proteome-the Human protein atlas as a resource for cancer research. Proteomics. 2012;12(13):2067–77. doi: 10.1002/pmic.201100504. [Asplund A, Edqvist PHD, Schwenk JM, et al. Antibodies for profiling the human proteome-the Human protein atlas as a resource for cancer research[J]. Proteomics, 2012, 12(13): 2067-77.] [DOI] [PubMed] [Google Scholar]
- 19.Cheng P. A prognostic 3-long noncoding RNA signature for patients with gastric cancer. J Cell Biochem. 2018;119(11):9261–9. doi: 10.1002/jcb.27195. [Cheng P. A prognostic 3-long noncoding RNA signature for patients with gastric cancer[J]. J Cell Biochem, 2018, 119(11): 9261-9.] [DOI] [PubMed] [Google Scholar]
- 20.Jemal A, Ward EM, Johnson CJ, et al. Annual report to the nation on the status of cancer, 1975-2014, featuring survival. J Natl Cancer Inst. 2017;109(9):djx030–7. doi: 10.1093/jnci/djx030. [Jemal A, Ward EM, Johnson CJ, et al. Annual report to the nation on the status of cancer, 1975-2014, featuring survival[J]. J Natl Cancer Inst, 2017, 109(9): djx030-7.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oliverri RS, Wetterslev J, Gluud C. Hepatocellular carcinoma. Lancet. 2012;380(9840):470–6. doi: 10.1016/S0140-6736(12)61285-9. [Oliverri RS, Wetterslev J, Gluud C. Hepatocellular carcinoma[J]. Lancet, 2012, 380(9840): 470 -6.] [DOI] [PubMed] [Google Scholar]
- 22.Weiskirchen R, Weiskirchen S, Tacke F. Organ and tissue fibrosis: molecular signals, cellular mechanisms and translational implications. MolAspects Med. 2019;65:2–15. doi: 10.1016/j.mam.2018.06.003. [Weiskirchen R, Weiskirchen S, Tacke F. Organ and tissue fibrosis: molecular signals, cellular mechanisms and translational implications[J]. MolAspects Med, 2019, 65: 2-15.] [DOI] [PubMed] [Google Scholar]
- 23.Simko F, Pelouch V, Torok J, et al. Protein remodeling of the heart ventricles in hereditary hypertriglyceridemic rat: effect of ACEinhibition. J Biomed Sci. 2005;12(1):103–11. doi: 10.1007/s11373-004-8173-9. [Simko F, Pelouch V, Torok J, et al. Protein remodeling of the heart ventricles in hereditary hypertriglyceridemic rat: effect of ACEinhibition[J]. J Biomed Sci, 2005, 12(1): 103-11.] [DOI] [PubMed] [Google Scholar]
- 24.Adamcová M, Pelouch V, Gersl V, et al. Protein profiling in daunorubicin-induced cardiomyopathy. Gen Physiol Biophys. 2003;22(3):411–9. [Adamcová M, Pelouch V, Gersl V, et al. Protein profiling in daunorubicin-induced cardiomyopathy[J]. Gen Physiol Biophys, 2003, 22(3): 411-9.] [PubMed] [Google Scholar]
- 25.Kolácná L, Bakesová J, Varga F, et al. Biochemical and biophysical aspects of collagen nanostructure in the extracellular matrix. Physiol Res. 2007;56(Suppl 1):S51–60. doi: 10.33549/physiolres.931302. [Kolácná L, Bakesová J, Varga F, et al. Biochemical and biophysical aspects of collagen nanostructure in the extracellular matrix[J]. Physiol Res, 2007, 56(Suppl 1): S51-60.] [DOI] [PubMed] [Google Scholar]
- 26.Shi R, Gao SS, Smith AH, et al. Superoxide-induced Type Ⅰ collagen secretion depends on prolyl 4-hydroxylases. Biochem Biophys Res Commun. 2020;529(4):1011–7. doi: 10.1016/j.bbrc.2020.07.002. [Shi R, Gao SS, Smith AH, et al. Superoxide-induced Type Ⅰ collagen secretion depends on prolyl 4-hydroxylases[J]. Biochem Biophys Res Commun, 2020, 529(4): 1011-7.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao Y, Han QC, Li J, et al. P4HA2 contributes to cervical cancer progression via inducing epithelial-mesenchymal transition. J Cancer. 2020;11(10):2788–99. doi: 10.7150/jca.38401. [Cao Y, Han QC, Li J, et al. P4HA2 contributes to cervical cancer progression via inducing epithelial-mesenchymal transition[J]. J Cancer, 2020, 11(10): 2788-99.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang CY, Saar V, Leung KL, et al. Human amyloid β peptide and tau co-expression impairs behavior and causes specific gene expression changes in Caenorhabditis elegans. Neurobiol Dis. 2018;109:88–101. doi: 10.1016/j.nbd.2017.10.003. [Wang CY, Saar V, Leung KL, et al. Human amyloid β peptide and tau co-expression impairs behavior and causes specific gene expression changes in Caenorhabditis elegans[J]. Neurobiol Dis, 2018, 109: 88- 101.] [DOI] [PubMed] [Google Scholar]
- 29.Chang KP, Yu JS, Chien KY, et al. Identification of PRDX4 and P4HA2 as metastasis-associated proteins in oral cavity squamous cell carcinoma by comparative tissue proteomics of microdissected specimens using iTRAQ technology. J Proteome Res. 2011;10(11):4935–47. doi: 10.1021/pr200311p. [Chang KP, Yu JS, Chien KY, et al. Identification of PRDX4 and P4HA2 as metastasis-associated proteins in oral cavity squamous cell carcinoma by comparative tissue proteomics of microdissected specimens using iTRAQ technology[J]. J Proteome Res, 2011, 10 (11): 4935-47.] [DOI] [PubMed] [Google Scholar]
- 30.Xiong GF, Deng L, Zhu JQ, et al. Prolyl-4-hydroxylase α subunit 2 promotes breast cancer progression and metastasis by regulating collagen deposition. BMC Cancer. 2014;14:1–8. doi: 10.1186/1471-2407-14-1. [Xiong GF, Deng L, Zhu JQ, et al. Prolyl-4-hydroxylase α subunit 2 promotes breast cancer progression and metastasis by regulating collagen deposition[J]. BMC Cancer, 2014, 14: 1-8.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mishra R, Nathani S, Varshney R, et al. Berberine reverses epithelialmesenchymal transition and modulates histone methylation in osteosarcoma cells. Mol Biol Rep. 2020;47(11):8499–511. doi: 10.1007/s11033-020-05892-8. [Mishra R, Nathani S, Varshney R, et al. Berberine reverses epithelialmesenchymal transition and modulates histone methylation in osteosarcoma cells[J]. Mol Biol Rep, 2020, 47(11): 8499-511.] [DOI] [PubMed] [Google Scholar]
- 32.Chong ZX, Yeap SK, Ho WY. Unraveling the roles of miRNAs in regulating epithelial-to-mesenchymal transition (EMT) in osteosarcoma. Pharmacol Res. 2021;172:105818–27. doi: 10.1016/j.phrs.2021.105818. [Chong ZX, Yeap SK, Ho WY. Unraveling the roles of miRNAs in regulating epithelial-to-mesenchymal transition (EMT) in osteosarcoma[J]. Pharmacol Res, 2021, 172: 105818-27.] [DOI] [PubMed] [Google Scholar]
- 33.Shintani Y, Maeda M, Chaika NN, et al. Collagen I promotes epithelial-to-mesenchymal transition in lung cancer cells via transforming growth factor-beta signaling. Am J Respir Cell Mol Biol. 2008;38(1):95–104. doi: 10.1165/rcmb.2007-0071OC. [Shintani Y, Maeda M, Chaika NN, et al. Collagen I promotes epithelial-to-mesenchymal transition in lung cancer cells via transforming growth factor-beta signaling[J]. Am J Respir Cell Mol Biol, 2008, 38(1): 95-104.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corti F, Nichetti F, Raimondi A, et al. Targeting the PI3K/AKT/ mTOR pathway in biliary tract cancers: a review of current evidences and future perspectives. Cancer Treat Rev. 2019;72:45–55. doi: 10.1016/j.ctrv.2018.11.001. [Corti F, Nichetti F, Raimondi A, et al. Targeting the PI3K/AKT/ mTOR pathway in biliary tract cancers: a review of current evidences and future perspectives[J]. Cancer Treat Rev, 2019, 72: 45-55.] [DOI] [PubMed] [Google Scholar]
- 35.Gasparri ML, Besharat ZM, Farooqi AA, et al. MiRNAs and their interplay with PI3K/AKT/mTOR pathway in ovarian cancer cells: a potential role in platinum resistance. J Cancer Res Clin Oncol. 2018;144(12):2313–8. doi: 10.1007/s00432-018-2737-y. [Gasparri ML, Besharat ZM, Farooqi AA, et al. MiRNAs and their interplay with PI3K/AKT/mTOR pathway in ovarian cancer cells: a potential role in platinum resistance[J]. J Cancer Res Clin Oncol, 2018, 144(12): 2313-8.] [DOI] [PMC free article] [PubMed] [Google Scholar]


