Abstract
Titanium dioxide (TiO2) nanoparticles (NPs) are one of the topmost widely used metallic oxide nanoparticles. Whether present in naked form or doped with metals or polymers, TiO2 NPs perform immensely important functions. However, the alteration in size and shape by doping results in improving the physical, chemical, and biological behaviour of TiO2 NPs. Hence, the differential effects of various TiO2 nanostructures including nanoflakes, nanoflowers, and nanotubes in various domains of biotechnology have been elucidated by researchers. Recently, the exponential growth of research activities regarding TiO2 NPs has been observed owing to their chemical stability, low toxicity, and multifaceted properties. Because of their enormous abundance, plants, humans, and environment are inevitably exposed to TiO2 NPs. These NPs play a significant role in improving agricultural attributes, removing environmental pollution, and upgrading the domain of nanomedicine. Therefore, the currently ongoing studies about the employment of TiO2 NPs in enhancement of different aspects of agriculture, environment, and medicine have been extensively discussed in this review.
Keywords: drug delivery, nanobiotechnology, nanofertilisers, nanosensors, phototherapy, TiO2 NPs
1. INTRODUCTION
Nanotechnology is the branch of science that uses materials at the nanometre scale. Nanobiotechnology is the rapidly emerging field involving interface between nanotechnology and biology. This is interdisciplinary field having applications in almost all areas of biotechnology [1, 2]. Titanium dioxide (TiO2) nanoparticles (NPs), often called titania, are the most exceptional NPs among all transition metal oxide nanostructures due to their distinctive behaviour. The four polymorphic forms in which the crystalline structure of TiO2 NPs exists are rutile (tetragonal) of 2.96 eV bandgap, anatase (tetragonal) of 3.2 eV bandgap, brookite (orthorhombic) of 3.02 eV bandgap, and TiO2‐B (monoclinic) of 3.69 eV band gap [3]. All forms of titania are found in zero dimension (0D), one dimension (1D), two dimensions (2D), and three dimensions (3D) and have promising applications in biotechnology. However, the literature suggests that anatase and rutile have greater biotechnological applications than brookite and TiO2‐B owing to the highly stable nature of the former [3, 4].
TiO2 NPs are very cost efficient and easy to synthesise on laboratory and industrial scale. Among the various methods of preparation, sol–gel, hydrothermal, and chemical vapour deposition are the most common methods for TiO2 NPs' synthesis by which the NPs having desired characteristics are obtained [5]. These NPs have been approved by the Food and Drug Administration (FDA) as the safe, biocompatible, highly reactive, and chemically stable substances. It is estimated that the global annual production of TiO2 NPs was 1,175,176 tons in 2012 [6] and it will reach up to 2.5 million metric tons in 2050, which will convert nearly 100% of the total TiO2 market into nano [7]. It has been estimated that the concentration of TiO2 NPs in wastewater treatment plants is 5–20 μg/L and is expected to increase gradually with the use of different NPs [8, 9, 10]. TiO2 NPs are strong oxidising agents capable of very high photocatalysis. Other than that, nanostructures of TiO2 have profound applications in the agriculture and food industry, food packaging, textile, energy, ceramics, cosmetics, medical devices, pharmacy, and theranostics of various diseases [11, 12]. These are excellent sanitisers and the remarkable antibacterial, UV protection, and catalytic activity of TiO2 NPs makes them effective to be used in medicine, agriculture, and environmental remediation. The significance of TiO2 NPs lies in the fact that they can solve problems and challenges related to the diverse scientific fields [13, 14].
The unique physico‐chemical and biological properties of TiO2 NPs make them potential candidates for scientific investigation regarding their tremendous functionalities and technological applications [3]. As the TiO2 NPs have been explored in different fields of science due to their versatility, this review is a novel attempt to acknowledge the readers with the recent advances in the inclusive usage of TiO2 nanostructures regarding the areas of medical biotechnology (bioimaging, drug delivery, phototherapy, antimicrobial activity, and tissue regeneration), agricultural biotechnology (nanopesticides, nanofertilisers, and plant tolerance), and environmental biotechnology (nanosensors and soil/water/air remediation). According to our knowledge, this is the first comprehensive report outlining the applications of TiO2 NPs in the interrelated areas of biomedicine, agriculture, and environmental remediation in a single document.
2. TiO2 NANOPARTICLES IN BIOMEDICINE
In recent times, there has been a steadily growing interest in using metallic oxide NPs in various biomedical domains [15]. TiO2 NPs are extensively studied due to their fascinating properties such as low toxicity, biological and chemical alertness, thin film transparency, and high chemical stability. Among other applications, TiO2 NPs' medical applications are remarkable, which may play an important role in the improvement of healthcare sector. For example, the excellent photocatalytic ability of photoexcited TiO2 NPs depicts the ability to kill cancer cells significantly, and it can also be utilised in genetic engineering as a nucleic acid endonuclease. Moreover, TiO2 NP‐based nanostructures and nanocomposites may act as candidate tools for various biomedical applications [16]. However, the toxicological profile of TiO2 NPs should also be considered under both in vitro and in vivo conditions [17]. So far, ample research work focussing on the TiO2 NPs' biomedical applications has been reported. The following subsection describes the potential role of TiO2 NPs in drug delivery, bioimaging, phototherapy, tissue engineering, and as antibacterial agents.
2.1. Bioimaging
There are a wide variety of imaging techniques that can be used in scientific research and for the biomedical purposes, including spectroscopy techniques such as (near) infrared (IR) spectroscopy and Raman spectroscopy, nuclear magnetic resonance (MRI), radioimaging using specific nuclides, computed tomography (CT) scanning, and more advanced scanning techniques that include laser ablation, ICP‐MS, MALDI‐MS, and so forth [18]. Improvement in diagnostic techniques leads to early treatment and recovery of patient. NPs in general and TiO2 NPs in particular are extensively studied and used in the diagnostic techniques including MRI and CT as contrast agents. TiO2 NPs are activated by irradiation, therefore, act as both diagnostic and therapeutic agents at the same time (Figure 1). In a study, image contrast properties of TiO2 NPs were assessed by means of MRI and CT scanner. A clear change in imaging was detected between the control and TiO2 NP samples on T2‐weighted images, showing that TiO2 NPs can possibly be expended as an innovative theranostic drug with radiosensitising capacity and radiological diagnostic ability because of chemical group modification on their surface [19, 20]. Also, TiO2 NPs have numerous beneficial photo‐physical characteristics, particularly a photocatalytic feature. The photocatalytic activity of TiO2 NPs has been investigated for use in diagnostic assays.
FIGURE 1.
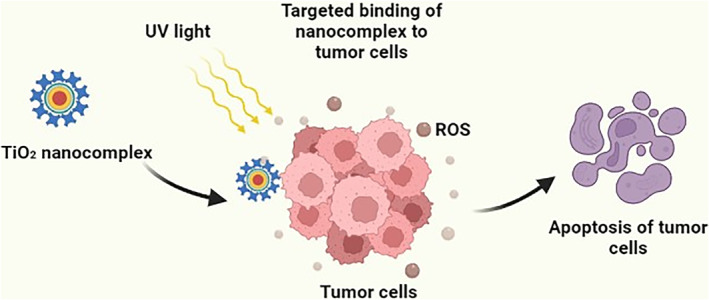
TiO2 nanoparticle‐based detection and killing of tumour cells
As per reports, the in situ labelling approaches for fluorescence microscopy to stain the TiO2 NPs taken up by the cells were studied in detail. Fluorescent biotin and fluorescent streptavidin were used to label the NPs before and after cellular uptake in the first approach. Whereas, in second approach, copper‐catalysed azide‐alkyne cycloaddition was used for labelling and detection of azide‐conjugated TiO2 NPs. Synchrotron X‐ray fluorescence microscopy (XFM) was used to detect TiO2 NPs. The results exhibiting TiO2 NPs by XFM displayed outstanding overlay with the site of optical fluorescence as detected by the confocal microscopy [21]. TiO2 NPs are prepared easily and modified, such as with europium (III), and TiO2 hollow nanoshells are feasible two‐photon nanoprobes. They cling to HeLa cervical cancer cells when coated with poly(ethylene imine) and hence detected [22]. According to the literature, mesoporous titania was coated on silver–silica core–shell NPs, resulting in an Ag@SiO2@mTiO2 nanoarchitecture. The metal core worked as a fluorescence enhancer. The particles were loaded with fluorescent flavin mononucleotide and the fluorescent cancer medication doxorubicin, and they were employed for simultaneous bimodal (fluorescence and surface‐enhanced Raman spectroscopy [SERS]) drug delivery imaging [23].
2.2. Drug delivery
Conventionally, routes of drug administration are oral, nasal, and parenteral, by which drug is distributed to the whole body. This limits the effectiveness of drug as well as causes the potential side effects. Thus, more efficient drug delivery systems are needed to overcome any drawbacks. Advancement in biotechnology, material science, and engineering approaches has incorporated the nanotechnology in biomedicine for targeted drug delivery. TiO2 NPs, owing to their unique electrochemical properties as well as controllable structures and biocompatibility, have been utilised in drug delivery processes [24]. Figure 2 represents the generalised process of drug delivery in living cells with and without TiO2 NPs. More specifically, the targeted drug delivery mechanism of TiO2 NPs involves the photocatalytic degradation of the organic compounds upon UV light excitation. When the energy absorbed from UV exceeds the band gap of TiO2 NPs, valence electrons are excited to the conduction band, forming an electron (e) and hole (h+) pair as well as active free radicals (OH and O2) that effectively decompose the organic compounds on the TiO2 NPs' surface, providing a theoretical foundation for light‐triggered drug release at a targeted site. It's worth noting that TiO2 NPs have been claimed to possess anticancer properties due to the generation of active free radicals on UV light exposure [25, 26].
FIGURE 2.
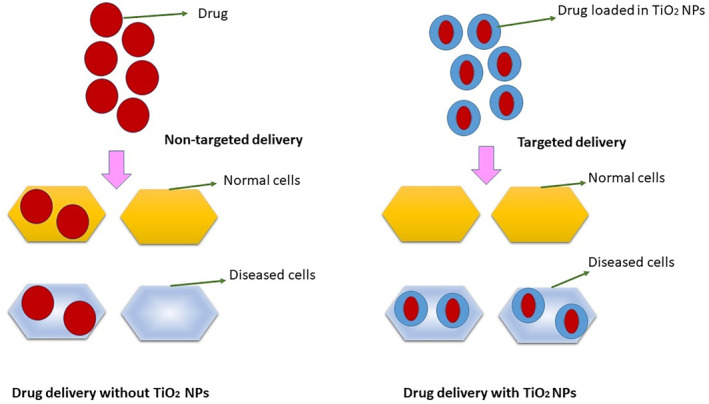
Diagrammatic representation of drug delivery with and without TiO2 nanoparticles
To make TiO2 NPs more biocompatible and to enhance their property of controlled drug release, these are coated with polyethylene glycol (PEG). TiO2 NPs were used as nanocarriers for the targeted drug delivery of an anticancer drug, paclitaxel. Although TiO2 NPs are stable and non‐toxic, their biocompatibility is enhanced by an attachment of PEG to their surface and folic acid (FA) grafted as a ligand for the targeted delivery of the drug. The characterisation studies revealed successful PEGylation and grafting of ligand to these NPs. Also, the drug‐loaded NPs held a significantly greater adsorption capability. Moreover, the in vitro release studies of paclitaxel from FA–PEG–TiO2 NPs showed an initial fast release followed by a sustained release phase [27].
Furthermore, an antitumour potential of TiO2 NPs loaded with paclitaxel was enhanced due to the incorporation of chitosan owing to its anti‐inflammatory and anti‐oxidant properties against osteosarcoma [28]. In another report, hollow TiO2 nanotubes were used along with iron oxide NPs as a nanocarrier through thermal annealing for magnetic guidance and drug delivery. Cytotoxic studies revealed that this nanocarrier was non‐toxic to HeLa cells at therapeutic concentrations (≤200 μg/ml). Under magnetic field gradient, adhesion and endocytosis to a layer of HeLa cells of nanotubes was observed. Two drugs, topoisomerase inhibitor camptothecin and oligonucleotides, for cell transfection were loaded in nanotubes exhibiting 90% and 100% killing and cellular uptake, respectively, hence suggesting the use of TiO2 NPs in therapeutics [29].
2.3. Phototherapy
Cancer is still one of the leading causes of death around the world and its treatment is also challenging. Primary treatment involves the surgery in combination with chemotherapy or radiotherapy. Chemotherapy faces challenges such as drug resistance or drug is inaccessible to the tumour cells, thus compromising its effectiveness [30]. Phototherapy, such as photodynamic therapy (PDT) and photothermal therapy (PTT), has become an emerging therapeutic technique for the treatment of different types of cancer because of its limited severity, high efficacy, and minimal side effects [31]. PDT is a comparatively recent therapeutic approach that has garnered attention in the last 3 decades. Its therapeutic principle needs the existence of a photosensitiser, proper light wavelength, and molecular oxygen. Photosensitiser and light are two adjustable elements among these three components, and the development of tumour‐specific photosensitiser is of interest to many chemists and pharmaceutical researchers. The photochemical, photobiological, and pharmacokinetic features of the photosensitiser influence the success of PDT therapy. Most strong photosensitisers contain a prolonged delocalised aromatic electron system, which permits them to absorb light effectively. In aqueous conditions, they quickly combine due to stacking and hydrophobic interactions. This leads to the aggregation, consequently leading to bioavailability and hampering of reactive oxygen species (ROS) generation [32].
Recently, NPs have been utilised to deliver photosensitisers for PDT, which is a good way to improve the targeting of photosensitisers [33]. In PDT, ROS generation takes place via stimulation of light absorbing photosensitisers causing cell damage. PDT is expended in the treatment of various malignancies and abnormal vasculatures [34]. Several studies are now underway to investigate the use of molybdenum oxide, TiO2, ZnO, and tungsten oxide NPs as photosensitisers in PDT [35]. Various reports have shown the promising results of using TiO2 NPs as photosensitisers or as conjugates to deliver photosensitisers in PDT and PTT as TiO2 NPs have unique catalytic activity. Moosavi and colleagues reported the photodynamic nitrogen‐doped titanium dioxide (N‐TiO2) NPs in conjugation with visible light. This novel PDT system showed not only the generation of ROS but also autophagy in leukaemia K562 cells. It implies that PDT with N‐TiO2 NPs is an efficient approach of priming autophagy via ROS generation. The potential of photo‐activated N‐TiO2 NPs to achieve desirable cellular outcomes constitutes a novel cancer cell treatment method [36]. In another study, scientists reported the combined effect of 2,2,6,6, tetramethylpiperidine‐N‐oxyl (TEMPO)‐coated TiO2 nanorods (NRs) for PDT. The sol–gel technique was employed for the synthesis of TiO2 NRs followed by TEMPO grafting via oxoammonium salt. It was reported that TEMPO‐grafted TiO2 NRs produced significant therapeutic response against human breast cancer cell line (MCF‐7) in combination with PDT under UV light as compared to TiO2 NRs in dark. Also, this nanocomposite had overcome the hindrance of multidrug resistance (MDR) alongside the PDT treatment [37].
Another phototherapy that employs NPs is PTT, which is a novel procedure to competently treat malignancies with no major limitation or side effect. In PTT, NPs convert the energy of photon into heat owing to their explicit physico‐chemical characteristics and generating hyperthermia in tumour tissues. A promising candidate with definite features for application in PTT of tumours is TiO2 NPs [38, 39]. TiO2 NPs along with a magnetic core make nano‐hybrid for imbedding an extensive range of theranostics qualities including magnetic‐guided and triggered therapeutic delivery systems. One such system has recently been developed, which is mesoporous TiO2‐coated Fe3O4 NPs. By employing combined production approach, the solvent thermal method was used to generate an amino‐functional magnetic core and porous shell formation via homogeneous precipitation of TiOSO4. To produce hollow TiO2 NPs, manufacture of iron oxide TiO2 core–shell nanocomposite is a benefit because of the easy magnetic core removal within the process [40].
2.4. Antimicrobial activity
Antibiotic overuse has resulted in the emergence of MDR bacterial strains, which is currently a source of concern for food safety and human health [41]. Metal oxide NPs have attracted the attention of researchers while looking for novel antibacterial compounds. One of the main contributing factors in antibiotic resistance is the formation of biofilms [42]. Therefore, research is shifted to the exploration of antimicrobial potential of metal and metal oxide NPs [43]. Because of their photocatalytic nature and the fact that they are chemically stable, non‐toxic, affordable, and Generally Recognised as Safe, TiO2 NPs have been regarded as an appealing antimicrobial agents [44]. Moreover, TiO2 NPs are one of the increasingly being used metal oxide NPs in biomedicine because of their antimicrobial activity [45].
Photocatalytic antimicrobial activity is exhibited by TiO2 NPs when irradiated with UV light (<385 nm). The effectiveness of TiO2 NPs' antimicrobial activity depends on the microbial cell surface thickness and the order is virus > bacterial wall > bacterial spore. In case of antibacterial activity, the generation of hydroxyl radicals as a response to photocatalysis by TiO2 NPs causes the oxidative stress on the cell membrane of bacteria. The photocatalytic activity of TiO2 NPs augments peroxidation of unsaturated phospholipids present in the plasma membrane, consequently damaging the bacterial membrane. It also interferes with vital biological mechanisms including respiration, oxidative phosphorylation reaction, and semi‐permeability [46]. The simplified mechanism of action of TiO2 NPs against bacteria has been illustrated in Figure 3.
FIGURE 3.
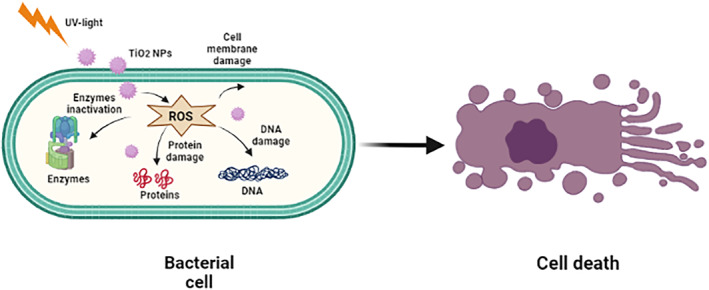
Antibacterial action mechanism of TiO2 nanoparticles
Among the major nosocomial infections, methicillin‐resistant Staphylococcus aureus (MRSA) is involved in broad‐spectrum infections and the emergence of bacterial resistance. According to the literature, the antibacterial activity of TiO2 NPs against MRSA was explored. A study was designed to evaluate the effect of TiO2 NPs against the biofilm formation by MRSA using tissue culture plate method. Total 30 isolates were taken and out of them, 22 were involved in strong biofilm formation and 2 were weak in the formation of biofilm. The TiO2 NPs (500 μg/ml) inhibited the growth of both strong and weak MRSA, thus showing that the TiO2 NPs are promising antibacterial candidates [47]. In another study, TiO2 NPs were used in combination with antibiotics including ceftazidime and cefotaxime against MDR Pseudomonas aeruginosa. The samples were isolated from sputum, pus, bronchoalveolar lavage, and endotracheal tract. On exposure to UV light for 1 h, a bactericidal effect was observed at >350 μg/ml. The minimum inhibitory concentration values of TiO2 NPs were observed six fold higher than the antibiotics. Hence, the combination of antibiotics and TiO2 NPs improved the antimicrobial activity [48].
The inclusion of antibacterial agents into synthetic polymer‐based nanocomposites has resulted in the creation of adaptable antibacterial materials suitable for a wide range of biomedical, packaging, and general‐purpose applications. However, the production of such materials using TiO2 NPs is difficult due to thermodynamic and kinetic hurdles that prevent inorganic and generally hydrophilic nanoparticles from dispersing in hydrophobic polymer matrices. Nevertheless, antimicrobial nanocomposites based on titania have received a lot of attention in recent years [49]. For example, Petica and co‐workers reported the synthesis of silver–titania nanocomposites by the electrochemical method for enhancement of photocatalytic characteristics and antifungal and antibacterial activity [50]. In another study, researchers developed the paraffin and silver‐coated titania NPs in polyethylene nanocomposite for food packaging. Addition of 3% and 5% of TiO2/Ag NPs into polyethylene with low density through melt‐blending nanocomposite films was performed and results revealed that 5% addition of TiO2/Ag NPs caused significant reduction of bacterial growth [51]. Thus, adding the TiO2 alone as well as in combination with the polymers reduces the microbial growth, therefore, preventing food spoilage and increasing the shelf‐life of food.
2.5. Tissue regeneration
Organ and tissue transplantation has been limited due to the non‐availability or lack of donor, tissue rejection, and use of immunosuppressants. This has increased the need and demand for tissue engineering and regenerative medicine [52]. Efforts have been shifted to the development of miniaturised artificial organs resembling the in vivo environment of the body. Nanoscale entities have shown better results as compared to macroscopic ones by providing good mechanical strength, delivery of bioactive agents, and monitoring cell activities [53]. Moreover, disadvantages and restraining issues, for instance, short half‐life, low solubility, and instability of bioactive molecules and contrast agents, have made the NPs as promising carriers for bioactive agents' delivery and monitoring for biomedical applications [54]. Table 1 shows the regeneration of different tissues using TiO2 NPs.
TABLE 1.
TiO2 nanocomposites used in tissue engineering
| Tissue | Hierarchical structure of TiO2 NPs | Concentration of TiO2 NPs | Polymer/hybrid | Characteristics/effects | Reference |
|---|---|---|---|---|---|
| Bone | Irregular | 1.0% | Chitosan | Improved density, osteogenesis, and vascularisation | [52] |
| Skin | Spherical | 0.5% | Gelatin | Angiogenesis, granulation, proliferation, antibacterial wound dressing | [55] |
| Bone | Nanowires | 1%–2% (w/w) | Poly(vinylidene fluoride‐trifluoroethylene) (P[VDF‐TrFE]) | Good mechanical strength, improved cell adherence and proliferation | [56] |
| Skin | Spherical | 1.0% | Gelatin composite | Stability of wound area, rearrangement of granulation tissue and collagen fibres | [57] |
| Tooth | Spherical clusters | 0.5% | Allyltriethoxysilane (ATES) | Microhardness, flexural strength | [58] |
| Tooth | Spherical | 1%–2% | Poly methyl methacrylate | Enhanced mechanical properties | [59] |
| Bone | Needle‐like | 0.2% | Poly(D,L‐lactide‐co‐glycolide) | Improved mechanical properties and wettability | [60] |
| Bone | ‐ | 1%–3% | Poly(vinyl alcohol) | Increase in the hydrophilicity and mechanical strength | [61] |
Abbreviation: NPs, nanoparticles.
Burn wounds are one of the major causes of morbidity and mortality around the world and regeneration of skin tissue is the most challenging issue. In a recent study, the dispersion of TiO2 NPs has been used to improve the burn wound healing process and regeneration of the tissue via interaction with blood serum proteins. In this study, it was proposed that TiO2 NPs are capable of adsorbing proteins by means of protective coating and then render them a robust capacity for the stimulation of coagulation of body fluid. In vitro and in vivo studies elucidated the initiation of cascade response and adherence of nanocomposite covering that prevented infection and inflammation with rapid diminution of wound area in comparison to control. Thus, the suspension of TiO2 NPs evidently improved damaged tissue regeneration along with considerable reduction in the formation of scar as well as skin colour differences [62].
TiO2 NPs have also been applied to enhance bone tissue regeneration in combination with chitosan. According to report, the chitosan hybrid with TiO2 NPs making a nano‐sponge scaffold for improved bone regeneration was investigated. Although chitosan is frequently used in tissue regeneration, its use is limited due to poor mechanical strength and non‐osteogenic inductivity. Thus, chitosan sponges were imbedded with TiO2 NPs, and morphological and crystallographic investigation displayed even distribution of NPs. Integrity of the nanocomposite remained intact upon addition of TiO2 NPs, which was confirmed by degradation studies. Biomineralisation, molecular, and cytotoxicity assays revealed that the addition of TiO2 NPs enhanced apatite formation, upregulation of regeneration genes, and biocompatibility. It clearly suggested chitosan and 50% TiO2 nano‐hybrid sponge as a potentially unique scaffold in the tissue engineering of bones [63].
3. TiO2 NANOPARTICLES IN AGRICULTURE
Agriculture is the core reason for human growth and the nation's economy; thus, it is crucial to create and innovate green technologies for sustainable crop sector development [64]. As per reports, in 2050, the world population is expected to rise to 10 billion, which will lead towards the rise in demand for food products. To meet the rising global food demand, the Food and Agriculture Organization (FAO) has predicted that between 2020 and 2056 the food production will be needed to get enhanced by 70% [65]. The existing modern technologies such as aquaculture system, photovoltaic green houses, synthetic biology, vertical farming, and fungicides etc., have brought significant advantages to agriculture by producing products with maximum yield and cultivation of out‐of‐season products but also caused considerable health and eco problems due to the inappropriate use of fertilisers and pesticides. Nanotechnology is an interdisciplinary fast emerging field in developing novel technical tools for maximum crop yield and protection with the strategy of improving plants' capacity to absorb maximum nutrients. Amongst various nanomaterials, extensive research has been directed towards the agricultural applications of TiO2‐based nanomaterials because of their unique structural configuration, chemical stability, hydrophilicity, and eco‐friendliness [66]. The following subsection demonstrates the applications of TiO2 NP‐based nanopesticides and nanofertilisers as well as the TiO2 NPs' interaction with crops leading to plant tolerance.
3.1. Nanopesticides
Crop protection from different diseases and pests presents an enduring challenge, which directs the development of innovative approaches and agents. Chemically designed pesticides are utilised to control or kill microbes, weeds, insects, and fungi. However, the excessive use of pesticides can cause serious health effects [67]. The use of NPs or nanoformulations of pesticides is more efficacious in contrast to the commercial formulations of pesticides. This might be the result of enhanced uptake of active ingredients and greater bioavailability with NPs, consequently leading to killing of infectious organisms [68].
TiO2 NPs are used to kill Spodoptera littoralis, the Egyptian cotton leaf worm. It is basically a pest having wide host range in plants that attacks certain field crops such as cotton and vegetables including tomato etc. In this context, an experiment was performed with six different concentrations (1000, 500, 250, 125, 62.5, and 31.25 ppm) of TiO2 NPs. In this study, the larvae were fed on TiO2‐treated leaves of cotton and 2 weeks post application, the mortality was detected. The results clearly showed that TiO2 NPs exhibited toxic action at all the concentrations applied against the larvae of S. littoralis [69]. Figure 4 shows the positive effects produced by TiO2‐based nanopesticides on plants.
FIGURE 4.
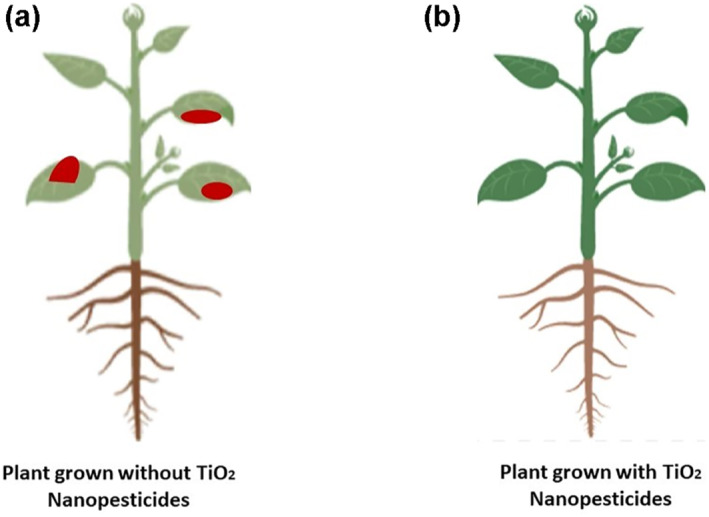
Plants grown (a) without TiO2 nanopesticides and (b) with TiO2 nanopesticides
Recently, TiO2 NPs were used alone and in combination with ZnO NPs to study their insecticidal effect against Bactericera cockerelli nymphs. The laboratory study and a greenhouse study were separately executed in tomato plant. The leaf immersion bioassay method was performed in laboratory experiment while direct plant spraying method was carried out under greenhouse conditions. Results indicated that the TiO2 NPs caused 99% and 100% mortality alone (at 100 ppm concentration) and in combination with ZnO NPs (at 250 ppm concentration), respectively, after 96h of treatment in laboratory. Whereas, 32% and 23% mortality was observed at 500 and 250 ppm concentration of TiO2 NPs and TiO2 + ZnO NPs, respectively, in the greenhouse experiment [70]. In another study, TiO2 NPs were prepared by green synthesis route using aqueous leaf extract of Pouteria campechiana and were analysed for insecticidal potential. It was demonstrated that at 900 μg/ml of TiO2 NPs, remarkable lethal activity was obtained against the larvae and pupa stages of Aedes aegypti [71].
TiO2 NPs when co‐doped with nitrogen (N) and fluoride (F) were utilised to inhibit fungal growth of Fusarium oxysporum in tomato. These NPs acted as antifungal agents in visible light. The colloidal form of NPs provided competent interactions of NPs with the cell wall of fungi owing to their appropriate surface chemistry. In fact, the synergistic effects were produced by the TiO2 NPs and N & F attached on their surface, producing stronger toxic influence on fungal strain, eventually killing it. The generation of ROS under visible light actually caused the disinfection of fungus. Thus, these NPs could be utilised for visible light‐induced bacterial and fungal disinfection [72].
In another study, the antibacterial aptitude of TiO2 NPs and silver (Ag)‐ and zinc (Zn)‐doped TiO2 NPs was evaluated for the underlying agent of bacterial spot disease in tomato, Xanthomonas perforans. The dose dependency and the photocatalytic activity were performed in vitro on glass cover slips that were coated with NPs. The known population of X. perforans was added on the cover slip, which were then illuminated with visible light. The in vitro and greenhouse studies showed that the doped TiO2 NPs at a concentration of 500–800 ppm had higher photocatalytic activity against X. perforans under visible light, which was obviously due to the combinatorial influence of TiO2 NPs with their doping agents, that is, Ag and Zn [73].
3.2. Nanofertilisers
Agricultural production can be enhanced with the introduction of fertilisers. To overcome the challenges such as pollution problems and use of nutrients with high efficiency, nanofertilisers serve as a best choice and might be the best alternative and more efficient tool than conventional fertilisers [74]. Also, they improve the condition of soil by reducing the toxic effects resulting from the overuse of conventional fertilisers [75].
The impact of nano TiO2 (a nanofertiliser) on spinach seeds was observed during its growth and development. The seed vigour and rate of germination showed enhanced effects with TiO2 NPs' treatment. Moreover, at 2.5% concentration of nano TiO2, the dry weight of plant, Rubisco activity, the formation of chlorophyll and the rate of photosynthesis were increased during growth stage [76]. To analyse the effects of TiO2 NPs on the development and growth of canola, a study was designed in which canola seeds were independently treated with various concentrations of TiO2 NPs and the impact of these treatments was focussed on seed and seedling vigour. It was inferred from the results that the higher concentration (2000 mg/L) of TiO2 NPs demonstrated enormous growth of seedling plumule and radicle [77].
In another study, the impact of TiO2 NPs' spray treatment on Zea mays was investigated. The TiO2 NPs' spray was applied and the two factors were mainly considered for treatment; in the first step, the growth of the plant was observed (growth of vegetative parts and the appearance of male and female flowers). Then, the second factor including chlorophyll, anthocyanins and carotenoids content etc., at various concentrations of TiO2 NPs was observed. The results clearly showed that the impact of TiO2 NP concentration 0.03% was noteworthy on chlorophyll (a & b), carotenoids, total chlorophyll (a + b), and anthocyanins. Besides, the nano TiO2 spray at the male and female flowers (reproductive stage) resulted in the greater amount of pigmentation as compared to control. Hence, it was concluded that the utilisation of TiO2 NPs can expedite the corn yield and several other parameters. Most importantly, TiO2 NPs produced a significant effect on photoreduction capabilities of photosystem II and electron transport chain (ETC), thus promoted photosynthesis by triggering the photochemical reaction of plant chloroplasts and subsequently enhanced the production of pigments [78].
Different agronomic species of plants have been exposed to different concentrations of nano TiO2 to examine the potential outcomes on germination, early seedling development, and other parameters such as root length etc. The seeds treated with TiO2 NPs have shown an enhanced rate of germination and an increase in the growth of seedlings and root length in different crop plants as explained in Table 2. In all of these studies, TiO2 NPs are either applied via foliar spray or given to seeds soaked in them and by soil medium. In the first case, the increased absorption of NPs by increasing the surface area of stomata of leaves might occur and later on, dissolution in the shoots leads to increase in growth parameters. While in the second case, enhancement of seed germination, plant growth, and crop yield might be due to the increased surface area of roots for absorption of NPs, which take part in the nutrients' uptake by acting as nutrient carriers. These effects are observed at optimum concentrations of TiO2 NPs and are dosage‐dependent. The NPs have been observed to produce negative effects mostly under high dosages while are beneficial at lower concentrations.
TABLE 2.
Influence of TiO2 nanofertilisers on different crops
| Plant | Concentration | Application method | Effects | Reference |
|---|---|---|---|---|
| Maize | 0, 100, 300, and 500 mg/L | Foliar spray | Increase in plant height, dry weight, and yield | [79] |
| Lettuce | 0, 25, 50, 75, and 100 mg/L | NPs in sandy soil | Fivefold increase in phosphorus (P) uptake and plant growth (shoot and root length) | [80] |
| Onion | 0, 100, 200, and 400 mg/L | Seeds soaked in NPs | Promote seed germination, maximum germination rate achieved at 100 mg/L | [81] |
| Cabbage | 0, 25, 50, and 100 mg/L | Seeds soaked in NPs | Promote seed germination and root growth | [82] |
| Wheat | 0, 30, 50, and 100 mg/kg | NPs in soil with phosphorus (P) | Promote plant growth and nutrient uptake | [83] |
| Cucumber | 0–4000 mg/L | Seeds soaked in NPs | Promote seed germination and root growth, >300% increase in root length at all concentrations | [84] |
| Barley | 0, 100, 200, and 300 mg/L | Foliar spray | Increase in crop yield | [85] |
Abbreviation: NP, nanoparticle.
Recently, TiO2 NPs synthesised by the green synthesis route using fruit peel extract of Citrus medica L. were used as nanofertilisers to enhance the yield of Capsicum annuum [86]. In another study, TiO2 NPs were applied via foliar spray to sunflower at a concentration of 2.6 mg/L. The results showed improved physiology and enhanced nutritional parameters such as oil content [87]. In an investigation, the coriander plants were sprayed with TiO2 NPs (2, 4, 6 ppm) that led to the increase in height of plant and other such physiological parameters. Moreover, significant rise of carotenoids, total sugars, phenols, and amino acids was observed [88].
3.3. Plant tolerance
The NPs of TiO2 are utilised broadly in different commercial items and plants. Despite their lavished utilisation, the investigation of the uptake of these NPs and their translocation in plants is restricted. Asli and Neumann examined the uptake of nano TiO2 and its translocation in Z. mays. The roots were excised having apices that were intact. The results showed that the NPs were not taken up through the cells of root, presumably because of their enormous size contrasted with the size of the diameter of root pore in the maize cell wall. This was appeared to diminish the water passage through the roots, thus causing diminished transpiration and growth of leaf [89]. A combination of SiO2 and TiO2 NPs in soybean augmented the uptake of fertiliser and water and incited the enzymatic antioxidant activity including catalase (CAT), superoxide dismutase (SOD), and peroxidase (POD). The synergism of SiO2–TiO2 nanocomposite increased the oxidative stress against which the enzymatic antioxidants were produced [90].
The high utilisation of TiO2 NPs has prompted their augmented delivery into the environment, where they might interact or associate with various plants and influence their physiological capacities [91, 92]. A study was designed to examine the response of different physiological parameters of Triticum aestivum L. with respect to the escalating amount of TiO2 NPs. The TiO2 NPs were applied to soil at a concentration of 0 (control), up to 100 mg/kg. The physiological parameters such as length of root and shoot, phosphorus (P) availability, chlorophyll content, biomass, and H2O2 production were then recorded. All investigations were repeated twice. After 2 months of the NPs' exposure, the length of root and shoot as well as P uptake by the plants was notably greater with increasing nano TiO2 concentration contrasted with the control; however, it was decreased at higher doses. The utilisation of NPs prompted chlorophyll content being greater than in the control; however, lesser content was seen at higher concentrations. The outcomes proposed that wheat could not tolerate much greater concentration of TiO2 due to the excessive production of H2O2. Actually, H2O2 acts as a regulator of growth and chlorophyll content. The rising concentration of TiO2 NPs increase the formation of H2O2 because it is an important stress‐signalling molecule, and hence the more oxidative stress means more H2O2 production while lesser growth and chlorophyll content [93].
TiO2 NPs play an important role in the growth of plants, particularly under exposure to abiotic stresses. In an interesting study, the impact of TiO2 NPs at different concentrations (0, 50, 100 and 200 mg/L) on the agronomic characteristics of Dracocephalum moldavica L. plants was explored that were grown under various dosages of salinity. Outcomes showed that all agronomical attributes were adversely influenced by all saltiness amounts; however, utilisation of TiO2 NPs alleviated those adverse effects. The treatments of TiO2 NPs on Moldavian, which was nurtured in the stress of salt settings, enhanced every single agronomical quality and increased the activity of antioxidant enzymes contrasted with the plants cultivated in soil that was devoid of TiO2 treatments under saltiness. The utilisation of TiO2 NPs brought down the quantity of H2O2 by reducing the oxidative stress and the quantity of essential oil was attained highest in plants treated with TiO2 NPs. In conclusion, the use of TiO2 NPs was found to fundamentally improve the impact of salinity in Dracocephalum moldavica L. plant [94].
Numerous studies have reported that the use of rutile TiO2 NPs can moderate the oxidative stress of plants. Addition of TiO2 NPs (50 mg/L) to the growth media reduced the growth of roots and shoots while enhanced enzymatic antioxidant activities (SOD, POD, and CAT) in maize tissues [95]. Another study reported that the application of TiO2 NPs (100–300 mg/kg) to Cd‐enriched soil effectively reduced the plant biomass and growth while increased the proline and malondialdehyde (MDA) content [96]. Therefore, TiO2 NPs have great application prospective in reducing oxidative stress in plants caused by the heavy metals. According to reports, the four types of TiO2 NPs (anatase, rutile with hydrophilic surface, rutile with hydrophobic surface, and pristine rutile) at 10–1000 mg/L concentration in rice plants effectively decreased the Pb content in plant roots and shoots [97].
Exposure medium plays a significant role in determining the plants' tolerance potential. Recently, in a study, TiO2 NPs (100 and 250 mg/L) were applied both through soil and foliar application in maize grown in Cd‐contaminated soil. Foliar application of TiO2 NPs inhibited Cd accumulation in maize and enhanced biomass production, while TiO2 NPs' application in soil upheld the accumulation of Cd in maize and substantially reduced the biomass. Foliar spraying of TiO2 NPs also improved SOD and glutathione S‐transferase (GST) enzyme activities, and galactose, aspartame acid, and other metabolic pathways were activated to alleviate abiotic stress in maize [98]. Figure 5 explains the interaction of TiO2 NPs with crop plants and associated mechanism.
FIGURE 5.
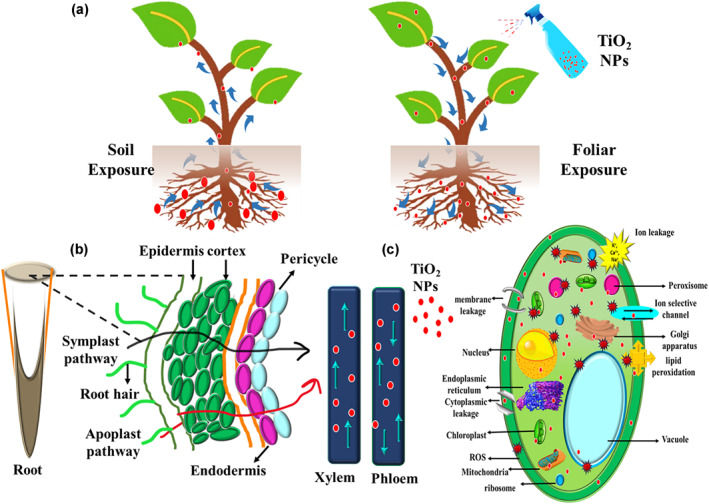
Interaction of TiO2 nanoparticles (NPs) with crop plants: (a) exposure of TiO2 NPs to the crops using soil medium and foliar spray; (b) TiO2 NPs entering the plant cells through apoplast and symplast pathways; (c) TiO2 NPs controlling the production of oxidative stress and its possible mechanism
4. TiO2 NPS IN ENVIRONMENTAL REMEDIATION
Technological advancement brought by rapid growth in world population and urbanisation and industrial evolution have led towards the drastic exploitation of natural resources such as water, air, and soil. Major causes of water and soil pollution are untreated industrial effluents, improper sewage water disposal, and haphazard application of pesticides and fertilisers. At present, the water and soil are polluted with lethal heavy metals, chlorinated compounds, and dyes. Air is loaded with abundant contaminants such as nitrogen oxides (NO), carbon monoxide (CO), volatile organic compounds, chlorofluorocarbons (CFCs), hydrocarbons etc. There is a substantial need for efficient technologies capable of tracking, identifying, and handling such kinds of contaminants in water, air, and soil [99]. In recent years, environmental nanotechnology has made tremendous developments in terms of environmental protection. Amongst the most promising contributions, environmental applications in an area of water/air remediation are significant. The unique properties of NPs such as their nanoscale size, large surface area to volume ratio, high flexibility for in situ and ex‐situ practices, and reluctance to eco‐factors make them suitable candidates for different environmental approaches. Different kinds of available nanomaterials and nanotools are used to remediate eco‐contaminants [100]. Among various materials, use of TiO2 NPs is increasing day by day as a remediating agent to clean water, purify air, and to decontaminate soil due to properties such as appropriate electronic band structure, high quantum efficiency, stability, and chemical inertness [101].
4.1. Nanosensors
Nanosensors are sensitive detecting devices having at least ∼100 nm as one of their sensing dimensions. These are instrumental for detecting and evaluating physical and chemical changes, observing biochemical and biomolecular alterations inside the cells, and measuring toxic environmental contaminants. Sensitive detection of pollutants is accredited to NPs' small size together with high surface to volume ratio for monitoring via nanosensor devices. Various kinds of nanostructured materials are utilised in the production of nanosensors, namely, nanoscale wires, carbon nanotubes (CNTs), graphene (G), polymers, biomaterials, thin films, metal and metal oxide NPs. Nanosensors function in the scale down, effective, accurate, and sensitive recognition of contaminants [102]. Analyte (sample, i.e. pollutants), bioreceptor (molecule that recognises the analyte, i.e. NPs), transducer (converts one form of energy to another, i.e. signaliser), electronics (processes/amplifies the transduced signal), and display (interpretation system, i.e. quantifier) are the basic components of biosensor. Various factors influencing the performance of nanosensors include selectivity and sensitivity of bioreceptor and linearity and reproducibility of response [103]. The process of nanosensing along with its various biological applications has been well explained in Figure 6.
FIGURE 6.
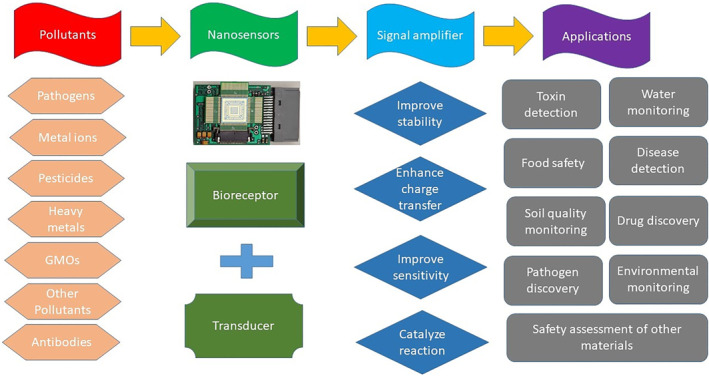
Process of nanosensing applied in biotechnology
TiO2 nanostructures possess unique physical and chemical characteristics such as non‐toxicity, strong oxidation ability, and high chemical inertness obtained at a relatively low cost. Based on this, TiO2 NP‐based nanosensors have been profoundly examined and employed for the detection of ions and substances [104, 105]. Henceforth, nowadays tremendous interest has been observed in TiO2 nanomaterial structures as well as their transduction principles and simulations for nanosensor applications [106, 107, 108, 109, 110]. In recent times, several new TiO2‐based nanomaterials with advanced compositions and structures have been employed for a variety of sensors. Based on different sensing targets or measurement principles, the TiO2 nanosensors can be stated as (1) TiO2‐based gas sensors, (2) TiO2‐based electrochemical sensors, and (3) TiO2‐based biosensors [111, 112, 113].
According to the report, a highly selective nanosensor, that is, thiazolylazopyrimidine‐doped titanium dioxide (TiO2‐TAP), was synthesised via diazo coupling reaction and surface modification reaction for colourimetric detection of Cu2+ in water samples. The novel nanosensor exhibited maximum sensitivity, high affinity, and selectivity for copper ions in aqueous media at pH 5.0. Moreover, the TiO2‐TAP revealed wide linear detection range for Cu2+ (0.01–12.5 μM) [114]. In a recent study, a novel approach was used for the detection of toxic metal (Pb) using carbon quantum dot (CQD) nanosensor with 0.070 μM sensitivity and selectivity, and its doping with TiO2 nanostructures to make Pb–CQDs–TiO2 (PCT) composite was later applied for the photocatalysis of industrial dyes. The adsorption ability of TiO2 NPs, light absorption capacity of Pb–CQDs, and photocatalytic ability of PCT were utilised. Using wet impregnation method, the Pb–CQDs solution was immersed in TiO2 to achieve Pb–CQDs–TiO2 (PCT) showing photocatalytic ability of 3.2–2.8 eV energy gap. Results illustrated ∼100% degradation efficiency for RBX dye and 1.8 μmols of CO2 release was noticed in 1 h [115]. As per the literature, the removal of Bi (III) ions using efficient, sensitive, and highly selective mesoporous TiO2 NP‐based sensing system was examined. The formation of TiO2 NPs and [Bi (DZ)3] complex led towards the successful removal of Bi (III) ions. TiO2 NPs of 174 m2/g surface area and 10 nm particle size played important role in [Bi (DZ)3] complex formation. Bi (III) ions detection limit was observed to be ∼1 ppb. Moreover, the colourimetric nanosensor depicted selective sensing performance for up to 5000 times in the presence of other competent anions and cations [116]. In another study, the sensing properties of CdTe quantum dot (QD)‐doped TiO2 nanotubes (TiO2 NTs) for polycyclic aromatic hydrocarbon (PAH) detection using fluorescence resonance energy transfer (FRET) were evaluated. FRET occurred between the CdTe QDs and PAHs, with CDTe QDs as donors and PAHs as receptors. The sensors' maximum sensitivity was found to be dependent upon the number of PAH rings having the highest sensitivity observed against benzopyrene (BaP). Results demonstrated that the proposed sensor could be used for efficient scanning of PAHs because the sensor (i) described a linear response to the log of BaP concentration having range of 400 nM–40 pM and (ii) exhibited 15 mP detection limit which is set by US‐EPA [117].
In another report, sensing potential of graphene‐doped anatase TiO2‐carbon paste (GTC) for electrochemical detection of phenol was elucidated. Investigation showed the efficient detection of phenol down to 3.6620 × 10−5 μM. GTC electrode demonstrated better selectivity and stability and predicted the corresponding reproducibility standard deviation to be 1.33% and 2.83%. Also, the electrode performance was found to be concentration‐dependent with the optimum electrode using G and TiO2 samples as lower as 0.015 and 0.3 g, respectively. A very low detection limit was obtained in this study, which is attributed to the graphene and TiO2 that were involved in acceleration of the process of charge transfer and surface reaction [118]. As per findings, sensing characteristics of ZIF‐8@ZnO/TiO2 1DTDPC (zeolitic‐imidazolate‐framework‐8, 1D) (top‐defect photonic crystalline structures) against carbon tetrachloride (CCl4) synthesised using spin‐coating technique were studied. ZIF‐8@ZnO/TiO2 1DTDPC represented excellent quality in terms of sensitivity (0.008 nm/ppm towards water vapours). Besides, the ZIF‐8@ZnO/TiO2 1DTDPC showed higher selectivity and sensitivity (0.05 nm/ppm) for CCl4 vapours. Moreover, at 320 ppm concentration, ∼300 ms was found to be the optical response time [119]. According to another report, a successful development of high performance nanosensor based on ZnO@TiO2 nanorods and their applications for n‐butanol detection was observed. Results revealed higher sensitivity, better selectivity, low detection limit of 133 ppb, and fast response recovery against n‐butanol investigation. The enhanced sensing capability of the developed nanosensor was attributed to the core‐shell nanostructures' heterojunction, maximum oxygen sorption owing to TiO2 nanoshells, and complete electron depletion having a Debye length comparable thickness [120].
As per data available, the role of anatase TiO2–CPE (carbon paste electrode) nanocomposite electrochemical sensor for cypermethrin detection was explored. Results showed maximum sensing performance with increased anatase TiO2 NPs' concentration of 2 w/w (CPE ∼ 3 w/w). The synthesised nanosensor exhibited 0.1 ppm detection limit, which is less than the cypermethrin residual limits in the environment (∼0.5 ppm) and in the food items (∼0.05–0.2 ppm). Also, the nanoelectrode exhibited remarkable stability with only 0.37% performance reduction (%RSD) for 11 times repeated measurement. The maximum sensing performance was ascribed to excellent physico‐chemical properties and surface chemistry of anatase TiO2 due to which greater electron transfer rate occurred in the CPE matrix [121]. According to the literature, sensing potential of CuO–TiO2 hybrid nanocomposite decorated on glass carbon electrode as electrochemical sensor for methyl parathion (pesticide) detection was investigated. Results illustrated lower detection limit of 1.21 ppb. Also, the as prepared nanosensor exhibited efficient sensing capability and maximum selectivity for methyl parathion. Additionally, the novel electrochemical sensor also showed effective sensing performance for the pesticide detection in actual ground water samples [122].
4.2. Soil remediation
The existence of hazardous compounds causes contamination of the naturally occurring soil. Heavy metals are the most common pollutants of the soil, especially when present at toxic level. There are different sources on which soil contamination depends including manufacturing, mining, and landfill sites especially those which are built to receive the wastes of different industries such as paint residues, electrical wastes, batteries etc., as well as industrial or municipal sludge. Thus, heavy metals are one of the challenging pollutants of the soil because they are considered as the non‐degradable substances that remain there once been introduced [123, 124]. Various studies have reported an interesting information related to nano‐TiO2, considering the soil remediation by the UV‐mediated degradation of the organic pollutants of soil [125]. Photodegradation of diphenyl arsenic acid (DPAA) by employing TiO2 NPs has enabled us to investigate that either nano‐TiO2 or the derivatives of TiO2 can wipe out the organic pollutants via the process of photocatalytic oxidation. The formation of DPAA usually results from the leakage of arsenic weapons and it also poses various adverse effects on human health. To optimise the removal, all the operational parameters required for removal of DPAA, which include dosage of TiO2 NPs, radiation time, light intensity, and also soil–water ratio, are studied well. It has also been observed that all the parameters mentioned above result in the removal efficacy of 82.7% of the DPAA. It has been reported that the DPAA is not completely converted by TiO2 NPs, rather its inorganic arsenic products are adsorbed by these NPs, hence playing crucial role in eradicating DPAA pollution [126].
In a report, the photocatalysis of nano‐TiO2, when used in combination with the pulse discharge plasma, required for the purpose to remove it from the contaminated soil was studied. The mechanism involved removal of p‐nitrophenol and then its degradation by the enhancement of pulse discharge voltage. The nitrophenols are termed as the bio‐refractory and the noxious organic compounds that are significantly used as both the intermediate as well as the raw materials to produce pharmaceuticals, dyes, rubber chemicals, pigments, wood preservatives, pesticides, and explosives. Wang et al. predicted that the pulsed discharge plasma could be the force used to drive the photocatalysis of nano‐TiO2. The report also revealed that with the use of this route, the p‐nitrophenol could be removed up to 88% in a succession of just 10 min [127]. Figure 7 depicts the mechanism of soil remediation by the TiO2‐based photocatalyst.
FIGURE 7.
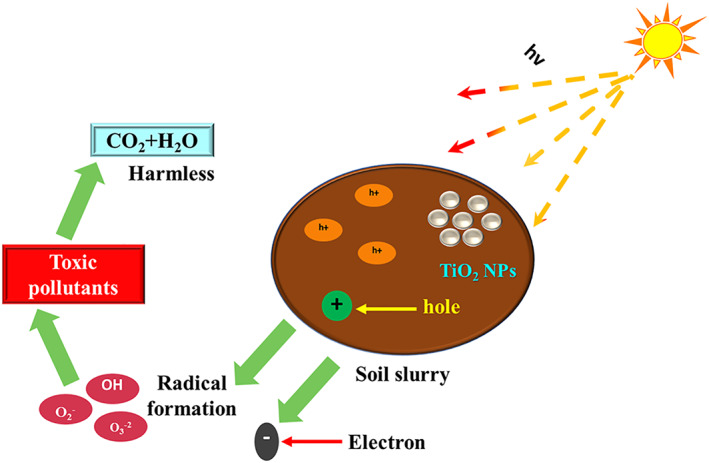
Schematic illustration of TiO2 nanoparticle‐based photocatalyst for soil remediation
A study related to the application of plant growth promoting rhizobacteria (PGPR) and TiO2 NPs, when combined, suggests the promotion of the phytoremediation of Cd‐contaminated soil. The percent of clay, sand, and silt in soil was 28%, 37%, and 35%, respectively. Soil had 0.47% N, 7.1 mg/kg of phosphorus, and its pH was 7.8. Different doses of the TiO2 NPs and the PGPR were used for the seedlings of Trifolium repens, both separately as well as in combination, with the purpose to analyse their effects on the uptake of Cd, growth of plants, and the content of chlorophyll present in plants. Thus, it was revealed that their combined application resulted in the enhanced plant growth with high chlorophyll content. It also reduced the amount of TiO2 NPs required for the phytoremediation of soils particularly polluted with heavy metals. It also promoted the growth of T. repens with increased Cd uptake in the Cd‐contaminated soil by the plants [128].
In a study, the association of biochar and TiO2 NPs was investigated extensively for the phytoremediation of soil contaminated with antimony. Soil pH was 7.7 and it had 1.12% N and 8.7 mg/kg of phosphorus. The percent of clay, sand, and silt was 28%, 37%, and 35%, respectively. Different concentrations of TiO2 and biochar were applied to the Sorghum bicolour seedlings individually and in combinations. The treatments were applied to study their impact on the growth of plants, the absorption of antimony and its accumulation, and the physiological response of the plants in antimony‐contaminated soil. The results demonstrated that the combination of biochar and TiO2 NPs had positive effects on the growth of plant. The accumulation of antimony was increased significantly with TiO2 and biochar combination. The results clearly revealed this technique to be favourable for the phytoremediation of heavy metal contaminated soil [129].
4.3. Water remediation
Clean and fresh water is essential for daily life and life cycle of living organisms. Due to different mankind activities, water is contaminated by physical, chemical (heavy metals, inorganic/organic etc.), and biological pollutants. Recently, nanotechnological approach for wastewater treatment using adsorption and photocatalytic technology has attained tremendous attention owing to its eco‐friendliness, sustainability, cost‐effectiveness, and other effective properties [130]. Among nanomaterials, TiO2‐based nanomaterials have achieved the core importance because of their unique physical and chemical features, remarkable biocompatibility, strong oxidation, and maximal photocatalytic properties [131, 132].
According to the report [133], a novel porous magnetic Ag/TiO2/Fe3O4@GO nanocomposite was synthesised and its photocatalytic ability for As (III) and As (V) was examined. Results showed optimum adsorption capacity of 91% at pH 5 and 20 mg sorbent concentration for 24 ppm A (III), 90 min duration, and at room temperature. pH plays a pivotal role in the adsorption technology for wastewater treatment application. At pH < 3, H3AsO3 are primary non‐anionic species and the As (III) adsorption on the adsorbent surface is lowered. While at pH > 8, the surface of the adsorbent becomes less positive and As (III) removal efficiency is reduced. Also, for As (V), 87% adsorption capacity was achieved at pH 3, 17 ppm A (V), 11 mg sorbent concentration, 30 min duration, and at room temperature. As (V) adsorption was also affected by varying the pH of the medium solution. Since As (V) existed in negative form H2AsO4 −, at acidic pH, H+ ions easily interacted with the negative charges of the adsorbent (Ag NPs) surface, TiO2 and Fe3O4 oxygen groups, and GO functional groups to make surface complexes. Overall, at lower pH, the electrostatic interaction of H2AsO4 − on the positive sites of the adsorbent resulted in maximum adsorption. Moreover, the nature of adsorption for As (III) and As (V) was best interpreted by Langmuir isotherms. Table 3 describes various examples of the application of TiO2‐based nanomaterials in wastewater treatment.
TABLE 3.
Different TiO2‐based nanomaterials applied in wastewater treatment
| TiO2‐based adsorbent | Sorbate | Optimum conditions | Removal (%) | Adsorption capacity (mg/g) | Isotherm model | Kinetics model | Reference |
|---|---|---|---|---|---|---|---|
| Ag/TiO2/Fe3O4@GO | As (III) | pH: 5 | 91 | ‐ | Langmuir | ‐ | [133] |
| Ag/TiO2/Fe3O4@GO | As (V) | pH: 3 | 87 | ‐ | Langmuir | ‐ | |
| PP@TiO2 | As (III) | ‐ | ‐ | 76.92 | ‐ | ‐ | [134] |
| [EMIM‐BF4] assisted GO/TiO2 nanocomposite | Cd (II) | pH: 7.5 | 69.36 | ‐ | ‐ | ‐ | [135] |
| Sorbent: 0.5 mg/L | |||||||
| Sorbate: 0.5 mg/L | |||||||
| Temp: 20 ± 2°C | |||||||
| Time: 40 min | |||||||
| [EMIM‐BF4] assisted GO/TiO2 nanocomposite | Pb (II) | pH: 3 | 89 | ‐ | ‐ | ‐ | |
| Sorbent: 0.5 mg/L | |||||||
| Sorbate: 0.5 mg/L | |||||||
| Temp: 20°C ± 2°C | |||||||
| Time: 40 min | |||||||
| Sodium modified TiO2 | Zn (II) | pH: 1–7.4 | ‐ | 93 | Freundlich | PSO | [136] |
| Sorbent: 50 mg | |||||||
| Time: 90 min | |||||||
| Sr (II) | pH: 8–10 | 2084 | |||||
| Sorbent: 50 mg | |||||||
| Time: 90 min | |||||||
| Ba (II) | Sorbent: 50 mg | 2746 | |||||
| Time: 90 min | |||||||
| Pristine TiO2 | Cr (VI) | pH: 5.36 | 100 | ‐ | ‐ | ‐ | [137] |
| Sorbent: 50 mg/L | |||||||
| Sorbate: 10 mg/L | |||||||
| Time: 60 min | |||||||
| Chitosan/g‐C3N4/TiO2 (CS/CNT) nanofibres | Cr (VI) | pH: 1–7 | ‐ | 165.3 | Langmuir | PSO | [138] |
| Sorbent: 10 mg/L | |||||||
| Sorbate: 20–800 mg/L | |||||||
| Temp: 24 h | |||||||
| Time: 0–1440 min | |||||||
| Ti3C2/TiO2 | Cr (VI) | pH: 2 | 99.34 | ‐ | ‐ | ‐ | [139] |
| Sorbent: 0.05 g/L | |||||||
| Sorbate: 50 mg/L | |||||||
| Time: 72 h |
In a study, TiO2 NPs along with the ZnO NPs were immobilised on a natural clay for the removal of ammonium ions from wastewater. The parameters adjusted were initial concentration, solution pH, agitation time, and adsorbent dosage. The adsorption process followed the pseudo‐second‐order kinetics and Langmuir model. The structure and surface properties of NPs were involved in regulating the adsorption capacity [140]. Another report showed the immobilisation of TiO2 NPs on bentonite and kaolin adsorbent for the removal of cationic polymer. The experimental conditions involved alteration in pH, ionic strength, and dosage. The rising pH was observed to improve the removal process from wastewater [141].
TiO2 NPs have shown very high photocatalytic activity as compared to other NPs [142]. Recently, Indira and colleagues prepared Ni–TiO2 nanoflakes using the leaf extract of Mukia madrasapatna and their photocatalytic potential in wastewater was analysed. Results indicated that the Congo red dye was degraded by UV illumination [143]. In another study, niobium‐doped TiO2 nanotubes (Nb–TNT) were developed via dip coating route for the catalytic degradation of organic pollutants and the results demonstrated excellent charge separation efficiency [144].
In a most recent study, TiO2 NPs were combined with a microalgae, Chlorella vulgaris, forming bio‐nano hybrid catalyst for downstream wastewater treatment. The photocatalytic behaviour of TiO2 NPs and sorption potential of C. vulgaris were combined. The synergistic effects of NPs and algal biomass enhanced the biosorption of copper ions (Cu2+) from 103 to 4000 mg/g and photodegradation of rhodamine B (RhB) in 1 h, increasing the kinetic constant from 8.7 to 10.7 × 10−2 min−1 [145].
4.4. Air remediation
With the growing world population, the industrial pollution and transportation will continue to increase causing environmental burden. Air pollution also has bad impact on the ecosystem. Today, the most important challenge is global warming, which is caused by different gases present in the atmosphere [146]. Various technologies have been developed to monitor, control, and eliminate the harmful gases from environment. Taking advantage of the properties of nanomaterials, the field of nanotechnology has been utilised as an effective treatment to control or eliminate air pollutants by using them as adsorbents and catalysts [147].
Nanocrystalline TiO2 photocatalysts formed by the process of sol–gel, which were then calcined at 100°C–800°C, were assessed for the deterioration of trichloroethylene (TCE) in indoor air. Catalysts were made up of pure rutile, pure anatase, and a combination of anatase/rutile that were immobilised on borosilicate glass as thick films and then introduced in a photocatalytic reactor. Higher photocatalytic activity was exhibited by the treated pure anatase TiO2 as compared to the commercial TiO2 (P‐25) in the presence of UV‐A radiation [148]. The action mechanism of TiO2 NP‐based photocatalyst is presented in Figure 8.
FIGURE 8.
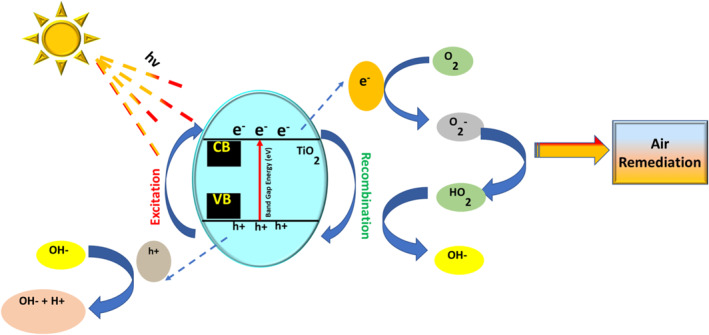
Schematic illustration of TiO2 nanoparticle‐based photocatalyst for air remediation
TiO2 NPs co‐doped with N‐F (NFT) powders, which were prepared through the process of spray pyrolysis (SP), were described by photoluminescence (PL) spectra and ultraviolet‐visible (UV‐Vis) absorption spectroscopy. For the purpose of evaluation of the photocatalytic properties of NFT powder, the decomposition of acetaldehyde was utilised as probe response and the results showed that under the influence of UV or visible irradiations, the NFT powder exhibited greater photocatalytic activity when compared with the commercial P‐25. These findings also showed the effectiveness of TiO2 nanocomposite against toluene and TCE [149].
As per findings, the TiO2 NP‐coated nano CaCO3 became able to prevent the sintering of nano CaCO3 and capture the CO2 effectively with the help of adsorption phase technique. It was a nano CaO‐based CO2 adsorbent. The durability and compactness of CO2 adsorption was enhanced by TiO2 NP coating [150]. Moreover, the photocatalytic degradation of greenhouse gases, viz., methane (CH4) and CO2 was done by the TiO2 NPs coated on stainless steel webnet. In a gas‐phase batch reactor, UV was irradiated on precursors and formate and acetate derivatives were formed as conversion products along with the degradation of CH4 and CO2 [151]. In another study, a hybrid nanomaterial Pt‐rGO‐TiO2 having a wide‐ranging light absorption wavelength (800–2500 nm) was developed. This well active and responsive photo‐thermal catalyst could decompose the volatile organic compounds (VOCs) under the influence of infrared (IR) radiations. The intensity of light could affect the rate and efficiency of Pt‐rGO‐TiO2 mixtures on toluene conversion with the production of CO2. When the intensity of IR radiations was 116 mW/cm2, the 14.1% of the photo‐thermal conversion efficiency of substantial toluene was achieved at a conversion rate of 95% with the production of 72% CO2. Moreover, the duration of stability was almost 50 h [152].
TiO2 NPs are being employed as photochemical deodorisers in Japan as they convert oxides and air pollutants to less lethal products like calcium nitrate (CaNO3) and carbon dioxide (CO2) [153]. Furthermore, titanium mesh filters have been developed to remove pollutants from cigarette smoke [154]. Toluene, an important volatile organic compound, can be treated with carbon © coated TiO2 NPs and the oxidation of indoor contaminants present in air by TiO2 NPs, helps in the cleansing of air, which is vital for smooth breathing of all living organisms [155].
5. CONCLUDING REMARKS AND FUTURE PERSPECTIVES
The biotechnological approaches of TiO2 NPs can be broadly categorised into medical nanobiotechnology, agricultural nanobiotechnology, and environmental nanobiotechnology. The employment of TiO2 NPs in medical nanobiotechnology can be divided into different areas, that is, bioimaging, drug delivery, phototherapy, antimicrobial potential, and tissue regeneration. The applications of TiO2 NPs in agricultural nanobiotechnology discussed in this review can be narrowed down to nanopesticides, nanofertilisers, and plant tolerance. TiO2 NPs' administration in environmental nanobiotechnology can be sectioned into nanosensors and the soil/water/air remediation. All of the sections are interrelated, for instance, nanosensors could be used for the monitoring and detection of plant or human pathogens as well as ecological toxicants.
Although several studies have reported the improvement in crop yield and quality on administration of TiO2 NPs, the toxic or negative impact of these NPs is also achieved at relatively higher dosages. Therefore, further investigations on the potential interactions of TiO2 NPs with toxic heavy metals are needed to improve our understanding of NPs–crop plants interactions for the safer applications of nanofertilisers in future. Moreover, the beneficial effects of TiO2 NPs as plant disease suppressing agents are dependent upon multiple factors, including physico‐chemical properties (size/morphology/charge/coating), concentration, duration of application, exposure dosage, plant species, and type of pathogen. Thus, the selection of appropriate concentration and application regime are critical for ensuring beneficial outcomes, also in case of biomedicine. Large‐scale application of TiO2 NPs could potentially lead to collateral damage in the terrestrial environment. Besides, the remediation potential of TiO2 NPs also depends on its surface properties and exposure medium. Regarding the domain of biomedicine, the detailed studies depicting photocatalytic performance, cytotoxicity, biodegradability, and biocompatibility of TiO2 NPs should be carried out to tackle challenges of translation of advanced nanomedical approaches from pre‐clinical to clinical settings. In addition, TiO2 NPs should be designed keeping in view of the biological microenvironment in order to get maximal biological response and negligible side effects in the human body.
In future, the planning, development, and implementation of nano‐enabled antiviral strategies should occur that would draw attention of a broad group of plant/human biologists, pathologists, and agricultural/biomedical engineers to collaboratively address the mounting challenges in the field of agriculture and biomedicine. The efforts should focus on exploring and establishing sustainable, environment friendly, long‐lasting, and orthogonal approaches that would optimise crop/human protection and forward efforts to achieve global food security and longevity of human beings. Furthermore, the evaluation of toxicity and toxicological pathways of TiO2 nanostructures is essential for the broad spectrum use of these NPs in different fields of biotechnology. In a nutshell, the inherent characteristics of TiO2 NPs must be improved to fill existing knowledge gaps and overcome future challenges in different functionalities of these NPs in biotechnology.
CONFLICT OF INTEREST
The authors declare that they have no competing interest.
PERMISSION TO REPRODUCE MATERIALS FROM OTHER SOURCES
None.
ACKNOWLEDGEMENT
Authors are obliged to the Sichuan University for providing all funding. This work was supported by the Sichuan Science and Technology Program (No. 2020YFH0008) and the National Key R & D Program of China (No. 2020YFF0426289).
Javed, R. , et al.: Diverse biotechnological applications of multifunctional titanium dioxide nanoparticles: an up‐to‐date review. IET Nanobiotechnol. 16(5), 171–189 (2022). 10.1049/nbt2.12085
Contributor Information
Rabia Javed, Email: rabia.javed@ymail.com.
Shen Tian, Email: cmu4h_ts1969@126.com.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Jamil, B. , et al.: Nanomaterials: toxicity, risk management and public perception. In: Nanomaterials: Ecotoxicity, Safety, and Public Perception, Chapter 14. Springer; (2019) [Google Scholar]
- 2. Javed, R. , et al.: Role of capping agents in the application of NPs in biomedicine and environmental remediation: recent trends and future prospects. J. Nanobiotechnol. 18, 1–15 (2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ali, I. , et al.: Recent advances in syntheses, properties and applications of TiO2 nanostructures. RSC Adv. 8, 30125–30147 (2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Reghunath, S. , Pinheiro, D. , Devi, S. : A review of hierarchial nanostructures of TiO2: advances and applications. Appl. Surf. Sci. Adv. 3, 100063 (2021) [Google Scholar]
- 5. Nyamukamba, P. , et al.: Synthetic methods for titanium dioxide nanoparticles: a review. In: Yang, D. (ed.) Titanium Dioxide ‐ Material for a Sustainable Environment, pp. 151–175. (2018) [Google Scholar]
- 6. Piccinno, F. , et al.: Industrial production quantities and uses of ten engineered nanomaterials in Europe and the world. J. Nanoparticle Res. 14, 1–11 (2012) [Google Scholar]
- 7. Robichaud, C.O. , et al.: Estimates of upper bounds and trends in nano‐TiO2 production as a basis for exposure assessment. Environ. Sci. Technol. 43, 4227–4233 (2009) [DOI] [PubMed] [Google Scholar]
- 8. Keller, A.A. , et al.: Stability and aggregation of metal oxide NPs in natural aqueous matrices. Environ. Sci. Technol. 44, 1962–1967 (2010) [DOI] [PubMed] [Google Scholar]
- 9. Keller, A.A. , et al.: Global life cycle releases of engineered nanomaterials. J. Nanoparticle Res. 15, 1–17 (2013) [Google Scholar]
- 10. Keller, A.A. , Lazareva, A. : Predicted releases of engineered nanomaterials: from global to regional to local. Environ. Sci. Technol. 1, 65–70 (2014) [Google Scholar]
- 11. Sani, M.A. , et al.: Titanium dioxide nanoparticles as multifunctional surface‐active materials for smart/active nanocomposite films. Adv. Colloid Interface Sci. 300, 102593 (2022) [DOI] [PubMed] [Google Scholar]
- 12. Rodríguez‐González, V. , et al.: Applications of photocatalytic titanium dioxide‐based nanomaterials in sustainable agriculture. J. Photochem. Photobiol. C Photochem. Rev. 40, 49–67 (2019) [Google Scholar]
- 13. Waghmode, M.S. , et al.: Studies on the titanium dioxide NPs: biosynthesis, applications and remediation. SN Appl. Sci. 1, 1–9 (2019) [Google Scholar]
- 14. Jafari, S. , et al.: Biomedical applications of TiO2 nanostructures: recent advances. Int. J. Nanomed. 15, 3447–3470 (2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McNamara, K. , Tofail, S.A.M. : NPs in biomedical applications. Adv. Phys. X. 2, 54–88 (2017) [Google Scholar]
- 16. Yin, Z.F. , et al.: Recent progress in biomedical applications of titanium dioxide. Phys. Chem. Chem. Phys. 15, 4844–4858 (2013) [DOI] [PubMed] [Google Scholar]
- 17. Shah, S.N. , et al.: Hazardous effects of titanium dioxide nanoparticles in ecosystem. Bioinorgan. Chem. Appl. 2017, 4101735 (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wolfbeis, O.S. : An overview of nanoparticles commonly used in fluorescent bioimaging. Chem. Soc. Rev. 44, 4743–4768 (2015) [DOI] [PubMed] [Google Scholar]
- 19. Akasaka, H. , et al.: Investigation of the potential of using TiO2 NPs as a contrast agent in computed tomography and magnetic resonance imaging. Appl. Nanosci. 10, 3143–3148 (2020) [Google Scholar]
- 20. Jalvo, B. , et al.: Antimicrobial and antibiofilm efficacy of self‐cleaning surfaces functionalized by TiO2 photocatalytic NPs against Staphylococcus aureus and Pseudomonas putida. J. Hazard Mater. 340, 160–170 (2017) [DOI] [PubMed] [Google Scholar]
- 21. Brown, K. , et al.: Intracellular in situ labeling of TiO2 NPs for fluorescence microscopy detection. Nano Res. 11, 464–476 (2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sandoval, S. , et al.: Europium‐doped TiO2 hollow nanoshells: two‐photon imaging of cell binding. Chem. Mater. 24, 4222–4230 (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang, W. , et al.: Mesoporous titania based yolk–shell nanoparticles as multifunctional theranostic platforms for SERS imaging and chemo‐photothermal treatment. Nanoscale. 6, 14514–14522 (2014) [DOI] [PubMed] [Google Scholar]
- 24. Wang, Q. , et al.: TiO2 nanotube platforms for smart drug delivery: a review. Int. J. Nanomed. 11, 4819 (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xu, J. , et al.: Photokilling cancer cells using highly cell‐specific antibody–TiO2 bioconjugates and electroporation. Bioelectrochemistry. 71, 217–222 (2007) [DOI] [PubMed] [Google Scholar]
- 26. Wang, T. , et al.: Potential application of functional porous TiO2 nanoparticles in light‐controlled drug release and targeted drug delivery. Acta Biomater. 13, 354–363 (2015) [DOI] [PubMed] [Google Scholar]
- 27. Venkatasubbu, G.D. , et al.: Folate targeted PEGylated titanium dioxide NPs as a nanocarrier for targeted paclitaxel drug delivery. Adv. Powder Technol. 24, 947–954 (2013) [Google Scholar]
- 28. Qu, Y. , et al.: Chitosan‐coated titanium dioxide‐embedded paclitaxel NPs enhance anti‐tumor efficacy against osteosarcoma. Front. Oncol. 10, 1837 (2020) [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29. Kafshgari, M.H. , et al.: Anodic titanium dioxide nanotubes for magnetically guided therapeutic delivery. Sci. Rep. 9, 1–8 (2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Javed, R. , Ahmad, M.A. , Ao, Q. : Theranostic applications of nanobiotechnology in cancer. In: Nanotheranostics, pp. 277–295. Springer; (2019) [Google Scholar]
- 31. Liu, P. , et al.: Concurrent photothermal therapy and photodynamic therapy for cutaneous squamous cell carcinoma by gold nanoclusters under a single NIR laser irradiation. J. Mater. Chem. B. 7, 6924–6933 (2019) [DOI] [PubMed] [Google Scholar]
- 32. Lucky, S.S. , Soo, K.C. , Zhang, Y. : Nanoparticles in photodynamic therapy. Chem. Rev. 115, 1990–2042 (2015) [DOI] [PubMed] [Google Scholar]
- 33. Youssef, Z. , et al.: The application of titanium dioxide, zinc oxide, fullerene, and graphene nanoparticles in photodynamic therapy. Cancer Nanotechnol. 8, 1–62 (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ziental, D. , et al.: Titanium dioxide NPs: prospects and applications in medicine. Nanomaterials. 10, 387 (2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sun, J. , et al.: Recent progress in metal‐based nanoparticles mediated photodynamic therapy. Molecules. 23, 1704 (2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Moosavi, M.A. , et al.: Photodynamic N‐TiO2 nanoparticle treatment induces controlled ROS‐mediated autophagy and terminal differentiation of leukemia cells. Sci. Rep. 6, 1–16 (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fakhar‐e‐Alam, M. , et al.: Synergistic effect of TEMPO‐coated TiO2 nanorods for PDT applications in MCF‐7 cell line model. Saudi J. Biol. Sci. 27, 3199–3207 (2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Behnam, M.A. , et al.: The application of titanium dioxide (TiO2) NPs in the photo‐thermal therapy of melanoma cancer model. Iran. J. Basic. Med. Sci. 21, 1133 (2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ou, G. , et al.: Photothermal therapy by using titanium oxide NPs. Nano Res. 9, 1236–1243 (2016) [Google Scholar]
- 40. Kafshgari, M.H. , Goldmann, W.H. : Insights into theranostic properties of titanium dioxide for nanomedicine. Nano‐Micro Lett. 12, 1–35 (2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Khan, M. , et al.: Controlled grafted poly (quaternized‐4‐vinylpyridine‐co‐acrylic acid) brushes attract bacteria for effective antimicrobial surfaces. J. Mater. Chem. B. 6, 3782–3791 (2018) [DOI] [PubMed] [Google Scholar]
- 42. Reygaert, W.C. : An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 4, 482–501 (2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ahmad, M.A. , et al.: Appraisal of comparative therapeutic potential of undoped and nitrogen‐doped titanium dioxide NPs. Molecules. 24, 3916 (2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de; Dicastillo, C.L. : Antimicrobial effect of titanium dioxide nanoparticles. In: Antimicrobial Resistance ‐ A One Health Perspective. Intech Open; (2020) [Google Scholar]
- 45. Sobha, K. , et al.: Emerging trends in nanobiotechnology. Biotechnol. Mol. Biol. Rev. 4, 1–12 (2010) [Google Scholar]
- 46. Hajipour, M.J. , et al.: Antibacterial properties of NPs. Trends Biotechnol. 30, 499–511 (2012) [DOI] [PubMed] [Google Scholar]
- 47. Jesline, A. , et al.: Antimicrobial activity of zinc and titanium dioxide NPs against biofilm‐producing methicillin‐resistant Staphylococcus aureus. Appl. Nanosci. 5, 157–162 (2015) [Google Scholar]
- 48. Arora, B. , Murar, M. , Dhumale, V. : Antimicrobial potential of TiO2 NPs against MDR Pseudomonas aeruginosa. J. Exp. Nanosci. 10, 819–827 (2015) [Google Scholar]
- 49. Kubacka, A. , et al.: Understanding the antimicrobial mechanism of TiO2‐based nanocomposite films in a pathogenic bacterium. Sci. Rep. 4, 4134 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Petica, A. , et al.: Synthesis and characterization of silver‐titania nanocomposites prepared by electrochemical method with enhanced photocatalytic characteristics, antifungal and antimicrobial activity. J. Mater. Res. Technol. 8, 41–53 (2019) [Google Scholar]
- 51. Hosseini Nasab, N. , Jalili, M.M. , Farrokhpay, S. : Application of paraffin and silver coated titania nanoparticles in polyethylene nanocomposite food packaging films. J. Appl. Polym. Sci. 135, 45913 (2018) [Google Scholar]
- 52. Salgado, A.J. , et al.: Tissue engineering and regenerative medicine: past, present, and future. Int. Rev. Neurobiol. 108, 1–33 (2013) [DOI] [PubMed] [Google Scholar]
- 53. Kumar, P. : Nano‐TiO2 doped chitosan scaffold for the bone tissue engineering applications. Int. J. Biomater. 6576157 (2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fathi‐Achachelouei, M. , et al.: Use of NPs in tissue engineering and regenerative medicine. Front. Bioeng. Biotechnol. 7, 113 (2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sivaranjani, V. , Philominathan, P. : Synthesis of titanium dioxide NPs using Moringa oleifera leaves and evaluation of wound healing activity. Wound Med. 12, 1–5 (2016) [Google Scholar]
- 56. Augustine, A. , et al.: Development of titanium dioxide nanowire incorporated poly (vinylidene fluoride–trifluoroethylene) scaffolds for bone tissue engineering applications. J. Mater. Sci. Mater. Med. 30, 1–13 (2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nikpasand, A. , Parvizi, M.R. : Evaluation of the effect of titanium dioxide NPs/gelatin composite on infected skin wound healing; an animal model study. Bull. Emerg. Trauma. 7, 366–372 (2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Xia, Y. , et al.: Nanoparticle‐reinforced resin‐based dental composites. J. Dent. 36, 450–455 (2008) [DOI] [PubMed] [Google Scholar]
- 59. Alamgir, M. , et al.: Development of PMMA/TiO2 nanocomposites as excellent dental materials. J. Mech. Sci. Technol. 33, 4755–4760 (2019) [Google Scholar]
- 60. Rasoulianboroujeni, M. , et al.: Development of 3D‐printed PLGA/TiO2 nanocomposite scaffolds for bone tissue engineering applications. Mater. Sci. Eng. C. 96, 105–113 (2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pattanashetti, N.A. , et al.: Development of nanofibrous scaffolds by varying the TiO2 content in crosslinked PVA for bone tissue engineering. New J. Chem. 44, 2111–2121 (2020) [Google Scholar]
- 62. Seisenbaeva, G.A. , et al.: Author correction: dispersion of TiO2 NPs improves burn wound healing and tissue regeneration through specific interaction with blood serum proteins. Sci. Rep. 8, 1–2 (2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ikono, R. , et al.: Enhanced bone regeneration capability of chitosan sponge coated with TiO2 NPs. Biotechnol. Rep. 24, e00350 (2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Raliya, R. , et al.: Nanofertilizer for precision and sustainable agriculture: current state and future perspectives. J. Agric. Food Chem. 66, 6487–6503 (2017) [DOI] [PubMed] [Google Scholar]
- 65. OECD, FAO : The Middle East and North Africa: prospects and challenges. In: OECD‐FAO Agricultural Outlook 2018‐2017, pp.67–108. OECD Publishing, Paris/Food and Agriculture Organization of the United States; (2018) [Google Scholar]
- 66. Rodríguez‐González, V. , et al.: Applications of photocatalytic titanium dioxide‐based nanomaterials in sustainable agriculture. J. Photochem. Photobiol. C Photochem. Rev. 40, 49–67 (2019) [Google Scholar]
- 67. Chhipa, H. : Nanofertilizers and nanopesticides for agriculture. Environ. Chem. Lett. 15, 15–22 (2017) [Google Scholar]
- 68. Balaure, P.C. , Gudovan, D. , Gudovan, L. : Nanopesticides: a new paradigm in crop protection. In: New Pesticides and Soil Sensors, pp. 129–192. Elsevier; (2017) [Google Scholar]
- 69. Shaker, A. , et al.: TiO2 NPs as an effective nanopesticide for cotton leaf worm. CIGR J. 61‐68 (2017) [Google Scholar]
- 70. Gutierrez‐Ramírez, J.A. , et al.: Insecticidal effect of zinc oxide and titanium dioxide nanoparticles against Bactericera cockerelli Sulc. (Hemiptera: Triozidae) on tomato Solanum lycopersicum. Agronomy. 11, 1460 (2021) [Google Scholar]
- 71. Narayanan, M. , et al.: Green synthesis and characterization of titanium dioxide nanoparticles using leaf extract of Pouteria campechiana and larvicidal and pupicidal activity on Aedes aegypti. Environ. Res. 200, 111333 (2021) [DOI] [PubMed] [Google Scholar]
- 72. Mukherjee, K. , et al.: TiO2 NPs co‐doped with nitrogen and fluorine as visible‐light‐activated antifungal agents. ACS Appl. Nano Mater. 3, 2016–2025 (2020) [Google Scholar]
- 73. Paret, M.L. , et al.: Photocatalysis: effect of light‐activated nanoscale formulations of TiO2 on Xanthomonas perforans and control of bacterial spot of tomato. Bacteriology. 103, 228–236 (2013) [DOI] [PubMed] [Google Scholar]
- 74. Bai, T. , et al.: Different physiological responses of C3 and C4 plants to nanomaterials. Environ. Sci. Pollut. Res. Int. 28, 25542–25551 (2021) [DOI] [PubMed] [Google Scholar]
- 75. Prasad, R. , Jain, V.K. , Varma, A. : Role of nanomaterials in symbiotic fungus growth enhancement. Curr. Sci. 99, 1189–1191 (2010) [Google Scholar]
- 76. Zheng, L. , et al.: Effect of nano‐TiO2 on strength of naturally aged seeds and growth of spinach. Biol. Trace Elem. Res. 104, 83–91 (2005) [DOI] [PubMed] [Google Scholar]
- 77. Mahmoodzadeh, H. , Nabavi, M. , Kashefi, H. : Effect of nanoscale titanium dioxide particles on the germination and growth of canola (Brassica napus). J. Ornam. Plants. 3, 25–32 (2013) [Google Scholar]
- 78. Morteza, E. , et al.: Study of photosynthetic pigments changes of maize (Zea mays L.) under nano TiO2 spraying at various growth stages. SpringerPlus. 2, 1–5 (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Moaveni, P. , Kheiri, T. : TiO2 nano particles affected on maize (Zea mays L). In: 2nd International Conference on Agricultural and Animal Science. IACSIT Press Singapore; (2011) [Google Scholar]
- 80. Hanif, H.U. , et al.: Phyto‐availability of phosphorus to Lactuca sativa in response to soil applied TiO2 NPs. Pak. J. Agri. Sci. 52, 177–182 (2015) [Google Scholar]
- 81. Haghighi, M. , da Silva, J.A.T. : The effect of N‐TiO2 on tomato, onion, and radish seed germination. J. Crop. Sci. Biotechnol. 17, 221–227 (2014) [Google Scholar]
- 82. Andersen, C.P. , et al.: Germination and early plant development of ten plant species exposed to titanium dioxide and cerium oxide NPs. Environ. Toxicol. Chem. 35, 2223–2229 (2016) [DOI] [PubMed] [Google Scholar]
- 83. Ullah, S. , et al.: Physiological and biochemical response of wheat (Triticum aestivum) to TiO2 NPs in phosphorous amended soil: a full life cycle study. J. Environ. Manag. 263, 110365 (2020) [DOI] [PubMed] [Google Scholar]
- 84. Servin, A.D. , et al.: Synchrotron micro‐XRF and micro‐XANES confirmation of the uptake and translocation of TiO2 NPs in cucumber (Cucumis sativus) plants. Environ. Sci. Technol. 46, 7637–7643 (2012) [DOI] [PubMed] [Google Scholar]
- 85. Moaveni, P. , et al.: Effect of TiO2 NPs spraying on barley (Hordem vulgare L.) under field condition. Adv. Environ. Biol. 2220‐2224 (2021) [Google Scholar]
- 86. Prakashraj, R. , et al.: Fabricated TiO2 nanofertilizers for foliar assimilation to enhance yield and disease resistance in Capsicum annuum L. J. Plant Growth Regul. (2021) [Google Scholar]
- 87. Kolencík, M. , et al.: Foliar application of low concentrations of titanium dioxide and zinc oxide nanoparticles to the common Sunflower under field conditions. Nanomaterials. 10, 1619 (2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Khater, M.S. : Effect of titanium nanoparticles (TiO2) on growth, yield and chemical constituents of coriander plants. Arab J. Nuclear Sci. Appl. 48, 187–194 (2015) [Google Scholar]
- 89. Asli, S. , Neumann, P.M. : Colloidal suspensions of clay or titanium dioxide NPs can inhibit leaf growth and transpiration via physical effects on root water transport. Plant Cell Environ. 32, 577–584 (2009) [DOI] [PubMed] [Google Scholar]
- 90. Changmei, L. , et al.: Research of the effect of nanometer materials on germination and growth enhancement of Glycine max and its mechanism. Soybean Sci. 21, 168–171 (2002) [Google Scholar]
- 91. Satti, S.H. , et al.: Titanium dioxide NPs elicited agro‐morphological and physico‐chemical modifications in wheat plants to control Bipolaris sorokiniana . PLoS One. 16, e0246880 (2021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Zhou, P. , et al.: Application of NPs alleviates heavy metals stress and promotes plant growth: an overview. Nanomaterials. 11, 26 (2021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Rafique, R. , et al.: Dose‐dependent physiological responses of Triticum aestivum L. to soil applied TiO2 NPs: alterations in chlorophyll content, H2O2 production, and genotoxicity. Agric. Ecosyst. Environ. 255, 95–101 (2018) [Google Scholar]
- 94. Gohari, G. , et al.: Titanium dioxide NPs (TiO2 NPs) promote growth and ameliorate salinity stress effects on essential oil profile and biochemical attributes of Dracocephalum moldavica. Sci. Rep. 10, 1–14 (2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Wang, Z. , et al.: Effect of nano‐rutile TiO2 and multiwalled carbon nanotubes on the growth of maize (Zea mays L.) seedlings and the relevant antioxidant response. Huang Jing Ke Xue. 31, 480–487 (2010) [PubMed] [Google Scholar]
- 96. Singh, J. , Lee, B.‐K. : Influence of nano‐TiO2 particles on the bioaccumulation of Cd in soybean plants (Glycine max): a possible mechanism for the removal of Cd from the contaminated soil. J. Environ. Manag. 170, 88–96 (2016) [DOI] [PubMed] [Google Scholar]
- 97. Cai, F. , et al.: Impact of TiO2 NPs on lead uptake and bioaccumulation in rice (Oryza sativa L.). Nanoimpact. 5, 101–108 (2017) [Google Scholar]
- 98. Lian, J. , et al.: Foliar spray of TiO2 NPs prevails over root application in reducing Cd accumulation and mitigating Cd‐induced phytotoxicity in maize (Zea mays L.). Chemosphere. 239, 124794 (2020) [DOI] [PubMed] [Google Scholar]
- 99. Ukaogo, P.O. , Ewuzie, U. , Onwuka, C.V. : Environmental pollution: causes, effects, and the remedies. In: Microorganisms for Sustainable Environment and Health, pp. 419–429. Elsevier; (2020) [Google Scholar]
- 100. Kabir, E. , et al.: Environmental impacts of nanomaterials. J. Environ. Manag. 225, 261–271 (2018) [DOI] [PubMed] [Google Scholar]
- 101. Haidera, A. , et al.: Exploring potential environmental applications of TiO2 NPs. Energy Proc. 119, 332–345 (2017) [Google Scholar]
- 102. Willner, M.R. , Vikesland, P.J. : Nanomaterial enabled sensors for environmental contaminants. J. Nanobiotechnol. 16, 1–16 (2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Bhalla, N. , et al.: Introduction to biosensors. Essays Biochem. 60, 1–8 (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Walter, M.G. , et al.: Solar water splitting cells. Chem. Rev. 110, 6446–6473 (2010) [DOI] [PubMed] [Google Scholar]
- 105. Konstantinou, I.K. , Albanis, T.A. : TiO2‐assisted photocatalytic degradation of azo dyes in aqueous solution: kinetic and mechanistic investigations: a review. Appl. Catal. B Environ. 49, 1–14 (2004) [Google Scholar]
- 106. Feng, D. , et al.: Multi‐layered mesoporous TiO2 thin films with large pores and highly crystalline frameworks for efficient photoelectrochemical conversion. J. Mater. Chem. A. 1, 1591–1599 (2013) [Google Scholar]
- 107. Khan, S.B. , et al.: Selective adsorption and determination of iron (III): Mn3O4/TiO2 composite nanosheets as marker of iron for environmental applications. Appl. Surf. Sci. 282, 46–51 (2013) [Google Scholar]
- 108. Rahman, M.M. , et al.: Hydrazine sensors development based on a glassy carbon electrode modified with a nanostructured TiO2 films by electrochemical approach. Microchim. Acta. 184, 2123–2129 (2017) [Google Scholar]
- 109. Subhan, M.A. , et al.: Enhanced photocatalytic activity and ultra‐sensitive benzaldehyde sensing performance of a SnO2.ZnO.TiO2 nanomaterial. RSC Adv. 8, 33048–33058 (2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Rakib, R.H. , et al.: Fabrication of a 3,4‐diaminotoluene sensor based on a TiO2‐Al2O3 nanocomposite synthesized by a fast and facile microwave irradiation method. Mater. Sci. 4, 12592–12600 (2019) [Google Scholar]
- 111. Chen, X. , et al.: Semiconductor‐based photocatalytic hydrogen generation. Chem. Rev. 110, 6503–6570 (2010) [DOI] [PubMed] [Google Scholar]
- 112. Grimes, C.A. : Synthesis and application of highly ordered arrays of TiO2 nanotubes. J. Mater. Chem. 17, 1451–1457 (2007) [Google Scholar]
- 113. Fujishima, A. , Zhang, X. , Tryk, D.A. : TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 63, 515–582 (2008) [Google Scholar]
- 114. Ghasemi, Z. , Mohammadi, A. : Sensitive and selective colorimetric detection of Cu (II) in water samples by thiazolylazopyrimidine‐functionalized TiO2 NPs. Spectrochim. Acta Mol. Biomol. Spectrosc. 239, 118554 (2020) [DOI] [PubMed] [Google Scholar]
- 115. Mehta, A. , et al.: Carbon quantum dots/TiO2 nanocomposite for sensing of toxic metals and photodetoxification of dyes with kill waste by waste concept. Mater. Des. 155, 485–493 (2018) [Google Scholar]
- 116. Faisal, M. , et al.: Mesoporous TiO2 based optical sensor for highly sensitive and selective detection and preconcentration of Bi (III) ions. Chem. Eng. J. 243, 509–516 (2014) [Google Scholar]
- 117. Yang, L. , et al.: Sensitive detection of polycyclic aromatic hydrocarbons using CdTe quantum dot‐modified TiO2 nanotube array through fluorescence resonance energy transfer. Environ. Sci. Technol. 44, 7884–7889 (2010) [DOI] [PubMed] [Google Scholar]
- 118. Nurdin, M. , et al.: Synthesis and electrochemical performance of graphene‐TiO2‐carbon paste nanocomposites electrode in phenol detection. Mater. Sci. 131, 104–110 (2019) [Google Scholar]
- 119. Zhan, K. , et al.: Carbon tetrachloride vapor sensing based on ZIF‐8@ ZnO/TiO2 one‐dimensional top‐defect photonic crystals. Sens. Actuators A Phys. 314, 112249 (2020) [Google Scholar]
- 120. Qi, Q. , et al.: Properties of humidity sensing ZnO nanorods‐base sensor fabricated by screen‐printing. Sensor. Actuator. B Chem. 133, 638–643 (2008) [Google Scholar]
- 121. Nurdin, M. , et al.: High performance cypermethrin pesticide detection using anatase TiO2‐carbon paste nanocomposites electrode. Microchem. J. 145, 756–761 (2019) [Google Scholar]
- 122. Tan, X. , et al.: Ultrasensitive electrochemical detection of methyl parathion pesticide based on cationic water‐soluble pillar[5]arene and reduced graphene nanocomposite. RSC Adv. 9, 345–353 (2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Paz‐Ferreiro, J. , et al.: Soil pollution and remediation. Int. J. Environ. Res. Publ. Health. 15, 1657 (2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Hao, Y. , et al.: Graphitic carbon nitride (gC3N4) alleviates cadmium‐induced phytotoxicity to rice (Oryza sativa L.). Environ. Sci. Pollut. Res. Int. 28, 21276–21284 (2021) [DOI] [PubMed] [Google Scholar]
- 125. Ahmari, H. , Heris, S.Z. , Khayyat, M.H. : The effect of titanium dioxide NPs and UV irradiation on photocatalytic degradation of imidaclopride. Environ. Technol. 39, 536–547 (2018) [DOI] [PubMed] [Google Scholar]
- 126. Wang, A. , et al.: Diphenylarsinic acid contaminated soil remediation by titanium dioxide (P25) photocatalysis: degradation pathway, optimization of operating parameters and effects of soil properties. Sci. Total Environ. 541, 348–355 (2016) [DOI] [PubMed] [Google Scholar]
- 127. Wang, T.C. , et al.: Plasma‐TiO2 catalytic method for high‐efficiency remediation of p‐nitrophenol contaminated soil in pulsed discharge. Environ. Sci. Technol. 45, 9301–9307 (2011) [DOI] [PubMed] [Google Scholar]
- 128. Zand, A.D. , Tabrizi, A.M. , Heir, A.V. : Application of titanium dioxide NPs to promote phytoremediation of Cd‐polluted soil: contribution of PGPR inoculation. Ann. Finance. 24, 171–189 (2020) [Google Scholar]
- 129. Zand, A.D. , Tabrizi, A.M. , Heir, A.V. : Co‐application of biochar and titanium dioxide NPs to promote remediation of antimony from soil by Sorghum bicolor: metal uptake and plant response. Heliyon. 6, e04669 (2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Adeel, M. , et al.: COVID‐19 and nanoscience in the developing world: rapid detection and remediation in wastewater. Nanomaterials. 11, 991 (2021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Irshad, M.A. , et al.: Synthesis, characterization and advanced sustainable applications of titanium dioxide NPs: a review. Ecotoxicol. Environ. Saf. 212, 111978 (2021) [DOI] [PubMed] [Google Scholar]
- 132. Zahra, Z. , et al.: Exposure route of TiO2 NPs from industrial applications to wastewater treatment and their impacts on the agro‐environment. Nanomaterials. 10, 1469 (2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Vahid, R. , et al.: Growth performance, blood metabolites and jejunum morphology of broiler chickens fed diets containing earthworm (Eisenia foetida) meal as a source of protein. Biology. 2, 2483–2494 (2014) [Google Scholar]
- 134. Poudel, B.R. , et al.: Agro‐waste derived biomass impregnated with TiO2 as a potential adsorbent for removal of as (III) from water. Catalysts. 10, 1125 (2020) [Google Scholar]
- 135. Siddeeg, S.M. : A novel synthesis of TiO2/GO nanocomposite for the uptake of Pb2+ and Cd2+ from wastewater. Mater. Res. Express. 7, 025038 (2020) [Google Scholar]
- 136. Mironyuk, I. , et al.: Sodium‐modified mesoporous TiO2: sol–gel synthesis, characterization and adsorption activity toward heavy metal cations. J. Mol. Liq. 316, 113840 (2020) [Google Scholar]
- 137. Sun, Y. , et al.: Simultaneous removal of colorless micropollutants and hexavalent chromium by pristine TiO2 under visible light: an electron transfer mechanism. Chem. Eng. J. 405, 126968 (2021) [Google Scholar]
- 138. Li, Q.‐H. , et al.: Enhancement of Cr (VI) removal efficiency via adsorption/photocatalysis synergy using electrospun chitosan/g‐C3N4/TiO2 nanofibers. Carbohydr. Polym. 253, 117200 (2021) [DOI] [PubMed] [Google Scholar]
- 139. Wang, H. , et al.: Facile synthesis of heterojunction of MXenes/TiO2 NPs towards enhanced hexavalent chromium removal. J. Colloid Interface Sci. 561, 46–57 (2020) [DOI] [PubMed] [Google Scholar]
- 140. Alshameri, A. , et al.: Adsorption of ammonium by different natural clay minerals: characterization, kinetics and adsorption isotherms. Appl. Clay Sci. 159, 83–93 (2018) [Google Scholar]
- 141. Konduri, M.K.R. , Fatehi, P. : Influence of pH and ionic strength on flocculation of clay suspensions with cationic xylan copolymer. Colloids Surf. A. 530, 20–32 (2017) [Google Scholar]
- 142. Nasir, J.A. , et al.: Recent developments and perspectives in CdS‐based photocatalysts for water splitting. J. Mater. Chem. A. 8, 20752–20780 (2020) [Google Scholar]
- 143. Indira, K. , et al.: Photocatalytic degradation of Congo red dye using nickel–titanium dioxide nanoflakes synthesized by Mukia madrasapatna leaf extract. Environ. Res. 202, 111647 (2021) [DOI] [PubMed] [Google Scholar]
- 144. Indira, K. , et al.: Synthesis of titanium/niobium oxide nanocomposite on top open bamboo like titanium dioxide nanotube for the catalytic degradation of organic pollutants. J. Environ. Chem. Eng. 9, 105400 (2021) [Google Scholar]
- 145. Blosi, M. , et al.: Chlorella vulgaris meets TiO2 NPs: effective sorbent/photocatalytic hybrid materials for water treatment application. J. Environ. Manag. 304, 114187 (2022) [DOI] [PubMed] [Google Scholar]
- 146. Cohen, A.J. , et al.: The global burden of disease due to outdoor air pollution. J. Toxicol. Environ. Health Part A. 68, 1301–1307 (2005) [DOI] [PubMed] [Google Scholar]
- 147. Khin, M.M. , et al.: A review on nanomaterials for environmental remediation. Energy Environ. Sci. 5, 8075–8109 (2012) [Google Scholar]
- 148. Puddu, V. , et al.: TiO2 photocatalyst for indoor air remediation: influence of crystallinity, crystal phase, and UV radiation intensity on trichloroethylene degradation. Appl. Catal. B Environ. 94, 211–218 (2010) [Google Scholar]
- 149. Li, D. , et al.: Visible‐light‐driven N−F−codoped TiO2 photocatalysts. 2. Optical characterization, photocatalysis, and potential application to air purification. Chem. Mater. 17, 2596–2602 (2005) [Google Scholar]
- 150. Wang, Y. , Zhu, Y.‐W. , Wu, S. : A new nano CaO‐based CO2 adsorbent prepared using an adsorption phase technique. Chem. Eng. J. 218, 39–45 (2013) [Google Scholar]
- 151. Merajin, M.T. , et al.: Photocatalytic conversion of greenhouse gases (CO2 and CH4) to high value products using TiO2 NPs supported on stainless steel webnet. Mater. Sci. 44, 239–246 (2013) [Google Scholar]
- 152. Li, J.‐J. , et al.: Efficient infrared light promoted degradation of volatile organic compounds over photo‐thermal responsive Pt‐rGO‐TiO2 composites. Appl. Catal. B Environ. 233, 260–271 (2018) [Google Scholar]
- 153. Fukuyama, J. : Odor pollution control for various odor emission sources in Japan. In: East Asia Workshop on Odor Measurement and Control Review, Office of Odor, Noise and Vibration. Environmental Management Bureau, Ministry of the Environment, Government of Japan; (2005) [Google Scholar]
- 154. Slimen, H. , et al.: Photocatalytic decomposition of cigarette smoke using a TiO2‐impregnated titanium mesh filter. Ind. Eng. Chem. Res. 51, 587–590 (2012) [Google Scholar]
- 155. Rezaee, A. , et al.: Photocatalytic decomposition of gaseous toluene by TiO2 NPs coated on activated carbon. Iran. J. Environ. Health Sci. Eng. 5, 305–310 (2008) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


