Summary
Trace metals are essential to all domains of life but toxic when found at high concentrations. While the importance of iron in host-pathogen interactions is firmly established, contemporary studies indicate that other trace metals, including manganese and zinc, are also critical to the infectious process. In this study, we sought to identify and characterize the zinc uptake system(s) of S. mutans, a keystone pathogen in dental caries and a causative agent of bacterial endocarditis. Different than other pathogenic bacteria, including several streptococci, that encode multiple zinc import systems, bioinformatic analysis indicated that the S. mutans core genome encodes a single, highly conserved, zinc importer commonly known as AdcABC. Inactivation of the genes coding for the metal-binding AdcA (ΔadcA) or both AdcC ATPase and AdcB permease (ΔadcCB) severely impaired the ability of S. mutans to grow under zinc-depleted conditions. Intracellular metal quantifications revealed that both mutants accumulated less zinc when grown in the presence of a sub-inhibitory concentration of a zinc-specific chelator. Notably, the ΔadcCB strain displayed a severe colonization defect in a rat oral infection model. Both Δadc strains were hypersensitive to high concentrations of manganese, showed reduced peroxide tolerance, and formed less biofilm in sucrose-containing media when cultivated in the presence of the lowest amount of zinc that support their growth, but not when zinc was supplied in excess. Collectively, this study identifies AdcABC as the only known high affinity zinc importer of S. mutans and provides preliminary evidence that zinc is a growth-limiting factor within the dental biofilm.
Keywords: Streptococcus mutans, dental caries, zinc, metal homeostasis, nutritional immunity
Introduction
The first-row d-block elements iron, manganese and zinc are known to be essential to most forms of life by serving structural, catalytic and regulatory functions to numerous biological processes. However, when in excess, these biometals are toxic such that their cellular flux and allocation must be tightly regulated. This Goldilocks paradox creates an opportunity for vertebrate hosts to deploy either metal sequestration or metal intoxication strategies to combat microbial infection; an active process known as nutritional immunity (Kehl-Fie & Skaar, 2010). Nutritional immunity strategies include mobilization of metal-chelating proteins to infected tissues such as iron-sequestering transferrin and lactoferrin, and neutrophil-secreted calprotectin which is responsible for restricting manganese, zinc and, in certain environments, iron availability (Nakashige, Zhang, Krebs, & Nolan, 2015; Zackular, Chazin, & Skaar, 2015). Paradoxically, the host can increase cytosolic zinc or mobilize copper or zinc into the phagolysosome creating a toxic environment for intracellular bacteria (Sheldon & Skaar, 2019). To overcome metal limitation during infection, bacteria rely on the expression of surface-associated metal importers with some organisms also secreting small metal-binding molecules (metallophores) that tightly bind trace metals that are then reinternalized via specific transporters (Palmer & Skaar, 2016). Importantly, in vivo studies have shown that metal import systems are critical to bacterial virulence (Fischer et al., 2016; Garcia, Brumbaugh, & Mobley, 2011; Koh et al., 2015; Mastropasqua et al., 2017; Ong, Berking, Walker, & McEwan, 2018).
While the central role of iron in host-pathogen interactions has been known for decades, the importance of manganese and zinc homeostasis to bacterial pathogenesis is a relatively newer development (Kehl-Fie & Skaar, 2010; Lonergan & Skaar, 2019). In bacteria, manganese is the co-factor of enzymes involved in DNA replication, central metabolism and critical to activation of oxidative stress responses of gram-positive bacteria (Juttukonda & Skaar, 2015). Zinc is the second most abundant metal co-factor and is incorporated into 5–6% of all bacterial proteins, including central metabolism enzymes, regulatory proteins and metalloproteases (Lonergan & Skaar, 2019; Rahman & Karim, 2018). In addition, zinc plays an additional role in host-pathogen interactions by stimulating innate and adaptive immune cell function and, as indicated above, through mobilization within phagosomes to intoxicate invading pathogens (Lonergan & Skaar, 2019; Sheldon & Skaar, 2019; Subramanian Vignesh & Deepe, 2016). Unlike iron and manganese, zinc does not undergo redox-cycling, but can form tighter and stabler interactions with metal ligands. As a result, zinc can occupy metal-binding residues of non-cognate metalloproteins inhibiting or reducing their activities, a process known as protein mismetallation (Chandrangsu & Helmann, 2016; Imlay, 2014).
A resident of dental plaque, Streptococcus mutans is a keystone pathogen in dental caries due to its ability to modify the oral biofilm architecture and environment in a way that facilitates the proliferation of acidogenic and aciduric bacteria at the expense of health-associated bacteria (Bowen, Burne, Wu, & Koo, 2018; Lemos et al., 2019). In addition to dental caries, S. mutans is a causative agent of infective endocarditis, a life-threatening infection of the endocardium (Pant et al., 2015). Once established in the oral biofilm, S. mutans metabolizes dietary carbohydrates, in particular sucrose, to produce an acidic biofilm matrix that is conducive to the growth of other cariogenic organisms (Lemos et al., 2019). Despite the importance of manganese and zinc to bacterial physiology, the mechanisms utilized by oral bacteria to maintain manganese and zinc homeostasis and their significance to polymicrobial and host-pathogen interactions in the oral cavity are poorly understood. Recently, we characterized the major manganese transporters of S. mutans and showed that maintenance of manganese homeostasis is critical for the expression of major virulence attributes of S. mutans, including the ability to tolerate acid and oxidative stresses and to form biofilms in a sucrose-dependent manner (Kajfasz et al., 2020). In this study, we sought to identify and characterize the zinc uptake system(s) of S. mutans and then determine the significance of zinc acquisition to the pathophysiology of S. mutans.
Results
AdcABC is the main transporter responsible for zinc uptake in S. mutans
In other streptococci, zinc acquisition is mediated by an ABC-type transporter known as AdcABC whereby AdcA is the zinc-binding lipoprotein, AdcB a membrane permease and AdcC a cytoplasmic ATPase (Bayle et al., 2011; Burcham et al., 2020; Loo, Mitrakul, Voss, Hughes, & Ganeshkumar, 2003; Ong et al., 2018). The adcABC genes are regulated by adcR, a metalloregulator from the MarR family, which is located immediately upstream and, depending on the streptococcal species, co-transcribed with adcCBA or adcCB (Fig. 1) (Reyes-Caballero et al., 2010). Through BLAST search analysis, we identified homologues of the adcR, adcA, adcB and adcC genes in the core genome of S. mutans. Similar to S. agalactiae and S. pyogenes, the gene coding for the zinc-binding AdcA lipoprotein is not co-localized with adcR, adcB and adcC being located as a monocistronic transcriptional unit elsewhere in the chromosome (Fig. 1). In addition, while the majority of streptococcal genomes encode two copies of adcA, dubbed adcA and adcAII (Bayle et al., 2011; Bersch et al., 2013; Brown et al., 2016; Burcham et al., 2020; Loisel et al., 2011; Loisel et al., 2008; Moulin et al., 2016), the genome of S. mutans encodes a single adcA gene, which is similar in size and more closely-related to the adcA gene genetically-linked to adcBC than to adcAII (Fig. 1). In most cases, the streptococcal adcAII is genetically coupled to metal-binding poly-histidine triad (pht) genes, which encode proteins that are thought to scavenge zinc outside the cell shuttling it to either AdcAII and, possibly, AdcA for internalization (Bersch et al., 2013; Loisel et al., 2011; Loisel et al., 2008; Zheng et al., 2011). While most streptococci encode at least one pht gene, bioinformatic analysis indicate that the core S. mutans genome lacks pht homologs. Similar to other streptococcal AdcA, the S. mutans AdcA has two zinc-binding domains that are characteristic of the genus: an N-terminal tertiary scaffold consisting of 3 histidine and 1 glutamic acid residue (H71, H149, H212, and E298) and a C-terminal ZinT domain consisting of three histidine residues (H461, H470 and H472) (Fig. 2 and S1). In addition, there are three additional conserved histidine residues (H71, H149 and H213) that aid in metal recruitment by AdcA (Cao et al., 2018).
Figure 1.
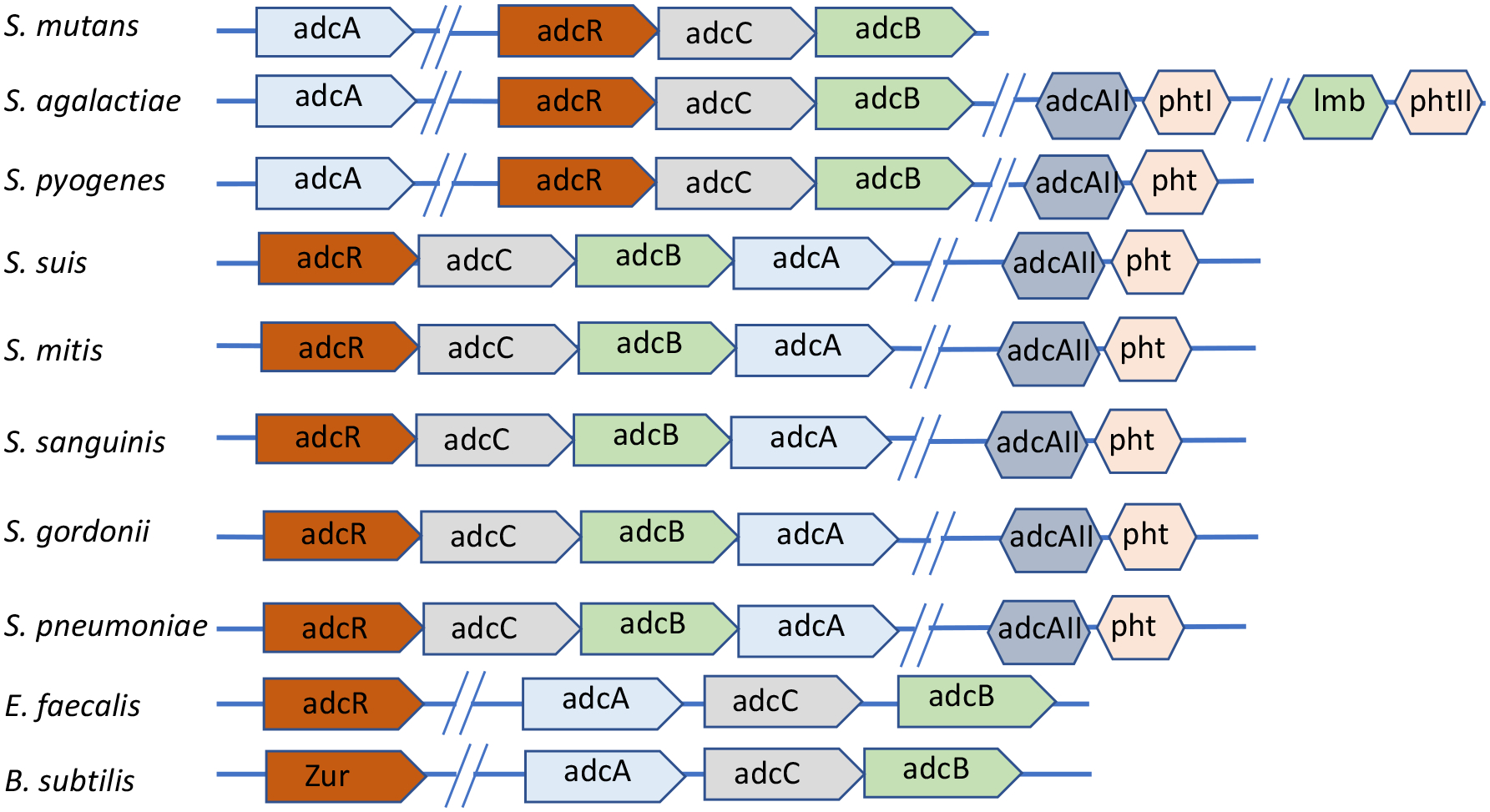
Genetic organization of the adcABC genes and other known zinc import systems in selected gram-positive bacteria.
Figure 2.
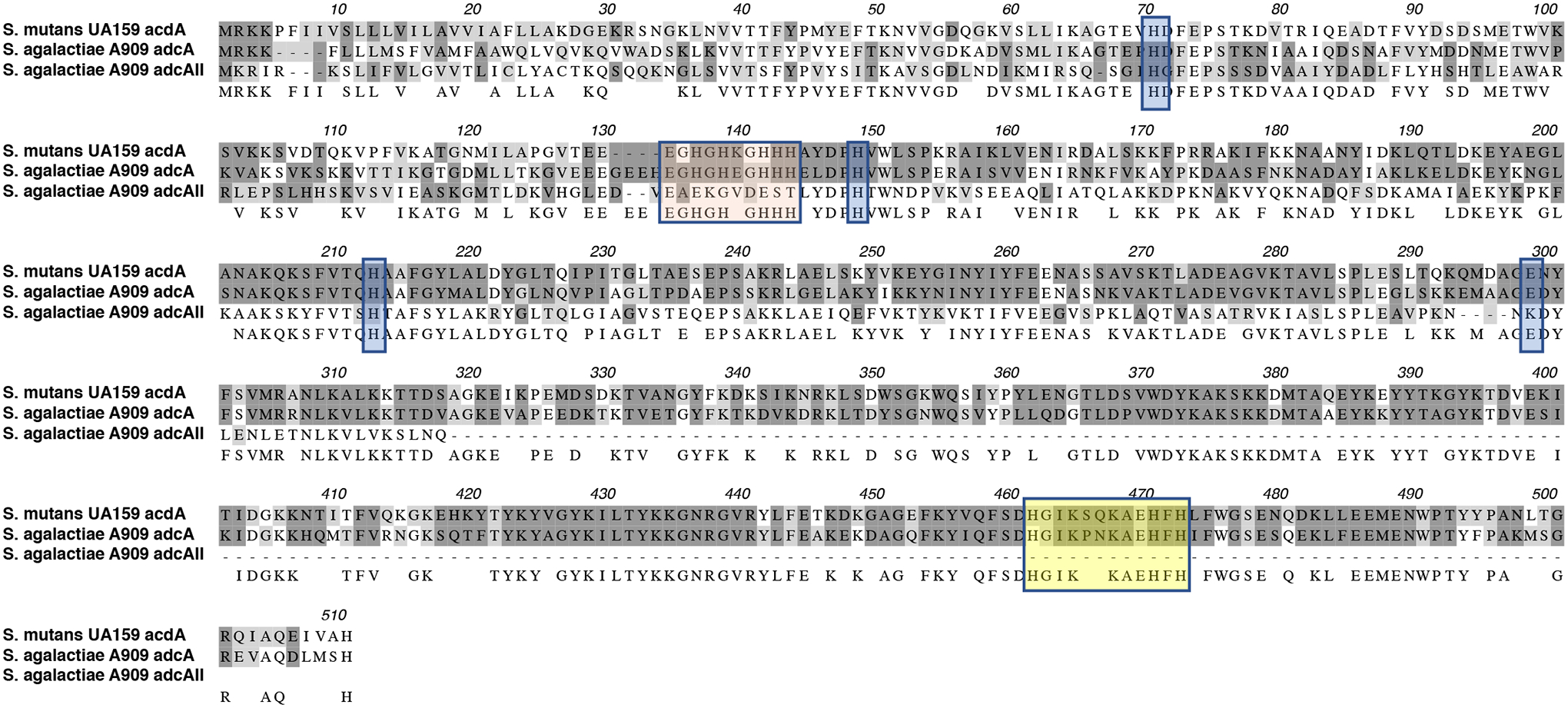
Alignment of AdcA of S. mutans with AdcA and AdcAII proteins of S. agalactiae. Dark and light grey shades represent identical and similar residues, respectively. Orange shaded residues are the N-terminal histidine rich metal-binding motif, yellow boxed residues depict the C-terminal ZinT domain while the glutamic acid and additional histidine residues that aid in metal recruitment are indicated in blue shades.
To evaluate the significance of zinc acquisition to the pathophysiology of S. mutans, we used UA159 as the background strain for the generation of mutants lacking either adcA (ΔadcA) or both adcC and adcB (ΔadcCB) genes and then tested their ability to grow under zinc-replete or zinc-depleted conditions. Both ΔadcA and ΔadcCB strains showed minimal growth in the chemically-defined FMC medium (Terleckyj, Willett, & Shockman, 1975) (Fig. 3A), which does not contain a source of zinc in its original recipe (<1 μM zinc), (Kajfasz et al., 2020). However, supplementation of the FMC medium with as little as 5 μM of ZnSO4 restored growth of both adc mutants (Fig. 3B). Next, we tested the ability of parent and mutant strains to grow in brain heart infusion (BHI), a complex media containing ~10 μM zinc (Kajfasz et al., 2020) that is routinely used to cultivate S. mutans in our laboratory. Based on the behavior of the mutant strains in zinc-supplemented FMC, it was not surprising that both the ΔadcA and ΔadcCB strains grew well in BHI (Fig. 3C). Next, we tested the ability of the ΔadcA and ΔadcCB strains to grow in BHI containing purified human calprotectin or in BHI containing the zinc-specific chelator N,N,N’,N’-Tetrakis(2-pyridylmethyl) ethylenediamine (TPEN) (Zhang et al., 2017). In agreement with the role of calprotectin and TPEN in zinc-sequestration, growth of ΔadcA and ΔadcCB was inhibited by calprotectin (Fig. 3D), or TPEN (Fig. 3E). Finally, the growth defect of the ΔadcCB strain under all zinc-depleted conditions were restored in the genetically-complemented ΔadcCBcomp strain (Fig. 3).
Figure 3.
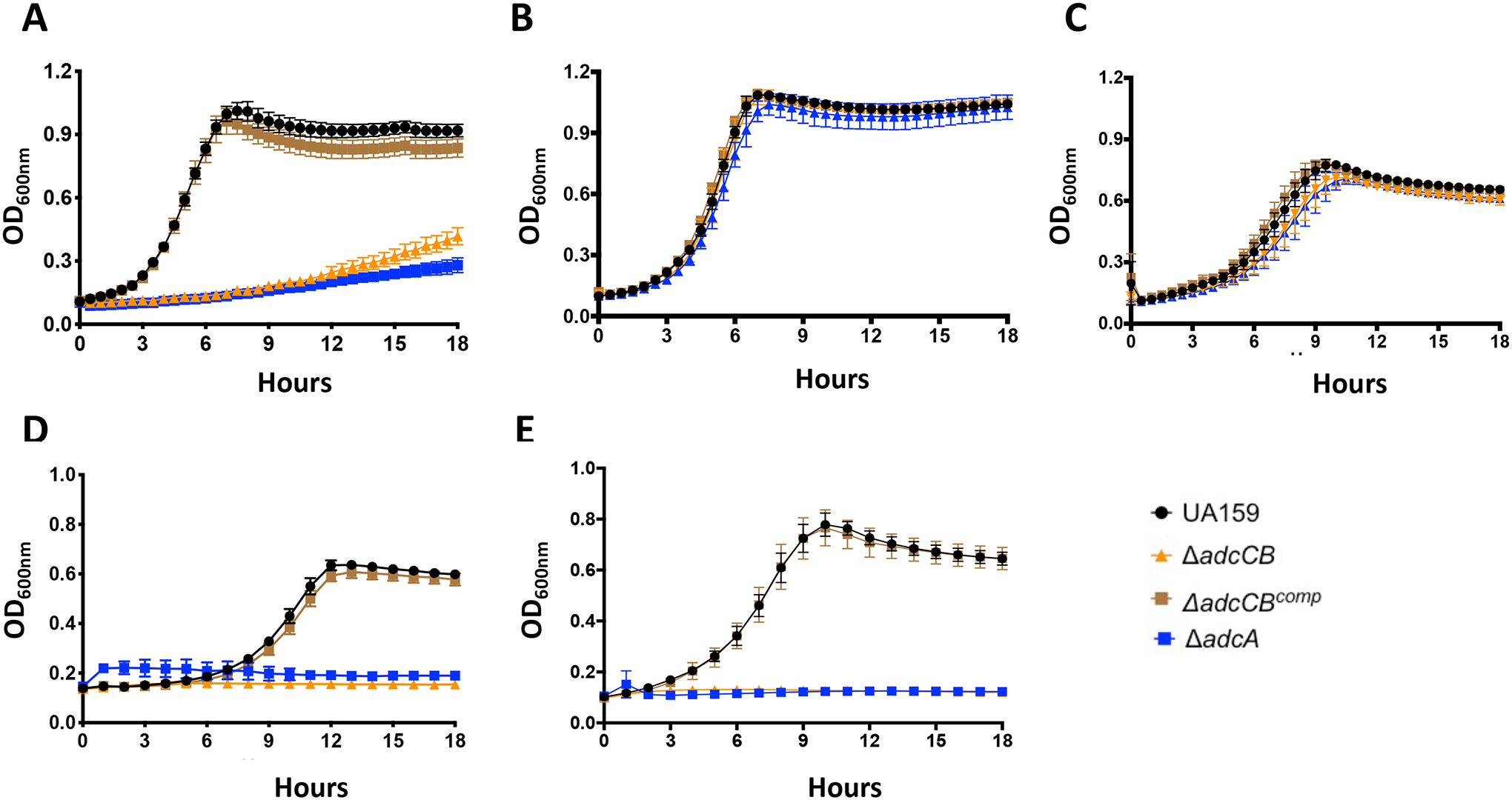
AdcABC mediates the growth of S. mutans under zinc-depleted conditions. Growth curves of UA159, ΔadcA, ΔadcCB or ΔadcCBcomp in (A) FMC, (B) FMC supplemented with 5 μM ZnSO4, (C) BHI, (D) CP/BHI medium containing 200 μg ml−1 of human calprotectin, and (E) BHI containing 10 μM TPEN. Curves shown represent average and standard deviation of at least five independent biological replicates.
While AdcAII and Pht-encoding genes are absent in S. mutans, a gene (smu2069) coding for a hypothetical protein with 37% identity and 60% similarity to the E.coli ZupT, a member of the zinc import ZIP family commonly found in gram-negative and in selected gram-positive bacteria (Grass, Wong, Rosen, Smith, & Rensing, 2002; Zackular et al., 2020) was identified through BLAST searches. To probe the possible role of Smu2069 in zinc uptake, we created a strain bearing a smu2069 deletion in UA159 (Δsmu2069) and in the ΔadcCB background thereby generating a ΔadcCBΔsmu2069 triple mutant. Inactivation of smu2069, alone or in combination with adcCB, did not affect zinc uptake as the Δsmu2069 strain was fully capable to grow in FMC without zinc supplementation and the triple mutant phenocopied the ΔadcCB strain (Fig. S2).
Next, we used inductively coupled plasma mass spectrometry (ICP-MS) to determine the intracellular concentration of selected metals (copper, iron, manganese and zinc) in the parent UA159 and derivative strains grown to mid logarithmic phase in BHI or in BHI containing 6 μM TPEN, a concentration permissible to the growth of the ΔadcCB and ΔadcA strains (data not shown). As compared to UA159, both ΔadcCB and ΔadcA mutants showed a relatively small, yet significant, reduction in intracellular zinc when grown in plain BHI (Fig. 4A). However, the addition of TPEN to the growth media further increased this difference with both ΔadcCB and ΔadcA strains showing ~3-fold reduction in intracellular zinc when compared to the parent strain (Fig. 4B). The addition of 6 μM TPEN to the growth media did not affect growth nor the intracellular zinc content of the parent strain confirming that AdcABC is a major factor in maintenance of intracellular zinc homeostasis under zinc-restricted conditions. Finally, intracellular copper and iron concentrations were not markedly different in parent and mutant strains (data not shown), but there were significant increases in intracellular manganese in ΔadcCB (~30% in BHI and ~20% in BHI+TPEN) with the ΔadcA strain showing a similar trend in BHI+TPEN (Fig. 4C–D). Collectively, these results strongly suggest that growth of S. mutans in zinc-restricted environments is primarily mediated by AdcABC.
Figure 4.

ICP-MS quantifications of intracellular zinc and manganese in the UA159, ΔadcA, ΔadcCB or ΔadcCBcomp strains. The bar graphs indicate zinc or manganese levels in cells grown in BHI (A and C) or BHI containing 6 μM TPEN (B and D). Data represent averages and standard deviations of three independent biological replicates. One-way ANOVA was used to compare the metal content of mutants and UA159 (*) and between ΔadcCB and ΔadcCBcomp (#). A p value <0.05 was considered significant.
The ΔadcCB mutant is hypersensitive to high manganese concentrations
The ICP-MS quantifications indicate that zinc:manganese ratio is drastically different in the Δadc strains when compared to the parent strain, particularly under TPEN-mediated zinc restriction. While zinc:manganese ratios of UA159 and Δadc strains grown in plain BHI was about 1:1 (1:0.7 for UA159 and ~1:1 for ΔadcA ΔadcCB), there was ~3 times more manganese than zinc (1:3 ratio) in the Δadc strains when grown in BHI+TPEN and a well-balanced 1:1 ratio for UA159. Interestingly, a notable increase in intracellular manganese was also observed in a S. pneumoniae double ΔadcAΔadcAII strain which, as expected, has a major defect in zinc uptake (Bayle et al., 2011). While manganese is an essential micronutrient to bacteria and lactic acid bacteria are notorious for having a high demand for manganese (Archibald, 1986), recent studies revealed that strains of S. pneumoniae, S. mutans and Enterococcus faecalis lacking the manganese exporter MntE can be intoxicated by manganese. This intoxication is likely attributed to cellular enzyme mismetallation, though more specific mechanisms such as disruption of the cell division cycle or dysregulation of the peroxide responsive regulator PerR have been proposed (Lam, Wong, Chong, & Kline, 2020; Martin, Lisher, Winkler, & Giedroc, 2017; O’Brien, Pastora, Stoner, & Spatafora, 2020; Turner et al., 2015). Using a plate titration assay, we showed that the ΔadcCB strain was hypersensitive to high concentrations of manganese (Fig. 5). This hypersensitivity was fully rescued by addition of 20 μM ZnSO4 to the growth media directly linking manganese toxicity to a disruption of zinc:manganese balance.
Figure 5.
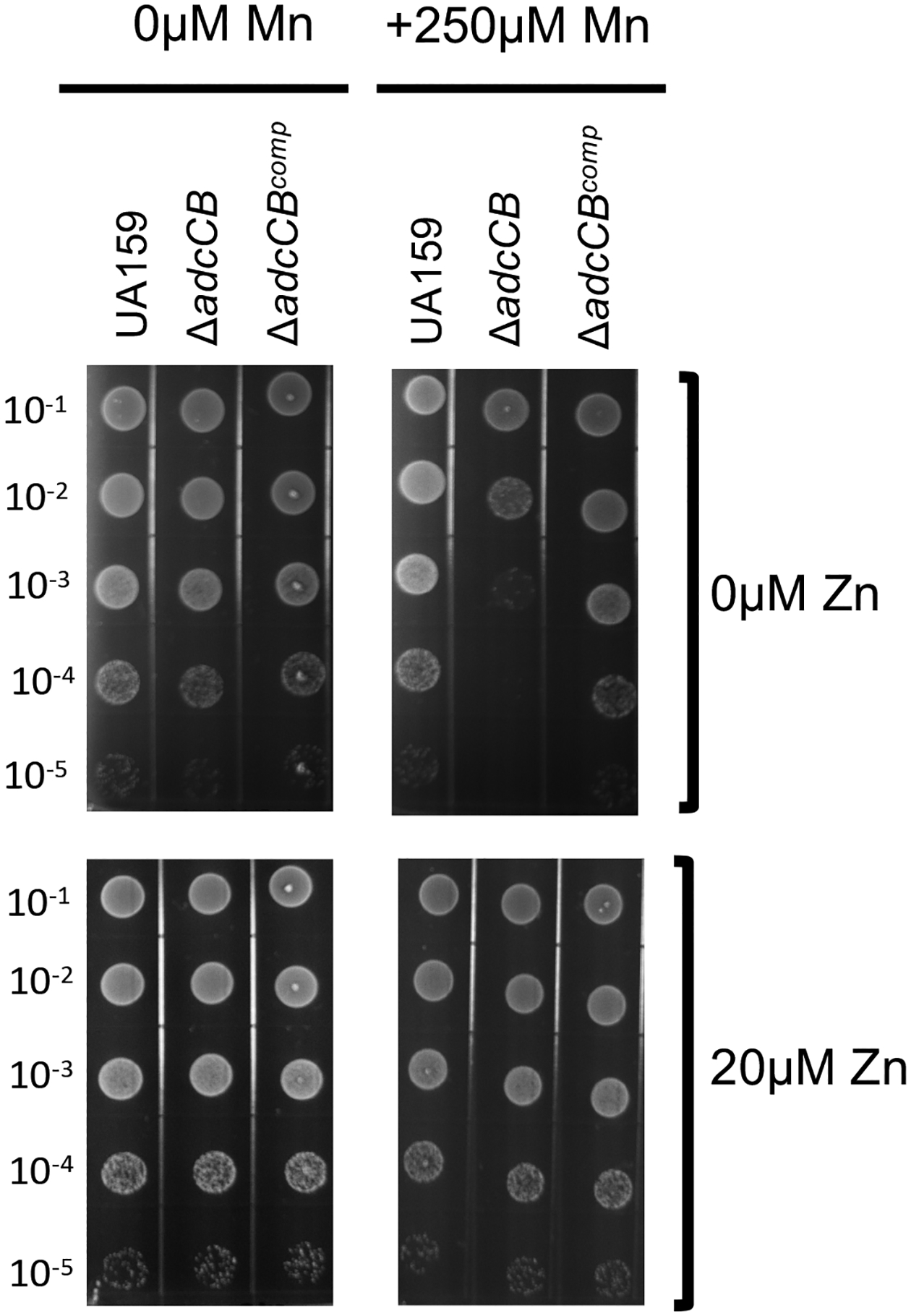
The ΔadcCB mutant strain is hypersensitive to manganese. Mid-log grown cultures of UA159, ΔadcCB or ΔadcCBcomp were serially diluted and spotted on BHI agar containing different concentrations of manganese or zinc as indicated in the figure labels.
The ΔadcCB strain showed an impaired ability to colonize the rat tooth surface
To determine the significance of zinc transport in oral infection by S. mutans, we utilized a rat oral colonization model to test the capacity of the ΔadcCB strain to colonize the teeth of rats fed a metal balanced diet (~1.25 nM ZnCO3) containing 12% sucrose to facilitate the establishment of S. mutans in dental plaque. As compared to the parent strain, the ΔadcCB strain was recovered in significantly fewer numbers (~2 logs) two weeks after the first day of infection, with no colonies recovered in two of the eight infected animals (Fig. 6A). Moreover, while S. mutans colonies accounted for 40 to 60% of the total flora recovered from animals infected with the parent strain, less than 1% of the recovered flora of animals infected with the ΔadcCB strain corresponded to S. mutans colonies (Fig. 6B). All in all, this result indicates that zinc is a growth-limiting factor within the oral biofilm and that the ability to scavenge environmental zinc via the AdcABC transporter is critical to the cariogenic potential of S. mutans.
Figure 6.
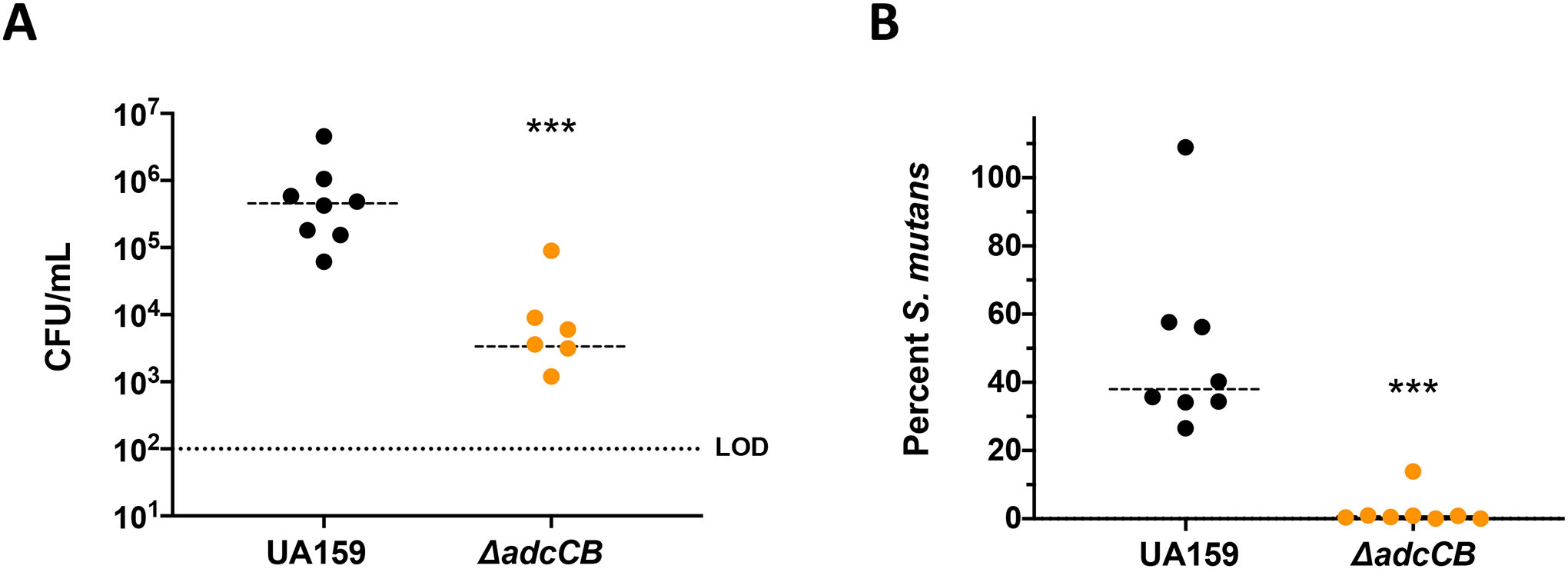
Colonization of S. mutans UA159 or ΔadcCB on the teeth of rats. (A) Total S. mutans colonies recovered from rat jaws by plating on MS agar, and (B) percentage of S. mutans colonies among total recovered flora plated on blood agar plates. The limit of detection for CFU is 102. (*) Indicates statistical significance (p = 0.0003 (A) and 0.0002 (B) by Mann-Whitney test).
Expression of two virulence-related traits were impaired in the ΔadcA and ΔadcCB strains
The ability to cope with environmental stresses, particularly low pH and oxidative stress, and to form robust biofilms in the presence of sucrose are key virulence traits of S. mutans (Bowen et al., 2018; Lemos et al., 2019). To investigate whether the oral colonization defect of the ΔadcCB strain could be linked to poor expression of one or more of these virulence attributes, we first assessed the ability of ΔadcA and ΔadcCB to tolerate acid or H2O2 stresses using both growth curve and disc diffusion assays. While the two mutants grew as well as the UA159 parent strain in media at low pH conditions (pH 6; data not shown), both showed larger growth inhibition zones when exposed to H2O2 in a disc diffusion assay (data not shown) and longer lag phase and slightly slower growth rates when grown in FMC supplemented with 5 μM ZnSO4, the minimal amount of zinc needed to support growth of the Δadc strains, in the presence of a sub-inhibitory concentration of H2O2 (Fig. 7A). Notably, increasing the final concentration of ZnSO4 from 5 to 20 μM abolished the increased H2O2 sensitivity of both mutants (Fig. 7B). Next, we tested the ability of the mutants to form biofilm on saliva-coated microtiter plate wells using 1% sucrose as the sugar source. After 24 hours of incubation, both ΔadcCB and ΔadcA showed a small but significant defect in biofilm formation when grown in FMC containing 5 μM ZnSO4 but not 20 μM ZnSO4 (Fig. 7C). Collectively, these results serve to further support the oral colonization defect of the ΔadcCB strain in the murine model.
Figure 7.

Expression of virulence-related attributes in the UA159, ΔadcA and ΔadcCB strains. (A) Growth in in the presence of a sub-inhibitory concentration of H2O2 (0.75 mM) in FMC supplemented with 5 μM (A) or 20 μM ZnSO4 (B). Curves shown represent average and standard deviation of at least five independent biological replicates. (C) 24-h biofilms formed on the surface of saliva-coated microtiter plate wells from cells grown in FMC supplemented with 1% sucrose in presence of varying amount of Zn. (*) Indicates statistical significance when compared to UA159 strain (p < 0.05, one-way ANOVA).
Discussion
Despite the critical importance of metals to bacterial virulence, little is known about the mechanisms of metal homeostasis in oral bacteria. In this report, we identified and characterized a high-affinity zinc transporter of S. mutans. Inactivation of adcA or adcCB rendered S. mutans unable to grow in the presence of zinc chelators, including calprotectin which can bind zinc in the picomolar range and is produced by host immune cells, especially neutrophils (Brophy, Hayden, & Nolan, 2012; Nakashige et al., 2015; Zackular et al., 2015). Different than other streptococci, the genome of S. mutans lacks a second copy of adcA or of polyhistidine triad (pht) genes. More recently, zinc uptake machineries that resemble siderophore-mediated iron uptake systems were identified in several bacteria including Staphylococcus aureus and Pseudomonas aeruginosa (Ghssein et al., 2016; Mastropasqua et al., 2017). This system is comprised of small molecules with high zinc affinity (zincophores) that are synthesized in the cytoplasm, exported and reinternalized as a zincophore-zinc complex by a dedicated import system (Grim et al., 2020). Despite evidence that zincophore-mediated uptake systems are produced in a wide array of bacteria, a zincophore biosynthetic gene cluster as well as zincophore export and import genes are absent in streptococcal genomes (Morey & Kehl-Fie, 2020). Collectively, our phenotypic characterizations and bioinformatic analysis indicate that the AdcABC transporter is the only known high-affinity zinc import system of S. mutans.
As the second most abundant trace metal in the human body, zinc is also present in oral fluids and tissues, including the tooth enamel surface, dental plaque and saliva. Independent reports have shown that zinc is detected in the low micromolar range in human saliva and at much higher concentrations in dental plaque, with some studies detecting millimolar levels of zinc in plaque samples (Lynch, 2011). However, the bioavailability of zinc in saliva or in dental plaque is unknown. Our in vivo study indicates that zinc is not readily available in dental plaque as the ability of the ΔadcCB strain to colonize the teeth of rats fed a zinc-balanced cariogenic diet was severely compromised (Fig. 6). Mammalian hosts utilize a number of mechanisms to restrict zinc access for invading microbes, from zinc tissue/cell reallocation, mediated by two families of zinc transporters, to zinc sequestration/chelation mechanisms via production of calprotectin (Zackular et al., 2015). While calprotectin can be present in oral fluids and tissues, particularly during inflammatory processes such as periodontitis, oral cancer and oropharyngeal candidiasis (Holmstrom et al., 2019; Sweet, Denbury, & Challacombe, 2001), there is no evidence of increases in salivary calprotectin levels in dental caries. Moreover, recent studies indicated that mildly acidic conditions compromise the ability of calprotectin to chelate manganese but not zinc (Rosen & Nolan, 2020). In addition, salivary glands synthesize metallothioneins, a family of low molecular weight cysteine-rich proteins that scavenge free radicals and can also chelate zinc and copper in tissues (Rahman & Karim, 2018). While the capacity of human metallothionein to restrict bacterial growth through metal sequestration is unclear, salivary metallothionein levels have been shown to increase after dental pulp injury in rats (Izumi, Eida, Matsumoto, & Inoue, 2007). To obtain preliminary insight into the host-derived zinc sequestration mechanisms in the oral cavity, we took advantage of the availability of saliva samples from caries-free and caries active subjects that had been previously collected for an ongoing clinical study (IRB201600851), and used ELISAs to measure the levels of calprotectin and metallothionein in these samples. While calprotectin was below detection levels in most samples, there was measurable quantities of metallothionein in the saliva samples from both subject groups (Fig. S3). However, the differences in the levels of metallothionein between the two groups were not significant suggesting that metallothionein levels do not increase in response to caries. Given the very low levels of calprotectin that we found in saliva and recent observations that the manganese-binding affinity of calprotectin is compromised at low pH (Rosen & Nolan, 2020), we speculate that zinc restriction in the oral cavity is primarily mediated by metallothioneins.
In addition to the host-driven mechanisms of metal sequestration, bacteria in polymicrobial biofilms such as dental plaque must also compete with the other oral residents for nutrients. Interestingly, a recent study showed that transcription of the S. mutans adcA and adcB genes was induced (about 6- and 3-fold, respectively) when S. mutans was co-cultured with Streptococcus A12, a health-associated peroxigenic oral streptococcus (Kaspar, Lee, Richard, Walker, & Burne, 2020). It should be noted that, in principle, peroxigenic streptococci are better equipped to scavenge zinc than S. mutans due to the concerted effort of AdcAII and Pht proteins in addition to the AdcABC system. Another important aspect to consider when it comes to zinc availability is the effect of pH on trace metal solubility. At acidic pH values (pH<6), most free zinc found in saliva is in the aquated Zn2+ form, but free zinc availability sharply decreases if the environmental pH increases above 6 (Rahman, Hossain, Pin, & Yahya, 2019). Thus, it is conceivable that as the plaque pH lowers as a result of bacterial metabolism, zinc availability to S. mutans might increase due to increased solubilization and concomitant reduction of acid-sensitive competitors. In addition, it is conceivable that net H2O2 production in dental plaque due to the presence of peroxigenic oral bacteria will stimulate metallothionein synthesis by salivary glands thereby limiting the availability of free zinc in the oral cavity. Studies to determine how host-pathogen and microbe-microbe interactions influence the ability of S. mutans to maintain zinc homeostasis in dental plaque will soon be underway.
While this study focused on the bacterial zinc acquisition mechanisms, it should be noted that, similar to other trace metals, excess zinc is poisonous to microbes. In addition to being essential to most forms of life, zinc is also recognized for having antimicrobial properties and to stimulate immune cell function such that zinc-containing products have been used in wound healing, as an adjuvant for the treatment of the common cold, and as antimicrobials. In the context of oral health, zinc has been incorporated into mouthwash and toothpaste formulations to assist in the control of calculus formation, gingivitis and halitosis, while the role of zinc as an anticaries agent is controversial (Barnes, Richter, Bastin, Lambert, & Xu, 2008; Bates & Navia, 1979; Giertsen, 2004; Harrap, Best, & Saxton, 1984; Li et al., 2015). A few studies have probed the consequences of high zinc concentrations to the physiology of oral streptococci, with in vitro studies showing that high zinc concentrations inhibit their metabolism (He, Pearce, & Sissons, 2002; Phan, Buckner, Sheng, Baldeck, & Marquis, 2004). Because the incorporation of zinc into routinely used oral health care products is predicted to disturb dental plaque ecology, future studies to determine the importance of environmental zinc in the caries process should also explore zinc excess conditions. From a translational standpoint, approaches to restrict the access of oral bacteria to zinc or, conversely, intoxicate them with zinc may prove effective in the control of dental caries.
Experimental procedures
Bacterial strains growth conditions
The S. mutans strains used in this study are listed in Table 1. Strains were routinely grown in brain heart infusion (BHI) medium at 37°C in a 5% CO2 incubator. When appropriate, spectinomycin (1 mg ml−1), kanamycin (1 mg ml−1) or erythromycin (10 μg ml−1) was added to the growth media. In addition, the chemically defined FMC medium (Terleckyj et al., 1975) was used to determine the minimal amounts of zinc that support growth of the mutant strains. Growth curves under different stress conditions and using media (BHI or FMC) with different amounts of zinc were obtained using an automated growth reader set to 37°C (Bioscreen C; Oy Growth Curves Ab, Ltd.) as described previously (Kajfasz et al., 2010). Briefly, overnight cultures grown in BHI were diluted 1:50 in to the appropriate medium in the wells of a microtiter plate with an overlay of sterile mineral oil added to each well to minimize oxidative stress. Growth in the presence of calprotectin requires the use of CP medium. To promote growth of S. mutans in the CP medium, BHI was prepared in CP Buffer such that the final concentration of the buffer components were: 12 mM Tris [pH 7.5], 60 mM NaCl, 1.8 mM CaCl2, 3 mM β-mercaptoethanol. To determine the sensitivity of the different strains to manganese, cultures were grown in BHI to an OD600 of 0.5, serially diluted and 10 μl of each dilution spotted onto BHI agar supplemented with 250 μM MnSO4 with or without additional zinc (20 μM ZnSO4). Plates were incubated for 24 hours at 37°C in a 5% CO2 incubator before they were photographed.
Table 1.
Bacterial strains and primers used in the study
| S. mutans strains | Relevant characteristics | Source |
|---|---|---|
| UA159 | Wild-type | ATCC |
| ΔadcA | adcA::Kan | This study |
| ΔadcCB | adcCB::Kan | This study |
| ΔadcCBcomp | adcCB::Kan, adcCB+ in pBGE | This study |
| Δsmu2069 | smu2069::Spec | This study |
| ΔadcCBΔsmu2069 | adcCB::Kan; smu2069::Spec | This study |
| Primers | Sequencea | Application |
| adcAdel5arm1 | GCA AGA TTA CGG TAG AAG ACA | adcA deletion |
| adcAdelarm1BamHI | TGA CTA ACA GAA GGG ATC CCG ATA ATA AA | adcA deletion |
| adcAdelarm2 BamHI | CCA GCT AAG GAT CCA GGC CGA | adcA deletion |
| adcAdel3arm2 | CCC CAA AAC CCT TCA TCC | adcA deletion |
| adcCBdel5arm1 | ACA AGA ATA GCG ACT GGA AA | adcCB deletion |
| adcCBdelarm1BamHI | GGA TTG ATG GGG ATC CTG GTG AAA | adcCB deletion |
| adcCBdelarm2 BamHI | GAT AAG ACC GGG ATC CAA CAG TAA TG | adcCB deletion |
| adcCBdel3arm2 | CCC ATG TCA TTA CTG TCC C | adcCB deletion |
| adcCBcompXbaI5’ | GCTCTAGACTTTGAAATCTTACCTTATCGTTGC | ΔadcCB comp. |
| adcCBcompBsrG3’ | CGCTGTACACTGTCTTTTCCCCAGCCTC | ΔadcCB comp. |
| smu2069del5arm1 | GGTTCTCCCTTACGGTCACGC | smu2069 deletion |
| smu2069delarm1SphI | CCAGCAAGCATGCGAGCAGTAAGTATAAAGGCA | smu2069 deletion |
| smu2069delarm2SphI | GGAGTTGCATGCATTTCTGGTATGCTTATCATGGCTG | smu2069 deletion |
| smu2069del3arm2 | GCTGCAATTCCGAGGTTCTTCC | smu2069 deletion |
Restriction sites are underlined.
Construction of mutant strains
Deletion strains lacking the adcA, adcCB or smu2069 genes were obtained using a PCR ligation mutagenesis approach (Lau, Sung, Lee, Morrison, & Cvitkovitch, 2002). Briefly, PCR fragments flanking the region to be deleted were ligated to a nonpolar kanamycin (adcA and adcCB) or spectinomycin (smu2069) resistance cassette and the ligation mixture used to transform S. mutans UA159 according to an established protocol (Lau et al., 2002). Mutant strains were selected on BHI agar supplemented with the appropriate antibiotic and confirmed by PCR analysis and DNA sequencing. To generate complemented strains, the full length adcRCB operon was amplified by PCR and cloned into the integration vector pBGE (Zeng & Burne, 2009) to yield plasmid pBGE-adcRCB. The plasmid was propagated in E. coli DH10B and used to transform the S. mutans ΔadcCB strain for integration at the gtfA locus. All primers used in this study are listed in Table 1.
ICP-MS analysis
The bacterial intracellular metal content was determined using Inductively Coupled Plasma Mass Spectrometry (ICP-MS). Briefly, cultures were grown in BHI or BHI containing 6 μM TPEN to an OD600 of 0.4, harvested by centrifugation at 4°C for 15 min at 4,000 rpm, washed in phosphate-buffered saline (PBS) supplemented with 0.2 mM EDTA to chelate extracellular divalent cations followed by a wash in PBS alone. The cell pellets were resuspended in HNO3 and metal composition was quantified using an Agilent 7900 ICP mass spectrometer. Metal concentrations were then normalized to total protein content as determined by the bicinchoninic acid (BCA) assay (Pierce).
Biofilm assay
The ability of the S. mutans strains to form biofilms on saliva-coated wells of polystyrene microtiter plates was assessed as described elsewhere (Kajfasz et al., 2020). The wells were first coated with 100 μl of sterile clarified and pooled human saliva (IRB# 201600877) for 30 min at room temperature. Strains were grown in BHI to an OD600 of 0.5 and diluted 1:100 in FMC containing 1% sucrose (FMC-S) and supplemented with 5 or 20 μM ZnSO4, and 200 μl of each bacterial suspension added to the saliva-coated wells. After incubation at 37°C in a 5% CO2 incubator for 24 hours, wells were washed twice with sterile water to remove planktonic and loosely bound bacteria, and adherent (biofilm) cells stained with 0.1% crystal violet for 15 min. The bound dye was eluted in a 33% acetic acid solution, and the total biofilm estimated by measuring the absorbance of the dissolved dye at 575 nm.
Rat tooth colonization assay
The ability of the mutant strains to colonize the teeth of rats was evaluated using an established model of dental caries (Miller et al., 2015) with some modifications. Briefly, specific pathogen-free Sprague-Dawley rat pups were purchased with their dams from Envigo Laboratories and screened for the presence of mutans streptococci by plating on mitis salivarius (MS) agar upon arrival. Prior to infection, pups and dams received 0.8 mg ml−1 sulfamethoxazole and 0.16 mg ml−1 trimethoprim in the drinking water for 3 days to suppress endogenous flora and facilitate infection. Pups aged 15 days and dams were taken off antibiotics on day 4, randomly placed into experimental groups, and infected for four consecutive days by means of cotton swab saturated with actively growing S. mutans UA159 or ΔadcCB cultures. During the days of infection, animals were fed a 12% sucrose powdered diet (ENVIGO diet, catalog # TD.190707) and 5% (wt/vol) sterile sucrose-water ad libitum, and the 12% sucrose diet with sterile water in the days after infection. The experiment proceeded for 10 additional days, at the end of which the animals were euthanized by CO2 asphyxiation, and the lower jaws surgically removed for microbiological assessment. Jaw sonicates were subjected to 10-fold serial dilution in PBS and plated on MS agar, BHI agar (supplemented with kanamycin for the ΔadcCB strain), and 5% sheeps’ blood agar to determine S. mutans counts. When grown on MS agar, S. mutans colonies are easily distinguished from non-S. mutans flora, particularly when viewed at 4x magnification. On MS agar, S. mutans form dark blue peaked colonies with an irregular margin that are difficult to remove from agar with a toothpick. The number of S. mutans recovered from the animals was expressed as CFU ml-1 of jaw sonicate. The S. mutans colonies counted on MS agar were divided by the total colonies counted on blood agar to determine the percent of S. mutans colonies recovered. This study was reviewed and approved by the University of Florida Institutional Animal Care and Use Committee (protocol # 201810421).
Statistical analysis
Data were analyzed using GraphPad Prism 9.0 software unless otherwise stated. Differences in intracellular metal content and biofilm formation were determined via ordinary one-way ANOVA. The rat colonization study was subjected to the Mann-Whitney U test. In all cases, p values < 0.05 were considered significant.
Supplementary Material
Acknowledgements
Purified calprotectin was provided by Walter Chazin at Vanderbilt University. This study was supported by NIH-NIDCR award DE019783 to J.A.L.
Footnotes
Conflicts of interests
The authors declare no conflict of interest.
References
- Archibald F (1986). Manganese: its acquisition by and function in the lactic acid bacteria. Crit Rev Microbiol, 13(1), 63–109. doi: 10.3109/10408418609108735 [DOI] [PubMed] [Google Scholar]
- Barnes VM, Richter R, Bastin D, Lambert P, & Xu T (2008). Dental plaque control effect of a zinc citrate dentifrice. J Clin Dent, 19(4), 127–130. [PubMed] [Google Scholar]
- Bates DG, & Navia JM (1979). Chemotherapeutic effect of zinc on Streptococcus mutans and rat dental caries. Arch Oral Biol, 24(10–11), 799–805. doi: 10.1016/0003-9969(79)90041-4 [DOI] [PubMed] [Google Scholar]
- Bayle L, Chimalapati S, Schoehn G, Brown J, Vernet T, & Durmort C (2011). Zinc uptake by Streptococcus pneumoniae depends on both AdcA and AdcAII and is essential for normal bacterial morphology and virulence. Mol Microbiol, 82(4), 904–916. doi: 10.1111/j.1365-2958.2011.07862.x [DOI] [PubMed] [Google Scholar]
- Bersch B, Bougault C, Roux L, Favier A, Vernet T, & Durmort C (2013). New insights into histidine triad proteins: solution structure of a Streptococcus pneumoniae PhtD domain and zinc transfer to AdcAII. PLoS One, 8(11), e81168. doi: 10.1371/journal.pone.0081168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen WH, Burne RA, Wu H, & Koo H (2018). Oral Biofilms: Pathogens, matrix, and polymicrobial interactions in microenvironments. Trends Microbiol, 26(3), 229–242. doi: 10.1016/j.tim.2017.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brophy MB, Hayden JA, & Nolan EM (2012). Calcium ion gradients modulate the zinc affinity and antibacterial activity of human calprotectin. J Am Chem Soc, 134(43), 18089–18100. doi: 10.1021/ja307974e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LR, Gunnell SM, Cassella AN, Keller LE, Scherkenbach LA, Mann B, Brown MW, Hill R, Fitzkee NC, Rosch JW, Tuomanen EI, & Thornton JA (2016). AdcAII of Streptococcus pneumoniae affects pneumococcal invasiveness. PLoS One, 11(1), e0146785. doi: 10.1371/journal.pone.0146785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burcham LR, Le Breton Y, Radin JN, Spencer BL, Deng L, Hiron A, Ransom MR, Mendonca J. da C., Belew AT, El-Sayed NM, McIver KS, & Kehl-Fie TE, Doran KS (2020). Identification of zinc-dependent mechanisms used by Group B Streptococcus to overcome calprotectin-mediated stress. mBio, 11(6). doi: 10.1128/mBio.02302-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao K, Li N, Wang H, Cao X, He J, Zhang B, He Q, Zhang G, & Sun X (2018). Two zinc-binding domains in the transporter AdcA from Streptococcus pyogenes facilitate high-affinity binding and fast transport of zinc. J Biol Chem, 293(16), 6075–6089. doi: 10.1074/jbc.M117.818997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrangsu P, & Helmann JD (2016). Intracellular Zn(II) intoxication leads to dysregulation of the PerR regulon resulting in heme toxicity in Bacillus subtilis. PLoS Genet, 12(12), e1006515. doi: 10.1371/journal.pgen.1006515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer F, Robbe-Saule M, Turlin E, Mancuso F, Michel V, Richaud P, Veyrier FJ, De Reuse H, & Vinella D (2016). Characterization in Helicobacter pylori of a nickel transporter essential for colonization that was acquired during evolution by gastric Helicobacter species. PLoS Pathog, 12(12), e1006018. doi: 10.1371/journal.ppat.1006018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia EC, Brumbaugh AR, & Mobley HL (2011). Redundancy and specificity of Escherichia coli iron acquisition systems during urinary tract infection. Infect Immun, 79(3), 1225–1235. doi: 10.1128/IAI.01222-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghssein G, Brutesco C, Ouerdane L, Fojcik C, Izaute A, Wang S, Hajjar C, Lobinski R, Lemaire D, Richaud P, Voulhoux R, Espaillat A, Cava F, Pignol D, Borezee-Durant E, & Arnoux P (2016). Biosynthesis of a broad-spectrum nicotianamine-like metallophore in Staphylococcus aureus. Science, 352(6289), 1105–1109. doi: 10.1126/science.aaf1018 [DOI] [PubMed] [Google Scholar]
- Giertsen E (2004). Effects of mouthrinses with triclosan, zinc ions, copolymer, and sodium lauryl sulphate combined with fluoride on acid formation by dental plaque in vivo. Caries Res, 38(5), 430–435. doi: 10.1159/000079623 [DOI] [PubMed] [Google Scholar]
- Grass G, Wong MD, Rosen BP, Smith RL, & Rensing C (2002). ZupT is a Zn(II) uptake system in Escherichia coli. J Bacteriol, 184(3), 864–866. doi: 10.1128/jb.184.3.864-866.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grim KP, Radin JN, Solorzano PKP, Morey JR, Frye KA, Ganio K, Neville SL, McDevitt CA, & Kehl-Fie TE (2020). Intracellular accumulation of staphylopine can sensitize Staphylococcus aureus to host-imposed zinc starvation by chelation-independent toxicity. J Bacteriol, 202(9). doi: 10.1128/JB.00014-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrap GJ, Best JS, & Saxton CA (1984). Human oral retention of zinc from mouthwashes containing zinc salts and its relevance to dental plaque control. Arch Oral Biol, 29(2), 87–91. doi: 10.1016/0003-9969(84)90110-9 [DOI] [PubMed] [Google Scholar]
- He G, Pearce EI, & Sissons CH (2002). Inhibitory effect of ZnCl(2) on glycolysis in human oral microbes. Arch Oral Biol, 47(2), 117–129. doi: 10.1016/s0003-9969(01)00093-0 [DOI] [PubMed] [Google Scholar]
- Holmstrom SB, Lira-Junior R, Zwicker S, Majster M, Gustafsson A, Akerman S, Klinge B, Svensson M, & Bostrom EA (2019). MMP-12 and S100s in saliva reflect different aspects of periodontal inflammation. Cytokine, 113, 155–161. doi: 10.1016/j.cyto.2018.06.036 [DOI] [PubMed] [Google Scholar]
- Imlay JA (2014). The mismetallation of enzymes during oxidative stress. J Biol Chem, 289(41), 28121–28128. doi: 10.1074/jbc.R114.588814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi T, Eida T, Matsumoto N, & Inoue H (2007). Immunohistochemical localization of metallothionein in dental pulp after cavity preparation of rat molars. Oral Surg Oral Med Oral Pathol Oral Radiol Endod, 104(4), e133–137. doi: 10.1016/j.tripleo.2007.04.023 [DOI] [PubMed] [Google Scholar]
- Juttukonda LJ, & Skaar EP (2015). Manganese homeostasis and utilization in pathogenic bacteria. Mol Microbiol, 97(2), 216–228. doi: 10.1111/mmi.13034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajfasz JK, Katrak C, Ganguly T, Vargas J, Wright L, Peters ZT, Spatafora GM, Abranches J, & Lemos JA (2020). Manganese uptake, mediated by SloABC and MntH, is essential for the fitness of Streptococcus mutans. mSphere, 5(1). doi: 10.1128/mSphere.00764-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajfasz JK, Rivera-Ramos I, Abranches J, Martinez AR, Rosalen PL, Derr AM, Quivey RG, & Lemos JA (2010). Two Spx proteins modulate stress tolerance, survival, and virulence in Streptococcus mutans. J Bacteriol, 192(10), 2546–2556. doi: 10.1128/JB.00028-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaspar JR, Lee K, Richard B, Walker AR, & Burne RA (2020). Direct interactions with commensal streptococci modify intercellular communication behaviors of Streptococcus mutans. ISME J. doi: 10.1038/s41396-020-00789-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehl-Fie TE, & Skaar EP (2010). Nutritional immunity beyond iron: a role for manganese and zinc. Curr Opin Chem Biol, 14(2), 218–224. doi: 10.1016/j.cbpa.2009.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh EI, Hung CS, Parker KS, Crowley JR, Giblin DE, & Henderson JP (2015). Metal selectivity by the virulence-associated yersiniabactin metallophore system. Metallomics, 7(6), 1011–1022. doi: 10.1039/c4mt00341a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam LN, Wong JJ, Chong KKL, & Kline KA (2020). Enterococcus faecalis manganese exporter MntE alleviates manganese toxicity and is required for mouse gastrointestinal colonization. Infect Immun, 88(6). doi: 10.1128/IAI.00058-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau PC, Sung CK, Lee JH, Morrison DA, & Cvitkovitch DG (2002). PCR ligation mutagenesis in transformable streptococci: application and efficiency. J Microbiol Methods, 49(2), 193–205. doi: 10.1016/s0167-7012(01)00369-4 [DOI] [PubMed] [Google Scholar]
- Lemos JA, Palmer SR, Zeng L, Wen ZT, Kajfasz JK, Freires IA, Abranches J, & Brady LJ (2019). The Biology of Streptococcus mutans. Microbiol Spectr, 7(1). doi: 10.1128/microbiolspec.GPP3-0051-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zhong Y, Jiang X, Hu D, Mateo LR, Morrison BM Jr., & Zhang YP (2015). Randomized clinical trial of the efficacy of dentifrices containing 1.5% arginine, an insoluble calcium compound and 1450 ppm fluoride over two years. J Clin Dent, 26(1), 7–12. [PubMed] [Google Scholar]
- Loisel E, Chimalapati S, Bougault C, Imberty A, Gallet B, Di Guilmi AM, Brown J, Vernet T, & Durmort C (2011). Biochemical characterization of the histidine triad protein PhtD as a cell surface zinc-binding protein of pneumococcus. Biochemistry, 50(17), 3551–3558. doi: 10.1021/bi200012f [DOI] [PubMed] [Google Scholar]
- Loisel E, Jacquamet L, Serre L, Bauvois C, Ferrer JL, Vernet T, Di Guilmi AM,. & Durmort C (2008). AdcAII, a new pneumococcal Zn-binding protein homologous with ABC transporters: biochemical and structural analysis. J Mol Biol, 381(3), 594–606. doi: 10.1016/j.jmb.2008.05.068 [DOI] [PubMed] [Google Scholar]
- Lonergan ZR, & Skaar EP (2019). Nutrient zinc at the host-pathogen interface. Trends Biochem Sci, 44(12), 1041–1056. doi: 10.1016/j.tibs.2019.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo CY, Mitrakul K, Voss IB, Hughes CV, & Ganeshkumar N (2003). Involvement of the adc operon and manganese homeostasis in Streptococcus gordonii biofilm formation. J Bacteriol, 185(9), 2887–2900. doi: 10.1128/jb.185.9.2887-2900.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch RJ (2011). Zinc in the mouth, its interactions with dental enamel and possible effects on caries; a review of the literature. Int Dent J, 61 Suppl 3, 46–54. doi: 10.1111/j.1875-595X.2011.00049.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JE, Lisher JP, Winkler ME, & Giedroc DP (2017). Perturbation of manganese metabolism disrupts cell division in Streptococcus pneumoniae. Mol Microbiol, 104(2), 334–348. doi: 10.1111/mmi.13630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastropasqua MC, D’Orazio M, Cerasi M, Pacello F, Gismondi A, Canini A, Canuti L, Consalvo A, Ciavardelli D, Chirullo B, Pasquali P, & Battistoni A (2017). Growth of Pseudomonas aeruginosa in zinc poor environments is promoted by a nicotianamine-related metallophore. Mol Microbiol, 106(4), 543–561. doi: 10.1111/mmi.13834 [DOI] [PubMed] [Google Scholar]
- Miller JH, Aviles-Reyes A, Scott-Anne K, Gregoire S, Watson GE, Sampson E, Progulski-Fox A, Koo H, Bowen WH, Lemos JA, & Abranches J (2015). The collagen binding protein Cnm contributes to oral colonization and cariogenicity of Streptococcus mutans OMZ175. Infect Immun, 83(5), 2001–2010. doi: 10.1128/IAI.03022-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey JR, & Kehl-Fie TE (2020). Bioinformatic mapping of opine-like zincophore biosynthesis in bacteria. mSystems, 5(4). doi: 10.1128/mSystems.00554-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulin P, Patron K, Cano C, Zorgani MA, Camiade E, Borezee-Durant E, Rosenau A, Mereghetti L, & Hiron A (2016). The Adc/Lmb system mediates zinc acquisition in Streptococcus agalactiae and contributes to bacterial growth and Survival. J Bacteriol, 198(24), 3265–3277. doi: 10.1128/JB.00614-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashige TG, Zhang B, Krebs C, & Nolan EM (2015). Human calprotectin is an iron-sequestering host-defense protein. Nat Chem Biol, 11(10), 765–771. doi: 10.1038/nchembio.1891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien J, Pastora A, Stoner A, & Spatafora G (2020). The S. mutans mntE gene encodes a manganese efflux transporter. Mol Oral Microbiol, 35(3), 129–140. doi: 10.1111/omi.12286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong CY, Berking O, Walker MJ, & McEwan AG (2018). New insights into the role of zinc acquisition and zinc tolerance in Group A Streptococcal infection. Infect Immun, 86(6). doi: 10.1128/IAI.00048-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer LD, & Skaar EP (2016). Transition metals and virulence in bacteria. Annu Rev Genet, 50, 67–91. doi: 10.1146/annurev-genet-120215-035146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pant S, Patel NJ, Deshmukh A, Golwala H, Patel N, Badheka A, Hirsch GA, & Mehta JL (2015). Trends in infective endocarditis incidence, microbiology, and valve replacement in the United States from 2000 to 2011. J Am Coll Cardiol, 65(19), 2070–2076. doi: 10.1016/j.jacc.2015.03.518 [DOI] [PubMed] [Google Scholar]
- Phan TN, Buckner T, Sheng J, Baldeck JD, & Marquis RE (2004). Physiologic actions of zinc related to inhibition of acid and alkali production by oral streptococci in suspensions and biofilms. Oral Microbiol Immunol, 19(1), 31–38. doi: 10.1046/j.0902-0055.2003.00109.x [DOI] [PubMed] [Google Scholar]
- Rahman MT, Hossain A, Pin CH, & Yahya NA (2019). Zinc and metallothionein in the development and progression of dental caries. Biol Trace Elem Res, 187(1), 51–58. doi: 10.1007/s12011-018-1369-z [DOI] [PubMed] [Google Scholar]
- Rahman MT, & Karim MM (2018). Metallothionein: a potential link in the regulation of zinc in nutritional immunity. Biol Trace Elem Res, 182(1), 1–13. doi: 10.1007/s12011-017-1061-8 [DOI] [PubMed] [Google Scholar]
- Reyes-Caballero H, Guerra AJ, Jacobsen FE, Kazmierczak KM, Cowart D, Koppolu UM, Scott RA, Winkler ME, & Giedroc DP (2010). The metalloregulatory zinc site in Streptococcus pneumoniae AdcR, a zinc-activated MarR family repressor. J Mol Biol, 403(2), 197–216. doi: 10.1016/j.jmb.2010.08.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen T, & Nolan EM (2020). Metal sequestration and antimicrobial activity of human calprotectin are pH-dependent. Biochemistry, 59(26), 2468–2478. doi: 10.1021/acs.biochem.0c00359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon JR, & Skaar EP (2019). Metals as phagocyte antimicrobial effectors. Curr Opin Immunol, 60, 1–9. doi: 10.1016/j.coi.2019.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian Vignesh K, & Deepe GS Jr. (2016). Immunological orchestration of zinc homeostasis: The battle between host mechanisms and pathogen defenses. Arch Biochem Biophys, 611, 66–78. doi: 10.1016/j.abb.2016.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet SP, Denbury AN, & Challacombe SJ (2001). Salivary calprotectin levels are raised in patients with oral candidiasis or Sjogren’s syndrome but decreased by HIV infection. Oral Microbiol Immunol, 16(2), 119–123. doi: 10.1034/j.1399-302x.2001.016002119.x [DOI] [PubMed] [Google Scholar]
- Terleckyj B, Willett NP, & Shockman GD (1975). Growth of several cariogenic strains of oral streptococci in a chemically defined medium. Infect Immun, 11(4), 649–655. doi: 10.1128/IAI.11.4.649-655.1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner AG, Ong CL, Gillen CM, Davies MR, West NP, McEwan AG, & Walker MJ (2015). Manganese homeostasis in group A Streptococcus is critical for resistance to oxidative stress and virulence. mBio, 6(2). doi: 10.1128/mBio.00278-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zackular JP, Chazin WJ, & Skaar EP (2015). Nutritional immunity: S100 proteins at the host-pathogen interface. J Biol Chem, 290(31), 18991–18998. doi: 10.1074/jbc.R115.645085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zackular JP, Knippel RJ, Lopez CA, Beavers WN, Maxwell CN, Chazin WJ, & Skaar EP (2020). ZupT facilitates Clostridioides difficile resistance to host-mediated nutritional immunity. mSphere, 5(2). doi: 10.1128/mSphere.00061-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L, & Burne RA (2009). Transcriptional regulation of the cellobiose operon of Streptococcus mutans. J Bacteriol, 191(7), 2153–2162. doi: 10.1128/JB.01641-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Ma XL, Wang YX, He CC, Tian K, Wang HG, An D, Xie HL, & Liu YQ (2017). TPEN, a specific Zn(2+) chelator, inhibits sodium dithionite and glucose deprivation (SDGD)-induced neuronal death by modulating apoptosis, glutamate signaling, and voltage-gated K(+) and Na(+) channels. Cell Mol Neurobiol, 37(2), 235–250. doi: 10.1007/s10571-016-0364-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B, Zhang Q, Gao J, Han H, Li M, Zhang J, Qi J, Yan J, & Gao GF (2011). Insight into the interaction of metal ions with TroA from Streptococcus suis. PLoS One, 6(5), e19510. doi: 10.1371/journal.pone.0019510 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


