Abstract
Small-molecule chemical drugs are of great significance for tumor-targeted and individualized therapies. However, the development of new small-molecule drugs, from basic experimental research and clinical trials to final application in clinical practice, is a long process that has a high cost. It takes at least 5 years for most drugs to be developed in the laboratory to prove their effectiveness and safety. Compared with the development of new drugs, repurposing traditional non-tumor drugs can be a shortcut. Metformin is a good model for a new use of an old drug. In recent years, the antitumor efficacy of metformin has attracted much attention. Epidemiological data and in vivo, and in vitro experiments have shown that metformin can reduce the incidence of cancer in patients with diabetes and has a strong antagonistic effect on metabolism-related tumors. Recent studies have shown that metformin can induce autophagy in esophageal cancer cells, mainly by inhibiting inflammatory signaling pathways. In recent years, studies have shown that the antitumor functions and mechanisms of metformin are multifaceted. The present study aims to review the application of metformin in tumor prevention and treatment.
Keywords: metformin, tumor prevention, treatment, esophageal cancer, repurpose
1. Introduction
In the field of antitumor therapy, small-molecule drug therapy is a targeted form of treatment with high specificity and few adverse reactions. Small-molecule drugs are called ‘biological missiles’ and are gradually becoming a hot spot in research, development and clinical application. Repurposing old small-molecule drugs is an active focus of research. Metformin is a typical ‘old medicine with new uses’. Metformin is a 1,1-dimethylbiguanide hydrochloride isolated from the legume Galega officinalis. It was first reported in 1957 as a hypoglycemic drug (1). Studies have found that metformin has weight loss, anti-aging, and anti-cardiovascular effects (2). In recent years, studies have reported an antitumoral effect of the drug, which has been confirmed in various malignant tumor cells such as liver (3), ovarian (4) and lung cancer cells. The present study summarizes knowledge on the anti-tumoral effects of metformin and its mechanism in order to provide evidence for the repurposing of this drug for tumor treatment.
Antitumoral advantages of small molecule drugs
Biomacromolecular drugs are considered one of the most promising fields in drug development in this century. Common biomacromolecular antitumor drugs include monoclonal antibodies, recombinant protein drugs and vaccines (5). These biomacromolecular drugs have some shortcomings in clinical use: i) Lack of selectivity for targeted lesion tissues in contrast to normal tissues, which may cause serious adverse reactions; ii) most drugs cannot enter cells to exert an antitumoral effect and iii) their mechanism of action on tissues, organs, cells and molecules within the body is frequently uncertain, therefore it is difficult to evaluate their efficacy (6). These factors lead to unsatisfactory clinical therapeutic effects of biomacromolecular drugs. By contrast, small-molecule drugs are more popular in the clinics because they may have better therapeutic efficacy and fewer adverse reactions. More importantly, they are relatively inexpensive (7). However, with the widespread application of these drugs, their shortcomings have also became prominent: Ease in development of drug resistance and multiple adverse reactions, resulting in reduced clinical efficacy. For example, gefitinib and erlotinib used in the treatment of non-small cell lung cancer (NSCLC) (8), imatinib in the treatment of chronic myeloid leukemia (8) and gastrointestinal stromal tumors (9) are reported to have several side effects and develop tumor resistance.
Among the numerous strategies available to improve tumor treatment (10), the development of new drugs is worth considering. However, this development requires a large amount of funding, a long testing period and large-scale and multi-center clinical trials. The economic and time costs of new drug research and development are huge, and it is difficult to improve the current status of tumor treatment in the short term. Therefore, exploring the functions and indications of existing non-antitumoral small-molecule drugs to evaluate their potential antitumoral efficacy, and therefore repurposing ‘old drugs’ is an important way to improve the clinical efficacy of antitumoral treatment.
Metformin, the chemical structure of which is shown in Fig. 1, is the first-line drug used clinically for type 2 diabetes. In recent years, it was discovered that the drug has antitumoral effects, which is a typical form of repurposing (11). In addition to metformin, there are a number of small-molecule drugs that have regained a ‘new life’ in the anti-cancer field, such as aspirin (12), thalidomide (13), statins (14), vitamin D (15) and green tea extract (16).
Figure 1.
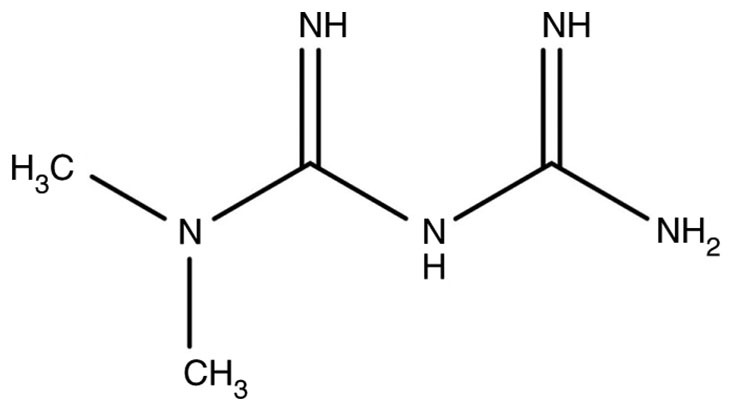
Chemical structure of metformin.
Metformin bioavailability
The cellular uptake and secretion rate of metformin largely depends on the expression of organic cation transporters and of multidrug and toxin extrusion proteins (17). The organic cation transporter, toxin extrusion protein and plasma membrane monoamine transporter distribute metformin to several tissues, such as the liver, kidneys and small intestine, and also mediate metformin metabolism and secretion. Oral metformin is mainly absorbed through the upper small intestine. Knockout mice for the toxin extrusion protein transporter show a significant reduction in the clearance and distribution of metformin, usually without an effect on tissue distribution or pharmacological effects (10). The bioavailability of oral metformin is 40-60% (18). Metformin uptake is dose-dependent, but saturable (19). In clinical trials, the plasma elimination half-life is ~5-6 h in patients with normal renal function under repeated metformin administration (20). Approximately 90% of oral metformin is secreted through the kidneys within 24 h. However, for patients with advanced cancer and gastrointestinal tumors with poor prognosis, the possibility of reaching high blood concentrations is low (21). In a cell line model, the half-inhibitory concentration (IC50) of metformin is between 5 and 20 mM. In breast cancer cell lines, the IC50 of metformin is increased and apoptosis and cell cycle arrest induced by metformin are decreased at high glucose levels compared with low glucose levels (22). The in vitro activation of cellular AMP-activated protein kinase (AMPK) requires at least 1 mM metformin in numerous cancer cell lines (23). This is equivalent to an intracellular concentration of 131 µM, which is close to the plasma concentration of metformin in mice receiving an intraperitoneal injection of 145 µM. Compared with oral metformin, the intraperitoneal injection can increase the bioavailability of metformin in mice. In particular, the metformin concentration in the blood, liver and kidneys increases, significantly (18). In mouse tumor models, the biodistribution of 11C-metformin administered intravenously shows relatively high tracer uptake in the kidneys and liver, and relatively low tracer uptake in the blood and tumors (24). 11C-metformin-positron emission tomography can be combined with mutation screening of metformin sensitivity-related genes and can be used clinically to identify metformin-sensitive tumors. In a rat model of lipopolysaccharide-induced systemic inflammation, the concentration of metformin varies between different brain regions, with the highest concentration of metformin in the pituitary gland and cerebrospinal fluid (~30 µM).
2. Metformin as an antitumor candidate drug
Multiple functions of metformin
Metformin was originally chemically synthesized in 1929 (25). It was first approved as a clinical drug in 1957 and has since been used to treat diabetes (26). In 1998, metformin was discovered to have a protective effect on the cardiovascular system (27). Since then, other functions of the drug have been discovered (Fig. 2). For example, metformin can be used in the adjuvant treatment of tuberculosis (28), and it is also used in the routine treatment of polycystic ovary syndrome (29). In addition, metformin can delay aging and improve the symptoms of non-alcoholic fatty liver (30), prevent and treat uveitis (31), reduce the prevalence of Parkinson's disease (32) and improve the intestinal flora imbalance in diabetic patients (33). Basic research has shown that metformin inhibits the expression of mammalian target of rapamycin (mTOR) by activating AMPK to improve rheumatoid arthritis (34). Metformin combined with risperidone can significantly improve fasting blood glucose, triglyceride levels, high-density lipoprotein levels, and body mass index in patients with schizophrenia complicated with metabolic syndrome, and effectively reduce the incidence of metabolic syndrome (35).
Figure 2.
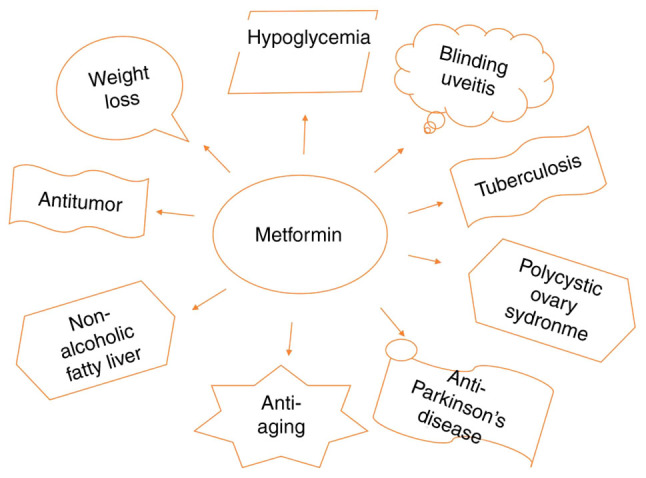
Multiple functions of metformin. In addition to hypoglycemia, metformin displays antitumor, anti-aging, anti-Parkinson's disease and weight loss effects.
Antitumoral effects of metformin
The basis of the antitumoral effect of metformin
In 1910, Maynard suggested that diabetes is associated with tumors (36). Since then, an increasing number of studies have shown that diabetes can increase the risk of various malignant tumors (37–39). Diabetes and tumors have common risk factors, including age, sex, diet and smoking and their onset is related to the insulin/insulin-like growth factor (IGF) pathway (40). Patients with diabetes are prone to develop tumors. At present, most studies suggest that hyperglycemia, hyperinsulinemia, IGF-1, DNA damage, inflammatory factors and obesity may be involved in the pathological process of diabetes-related tumors (41–43). Adequate blood sugar control is beneficial in reducing the risk of malignant tumors. Obese patients with type 2 diabetes have a higher risk of developing cancer such as liver (44), biliary tract (45), pancreatic (46), colorectal (47), kidney (48), bladder (49), breast (50) and endometrial cancer (51). Insulin resistance leads to unsatisfactory treatment effects in diabetes, and is a chronic, non-specific inflammatory process. Insulin resistance not only leads to an increase in insulin and IGFs, but also promotes mitosis (52), activates the PI3K/AKT cell proliferation signaling pathway, promotes tumor cell growth (53) and aggravates the tumor inflammatory response to promote tumor invasion and metastasis.
Studies have reported that metformin has an effect on tumors of non-obese patients. Metformin can inhibit growth and promote differentiation of ovarian (54), endometrial (55), and breast cancer (56), thereby reducing the risk and mortality of these tumors. Jayalath et al (44) found that metformin reduced the level of serum prostate-specific antigen and delayed the progression of prostate cancer. In addition, metformin increases the sensitivity of patients with colorectal cancer (especially those with diabetes) to radiotherapy and improved the progression-free survival of patients with NSCLC (57). Additionally, metformin inhibits metastasis of malignant gliomas (58).
Epidemiological research on the antitumoral effect of metformin
In recent years, epidemiological data have shown that diabetes increased the risk of a number of tumors. The cancer mortality rate in patients with diabetes is 1.41 times that of patients without diabetes (59). Studies have shown that metformin reduced the incidence of cancer by 30-50%, especially pancreatic cancer (60), hepatocellular carcinoma (61) and colon cancer (62). Febbraro et al (63) conducted a retrospective cohort study on 341 patients with ovarian cancer and found that, the 5-year progression-free survival rate of diabetic patients taking metformin was higher than diabetic patients who did not take metformin and non-diabetic patients., but the 5-year overall survival rates were not significantly different. Xu et al (64) reported that the pathological complete response rate of patients with diabetes and breast cancer taking metformin was 24%, which was higher than that of patients not taking metformin (8%) and also higher than that of patients without diabetes taking metformin (16%), suggesting that metformin may improve the prognosis of breast cancer in patients with diabetes. Wang et al (65) proved through randomized controlled trials that for patients with breast cancer but without diabetes, metformin could improve the prognosis of breast cancer by reducing blood insulin and testosterone levels. Tseng (66) showed that metformin significantly reduced the incidence of prostate cancer in men with type 2 diabetes mellitus. In a follow-up study of patients with newly developed diabetes between 1998 and 2002, as of the end of 2009, the incidence of prostate cancer in patients with diabetes taking metformin was 239.42/100,000 per year, while the incidence of non-users was 737.10/100,000 person-years, and the adjusted risk odds ratio was 0.467 (95% CI, 0.446-0.488). A comparison of the survival of 750 patients with stage 4 NSCLC aged 65-80 years old found that 61% of the patients were already taking metformin when they were diagnosed with lung cancer (67). The median survival time of the metformin group was 5 months, while that of the non-users was 3 months with a statistically significant difference (67). Studies on the risk of thyroid cancer in patients with type 2 diabetes taking metformin have also shown that metformin could reduce the incidence of thyroid cancer (68). The incidence rate of patients taking metformin was 24.09/100,000 person-years, while the incidence rate of those not taking metformin was 87.33/100,000 person-years, with an adjusted risk ratio of 0.683 (95% CI, 0.598-0.780) (68). Other studies have also shown that metformin decreases the risk of esophageal adenocarcinoma (69) and endometrial cancer (70). Bowker et al (71) conducted a five-year follow-up of 10,309 newly-diagnosed patients with type 2 diabetes, and found that the tumor-related mortality of patients taking metformin was significantly lower than that of patients using sulfonylurea-type hypoglycemic drugs or insulin. Another meta-analysis on 13,008 patients with type 2 diabetes and tumors showed that the survival rate of tumor patients using metformin was significantly higher than that of non-users, and cancer-related mortality was significantly lower than that of non-users (72). Metformin is likely to inhibit tumor progression in patients with type 2 diabetes and can reduce the progression risk of patients with tumors and tumor-related mortality, thereby improving patient survival.
Experimental research on metformin antitumoral activity
Emerging evidence has shown that metformin can inhibit the growth of several types of cancer cells in vitro (73–75). At the same time, its antitumoral effect in a mouse tumor model has also been confirmed (76). Huang et al (76) reported that PTEN knockout mice fed with 300 mg/kg/day metformin had delayed tumorigenesis, and that metformin effectively inhibits growth of colon polyps. In addition to inhibiting spontaneous and carcinogen-induced tumors, metformin could inhibit allograft tumors. Phoenix et al (77) implanted tumor cells cultured in a high-sugar and high-insulin environment into mice, and found that the administration of metformin at a dose 40 times higher than the clinical dose effectively reduces the growth rate of tumors. Anisimov et al (78) found that metformin significantly inhibited tumor growth in HER2/neu transgenic mice and increased their average life span. Memmott et al (79) used the tobacco carcinogen NNK to generate an A/J mouse lung cancer model and found that metformin could delay tumorigenesis and reduced tumor burden in mice. It was hypothesized that the antitumoral effect of metformin was related to the downregulation of insulin and IGF-1 receptor. The AKT receptor pathway was also involved. Metformin could indirectly reduce the expression of mTOR (the ‘master factor’ of protein synthesis in cells) in the mouse lung tissue through the AKT pathway, leading to the inhibition of tumor cell growth.
Antitumoral mechanism of metformin
The protein kinase (AMPK)/mTOR pathway
AMPK is one of the main regulators of cell energy status and key cellular processes such as lipid and glucose metabolism, cell growth, autophagy and apoptosis. This enzyme maintains mitochondrial homeostasis and is activated when the ratio of AMP or ADP to ATP increases, and compensates for energy loss by upregulating glycolysis (80). Tumors such as breast (81), pancreatic (82) and prostate cancer (83) are related to obesity, which in turn is associated with the AMPK/mTOR metabolic pathway (84). AMPK is the cell's ‘energy receptor’ being activated when energy is lacking and inhibited when energy is excessive. After AMPK is activated, it can regulate multiple pathways, including the mTOR pathway, thus dominating intracellular protein synthesis (84). Most studies have shown that metformin inhibits obesity-related tumors through the AMPK/mTOR pathway (85,86). In this pathway, AMPK activation by metformin requires an interaction between the regulatory factor liver kinase B-1 and inositol polyphosphate multikinase (87). In addition, the lysosomal pathway is essential for metformin to activate AMPK (88). Metformin activates AMPK through phosphorylation, which in turn downregulates the expression of key adipogenic transcription factor sterol regulatory elements combined with transcription factor 1, leading to the downregulation of lipogenic enzymes, such as Fas cell surface death receptors (89). Metformin directly and indirectly activates AMPK. AMPK can block proliferation and metastasis of tumor cells by compromising mTOR. Tumor cells cannot survive because they cannot synthesize proteins normally (90) (Fig. 3).
Figure 3.
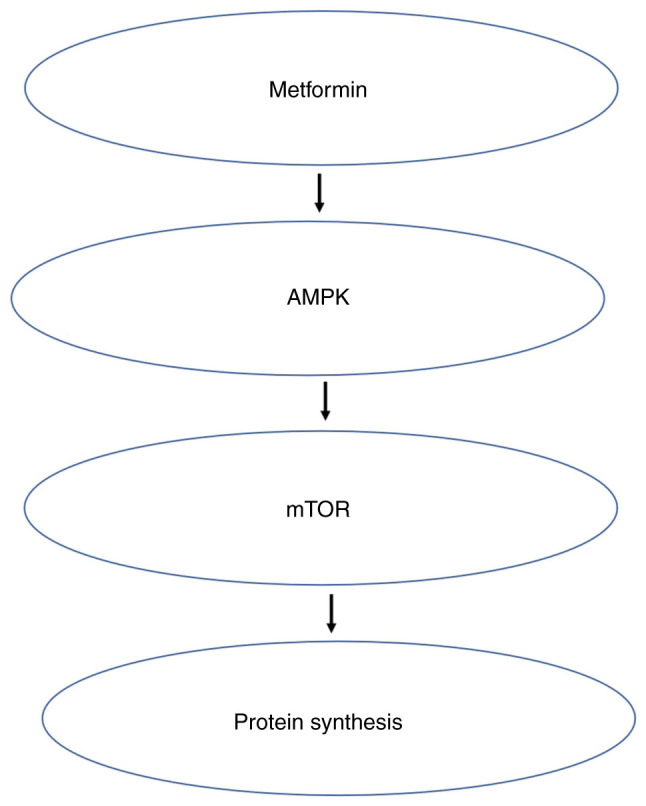
Anti-neoplastic activity of metformin via inhibition of AMPK/mTOR pathway. Inhibition of AMPK/mTOR pathway leading to the arrest of protein synthesis is a major anti- neoplastic activity of metformin.
Metformin does not act only on AMPK to function. Compared with normal cells, cancer cells have a higher ATP demand. Metformin can reversibly inhibit NADH dehydrogenase (mitochondrial complex I) activity of the respiratory chain and exert its role as an inhibitor of oxidative phosphorylation at the molecular level, thereby inhibiting ATP production. Metformin accumulates in the mitochondrial matrix in the presence of a polarized mitochondrial membrane potential. By inhibiting the effective coupling of redox and proton transfer domains, it reversibly inhibits the NADH dehydrogenase (mitochondrial complex I) of the respiratory chain (91), thereby inhibiting ATP production.
Inflammatory pathways and cell death induction
Metformin also has antitumoral effects on tumors of non-obese patients and can cause tumor cell death. Cell death can be divided into programmed and unprogrammed cell death, the latter of which is also known as necrosis. According to the Clarke morphological classification, programmed cell death can be divided into: i) Apoptosis); ii) autophagic cell death and iii) necrosis-like programmed cell death. Feng et al (92) found that metformin inhibited tumors by inducing tumor cell apoptosis and autophagy, in the treatment of esophageal cancer. Metformin-mediated apoptosis of esophageal cancer cells was subtle, and the main mechanism of cell death was autophagy. Additionally, it was found that AMPK was not the only pathway involved in metformin-mediated esophageal cancer cell apoptosis and autophagy.
Several metformin effects do not rely on AMPK mechanisms, these are known as the pleiotropic effect of metformin in tumor treatment (93–95). Biochemical studies have shown that the redox shuttle enzyme mitochondrial glycerol-3-phosphate dehydrogenase (GPDH) was another major target (96). The drug reduced the redox state of mitochondria (in the plasma and liver) and limited the conversion of lactic acid to glycerol and glucose, thereby reducing liver gluconeogenesis. Mitochondrial GPDH was overexpressed in the tumoral thyroid compared with that of normal thyroid tissues. Thyroid cancer cells with high mitochondrial GPDH expression were more susceptible to the inhibitory effects of metformin during proliferation (97). Hexokinase II is attached to the outer mitochondrial membrane and is highly expressed in cancer cells. Cell experiments and molecular simulation studies have shown that metformin inhibited the activity of hexokinase II by occupying the binding site of glucose-6-phosphate, and directly impaired glucose metabolism (98). This induced the dissociation of hexokinase II from mitochondria, leading to the activation of apoptotic signals.
Esophageal cancer was an inflammatory tumor (99). The Stat3 gene product is at the intersection of many cancer and inflammatory signaling pathways (100). Therefore, it was possible that the mechanism of action of metformin in esophageal cancer was related to Stat3. Feng et al (92) showed that the treatment of esophageal cancer by metformin activated the STAT3 pathway, especially the STAT3/Bcl-2/ATG network signaling pathway, promoted apoptosis and autophagy, in which autophagy had a protective effect on the metformin-mediated apoptosis, inducing an inhibition of tumor growth (Fig. 4). The results clarified the target of metformin in the treatment of esophageal cancer, revealed the possibility of combining autophagy inhibitors to enhance the clinical efficacy of metformin, and laid the foundation for the optimal design of esophageal cancer treatment options.
Figure 4.
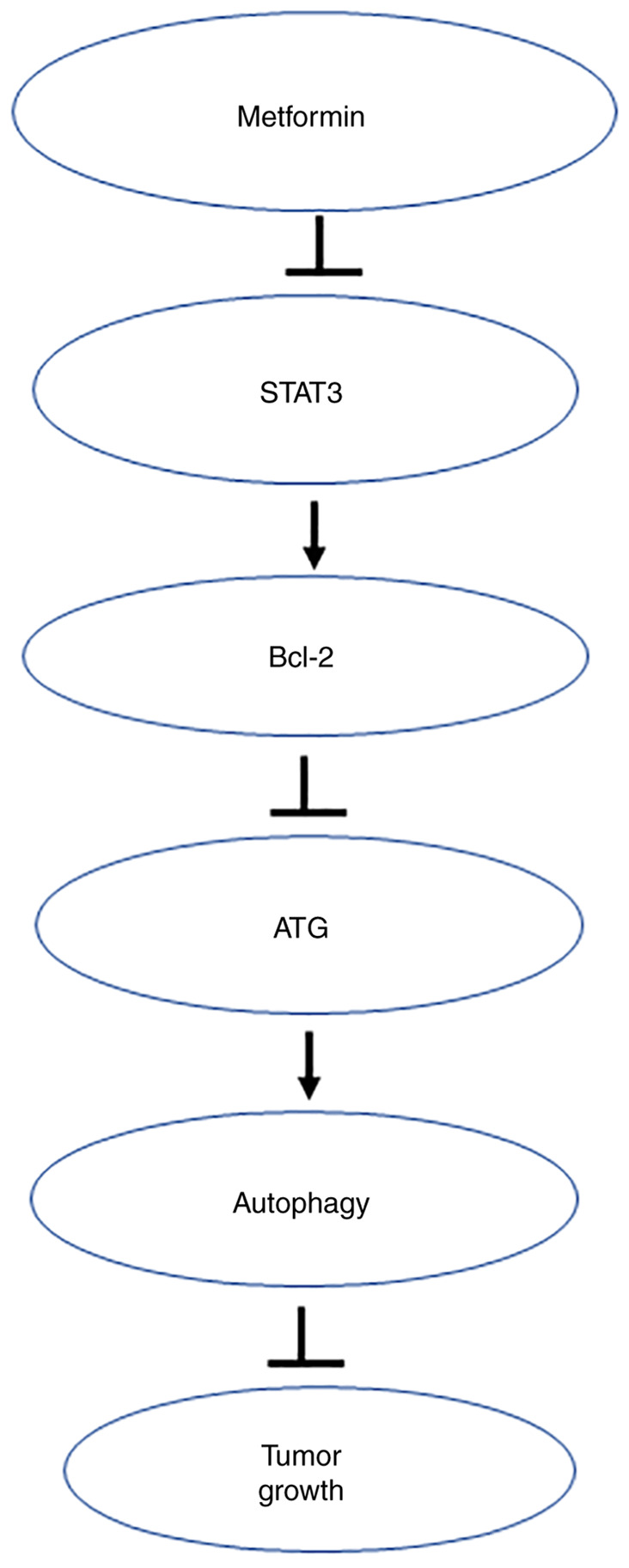
Metformin induces esophageal cancer cell autophagy via the STAT3/Bcl-2/ATG pathway. Metformin inhibits STAT3 expression to decrease Bcl-2 protein expression, which upregulates expression of autophagy marker ATG, thus inducing autophagy to slow tumor growth of esophageal cancer.
Other antitumoral effects of metformin
Other metformin antitumoral effects were presented in a recent study that reported that metformin had the potential to block or delay all kinds of malignant biological behaviors, such as cell cycle arrest, sensitivity to radiotherapy and chemotherapy, and inhibition of proliferation and differentiation of cancer stem cells (101).
3. Clinical trials on metformin in the treatment of tumors
The short-term randomized clinical trial by Higurashi et al (102) showed that metformin could reduce abnormal colorectal polyps by 40% compared with the control group. Currently, several clinical trials (such as NCT04559308, NCT04387630 and NCT03137186) on cancer treatment with metformin have been initiated. Most ongoing clinical trials are based on the evaluation of changes in biomarkers, including insulin levels, AKT/mTOR signals and Ki67 staining. In addition, the clinical benefit of metformin, which uses response and survival rates as indicators, have also been studied, and retrospective studies have used cancer chemotherapy response and survival time as indicators, proving that metformin has potential clinical benefits (103,104).
Our research group adopted a short-term preoperative metformin neoadjuvant treatment for esophageal squamous cell carcinoma and analyzed the changes in the proliferation and apoptosis indices of cancer tissues of patients with metformin. Preliminary results confirmed that cancer proliferation is inhibited by the short-term administration of metformin before surgery, indicating that the patient can benefit from short-term sustained-release of metformin treatment before surgery (105). The main side effect of metformin in patients is gastrointestinal discomfort. These results provide a theoretical basis for further application of metformin in the treatment of esophageal cancer.
The results of a meta-analysis showed that for patients with type 2 diabetes, the effect on blood sugar control was not sufficient when using metformin alone and that metformin combined with sulfonylurea hypoglycemic agents could improve blood sugar control and reduced cardiovascular disease mortality in patients with type 2 diabetes (106). Recent research has shown that metformin combined with chemotherapy drugs can significantly decrease local recurrence in patients with diabetes and NSCLC (107). In addition, compared with VEGF-A inhibitors alone, metformin combined with VEGF-A inhibitors is more effective in inhibiting tumor growth (108), indicating that the combined application of metformin can be a promising route to increase its antitumoral efficacy.
4. Conclusions
Metformin is a small-molecule drug with multiple pharmacological functions. Although research on metformin as an antitumoral drug is still in its preliminary stage, its potential antitumoral efficacy has made people look forward to studying metformin. Based on current research, it is hypothesized that metformin combined with radiotherapy and chemotherapy can enhance the clinical efficacy against tumors, thereby benefiting patients. Further research on metformin should be carried out on the two following aspects: i) Improvement of the molecular structure and pharmaceutical form of metformin, such as modifying tablets into other pharmaceutical forms to produce targeted drugs to improve efficacy and reduce adverse reactions, or coupling certain active groups to the chemical structure of metformin to increase its specificity and effective concentration; ii) evaluation of the combination of metformin with other drugs to improve efficacy. In both cases, the ultimate goal of researchers is to fully understand and optimize the antitumoral effect of metformin in clinical practice and benefit patients.
Acknowledgements
Not applicable.
Funding Statement
This study was supported by the following grants: Xianning high-level personnel project (grant no. 3) and Hubei University of Science and Technology developing project (grant no. 2019-21GP04) for NZF, Hubei University of Science and Technology developing (grant no. 2019-20X018) and pharmacy project (grant no. 2019-20YZ03) for WZ, Hubei University of Science and Technology doctor initiation project (grant no. BK1431) and Hubei Department of Education direction project (grant no. B201771) for WHN, National Natural Science Foundation of China Youth Science Foundation project (grant no. 81902937) for SYL.
Availability of data and materials
Not applicable.
Authors' contributions
HZha, ZN, TS and YC designed the study. HW, DH, HZho, ZW, FW, LW and QW wrote the manuscript. YS, ZN, XS, YR, QH and HZha revised the manuscript. Data authentication is not applicable. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Nasri H, Rafieian-Kopaei M. Metformin: Current knowledge. J Res Med Sci. 2014;19:658–664. [PMC free article] [PubMed] [Google Scholar]
- 2.Top WMC, Kooy A, Stehouwer CDA. Metformin: A narrative review of its potential benefits for cardiovascular disease, cancer and dementia. Pharmaceuticals (Basel) 2022;15:312. doi: 10.3390/ph15030312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kramer JR, Natarajan Y, Dai J, Yu X, Li L, El-Serag HB, Kanwal F. Effect of diabetes medications and glycemic control on risk of hepatocellular cancer in patients with nonalcoholic fatty liver disease. Hepatology. 2021;15:32244. doi: 10.1002/hep.32244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gralewska P, Gajek A, Marczak A, Rogalska A. Metformin affects olaparib sensitivity through induction of apoptosis in epithelial ovarian cancer cell lines. Int J Mol Sci. 2021;22:10557. doi: 10.3390/ijms221910557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsogas FK, Majerczyk D, Hart PC. Possible role of metformin as an immune modulator in the tumor microenvironment of ovarian cancer. Int J Mol Sci. 2021;22:867. doi: 10.3390/ijms22020867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adeyemi SA, Choonara YE. Current advances in cell therapeutics: A biomacromolecules application perspective. Expert Opin Drug Deliv. 2022;12:1–18. doi: 10.1080/17425247.2022.2064844. [DOI] [PubMed] [Google Scholar]
- 7.Liao M, Qin R, Huang W, Zhu HP, Peng F, Han B, Liu B. Targeting regulated cell death (RCD) with small-molecule compounds in triple-negative breast cancer: A revisited perspective from molecular mechanisms to targeted therapies. J Hematol Oncol. 2022;15:44. doi: 10.1186/s13045-022-01260-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marciano O, Mehazri L, Shpungin S, Varvak A, Zacksenhaus E, Nir U. Fer and fert govern mitochondrial susceptibility to metformin and hypoxic stress in colon and lung carcinoma cells. Cells. 2021;10:97. doi: 10.3390/cells10010097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohammadi S, Arefnezhad R, Danaii S, Yousefi M. New insights into the core Hippo signaling and biological macromolecules interactions in the biology of solid tumors. Biofactors. 2020;46:514–530. doi: 10.1002/biof.1634. [DOI] [PubMed] [Google Scholar]
- 10.Kim YS, Hwang KA, Go RE, Kim CW, Choi KC. Gene therapy strategies using engineered stem cells for treating gynecologic and breast cancer patients (Review) Oncol Rep. 2015;33:2107–2112. doi: 10.3892/or.2015.3846. [DOI] [PubMed] [Google Scholar]
- 11.Hotta K, Kiura K, Ueoka H, Tabata M, Fujiwara K, Kozuki T, Okada T, Hisamoto A, Tanimoto M. Effect of gefitinib (‘Iressa’, ZD1839) on brain metastases in patients with advanced non-small-cell lung cancer. Lung Cancer. 2004;46:255–261. doi: 10.1016/j.lungcan.2004.04.036. [DOI] [PubMed] [Google Scholar]
- 12.Lankheet NA, Schaake EE, Burgers SA, van Pel R, Beijnen JH, Huitema ADR, Klomp H, NEL Study Group Concentrations of erlotinib in tumor tissue and plasma in non-small-cell lung cancer patients after neoadjuvant therapy. Clin Lung Cancer. 2015;16:320–324. doi: 10.1016/j.cllc.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 13.Zitvogel L, Rusakiewicz S, Routy B, Ayyoub M, Kroemer G. Immunological off-target effects of imatinib. Nat Rev Clin Oncol. 2016;13:431–446. doi: 10.1038/nrclinonc.2016.41. [DOI] [PubMed] [Google Scholar]
- 14.Joensuu H. Treatment of inoperable gastrointestinal stromal tumor (GIST) with imatinib (Glivec, Gleevec) Med Klin (Munich) 2002;15((Suppl 1)):S28–S30. [PubMed] [Google Scholar]
- 15.Cohen C, Pop D, Icard P, Berthet JP, Venissac N, Mouroux J. Is there a place for thoracoscopic enucleation of esophageal gastrointestinal stromal tumors? Thorac Cardiovasc Surg. 2019;67:585–588. doi: 10.1055/s-0038-1670662. [DOI] [PubMed] [Google Scholar]
- 16.Chen YH, Yang SF, Yang CK, Tsai HD, Chen TH, Chou MC, Hsiao YH. Metformin induces apoptosis and inhibits migration by activating the AMPK/p53 axis and suppressing PI3K/AKT signaling in human cervical cancer cells. Mol Med Rep. 2021;23:88. doi: 10.3892/mmr.2020.11725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng L, Lv W, Zhou Y, Lin X, Yao J. Progress on the mechanism for aspirin's anti-tumor effects. Curr Drug Targets. 2021;22:105–111. doi: 10.2174/1389450121999201013152931. [DOI] [PubMed] [Google Scholar]
- 18.Pereira PMR, Mandleywala K, Ragupathi A, Lewis JS. Acute statin treatment improves antibody accumulation in EGFR- and PSMA-expressing tumors. Clin Cancer Res. 2020;26:6215–6229. doi: 10.1158/1078-0432.CCR-20-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pereira A, de Souza Lima ML, da Silva-Junior AA, Silva EDS, de Araújo Júnior RF, Martins AA, Alves JSF, de Santana Oliveira A, Ferreira LDS, de Araújo Costa ECT, et al. In vitro-in vivo availability of metformin hydrochloride-PLGA nanoparticles in diabetic rats in a periodontal disease experimental model. Pharm Biol. 2021;59:1576–1584. doi: 10.1080/13880209.2021.2002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horas K, van Herck U, Maier GS, Maus U, Harrasser N, Jakob F, Weissenberger M, Arnholdt J, Holzapfel BM, Rudert M. Does vitamin D deficiency predict tumour malignancy in patients with bone tumours? Data from a multi-center cohort analysis. J Bone Oncol. 2020;25:100329. doi: 10.1016/j.jbo.2020.100329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeng L, Holly JM, Perks CM. Effects of physiological levels of the green tea extract epigallocatechin-3-gallate on breast cancer cells. Front Endocrinol (Lausanne) 2014;5:61. doi: 10.3389/fendo.2014.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Varghese S, Samuel SM, Varghese E, Kubatka P, Büsselberg D. High glucose represses the anti-proliferative and pro-apoptotic effect of metformin in triple negative breast cancer cells. Biomolecules. 2019;9:16. doi: 10.3390/biom9010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawakita E, Yang F, Kumagai A, Takagaki Y, Kitada M, Yoshitomi Y, Ikeda T, Nakamura Y, Ishigaki Y, Kanasaki K, Koya D. Metformin mitigates DPP-4 inhibitor-induced breast cancer metastasis via suppression of mTOR signaling. Mol Cancer Res. 2021;19:61–73. doi: 10.1158/1541-7786.MCR-20-0115. [DOI] [PubMed] [Google Scholar]
- 24.Stage TB, Brøsen K, Christensen MM. A comprehensive review of drug-drug interactions with metformin. Clin Pharmacokinet. 2015;54:811–824. doi: 10.1007/s40262-015-0270-6. [DOI] [PubMed] [Google Scholar]
- 25.Bian X, Jiang L, Gan Z, Zhang L, Cai L, Hu X. A glimepiride-metformin multidrug crystal: Synthesis, crystal structure analysis, and physicochemical properties. Molecules. 2019;24:3786. doi: 10.3390/molecules24203786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scheen AJ. Clinical pharmacokinetics of metformin. Clin Pharmacokinet. 1996;30:359–371. doi: 10.2165/00003088-199630050-00003. [DOI] [PubMed] [Google Scholar]
- 27.Palumbo PJ. Metformin: Effects on cardiovascular risk factors in patients with non-insulin-dependent diabetes mellitus. J Diabetes Complications. 1998;12:110–119. doi: 10.1016/S1056-8727(97)00053-6. [DOI] [PubMed] [Google Scholar]
- 28.Mbara KC, Mato PE, Driver C, Nzuza S, Mkhombo NT, Gcwensa SK, Mcobothi EN, Owira PM. Metformin turns 62 in pharmacotherapy: Emergence of non-glycaemic effects and potential novel therapeutic applications. Eur J Pharmacol. 2021;898:173934. doi: 10.1016/j.ejphar.2021.173934. [DOI] [PubMed] [Google Scholar]
- 29.Notaro ALG, Neto FTL. The use of metformin in women with polycystic ovary syndrome: An updated review. J Assist Reprod Genet. 2022;14:573–579. doi: 10.1007/s10815-022-02429-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu Y, Yu L, Cheng N, Liu X, Fang C, Liu S, Zhu L. Exercise alleviates the apolipoprotein A5-toll-like receptor 4 axis impairment in mice with high-fat diet-induced non-alcoholic steatohepatitis. Front Physiol. 2021;12:783341. doi: 10.3389/fphys.2021.783341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kalariya NM, Shoeb M, Ansari NH, Srivastava SK, Ramana KV. Antidiabetic drug metformin suppresses endotoxin-induced uveitis in rats. Invest Ophthalmol Vis Sci. 2012;53:3431–3440. doi: 10.1167/iovs.12-9432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Agostini F, Masato A, Bubacco L, Bisaglia M. Metformin repurposing for parkinson disease therapy: Opportunities and challenges. Int J Mol Sci. 2021;23:398. doi: 10.3390/ijms23010398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma X, Xiao W, Li H, Pang P, Xue F, Wan L, Pei L, Yan H. Metformin restores hippocampal neurogenesis and learning and memory via regulating gut microbiota in the obese mouse model. Brain Behav Immun. 2021;95:68–83. doi: 10.1016/j.bbi.2021.02.011. [DOI] [PubMed] [Google Scholar]
- 34.Kim JW, Choe JY, Park SH. Metformin and its therapeutic applications in autoimmune inflammatory rheumatic disease. Korean J Intern Med. 2022;37:13–26. doi: 10.3904/kjim.2021.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siskind D, Friend N, Russell A, McGrath JJ, Lim C, Patterson S, Flaws D, Stedman T, Moudgil V, Sardinha S. CoMET: A protocol for a randomised controlled trial of co-commencement of METformin as an adjunctive treatment to attenuate weight gain and metabolic syndrome in patients with schizophrenia newly commenced on clozapine. BMJ Open. 2018;8:e021000. doi: 10.1136/bmjopen-2017-021000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taylor A, Siddiqui MK, Ambery P, Armisen J, Challis BG, Haefliger C, Pearson ER, Doney ASF, Dillon JF, Palmer CNA. Metabolic dysfunction-related liver disease as a risk factor for cancer. BMJ Open Gastroenterol. 2022;9:e000817. doi: 10.1136/bmjgast-2021-000817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sousa AP, Costa R, Alves MG, Soares R, Baylina P, Fernandes R. The impact of metabolic syndrome and type 2 diabetes mellitus on prostate cancer. Front Cell Dev Biol. 2022;10:843458. doi: 10.3389/fcell.2022.843458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang J, Nishihara R, Zhang X, Ogino S, Qian ZR. Energy sensing pathways: Bridging type 2 diabetes and colorectal cancer? J Diabetes Complications. 2017;31:1228–1236. doi: 10.1016/j.jdiacomp.2017.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kabat GC, Kim M, Chlebowski RT, Khandekar J, Ko MG, McTiernan A, Neuhouser ML, Parker DR, Shikany JM, Stefanick ML, et al. A longitudinal study of the metabolic syndrome and risk of postmenopausal breast cancer. Cancer Epidemiol Biomarkers Prev. 2009;18:2046–2053. doi: 10.1158/1055-9965.EPI-09-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang X, Ding S. The biological and pharmacological connections between diabetes and various types of cancer. Pathol Res Pract. 2021;227:153641. doi: 10.1016/j.prp.2021.153641. [DOI] [PubMed] [Google Scholar]
- 41.Cook A, Cowan C. StemBook. Harvard Stem Cell Institute; Cambridge, MA: 2008. Adipose. [PubMed] [Google Scholar]
- 42.Menendez JA, Vazquez-Martin A, Ortega FJ, Fernandez-Real JM. Fatty acid synthase: Association with insulin resistance, type 2 diabetes, and cancer. Clin Chem. 2009;55:425–438. doi: 10.1373/clinchem.2008.115352. [DOI] [PubMed] [Google Scholar]
- 43.LeRoith D, Novosyadlyy R, Gallagher EJ, Lann D, Vijayakumar A, Yakar S. Obesity and type 2 diabetes are associated with an increased risk of developing cancer and a worse prognosis; epidemiological and mechanistic evidence. Exp Clin Endocrinol Diabetes. 2008;116((Suppl 1)):S4–S6. doi: 10.1055/s-2008-1081488. [DOI] [PubMed] [Google Scholar]
- 44.Jayalath VH, Ireland C, Fleshner NE, Hamilton RJ, Jenkins DJ. The relationship between metformin and serum prostate-specific antigen levels. Prostate. 2016;76:1445–1453. doi: 10.1002/pros.23228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park JH, Hong JY, Park YS, Kang G, Han K, Park JO. Association of prediabetes, diabetes, and diabetes duration with biliary tract cancer risk: A nationwide cohort study. Metabolism. 2021;123:154848. doi: 10.1016/j.metabol.2021.154848. [DOI] [PubMed] [Google Scholar]
- 46.Zhang AMY, Chu KH, Daly BF, Ruiter T, Dou Y, Yang JCC, de Winter TJJ, Chhuor J, Wang S, Flibotte S, et al. Effects of hyperinsulinemia on pancreatic cancer development and the immune microenvironment revealed through single-cell transcriptomics. Cancer Metab. 2022;10:5. doi: 10.1186/s40170-022-00282-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu L, Koo S, McPherson S, Hull MA, Rees CJ, Sharp L. Systematic review and meta-analysis: Associations between metabolic syndrome and colorectal neoplasia outcomes. Colorectal Dis. 2022;13:16092. doi: 10.1111/codi.16092. [DOI] [PubMed] [Google Scholar]
- 48.Nayak S, Rathore V, Bharati J, Sahu KK. Extending the ambit of SGLT2 inhibitors beyond diabetes: A review of clinical and preclinical studies on non-diabetic kidney disease. Expert Rev Clin Pharmacol. 2021;14:1513–1526. doi: 10.1080/17512433.2021.2028620. [DOI] [PubMed] [Google Scholar]
- 49.Lam BQ, Srivastava R, Morvant J, Shankar S, Srivastava RK. Association of diabetes mellitus and alcohol abuse with cancer: Molecular mechanisms and clinical significance. Cells. 2021;10:3077. doi: 10.3390/cells10113077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Srinivasan M, Arzoun H, Gk LB, Thangaraj SR. A systematic review: Does insulin resistance affect the risk and survival outcome of breast cancer in women? Cureus. 2022;14:e21712. doi: 10.7759/cureus.21712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lemon LS, Orr B, Modugno F, Buckanovich RJ, Coffman L, Edwards RP, Taylor S. Metformin and survival: Is there benefit in a cohort limited to diabetic women with endometrial, breast, or ovarian cancer? Gynecol Oncol. 2022;165:60–66. doi: 10.1016/j.ygyno.2022.01.022. [DOI] [PubMed] [Google Scholar]
- 52.Choi E, Kikuchi S, Gao H, Brodzik K, Nassour I, Yopp A, Singal AG, Zhu H, Yu H. Mitotic regulators and the SHP2-MAPK pathway promote IR endocytosis and feedback regulation of insulin signaling. Nat Commun. 2019;10:1473. doi: 10.1038/s41467-019-09318-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang L, Zhang B, Zheng W, Kang M, Chen Q, Qin W, Li C, Zhang Y, Shao Y, Wu Y. Exosomes derived from pancreatic cancer cells induce insulin resistance in C2C12 myotube cells through the PI3K/Akt/FoxO1 pathway. Sci Rep. 2017;7:5384. doi: 10.1038/s41598-017-05541-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang X, Huang M, Zhang Q, Chen J, Li J, Han Q, Zhang L, Li J, Liu S, Ma Y, et al. Metformin antagonizes ovarian cancer cells malignancy through MSLN mediated IL-6/STAT3 signaling. Cell Transplant. 2021;30:9636897211027819. doi: 10.1177/09636897211027819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rho SB, Byun HJ, Kim BR, Lee CH. Knockdown of LKB1 sensitizes endometrial cancer cells via AMPK activation. Biomol Ther. 2021;29:650–657. doi: 10.4062/biomolther.2021.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheng T, Wang C, Lu Q, Cao Y, Yu W, Li W, Liu B, Gao X, Lü J, Pan X. Metformin inhibits the tumor-promoting effect of low-dose resveratrol, and enhances the anti-tumor activity of high-dose resveratrol by increasing its reducibility in triple negative breast cancer. Free Radic Biol Med. 2022;180:108–120. doi: 10.1016/j.freeradbiomed.2022.01.010. [DOI] [PubMed] [Google Scholar]
- 57.Eze C, Belka C, Manapov F. Forging a path for metformin use in inoperable locally advanced non-small cell lung cancer. JAMA Oncol. 2021;7:1341–1342. doi: 10.1001/jamaoncol.2021.2316. [DOI] [PubMed] [Google Scholar]
- 58.Mazurek M, Litak J, Kamieniak P, Kulesza B, Jonak K, Baj J, Grochowski C. Metformin as potential therapy for high-grade glioma. Cancers (Basel) 2020;12:210. doi: 10.3390/cancers12010210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao A, Xiao H, Zhu Y, Liu S, Zhang S, Yang Z, Du L, Li X, Niu X, Wang C, et al. Omentin-1: A newly discovered warrior against metabolic related diseases. Expert Opin Ther Targets. 2022;10:275–289. doi: 10.1080/14728222.2022.2037556. [DOI] [PubMed] [Google Scholar]
- 60.Eibl G, Rozengurt E. Metformin: Review of epidemiology and mechanisms of action in pancreatic cancer. Cancer Metastasis Rev. 2021;40:865–878. doi: 10.1007/s10555-021-09977-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang CP, Kuhn J, Shah DP, Schmidt S, Lam YWF, MacCarthy D, Tenner L, Ramirez AG. Metformin modifies disparity in hepatocellular carcinoma incidence in men with type 2 diabetes but without chronic liver diseases. Cancer Med. 2019;8:3206–3215. doi: 10.1002/cam4.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Erkinantti S, Marttila M, Sund R, Arffman M, Urpilainen E, Puistola U, Hautakoski A, Karihtala P, Läärä E, Jukkola A. Association of metformin, other antidiabetic medications, and statins with incidence of colon cancer in patients with type 2 diabetes. Clin Colorectal Cancer. 2021;20:e113–e119. doi: 10.1016/j.clcc.2020.11.003. [DOI] [PubMed] [Google Scholar]
- 63.Febbraro T, Lengyel E, Romero IL. Old drug, new trick: Repurposing metformin for gynecologic cancers? Gynecol Oncol. 2014;135:614–621. doi: 10.1016/j.ygyno.2014.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu H, Chen K, Jia X, Tian Y, Dai Y, Li D, Xie J, Tao M, Mao Y. Metformin use is associated with better survival of breast cancer patients with diabetes: A meta-analysis. Oncologist. 2015;20:1236–1244. doi: 10.1634/theoncologist.2015-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang Q, Ma X, Long J, Du X, Pan B, Mao H. Metformin and survival of women with breast cancer: A meta-analysis of randomized controlled trials. J Clin Pharm Ther. 2021;16:13500. doi: 10.1111/jcpt.13500. [DOI] [PubMed] [Google Scholar]
- 66.Tseng CH. Metformin significantly reduces incident prostate cancer risk in Taiwanese men with type 2 diabetes mellitus. Eur J Cancer. 2014;50:2831–2837. doi: 10.1016/j.ejca.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 67.Brancher S, Støer NC, Weiderpass E, Damhuis RAM, Johannesen TB, Botteri E, Strand TE. Metformin use and lung cancer survival: A population-based study in Norway. Br J Cancer. 2021;124:1018–1025. doi: 10.1038/s41416-020-01186-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tseng CH. Metformin reduces thyroid cancer risk in taiwanese patients with type 2 diabetes. PLoS One. 2014;9:e109852. doi: 10.1371/journal.pone.0109852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Agrawal S, Patel P, Agrawal A, Makhijani N, Markert R, Deidrich W. Metformin use and the risk of esophageal cancer in Barrett esophagus. South Med J. 2014;107:774–779. doi: 10.14423/SMJ.0000000000000212. [DOI] [PubMed] [Google Scholar]
- 70.Becker C, Jick SS, Meier CR, Bodmer M. Metformin and the risk of endometrial cancer: A case-control analysis. Gynecol Oncol. 2013;129:565–569. doi: 10.1016/j.ygyno.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 71.Bowker SL, Majumdar SR, Veugelers P, Johnson JA. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care. 2006;29:254–258. doi: 10.2337/diacare.29.02.06.dc05-1558. [DOI] [PubMed] [Google Scholar]
- 72.Gandini S, Puntoni M, Heckman-Stoddard BM, Dunn BK, Ford L, DeCensi A, Szabo E. Metformin and cancer risk and mortality: A systematic review and meta-analysis taking into account biases and confounders. Cancer Prev Res (Phila) 2014;7:867–885. doi: 10.1158/1940-6207.CAPR-13-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu Z, Wu L, Zou L, Wang M, Liu X. Metformin induces myeloma cells necrosis and apoptosis and it is considered for therapeutic use. J Chemother. 2022;15:1–11. doi: 10.1080/1120009X.2022.2062895. [DOI] [PubMed] [Google Scholar]
- 74.Teixeira SF, Guimarães IDS, Madeira KP, Daltoé RD, Silva IV, Rangel LB. Metformin synergistically enhances antiproliferative effects of cisplatin and etoposide in NCI-H460 human lung cancer cells. J Bras Pneumol. 2013;39:644–649. doi: 10.1590/S1806-37132013000600002. (In English, Portuguese) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lin F, Yan W, Wen T, Wu GY. Metformin induces apoptosis in hepatocellular carcinoma Huh-7 cells in vitro and its mechanism. Zhonghua Zhong Liu Za Zhi. 2013;35:742–746. (In Chinese) [PubMed] [Google Scholar]
- 76.Huang X, Hong X, Wang J, Sun T, Yu T, Yu Y, Fang J, Xiong H. Metformin elicits antitumour effect by modulation of the gut microbiota and rescues Fusobacterium nucleatum-induced colorectal tumourigenesis. EBioMedicine. 2020;61:103037. doi: 10.1016/j.ebiom.2020.103037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Phoenix KN, Vumbaca F, Claffey KP. Therapeutic metformin/AMPK activation promotes the angiogenic phenotype in the ERalpha negative MDA-MB-435 breast cancer model. Breast Cancer Res Treat. 2009;113:101–111. doi: 10.1007/s10549-008-9916-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Anisimov VN, Egormin PA, Piskunova TS, Popovich IG, Tyndyk ML, Yurova MN, Zabezhinski MA, Anikin IV, Karkach AS, Romanyukha AA. Metformin extends life span of HER-2/neu transgenic mice and in combination with melatonin inhibits growth of transplantable tumors in vivo. Cell Cycle. 2010;9:188–197. doi: 10.4161/cc.9.1.10407. [DOI] [PubMed] [Google Scholar]
- 79.Memmott RM, Mercado JR, Maier CR, Kawabata S, Fox SD, Dennis PA. Metformin prevents tobacco carcinogen-induced lung tumorigenesis. Cancer Prev Res (Phila) 2010;3:1066–1076. doi: 10.1158/1940-6207.CAPR-10-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang S, Lachance BB, Mattson MP, Jia X. Glucose metabolic crosstalk and regulation in brain function and diseases. Prog Neurobiol. 2021;204:102089. doi: 10.1016/j.pneurobio.2021.102089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dai JZ, Wang YJ, Chen CH, Tsai IL, Chao YC, Lin CW. YAP dictates mitochondrial redox homeostasis to facilitate obesity-associated breast cancer progression. Adv Sci. 2022;18:e2103687. doi: 10.1002/advs.202103687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fleming JB, Gonzalez RJ, Petzel MQ, Lin E, Morris JS, Gomez H, Lee JE, Crane CH, Pisters PWT, Evans DB. Influence of obesity on cancer-related outcomes after pancreatectomy to treat pancreatic adenocarcinoma. Arch Surg. 2009;144:216–221. doi: 10.1001/archsurg.2008.580. [DOI] [PubMed] [Google Scholar]
- 83.Purcell SA, Oliveira CLP, Mackenzie M, Robson P, Lewis J, Prado CM. Body composition and prostate cancer risk: A systematic review of observational studies. Adv Nutr. 2021;16:153. doi: 10.1093/advances/nmab153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gong L, Wang Z, Zhang Z. Sestrin2 as a potential target for regulating metabolic-related diseases. Front Endocrinol (Lausanne) 2021;12:751020. doi: 10.3389/fendo.2021.751020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zell JA, McLaren CE, Morgan TR, Lawson MJ, Rezk S, Albers CG, Chen WP, Carmichael JC, Chung J, Richmond E, et al. A phase IIa trial of metformin for colorectal cancer risk reduction among individuals with history of colorectal adenomas and elevated body mass index. Cancer Prev Res (Phila) 2020;13:203–212. doi: 10.1158/1940-6207.CAPR-18-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Saini N, Yang X. Metformin as an anti-cancer agent: Actions and mechanisms targeting cancer stem cells. Acta Biochim Biophys Sin (Shanghai) 2018;50:133–143. doi: 10.1093/abbs/gmx106. [DOI] [PubMed] [Google Scholar]
- 87.Bang S, Chen Y, Ahima RS, Kim SF. Convergence of IPMK and LKB1-AMPK signaling pathways on metformin action. Mol Endocrinol. 2014;28:1186–1193. doi: 10.1210/me.2014-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Najafov A, Luu HS, Mookhtiar AK, Mifflin L, Xia HG, Amin PP, Ordureau A, Wang H, Yuan J. RIPK1 promotes energy sensing by the mTORC1 pathway. Mol Cell. 2021;81:370–385. doi: 10.1016/j.molcel.2020.11.008. [DOI] [PubMed] [Google Scholar]
- 89.Kim BH, Han S, Lee H, Park CH, Chung YM, Shin K, Lee HG, Ye SK. Metformin enhances the anti-adipogenic effects of atorvastatin via modulation of STAT3 and TGF-β/Smad3 signaling. Biochem Biophys Res Commun. 2015;456:173–178. doi: 10.1016/j.bbrc.2014.11.054. [DOI] [PubMed] [Google Scholar]
- 90.Xu S, Lam SK, Cheng PN, Ho JC. Recombinant human arginase induces apoptosis through oxidative stress and cell cycle arrest in small cell lung cancer. Cancer Sci. 2018;109:3471–3482. doi: 10.1111/cas.13782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cameron AR, Logie L, Patel K, Erhardt S, Bacon S, Middleton P, Harthill J, Forteath C, Coats JT, Kerr C, et al. Metformin selectively targets redox control of complex I energy transduction. Redox Biol. 2018;14:187–197. doi: 10.1016/j.redox.2017.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Feng Y, Ke C, Tang Q, Dong H, Zheng X, Lin W, Ke J, Huang J, Yeung SCJ, Zhang H. Metformin promotes autophagy and apoptosis in esophageal squamous cell carcinoma by downregulating Stat3 signaling. Cell Death Dis. 2014;5:e1088. doi: 10.1038/cddis.2014.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Janjetovic K, Vucicevic L, Misirkic M, Vilimanovich U, Tovilovic G, Zogovic N, Nikolic Z, Jovanovic S, Bumbasirevic V, Trajkovic V, Harhaji-Trajkovic L. Metformin reduces cisplatin-mediated apoptotic death of cancer cells through AMPK-independent activation of Akt. Eur J Pharmacol. 2011;651:41–50. doi: 10.1016/j.ejphar.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 94.Athreya AP, Kalari KR, Cairns J, Gaglio AJ, Wills QF, Niu N, Weinshilboum R, Iyer RK, Wang L. Model-based unsupervised learning informs metformin-induced cell-migration inhibition through an AMPK-independent mechanism in breast cancer. Oncotarget. 2017;8:27199–27215. doi: 10.18632/oncotarget.16109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yenmiş G, Beşli N, Saraç EY, Emre FS, Şenol K, Kanıgür G. Metformin promotes apoptosis in primary breast cancer cells by downregulation of cyclin D1 and upregulation of P53 through an AMPK-alpha independent mechanism. Turk J Med Sci. 2021;51:826–834. doi: 10.3906/sag-1908-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Calza G, Nyberg E, Mäkinen M, Soliymani R, Cascone A, Lindholm D, Barborini E, Baumann M, Lalowski M, Eriksson O. Lactate-induced glucose output is unchanged by metformin at a therapeutic concentration-a mass spectrometry imaging study of the perfused rat liver. Front Pharmacol. 2018;9:141. doi: 10.3389/fphar.2018.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Thakur S, Daley B, Gaskins K, Vasko VV, Boufraqech M, Patel D, Sourbier C, Reece J, Cheng SY, Kebebew E, et al. Metformin targets mitochondrial glycerophosphate dehydrogenase to control rate of oxidative phosphorylation and growth of thyroid cancer in vitro and in vivo. Clin Cancer Res. 2018;24:4030–4043. doi: 10.1158/1078-0432.CCR-17-3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Salani B, Marini C, Rio AD, Ravera S, Massollo M, Orengo AM, Amaro A, Passalacqua M, Maffioli S, Pfeffer U, et al. Metformin impairs glucose consumption and survival in Calu-1 cells by direct inhibition of hexokinase-II. Sci Rep. 2013;3:2070. doi: 10.1038/srep02070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jiang Y, Xu D, Song H, Qiu B, Tian D, Li Z, Ji Y, Wang J. Inflammation and nutrition-based biomarkers in the prognosis of oesophageal cancer: A systematic review and meta-analysis. BMJ Open. 2021;11:e048324. doi: 10.1136/bmjopen-2020-048324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.El-Tanani M, Al Khatib AO, Aladwan SM, Abuelhana A, McCarron PA, Tambuwala MM. Importance of STAT3 signalling in cancer, metastasis and therapeutic interventions. Cell Signal. 2022;92:110275. doi: 10.1016/j.cellsig.2022.110275. [DOI] [PubMed] [Google Scholar]
- 101.Zahra MH, Afify SM, Hassan G, Nawara HM, Kumon K, Seno A, Seno M. Metformin suppresses self-renewal and stemness of cancer stem cell models derived from pluripotent stem cells. Cell Biochem Funct. 2021;39:896–907. doi: 10.1002/cbf.3661. [DOI] [PubMed] [Google Scholar]
- 102.Higurashi T, Hosono K, Takahashi H, Komiya Y, Umezawa S, Sakai E, Uchiyama T, Taniguchi L, Hata Y, Uchiyama S, et al. Metformin for chemoprevention of metachronous colorectal adenoma or polyps in post-polypectomy patients without diabetes: A multicentre double-blind, placebo-controlled, randomised phase 3 trial. Lancet Oncol. 2016;17:475–483. doi: 10.1016/S1470-2045(15)00565-3. [DOI] [PubMed] [Google Scholar]
- 103.Arrieta O, Zatarain-Barrón ZL, Turcott JG, Barrón F, Yendamuri S, Cardona AF, Rosell R. Association of BMI with benefit of metformin plus epidermal growth factor receptor-tyrosine kinase inhibitors in patients with advanced lung adenocarcinoma: A secondary analysis of a phase 2 randomized clinical trial. JAMA Oncol. 2022;8:477–479. doi: 10.1001/jamaoncol.2021.7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pusceddu S, Vernieri C, Di Maio M, Prinzi N, Torchio M, Corti F, Coppa J, Buzzoni R, Bartolomeo MD, Milione M, et al. Impact of diabetes and metformin use on enteropancreatic neuroendocrine tumors: Post hoc analysis of the CLARINET study. Cancers (Basel) 2021;14:69. doi: 10.3390/cancers14010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang S, Lin Y, Xiong X, Wang L, Guo Y, Chen Y, Chen S, Wang G, Lin P, Chen H, et al. Low-dose metformin reprograms the tumor immune microenvironment in human esophageal cancer: Results of a phase II clinical trial. Clin Cancer Res. 2020;26:4921–4932. doi: 10.1158/1078-0432.CCR-20-0113. [DOI] [PubMed] [Google Scholar]
- 106.Maruthur NM, Tseng E, Hutfless S, Wilson LM, Suarez-Cuervo C, Berger Z, Chu Y, Iyoha E, Segal JB, Bolen S. Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes: A systematic review and meta-analysis. Ann Intern Med. 2016;164:740–751. doi: 10.7326/M15-2650. [DOI] [PubMed] [Google Scholar]
- 107.Wink KC, Belderbos JS, Dieleman EM, Rossi M, Rasch CRN, Damhuis RAM, Houben RMA, Troost EGC. Improved progression free survival for patients with diabetes and locally advanced non-small cell lung cancer (NSCLC) using metformin during concurrent chemoradiotherapy. Radiother Oncol. 2016;118:453–459. doi: 10.1016/j.radonc.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 108.Martin MJ, Hayward R, Viros A, Marais R. Metformin accelerates the growth of BRAF V600E-driven melanoma by upregulating VEGF-A. Cancer Discov. 2012;2:344–355. doi: 10.1158/2159-8290.CD-11-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


